Submitted:
07 March 2024
Posted:
26 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. MLT as an Anti-Oxidant
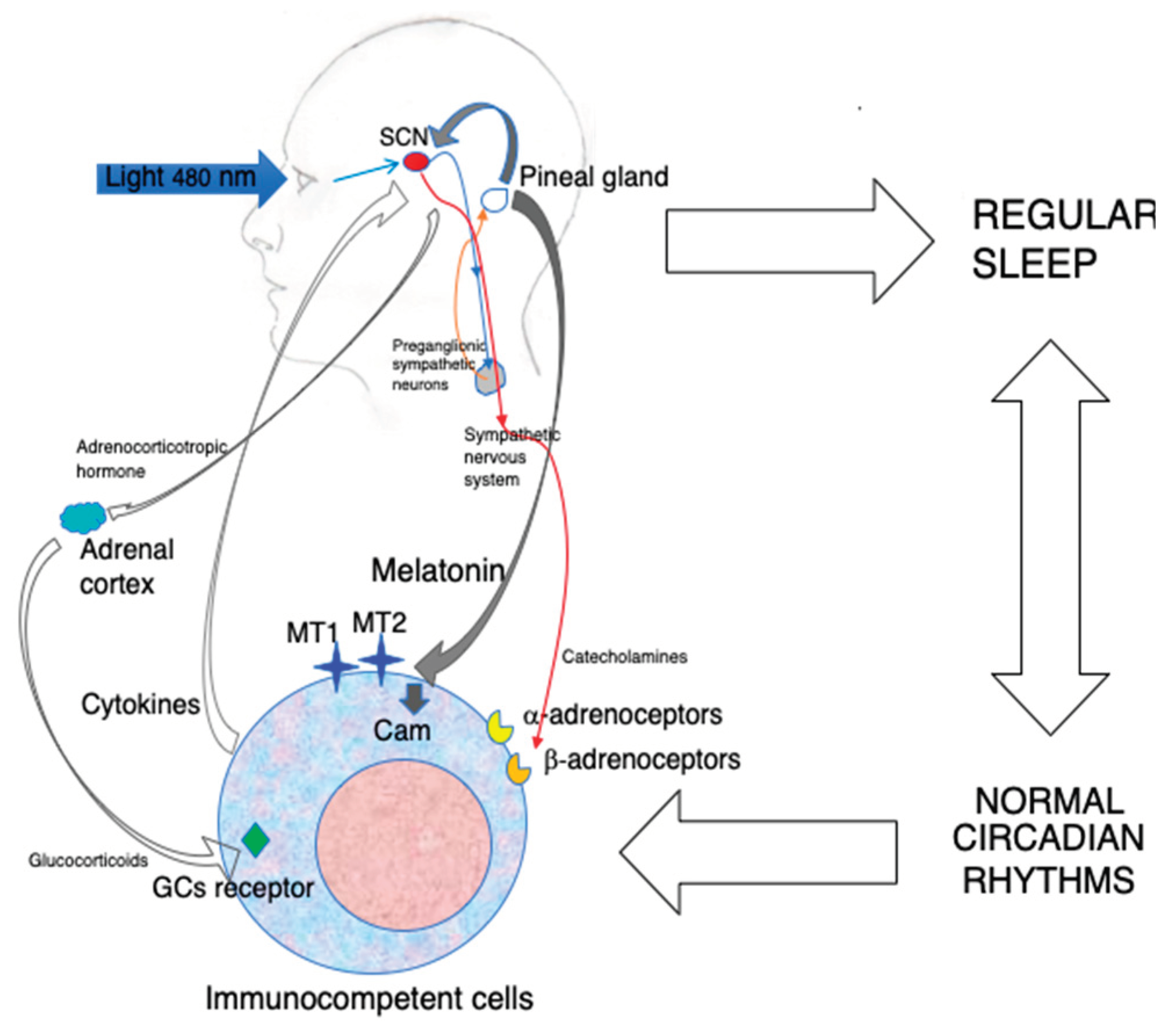
3. Melatonin and the Immune System
4. MLT and Viral Infections
5. MLT and Bacterial Infections
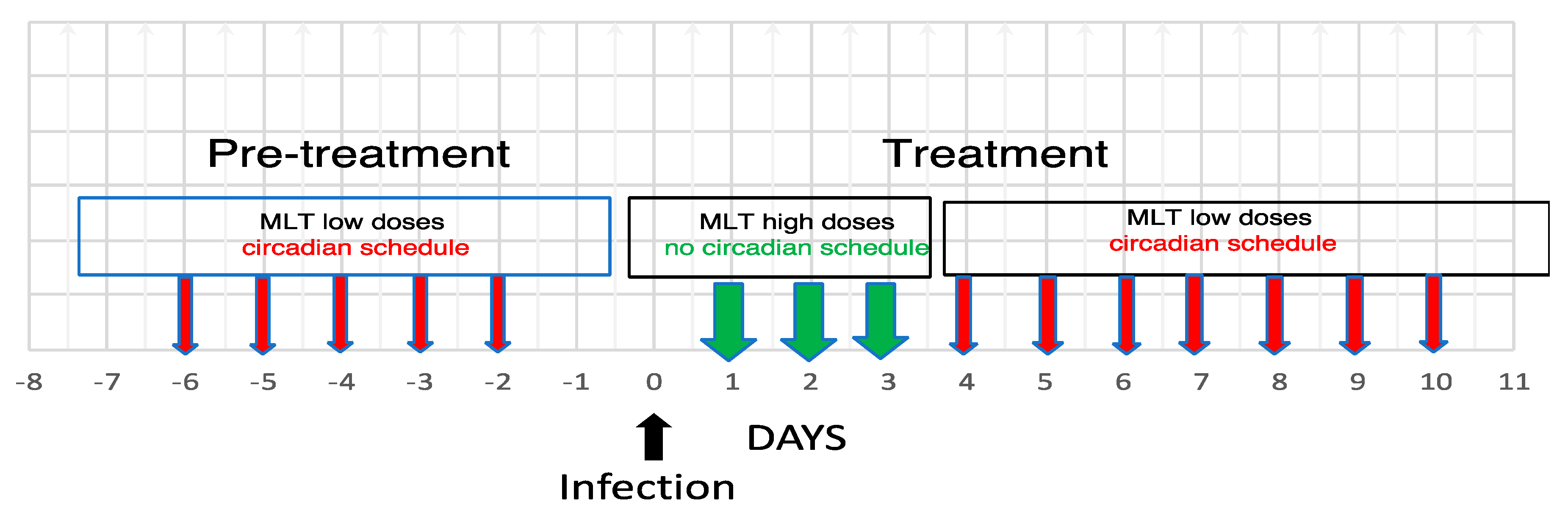
6. MLT and Parasitic Infections
6.1. Malaria
6.2. Trypanosomiasis
6.2. Leishmaniases
6.2. Toxoplasmosis
6. Conclusion
Conflicts of Interest
References
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front Endocrinoly 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Diaz-Casado, M.; Lima-Cabello, E.; Lopez, L.; Rosales-Corral, S.; Tan, D.; Reiter, R. Extra-pineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Kennaway, D. J. The mammalian gastro-intestinal tract is a NOT a major extra-pineal source of melatonin. J Pineal Res 2023, 75, e12906. [Google Scholar] [CrossRef] [PubMed]
- Carretero, V.J.; Ramos, E.; Segura-Chama, P.; Hernández, A.; Baraibar, A.M.; Álvarez-Merz, I.; Muñoz, F.L.; Egea, J.; Solís, J.M.; Romero, A.; Hernández-Guijo, J.M. Non-Excitatory Amino Acids, Melatonin, and Free Radicals: Examining the Role in Stroke and Aging. Antioxidants (Basel, Switzerland) 2023, 12, 1844. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: exceeding expectations. Physiology (Bethesda, Md.), 2014, 29, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Kalsbeek, A.; Yi, C.X.; Cailotto, C.; la Fleur, S.E.; Fliers, E.; Buijs, R.M. (2011). Mammalian clock output mechanisms. Essays in biochemistry 2014, 49, 137–151. [Google Scholar] [CrossRef]
- Reppert, S.M.; Weaver, D.R.; Ebisawa, T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 1994, 13, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R.; Ebisawa, T.; Mahle, C.D.; Kolakowski, L.F.; Jr. Cloning of a melatonin-related receptor from human pituitary. FEBS letters 1996, 386, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Jockers, R.; Maurice, P.; Boutin, J.A.; Delagrange, P. Melatonin receptors, heterodimerization, signal transduction and binding sites: what's new? British journal of pharmacology, 2008, 154, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Levoye, A.; Dam, J.; Ayoub, M.A.; Guillaume, J.L.; Couturier, C.; Delagrange, P.; Jockers, R. The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. The EMBO J 2006, 25, 3012–3023. [Google Scholar] [CrossRef] [PubMed]
- Boutin, J. A. Quinone reductase 2 as a promising target of melatonin therapeutic actions. Expert opinion on therapeutic targets 2016, 20, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Argueta, J.; Solís-Chagoyán, H.; Estrada-Reyes, R.; Constantino-Jonapa, L.A.; Oikawa-Sala, J.; Velázquez-Moctezuma, J.; Benítez-King, G. Further Evidence of the Melatonin Calmodulin Interaction: Effect on CaMKII Activity. Int J Mol Sci 2022, 23, 2479. [Google Scholar] [CrossRef] [PubMed]
- Targhazeh, N.; Reiter, R.J.; Rahimi, M.; Qujeq, D.; Yousefi, T.; Shahavi, M.H.; Mir, S.M. Oncostatic activities of melatonin: Roles in cell cycle, apoptosis, and autophagy. Biochimie 2022, 200, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Kang, J.; Fan, W.; He, H.; Huang, F. ROR: Nuclear Receptor for Melatonin or Not? Molecules (Basel, Switzerland) 2021, 26, 2693. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: under promises but over delivers. J Pin Res 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Reiter, R.J. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef] [PubMed]
- Boutin, J.A.; Kennaway, D.J.; Jockers, R. Melatonin: Facts, Extrapolations and Clinical Trials. Biomolecules 2023, 13, 943. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Pandi-Perumal, S.R. Melatonin, a potent agent in antioxidative defense: actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutrition & metabolism 2005, 2, 22. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Andolina, G.; Cannata, A.; Costanzo, M.G.; Rizzo, V.; Currò, M.; Ientile, R.; Caccamo, D. Is Melatonin the Cornucopia of the 21st Century? Antioxidants 2020, 9, 1088. [Google Scholar] [CrossRef] [PubMed]
- Csaba, G.; Bodoky, M.; Fischer, J.; Acs, T. The effect of pinealectomy and thymectomy on the immune capacity of the rat. Experientia 1966, 22, 168–169. [Google Scholar] [CrossRef] [PubMed]
- Janković, B.D.; Isaković, K.; Petrović, S. Effect of pinealectomy on immune reactions in the rat. Immunology 1970, 1970 18, 1–6. [Google Scholar]
- Maestroni, G.J.; Conti, A.; Pierpaoli, W. Role of the pineal gland in immunity. Circadian synthesis and release of melatonin modulates the antibody response and antagonizes the immunosuppressive effect of corticosterone. J. Neuroimmunol. 1986, 13, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, G.J.; Conti, A.; Pierpaoli, W. Role of the pineal gland in immunity: II. Melatonin enhances the antibody response via an opiatergic mechanism. Clinical Exp Immunol 1987, 68, 384–391. [Google Scholar]
- Maestroni, G.J.; Conti, A.; Pierpaoli, W. Role of the pineal gland in immunity. III. Melatonin antagonizes the immunosuppressive effect of acute stress via an opiatergic mechanism. Immunology 1988, 63, 465–469. [Google Scholar] [PubMed]
- Moore, C.B.; Siopes, T.D. Melatonin enhances cellular and humoral immune responses in the Japanese quail (Coturnix coturnix japonica) via an opiatergic mechanism. Gen Comp Endocrinol 2003, 131, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Majewski, P.; Dziwinski, T.; Pawlak, J.; Waloch, M.; Skwarlo-Sonta, K. (2005). Anti-inflammatory and opioid-mediated effects of melatonin on experimental peritonitis in chickens. Life Sci 2005, 76, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Liebmann, P.M.; Wölfler, A.; Felsner, P.; Hofer, D.; Schauenstein, K. Melatonin and the immune system. Intern arch aller Immunol 1997, 112, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Calvo, J.R.; Karbownik, M.; Qi, W.; Tan, D.X. Melatonin and its relation to the immune system and inflammation. Annals NY Acad Sci 2000, 917, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.B.; Siopes, T.D. Melatonin enhances cellular and humoral immune responses in the Japanese quail (Coturnix coturnix japonica) via an opiatergic mechanism. Gen Comp Endocrinol 2003, 131, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Esquifino, A.I.; Srinivasan, V.; Pandi-Perumal, S.R. Melatonin and the immune system in aging. Neuroimmunomodulation 2008, 15, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: buffering the immune system. Int J Mol Sci 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neuro-Chirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int J Mol Sci 2019, 20, 1223. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.; Murray, P.J. Tryptophan and indole metabolism in immune regulation. Curr Opin Immunol 2021, 70, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, M.; Moazamiyanfar, R.; Dakkali, M.S.; Rezaei, S.; Rastegar-Pouyani, N.; Jafarzadeh, E.; Mouludi, K.; Khodamoradi, E.; Taeb, S.; Najafi, M. Modulation of the immune system by melatonin; implications for cancer therapy. Int Immunopharmacol 2022, 108, 108890. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Redox Biology of Melatonin: Discriminating Between Circadian and Noncircadian Functions. Antioxid Redox Signal 2022, 37, 704–725. [Google Scholar] [CrossRef] [PubMed]
- García-García, A.; Méndez-Ferrer, S. The Autonomic Nervous System Pulls the Strings to Coordinate Circadian HSC Functions. Front Immunol. 20 May. [CrossRef]
- Shimba, A.; Ikuta, K. Glucocorticoids Regulate Circadian Rhythm of Innate and Adaptive Immunity. Front Immunol 2020, 11, 2143. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian control of the immune system. Nat Rev Immunol 2013, 13, 190–198. [Google Scholar] [CrossRef]
- Holtkamp, S.J.; Ince, L.M.; Barnoud, C.; Schmitt, M.T.; Sinturel, F.; Pilorz, V.; Pick, R.; Jemelin, S.; Mühlstädt, M.; Boehncke, W.H.; Weber, J.; Laubender, D.; Philippou-Massier, J.; Chen, C.S.; Holtermann, L.; Vestweber, D.; Sperandio, M.; Schraml, B.U.; Halin, C.; Dibner, C.; … Scheiermann, C. Circadian clocks guide dendritic cells into skin lymphatics. Nat Immunol 2021, 22, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Pevet, P.; Challet, E. Melatonin: both master clock output and internal time-giver in the circadian clocks network. J Physiol Paris 2011, 105, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.C.; Pandi-Perumal, S.R.; Esquifino, A.I.; Cardinali, D.P.; Maestroni, G.J. The role of melatonin in immuno-enhancement: potential application in cancer. Intern J Exp Pathol 2006, 87, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Csaba, G. The pineal regulation of the immune system: 40 years since the discovery. Acta Microbiol Immunol Hung 2013, 60, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G. Melatonin, BAG-1 and cortisol circadian interactions in tumor pathogenesis and patterned immune responses. Expl Target Antitumor Ther 2023, 4, 962–993. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Haldar, C. Physiological crosstalk between melatonin and glucocorticoid receptor modulates T-cell mediated immune responses in a wild tropical rodent, Funambulus pennanti. J Steroid Biochem Mol Bio 2013, 2013 134, 23–36. [Google Scholar] [CrossRef]
- Hardeland, R. (2019). Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int J Mol Sci 2019, 20, 1223. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, S.Y.; Sun, J.H.; Zhang, H.C.; Cai, Q.L.; Gao, C.; Li, L.; Cao, J.; Xu, F.; Zhou, Y.; Guan, C.X.; Jin, S.W.; Deng, J.; Fang, X.M.; Jiang, J.X.; Zeng, L. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options. Mil Med Res 2022, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Opposite effects of melatonin in different systems and under different conditions. Curr. Top. Biochem. Res 2016, 17, 57–69. [Google Scholar]
- Markus, R.P.; Fernandes, P.A.; Kinker, G.S.; da Silveira Cruz-Machado, S.; Marçola, M. Immune-pineal axis - acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. Br J Pharm 2018, 175, 3239–3250. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, G.J. Neurohormones and catecholamines as functional components of the bone marrow microenvironment. Ann NY Acad Sci 2000, 917, 29–37. [Google Scholar] [CrossRef]
- Lissoni, P.; Paolorossi, F.; Ardizzoia, A. : Barni, S., Chilelli, M.; Mancuso, M., Tancini, G.,; Conti, A.; Maestroni, G.J. A randomized study of chemotherapy with cisplatin plus etoposide versus chemoendocrine therapy with cisplatin, etoposide and the pineal hormone melatonin as a first-line treatment of advanced non-small cell lung cancer patients in a poor clinical state. J Pineal Res 1997, 23(1), 15–19. [CrossRef]
- Golan, K.; Kumari, A.; Kollet, O.; Khatib-Massalha, E.; Subramaniam, M.D.; Ferreira, Z.S.; Avemaria, F.; Rzeszotek, S.; García-García, A.; Xie, S.; Flores-Figueroa, E.; Gur-Cohen, S.; Itkin, T.; Ludin-Tal, A.; Massalha, H.; Bernshtein, B.; Ciechanowicz, A.K.; Brandis, A.; Mehlman, T.; Bhattacharya, S.; … Lapidot, T. Daily Onset of Light and Darkness Differentially Controls Hematopoietic Stem Cell Differentiation and Maintenance. Cell stem cell. [CrossRef]
- Mafi, A.; Rezaee, M.; Hedayati, N.; Hogan, S.D.; Reiter, R.J.; Aarabi, M.H.; Asemi, Z. Melatonin and 5-fluorouracil combination chemotherapy: opportunities and efficacy in cancer therapy. Cell Comm Signal : CCS. [CrossRef]
- Maestroni, G. Circadian regulation of the immune-hematopoietic system. Expl Neurosci 2023, 2, 123–139. [Google Scholar] [CrossRef]
- Maestroni, G.J.; Conti, A.; Pierpaoli, W. (1988). Pineal melatonin, its fundamental immunoregulatory role in aging and cancer. Ann NY Acad Sci 1988, 521, 140–148. [CrossRef]
- Ben-Nathan, D.; Maestroni, G.J.; Lustig, S.; Conti, A. Protective effects of melatonin in mice infected with encephalitis viruses. Archi Virol 1995, 140, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, E.; Valero-Fuenmayor, N.; Pons, H.; Chacín-Bonilla, L. Melatonin protects mice infected with Venezuelan equine encephalomyelitis virus. Cell Mol Life Sci 1997, 53, 430–434. [Google Scholar] [CrossRef]
- Valero, N.; Bonilla, E.; Pons, H.; Chacin-Bonilla, L.; Añez, F.; Espina, L.M.; Medina-Leendertz, S.; García Tamayo, J. Melatonin induces changes to serum cytokines in mice infected with the Venezuelan equine encephalomyelitis virus. Trans R Soc Trop Med Hyg 2002, 96, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, G.; Nigro, L.; Palermo, F.; Leto, D.; Pomerantz, R.J.; Cacopardo, B. Reduction of serum melatonin levels in HIV-1-infected individuals' parallel disease progression: correlation with serum interleukin-12 levels. Infection 2003, 31, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Cao, X.J.; Wei, W. Melatonin decreases TLR3-mediated inflammatory factor expression via inhibition of NF-kappa B activation in respiratory syncytial virus-infected RAW264.7 macrophages. J Pineal Res 2008, 45, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Cao, X.J.; Liu, W.; Shi, X.Y.; Wei, W. Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice. J Pineal Res 2010, 48, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Laliena, A.; San Miguel, B.; Crespo, I.; Alvarez, M.; González-Gallego, J.; Tuñón, M.J. Melatonin attenuates inflammation and promotes regeneration in rabbits with fulminant hepatitis of viral origin. J Pineal Res 2012, 53, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Crespo, I.; Fernández-Palanca, P.; San-Miguel, B.; Álvarez, M.; González-Gallego, J.; Tuñón, M.J. Melatonin modulates mitophagy, innate immunity and circadian clocks in a model of viral-induced fulminant hepatic failure. J Cell Mol Med 2020, 24, 7625–7636. [Google Scholar] [CrossRef] [PubMed]
- Junaid, A.; Tang, H.; van Reeuwijk, A.; Abouleila, Y.; Wuelfroth, P.; van Duinen, V.; Stam, W.; van Zonneveld, A.J.; Hankemeier, T.; Mashaghi, A. Ebola Hemorrhagic Shock Syndrome-on-a-Chip. iScience 2020, 23, 100765. [Google Scholar] [CrossRef] [PubMed]
- Bahrampour Juybari, K.; Pourhanifeh, M.H.; Hosseinzadeh, A.; Hemati, K.; Mehrzadi, S. (2020). Melatonin potentials against viral infections including COVID-19: Current evidence and new findings. Virus Res 2020, 287, 198108. [Google Scholar] [CrossRef] [PubMed]
- Sehirli, A.O.; Sayiner, S.; Serakinci, N. Role of melatonin in the treatment of COVID-19; as an adjuvant through cluster differentiation 147 (CD147). Mol Biol Rep 2020, 47, 8229–8233. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.T.; Atrakji, D.M.Q.Y.M.A.A.; Mehuaiden, D.A.K. The Effect of Melatonin on Thrombosis, Sepsis and Mortality Rate in COVID-19 Patients. Inter J Inf Dis , 2022, 114, 79–84. [CrossRef]
- Loh, D.; Reiter, R.J. Melatonin: Regulation of Viral Phase Separation and Epitranscriptomics in Post-Acute Sequelae of COVID-19. Int J Mol Sci 2022, 23, 8122. [Google Scholar] [CrossRef] [PubMed]
- Farnoosh, G.; Akbariqomi, M.; Badri, T.; Bagheri, M.; Izadi, M.; Saeedi-Boroujeni, A.; Rezaie, E.; Ghaleh, H.E.G.; Aghamollaei, H.; Fasihi-Ramandi, M.; Hassanpour, K.; Alishiri, G. Efficacy of a Low Dose of Melatonin as an Adjunctive Therapy in Hospitalized Patients with COVID-19: A Randomized, Double-blind Clinical Trial. Arch Med Res 2022, 53, 79–85. [Google Scholar] [CrossRef]
- Ameri, A.; Frouz Asadi, M.; Ziaei, A.; Vatankhah, M.; Safa, O.; Kamali, M.; Fathalipour, M.; Mahmoodi, M.; Hassanipour, S. (2023). Efficacy and safety of oral melatonin in patients with severe COVID-19: a randomized controlled trial. Inflammopharmacology 2023, 31(1), 265–274. [CrossRef]
- Sánchez-Rico, M.; de la Muela, P.; Herrera-Morueco, J.J.; Geoffroy, P.A.; Limosin, F.; Hoertel, N. ; AP-HP/Université de Paris/INSERM COVID-19 Research Collaboration/AP-HP COVID CDR Initiative/Entrepôt de Données de Santé AP-HP Consortium Melatonin does not reduce mortality in adult hospitalized patients with COVID-19: a multicenter retrospective observational study. J Travel Med, 2022, 29(3), taab195.
- https://doi.org/10.1093/jtAlizadeh, N.; Dianatkhah, M.; Alimohamadi, Y.; Moradi, H.; Akbarpour, S.; Akrami, M.; Mansouri, F.; Faraji, N.; Rezaie, Z.; Alizadeh, M.; Hosamirudsari, H. High dose melatonin as an adjuvant therapy in intubated patients with COVID-19: A randomized clinical trial. J Taibah Uni Med Sci 2022,17(3), 454–460. https://doi.org/10.1016/j.jtumed.2022.04.012.
- Darban, M.; Malek, F.; Memarian, M.; Gohari, A.; Kiani, A.; Emadi, A.; Bagheri, B. Efficacy of high dose vitamin C, melatonin and zinc in Iranian patients with acute respiratory syndrome due to coronavirus infection: a pilot randomized trial. J. Cell. Mol. Anesth 2021, 6, 164–167. [Google Scholar]
- Hing-Hwa,H.;, Ching-Len,L.; Shyi-Jou,C.; Li-Ge,S.; Li,L.;, Yuan-Wu,C.;, Chia-Pi,C.; Huey-Kang,S.; Shih-Ta,S.;, Gu-Jiun, L. Melatonin possesses an anti-influenza potential through its immune modulatory effect,J Fun Foods 2019, 58, 189-198. [CrossRef]
- Xu, M.M.; Kang, J.Y.; Ji, S.; Wei, Y.Y.; Wei, S.L.; Ye, J.J.; Wang, Y.G.; Shen, J.L.; Wu, H.M.; Fei, G.H. Melatonin Suppresses Macrophage M1 Polarization and ROS-Mediated Pyroptosis via Activating ApoE/LDLR Pathway in Influenza A-Induced Acute Lung Injury. Ox Med Cell Longev 2022, 2022, 2520348. [Google Scholar] [CrossRef] [PubMed]
- Huo, C.; Tang, Y.; Li, X.; Han, D.; Gu, Q.; Su, R.; Liu, Y.; Reiter, R.J.; Liu, G.; Hu, Y.; Yang, H. Melatonin alleviates lung injury in H1N1-infected mice by mast cell inactivation and cytokine storm suppression. PLoS pathogens 2023, 19, e1011406. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, G. J. Melatonin as a therapeutic agent in experimental endotoxic shock. J Pineal Res. [CrossRef]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Mohammed, Y.H.; Marik, P.E. Melatonin for the treatment of sepsis: the scientific rationale. J Thoracic Dis 2020, 12 (Suppl 1), S54–S65. [Google Scholar] [CrossRef] [PubMed]
- Poggi, C.; Dani, C. Sepsis and Oxidative Stress in the Newborn: From Pathogenesis to Novel Therapeutic Targets. Oxid Med Cell Long 2018, 9390140. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, W.; Kwak, M.; Zhang, L.; Lee, P.C.W.; Jin, J.O. Protective Effect of Melatonin Against Polymicrobial Sepsis Is Mediated by the Anti-bacterial Effect of Neutrophils. Front Immunol 2019, 10, 1371. [Google Scholar] [CrossRef] [PubMed]
- Bishayi, B.; Adhikary, R.; Nandi, A.; Sultana, S. Beneficial Effects of Exogenous Melatonin in Acute Staphylococcus aureus and Escherichia coli Infection-Induced Inflammation and Associated Behavioral Response in Mice After Exposure to Short Photoperiod. Inflammation 2016, 39, 2072–2093. [Google Scholar] [CrossRef]
- Du, L.; Liu, B.; Han, Z.; Xia, Y.; Wu, M.; Liu, S. Melatonin shapes bacterial clearance function of porcine macrophages during enterotoxigenic Escherichia coli infection. Anim Nut 2022, 11, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xu, S.; Wu, H.; Liu, J.; Wang, Y.; Zhu, G. (2022). Melatonin Is Neuroprotective in Escherichia coli Meningitis Depending on Intestinal Microbiota. Int J Mol Sci 2023, 24, 298. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, P. Insight of Melatonin: The Potential of Melatonin to Treat Bacteria-Induced Mastitis. Antioxidants (Basel, Switzerland), 2022, 11, 1107. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liu, J.; Zhao, X.; Yang, W. Melatonin ameliorates lung cell inflammation and apoptosis caused by Klebsiella pneumoniae via AMP-activated protein kinase. Inflammopharmacol 2022, 30, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, T.; Lv, X.; Sun, N. , Li, F.; Luo, L.; Zhuge, X.; Huang, J.; Wang, L. (A Potential Nontraditional Approach To Combat tmexCD1-toprJ1-Mediated Tigecycline Resistance: Melatonin as a Synergistic Adjuvant of Tigecycline. Antimicr Agent Chemother 2023, 67, e0004723. [Google Scholar] [CrossRef]
- Huang, H.; Mehta, A.; Kalmanovich, J.; Anand, A.; Bejarano, M.C.; Garg, T.; Khan, N.; Tonpouwo, G.K.; Shkodina, A.D.; Bardhan, M. Immunological and inflammatory effects of infectious diseases in circadian rhythm disruption and future therapeutic directions. Mol Biol Rep 2023, 50, 3739–3753. [Google Scholar] [CrossRef]
- Singh, M.K.; Dias, B.K.M.; Garcia, C.R. S Role of Melatonin in the Synchronization of Asexual Forms in the Parasite Plasmodium falciparum. Biomolecules 2020, 10, 1243. [Google Scholar] [CrossRef] [PubMed]
- Ataide, B.J.A.; Kauffmann, N.; Mendes, N.S.F.; Torres, M.L.M.; Dos Anjos, L.M.; Passos, A.D.C.F.; de Moraes, S.A.S.; Batista, E.J.O.; Herculano, A.M.; Oliveira, K.R.H.M. (Melatonin Prevents Brain Damage and Neurocognitive Impairment Induced by Plasmodium Berghei ANKA Infection in Murine Model of Cerebral Malaria. Front Cell Infect Microbiol 2020, 10, 541624. [Google Scholar] [CrossRef] [PubMed]
- Bagnaresi, P.; Alves, E.; Borges da Silva, H.; Epiphanio, S.; Mota, M.M.; Garcia, C.R. (Unlike the synchronous Plasmodium falciparum and P. chabaudi infection, the P. berghei and P. yoelii asynchronous infections are not affected by melatonin. Int J Gen Med. [CrossRef]
- Bocchi, E.A.; Bestetti, R.B.; Scanavacca, M.I.; Cunha Neto, E.; Issa, V.S. Chronic Chagas Heart Disease Management: From Etiology to Cardiomyopathy Treatment J Am Coll Cardiol 2017, 70, 1510–1524. [CrossRef]
- Santello, F.H.; Frare, E.O.; dos Santos, C.D.; Toldo, M.P.; Kawasse, L.M.; Zucoloto, S.; do Prado, J.C. Jr. Melatonin treatment reduces the severity of experimental Trypanosoma cruzi infection. J Pineal Res 2007, 42, 359–363. [Google Scholar] [CrossRef]
- Santello, F.H.; Frare, E.O.; dos Santos, C.D.; Caetano, L.C.; Alonso Toldo, M.P.; do Prado, J.C. , Jr. Suppressive action of melatonin on the TH-2 immune response in rats infected with Trypanosoma cruzi. J Pineal Res 2008, 45(3), 291–296. [CrossRef]
- Santello, F.H.; Frare, E.O.; Caetano, L.C.; AlonsoToldo, M.P.; do Prado, J.C. Jr Melatonin enhances pro-inflammatory cytokine levels and protects against Chagas disease. J Pineal Res 2008, 45, 79–85. [Google Scholar] [CrossRef]
- Brazão, V.; Del Vecchio Filipin, M.; Santello, F.H.; Caetano, L.C.; Abrahão, A.A.; Toldo, M.P.; do Prado, J.C. Jr.Melatonin and zinc treatment: distinctive modulation of cytokine production in chronic experimental Trypanosoma cruzi infection. Cytokine 2011, 56(3), 627–632. [CrossRef]
- Providello, M.V. , Portapilla, G.B., Oliveira, P.A.S., da Silva, C.B.P., Anchieta, N.F., Tirapelli, C.R., de Albuquerque, S. Melatonin decreases circulating Trypanosoma cruzi load with no effect on tissue parasite replication. Can J Physiol Pharmacol 2021, 99, 795–802. [Google Scholar] [CrossRef]
- Hunter, F.K.; Butler, T.D.; Gibbs, J.E. Circadian rhythms in immunity and host-parasite interactions. Parasite Immunol 2022, 44, e12904. [Google Scholar] [CrossRef]
- Grassi-Zucconi, G.; Semprevivo, M.; Mocaer, E.; Kristensson, K.; Bentivoglio, M. Melatonin and its new agonist S-20098 restore synchronized sleep fragmented by experimental trypanosome infection in the rat. Brain Res Bull 1996, 39, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Elmahallawy, E.K.; Jiménez-Aranda, A.; Martínez, A.S.; Rodriguez-Granger, J.; Navarro-Alarcón, M.; Gutiérrez-Fernández, J.; Agil, A. Activity of melatonin against Leishmania infantum promastigotes by mitochondrial dependent pathway. Chemico-biol Interac 2014, 220, 84–93. [CrossRef]
- Laranjeira-Silva, M.F.; Zampieri, R.A.; Muxel, S.M.; Floeter-Winter, L.M.; Markus, R.P. Melatonin attenuates Leishmania (L.) amazonensis infection by modulating arginine metabolism. J Pineal Res 2015, 59(4), 478–487. [CrossRef]
- Fernandes, J.C.R.; Aoki, J.I.; Maia Acuña, S.; Zampieri, R.A.; Markus, R.P.; Floeter-Winter, L.M.; Muxel, S.M. Melatonin and Leishmania amazonensis Infection Altered miR-294, miR-30e, and miR-302d Impacting on Tnf, Mcp-1, and Nos2 Expression. Front Cell Inf Microbiol 2019, 9, 60. [Google Scholar] [CrossRef]
- Parvez, S.; Yadagiri, G.; Arora, K.; Javaid, A.; Kushwaha, A.K.; Singh, O.P.; Sundar, S.; Mudavath, S.L. (Coalition of Biological Agent (Melatonin) With Chemotherapeutic Agent (Amphotericin B) for Combating Visceral Leishmaniasis via Oral Administration of Modified Solid Lipid Nanoparticles. ACS Bio,mater Sci Eng 2023, 9(6), 2902–2910. [CrossRef]
- Szewczyk-Golec, K.; Pawłowska, M.; Wesołowski, R.; Wróblewski, M.; Mila-Kierzenkowska, C. Oxidative Stress as a Possible Target in the Treatment of Toxoplasmosis: Perspectives and Ambiguities. Int J Mol Sci 2021, 22, 5705. [Google Scholar] [CrossRef] [PubMed]
- Avunduk, A.M.; Avunduk, M.C.; Baltaci, A.K.; Moğulkoç, R. Effect of melatonin and zinc on the immune response in experimental Toxoplasma retinochoroiditis. Ophthalmologica. Int J Ophtal. 2007, 221, 421–425. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Bediz, C.S.; Mogulkoc, R.; Kurtoglu, E.; Pekel, A. Effect of zinc and melatonin supplementation on cellular immunity in rats with toxoplasmosis. Biol Trace Elem Res 2003, 96(1-3), 237–245. [CrossRef]
- Baltaci, A.K.; Mogulkoc, R.; Bediz, C.S. , Pekel, A.Effects of zinc deficiency and pinealectomy on cellular immunity in rats infected with Toxoplasma gondii. Biol Trace Elem Res 2005, 104, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.I.; Dos Santos, T.A.T.; de Souza, W.; DaMatta, R.A.; Seabra, S.H. Treatment with melatonin induces a reduction of Toxoplasma gondii development in LLC-MK2 cells. Parasitol Res 2020, 119, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, T.; Sharma, S.; Kumar, M.; Hussain, M.A.; Chauhan, N.; Kalia, I.; Sahu, A.K.; Rana, V.S.; Bharti, R.; Haldar, A.K.; Singh, A.P.; Mazumder, S. Tryptophan-kynurenine pathway attenuates β-catenin-dependent pro-parasitic role of STING-TICAM2-IRF3-IDO1 signalosome in Toxoplasma gondii infection. Cell Death Dis 2019, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Alsayed, A. R.; Abuawad, A.; Daoud, S.; Mahmod, A.I. Melatonin in Cancer Treatment: Current Knowledge and Future Opportunities. Molecules 2021, 26, 2506. [CrossRef]
| Pathogen | Species | MLT dose | Treatment | Outcome | Ref. |
|---|---|---|---|---|---|
| EMCV | Mice | 1ug /mouse | 10 days at 4 pm | Reversal of stress -induced death | [55] |
| SFV | Mice | 500 ug /kg | From 3 days before until 10 days after infection at 4 pm | Increased survival and decreased viremia | [56] |
| aWNV | Mice | 5ug /mouse | From 2 days before until 8 days after infection at 4 pm | Reduced mortality | [56] |
| VEEV | MIce | 1 mg/kg | From 3 days before until 10 days after infection at 6 pm | Increased survival, decreased viremia, increased antibody response | [57] |
| RSV | Mice | 5mg/kg | Twice daily for 3 days | Reduced oxidative damage of the lung | [61] |
| RHDV | Rabbits | 20 mg/kg | 0, 12, 24 h after infection | Decreased mitophagy, inflammation and innate immunity | [62,63] |
| H1N1 | Mice | 3, 10, 30 mg/kg | Pretreatment for 3 days before infection | Decreased lung injury by inhibition of mast cells and cytokine storm | [76] |
| H1N1 | Mice | 200 mg/kg | 6 h before and 2,4 and 6 days post infection | Inhibition of pro-inflammatory cytokines and stimulation of IL-10; Synergy with an antiviral drug | [74] |
| H3N2 | Mice | 30 mg/kg | 7 days at 6 pm | Attenuated pulmonary damage, leukocyte infiltration and edema | [75] |
| Pathogen | Species | MLT dose | Treatment | Outcome | Ref. |
|---|---|---|---|---|---|
| Lethal dose of LPS | Mice | 1,2,3,4,5,10 mg/kg | 3 or 6 h after LPS injection | 2,3,4, 5 mg/kg reduced mortality and NO synthesis | [77] |
| Sepsis | HumanNewborns | 2 x 10 mg | Oral administration within 12 hours after diagnosis | Increased survival and improved clinical status | [78] |
| Sepsis | Human newborns | 20 mg/kg | One injection plus antibiotics | Increased survival and improved clinical status | [79] |
| Polymicrobial sepsis | Mice | 50 mg/kg | Two doses , 30 min before and 30 min after cecal ligation puncture | Protection of mice by induction of neutrophil extracellular trap | [80] |
| Staphylococcus aureus, Escherichia coli | Mice | 10mg/kg | Once daily for 7 days | Improved clearance of bacteria from blood, reduced iNOS, plasma C-reactive protein, COX2 expression in the hypothalamus . | [81] |
| Escherichia coli | Mice | 30mg/kg | Pretreatment for 7 consecutive days before infection | Prevention of and protection from bacterial meningitis by modulating the intestinal microbiota | [83] |
| Tigecyclin resistant Klebsiella pneumoniae | Mice | 50 mg/kg | One dose after infection | Restoring tigecycline activity | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





