Submitted:
23 February 2024
Posted:
26 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Description of dataset
2.1.1. Subjects
2.1.2. MRI Image Acquisition
2.2. Image Processing and Analysis
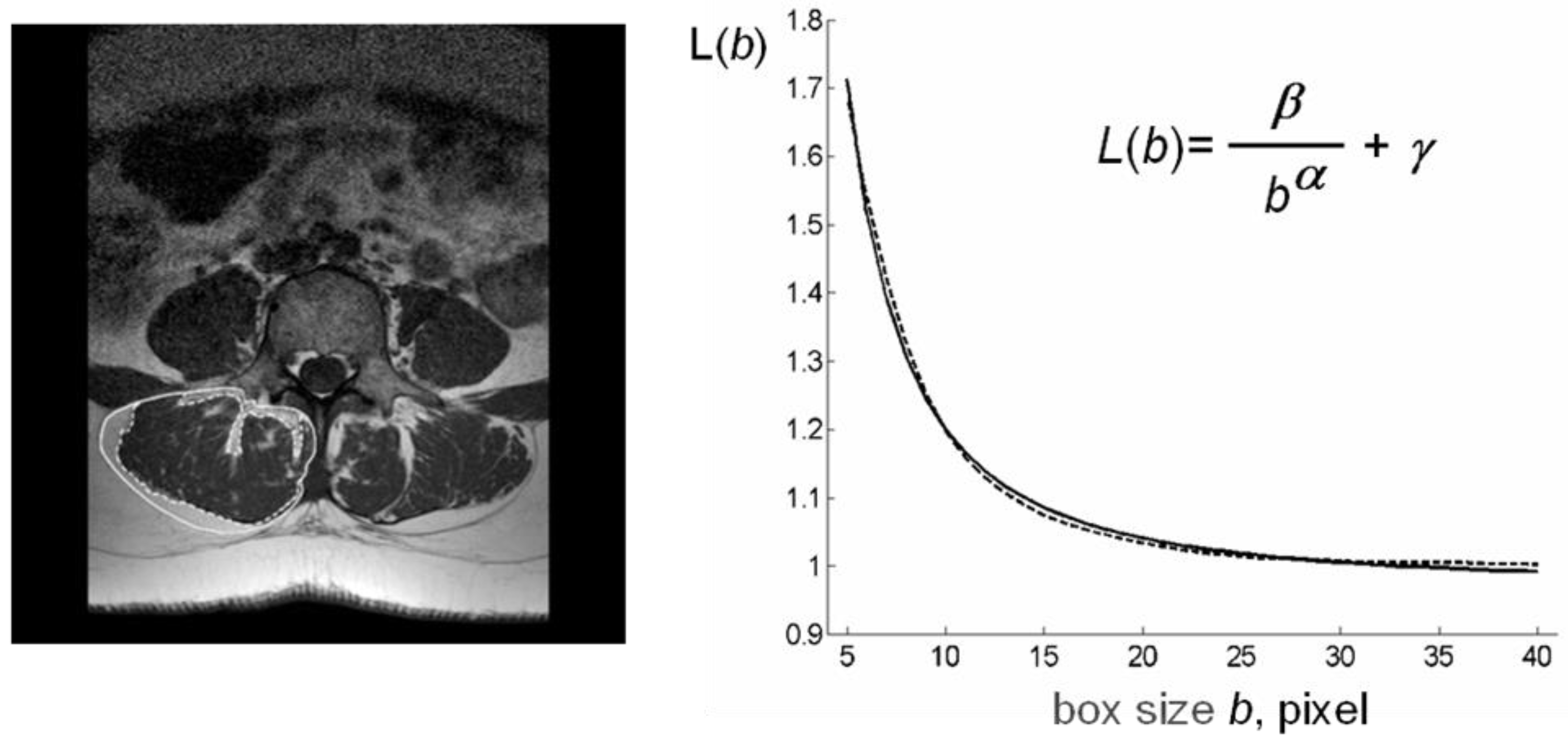
2.2.1. Analysis of MR Images
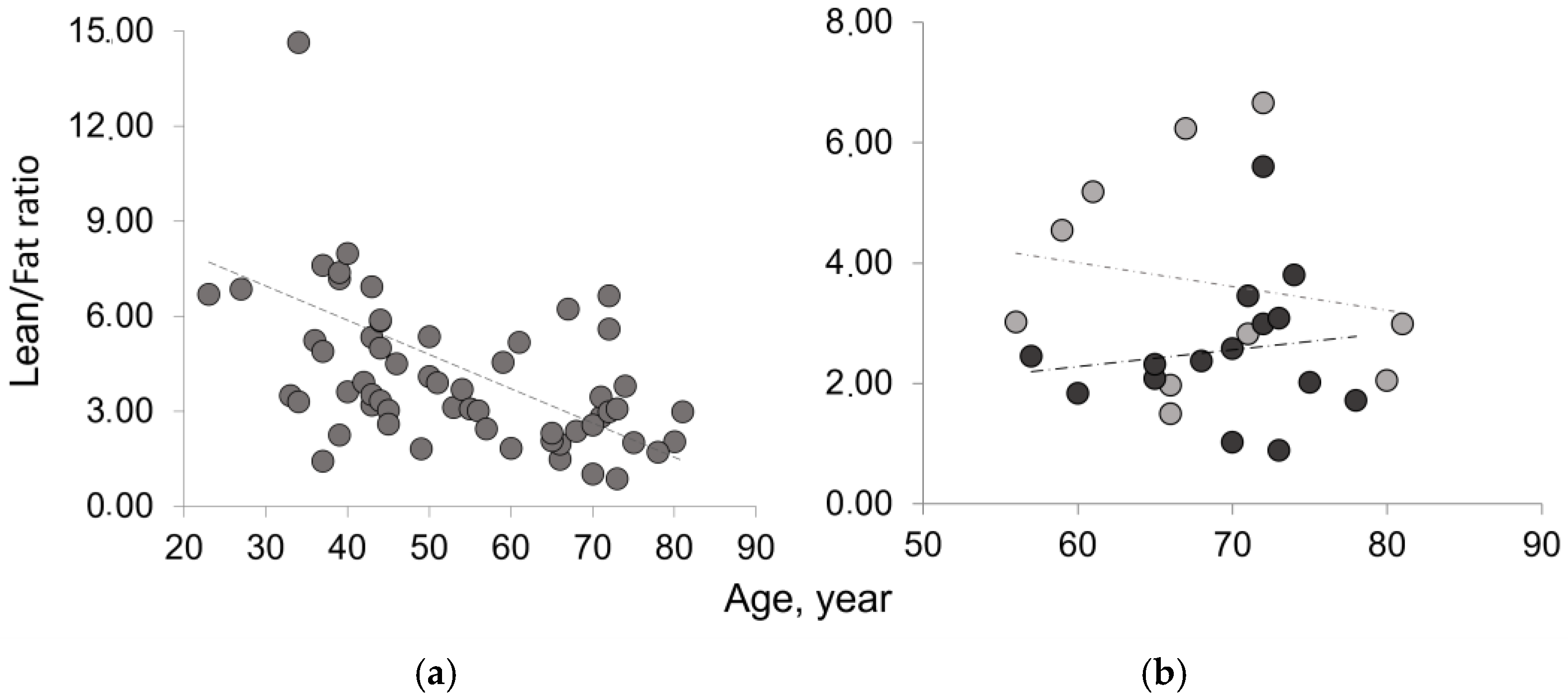
2.2.2. Computation of Index ρ (Lean/Fat Ratio)
- let τ1 be the index of the relative maximum of H that is closest to i=0;
- let τ2 be the index of the relative maximum of H that is closest to i=255;
- let τ=(τ1+τ2)/2;
-
do
- ◦
- τold=τ;
- ◦
- let τ1 be the weighted average of gray levels less than τ with weights H(i), i=0, 1, …, τ-1;
- ◦
- let τ2 be the weighted average of gray levels greater than τ with weights H(i), i=τ+1, τ+2, …, 255;
- ◦
- let τ=(τ1+τ2)/2;
- nF=0;
- nL=0;
-
for every pixel p in the ROI,
- ◦
- let Rj be the sub-image containing p, and dj=|cj-p|;
- ◦
- let Rl, l =1, 2,…, L be all the sub-images such that |cl-p|<|cj-cl| and |cl-p|<d;
- ◦
- let dl=|cl-p|, l=1, 2,…, L and
- ◦
- if GL(p)> τ, increase nF by 1, else increase nL by 1.
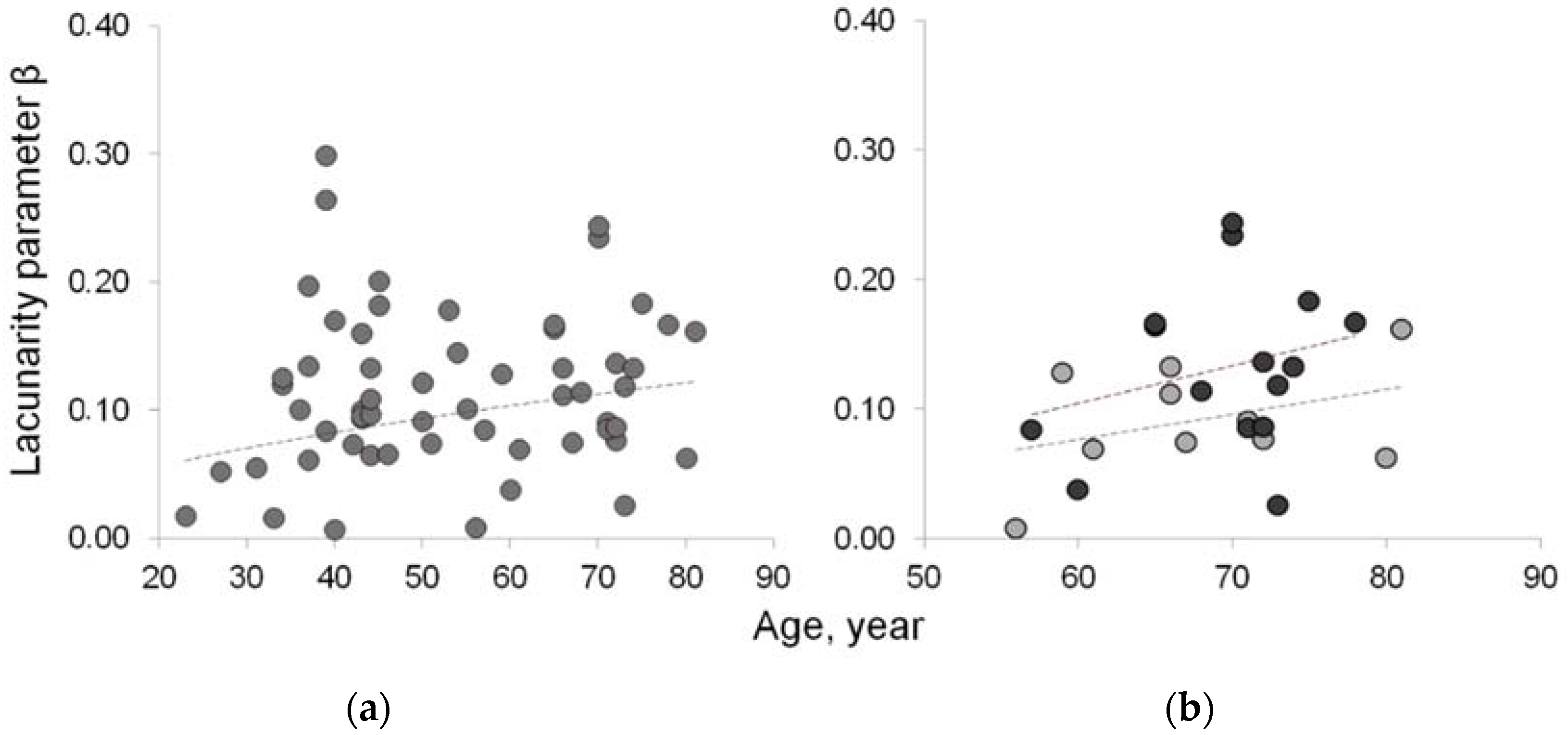
2.2.3. Computation of index β (lacunarity)
2.2.4. Computation of index µ (succolarity)
3. Results
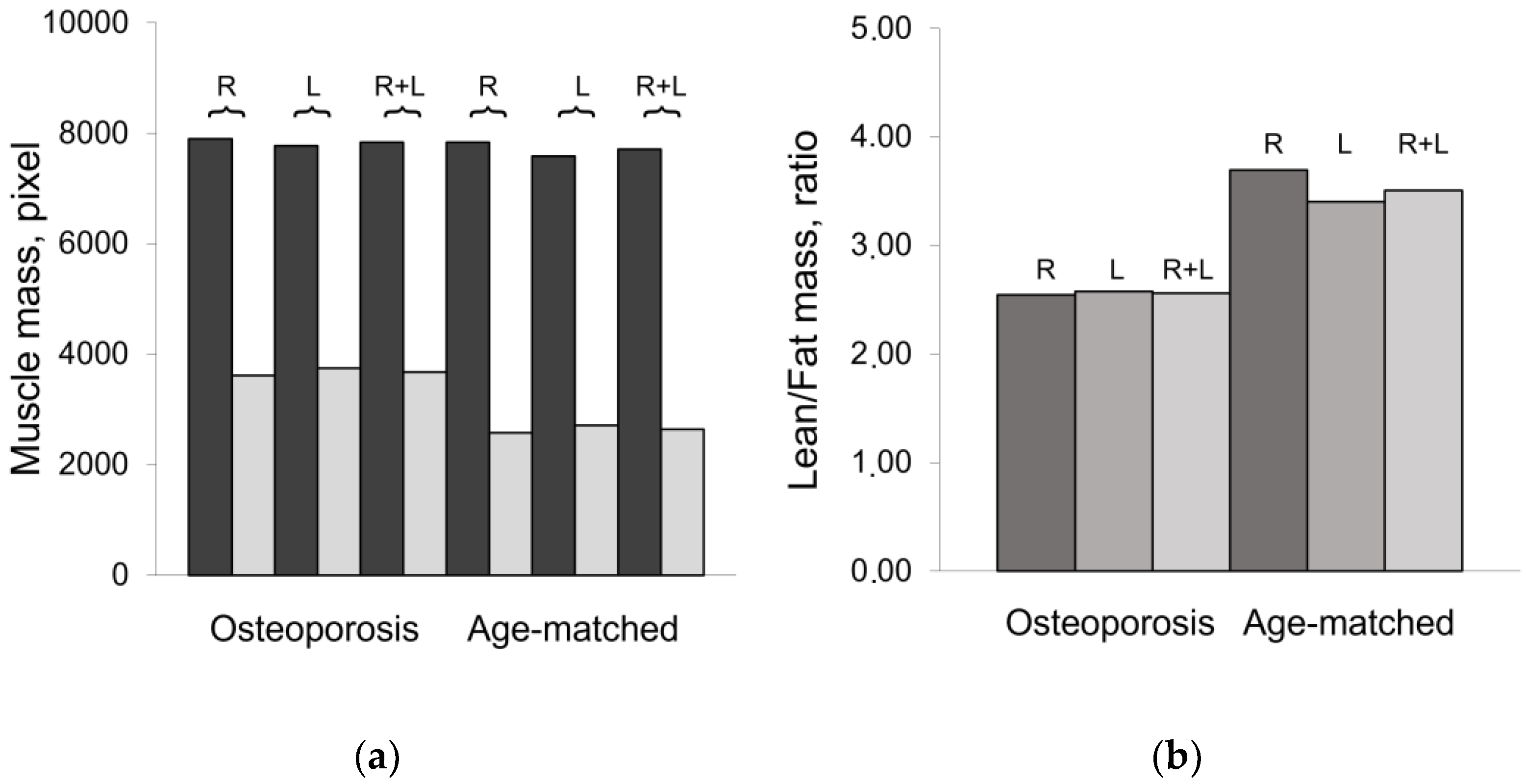
3.1. Mass composition of paraspinal muscles
3.2. Fractal features of paraspinal muscle
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, W.J. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am. J. Clin. Nutr. 2010, 91, 1123S–1127S. [Google Scholar] [CrossRef]
- Evans, W.J.; Paolisso, G.; Abbatecola, A.M.; Corsonello, A.; Bustacchini, S.; Strollo, F.; Lattanzio, F. Frailty and muscle metabolism dysregulation in the elderly. Biogerontol. 2010, 11, 527–536. [Google Scholar] [CrossRef]
- Russ, D.W.; Grandy, J.S.; Toma, K.; Ward, C.W. Ageing, but not yet senescent, rats exhibit reduced muscle quality and sarcoplasmic reticulum function. Acta Physiol. 2011, 201, 391–403. [Google Scholar] [CrossRef]
- Seene, T.; Kaasik, P.; Riso, E.M. Review on aging, unloading and reloading: changes in skeletal muscle quantity and quality. Arch. Gerontol. Geriatr. 2012, 54, 374–380. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Summary comments: epidemiological and methodological problems in determining nutritional status of older persons. Am. J. Clin. Nutr. 1989, 50, 1231–1233. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef]
- Delmonico, M.J.; Harris, T.B.; Lee, J.S.; Visser, M.; Nevitt, M.; Kritchevsky, S.B.; et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J. Am. Geriatr. Soc. 2007, 55, 769–774. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; et al. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Hiona, A.; Leeuwenburgh, C. The role of mitochondrial DNA mutations in aging and sarcopenia: implications for the mitochondrial vicious cycle theory of aging. Exp. Gerontol. 2008, 43, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Leeuwenburgh, C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp. Gerontol. 2006, 41, 1234–1238. [Google Scholar] [CrossRef]
- Fearon, K.; Evans, W.J.; Anker, S.D. Myopenia—a new universal term for muscle wasting. J. Cachexia Sarcopenia Muscle. 2011, 2, 1–3. [Google Scholar] [CrossRef]
- Clark, B.C.; Manini, T.M. Functional consequences of sarcopenia and dynapenia in the elderly. Curr. Opin. Clin. Nutr. Metab. Care. 2010, 13, 271–276. [Google Scholar] [CrossRef]
- Gonzales, E.; Messi, M.L.; Delbono, O. The specific force of single intact extensor digitorum longus and soleus mouse muscle fibers declines with aging. J. Membr. Biol. 2000, 178, 175–183. [Google Scholar] [CrossRef]
- Stackhouse, S.K.; Stevens, J.E.; Lee, S.C.; Pearce, K.M.; Snyder-Mackler, L.; Binder-Macleod, S.A. Maximum voluntary activation in nonfatigued and fatigued muscle of young and elderly individuals. Phys. Ther. 2001, 81, 1102–1109. [Google Scholar] [CrossRef]
- Weisleder, N.; Brotto, M.; Komazaki, S.; Pan, Z.; Zhao, X.; Nosek, T.; et al. Muscle aging is associated with compromised Ca2+ spark signaling and segregated intracellular Ca2+ release. J. Cell. Biol. 2006, 174, 639–645. [Google Scholar] [CrossRef]
- Delmonico, M.J.; Harris, T.B.; Visser, M. , Park, S.W.; Conroy, M.B.; Valasquez-Mieyer, P.; et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Adamek, M.; Gonzalez, M.C.; Gia, G.; Thomas, D.M. Assessing skeletal muscle mass: historical overview and state of the art. J. Cachexia Sarcopenia Muscle. 2014, 5, 9–18. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Heskamp, L.; Okkersen, K.; van Nimwegen, M.; Ploegmakers, M.J.; Bassez, G.; Deux, J-F. ; et al. Quantitative Muscle MRI Depicts Increased Muscle Mass after a Behavioral Change in Myotonic Dystrophy Type 1. Radiol. 2020, 297, 132–142. [Google Scholar] [CrossRef]
- Goutallier, D.; Postel, J.M.; Bernageau, J.; Lavau, L.; Voisin, M.C. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin. Orthop. Relat. Res. 1994, 304, 78–83. [Google Scholar] [CrossRef]
- Fortin, M.; Lazáry, À.; Varga, P.P.; Battié, M.C. Association between paraspinal muscle morphology, clinical symptoms and functional status in patients with lumbar spinal stenosis. Eur. Spine J. 2017, 26, 2543–2551. [Google Scholar] [CrossRef]
- Battaglia, P.J.; Maeda, Y.; Welk, A.; Hough, B. , Kettner, N. Reliability of the Goutallier classification in quantifying muscle fatty degeneration in the lumbar multifidus using magnetic resonance imaging. J. Manipulative Physiol. Ther. 2014, 37, 190–197. [Google Scholar] [CrossRef]
- Tamai, K.; Chen, J.; Stone, M.; Arakelyan, A.; Paholpak, P.; Nakamura, H.; et al. The evaluation of lumbar paraspinal muscle quantity and quality using the Goutallier classification and lumbar indentation value. Eur. Spine J. 2018, 27, 1005–1012. [Google Scholar] [CrossRef]
- Fortin, M.; Battie, M.C. Quantitative paraspinal muscle measurements: intersoftware reliability and agreement using OsiriX and ImageJ. Phys. Ther. 2012, 92, 853–864. [Google Scholar] [CrossRef]
- Addison, O.; Marcus, R.L.; LaStayo, P.C.; Ryan, A.S. Intermuscular Fat: A Review of the Consequences and Causes. Int. J. Endocrinol. 2014, 2014, 309570. [Google Scholar] [CrossRef]
- Zaia, A.; Eleonori, R.; Maponi, P.; Rossi, R.; Murri, R. Medical imaging and osteoporosis: Fractal’s lacunarity analysis of trabecular bone in MR images. In Proceedings of the Eighteenth IEEE Symposium on Computer-Based Medical Systems (CBMS 2005), Dublin, Ireland, 23–24 June 2005; IEEE, 2005; pp. 3–8. [Google Scholar] [CrossRef]
- Zaia, A.; Eleonori, R.; Maponi, P.; Rossi, R.; Murri, R. MR imaging and osteoporosis: Fractal lacunarity analysis of trabecular bone. IEEE Trans. Inf. Technol. Biomed. 2006, 10, 484–489. [Google Scholar] [CrossRef]
- Zaia, A.; Rossi, R.; Egidi, N.; Maponi, P. Fractal’s lacunarity analysis of trabecular bone in MR images. In Computational Vision and Medical Image Processing; Tavares, J., Jorge, N., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 421–426. [Google Scholar]
- Zaia, A. Fractal lacunarity of trabecular bone and magnetic resonance imaging: New perspectives for osteoporotic fracture risk assessment. World. J. Orthop. 2015, 6, 221–235. [Google Scholar] [CrossRef]
- Mandelbrot, B.B. A Fractal’s Lacunarity, and how it can be Tuned and Measured. In: Nonnenmacher TF, Losa GA, Weibel ER, editors. Fractals in Biology and Medicine. Birkhauser Press: Basel, Switzerland, 1993; pp. 8-21.
- Plotnick, R.E.; Gardner, R.H.; Hargrove, W.W. , Prestegaard, K.; Perlmutter, M. Lacunarity analysis: A general technique for the analysis of spatial patterns. Phys. Rev. E. 1996, 53, 5461–5468. [Google Scholar] [CrossRef]
- Mandelbrot, B.B. The Fractal Geometry of Nature. In: Trees and the Diameter Exponent. WH Freeman: New York, USA, 1977; pp 156-165.
- Metze, K.; Adam, R.; Florindo, J.B. The fractal dimension of chromatin - a potential molecular marker for carcinogenesis, tumor progression and prognosis. Expert Rev. Mol. Diagn. 2019, 19, 299–312. [Google Scholar] [CrossRef]
- de Melo, R.H.C.; Conci, A. How Succolarity could be used as another fractal measure in image analysis. Telecommun. Syst. 2013, 52, 1643–1655. [Google Scholar] [CrossRef]
- de Melo, R.H.C.; Conci, A. Succolarity: Defining a method to calculate this fractal measure. 2008 In Proceedings of the 15th International Conference on Systems, Signals and Image Processing, Bratislava, Slovakia, 25-28 June 2008, pp. 291–294. [CrossRef]
- Allain, C.; Cloitre, M. Characterizing the lacunarity of random and deterministic fractal sets. Phys. Rev. A. 1991, 44, 3552–3558. [Google Scholar] [CrossRef]
- Shur, N.F.; Creedon, L.; Skirrow, S.; Atherton, P.J.; MacDonald, I.A.; Lund, J.; Greenhaff, P.L. Age-related changes in muscle architecture and metabolism in humans: The likely contribution of physical inactivity to age-related functional decline. Ageing Res. Rev. 2021, 68, 101344. [Google Scholar] [CrossRef]
- Volpi, E.; Nazemi, R.; Fujita, S. Muscle tissue changes with aging. Curr. Opin. Clin. Nutr. Metab. Care. 2004, 7, 405–410. [Google Scholar] [CrossRef]
- Pang, J.; Tu, F.; Han, Y.; Zhang, E.; Zhang, Y.; Zhang, T. Age-related change in muscle strength, muscle mass, and fat mass between the dominant and non-dominant upper limbs. Front. Public. Health. 2023, 11, 1284959. [Google Scholar] [CrossRef]
- Budui, S.L.; Rossi, A.P.; Zamboni, M. The pathogenetic bases of sarcopenia. Clin. Cases Miner. Bone Metab. 2015, 12, 22–26. [Google Scholar] [CrossRef]
- Scicchitano, B.M.; Pelosi, L.; Sica, G.; Musarò, A. The physiopathologic role of oxidative stress in skeletal muscle. Mech. Aging Dev. 2017, 170, 37–44. [Google Scholar] [CrossRef]
- Johnson, T.E. Recent results: Biomarkers of aging. Exp. Gerontol. 2006, 41, 1243–1246. [Google Scholar] [CrossRef]
- Wagner, K-H. ; Cameron-Smith, D.; Wessner, B.; Franzke, B. Biomarkers of Aging: From Function to Molecular Biology. Nutrients. 2016, 8, 338. [Google Scholar] [CrossRef]
- Burkle, A.; Moreno-Villanueva, M.; Bernhard, J.; Blasco, M.; Zondag, G.; Hoeijmakers, J.H.; et al. Mark-age biomarkers of ageing. Mech. Ageing Dev. 2015, 151, 2–12. [Google Scholar] [CrossRef]
- Piantanelli, L.; Rossolini, G.; Basso, A.; Piantanelli, A.; Malavolta, M.; Zaia, A. Use of mathematical models of survivorship in the study of biomarkers of aging: The role of heterogeneity. Mech. Ageing Dev. 2001, 122, 1461–1475. [Google Scholar] [CrossRef]
- Zaia, A. Osteoporosis and fracture risk: New perspectives for early diagnosis and treatment assessment. In Osteoporosis: Etiology, Diagnosis and Treatment; Mattingly, B.E., Pillare, A.C., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2009; pp. 267–290. [Google Scholar]
- Pettersson, M. Complexity and Evolution; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Zaia, A.; Maponi, P. Mitochondrial DNA Profiling by Fractal Lacunarity to Characterize the Senescent Phenotype as Normal Aging or Pathological Aging. Fractal Fract. 2022, 6, 219. [Google Scholar] [CrossRef]
- Lipsitz, L.A.; Goldberger, A.L. Loss of ‘complexity’ and aging: Potential applications of fractals and chaos theory to senescence. JAMA 1992, 267, 1806–1809. [Google Scholar] [CrossRef]
- Grassberger, A.; Procaccia, I. Measuring the strangeness of strange attractors. Phys. D 1983, 9, 189–208. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Rigney, D.R.; West, B.J. Chaos and fractals in human physiology. Sci. Am. 1990, 262, 42–49. [Google Scholar] [CrossRef]
- Goldberger, A.L. Non-linear dynamics for clinicians: Chaos theory, fractals, and complexity at the bedside. Lancet 1996, 347, 1312–1314. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef]
- Mandelli, F.; Nüesch, C.; Zhang, Y.; Halbeisen, F.; Schären, S.; Mündermann, A.; Netzer, C. Assessing Fatty Infiltration of Paraspinal Muscles in Patients With Lumbar Spinal Stenosis: Goutallier Classification and Quantitative MRI Measurements. Front. Neurol. 2021, 12, 656487. [Google Scholar] [CrossRef]
- Coelho, A.L.V.; Lima, C.A.M. Assessing fractal dimension methods as feature extractors for EMG signal classification. Eng. Appl. Artif. Intell. 2014, 36, 81–98. [Google Scholar] [CrossRef]
- Zaia, A.; Rossi, R.; Galeazzi, R.; Sallei, M.; Maponi, P.; Scendoni, P. Fractal lacunarity of trabecular bone in vertebral MRI to predict osteoporotic fracture risk in over-fifties women. The LOTO study. BMC Musculoskelet. Disord. 2021, 22, 108. [Google Scholar] [CrossRef]
- Zaia, A.; Maponi, P.; Sallei, M.; Galeazzi, R.; Scendoni, P. Measuring Drug Therapy Effect on Osteoporotic Fracture Risk by Trabecular Bone Lacunarity: The LOTO Study. Biomedicines 2023, 11, 781. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, P.; Bendix, T.; Sorensen, J.S.; Korsholm, L.; Leboeuf-Yde, C. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med. 2007, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Cha, J.G.; Kim, Y.; Kim, Y.I.; Shin, B.J. Quantitative analysis of back muscle degeneration in the patients with the degenerative lumbar flat back using a digital image analysis: comparison with the normal controls. Spine. 2008, 33, 318–325. [Google Scholar] [CrossRef] [PubMed]
| Subjects | Number | Age Range | Mean Age 1 | Median Age |
|---|---|---|---|---|
| All | 59 | 23 - 81 | 53 ± 15 | 50 |
| Young | 15 | 23 - 40 | 35 ± 5 | 37 |
| Pre-menopause | 15 | 42 - 50 | 45 ± 3 | 44 |
| Post-menopause | 14 | 51 - 81 | 64 ± 10 | 64 |
| Osteoporosis 2 | 15 | 57 - 78 | 70 ± 6 | 71 |
| Age-matched 3 | 10 | 56 - 81 | 68 ± 8 | 67 |
| Subjects | TotCSA | LeanCSA* | FatCSA† | Lean/Fat Ratio |
|---|---|---|---|---|
| All | 11114 ± 1858 | 8507 ± 2608 (77%)* | 1925 ± 1153 (23%) | 4.40 ± 3.53 |
| Young | 11222 ± 1939 | 9364 ± 1929 (84%) | 1858 ± 1015 (16%) | 7.17 ± 5.73 |
| Pre-menopause | 10652 ± 1686 | 8481 ± 1105 (80%) | 2171 ± 838 (20%) | 4.34 ± 1.35 |
| Menopause | 11062 ± 2043 | 8332 ± 1609 (75%) | 2730 ± 1226 (25%) | 3.50 ± 1.54 |
| Age-matched 1 | 10356 ± 1215 | 7714 ± 1364 (75%) | 2642 ± 1018 (25%) | 3.51 ± 1.87 |
| Osteoporosis 2 | 11517 ± 1865 | 7837 ± 1449 (68%) | 3680 ± 1315 (32%) | 2.52 ± 1.34 |
| Subjects | Young | pre-Menopause | Menopause | Age-matched | Osteoporosis | P value1 |
|---|---|---|---|---|---|---|
| Lean mass2 | 8586 ± 1778 | 7889 ± 1002 | 7277 ± 1638 | 6581 ± 1024 | 6890 ± 1402 | 0.286 |
| Fatty mass2 | 2234 ± 756 | 2444 ± 732 | 2894 ± 936 | 2813 ± 1041 | 3627 ± 1114 | 0.046 |
| Lean/Fat ratio ρ | 4.21 ± 1.33 | 3.57 ± 0.68 | 2.70 ± 0.76 | 2.61 ± 0.89 | 2.08 ± 0.67 | 0.057 |
| Lacunarity α | 0.900 ± 0.403 | 0.572 ± 0.350 | 0.639 ± 0.303 | 0.672 ± 0.316 | 0.639 ± 0.420 | 0.420 |
| Lacunarity β | 0.062 ± 0.039 | 0.113 ± 0.040 | 0.101 ± 0.044 | 0.092 ± 0.041 | 0.148 ± 0.062 | 0.012 |
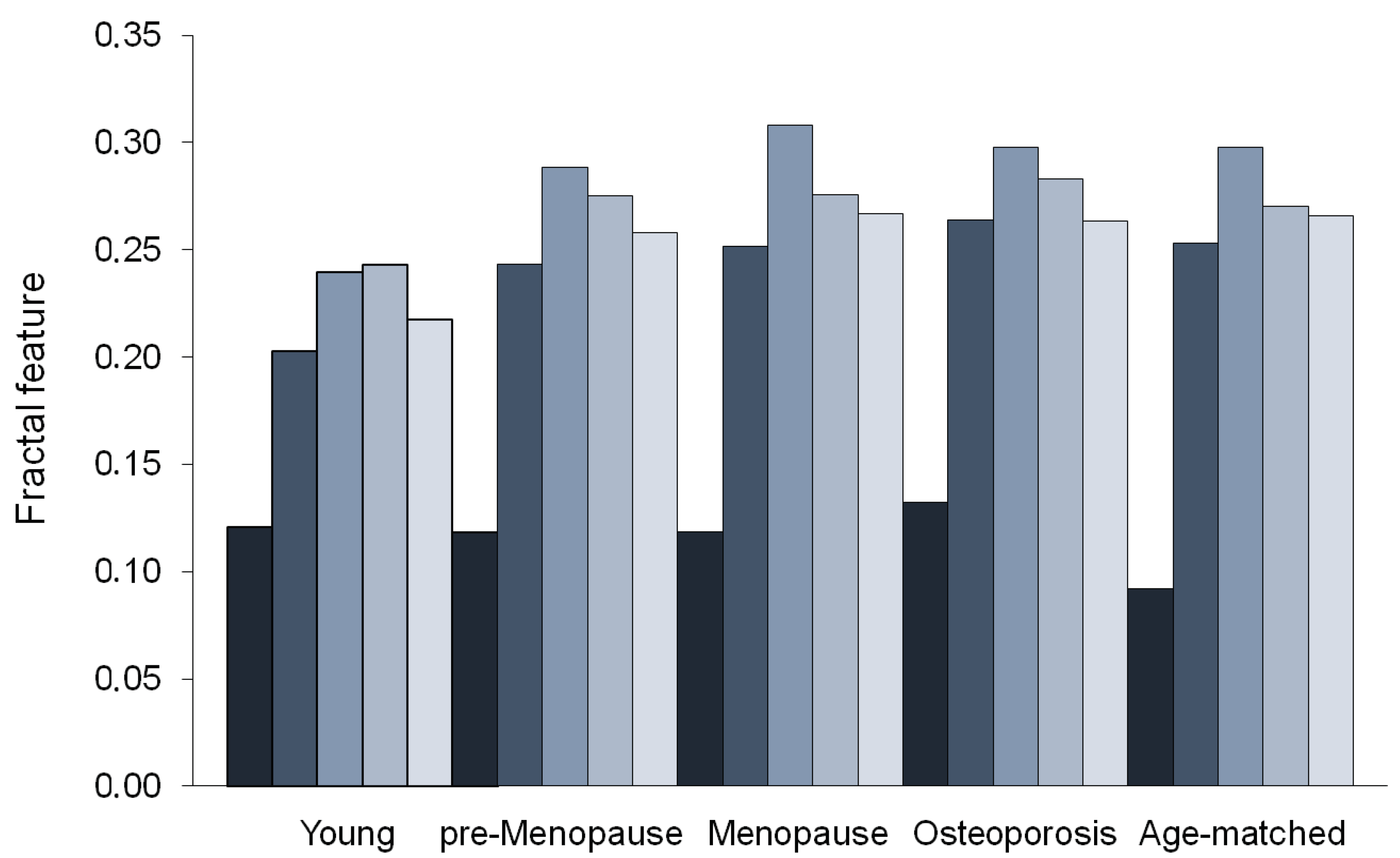
| Succolarity µ↑ | 0.203 ± 0.075 | 0.243 ± 0.047 | 0.252 ± 0.070 | 0.253 ± 0.084 | 0.264 ± 0.077 | 0.371 |
| Succolarity µ → | 0.240 ± 0.085 | 0.288 ± 0.050 | 0.308 ± 0.070 | 0.298 ± 0.077 | 0.298 ± 0.080 | 0.499 |
| Succolarity µ ↓ | 0.243 ± 0.096 | 0.275 ± 0.042 | 0.276 ± 0.072 | 0.270 ± 0.086 | 0.283 ± 0.086 | 0.361 |
| Succolarity µ ← | 0.218 ± 0.080 | 0.258 ± 0.042 | 0.267 ± 0.071 | 0.266 ± 0.084 | 0.263 ± 0.079 | 0.470 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





