Submitted:
23 February 2024
Posted:
24 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
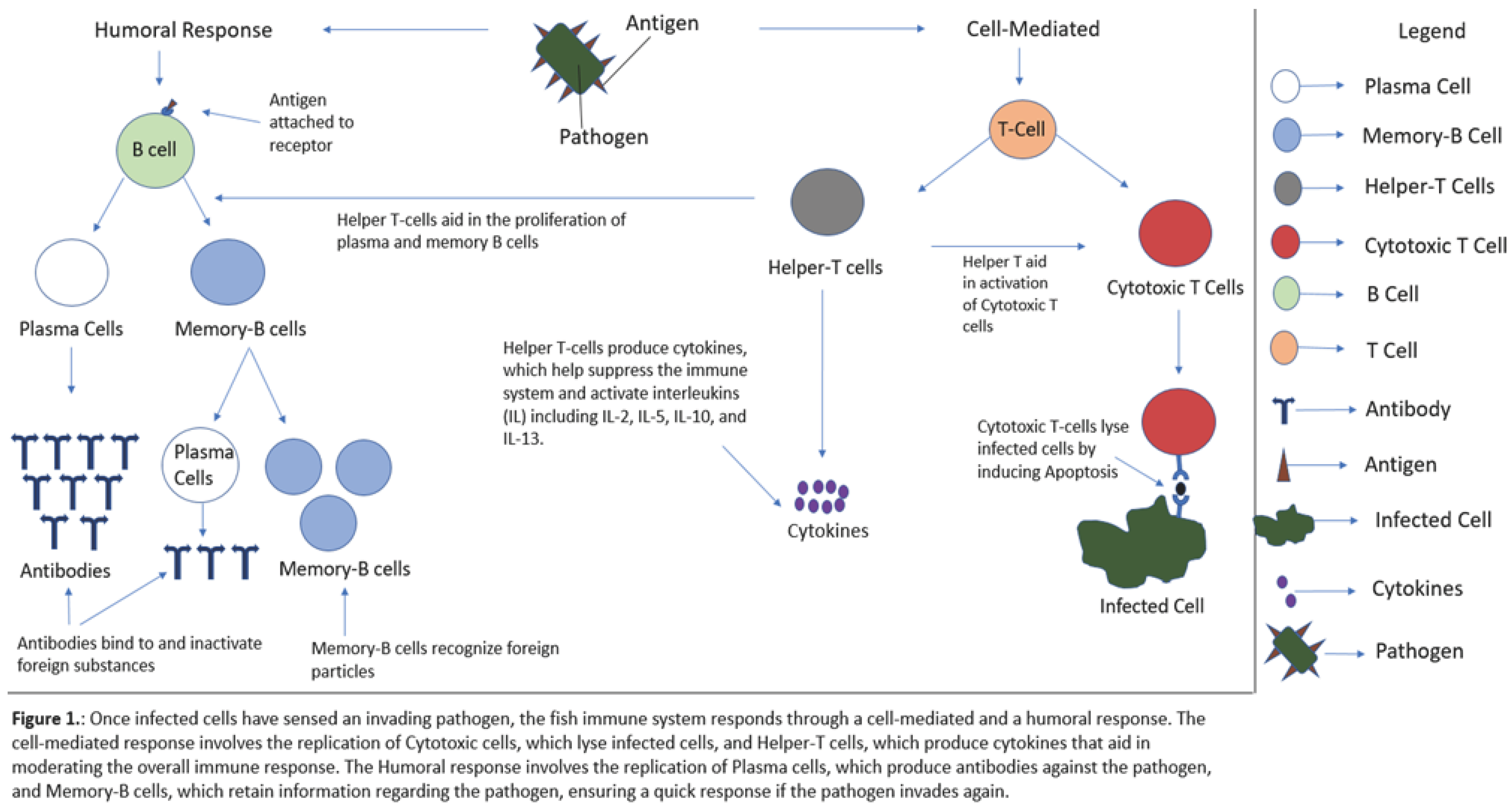
2. Fish Immune System
3. Bacterial, Viral, and Parasitic Diseases in Fish
4. Current Licensed Vaccines for Bacterial Diseases
4.1. Edwardsiellosis in Fish
4.2. Enteric Septicemia of Catfish
4.3. Bacterial Kidney Disease:
4.4. Flavobacteriosis/Columnaris
4.5. Furunculosis
5. Current Licensed Vaccines for Viral Diseases
Koi Herpes Virus (KHV)
Infectious hematopoietic necrosis virus (IHNV)
Red Sea Bream Iridovirus (RSIV)
Salmonid Alphavirus (SAV)
Infectious Pancreatic Necrosis Virus (IPNV)
Infectious Salmon Anemia (ISA)
Tilapia Lake Virus
5.1. Current Licensed Vaccines for Parasitic Diseases
5.2. Challenges and Limitations in Developing Vaccines for Fish
6. Summary and Conclusions
Acknowledgments
References
- A. Adams, Progress, challenges and opportunities in fish vaccine development, Fish & shellfish immunology 90 (2019) 210-214. [CrossRef]
- D.W. Wanja, P.G. Mbuthia, R.M. Waruiru, J.M. Mwadime, L.C. Bebora, P.N. Nyaga, H.A. Ngowi, Fish Husbandry Practices and Water Quality in Central Kenya: Potential Risk Factors for Fish Mortality and Infectious Diseases, Veterinary medicine international 2020 (2020) 6839354. [CrossRef]
- A.K. Dhar, S.K. Manna, F.C. Thomas Allnutt, Viral vaccines for farmed finfish, Virusdisease 25(1) (2014) 1-17. [CrossRef]
- W. Surachetpong, S.R.K. Roy, P. Nicholson, Tilapia lake virus: The story so far, Journal of Fish Diseases 43(10) (2020) 1115-1132. [CrossRef]
- F.A.O. Fisheries, Aquaculture Department, The state of world fisheries and aquaculture (2012) 1-153.
- A. Assefa, F. Abunna, Maintenance of Fish Health in Aquaculture: Review of Epidemiological Approaches for Prevention and Control of Infectious Disease of Fish, Veterinary Medicine International 2018 (2018) 5432497. [CrossRef]
- E.I. Broughton, D.G. Walker, Policies and practices for aquaculture food safety in China, Food Policy 35(5) (2010) 471-478. [CrossRef]
- G.T. Rijkers, A.G. Teunissen, R. Van Oosterom, W.B. Van Muiswinkel, The immune system of cyprinid fish. The immunosuppressive effect of the antibiotic oxytetracycline in carp (Cyprinus carpio L.), Aquaculture 19(2) (1980) 177-189. [CrossRef]
- M.A. Salam, M.Y. Al-Amin, M.T. Salam, J.S. Pawar, N. Akhter, A.A. Rabaan, M.A.A. Alqumber, Antimicrobial Resistance: A Growing Serious Threat for Global Public Health, Healthcare (Basel, Switzerland) 11(13) (2023). [CrossRef]
- J. Ma, T.J. Bruce, E.M. Jones, K.D. Cain, A Review of Fish Vaccine Development Strategies: Conventional Methods and Modern Biotechnological Approaches, Microorganisms 7(11) (2019). [CrossRef]
- M. Horzinek, V. Schijns, M. Denis, P. Desmettre, L. Babiuk, General description of vaccines, Veterinary Vaccinology. Amsterdam: Elsevier (1997) 131-52.
- V.f. Aquaculture, USDA, AMS, LPS Agricultural Analytics Division for the USDA National Organic Program, USDA (2014).
- Y. Du, X. Hu, L. Miao, J. Chen, Current status and development prospects of aquatic vaccines, Frontiers in immunology 13 (2022). [CrossRef]
- M. Guo, C. Li, An overview of cytokine used as adjuvants in fish: current state and future trends, Reviews in Aquaculture 13(2) (2021) 996-1014. [CrossRef]
- C. Secombes, Will advances in fish immunology change vaccination strategies?, Fish & shellfish immunology 25(4) (2008) 409-16. [CrossRef]
- C. Langevin, E. Aleksejeva, G. Passoni, N. Palha, J.-P. Levraud, P. Boudinot, The Antiviral Innate Immune Response in Fish: Evolution and Conservation of the IFN System, Journal of Molecular Biology 425(24) (2013) 4904-4920. [CrossRef]
- E.R. Verrier, C. Langevin, C. Tohry, A. Houel, V. Ducrocq, A. Benmansour, E. Quillet, P. Boudinot, Genetic resistance to rhabdovirus infection in teleost fish is paralleled to the derived cell resistance status, PLoS One 7(4) (2012) e33935-e33935. [CrossRef]
- B. Swain, C.T. Powell, R. Curtiss, Virulence, immunogenicity and live vaccine potential of aroA and phoP mutants of Edwardsiella piscicida in zebrafish, Microbial Pathogenesis 162 (2022) 105355. [CrossRef]
- B. Swain, C.T. Powell, R. Curtiss, Pathogenicity and immunogenicity of Edwardsiella piscicida ferric uptake regulator (fur) mutations in zebrafish, Fish & Shellfish Immunology 107 (2020) 497-510. [CrossRef]
- B. Swain, V.A. Campodonico, R. Curtiss, Recombinant Attenuated Edwardsiella piscicida Vaccine Displaying Regulated Lysis to Confer Biological Containment and Protect Catfish against Edwardsiellosis, Vaccines 11(9) (2023) 1470.
- C.A. Shoemaker, P.H. Klesius, J.J. Evans, C.R. Arias, Use of Modified Live Vaccines in Aquaculture, Journal of the World Aquaculture Society 40(5) (2009) 573-585. [CrossRef]
- R. Gudding, W.B. Van Muiswinkel, A history of fish vaccination: Science-based disease prevention in aquaculture, Fish & Shellfish Immunology 35(6) (2013) 1683-1688. [CrossRef]
- B. Swain, C.T. Powell, R. Curtiss, Construction and Evaluation of Recombinant Attenuated Edwardsiella piscicida Vaccine (RAEV) Vector System Encoding Ichthyophthirius multifiliis (Ich) Antigen IAG52B, Frontiers in Immunology 12 (2022). [CrossRef]
- P.R. Rauta, B. Nayak, S. Das, Immune system and immune responses in fish and their role in comparative immunity study: a model for higher organisms, Immunology letters 148(1) (2012) 23-33. [CrossRef]
- N.C. Smith, M.L. Rise, S.L. Christian, A Comparison of the Innate and Adaptive Immune Systems in Cartilaginous Fish, Ray-Finned Fish, and Lobe-Finned Fish, Frontiers in immunology 10 (2019) 2292. [CrossRef]
- G.Á. Aristizábal B, Innate immune system." In Autoimmunity: From Bench to Bedside, El Rosario University Press Chapter 2 (2013).
- B. Magnadóttir, Innate immunity of fish (overview), Fish & shellfish immunology 20(2) (2006) 137-51. [CrossRef]
- S. Mutoloki, H.M. Munang'andu, Ø. Evensen, Oral Vaccination of Fish - Antigen Preparations, Uptake, and Immune Induction, Front Immunol 6 (2015) 519-519. [CrossRef]
- B. Swain, M. Samanta, M. Basu, P. Panda, B.R. Sahoo, N.K. Maiti, B.K. Mishra, A.E. Eknath, Molecular characterization, inductive expression and mechanism of interleukin-10 gene induction in the Indian major carp, catla (Catla catla), Aquaculture Research 43(6) (2012) 897-907. [CrossRef]
- J. Playfair, Living with Germs: In sickness and in health, 2004.
- C.A. Janeway, Paul Travers, Mark Walport, and Donald J. Capra., Immunobiology, UK: Garland Science: Taylor & Francis Group 5th edition (2001).
- J. Gregory, Taylor, Dennis., Fosbery, Richard., Jones, Mary, Cambridge International AS and A Level Biology Coursebook with CD-ROM. United Kingdom, Cambridge University Press (2014).
- T. Nakanishi, Y. Shibasaki, Y. Matsuura, T Cells in Fish, Biology (Basel) 4(4) (2015) 640-663. [CrossRef]
- M. Yamasaki, K. Araki, T. Nakanishi, C. Nakayasu, Y. Yoshiura, T. Iida, A. Yamamoto, Adaptive immune response to Edwardsiella tarda infection in ginbuna crucian carp, Carassius auratus langsdorfii, Veterinary immunology and immunopathology 153(1-2) (2013) 83-90. [CrossRef]
- Q. Wang, W. Ji, Z. Xu, Current use and development of fish vaccines in China, Fish & shellfish immunology 96 (2020) 223-234. [CrossRef]
- B. Swain, C.T. Powell, R. Curtiss, 3rd, Pathogenicity and immunogenicity of Edwardsiella piscicida ferric uptake regulator (fur) mutations in zebrafish, Fish Shellfish Immunol 107(Pt B) (2020) 497-510. [CrossRef]
- Y. Yu, Q. Wang, Z. Huang, L. Ding, Z. Xu, Immunoglobulins, Mucosal Immunity and Vaccination in Teleost Fish, Front Immunol 11 (2020) 567941. [CrossRef]
- S.P. Wamala, K.K. Mugimba, S. Mutoloki, Ø. Evensen, R. Mdegela, D.K. Byarugaba, H. Sørum, Occurrence and antibiotic susceptibility of fish bacteria isolated from Oreochromis niloticus (Nile tilapia) and Clarias gariepinus (African catfish) in Uganda, Fisheries and Aquatic Sciences 21(1) (2018) 6. [CrossRef]
- M.K. Viršek, M.N. Lovšin, Š. Koren, A. Kržan, M. Peterlin, Microplastics as a vector for the transport of the bacterial fish pathogen species Aeromonas salmonicida, Marine Pollution Bulletin 125(1) (2017) 301-309. [CrossRef]
- S. Kumari, Freshwater and marine water fish diseases: A review, Int J Fish Aquat Stud 8(4) (2020) 4.
- L. Gui, V.G. Chinchar, Q. Zhang, Molecular basis of pathogenesis of emerging viruses infecting aquatic animals, Aquaculture and Fisheries 3(1) (2018) 1-5. [CrossRef]
- J.C. Leong, Fish Viruses, Encyclopedia of Virology (2008) 227-234. [CrossRef]
- L.B. Hølvold, A.I. Myhr, R.A. Dalmo, Strategies and hurdles using DNA vaccines to fish, Veterinary research 45(1) (2014) 21-21. [CrossRef]
- F. Athanasopoulou, I. Pappas, K. Bitchava, An overview of the treatments of parasitic disease in Mediterranean aquaculture, Options Méditerranéennes 86 (2009).
- R.Y. Ruth Francis-Floyd, and Deborah Pouder, Ichthyophthirius multifiliis (White Spot) Infections in Fish, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida (2018).
- Bridging America and Russia with shared perspectives on aquatic animal health: Proceedings of the Third Bilateral Conference Between the United States and Russia, Aquatic Animal Health 2009, Khaled bin Sultan Living Oceans Foundation, Landover, MD, 2011.
- A.E. Toranzo, J.L. Romalde, B. Magariños, J.L. Barja, Present and future of aquaculture vaccines against fish bacterial diseases, in: B. Basurco, C. Rogers (Eds.), The use of veterinary drugs and vaccines in Mediterranean aquaculture, Zaragoza : CIHEAM2009, pp. 155–176.
- N. Bujan, A. Toranzo, B. Magarinos, Edwardsiella piscicida: a significant bacterial pathogen of cultured fish, Diseases of Aquatic Organisms 131 (2018). [CrossRef]
- K.Y. Leung, Q. Wang, Z. Yang, B.A. Siame, Edwardsiella piscicida: A versatile emerging pathogen of fish, Virulence 10(1) (2019) 555-567. [CrossRef]
- S.B. Park, T. Aoki, T.S. Jung, Pathogenesis of and strategies for preventing Edwardsiella tarda infection in fish, Veterinary Research 43(1) (2012) 67. [CrossRef]
- B. Swain, C.T. Powell, R. Curtiss, Virulence, immunogenicity and live vaccine potential of aroA and phoP mutants of Edwardsiella piscicida in zebrafish, Microbial Pathogenesis (2021) 105355. [CrossRef]
- M. Sayed, O. Ozdemir, M. Essa, A. Olivier, A. Karsi, M.L. Lawrence, H. Abdelhamed, Virulence and live vaccine potential of Edwardsiella piscicida phoP and phoQ mutants in catfish against edwardsiellosis, J Fish Dis 44(9) (2021) 1463-1474. [CrossRef]
- W. Yang, L. Wang, L. Zhang, J. Qu, Q. Wang, Y. Zhang, An invasive and low virulent Edwardsiella tarda esrB mutant promising as live attenuated vaccine in aquaculture, Appl Microbiol Biotechnol 99(4) (2015) 1765-77. [CrossRef]
- B. Swain, C.T. Powell, R. Curtiss, 3rd, Construction and Evaluation of Recombinant Attenuated Edwardsiella piscicida Vaccine (RAEV) Vector System Encoding Ichthyophthirius multifiliis (Ich) Antigen IAG52B, Frontiers in immunology 12 (2021) 802760. [CrossRef]
- A.O. Kordon, H. Abdelhamed, H. Ahmed, W. Baumgartner, A. Karsi, L.M. Pinchuk, Assessment of the Live Attenuated and Wild-Type Edwardsiella ictaluri-Induced Immune Gene Expression and Langerhans-Like Cell Profiles in the Immune-Related Organs of Catfish, Front Immunol 10 (2019) 392. [CrossRef]
- H. Abdelhamed, M.L. Lawrence, A. Karsi, Development and Characterization of a Novel Live Attenuated Vaccine Against Enteric Septicemia of Catfish, Front Microbiol 9 (2018) 1819-1819. [CrossRef]
- D.J. Wise, T.E. Greenway, T.S. Byars, M.J. Griffin, L.H. Khoo, Oral Vaccination of Channel Catfish against Enteric Septicemia of Catfish Using a Live Attenuated Edwardsiella ictaluri Isolate, Journal of aquatic animal health 27(2) (2015) 135-43. [CrossRef]
- N. Dahal, H. Abdelhamed, J. Lu, A. Karsi, M.L. Lawrence, Tricarboxylic Acid Cycle and One-Carbon Metabolism Pathways Are Important in Edwardsiella ictaluri Virulence, PLoS One 8(6) (2013) e65973. [CrossRef]
- S.W. Nho, H. Abdelhamed, A. Karsi, M.L. Lawrence, Improving safety of a live attenuated Edwardsiella ictaluri vaccine against enteric septicemia of catfish and evaluation of efficacy, Veterinary Microbiology 210 (2017) 83-90. [CrossRef]
- M.R. Delghandi, M. El-Matbouli, S. Menanteau-Ledouble, Renibacterium salmoninarum-The Causative Agent of Bacterial Kidney Disease in Salmonid Fish, Pathogens 9(10) (2020) 845. [CrossRef]
- D.G. Elliott, G.D. Wiens, K.L. Hammell, L.D. Rhodes, Vaccination against Bacterial Kidney Disease, Fish Vaccination2014, pp. 255–272. [CrossRef]
- O. Brynildsrud, E.J. Feil, J. Bohlin, S. Castillo-Ramirez, D. Colquhoun, U. McCarthy, I.M. Matejusova, L.D. Rhodes, G.D. Wiens, D.W. Verner-Jeffreys, Microevolution of Renibacterium salmoninarum: evidence for intercontinental dissemination associated with fish movements, Isme j 8(4) (2014) 746-56. [CrossRef]
- I. Matejusova, N. Bain, D.J. Colquhoun, E.J. Feil, U. McCarthy, D. McLennan, M. Snow, D. Verner-Jeffreys, I.S. Wallace, S.J. Weir, M. Hall, Multilocus variable-number tandem-repeat genotyping of Renibacterium salmoninarum, a bacterium causing bacterial kidney disease in salmonid fish, BMC Microbiology 13(1) (2013) 285. [CrossRef]
- J. Bethke, M. Poblete-Morales, R. Irgang, A. Yáñez, R. Avendaño-Herrera, Iron acquisition and siderophore production in the fish pathogen Renibacterium salmoninarum, J Fish Dis 39(11) (2016) 1275-1283. [CrossRef]
- T. Zhou, Z. Yuan, S. Tan, Y. Jin, Y. Yang, H. Shi, W. Wang, D. Niu, L. Gao, W. Jiang, D. Gao, Z. Liu, A Review of Molecular Responses of Catfish to Bacterial Diseases and Abiotic Stresses, Front Physiol 9 (2018) 1113-1113. [CrossRef]
- H. Mohammed, O. Olivares-Fuster, S. LaFrentz, C.R. Arias, New attenuated vaccine against columnaris disease in fish: choosing the right parental strain is critical for vaccine efficacy, Vaccine 31(45) (2013) 5276-80. [CrossRef]
- G. Rathore, Bacterial Vaccines for Fishes: Current Status, PROPHYLAXIS IN AQUACULTURE (2017) 2.
- S. Bravo, Environmental impacts and management of veterinary medicines in aquaculture: the case of salmon aquaculture in Chile, Improving biosecurity through prudent and responsible use of veterinary medicines in aquatic food production (2012) 11.
- A. Gavriilidou, J. Gutleben, D. Versluis, F. Forgiarini, M.W.J. van Passel, C.J. Ingham, H. Smidt, D. Sipkema, Comparative genomic analysis of Flavobacteriaceae: insights into carbohydrate metabolism, gliding motility and secondary metabolite biosynthesis, BMC Genomics 21(1) (2020) 569. [CrossRef]
- F. Dumetz, S.E. Lapatra, E. Duchaud, S. Claverol, M. Le Hénaff, The Flavobacterium psychrophilum OmpA, an outer membrane glycoprotein, induces a humoral response in rainbow trout, J Appl Microbiol 103(5) (2007) 1461-70. [CrossRef]
- J. Bøgwald, R.A. Dalmo, Review on Immersion Vaccines for Fish: An Update 2019, Microorganisms 7(12) (2019) 627. [CrossRef]
- P.S. Sudheesh, K.D. Cain, Prospects and challenges of developing and commercializing immersion vaccines for aquaculture, International Biology Review 1(1) (2017).
- J. Ma, T.J. Bruce, P.S. Sudheesh, C. Knupp, T.P. Loch, M. Faisal, K.D. Cain, Assessment of cross-protection to heterologous strains of Flavobacterium psychrophilum following vaccination with a live-attenuated coldwater disease immersion vaccine, J Fish Dis 42(1) (2019) 75-84. [CrossRef]
- T.J. Bruce, J. Ma, C. Knupp, T.P. Loch, M. Faisal, K.D. Cain, Cross-protection of a live-attenuated Flavobacterium psychrophilum immersion vaccine against novel Flavobacterium spp. and Chryseobacterium spp. strains, J Fish Dis 43(8) (2020) 915-928. [CrossRef]
- M.D. Fast, B. Tse, J.M. Boyd, S.C. Johnson, Mutations in the Aeromonas salmonicida subsp. salmonicida type III secretion system affect Atlantic salmon leucocyte activation and downstream immune responses, Fish Shellfish Immunol 27(6) (2009) 721-8. [CrossRef]
- B.K. Gudmundsdottir, Bjornsdottir, B., Aeromonas salmonicida and A. hydrophila., 2017. [CrossRef]
- L.M. Braden, S.K. Whyte, A.B.J. Brown, C.V. Iderstine, C. Letendre, D. Groman, J. Lewis, S.L. Purcell, T. Hori, M.D. Fast, Vaccine-Induced Protection Against Furunculosis Involves Pre-emptive Priming of Humoral Immunity in Arctic Charr, Frontiers in Immunology 10(120) (2019). [CrossRef]
- P. Vanden Bergh, M. Heller, S. Braga-Lagache, J. Frey, The Aeromonas salmonicida subsp. salmonicida exoproteome: determination of the complete repertoire of Type-Three Secretion System effectors and identification of other virulence factors, Proteome Sci 11(1) (2013) 42. [CrossRef]
- H. Mikkelsen, M.B. Schrøder, V. Lund, Vibriosis and atypical furunculosis vaccines; efficacy, specificity and side effects in Atlantic cod, Gadus morhua L, Aquaculture 242(1-4) (2004) 81-91. [CrossRef]
- G. Rørstad, P.M. Aasjord, B. Robertsen, Adjuvant effect of a yeast glucan in vaccines against furunculosis in Atlantic salmon (Salmo salar L.), Fish & Shellfish Immunology 3(3) (1993) 179-190. [CrossRef]
- A. Toranzo, J. Romalde, B. Magariños, J. Barja, Present and future of aquaculture vaccines against fish bacterial diseases, Options Mediterraneennes 86 (2009) 155-176.
- T. Erkinharju, M.R. Lundberg, E. Isdal, I. Hordvik, R.A. Dalmo, T. Seternes, Studies on the antibody response and side effects after intramuscular and intraperitoneal injection of Atlantic lumpfish (Cyclopterus lumpus L.) with different oil-based vaccines, J Fish Dis 40(12) (2017) 1805-1813. [CrossRef]
- M.H. Marana, D. Sepúlveda, D. Chen, A. Al-Jubury, R.M. Jaafar, P.W. Kania, N.H. Henriksen, B. Krossøy, I. Dalsgaard, N. Lorenzen, K. Buchmann, A pentavalent vaccine for rainbow trout in Danish aquaculture, Fish Shellfish Immunol 88 (2019) 344-351. [CrossRef]
- K.R. Villumsen, P.W. Kania, D. Christensen, E.O. Koppang, A.M. Bojesen, Injection Vaccines Formulated with Nucleotide, Liposomal or Mineral Oil Adjuvants Induce Distinct Differences in Immunogenicity in Rainbow Trout, Vaccines (Basel) 8(1) (2020). [CrossRef]
- R. Hoare, S.J. Jung, T.P.H. Ngo, K. Bartie, J. Bailey, K.D. Thompson, A. Adams, Efficacy and safety of a non-mineral oil adjuvanted injectable vaccine for the protection of Atlantic salmon (Salmo salar L.) against Flavobacterium psychrophilum, Fish Shellfish Immunol 85 (2019) 44-51. [CrossRef]
- H. Tziouvas, a.P. Varvarigos, Intensity scale of side effects in European sea bass (Dicentrarchus labrax) post intraperitoneal injection with commercial oil-adjuvanted vaccines Bulletin of the European Association of Fish Pathologist 41(3) (2021) 8. [CrossRef]
- J.C. Thornton, R.A. Garduq o, W.W. Kay, The development of live vaccines for furunculosis lacking the A-layer and O-antigen of Aeromonas salmonicida, Journal of Fish Diseases 17 (1994) 195-204. [CrossRef]
- S. Menanteau-Ledouble, M. El-Matbouli, Antigens of Aeromonas salmonicida subsp. salmonicida specifically induced in vivo in Oncorhynchus mykiss, Journal of fish diseases 39(8) (2016) 1015-1019. [CrossRef]
- X.-D. Ling, W. Dong, Y. Zhang, J. Hu, J.-x. Liu, X. Zhao, A recombinant adenovirus targeting typical Aeromonas salmonicida induces an antibody-mediated adaptive immune response after immunization of rainbow trout, Microbial pathogenesis 133 (2019) 103559. [CrossRef]
- S.M. Bergmann, Y. Jin, K. Franzke, B. Grunow, Q. Wang, S. Klafack, Koi herpesvirus (KHV) and KHV disease (KHVD) – a recently updated overview, Journal of Applied Microbiology 129(1) (2020) 98-103. [CrossRef]
- L. Schröder, S. Klafack, S.M. Bergmann, D. Fichtner, Y. Jin, P.Y. Lee, D. Höper, T.C. Mettenleiter, W. Fuchs, Generation of a potential koi herpesvirus live vaccine by simultaneous deletion of the viral thymidine kinase and dUTPase genes, J Gen Virol 100(4) (2019) 642-655. [CrossRef]
- L.-C. Cui, X.-T. Guan, Z.-M. Liu, C.-Y. Tian, Y.-G. Xu, Recombinant lactobacillus expressing G protein of spring viremia of carp virus (SVCV) combined with ORF81 protein of koi herpesvirus (KHV): A promising way to induce protective immunity against SVCV and KHV infection in cyprinid fish via oral vaccination, Vaccine 33(27) (2015) 3092-3099. [CrossRef]
- F. Hu, Y. Li, Q. Wang, G. Wang, B. Zhu, Y. Wang, W. Zeng, J. Yin, C. Liu, S.M. Bergmann, C. Shi, Carbon nanotube-based DNA vaccine against koi herpesvirus given by intramuscular injection, Fish & Shellfish Immunology 98 (2020) 810-818. [CrossRef]
- M.M.D. Peñaranda, S.E. LaPatra, G. Kurath, Specificity of DNA vaccines against the U and M genogroups of infectious hematopoietic necrosis virus (IHNV) in rainbow trout (Oncorhynchus mykiss), Fish & Shellfish Immunology 31(1) (2011) 43-51. [CrossRef]
- E.T. Larragoite, L. Tacchi, S.E. LaPatra, I. Salinas, An attenuated virus vaccine appears safe to the central nervous system of rainbow trout (Oncorhynchus mykiss) after intranasal delivery, Fish Shellfish Immunol 49 (2016) 351-4. [CrossRef]
- L. Tang, H. Kang, K. Duan, M. Guo, G. Lian, Y. Wu, Y. Li, S. Gao, Y. Jiang, J. Yin, M. Liu, Effects of Three Types of Inactivation Agents on the Antibody Response and Immune Protection of Inactivated IHNV Vaccine in Rainbow Trout, Viral Immunol 29(7) (2016) 430-5. [CrossRef]
- R.N. Rouxel, C. Tafalla, E. Mérour, E. Leal, S. Biacchesi, M. Brémont, D.S. Lyles, Attenuated Infectious Hematopoietic Necrosis Virus with Rearranged Gene Order as Potential Vaccine, Journal of Virology 90(23) (2016) 10857-10866. [CrossRef]
- A. Long, J. Richard, L. Hawley, S.E. LaPatra, K.A. Garver, Transmission potential of infectious hematopoietic necrosis virus in APEX-IHN®-vaccinated Atlantic salmon, Dis Aquat Organ 122(3) (2017) 213-221. [CrossRef]
- C.Y. Yong, H.K. Ong, H.C. Tang, S.K. Yeap, A.R. Omar, K.L. Ho, W.S. Tan, Infectious hematopoietic necrosis virus: advances in diagnosis and vaccine development, PeerJ 7 (2019) e7151. [CrossRef]
- C.M. Caipang, T. Takano, I. Hirono, T. Aoki, Genetic vaccines protect red seabream, Pagrus major, upon challenge with red seabream iridovirus (RSIV), Fish Shellfish Immunol 21(2) (2006) 130-8. [CrossRef]
- K. Nakajima, Y. Maeno, A. Honda, K. Yokoyama, T. Tooriyama, S. Manabe, Effectiveness of a vaccine against red sea bream iridoviral disease in a field trial test, Dis Aquat Organ 36(1) (1999) 73-5. [CrossRef]
- M.H. Jung, C. Nikapitiya, S.J. Jung, DNA vaccine encoding myristoylated membrane protein (MMP) of rock bream iridovirus (RBIV) induces protective immunity in rock bream (Oplegnathus fasciatus), Vaccine 36(6) (2018) 802-810. [CrossRef]
- S.Y. Oh, M.J. Oh, T. Nishizawa, Potential for a live red seabream iridovirus (RSIV) vaccine in rock bream Oplegnathus fasciatus at a low rearing temperature, Vaccine 32(3) (2014) 363-8. [CrossRef]
- S. Senapin, H.T. Dong, W. Meemetta, W. Gangnonngiw, P. Sangsuriya, R. Vanichviriyakit, M. Sonthi, B. Nuangsaeng, Mortality from scale drop disease in farmed Lates calcarifer in Southeast Asia, J Fish Dis 42(1) (2019) 119-127. [CrossRef]
- K. Thanasaksiri, K. Fukuda, S. Tsubone, H. Miyadai, T. Murakami, A. Murakami, R. Takano, Efficacy of a bivalent inactivated vaccine against red seabream iridovirus and Streptococcus iniae in red seabream, Pagrus major, Aquaculture 492 (2018) 132-136. [CrossRef]
- I. Deperasińska, P. Schulz, A.K. Siwicki, Salmonid Alphavirus (SAV), J Vet Res 62(1) (2018) 1-6. [CrossRef]
- D.A. Graham, H.R. Rowley, P. Frost, Cross-neutralization studies with salmonid alphavirus subtype 1-6 strains: results with sera from experimental studies and natural infections, J Fish Dis 37(8) (2014) 683-91. [CrossRef]
- B. Bang Jensen, A.B. Kristoffersen, C. Myr, E. Brun, Cohort study of effect of vaccination on pancreas disease in Norwegian salmon aquaculture, Dis Aquat Organ 102(1) (2012) 23-31. [CrossRef]
- C.J. Chang, J. Gu, B. Robertsen, Protective effect and antibody response of DNA vaccine against salmonid alphavirus 3 (SAV3) in Atlantic salmon, J Fish Dis 40(12) (2017) 1775-1781. [CrossRef]
- C.J. Chang, J. Gu, B. Robertsen, Protective effect and antibody response of DNA vaccine against salmonid alphavirus 3 (SAV3) in Atlantic salmon, Journal of Fish Diseases 40(12) (2017) 1775-1781. [CrossRef]
- C. Collins, K. Lester, J. Del-Pozo, B. Collet, Non-Lethal Sequential Individual Monitoring of Viremia in Relation to DNA Vaccination in Fish-Example Using a Salmon Alphavirus DNA Vaccine in Atlantic Salmon Salmo salar, Vaccines (Basel) 9(2) (2021). [CrossRef]
- J. Pajdak-Czaus, P. Schulz, E. Terech-Majewska, W. Szweda, A.K. Siwicki, A. Platt-Samoraj, Influence of Infectious Pancreatic Necrosis Virus and Yersinia ruckeri Co-Infection on a Non-Specific Immune System in Rainbow Trout (Oncorhynchus mykiss), Animals (Basel) 11(7) (2021) 1974. [CrossRef]
- S. Kanrar, A.K. Dhar, Complete Genome Sequence of a Novel Mutant Strain of Vibrio parahaemolyticus from Pacific White Shrimp (Penaeus vannamei), Genome Announcements 6(24) (2018) e00497-18. [CrossRef]
- N.A. Ballesteros, S. Rodriguez Saint-Jean, S.I. Perez-Prieto, Food pellets as an effective delivery method for a DNA vaccine against infectious pancreatic necrosis virus in rainbow trout (Oncorhynchus mykiss, Walbaum), Fish & Shellfish Immunology 37(2) (2014) 220-228. [CrossRef]
- S. Ahmadivand, M. Soltani, M. Behdani, Ø. Evensen, E. Alirahimi, E. Soltani, R. Hassanzadeh, J. Ashrafi-Helan, VP2 (PTA motif) encoding DNA vaccine confers protection against lethal challenge with infectious pancreatic necrosis virus (IPNV) in trout, Molecular Immunology 94 (2018) 61-67. [CrossRef]
- F.S. Kibenge, K. Munir, M.J. Kibenge, T. Joseph, E. Moneke, Infectious salmon anemia virus: causative agent, pathogenesis and immunity, Anim Health Res Rev 5(1) (2004) 65-78. [CrossRef]
- F.S. Kibenge, Kibenge, M. J., Joseph, T., & McDougall, J. , The development of infectious salmon anemia virus vaccines in Canada, In Miller, Otis; Cipriano, Rocco C., tech. coords. International response to infectious salmon anemia: prevention, control, and eradication: proceedings of a symposium, US Department of Agriculture, Animal and Plant Health Inspection Service; US Department of the Interior, US Geological Survey; US Department of Commerce, National Marine Fisheries Service, New Orleans, LA. Tech. Bull. 1902. Washington, DC, 2003, pp. 39–49.
- A. Wolf, K. Hodneland, P. Frost, S. Braaen, E. Rimstad, A hemagglutinin-esterase-expressing salmonid alphavirus replicon protects Atlantic salmon (Salmo salar) against infectious salmon anemia (ISA), Vaccine 31(4) (2013) 661-9. [CrossRef]
- C.J. Chang, B. Sun, B. Robertsen, Adjuvant activity of fish type I interferon shown in a virus DNA vaccination model, Vaccine 33(21) (2015) 2442-8. [CrossRef]
- M.S. Ahasan, W. Keleher, C. Giray, B. Perry, W. Surachetpong, P. Nicholson, L. Al-Hussinee, K. Subramaniam, T.B. Waltzek, Genomic Characterization of Tilapia Lake Virus Isolates Recovered from Moribund Nile Tilapia (Oreochromis niloticus) on a Farm in the United States, Microbiol Resour Announc 9(4) (2020) e01368-19. [CrossRef]
- M. Abbadi, A. Basso, L. Biasini, R. Quartesan, A. Buratin, N. Davidovich, A. Toffan, Tilapia lake virus: A structured phylogenetic approach, Frontiers in genetics 14 (2023) 1069300. [CrossRef]
- M.D. Jansen, H.T. Dong, C.V. Mohan, Tilapia lake virus: a threat to the global tilapia industry?, Reviews in Aquaculture 11(3) (2019) 725-739. [CrossRef]
- Y. Thawornwattana, H.T. Dong, K. Phiwsaiya, P. Sangsuriya, S. Senapin, P. Aiewsakun, Tilapia lake virus (TiLV): Genomic epidemiology and its early origin, Transboundary and Emerging Diseases 68(2) (2021) 435-444. [CrossRef]
- W. Zeng, Y. Wang, H. Hu, Q. Wang, S.M. Bergmann, Y. Wang, B. Li, Y. Lv, H. Li, J. Yin, Y. Li, Cell Culture-Derived Tilapia Lake Virus-Inactivated Vaccine Containing Montanide Adjuvant Provides High Protection against Viral Challenge for Tilapia, Vaccines (Basel) 9(2) (2021). [CrossRef]
- T.T. Mai, P. Kayansamruaj, C. Soontara, P. Kerddee, D.-H. Nguyen, S. Senapin, J.Z. Costa, J. del-Pozo, K.D. Thompson, C. Rodkhum, H.T. Dong, Immunization of Nile Tilapia (Oreochromis niloticus) Broodstock with Tilapia Lake Virus (TiLV) Inactivated Vaccines Elicits Protective Antibody and Passive Maternal Antibody Transfer, Vaccines 10(2) (2022) 167. [CrossRef]
- S. Shivam, M. El-Matbouli, G. Kumar, Development of Fish Parasite Vaccines in the OMICs Era: Progress and Opportunities, Vaccines 9(2) (2021) 179. [CrossRef]
- L.T. Barrett, F. Oppedal, N. Robinson, T. Dempster, Prevention not cure: a review of methods to avoid sea lice infestations in salmon aquaculture, Reviews in Aquaculture 12(4) (2020) 2527-2543. [CrossRef]
- A. Sitjà-Bobadilla, Living off a fish: a trade-off between parasites and the immune system, Fish & shellfish immunology 25 4 (2008) 358-72. [CrossRef]
- R.A. Khan, Host-Parasite Interactions in Some Fish Species, Journal of Parasitology Research 2012 (2012) 237280. [CrossRef]
- A. Sfacteria, M. Brines, U. Blank, The mast cell plays a central role in the immune system of teleost fish, Mol Immunol 63(1) (2015) 3-8. [CrossRef]
- B.S. Dezfuli, T. Bo, M. Lorenzoni, A.P. Shinn, L. Giari, Fine structure and cellular responses at the host-parasite interface in a range of fish-helminth systems, Vet Parasitol 208(3-4) (2015) 272-9. [CrossRef]
- M.N. Faber, J.W. Holland, C.J. Secombes, Vaccination strategies and IgM responses against PKD in rainbow trout, Fish & Shellfish Immunology 91 (2019) 423. [CrossRef]
- Y. Carpio, L. Basabe, J. Acosta, A. Rodríguez, A. Mendoza, A. Lisperger, E. Zamorano, M. González, M. Rivas, S. Contreras, D. Haussmann, J. Figueroa, V.N. Osorio, G. Asencio, J. Mancilla, G. Ritchie, C. Borroto, M.P. Estrada, Novel gene isolated from Caligus rogercresseyi: A promising target for vaccine development against sea lice, Vaccine 29(15) (2011) 2810-2820. [CrossRef]
- P. Guragain, M. Tkachov, A.S. Båtnes, Y. Olsen, P. Winge, A.M. Bones, Principles and Methods of Counteracting Harmful Salmon–Arthropod Interactions in Salmon Farming: Addressing Possibilities, Limitations, and Future Options, Frontiers in Marine Science 8(965) (2021). [CrossRef]
- J.Y. Yao, X.M. Yuan, Y. Xu, W.L. Yin, L.Y. Lin, X.Y. Pan, G.L. Yang, C.F. Wang, J.Y. Shen, Live recombinant Lactococcus lactis vaccine expressing immobilization antigen (i-Ag) for protection against Ichthyophthirius multifiliis in goldfish, Fish Shellfish Immunol 58 (2016) 302-308. [CrossRef]
- L.v.G. Jørgensen, The fish parasite Ichthyophthirius multifiliis – Host immunology, vaccines and novel treatments, Fish & Shellfish Immunology 67 (2017) 586-595. [CrossRef]
- L. von Gersdorff Jørgensen, P.W. Kania, K.J. Rasmussen, A.H. Mattsson, J. Schmidt, A. Al-Jubury, A. Sander, A. Salanti, K. Buchmann, Rainbow trout (Oncorhynchus mykiss) immune response towards a recombinant vaccine targeting the parasitic ciliate Ichthyophthirius multifiliis, J Fish Dis 40(12) (2017) 1815-1821. [CrossRef]
- M. Kang, F. Feng, Y. Wang, L. Guo, L. Chen, K. Chen, Advances in Research into Oral Vaccines for Fish, 2018.
- C.W.E. Embregts, M. Forlenza, Oral vaccination of fish: Lessons from humans and veterinary species, Developmental & Comparative Immunology 64 (2016) 118-137. [CrossRef]
- H.M. Munang'andu, S. Mutoloki, Ø. Evensen, An Overview of Challenges Limiting the Design of Protective Mucosal Vaccines for Finfish, Front Immunol 6 (2015) 542-542. [CrossRef]
- M.R. Neutra, P.A. Kozlowski, Mucosal vaccines: the promise and the challenge, Nature Reviews Immunology 6(2) (2006) 148-158. [CrossRef]
- Z. Cao, S. Liu, H. Nan, K. Zhao, X. Xu, G. Wang, H. Ji, H. Chen, Immersion immunization with recombinant baculoviruses displaying cyprinid herpesvirus 2 membrane proteins induced protective immunity in gibel carp, Fish & shellfish immunology 93 (2019) 879-887. [CrossRef]
- C. Shoemaker, P. Klesius, J. Evans, C. Arias, Use of Modified Live Vaccines in Aquaculture, J World Aquac Soc 40 (2009). [CrossRef]
- S. Li, H. Xie, Z. Yan, B. Li, P. Wu, X. Qian, X. Zhang, J. Wu, J. Liu, X. Zhao, Development of a live vector vaccine against infectious hematopoietic necrosis virus in rainbow trout, Fish & shellfish immunology 89 (2019) 516-524. [CrossRef]
- J.L. Clarke, M.T. Waheed, A.G. Lössl, I. Martinussen, H. Daniell, How can plant genetic engineering contribute to cost-effective fish vaccine development for promoting sustainable aquaculture?, Plant molecular biology 83(1-2) (2013) 33-40. [CrossRef]
- M. Yimer, S. Tesfaye, B. Birhanu, Present Status and Future Prospects of Fish Vaccination: A Review, Journal of Veterinary Science & Technology 07 (2016). [CrossRef]
- C. Flores-Kossack, R. Montero, B. Köllner, K. Maisey, Chilean aquaculture and the new challenges: Pathogens, immune response, vaccination and fish diversification, Fish Shellfish Immunol 98 (2020) 52-67. [CrossRef]
| Name of the Diseases | Causative Agent | Fish it Affects |
|---|---|---|
| Bacterial | ||
| Atypical furunculosis | Aeromonas salmonicida | Salmonids |
| Motile aeromonid septicemia | Aeromonas hydrophila, A. caviae, A. sobria | Catfish, salmonids, cyprinids |
| Vibriosis | Vibrio anguillarum, V. ordalii | Marine Fish including: Salmonids, Yellowtail, Halibut, Amberjack |
| Enteric septicemia | Edwardsiella ictaluri | Catfish |
| Edwardsiellosis | Edwardsiella piscicida | Catfish, Striped Bass, Tilapia, Sea bream |
| Enteric septicemia | Edwardsiella tarda | Catfish, eel, hirame |
| Tuberculosis | Mycobacterium marinum, M. fortuitum, M. chelonae | Sea bass, tropical aquarium fish |
| Streptococcosis | Streptococcus parauberis | Turbot |
| Streptococcosis | Streptococcus phocae | Atlantic salmon |
| Flavobacteriosis | Flavobacterium psychrophilum | Salmonids, freshwater fish |
| Rainbow trout fry syndrome | Flavobacterium psychrophilum | Salmonids, freshwater Fish |
| Viral | ||
| Tilapia Lake Virus | Tilapia Tilapinevirus | Tilapia and hybrid tilapia fish |
| Infectious Hemorrhagic necrosis | Rhabdovirus | Snakehead, carp, barbs |
| Infectious salmon anemia | Orthomyxovirus | Atlantic salmon |
| Infectious pancreatic necrosis | Birnavirus | Salmonids, Sea brass, Sea bream, Pacific Cod |
| Koi Herpes Virus | Herpesvirus | Cyprinid Fish |
| Viral nervous necrosis | Betanovirus | Marine fish species including Sea brass, groupers, halibut |
| Salmonid Alphavirus | Alphavirus | Altantic Salmon, Rainbow Trout |
| Iridoviral disease | Iridovirus | Amberjack, Yellowtail, Red Sea Bream |
| Costiasis | Ichthyobodo necotor | Several Freshwater and Saltwater fish |
| Salmon Poisoning disease | Nanophyetus salmincola | Salmon, several Freshwater Fish |
| White Spot | Ichthyophthirius mulifiliis | Freshwater Fish |
| Sea Lice | Lepeophtheirus solmonis | Marine Salmonids |
| Whirling Disease | Myxobolus cerebralis | Trout, Salmon, Whitefish |
| Myxosporeans | Myxobolus genera | Freshwater and Marine Fish |
| Microsporean | Pleistophora genera | Freshwater and Marine Fish |
| Disease | Pathogen | Host | Type of Vaccine | Route of delivery | Trade Name | Country |
|---|---|---|---|---|---|---|
| Enteric septicaemia of catfish (ESC) | Edwardsiella ictaluri | catfish | Live attenuated | Immersion | Aquavac-ESC | US |
| Bacterial Kidney Disease (BKD) | Renibacterium salmoninarum | salmonids | Live, Attenuated | IP | Renogen | US Canada Chile |
| Flavobacteriosis/ Columnaris |
Flavobacterium columnare Flavobacterium maritimus |
cyprinids, salmonids, catfish, carp | Live, Attenuated | Immersion | Aquavac-Col | US Canada Chile |
| Inactivated | IP | Alpha Ject® IPNVFlevo 0.025 | Chile | |||
| Killed bacterin | Immersion | FryVacc 1 | US Canada |
|||
| FryVacc 2 | Chile | |||||
| Furunculosis | Aeromonas salmonicida | Atlantic Salmon & Rainbow Trout |
Inactivated, oil-based | IP | AlphaJect 3000 | Denmark Finland Iceland Ireland Norway Sweden |
| Alpha Ject® 2.2 | UK | |||||
| Alpha Ject® 4-1, Alpha Ject® 5-1 | Chile | |||||
| Alpha Ject® 6-2 | Norway The Faroe Islands |
|||||
| Alpha Ject® micro 7 ILA | Norway The Faroe Islands |
|||||
| Subunit Vaccine | IP | Norvax® Minova 6 | Norway | |||
| Inactivated Bacterin | IP | AquaVac-FNM | UK Ireland Spain France |
|||
| Killed Bacterin | IP | Lipogen Forte, Furogen Dip, Forte VI | US Canada |
|||
| Streptococcosis | Streptococcus iniae | tilapia and seabass | Inactivated | IP or Bath | Norvax Strep Si, Aquavac Strep Sa | Vietnam Honduras Indonesia |
| tilapia | Killed | IP | Aquavac-Garvetil | Honduras Venezuela Ecuador The Philippines Indonesia |
||
|
Streptococcus agalactiae |
tilapia | Inactivated | IP | AlphaJect® micro1 TiLa | Brazil Colombia Honduras Indonesia Panama |
|
|
Streptococcus parauberis |
Turbot | Inactivated | IP | Icthiovac-STR | Spain | |
| Vibriosis |
V. anguillarum V. ordalii |
Atlantic Salmon | Inactivated, Oil-based | IP | Alpha Ject® micro 7 ILA, Alpha Ject® 6-2 | Norway The Faroe Islands |
| Inactivated, oil-based | IP | Alpha Ject® 5-1, Alpha Ject® 4-1, | Chile | |||
| Inactivated, oil-based | IP | Alpha Ject® Micro-4 | Canada | |||
| Subunit Vaccine | IP | Norvax® Minova 6 | Norway | |||
| Inactivated, Oil-based | IP | Alpha Ject® micro 6 | Ireland UK The Faroe Islands Norway |
|||
| Sea bass | Inactivated, Oil-based | IP | Alpha Ject® micro 2000 | Croatia Spain Greece France |
||
| Atlantic salmon | Inactivated, oil-based | IP | Alpha Ject® Micro-3 | Chile | ||
| Atlantic aalmon & Rainbow trout |
Inactivated, oil-based | IP | Alpha Ject® 5-3 | Iceland Norway |
||
| Atlantic salmon & Rainbow trout |
Inactivated, oil-based | IP | AlphaJect 3000 | Denmark Finland Iceland Ireland Norway Sweden |
||
| Sea bass | Inactivated, oil-based | Dip | ALPHA DIP® Vib | Croatia Cyprus Greece Italy Portugal Spain |
||
| Sea bass | Inactivated, oil-based | Bath/ Immersion |
ALPHA DIP® Vibrio | Turkey | ||
| Atlantic salmon | Inactivated, oil-based | IP | Alpha Ject® 2-2 | UK | ||
| Salmonids | Killed bacterin | IP | Furogen Dip, Forte VI, Lipogen Forte |
US Canada |
||
| Salmonids | Killed bacterin | Bath/ Immersion |
Vibrogen-2 | US Canada |
||
| European sea bass | Inactivated bacterin | IP | AquaVac Vibrio Pasteurella | Greece Middle East |
||
| Rainbow trout | Inactivated bacterin | Oral/ Immersion |
AquaVac Vibrio, AquaVac Vibrio Oral Boost | Finland UK Ireland Spain Greece |
| Virus | Type of Virus (RNA/DNA) | Fish Host | Trade Name (if applicable) | Type of Vaccine | Delivery Method | Licensed for use in the following countries: | Description |
|---|---|---|---|---|---|---|---|
| SAV | RNA | Atlantic Salmon | Norvax Compact PD | Inactivated | Intraperitoneal Injection | Norway Chile UK |
A monovalent vaccine which contains an inactivated strain of SAV subtype 1. |
| SAV | RNA | Atlantic Salmon | Aquavac PD7 | Inactivated | Intraperitoneal Injection | Norway | A polyvalent vaccine which contains seven strains to protect against pancreatic disease, infectious pancreatic necrosis, furunculosis, cold-water vibriosis, vibriosis and winter ulcers.Specifically, to protect against SAV, it contains an inactivated strain of SAV subtype 1. |
| SAV | RNA | Atlantic Salmon | Aquavac PD3 | Inactivated | Intraperitoneal Injection | UK | A polyvalent vaccine which contains an inactivated strain of SAV subtype 1, as well as infectious pancreatic necrosis and furunculosis. |
| SAV | RNA | Atlantic Salmon | Alphaject Micro 1 PD | Inactivated | Intraperitoneal Injection | UK Norway |
A monovalent vaccine which contains the inactivated SAV subtype 3, the SAV strain most dominant in Norway. |
| IPNV | RNA | Atlantic Salmon, Rainbow Trout | AlphaJect 1000 | Inactivated | Intraperitoneal Injection | Chile Norway UK | A monovalent vaccine containing an inactivated form of the virus. |
| IPNV | RNA | Atlantic Salmon | Birnagen Forte | Inactivated | Intraperitoneal Injection | Canada UK |
A monovalent vaccine containing inactivated bacterins and virulins |
| IPNV | RNA | Atlantic Salmon | Aquavac IPN Oral | Recombinant | Oral | US Canada Chile Middle East |
A monovalent vaccine containing capsid proteins VP2 and VP3 |
| IPNV | RNA | Atlantic Salmon, Pacific Salmon, Chinook Salmon, Rainbow Trou | Blueguard IPNV Oral | Inactivated | Oral | Chile | A monovalent vaccine containing 2 inactivated strains of IPNV |
| IPNV | RNA | Rainbow Trout, Atlantic Salmon, Pacific Salmon, Chinook Salmon |
Blueguard IPN Inyectable | Inactivated | Intraperitoneal Injection | Chile | A monovalent vaccine containing 2 strains of inactivated IPNV |
| IPNV | RNA | Atlantic Salmon | AlphaJect IPNV-Flavo 0.025 | Inactivated | Intraperitoneal Injection | Chile | A bivalent vaccine protecting against IPNV and Flavobacteriosis. |
| IPNV | RNA | Atlantic Salmon, Pacific Salmon, Rainbow Trout | AlphaJect Micro 2 | Inactivated | Intraperitoneal Injection | Chile | A bivalent vaccine protecting against IPNV and SRS |
| IPNV | RNA | Atlantic Salmon | AlphaJect 2-2 | Inactivated | Intraperitoneal Injection | UK | A bivalent vaccine protecting against IPNV and Furunculosis. |
| IPNV | RNA | Atlantic Salmon | AlphaJect Micro 3 | Inactivated | Intraperitoneal Injection | Chile | A trivalent vaccine protecting against IPNV, SRS, and Vibriosis. |
| IPNV | RNA | Atlantic Salmon Rainbow Trout |
Blueguard SRS+IPN+Vibrio | Inactivated | Intraperitoneal Injection | Chile | A trivalent vaccine which includes 2 strains of inactivated IPNV and inactivated bacterins to protect against SRS and Vibrio |
| IPNV | RNA | Atlantic Salmon | AlphaJect 4-1 | Inactivated | Intraperitoneal Injection | Chile | A polyvalent vaccine protecting against Furunculosis, SRS, Vibriosis, and IPNV |
| IPNV | RNA | Atlantic Salmon | Pentium Forte Plus | Inactivated | Intraperitoneal Injection | Norway | Contains inactivated whole virus of IPNV, and also protects against Furunculosis, Classical Vibriosis, coldwater vibriosis, and Winter Ulcer. |
| IPNV | RNA | Atlantic Salmon | Norvax Minova 6 | Subunit, inactivated | Intraperitoneal Injection | UK Norway |
A multivalent vaccine which protects against Furunculosis, classical vibriosis, coldwater vibriosis, wound disease and IPNV. It contains a subunit VP2 capsid protein. |
| IPNV | RNA | Atlantic Salmon | AlphaJect Micro 6 | Inactivated | Intraperitoneal Injection | Norway United Kingdom The Faroe Islands Ireland |
A multivalent vaccine protecting against Furunculosis, Vibriosis, Coldwater vibriosis, Winter sore, and IPNV |
| IPNV | RNA | Atlantic Salmon | AlphaJect 6-2 | Inactivated | Intraperitoneal Injection | Norway The Faroe Islands |
A polyvalent vaccine protecting against Furunculosis, Vibriosis. Coldwater vibriosis, Winter sore, and IPNV. |
| IPNV and ISA | RNA | Atlantic Salmon | AlphaJect Micro 4-2 | Inactivated | Intraperitoneal Injection | Chile | A multivalent vaccine protecting against IPNV, Infectious Salmon Anemia (ISA), Vibriosis, and Furunculosis. |
| IPNV and ISA | RNA | Atlantic Salmon | AlphaJect 5-1 | Inactivated | Intraperitoneal Injection | Chile | A polyvalent vaccine protecting against Furunculosis, SRS, Vibriosis, ISA, and IPNV |
| IPNV and ISA | RNA | Atlantic Salmon | AlphaJect Micro 7 | Inactivated | Intraperitoneal Injection | Norway The Faroe Islands |
A multivalent vaccine protecting against, Furunculosis, Vibriosis, Coldwater vibriosis, Winter sore, IPNV, and (ISA). |
| IPNV and ISA | RNA | Atlantic Salmon | Blueguard SRS+IPN+VO+ISA | Subunit and Inactivated | Intraperitoneal Injection | Chile | A polyvalent vaccine containing subunit ISA, inactivated IPNV strain, and bacterins. It protects against ISA, IPNV, SRS, and Vibriosis. |
| IPNV and ISA | RNA | Altantic Salmon | Blueguard IPN+SRS+AS+VO+ISA inyectable | Subunit and Inactivated | Intraperitoneal Injection | Chile | A polyvalent vaccine containing subunit ISA, inactivated IPNV strain, and bacterins. It protects against ISA, IPN, SRS, vibriosis, and furunculosis. |
| ISA | RNA | Atlantic Salmon | AlphaJect Micro 1 ISA | Inactivated | Intraperitoneal Injection | Chile | A monovalent vaccine that includes an inactivated strain of ISA. |
| ISA | RNA | Salmonids | Forte VII | Inactivated | Intraperitoneal Injection | Canada | A polyvalent vaccine which contains inactivated ISA and bacterin. It protects against ISA, Furunculosis, and Vibriosis. |
| Koi Herpes Virus (KHV) | DNA | Cyprinid Fish | KV-3 | Attenuated | Immersion/Injection | Isreal, USA | A live, attenuated monovalent vaccine that protects against KHV. |
| RSIV | DNA | Red Sea Bream, along with cultured marine fish such as yellowtail and sea brass | n.a | Formalin | Intraperitoneal | Japan | A monovalent formalin-based vaccine that fights against RSIV. This was the first vaccine made against the virus. |
| RSIV | DNA | Red Sea Bream, along with cultured marine fish such as yellowtail and sea brass | AQUAVAC® IridoV | Formalin, oil-adjuvant | Intraperitoneal | Singapore | A monovalent vaccine with an inactivated strain of RSIV which targets tilapia and Asian Sea Bass. |
| IHNV | RNA | Salmonids including rainbow trout, steelhead trout and Atlantic Salmon | Apex-IHN | DNA | Intramuscular Injection | Canada, USA | A DNA plasmid vaccine targeting IHNV in salmonids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





