Submitted:
21 February 2024
Posted:
22 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Drug Delivery Using Polymeric Nanoparticles
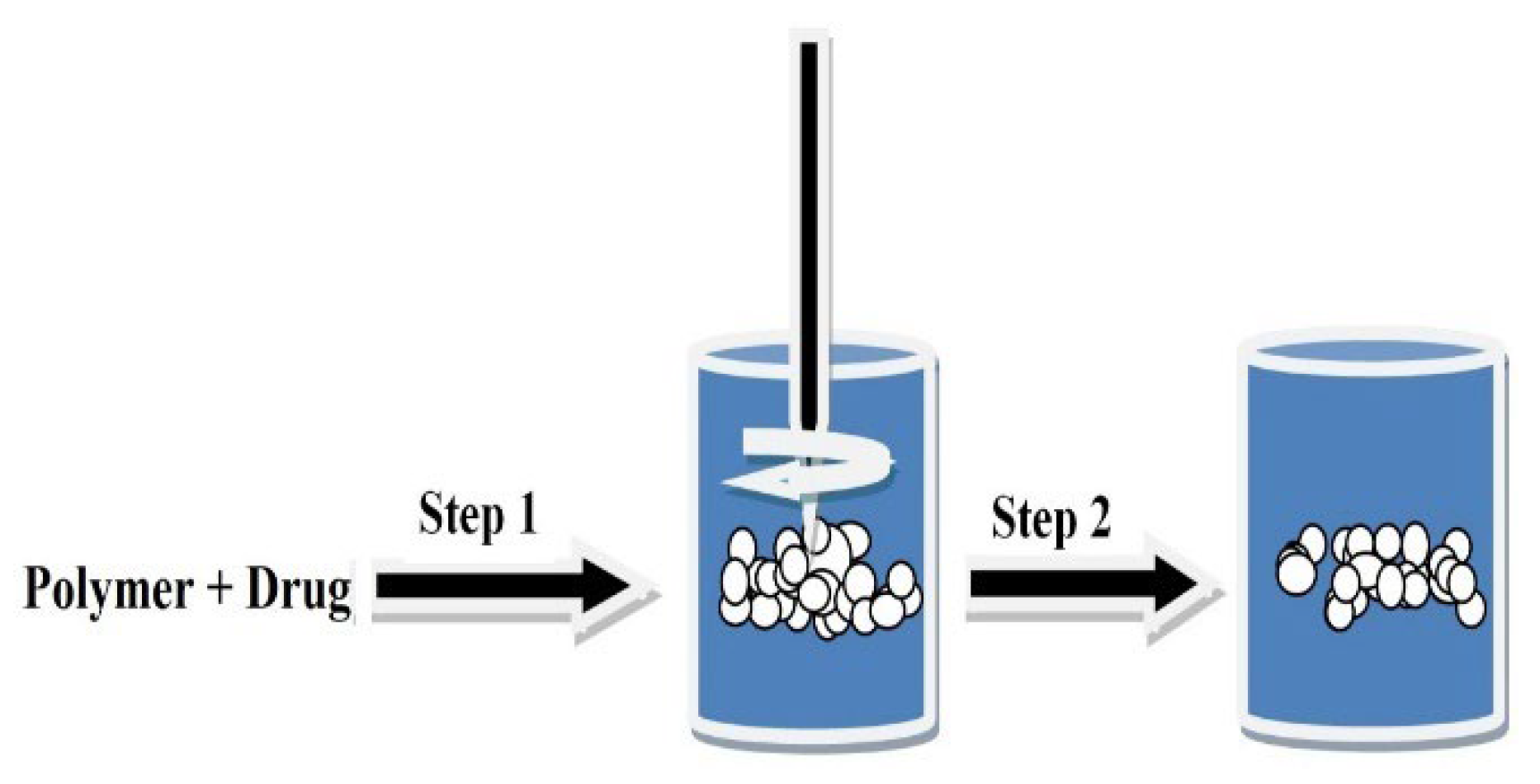
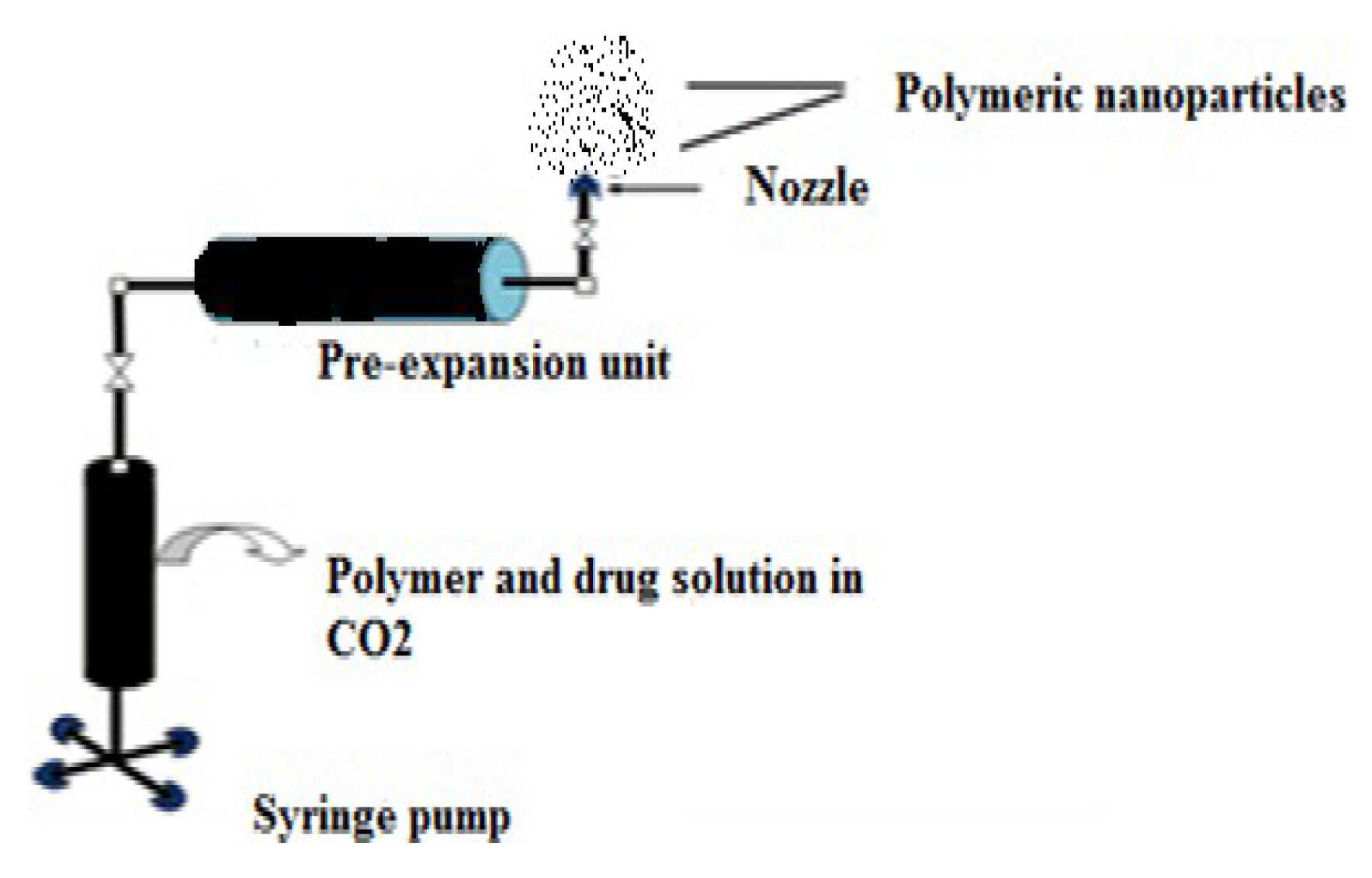
- Preparation of Polymeric Nanoparticles (PNPs)
- Solvent Evaporation Technique
- Micro-Emulsion Polymerization Technique
- Supercritical Fluid (SCF) Technology
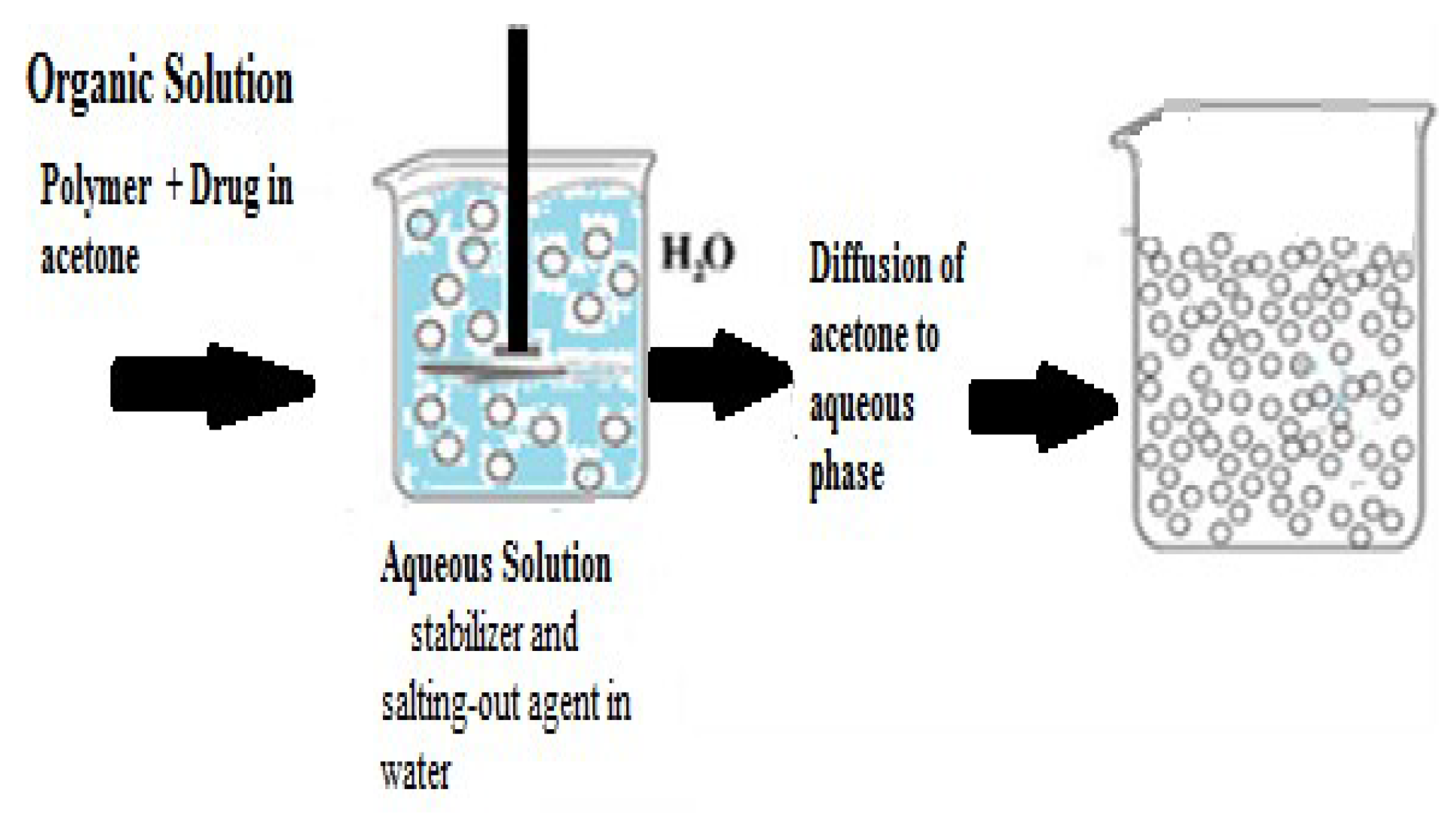
- Salting-Out Technique
- a)
- Dissolution: The polymer and drug are initially dissolved in a solvent such as acetone.
- b)
- Emulsification: The solvent containing the dissolved polymer and drug is then emulsified into an aqueous gel. This gel contains a salting-out agent such as magnesium chloride, calcium chloride, magnesium acetate, or non-electrolytes like sucrose. Additionally, a colloidal stabilizer such as polyvinyl pyrrolidone or hydroxy ethyl cellulose may be included.
- c)
- Formation of Nano-spheres: The oil/water emulsion is diluted with water or an aqueous solution to enhance the diffusion of acetone into the aqueous phase. This induces the formation of nanoparticles.
- d)
- Elimination of Solvent and Salting-Out Agent: Finally, both the solvent and the salting-out agent are removed through cross-flow filtration. Thus, designing an effective drug delivery system using nanoparticles requires careful consideration of several factors such as: the quality and reliability, controlled drug release profile, and recognition of disease affected part [14,18].
- Designing of Targeted PNP’s towards Specific Tumor Cells
- a)
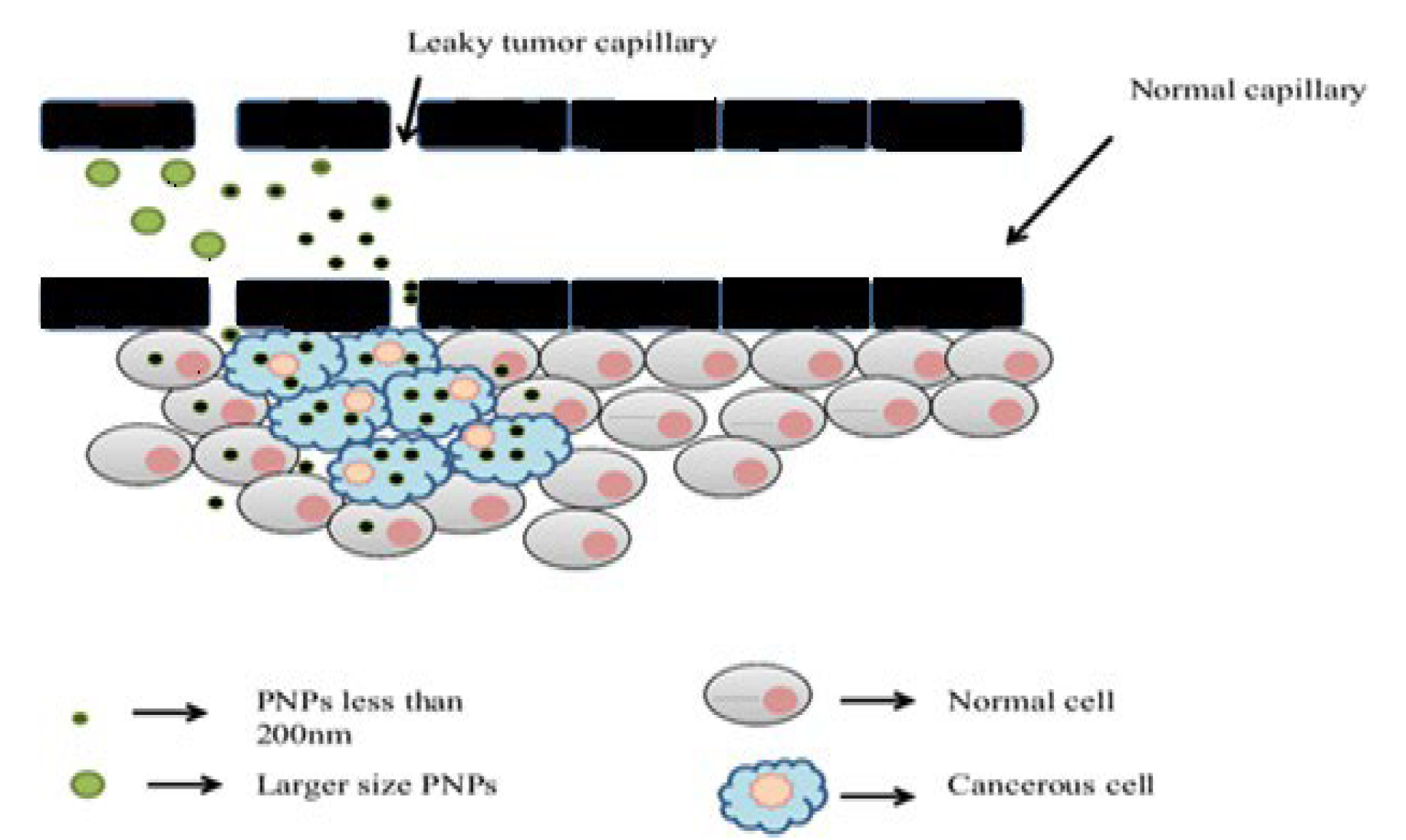
- Passive Targeting: this involves the transport and accumulation of PNPs through leaky tumor capillary fenestrations into the tumor interstitium via the enhanced permeability and retention (EPR) effect (Figure 4) [6]. This mechanism does not involve specific targeting ligands and relies on passive diffusion to tumor tissues [19].
- b)
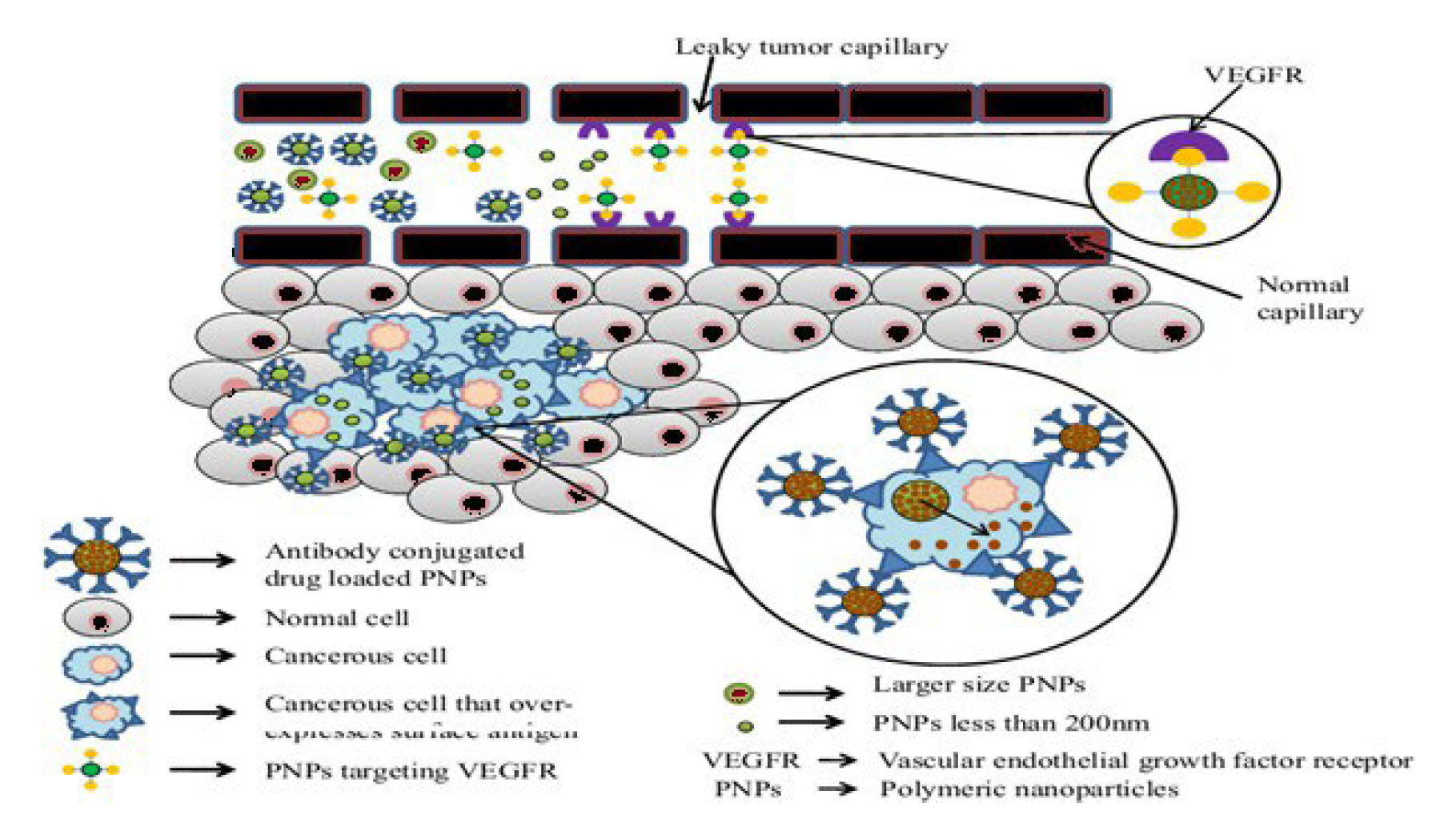
- Ligand-Based Targeting of PNP’s (Active Targeting): Active targeting mechanisms involve the recognition of ligands by specific receptors on tumor cells, leading to receptor-mediated endocytosis. Several ligands have been explored for targeted drug delivery: Thus, ligands stand for a diverse class of molecules that can be exploited for targeted drug delivery because the ligand-receptor complex is the result of a specific molecular interaction that requires structural complementarity [5]. Design of actively-targeted PNP drug carriers is a complex process because the ligand conjugation chemistry, the NP architecture, the types of ligands available, route of administration and protein binding nature of the PNP’s all contribute to the success of the process and should be taken into account during designing [14].
- c)
- The formulation of a targeted drug delivery system relies on understanding the specific processes and characteristics of the disease state. This involves identifying key receptors, antigens, or binding domains associated with the disease, which can serve as targets for the drug delivery system. Once these targets are identified, ligands that can interact specifically with these targets are selected to enable receptor-mediated endocytosis. This interaction facilitates the uptake of the drug-loaded nanoparticles into the target cells, enhancing the therapeutic efficacy while minimizing off-target effects (Figure 5) [20].
- Antibodies and Antibody Fragments
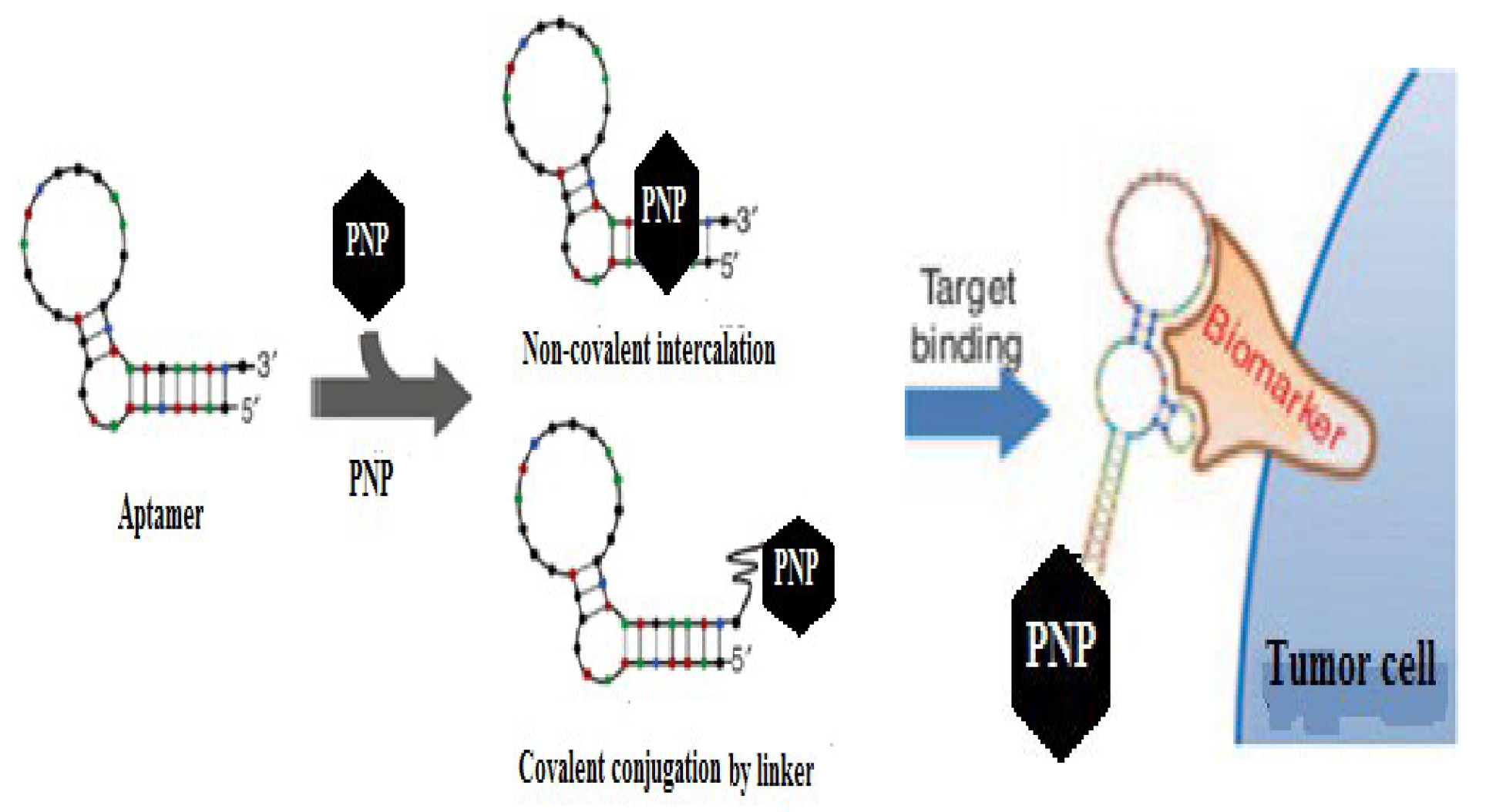
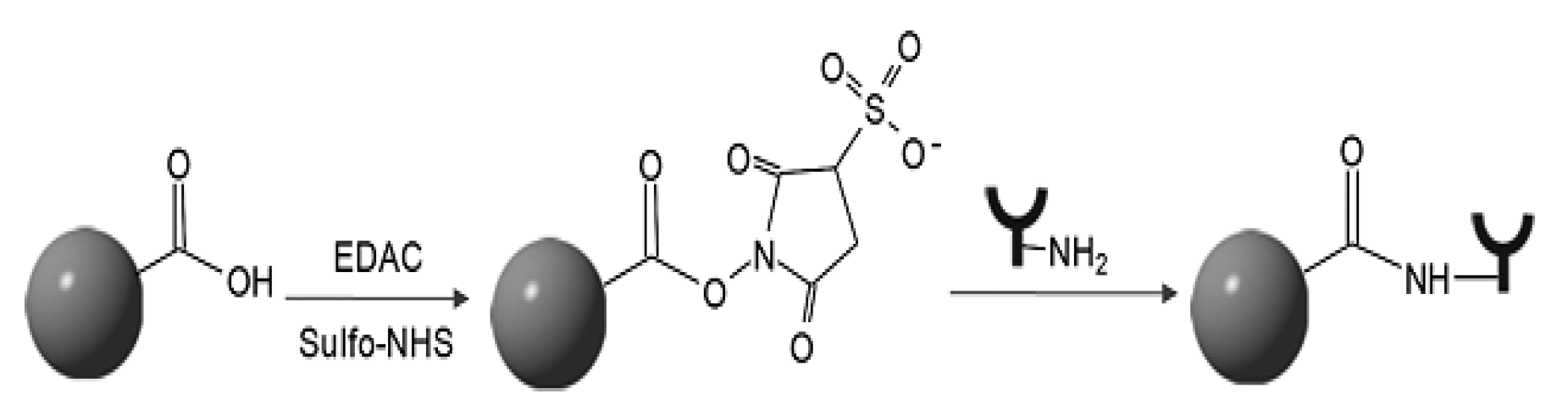
- Mechanisms of Ligand-PNP Coupling
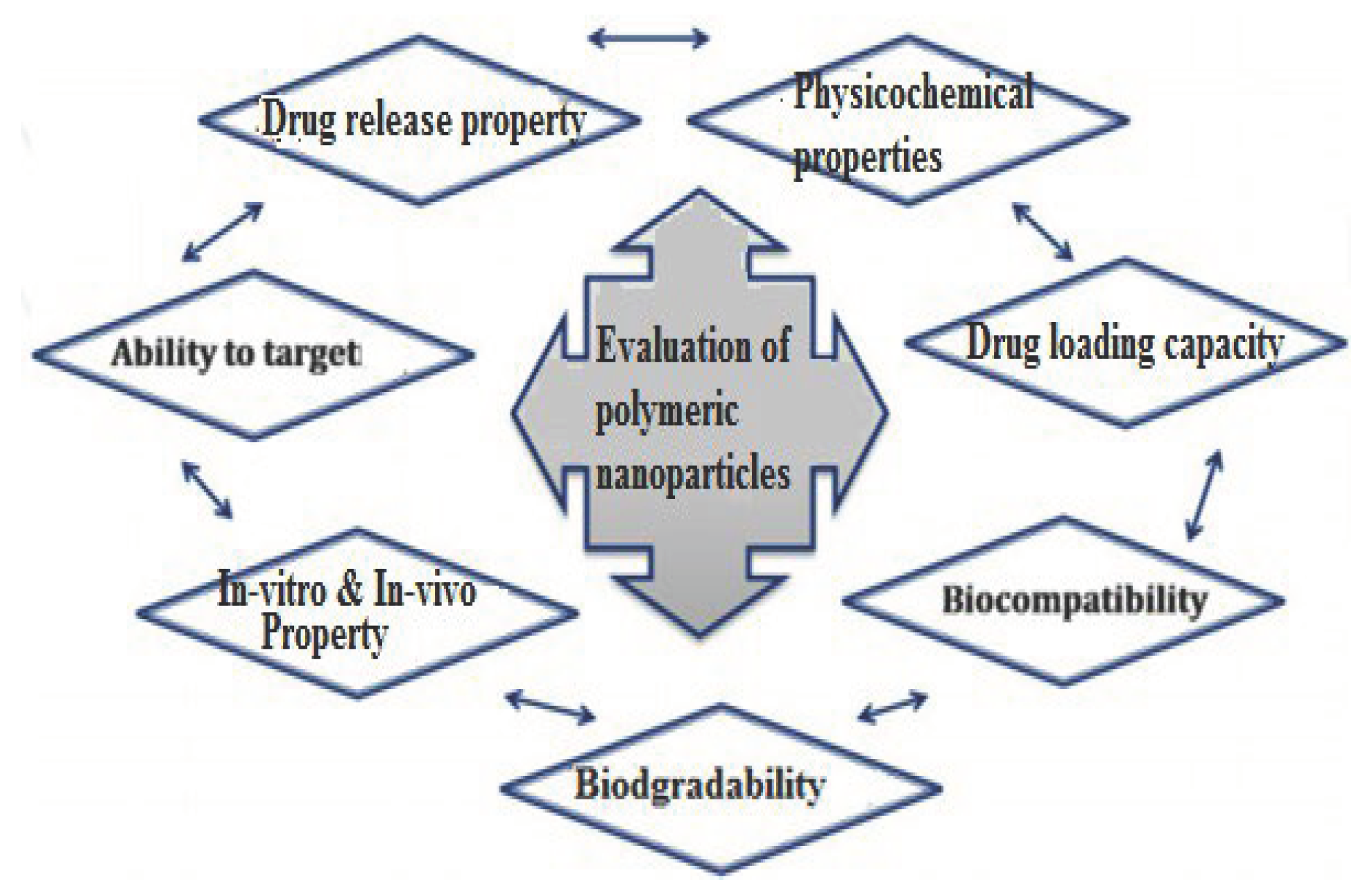
- Characterization of Polymeric Nanoparticles (PNPs)
- Applications of Targeted PNP’s in Different Tumors
- Breast Cancer
- Liver and Spleen Cancers
- Lung Cancer
- Colon Cancer
- Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Annex.1. Commercially available ligands and their targets for different types of target molecule expressing tumor cells.
| Ligand type | Target molecule or receptor | Site of target |
| Antibody fragments Antigen binding fragments (fab) Single chain variablefragments(scFv) |
Human epidermal receptor (HER-1,HER-2,HER-3 and HER-4) |
Breast, ovarian, bladder, prostate, head and neck tumors. |
Monoclonal antibodies
|
-Vascular endothelial growth factor receptor (VEGFR) -Epidermal growth factor receptor(EGFR) |
Colorectal tumor, breast tumor, prostate tumor and lung tumor [5]. |
| - Transferrin(Tf) -Low density lipoproteins(LDL) -Cell |
Transferring receptor 1 and 2 LDL- receptors | -Brain tumor, breast tumor, prostate tumor and squamous cell carcinomas and other tumors [14]. |
| A10 aptamer | Prostate specific membrane antigen (PSMA) | Prostate tumor |
| Vitamins (Folic acid, riboflavin and biotin) | Vitamin receptors (e.g folic acid receptor) | Colon, lung, Uterus, prostate, and brain tumors |
| Arginine-Glycine-Aspartic acid(RGD) or LDV |
α vβ3, αvβ5 integrin receptors | tumor-associated vascular endothelial cells [21]. |
Annex 2: Summary of types of reactions and functional groups used for PNP-ligand coupling.
| Types of Reactions | Functional Groups at Nano-carrier Surface | Functional Groups at Targeting Ligands |
| Electrophilic addition of thiol to alkene | Maleimide Carboxylic acid Pyridyldithiopropionate(PDP) Vinylsulfone |
Thiol Thiol Maleimide Thiol |
| Nucleophilic acyl substitution Reaction |
Carboxylic acid Amine P-nitrophenyl carbonyl |
Amine Amine Amine |
| Hydrazide copling Disulfide exchange Biotin-streptavidin Diel’s-Alder Click-chemistry |
Hydrazide PDP Biotin Furan Azide |
Aldehayde Thiol Streptavidin Malemide Alkyne |
References
- Panchangam, R.B.S.; Dutta, T. Engineered Nanoparticles for the Delivery of Anticancer Therapeutics. J Pharm Drug Deliv Res. 2015, 4(1), 1–16. [Google Scholar]
- Theresa, M.A. Ligand targeted therapeutics in anticancer therapy. Nature Publishing Group. 2002, 2, 750–763. [Google Scholar]
- Subhra, M. Polyelectrolyte based Nano-approaches for Cancer therapy or diagnostics. Statistical and Biological Physics. 2010, 3, 12–247. [Google Scholar]
- Malcolm, R.A. Cancer. Nature publishing group 2010, 5, 4–8. [Google Scholar]
- Deepak, K.; Deepti, J.; Vivek, S.; et al. Cancer Therapeutics, Opportunities, Challenges and Advances in Drug Delivery. Journal of Applied Pharmaceutical Science 2011, 1(9), 01–10. [Google Scholar]
- Meng, S.; Jiao, L.; Molly, S.S. Organic nanoscale drug carriers coupled with ligands for targeted drug delivery in cancer. Journal of Material Chemistry 2009, 19, 5485–5498. [Google Scholar]
- Monika Tk Justyna, M.; Lucyna, M.; et al. Toward a magic or imaginary bullet? Ligands for drug targeting to cancer cells: principles, hopes, and challenges. International Journal of Nano medicine 2015, 10, 1399–1414. [Google Scholar]
- Archana, S.; Jinjun, S.; Suresh, G.; et al. Nanoparticles for Targeted and Temporally Controlled Drug Delivery. Nanostructure Science and Technology 2012, 4, 9–29. [Google Scholar]
- Eun, K.L.; Eunji, J.; Kwangyeol, L.; et al. Delivery of Cancer Therapeutics Using Nanotechnology. Pharmaceutics. 2013, 5, 294–317. [Google Scholar]
- Nagavarma, B.V.; Hemant, k.S.; Ayaz, A.; et al. Different techniques for preparation of polymeric nanoparticles. Asian J Pharm Clin Res 2012, 5(3), 16–23. [Google Scholar]
- A Holgado, M.; Martín-B, L.; Álvarez-F, J. Drug Targeting to Cancer by Nanoparticles Surface Functionalized with Special Biomolecules. Bentham Science Publishers 2012, 19(19), 3188–3195. [Google Scholar] [CrossRef]
- Zhuo (Georgia), C. Small-molecule delivery by nanoparticles for anticancer therapy. Trends in Molecular Medicine 2010, 16(12), 594–601. [Google Scholar]
- C. Tuba, Ş.T.; Zerrin, S.B.; Ulya, B. preparation of polymeric nanoparticles using different stabilizing agents. J Fac Pharm Ankara 2009, 38(4), 257–268. [Google Scholar]
- Ida, I.M.; Suguna, S.; Nurul Asmak, M.L. Designing Polymeric Nanoparticles for Targeted Drug Delivery System. Journal of nanomedicine 2010, 11, 287–313. [Google Scholar]
- S,Ajay V. Polymer Nanoparticles: Newer Strategies towards Targeted CancerTherapy. JPhysChemBiophys 2013, 3(4), 2161–0398.
- Ketan, T.S.; Anuradha, K.G.; Jignasa, K.S. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharmaceutics. 2012, 1–10. [Google Scholar]
- Varun, R.V.; Venkateshwarlu, L.; Srikanth, L. solubility enhancement techniques. International journal of pharmaceutical Sciences Review and Research 2010, 5(1), 41–51. [Google Scholar]
- Devasier, B.; Sanghyo, K. Polymer Nanoparticles for Smart Drug Delivery. Licensee InTech 2014, 258–296. [Google Scholar]
- You Han Bae, Kinam P. Targeted drug delivery to tumors: Myths, reality and possibility. Journal of Controlled Release 2011, 153, 198–205. [Google Scholar] [CrossRef]
- Nicolas, B.; Jun, W.u.; Xiaoyang, X.u.; et al. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Advanced Drug Delivery Reviews 2013, 1–24. [Google Scholar]
- Saul, J.M.; Annapragada, A.V.; Bellamkonda, R.V. A dual-ligand approach for enhancing targeting selectivity of therapeutic nanocarriers. Journal of Controlled Release 2006, 114, 277–287. [Google Scholar] [CrossRef]
- Tang, M.F.; Lei, L.; Rong, S.G.; Wen Lin, H. Recent progress in nanotechnology for cancer therapy. Chinese Journal of Cancer 2010, 29(9), 775–780. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Rajabi, M.; Mousa, S.A. Multifunctional Nanomaterials and Their Applications in Drug Delivery and Cancer Therapy. Nanomaterials 2015, 5, 1690–1703. [Google Scholar] [CrossRef]
- Hongguang, S.; Xun, Z.; Patrick, Y.L.; et al. Oligonucleotide Aptamers: New Tools for Targeted Cancer Therapy. The American Society of Gene and Cell Therapy 2014, 3, 1–14. [Google Scholar]
- and Vinga D, S. Polymer-Nanoparticle Composites: From Synthesis to ModernApplications. Materials 2010, 3, 3468–3517. [Google Scholar]
- Bin, W.; Jenna, M.R.; Rabe’e, C.; et al. Towards a targeted multi-drug delivery approach to improve therapeutic efficacy in breast cancer. Expert Opinion Drug Delivery 2010, 7(10), 1159–1173. [Google Scholar]
- Biswajit, M.; Bhabani, S.S.; Laboni, M. Potentials and Challenges of Active Targeting at the Tumor Cells by Engineered Polymeric Nanoparticles. Current Pharmaceutical Biotechnology 2013, 14(15), 1250–1263. [Google Scholar]
- Wang, A.Z.; Frank, G.u.; Zhang, L.; et al. Biofunctionalized targeted nanoparticles for therapeutic applications. Expert Opin. Biol. Ther. 2008, 8(8), 1063–1070. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; et al. PLGA-based nanoparticles: An overview of biomedical applications. Journal of Controlled Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Mohanraj, V.J.; Chen, Y. Nanoparticles—A Review. Tropical Journal of Pharmaceutical Research 2006, 5(1), 561–573. [Google Scholar] [CrossRef]
- Blanco, M.D.; Teijón, C.; Olmo, R.M.; et al. Targeted nanoparticles for Cancer Therapy. License intech 2012, 7, 241–278. [Google Scholar]
- Priya, R.; Vasugi, R. Treatment of colorectal cancer using nanotechnology. International Journal of Pharmacy & Technology 2015, 7(2), 8977–8985. [Google Scholar]


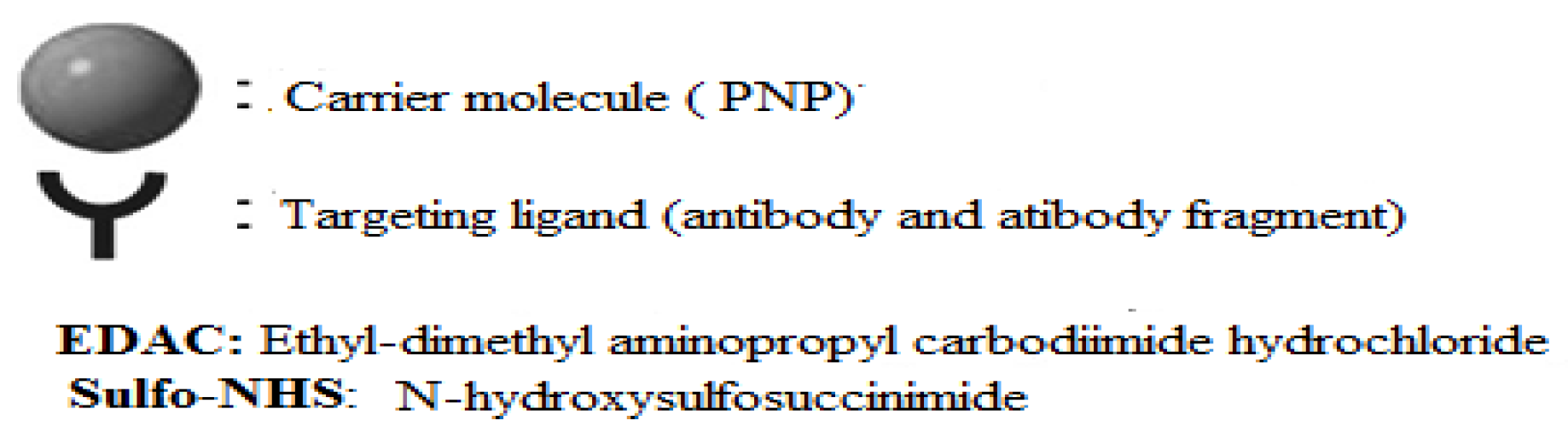
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




