Submitted:
21 February 2024
Posted:
21 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Collection of plants
2.2. Sample preparation
2.3. Acetylcholinesterase inhibitory activity
2.4. Free radical scavenging activity
2.5. Total phenolics
2.6. Total flavonoids
2.7. Total terpenoids
2.8. Total alkaloids
2.9. Total tannins
2.10. Statistical analysis
3. Results and Discussion
3.1. Acetylcholinesterase inhibitory activity of selected medicinal plants
3.2. Antioxidant potential of selected medicinal plants
3.3. Phytochemical composition of selected medicinal plants
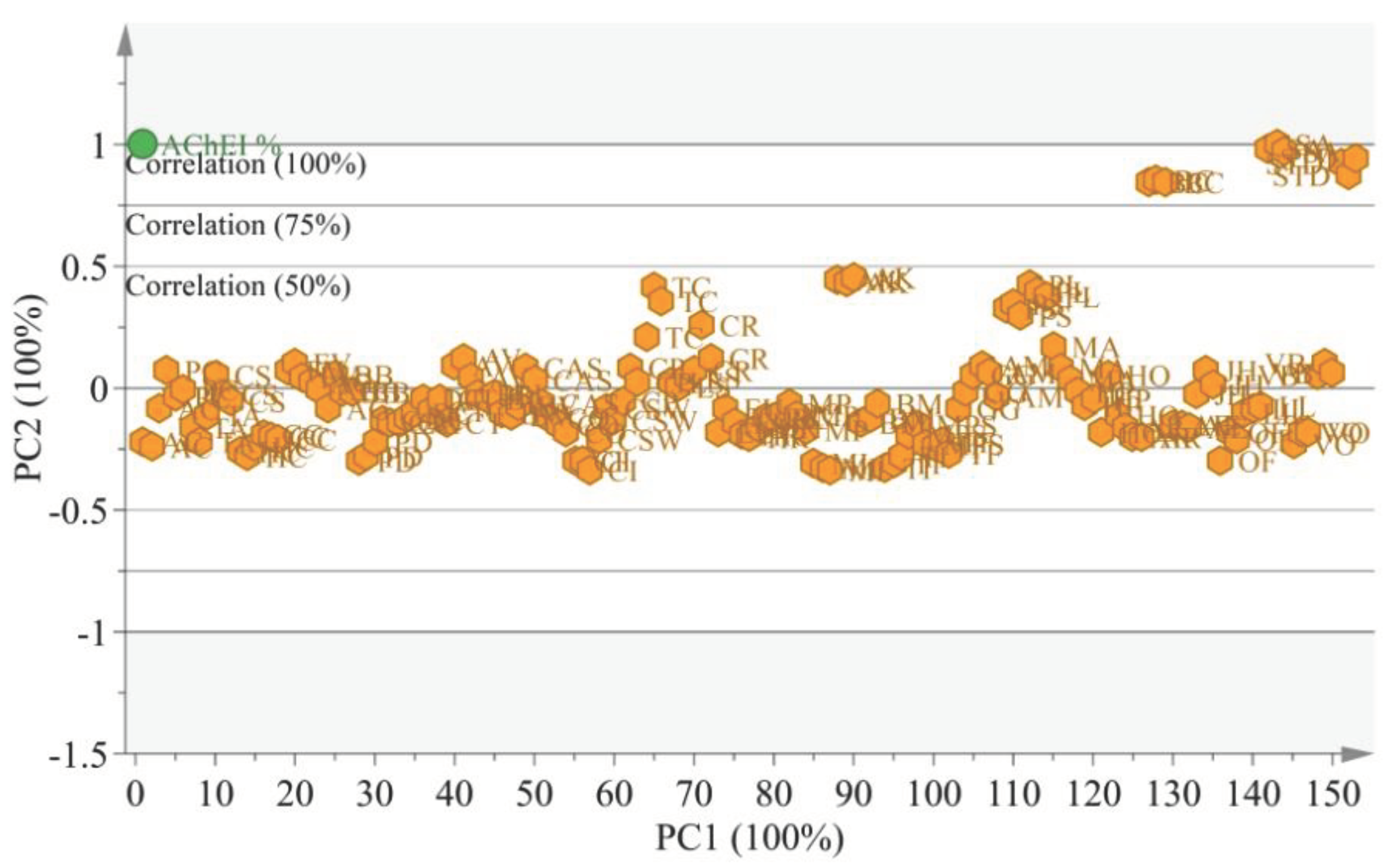
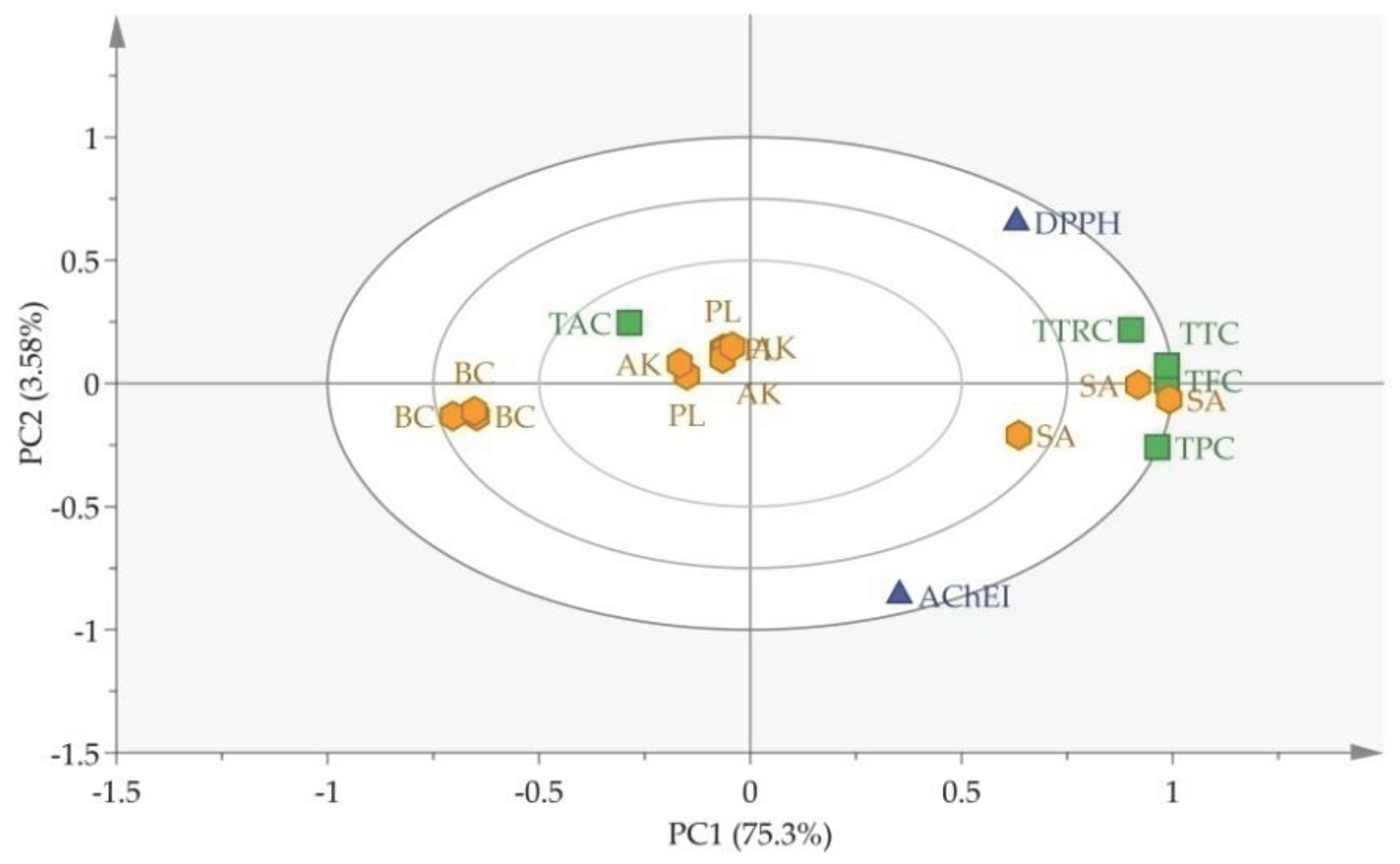
3.4. Chemometric analysis
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bortolami, M.; Rocco, D.; Messore, A.; Di-Santo, R.; Costi, R.; Madia, V.N.; Scipione, L.; Pandolfi, F. Acetylcholinesterase inhibitors for the treatment of Alzheimer’s disease-a patent review (2016-present). Expert Opinion on Therapeutic Patents 2021. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Identification and characterization of anthocyanins and non-anthocyanin phenolics from Australian native fruits and their antioxidant, antidiabetic, and anti-Alzheimer potential. Food Res. Int. 2022, 162, 111951. [Google Scholar] [CrossRef]
- Arya, A.; Chahal, R.; Rao, R.; Rahman, M.H.; Kaushik, D.; Akhtar, M.F.; Saleem, A.; Khalifa, S.M.; El-Seedi, H.R.; Kamel, M.; Albadrani, G.M. Acetylcholinesterase Inhibitory Potential of Various Sesquiterpene Analogues for Alzheimer’s Disease Therapy. Biomolecules. 2021, 11, 350. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007, 14, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Alzobaidi, N. Quasimi, H.; Emad, N.A.; Alhalmi, A.; Naqvi, M. Bioactive Compounds and Traditional Herbal Medicine: Promising Approaches for the Treatment of Dementia. Degener.Neurol. Neuromuscul Dis. 2021, 11, 1–14. [Google Scholar] [PubMed]
- Mukherjee, P.K.; Kumar, V.; Houghton, P.J. Screening of Indian medicinal plants for acetylcholinesterase inhibitory activity. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives 2007, 21, 1142–1145. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, Y.; Yin, W.; Li, J.; Wang, W.; Bai, F.; Xu, S.; Gong, Q.; Peng, T.; Hong, Y.; Zhang, D. Kinetics-Driven Drug Design Strategy for Next-Generation Acetylcholinesterase Inhibitors to Clinical Candidate. J. Med. Chem. 2021, 64, 1844–1855. [Google Scholar] [CrossRef]
- Kiani, H.S.; Ali, B.; Al-Sadoon, M.K.; Al-Otaibi, H.S.; Ali, A. Lc-ms/ms and gc-ms identification of metabolites from the selected herbs and spices, their antioxidant, anti-diabetic potential, and chemometric analysis. Processes 2023, 11, 2721. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Characterization, antioxidant potential, and pharmacokinetics properties of phenolic compounds from native Australian herbs and fruits. Plants 2023, 12, 993. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Antioxidant, alpha-glucosidase inhibition activities, in silico molecular docking and pharmacokinetics study of phenolic compounds from native Australian fruits and spices. Antioxidants 2023, 12, 254. [Google Scholar] [CrossRef]
- Yizibula, M.; Wusiman, Z.; Abudouhalike, N.; Maimaitiming, B. Cognitive enhancement and neuroprotective effects of a traditional Chinese herbal compound medicine on Aβ1–42 induced Alzheimer’s disease in rats. Folia Neuropathol. 2020, 58, 365–376. [Google Scholar] [CrossRef]
- Gregory, J.; Vengalasetti, Y.V.; Bredesen, D.E.; Rao, R.V. Neuroprotective Herbs for the Management of Alzheimer’s Disease. Biomolecules. 2021, 11, 543. [Google Scholar] [CrossRef]
- Derakhshan, A.R. Natural Treatments for Fissure in Ano Used by Traditional Persian Scholars, Razi (Rhazes) and Ibn Sina (Avicenna). Evid. Based Complement. Alternat. Med. 2017, 22, 324–333. [Google Scholar] [CrossRef]
- Adsersen, A.; Gauguin, B.; Gudiksen, L.; Jäger, A.K. Screening of plants used in Danish folk medicine to treat memory dysfunction for acetylcholinesterase inhibitory activity. J. Ethnopharmacol. 2006, 104, 418–422. [Google Scholar] [CrossRef]
- Guzman-Martinez, L.; Calfío, C.; Farias, G.A.; Vilches, C.; Prieto, R.; Maccioni, R.B. New Frontiers in the Prevention, Diagnosis, and Treatment of Alzheimer’s Disease. J. Alzheimer’s Dis.2021, Preprint, 1-13.
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Shah, M.S.; Najam-ul-Haq, M.; Shah, H.S.; Rizvi, S.U.F.; Iqbal, J. Quinoline containing chalcone derivatives as cholinesterase inhibitors and their in silico modeling studies. Comput.Biol.Chem. 2018, 76, 310–317. [Google Scholar] [CrossRef]
- Afsar, V.; Reddy, Y.M.; Saritha, K. In vitro antioxidant activity and anti-inflammatory activity of methanolic leaf extract of Boswellia serrata. Int. J. Life Sci. Biotechnol. Pharma. Res. 2012, 1, 15–23. [Google Scholar]
- Siddiqui, N.; Rauf, A.; Latif, A.; Mahmood, Z. Spectrophotometric determination of the total phenolic content, spectral and fluorescence study of the herbal Unani drug Gul-e-Zoofa (Nepeta bracteata Benth). J. Taibah Univ. Medical Sci. 2017, 12, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Minh, L.V.; Trieu, L.H.; Bui, L.M.; Lam, T.D.; Hieu, V.Q.; Khang, T.V.; Trung, L.N.Y. Evaluation of total polyphenol content, total flavonoid content, and antioxidant activity of Plectranthus amboinicus leaves. in IOP Conference Series: Materials Science and Engineering. 2020. IOP Publishing.
- Ghorai, N.; Chakraborty, S.; Gucchait, S.; Saha, S.K.; Biswas, S. Estimation of total Terpenoids concentration in plant tissues using a monoterpene, Linalool as standard reagent. Protoc. Exch. 2012, 5, 1038. [Google Scholar] [CrossRef]
- Shamsa, F.; Monsef, H.; Ghamooshi, R.; Verdian-rizi, M. Spectrophotometric determination of total alkaloids in some Iranian medicinal plants. Thai. J. Pharm. Sci. 2008, 32, 17–20. [Google Scholar]
- Ali, A.; Wu, H.; Ponnampalam, E.N.; Cottrell, J.J.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive profiling of most widely used spices for their phenolic compounds through LC-ESI-QTOF-MS2 and their antioxidant potential. Antioxidants 2021, 10, 721. [Google Scholar] [CrossRef]
- Jahangir, M.; Abdel-Farid, I.B.; Choi, Y.H.; Verpoorte, R. Metal ion-inducing metabolite accumulation in Brassica rapa. J. Plant Physiol. 2008, 165, 1429–1437. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H. Principles and procedures of statistics, a biometrical approach. 1980: McGraw-Hill Kogakusha, Ltd.
- Zahid, H.F.; Ali, A.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Identification of phenolics profile in freeze-dried apple peel and their bioactivities during in vitro digestion and colonic fermentation. Int. J. Mol. Sci. 2023, 24, 1514. [Google Scholar] [CrossRef]
- Mohammed, S.; Manan, F.A. Aalysis of total phenolics, tannins and flavonoids from Moringa oleifera seed extract. J. Chem. Pharm. Res. 2015, 7, 132–135. [Google Scholar]
- Asem, N.; Abdul-Gapar, N.A.; Abd-Hapit, N.H.; Omar, E.A. Correlation between total phenolic and flavonoid contents with antioxidant activity of Malaysian stingless bee propolis extract. J. Apic. Res. 2020, 59, 437–442. [Google Scholar] [CrossRef]
- Malik, S.K. Qualtitative and quantitative estimation of terpenoid contents in some important plants of Punjab, Pakistan. Pak. J. Sci. 2017, 69. [Google Scholar]
- Harman-Ware, A.E.; Sykes, R.; Peter, G.F.; Davis, M. Determination of terpenoid content in pine by organic solvent extraction and fast-GC analysis. Front. Energy Res. 2016, 4, 2. [Google Scholar] [CrossRef]
- John, B.I.J.U; Sulaiman, C.T.; George, S.; Reddy, V.R.K. Spectrophotometric estimation of total alkaloids in selected Justicia species. Int. J. Pharm. Sci. 2014, 6, 647–648. [Google Scholar]
- Mohammad, A.; Syed, B.; Malay, B.; Kumar, B. Effect of Santalum album Linn on memory enhancing activity on mice. J. Chem. Pharm. Sci. 2010, 3, 172–177. [Google Scholar]
- Sindhu, R.K.; Upma, K.A.; Arora, S. Santalum album linn: a review on morphology, phytochemistry and pharmacological aspects. Intl. J. Pharm. Tech. Res. 2010, 2, 914–919. [Google Scholar]
- Kumar, D.N.; Alex, S.A.; Kumar, R.S.S.; Chandrasekaran, N.; Mukherjee, A. Acetylcholinesterase inhibition-based ultrasensitive fluorometric detection of malathion using unmodified silver nanoparticles. Colloids Surf. A: Physicochem. Eng. Asp. 2015, 485, 111–117. [Google Scholar] [CrossRef]
- Mehvish, S.; Barkat, M.Q. Phytochemical And Antioxidant Screening of Amomum Subulatum, Elettaria Cardamomum, Emblica Officinalis, Rosa Damascene, Santalum album and Valeriana officinalis And Their Effect On Stomach, Liver And Heart. Matrix Sci. Medica. 2018, 2, 28–33. [Google Scholar] [CrossRef]
- Saleem, Z.M.; Ahmed, S.; Hasan, M.M. Phaseolus lunatus linn: Botany, medicinal uses, phytochemistry and pharmacology. World J. Pharm. Pharm. Sci. 2016, 5, 87–93. [Google Scholar]
- Granito, M.; Brito, Y.; Torres, A. Chemical composition, antioxidant capacity and functionality of raw and processed Phaseolus lunatus. J. Sci. Food Agric. 2007, 87, 2801–2809. [Google Scholar] [CrossRef]
- Maroyi, A. Acacia karroo Hayne: Ethnomedicinal uses, phytochemistry and pharmacology of an important medicinal plant in southern Africa. Asian Pac. J. Trop. Med. 2017, 10, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Kumar, B.; Singh, D.K.; Luqman, S.; Singh, M.; Singh, A. Antioxidant and Choline Esterase Inhibitory Activity of Phenolic Rich Extracts from Bombax ceiba L. Flowers. Free Radic. Antioxid. 2018, 8. [Google Scholar] [CrossRef]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
| S. No | Code | Family Name | English Name | Scientific Name | Part Used | AChEI % |
|---|---|---|---|---|---|---|
| 1 | S87 | Acoraceae | Sweet Flag, Calamus | Acorus calamus | RT | 14.6±5.3 |
| 2 | S92 | Apiaceae | Dill, True dill | Anethum graveolens | SD | 24.4±8.3 |
| 3 | S3 | Apiaceae | Black cumin | Bunium bulbocastanum | SD | 26.0±2.5 |
| 4 | S94 | Apiaceae | Indian pennywort | Centella asiatica | WP | 17.1±0.4 |
| 5 | S158 | Apiaceae | Coriander, Chinese parsley | Coriandrum sativum | SD | 25.1±5.4 |
| 6 | S20 | Apiaceae | Cumin Seed | Cuminum cyminum | SD | 13.5±0.8 |
| 7 | S188 | Apiaceae | Wild Carrot, Bishop’s lace | Daucus carota | SD | 20.2±7.3 |
| 8 | S106 | Apiaceae | Fennel Seed | Foeniculum vulgare | SD | 30.1±1.6 |
| 9 | S44 | Apiaceae | Hogweed, Tookar | Heracleum candicans | TB | 9.6±3.1 |
| 10 | S145 | Apiaceae | Parsley | Petroselinum crispum | SD | 26.4±8.5 |
| 11 | S73 | Apiaceae | Sweet Cumin, Pimpenella | Pimpinella diversifolia | SD | 9.3±5.2 |
| 12 | S16 | Apiaceae | Ajowan | Trachyspermum ammi | SD | 15.6±8.3 |
| 13 | S177 | Asclepiadaceae | Bitter Cress | Caralluma tuberculata | WP | 20.0±3.1 |
| 14 | S136 | Asphodelaceae | Aloe vera | Aloe vera | LV | 31.1±6.3 |
| 15 | S40 | Berberidaceae | Tree Turmeric, Indian Lycium | Berberis lyceum | FR | 23.7±0.7 |
| 16 | S154 | Berberidaceae | Barberry | Berberis vulgaris | FR | 19.8±1.3 |
| 17 | S162 | Caesalpiniaceae | Jasmeejaz | Cassia absus | SD | 26.8±8.3 |
| 18 | S45 | Caesalpiniaceae | Cloves | Cassia occidentalis | WP | 16.1±1.8 |
| 19 | S43 | Cannabinaceae | Canna lilly, Marijuana, Arrowroot | Cannabis sativa | WP | 16.9±4.1 |
| 20 | S169 | Cannaceae | Canna lilly, Arrowroot | Canna indica | WP | 6.7±1.3 |
| 21 | S6 | Caricaceae | Papaya | Carica papaya | PU | 26.7±4.5 |
| 22 | S79 | Combretaceae | French lavender | Lavandula stoechas | FW | 27.2±1.1 |
| 23 | S26 | Combretaceae | Black myrobalan, Chebulic myrobalan | Terminalia chebula | SD | 45.7±6.6 |
| 24 | S197 | Cyperaceae | Nut-grass | Cyperus rotundus | RZ | 34.9±6.1 |
| 25 | S52 | Elaeagnaceae | Autumn olive, Spreading oleaster | Elaeagnus umbellate | FR | 17.2±7.9 |
| 26 | S23 | Elaeagnaceae | Sea buckthorns | Hippophae rhamnoides | SD | 14.3±1.3 |
| 27 | S172 | Euphorbiaceae | Kamala tree | Mallotus philippensis | FR | 18.5±13.3 |
| 28 | S103 | Euphorbiaceae | Castor oil plant | Ricinus cummunis | FR | 18.4±0.7 |
| 29 | S216 | Fabaceae | Acacia Tree Bark | Acacia karoo | ST | 52.9±0.6 |
| 30 | S39 | Fabaceae | Bear’s breeches | Atylosia mollis | SD | 28.0±4.2 |
| 31 | S196 | Fabaceae | Sacred Tree | Butea monosperma | FW | 19.0±2.7 |
| 32 | S48 | Fabaceae | Liquorice | Glycyrrhiza glabra | RT | 24.9±4.2 |
| 33 | S173 | Fabaceae | White sweet clover | Melilotus albus | WP | 31.4±4.4 |
| 34 | S59 | Fabaceae | Blue sweet clover | Melilotus indica | WP | 5.7±0.7 |
| 35 | S64 | Fabaceae | Velvet Beans, Cowhage, Cow-itch | Mucuna pruriens | SD | 13.9±2.2 |
| 36 | S156 | Fabaceae | Lima Seed, Sieva bean | Phaseolus lunatus | SD | 50.1±1.5 |
| 37 | S85 | Fabaceae | Red Sandalwood | Pterocarpus santalinus | BR | 45.7±1.5 |
| 38 | S127 | Fabaceae | Tamarind | Tamarindus indica | FR | 7.1±4.8 |
| 39 | S69 | Fabaceae | Fenugreek | Trigonella foenum-graecum | SD | 10.7±1.3 |
| 40 | S160 | Hyperiaceae | St. John’s wort | Hypericum oblongifolium | LV | 20.7±7.5 |
| 41 | S54 | Hyperiaceae | Perforate St John’s-wort | Hypericum perforatum | WP | 23.1±1.8 |
| 42 | S191 | Malvaceae | Lady’s finger, Okra | Abelmoschus esculentus | SD | 16.3±0.3 |
| 43 | S34 | Malvaceae | Hollyhocks | Alcea rosea | FW | 14.1±1.5 |
| 44 | S142 | Malvaceae | Silk-cotton tree | Bombax ceiba | RT | 77.8±0.3 |
| 45 | S185 | Oleaceae | Yellow jasmine | Jasminum humile | LV | 26.9±2.9 |
| 46 | S27 | Oleaceae | Broad leaf privet, Glossy privet | Ligustrum lucidum | LV | 20.1±0.8 |
| 47 | S60 | Oleaceae | Indian olive, Wild olive | Olea ferruginea | SD | 11.5±3.5 |
| 48 | S77 | Santalaceae | White sandalwood, Sandal peel | Santalum album | BR | 86.2±0.8 |
| 49 | S82 | Violaceae | Mountain violet, Showy violet | Viola betonicifolia | WP | 30.2±1.4 |
| 50 | S83 | Violaceae | Sweet violet, Viola | Viola odorata | WP | 13.7±1.8 |
| Galatamine hydrobromide (0.1mM) | 81.8±2.09 | |||||
| Sample | DPPH (%) | TFC (mg QE/g) | TPC (mg GAE/g) | TTRC (mg LE/g) | TAC (mg BE/g) | TTC (mg GAE/g) |
|---|---|---|---|---|---|---|
| PL | 90.9±0.2a | 16.1±1.6b | 18.7±1.6b | 11.2±1.2c | 1.8±0.1b | 24.6±0.6b |
| AK | 88.6±1.1bc | 16.84±2.8b | 15.9±3.2b | 16.3±1.0b | 4.7±2.0a | 25.1±2.5b |
| BC | 61.4±2.1d | 7.18±0.2c | 3.9±0.2c | 6.0±0.5d | 2.7±0.3b | 17.9±0.6c |
| SA | 87.1±0.2c | 31.12±2.1a | 71.94±2.4a | 19.9±2.5a | 1.9±0.5b | 34.1±2.0a |
| AA | 91.7±0.7a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





