1. Introduction
Transpulmonary thermodilution is a technique for hemodynamic monitoring of critically ill patients that has gained great interest in recent years. It measures cardiac output (CO), stroke volume (SV), but also calculates variables related with volume status, cardiac contractility, dynamic predictors of volume response, and extravascular lung water (ELWI) [
1,
2,
3,
4,
5].
One of the hemodynamic variables that it measures is the global end diastolic indexed volume (GEDVI), which provides the volume of the 4 cardiac chambers and large intrathoracic vessels during diastole. It is a static variable of cardiac preload [
5,
6,
7,
8,
9,
10].
Another calculated variable is the global ejection fraction (GEF). It provides information regarding cardiac function. GEF reflects the relationship between SV and the volume of the 4 cardiac chambers and large intrathoracic vessels. It assumes that the overall end-diastolic volume (GEDV) is equal to GEDV /4. This is an approximation since the volume of each of the cardiac chambers is different.
It has been described that impaired GEF values have good correlation with LV ejection fractions below 40 - 30%. Also has been described that GEF adequately track directional changes in ejection fraction when inotropic drugs are being used [11–16,19].
GEF and GEDVI calculations are performed simultaneously from the same CO measurement. The formula that calculates GEDV includes the CO, and the calculation of the GEF includes the SV, therefore there could be a mathematical coupling between both [18].
The aim of our work was to evaluate whether impairment in GEDVI and SV may constitute a limitation for the interpretation of GEF in hypovolemic patients. A secondary objective was to determine factors independently associated with impaired GEF.
2. Materials and Methods
A retrospective study was conducted on a database of patients with shock who received hemodynamic monitoring with transpulmonary thermodilution by means of VolumeView/EV1000™ system (Edwards Lifesciences, Irvine CA, USA) from 2016 to 2023 in a 24-bed medical-surgical intensive care unit (UCI) in Argentina.
The following variables were recorded: sex, age, comorbidities, APACHE II and SOFA scores during the first 24 hours of hospitalization, shock etiology, ICU length of stay, mortality, mean arterial pressure (MAP), heart rate (HR), cardiac index (CI), stroke volume (SV), indexed global end-diastolic volume (GEDVI), indexed systemic vascular resistance (IRVSi) and extravascular lung water (ELWI). Hemodynamic variables were recorded during the first measurement of transpulmonary thermodilution.
Shock was defined as the need of norepinephrine infusion to keep at least TAM of 65 mmHg plus at least 1 sign of tissue hypoperfusion. We defined hypovolemia as SV values less than 60 mL and GEDVI less than 680 mL/m2. Patients were classified according to GEF, GEDVI, and SV values.
To determine correlation between GEF with SV, or GEF with GEDVI Pearson's coefficient was used.
Statistical analysis was performed by means of Mann Whitney test for continuous variables of abnormal distribution and student t test for continuous variables of normal distribution. For categorical variables, Pearson's Chi² test was used. To determine factors independently associated with the presence of impaired GEF, binary logistic regression was performed. We included all variables with a p< value of 0.10 in the univariate analysis and those that were physiologically relevant.
3. Results
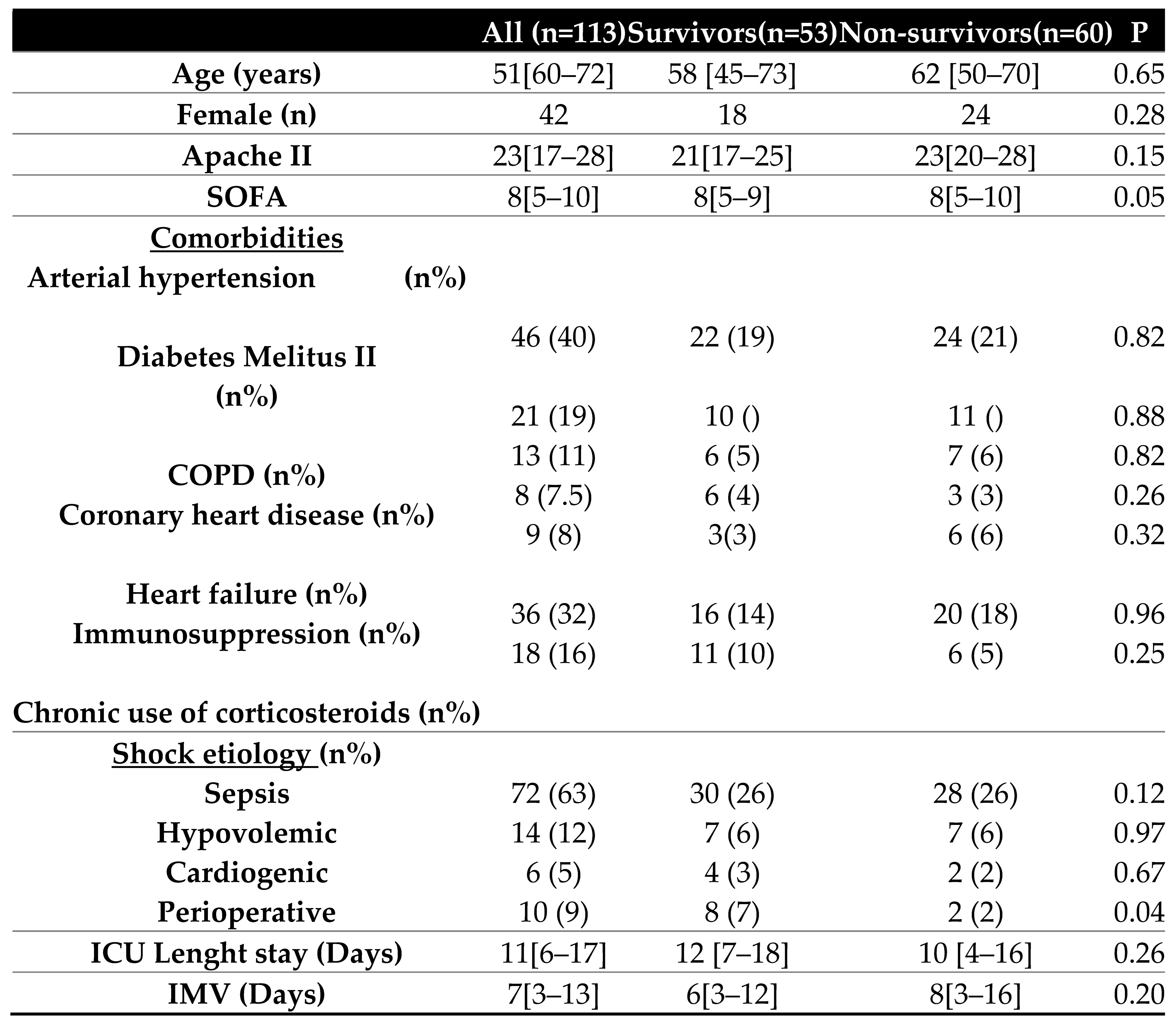
We included 113 patients with shock who received hemodynamic monitoring by transpulmonary thermodilution. The most frequent cause of shock that triggered the placement of transpulmonary thermodilution monitoring was sepsis (63%). There were no statistically significant differences in the severity or epidemiological characteristics of the patients. (
Table 1).
The haemodynamic characteristics of the first measurement with transpulmonary thermodilution are shown in
Table 2a. Statistically significant differences were detected between survivors and non-survivors in GEF (21[16–28] versus 18[11–26]; p= 0.01) and in MAP (79[67–93] mmHg versus 73[62–88] mmHg; (p= 0.00)) 38(34%) patients had hypovolemia. 44(39%) of them had depressed GEF values.
Hypovolemic patients had lower GEF values than non-hypovolemic patients (16 [12–22] vs 20 [15–27]; p=0.01) (
Table 2.b) Figura 1.
Figure 1.
GEF according to blood volume status.
Figure 1.
GEF according to blood volume status.
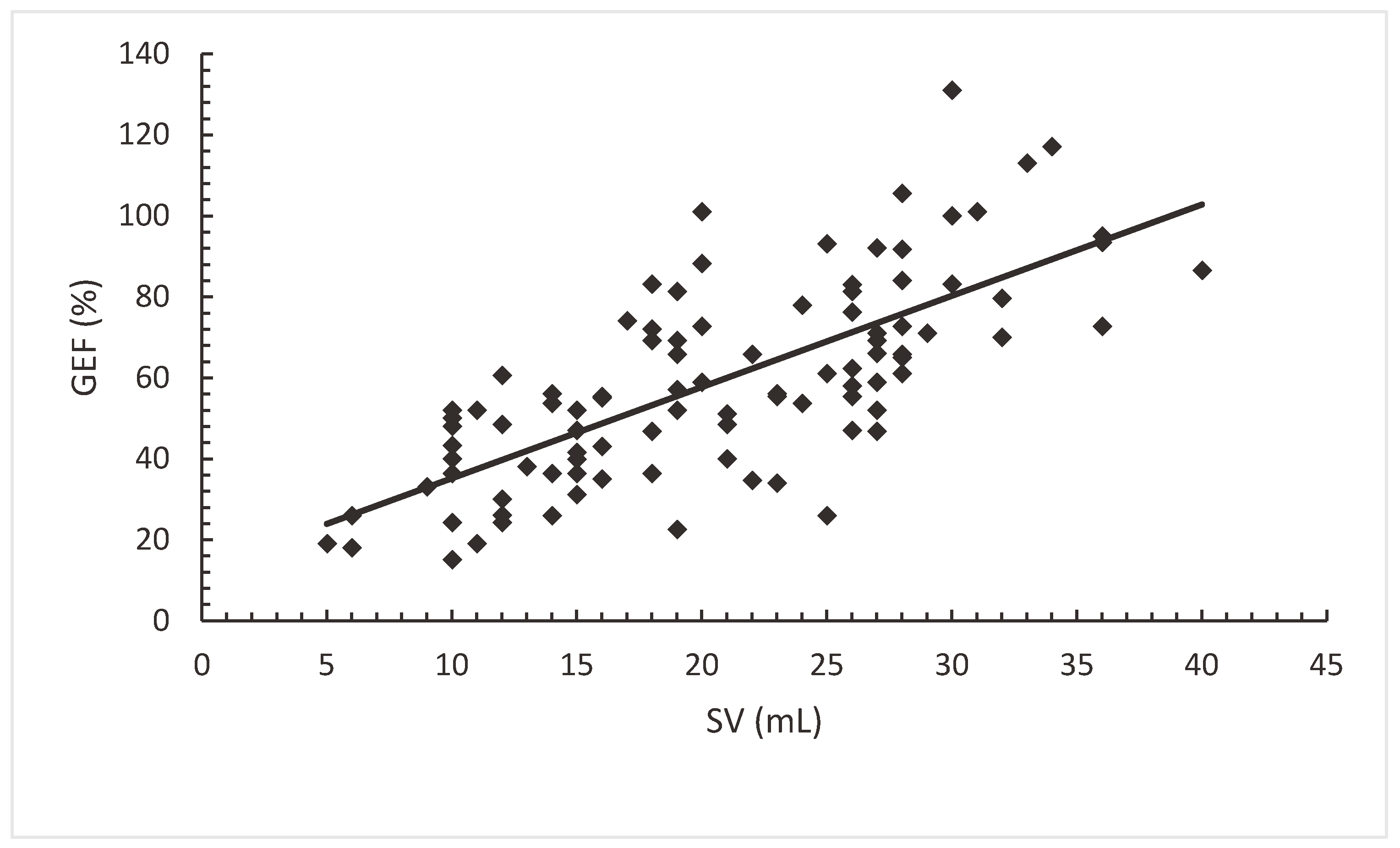
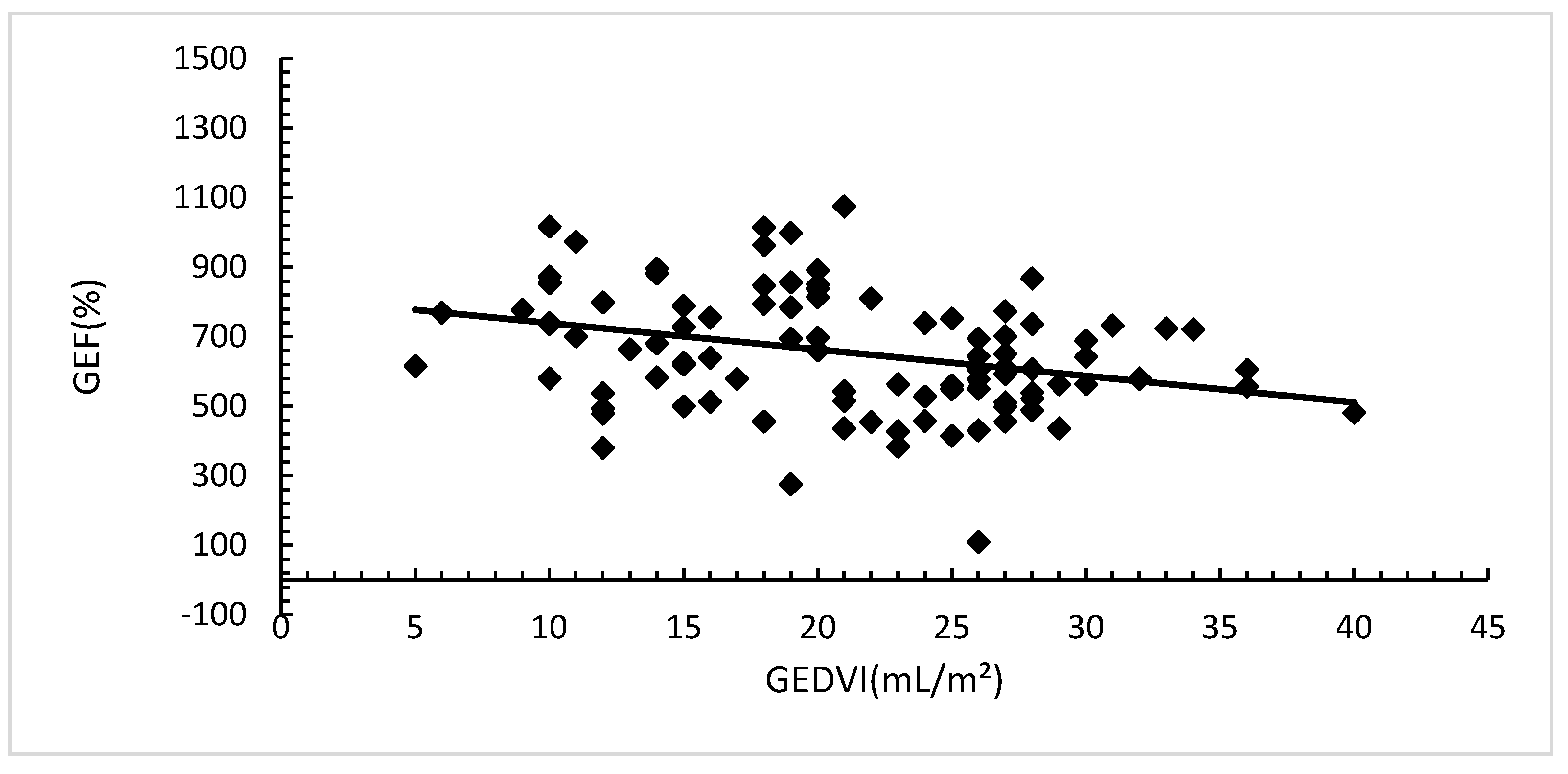
Both SV and GEDVI had a statistically significant correlation with GEF, SV (Pearson's r 0.74; (p=0.00).
Figure 2a) and GEDVI (Pearson's r -0.25; (p=0.00).
Figure 2b).
Transthoracic echocardiogram was performed in 72(64%) patients, 36(31%) of them had depressed GEF values, however in only 19(17%) patients depressed left ventricular ejection fractions by echocardiogram coexist.
The only factors independently associated with the development of low GEF were low SV and low GEDVI.
Table 3.
4. Discusión
Our main finding is that in the population of hypovolemic patients, GEF values are lower than in non-hypovolemic patients, therefore; they can’t be interpreted in isolation because it could lead to misinterpretations. Preload conditions determine cardiac ejection fraction, i.e patients with low preload could have low cardiac function and CO. While this statement may not be novel, as it is physiologically known, it has not previously been highlighted as a limitation that underestimates cardiac function measured by TDTP, to the best of our knowledge.
The correlation between SV and GEF was strong, and between GEDVI and GEF was week. It could be due to mathematical coupling in the formulas that are used to make the calculations. GEDV is calculated by the formula GEDV= CO x MTt x f (S1/* S2) (MTt: mean transit time. S1 and S2 are respectively the maximum ascending and descending slopes of the transpulmonary thermodilution curve and f is a proprietary function). The calculation of GEF is done using the formula SV*4 / GEDV. GEF and GEDVI calculations are performed simultaneously from the same CO measurement, so there is a risk of mathematical coupling [12,18,19].
One way to address this limitation could be to assess, at the same time, fluid response and other preload parameters such as PVC or GEDVI, or to perform echocardiography. It should be borne in mind that GEF is a parameter of overall cardiac function, not cardiac contractility.
The retrospective nature of our study constitutes its main limitation, for this reason we could not establish the volume response of the patients included, since it was impossible for us to determine if the appropriate conditions for the interpretation of VVS were met. We also did not assess whether the GEF was modified after correcting hypovolemia. In adittion, we did not perform simultaneous measurements de thermodilution and the echocardiogram, but the latter was performed within 24 hours, and was only performed in part of the patients studied.
5. Conclusions
Hypovolemic patients frequently show low GEF values. We found correlation between SV and GEDVI with GEF. The factors independently associated with low GEF were low SV, low GEDVI. This could constitute a limitation in the interpretation of GEF and cardiac pump function. Hypovolemic patients could have low GEF without implying disorders in cardiac contractility.
Author Contributions
Gonzalez Cecilia contributed to conception and design of the study, performed the main statistical analysis and wrote the first draft of this paper. Rodriguez Louzan Jesica contributed to conception and design of the study, helped collect the data, contributed to the analysis of the data. Traverso Agustin helped collect the data, contributed to the analysis of the data. Giordana Agostina helped collect the data, contributed to the analysis of the data. Papini Emanuele helped collect the data, contributed to the analysis of the data. Latasa Diana helped collect the data, contributed to the analysis of the data. Lovesio Carlos is the study lead and guarantor for this paper. Dubin Arnaldo contributed to conception and design of the study and revised it critically for important intellectual content, proofread and helped write the draft. All authors read and approved the final manuscript.
Funding
This research received no external funding.
Acknowledgments
We also want to thank Dr. Vanina Kannore Edul, who by example gives us permanent teachings of camaraderie of the scientific spirit and love for science and knowledge.
Conflicts of Interest
The authors declare no conflict of interest.
Ethics approval and consent to participate
Ethical review and approval were waived for this study, due to the it was considered as posing minimal risk to the study participants because of its retrospective design.
References
- Sakka, S.G.; Reuter, D.A.; Perel, A. The transpulmonary thermodilution technique. J. Clin. Monit. Comput. 2012, 26, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Persichini, R.; Ktari, M.; Jozwiak, M.; Richard, C.; Teboul, J.L. Precision of the transpulmonary thermodilution measurements. Crit. Care. 2011, 15, R204. [Google Scholar] [CrossRef] [PubMed]
- Stetz, C.W.; Miller, R.G.; Kelly, G.E.; Raffin, T.A. Reliability of the thermodilution method in the determination of cardiac output in clinical practice. Am. Rev. Respir. Dis. 1982, 126, 1001–1004. [Google Scholar] [PubMed]
- Kiefer, N.; Hofer, C.K.; Marx, G.; Geisen, M.; Giraud, R.; Siegenthaler, N.; Hoeft, A.; Bendjelid, K.; Rex, S. Clinical validation of a new thermodilution system for the assessment of cardiac output and volumetric parameters. Crit. Care. 2012, 16, R98. [Google Scholar] [CrossRef]
- Bendjelid, K.; Giraud, R.; Siegenthaler, N.; Michard, F. Validation of a new transpulmonary thermodilution system to assess global end-diastolic volume and extravascular lung water. Crit. Care. 2010, 14, R209. [Google Scholar] [CrossRef] [PubMed]
- Michard, F.; Alaya, S.; Zarka, V.; Bahloul, M.; Richard, C.; Teboul, J.L. Global end diastolic volume as an indicator of cardiac preload in patients with septic shock. Chest. 2003, 124, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- McLuckie, A.; Bihari, D. Investigating the relationship between intrathoracic blood volume index and cardiac index. Intensive Care Med. 2000, 26, 1376–1378. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Berneau, J.B.; Luyt, C.E.; Trouillet, J.L. Estimation of left ventricular systolic function by single transpulmonary thermodilution. Intensive Care Med. 2004, 30, 1377–1383. [Google Scholar] [CrossRef]
- Jabot, J.; Monnet, X.; Lamia, B.; Chemla, D.; Richard, C.; Teboul, J.L. Cardiac function index provided by transpulmonary thermodilution behaves as an indicator of left ventricular systolic function. Crit. Care Med. 2009, 37, 2913–2918. [Google Scholar] [CrossRef] [PubMed]
- Trepte, C.J.; Eichhorn, V.; Haas, S.A.; Richter, H.P.; Goepfert, M.S.; Kubitz, J.C.; Goetz, A.E.; Reuter, D.A. Thermodilution-derived indices for assessment of left and right ventricular cardiac function in normal and impaired cardiac function. Crit. Care Med. 2011, 39, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Perny, J.; Kimmoun, A.; Perez, P.; Levy, B. Evaluation of cardiac function index as measured by transpulmonary thermodilution as an indicator of left ventricular ejection fraction in cardiogenic shock. Biomed. Res. Int. 2014, 2014, 598029. [Google Scholar] [CrossRef] [PubMed]
- Robotham, J.L.; Takata, M.; Berman, M.; Harasawa, Y. Ejection fraction revisited. Anesthesiology. 1991, 74, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Beitz, A.; Berbara, H.; Mair, S.; Henschel, B.; Lahmer, T.; Rasch, S.; Schmid, R.; Huber, W. Consistency of cardiac function index and global ejection fraction with global end-diastolic volume in patients with femoral central venous access for transpulmonary thermodilution: a prospective observational study. J. Clin. Monit. Comput; 2017, 31, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Belda, F.; Aguilar, G.; Jover, J.L.; Ferrando, C.; Postigo, S.; Aznárez, B. Validación clínica de la valoración mínimamente invasiva de la función sistólica. Rev. Esp. Anestesiol. Reanim. 2010, 57, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Hilty, M.P.; Franzen, D.P.; Wyss, C.; Biaggi, P.; Maggiorini, M. Validation of transpulmonary thermodilution variables in hemodynamically stable patients with heart diseases. Ann. Intensive Care (2017) 7:86. J Clin Monit Comput 2019 Apr;33(2):233-239. [CrossRef]
- Nakwan, N.; Chichareon, P.; Khwannimit, B. A comparison of ventricular systolic function indices provided by VolumeView/EV1000™ and left ventricular ejection fraction by echocardiography among septic shock patients. J. Clin. Monit. Comput. 2019, 33, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Meybohm, P.; Gruenewald, M.; Renner, J.; Maracke, M.; Rossee, S.; Hocker, J.; Hagelstein, S.; Zacharowski, K.; Bein, B. Assessment of left ventricular systolic function during acute myocardial ischemia: a comparison of transpulmonary thermodilution and transesophageal echocardiography. Minerva Anestesiol. 2011, 77, 132–141. [Google Scholar] [PubMed]
- Walsh TS, Lee A: Mathematical coupling in medical research: Lessons from studies of oxygen kinetics. Br. J. Anaesth. 1998, 81, 118–120. [CrossRef] [PubMed]
- Transpulmonary thermodilution: advantages and limits. Monnet Teboul Crit. Care 2017, 21, 147. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).