Submitted:
19 February 2024
Posted:
20 February 2024
You are already at the latest version
Abstract
Keywords:
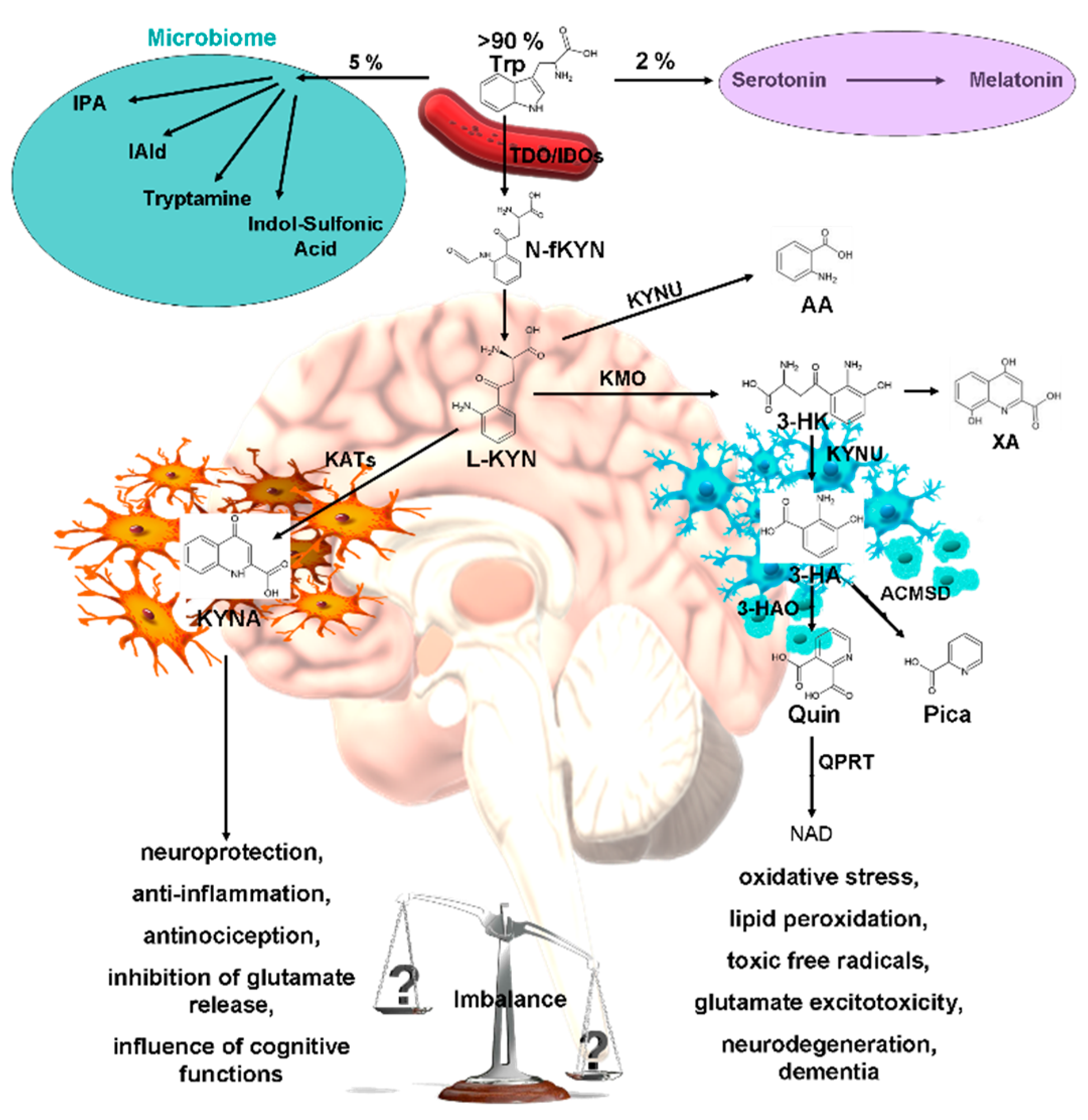
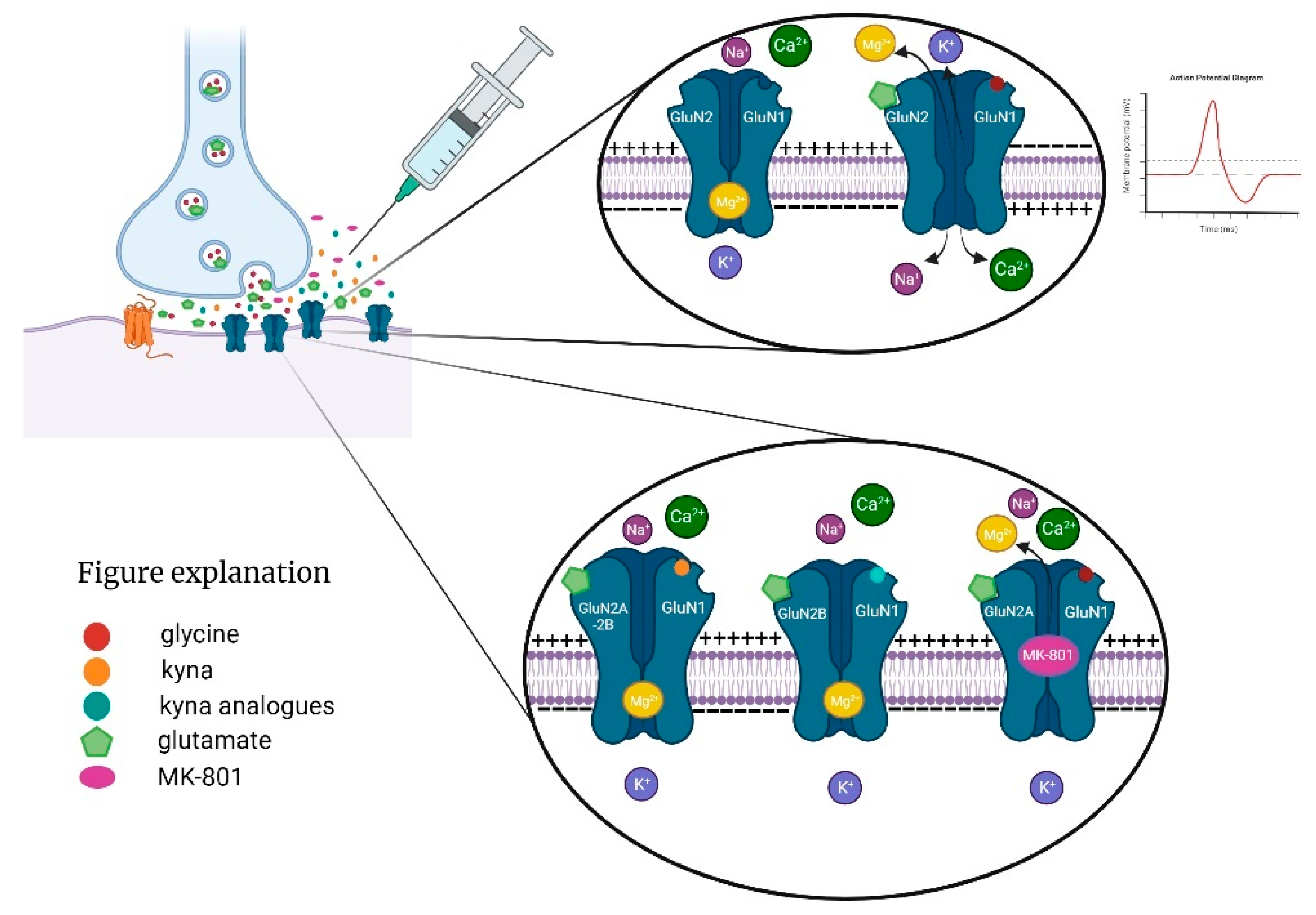
1. Introduction
2. Results
2.2. Behavioral Tests
2.2.1. Pilot Study

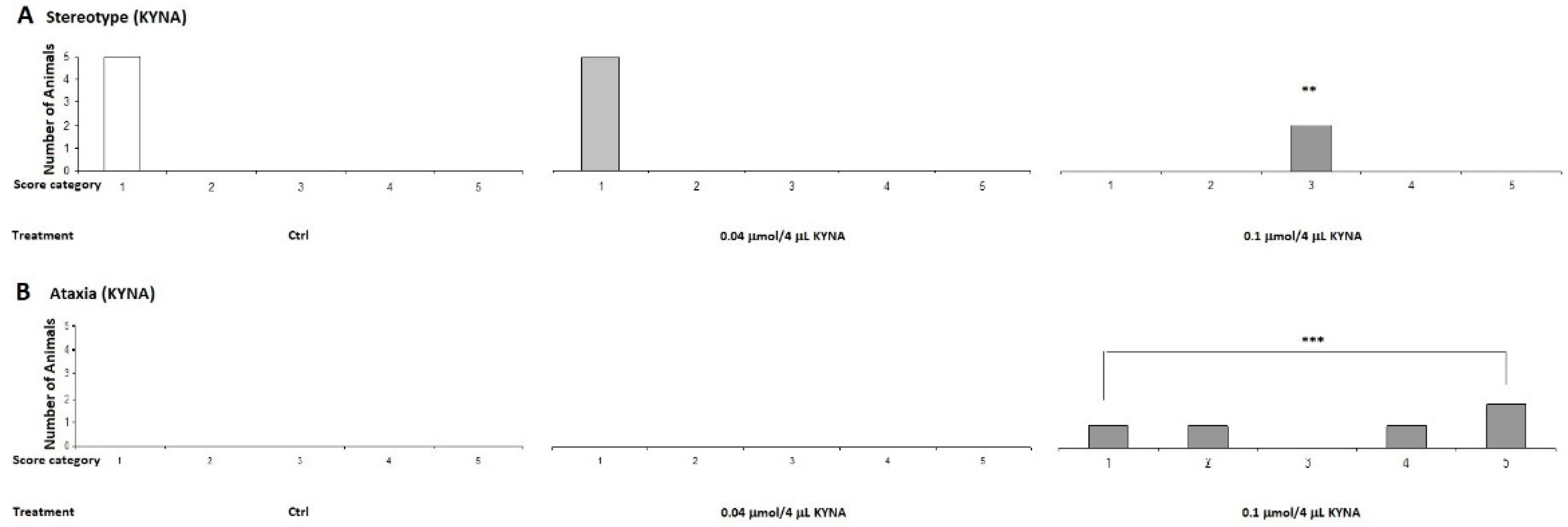
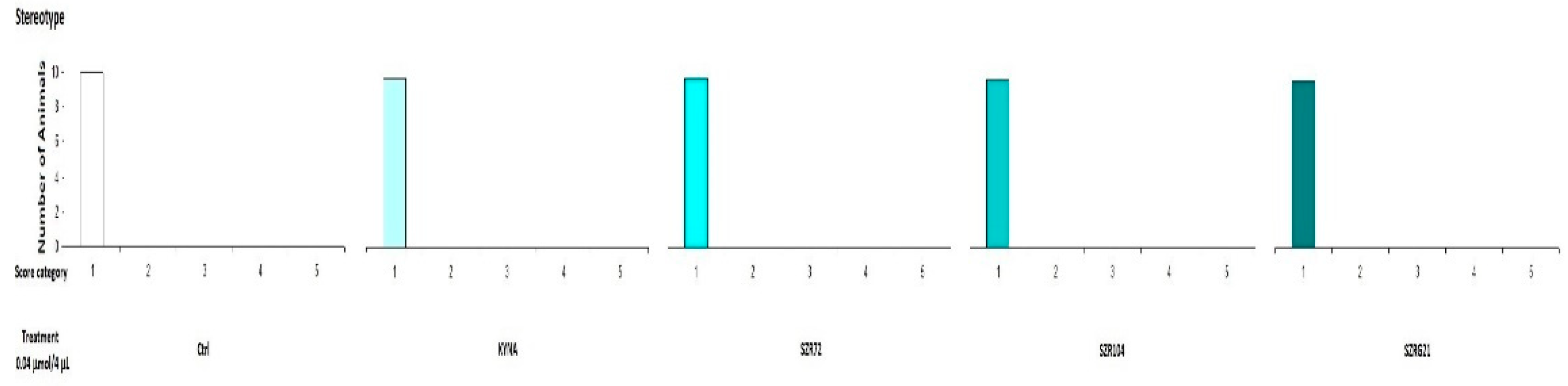
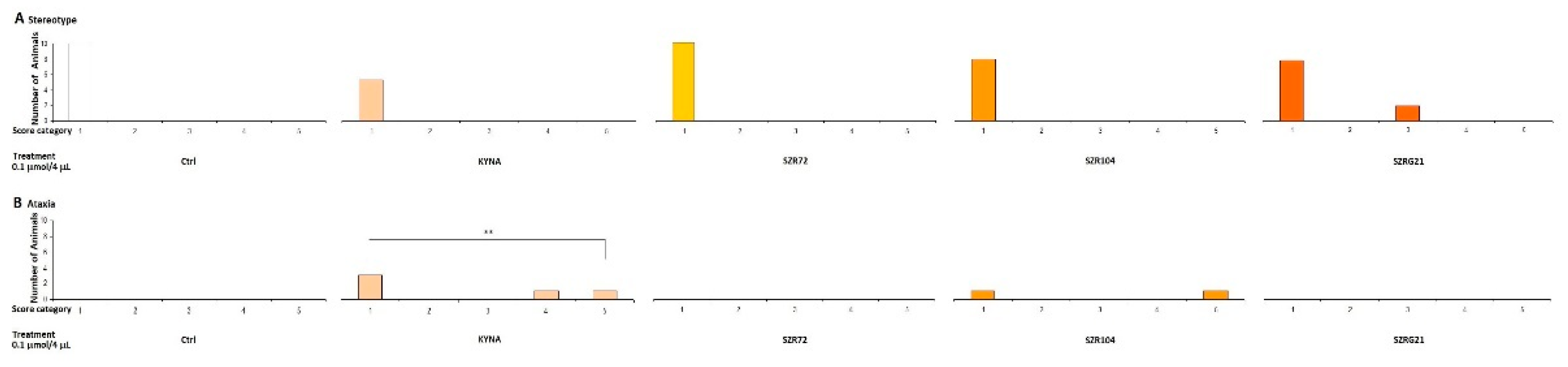
2.2.2. Stereotype/Ataxia Test
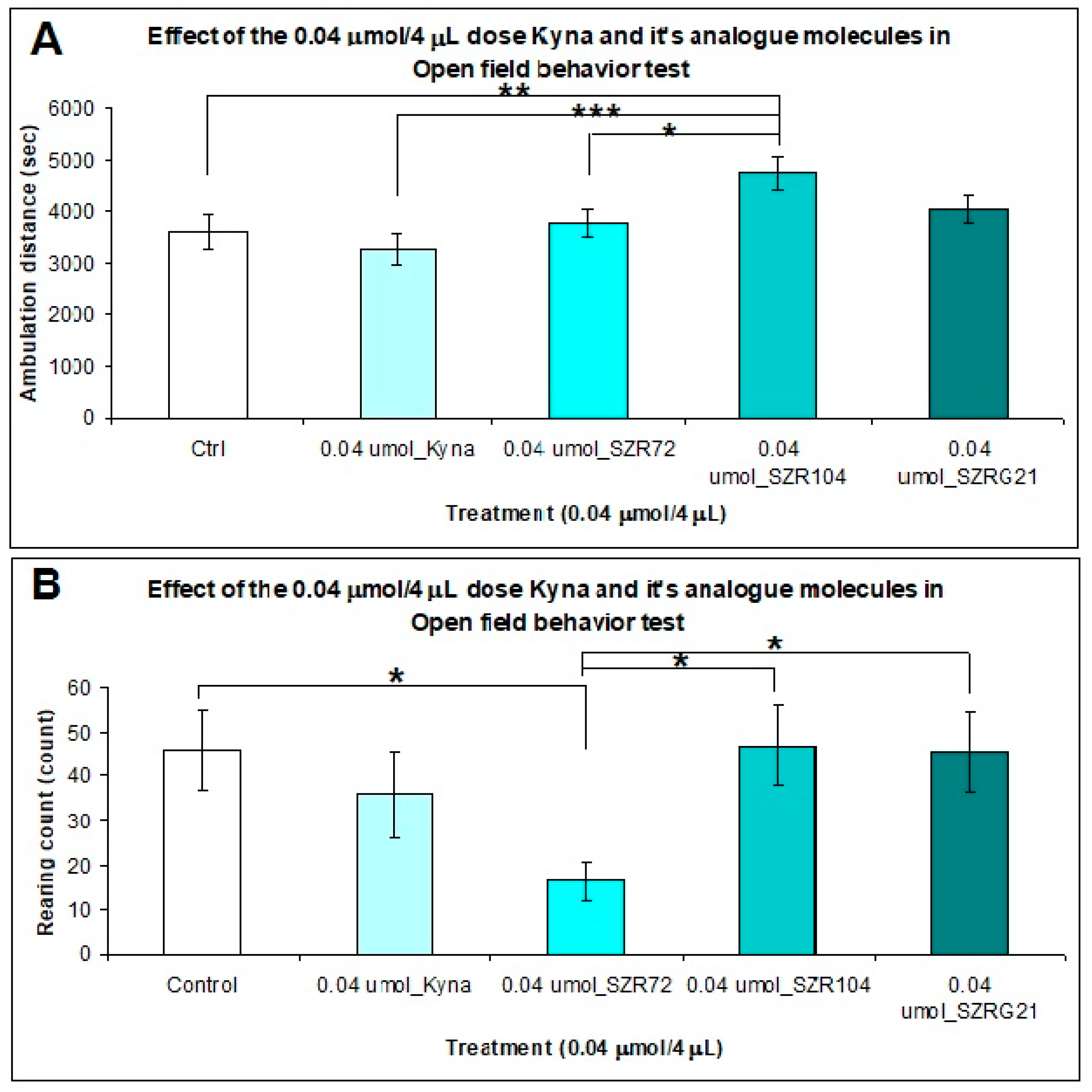
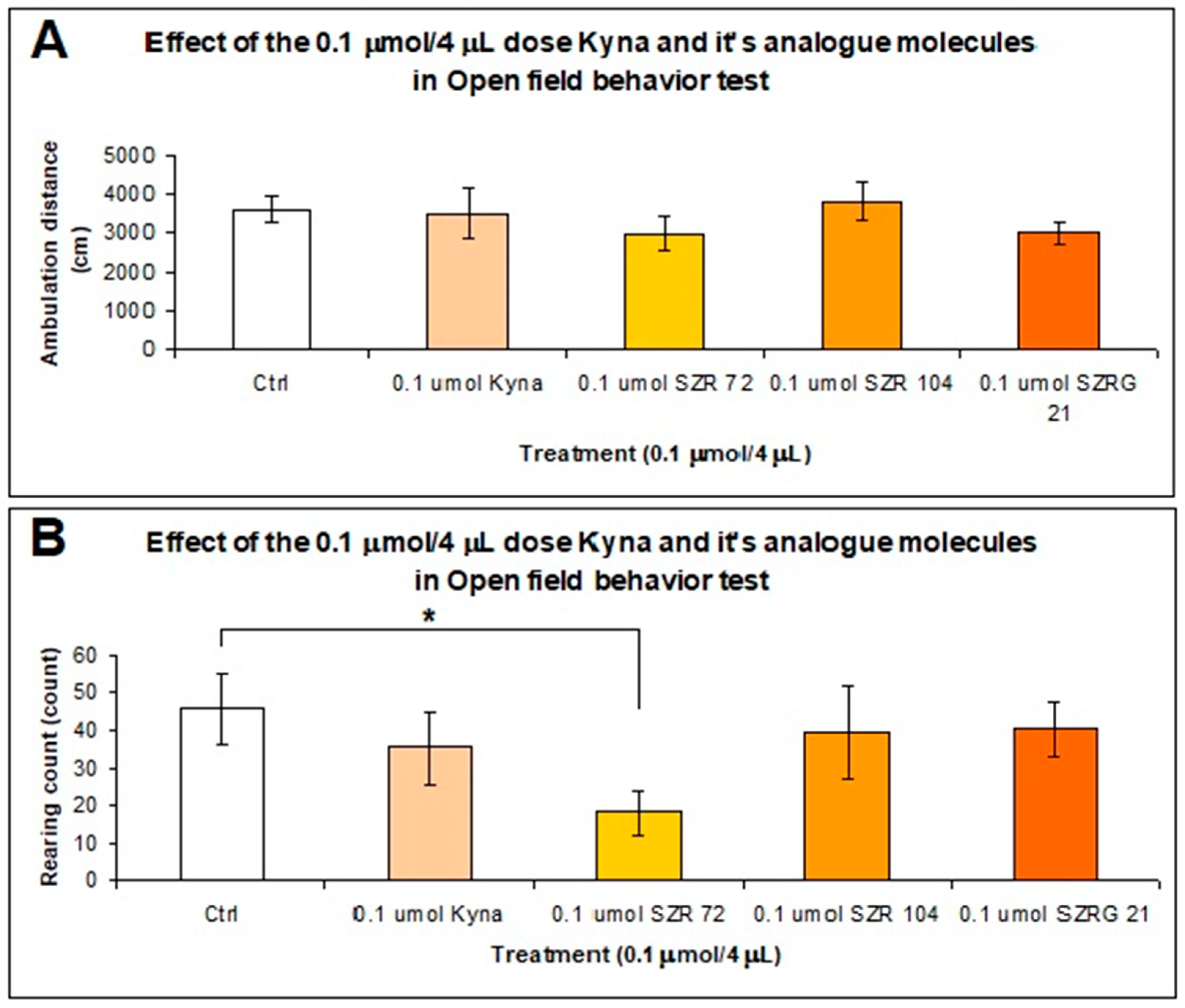
2.2.3. Open Field (OF) Test
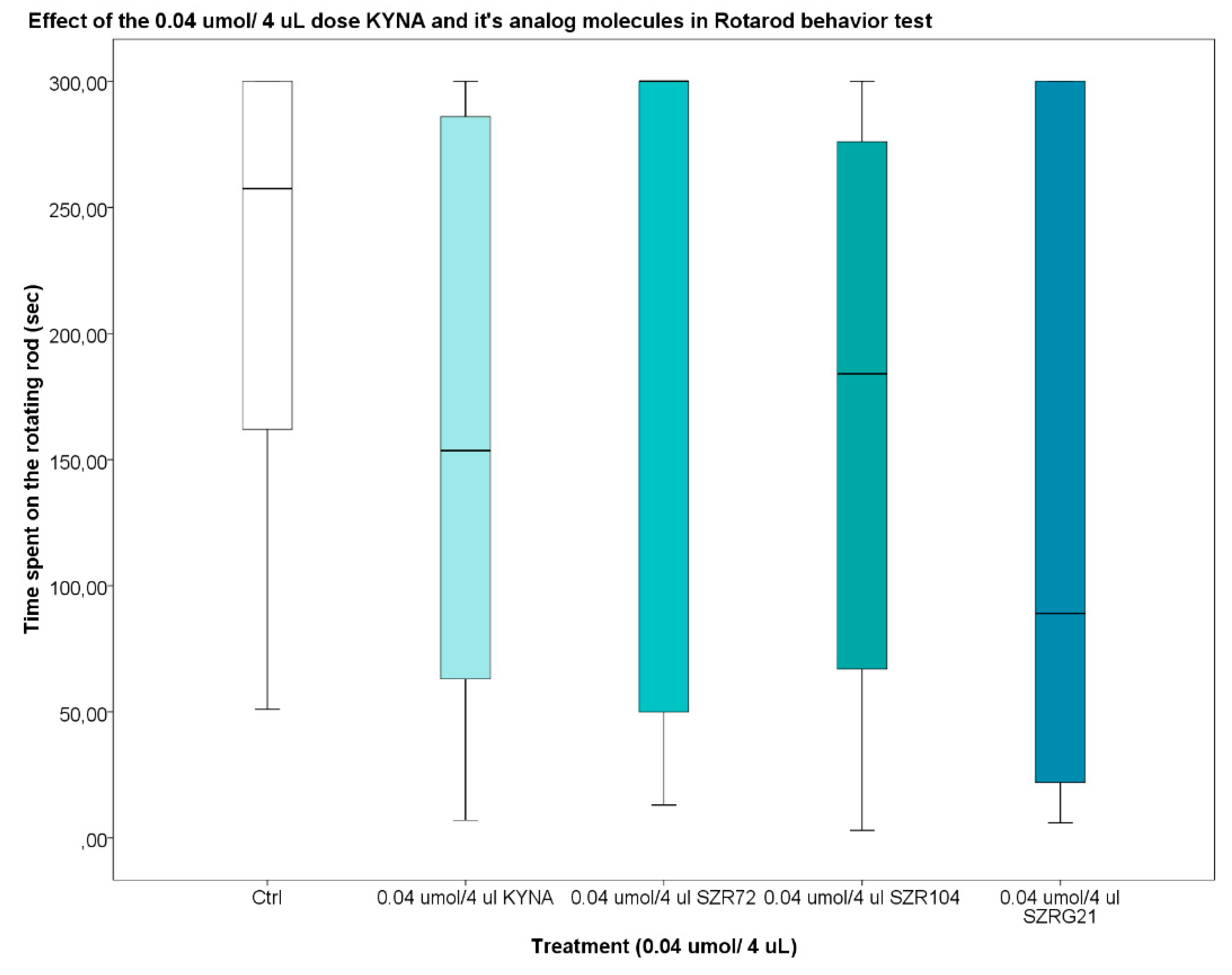
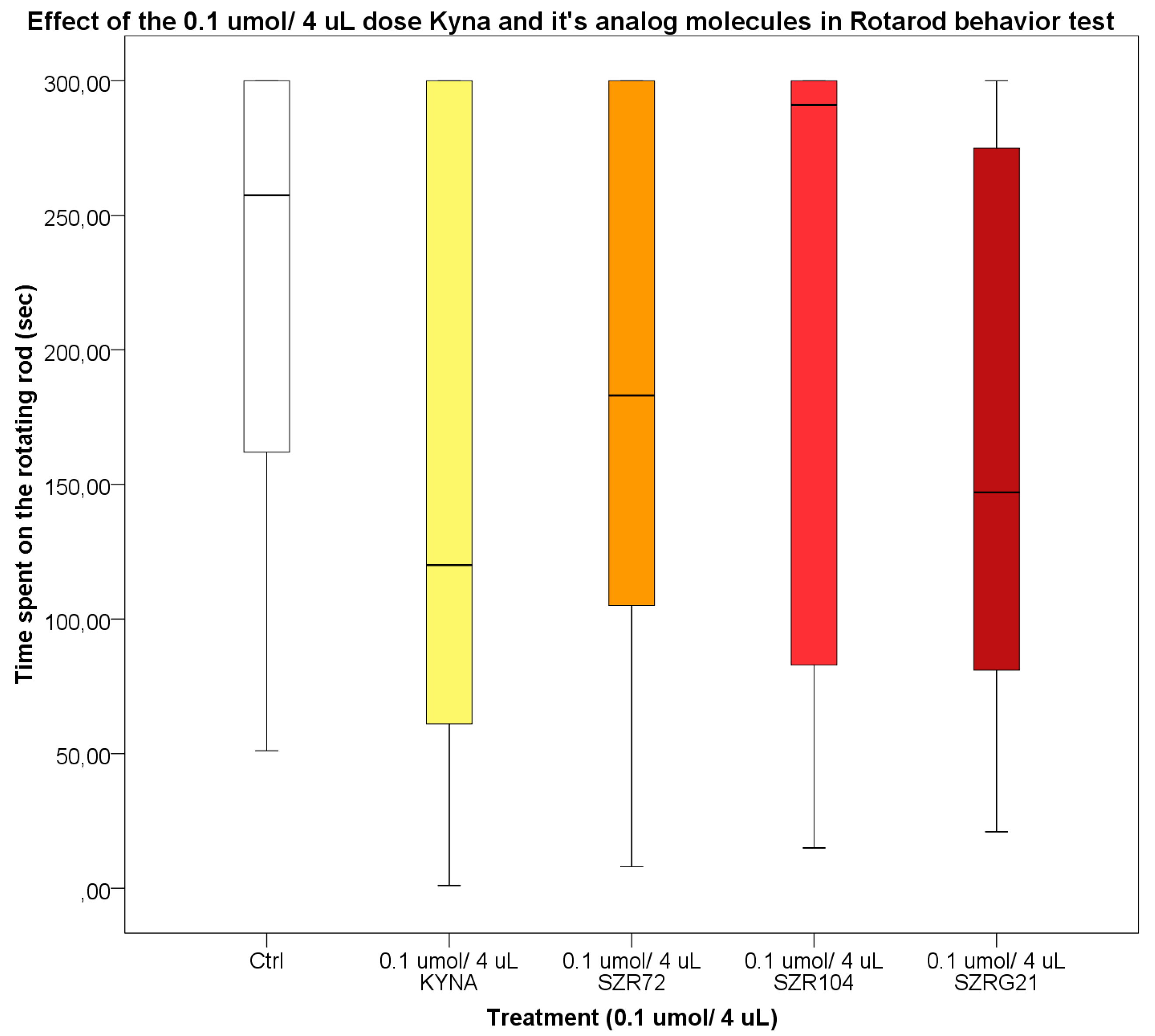
2.2.4. Rotarod (RR) Test
3. Discussion
4. Materials and Methods
4.1. Materials
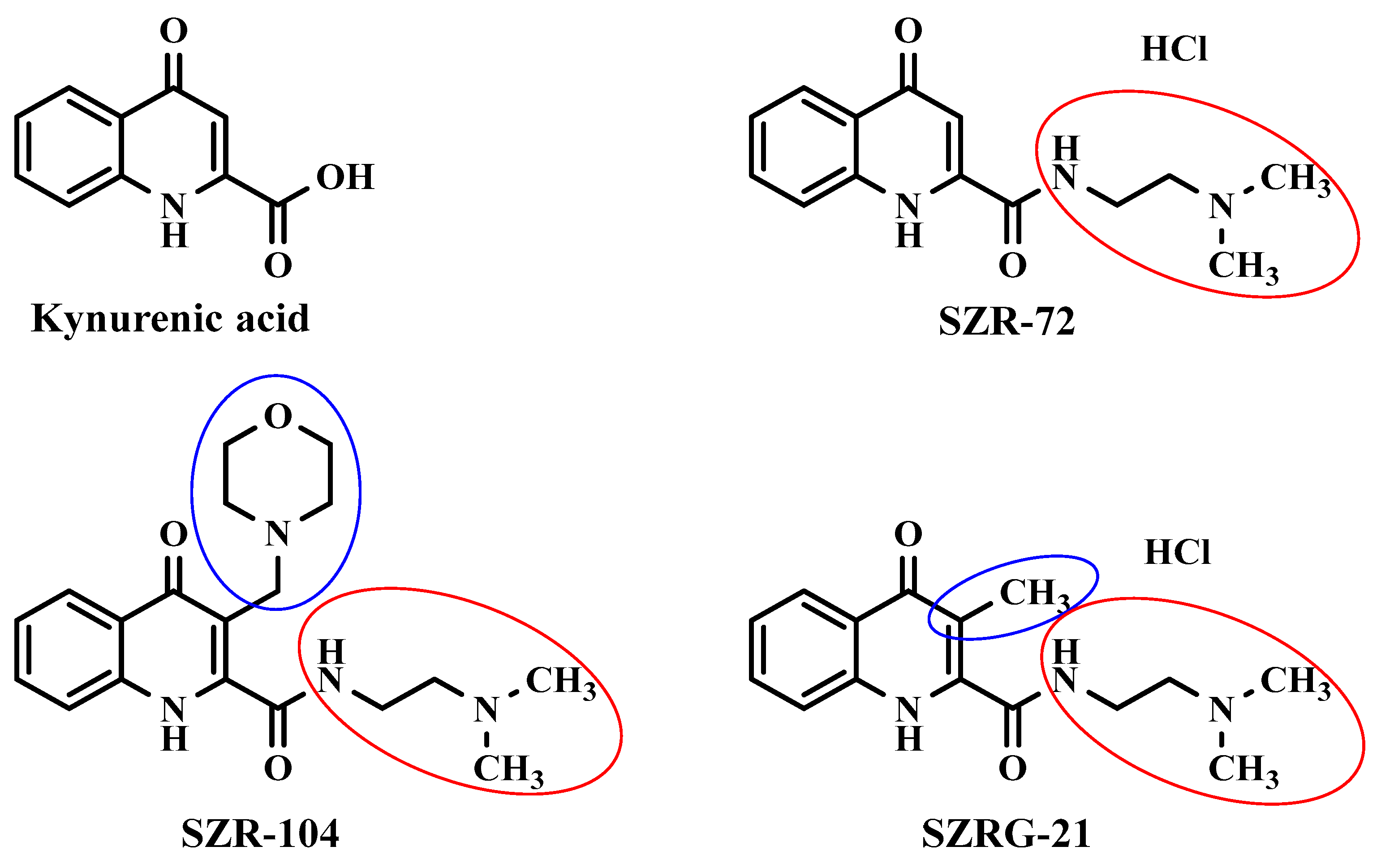
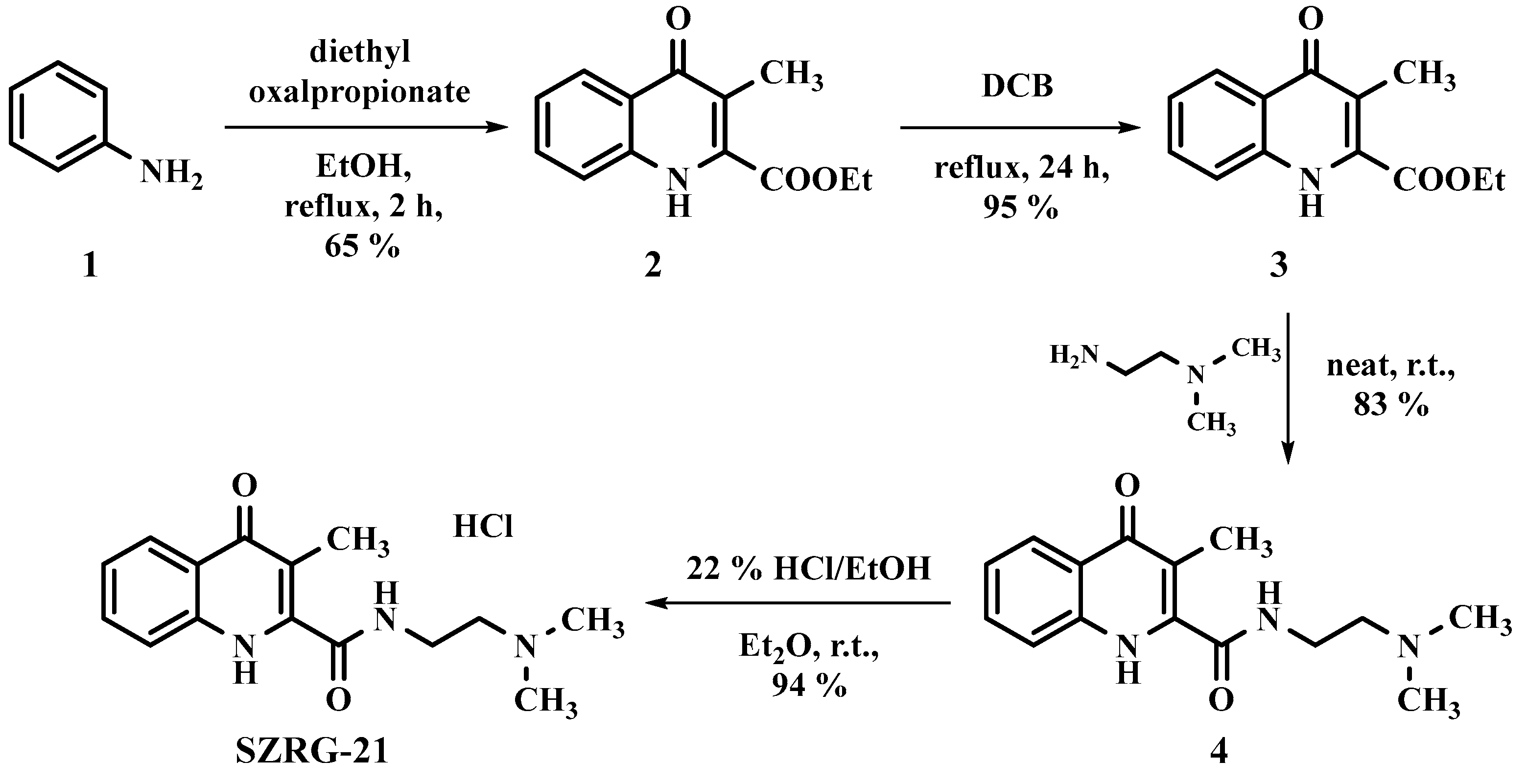
4.2. Kynurenic Acid Analogs Synthesis
4.3. Animals
4.4. Surgery
4.5. Behavioral Tests
4.5.1. Pilot Study
4.5.2. Stereotype Behavior and Ataxia
4.5.3. Open Field (OF) Test
4.5.4. Rotarod (RR) Test
4.6. Statistical Analysis
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | anthranilic acid |
| Acetyl-CoA | acetyl coenzyme A |
| ACMS | 2-amino-3-carboxymuconate semialdehyde |
| ACMSD | 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AMS | 2-aminomuconic-6-semialdehyde |
| AMSD | 2-aminomuconate semialdehyde dehydrogenase |
| AD | Alzheimer's disease |
| BBB | blood–brain barrier |
| CA | cinnabarinic acid |
| GPR35 | G-protein-coupled receptor 35 |
| CNS | central nervous system |
| 5-HT | serotonin |
| IDOs | indoleamine 2,3-dioxygenases |
| 3-HAA | 3-hydroxyanthranilic acid |
| 3-HK | 3-hydroxy-L-kynurenine |
| 3-HAO | 3-hydroxyanthranilate oxidase |
| KAT | kynurenine aminotransferase |
| KFA | kynurenine formamidase |
| KI | knock-in |
| KMO | kynurenine 3-monooxygenase |
| KO | knockout |
| KYN | kynurenine |
| KYNA | kynurenic acid |
| KYNU | kynureninase |
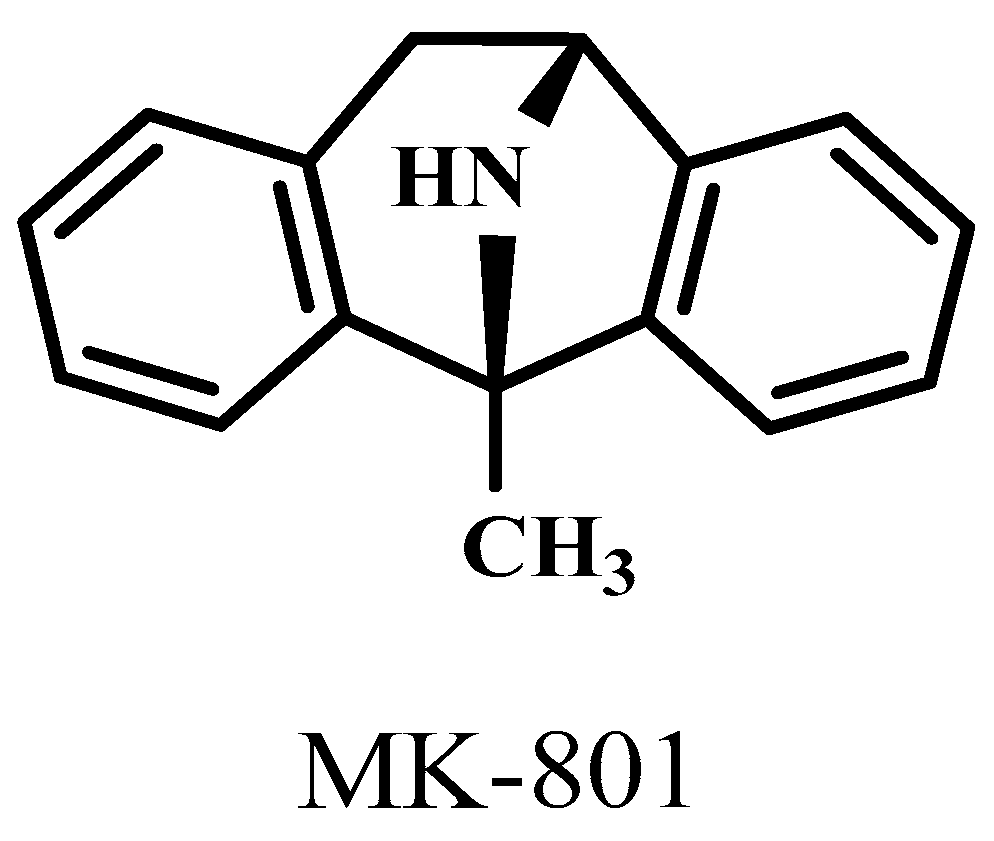
| MK-801 | 1-methyl-8-azabicyclo[3.2.1]octane |
| NADH | nicotinamide adenine dinucleotide + hydrogen |
| N-fKYN | N-formyl-kynurenine |
| NMDA | N-methyl-D-aspartic acid |
| PC | picolinic acid |
| PD | Parkinson's disease |
| PLP | pyridoxal 5’-phosphate |
| QPRT | quinolinate phosphoribosyltransferase |
| QUIN | quinolinic acid |
| SZR72 | diethyl 2-methyl-3-(phenylamino)maleate |
| SZR-104 | N-(2-(dimethylamino)ethyl)-4-oxo-1,4-dihydroquinazoline-2-carboxamide |
| SZRG-21 | N-(2-(dimethylamino)ethyl)-4-oxo-1,4-dihydroquinazoline-2-carboxamide hydrochloride |
| TDO | tryptophan 2,3-dioxygenase |
| Trp | Tryptophan |
| XA | xanthurenic acid |
References
- Derryberry, D.; Tucker, D.M. Neural mechanisms of emotion. J Consult Clin Psychol 1992, 60, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, L. A Network Model of the Emotional Brain. Trends Cogn Sci 2017, 21, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Di Rienzo, F.; El Hoyek, N.; Guillot, A. Autonomic nervous system correlates in movement observation and motor imagery. Front Hum Neurosci 2013, 7, 415. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Körtési, T.; Szok, D.; Tajti, J.; Vécsei, L. From CGRP to PACAP, VIP, and Beyond: Unraveling the Next Chapters in Migraine Treatment. Cells 2023, 12, 2649. [Google Scholar] [CrossRef] [PubMed]
- Tajti, J.; Szok, D.; Csáti, A.; Szabó, Á.; Tanaka, M.; Vécsei, L. Exploring novel therapeutic targets in the common pathogenic factors in migraine and neuropathic pain. International Journal of Molecular Sciences 2023, 24, 4114. [Google Scholar] [CrossRef]
- Zigmond, M.J.; Wiley, C.A.; Chesselet, M.-F. Neurobiology of brain disorders: biological basis of neurological and psychiatric disorders; Academic press: 2022.
- Bors, L.A.; Erdő, F. Overcoming the blood–brain barrier. challenges and tricks for CNS drug delivery. Scientia Pharmaceutica 2019, 87, 6. [Google Scholar] [CrossRef]
- Abbott, N.J. Blood–brain barrier structure and function and the challenges for CNS drug delivery. Journal of inherited metabolic disease 2013, 36, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Barchet, T.M.; Amiji, M.M. Challenges and opportunities in CNS delivery of therapeutics for neurodegenerative diseases. Expert opinion on drug delivery 2009, 6, 211–225. [Google Scholar] [CrossRef]
- Alahmari, A. Blood-brain barrier overview: structural and functional correlation. Neural Plasticity 2021, 2021. [Google Scholar] [CrossRef]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-brain barrier dysfunction amplifies the development of neuroinflammation: understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction. Frontiers in cellular neuroscience 2021, 15, 661838. [Google Scholar] [CrossRef]
- Zinserling, V. Defense Mechanisms and Local Immunity of the Brain. In Infectious Lesions of the Central Nervous System; Springer: 2022; pp. 5–24.
- Fong, C.W. Permeability of the blood–brain barrier: molecular mechanism of transport of drugs and physiologically important compounds. The Journal of membrane biology 2015, 248, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Seelig, A. The role of size and charge for blood–brain barrier permeation of drugs and fatty acids. Journal of molecular neuroscience 2007, 33, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Begley, D.J. Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Ther 2004, 104, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Segal, M.; Zlokovic, B.V. The blood-brain barrier, amino acids and peptides; Springer Science & Business Media: 2012.
- Oldendorf, W.H. The blood-brain barrier. Experimental eye research 1977, 25, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, C.; Wang, L.; Chen, Y. A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment. Drug delivery 2019, 26, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M. Molecular anatomy of the brain endothelial barrier: an overview of the distributional features. Curr Med Chem 2007, 14, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Witt, K.A.; Gillespie, T.J.; Huber, J.D.; Egleton, R.D.; Davis, T.P. Peptide drug modifications to enhance bioavailability and blood-brain barrier permeability. Peptides 2001, 22, 2329–2343. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Wang, Y.; Chen, Y.; Xing, S.; Liao, Q.; Chen, Y.; Li, Q.; Li, W.; Sun, H. Strategies for structural modification of small molecules to improve blood–brain barrier penetration: a recent perspective. Journal of Medicinal Chemistry 2021, 64, 13152–13173. [Google Scholar] [CrossRef]
- Chung, S.; Yi, Y.; Ullah, I.; Chung, K.; Park, S.; Lim, J.; Kim, C.; Pyun, S.-H.; Kim, M.; Kim, D. Systemic Treatment with Fas-Blocking Peptide Attenuates Apoptosis in Brain Ischemia. International Journal of Molecular Sciences 2024, 25, 661. [Google Scholar] [CrossRef]
- Peraro, L.; Kritzer, J.A. Emerging methods and design principles for cell-penetrant peptides. Angewandte Chemie International Edition 2018, 57, 11868–11881. [Google Scholar] [CrossRef]
- Malakoutikhah, M.; Prades, R.; Teixido, M.; Giralt, E. N-methyl phenylalanine-rich peptides as highly versatile blood− brain barrier shuttles. Journal of medicinal chemistry 2010, 53, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Navarro, M.; Teixido, M.; Giralt, E. Jumping hurdles: peptides able to overcome biological barriers. Accounts of chemical research 2017, 50, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.S.; Ghorpade, A.; Labhasetwar, V. Targeting anti-HIV drugs to the CNS. Expert opinion on drug delivery 2009, 6, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.; Rosch, J.; Putnam, D. Concepts, technologies, and practices for drug delivery past the blood–brain barrier to the central nervous system. Journal of Controlled Release 2016, 240, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Morofuji, Y.; Nakagawa, S. Drug development for central nervous system diseases using in vitro blood-brain barrier models and drug repositioning. Current pharmaceutical design 2020, 26, 1466–1485. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Török, N.; Vécsei, L. Are 5-HT1 receptor agonists effective anti-migraine drugs? Expert Opinion on Pharmacotherapy 2021, 22, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.; Hernández-Ruiz, J. Melatonin: synthesis from tryptophan and its role in higher plant. In Amino acids in higher plants; CAB International Wallingford UK: 2015; pp. 390–435.
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Frontiers in cellular and infection microbiology 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Barik, S. The uniqueness of tryptophan in biology: properties, metabolism, interactions and localization in proteins. International journal of molecular sciences 2020, 21, 8776. [Google Scholar] [CrossRef] [PubMed]
- Peyrot, F.; Ducrocq, C. Potential role of tryptophan derivatives in stress responses characterized by the generation of reactive oxygen and nitrogen species. Journal of pineal research 2008, 45, 235–246. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, D.; Song, P.; Zou, M.-H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front Biosci (Landmark Ed) 2015, 20, 1116–1143. [Google Scholar] [PubMed]
- Badawy, A.A.-B. Kynurenine pathway and human systems. Experimental Gerontology 2020, 129, 110770. [Google Scholar] [CrossRef] [PubMed]
- Ruddick, J.P.; Evans, A.K.; Nutt, D.J.; Lightman, S.L.; Rook, G.A.; Lowry, C.A. Tryptophan metabolism in the central nervous system: medical implications. Expert reviews in molecular medicine 2006, 8, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Dezsi, L.; Tuka, B.; Martos, D.; Vecsei, L. Alzheimer’s disease, astrocytes and kynurenines. Current Alzheimer Research 2015, 12, 462–480. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Spekker, E.; Polyák, H.; Tóth, F.; Vécsei, L. Mitochondrial impairment: A common motif in neuropsychiatric presentation? The link to the tryptophan–kynurenine metabolic system. Cells 2022, 11, 2607. [Google Scholar] [CrossRef] [PubMed]
- Muneer, A. Kynurenine pathway of tryptophan metabolism in neuropsychiatric disorders: pathophysiologic and therapeutic considerations. Clinical Psychopharmacology and Neuroscience 2020, 18, 507. [Google Scholar] [CrossRef] [PubMed]
- Kindler, J.; Lim, C.K.; Weickert, C.S.; Boerrigter, D.; Galletly, C.; Liu, D.; Jacobs, K.R.; Balzan, R.; Bruggemann, J.; O’Donnell, M. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Molecular psychiatry 2020, 25, 2860–2872. [Google Scholar] [CrossRef] [PubMed]
- Iwaoka, K.; Otsuka, C.; Maeda, T.; Yamahara, K.; Kato, K.; Takahashi, K.; Takahashi, K.; Terayama, Y. Impaired metabolism of kynurenine and its metabolites in CSF of parkinson’s disease. Neuroscience Letters 2020, 714, 134576. [Google Scholar] [CrossRef] [PubMed]
- Baran, H.; Jellinger, K.; Deecke, L. Kynurenine metabolism in Alzheimer's disease. Journal of neural transmission 1999, 106, 165–181. [Google Scholar] [CrossRef]
- Tan, L.; Yu, J.-T.; Tan, L. The kynurenine pathway in neurodegenerative diseases: mechanistic and therapeutic considerations. Journal of the neurological sciences 2012, 323, 1–8. [Google Scholar] [CrossRef]
- Urbańska, E.M.; Chmiel-Perzyńska, I.; Perzyński, A.; Derkacz, M.; Owe-Larsson, B. Endogenous kynurenic acid and neurotoxicity. Handbook of neurotoxicity 2021, 1–31. [Google Scholar]
- Sharma, R.; Razdan, K.; Bansal, Y.; Kuhad, A. Rollercoaster ride of kynurenines: Steering the wheel towards neuroprotection in Alzheimer’s disease. Expert Opinion on Therapeutic Targets 2018, 22, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. Monitoring the kynurenine system: Concentrations, ratios or what else? Advances in Clinical and Experimental Medicine 2021, 30, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Polyák, H.; Galla, Z.; Nánási, N.; Cseh, E.K.; Rajda, C.; Veres, G.; Spekker, E.; Szabó, Á.; Klivényi, P.; Tanaka, M. The tryptophan-kynurenine metabolic system is suppressed in cuprizone-induced model of demyelination simulating progressive multiple sclerosis. Biomedicines 2023, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of kynurenine pathway in oxidative stress during neurodegenerative disorders. Cells 2021, 10, 1603. [Google Scholar] [CrossRef] [PubMed]
- Barone, P. The ‘Yin’and the ‘Yang’of the kynurenine pathway: excitotoxicity and neuroprotection imbalance in stress-induced disorders. Behavioural pharmacology 2019, 30, 163–186. [Google Scholar] [CrossRef]
- Savitz, J.; Drevets, W.C.; Smith, C.M.; Victor, T.A.; Wurfel, B.E.; Bellgowan, P.S.; Bodurka, J.; Teague, T.K.; Dantzer, R. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology 2015, 40, 463–471. [Google Scholar] [CrossRef]
- Maddison, D.C.; Giorgini, F. The kynurenine pathway and neurodegenerative disease. In Proceedings of the Seminars in cell & developmental biology; 2015; pp. 134–141. [Google Scholar]
- Pérez-De La Cruz, V.; Königsberg, M.; Santamaría, A. Kynurenine pathway and disease: an overview. CNS Neurol Disord Drug Targets 2007, 6, 398–410. [Google Scholar] [PubMed]
- Stone, T.W.; Darlington, L.G. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. British journal of pharmacology 2013, 169, 1211–1227. [Google Scholar] [CrossRef]
- Tao, X.; Yan, M.; Wang, L.; Zhou, Y.; Wang, Z.; Xia, T.; Liu, X.; Pan, R.; Chang, Q. Homeostasis imbalance of microglia and astrocytes leads to alteration in the metabolites of the kynurenine pathway in LPS-induced depressive-like mice. International journal of molecular sciences 2020, 21, 1460. [Google Scholar] [CrossRef]
- Fujigaki, H.; Yamamoto, Y.; Saito, K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology 2017, 112, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Sorgdrager, F.J.H.; Naudé, P.J.W.; Kema, I.P.; Nollen, E.A.; Deyn, P.P. Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target. Front Immunol 2019, 10, 2565. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov 2019, 18, 379–401. [Google Scholar] [CrossRef] [PubMed]
- Ostapiuk, A.; Urbanska, E.M. Kynurenic acid in neurodegenerative disorders-unique neuroprotection or double-edged sword? CNS Neurosci Ther 2022, 28, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Tutakhail, A.; Boulet, L.; Khabil, S.; Nazari, Q.A.; Hamid, H.; Coudoré, F. Neuropathology of kynurenine pathway of tryptophan metabolism. Current pharmacology reports 2020, 6, 8–23. [Google Scholar] [CrossRef]
- Gong, X.; Chang, R.; Zou, J.; Tan, S.; Huang, Z. The role and mechanism of tryptophan–kynurenine metabolic pathway in depression. Reviews in the Neurosciences 2023, 34, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Deora, G.S.; Kantham, S.; Chan, S.; Dighe, S.N.; Veliyath, S.K.; McColl, G.; Parat, M.O.; McGeary, R.P.; Ross, B.P. Multifunctional Analogs of Kynurenic Acid for the Treatment of Alzheimer's Disease: Synthesis, Pharmacology, and Molecular Modeling Studies. ACS Chem Neurosci 2017, 8, 2667–2675. [Google Scholar] [CrossRef] [PubMed]
- Bratek-Gerej, E.; Ziembowicz, A.; Godlewski, J.; Salinska, E. The mechanism of the neuroprotective effect of kynurenic acid in the experimental model of neonatal hypoxia–ischemia: The link to oxidative stress. Antioxidants 2021, 10, 1775. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Lennon, M.J.; Lim, C.K.; Jacobs, K.; Guillemin, G.J.; Brew, B.J. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 2017, 112, 373–388. [Google Scholar] [CrossRef]
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the etiological links behind neurodegenerative diseases: Inflammatory cytokines and bioactive kynurenines. International Journal of Molecular Sciences 2020, 21, 2431. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune influencers in action: metabolites and enzymes of the tryptophan-kynurenine metabolic pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Török, N.; Török, R.; Molnár, K.; Szolnoki, Z.; Somogyvári, F.; Boda, K.; Tanaka, M.; Klivényi, P.; Vécsei, L. Single Nucleotide Polymorphisms of Indoleamine 2, 3-Dioxygenase 1 Influenced the Age Onset of Parkinson's Disease. Frontiers in Bioscience-Landmark 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vecsei, L. Monitoring the redox status in multiple sclerosis. Biomedicines 2020, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G.F. Genetic and hormonal regulation of tryptophan–kynurenine metabolism: implications for vascular cognitive impairment, major depressive disorder, and aging. Annals of the New York Academy of Sciences 2007, 1122, 35–49. [Google Scholar] [CrossRef]
- Battaglia, M.R.; Di Fazio, C.; Battaglia, S. Activated Tryptophan-Kynurenine metabolic system in the human brain is associated with learned fear. Frontiers in Molecular Neuroscience 2023, 16, 1217090. [Google Scholar] [CrossRef] [PubMed]
- Borgomaneri, S.; Vitale, F.; Battaglia, S.; de Vega, M.; Avenanti, A. Task-related modulation of motor response to emotional bodies: A TMS motor-evoked potential study. Cortex 2024, 171, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Tortora, F.; Hadipour, A.L.; Battaglia, S.; Falzone, A.; Avenanti, A.; Vicario, C.M. The role of Serotonin in fear learning and memory: a systematic review of human studies. Brain Sciences 2023, 13, 1197. [Google Scholar] [CrossRef]
- Battaglia, S.; Nazzi, C.; Thayer, J.F. Heart's tale of trauma: Fear-conditioned heart rate changes in post-traumatic stress disorder. Acta Psychiatr Scand 2023, 148, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Talarowska, M.; Galecki, P. Cognition and emotions in recurrent depressive disorders-the role of inflammation and the kynurenine pathway. Current Pharmaceutical Design 2016, 22, 955–962. [Google Scholar] [CrossRef]
- Ren, W.H.; Guo, J.D.; Cao, H.; Wang, H.; Wang, P.F.; Sha, H.; Ji, R.R.; Zhao, Z.Q.; Zhang, Y.Q. Is endogenous d-serine in the rostral anterior cingulate cortex necessary for pain-related negative affect? Journal of neurochemistry 2006, 96, 1636–1647. [Google Scholar] [CrossRef]
- Javelle, F. Impulsivity and its biomarkers: A focus on the tryptophan pathways and the moderating effects of physical exercise. 2021.
- Lasoń, W.; Budziszewska, B.; Basta-Kaim, A.; Kubera, M.; Maes, M. New trends in the neurobiology and pharmacology of affective disorders. Pharmacological Reports 2013, 65, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Harrison, B.J.; Fullana, M.A. Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both? A commentary on subregional contributions. Molecular Psychiatry 2022, 27, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Garofalo, S.; di Pellegrino, G.; Starita, F. Revaluing the role of vmPFC in the acquisition of Pavlovian threat conditioning in humans. Journal of Neuroscience 2020, 40, 8491–8500. [Google Scholar] [CrossRef] [PubMed]
- Borgomaneri, S.; Battaglia, S.; Garofalo, S.; Tortora, F.; Avenanti, A.; di Pellegrino, G. State-dependent TMS over prefrontal cortex disrupts fear-memory reconsolidation and prevents the return of fear. Current Biology 2020, 30, 3672–3679. [Google Scholar] [CrossRef] [PubMed]
- Samavati, R.; Zádor, F.; Szűcs, E.; Tuka, B.; Martos, D.; Veres, G.; Gáspár, R.; Mándity, I.M.; Fülöp, F.; Vécsei, L. Kynurenic acid and its analogue can alter the opioid receptor G-protein signaling after acute treatment via NMDA receptor in rat cortex and striatum. Journal of the Neurological Sciences 2017, 376, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Morales-Puerto, N.; Gimenez-Gomez, P.; Perez-Hernandez, M.; Abuin-Martinez, C.; de Biedma-Elduayen, L.G.; Vidal, R.; Gutiérrez-López, M.D.; O'Shea, E.; Colado, M.I. Addiction and the kynurenine pathway: A new dancing couple? Pharmacology & therapeutics 2021, 223, 107807. [Google Scholar]
- Klausing, A.D. Effects of Acute Stress on Discriminative Fear Conditioning: A Key Role of Kynurenic Acid in the Medial Prefrontal Cortex. University of Maryland, Baltimore, 2020.
- Meier, T.B.; Drevets, W.C.; Wurfel, B.E.; Ford, B.N.; Morris, H.M.; Victor, T.A.; Bodurka, J.; Teague, T.K.; Dantzer, R.; Savitz, J. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain, behavior, and immunity 2016, 53, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, V.; Costafreda, S.G.; Rimmer, R.M.; Rasenick, M.M.; Marangell, L.B.; Fu, C.H. Brain-derived neurotrophic factor association with amygdala response in major depressive disorder. Journal of affective disorders 2020, 267, 103–106. [Google Scholar] [CrossRef]
- Vecchiarelli, H.A.; Gandhi, C.P.; Hill, M.N. Acute psychological stress modulates the expression of enzymes involved in the kynurenine pathway throughout corticolimbic circuits in adult male rats. Neural Plasticity 2016, 2016. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Lőrinczi, B.; Szatmári, I.; Fülöp, F.; Vécsei, L. Antidepressant-like Effects of Kynurenic Acid Analogues. In Proceedings of the Presented at the 1st International Electronic Conference on Biomedicine; 2021; p. 26. [Google Scholar]
- Battaglia, S.; Orsolini, S.; Borgomaneri, S.; Barbieri, R.; Diciotti, S.; di Pellegrino, G. Characterizing cardiac autonomic dynamics of fear learning in humans. Psychophysiology 2022, 59, e14122. [Google Scholar] [CrossRef]
- Battaglia, S.; Di Fazio, C.; Vicario, C.M.; Avenanti, A. Neuropharmacological modulation of N-methyl-D-aspartate, noradrenaline and endocannabinoid receptors in fear extinction learning: Synaptic transmission and plasticity. International Journal of Molecular Sciences 2023, 24, 5926. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S. Neurobiological advances of learned fear in humans. Advances in Clinical and Experimental Medicine 2022, 31, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Bohár, Z.; Vécsei, L. Are kynurenines accomplices or principal villains in dementia? Maintenance of kynurenine metabolism. Molecules 2020, 25, 564. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.Y.; Lovejoy, D.B.; Guillemin, G.J.; Kozak, R.; Stone, T.W.; Koola, M.M. Galantamine-memantine combination and kynurenine pathway enzyme inhibitors in the treatment of neuropsychiatric disorders. Complex psychiatry 2021, 7, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Geng, X. Research progress on the kynurenine pathway in the prevention and treatment of Parkinson’s disease. Journal of Enzyme Inhibition and Medicinal Chemistry 2023, 38, 2225800. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, M.; Chen, X.; Zhang, R.; Le, A.; Hong, M.; Zhang, Y.; Jia, L.; Zang, W.; Jiang, C. Tryptophan Metabolism in Central Nervous System Diseases: Pathophysiology and Potential Therapeutic Strategies. Aging and Disease 2023, 14, 858. [Google Scholar] [CrossRef]
- Sheibani, M.; Shayan, M.; Khalilzadeh, M.; Soltani, Z.E.; Jafari-Sabet, M.; Ghasemi, M.; Dehpour, A.R. Kynurenine pathway and its role in neurologic, psychiatric, and inflammatory bowel diseases. Molecular Biology Reports 2023, 50, 10409–10425. [Google Scholar] [CrossRef]
- S Jayawickrama, G.; R Sadig, R.; Sun, G.; Nematollahi, A.; A Nadvi, N.; R Hanrahan, J.; D Gorrell, M.; Bret Church, W. Kynurenine aminotransferases and the prospects of inhibitors for the treatment of schizophrenia. Current Medicinal Chemistry 2015, 22, 2902–2918. [Google Scholar] [CrossRef]
- Kloog, Y.; Lamdani-Itkin, H.; Sokolovsky, M. The glycine site of the N-methyl-D-aspartate receptor channel: differences between the binding of HA-966 and of 7-chlorokynurenic acid. J Neurochem 1990, 54, 1576–1583. [Google Scholar] [CrossRef]
- Mok, M.S.; Fricker, A.-C.; Weil, A.; Kew, J.N. Electrophysiological characterisation of the actions of kynurenic acid at ligand-gated ion channels. Neuropharmacology 2009, 57, 242–249. [Google Scholar]
- Prescott, C.; Weeks, A.M.; Staley, K.J.; Partin, K.M. Kynurenic acid has a dual action on AMPA receptor responses. Neurosci Lett 2006, 402, 108–112. [Google Scholar] [CrossRef]
- Rózsa, E.; Robotka, H.; Vécsei, L.; Toldi, J. The Janus-face kynurenic acid. J Neural Transm (Vienna) 2008, 115, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Gladding, C.M.; Raymond, L.A. Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Mol Cell Neurosci 2011, 48, 308–320. [Google Scholar] [CrossRef]
- Petralia, R.S. Distribution of extrasynaptic NMDA receptors on neurons. The Scientific World Journal 2012, 2012. [Google Scholar] [CrossRef]
- Lener, M.S.; Niciu, M.J.; Ballard, E.D.; Park, M.; Park, L.T.; Nugent, A.C.; Zarate Jr, C.A. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biological psychiatry 2017, 81, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Pribiag, H.; Jefferson, S.J.; Shorey, M.; Fuchs, T.; Stellwagen, D.; Luscher, B. Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric acidergic deficits and ketamine treatment. Biological psychiatry 2016, 80, 457–468. [Google Scholar] [CrossRef]
- Kasper, J.M.; McCue, D.L.; Milton, A.J.; Szwed, A.; Sampson, C.M.; Huang, M.; Carlton, S.; Meltzer, H.Y.; Cunningham, K.A.; Hommel, J.D. Gamma-Aminobutyric Acidergic Projections From the Dorsal Raphe to the Nucleus Accumbens Are Regulated by Neuromedin U. Biol Psychiatry 2016, 80, 878–887. [Google Scholar] [CrossRef]
- Adeel, M.; Chen, C.-C.; Lin, B.-S.; Chen, H.-C.; Liou, J.-C.; Li, Y.-T.; Peng, C.-W. Safety of Special Waveform of Transcranial Electrical Stimulation (TES): In Vivo Assessment. International journal of molecular sciences 2022, 23, 6850. [Google Scholar] [CrossRef]
- Poles, M.Z.; Nászai, A.; Gulácsi, L.; Czakó, B.L.; Gál, K.G.; Glenz, R.J.; Dookhun, D.; Rutai, A.; Tallósy, S.P.; Szabó, A.; et al. Kynurenic Acid and Its Synthetic Derivatives Protect Against Sepsis-Associated Neutrophil Activation and Brain Mitochondrial Dysfunction in Rats. Front Immunol 2021, 12, 717157. [Google Scholar] [CrossRef] [PubMed]
- Szabo, M.; Lajkó, N.; Dulka, K.; Szatmári, I.; Fülöp, F.; Mihály, A.; Vécsei, L.; Gulya, K. Kynurenic Acid and Its Analog SZR104 Exhibit Strong Antiinflammatory Effects and Alter the Intracellular Distribution and Methylation Patterns of H3 Histones in Immunochallenged Microglia-Enriched Cultures of Newborn Rat Brains. International Journal of Molecular Sciences 2022, 23, 1079. [Google Scholar] [CrossRef]
- Gregorio, F.; Battaglia, S. Advances in EEG-based functional connectivity approaches to the study of the central nervous system in health and disease. Advances in Clinical and Experimental Medicine: Official Organ Wroclaw Medical University.
- Marx, W.; McGuinness, A.J.; Rocks, T.; Ruusunen, A.; Cleminson, J.; Walker, A.J.; Gomes-da-Costa, S.; Lane, M.; Sanches, M.; Diaz, A.P. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: a meta-analysis of 101 studies. Molecular psychiatry 2021, 26, 4158–4178. [Google Scholar] [CrossRef]
- Erhardt, S.; Schwieler, L.; Imbeault, S.; Engberg, G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology 2017, 112, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Nazzi, C.; Thayer, J.F. Fear-induced bradycardia in mental disorders: Foundations, current advances, future perspectives. Neurosci Biobehav Rev 2023, 149, 105163. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Thayer, J.F. Functional interplay between central and autonomic nervous systems in human fear conditioning. Trends Neurosci 2022, 45, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Serio, G.; Scarpazza, C.; D'Ausilio, A.; Borgomaneri, S. Frozen in (e) motion: How reactive motor inhibition is influenced by the emotional content of stimuli in healthy and psychiatric populations. Behaviour Research and Therapy 2021, 146, 103963. [Google Scholar] [CrossRef] [PubMed]
- Borgomaneri, S.; Battaglia, S.; Sciamanna, G.; Tortora, F.; Laricchiuta, D. Memories are not written in stone: Re-writing fear memories by means of non-invasive brain stimulation and optogenetic manipulations. Neuroscience & Biobehavioral Reviews 2021, 127, 334–352. [Google Scholar]
- Borgomaneri, S.; Battaglia, S.; Avenanti, A.; di Pellegrino, G. Don't hurt me no more: State-dependent transcranial magnetic stimulation for the treatment of specific phobia. Journal of affective disorders 2021, 286, 78–79. [Google Scholar] [CrossRef]
- Almeida, P.G.; Nani, J.V.; Oses, J.P.; Brietzke, E.; Hayashi, M.A. Neuroinflammation and glial cell activation in mental disorders. Brain, Behavior, & Immunity-Health 2020, 2, 100034. [Google Scholar]
- Battaglia, S.; Cardellicchio, P.; Di Fazio, C.; Nazzi, C.; Fracasso, A.; Borgomaneri, S. Stopping in (e) motion: Reactive action inhibition when facing valence-independent emotional stimuli. Frontiers in Behavioral Neuroscience 2022, 16, 998714. [Google Scholar] [CrossRef]
- Di Gregorio, F.; La Porta, F.; Petrone, V.; Battaglia, S.; Orlandi, S.; Ippolito, G.; Romei, V.; Piperno, R.; Lullini, G. Accuracy of EEG biomarkers in the detection of clinical outcome in disorders of consciousness after severe acquired brain injury: preliminary results of a pilot study using a machine learning approach. Biomedicines 2022, 10, 1897. [Google Scholar] [CrossRef]
- Battaglia, S.; Cardellicchio, P.; Di Fazio, C.; Nazzi, C.; Fracasso, A.; Borgomaneri, S. The influence of vicarious fear-learning in “infecting” reactive action inhibition. Frontiers in Behavioral Neuroscience 2022, 16, 946263. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 2012, 13, 465–477. [Google Scholar] [CrossRef]
- nrn3257 [pii].
- Veres, G.; Fejes-Szabo, A.; Zadori, D.; Nagy-Grocz, G.; Laszlo, A.M.; Bajtai, A.; Mandity, I.; Szentirmai, M.; Bohar, Z.; Laborc, K.; et al. A comparative assessment of two kynurenic acid analogs in the formalin model of trigeminal activation: a behavioral, immunohistochemical and pharmacokinetic study. J Neural Transm (Vienna) 2017, 124, 99–112. [Google Scholar] [CrossRef] [PubMed]
- 10.1007/s00702-016-1615-5 [pii].
- Tanaka, M.; Chen, C. Towards a mechanistic understanding of depression, anxiety, and their comorbidity: perspectives from cognitive neuroscience. Frontiers in behavioral neuroscience 2023, 17. [Google Scholar] [CrossRef] [PubMed]
- Quadt, L.; Critchley, H.; Nagai, Y. Cognition, emotion, and the central autonomic network. Auton Neurosci 2022, 238, 102948. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.D.; Todd, R.M. The self-regulating brain: Cortical-subcortical feedback and the development of intelligent action. Cognitive Development 2007, 22, 406–430. [Google Scholar] [CrossRef]
- Maiese, M. Embodiment, emotion, and cognition; Springer: 2010.
- Salim, S. Oxidative Stress and the Central Nervous System. J Pharmacol Exp Ther 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Lucas, S.M.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease. Br J Pharmacol 2006, 147 Suppl 1, S232–240. [Google Scholar] [CrossRef]
- Critchley, H.D.; Eccles, J.; Garfinkel, S.N. Interaction between cognition, emotion, and the autonomic nervous system. In Handbook of clinical neurology; Elsevier: 2013; Volume 117, pp. 59–77.
- Šumec, R.; Rektorová, I.; Jech, R.; Menšíková, K.; Roth, J.; Růžička, E.; Sochorová, D.; Dušek, L.; Kaňovský, P.; Rektor, I. Motion and emotion: anxiety–axial connections in Parkinson’s disease. Journal of neural transmission 2017, 124, 369–377. [Google Scholar] [CrossRef]
- Jawer, M.A. Sensitive soul: The unseen role of emotion in extraordinary states; Simon and Schuster: 2020.
- Bellettato, C.M.; Scarpa, M. Possible strategies to cross the blood-brain barrier. Ital J Pediatr 2018, 44, 131. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Drug delivery systems, CNS protection, and the blood brain barrier. BioMed research international 2014, 2014. [Google Scholar] [CrossRef]
- Gabathuler, R. Approaches to transport therapeutic drugs across the blood–brain barrier to treat brain diseases. Neurobiology of disease 2010, 37, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Gawdi, R.; Shumway, K.R.; Emmady, P.D. Physiology, Blood Brain Barrier. StatPearls.
- Copyright © 2023, StatPearls Publishing LLC.: Treasure Island (FL), 2023.
- Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Brain endothelial cell-cell junctions: how to "open" the blood brain barrier. Curr Neuropharmacol 2008, 6, 179–192. [Google Scholar] [CrossRef]
- Sanchez-Covarrubias, L.; Slosky, L.M.; Thompson, B.J.; Davis, T.P.; Ronaldson, P.T. Transporters at CNS barrier sites: obstacles or opportunities for drug delivery? Current pharmaceutical design 2014, 20, 1422–1449. [Google Scholar] [CrossRef]
- De Boer, A.; Van Der Sandt, I.; Gaillard, P. The role of drug transporters at the blood-brain barrier. Annual review of pharmacology and toxicology 2003, 43, 629–656. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Neuhaus, W.; Dandekar, T.; Förster, C. Analysing molecular polar surface descriptors to predict blood-brain barrier permeation. Int J Comput Biol Drug Des 2013, 6, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Betz, A.L.; Firth, J.A.; Goldstein, G.W. Polarity of the blood-brain barrier: distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain research 1980, 192, 17–28. [Google Scholar] [CrossRef]
- Janicka, M.; Sztanke, M.; Sztanke, K. Predicting the blood-brain barrier permeability of new drug-like compounds via HPLC with various stationary phases. Molecules 2020, 25, 487. [Google Scholar] [CrossRef]
- Sánchez-Dengra, B.; González-Álvarez, I.; Bermejo, M.; González-Álvarez, M. Access to the CNS: Strategies to overcome the BBB. International Journal of Pharmaceutics 2023, 122759. [Google Scholar] [CrossRef]
- Liu, H.-M.; Liu, X.-F.; Yao, J.-L.; Wang, C.-L.; Yu, Y.; Wang, R. Utilization of combined chemical modifications to enhance the blood-brain barrier permeability and pharmacological activity of endomorphin-1. Journal of Pharmacology and Experimental Therapeutics 2006, 319, 308–316. [Google Scholar] [CrossRef]
- Cai, Q.; Wang, L.; Deng, G.; Liu, J.; Chen, Q.; Chen, Z. Systemic delivery to central nervous system by engineered PLGA nanoparticles. American journal of translational research 2016, 8, 749. [Google Scholar] [PubMed]
- Li, D.; Yu, S.; Long, Y.; Shi, A.; Deng, J.; Ma, Y.; Wen, J.; Li, X.; Liu, S.; Zhang, Y.; et al. Tryptophan metabolism: Mechanism-oriented therapy for neurological and psychiatric disorders. Front Immunol 2022, 13, 985378. [Google Scholar] [CrossRef] [PubMed]
- Myint, A.M. Kynurenines: from the perspective of major psychiatric disorders. The FEBS journal 2012, 279, 1375–1385. [Google Scholar] [CrossRef]
- Müller, N.; Myint, A.-M.; Schwarz, M.J. The impact of neuroimmune dysregulation on neuroprotection and neurotoxicity in psychiatric disorders-relation to drug treatment. Dialogues in clinical neuroscience 2009, 11, 319–332. [Google Scholar] [CrossRef]
- Mithaiwala, M.N.; Santana-Coelho, D.; Porter, G.A.; O'Connor, J.C. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.; Outeiro, T.F.; Scrutton, N.S.; Giorgini, F. The causative role and therapeutic potential of the kynurenine pathway in neurodegenerative disease. Journal of Molecular Medicine 2013, 91, 705–713. [Google Scholar] [CrossRef]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front Immunol 2017, 8, 1957. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Singh, T.G.; Prabhakar, N.K.; Mannan, A. Kynurenine Metabolism and Alzheimer's Disease: The Potential Targets and Approaches. Neurochem Res 2022, 47, 1459–1476. [Google Scholar] [CrossRef]
- Martin, K.S.; Azzolini, M.; Ruas, J.L. The kynurenine connection: How exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. American Journal of Physiology-Cell Physiology.
- González Esquivel, D.; Ramírez-Ortega, D.; Pineda, B.; Castro, N.; Ríos, C.; Pérez de la Cruz, V. Kynurenine pathway metabolites and enzymes involved in redox reactions. Neuropharmacology 2017, 112, 331–345. [Google Scholar] [CrossRef]
- Debnath, N.; Kumar, R.; Kumar, A.; Mehta, P.K.; Yadav, A.K. Gut-microbiota derived bioactive metabolites and their functions in host physiology. Biotechnology and Genetic Engineering Reviews 2021, 37, 105–153. [Google Scholar] [CrossRef]
- Joisten, N.; Ruas, J.L.; Braidy, N.; Guillemin, G.J.; Zimmer, P. The kynurenine pathway in chronic diseases: a compensatory mechanism or a driving force? Trends Mol Med 2021, 27, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guillemin, G.J. Kynurenine pathway metabolites in humans: disease and healthy states. International journal of tryptophan research 2009, 2, IJTR. S2097. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Guillemin, G.J. Microorganisms, tryptophan metabolism, and kynurenine pathway: a complex interconnected loop influencing human health status. International journal of tryptophan research 2019, 12, 1178646919852996. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Sun-Waterhouse, D.; Cui, C. The therapeutic potential of diet on immune-related diseases: based on the regulation on tryptophan metabolism. Crit Rev Food Sci Nutr 2022, 62, 8793–8811. [Google Scholar] [CrossRef] [PubMed]
- Günther, J.; Fallarino, F.; Fuchs, D.; Wirthgen, E. Immunomodulatory roles of tryptophan metabolites in inflammation and cancer. Frontiers in Immunology, 2020; 11, 1497. [Google Scholar]
- Turski, M.P.; Turska, M.; Paluszkiewicz, P.; Parada-Turska, J.; Oxenkrug, G.F. Kynurenic acid in the digestive system–-new facts, new challenges. International Journal of Tryptophan Research 2013, 6, IJTR. S12536. [Google Scholar] [CrossRef]
- Török, N.; Tanaka, M.; Vécsei, L. Searching for peripheral biomarkers in neurodegenerative diseases: the tryptophan-kynurenine metabolic pathway. International journal of molecular sciences 2020, 21, 9338. [Google Scholar] [CrossRef]
- Balogh, L.; Tanaka, M.; Török, N.; Vécsei, L.; Taguchi, S. Crosstalk between existential phenomenological psychotherapy and neurological sciences in mood and anxiety disorders. Biomedicines 2021, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Bohár, Z.; Martos, D.; Telegdy, G.; Vécsei, L. Antidepressant-like effects of kynurenic acid in a modified forced swim test. Pharmacological Reports 2020, 72, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R. The kynurenine pathway of tryptophan degradation as a drug target. Curr Opin Pharmacol 2004, 4, 12–17. [Google Scholar] [CrossRef]
- Balla, Z.; Kormányos, E.S.; Kui, B.; Bálint, E.R.; Fűr, G.; Orján, E.M.; Iványi, B.; Vécsei, L.; Fülöp, F.; Varga, G.; et al. Kynurenic Acid and Its Analogue SZR-72 Ameliorate the Severity of Experimental Acute Necrotizing Pancreatitis. Front Immunol 2021, 12, 702764. [Google Scholar] [CrossRef]
- Gellért, L.; Varga, D.; Ruszka, M.; Toldi, J.; Farkas, T.; Szatmári, I.; Fülöp, F.; Vécsei, L.; Kis, Z. Behavioural studies with a newly developed neuroprotective KYNA-amide. J Neural Transm (Vienna) 2012, 119, 165–172. [Google Scholar] [CrossRef]
- Vámos, E.; Párdutz, A.; Varga, H.; Bohár, Z.; Tajti, J.; Fülöp, F.; Toldi, J.; Vécsei, L. l-kynurenine combined with probenecid and the novel synthetic kynurenic acid derivative attenuate nitroglycerin-induced nNOS in the rat caudal trigeminal nucleus. Neuropharmacology 2009, 57, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Mándi, Y.; Endrész, V.; Mosolygó, T.; Burián, K.; Lantos, I.; Fülöp, F.; Szatmári, I.; Lőrinczi, B.; Balog, A.; Vécsei, L. The Opposite Effects of Kynurenic Acid and Different Kynurenic Acid Analogs on Tumor Necrosis Factor-α (TNF-α) Production and Tumor Necrosis Factor-Stimulated Gene-6 (TSG-6) Expression. Front Immunol 2019, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Demeter, I.; Nagy, K.; Gellért, L.; Vécsei, L.; Fülöp, F.; Toldi, J. A novel kynurenic acid analog (SZR104) inhibits pentylenetetrazole-induced epileptiform seizures. An electrophysiological study: special issue related to kynurenine. Journal of Neural Transmission 2012, 119, 151–154. [Google Scholar] [CrossRef]
- Martos, D.; Tuka, B.; Tanaka, M.; Vécsei, L.; Telegdy, G. Memory enhancement with kynurenic acid and its mechanisms in neurotransmission. Biomedicines 2022, 10, 849. [Google Scholar] [CrossRef]
- Benson, H.A.; Roberts, M.S. Challenges and Innovations of Controlled Drug Delivery. Fundamentals of Drug Delivery 2021, 1–14. [Google Scholar]
- McCarson, K.E. Strategies for behaviorally phenotyping the transgenic mouse. Transgenic Mouse: Methods and Protocols.
- Sukoff Rizzo, S.J.; Crawley, J.N. Behavioral phenotyping assays for genetic mouse models of neurodevelopmental, neurodegenerative, and psychiatric disorders. Annual Review of Animal Biosciences 2017, 5, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Török, N.; Tóth, F.; Szabó, Á.; Vécsei, L. Co-players in chronic pain: neuroinflammation and the tryptophan-kynurenine metabolic pathway. Biomedicines 2021, 9, 897. [Google Scholar] [CrossRef]
- Molnár, K.; Lőrinczi, B.; Fazakas, C.; Szatmári, I.; Fülöp, F.; Kmetykó, N.; Berkecz, R.; Ilisz, I.; Krizbai, I.A.; Wilhelm, I. Szr-104, a novel kynurenic acid analogue with high permeability through the blood–brain barrier. Pharmaceutics 2021, 13, 61. [Google Scholar] [CrossRef]
- Castillo-Mariqueo, L.; Giménez-Llort, L. Impact of Behavioral Assessment and Re-Test as Functional Trainings That Modify Survival, Anxiety and Functional Profile (Physical Endurance and Motor Learning) of Old Male and Female 3xTg-AD Mice and NTg Mice with Normal Aging. Biomedicines 2022, 10, 973. [Google Scholar] [CrossRef]
- Lee, E.C.; Hong, D.-Y.; Lee, D.-H.; Park, S.-W.; Lee, J.Y.; Jeong, J.H.; Kim, E.-Y.; Chung, H.-M.; Hong, K.-S.; Park, S.-P. Inflammation and rho-associated protein kinase-induced brain changes in vascular dementia. Biomedicines 2022, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Belozertseva, I.V.; Tamkovich, N.V.; Baranov, K.O.; Kudryavtseva, N.N. Chronic Lithium Treatment affects anxious behaviors and theExpression of serotonergic genes in Midbrain Raphe nuclei of defeated male mice. Biomedicines 2021, 9, 1293. [Google Scholar] [CrossRef] [PubMed]
- Vila-Merkle, H.; González-Martínez, A.; Campos-Jiménez, R.; Martínez-Ricós, J.; Teruel-Martí, V.; Blasco-Serra, A.; Lloret, A.; Celada, P.; Cervera-Ferri, A. The Oscillatory Profile Induced by the Anxiogenic Drug FG-7142 in the Amygdala–Hippocampal Network Is Reversed by Infralimbic Deep Brain Stimulation: Relevance for Mood Disorders. Biomedicines 2021, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Santana-Santana, M.; Bayascas, J.-R.; Giménez-Llort, L. Fine-Tuning the PI3K/Akt Signaling Pathway Intensity by Sex and Genotype-Load: Sex-Dependent Homozygotic Threshold for Somatic Growth but Feminization of Anxious Phenotype in Middle-Aged PDK1 K465E Knock-In and Heterozygous Mice. Biomedicines 2021, 9, 747. [Google Scholar] [CrossRef] [PubMed]
- Muntsant, A.; Giménez-Llort, L. Genotype Load Modulates Amyloid Burden and Anxiety-Like Patterns in Male 3xTg-AD Survivors despite Similar Neuro-Immunoendocrine, Synaptic and Cognitive Impairments. Biomedicines 2021, 9, 715. [Google Scholar] [CrossRef]
- Giménez-Llort, L.; Marin-Pardo, D.; Marazuela, P.; Hernández-Guillamón, M. Survival Bias and Crosstalk between Chronological and Behavioral Age: Age-and Genotype-Sensitivity Tests Define Behavioral Signatures in Middle-Aged, Old, and Long-Lived Mice with Normal and AD-Associated Aging. Biomedicines 2021, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Samra, A.I.; Kamel, A.S.; Abdallah, D.M.; El Fattah, M.A.A.; Ahmed, K.A.; El-Abhar, H.S. Preclinical Evidence for the Role of the Yin/Yang Angiotensin System Components in Autism Spectrum Disorder: A Therapeutic Target of Astaxanthin. Biomedicines 2023, 11, 3156. [Google Scholar] [CrossRef] [PubMed]
- Canfora, F.; Ottaviani, G.; Calabria, E.; Pecoraro, G.; Leuci, S.; Coppola, N.; Sansone, M.; Rupel, K.; Biasotto, M.; Di Lenarda, R. Advancements in Understanding and Classifying Chronic Orofacial Pain: Key Insights from Biopsychosocial Models and International Classifications (ICHD-3, ICD-11, ICOP). Biomedicines 2023, 11, 3266. [Google Scholar] [CrossRef]
- Cruz-Martínez, Y.; Aguilar-Ponce, L.; Romo-Araiza, A.; Chávez-Guerra, A.; Martiñón, S.; Ibarra-García, A.P.; Arias-Santiago, S.; Gálvez-Susano, V.; Ibarra, A. Supplementation with a Symbiotic Induced Neuroprotection and Improved Memory in Rats with Ischemic Stroke. Biomedicines 2024, 12, 209. [Google Scholar] [CrossRef]
- Baliellas, D.E.; Barros, M.P.; Vardaris, C.V.; Guariroba, M.; Poppe, S.C.; Martins, M.F.; Pereira, Á.A.; Bondan, E.F. Propentofylline Improves Thiol-Based Antioxidant Defenses and Limits Lipid Peroxidation following Gliotoxic Injury in the Rat Brainstem. Biomedicines 2023, 11, 1652. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaokun, O.O. The Beneficial Role of Apigenin against Cognitive and Neurobehavioural Dysfunction: A Systematic Review of Preclinical Investigations. Biomedicines 2024, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, G.; Yi, J.; Huang, Y. Intraoperative Hypothermia Induces Vascular Dysfunction in the CA1 Region of Rat Hippocampus. Brain Sciences 2022, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wei, S.; Low, S.W.; Poore, C.P.; Lee, A.T.; Nilius, B.; Liao, P. TRPM4 Blocking Antibody Protects Cerebral Vasculature in Delayed Stroke Reperfusion. Biomedicines 2023, 11. [Google Scholar] [CrossRef]
- Salafutdinov, II; Gatina, D.Z.; Markelova, M.I.; Garanina, E.E.; Malanin, S.Y.; Gazizov, I.M.; Izmailov, A.A.; Rizvanov, A.A.; Islamov, R.R.; Palotás, A.; Safiullov, Z.Z. A Biosafety Study of Human Umbilical Cord Blood Mononuclear Cells Transduced with Adenoviral Vector Carrying Human Vascular Endothelial Growth Factor cDNA In Vitro. Biomedicines 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Gaebler, A.J.; Finner-Prével, M.; Sudar, F.P.; Langer, F.H.; Keskin, F.; Gebel, A.; Zweerings, J.; Mathiak, K. The interplay between vitamin D, exposure of anticholinergic antipsychotics and cognition in schizophrenia. Biomedicines 2022, 10, 1096. [Google Scholar] [CrossRef] [PubMed]
- Simonato, M.; Dall’Acqua, S.; Zilli, C.; Sut, S.; Tenconi, R.; Gallo, N.; Sfriso, P.; Sartori, L.; Cavallin, F.; Fiocco, U. Tryptophan metabolites, cytokines, and fatty acid binding protein 2 in myalgic encephalomyelitis/chronic fatigue syndrome. Biomedicines 2021, 9, 1724. [Google Scholar] [CrossRef]
- Panov, G.; Dyulgerova, S.; Panova, P. Cognition in Patients with Schizophrenia: Interplay between Working Memory, Disorganized Symptoms, Dissociation, and the Onset and Duration of Psychosis, as Well as Resistance to Treatment. Biomedicines 2023, 11, 3114. [Google Scholar] [CrossRef]
- de Oliveira, M.; Santinelli, F.B.; Lisboa-Filho, P.N.; Barbieri, F.A. The blood concentration of metallic nanoparticles is related to cognitive performance in people with multiple sclerosis: An exploratory analysis. Biomedicines 2023, 11, 1819. [Google Scholar] [CrossRef]
- Rajkumar, R.P. Biomarkers of Neurodegeneration in Post-Traumatic Stress Disorder: An Integrative Review. Biomedicines 2023, 11, 1465. [Google Scholar] [CrossRef]
- Stojsavljević, A.; Lakićević, N.; Pavlović, S. Mercury and Autism Spectrum Disorder: Exploring the Link through Comprehensive Review and Meta-Analysis. Biomedicines 2023, 11, 3344. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Kasselimis, D.; Laskaris, N.; Potagas, C. Unraveling the Thread of Aphasia Rehabilitation: A Translational Cognitive Perspective. Biomedicines 2023, 11, 2856. [Google Scholar] [CrossRef]
- Zakia, H.; Iskandar, S. Case report: Depressive disorder with peripartum onset camouflages suspected intracranial tuberculoma. Frontiers in Psychiatry 2022, 13, 932635. [Google Scholar] [CrossRef]
- Liu, M.; Xie, X.; Xie, J.; Tian, S.; Du, X.; Feng, H.; Zhang, H. Early-onset Alzheimer’s disease with depression as the first symptom: a case report with literature review. Frontiers in Psychiatry 2023, 14, 1192562. [Google Scholar] [CrossRef]
- Komatsu, H.; Watanabe, E.; Fukuchi, M. Psychiatric neural networks and precision therapeutics by machine learning. Biomedicines 2021, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Koul, A.M.; Ahmad, F.; Bhat, A.; Aein, Q.-u.; Ahmad, A.; Reshi, A.A.; Kaul, R.-u.-R. Unraveling Down Syndrome: From Genetic Anomaly to Artificial Intelligence-Enhanced Diagnosis. Biomedicines 2023, 11, 3284. [Google Scholar] [CrossRef] [PubMed]
- Ikonnikova, A.; Anisimova, A.; Galkin, S.; Gunchenko, A.; Abdukhalikova, Z.; Filippova, M.; Surzhikov, S.; Selyaeva, L.; Shershov, V.; Zasedatelev, A. Genetic Association Study and Machine Learning to Investigate Differences in Platelet Reactivity in Patients with Acute Ischemic Stroke Treated with Aspirin. Biomedicines 2022, 10, 2564. [Google Scholar] [CrossRef] [PubMed]
- Rassler, B.; Blinowska, K.; Kaminski, M.; Pfurtscheller, G. Analysis of Respiratory Sinus Arrhythmia and Directed Information Flow between Brain and Body Indicate Different Management Strategies of fMRI-Related Anxiety. Biomedicines 2023, 11, 1028. [Google Scholar] [CrossRef]
- Wasko, M.J.; Pellegrene, K.A.; Madura, J.D.; Surratt, C.K. A role for fragment-based drug design in developing novel lead compounds for central nervous system targets. Frontiers in neurology 2015, 6, 197. [Google Scholar] [CrossRef]
- Klon, A.E. Computational models for central nervous system penetration. Current Computer-Aided Drug Design 2009, 5, 71–89. [Google Scholar] [CrossRef]
- Ghose, A.K.; Herbertz, T.; Hudkins, R.L.; Dorsey, B.D.; Mallamo, J.P. Knowledge-based, central nervous system (CNS) lead selection and lead optimization for CNS drug discovery. ACS chemical neuroscience 2012, 3, 50–68. [Google Scholar] [CrossRef]
- Koszła, O.; Stępnicki, P.; Zięba, A.; Grudzińska, A.; Matosiuk, D.; Kaczor, A.A. Current Approaches and Tools Used in Drug Development against Parkinson's Disease. Biomolecules 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Nisha, C.M.; Silakari, C.; Sharma, I.; Anusha, K.; Gupta, N.; Nair, P.; Tripathi, T.; Kumar, A. Current and novel therapeutic molecules and targets in Alzheimer's disease. J Formos Med Assoc 2016, 115, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Yuen, J.; Kouzani, A.Z.; Berk, M.; Tye, S.J.; Rusheen, A.E.; Blaha, C.D.; Bennet, K.E.; Lee, K.H.; Shin, H.; Kim, J.H. Deep brain stimulation for addictive disorders—where are we now? Neurotherapeutics 2022, 19, 1193–1215. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, L.L.; Pantovic, M.; Clingo, M.; Fischer, K.; Jalene, S.; Landers, M.; Mari, Z.; Poston, B. A Single Application of Cerebellar Transcranial Direct Current Stimulation Fails to Enhance Motor Skill Acquisition in Parkinson’s Disease: A Pilot Study. Biomedicines 2023, 11, 2219. [Google Scholar] [CrossRef]
- Senevirathne, D.K.L.; Mahboob, A.; Zhai, K.; Paul, P.; Kammen, A.; Lee, D.J.; Yousef, M.S.; Chaari, A. Deep Brain Stimulation beyond the Clinic: Navigating the Future of Parkinson’s and Alzheimer’s Disease Therapy. Cells 2023, 12, 1478. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Wang, W.-L.; Shieh, Y.-H.; Peng, H.-Y.; Ho, C.-S.; Tsai, H.-C. Case Report: Low-Frequency Repetitive Transcranial Magnetic Stimulation to Dorsolateral Prefrontal Cortex and Auditory Cortex in a Patient With Tinnitus and Depression. Frontiers in Psychiatry 2022, 13, 847618. [Google Scholar] [CrossRef] [PubMed]
- Rymaszewska, J.; Wieczorek, T.; Fila-Witecka, K.; Smarzewska, K.; Weiser, A.; Piotrowski, P.; Tabakow, P. Various neuromodulation methods including Deep Brain Stimulation of the medial forebrain bundle combined with psychopharmacotherapy of treatment-resistant depression—Case report. Frontiers in Psychiatry 2023, 13, 3014. [Google Scholar] [CrossRef] [PubMed]
- Schindler, E.A.D. Psychedelics in the Treatment of Headache and Chronic Pain Disorders. Curr Top Behav Neurosci 2022, 56, 261–285. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Tao, Q.; Niu, X.; Zhang, M.; Gao, X.; Yang, Z.; Yu, M.; Wang, W.; Han, S.; Cheng, J. Meta-analysis of structural and functional brain abnormalities in cocaine addiction. Frontiers in Psychiatry 2022, 13, 927075. [Google Scholar] [CrossRef]
- Borbély, K.; Emri, M.; Kenessey, I.; Tóth, M.; Singer, J.; Barsi, P.; Vajda, Z.; Pál, E.; Tóth, Z.; Beyer, T. Pet/Mri in the presurgical evaluation of patients with epilepsy: a concordance analysis. Biomedicines 2022, 10, 949. [Google Scholar] [CrossRef]
- Okanda Nyatega, C.; Qiang, L.; Jajere Adamu, M.; Bello Kawuwa, H. Altered striatal functional connectivity and structural dysconnectivity in individuals with bipolar disorder: a resting state magnetic resonance imaging study. Frontiers in Psychiatry 2022, 13, 1054380. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, Y.; Hong, Y.; Huo, J.; Chang, T.; Wang, H.; Huang, Y.; Li, W.; Zhang, Y. Altered brain activities in mesocorticolimbic pathway in primary dysmenorrhea patients of long-term menstrual pain. Frontiers in Neuroscience 2023, 17, 1098573. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yang, B.; Wang, H.; Zeng, Y.; Xin, J.; Li, X. The non-linear correlation between the volume of cerebral white matter lesions and incidence of bipolar disorder: A secondary analysis of data from a cross-sectional study. Frontiers in Psychiatry 2023, 14, 1149663. [Google Scholar] [CrossRef]
- Adamu, M.J.; Qiang, L.; Nyatega, C.O.; Younis, A.; Kawuwa, H.B.; Jabire, A.H.; Saminu, S. Unraveling the pathophysiology of schizophrenia: insights from structural magnetic resonance imaging studies. Frontiers in Psychiatry 2023, 14, 1188603. [Google Scholar] [CrossRef]
- Nyatega, C.O.; Qiang, L.; Adamu, M.J.; Kawuwa, H.B. Gray matter, white matter and cerebrospinal fluid abnormalities in Parkinson’s disease: A voxel-based morphometry study. Frontiers in Psychiatry 2022, 13, 1027907. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-H.; Kim, S.-H.; Han, C.; Jeong, H.-G.; Lee, M.-S.; Kim, J. Antidepressant-induced mania in panic disorder: a single-case study of clinical and functional connectivity characteristics. Frontiers in Psychiatry 2023, 14, 1205126. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, R.; DeSouza, J.F.; Shen, Y.; Zhang, H.; Zhu, C.; Huang, P.; Wang, C. Differential responses from the left postcentral gyrus, right middle frontal gyrus, and precuneus to meal ingestion in patients with functional dyspepsia. Frontiers in Psychiatry 2023, 14, 1184797. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cao, Y.; Deng, G.; Fang, J.; Qiu, C. Transient splenial lesion syndrome in bipolar-II disorder: a case report highlighting reversible brain changes during hypomanic episodes. Frontiers in Psychiatry 2023, 14. [Google Scholar] [CrossRef]
- Forrest, M.P.; Parnell, E.; Penzes, P. Dendritic structural plasticity and neuropsychiatric disease. Nat Rev Neurosci 2018, 19, 215–234. [Google Scholar] [CrossRef]
- Salvaggio, F.; Hodgkinson, J.T.; Carro, L.; Geddis, S.M.; Galloway, W.R.; Welch, M.; Spring, D.R. The synthesis of quinolone natural products from pseudonocardia sp. 2016.
- Piochon, M.; Coulon, P.M.; Caulet, A.; Groleau, M.-C.; Déziel, E.; Gauthier, C. Synthesis and antimicrobial activity of Burkholderia-related 4-hydroxy-3-methyl-2-alkenylquinolines (HMAQs) and their N-oxide counterparts. Journal of Natural Products 2020, 83, 2145–2154. [Google Scholar] [CrossRef]
- Nam, D.H.; Lee, K.S.; Kim, S.H.; Kim, S.M.; Jung, S.Y.; Chung, S.H.; Kim, H.J.; Kim, N.D.; Jin, C.; Lee, Y.S. Design and synthesis of 4-quinolinone 2-carboxamides as calpain inhibitors. Bioorganic & medicinal chemistry letters 2008, 18, 205–209. [Google Scholar]
- Vyas, V.K.; Variya, B.; Ghate, M.D. Design, synthesis and pharmacological evaluation of novel substituted quinoline-2-carboxamide derivatives as human dihydroorotate dehydrogenase (hDHODH) inhibitors and anticancer agents. European Journal of Medicinal Chemistry 2014, 82, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jung, J.S.; Moon, Y.S.; Song, D.K. Central or peripheral norepinephrine depletion enhances MK-801-induced plasma corticosterone level in mice. Prog Neuropsychopharmacol Biol Psychiatry 2009, 33, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Vécsei, L.; Giménez-Llort, L. Emerging translational research in neurological and psychiatric diseases: from in vitro to in vivo models. 2023; 24, 15739. [Google Scholar]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Preclinical modeling in depression and anxiety: Current challenges and future research directions. Adv. Clin. Exp. Med 2023, 32, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, R.D.; Fessler, R.G.; Meltzer, H.Y. Behavioral rating scales for assessing phencyclidine-induced locomotor activity, stereotyped behavior and ataxia in rats. Eur J Pharmacol 1979, 59, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Contreras, P.C. D-serine antagonized phencyclidine- and MK-801-induced stereotyped behavior and ataxia. Neuropharmacology 1990, 29, 291–293. [Google Scholar] [CrossRef]
- Lim, C.J.M.; Platt, B.; Janhunen, S.K.; Riedel, G. Comparison of automated video tracking systems in the open field test: ANY-Maze versus EthoVision XT. J Neurosci Methods 2023, 397, 109940. [Google Scholar] [CrossRef] [PubMed]
- Stanford, S.C. The Open Field Test: reinventing the wheel. J Psychopharmacol 2007, 21, 134–135. [Google Scholar] [CrossRef]
- Walsh, R.N.; Cummins, R.A. The Open-Field Test: a critical review. Psychol Bull 1976, 83, 482–504. [Google Scholar] [CrossRef]
- Pritchett, K.; Mulder, G.B. The rotarod. Contemp Top Lab Anim Sci 2003, 42, 49. [Google Scholar]
- Osmon, K.J.; Vyas, M.; Woodley, E.; Thompson, P.; Walia, J.S. Battery of Behavioral Tests Assessing General Locomotion, Muscular Strength, and Coordination in Mice. J Vis Exp 2018. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




