Submitted:
20 February 2024
Posted:
20 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Liquid-liquid extraction experiments
2.2.2. Liquid membrane experiments
3. Results and discussion
3.1. Solvent extraction experiments
3.2. Supported liquid membrane experiments
Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, G. Wang, Z.; Zhang, W.; He, H.; Zhang, Y.; Deng, X.; Li, W. MIL-161 metal–organic framework for efficient Au(III) recovery from secondary resources: performance, mechanism, and DFT calculations. Molecules 2023, 28, 5459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, Q.; Yang, Y.; Zhang, Y.; Qiu, Y.; Chen, Y.; Jin, X.; Yang, X.; Wang, S. Ultra-high adsorption capacity and selectivity of photo-enhanced sulfur-rich M2S3 (M[dbnd]Bi and Sb) for gold recovery from electronic wastewater. J. Water Proc. Eng. 2024, 57, 104572. [Google Scholar] [CrossRef]
- Hubicki, Z.; Zinkowska, K.; Wójcik, G. A new impregnated adsorbent for noble metal ion sorption. Molecules 2023, 28, 6040. [Google Scholar] [CrossRef]
- Thombre, A.V.; Kundu, D. Ionic liquid promoted extraction of gold(III) from electronic waste: a modeling study. Sep. Sci. Technol. 2023, 58, 2641–2654. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Robla, J.I. On the use of pseudo-protic ionic liquids to extract gold(III) from HCl solutions. Int. J. Molec. Sci. 2023, 24, 6305. [Google Scholar] [CrossRef]
- Ansari, S.A. ; Mohapatra, P,K. Diglycolamides as highly efficient carrier ligands for actinide transport in supported liquid membranes. Desalin. Water Treat. 2023. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Tenório, J.A.S.; Espinosa, D.C.R. Separation of critical metals by membrane technology under a circular economy framework: a review of the state-of-the-art. Processes 2023, 11, 1256. [Google Scholar] [CrossRef]
- Kaczorowska, M.A. The latest achievements of liquid membranes for rare earth elements recovery from aqueous solutions—a mini review. Membranes 2023, 13, 839. [Google Scholar] [CrossRef] [PubMed]
- Kostanyan, A.E.; Voshkin, A.A.; Belova, V.V.; Zakhodyaeva, Y.A. Modelling and comparative analysis of different methods of liquid membrane separations. Membranes 2023, 13, 554. [Google Scholar] [CrossRef] [PubMed]
- Rzelewska-Piekut, M.; Regel-Rosocka, M. Liquid membranes for separation of metal ions from wastewaters. Phys. Sci. Rev. 2023, 8, 937–982. [Google Scholar] [CrossRef]
- Wang, B.; Wang, H.; Yan, J.; Wang, Y.; Xu, T. Research progress of polymer inclusion membrane in metal separation and recovery. Huagong Jinzhan/Chem. Ind. Eng. Prog. 3990. [Google Scholar] [CrossRef]
- Farah, M.; Giralt, J.; Stüber, F.; Font, J.; Fabregat, A.; Fortuny, A. Hollow fiber liquid membrane: a promising approach for elimination of pharmaceutical compounds from wastewater. J. Environ. Chem. Eng. 2023, 11, 111544. [Google Scholar] [CrossRef]
- Farah, M.; Giralt, J.; Stüber, F.; Font, J.; Fabregat,A. ; Fortuny, A. Intensification of diclofenac removal through supported liquid membrane and ozonation. Environ. Technol. Innov. 2024, 33, 103469. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Robla, J.I. Transport of chromium(VI) across a aupported liquid membrane containing Cyanex 921 or Cyanex 923 dissolved in Solvesso 100 as carrier phase: estimation of diffusional parameters. Membranes 2023, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Dhiman, S.; Gupta, H. . Indium extraction from nitrate medium using Cyphos ionic liquid 104 and its mathematical modeling. Environ. Sci. Pollut. Res. 1073. [Google Scholar] [CrossRef]
- Middleton, A.; Hsu-Kim, H. Separation of rare-earth elements by supported liquid membranes: impacts of soluble iron, aluminum, and pH in low-grade feedstocks. ACS ES T Eng. 2023, 3, 1197–1204. [Google Scholar] [CrossRef]
- Kadhim, N.R.; Abbar, A.H.; Flayeh, H.M. Removal of copper from a simulated wastewater by electromembrane extraction technique using a novel electrolytic cell provided with a flat polypropylene membrane infused with 1-octanol and DEHP as a carrier. Case Stud. Chem. Environ. Eng. 2023, 8, 100430. [Google Scholar] [CrossRef]
- Kadhim, N.R.; Flayeh, H.M.; Abbar, A.H. Zinc (II) removal from simulated wastewater by electro-membrane extraction approach: adopting an electrolysis cell with a flat sheet supported liquid membrane. J. Electrochem. Sci. Eng. 2023, 13, 1097–1112. [Google Scholar] [CrossRef]
- Kadhim, N.R.; Flayeh, H.M.; Abbar, A.H. A new approach for cobalt (II) removal from simulated wastewater using electro membrane extraction with a flat sheet supported liquid membrane. Heliyon 2023, 9, e22343. [Google Scholar] [CrossRef]
- Suren, S.; Punyain, W.; Maneeintr, K.; Nootong, K.; Pancharoen, U. The simultaneous elimination of arsenic and mercury ions via hollow fiber supported liquid membrane and their reaction mechanisms: experimental and modeling based on DFT and generating function. Arabian J. Chem. 2023, 16, 104501. [Google Scholar] [CrossRef]
- Traiwongsa, N.; Suren, S.; Pancharoen, U.; Nootong, K.; Maneeintr, K.; Punyain, W.; Lothongkum, A.W. Mechanisms of mercury ions separation by non-toxic organic liquid membrane via DFT, thermodynamics, kinetics and mass transfer model. J. Ind. Eng. Chem. 2023, 117, 522–537. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Robla, J.I. Iron control in liquid effluents: pseudo-emulsion based hollow fiber membrane with strip dispersion technology with pseudo-protic ionic liquid (RNH3+HSO4−) as mobile carrier. Membranes 2023, 13, 723. [Google Scholar] [CrossRef] [PubMed]
- Alguacil, F.J.; Robla, J.I. Treatment of stainless steel rinse waters using non-dispersive extraction and strip dispersion membrane technology. Membranes 2023, 13, 902. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, J.; Biswas, A.; Fang, W.; Chen, L. Acid mine wastewater treatment: a scientometrics review. J. Water Proc. Eng. 2024, 57, 104713. [Google Scholar] [CrossRef]
- Pacary, V.; Burdet, F.; Duchesne, M.T. Experimental and modeling of extraction of lanthanides in system HNO3-TEDGA-{DMDOHEMA-HDEHP}. Proc. Chem. 2012, 7, 328–333. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, R.; Liu, X.; Li, J.; Wu, R.; Yang, Y. Study on extraction of Rh(III) by DABCO-based ionic liquid from hydrochloric acid medium. Sep. Purif. Technol. 2023, 324, 124578. [Google Scholar] [CrossRef]
- Li, H.; Mabhiza, T.; Ning, Y.; Yu, Z.; Lv, C.; Shen, X.; Wei, G.; Qu, J. Solvent extraction and recovery of beryllium from hydrochloric acid solution with naphthenic acid. Sep. Purif. Technol. 2024, 331, 125594. [Google Scholar] [CrossRef]
- Dong, H.; Ci, E.; Zhao, T.; Chen, P.; Liu, F.; Hu, G.; Yang, L. Hydrophobic deep eutectic solvents as the green media for highly efficient extraction of Cr(VI) over a broad pH range and low oil-water ratio. Sep. Purif. Technol. 2024, 334, 126104. [Google Scholar] [CrossRef]
- Diamond, R.M. The solvent extraction behavior of inorganic compounds. III. Variation of the distribution quotient with metal ion concentration. J. Phys. Chem. 1957, 61, 75–81. [Google Scholar] [CrossRef]
- Bohrer, M. P. Diffusional boundary layer resistance for membrane transport. Ind. Eng. Chem. Fundam. 1983, 22, 72–78. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Martinez, S. Permeation of iron(III) by an immobilised liquid membrane using Cyanex 923 as mobile carrier. J. Membr. Sci. 2000, 176, 249–255. [Google Scholar] [CrossRef]
- Pavón, S.; Fortuny, A.; Coll, M. T.; Bertau, M.; Sastre, A. M. Permeability dependencies on the carrier concentration and membrane viscosity for Y(III) and Eu(III) transport by using liquid membranes. Sep. Purif. Technol. 2020, 239, 116573. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Alonso, M.; Lopez, F.; Lopez-Delgado, A. Uphill permeation of Cr(VI) using Hostarex A327 as ionophore by membrane-solvent extraction processing. Chemosphere 2008, 72, 684–689. [Google Scholar] [CrossRef] [PubMed]
- El Aamrani, F.Z.; Kumar, A.; Beyer, L.; Cortina, J.L.; Sastre, A.M. Uphill permeation model of gold(III) and its separation from base metals using thioures derivatives as ionophores across a liquid membrane. Hydrometallurgy 1998, 50, 315–330. [Google Scholar] [CrossRef]
- Gupta, S.K.; Rathore, N.S.; Sonawane, J.V.; Pabby, A.K.; Janardan, P.; Changrani, R.D.; Dey, P.K. Dispersion-free solvent extraction of U(VI) in macro amount from nitric acid solutions using hollow fiber contactors. J. Membr. Sci. 2007, 300, 131–136. [Google Scholar] [CrossRef]
- Sastre, A.M.; Madi, A. , Cortina, J.L.; Miralles, N. Modelling of mass transfer in facilitated supported liquid membrane transport of gold(III) using phospholene derivatives a s carriers. J. Membr. Sci. [CrossRef]
- Haghighi, H.K.; Irannajad, M. ; Fortuny, A,; Sastre, A.M. Mathematical modeling on non-dispersive extraction of germanium from aqueous solutions using Aliquat 336. Water Sci. Technol. 2489. [Google Scholar] [CrossRef]
- Alguacil, F.J. Mechanistic investigation of facilitated transport of gold(III) from HCl media using ionic liquid Cyphos IL102 as carrier across a supported liquid membrane. Gold Bull. 2019, 52, 145–151. [Google Scholar] [CrossRef]
- Huang, T.-C.; Juang, R.-S. Rate and mechanism of divalent metal transport through supported liquid membrane containing di(2-ethylhexyl) phosphoric acid as a mobile carrier. J. Chem. Technol. Biotechnol. 1998, 42, 3–17. [Google Scholar] [CrossRef]
- De Gyves, J.; De San Miguel, E.R. Metals separations by supported liquid membranes, Ind. Eng. Chem. Res. 1999, 38, 2182–2202–2202. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Adeva, P.; Alonso, M. Processing of residual gold(III) solutions via ion exchange. Gold. Bull. 2005, 38, 9–13. [Google Scholar] [CrossRef]
- Oestreicher, V.; García, C.S.; Soler-Illia, G.J.A.A.; Angelomé, P.C. Gold recycling at laboratory scale: from nanowaste to nanospheres. ChemSusChem 2019, 12, 4882–4888. [Google Scholar] [CrossRef] [PubMed]
- Layek, K. Hydroxyapatite supported gold nanoparticles catalyzed efficient catalysis for the reduction of nitroarenes and degradation of azo dyes. Catal. Surv. Asia 2024, 27, 349–362. [Google Scholar] [CrossRef]
- Cheng, C.-W.; Lee, S.-Y.; Zhan, S.-Q.; Huang, C.-L.; Chen, T.-Y.; Yuan, J.-M.P.; Huang, S.-T. Chiu, C.-M-: Liang, J.-Y. The effect of photolysis of sodium citrate treated with gold chloride using coloured light on the generation of gold nanoparticles and the repression of WiDr colon cancer cells. J. Photochem. Photobiol. B: Biology 2024, 251, 112844. 251,. [CrossRef]
- Hiral, V.; Kokila, P.; Jyotindra, M. Biogenic synthesis of gold nanoparticles using bark extract of Bauhinia variegata: antibacterial and in vitro anticancer study. Res. J. Chem. Environ. [CrossRef]
- Ajayi, R.F.; Nqunqa, S.; Ngema, N.P.P.; Barry, S.C.L.; Feleni, U.; Mulaudzi, T. UV–Vis detection of E. coli 0157:H7 using Vitis vinifera and Musa paradaisica modified Au-NPs. MethodsX, 1025. [Google Scholar] [CrossRef]
| Time, min | F |
|---|---|
| 1 2 5 10 15 |
0.71 0.98 1 1 1 |
| [Au]0, g/L | % Extraction |
|---|---|
| 5 15 25 35 50 75 100 150 200 |
98 98 98 98 98 97 96 93 87 |
| [2-ethylhexanol], % v/v | % Extraction |
|---|---|
| 25 35 50 60 70 75 80 90 undiluted |
57 75 88 92 94 95 96 98 98 |
| Element | % Extraction | D | SF |
|---|---|---|---|
| Au(III) Cu(II) Zn(II) Ni(II) |
98 21 11 12 |
69 0.25 0.12 0.13 |
276 575 530 |
| [Au]0:[Cu]0 | DAu | DCu | SF |
|---|---|---|---|
| 2:1 3:1 6:1 18:1 |
69 69 69 69 |
0.045 0.047 0.044 0.049 |
1533 1468 1568 1408 |
| Stirring speed, min-1 | KO·103, cm/s |
|---|---|
| 600 800 1000 1200 1400 1600 |
0.95 2.6 5.7 5.7 5.5 4.9 |
| [HCl], M | KO·103 , cm/s |
| 0.5 1 3 6 |
3.8 4.7 5.7 5.7 |
| [carrier], % v/v | KO·103, cm/s | a% Gold recovery |
|---|---|---|
| 10 25 35 45 50 65 75 undiluted |
1.2 2.7 4.0 5.6 5.7 5.5 3.0 1.2 |
95 95 94 93 92 93 90 89 |
| [Au]0, g/L | KO·103, cm/s | J, mol/cm2s | a% Gold recovery |
|---|---|---|---|
| 0.01 0.02 0.04 0.06 0.08 0.1 |
5.7 4.5 3.0 2.2 1.7 1.4 |
2.9 4.6 6.0 6.6 6.8 7.0 |
95 93 94 93 94 95 |
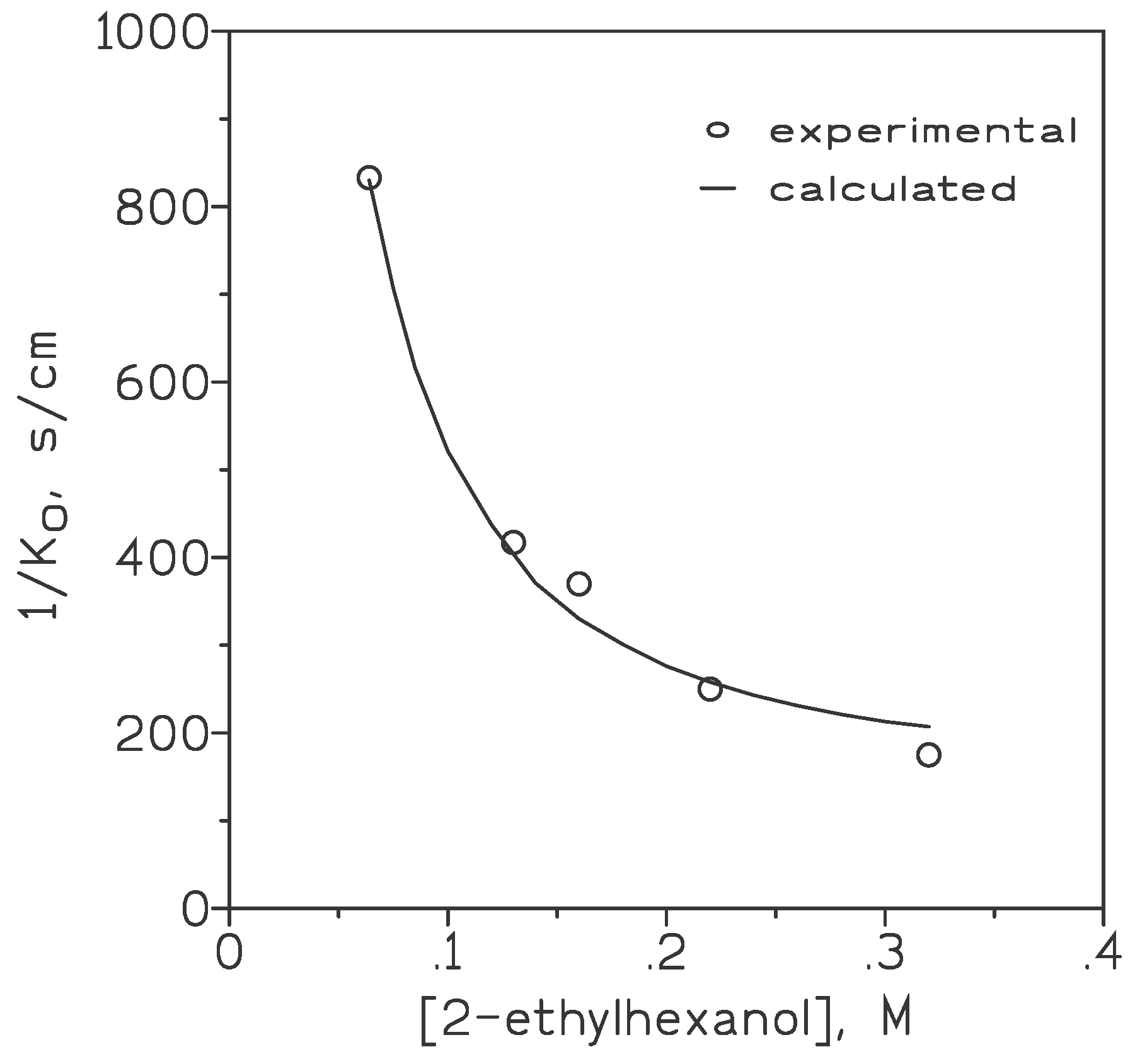
| Experimental condition | RT, s/cm | aRT, s/cm | %Rfo | %Rmo |
|---|---|---|---|---|
| Carrier 10-50% v/v Gold: 0.01-0.1 g/L HCl 6 M |
370-175 175-714 175 |
147 147 147 |
40-84 84-21 84 |
60-16 16-79 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





