Submitted:
15 February 2024
Posted:
16 February 2024
You are already at the latest version
Abstract
Keywords:
Introduction
2. Methods
2.1. Materials
2.2. Synthesis technique
2.2. Characterization methods
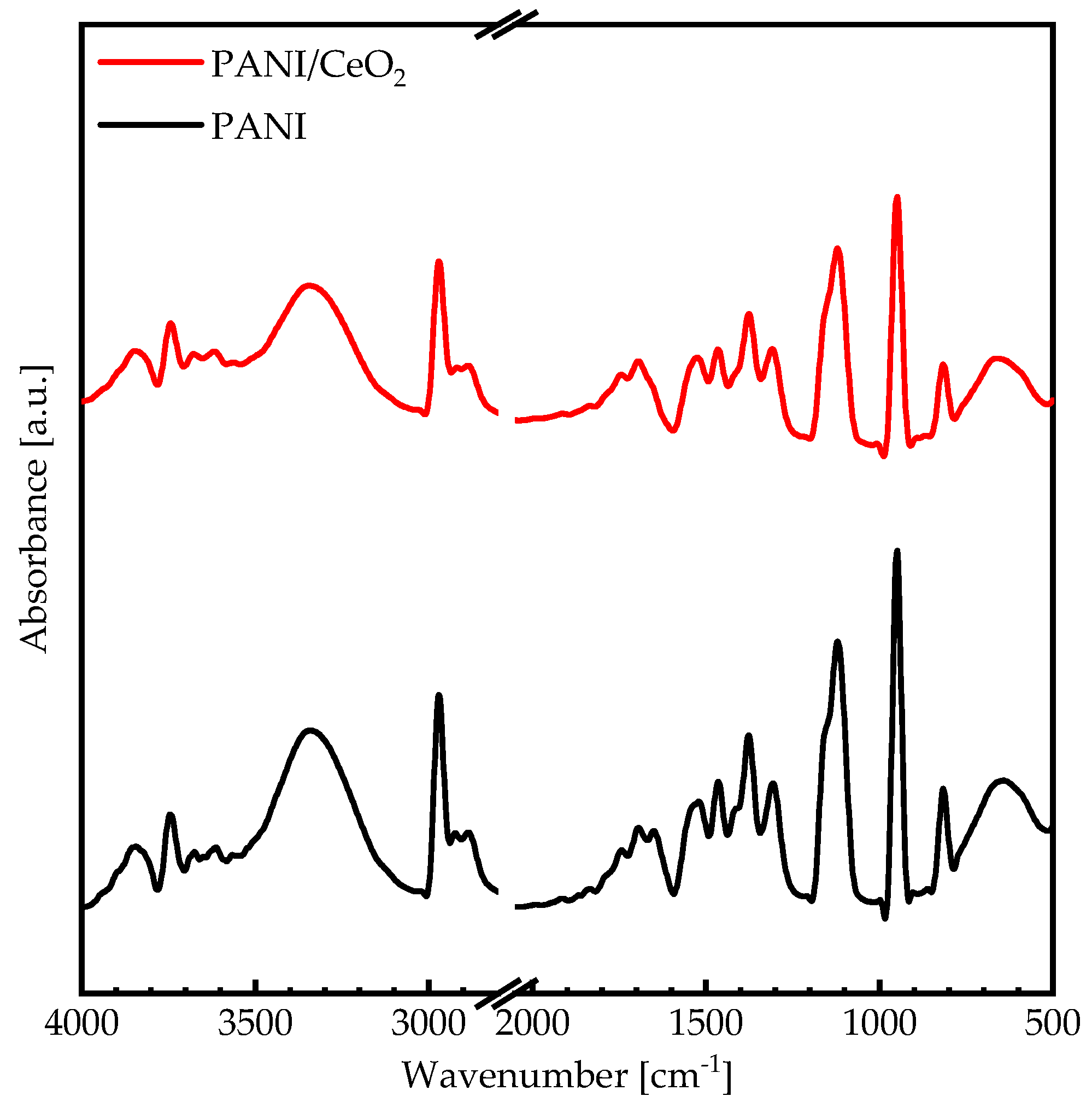
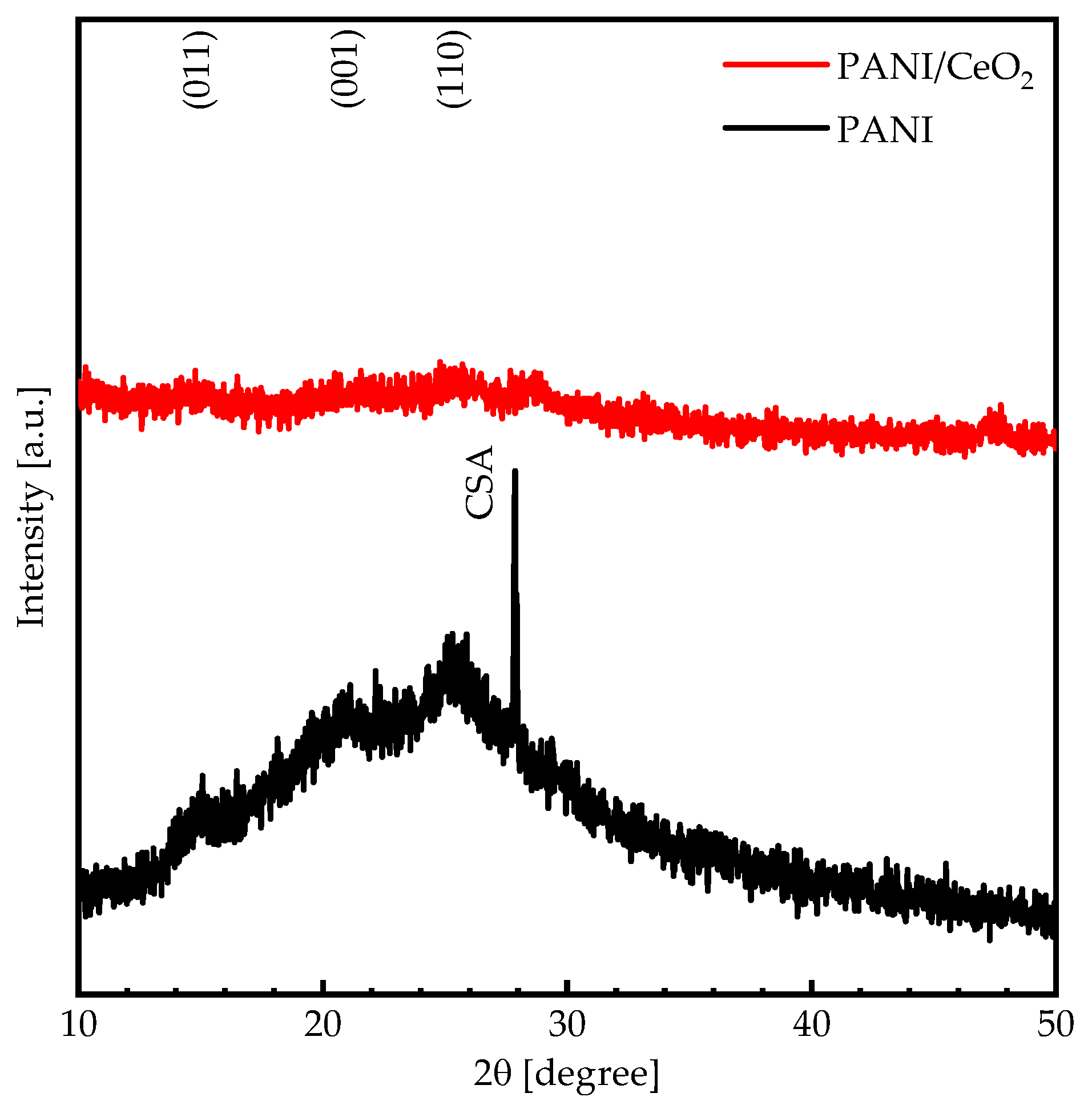
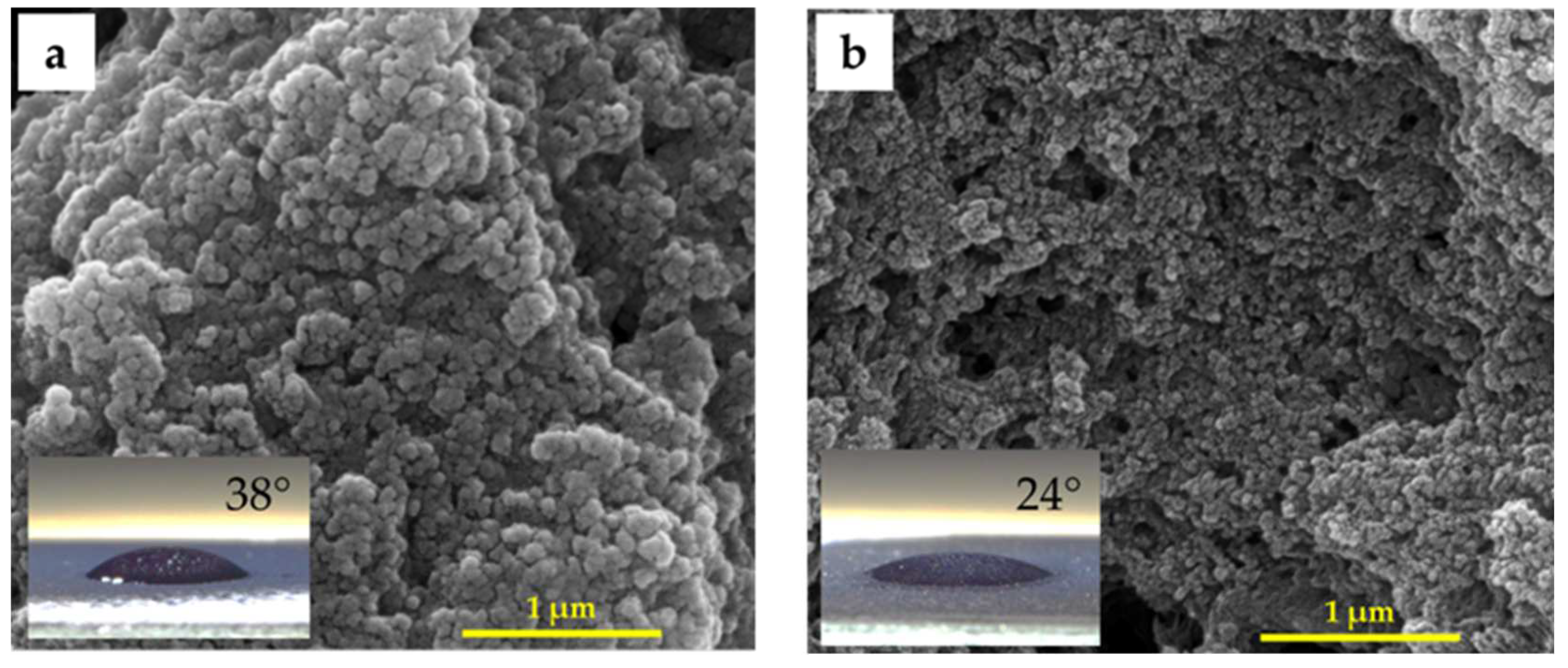
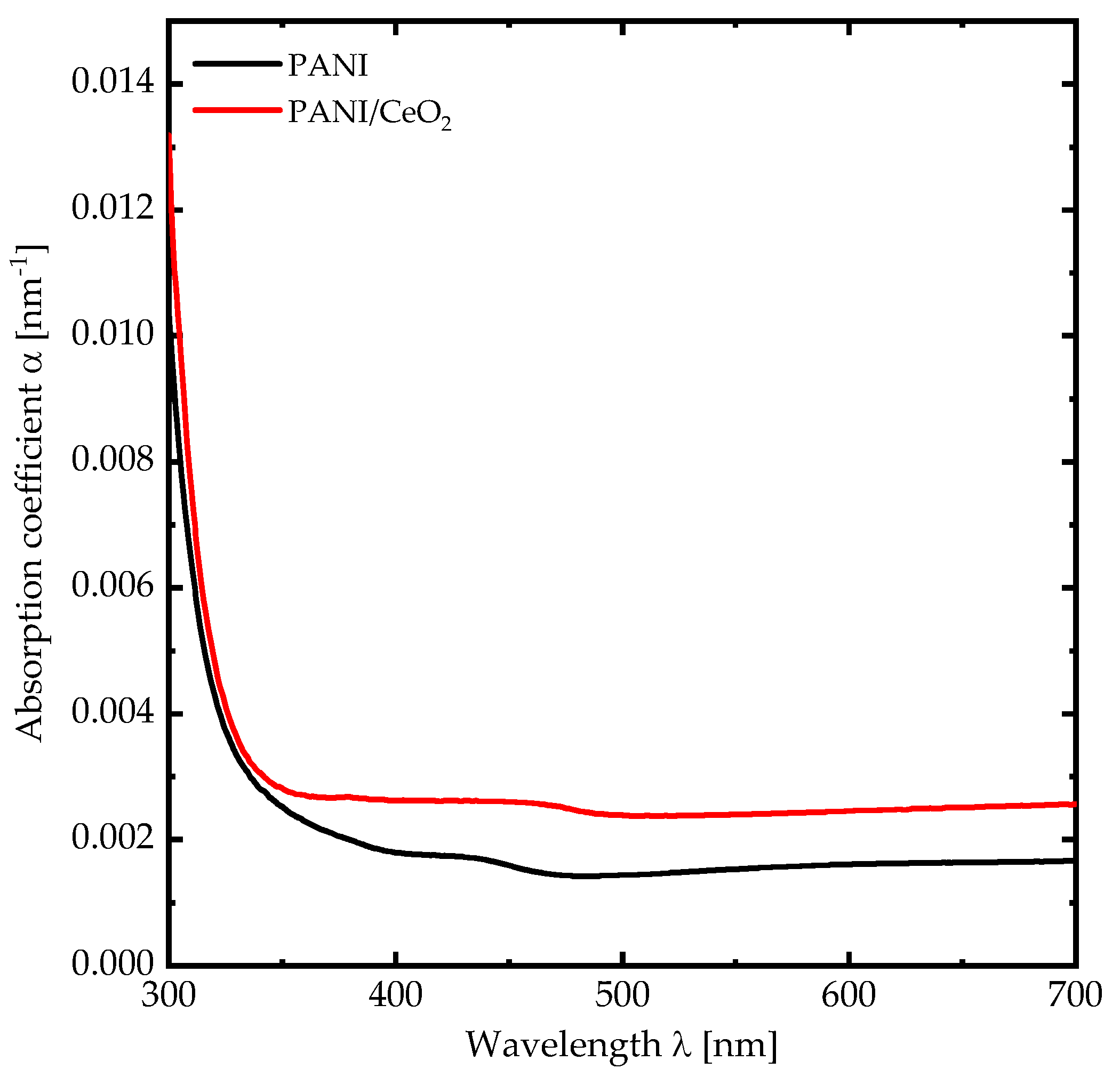
3. Results and Discussion
4. Conclusions
Author Contributions
Data Availability Statement
Conflicts of Interest
Data availability
Acknowledgments
References
- Ramezanzadeh, B.; Moghadam, M.M.; Shohani, N.; Mahdavian, M. Effects of highly crystalline and conductive polyaniline/graphene oxide composites on the corrosion protection performance of a zinc-rich epoxy coating. Chemical Engineering Journal 2017, 320, 363–375. [Google Scholar] [CrossRef]
- Taheri, N.N.; Ramezanzadeh, B.; Mahdavian, M.; Bahlakeh, G. In-situ synthesis of Zn doped polyaniline on graphene oxide for inhibition of mild steel corrosion in 3.5 wt.% chloride solution. Journal of industrial and engineering chemistry 2018, 63, 322–339. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Tsai, C.-H.; Huang, H.-L.; Ho, K.-S.; Lin, I.; Wang, Y.-F. Fabrication of polyaniline coated plasma modified polypropylene filter for antibioaerosol application. Aerosol and Air Quality Research 2016, 16, 1911–1921. [Google Scholar] [CrossRef]
- Qiu, G.; Zhu, A.; Zhang, C. Hierarchically structured carbon nanotube–polyaniline nanobrushes for corrosion protection over a wide pH range. Rsc Advances 2017, 7, 35330–35339. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Polyaniline-cerium oxide (PAni-CeO2) coated graphene oxide for enhancement of epoxy coating corrosion protection performance on mild steel. Corrosion Science 2018, 137, 111–126. [Google Scholar] [CrossRef]
- Hayatgheib, Y.; Ramezanzadeh, B.; Kardar, P.; Mahdavian, M. A comparative study on fabrication of a highly effective corrosion protective system based on graphene oxide-polyaniline nanofibers/epoxy composite. Corrosion Science 2018, 133, 358–373. [Google Scholar] [CrossRef]
- Abdeslam, M.; Hichem, B.; Youcef, H. Synthesis of polyaniline/CeO2 nanocomposites as inhibitor coating on zinc: Evaluation of the corrosion behaviour. Proceedings of International Conference on Scientific and Innovative Studies; pp. 282–288.
- Hosseini, M.G.; Aboutalebi, K. Enhancement the anticorrosive resistance of epoxy coatings by incorporation of CeO2@ polyaniline@ 2-mercaptobenzotiazole nanocomposite. Synthetic Metals 2019, 250, 63–72. [Google Scholar] [CrossRef]
- Montemor, M.; Ferreira, M. Cerium salt activated nanoparticles as fillers for silane films: Evaluation of the corrosion inhibition performance on galvanised steel substrates. Electrochimica Acta 2007, 52, 6976–6987. [Google Scholar] [CrossRef]
- Calado, L.M.; Taryba, M.G.; Carmezim, M.J.; Montemor, M.F. Self-healing ceria-modified coating for corrosion protection of AZ31 magnesium alloy. Corrosion Science 2018, 142, 12–21. [Google Scholar] [CrossRef]
- Lei, Y.; Qiu, Z.; Tan, N.; Du, H.; Li, D.; Liu, J.; Liu, T.; Zhang, W.; Chang, X. Polyaniline/CeO2 nanocomposites as corrosion inhibitors for improving the corrosive performance of epoxy coating on carbon steel in 3.5% NaCl solution. Progress in Organic Coatings 2020, 139, 105430. [Google Scholar] [CrossRef]
- Daikh, S.; Zeggai, F.; Bellil, A.; Benyoucef, A. Chemical polymerization, characterization and electrochemical studies of PANI/ZnO doped with hydrochloric acid and/or zinc chloride: differences between the synthesized nanocomposites. Journal of Physics and Chemistry of Solids 2018, 121, 78–84. [Google Scholar] [CrossRef]
- Kulkarni, M.V.; Viswanath, A.K.; Marimuthu, R.; Seth, T. Spectroscopic, transport, and morphological studies of polyaniline doped with inorganic acids. Polymer Engineering & Science 2004, 44, 1676–1681. [Google Scholar]
- Chamroukhi, H.; Hamed, Z.B.; Telfah, A.; Bassou, M.; Zeinert, A.; Hergenröder, R.; Bouchriha, H. Optical and structural properties enhancement of hybrid nanocomposites thin films based on polyaniline doped with Zinc Oxide embedded in bimodal mesoporous silica (ZnO@ SiOX) nanoparticles. Optical Materials 2018, 84, 703–713. [Google Scholar] [CrossRef]
- Mazzeu, M.A.C.; Faria, L.K.; Cardoso, A.d.M.; Gama, A.M.; Baldan, M.R.; Gonçalves, E.S. Structural and morphological characteristics of polyaniline synthesized in pilot scale. Journal of Aerospace Technology and Management 2017, 9, 39–47. [Google Scholar] [CrossRef]
- Diggikar, R.S.; Deshmukh, S.P.; Thopate, T.S.; Kshirsagar, S.R. Performance of polyaniline nanofibers (PANI NFs) as PANI NFs-silver (Ag) nanocomposites (NCs) for energy storage and antibacterial applications. ACS Omega 2019, 4, 5741–5749. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D. Determination of crystal structure of polyaniline and substituted polyanilines through powder X-ray diffraction analysis. Polymer Testing 2008, 27, 851–857. [Google Scholar] [CrossRef]
- Dhahri, I.; Ellouze, M.; Labidi, S.; Al-Bataineh, Q.M.; Etzkorn, J.; Guermazi, H.; Telfah, A.; Tavares, C.J.; Hergenröder, R.; Appel, T. Optical and structural properties of ZnO NPs and ZnO–Bi2O3 nanocomposites. Ceramics International 2022, 48, 266–277. [Google Scholar] [CrossRef]
- Hassanien, A.; Abdelhaleem, S.; Ahmad, R.; Schuster, M.; Moustafa, S.; Distaso, M.; Peukert, W.; Wellmann, P. Effect of fast annealing on structural characteristics and optical properties of Cu2ZnSnS4 absorber films deposited by doctor-blade technique. Journal of Nanoelectronics and Optoelectronics 2019, 14, 1394–1400. [Google Scholar] [CrossRef]
- Al-Bataineh, Q.M.; Shpacovitch, V.; Sadiq, D.; Telfah, A.; Hergenröder, R. Surface Plasmon Resonance Sensitivity Enhancement Based on Protonated Polyaniline Films Doped by Aluminum Nitrate. Biosensors 2022, 12, 1122. [Google Scholar] [CrossRef]
- Hassanien, A.S.; Neffati, R.; Aly, K. Impact of Cd-addition upon optical properties and dispersion parameters of thermally evaporated CdxZn1-xSe films: discussions on bandgap engineering, conduction and valence band positions. Optik 2020, 212, 164681. [Google Scholar] [CrossRef]
- Hassanien, A.; Atta, A.; Ward, A.A.; Ahmed, E.M.; Alsubaie, A.; El-Nahass, M.; Altalhi, T. Investigation of structural, electrical and optical properties of chitosan/fullerene composites. Materials Research Express 2019, 6, 125304. [Google Scholar] [CrossRef]
- Al-Gharram, M.; Jum'h, I.; Telfah, A.; Al-Hussein, M. Highly crystalline conductive electrodeposited films of PANI-CSA/CoFe2O4 nanocomposites. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2021, 628, 127342. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, S.; Hong, R. Graphene oxide/polyaniline nanocomposites used in anticorrosive coatings for environmental protection. Coatings 2020, 10, 1215. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Don, T.-M.; Wong, C.-J.; Meng, F.-C.; Lin, Y.-J.; Lee, S.-Y.; Lee, C.-F.; Chiu, W.-Y. Improvement of mechanical properties and anticorrosion performance of epoxy coatings by the introduction of polyaniline/graphene composite. Surface and Coatings Technology 2019, 374, 1128–1138. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Mohamad, A.B.; Kadhum, A.A.H.; Shaker, L.M.; Isahak, W.N.R.W.; Takriff, M.S. Experimental and theoretical study on the corrosion inhibition of mild steel by nonanedioic acid derivative in hydrochloric acid solution. Scientific Reports 2022, 12, 4705. [Google Scholar] [CrossRef]
- Almashhadani, H. Synthesis of a CoO–ZnO nanocomposite and its study as a corrosion protection coating for stainless steel in saline solution. Int. J. Corros. Scale Inhib 2021, 10, 1294–1306. [Google Scholar]
- Telfah, A.; Kalfe-Yildiz, A.; Al Bataineh, Q.M.; Jum'h, I.; Tavares, C.J.; Hergenröder, R. Thermal-dependent morphological evolution effect on ion transportation in polyethylene oxide films. Polymer 2023, 288, 126440. [Google Scholar] [CrossRef]
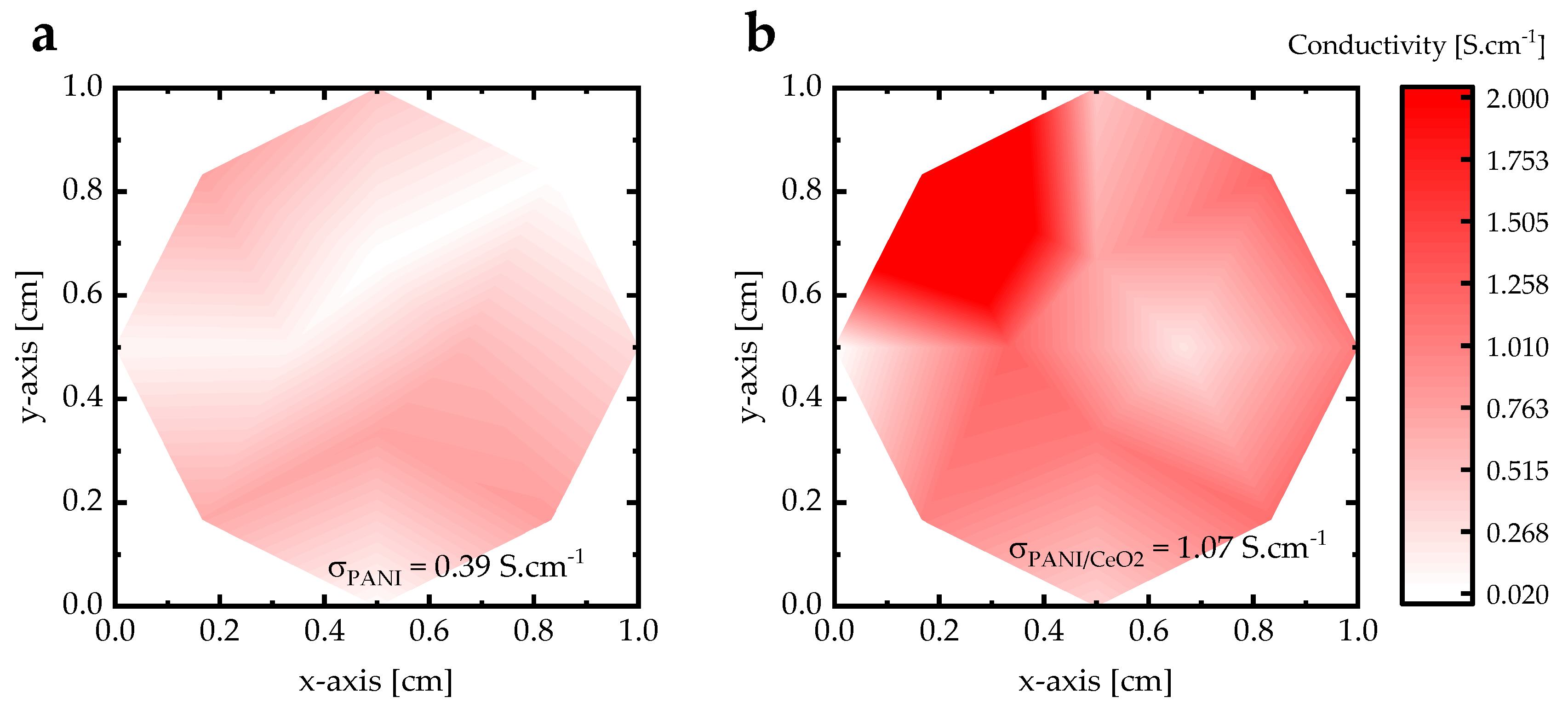
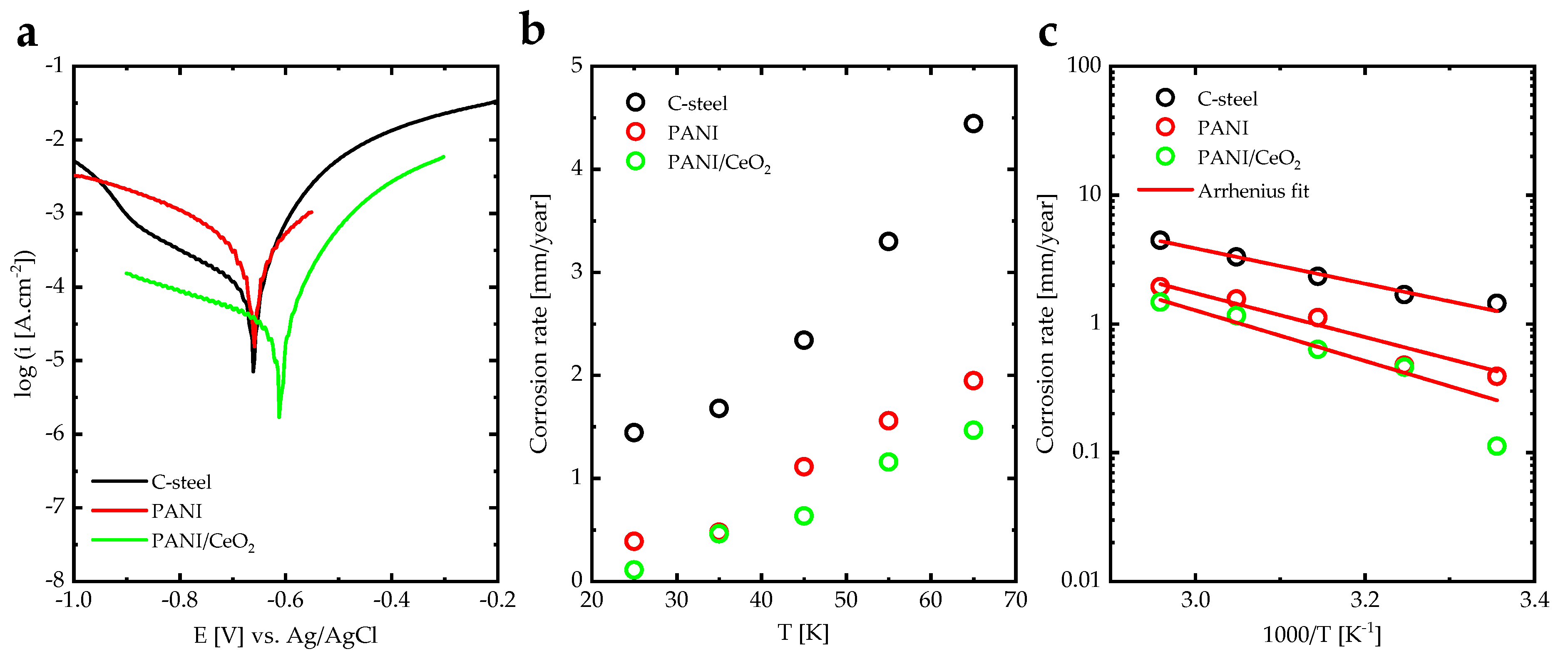
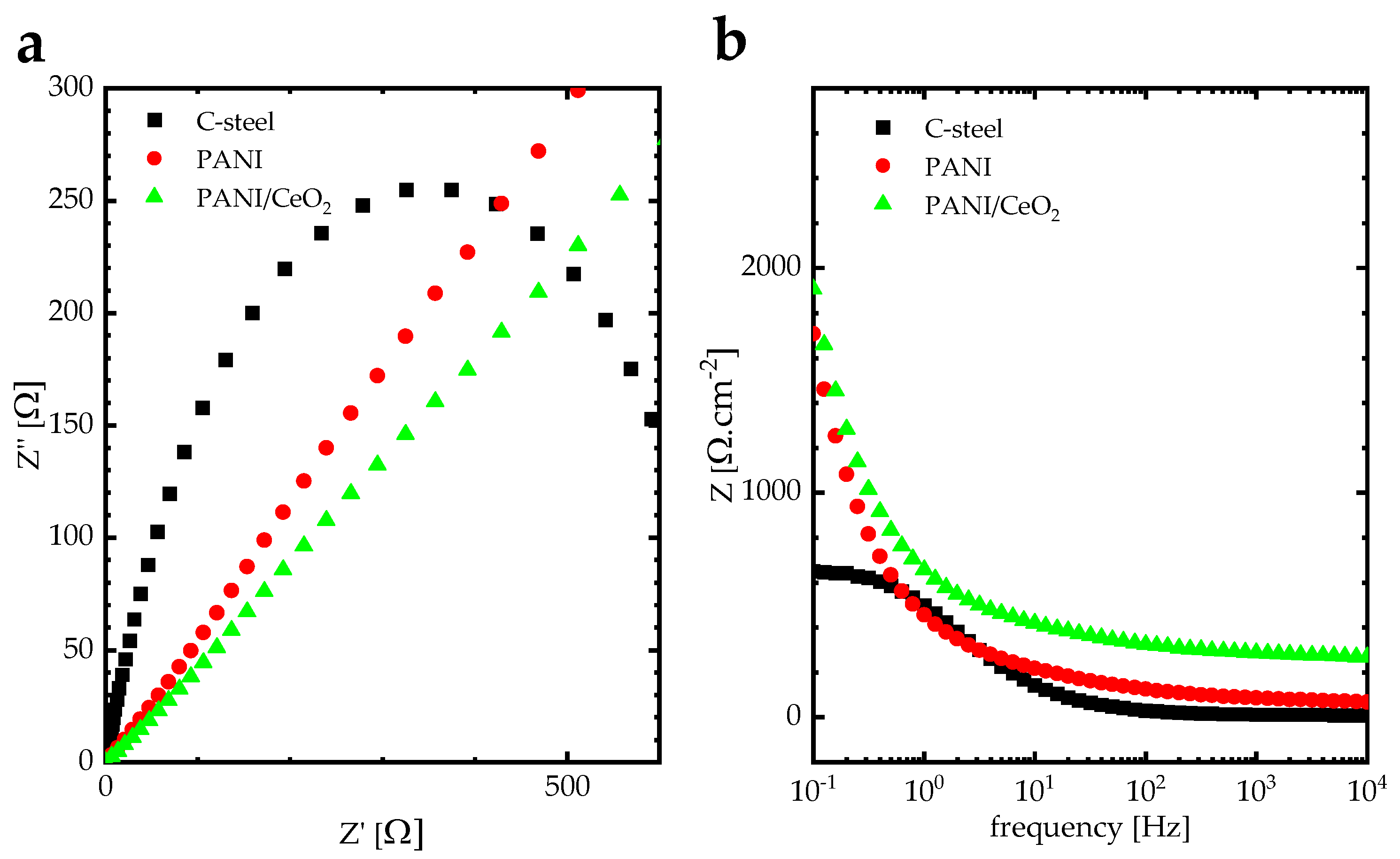
| C-Steel | PANI | PANI/CeO2 | |
|---|---|---|---|
| CR [mm/year] | 1.445 | 0.390 | 0.112 |
| (%) | -- | 72.90 | 92.25 |
| [eV] | 0.27 | 0.34 | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





