Submitted:
12 February 2024
Posted:
13 February 2024
You are already at the latest version
Abstract
Keywords:
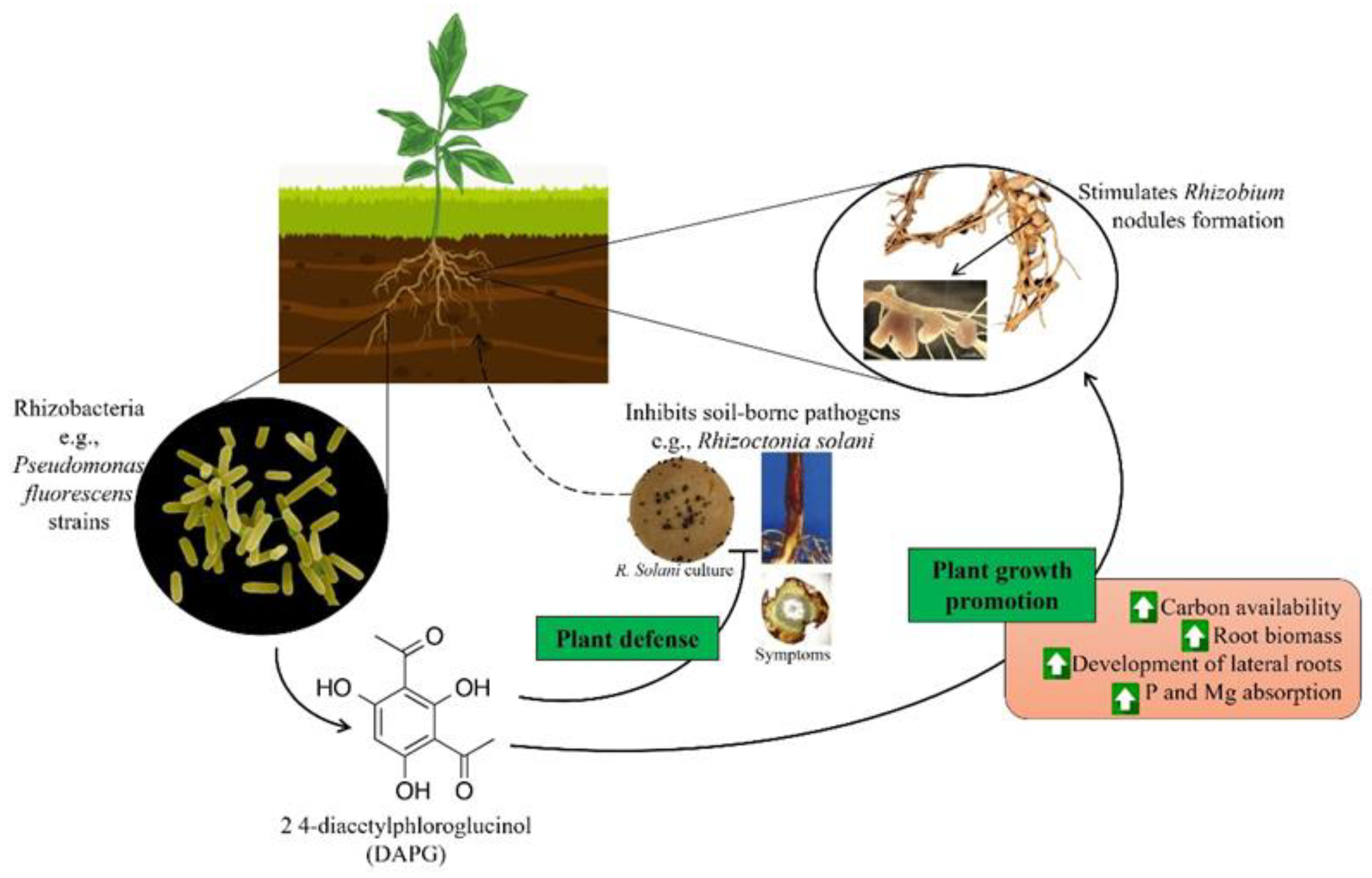
1. Introduction
2. Siderophore production
3. Production of antimicrobial compounds
4. Induced Systemic Resistance (ISR) and Systemic Acquired Resistance (SAR)
5. Quorum Sensing System (QS)
6. Antibiotic enzyme Production
7. Conclusions
References
- Shafique, H. A.; Sultana, V.; Ehteshamul-Haque, S.; Athar, M. Management of Soil-Borne Diseases of Organic Vegetables. J. Plant Prot. Res. 2016, 56, 221–230. [Google Scholar] [CrossRef]
- Ownley, B. H.; Benson, D. M. Soilborne Plant Pathogens. In Plant Pathology Concepts and Laboratory Exercises; Ownley, B. H., Trigiano, R. N., Eds.; CRC Press Taylor & Francis: Boca Raton, 2017; p. 400. [Google Scholar]
- Haichar, F. el Z.; Santaella, C.; Heulin, T.; Achouak, W. Root Exudates Mediated Interactions Belowground. Soil Biol. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Steinauer, K.; Chatzinotas, A.; Eisenhauer, N. Root Exudate Cocktails: The Link between Plant Diversity and Soil Microorganisms? Ecol. Evol. 2016, 6, 7387–7396. [Google Scholar] [CrossRef]
- Haas, D.; Défago, G. Biological Control of Soil-Borne Pathogens by Fluorescent Pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Agrios, G. N. Plant Pathology, 5th ed.; Elsevier academic press: San Diego, 2005; Volume 9780080473. [Google Scholar] [CrossRef]
- Vidhyasekaran, P. Concise Encyclopedia of Plant Pathology, 1st ed.; Food Products Press: Binghamton, 2004. [Google Scholar]
- Halila, M. H.; Strange, R. N. Identification of the Causal Agent of Wilt of Chickpea in Tunisia as Fusarium Oxysporum f. Sp. Ciceri Race 0. Phytopathol. Mediterr. 1996, 35, 67–74. [Google Scholar]
- Jones, J. P.; Woltz, S. S.; Scott, J. W. Fusarium Crown Rot of Tomato: Some Factors Affecting Disease Development. In Proceedings of the Florida tomato institute SS-VEG-01; Stall, W.., Ed.; Veg Crops Dept: Miami, 1991; pp. 74–79. [Google Scholar]
- Hibar, K.; Daami-Remadi, M.; El Mahjoub, M. Induction of Resistance in Tomato Plants against Fusarium Oxysporum f. Sp. Radicis-Lycopersici by Trichoderma Spp. Tunis. J. Plant Prot. 2007, 2, 47–58. [Google Scholar]
- Jarvis, W. R.; Thorpe, H. J.; Meloche, R. B. Survey of Greenhouse Management Practices in Essex County, Ontario, in Relation to Fusarium Foot and Root Rot of Tomato. Plant Dis. 1983, 67, 38–40. [Google Scholar] [CrossRef]
- Hassan, H. A. Biology and Integrated Control of Tomato Wilt Caused by Fusarium Oxysporum Lycopersici: A Comprehensive Review under the Light of Recent Advancements. J. Bot. Res. 2020, 3. [Google Scholar] [CrossRef]
- Oerke, E. C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Ristaino, J. B. Tracking Historic Migrations of the Irish Potato Famine Pathogen, Phytophthora Infestans. Microbes Infect. 2002, 4, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Dohroo, N. Diseases of Ginger. In Ginger, the genus Zingiber; Ravindran, P.., Nirmal Babu, K., Eds.; CRC Press Taylor & Francis: Boca Raton, 2005; pp. 305–340. [Google Scholar]
- Bickel, J. T.; Koehler, A. M. Review of Pythium Species Causing Damping-Off in Corn. Plant Heal. Prog. 2021, 22, 1–7. [Google Scholar] [CrossRef]
- Annou, M. M.; Wailes, E. J.; Thomsen, M. R. A Dynamic Decision Model of Technology Adoption under Uncertainty: Case of Herbicide-Resistant Rice. J. Agric. Appl. Econ. 2005, 37, 161–172. [Google Scholar] [CrossRef]
- Jayaweera, D. P.; Ray, R. V. Yield Loss and Integrated Disease Control of Rhizoctonia Solani AG2-1 Using Seed Treatment and Sowing Rate of Oilseed Rape. Plant Dis. 2023, 107, 1159–1165. [Google Scholar] [CrossRef]
- Mukhtar, T.; Kayani, M. Z. Comparison of the Damaging Effects of Meloidogyne Incognita on a Resistant and Susceptible Cultivar of Cucumber. Bragantia 2020, 79, 83–93. [Google Scholar] [CrossRef]
- Subedi, S.; Thapa, B.; Shrestha, J. Root-Knot Nematode (Meloidogyne Incognita) and Its Management: A Review. J. Agric. Nat. Resour. 2020, 3, 21–31. [Google Scholar] [CrossRef]
- Raymaekers, K.; Ponet, L.; Holtappels, D.; Berckmans, B.; Cammue, B. P. A. Screening for Novel Biocontrol Agents Applicable in Plant Disease Management – A Review. Biol. Control 2020, 144. [Google Scholar] [CrossRef]
- Enebe, M. C.; Babalola, O. O. The Impact of Microbes in the Orchestration of Plants’ Resistance to Biotic Stress: A Disease Management Approach. Appl. Microbiol. Biotechnol. 2019, 103, 9–25. [Google Scholar] [CrossRef]
- Panth, M.; Hassler, S. C.; Baysal-Gurel, F. Methods for Management of Soilborne Diseases in Crop Production. Agric. 2020, 10. [Google Scholar] [CrossRef]
- Ueki, A.; Kaku, N.; Ueki, K. Role of Anaerobic Bacteria in Biological Soil Disinfestation for Elimination of Soil-Borne Plant Pathogens in Agriculture. Appl. Microbiol. Biotechnol. 2018, 102, 6309–6318. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, C. M.; Tariq Mahmood, M.; Ahmad, M.; Ali, I.; Kaukab, S.; Shafiq, M.; Saleem, M.; Iqbal, U. Fusarium Wilt’s Pathogenic Studies and Disease Management: A Review. Genet. Mol. Res. 2020, 19. [Google Scholar]
- Katan, J. Diseases Caused by Soilborne Pathogens: Biology, Management and Challenges. J. Plant Pathol. 2017, 99, 305–315. [Google Scholar]
- Yuen, G. Y.; Schroth, M. N.; Weinhold, A. R.; Hancock, J. G. Effects of Soil Fumigation with Methyl Bromide and Chloropicrin on Root Health and Yield of Strawberry. Plant Dis. 1991, 75, 416–420. [Google Scholar] [CrossRef]
- White, P. J.; Brown, P. H. Plant Nutrition for Sustainable Development and Global Health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef]
- Sugiyama, A.; Vivanco, J. M.; Jayanty, S. S.; Manter, D. K. Pyrosequencing Assessment of Soil Microbial Communities in Organic and Conventional Potato Farms. Plant Dis. 2010, 94, 1329–1335. [Google Scholar] [CrossRef]
- Qing, W.; Daqi, Z.; Wensheng, F.; Yuan, L. I.; Qiuxia, W.; Dongdong, Y. A. N.; Aocheng, C. A. O. Research Progress on the Effect of Soil Fumigation on Soil Nitrogen Cycles and Functional Microorganisms. Chinese J. Pestic. Sci. 2021, 23, 1063–1072. [Google Scholar]
- Larson, S.; Capel, P.; Majewski, M. Pesticides in Surface Waters-Distribution, Trends, and Governing Factors. In Series of Pesticides in Hydrologic System; Gillion, R., Ed.; Ann Arbor Press: Chelsea, Michigan, 1997. [Google Scholar]
- Buccini, J. The Development of a Global Treaty on Persistent Organic Pollutants (POPs). In Tha handbook of environmental Chemistry; Barceló, D., Kostianoy, A.., Eds.; Springer Science & Business Media, 2003; pp 13–30.
- Sadana, D.; Didwania, N. Integrated Disease Management of Bull’s Eye Pathogen Infecting Lycopersicon Esculentum (Tomato). J. Microbiol. Biotechnol. Food Sci. 2019, 9, 58–62. [Google Scholar] [CrossRef]
- Sukul, P. Enzymatic Activities and Microbial Biomass in Soil as Influenced by Metalaxyl Residues. Soil Biol. Biochem. 2006, 38, 320–326. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, L.; Wang, X.; Wang, J.; Wang, J. Impact of Repeated Applications of Metalaxyl on Its Dissipation and Microbial Community in Soil. Water. Air. Soil Pollut. 2015, 226, 1–14. [Google Scholar] [CrossRef]
- Yen, J. H.; Chang, J. S.; Huang, P. J.; Wang, Y. S. Effects of Fungicides Triadimefon and Propiconazole on Soil Bacterial Communities. J. Environ. Sci. Heal. - Part B Pestic. Food Contam. Agric. Wastes 2009, 44, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Tang, F. F.; Zhou, W.; Cao, Z. Y.; Wang, D. D.; Liu, K. L.; Wu, X. W.; Yu, Y. L. Persistence of Repeated Triadimefon Application and Its Impact on Soil Microbial Functional Diversity. J. Environ. Sci. Heal. - Part B Pestic. Food Contam. Agric. Wastes 2012, 47, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, B.; Wang, Q.; Li, Y.; Fang, W.; Yan, D.; Guo, M.; Cao, A. Effect of Fumigation with Chloropicrin on Soil Bacterial Communities and Genes Encoding Key Enzymes Involved in Nitrogen Cycling. Environ. Pollut. 2017, 227, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.; Rigby, M.; Burkholder, J. .; Fernandez, R. P.; Froidevaux, L.; Hall, B.; Hossaini, R.; Saito, T.; Vollmer, M. K.; Yao, B. Update on Ozone-Depleting Substances (ODSs) and Other Gases of Interest to the Montreal Protocol. In Scientific Assessment of Ozone Depletion; Liang, Q., Reimann, S., Eds.; World Meteorological Organization, 2019; pp 1–91.
- Agostini, M. G.; Roesler, I.; Bonetto, C.; Ronco, A. E.; Bilenca, D. Pesticides in the Real World: The Consequences of GMO-Based Intensive Agriculture on Native Amphibians. Biol. Conserv. 2020, 241, 108355. [Google Scholar] [CrossRef]
- Tong, L.; Nieh, J. C.; Tosi, S. Combined Nutritional Stress and a New Systemic Pesticide (Flupyradifurone, Sivanto®) Reduce Bee Survival, Food Consumption, Flight Success, and Thermoregulation. Chemosphere 2019, 237, 124408. [Google Scholar] [CrossRef] [PubMed]
- Straub, L.; Villamar-Bouza, L.; Bruckner, S.; Chantawannakul, P.; Kolari, E.; Maitip, J.; Vidondo, B.; Neumann, P.; Williams, G. R. Negative Effects of Neonicotinoids on Male Honeybee Survival, Behaviour and Physiology in the Field. J. Appl. Ecol. 2021, 58, 2515–2528. [Google Scholar] [CrossRef]
- Toledo-Hernández, E.; Peña-Chora, G.; Hernández-Velázquez, V. M.; Lormendez, C. C.; Toribio-Jiménez, J.; Romero-Ramírez, Y.; León-Rodríguez, R. The Stingless Bees (Hymenoptera: Apidae: Meliponini): A Review of the Current Threats to Their Survival. Apidologie 2022, 53. [Google Scholar] [CrossRef]
- Hamadamin, A. Y.; Hassan, K. I. Gas Chromatography–Mass Spectrometry Based Sensitive Analytical Approach to Detect and Quantify Non-Polar Pesticides Accumulated in the Fat Tissues of Domestic Animals. Saudi J. Biol. Sci. 2020, 27, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Boudh, S.; Singh, J. S. Pesticide Contamination: Environmental Problems and Remediation Strategies. In Emerging and Eco-friendly Approaches for Waste Management; Bharagava, R. N., Chowdhary, P., Eds.; Springer, 2018; pp 245–269. [CrossRef]
- Rajiv, K. S.; George, H.; Upendra, P.; Chandrajeet, K. Embarking on Second Green Revolution by Vermiculture for Production of Chemical Free Organic Foods, Protection of Crops and Farm Soils and Elimination of Deadly Agrochemicals from Earth: Meeting the Challenges of Food Security of 21st Century by Earthworm. In Agricultural Research Updates; Gorawala, P., Mandhatri, S., Eds.; Nova Science Publishers Inc: New York, 2016; pp. 1–49. [Google Scholar]
- U.S. Environmental Proteccion Agency. Waste minimization priority chemicals and chemical fact sheets website. Priority Chemicals. Available online: https://archive.epa.gov/epawaste/hazard/wastemin/web/html/priority.html (accessed on 18 June 2020).
- Wu, S.; Jin, C.; Wang, Y.; Fu, Z.; Jin, Y. Exposure to the Fungicide Propamocarb Causes Gut Microbiota Dysbiosis and Metabolic Disorder in Mice. Environ. Pollut. 2018, 237, 775–783. [Google Scholar] [CrossRef]
- Sturz, A. V; Christie, B. R.; Nowak, J. Bacterial Endophytes: Potential Role in Developing Sustainable Systems of Crop Production. CRC. Crit. Rev. Plant Sci. 2000, 19, 1–30. [Google Scholar] [CrossRef]
- Bouzoumita, A.; Metoui, M.; Jemni, M.; Kabaeir, N.; Belhouchette, K.; Ferchichi, A. The Efficacy of Various Bacterial Organisms for Biocontrol of Fusarium Root Rot of Olive in Tunisia. Polish J. Environ. Stud. 2020, 29, 11–16. [Google Scholar] [CrossRef]
- Sajeena, A.; Nair, D. S.; Sreepavan, K. Non-Pathogenic Fusarium Oxysporum as a Biocontrol Agent. Indian Phytopathol. 2020, 73, 177–183. [Google Scholar] [CrossRef]
- Barriuso, J.; Ramos Solano, B.; Lucas, J. A.; Lobo, A. P.; García-Villaraco, A.; Gutiérrez Mañero, F. J. Ecology, Genetic Diversity and Screening Strategies of Plant Growth Promoting Rhizobacteria (PGPR). In Plant-Bacteria Interactions: Strategies and Techniques to Promote Plant Growth; Ahmad, I., Pitchel, J., Hayat, S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA, 2008; pp 1–17. [CrossRef]
- Bhattacharjee, R. B.; Singh, A.; Mukhopadhyay, S. N. Use of Nitrogen-Fixing Bacteria as Biofertiliser for Non-Legumes: Prospects and Challenges. Appl. Microbiol. Biotechnol. 2008, 80, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M.; Bahmanyar, M. A.; Pirdashti, H.; Esmaili, M. A. Effect of Phosphate Solubilization Microorganisms (PSM) and Plant Growth Promoting Rhizobacteria (PGPR) on Yield and Yield Components of Corn (Zea Mays L.). World Acad. Sci. Eng. Technol. 2009, 49, 90–92. [Google Scholar]
- Ngalimat, M. S.; Hata, E. M.; Zulperi, D.; Ismail, S. I.; Ismail, M. R.; Zainudin, N. A. I. M.; Saidi, N. B.; Yusof, M. T. Plant Growth-Promoting Bacteria as an Emerging Tool to Manage Bacterial Rice Pathogens. Microorganisms 2021, 9, 1–23. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S. N.; Kalam, S.; Sayyed, R. Z.; Reddy, M. S.; Enshasy, H. El. Plant Growth Promoting Rhizobacteria (Pgpr) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1–20. [Google Scholar] [CrossRef]
- ALKahtani, M. D. F.; Fouda, A.; Attia, K. A.; Al-Otaibi, F.; Eid, A. M.; El-Din Ewais, E.; Hijri, M.; St-Arnaud, M.; El-Din Hassan, S.; Khan, N.; Hafez, Y. M.; Abdelaal, K. A. A. Isolation and Characterization of Plant Growth Promoting Endophytic Bacteria from Desert Plants and Their Application as Bioinoculants for Sustainable Agriculture. Agronomy 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Kloepper, J. W.; Lifshitz, R.; Zablotovicz, R. M. Free-Living Bacteria Inocula for Enhancing Crop Productivity. Trends Biotechnol. 1989, 7, 39–44. [Google Scholar] [CrossRef]
- Berg, G.; Smalla, K. Plant Species and Soil Type Cooperatively Shape the Structure and Function of Microbial Communities in the Rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef]
- Jiao, X.; Takishita, Y.; Zhou, G.; Smith, D. L. Plant Associated Rhizobacteria for Biocontrol and Plant Growth Enhancement. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Katiyar, V.; Goel, R. Siderophore Mediated Plant Growth Promotion at Low Temperature by Mutant of Fluorescent Pseudomonad. Plant Growth Regul. 2004, 42, 239–244. [Google Scholar] [CrossRef]
- Brazelton, J. N.; Pfeufer, E. E.; Sweat, T. A.; McSpadden Gardener, B. B.; Coenen, C. 2,4-Diacetylphloroglucinol Alters Plant Root Development. Mol. Plant-Microbe Interact. 2008, 21, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Shi, C. L.; Park, H. B.; Lee, J. S.; Ryu, S.; Ryu, C. M. Inhibition of Primary Roots and Stimulation of Lateral Root Development in Arabidopsis Thaliana by the Rhizobacterium Serratia Marcescens 90-166 Is through Both Auxin-Dependent and -Independent Signaling Pathways. Mol. Cells 2010, 29, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Liliane, T. N.; Charles, M. S. Factors Affecting Yield of Crops. In Agronomy climate change and food security; Amanullah, Ed.; InTechOpen: London, 2020; pp. 9–25. [Google Scholar]
- Ficke, A.; Cowger, C.; Bergstrom, G.; Brodal, G. Understanding Yield Loss and Pathogen Biology to Improve Disease Management: Septoria Nodorum Blotch - A Case Study in Wheat. Plant Dis. 2018, 102, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Brittenham, G. . New Advances in Iron Metabolism, Iron Deficiency, and Iron Overload. Curr. Opin. Hematol. 1994, 1, 101–106. [Google Scholar] [PubMed]
- Pierre, J. L.; Fontecave, M.; Crichton, R. R. Chemistry for an Essential Biological Process: The Reduction of Ferric Iron. BioMetals 2002, 15, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Miller, G. W.; Huang, I. J.; Welkie, G. W.; Pushnik, J. C. Function of Iron in Plants with Special Emphasis on Chloroplasts and Photosynthetic Activity. In Iron Nutrition in Soils and Plants; Abadia, J., Ed.; Kluwer Academic Publishers: Dordrecht, 1995; pp. 19–28. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Sun, W.; Wang, T. The Adaptive Mechanism of Plants to Iron Deficiency via Iron Uptake, Transport, and Homeostasis. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Tsai, H. H.; Schmidt, W. One Way. Or Another? Iron Uptake in Plants. New Phytol. 2017, 214, 500–505. [Google Scholar] [CrossRef]
- Ansari, R.A.; Mahmood, I.; Rizvi, R.; Sumbul, A.; Safiuddin. Augmentation of Soil Health and Crop Productivity. In Probiotics in Agroecosystem; Kumar, V., Kumar, M., Sharma, S., Prasad, R., Eds.; Springer: Singapore, 2017; pp. 291–312. [Google Scholar] [CrossRef]
- Timofeeva, A. M.; Galyamova, M. R.; Sedykh, S. E. Bacterial Siderophores: Classification, Biosynthesis, Perspectives of Use in Agriculture. Plants 2022, 11. [Google Scholar] [CrossRef]
- Nithyapriya, S.; Lalitha, S.; Sayyed, R. Z.; Reddy, M. S.; Dailin, D. J.; El Enshasy, H. A.; Luh Suriani, N.; Herlambang, S. Production, Purification, and Characterization of Bacillibactin Siderophore of Bacillus Subtilis and Its Application for Improvement in Plant Growth and Oil Content in Sesame. Sustain. 2021, 13. [Google Scholar] [CrossRef]
- Yu, S.; Teng, C.; Bai, X.; Liang, J.; Song, T.; Dong, L.; Jin, Y.; Qu, J. Optimization of Siderophore Production by Bacillus Sp. PZ-1 and Its Potential Enhancement of Phytoextration of PB from Soil. J. Microbiol. Biotechnol. 2017, 27, 1500. [Google Scholar] [CrossRef]
- Rizzi, A.; Roy, S.; Bellenger, J. P.; Beauregard, P. B. Iron Homeostasis in Bacillus Subtilis Requires Siderophore Production and Biofilm Formation. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Doshi, H. .; Thakur, M.. Bacillus Spp.: A Prolific Siderophore Producer. In Bacilli and Agrobiotechnology; Rahman, M., Pandey, P., Jha, C., Aeron, A., Eds.; Springer, 2016; pp 309–323. [CrossRef]
- Aznar, A.; Dellagi, A. New Insights into the Role of Siderophores as Triggers of Plant Immunity: What Can We Learn from Animals? J. Exp. Bot. 2015, 66, 3001–3010. [Google Scholar] [CrossRef]
- Sadeghi, A.; Karimi, E.; Dahaji, P. A.; Javid, M. G.; Dalvand, Y.; Askari, H. Plant Growth Promoting Activity of an Auxin and Siderophore Producing Isolate of Streptomyces under Saline Soil Conditions. World J. Microbiol. Biotechnol. 2012, 28, 1503–1509. [Google Scholar] [CrossRef]
- Verma, V. C.; Singh, S. K.; Prakash, S. Bio-Control and Plant Growth Promotion Potential of Siderophore Producing Endophytic Streptomyces from Azadirachta Indica A. Juss. J. Basic Microbiol. 2011, 51, 550–556. [Google Scholar] [CrossRef]
- Paramanandham, P.; Rajkumari, J.; Pattnaik, S.; Busi, S. Biocontrol Potential against Fusarium Oxysporum f. Sp. Lycopersici and Alternaria Solani and Tomato Plant Growth Due to Plant Growth–Promoting Rhizobacteria. Int. J. Veg. Sci. 2017, 23, 294–303. [Google Scholar] [CrossRef]
- Siddiqui, Z. A.; Baghel, G.; Akhtar, M. S. Biocontrol of Meloidogyne Javanica by Rhizobium and Plant Growth-Promoting Rhizobacteria on Lentil. World J. Microbiol. Biotechnol. 2007, 23, 435–441. [Google Scholar] [CrossRef]
- Dimopoulou, A.; Theologidis, I.; Benaki, D.; Koukounia, M.; Zervakou, A.; Tzima, A.; Diallinas, G.; Hatzinikolaou, D. G.; Skandalis, N. Direct Antibiotic Activity of Bacillibactin Broadens the Biocontrol Range of Bacillus Amyloliquefaciens MBI600. mSphere 2021, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rais, A.; Shakeel, M.; Hafeez, F. Y.; Hassan, M. N. Plant Growth Promoting Rhizobacteria Suppress Blast Disease Caused by Pyricularia Oryzae and Increase Grain Yield of Rice. BioControl 2016, 61, 769–780. [Google Scholar] [CrossRef]
- Ait-Kaki, A.; Kacem-Chaouche, N.; Ongena, M.; Kara-Ali, M.; Dehimat, L.; Kahlat, K.; Thonart, P. In Vitro and in Vivo Characterization of Plant Growth Promoting Bacillus Strains Isolated from Extreme Environments of Eastern Algeria. Appl. Biochem. Biotechnol. 2014, 172, 1735–1746. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M. N.; Osborn, A. M.; Hafeez, F. Y. Molecular and Biochemical Characterization of Surfactin Producing Bacillus Species Antagonistic to Colletotrichum Falcatum Went Causing Sugarcane Red Rot. African J. Microbiol. Res. 2010, 4, 2137–2142. [Google Scholar]
- Caulier, S.; Gillis, A.; Colau, G.; Licciardi, F.; Liépin, M.; Desoignies, N.; Modrie, P.; Legrève, A.; Mahillon, J.; Bragard, C. Versatile Antagonistic Activities of Soil-Borne Bacillus Spp. and Pseudomonas Spp. against Phytophthora Infestans and Other Potato Pathogens. Front. Microbiol. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Li, S.; Li, S. Y.; Zhang, H.; Jiang, M. The Siderophore-Producing Bacterium, Bacillus Siamensis Gxun-6, Has an Antifungal Activity against Fusarium Oxysporum and Promotes the Growth of Banana. Egypt. J. Biol. Pest Control 2022, 32. [Google Scholar] [CrossRef]
- Kamensky, M.; Ovadis, M.; Chet, I.; Chernin, L. Soil-Borne Strain IC14 of Serratia Plymuthica with Multiple Mechanisms of Antifungal Activity Provides Biocontrol of Botrytis Cinerea and Sclerotinia Sclerotiorum Diseases. Soil Biol. Biochem. 2003, 35, 323–331. [Google Scholar] [CrossRef]
- Khoa, N. Đ.; Giàu, N. Đ. N.; Tuấn, T. Q. Effects of Serratia Nematodiphila CT-78 on Rice Bacterial Leaf Blight Caused by Xanthomonas Oryzae Pv. Oryzae. Biol. Control 2016, 103, 1–10. [Google Scholar] [CrossRef]
- Tortora, M. L.; Díaz-Ricci, J. C.; Pedraza, R. O. Azospirillum Brasilense Siderophores with Antifungal Activity against Colletotrichum Acutatum. Arch. Microbiol. 2011, 193, 275–286. [Google Scholar] [CrossRef]
- Bendaha, M. E. A.; Belaouni, H. A. Tomato Growth and Resistance Promotion by Enterobacter Hormaechei Subsp. Steigerwaltii EB8D. Arch. Phytopathol. Plant Prot. 2019, 52, 318–332. [Google Scholar] [CrossRef]
- Naureen, Z.; Hafeez, F. Y.; Hussain, J.; Harrasi, A. Al; Bouqellah, N.; Roberts, M. R. Suppression of Incidence of Rhizoctonia Solani in Rice By Siderophore Producing Rhizobacterial Strains Based on Competition for Iron. Eur. Sci. J. 2015, 11, 186–207. [Google Scholar]
- Gechemba, O. R.; Julius, M.; Makonde, H. M.; Bubambula, N. L. M.; Matiru, V. N. Potentially Beneficial Rhizobacteria Associated with Banana Plants in Juja Kenya. Res. Rev. J. Microbiol. Biotechnol. 2016, 5, 19–25. [Google Scholar]
- Ahemad, M.; Khan, M. S. Effects of Insecticides on Plant-Growth-Promoting Activities of Phosphate Solubilizing Rhizobacterium Klebsiella Sp. Strain PS19. Pestic. Biochem. Physiol. 2011, 100, 51–56. [Google Scholar] [CrossRef]
- El_Komy, M. H.; Hassouna, M. G.; Abou-Taleb, E. M.; Al-Sarar, A. S.; Abobakr, Y. A Mixture of Azotobacter, Azospirillum, and Klebsiella Strains Improves Root-Rot Disease Complex Management and Promotes Growth in Sunflowers in Calcareous Soil. Eur. J. Plant Pathol. 2020, 156, 713–726. [Google Scholar] [CrossRef]
- Liu, X.; Jia, J.; Atkinson, S.; Cámara, M.; Gao, K.; Li, H.; Cao, J. Biocontrol Potential of an Endophytic Serratia Sp. G3 and Its Mode of Action. World J. Microbiol. Biotechnol. 2010, 26, 1465–1471. [Google Scholar] [CrossRef]
- Thomas, M. S. Iron Acquisition Mechanisms of the Burkholderia Cepacia Complex. BioMetals 2007, 20, 431–452. [Google Scholar] [CrossRef] [PubMed]
- Araújo, W. L.; Creason, A. L.; Mano, E. T.; Camargo-Neves, A. A.; Minami, S. N.; Chang, J. H.; Loper, J. E. Genome Sequencing and Transposon Mutagenesis of Burkholderia Seminalis TC3.4.2R3 Identify Genes Contributing to Suppression of Orchid Necrosis Caused by B. Gladioli. Mol. Plant-Microbe Interact. 2016, 29, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, V. C.; Barbosa, L. de O.; Andrade, J. P.; Soares, A. C. F.; de Souza, J. T.; Marbach, P. A. S. Burkholderia Isolates from a Sand Dune Leaf Litter Display Biocontrol Activity against the Bole Rot Disease of Agave Sisalana. Biol. Control 2017, 112, 41–48. [Google Scholar] [CrossRef]
- Pellegrini, M.; Pagnani, G.; Bernardi, M.; Mattedi, A.; Spera, D. M.; Del Gallo, M. Cell-Free Supernatants of Plant Growth-Promoting Bacteria: A Review of Their Use as Biostimulant and Microbial Biocontrol Agents in Sustainable Agriculture. Sustainability 2020, 12, 1–22. [Google Scholar] [CrossRef]
- Sayyed, R. Z.; Chincholkar, S. B. Siderophore-Producing Alcaligenes Feacalis Exhibited More Biocontrol Potential Vis-à-Vis Chemical Fungicide. Curr. Microbiol. 2009, 58, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C. Microbial Siderophores: Production, Detection and Application in Agriculture and Environment. Endocytobiosis Cell Res. 2016, 27, 7–16. [Google Scholar]
- Soares, E. V. Perspective on the Biotechnological Production of Bacterial Siderophores and Their Use. Appl. Microbiol. Biotechnol. 2022, 106, 3985–4004. [Google Scholar] [CrossRef]
- Tariq, M.; Noman, M.; Ahmed, T.; Hameed, A.; Manzoor, N.; Zafar, M. Antagonistic Features Displayed by Plant Growth Promoting Rhizobacteria (PGPR): A Review. J. Plant Sci. Phytopathol. 2017, 1, 038–043. [Google Scholar] [CrossRef]
- Prasad, K. Utilization of Siderophore Producing Plant Growth Promoting Rhizobacteria to Improve Crucial Nourishment and Management of Phytopathogen in Cash Crops for Sustainable Development. Am. J. Sci. Eng. Res. 2022, 5, 15–23. [Google Scholar]
- Shafi, J.; Tian, H.; Ji, M. Bacillus Species as Versatile Weapons for Plant Pathogens: A Review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Ghazy, N.; El-Nahrawy, S. Siderophore Production by Bacillus Subtilis MF497446 and Pseudomonas Koreensis MG209738 and Their Efficacy in Controlling Cephalosporium Maydis in Maize Plant. Arch. Microbiol. 2021, 203, 1195–1209. [Google Scholar] [CrossRef]
- Sasirekha, B.; Srividya, S. Siderophore Production by Pseudomonas Aeruginosa FP6, a Biocontrol Strain for Rhizoctonia Solani and Colletotrichum Gloeosporioides Causing Diseases in Chilli. Agric. Nat. Resour. 2016, 50, 250–256. [Google Scholar] [CrossRef]
- Dimkpa, C. O.; Merten, D.; Svatoš, A.; Büchel, G.; Kothe, E. Metal-Induced Oxidative Stress Impacting Plant Growth in Contaminated Soil Is Alleviated by Microbial Siderophores. Soil Biol. Biochem. 2009, 41, 154–162. [Google Scholar] [CrossRef]
- Becker, J. O.; Cook, R. J. Role of Siderophores in Suppression of Pythium Species and Production of Increased-Growth Response of Wheat by Fluorescent Pseudomonads. Phytopathology, 1988, 78, 778–782. [Google Scholar] [CrossRef]
- Sharma, A.; Johri, B. N.; Sharma, A. K.; Glick, B. R. Plant Growth-Promoting Bacterium Pseudomonas Sp. Strain GRP3 Influences Iron Acquisition in Mung Bean (Vigna Radiata L. Wilzeck). Soil Biol. Biochem. 2003, 35, 887–894. [Google Scholar] [CrossRef]
- Syed, A.; Elgorban, A. M.; Bahkali, A. H.; Eswaramoorthy, R.; Iqbal, R. K.; Danish, S. Metal-Tolerant and Siderophore Producing Pseudomonas Fluorescence and Trichoderma Spp. Improved the Growth, Biochemical Features and Yield Attributes of Chickpea by Lowering Cd Uptake. Sci. Rep. 2023, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J. W.; Leong, J.; Teintze, M.; Schroth, M. N. Enhanced Plant Growth by Siderophores Produced by Plant Growth-Promoting Rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Rungin, S.; Indananda, C.; Suttiviriya, P.; Kruasuwan, W.; Jaemsaeng, R.; Thamchaipenet, A. Plant Growth Enhancing Effects by a Siderophore-Producing Endophytic Streptomycete Isolated from a Thai Jasmine Rice Plant (Oryza Sativa L. Cv. KDML105). Antonie van Leeuwenhoek, Int. J. Gen. Mol. Microbiol. 2012, 102, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ai, C.; Xin, L.; Zhou, G. The Siderophore-Producing Bacterium, Bacillus Subtilis CAS15, Has a Biocontrol Effect on Fusarium Wilt and Promotes the Growth of Pepper. Eur. J. Soil Biol. 2011, 47, 138–145. [Google Scholar] [CrossRef]
- Jikare, A. M.; Chavan, M. D. SiderophorepProduced by Bacillus Shackletonii. Gn-09 and Showed Its Plant Growth Promoting Activity. Int. J. Pharm. Biol. Sci. 2013, 3, 198–202. [Google Scholar]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The Significance of Bacillus Spp. In Disease Suppression and Growth Promotion of Field and Vegetable Crops. Microorganisms 2020, 8, 1–19. [Google Scholar] [CrossRef]
- Zhang, S.; Deng, Z.; Borham, A.; Ma, Y.; Wang, Y.; Hu, J.; Wang, J.; Bohu, T. Significance of Soil Siderophore-Producing Bacteria in Evaluation and Elevation of Crop Yield. Horticulturae 2023, 9, 1–10. [Google Scholar] [CrossRef]
- Santoyo, G.; del Orozco-Mosqueda, M. C.; Govindappa, M. Mechanisms of Biocontrol and Plant Growth-Promoting Activity in Soil Bacterial Species of Bacillus and Pseudomonas: A Review. Biocontrol Sci. Technol. 2012, 22, 855–872. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant Growth Promoting Bacteria in Agriculture: Two Sides of a Coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Niu, B.; Wang, W.; Yuan, Z.; Sederoff, R. R.; Sederoff, H.; Chiang, V. L.; Borriss, R. Microbial Interactions Within Multiple-Strain Biological Control Agents Impact Soil-Borne Plant Disease. Front. Microbiol. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Silby, M. W.; Winstanley, C.; Godfrey, S. A. C.; Levy, S. B.; Jackson, R. W. Pseudomonas Genomes: Diverse and Adaptable. FEMS Microbiol. Rev. 2011, 35, 652–680. [Google Scholar] [CrossRef]
- Wang, X.; Mavrodi, D. V.; Ke, L.; Mavrodi, O. V.; Yang, M.; Thomashow, L. S.; Zheng, N.; Weller, D. M.; Zhang, J. Biocontrol and Plant Growth-Promoting Activity of Rhizobacteria from Chinese Fields with Contaminated Soils. Microb. Biotechnol. 2015, 8, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Nandi, M.; Selin, C.; Brawerman, G.; Fernando, W. G. D.; de Kievit, T. Hydrogen Cyanide, Which Contributes to Pseudomonas Chlororaphis Strain PA23 Biocontrol, Is Upregulated in the Presence of Glycine. Biol. Control 2017, 108, 47–54. [Google Scholar] [CrossRef]
- Strano, C. P.; Bella, P.; Licciardello, G.; Caruso, A.; Catara, V. Role of Secondary Metabolites in the Biocontrol Activity of Pseudomonas Corrugata and Pseudomonas Mediterranea. Eur. J. Plant Pathol. 2017, 149, 103–115. [Google Scholar] [CrossRef]
- Biessy, A.; Filion, M. Phloroglucinol Derivatives in Plant-Beneficial Pseudomonas Spp.: Biosynthesis, Regulation, and Functions. Metabolites 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Biessy, A.; Filion, M. Phenazines in Plant-Beneficial Pseudomonas Spp.: Biosynthesis, Regulation, Function and Genomics. Environ. Microbiol. 2018, 20, 3905–3917. [Google Scholar] [CrossRef]
- Serafim, B.; Bernardino, A. R.; Freitas, F.; Torres, C. A. V. Recent Developments in the Biological Activities, Bioproduction, and Applications of Pseudomonas Spp. Phenazines. Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Oni, F. E.; Geudens, N.; Onyeka, J. T.; Olorunleke, O. F.; Salami, A. E.; Omoboye, O. O.; Arias, A. A.; Adiobo, A.; De Neve, S.; Ongena, M.; Martins, J. C.; Höfte, M. Cyclic Lipopeptide-Producing Pseudomonas Koreensis Group Strains Dominate the Cocoyam Rhizosphere of a Pythium Root Rot Suppressive Soil Contrasting with P. Putida Prominence in Conducive Soils. Environ. Microbiol. 2020, 22, 5137–5155. [Google Scholar] [CrossRef] [PubMed]
- Geudens, N.; Martins, J. C. Cyclic Lipodepsipeptides from Pseudomonas Spp. - Biological Swiss-Army Knives. Front. Microbiol. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Bajpai, A.; Singh, B.; Joshi, S.; Johri, B. N. Production and Characterization of an Antifungal Compound from Pseudomonas Protegens Strain W45. Proc. Natl. Acad. Sci. India Sect. B - Biol. Sci. 2018, 88, 1081–1089. [Google Scholar] [CrossRef]
- Hansen, M. L.; Wibowo, M.; Jarmusch, S. A.; Larsen, T. O.; Jelsbak, L. Sequential Interspecies Interactions Affect Production of Antimicrobial Secondary Metabolites in Pseudomonas Protegens DTU9.1. ISME J. 2022, 16, 2680–2690. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, A.; De Stradis, A.; Lo Cantore, P.; Iacobellis, N. S. Biocide Effects of Volatile Organic Compounds Produced by Potential Biocontrol Rhizobacteria on Sclerotinia Sclerotiorum. Front. Microbiol. 2015, 6, 1056. [Google Scholar] [CrossRef]
- Kalantari, S.; Marefat, A.; Naseri, B.; Hemmati, R. Improvement of Bean Yield and Fusarium Root Rot Biocontrol Using Mixtures of Bacillus, Pseudomonas and Rhizobium. Trop. Plant Pathol. 2018, 43, 499–505. [Google Scholar] [CrossRef]
- Mehmood, N.; Saeed, M.; Zafarullah, S.; Hyder, S.; Rizvi, Z. F.; Gondal, A. S.; Jamil, N.; Iqbal, R.; Ali, B.; Ercisli, S.; Kupe, M. Multifaceted Impacts of Plant-Beneficial Pseudomonas Spp. in Managing Various Plant Diseases and Crop Yield Improvement. ACS Omega 2023, 8, 22296–22315. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Santoyo, G. Action Mechanisms, Biodiversity, and Omics Approaches in Biocontrol and Plant Growth-Promoting Pseudomonas: An Updated Review. Biocontrol Sci. Technol. 2022, 32, 527–550. [Google Scholar] [CrossRef]
- Raio, A.; Puopolo, G. Pseudomonas Chlororaphis Metabolites as Biocontrol Promoters of Plant Health and Improved Crop Yield. World J. Microbiol. Biotechnol. 2021, 37, 1–8. [Google Scholar] [CrossRef]
- Liu, K.; Newman, M.; McInroy, J. A.; Hu, C. H.; Kloepper, J. W. Selection and Assessment of Plant Growth-Promoting Rhizobacteria for Biological Control of Multiple Plant Diseases. Phytopathology 2017, 107, 928–936. [Google Scholar] [CrossRef]
- Vitullo, D.; Di Pietro, A.; Romano, A.; Lanzotti, V.; Lima, G. Role of New Bacterial Surfactins in the Antifungal Interaction between Bacillus Amyloliquefaciens and Fusarium Oxysporum. Plant Pathol. 2012, 61, 689–699. [Google Scholar] [CrossRef]
- Béchet, M.; Caradec, T.; Hussein, W.; Abderrahmani, A.; Chollet, M.; Leclére, V.; Dubois, T.; Lereclus, D.; Pupin, M.; Jacques, P. Structure, Biosynthesis, and Properties of Kurstakins, Nonribosomal Lipopeptides from Bacillus Spp. Appl. Microbiol. Biotechnol. 2012, 95, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Chenniappan, C.; Narayanasamy, M.; Daniel, G. M.; Ramaraj, G. B.; Ponnusamy, P.; Sekar, J.; Vaiyapuri Ramalingam, P. Biocontrol Efficiency of Native Plant Growth Promoting Rhizobacteria against Rhizome Rot Disease of Turmeric. Biol. Control 2019, 129, 55–64. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, C. K.; Ma, L.; Zhang, K. Q.; Duan, C. Q.; Mo, M. H. Characterisation of Volatiles Produced from Bacillus Megaterium YFM3.25 and Their Nematicidal Activity against Meloidogyne Incognita. Eur. J. Plant Pathol. 2010, 126, 417–422. [Google Scholar] [CrossRef]
- Peng, D.; Chai, L.; Wang, F.; Zhang, F.; Ruan, L.; Sun, M. Synergistic Activity between Bacillus Thuringiensis Cry6Aa and Cry55Aa Toxins against Meloidogyne Incognita. Microb. Biotechnol. 2011, 4, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Vial, L.; Groleau, M. C.; Dekimpe, V.; Deziel, E. Burkholderia Diversity and Versatility: An Inventory of the Extracellular Products. J. Microbiol. Biotechnol. 2007, 17, 1407–1428. [Google Scholar] [CrossRef]
- da Silva Araújo, D. F.; Araújo, W. L.; Eberlin, M. N. Potential of Burkholderia Seminalis TC3.4.2R3 as Biocontrol Agent Against Fusarium Oxysporum Evaluated by Mass Spectrometry Imaging. J. Am. Soc. Mass Spectrom. 2017, 28, 901–907. [Google Scholar] [CrossRef]
- Dey, S.; Dutta, P.; Majumdar, S. Biological Control of Macrophomina Phaseolina in Vigna Mungo L. by Endophytic Klebsiella Pneumoniae HR1. Jordan J. Biol. Sci. 2019, 12, 219–227. [Google Scholar] [CrossRef]
- Mandal, S. M.; Sharma, S.; Pinnaka, A. K.; Kumari, A.; Korpole, S. Isolation and Characterization of Diverse Antimicrobial Lipopeptides Produced by Citrobacter and Enterobacter. BMC Microbiol. 2013, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial Features of Plant Growth-Promoting Rhizobacteria for Improving Plant Growth and Health in Challenging Conditions: A Methodical Review. Sci. Total Environ. 2020, 743. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Kabir, S.; Shabbir, U.; Batool, R. Plant Growth Promoting Rhizobacteria in Sustainable Agriculture: From Theoretical to Pragmatic Approach. Symbiosis 2019, 78, 115–123. [Google Scholar] [CrossRef]
- Aeron, A.; Khare, E.; Jha, C. K.; Meena, V. S.; Aziz, S. M. A.; Islam, M. T.; Kim, K.; Meena, S. K.; Pattanayak, A.; Rajashekara, H.; Dubey, R. C.; Maurya, B. R.; Maheshwari, D. K.; Saraf, M.; Choudhary, M.; Verma, R.; Meena, H. N.; Subbanna, A. R. N. S.; Parihar, M.; Shukla, S.; Muthusamy, G.; Bana, R. S.; Bajpai, V. K.; Han, Y. K.; Rahman, M.; Kumar, D.; Singh, N. P.; Meena, R. K. Revisiting the Plant Growth-Promoting Rhizobacteria: Lessons from the Past and Objectives for the Future. Arch. Microbiol. 2020, 202, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Julian, W. T.; Vasilchenko, A. V.; Shpindyuk, D. D.; Poshvina, D. V.; Vasilchenko, A. S. Bacterial-Derived Plant Protection Metabolite 2,4-Diacetylphloroglucinol: Effects on Bacterial Cells at Inhibitory and Subinhibitory Concentrations. Biomolecules 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Stepanov, A. A.; Poshvina, D. V.; Vasilchenko, A. S. 2,4-Diacetylphloroglucinol Modulates Candida Albicans Virulence. J. Fungi 2022, 8. [Google Scholar] [CrossRef]
- Maketon, C.; Fortuna, A. M.; Okubara, P. A. Cultivar-Dependent Transcript Accumulation in Wheat Roots Colonized by Pseudomonas Fluorescens Q8r1-96 Wild Type and Mutant Strains. Biol. Control 2012, 60, 216–224. [Google Scholar] [CrossRef]
- Naseby, D. C.; Lynch, J. M. Impact of Wild-type and Genetically Modified Pseudomonas Fluorescens on Soil Enzyme Activities and Microbial Population Structure in the Rhizosphere of Pea. Mol. Ecol. 1998, 75, 617–625. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Renoud, S.; Padilla, R.; Walker, V.; Muller, D.; PrigentCombaretlt, C. Differential Contribution of Plant-Beneficial Functions from Pseudomonas Kionensis Fl 13 to Root System Architecture Alterations in Arabidopsis Thaliana and Zea Mays. Mol. Plant-Microbe Interact. 2018, 31, 212–223. [Google Scholar] [CrossRef]
- De Leij, F. A. A. M.; Dixon-Hardy, J. E.; Lynch, J. M. Effect of 2,4-Diacetylphloroglucinol-Producing and Non-Producing Strains of Pseudomonas Fluorescens on Root Development of Pea Seedlings in Three Different Soil Types and Its Effect on Nodulation by Rhizobium. Biol. Fertil. Soils 2002, 35, 114–121. [Google Scholar] [CrossRef]
- Raudales, R. E.; Stone, E.; McSpadden Gardener, B. B. Seed Treatment with 2,4-Diacetylphloroglucinol-Producing Pseudomonads Improves Crop Health in Low-PH Soils by Altering Patterns of Nutrient Uptake. Phytopathology 2009, 99, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.; De Leij, F. A. A. M.; Lynch, J. M. Plant Mediated Interactions between Pseudomonas Fluorescens, Rhizobium Leguminosarum and Arbuscular Mycorrhizae on Pea. Lett. Appl. Microbiol. 1998, 26, 311–316. [Google Scholar] [CrossRef]
- Lugtenberg, B. J. J.; Bloemberg, G. V.; Van Brussel, A. A. N.; Kijne, J. W.; Thomas-Oates, J. E.; Spaink, H. P. Signals Involved in Nodulation and Nitrogen Fixation. In Nitrogen Fixation: Fundamentals and Applicantions; Tikhonovich, I. A., Provorov, N. A., Romanov, V. I., Newton, W. E., Eds.; Springer: Dordrecht, 1995; pp. 37–48. [Google Scholar] [CrossRef]
- Combes-Meynet, E.; Pothier, J. F.; Moënne-Loccoz, Y.; Prigent-Combaret, C. The Pseudomonas Secondary Metabolite 2,4-Diacetylphloroglucinol Is a Signal Inducing Rhizoplane Expression of Azospirillum Genes Involved in Plant-Growth Promotion. Mol. Plant-Microbe Interact. 2011, 24, 271–284. [Google Scholar] [CrossRef]
- Kim, H. S.; Sang, M. K.; Jung, H. W.; Jeun, Y. C.; Myung, I. S.; Kim, K. D. Identification and Characterization of Chryseobacterium Wanjuense Strain KJ9C8 as a Biocontrol Agent of Phytophthora Blight of Pepper. Crop Prot. 2012, 32, 129–137. [Google Scholar] [CrossRef]
- Park, M. S.; Jung, S. R.; Lee, K. H.; Lee, M. S.; Do, J. O.; Kim, S. B.; Bae, K. S. Chryseobacterium Soldanellicola Sp. Nov. and Chryseobacterium Taeanense Sp. Nov., Isolated from Roots of Sand-Dune Plants. Int. J. Syst. Evol. Microbiol. 2006, 56, 433–438. [Google Scholar] [CrossRef]
- Anand, A.; Chinchilla, D.; Tan, C.; Mène-Saffrané, L.; L’haridon, F.; Weisskopf, L. Contribution of Hydrogen Cyanide to the Antagonistic Activity of Pseudomonas Strains against Phytophthora Infestans. Microorganisms 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Voisard, C.; Keel, C.; Haas, D.; Dèfago, G. Cyanide Production by Pseudomonas Fluorescens Helps Suppress Black Root Rot of Tobacco under Gnotobiotic Conditions. EMBO J. 1989, 8, 351–358. [Google Scholar] [CrossRef]
- Sehrawat, A.; Sindhu, S. S.; Glick, B. R. Hydrogen Cyanide Production by Soil Bacteria: Biological Control of Pests and Promotion of Plant Growth in Sustainable Agriculture. Pedosphere 2022, 32, 15–38. [Google Scholar] [CrossRef]
- Rijavec, T.; Lapanje, A. Hydrogen Cyanide in the Rhizosphere: Not Suppressing Plant Pathogens, but Rather Regulating Availability of Phosphate. Front. Microbiol. 2016, 7, 1785. [Google Scholar] [CrossRef]
- Ryu, C. M.; Faragt, M. A.; Hu, C. H.; Reddy, M. S.; Wei, H. X.; Paré, P. W.; Kloepper, J. W. Bacterial Volatiles Promote Growth in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 4927–4932. [Google Scholar] [CrossRef]
- Kai, M.; Haustein, M.; Molina, F.; Petri, A.; Scholz, B.; Piechulla, B. Bacterial Volatiles and Their Action Potential. Appl. Microbiol. Biotechnol. 2009, 81, 1001–1012. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, J.; Li, C.; Ma, Y. Antifungal and Plant Growth Promotion Activity of Volatile Organic Compounds Produced by Bacillus Amyloliquefaciens. Microbiologyopen 2019, 8, 1–14. [Google Scholar] [CrossRef]
- Pest Management Regulatory Agency. Registration Decision RD2014-08, Bacillus subtilis strain GB03; Government of Canada. [CrossRef]
- Zhu, L.; Huang, J.; Lu, X.; Zhou, C. Development of Plant Systemic Resistance by Beneficial Rhizobacteria: Recognition, Initiation, Elicitation and Regulation. Front. Plant Sci. 2022, 13, 1–16. [Google Scholar] [CrossRef]
- Lü, P.; Liu, Y.; Yu, X.; Shi, C. L.; Liu, X. The Right Microbe-Associated Molecular Patterns for Effective Recognition by Plants. Front. Microbiol. 2022, 13, 1–10. [Google Scholar] [CrossRef]
- Tonelli, M. L.; Figueredo, M. S.; Rodríguez, J.; Fabra, A.; Ibañez, F. Induced Systemic Resistance -like Responses Elicited by Rhizobia. Plant Soil 2020, 448, 1–14. [Google Scholar] [CrossRef]
- Van der Ent, S.; Van Wees, S. C. M.; Pieterse, C. M. J. Jasmonate Signaling in Plant Interactions with Resistance-Inducing Beneficial Microbes. Phytochemistry 2009, 70, 1581–1588. [Google Scholar] [CrossRef]
- Altinok, H.; Yildiz, H. Induced Systemic Resistance By Plant Growth-Promoting Rhizobacteria in Control of Plant Diseases. Curr. Trends Nat. Sci. 2019, 8, 125–133. [Google Scholar]
- Pozo, M. J.; Van Der Ent, S.; Van Loon, L. C.; Pieterse, C. M. J. Transcription Factor MYC2 Is Involved in Priming for Enhanced Defense during Rhizobacteria-Induced Systemic Resistance in Arabidopsis Thaliana. New Phytol. 2008, 180, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants 2022, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Molina, J.; Proietti, S.; Pollier, J.; Orozco-Freire, W.; Ramirez-Villacis, D.; Leon-Reyes, A. Induced Tolerance to Abiotic and Biotic Stresses of Broccoli and Arabidopsis after Treatment with Elicitor Molecules. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Maithani, D.; Singh, H.; Sharma, A. Stress Alleviation in Plants Using SAR and ISR: Current Views on Stress Signaling Network. In Microbes and Signaling Biomolecules Against Plant Stress. Rhizosphere Biology; Sharma, A., Ed.; Springer: Singapore, 2021; pp. 7–36. [Google Scholar] [CrossRef]
- Lucas, J. A.; García-Cristobal, J.; Bonilla, A.; Ramos, B.; Gutierrez-Mañero, J. Beneficial Rhizobacteria from Rice Rhizosphere Confers High Protection against Biotic and Abiotic Stress Inducing Systemic Resistance in Rice Seedlings. Plant Physiol. Biochem. 2014, 82, 44–53. [Google Scholar] [CrossRef]
- Davletova, S.; Rizhsky, L.; Liang, H.; Shengqiang, Z.; Oliver, D. J.; Coutu, J.; Shulaev, V.; Schlauch, K.; Mittler, R. Cytosolic Ascorbate Peroxidase 1 Is a Central Component of the Reactive Oxygen Gene Network of Arabidopsis. Plant Cell 2005, 17, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Arkhipov, A.; Carvalhais, L. C.; Schenk, P. M. PGPR Control Phytophthora Capsici in Tomato through Induced Systemic Resistance, Early Hypersensitive Response and Direct Antagonism in a Cultivar-Specific Manner. Eur. J. Plant Pathol. 2023, 167, 811–832. [Google Scholar] [CrossRef]
- Li, W.; Lee, S. Y.; Cho, Y. J.; Ghim, S. Y.; Jung, H. Y. Mediation of Induced Systemic Resistance by the Plant Growth-Promoting Rhizobacteria Bacillus Pumilus S2-3-2. Mol. Biol. Rep. 2020, 47, 8429–8438. [Google Scholar] [CrossRef] [PubMed]
- Singh, R. P.; Jha, P. N. The Multifarious PGPR Serratia Marcescens CDP-13 Augments Induced Systemic Resistance and Enhanced Salinity Tolerance of Wheat (Triticum Aestivum L.). PLoS One 2016, 11, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Samaras, A.; Kamou, N.; Tzelepis, G.; Karamanoli, K.; Menkissoglu-Spiroudi, U.; Karaoglanidis, G. S. Root Transcriptional and Metabolic Dynamics Induced by the Plant Growth Promoting Rhizobacterium (PGPR) Bacillus Subtilis Mbi600 on Cucumber Plants. Plants 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Samaras, A.; Roumeliotis, E.; Ntasiou, P.; Karaoglanidis, G. Bacillus Subtilis Mbi600 Promotes Growth of Tomato Plants and Induces Systemic Resistance Contributing to the Control of Soilborne Pathogens. Plants 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Liu, Y.; Xu, Y.; Zhang, G.; Shen, Q.; Zhang, R. Exploring Elicitors of the Beneficial Rhizobacterium Bacillus Amyloliquefaciens SQR9 to Induce Plant Systemic Resistance and Their Interactions with Plant Signaling Pathways. Mol. Plant-Microbe Interact. 2018, 31, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, N.; Khan, M. M. A.; Moinuddin; Idrees, M.; Aftab, T. Exogenous Salicylic Acid Stimulates Physiological and Biochemical Changes to Improve Growth, Yield and Active Constituents of Fennel Essential Oil. Plant Growth Regul. 2012, 68, 281–291. [Google Scholar] [CrossRef]
- Aftab, T.; Masroor, M.; Khan, A.; Idrees, M.; Naeem, M.; Moinuddin. Salicylic Acid Acts as Potent Enhancer of Growth, Photosynthesis and Artemisinin Production in Artemisia Annua L. J. Crop Sci. Biotechnol. 2010, 13, 183–188. [Google Scholar] [CrossRef]
- Martín-Mex, R.; Nexticapan-Garcéz, Á.; Villanueva-Couoh, E.; Uicab-Quijano, V.; Vergara-Yoisura, S.; Larqué-Saavedra, A. Salicylic Acid Stimulates Flowering in Micropopagated Gloxinia Plants. Rev. Fitotec. Mex. 2015, 38, 115–118. [Google Scholar] [CrossRef]
- Ueda, J.; Miyamoto, K.; Sato, T.; Momotani, Y. Identification of Jasmonic Acid from Euglena Gracilis z as a Plant Growth Regulator. Agric. Biol. Chem. 1991, 55, 275–276. [Google Scholar] [CrossRef]
- Ravnikar, M.; Vilhar, B.; Gogala, N. Stimulatory Effects of Jasmonic Acid on Potato Stem Node and Protoplast Culture. J. Plant Growth Regul. 1992, 11, 29–33. [Google Scholar] [CrossRef]
- Ravnikar, M.; Žel, J.; Plaper, I.; Špacapan, A. Jasmonic Acid Stimulates Shoot and Bulb Formation of Garlic in Vitro. J. Plant Growth Regul. 1993, 12, 73–77. [Google Scholar] [CrossRef]
- Van Peer, R.; Niemann, G. J.; Schippers, B. Induced Resistance and Phytoalexin Accumulation in Biological Control of Fusarium Wilt of Carnation by Pseudomonas Sp. Strain WCS417r. Phytopathology 1991, 728–734. [Google Scholar] [CrossRef]
- Spence, C.; Alff, E.; Johnson, C.; Ramos, C.; Donofrio, N.; Sundaresan, V.; Bais, H. Natural Rice Rhizospheric Microbes Suppress Rice Blast Infections. BMC Plant Biol. 2014, 14, 1–17. [Google Scholar] [CrossRef]
- Nandakumar, R.; Babu, S.; Viswanathan, R.; Raguchander, T.; Samiyappan, R. Induction of Systemic Resistance in Rice against Sheath Blight Disease by Pseudomonas Fluorescens. Soil Biol. Biochem. 2001, 33, 603–612. [Google Scholar] [CrossRef]
- Fatima, S.; Anjum, T. Plant Pathology Potential of Rhizospheric Pseudomonas Strains To Manage Fusarium Wilt of Tomato. J. Agric. Res. 2017, 55, 525–536. [Google Scholar]
- Yan, Z.; Reddy, M. S.; Ryu, C. M.; McInroy, J. A.; Wilson, M.; Kloepper, J. W. Induced Systemic Protection against Tomato Late Blight Elicited by Plant Growth-Promoting Rhizobacteria. Phytopathology 2002, 92, 1329–1333. [Google Scholar] [CrossRef]
- Kloepper, J. W.; Ryu, C. M.; Zhang, S. Induced Systemic Resistance and Promotion of Plant Growth by Bacillus Spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M. N.; Namood-e-Sahar; Zia-Ul-Husnain Shah, S.; Afghan, S.; Hafeez, F.Y. Suppression of Red Rot Disease by Bacillus Sp. Based Biopesticide Formulated in Non-Sterilized Sugarcane Filter Cake. BioControl 2015, 60, 691–702. [Google Scholar] [CrossRef]
- Li, H.; Ding, X.; Wang, C.; Ke, H.; Wu, Z.; Wang, Y.; Liu, H.; Guo, J. Control of Tomato Yellow Leaf Curl Virus Disease by Enterobacter Asburiae BQ9 as a Result of Priming Plant Resistance in Tomatoes. Turkish J. Biol. 2016, 40, 150–159. [Google Scholar] [CrossRef]
- Fukami, J.; Ollero, F. J.; Megías, M.; Hungria, M. Phytohormones and Induction of Plant-Stress Tolerance and Defense Genes by Seed and Foliar Inoculation with Azospirillum Brasilense Cells and Metabolites Promote Maize Growth. AMB Express 2017, 7. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Höfte, M. Using Serratia Plymuthica to Control Fungal Pathogens of Plants. CAB Rev. 2007, 2. [Google Scholar] [CrossRef]
- Liu, D.; Chen, L.; Zhu, X.; Wang, Y.; Xuan, Y.; Liu, X.; Chen, L.; Duan, Y. Klebsiella Pneumoniae SnebYK Mediates Resistance against Heterodera Glycines and Promotes Soybean Growth. Front. Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Ji, S. H.; Gururani, M. A.; Chun, S. C. Isolation and Characterization of Plant Growth Promoting Endophytic Diazotrophic Bacteria from Korean Rice Cultivars. Microbiol. Res. 2014, 169, 83–98. [Google Scholar] [CrossRef]
- Wei, G.; Kloepper, J. W.; Tuzun, S. Induction of Systemic Resistance of Cucumber to Colletotrichum Orbiculare by Select Strains of Plant Growth-Promoting Rhizobacteria. Phytopathology 1991, 81, 1508–1512. [Google Scholar] [CrossRef]
- Majdura, J.; Jankiewicz, U.; Gałązka, A.; Orzechowski, S. The Role of Quorum Sensing Molecules in Bacterial–Plant Interactions. Metabolites 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Rtis-Flores, C. A.; Loeza-Lara, P. D.; Orozco-Mosqueda, M. D. C.; Glick, B. R. Rhizosphere Colonization Determinants by Plant Growth-Promoting Rhizobacteria (PGPR). Biology (Basel). 2021, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Singh, P.; Sarmah, B. K.; Nandi, S. P. Quorum Sensing: Its Role in Microbial Social Networking. Res. Microbiol. 2020, 171, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, R.; Givskov, M.; Michiels, C. W. Quorum Sensing in Serratia. FEMS Microbiol. Rev. 2007, 31, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.; Torres, M.; Blanco, L.; Béjar, V.; Sampedro, I.; Llamas, I. Plant Growth-Promoting Activity and Quorum Quenching-Mediated Biocontrol of Bacterial Phytopathogens by Pseudomonas Segetis Strain P6. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Gislason, A. S.; Becker, M.; Belmonte, M. F.; Fernando, W. G. D.; de Kievit, T. R. Investigation of the Quorum-Sensing Regulon of the Biocontrol Bacterium Pseudomonas Chlororaphis Strain PA23. PLoS One 2020, 15, e0226232. [Google Scholar] [CrossRef] [PubMed]
- Schuhegger, R.; Ihring, A.; Gantner, S.; Bahnweg, G.; Knappe, C.; Vogg, G.; Hutzler, P.; Schmid, M.; Van Breusegem, F.; Eberl, L.; Hartmann, A.; Langebartels, C. Induction of Systemic Resistance in Tomato by N-Acyl-L-Homoserine Lactone-Producing Rhizosphere Bacteria. Plant, Cell Environ. 2006, 29, 909–918. [Google Scholar] [CrossRef]
- Liu, X.; Bimerew, M.; Ma, Y.; Müller, H.; Ovadis, M.; Eberl, L.; Berg, G.; Chernin, L. Quorum-Sensing Signaling Is Required for Production of the Antibiotic Pyrrolnitrin in a Rhizospheric Biocontrol Strain of Serratia Plymuthica. FEMS Microbiol. Lett. 2007, 270, 299–305. [Google Scholar] [CrossRef]
- Pang, Y.; Liu, X.; Ma, Y.; Chernin, L.; Berg, G.; Gao, K. Induction of Systemic Resistance, Root Colonisation and Biocontrol Activities of the Rhizospheric Strain of Serratia Plymuthica Are Dependent on N-Acyl Homoserine Lactones. Eur. J. Plant Pathol. 2009, 124, 261–268. [Google Scholar] [CrossRef]
- Rankl, S.; Gunsé, B.; Sieper, T.; Schmid, C.; Poschenrieder, C.; Schröder, P. Microbial Homoserine Lactones (AHLs) Are Effectors of Root Morphological Changes in Barley. Plant Sci. 2016, 253, 130–140. [Google Scholar] [CrossRef]
- Moshynets, O. V.; Babenko, L. M.; Rogalsky, S. P.; Iungin, O. S.; Foster, J.; Kosakivska, I. V.; Potters, G.; Spiers, A. J. Priming Winter Wheat Seeds with the Bacterial Quorum Sensing Signal N-Hexanoyl-L-Homoserine Lactone (C6-HSL) Shows Potential to Improve Plant Growth and Seed Yield. PLoS One 2019, 14, 1–17. [Google Scholar] [CrossRef]
- Shrestha, A.; Grimm, M.; Ojiro, I.; Krumwiede, J.; Schikora, A. Impact of Quorum Sensing Molecules on Plant Growth and Immune System. Front. Microbiol. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Hartmann, A.; Klink, S.; Rothballer, M. Importance of N-Acyl-Homoserine Lactone-Based Quorum Sensing and Quorum Quenching in Pathogen Control and Plant Growth Promotion. Pathogens 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Barriuso, J.; Ramos Solano, B.; Fray, R. G.; Cámara, M.; Hartmann, A.; Gutiérrez Mañero, F. J. Transgenic Tomato Plants Alter Quorum Sensing in Plant Growth-Promoting Rhizobacteria. Plant Biotechnol. J. 2008, 6, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yang, X. Y.; Li, Y.; Liu, F.; Cao, X. Y.; Jia, Z. H.; Song, S. S. N-3-Oxo-Hexanoyl-Homoserine Lactone, a Bacterial Quorum Sensing Signal, Enhances Salt Tolerance in Arabidopsis and Wheat. Bot. Stud. 2020, 61. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M. S.; Arshad, A.; Rajput, L.; Fatima, K.; Ullah, S.; Ahmad, M.; Imran, A. Growth-Stimulatory Effect of Quorum Sensing Signal Molecule N-Acyl-Homoserine Lactone-Producing Multi-Trait Aeromonas Spp. on Wheat Genotypes Under Salt Stress. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Von Rad, U.; Klein, I.; Dobrev, P. I.; Kottova, J.; Zazimalova, E.; Fekete, A.; Hartmann, A.; Schmitt-Kopplin, P.; Durner, J. Response of Arabidopsis Thaliana to N-Hexanoyl-DL-Homoserine-Lactone, a Bacterial Quorum Sensing Molecule Produced in the Rhizosphere. Planta 2008, 229, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, M.; Jia, Z.; Liu, F.; Ma, H.; Huang, Y.; Song, S. AtMYB44 Positively Regulates the Enhanced Elongation of Primary Roots Induced by N-3-Oxo-Hexanoyl-Homoserine Lactone in Arabidopsis Thaliana. Mol. Plant-Microbe Interact. 2016, 29, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Bian, Z.; Jia, Z.; Zhao, Q.; Song, S. The GCR1 and GPA1 Participate in Promotion of Arabidopsis Primary Root Elongation Induced by N-Acyl-Homoserine Lactones, the Bacterial Quorum-Sensing Signals. Mol. Plant-Microbe Interact. 2012, 25, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Castro, R.; Díaz-Pérez, C.; Martínez-Trujillo, M.; Del Río, R. E.; Campos-García, J.; López-Bucio, J. Transkingdom Signaling Based on Bacterial Cyclodipeptides with Auxin Activity in Plants. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 7253–7258. [Google Scholar] [CrossRef]
- Ortíz-Castro, R.; Martínez-Trujillo, M.; López-Bucio, J. N-Acyl-L-Homoserine Lactones: A Class of Bacterial Quorum-Sensing Signals Alter Post-Embryonic Root Development in Arabidopsis Thaliana. Plant, Cell Environ. 2008, 31, 1497–1509. [Google Scholar] [CrossRef]
- Hanif, M. K.; Malik, K. A.; Hameed, S.; Saddique, M. J.; Ayesha; Fatima, K. ; Naqqash, T.; Majeed, A.; Iqbal, M. J.; Imran, A. Growth Stimulatory Effect of AHL Producing Serratia Spp. from Potato on Homologous and Non-Homologous Host Plants. Microbiol. Res. 2020, 238, 126506. [Google Scholar] [CrossRef]
- Jadhav, H.; Shaikh, S.; Sayyed, R. Role of Hydrolytic Enzymes of Rhizoflora in Biocontrol of Fungal Phytopathogens: An Overview. In Rhizotrophs: Plant Growth Promotion to Bioremediation; Mehnaz, S., Ed.; Springer: Singapore, 2017; pp. 183–203. [Google Scholar] [CrossRef]
- Khalil, M. S. M.; Hassan, M. H. A. R.; Mahmoud, A. F.; Morsy, K. M. M. Involvement of Secondary Metabolites and Extracellular Lytic Enzymes Produced by Plant Growth Promoting Rhizobacteria in Inhibiting the Soilborne Pathogens in Faba Bean Plants. J. Trop. Plant Pests Dis. 2022, 22, 100–108. [Google Scholar] [CrossRef]
- Polonio, Á.; Vida, C.; de Vicente, A.; Cazorla, F. M. Impact of Motility and Chemotaxis Features of the Rhizobacterium Pseudomonas Chlororaphis PCL1606 on Its Biocontrol of Avocado White Root Rot. Int. Microbiol. 2017, 20, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Pan, R.; Shen, Y.; Yuan, J.; Wang, L.; Luo, X.; Raza, W.; Ling, N.; Huang, Q.; Shen, Q. Development of a Novel Bio-Organic Fertilizer for Plant Growth Promotion and Suppression of Rhizome Rot in Ginger. Biol. Control 2017, 114, 97–105. [Google Scholar] [CrossRef]
- Mishra, P.; Mishra, J.; Dwivedi, S. K.; Arora, N. K. Microbial Enzymes in Biocontrol of Phytopathogens. In Microbial Enzymes: Roles and Applications in Industries. Microorganisms for Sustainability; Arora, N., Mishra, J., Mishra, V., Eds.; Springer: Singapore, 2020; pp. 259–285. [Google Scholar] [CrossRef]
- Adhav, H.; Shaikh, S.; Sayyed, R. Role of Hydrolytic Enzymes of Rhizoflora in Biocontrol of Fungal Phytopathogens: An Overview. In Rhizotrophs: Plant Growth Promotion to Bioremediation. Microorganisms for Sustainability; Mehnaz, S., Ed.; Springer: Singapore, 2017; pp. 183–203. [Google Scholar] [CrossRef]
- Reddy, E. C.; Reddy, G. S.; Goudar, V.; Sriramula, A.; Swarnalatha, G. V; Al Tawaha, A. R. M.; Sayyed, R.Z. Hydrolytic Enzyme Producing Plant Growth-Promoting Rhizobacteria (PGPR) in Plant Growth Promotion and Biocontrol. In Secondary Metabolites and Volatiles of PGPR in Plant-Growth Promotion; Sayyed, R., Uarrota, V., Eds.; Springer, 2022; pp. 303–312. [Google Scholar] [CrossRef]
- Yoshimi, A.; Miyazawa, K.; Abe, K. Function and Biosynthesis of Cell Wall α-1,3-Glucan in Fungi. J. Fungi 2017, 3, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ueki, A.; Takehara, T.; Ishioka, G.; Kaku, N.; Ueki, K. Production of β-1,3-Glucanase and Chitosanase from Clostridial Strains Isolated from the Soil Subjected to Biological Disinfestation. AMB Express 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Suyotha, W.; Yano, S.; Wakayama, M. α-1,3-Glucanase: Present Situation and Prospect of Research. World J. Microbiol. Biotechnol. 2016, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gomma, M. A.; Radwan, F. I.; Kandil, E. E.; Gharib, A. F. Effect of Zinc Application on Growth and Yield of Rice ( Oryza Sativa L.) Effect of Zinc Application on Growth and Yield of Rice ( Oryza Sativa L.). Middle East J. Appl. Sci. 2015, 5, 913–919. [Google Scholar] [CrossRef]
- de Souza, R.; Ambrosini, A.; Passaglia, L. M. P. Plant Growth-Promoting Bacteria as Inoculants in Agricultural Soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Kalbe, C.; Marten, P.; Berg, G. Strains of the Genus Serratia as Beneficial Rhizobacteria of Oilseed Rape with Antifungal Properties. Microbiol. Res. 1996, 151, 433–439. [Google Scholar] [CrossRef]
- Zia, M. A.; Yasmin, H.; Shair, F.; Jabeen, Z.; Mumtaz, S.; Hayat, Z.; Shah, S. Z. ul H.; Afghan, S.; Hafeez, F. Y.; Hassan, M. N. Glucanolytic Rhizobacteria Produce Antifungal Metabolites and Elicit ROS Scavenging System in Sugarcane. Sugar Tech 2019, 21, 244–255. [Google Scholar] [CrossRef]
- Sayyed, R. Z.; Uarrota, V. G. Hydrolytic Enzyme Producing Plant Growth-Promoting Rhizobacteria (PGPR) in Plant Growth Promotion and Biocontrol. In Secondary Metabolites and Volatiles of PGPR in Plant-Growth Promotion; Sayyed, R., Uarrota, V., Eds.; Springer, 2022; pp. 303–312. [Google Scholar] [CrossRef]
- Perrot, T.; Pauly, M.; Ramírez, V. Emerging Roles of β-Glucanases in Plant Development and Adaptative Responses. Plants 2022, 11. [Google Scholar] [CrossRef]
- Dukare, A.; Paul, S.; Arambam, A. Isolation and Efficacy of Native Chitinolytic Rhizobacteria for Biocontrol Activities against Fusarium Wilt and Plant Growth Promotion in Pigeon Pea (Cajanus Cajan L.). Egypt. J. Biol. Pest Control 2020, 30, 1–12. [Google Scholar] [CrossRef]
- Mohammed, A. F. Optimization of Cellulase and Chitinase Enzymes Production by Plant Growth Promoting Rhizobacteria. Nov. Res. Microbiol. J. 2020, 4, 641–652. [Google Scholar] [CrossRef]
- Yuliar; Kartadi, S. F.; Salmah, A. Combined Use of Enterobacter Cloacae MB20 and the Microelements of Copper and Manganese to Control Damping-off of Tomato. Earth Environ. Sci. 2019, 308, 012025. [Google Scholar] [CrossRef]
- Singh, S. P.; Gaur, R. Evaluation of Antagonistic and Plant Growth Promoting Activities of Chitinolytic Endophytic Actinomycetes Associated with Medicinal Plants against Sclerotium Rolfsii in Chickpea. J. Appl. Microbiol. 2016, 121, 506–518. [Google Scholar] [CrossRef]
- Kim, H. J.; Choi, H. S.; Yang, S. Y.; Kim, I. S.; Yamaguchi, T.; Sohng, J. K.; Park, S. K.; Kim, J. C.; Lee, C. H.; Gardener, B. M.; Kim, Y. C. Both Extracellular Chitinase and a New Cyclic Lipopeptide, Chromobactomycin, Contribute to the Biocontrol Activity of Chromobacterium Sp. C61. Mol. Plant Pathol. 2014, 15, 122–132. [Google Scholar] [CrossRef]
- Gongora, C. E.; Broadway, R. M. Plant Growth and Development Influenced by Transgenic Insertion of Bacterial Chitinolytic Enzymes. Mol. Breed. 2002, 9, 123–135. [Google Scholar] [CrossRef]
- Fitza, K. N. E.; Payn, K. G.; Steenkamp, E. T.; Myburg, A. A.; Naidoo, S. Chitosan Application Improves Resistance to Fusarium Circinatum in Pinus Patula. South African J. Bot. 2013, 85, 70–78. [Google Scholar] [CrossRef]
- Zong, H.; Li, K.; Liu, S.; Song, L.; Xing, R.; Chen, X.; Li, P. Improvement in Cadmium Tolerance of Edible Rape (Brassica Rapa L.) with Exogenous Application of Chitooligosaccharide. Chemosphere 2017, 181, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E. Z. Microbial Chitinases: Properties, Enhancement and Potential Applications. Protoplasma 2021, 258, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Brar, A.; Yadav, M.; Chawade, A.; Vivekanand, V.; Pareek, N. Chitinases—Potential Candidates for Enhanced Plant Resistance towards Fungal Pathogens. Agric. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Billot, R.; Plener, L.; Jacquet, P.; Elias, M.; Chabrière, E.; Daudé, D. Engineering Acyl-Homoserine Lactone-Interfering Enzymes toward Bacterial Control. J. Biol. Chem. 2020, 295, 12993–13007. [Google Scholar] [CrossRef]
- de Leon, V.; Orr, K.; Stelinski, L. L.; Mandadi, K.; Ibanez-Carrasco, F. Inoculation of Tomato With Plant Growth Promoting Rhizobacteria Affects the Tomato—Potato Psyllid—Candidatus Liberibacter Solanacearum Interactions. J. Econ. Entomol. 2023, 116, 379–388. [Google Scholar] [CrossRef]
- Mumtaz, M. Z.; Ahmad, M.; Zafar-Ul-Hye, M.; Saqib, M.; Akhtar, M. F. U. Z.; Zaheer, M. S. Seed-Applied Zinc-Solubilising Bacillus Biofertilisers Improve Antioxidant Enzyme Activities, Crop Productivity, and Biofortification of Maize. Crop Pasture Sci. 2022, 73, 503–514. [Google Scholar] [CrossRef]
- Saral, A.; Kanekar, S.; Koul, K. K.; Bhagyawant, S. S. Plant Growth Promoting Bacteria Induce Anti-Quorum-Sensing Substances in Chickpea Legume Seedling Bioassay. Physiol. Mol. Biol. Plants 2021, 27, 1577–1595. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, R.; Elias, M. Quorum Quenching Enzymes and Their Effects on Virulence, Biofilm, and Microbiomes: A Review of Recent Advances. Expert Rev. Anti. Infect. Ther. 2020, 18, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Bzdrenga, J.; Daudé, D.; Rémy, B.; Jacquet, P.; Plener, L.; Elias, M.; Chabrière, E. Biotechnological Applications of Quorum Quenching Enzymes. Chem. Biol. Interact. 2017, 267, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Achari, G. A.; Ramesh, R. Characterization of Quorum Quenching Enzymes from Endophytic and Rhizosphere Colonizing Bacteria. Biocatal. Agric. Biotechnol. 2018, 13, 20–24. [Google Scholar] [CrossRef]
- Mehmood, A.; Liu, G.; Wang, X.; Meng, G.; Wang, C.; Liu, Y. Fungal Quorum-Sensing Molecules and Inhibitors with Potential Antifungal Activity: A Review. Molecules 2019, 24, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M. A.; Santoyo, G. Unlocking the Potential of Plant Growth-Promoting Rhizobacteria on Soil Health and the Sustainability of Agricultural Systems. J. Environ. Manage. 2020, 273. [Google Scholar] [CrossRef]
- Kumar, V. K. K.; Reddy, M. S.; Kloepper, J. W.; Yellareddygari, S. K. R.; Lawrence, K. S.; Zhou, X. G.; Sudini, H.; Miller, M. E.; Podile, A. .; Reddy, E..; Niranjana, S. R.; Nayaka, C. S. Plant Growth-Promoting Activities of Bacillus Subtilis Mbi 600 (Integral®) and Its Compatibility With Commonly Used Fungicides in Rice Sheath Blight Management. Int. J. Microbiol. Res. 2011, 3, 120–130. [Google Scholar] [CrossRef]
- Jang, S.; Choi, S. K.; Zhang, H.; Zhang, S.; Ryu, C. M.; Kloepper, J. W. History of a Model Plant Growth-Promoting Rhizobacterium, Bacillus Velezensis GB03: From Isolation to Commercialization. Front. Plant Sci. 2023, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mehnaz, S. An Overview of Globally Available Bioformulations. In Bioformulations: for Sustainable Agriculture; Arora, N., Mehnaz, S., Balestrini, R., Eds.; Springer: New Delhi, 2016; pp. 267–281. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




