1. Introduction
For several years, supramolecular chemistry has been successfully applied to controlled drug delivery systems that enable the continuous release at a controlled rate of small amounts of ingredients at a target part of the body. Controlled delivery allows plasma levels of the drug to be maintained for the desired time and its effects to be achieved safely and efficiently [
1]. Among the macromolecules which have been used are cucurbit[n]urils [
2], tetrapyrazolic macrocycles [
3], calixarenes [
4,
5], resorcinarenes [
6], crown ethers [
7], phthalocyanines [
8], and cyclodextrins [
9], among others. In the last case, cyclodextrins have the advantage of being an alternative in the formulation of drugs with poorly soluble active ingredients, due to their solubility in water and their ability to form inclusion complexes with them. In addition, these can remove the unpleasant taste of the drug by forming inclusion complexes, thus facilitating its administration to the patient [
10], improve their stability [
11], and reduce the loss of volatile compounds [
12].
Cyclodextrins are cyclic oligosaccharides that have a hydrophilic exterior and a hydrophobic inner cavity. Due to their shape, these compounds allow the interaction of hydrophobic guests with the inner cavity of the macrocycle by forming inclusion complexes, which improves the solubility and bioavailability of poorly water-soluble drugs [
10].
An interesting example is found with the administration of hydrocortisone, which presents a low solubility in aqueous solutions. Hydrocortisone is a steroid hormone, specifically glucocorticoid, which calms the body’s immune response, reducing pain, itching and inflammation, so it can be used in multiple applications, such as the colon inflammation treatment [
13], to treat osteoarthritis [
14], atopic dermatitis therapy [
15] and in the treatment of aphthous ulcer, among others [
16]. Hydrocortisone is fairly soluble in water, so this work seeks to find alternative methods of formulation, through the formation of inclusion complexes with cyclodextrins, that help to improve the solubility of the drug. The results obtained are discussed in terms of host-guest interactions.
Continuing with our research on polyhydroxylated platforms [
17,
18,
19,
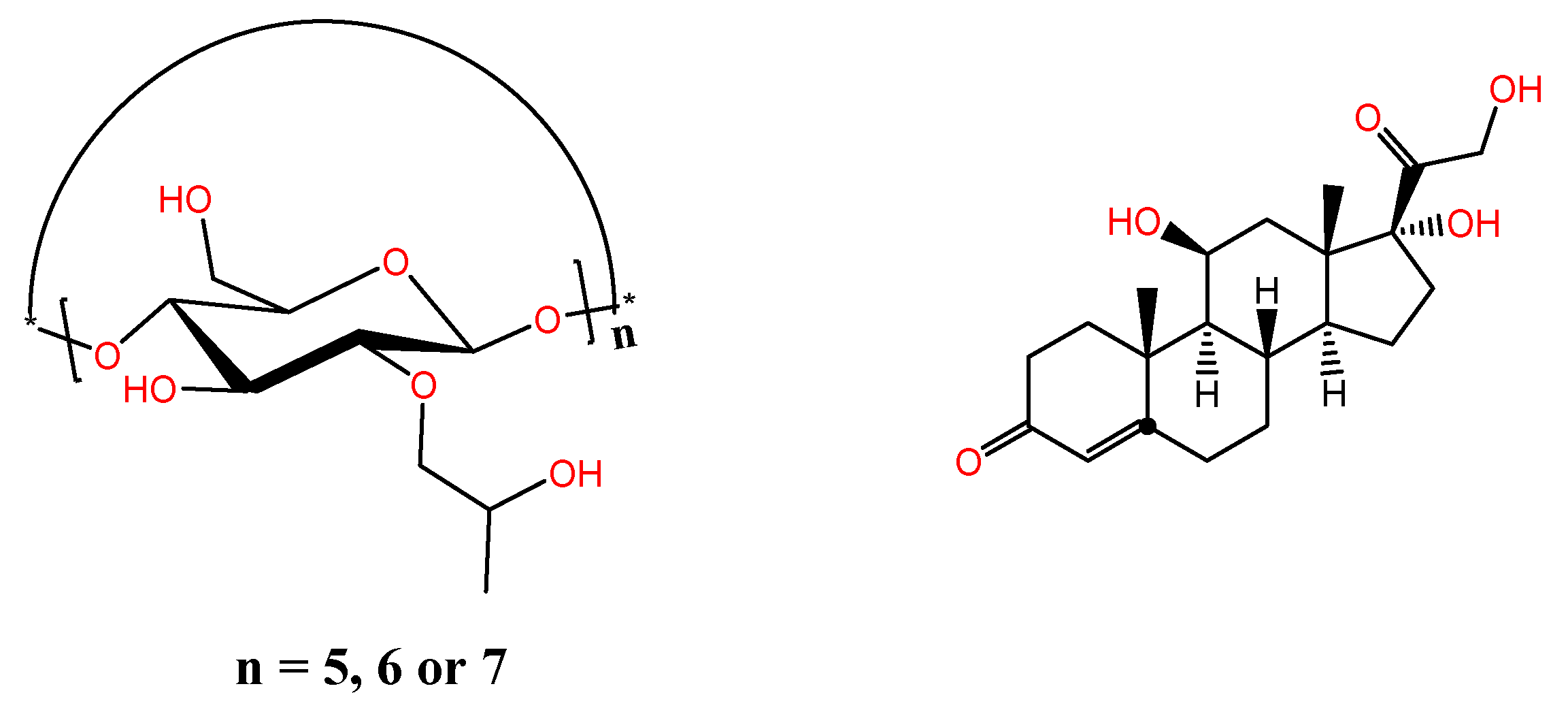
20], in this article the complexing capacity of three macrocycles systems: 2-hydroxypropyl-α-cyclodextrin, 2-hydroxypropyl-β-cyclodextrin and 2-hydroxypropyl-γ-cyclodextrin, with hydrocortisone (
Figure 1) were evaluated. The formed complexes were analyzed by using FT-IR and
1H-NMR spectroscopy. Their properties in aqueous solution were studied at two temperatures, 298.15 and 310.15 K, and the study of their volumetric properties was carried out in order to evaluate both the role and the effect of the structure of the solute complexes on the solvent structure. Finally, the results were interpreted in terms of solute-solute, solvent-solvent and solute-solvent interactions.
2. Results and Discussion
For the present study, the experiments were carried out in several stages. The first step involved the evaluation of the solubility of hydrocortisone in aqueous solutions containing each of the cyclodextrins studied. From these tests we found that hydrocortisone was not soluble in the aqueous solution of 2-hydroxypropyl-α-cyclodextrin at any concentration. As a consequence, the formation of HC/2-HP-alfa-CD complex would be excluded. In this preliminary stage, the possible interactions were also evaluated, using FT-IR spectroscopy on the solid state mixtures obtained, with respect to the starting materials. In the second stage, the spectroscopic characterization was performed in solution using techniques including 1H-NMR and mass spectrometry. Finally, the volumetric properties were analyzed by means of density measurements.
2.1. Spectroscopics Studies
2.1.1. FT-IR Analysis
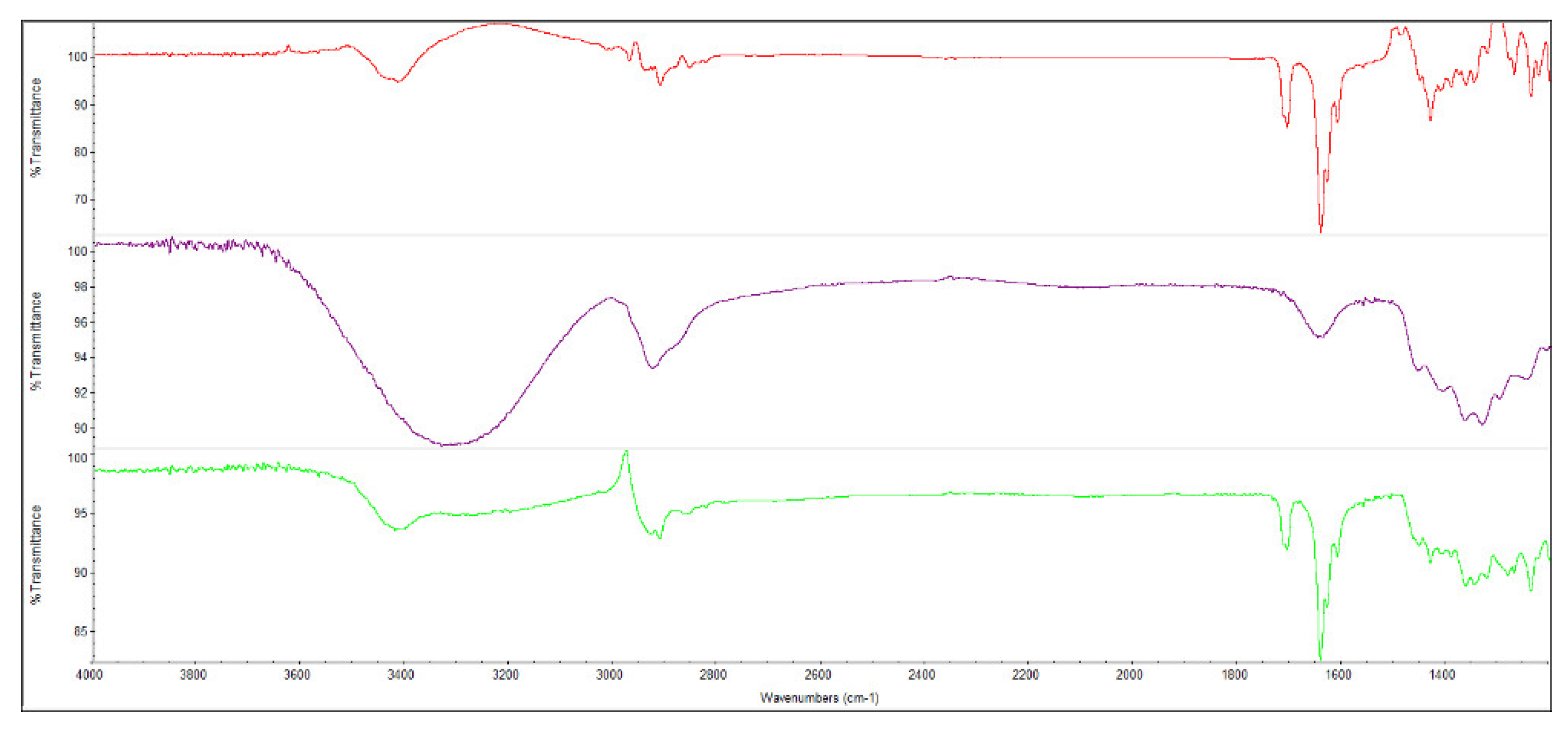
FT-IR spectra were performed, in a scanning range of 600-4000 cm
–1, for the starting materials as well as for the mixtures obtained. In this sense, the FT-IR of hydrocortisone (red line in
Figure 2) agreed with the organic functionalities present, being the main features observed the stretching of the O-H and C-O groups at 3355 cm
–1 and 1110 cm
–1, respectively, whereas the C=O of the ketone groups can be seen at 1692 cm
–1. The bands of stretching C-H and the alkene function (stretching =C-H) can be seen at 2955 cm
–1 and 3030 cm
−1, respectively. The spectroscopy data agree with the information reported in the literature [
21].
The FT-IR spectrum of 2-hydroxypropyl-β-cyclodextrin (purple line in
Figure 2) showed several peaks: a broad peak at 3256 cm
–1 corresponding to the hydroxyl groups of the polyhydroxylated platform, two peaks at 2960 cm
–1, which correspond to the C-H bonds in the aliphatic rings, and another one at 1050 cm
–1 from the C-O bond. Similar results are found in the literature [
22,
23]. The spectroscopic analysis of the hydrocortisone complexes with each of these three cyclodextrins, was performed using mixtures of hydrocortisone + cyclodextrin with 1:1 stoichiometry prepared based on the results obtained in previous works with similar systems [
24,
25,
26].
As it can be seen in the IR spectra (
Figure S5 of Supplementary Material), the spectrum of the equimolar mixture of hydrocortisone with 2-hydroxypropyl-α-cyclodextrin exhibits either the peaks corresponding to the pure hydrocortisone sample (at 3355-2950 cm
–1) or the peaks corresponding to 2-hydroxypropyl-α-cyclodextrin (at 3150 cm
–1), and no shifting is observed. This result indicates that in this case there is no a significant interaction, a valid explanation could be that the 2-hydroxypropyl-α-cyclodextrin cavity is not large enough to accommodate the hydrocortisone molecule. In accordance with this result, subsequent studies were carried out only with 2-hydroxypropyl-β-cyclodextrin and 2-hydroxypropyl-γ-cyclodextrin in aqueous solutions.
The FT-IR spectrum of the equimolar mixture of hydrocortisone with 2-hydroxypropyl-β-cyclodextrin (green line in
Figure 2) showed some peaks consistent with the hydrocortisone patterns, but also showed important changes in some signals, particularly in the 3000-3500 region (OH band) and in the carbonyl bands (1640-1710 cm
−1). This fact could indicate the existence of an interaction between hydrocortisone and 2-hydroxypropyl-β-cyclodextrin that would give rise to an inclusion complex. A similar result was observed for the equimolar mixture of hydrocortisone with 2-hydroxypropyl-γ-cyclodextrin.
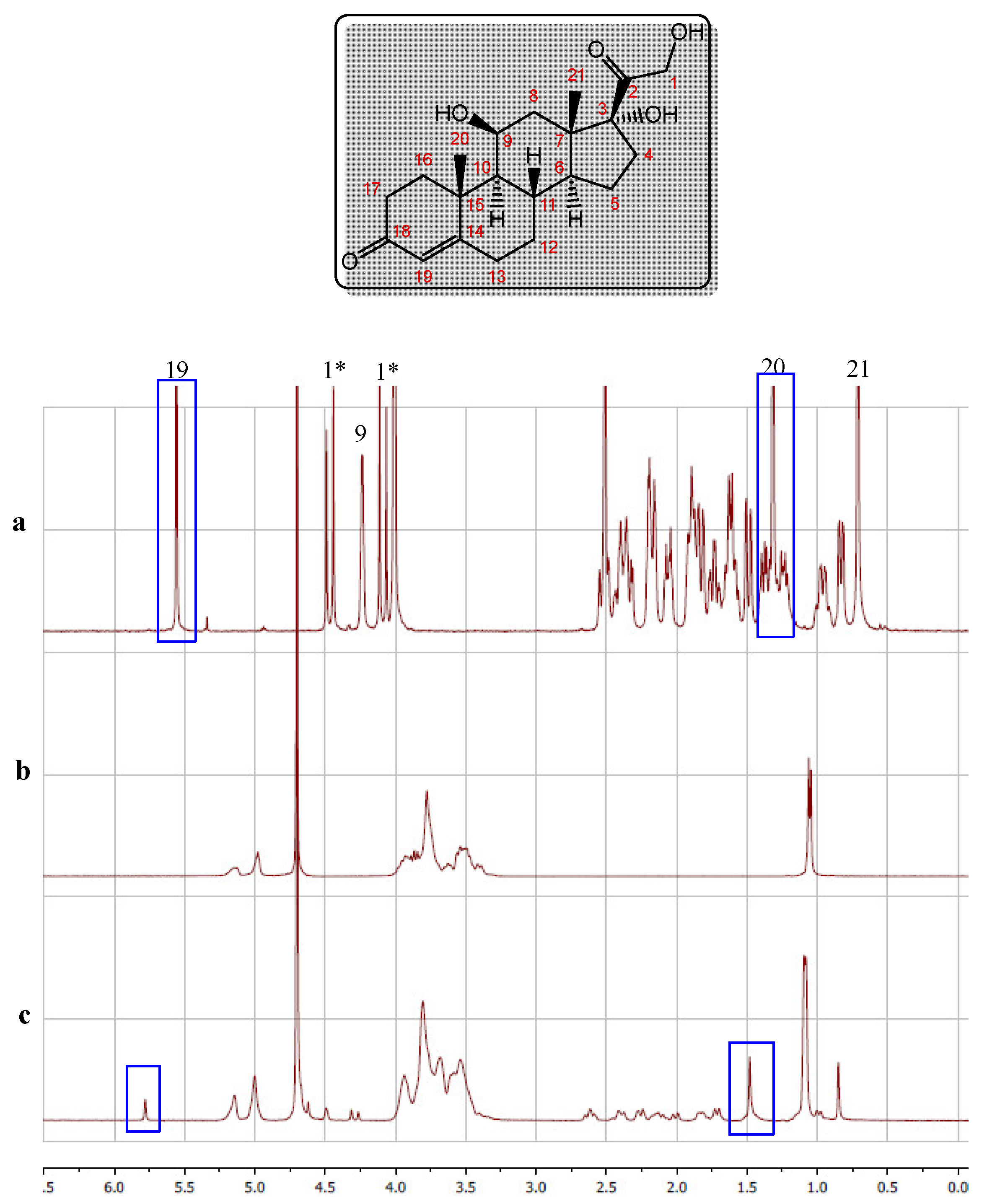
2.1.2.1. H-NMR Studies
For the present study,
1H-NMR spectroscopy was chosen because it provides information to investigate the molecular interactions that take place in solution and it is relevant to determine the mode of inclusion of the possible complexes formed. The
1H-NMR spectrum of hydrocortisone was first analyzed. The individual assignments of the protons were made based on their positions, multiplicities, integral values, and the comparison of these spectral data with those reported in the literature, finding that all the patterns were consistent with the structure of the expected compound; the signal assignment is shown in
Figure 3a [
27,
28]. Next, the
1H-NMR spectrum of 2-hydroxypropyl-β-cyclodextrin was obtained, and its main signals are shown in
Figure 3b. The signals in this spectrum exhibited a pattern which is in agreement with the structure of this polyhydroxylated platform [
29,
30].
The next step consisted in obtaining the
1H-NMR spectrum of an equimolar mixture of hydrocortisone and 2-hydroxypropyl-β-cyclodextrin. The signals in the spectrum of the mixture were compared with those of the spectrum of hydrocortisone, observing an important shift concerning the corresponding signals in hydrocortisone. These changes in the chemical shifts seem to be caused by the formation of the complex. As shown in
Figure 3c, the fact that several signals in the spectrum are markedly shifted between the HP-CD protons and the protons (H9, H19 and H20), together with the small effect of interaction with other protons, suggests that the ring of the ketone group could be included in the cavity of the 2-hydroxypropyl-β-cyclodextrin.
The procedure used to evaluate the interaction of hydrocortisone with 2-hydroxypropyl-γ-cyclodextrin was basically the same. The signals evaluated in the spectrum of the complex with hydrocortisone were the same (H9, H19 and H20) and the results found were similar, although the displacements were slightly smaller. The comparison between de displacement of the H9, H19 and H20 signals observed for hydrocortisone in the two complexes showed that the one found in the case of 2-hydroxypropyl-β-cyclodextrin is slightly larger than that observed in the presence of 2-hydroxypropyl-γ-cyclodextrin, suggesting that the hydrocortisone complex with 2-hydroxypropyl-β-cyclodextrin is a little more stable than the complex with 2-hydroxypropyl-γ-cyclodextrin. To confirm this behavior, the stability of the complexes formed was measured through the variation of the
1H-NMR chemical shift of a single proton resonance in the hydrocortisone (H19), as a function of the molar ratio between hydrocortisone and cyclodextrin (
Figures S8 and S9 in the Supplementary Material). The fitting of the experimental data to the theoretical curves was performed using the Hyp-NMR2008 program. In this way, values for the binding constants were determined from the chemical shifts measured, with an error of 0.01, in the NMR spectra. Thus, for the complex between hydrocortisone and 2-hydroxypropyl-β-cyclodextrin a value of log β= 1.28 (19.05 M
−1) was estimated, while for the union between hydrocortisone and 2-hydroxypropyl-γ-cyclodextrin was slightly smaller, log β= 1.26 (18.20 M
−1). The standard deviation values, σ, corresponding to the best fit for hydrocortisone + 2-hydroxypropyl-β-cyclodextrin and hydrocortisone + 2-hydroxypropyl-γ-cyclodextrin, were 0.8 and 1.3, respectively.
2.2. Analysis of Volumetric Properties
2.2.1. Binary Systems: Cyclodextrin + Water
The apparent molar volume,
Vϕ (in cm
3·mol
–1), of 2-hydroxypropyl-β-cyclodextrin (2-HP-β-CD) and 2-hydroxypropyl-γ-cyclodextrin (2-HP-γ-CD) in water were obtained from their corresponding density values using the equation:
where
is the molar mass of the solute (1380 g·mol
–1 and 1580 g·mol
–1 for 2-HP-β-CD and 2-HP-γ-CD, respectively),
ρ0 is the density of pure water and
ρ is the density of the corresponding aqueous solution at molal concentration,
m. The values of density and apparent molar volume, at different concentrations and at temperatures of 298.15 K and 310.15 K, for the aqueous solutions of 2-HP-β-CD and 2-HP-γ-CD are shown in
Table 1 and
Table 2, respectively.
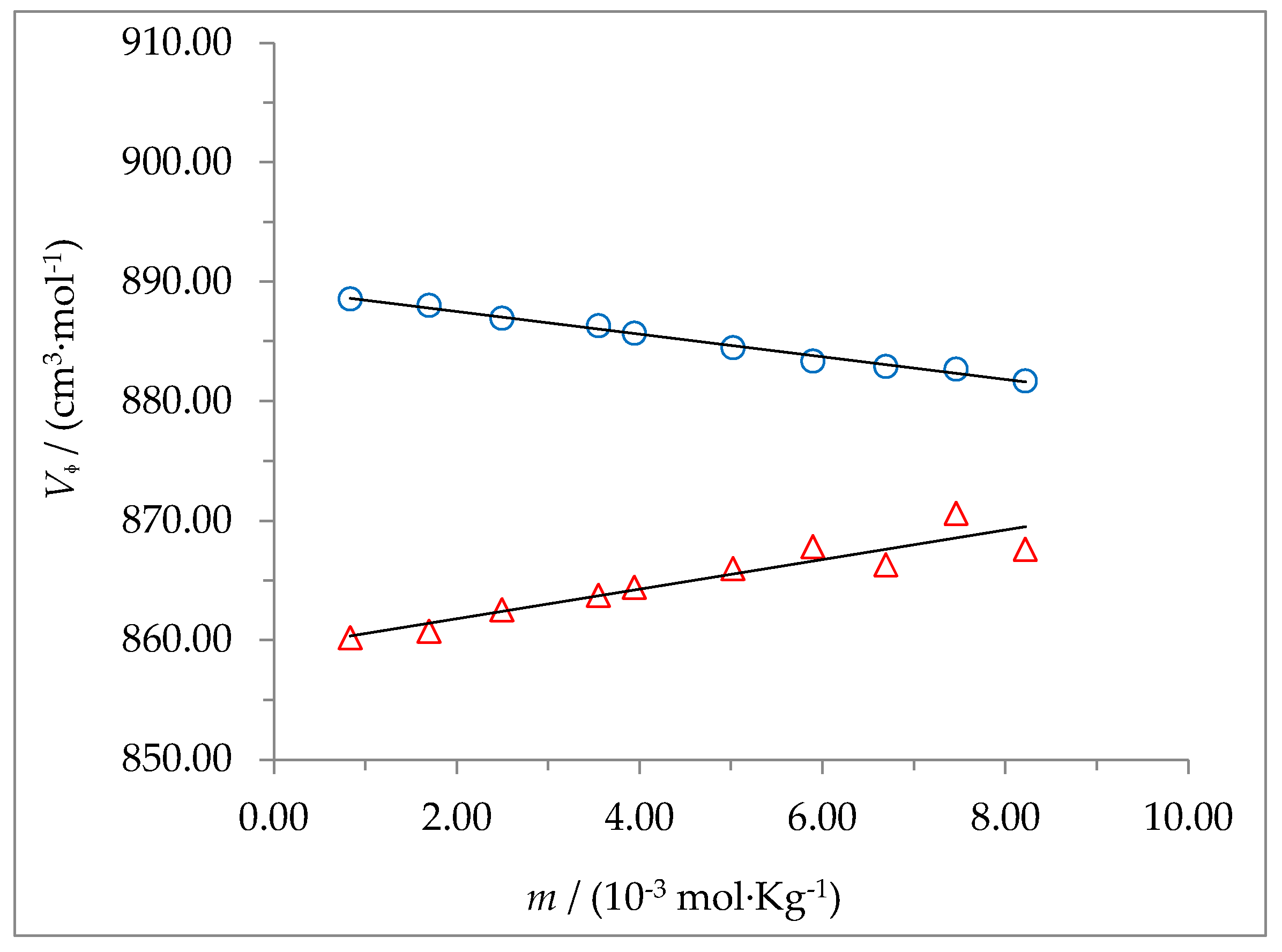
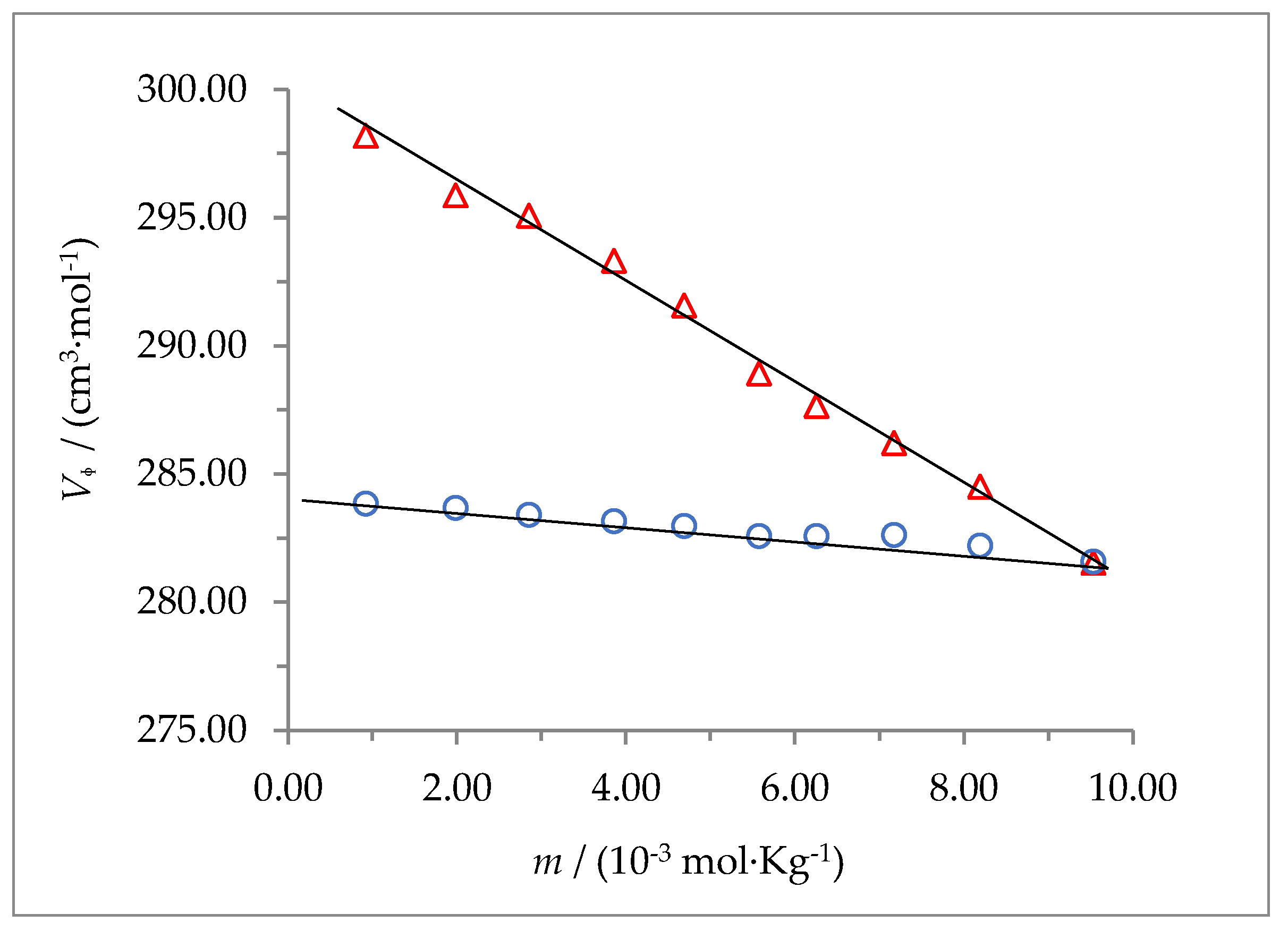
The dependence of
on
m is shown in
Figure 4 and
Figure 5 for 2-HP-β-CD and 2-HP-γ-CD, respectively. The apparent molar volumes were correlated with molality by using the least squares method to find the best fit for the data. The results follow a linear behavior thar can be described by the equation:
The independent term of this equation is the limiting value of the apparent molar volume at infinitesimal concentration, which corresponds to that of the partial molar volume of the solute at infinitesimal concentration,
. This value was obtained by extrapolation. The partial molar volume of the solute provides information regarding solute-solvent interactions in solution, and the experimental slope
, which is an empirical parameter, can be related to solute-solute interactions in solution [
31]. The values of
and
for both cyclodextrins are shown in
Table 3, together with their uncertainties, at the working temperatures.
Figure 4 shows that in the case of 2-HP-β-CD,
increase with the concentration (
BV > 0) at the temperature of 298.15 K, but it decreases (
BV < 0) with the concentration at 310.15 K; on the contrary,
Figure 5 shows that for 2-HP-γ-CD,
increase with the concentration (
BV > 0) at the two temperatures studied. Positive values of
BV have been related to destructive overlap in solvation cells of solute (decreased degree of solvent structuring) and negative values have been related to cooperative overlap (increased degree of solvent structuring) [
32]. The
BV values found for these solutes suggest a decrease in the degree of structuring of the solvent for 2-HP-γ-CD, independent of the temperature, while for 2-HP-β-CD the increase in temperature causes that the decreasing effect observed at 298.15 K to change until it becomes an increase in the degree of structuring of the solvent when the temperature rises up to 310.15 K. The standard partial molar volume value found for 2-HP-β-CD is in good agreement with those reported by other authors [
11,
31]. No information has been found for 2-HP-γ-CD. The
value for 2-HP-γ-CD is considerably larger than that of 2-HP-β-CD at both temperatures, which is expected considering that 2-HP-γ-CD has one D-(+)-glucose ring more than 2-HP-β-CD.
2.2.1. Ternary Systems: 2-HP-β-CD + Water + HC and 2-HP-γ-CD + Water +HC
It is important to note that hydocortisone, like approximately 50% of pharmaceutical drugs, is poorly water soluble, which is associated with low oral bioavailability and low saturation solubility [
33]; for this reason, it was not possible to measure its properties in water and the evaluation of its behavior with cyclodextrins was followed by other techniques described in this work. However, taking advantage of the fact that cyclodextrins significantly improve the solubility of this drug in the range of concentrations and temperatures studied, the volumetric properties of the systems 2-HP-β-CD + water + HC and 2-HP-γ-CD + water +HC were evaluated. The values obtained by considering HC as the solute and as solvent the mixtures of 2-HP-β-CD + water, in the first case, and of 2-HP-γ-CD + water, in second case, in a 1:1 ratio, are shown in
Table 4 and
Table 5, respectively.
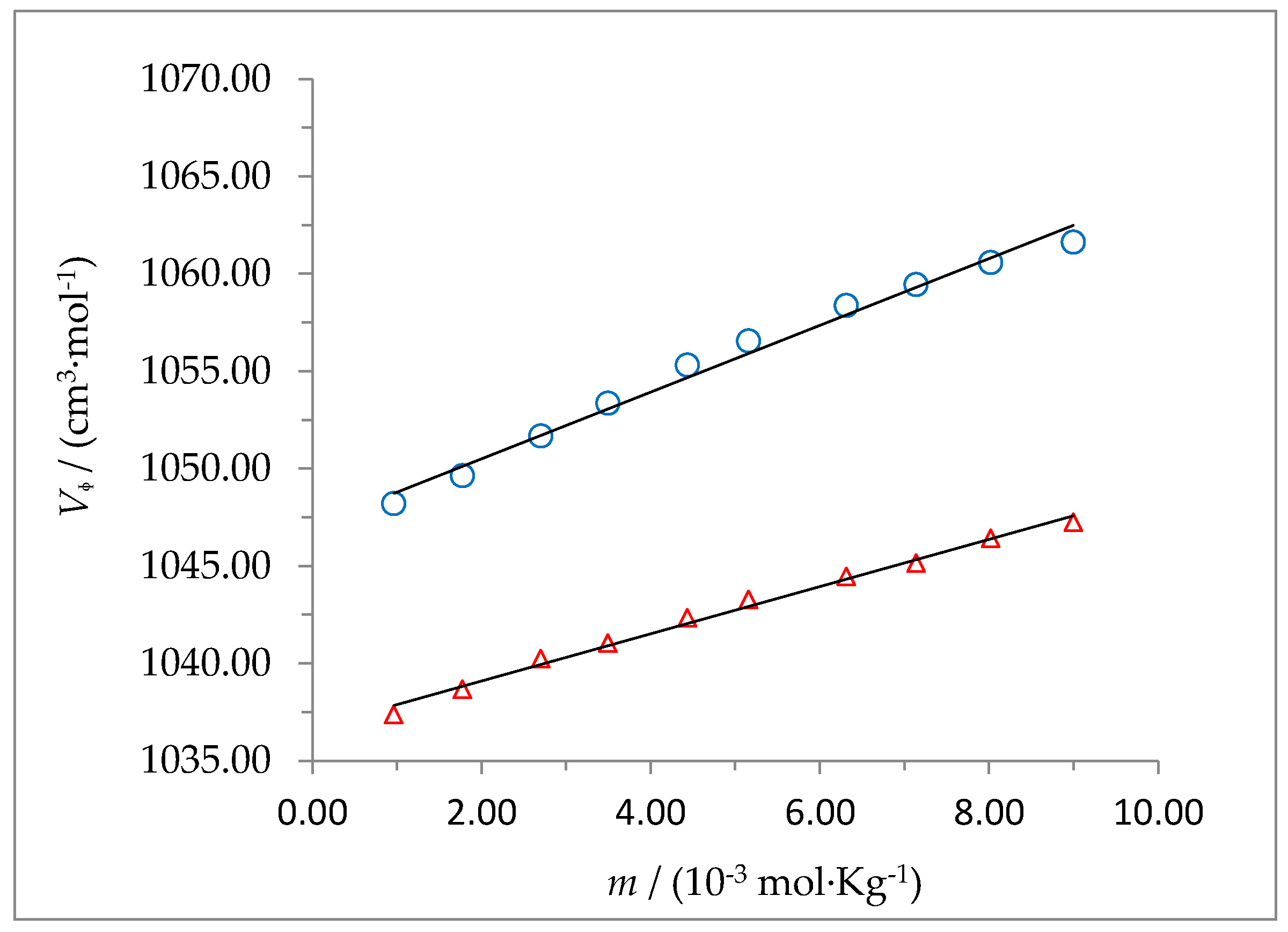
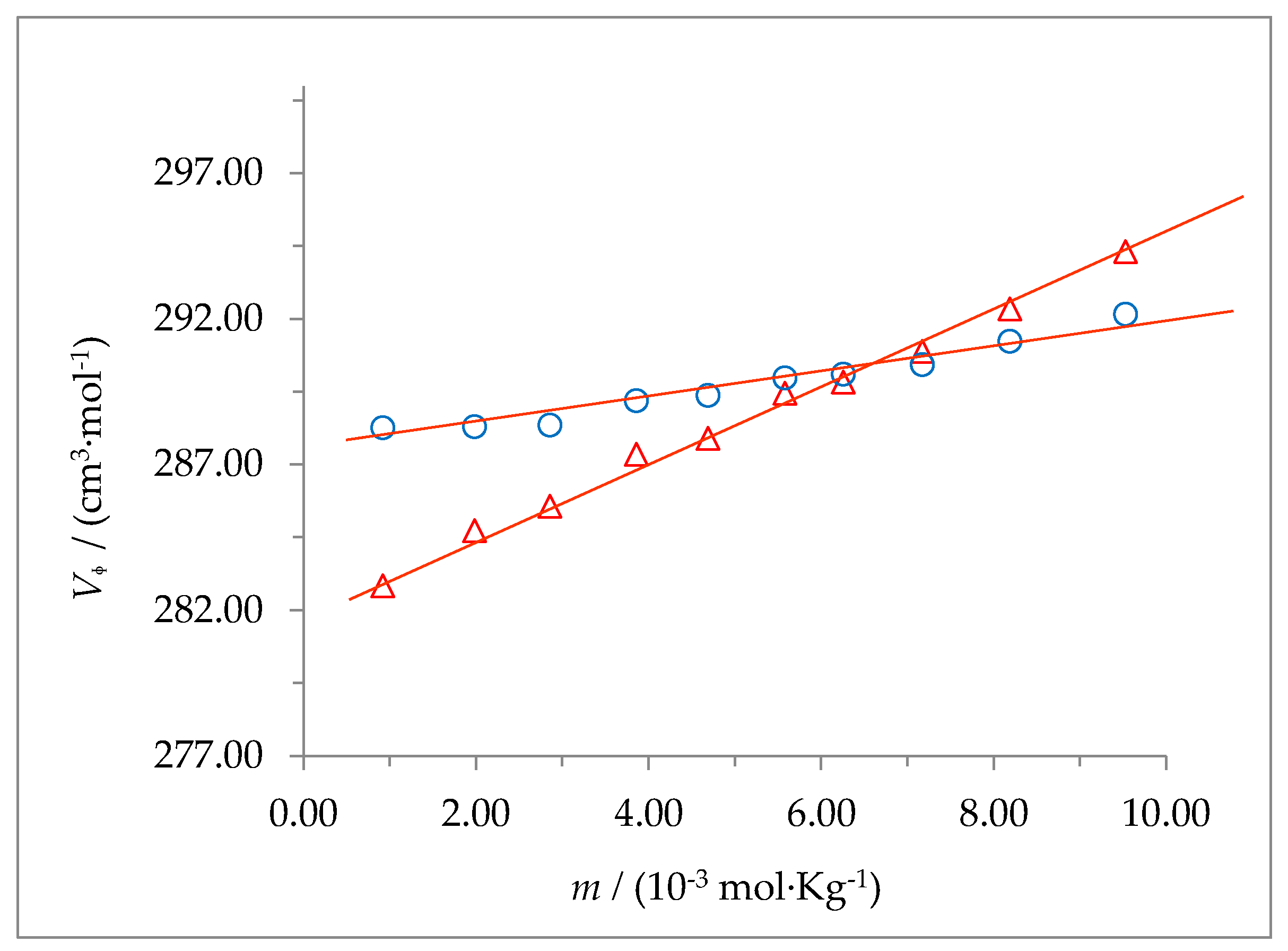
The dependence of
Vϕ on
m was also analyzed using equation 2 and is shown in
Figure 6 and
Figure 7 for the systems 2-HP-β-CD + water + HC and 2-HP-γ-CD + water + HC, respectively, at 298.15 and 310.15 K. The values of
and
for the ternary systems indicated above, together with their respective uncertainties, are shown in
Table 6 at the working temperatures.
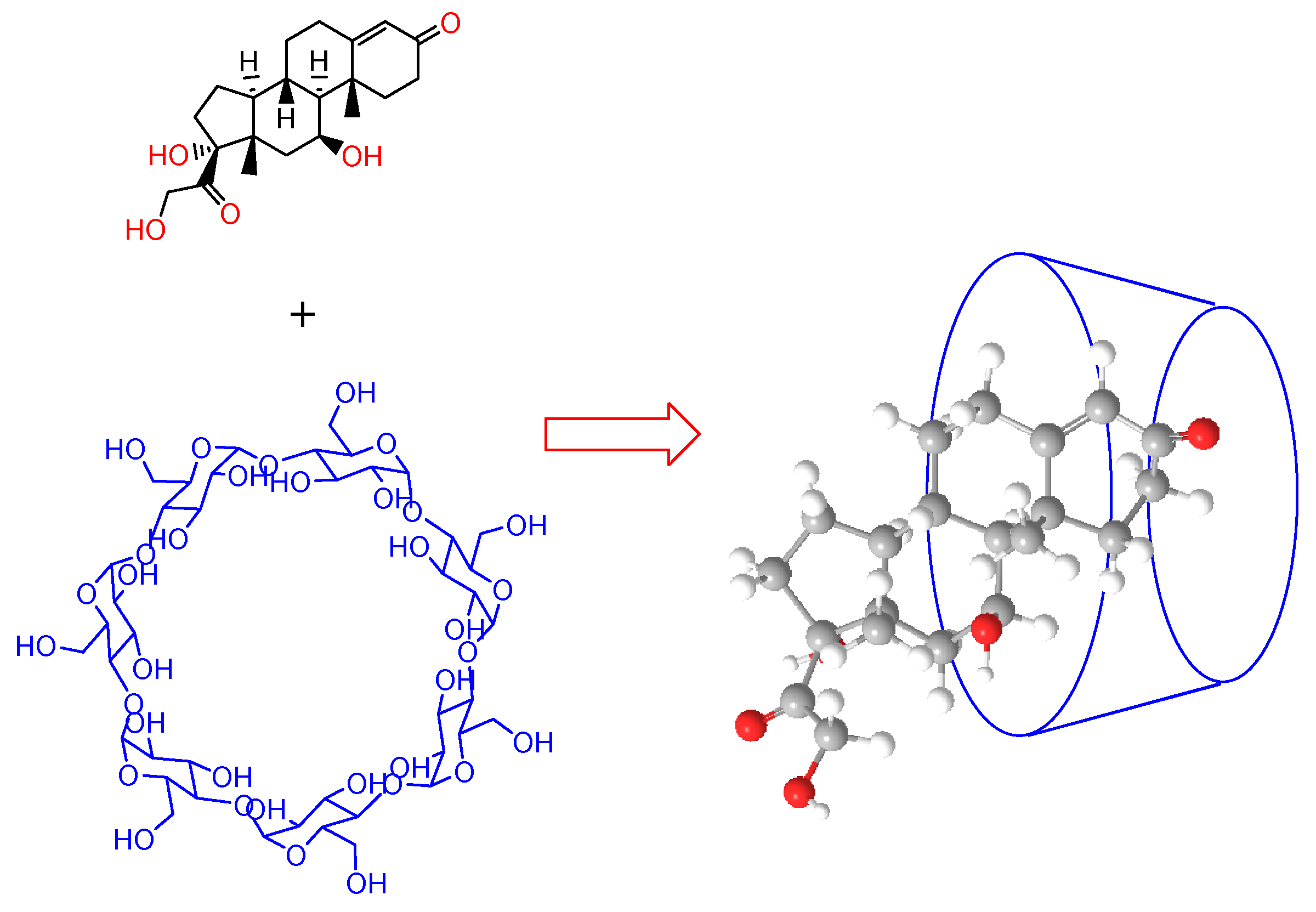
The drastic change of
at both temperatures, as a consequence of the presence of hydrocortisone in the solution, suggests the formation of a host-guest complex. This agrees with the fact, observed in this work using other techniques such as
1H-NMR, that hydrocortisone (which is poorly soluble in water when found alone) is solubilized in water due to the presence of 2-HP-β-CD or 2-HP-γ-CD. The application of the Hyp-NMR program shown that the percentage of free HC in the solution is close to zero, which means that all the HC is solubilized because it is forming part of a complex with the cyclodextrin. A possible explanation may be that the hydroxyl groups located on the narrower side of the truncated cone (primary) and those on the opposite side, at the wider end (secondary), of the cyclodextrins interact with the carbonyl and the hydroxyl groups of hydrocortisone when it enters the cone, breaking hydrogen bonds between the macrocycle and the solvent (as it is known, interactions are competitive with each other; that is, if the solute-solute interaction increases, it does so at the expense of a decrease in the solute-solvent interaction and vice versa). This fact results in a lower number of water molecules around the complex compared to the free cyclodextrin. The fact that the value for
(2-HP-γ-CD system + water + HC) is lower than that of
(2-HP-β-CD system + water + HC), at both temperatures, despite the fact that 2-HP-γ-CD has one more unit of D-(+)-glucose, suggests that the effect of decreasing the number of water molecules around the complex formed is greater for 2-HP-γ-CD. This effect is smaller when the temperature increases. Once inside the cavity, hydrocortisone presents hydrophobic interactions with the glucose rings of cyclodextrins, as was evidenced by the
1H-NMR technique (
Figure 3c).
Figure 8 presents a proposal of the hydrocortisone-2-hydroxypropyl-β-cyclodextrin complex.
On the other hand, the increase in the concentration of solute in the system causes an additional effect (although smaller) on the structure of the solvent. Thus, the decrease with concentration observed in the
Vϕ value for the 2-HP-β-CD + water + HC system, at both temperatures (
Figure 6), suggests an increase of the solute-solute interaction and, consequently, an increase in the degree of solvent structure around the solute complex molecule, as the number of solute molecules in the system increases. On the contrary, for the 2-HP-γ-CD + water + HC system, the increase with concentration observed in the
Vᶲ value (
Figure 7) suggests a decrease in the degree of solvent structure in the solution as the concentration of the solute in the system increases.
3. Materials and Methods
3.1. General Information
All reagents and solvents used in the present study were of analytical grade and they were used without further purification. 2-hydroxypropyl-β-cyclodextrin (95% purity, 18.2% water mass fraction) was provided by Sigma-Aldrich, and 2-hydroxypropyl-γ-cyclodextrin (95% purity, 10% water mass fraction) and hydorcortisone (98% purity) were provided by Fluka. These substances were stored in a desiccator over silica gel. Milli Q water (κ = 5.6 × 10−8 S cm−1) was used as solvent.
3.2. FT-IR Analysis
IR spectra were recorded on a Thermo Fisher Scientific Nicolet iS10 FT-IR spectrometer with the Monolithic Diamond ATR accessory and absorption in cm–1. The sample preparation of each of the systems under study was carried out by mixing equimolar amounts of HC and 2-HP-CD (α, β or γ) dissolved in 5 mL of water. The resulting mixture was stirred for 1 hour, at room temperature, then the solvent was evaporated and finally the IR spectrum of the resulting solid sample was obtained.
3.3. 1H-NMR Analysis
1H-NMR spectra were recorded in DMSO-d6 at 400 MHz using a Bruker Avance 400 instrument. Chemical shifts are reported in ppm, using the residual solvent signal as a reference. A total of seven samples were prepared, four of them are reference samples: the guest (hydrocortisone) and the three cyclodextrins: 2-hydroxypropyl-α-cyclodextrin, 2-hydroxypropyl-β-cyclodextrin and 2-hydroxypropyl-γ-cyclodextrin. The remaining 3 samples consisted of mixtures of a fixed amount of the respective cyclodextrin (20 mg in 600 μL), and the hydrocortisone solution (0.27 M) until a 1:1 cyclodextrin-hydrocortisone molar ratio was reached. The total volume of solution prepared was, in all cases, 700 μL.
The binding capacity of cyclodextrins with hydrocortisone was studied by 1H-NMR. To do this, variable amounts of hydrocortisone were added to the solution of the host macrocycle (2-HP-β-CD or 2-HP-γ-CD). After each addition, the 1H-NMR spectrum was recorded. The guest and host concentrations in the solution were corrected for each addition. Binding constants for the complexes were determined using the HYPERQUAD program.
3.4. Density Measurements
Densities were measured with an Anton Paar DMA 5000 densimeter, which uses the vibrating U tube principle (operating at a frequency of 3 MHz). It has a sensitivity of 1 × 10–6 g∙cm–3 and a reproducibility of ±5 × 10–6 g.cm–3 in the ranges of (0-90)°C of temperature and (0-1.0) MPa of pressure. All measurements were performed at local atmospheric pressure (101 kPa). This densimeter is equipped with a Peltier-type thermostatic unit that guarantees a temperature control of ±0.005 K. Before each series of measurements, verification of the instrument was carried out by using successively dry air and degassed-ultrapure water (Milli-Q quality) at 293.15 K according to the manufacturer’s recommendations.
Solutions were prepared by weighing their components: Millipore-Q water (specific conductivity 5.6 × 10
−6 S m
−1) and the solute(s), using a Mettler AE240 analytical balance that has a precision of 1 × 10
−5 g in the range used. The water content of the solutes was taken into account to calculate the concentrations of the solutions. All solutions were degassed before each experiment. The reported density data are the average of at least three independent measurements that were reproducible within 1 × 10
−3 kg m
−3, with an uncertainty of 0.150 kg m
−3 [
34].
4. Conclusions
Based on the solubility and FT-IR evaluations as well as the results of density and 1H-NMR, reported in this work, the complexing capacity of three macrocycle systems was studied: 2-hydroxypropyl-α-cyclodextrin, 2-hydroxypropyl-β-cyclodextrin and 2-hydroxypropyl-γ-cyclodextrin, with hydrocortisone. From the analysis of this information, it is clear that hydrocortisone interacts with 2-hydroxypropyl-β-cyclodextrin and with 2-hydroxypropyl-γ-cyclodextrin to form HC–CD complexes, either in solution or in the solid state, while the interaction of hydrocortisone with 2-hydroxypropyl-α-cyclodextrin was not evident in either solution or in solid state. The results observed by NMR suggested that the ring of the ketone group of hydrocortisone could enter the cavity of the cyclodextrin, in which case hydrogen bonding interactions between the hydroxyl groups of the cyclic system of cyclodextrin and the carbonyl group of the steroid hormone would predominate. Additionally, the complex can also be stabilized by hydrophobic interactions with the glucose rings. Finally, density measurements show the drastic change of for both solutes at the two temperatures (298.15K or 310.15K) in the presence of hydrocortisone, confirming the formation of host-guest, hydrocortisone-cyclodextrin, complexes.
Supplementary Materials
The supporting information can be downloaded at:
Author Contributions
Conceptualization, M.M., E.S., C.M.R. and M.A.E.; Data curation, M.M., E.S., C.M.R. and M.A.E.; Formal analysis, M.M., E.S. C.M.R. and M.A.E.; Investigation, M.M. and E.S.; Methodology, M.M. and E.S.; Project administration, M.A.E.; Resources, M.A.E.; Software, E.S. and M.M; Supervision, M.A.E.; Validation, M.M., E.S. C.M.R. and M.A.E.; Visualization, M.M., E.S. C.M.R. and M.A.E.; Writing—original draft, M.M. and E.S.; Writing—review and editing, C.M.R. and M.A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive external funding
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yun-Da, Y.; Yuan-Yuan, C.; Cheng-Xiong, Y. Construction of cyclodextrin microporous organic network based drug delivery platform for controllable release and targeting delivery of doxorubicin. Chem. Eng. J. Adv. 2023, 14, 100487. [Google Scholar]
- Abuhasan, M. O.; El-Barghouthi, M. I.; Bodoor, K.; Monem, A.; Assaf, K. I. Molecular recognition of tripeptides containing tryptophan by cucurbit [8]uril: A computational study. Arabian J. Chem. 2023, 16, 104819. [Google Scholar] [CrossRef]
- Harit, T.; Bellaouchi, R.; Mokhtari, C.; El Bali, B.; Asehraou, A.; Malek, F. New generation of tetrapyrazolic macrocycles: Synthesis and examinaton of their complexation properties and antibacterial activity. Tetrahedron 2017, 73, 5138–5143. [Google Scholar] [CrossRef]
- Español, E.S.; Villamil, M.M. Calixarenes: Generalities and their role in improving the solubility, biocompatibility, stability, bioavailability, detection, and transport of biomolecules. Biomolecules. 2019, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Kashapov, R.; Kashapov, N.; Razuvayeva, Y.; Ziganshina, A.; Salnikov, V.; Zakharova, L. Green-step assembly of the supramolecular amphiphile constructed by sodium carboxymethyl cellulose and calixarene for facile loading of hydrophobic food bioactive compounds. Food Chem. 2023, 424, 136293. [Google Scholar] [CrossRef]
- Syakaev, V.V.; Morozova, J.E.; Bogdanov, A.V.; Shalaeva, Y.V.; Ermakova, A.M.; Voloshina, A.D.; Zobov, V.V.; Nizameev, I.R.; Kadirov, M.K.; Morinov, V.F.; Kanovalol, A.I. Solubilization of azo-dye-modified isatin derivative by amphiphilic carboxyresorcinarenes: The effect of macrocycle structure on the supramolecular association. Colloids Surf. A. 2018, 553, 368–377. [Google Scholar] [CrossRef]
- Ghosh, T.; Das, A.K. Dynamic boronate esters cross-linked guanosine hydrogels: A promising biomaterial for emergent applications. Coord. Chem. Rev. 2023, 488, 215170. [Google Scholar] [CrossRef]
- TitCeron, C.; Calori, I. R.; Marques, E.; Tedesco, A.C. Evaluation of aluminum phthalocyanine chloride and DNA interactions for the design of an advanced drug delivery system in photodynamic therapy. Spectrochim. Acta, Part A. 2018, 201, 242–248. [Google Scholar]
- Garcia, A.; Leonardi, D.; Lamas, C.M. Promising applications in drug delivery systems of a novel β-cyclodextrin derivative obtained by green synthesis. Bioorg. Med. Chem. Lett. 2016, 26, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Celebioglu, A.; Uyar, T. Orally fast-dissolving drug delivery systems for pediatrics: Nanofibrous oral strips from isoniazid/cyclodextrin inclusion complexes. J. Drug Delivery Sci. Technol. 2023, 85, 104584. [Google Scholar] [CrossRef]
- Santos, C.; Ribeiro, A.C.; Esteso, M.A. Drug Delivery Systems: Study of Inclusion Complex Formation between Methylxanthines and Cyclodextrins and Their Thermodynamic and Transport Properties. Biomolecules. 2019, 9, 196. [Google Scholar] [CrossRef]
- Peng-Wei, X.; Xiao-Fan, Y.; Hang, L.; Zhu, Y.; Zhao, B. Preparation, characterization, and physicochemical property of the inclusion complexes of Cannabisin A with β-cyclodextrin and hydroxypropyl-β-cyclodextrin. J. Mol. Struct. 2023, 1272, 134168. [Google Scholar]
- Singh, B.; Mohan, M.; Kumar, R. Synthesis of hydrocortisone containing dietary fiber almond gum-based hydrogels as sustained drug delivery carriers for use in colon inflammation. Food hydrocolloids for health 2022, 2, 100057. [Google Scholar] [CrossRef]
- Jahanbekam, S.; Mozafari, N.; Bagheri-Alamooti, A.; Mohammadi-Samadi, S.; Daneshamouz, S.; Helari, R.; Azarpira, N.; Ashrafi, H.; Azadi, A. Ultrasound-responsive hyaluronic acid hydrogel of hydrocortisone to treat osteoarthritis. Int. J. Biol. Macromol. 2023, 240, 124449. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Pan, M.; Lei, M.; Zhou, X.; Hu, D.; Liu, Q.; Chen, Yu.; Li, W.; Qian, Z. A novel composite of micelles and hydrogel for improving skin delivery of hydrocortisone and application in atopic dermatitis therapy. Appl. Mater. Today 2020, 19, 100593. [Google Scholar] [CrossRef]
- Sanjana, A.; Ahmed, M.G.; Gowda, Bh.J. Preparation and evaluation of in-situ gels containing hydrocortisone for the treatment of aphthous ulcer. J. Oral Biol. Craniofac. Res., 2021, 11, 269–276. [Google Scholar] [CrossRef]
- Casas-Hinestroza, J.L.; Pérez-Redondo, A.; Maldonado, M. Inclusion complexation between neurotransmitters with polyacetylated calix [4]pyrogallolarenes: 1H-NMR and crystallographic analysis, Res. Chem. Intermed. 2022, 48, 3091–3107. [Google Scholar] [CrossRef]
- Maldonado, M.; Sanabria, E.; Velasquez-Silva, A.; Casas-Hinestroza, J.L.; Esteso, M.A. Comparative study of the volumetric properties of three regioisomers of diazoted C-tetra(propyl)resorcin [4]arene in DMSO at various temperaturas. J. Mol. Liq. 2021, 325, 115252. [Google Scholar] [CrossRef]
- Castillo-Aguirre, A.; Maldonado, M.; Esteso, M.A. Removal of Toxic Metal Ions Using Poly(BuMA–co–EDMA) Modified with C-Tetra(nonyl)calix [4]resorcinarene. Toxics, 2020, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Aguirre, A.; Esteso, M.A.; Maldonado, M. Resorcin [4]arenes: Generalities and their role in the modification and detection of amino acids, Curr. Org. Chem. 2020, 24, 2412–2425. [Google Scholar] [CrossRef]
- Baulsir, C.F.; Simler, R.J. Design and evaluation of IR sensors for pharmaceutical sensing. Adv. Drug Delivery Rev. 1996, 21, 191–203. [Google Scholar] [CrossRef]
- Namazi, H.; Hamrahloo, Y.T. Novel PH sensitive nanocarrier agents based on citric acid dendrimers containing conjugated β-cyclodextrins. Adv. Pharm. Bull. 2011, 1, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Dickert, F.L.; Landgraf, S.; Sikorski, R. Host-guest chemistry and chemical sensors: FT-IR-analysis and spectra simulation of CDCI3 inclusion. J. Mol. Model. 2000, 6, 491–497. [Google Scholar] [CrossRef]
- Khomutov, S.; Donova, M.V. Nanodimer cyclodextrin ligands with high affinity to steroids. J. Inclusion Phenom. Macrocyclic Chem. 2011, 70, 353–357. [Google Scholar] [CrossRef]
- Cai, W.; Sun, T.; Liu, P.; Chipot, C.; Shao, X. Inclusion mechanism of steroid drugs into β-cyclodextrins. insights from free energy calculations. J. Phy. Chem. B. 2009, 113, 7836–7843. [Google Scholar] [CrossRef] [PubMed]
- Al-Sou’od, K.A. Investigation of the hydrocortisone-β-cyclodextrin complex by phase solubility method: Some theoretical and practical considerations. J. Solution Chem. 2008, 37, 119–133. [Google Scholar] [CrossRef]
- Chen, R.; Wang, J.; Liao, C.; Ma, N.; Zhang, L.; Wang, X. 1H NMR studies on serum metabonomic changes over time in a kidney-Yang deficiency syndrome model. RSC Adv. 2017, 7, 34251–34261. [Google Scholar] [CrossRef]
- Wechter, W.J.; Slomp, G.; MacKellar, F.A.; Wiechert, R.; Kerb, U. Configuration and conformation of the A-ring of 1-methyl steroids. Tetrahedron 1965, 21, 1625–1634. [Google Scholar] [CrossRef]
- Xu, P.-W.; Yuan, X.-F.; Li, H.; Zhu, Y.; Zhao, B. Preparation, characterization, and physicochemical property of the inclusion complexes of Cannabisin A with β-cyclodextrin and hydroxypropyl-β-cyclodextrin. J. Mol. Struct. 2023, 1272, 134168. [Google Scholar] [CrossRef]
- Mokhtar, M.S.; Suliman, F.O.; Elbashir, A.A. Atrazine and ametryne inclusion complexes with 2-hydroxypropyl-β/γ-cyclodextrin: Spectroscopic studies and molecular dynamics simulation. J. Mol. Struct. 2019, 1179, 161–170. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, R.; Xu, J.; Du, W.; Wang, X.; Fang, W. Thermodynamic (volume and viscosity) of amino acids in aqueous hydroxypropyl-b-cyclodextrin solutions at different temperaturas. J. Chem. Thermodyn. 2017, 113, 388–393. [Google Scholar] [CrossRef]
- Riveros, D. C.; Hefter, G.; Vargas, E. Thermodynamic evidence for nano-heterogeneity in solutions of the macrocycle C-butylresorcin [4]arene in non-aqueous solvents. J. Chem. Thermodyn. 2018, 125, 250–256. [Google Scholar] [CrossRef]
- Ali, S.W.; Sharma, V. Drug nanocrystals: emerging trends in pharmaceutical industries. Ind. Appl. Nanocrystals, 2022, 97-115.
- Taylor, B.N.; Kuyatt, C.E. Guidelines for Evaluating and Expressing the Uncertainty of NIST Measurement Results; NIST Technical Note 1297; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1994. [Google Scholar]
Figure 1.
Hydroxypropyl-cyclodextrins {n = 5(α), 6 (β) and 7(γ)} and hydrocortisone.
Figure 1.
Hydroxypropyl-cyclodextrins {n = 5(α), 6 (β) and 7(γ)} and hydrocortisone.
Figure 2.
FT-IR of hydrocortisone (red,

),
2-hydroxypropyl-β-cyclodextrin (purple,

) and equimolar mixture of
hydrocortisone and 2-hydroxypropyl-β-cyclodextrin (green,

).
Figure 2.
FT-IR of hydrocortisone (red,

),
2-hydroxypropyl-β-cyclodextrin (purple,

) and equimolar mixture of
hydrocortisone and 2-hydroxypropyl-β-cyclodextrin (green,

).
Figure 3.
1H-NMR of hydrocortisone (a), 2-hydroxypropyl-β-cyclodextrin (b) and equimolar mixture of hydrocortisone and 2-hydroxypropyl-β-cyclodextrin (c).
Figure 3.
1H-NMR of hydrocortisone (a), 2-hydroxypropyl-β-cyclodextrin (b) and equimolar mixture of hydrocortisone and 2-hydroxypropyl-β-cyclodextrin (c).
Figure 4.
Apparent molar volumes of 2-hydroxypropyl-β-cyclodextrin in aqueous solution at (Δ) 298.15, and (○) 310.15 K.
Figure 4.
Apparent molar volumes of 2-hydroxypropyl-β-cyclodextrin in aqueous solution at (Δ) 298.15, and (○) 310.15 K.
Figure 5.
Apparent molar volumes of 2-hydroxypropyl-γ-cyclodextrin in aqueous solution at (Δ) 298.15, and (○) 310.15 K.
Figure 5.
Apparent molar volumes of 2-hydroxypropyl-γ-cyclodextrin in aqueous solution at (Δ) 298.15, and (○) 310.15 K.
Figure 6.
Apparent molar volumes of the 2-HP-β-CD + water + HC system at (Δ) 298.15, and (○) 310.15 K.
Figure 6.
Apparent molar volumes of the 2-HP-β-CD + water + HC system at (Δ) 298.15, and (○) 310.15 K.
Figure 7.
Apparent molar volumes of the 2-HP-γ-CD + water + HC system at (Δ) 298.15 and (○) 310.15 K.
Figure 7.
Apparent molar volumes of the 2-HP-γ-CD + water + HC system at (Δ) 298.15 and (○) 310.15 K.
Figure 8.
Proposed interaction for the hydrocortisone-2-hydroxypropyl-β-cyclodextrin complex.
Figure 8.
Proposed interaction for the hydrocortisone-2-hydroxypropyl-β-cyclodextrin complex.
Table 1.
Densities and apparent molar volumes for aqueous solutions of 2-HP-β-CD as a function of molality, at temperatures 298.15 K and 310.15 K.
Table 1.
Densities and apparent molar volumes for aqueous solutions of 2-HP-β-CD as a function of molality, at temperatures 298.15 K and 310.15 K.
| |
T = 298.15 K |
T = 310.15 K |
m(2-HP-β-CD)/
(10–3 mol·kg–1) |
ρ/
(g·cm–3) |
Vϕ/
(cm3· mol–1) |
ρ/
(g·cm–3) |
Vϕ/
(cm3· mol–1) |
| 0.00000 |
0.998203 |
- |
0.993340 |
- |
| 0.83453 |
0.998637 |
860.2 |
0.993752 |
888.6 |
| 1.69900 |
0.999085 |
860.7 |
0.994179 |
888.0 |
| 2.49719 |
0.999494 |
862.5 |
0.994575 |
886.9 |
| 3.55141 |
1.000033 |
863.8 |
0.995097 |
886.3 |
| 3.94013 |
1.000230 |
864.4 |
0.995291 |
885.7 |
| 5.02342 |
1.000777 |
866.0 |
0.995831 |
884.5 |
| 5.89585 |
1.001211 |
867.8 |
0.996268 |
883.3 |
| 6.69002 |
1.001624 |
866.3 |
0.996663 |
882.9 |
| 7.46110 |
1.001984 |
870.6 |
0.997045 |
882.7 |
| 8.21760 |
1.002389 |
867.6 |
0.997426 |
881.7 |
Table 2.
Densities and apparent molar volumes for aqueous solutions of 2-HP-γ-CD as a function of molality, at temperatures 298.15 K and 310.15 K.
Table 2.
Densities and apparent molar volumes for aqueous solutions of 2-HP-γ-CD as a function of molality, at temperatures 298.15 K and 310.15 K.
| |
T = 298.15 K |
T = 310.15 K |
m(2-HP-γ-CD)/
(10–3 mol·kg–1) |
ρ/
(g·cm–3) |
Vϕ/
(cm3· mol–1) |
ρ/
(g·cm–3) |
Vϕ/
(cm3· mol–1) |
| 0.963253 |
0.998729 |
1037.4 |
0.993859 |
1048.2 |
| 1.775605 |
0.999167 |
1038.7 |
0.994290 |
1049.6 |
| 2.702434 |
0.999663 |
1040.2 |
0.994777 |
1051.7 |
| 3.495164 |
1.000086 |
1041.1 |
0.995190 |
1053.4 |
| 4.432124 |
1.000582 |
1042.3 |
0.995674 |
1055.3 |
| 5.157489 |
1.000964 |
1043.3 |
0.996047 |
1056.6 |
| 6.310388 |
1.001569 |
1044.5 |
0.996636 |
1058.4 |
| 7.136245 |
1.002001 |
1045.2 |
0.997056 |
1059.5 |
| 8.019974 |
1.002457 |
1046.4 |
0.997503 |
1060.6 |
| 8.991847 |
1.002960 |
1047.2 |
0.997993 |
1061.6 |
Table 3.
Limiting partial molar volumes, , and experimental slopes, , for 2-HP-β-CD and 2-HP-γ-CD in water, at temperatures 298.15 K and 310.15 K. (Eq. 2).
Table 3.
Limiting partial molar volumes, , and experimental slopes, , for 2-HP-β-CD and 2-HP-γ-CD in water, at temperatures 298.15 K and 310.15 K. (Eq. 2).
| |
T = 298.15K |
T = 310.15K |
| |
|
Bv
|
|
Bv
|
| 2-HP-β-CD |
859.3(±0.4) |
1244.7(±113) |
889.4(±0.1) |
–950.6 (±30) |
| 2-HP-γ-CD |
1036.7(±0.2) |
1212(±39) |
1047.1(±0.2) |
1712.6 (±60) |
Table 4.
Molality, density and apparent molar volume for 2-HP-β-CD + water + HC, at temperatures 298.15 K and 310.15 K.
Table 4.
Molality, density and apparent molar volume for 2-HP-β-CD + water + HC, at temperatures 298.15 K and 310.15 K.
| |
T = 298.15 K |
T = 310.15 K |
m(2-HP-β-CD)/
(10–3 mol·Kg–1) |
ρ/
(g·cm–3) |
Vϕ/
(cm3· mol–1) |
ρ/
(g·cm–3) |
Vϕ/
(cm3· mol–1) |
| 0.914009 |
0.998696 |
298.2 |
0.993825 |
283.8 |
| 1.977355 |
0.999217 |
295.9 |
0.994337 |
283.7 |
| 2.847149 |
0.999686 |
295.1 |
0.994803 |
283.4 |
| 3.852493 |
1.000299 |
293.3 |
0.995406 |
283.2 |
| 4.680349 |
1.000561 |
291.6 |
0.995667 |
283.0 |
| 5.571206 |
1.001185 |
288.9 |
0.996280 |
282.6 |
| 6.249229 |
1.001676 |
287.7 |
0.996771 |
282.6 |
| 7.168652 |
1.002167 |
286.2 |
0.997239 |
282.6 |
| 8.182700 |
1.002617 |
284.5 |
0.997705 |
282.2 |
| 9.523399 |
1.003153 |
281.5 |
0.998199 |
281.6 |
Table 5.
Molality, density and apparent molar volume for 2-HP-γ-CD + water + HC, at temperatures 298.15 K and 310.15 K.
Table 5.
Molality, density and apparent molar volume for 2-HP-γ-CD + water + HC, at temperatures 298.15 K and 310.15 K.
| |
T = 298.15 K |
T = 310.15 K |
m2-HP-γ-CD/
(10–3 mol·Kg–1) |
ρ/
(g·cm–3) |
Vϕ/
(cm3· mol–1) |
ρ/
(g·cm–3) |
Vϕ/
(cm3· mol–1) |
| 0.914009 |
0.998804 |
282.8 |
0.993928 |
288.3 |
| 1.977355 |
0.999321 |
284.7 |
0.994439 |
288.3 |
| 2.847149 |
0.999881 |
285.6 |
0.994991 |
288.4 |
| 3.852493 |
1.000372 |
287.4 |
0.995476 |
289.2 |
| 4.680349 |
1.000925 |
287.9 |
0.996020 |
289.4 |
| 5.571206 |
1.001365 |
289.4 |
0.996455 |
290.0 |
| 6.249229 |
1.002015 |
289.8 |
0.997092 |
290.1 |
| 7.168652 |
1.002505 |
290.9 |
0.997576 |
290.4 |
| 8.182700 |
1.003025 |
292.3 |
0.998089 |
291.2 |
| 9.523399 |
1.003601 |
294.3 |
0.998665 |
292.2 |
Table 6.
Limiting partial molar volumes, , and experimental slopes, , for the systems 2-HP-β-CD + water + HC and 2-HP-γ-CD + water + HC, at temperatures 298.15 K and 310.15 K.
Table 6.
Limiting partial molar volumes, , and experimental slopes, , for the systems 2-HP-β-CD + water + HC and 2-HP-γ-CD + water + HC, at temperatures 298.15 K and 310.15 K.
| |
T = 298.15K |
T = 310.15K |
| |
|
Bv
|
|
Bv
|
| 2-HP-β-CD + water + HC |
300.2(±0.6) |
–1947.7(±89) |
284.1(±0.2) |
–245.8 (±27) |
| 2-HP-γ-CD + water + HC |
282.0(±0.1) |
1279(±27) |
287.4(±0.1) |
462.6 (±32) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
 ),
2-hydroxypropyl-β-cyclodextrin (purple,
),
2-hydroxypropyl-β-cyclodextrin (purple,  ) and equimolar mixture of
hydrocortisone and 2-hydroxypropyl-β-cyclodextrin (green,
) and equimolar mixture of
hydrocortisone and 2-hydroxypropyl-β-cyclodextrin (green,  ).
).
 ),
2-hydroxypropyl-β-cyclodextrin (purple,
),
2-hydroxypropyl-β-cyclodextrin (purple,  ) and equimolar mixture of
hydrocortisone and 2-hydroxypropyl-β-cyclodextrin (green,
) and equimolar mixture of
hydrocortisone and 2-hydroxypropyl-β-cyclodextrin (green,  ).
).