1. Introduction
Ensuring adequate canal cleanliness and debridement is essential for successful endodontic treatment, allowing infection control, promoting tissue healing and increasing long-term preservation of the tooth by reducing the potential risks of reinfection [
1]. Even if root canal obturation can help entombing bacteria and some filling materials exhibit short-term antibacterial properties, tissue remnants inside root canal can be a potential source of food for remaining bacteria or more likely, for bacteria re-infecting the endodontic space [
2]. Moreover, removing organic and inorganic debris allows for better adaptation of the filling material to the canal walls, which promotes a more effective seal, thus preventing reinfection of the obturated canals [
3]. Overall, proper canal debridement directly impacts on both short-term and long-term clinical outcomes.
Clinicians are aware that the success of endodontic treatment heavily relies on the cleanliness and debridement of the root canal system, which is not easy due to complex canal anatomy and limitations related to the use of instruments mainly designed to shape round canals [
4,
5]. Therefore, they look for improvements in the shaping and cleaning procedures, with new material and techniques, aiming at improving removal of pulp tissue, bacteria and infected debris [
6,
7,
8].
However, despite its crucial clinical relevance, assessing canal cleanliness in vivo is not a simple, well-defined and objective procedure [
9,
10]. It typically involves methods like visual inspection using magnification, detection of debris or dirt on instruments or paper points, which are based mainly on individual judgement. Despite progress in digital imaging techniques, currently radiographs or cone-beam computed tomography (CBCT) cannot detect and evaluate debris and tissue inside canals and canal morphology. Microbiological sampling techniques to detect bacterial presence have also been proposed as an alternative means to decide when shaping and cleaning procedures in vivo are completed, suggesting when they can obturate properly disinfected canals. However, these microbial culturing techniques that quantify bacterial presence (and not residual debris) are not simple to use in a clinical environment and are currently used mostly for in vitro studies [
11,
12,
13].
Assessing canal cleanliness in vitro often involves techniques such as scanning electron microscopy (SEM) to visualize the surface of the canal walls for debris and biofilm, and chemical analyses to detect residual organic or inorganic materials [
14,
15]. These methods can provide valuable images of the cleanliness of root canal systems in laboratory settings, but there is a limitation related to the fact that it is not easy and/or reliable to evaluate and count debris taking in considerations all the canal walls surfaces. Using digital imaging analysis software may help to quantify debris removal more precisely and consistently, but still it is a procedure usually limited to small portions of the canal space. The same problem can also be a limit of histological studies, which usually show only small part of the canals [
16].
More recently techniques like fluorescence-based imaging or spectroscopy have been proposed as an aid in detecting bacteria and residual organic material [
17]. These technologies can help assessing the cleanliness during endodontic procedures and could be used both in vitro and in vivo.
The aim of the present study was to evaluate the in vitro sensitivity and precision of a new device to quantify the presence of organic debris inside an artificial root canal.
2. Materials and Methods
2.1. Specimen Selection
Five single channel endodontic transparent resin training blocks were selected. The artificial canals (SystemB blocks, Kerr, Glendora, CA, USA) were designed for evaluation of root canal filling techniques and consequently their dimensions were approximately .06 tapered with apical size 25. Such transparent blocks were also chosen to visually check the cleanliness and dirt of the specimens. A 60°curvature was present in the apical third with a radius of 5 mm, thus allowing proper insertion of needles and other irrigating devices (
Figure 1). Moreover, the artificially prepared canals were selected to avoid variation in canal shaper and in debris production generated by mechanical instrumentation. Therefore, there was no need to instrument or prepare the artificial canals and the only variables were related to artificial contaminations and subsequent debridement. The blocks were not instrumented to avoid the creation of debris, revealed from a preliminary analysis. Sample size was determined by Power Analysis and calculated based on preliminary data obtained after 4 initial measurements with a power of 80% and a 0.05 alpha type error (G*Power, Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany). With an effect size of 1.48, sample calculation was 3 and consequently a total number of 5 artificial blocks was considered sufficient to provide significant data.
2.2. Device
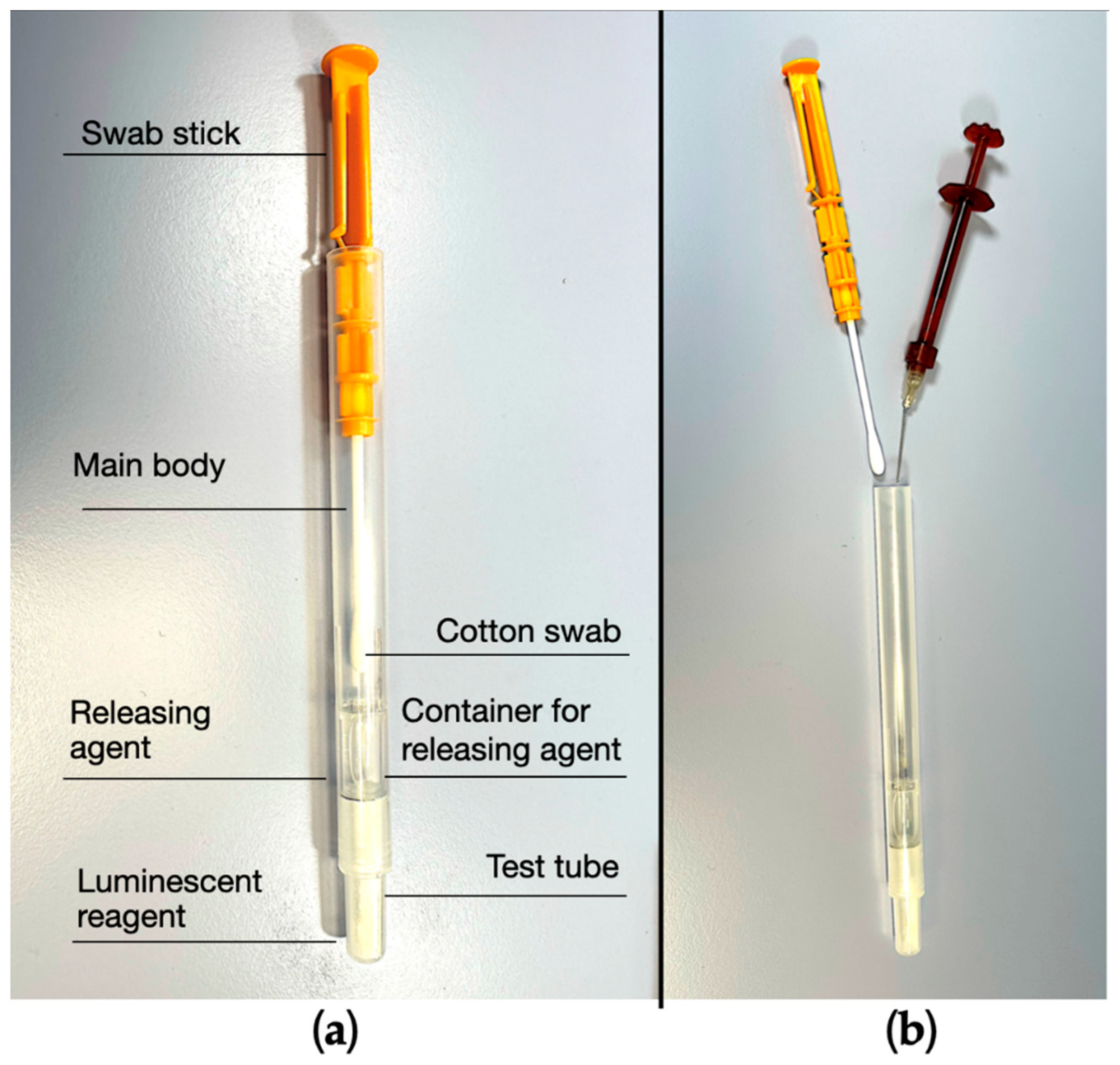
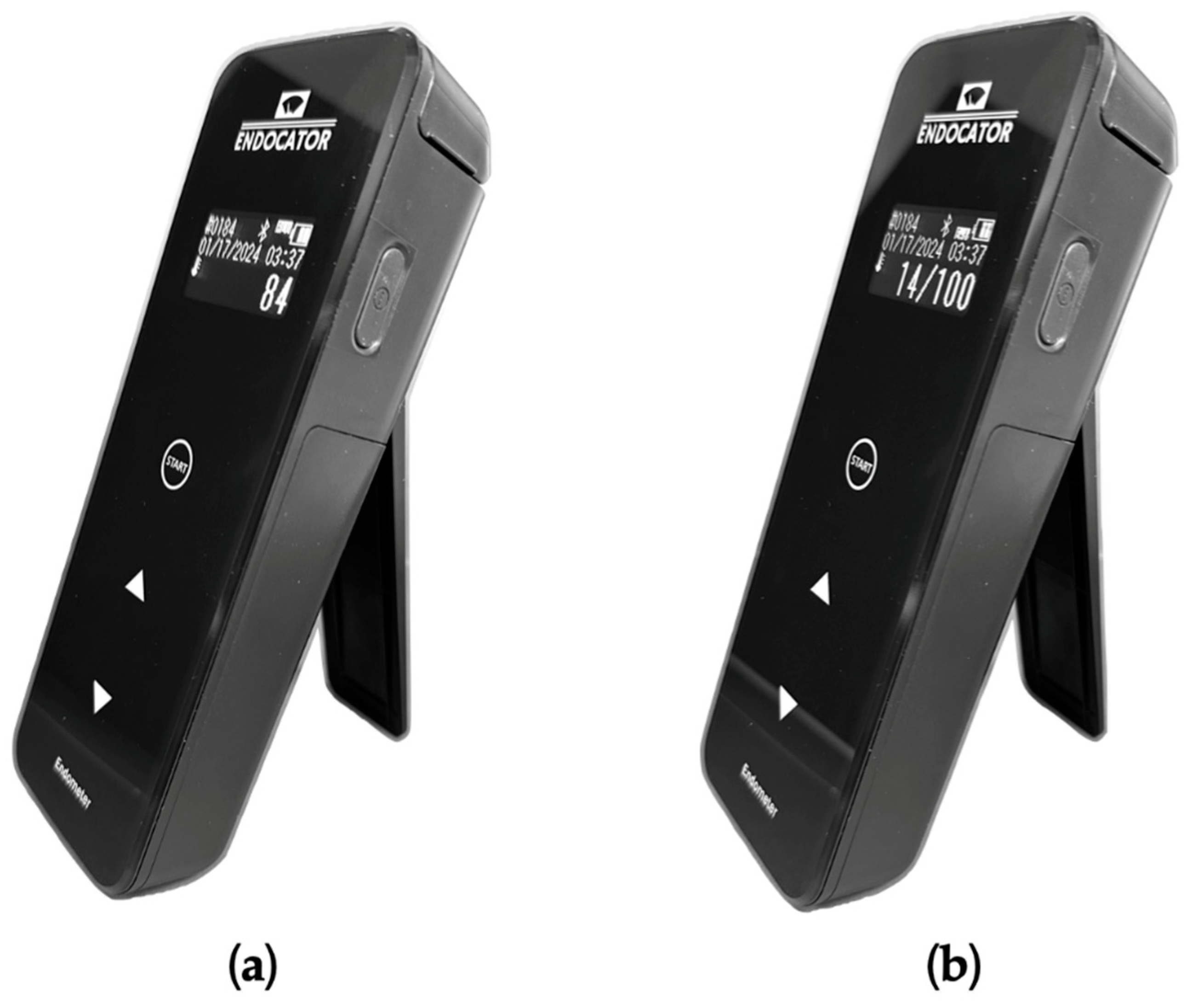
The analyzed procedure is based on the use of 1 device consisting of a dedicated swab (Endotester, Endocator Inc, Aptos, CA, USA) and a luminometer (Endocator, Endocator Inc, Aptos, CA, USA) (
Figure 2 and
Figure 3). Endotester is containing the swab and the reagent for testing of a root canal (
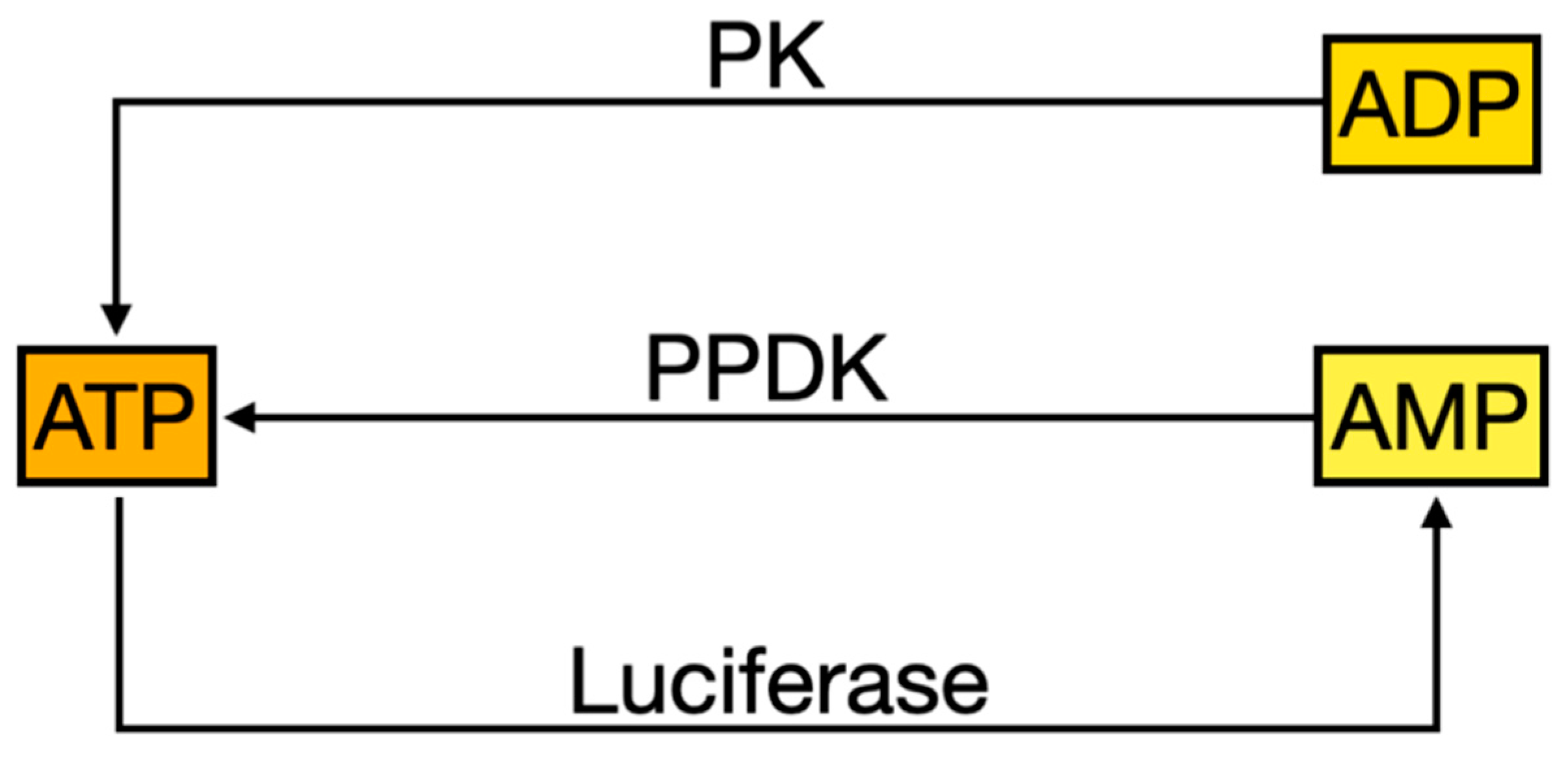
Figure 2). Endotester uses an enzyme cycling method based on a combination of luminescent reactions from firefly luciferase, pyruvate, orthophosphate dikinase (PPDK) and pyruvate kinase (PK). This method produces a given amount of luminescence that is proportional to the amounts of adenosine triphosphate (ATP), adenosine diphosphate (ADP) and adenosine monophosphate (AMP) present. ATP is a source of energy necessary for various forms of life that are present in organic residues, such as microorganisms and biological substances that originate from other living organisms. This ATP monitoring system allows you to measure and detect organic residues at high speed and high sensitivity by detecting ATP using luciferase, which is why it is widely used in determining cleanliness levels. However, conventional ATP monitoring system is insufficient because ADP and AMP generated from ATP degradation are completely overlooked. A new ATP + ADP + AMP monitoring system as shown in
Figure 4. This method definitely enables highly sensitive analyses of a wider range of organic residues. This kit is a simple integrated testing instrument that contains both the test reagent and the swab device required for testing cleanliness levels (
Figure 2). The luminescence is measured by the Endocator (
Figure 3).
2.3. Samples and Sampling Procedure
All the samples were collected from blocks, that were not instrumented and/or cleaned because from a preliminary analysis we found that the blocks provided by the manufacturer inside their plastic packaging already contained some organic contamination, even if there was no visual sign of any debris or manufacturing remnants inside the canal (
Figure 1).
To test the efficacy and reliability of this novel device in vitro, for each canal 3 consecutive measurements were taken and compared. The null hypothesis was that there should be no significant difference amongst the 5 samples and there should be no significant difference between the three consecutive measurements on the same samples.
The sampling procedure strictly followed the instruction of use (IFU) provided by the manufacturers. An endodontic needle mounted on a syringe was inserted into the channel to rinse with 1 ml distilled water. A delicate up and down movement of the needle was performed to agitate the irrigating solution inside the artificial canal. Then, the needle tip was positioned in the apical third (3 mm from the apex) and the irrigating solution was collected and transferred to the Endotester. For each procedure a single sterile needle and syringe were used to avoid any type of cross contamination.
The swab stick was then removed from the main body and 1-2 drops of the sample liquid were released by the needle inside the upper part of the tube main body (
Figures 2B). The swab stick was reinserted in the tube and moved to ensure proper absorption of the sample liquid. Then the swab stick was completely inserted inside the casing to mix the sample solution with the releasing reagent surfactant (Benzalkonium chloride) and the luminescent reagent (Luciferin, Luciferase, Magnesium acetate, Phosphoenolpyruvic acid, Pyrophosphoric acid, PPDK, PK). Correct mixing of the two components was ensured by shaking the Endotester casing for at least 10 seconds and allow visual assessment of dissolution of the luminescent reagent with the sample solution. Finally, the Endotester was inserted into the Endocator to measure the generated luminescence. The outcomes were displayed according to 2 different measuring scale, each of them chosen by the examiner (
Figure 3): Endoscore (ES) and Relative Light Unit (RLU). The measurements were displayed after 10 seconds, and the overall procedure was completed in less than 1 minute. All the measurements were performed by one trained operator to eliminate variables amongst examiners.
2.4. Outcomes
ES is a 0 to 100 analogic scale, where 0 correspond to absence of organic material and 100 to dirty channel. RLU is a continuous scale, the higher the score the higher is the amount of organic material collected, with values ranging from 0 to more than 600000. No information about the correlation between the two scales was provided by the manufacturer. In the IFU, only for the ES the following values were suggested: 0-30 is clean, 31-60 is contaminated, and 61-100 is dirty.
2.5. Statistical Analysis
All the data were recorded using both scales (ES and RLU) and were than divided in 6 groups: A, B and C representing the first, second and third sampling for each block, according to ES, and D, E and F representing the first, second and third sampling for each block, according to RLU.
Descriptive analysis was performed to determine mean and standard deviation (SD) of the findings for the 6 groups. Paired T-test with Bonferroni correction was executed to find out significant differences (p<0.05) between the 6 groups per score type. Statistical analysis was undertaken using SPSS (SPSS, v25.0 for Windows, SPSS Inc Chicago, IL, USA).
3. Results
Descriptive results are shown in
Table 1. Mean and SD of ES/RLU for first, second and third sampling resulted to be 31±6.32/263.8±88.23, 25.8±8.87/210.8±139.74 and 17.8±4.81/110.8±37.51 (A/D, B/E and C/F).
When comparing the first 5 measurements, no significant difference was noted amongst the 5 specimens. However, all specimens showed a debris contamination which demonstrated a high sensitivity of the device. Same non-significant differences were noted when comparing the second and third measurements.
For each canal, the score comparison between the three consecutive samplings showed statistically significant difference only between the first and third measurements both for ES (p=0.00115999) and RLU (p=0.00532749).
4. Discussion
The present study was conducted in a controlled laboratory environment, trying to reduce all possible variables related to cross contamination and sampling (no differences in canal anatomy and/or endodontic procedures, since all tests were performed in non-instrumented wide canal, which allowed proper needle insertion at the desired length). Measurements were performed by a skilled operator and a skilled assistant to minimize any procedural error. Results show that the Endocator device is rapid, simple to use and provides precise measurements, detecting the organic components present or left inside an artificial canal. The device exhibits high sensitivity, because it can check minimal, non-visible, organic debris contamination, as shown in new canals that were not instrumented or contaminated by pulp or other organic tissues. The high sensitivity is demonstrated by the fact that a new, clean canal (not used or artificially contaminated) provides RLU values ranging from 168 to 366 (for the first measurements), which are very small values compared to the measuring scale which allows maximum RLU at 450000. In the other modality (ES) the highest values are defined as 100 Over, and values for the first measurements from the present study ranged from 24 to 38 for the first initial measurement.
Such differences promote the use of RLU in laboratory testing, because if provides a wider, more accurate range of values, and consequently more precise comparisons. The suggested clinical ES values (0-30 clean, 31-60 contaminated, and 61-100 dirty) should be reevaluated with further studies. In the present study, when using the proposed “clinical score”, 2 samples showed contamination values after the first measurements, 1 of them also after the second measurement and none of them after the third one. Such differences, however, are based on an empirical scale, which need to be validated, also because it is not supported by the data of the present study.
Results from the present study showed that, when comparing differences amongst the 5 canals, there was no significant difference amongst the data, when analyzing the same measuring step (first, second and third), showing the precision and reliability of the test (p<0.05). Such results show that the Endocator could be a valid and predictable device for objective evaluation of canal cleanliness using an easy, not expensive, non-distractive methodology.
Precision of Endocator was also confirmed by differences between first, second and third measurements performed on the same canal. In such cases, to make the measurements some irrigating solution (distilled water) and some activation (using the needle with up and down motion) were added, as a consequence of the sampling technique. In all tested canals there was a reduction of the canal contamination in the following measurements and the device was able to detect it: in fact, a statistically significant difference was noted in all specimens between the first and third measurements (p=0.001 and 0.005 for the ES and RLU values, respectively), even if no visible sign of contamination could be visually detected. These results also show the future importance of preliminary testing of artificial canals to be used in vitro study before assessing quality of canal debridement provided by different techniques and materials.
The differences shown in each sample by consecutive measurement could be also very useful in performing in vitro studies about cleaning when the different methodologies are implemented during use [
18]. Traditional microscopic or histological studies can only show the results of a methodology, while the Endocator can evaluate the different steps inside a procedure [
19,
20,
21,
22]. For example, it may allow to quantify how a technique can be improved by adding more steps or increasing time or volumes of irrigants.
The high sensitivity of RLU values is an extremely positive factor in providing accurate measurements both in vitro and we expect similar results in vivo, which makes the device very useful for experimental research and clinical cases. In clinical cases, the device can display the amount of contamination left inside the canal and then the dentist can choose whether the final cleaning procedure should be implemented or not. On the other hand, such a high sensitivity requires to be very careful in the sampling procedure to avoid any cross contamination related to needles, syringes, gloves etc. Further studies will be necessary to improve or standardize the sampling procedure in terms of quantity of solution to be collected, depth of needle insertion and possible influence of relevant amounts of blood, exudate or chemicals in the sample liquid.
5. Conclusions
Within the limitations of the present study, it is possible to conclude that Endocator was able to determine small variations of canal contamination in a controlled laboratory environment, showing precise and reliable measurements. These findings suggest the possible use of the Endocator for in vitro comparative studies amongst different irrigation techniques and materials with an objective, non-distractive methodology. Further studies are however necessary to determine its use in clinical studies or clinical practice, because the technique is very sensitive, and there is a potential risk that differences in the sampling technique may affect results. So, the proposed ES scale should be also validated.
Author Contributions
Conceptualization, L.A., G.G. and L.T.; methodology, L.A, G.G. and M.G.; validation, R.C., C.A. and A.Z.; formal analysis, G.G, R.C. and A.Z.; investigation, L.A., G.G. and M.G.; resources, L.T.; data curation, G.G., C.A and R.C..; writing—original draft preparation, L.A., L.T and M.G.; writing—review and editing, C.A., A.Z; visualization, G.G.; supervision, M.G., G.G., L.T. and C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study did not require ethical approval.
Informed Consent Statement
The study did not involve humans.
Data Availability Statement
Data available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ali, A.; Bhosale, A.; Pawar, S.; Kakti, A.; Bichpuriya, A.; Agwan, M. A.; Agwan, A. S. Current Trends in Root Canal Irrigation. Cureus 2022, 14 (5), e24833. [CrossRef]
- Nair, P. N. R. On the Causes of Persistent Apical Periodontitis: A Review. Int. Endod. J. 2006, 39 (4), 249–281. [CrossRef]
- Gambarini, G.; Testarelli, L.; Pongione, G.; Gerosa, R.; Gagliani, M. Radiographic and Rheological Properties of a New Endodontic Sealer. Aust. Endod. J. 2006, 32 (1), 31–34. [CrossRef]
- Valenti-Obino, F.; Nardo, D. D.; Quero, L.; Miccoli, G.; Gambarini, G.; Testarelli, L.; Galli, M. Symmetry of Root and Root Canal Morphology of Mandibular Incisors: A Cone-Beam Computed Tomography Study in Vivo. J. Clin. Exp. Dent. 2019, 11 (6), e527–e533. [CrossRef]
- Zanza, A.; D’Angelo, M.; Reda, R.; Gambarini, G.; Testarelli, L.; Nardo, D. D. An Update on Nickel-Titanium Rotary Instruments in Endodontics: Mechanical Characteristics, Testing and Future Perspective—An Overview. Bioengineering 2021, 8 (12), 218. [CrossRef]
- Plotino, G.; Grande, N. M.; Tocci, L.; Testarelli, L.; Gambarini, G. Influence of Different Apical Preparations on Root Canal Cleanliness in Human Molars: A SEM Study. J. Oral Maxillofac. Res. 2014, 5 (2), e4. [CrossRef]
- Akçay, A.; Gorduysus, M.; Gorduysus, M. O.; Annamma, L. M.; Müftüoglu, S. A Comparative Evaluation of the Cleaning Efficacy of Five Different Root Canal Irrigation Devices: A Histological Study. Eur. J. Dent. 2023. [CrossRef]
- Neto, R. S. de O.; Lima, L. A. de S.; Titato, P. C. G.; Andrade, F. B. de; Vivan, R. R.; Alcalde, M. P.; Duarte, M. A. H. Effectiveness of a New Endodontic Irrigation System for Removing Smear Layer and Dissolving Simulated Organic Matter. Clin. Oral Investig. 2023, 28 (1), 10. [CrossRef]
- Nardo, D. D.; Gambarini, G.; Capuani, S.; Testarelli, L. Nuclear Magnetic Resonance Imaging in Endodontics: A Review. J. Endod. 2018, 44 (4), 536–542. [CrossRef]
- Tashkandi, N.; Alghamdi, F. Effect of Chemical Debridement and Irrigant Activation on Endodontic Treatment Outcomes: An Updated Overview. Cureus 2022, 14 (1), e21525. [CrossRef]
- Barbosa-Ribeiro, M.; De-Jesus-Soares, A.; Zaia, A. A.; Ferraz, C. C. R.; Almeida, J. F. A.; Gomes, B. P. F. A. Quantification of Lipoteichoic Acid Contents and Cultivable Bacteria at the Different Phases of the Endodontic Retreatment. J. Endod. 2016, 42 (4), 552–556. [CrossRef]
- Higuchi, N.; Hayashi, J.; Fujita, M.; Iwamura, Y.; Sasaki, Y.; Goto, R.; Ohno, T.; Nishida, E.; Yamamoto, G.; Kikuchi, T.; Mitani, A.; Fukuda, M. Photodynamic Inactivation of an Endodontic Bacteria Using Diode Laser and Indocyanine Green-Loaded Nanosphere. Int. J. Mol. Sci. 2021, 22 (16), 8384. [CrossRef]
- Zeng, C.; Hu, P.; Egan, C. P.; Bergeron, B. E.; Tay, F.; Ma, J. Bacteria Debridement Efficacy of Two Sonic Root Canal Irrigant Activation Systems. J. Dent. 2024, 140, 104770. [CrossRef]
- Gambarini, G. Shaping and Cleaning the Root Canal System: A Scanning Electron Microscopic Evaluation of a New Instrumentation and Irrigation Technique. J. Endod. 1999, 25 (12), 800–803. [CrossRef]
- Plotino, G.; Grande, N. M.; Mercade, M.; Cortese, T.; Staffoli, S.; Gambarini, G.; Testarelli, L. Efficacy of Sonic and Ultrasonic Irrigation Devices in the Removal of Debris from Canal Irregularities in Artificial Root Canals. J. Appl. Oral Sci. 2019, 27, e20180045. [CrossRef]
- Bago, I.; Đurin, A.; Kanižaj, D.; Vuletić, L. B.; Zdrilić, I. V.; Anić, I. The Efficacy of a Novel SWEEPS Laser-Activated Irrigation Compared to Ultrasonic Activation in the Removal of Pulp Tissue from an Isthmus Area in the Apical Third of the Root Canal. Lasers Méd. Sci. 2023, 38 (1), 189. [CrossRef]
- Lee, E.-S.; Jong, E. de J. de; Kim, E.; Kim, B.-I. Real-Time Optical Detection of Endodontic Infection Using Bacterial Autofluorescence. J. Dent. 2023, 136, 104600. [CrossRef]
- Haapasalo, M.; Shen, Y.; Qian, W.; Gao, Y. Irrigation in Endodontics. Dent. Clin. North Am. 2010, 54 (2), 291–312. [CrossRef]
- Salman, M. I.; Baumann, M. A.; Hellmich, M.; Roggendorf, M. J.; Termaat, S. SEM Evaluation of Root Canal Debridement with Sonicare CanalBrush Irrigation. Int. Endod. J. 2010, 43 (5), 363–369. [CrossRef]
- Li, Q.; Zhang, Q.; Zou, X.; Yue, L. Evaluation of Four Final Irrigation Protocols for Cleaning Root Canal Walls. Int. J. Oral Sci. 2020, 12 (1), 29. [CrossRef]
- Iandolo, A.; Amato, A.; Pisano, M.; Sangiovanni, G.; Abdellatif, D.; Fornara, R.; Simeone, M. Histological Evaluation of Root Canals by Performing a New Cleaning Protocol “RUA” in Endodontic Surgery. Dent. J. 2023, 11 (3), 78. [CrossRef]
- Özlek, E.; Acikgoz, E.; Gökkaya, N. Z.; Taşan, A.; Altındağ, F. Histological Evaluation of the Debris Removal Efficiency of Activation of Sodium Hypochlorite Solution at Different Concentrations. BMC Oral Heal. 2023, 23 (1), 528. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).