Submitted:
07 February 2024
Posted:
08 February 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. The TRAIL System:
2.1. TRAIL-Induced Cell Death:
2.2. Comparison of the Proximal Regulatory Mechanisms Governing TRAIL-Induced Cell Death with Other TNFRSF Members
3. Physiological and physiopathological functions of TRAIL:
3.1. In Immune System:
3.2. In Diseases:
4. Signalling Machinery Associated with TRAIL Non-Canonical Transduction:
4.1. Lessons from Fas/CD95 induced non-canonical signalling (secondary complex):
4.1. Calcium Signalling Inducing Cell Motility and Metastasis:
4.2. Nuclear DR5 Regulates Both Proliferation and Metastasis:
4.3. Caspase-8 contribution in TRAIL non-canonical signalling:
4.4. TRAIL Induce Cancer Metastasis after uPA and c-cbl Regulation:
5. Conclusion and Perspectives:
Acknowledgments
References
- Wiley, S.R.; Schooley, K.; Smolak, P.J.; Din, W.S.; Huang, C.P.; Nicholl, J.K.; Sutherland, G.R.; Smith, T.D.; Rauch, C.; Smith, C.A. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995, 3, 673–682, Research Support, Non-U.S. Gov’t. [Google Scholar] [CrossRef] [PubMed]
- Pitti, R.M.; Marsters, S.A.; Ruppert, S.; Donahue, C.J.; Moore, A.; Ashkenazi, A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. The Journal of biological chemistry 1996, 271, 12687–12690. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; O’Rourke, K.; Chinnaiyan, A.M.; Gentz, R.; Ebner, R.; Ni, J.; Dixit, V.M. The receptor for the cytotoxic ligand TRAIL. Science 1997, 276, 111–113. [Google Scholar] [CrossRef]
- MacFarlane, M.; Ahmad, M.; Srinivasula, S.M.; Fernandes-Alnemri, T.; Cohen, G.M.; Alnemri, E.S. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. The Journal of biological chemistry 1997, 272, 25417–25420. [Google Scholar] [CrossRef]
- Walczak, H.; Degli-Esposti, M.A.; Johnson, R.S.; Smolak, P.J.; Waugh, J.Y.; Boiani, N.; Timour, M.S.; Gerhart, M.J.; Schooley, K.A.; Smith, C.A.; et al. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. Embo J 1997, 16, 5386–5397. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Bodmer, J.L.; Thome, M.; Hofmann, K.; Holler, N.; Tschopp, J. Characterization of two receptors for TRAIL. FEBS letters 1997, 416, 329–334. [Google Scholar] [CrossRef]
- Schneider, P.; Olson, D.; Tardivel, A.; Browning, B.; Lugovskoy, A.; Gong, D.; Dobles, M.; Hertig, S.; Hofmann, K.; Van Vlijmen, H.; et al. Identification of a new murine tumor necrosis factor receptor locus that contains two novel murine receptors for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). The Journal of biological chemistry 2003, 278, 5444–5454. [Google Scholar] [CrossRef]
- Wu, G.S.; Burns, T.F.; Zhan, Y.; Alnemri, E.S.; El-Deiry, W.S. Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer research 1999, 59, 2770–2775. [Google Scholar]
- Boldin, M.P.; Mett, I.L.; Varfolomeev, E.E.; Chumakov, I.; Shemer-Avni, Y.; Camonis, J.H.; Wallach, D. Self-association of the “death domains” of the p55 tumor necrosis factor (TNF) receptor and Fas/APO1 prompts signaling for TNF and Fas/APO1 effects. The Journal of biological chemistry 1995, 270, 387–391. [Google Scholar] [CrossRef]
- Boldin, M.P.; Varfolomeev, E.E.; Pancer, Z.; Mett, I.L.; Camonis, J.H.; Wallach, D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. The Journal of biological chemistry 1995, 270, 7795–7798. [Google Scholar] [CrossRef]
- Feinstein, E.; Kimchi, A.; Wallach, D.; Boldin, M.; Varfolomeev, E. The death domain: a module shared by proteins with diverse cellular functions. Trends Biochem Sci 1995, 20, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K. The modular nature of apoptotic signaling proteins. Cell Mol Life Sci 1999, 55, 1113–1128. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, L.A.; Ayres, T.M.; Wong, G.H.; Goeddel, D.V. A novel domain within the 55 kd TNF receptor signals cell death. Cell 1993, 74, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Nagata, S. A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. The Journal of biological chemistry 1993, 268, 10932–10937, Comparative Study Research Support, 52 Non-U.S. Gov’t. [Google Scholar] [CrossRef]
- Merino, D.; Lalaoui, N.; Morizot, A.; Solary, E.; Micheau, O. TRAIL in cancer therapy: present and future challenges. Expert opinion on therapeutic targets 2007, 11, 1299–1314. [Google Scholar] [CrossRef] [PubMed]
- Merino, D.; Lalaoui, N.; Morizot, A.; Schneider, P.; Solary, E.; Micheau, O. Differential inhibition of TRAIL-mediated DR5-DISC formation by decoy receptors 1 and 2. Mol Cell Biol 2006, 26, 7046–7055. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, J.P.; Marsters, S.A.; Pitti, R.M.; Gurney, A.; Skubatch, M.; Baldwin, D.; Ramakrishnan, L.; Gray, C.L.; Baker, K.; Wood, W.I.; et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 1997, 277, 818–821. [Google Scholar] [CrossRef]
- Pitti, R.M.; Marsters, S.A.; Lawrence, D.A.; Roy, M.; Kischkel, F.C.; Dowd, P.; Huang, A.; Donahue, C.J.; Sherwood, S.W.; Baldwin, D.T.; et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature 1998, 396, 699–703. [Google Scholar] [CrossRef]
- Pan, G.; Ni, J.; Yu, G.; Wei, Y.F.; Dixit, V.M. TRUNDD, a new member of the TRAIL receptor family that antagonizes TRAIL signalling. FEBS letters 1998, 424, 41–45. [Google Scholar] [CrossRef]
- Pan, G.; Ni, J.; Wei, Y.F.; Yu, G.; Gentz, R.; Dixit, V.M. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 1997, 277, 815–818. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, M.; Sun, W.; Yang, S.; Su, Y.; Zhang, H.; Liu, C.; Li, X.; Lin, L.; Kim, S.; et al. Reduction of decoy receptor 3 enhances TRAIL-mediated apoptosis in pancreatic cancer. PLoS One 2013, 8, e74272. [Google Scholar] [CrossRef] [PubMed]
- Degli-Esposti, M.A.; Smolak, P.J.; Walczak, H.; Waugh, J.; Huang, C.P.; DuBose, R.F.; Goodwin, R.G.; Smith, C.A. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med 1997, 186, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.S.; Huang, Y.; Fernandez-Salas, E.A.; El-Deiry, W.S.; Friess, H.; Amundson, S.; Yin, J.; Meltzer, S.J.; Holbrook, N.J.; Fornace, A.J., Jr. The antiapoptotic decoy receptor TRID/TRAIL-R3 is a p53-regulated DNA damage-inducible gene that is overexpressed in primary tumors of the gastrointestinal tract. Oncogene 1999, 18, 4153–4159. [Google Scholar] [CrossRef] [PubMed]
- Degli-Esposti, M.A.; Dougall, W.C.; Smolak, P.J.; Waugh, J.Y.; Smith, C.A.; Goodwin, R.G. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity 1997, 7, 813–820. [Google Scholar] [CrossRef]
- Toscano, F.; Fajoui, Z.E.; Gay, F.; Lalaoui, N.; Parmentier, B.; Chayvialle, J.A.; Scoazec, J.Y.; Micheau, O.; Abello, J.; Saurin, J.C. P53-mediated upregulation of DcR1 impairs oxaliplatin/TRAIL-induced synergistic anti-tumour potential in colon cancer cells. Oncogene 2008, 27, 4161–4171. [Google Scholar] [CrossRef]
- Morizot, A.; Merino, D.; Lalaoui, N.; Jacquemin, G.; Granci, V.; Iessi, E.; Lanneau, D.; Bouyer, F.; Solary, E.; Chauffert, B.; et al. Chemotherapy overcomes TRAIL-R4-mediated TRAIL resistance at the DISC level. Cell Death Differ 2011, 18, 700–711. [Google Scholar] [CrossRef]
- Lalaoui, N.; Morle, A.; Merino, D.; Jacquemin, G.; Iessi, E.; Morizot, A.; Shirley, S.; Robert, B.; Solary, E.; Garrido, C.; Micheau, O. TRAIL-R4 promotes tumor growth and resistance to apoptosis in cervical carcinoma HeLa cells through AKT. PLoS One 2011, 6, e19679, Research Support, Non-U.S. Gov’t. [Google Scholar] [CrossRef]
- Lalaoui, N.; Merino, D.; Morizot, A.; Jacquemin, G.; Granci, V.; Iessi, E.; Solary, E.; Micheau, O. DcR2 PROTECTS CANCER CELLS FROM TRAIL-INDUCED APOPTOSIS BY ACTIVATING Akt. Advances in Tnf Family Research 2011, 691, 745–745. [Google Scholar]
- Emery, J.G.; McDonnell, P.; Burke, M.B.; Deen, K.C.; Lyn, S.; Silverman, C.; Dul, E.; Appelbaum, E.R.; Eichman, C.; DiPrinzio, R.; et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. The Journal of biological chemistry 1998, 273, 14363–14367. [Google Scholar] [CrossRef]
- Neumann, S.; Hasenauer, J.; Pollak, N.; Scheurich, P. Dominant negative effects of tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) receptor 4 on TRAIL receptor 1 signaling by formation of heteromeric complexes. The Journal of biological chemistry 2014, 289, 16576–16587. [Google Scholar] [CrossRef]
- Rimondi, E.; Secchiero, P.; Quaroni, A.; Zerbinati, C.; Capitani, S.; Zauli, G. Involvement of TRAIL/TRAIL-receptors in human intestinal cell differentiation. Journal of cellular physiology 2006, 206, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Gunalp, S.; Helvaci, D.G.; Oner, A.; Bursali, A.; Conforte, A.; Guner, H.; Karakulah, G.; Szegezdi, E.; Sag, D. TRAIL promotes the polarization of human macrophages toward a proinflammatory M1 phenotype and is associated with increased survival in cancer patients with high tumor macrophage content. Front Immunol 2023, 14, 1209249. [Google Scholar] [CrossRef] [PubMed]
- Loeuillard, E.; Li, B.; Stumpf, H.E.; Yang, J.; Willhite, J.; Tomlinson, J.L.; Wang, J.; Rohakhtar, F.R.; Simon, V.A.; Graham, R.P.; et al. Noncanonical TRAIL Signaling Promotes Myeloid-Derived Suppressor Cell Abundance and Tumor Progression in Cholangiocarcinoma. bioRxiv 2023. [Google Scholar] [CrossRef]
- Toffoli, B.; Tonon, F.; Tisato, V.; Zauli, G.; Secchiero, P.; Fabris, B.; Bernardi, S. TRAIL/DR5 pathway promotes AKT phosphorylation, skeletal muscle differentiation, and glucose uptake. Cell death & disease 2021, 12. [Google Scholar] [CrossRef]
- von Karstedt, S.; Conti, A.; Nobis, M.; Montinaro, A.; Hartwig, T.; Lemke, J.; Legler, K.; Annewanter, F.; Campbell, A.D.; Taraborrelli, L.; et al. Cancer cell-autonomous TRAIL-R signaling promotes KRAS-driven cancer progression, invasion, and metastasis. Cancer cell 2015, 27, 561–573. [Google Scholar] [CrossRef]
- Grosse-Wilde, A.; Voloshanenko, O.; Bailey, S.L.; Longton, G.M.; Schaefer, U.; Csernok, A.I.; Schutz, G.; Greiner, E.F.; Kemp, C.J.; Walczak, H. TRAIL-R deficiency in mice enhances lymph node metastasis without affecting primary tumor development. J Clin Invest 2008, 118, 100–110. [Google Scholar] [CrossRef]
- Steitz, A.M.; Schroder, C.; Knuth, I.; Keber, C.U.; Sommerfeld, L.; Finkernagel, F.; Jansen, J.M.; Wagner, U.; Muller-Brusselbach, S.; Worzfeld, T.; et al. TRAIL-dependent apoptosis of peritoneal mesothelial cells by NK cells promotes ovarian cancer invasion. iScience 2023, 26. [Google Scholar] [CrossRef]
- Trauzold, A.; Siegmund, D.; Schniewind, B.; Sipos, B.; Egberts, J.; Zorenkov, D.; Emme, D.; Roder, C.; Kalthoff, H.; Wajant, H. TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene 2006, 25, 7434–7439. [Google Scholar] [CrossRef]
- Azijli, K.; Yuvaraj, S.; Peppelenbosch, M.P.; Wurdinger, T.; Dekker, H.; Joore, J.; van Dijk, E.; Quax, W.J.; Peters, G.J.; de Jong, S.; Kruyt, F.A. Kinome profiling of non-canonical TRAIL signaling reveals RIP1-Src-STAT3-dependent invasion in resistant non-small cell lung cancer cells. Journal of cell science 2012, 125 Pt 19, 4651–4661. [Google Scholar] [CrossRef]
- Ishimura, N.; Isomoto, H.; Bronk, S.F.; Gores, G.J. Trail induces cell migration and invasion in apoptosis-resistant cholangiocarcinoma cells. American journal of physiology 2006, 290, G129–136. [Google Scholar] [CrossRef]
- Xiao, C.; Rui, Y.; Zhou, S.; Huang, Y.; Wei, Y.; Wang, Z. TNF-related apoptosis-inducing ligand (TRAIL) promotes trophoblast cell invasion via miR-146a-EGFR/CXCR4 axis: A novel mechanism for preeclampsia? Placenta 2020, 93, 8–16. [Google Scholar] [CrossRef]
- Vanamee, E.S.; Faustman, D.L. On the TRAIL of Better Therapies: Understanding TNFRSF Structure-Function. Cells 2020, 9, 764. [Google Scholar] [CrossRef] [PubMed]
- Varfolomeev, E.; Maecker, H.; Sharp, D.; Lawrence, D.; Renz, M.; Vucic, D.; Ashkenazi, A. Molecular determinants of kinase pathway activation by Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. The Journal of biological chemistry 2005, 280, 40599–40608. [Google Scholar] [CrossRef]
- Trauzold, A.; Wermann, H.; Arlt, A.; Schutze, S.; Schafer, H.; Oestern, S.; Roder, C.; Ungefroren, H.; Lampe, E.; Heinrich, M.; et al. CD95 and TRAIL receptor-mediated activation of protein kinase C and NF-kappaB contributes to apoptosis resistance in ductal pancreatic adenocarcinoma cells. Oncogene 2001, 20, 4258–4269. [Google Scholar] [CrossRef]
- Wajant, H. TRAIL and NFkappaB signaling--a complex relationship. Vitamins and hormones 2004, 67, 101–132. [Google Scholar]
- Shetty, S.; Gladden, J.B.; Henson, E.S.; Hu, X.; Villanueva, J.; Haney, N.; Gibson, S.B. Tumor necrosis factor-related apoptosis inducing ligand (TRAIL) up-regulates death receptor 5 (DR5) mediated by NFkappaB activation in epithelial derived cell lines. Apoptosis 2002, 7, 413–420. [Google Scholar] [CrossRef]
- Zhang, L.; Dittmer, M.R.; Blackwell, K.; Workman, L.M.; Hostager, B.; Habelhah, H. TRAIL activates JNK and NF-kappaB through RIP1-dependent and -independent pathways. Cell Signal 2015, 27, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Thome, M.; Burns, K.; Bodmer, J.L.; Hofmann, K.; Kataoka, T.; Holler, N.; Tschopp, J. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity 1997, 7, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.L.; Maeda, S.; Hsu, L.C.; Yagita, H.; Karin, M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer cell 2004, 6, 297–305. [Google Scholar] [CrossRef]
- Tang, W.; Wang, W.; Zhang, Y.; Liu, S.; Liu, Y.; Zheng, D. TRAIL receptor mediates inflammatory cytokine release in an NF-kappaB-dependent manner. Cell Res 2009, 19, 758–767. [Google Scholar] [CrossRef]
- Geismann, C.; Erhart, W.; Grohmann, F.; Schreiber, S.; Schneider, G.; Schafer, H.; Arlt, A. TRAIL/NF-kappaB/CX3CL1 Mediated Onco-Immuno Crosstalk Leading to TRAIL Resistance of Pancreatic Cancer Cell Lines. Int J Mol Sci 2018, 19, 1661. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, P.; Higgins, C.A.; Najda, Z.; Longley, D.B.; Martin, S.J. cFLIP(L) acts as a suppressor of TRAIL- and Fas-initiated inflammation by inhibiting assembly of caspase-8/FADD/RIPK1 NF-kappaB-activating complexes. Cell Rep 2023, 42, 113476. [Google Scholar] [CrossRef] [PubMed]
- Imamura, R.; Konaka, K.; Matsumoto, N.; Hasegawa, M.; Fukui, M.; Mukaida, N.; Kinoshita, T.; Suda, T. Fas ligand induces cell-autonomous NF-kappaB activation and interleukin-8 production by a mechanism distinct from that of tumor necrosis factor-alpha. The Journal of biological chemistry 2004, 279, 46415–46423. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kim, E.J.; Suk, K.; Lee, W.H. Stimulation of Fas (CD95) induces production of pro-inflammatory mediators through ERK/JNK-dependent activation of NF-kappaB in THP-1 cells. Cellular immunology 2011, 271, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, F.; Teng, F.; Zhang, M. Fas/FasL Complex Promotes Proliferation and Migration of Brain Endothelial Cells Via FADD-FLIP-TRAF-NF-kappaB Pathway. Cell Biochem Biophys 2015, 71, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Kreuz, S.; Siegmund, D.; Rumpf, J.J.; Samel, D.; Leverkus, M.; Janssen, O.; Hacker, G.; Dittrich-Breiholz, O.; Kracht, M.; Scheurich, P.; Wajant, H. NFkappaB activation by Fas is mediated through FADD, caspase-8, and RIP and is inhibited by FLIP. J Cell Biol 2004, 166, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, B.C.; Legembre, P.; Pietras, E.; Bubici, C.; Franzoso, G.; Peter, M.E. CD95 ligand induces motility and invasiveness of apoptosis-resistant tumor cells. EMBO J 2004, 23, 3175–3185. [Google Scholar] [CrossRef]
- Legembre, P.; Barnhart, B.C.; Zheng, L.; Vijayan, S.; Straus, S.E.; Puck, J.; Dale, J.K.; Lenardo, M.; Peter, M.E. Induction of apoptosis and activation of NF-kappaB by CD95 require different signalling thresholds. EMBO reports 2004, 5, 1084–1089, Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. [Google Scholar] [CrossRef]
- Kawakubo, T.; Okamoto, K.; Iwata, J.; Shin, M.; Okamoto, Y.; Yasukochi, A.; Nakayama, K.I.; Kadowaki, T.; Tsukuba, T.; Yamamoto, K. Cathepsin E prevents tumor growth and metastasis by catalyzing the proteolytic release of soluble TRAIL from tumor cell surface. Cancer research 2007, 67, 10869–10878, Research Support, Non-U.S. Gov’t. [Google Scholar] [CrossRef]
- Yagolovich, A.V.; Artykov, A.A.; Karmakova, T.A.; Vorontsova, M.S.; Pankratov, A.A.; Andreev-Andrievsky, A.A.; Dolgikh, D.A.; Kirpichnikov, M.P.; Gasparian, M.E. Genetically Modified DR5-Specific TRAIL Variant DR5-B Revealed Dual Antitumor and Protumoral Effect in Colon Cancer Xenografts and an Improved Pharmacokinetic Profile. Transl Oncol 2020, 13, 100762. [Google Scholar] [CrossRef]
- Chen, L.; Park, S.M.; Tumanov, A.V.; Hau, A.; Sawada, K.; Feig, C.; Turner, J.R.; Fu, Y.X.; Romero, I.L.; Lengyel, E.; Peter, M.E. CD95 promotes tumour growth. Nature 2010, 465, 492–496. [Google Scholar] [CrossRef]
- Henry, C.M.; Martin, S.J. Caspase-8 Acts in a Non-enzymatic Role as a Scaffold for Assembly of a Pro-inflammatory “FADDosome” Complex upon TRAIL Stimulation. Molecular cell 2017, 65, 715–729.e715. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.P.; O’Connor, H.; Henry, C.M.; Davidovich, P.; Clancy, D.M.; Albert, M.L.; Cullen, S.P.; Martin, S.J. TRAIL Receptors Serve as Stress-Associated Molecular Patterns to Promote ER-Stress-Induced Inflammation. Dev Cell 2020, 52, 714–730.e715. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Kim, H.S.; Kim, H.Y.; Kang, M.J.; Jung, H.; Oh, Y.; Kim, D.; Koh, J.; Cho, S.Y.; Jeon, Y.K.; et al. Soluble Fas ligand drives autoantibody-induced arthritis by binding to DR5/TRAIL-R2. Elife 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, S.; Infante-Duarte, C.; Seeger, B.; Zipp, F. Regulation of soluble and surface-bound TRAIL in human T cells, B cells, and monocytes. Cytokine 2003, 24, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Kamohara, H.; Matsuyama, W.; Shimozato, O.; Abe, K.; Galligan, C.; Hashimoto, S.; Matsushima, K.; Yoshimura, T. Regulation of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) and TRAIL receptor expression in human neutrophils. Immunology 2004, 111, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Matsuzaki, A.; Suminoe, A.; Hattori, H.; Hara, T. Neutrophil-derived TNF-related apoptosis-inducing ligand (TRAIL): a novel mechanism of antitumor effect by neutrophils. Cancer research 2004, 64, 1037–1043. [Google Scholar] [CrossRef]
- Simons, M.P.; Leidal, K.G.; Nauseef, W.M.; Griffith, T.S. TNF-related apoptosis-inducing ligand (TRAIL) is expressed throughout myeloid development, resulting in a broad distribution among neutrophil granules. Journal of leukocyte biology 2008, 83, 621–629. [Google Scholar] [CrossRef]
- Fanger, N.A.; Maliszewski, C.R.; Schooley, K.; Griffith, T.S. Human dendritic cells mediate cellular apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J Exp Med 1999, 190, 1155–1164. [Google Scholar] [CrossRef]
- Cartland, S.P.; Genner, S.W.; Martinez, G.J.; Robertson, S.; Kockx, M.; Lin, R.C.; O’Sullivan, J.F.; Koay, Y.C.; Manuneedhi Cholan, P.; Kebede, M.A.; et al. TRAIL-Expressing Monocyte/Macrophages Are Critical for Reducing Inflammation and Atherosclerosis. iScience 2019, 12, 41–52. [Google Scholar] [CrossRef]
- Griffith, T.S.; Wiley, S.R.; Kubin, M.Z.; Sedger, L.M.; Maliszewski, C.R.; Fanger, N.A. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med 1999, 189, 1343–1354. [Google Scholar] [CrossRef]
- Tecchio, C.; Huber, V.; Scapini, P.; Calzetti, F.; Margotto, D.; Todeschini, G.; Pilla, L.; Martinelli, G.; Pizzolo, G.; Rivoltini, L.; Cassatella, M.A. IFNalpha-stimulated neutrophils and monocytes release a soluble form of TNF-related apoptosis-inducing ligand (TRAIL/Apo-2 ligand) displaying apoptotic activity on leukemic cells. Blood 2004, 103, 3837–3844. [Google Scholar] [CrossRef] [PubMed]
- Halaas, O.; Vik, R.; Ashkenazi, A.; Espevik, T. Lipopolysaccharide induces expression of APO2 ligand/TRAIL in human monocytes and macrophages. Scandinavian journal of immunology 2000, 51, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chen, S.L.; Shih, S.C.; Chang, S.J.; Yang, S.L.; Hsieh, J.W.; Cheng, H.C.; Chen, L.J.; Tsao, Y.P. Pigment epithelium-derived factor (PEDF) promotes tumor cell death by inducing macrophage membrane tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). The Journal of biological chemistry 2011, 286, 35943–35954. [Google Scholar] [CrossRef]
- Johnsen, A.C.; Haux, J.; Steinkjer, B.; Nonstad, U.; Egeberg, K.; Sundan, A.; Ashkenazi, A.; Espevik, T. Regulation of APO-2 ligand/trail expression in NK cells-involvement in NK cell-mediated cytotoxicity. Cytokine 1999, 11, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Zamai, L.; Ahmad, M.; Bennett, I.M.; Azzoni, L.; Alnemri, E.S.; Perussia, B. Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med 1998, 188, 2375–2380. [Google Scholar] [CrossRef]
- Mirandola, P.; Ponti, C.; Gobbi, G.; Sponzilli, I.; Vaccarezza, M.; Cocco, L.; Zauli, G.; Secchiero, P.; Manzoli, F.A.; Vitale, M. Activated human NK and CD8+ T cells express both TNF-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors but are resistant to TRAIL-mediated cytotoxicity. Blood 2004, 104, 2418–2424. [Google Scholar] [CrossRef]
- Beraza, N.; Malato, Y.; Sander, L.E.; Al-Masaoudi, M.; Freimuth, J.; Riethmacher, D.; Gores, G.J.; Roskams, T.; Liedtke, C.; Trautwein, C. Hepatocyte-specific NEMO deletion promotes NK/NKT cell- and TRAIL-dependent liver damage. J Exp Med 2009, 206, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Nishihori, Y.; Kato, K.; Tanaka, M.; Okamoto, T.; Hagiwara, S.; Araki, N.; Kogawa, K.; Kuribayashi, K.; Nakamura, K.; Niitsu, Y. Interleukin-2 gene transfer potentiates the alpha-galactosylceramide-stimulated antitumor effect by the induction of TRAIL in NKT and NK cells in mouse models of subcutaneous and metastatic carcinoma. Cancer Biol Ther 2009, 8, 1763–1770. [Google Scholar] [CrossRef]
- Smyth, M.J.; Cretney, E.; Takeda, K.; Wiltrout, R.H.; Sedger, L.M.; Kayagaki, N.; Yagita, H.; Okumura, K. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon gamma-dependent natural killer cell protection from tumor metastasis. J Exp Med 2001, 193, 661–670. [Google Scholar] [CrossRef]
- Nieda, M.; Nicol, A.; Koezuka, Y.; Kikuchi, A.; Lapteva, N.; Tanaka, Y.; Tokunaga, K.; Suzuki, K.; Kayagaki, N.; Yagita, H.; et al. TRAIL expression by activated human CD4(+)V alpha 24NKT cells induces in vitro and in vivo apoptosis of human acute myeloid leukemia cells. Blood 2001, 97, 2067–2074. [Google Scholar] [CrossRef]
- Gomez-Santos, L.; Luka, Z.; Wagner, C.; Fernandez-Alvarez, S.; Lu, S.C.; Mato, J.M.; Martinez-Chantar, M.L.; Beraza, N. Inhibition of natural killer cells protects the liver against acute injury in the absence of glycine N-methyltransferase. Hepatology (Baltimore, Md 2012, 56, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, A.; Barreyro, F.J.; Bronk, S.F.; Werneburg, N.W.; Mott, J.L.; Akazawa, Y.; Masuoka, H.C.; Howe, C.L.; Gores, G.J. TRAIL mediates liver injury by the innate immune system in the bile duct-ligated mouse. Hepatology (Baltimore, Md 2008, 47, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Metelitsa, L.S.; Weinberg, K.I.; Emanuel, P.D.; Seeger, R.C. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia 2003, 17, 1068–1077. [Google Scholar] [CrossRef]
- Teng, M.W.; Westwood, J.A.; Darcy, P.K.; Sharkey, J.; Tsuji, M.; Franck, R.W.; Porcelli, S.A.; Besra, G.S.; Takeda, K.; Yagita, H.; et al. Combined natural killer T-cell based immunotherapy eradicates established tumors in mice. Cancer research 2007, 67, 7495–7504. [Google Scholar] [CrossRef]
- Stelma, F.; de Niet, A.; Tempelmans Plat-Sinnige, M.J.; Jansen, L.; Takkenberg, R.B.; Reesink, H.W.; Kootstra, N.A.; van Leeuwen, E.M. Natural Killer Cell Characteristics in Patients With Chronic Hepatitis B Virus (HBV) Infection Are Associated With HBV Surface Antigen Clearance After Combination Treatment With Pegylated Interferon Alfa-2a and Adefovir. J Infect Dis 2015, 212, 1042–1051. [Google Scholar] [CrossRef]
- Peteranderl, C.; Morales-Nebreda, L.; Selvakumar, B.; Lecuona, E.; Vadasz, I.; Morty, R.E.; Schmoldt, C.; Bespalowa, J.; Wolff, T.; Pleschka, S.; et al. Macrophage-epithelial paracrine crosstalk inhibits lung edema clearance during influenza infection. J Clin Invest 2016, 126, 1566–1580. [Google Scholar] [CrossRef]
- Azam, S.; Manzoor, S.; Imran, M.; Ashraf, J.; Ashraf, S.; Resham, S.; Ghani, E. Role of interferon gamma and tumor necrosis factor-related apoptosis-inducing ligand receptor 1 single nucleotide polymorphism in natural clearance and treatment response of HCV infection. Viral immunology 2015, 28, 222–228. [Google Scholar] [CrossRef]
- Seyman, D.; Yalcin, A.D.; Oztoprak, N.; Genc, G.E.; Ozen, N.S.; Kizilates, F.; Berk, H.; Gumuslu, S. Soluble TRAIL levels decreased in chronic hepatitis C treatment with pegylated interferon alpha plus ribavirin: association with viral responses. Int J Clin Exp Med 2014, 7, 5650–5656. [Google Scholar] [PubMed]
- Gyurkovska, V.; Ivanovska, N. Distinct roles of TNF-related apoptosis-inducing ligand (TRAIL) in viral and bacterial infections: from pathogenesis to pathogen clearance. Inflammation research: official journal of the European Histamine Research Society... [et al.] 2016, 65, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Cardoso Alves, L.; Berger, M.D.; Koutsandreas, T.; Kirschke, N.; Lauer, C.; Sporri, R.; Chatziioannou, A.; Corazza, N.; Krebs, P. Non-apoptotic TRAIL function modulates NK cell activity during viral infection. EMBO reports 2020, 21, e48789. [Google Scholar] [CrossRef]
- Sato, K.; Hida, S.; Takayanagi, H.; Yokochi, T.; Kayagaki, N.; Takeda, K.; Yagita, H.; Okumura, K.; Tanaka, N.; Taniguchi, T.; Ogasawara, K. Antiviral response by natural killer cells through TRAIL gene induction by IFN-alpha/beta. Eur J Immunol 2001, 31, 3138–3146. [Google Scholar] [CrossRef]
- Warke, R.V.; Martin, K.J.; Giaya, K.; Shaw, S.K.; Rothman, A.L.; Bosch, I. TRAIL is a novel antiviral protein against dengue virus. J Virol 2008, 82, 555–564. [Google Scholar] [CrossRef]
- Verma, S.; Loewendorf, A.; Wang, Q.; McDonald, B.; Redwood, A.; Benedict, C.A. Inhibition of the TRAIL death receptor by CMV reveals its importance in NK cell-mediated antiviral defense. PLoS pathogens 2014, 10. [Google Scholar] [CrossRef]
- Stacey, M.A.; Marsden, M.; Pham, N.T.; Clare, S.; Dolton, G.; Stack, G.; Jones, E.; Klenerman, P.; Gallimore, A.M.; Taylor, P.R.; et al. Neutrophils recruited by IL-22 in peripheral tissues function as TRAIL-dependent antiviral effectors against MCMV. Cell host & microbe 2014, 15, 471–483. [Google Scholar] [CrossRef]
- Smith, W.; Tomasec, P.; Aicheler, R.; Loewendorf, A.; Nemcovicova, I.; Wang, E.C.; Stanton, R.J.; Macauley, M.; Norris, P.; Willen, L.; et al. Human cytomegalovirus glycoprotein UL141 targets the TRAIL death receptors to thwart host innate antiviral defenses. Cell host & microbe 2013, 13, 324–335. [Google Scholar] [CrossRef]
- Schuster, I.S.; Wikstrom, M.E.; Brizard, G.; Coudert, J.D.; Estcourt, M.J.; Manzur, M.; O’Reilly, L.A.; Smyth, M.J.; Trapani, J.A.; Hill, G.R.; et al. TRAIL+ NK cells control CD4+ T cell responses during chronic viral infection to limit autoimmunity. Immunity 2014, 41, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.; Brunetto, M.; Reynolds, G.; Christophides, T.; Kennedy, P.T.; Lampertico, P.; Das, A.; Lopes, A.R.; Borrow, P.; Williams, K.; et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med 2007, 204, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Smyth, M.J.; Cretney, E.; Hayakawa, Y.; Yamaguchi, N.; Yagita, H.; Okumura, K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in NK cell-mediated and IFN-gamma-dependent suppression of subcutaneous tumor growth. Cellular immunology 2001, 214, 194–200. [Google Scholar] [CrossRef]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. TNF-related apoptosis inducing ligand (TRAIL) and its receptors in tumor surveillance and cancer therapy. Apoptosis 2002, 7, 449–459. [Google Scholar] [CrossRef]
- Takeda, K.; Smyth, M.J.; Cretney, E.; Hayakawa, Y.; Kayagaki, N.; Yagita, H.; Okumura, K. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J Exp Med 2002, 195, 161–169. [Google Scholar] [CrossRef]
- Takeda, K.; Yamaguchi, N.; Akiba, H.; Kojima, Y.; Hayakawa, Y.; Tanner, J.E.; Sayers, T.J.; Seki, N.; Okumura, K.; Yagita, H.; Smyth, M.J. Induction of Tumor-specific T Cell Immunity by Anti-DR5 Antibody Therapy. J Exp Med 2004, 199, 437–448. [Google Scholar] [CrossRef]
- Takeda, K.; Cretney, E.; Hayakawa, Y.; Ota, T.; Akiba, H.; Ogasawara, K.; Yagita, H.; Kinoshita, K.; Okumura, K.; Smyth, M.J. TRAIL identifies immature natural killer cells in newborn mice and adult mouse liver. Blood 2005, 105, 2082–2089. [Google Scholar] [CrossRef]
- Anees, M.; Horak, P.; Schiefer, A.I.; Vanhara, P.; El-Gazzar, A.; Perco, P.; Kiesewetter, B.; Mullauer, L.; Streubel, B.; Raderer, M.; Krainer, M. The potential evasion of immune surveillance in mucosa associated lymphoid tissue lymphoma by DcR2-mediated up-regulation of nuclear factor-kappaB. Leukemia & lymphoma 2015, 56, 1440–1449. [Google Scholar] [CrossRef]
- Cassioli, C.; Baldari, C.T. The Expanding Arsenal of Cytotoxic T Cells. Front Immunol 2022, 13, 883010. [Google Scholar] [CrossRef]
- Kemp, T.J.; Ludwig, A.T.; Earel, J.K.; Moore, J.M.; Vanoosten, R.L.; Moses, B.; Leidal, K.; Nauseef, W.M.; Griffith, T.S. Neutrophil stimulation with Mycobacterium bovis bacillus Calmette-Guerin (BCG) results in the release of functional soluble TRAIL/Apo-2L. Blood 2005, 106, 3474–3482. [Google Scholar] [CrossRef] [PubMed]
- Shamili, F.H.; Bayegi, H.R.; Salmasi, Z.; Sadri, K.; Mahmoudi, M.; Kalantari, M.; Ramezani, M.; Abnous, K. Exosomes derived from TRAIL-engineered mesenchymal stem cells with effective anti-tumor activity in a mouse melanoma model. Int J Pharm 2018, 549, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Schmaltz, C.; Alpdogan, O.; Kappel, B.J.; Muriglan, S.J.; Rotolo, J.A.; Ongchin, J.; Willis, L.M.; Greenberg, A.S.; Eng, J.M.; Crawford, J.M.; et al. T cells require TRAIL for optimal graft-versus-tumor activity. Nat Med 2002, 8, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Zamai, L.; Del Zotto, G.; Buccella, F.; Galeotti, L.; Canonico, B.; Luchetti, F.; Papa, S. Cytotoxic functions and susceptibility to apoptosis of human CD56(bright) NK cells differentiated in vitro from CD34(+) hematopoietic progenitors. Cytometry A 2012, 81, 294–302. [Google Scholar] [CrossRef]
- Sur, S.Y.; Lim, G.H.; Park, S.M.; Seo, K.W.; Youn, H.Y. Anti-tumor Effect of Activated Canine B Cells With Interleukin-21 and Anti-B Cell Receptor. Anticancer research 2023, 43, 4007–4014. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, A.A.; Peters, E.; Vodegel, D.; Steenmans, D.; Raimo, M.; Gibbs, S.; de Gruijl, T.D.; Duru, A.D.; Spanholtz, J.; Georgoudaki, A.M. Early TRAIL-engagement elicits potent multimodal targeting of melanoma by CD34(+) progenitor cell-derived NK cells. iScience 2023, 26, 107078. [Google Scholar] [CrossRef]
- Smyth, M.J.; Hayakawa, Y.; Takeda, K.; Yagita, H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer 2002, 2, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Labrada, A.; Pesini, C.; Santiago, L.; Hidalgo, S.; Calvo-Perez, A.; Onate, C.; Andres-Tovar, A.; Garzon-Tituana, M.; Uranga-Murillo, I.; Arias, M.A.; et al. All About (NK Cell-Mediated) Death in Two Acts and an Unexpected Encore: Initiation, Execution and Activation of Adaptive Immunity. Front Immunol 2022, 13, 896228. [Google Scholar] [CrossRef] [PubMed]
- Asher, A.; Mule, J.J.; Reichert, C.M.; Shiloni, E.; Rosenberg, S.A. Studies on the anti-tumor efficacy of systemically administered recombinant tumor necrosis factor against several murine tumors in vivo. J Immunol 1987, 138, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, J.; Watanabe-Fukunaga, R.; Adachi, M.; Matsuzawa, A.; Kasugai, T.; Kitamura, Y.; Itoh, N.; Suda, T.; Nagata, S. Lethal effect of the anti-Fas antibody in mice. Nature 1993, 364, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Bonavida, B.; Ng, C.P.; Jazirehi, A.; Schiller, G.; Mizutani, Y. Selectivity of TRAIL-mediated apoptosis of cancer cells and synergy with drugs: the trail to non-toxic cancer therapeutics (review). Int J Oncol 1999, 15, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Pollack, I.F.; Erff, M.; Ashkenazi, A. Direct stimulation of apoptotic signaling by soluble Apo2l/tumor necrosis factor-related apoptosis-inducing ligand leads to selective killing of glioma cells. Clin Cancer Res 2001, 7, 1362–1369. [Google Scholar] [PubMed]
- Ashkenazi, A.; Pai, R.C.; Fong, S.; Leung, S.; Lawrence, D.A.; Marsters, S.A.; Blackie, C.; Chang, L.; McMurtrey, A.E.; Hebert, A.; et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 1999, 104, 155–162. [Google Scholar] [CrossRef]
- Kelley, S.K.; Harris, L.A.; Xie, D.; Deforge, L.; Totpal, K.; Bussiere, J.; Fox, J.A. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther 2001, 299, 31–38. [Google Scholar]
- Herbst, R.S.; Mendolson, D.S.; Ebbinghaus, S.; Gordon, M.S.; O’Dwyer, M.; Lieberman, G.; Ing, J.; Kurzrock, R.; Novotny, W.; Eckhardt, S.G. A phase I safety and pharmacokinetic (PK) study of recombinant Apo2L/TRAIL, an apoptosis-inducing protein in patients with advanced cancer. J Clin Oncol 2006, 24, abstr 3013, ASCO Annual Meeting Proceedings. [Google Scholar] [CrossRef]
- Ichikawa, K.; Liu, W.; Zhao, L.; Wang, Z.; Liu, D.; Ohtsuka, T.; Zhang, H.; Mountz, J.D.; Koopman, W.J.; Kimberly, R.P.; Zhou, T. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med 2001, 7, 954–960. [Google Scholar] [CrossRef]
- Walczak, H.; Miller, R.E.; Ariail, K.; Gliniak, B.; Griffith, T.S.; Kubin, M.; Chin, W.; Jones, J.; Woodward, A.; Le, T.; et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 1999, 5, 157–163. [Google Scholar] [CrossRef]
- French, L.E.; Tschopp, J. The TRAIL to selective tumor death. Nat Med 1999, 5, 146–147. [Google Scholar] [CrossRef]
- Kurbanov, B.M.; Geilen, C.C.; Fecker, L.F.; Orfanos, C.E.; Eberle, J. Efficient TRAIL-R1/DR4-mediated apoptosis in melanoma cells by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J Invest Dermatol 2005, 125, 1010–1019, Research Support, Non-U.S. Gov’t. [Google Scholar] [CrossRef]
- Strater, J.; Hinz, U.; Walczak, H.; Mechtersheimer, G.; Koretz, K.; Herfarth, C.; Moller, P.; Lehnert, T. Expression of TRAIL and TRAIL receptors in colon carcinoma: TRAIL-R1 is an independent prognostic parameter. Clin Cancer Res 2002, 8, 3734–3740. [Google Scholar]
- Spierings, D.C.; de Vries, E.G.; Timens, W.; Groen, H.J.; Boezen, H.M.; de Jong, S. Expression of TRAIL and TRAIL death receptors in stage III non-small cell lung cancer tumors. Clin Cancer Res 2003, 9, 3397–3405. [Google Scholar]
- Spierings, D.C.; de Vries, E.G.; Vellenga, E.; van den Heuvel, F.A.; Koornstra, J.J.; Wesseling, J.; Hollema, H.; de Jong, S. Tissue distribution of the death ligand TRAIL and its receptors. J Histochem Cytochem 2004, 52, 821–831. [Google Scholar] [CrossRef]
- Daniels, R.A.; Turley, H.; Kimberley, F.C.; Liu, X.S.; Mongkolsapaya, J.; Ch’En, P.; Xu, X.N.; Jin, B.Q.; Pezzella, F.; Screaton, G.R. Expression of TRAIL and TRAIL receptors in normal and malignant tissues. Cell Res 2005, 15, 430–438. [Google Scholar] [CrossRef]
- Sanlioglu, A.D.; Korcum, A.F.; Pestereli, E.; Erdogan, G.; Karaveli, S.; Savas, B.; Griffith, T.S.; Sanlioglu, S. TRAIL death receptor-4 expression positively correlates with the tumor grade in breast cancer patients with invasive ductal carcinoma. Int J Radiat Oncol Biol Phys 2007, 69, 716–723. [Google Scholar] [CrossRef]
- Ganten, T.M.; Sykora, J.; Koschny, R.; Batke, E.; Aulmann, S.; Mansmann, U.; Stremmel, W.; Sinn, H.P.; Walczak, H. Prognostic significance of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor expression in patients with breast cancer. J Mol Med (Berl) 2009, 87, 995–1007. [Google Scholar] [CrossRef]
- Chen, S.M.; Sun, H.; Liu, Y.F.; Ma, J.; Zhang, Q.T.; Zhu, J.; Li, T. Expression of TRAIL and its receptor DR5 and their significance in acute leukemia cells. Genetics and molecular research: GMR 2015, 14, 18562–18568. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, F.; Kruger, S.; Roder, C.; Trauzold, A.; Rocken, C.; Kalthoff, H. The expression of death receptor systems TRAIL-R1/-R2/-R4, CD95 and TNF-R1 and their cognate ligands in pancreatic ductal adenocarcinoma. Histol Histopathol 2019, 34, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Ravi, R.; Bedi, A. Requirement of BAX for TRAIL/Apo2L-induced apoptosis of colorectal cancers: synergism with sulindac-mediated inhibition of Bcl-x(L). Cancer research 2002, 62, 1583–1587. [Google Scholar] [PubMed]
- Willms, A.; Schittek, H.; Rahn, S.; Sosna, J.; Mert, U.; Adam, D.; Trauzold, A. Impact of p53 status on TRAIL-mediated apoptotic and non-apoptotic signaling in cancer cells. PLoS One 2019, 14, e0214847. [Google Scholar] [CrossRef]
- Micheau, O.; Shirley, S.; Dufour, F. Death receptors as targets in cancer. British Journal of Pharmacology 2013, 169, 1723–1744. [Google Scholar] [CrossRef] [PubMed]
- Naoum, G.E.; Buchsbaum, D.J.; Tawadros, F.; Farooqi, A.; Arafat, W.O. Journey of TRAIL from Bench to Bedside and its Potential Role in Immuno-Oncology. Oncol Rev 2017, 11, 332. [Google Scholar] [CrossRef]
- Smyth, M.J.; Takeda, K.; Hayakawa, Y.; Peschon, J.J.; van den Brink, M.R.; Yagita, H. Nature’s TRAIL-On a Path to Cancer Immunotherapy. Immunity 2003, 18, 1–6. [Google Scholar] [CrossRef]
- Stuckey, D.W.; Shah, K. TRAIL on trial: preclinical advances in cancer therapy. Trends Mol Med 2013, 19, 685–694. [Google Scholar] [CrossRef]
- Di Cristofano, F.; George, A.; Tajiknia, V.; Ghandali, M.; Wu, L.; Zhang, Y.; Srinivasan, P.; Strandberg, J.; Hahn, M.; Sanchez Sevilla Uruchurtu, A.; et al. Therapeutic targeting of TRAIL death receptors. Biochemical Society transactions 2023, 51, 57–70. [Google Scholar] [CrossRef]
- Bodmer, J.L.; Holler, N.; Reynard, S.; Vinciguerra, P.; Schneider, P.; Juo, P.; Blenis, J.; Tschopp, J. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nature cell biology 2000, 2, 241–243. [Google Scholar] [CrossRef]
- Kischkel, F.C.; Lawrence, D.A.; Chuntharapai, A.; Schow, P.; Kim, K.J.; Ashkenazi, A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity 2000, 12, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.B.; de Vries, E.; Tait, S.W.; Bontjer, I.; Borst, J. TRAIL receptor and CD95 signal to mitochondria via FADD, caspase-8/10, Bid, and Bax but differentially regulate events downstream from truncated Bid. The Journal of biological chemistry 2002, 277, 40760–40767. [Google Scholar] [CrossRef] [PubMed]
- Sprick, M.R.; Weigand, M.A.; Rieser, E.; Rauch, C.T.; Juo, P.; Blenis, J.; Krammer, P.H.; Walczak, H. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 2000, 12, 599–609. [Google Scholar] [CrossRef]
- Muzio, M.; Stockwell, B.R.; Stennicke, H.R.; Salvesen, G.S.; Dixit, V.M. An induced proximity model for caspase-8 activation. The Journal of biological chemistry 1998, 273, 2926–2930. [Google Scholar] [CrossRef]
- Boatright, K.M.; Deis, C.; Denault, J.B.; Sutherlin, D.P.; Salvesen, G.S. Activation of caspases-8 and -10 by FLIP(L). Biochem J 2004, 382 Pt 2, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Boatright, K.M.; Renatus, M.; Scott, F.L.; Sperandio, S.; Shin, H.; Pedersen, I.M.; Ricci, J.E.; Edris, W.A.; Sutherlin, D.P.; Green, D.R.; Salvesen, G.S. A unified model for apical caspase activation. Molecular cell 2003, 11, 529–541. [Google Scholar] [CrossRef]
- Stennicke, H.R.; Jurgensmeier, J.M.; Shin, H.; Deveraux, Q.; Wolf, B.B.; Yang, X.; Zhou, Q.; Ellerby, H.M.; Ellerby, L.M.; Bredesen, D.; et al. Pro-caspase-3 is a major physiologic target of caspase-8. The Journal of biological chemistry 1998, 273, 27084–27090. [Google Scholar] [CrossRef]
- Martin, S.J.; Green, D.R. Protease activation during apoptosis: death by a thousand cuts? Cell 1995, 82, 349–352. [Google Scholar] [CrossRef]
- Matveeva, A.; Fichtner, M.; McAllister, K.; McCann, C.; Sturrock, M.; Longley, D.B.; Prehn, J.H.M. Heterogeneous responses to low level death receptor activation are explained by random molecular assembly of the Caspase-8 activation platform. PLoS Comput Biol 2019, 15, e1007374. [Google Scholar] [CrossRef]
- Spencer, S.L.; Gaudet, S.; Albeck, J.G.; Burke, J.M.; Sorger, P.K. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature 2009, 459, 428–432. [Google Scholar] [CrossRef]
- Scaffidi, C.; Schmitz, I.; Zha, J.; Korsmeyer, S.J.; Krammer, P.H.; Peter, M.E. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. The Journal of biological chemistry 1999, 274, 22532–22538. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Tada-Oikawa, S.; Uchida, A.; Kawanishi, S. TRAIL causes cleavage of bid by caspase-8 and loss of mitochondrial membrane potential resulting in apoptosis in BJAB cells. Biochem Biophys Res Commun 1999, 265, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Walczak, H.; Bouchon, A.; Stahl, H.; Krammer, P.H. Tumor necrosis factor-related apoptosis-inducing ligand retains its apoptosis-inducing capacity on Bcl-2- or Bcl-xL-overexpressing chemotherapy-resistant tumor cells. Cancer research 2000, 60, 3051–3057, Research Support, Non-U.S. Gov’t. [Google Scholar] [CrossRef]
- Gazitt, Y.; Shaughnessy, P.; Montgomery, W. Apoptosis-induced by TRAIL AND TNF-alpha in human multiple myeloma cells is not blocked by BCL-2. Cytokine 1999, 11, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Keogh, S.A.; Walczak, H.; Bouchier-Hayes, L.; Martin, S.J. Failure of Bcl-2 to block cytochrome c redistribution during TRAIL-induced apoptosis. FEBS letters 2000, 471, 93–98. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, H.; Lawrence, D.; Varfolomeev, E.; Totpal, K.; Morlan, J.; Schow, P.; Fong, S.; Schwall, R.; Sinicropi, D.; Ashkenazi, A. Tumor-cell resistance to death receptor--induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med 2002, 8, 274–281. [Google Scholar] [CrossRef]
- Hinz, S.; Trauzold, A.; Boenicke, L.; Sandberg, C.; Beckmann, S.; Bayer, E.; Walczak, H.; Kalthoff, H.; Ungefroren, H. Bcl-XL protects pancreatic adenocarcinoma cells against CD95- and TRAIL-receptor-mediated apoptosis. Oncogene 2000, 19, 5477–5486. [Google Scholar] [CrossRef]
- Guo, B.C.; Xu, Y.H. Bcl-2 over-expression and activation of protein kinase C suppress the trail-induced apoptosis in Jurkat T cells. Cell Res 2001, 11, 101–106. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, J.; O’Neill, K.L.; Gurumurthy, C.B.; Quadros, R.M.; Tu, Y.; Luo, X. Cleavage by Caspase 8 and Mitochondrial Membrane Association Activate the BH3-only Protein Bid during TRAIL-induced Apoptosis. The Journal of biological chemistry 2016, 291, 11843–11851. [Google Scholar] [CrossRef]
- Desagher, S.; Osen-Sand, A.; Nichols, A.; Eskes, R.; Montessuit, S.; Lauper, S.; Maundrell, K.; Antonsson, B.; Martinou, J.C. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol 1999, 144, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Zhao, Y.; Barber, M.J.; Kuharsky, D.K.; Yin, X.M. Bid-induced cytochrome c release is mediated by a pathway independent of mitochondrial permeability transition pore and Bax. The Journal of biological chemistry 2000, 275, 39474–39481. [Google Scholar] [CrossRef]
- Korsmeyer, S.J.; Wei, M.C.; Saito, M.; Weiler, S.; Oh, K.J.; Schlesinger, P.H. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ 2000, 7, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Chinnaiyan, A.M. The apoptosome: heart and soul of the cell death machine. Neoplasia (New York, N.Y 1999, 1, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Li, Y.; Liu, X.; Wang, X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. The Journal of biological chemistry 1999, 274, 11549–11556. [Google Scholar] [CrossRef] [PubMed]
- Acehan, D.; Jiang, X.; Morgan, D.G.; Heuser, J.E.; Wang, X.; Akey, C.W. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Molecular cell 2002, 9, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Pop, C.; Timmer, J.; Sperandio, S.; Salvesen, G.S. The apoptosome activates caspase-9 by dimerization. Molecular cell 2006, 22, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Holler, N.; Zaru, R.; Micheau, O.; Thome, M.; Attinger, A.; Valitutti, S.; Bodmer, J.L.; Schneider, P.; Seed, B.; Tschopp, J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 2000, 1, 489–495. [Google Scholar] [CrossRef]
- Meurette, O.; Rebillard, A.; Huc, L.; Le Moigne, G.; Merino, D.; Micheau, O.; Lagadic-Gossmann, D.; Dimanche-Boitrel, M.T. TRAIL induces receptor-interacting protein 1-dependent and caspase-dependent necrosis-like cell death under acidic extracellular conditions. Cancer research 2007, 67, 218–226. [Google Scholar] [CrossRef]
- Jouan-Lanhouet, S.; Arshad, M.I.; Piquet-Pellorce, C.; Martin-Chouly, C.; Le Moigne-Muller, G.; Van Herreweghe, F.; Takahashi, N.; Sergent, O.; Lagadic-Gossmann, D.; Vandenabeele, P.; et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ 2012, 19, 2003–2014. [Google Scholar] [CrossRef]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nature chemical biology 2005, 1, 112–119. [Google Scholar] [CrossRef]
- Degterev, A.; Hitomi, J.; Germscheid, M.; Ch’en, I.L.; Korkina, O.; Teng, X.; Abbott, D.; Cuny, G.D.; Yuan, C.; Wagner, G.; et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nature chemical biology 2008, 4, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Wickliffe, K.E.; Dugger, D.L.; Maltzman, A.; Roose-Girma, M.; Dohse, M.; Komuves, L.; Webster, J.D.; Dixit, V.M. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature 2019, 574, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Micheau, O.; Thome, M.; Schneider, P.; Holler, N.; Tschopp, J.; Nicholson, D.W.; Briand, C.; Grutter, M.G. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. The Journal of biological chemistry 2002, 277, 45162–45171. [Google Scholar] [CrossRef] [PubMed]
- Mompean, M.; Li, W.; Li, J.; Laage, S.; Siemer, A.B.; Bozkurt, G.; Wu, H.; McDermott, A.E. The Structure of the Necrosome RIPK1-RIPK3 Core, a Human Hetero-Amyloid Signaling Complex. Cell 2018, 173, 1244–1253. [Google Scholar] [CrossRef]
- Wu, X.; Ma, Y.; Zhao, K.; Zhang, J.; Sun, Y.; Li, Y.; Dong, X.; Hu, H.; Liu, J.; Wang, J.; et al. The structure of a minimum amyloid fibril core formed by necroptosis-mediating RHIM of human RIPK3. Proc Natl Acad Sci U S A 2021, 118. [Google Scholar] [CrossRef]
- Cho, Y.S.; Challa, S.; Moquin, D.; Genga, R.; Ray, T.D.; Guildford, M.; Chan, F.K. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009, 137, 1112–1123. [Google Scholar] [CrossRef]
- Zhang, D.W.; Shao, J.; Lin, J.; Zhang, N.; Lu, B.J.; Lin, S.C.; Dong, M.Q.; Han, J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009, 325, 332–336. [Google Scholar] [CrossRef]
- Orozco, S.; Yatim, N.; Werner, M.R.; Tran, H.; Gunja, S.Y.; Tait, S.W.; Albert, M.L.; Green, D.R.; Oberst, A. RIPK1 both positively and negatively regulates RIPK3 oligomerization and necroptosis. Cell Death Differ 2014, 21, 1511–1521. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X.; Wang, X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012, 148, (1–2). [Google Scholar] [CrossRef] [PubMed]
- Bertrand, M.J.; Vandenabeele, P. The Ripoptosome: death decision in the cytosol. Molecular cell 2011, 43, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, L.; Su, L.; Rizo, J.; Liu, L.; Wang, L.F.; Wang, F.S.; Wang, X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Molecular cell 2014, 54, 133–146. [Google Scholar] [CrossRef]
- Zhao, J.; Jitkaew, S.; Cai, Z.; Choksi, S.; Li, Q.; Luo, J.; Liu, Z.G. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A 2012, 109, 5322–5327. [Google Scholar] [CrossRef]
- Dondelinger, Y.; Declercq, W.; Montessuit, S.; Roelandt, R.; Goncalves, A.; Bruggeman, I.; Hulpiau, P.; Weber, K.; Sehon, C.A.; Marquis, R.W.; et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep 2014, 7, 971–981. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Kroemer, G. MLKL regulates necrotic plasma membrane permeabilization. Cell Res 2014, 24, 139–140. [Google Scholar] [CrossRef]
- Murphy, J.M.; Vince, J.E. Post-translational control of RIPK3 and MLKL mediated necroptotic cell death. F1000Research 2015, 4. [Google Scholar] [CrossRef]
- Wike-Hooley, J.L.; Haveman, J.; Reinhold, H.S. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol 1984, 2, 343–366. [Google Scholar] [CrossRef]
- Barja de Quiroga, G. Hypothesis that the acidification of a tissue which takes place during ischemia can lead to tissue hyperoxia during reperfusion due to the Bohr effect. Free radical biology & medicine 1990, 8, 487–489. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Gan, I.; Pavlosky, A.; Huang, X.; Fuhrmann, B.; Jevnikar, A.M. Intracellular pH Regulates TRAIL-Induced Apoptosis and Necroptosis in Endothelial Cells. J Immunol Res 2017, 2017, 1503960. [Google Scholar] [CrossRef]
- Bogdanov, A.; Bogdanov, A.; Chubenko, V.; Volkov, N.; Moiseenko, F.; Moiseyenko, V. Tumor acidity: From hallmark of cancer to target of treatment. Front Oncol 2022, 12, 979154. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.; Camisaschi, C.; Berzi, A.; Ferro, S.; Lugini, L.; Triulzi, T.; Tuccitto, A.; Tagliabue, E.; Castelli, C.; Rivoltini, L. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Seminars in cancer biology 2017, 43, 74–89. [Google Scholar] [CrossRef]
- Lu, M.; Lawrence, D.A.; Marsters, S.; Acosta-Alvear, D.; Kimmig, P.; Mendez, A.S.; Paton, A.W.; Paton, J.C.; Walter, P.; Ashkenazi, A. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science 2014, 345, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Lawrence, D.A.; Ashkenazi, A.; Walter, P. Confirming a critical role for death receptor 5 and caspase-8 in apoptosis induction by endoplasmic reticulum stress. Cell Death Differ 2018, 25, 1530–1531. [Google Scholar] [CrossRef] [PubMed]
- Dufour, F.; Rattier, T.; Constantinescu, A.A.; Zischler, L.; Morle, A.; Ben Mabrouk, H.; Humblin, E.; Jacquemin, G.; Szegezdi, E.; Delacote, F.; et al. TRAIL receptor gene editing unveils TRAIL-R1 as a master player of apoptosis induced by TRAIL and ER stress. Oncotarget 2017, 8, 9974–9985. [Google Scholar] [CrossRef]
- Iurlaro, R.; Puschel, F.; Leon-Annicchiarico, C.L.; O’Connor, H.; Martin, S.J.; Palou-Gramon, D.; Lucendo, E.; Munoz-Pinedo, C. Glucose Deprivation Induces ATF4-Mediated Apoptosis through TRAIL Death Receptors. Mol Cell Biol 2017, 37. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.K.; Lawrence, D.A.; Lu, M.; Tan, J.; Harnoss, J.M.; Marsters, S.A.; Liu, P.; Sandoval, W.; Martin, S.E.; Ashkenazi, A. Coordination between Two Branches of the Unfolded Protein Response Determines Apoptotic Cell Fate. Molecular cell 2018, 71, 629–636.e625. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Marsters, S.A.; Ashkenazi, A.; Walter, P. Misfolded proteins bind and activate death receptor 5 to trigger apoptosis during unresolved endoplasmic reticulum stress. Elife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Fundora, K.A.; Hamamoto, K.; Opozda, D.M.; Liang, X.; Liu, X.; Zhang, J.; Uzun, Y.; Takahashi, Y.; Wang, H.G. ER stress elicits non-canonical CASP8 (caspase 8) activation on autophagosomal membranes to induce apoptosis. Autophagy 2023, 1–16. [Google Scholar] [CrossRef]
- Favaro, F.; Both, D.; Derks, I.A.M.; Spaargaren, M.; Munoz-Pinedo, C.; Eldering, E. Negligible role of TRAIL death receptors in cell death upon endoplasmic reticulum stress in B-cell malignancies. Oncogenesis 2023, 12, 6. [Google Scholar] [CrossRef]
- Glab, J.A.; Doerflinger, M.; Nedeva, C.; Jose, I.; Mbogo, G.W.; Paton, J.C.; Paton, A.W.; Kueh, A.J.; Herold, M.J.; Huang, D.C.; et al. DR5 and caspase-8 are dispensable in ER stress-induced apoptosis. Cell Death Differ 2017, 24, 944–950. [Google Scholar] [CrossRef]
- Havell, E.A.; Fiers, W.; North, R.J. The antitumor function of tumor necrosis factor (TNF), I. Therapeutic action of TNF against an established murine sarcoma is indirect, immunologically dependent, and limited by severe toxicity. J Exp Med 1988, 167, 1067–1085. [Google Scholar] [CrossRef]
- North, R.J.; Havell, E.A. The antitumor function of tumor necrosis factor (TNF) II. Analysis of the role of endogenous TNF in endotoxin-induced hemorrhagic necrosis and regression of an established sarcoma. J Exp Med 1988, 167, 1086–1099. [Google Scholar] [CrossRef]
- Rensing-Ehl, A.; Frei, K.; Flury, R.; Matiba, B.; Mariani, S.M.; Weller, M.; Aebischer, P.; Krammer, P.H.; Fontana, A. Local Fas/APO-1 (CD95) ligand-mediated tumor cell killing in vivo. Eur J Immunol 1995, 25, 2253–2258. [Google Scholar] [CrossRef]
- Itoh, N.; Yonehara, S.; Ishii, A.; Yonehara, M.; Mizushima, S.; Sameshima, M.; Hase, A.; Seto, Y.; Nagata, S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 1991, 66, 233–243. [Google Scholar] [CrossRef]
- Suda, T.; Takahashi, T.; Golstein, P.; Nagata, S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 1993, 75, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Leist, M.; Gantner, F.; Bohlinger, I.; Germann, P.G.; Tiegs, G.; Wendel, A. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-alpha requires transcriptional arrest. J Immunol 1994, 153, 1778–1788. [Google Scholar] [CrossRef] [PubMed]
- Nio, Y.; Zighelboim, J.; Berek, J.; Bonavida, B. Cycloheximide-induced modulation of TNF-mediated cytotoxicity in sensitive and resistant ovarian tumor cells. Cancer chemotherapy and pharmacology 1990, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H.; Haas, E.; Schwenzer, R.; Muhlenbeck, F.; Kreuz, S.; Schubert, G.; Grell, M.; Smith, C.; Scheurich, P. Inhibition of death receptor-mediated gene induction by a cycloheximide-sensitive factor occurs at the level of or upstream of Fas-associated death domain protein (FADD). The Journal of biological chemistry 2000, 275, 24357–24366. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Suda, T.; Yatomi, T.; Nakamura, N.; Nagata, S. Lethal effect of recombinant human Fas ligand in mice pretreated with Propionibacterium acnes. J Immunol 1997, 158, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, V.; Freudenberg, M.A.; Galanos, C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. J Exp Med 1987, 165, 657–663. [Google Scholar] [CrossRef]
- Schuchmann, M.; Varfolomeev, E.E.; Hermann, F.; Rueckert, F.; Strand, D.; Koehler, H.; Strand, S.; Lohse, A.W.; Wallach, D.; Galle, P.R. Dominant negative MORT1/FADD rescues mice from CD95 and TNF-induced liver failure. Hepatology (Baltimore, Md 2003, 37, 129–135. [Google Scholar] [CrossRef]
- Eichacker, P.Q.; Hoffman, W.D.; Farese, A.; Banks, S.M.; Kuo, G.C.; MacVittie, T.J.; Natanson, C. TNF but not IL-1 in dogs causes lethal lung injury and multiple organ dysfunction similar to human sepsis. Journal of applied physiology 1991, 71, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, L.B.; Emerson, T.E., Jr.; Taylor, F.B., Jr.; Chang, A.C.; Duerr, M.; Peer, G.T.; Flournoy, D.J.; White, G.L.; Kosanke, S.D.; Murray, C.K.; et al. Lethal Staphylococcus aureus-induced shock in primates: prevention of death with anti-TNF antibody. J Trauma 1992, 33, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Micheau, O.; Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003, 114, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Huang, J.; Shu, H.B.; Baichwal, V.; Goeddel, D.V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 1996, 4, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Kelliher, M.A.; Grimm, S.; Ishida, Y.; Kuo, F.; Stanger, B.Z.; Leder, P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity 1998, 8, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Ting, A.T.; Bertrand, M.J.M. More to Life than NF-kappaB in TNFR1 Signaling. Trends Immunol 2016, 37, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Shu, H.B.; Pan, M.G.; Goeddel, D.V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 1996, 84, 299–308. [Google Scholar] [CrossRef]
- Hsu, H.; Xiong, J.; Goeddel, D.V. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 1995, 81, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Varfolomeev, E.E.; Schuchmann, M.; Luria, V.; Chiannilkulchai, N.; Beckmann, J.S.; Mett, I.L.; Rebrikov, D.; Brodianski, V.M.; Kemper, O.C.; Kollet, O.; et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 1998, 9, 267–276. [Google Scholar] [CrossRef]
- Boldin, M.P.; Goncharov, T.M.; Goltsev, Y.V.; Wallach, D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell 1996, 85, 803–815. [Google Scholar] [CrossRef]
- Chhibber-Goel, J.; Coleman-Vaughan, C.; Agrawal, V.; Sawhney, N.; Hickey, E.; Powell, J.C.; McCarthy, J.V. gamma-Secretase Activity Is Required for Regulated Intramembrane Proteolysis of Tumor Necrosis Factor (TNF) Receptor 1 and TNF-mediated Pro-apoptotic Signaling. The Journal of biological chemistry 2016, 291, 5971–5985. [Google Scholar] [CrossRef]
- Chan, F.K.; Chun, H.J.; Zheng, L.; Siegel, R.M.; Bui, K.L.; Lenardo, M.J. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science 2000, 288, 2351–2354, Comment Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. [Google Scholar] [CrossRef] [PubMed]
- Albogami, S.; Todd, I.; Negm, O.; Fairclough, L.C.; Tighe, P.J. Mutations in the binding site of TNFR1 PLAD reduce homologous interactions but can enhance antagonism of wild-type TNFR1 activity. Immunology 2021, 164, 637–654. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Zhao, L.; Zheng, Y.; Belfetmi, A.; Cai, T.; Xu, B.; Heyninck, K.; Van Den Heede, K.; Buyse, M.A.; Fontana, P.; et al. Autoinhibitory structure of preligand association state implicates a new strategy to attain effective DR5 receptor activation. Cell Res 2023, 33, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Clancy, L.; Mruk, K.; Archer, K.; Woelfel, M.; Mongkolsapaya, J.; Screaton, G.; Lenardo, M.J.; Chan, F.K. Preligand assembly domain-mediated ligand- independent association between TRAIL receptor 4 (TR4) and TR2 regulates TRAIL-induced apoptosis. Proc Natl Acad Sci U S A 2005, 102, 18099–18104. [Google Scholar] [CrossRef]
- Deng, G.M.; Liu, L.; Tsokos, G.C. Targeted tumor necrosis factor receptor I preligand assembly domain improves skin lesions in MRL/lpr mice. Arthritis Rheum 2010, 62, 2424–2431. [Google Scholar] [CrossRef]
- Deng, G.M.; Zheng, L.; Chan, F.K.; Lenardo, M. Amelioration of inflammatory arthritis by targeting the pre-ligand assembly domain of tumor necrosis factor receptors. Nat Med 2005, 11, 1066–1072. [Google Scholar] [CrossRef]
- Wang, Y.L.; Chou, F.C.; Chen, S.J.; Lin, S.H.; Chang, D.M.; Sytwu, H.K. Targeting pre-ligand assembly domain of TNFR1 ameliorates autoimmune diseases - an unrevealed role in downregulation of Th17 cells. J Autoimmun 2011, 37, 160–170. [Google Scholar] [CrossRef]
- Micheau, O.; Rizzi, M.; Smulski, C.R. Editorial: TNFR Superfamily Oligomerization and Signaling. Front Cell Dev Biol 2021, 9, 682472. [Google Scholar] [CrossRef]
- Vanamee, E.S.; Faustman, D.L. The benefits of clustering in TNF receptor superfamily signaling. Front Immunol 2023, 14, 1225704. [Google Scholar] [CrossRef]
- Pan, L.; Fu, T.M.; Zhao, W.; Zhao, L.; Chen, W.; Qiu, C.; Liu, W.; Liu, Z.; Piai, A.; Fu, Q.; et al. Higher-Order Clustering of the Transmembrane Anchor of DR5 Drives Signaling. Cell 2019, 176, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Valley, C.C.; Lewis, A.K.; Mudaliar, D.J.; Perlmutter, J.D.; Braun, A.R.; Karim, C.B.; Thomas, D.D.; Brody, J.R.; Sachs, J.N. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces death receptor 5 networks that are highly organized. The Journal of biological chemistry 2012, 287, 21265–21278. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.K.; Valley, C.C.; Peery, S.L.; Brummel, B.; Braun, A.R.; Karim, C.B.; Sachs, J.N. Death Receptor 5 Networks Require Membrane Cholesterol for Proper Structure and Function. J Mol Biol 2016, 428, 4843–4855. [Google Scholar] [CrossRef]
- Zhao, L.; Fu, Q.; Pan, L.; Piai, A.; Chou, J.J. The Diversity and Similarity of Transmembrane Trimerization of TNF Receptors. Front Cell Dev Biol 2020, 8, 569684. [Google Scholar] [CrossRef] [PubMed]
- Frazzette, N.; Cruz, A.C.; Wu, X.; Hammer, J.A.; Lippincott-Schwartz, J.; Siegel, R.M.; Sengupta, P. Super-Resolution Imaging of Fas/CD95 Reorganization Induced by Membrane-Bound Fas Ligand Reveals Nanoscale Clustering Upstream of FADD Recruitment. Cells 2022, 11, 1908. [Google Scholar] [CrossRef] [PubMed]
- Scott, F.L.; Stec, B.; Pop, C.; Dobaczewska, M.K.; Lee, J.J.; Monosov, E.; Robinson, H.; Salvesen, G.S.; Schwarzenbacher, R.; Riedl, S.J. The Fas-FADD death domain complex structure unravels signalling by receptor clustering. Nature 2009, 457, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Salvesen, G.S.; Riedl, S.J. Structure of the Fas/FADD complex: a conditional death domain complex mediating signaling by receptor clustering. Cell cycle (Georgetown, Tex 2009, 8, 2723–2727. [Google Scholar] [CrossRef]
- Ho, K.L.; Harrington, H.A. Bistability in apoptosis by receptor clustering. PLoS Comput Biol 2010, 6, e1000956. [Google Scholar] [CrossRef]
- Micheau, O. Posttranslational Modifications and Death Receptor Signalling. In TRAIL, Fas Ligand, TNF and TLR3 in Cancer, Micheau, O. Ed.; Springer International Publishing, 2017; pp 247-290. [CrossRef]
- Micheau, O. Regulation of TNF-Related Apoptosis-Inducing Ligand Signaling by Glycosylation. Int J Mol Sci 2018, 19, 715. [Google Scholar] [CrossRef]
- Wagner, K.W.; Punnoose, E.A.; Januario, T.; Lawrence, D.A.; Pitti, R.M.; Lancaster, K.; Lee, D.; von Goetz, M.; Yee, S.F.; Totpal, K.; et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med 2007, 13, 1070–1077. [Google Scholar] [CrossRef]
- Jiang, Y.; Wen, T.; Yan, R.; Kim, S.R.; Stowell, S.R.; Wang, W.; Wang, Y.; An, G.; Cummings, R.D.; Ju, T. O-glycans on death receptors in cells modulate their sensitivity to TRAIL-induced apoptosis through affecting on their stability and oligomerization. FASEB J 2020, 34, 11786–11801. [Google Scholar] [CrossRef]
- Dufour, F.; Rattier, T.; Shirley, S.; Picarda, G.; Constantinescu, A.A.; Morle, A.; Zakaria, A.B.; Marcion, G.; Causse, S.; Szegezdi, E.; et al. N-glycosylation of mouse TRAIL-R and human TRAIL-R1 enhances TRAIL-induced death. Cell Death Differ 2017, 24, 500–510. [Google Scholar] [CrossRef]
- Estornes, Y.; Dondelinger, Y.; Weber, K.; Bruggeman, I.; Peall, A.; MacFarlane, M.; Lebecque, S.; Vandenabeele, P.; Bertrand, M.J.M. N-glycosylation of mouse TRAIL-R restrains TRAIL-induced apoptosis. Cell death & disease 2018, 9. [Google Scholar] [CrossRef]
- Shatnyeva, O.M.; Kubarenko, A.V.; Weber, C.E.; Pappa, A.; Schwartz-Albiez, R.; Weber, A.N.; Krammer, P.H.; Lavrik, I.N. Modulation of the CD95-induced apoptosis: the role of CD95 N-glycosylation. PLoS One 2011, 6, e19927, Research Support, Non-U.S. Gov’t. [Google Scholar] [CrossRef]
- Yoshida, T.; Shiraishi, T.; Horinaka, M.; Wakada, M.; Sakai, T. Glycosylation modulates TRAIL-R1/death receptor 4 protein: different regulations of two pro-apoptotic receptors for TRAIL by tunicamycin. Oncology reports 2007, 18, 1239–1242. [Google Scholar] [CrossRef]
- Corti, A.; Merli, S.; Bagnasco, L.; D’Ambrosio, F.; Marino, M.; Cassani, G. Identification of two forms (31-33 and 48 kD) of the urinary soluble p55 tumor necrosis factor receptor that are differentially N- and O-glycosylated. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research 1995, 15, 143–152. [Google Scholar] [CrossRef]
- de Vreede, G.; Morrison, H.A.; Houser, A.M.; Boileau, R.M.; Andersen, D.; Colombani, J.; Bilder, D. A Drosophila Tumor Suppressor Gene Prevents Tonic TNF Signaling through Receptor N-Glycosylation. Dev Cell 2018, 45, 595–605. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, W.; Liu, S.; Chi, J.; Zhang, J.; Sui, A.; Wang, L.; Liang, Z.; Li, D.; Chen, Y.; Niu, H. N-Acetyl-Glucosamine Sensitizes Non-Small Cell Lung Cancer Cells to TRAIL-Induced Apoptosis by Activating Death Receptor 5. Cell Physiol Biochem 2018, 45, 2054–2070. [Google Scholar] [CrossRef]
- Zhang, B.; van Roosmalen, I.A.M.; Reis, C.R.; Setroikromo, R.; Quax, W.J. Death receptor 5 is activated by fucosylation in colon cancer cells. FEBS J 2019, 286, 555–571. [Google Scholar] [CrossRef]
- Jeon, M.Y.; Seo, S.U.; Woo, S.M.; Min, K.J.; Byun, H.S.; Hur, G.M.; Kang, S.C.; Kwon, T.K. Oridonin enhances TRAIL-induced apoptosis through GALNT14-mediated DR5 glycosylation. Biochimie 2019, 165, 108–114. [Google Scholar] [CrossRef]
- Peter, M.E.; Hellbardt, S.; Schwartz-Albiez, R.; Westendorp, M.O.; Walczak, H.; Moldenhauer, G.; Grell, M.; Krammer, P.H. Cell surface sialylation plays a role in modulating sensitivity towards APO-1-mediated apoptotic cell death. Cell Death Differ 1995, 2, 163–171. [Google Scholar]
- Liu, Z.; Swindall, A.F.; Kesterson, R.A.; Schoeb, T.R.; Bullard, D.C.; Bellis, S.L. ST6Gal-I regulates macrophage apoptosis via alpha2-6 sialylation of the TNFR1 death receptor. The Journal of biological chemistry 2011, 286, 39654–39662. [Google Scholar] [CrossRef]
- Swindall, A.F.; Bellis, S.L. Sialylation of the Fas death receptor by ST6Gal-I provides protection against Fas-mediated apoptosis in colon carcinoma cells. The Journal of biological chemistry 2011, 286, 22982–22990, Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. [Google Scholar] [CrossRef]
- Holdbrooks, A.T.; Britain, C.M.; Bellis, S.L. ST6Gal-I sialyltransferase promotes tumor necrosis factor (TNF)-mediated cancer cell survival via sialylation of the TNF receptor 1 (TNFR1) death receptor. The Journal of biological chemistry 2018, 293, 1610–1622. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Song, Y.K.; Song, J.J.; Siervo-Sassi, R.R.; Kim, H.R.; Li, L.; Spitz, D.R.; Lokshin, A.; Kim, J.H. Reconstitution of galectin-3 alters glutathione content and potentiates TRAIL-induced cytotoxicity by dephosphorylation of Akt. Exp Cell Res 2003, 288, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Oka, N.; Nakahara, S.; Takenaka, Y.; Fukumori, T.; Hogan, V.; Kanayama, H.O.; Yanagawa, T.; Raz, A. Galectin-3 inhibits tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by activating Akt in human bladder carcinoma cells. Cancer research 2005, 65, 7546–7553. [Google Scholar] [CrossRef]
- Lin, C.I.; Whang, E.E.; Abramson, M.A.; Donner, D.B.; Bertagnolli, M.M.; Moore, F.D., Jr.; Ruan, D.T. Galectin-3 regulates apoptosis and doxorubicin chemoresistance in papillary thyroid cancer cells. Biochem Biophys Res Commun 2009, 379, 626–631. [Google Scholar] [CrossRef]
- Mazurek, N.; Byrd, J.C.; Sun, Y.; Ueno, S.; Bresalier, R.S. A galectin-3 sequence polymorphism confers TRAIL sensitivity to human breast cancer cells. Cancer 2011, 117, 4375–4380. [Google Scholar] [CrossRef]
- Mazurek, N.; Byrd, J.C.; Sun, Y.; Hafley, M.; Ramirez, K.; Burks, J.; Bresalier, R.S. Cell-surface galectin-3 confers resistance to TRAIL by impeding trafficking of death receptors in metastatic colon adenocarcinoma cells. Cell Death Differ 2012, 19, 523–533, Research Support, N.I.H., Extramural. [Google Scholar] [CrossRef]
- Saksida, T.; Nikolic, I.; Vujicic, M.; Nilsson, U.J.; Leffler, H.; Lukic, M.L.; Stojanovic, I.; Stosic-Grujicic, S. Galectin-3 deficiency protects pancreatic islet cells from cytokine-triggered apoptosis in vitro. Journal of cellular physiology 2013, 228, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, R.R.; Yu, Z.J.; Liang, H.; Shen, S.; Kan, Q. Galectin-1 Modulates the Survival and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) Sensitivity in Human Hepatocellular Carcinoma Cells. Cancer biotherapy & radiopharmaceuticals 2015, 30, 336–341. [Google Scholar] [CrossRef]
- Lee, H.; Oh, Y.; Jeon, Y.J.; Lee, S.Y.; Kim, H.; Lee, H.J.; Jung, Y.K. DR4-Ser424 O-GlcNAcylation Promotes Sensitization of TRAIL-Tolerant Persisters and TRAIL-Resistant Cancer Cells to Death. Cancer research 2019, 79, 2839–2852. [Google Scholar] [CrossRef]
- Yang, S.Z.; Xu, F.; Yuan, K.; Sun, Y.; Zhou, T.; Zhao, X.; McDonald, J.M.; Chen, Y. Regulation of pancreatic cancer TRAIL resistance by protein O-GlcNAcylation. Laboratory investigation; a journal of technical methods and pathology 2020, 100, 777–785. [Google Scholar] [CrossRef]
- Xue, J.; Pan, X.; Peng, T.; Duan, M.; Du, L.; Zhuang, X.; Cai, X.; Yi, X.; Fu, Y.; Li, S. Auto Arginine-GlcNAcylation Is Crucial for Bacterial Pathogens in Regulating Host Cell Death. Front Cell Infect Microbiol 2020, 10, 197. [Google Scholar] [CrossRef]
- Xue, J.; Hu, S.; Huang, Y.; Zhang, Q.; Yi, X.; Pan, X.; Li, S. Arg-GlcNAcylation on TRADD by NleB and SseK1 Is Crucial for Bacterial Pathogenesis. Front Cell Dev Biol 2020, 8, 641. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Yao, Q.; Li, L.; Dong, N.; Rong, J.; Gao, W.; Ding, X.; Sun, L.; Chen, X.; et al. Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains. Nature 2013, 501, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Kim, Y.; Ji, S.; Kim, H.B.; Jung, H.; Yi, E.C.; Lee, Y.H.; Shin, I.; Yang, W.H.; Cho, J.W. O-GlcNAcylation of RIPK1 rescues red blood cells from necroptosis. Front Immunol 2023, 14, 1160490. [Google Scholar] [CrossRef] [PubMed]
- Rossin, A.; Derouet, M.; Abdel-Sater, F.; Hueber, A.O. Palmitoylation of the TRAIL receptor DR4 confers an efficient TRAIL-induced cell death signalling. Biochem J 2009, 419, 185–192, 182 p following 192. [Google Scholar] [CrossRef] [PubMed]
- Chakrabandhu, K.; Herincs, Z.; Huault, S.; Dost, B.; Peng, L.; Conchonaud, F.; Marguet, D.; He, H.T.; Hueber, A.O. Palmitoylation is required for efficient Fas cell death signaling. EMBO J 2007, 26, 209–220, Research Support, Non-U.S. Gov’t. [Google Scholar] [CrossRef] [PubMed]
- Feig, C.; Tchikov, V.; Schutze, S.; Peter, M.E. Palmitoylation of CD95 facilitates formation of SDS-stable receptor aggregates that initiate apoptosis signaling. EMBO J 2007, 26, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Rossin, A.; Durivault, J.; Chakhtoura-Feghali, T.; Lounnas, N.; Gagnoux-Palacios, L.; Hueber, A.O. Fas palmitoylation by the palmitoyl acyltransferase DHHC7 regulates Fas stability. Cell Death Differ 2015, 22, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Zingler, P.; Sarchen, V.; Glatter, T.; Caning, L.; Saggau, C.; Kathayat, R.S.; Dickinson, B.C.; Adam, D.; Schneider-Brachert, W.; Schutze, S.; Fritsch, J. Palmitoylation is required for TNF-R1 signaling. Cell Commun Signal 2019, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Anel, A.; Bosque, A.; Naval, J.; Pineiro, A.; Larrad, L.; Alava, M.A.; Martinez-Lorenzo, M.J. Apo2L/TRAIL and immune regulation. Front Biosci 2007, 12, 2074–2084. [Google Scholar] [CrossRef]
- Bossi, F.; Bernardi, S.; Zauli, G.; Secchiero, P.; Fabris, B. TRAIL modulates the immune system and protects against the development of diabetes. J Immunol Res 2015, 2015, 680749. [Google Scholar] [CrossRef] [PubMed]
- Sag, D.; Ayyildiz, Z.O.; Gunalp, S.; Wingender, G. The Role of TRAIL/DRs in the Modulation of Immune Cells and Responses. Cancers (Basel) 2019, 11, 1469. [Google Scholar] [CrossRef]
- Burgaletto, C.; Munafo, A.; Di Benedetto, G.; De Francisci, C.; Caraci, F.; Di Mauro, R.; Bucolo, C.; Bernardini, R.; Cantarella, G. The immune system on the TRAIL of Alzheimer’s disease. J Neuroinflammation 2020, 17, 298. [Google Scholar] [CrossRef] [PubMed]
- Cardoso Alves, L.; Corazza, N.; Micheau, O.; Krebs, P. The multifaceted role of TRAIL signaling in cancer and immunity. FEBS J 2021, 288, 5530–5554. [Google Scholar] [CrossRef]
- Harith, H.H.; Morris, M.J.; Kavurma, M.M. On the TRAIL of obesity and diabetes. Trends Endocrinol Metab 2013, 24, 578–587. [Google Scholar] [CrossRef]
- Remuzgo-Martinez, S.; Genre, F.; Lopez-Mejias, R.; Ubilla, B.; Mijares, V.; Pina, T.; Corrales, A.; Blanco, R.; Martin, J.; Llorca, J.; Gonzalez-Gay, M.A. Expression of osteoprotegerin and its ligands, RANKL and TRAIL, in rheumatoid arthritis. Scientific reports 2016, 6, 29713. [Google Scholar] [CrossRef]
- Gao, S.; Fang, Y.; Tu, S.; Chen, H.; Shao, A. Insight into the divergent role of TRAIL in non-neoplastic neurological diseases. J Cell Mol Med 2020, 24, 11070–11083. [Google Scholar] [CrossRef]
- Kelland, E.; Patil, M.S.; Patel, S.; Cartland, S.P.; Kavurma, M.M. The Prognostic, Diagnostic, and Therapeutic Potential of TRAIL Signalling in Cardiovascular Diseases. Int J Mol Sci 2023, 24, 6725. [Google Scholar] [CrossRef] [PubMed]
- Bosque, A.; Pardo, J.; Martinez-Lorenzo, M.J.; Lasierra, P.; Larrad, L.; Marzo, I.; Naval, J.; Anel, A. Human CD8+ T cell blasts are more sensitive than CD4+ T cell blasts to regulation by APO2L/TRAIL. Eur J Immunol 2005, 35, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Yamaguchi, N.; Nakayama, M.; Eto, H.; Okumura, K.; Yagita, H. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: A novel mechanism for the antitumor effects of type I IFNs. J Exp Med 1999, 189, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Badovinac, V.P.; Messingham, K.A.; Griffith, T.S.; Harty, J.T. TRAIL deficiency delays, but does not prevent, erosion in the quality of “helpless” memory CD8 T cells. J Immunol 2006, 177, 999–1006. [Google Scholar] [CrossRef]
- Janssen, E.M.; Droin, N.M.; Lemmens, E.E.; Pinkoski, M.J.; Bensinger, S.J.; Ehst, B.D.; Griffith, T.S.; Green, D.R.; Schoenberger, S.P. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 2005, 434, 88–93. [Google Scholar] [CrossRef]
- Sacks, J.A.; Bevan, M.J. TRAIL deficiency does not rescue impaired CD8+ T cell memory generated in the absence of CD4+ T cell help. J Immunol 2008, 180, 4570–4576. [Google Scholar] [CrossRef]
- Wolkers, M.C.; Gerlach, C.; Arens, R.; Janssen, E.M.; Fitzgerald, P.; Schumacher, T.N.; Medema, J.P.; Green, D.R.; Schoenberger, S.P. Nab2 regulates secondary CD8+ T-cell responses through control of TRAIL expression. Blood 2012, 119, 798–804. [Google Scholar] [CrossRef]
- Zhang, X.R.; Zhang, L.Y.; Devadas, S.; Li, L.; Keegan, A.D.; Shi, Y.F. Reciprocal expression of TRAIL and CD95L in Th1 and Th2 cells: role of apoptosis in T helper subset differentiation. Cell Death Differ 2003, 10, 203–210. [Google Scholar] [CrossRef]
- Martinez-Lorenzo, M.J.; Alava, M.A.; Gamen, S.; Kim, K.J.; Chuntharapai, A.; Pineiro, A.; Naval, J.; Anel, A. Involvement of APO2 ligand/TRAIL in activation-induced death of Jurkat and human peripheral blood T cells. Eur J Immunol 1998, 28, 2714–2725. [Google Scholar] [CrossRef]
- Hamilton, S.E.; Wolkers, M.C.; Schoenberger, S.P.; Jameson, S.C. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol 2006, 7, 475–481. [Google Scholar] [CrossRef]
- Sedger, L.M.; Katewa, A.; Pettersen, A.K.; Osvath, S.R.; Farrell, G.C.; Stewart, G.J.; Bendall, L.J.; Alexander, S.I. Extreme lymphoproliferative disease and fatal autoimmune thrombocytopenia in FasL- and TRAIL-double deficient mice. Blood 2010. [Google Scholar] [CrossRef]
- Takeda, K.; Hayakawa, Y.; Smyth, M.J.; Kayagaki, N.; Yamaguchi, N.; Kakuta, S.; Iwakura, Y.; Yagita, H.; Okumura, K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med 2001, 7, 94–100. [Google Scholar] [CrossRef]
- Pimentel, J.M.; Zhou, J.Y.; Wu, G.S. The Role of TRAIL in Apoptosis and Immunosurveillance in Cancer. Cancers (Basel) 2023, 15, 2752. [Google Scholar] [CrossRef]
- Chaperot, L.; Blum, A.; Manches, O.; Lui, G.; Angel, J.; Molens, J.P.; Plumas, J. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J Immunol 2006, 176, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Griffith, T.S.; Brincks, E.L.; Gurung, P.; Kucaba, T.A.; Ferguson, T.A. Systemic immunological tolerance to ocular antigens is mediated by TRAIL-expressing CD8+ T cells. J Immunol 2011, 186, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.A.; Ni, J.; Pan, G.; Ruben, S.M.; Wei, Y.F.; Pace, J.L.; Hunt, J.S. TRAIL (Apo-2L) and TRAIL receptors in human placentas: implications for immune privilege. J Immunol 1999, 162, 6053–6059. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, A.K.; Sattler, A.; Hahn, E.M.; Hering, N.A.; Arndt, M.; Lauscher, J.C.; Speichinger-Hillenberg, F.; Kotsch, K.; Berg, A.K.; Beyer, K. Immune Phenotypic Characterization of a TRAIL-Knockout Mouse. Cancers (Basel) 2023, 15, 1475. [Google Scholar] [CrossRef]
- Delacher, M.; Schmidleithner, L.; Simon, M.; Stuve, P.; Sanderink, L.; Hotz-Wagenblatt, A.; Wuttke, M.; Schambeck, K.; Ruhland, B.; Hofmann, V.; et al. The effector program of human CD8 T cells supports tissue remodeling. J Exp Med 2024, 221. [Google Scholar] [CrossRef]
- Chyuan, I.T.; Tsai, H.F.; Wu, C.S.; Hsu, P.N. TRAIL suppresses gut inflammation and inhibits colitogeic T-cell activation in experimental colitis via an apoptosis-independent pathway. Mucosal Immunol 2019, 12, 980–989. [Google Scholar] [CrossRef]
- Chyuan, I.T.; Tsai, H.F.; Liao, H.J.; Wu, C.S.; Hsu, P.N. An apoptosis-independent role of TRAIL in suppressing joint inflammation and inhibiting T-cell activation in inflammatory arthritis. Cell Mol Immunol 2018, 15, 846–857. [Google Scholar] [CrossRef]
- Song, K.; Chen, Y.; Goke, R.; Wilmen, A.; Seidel, C.; Goke, A.; Hilliard, B.; Chen, Y. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation and cell cycle progression. J Exp Med 2000, 191, 1095–1104. [Google Scholar] [CrossRef]
- Chyuan, I.T.; Hsu, P.N. TRAIL regulates T cell activation and suppresses inflammation in autoimmune diseases. Cell Mol Immunol 2020, 17, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Cao, Z.; Wolf, J.M.; Van Antwerp, M.; Baker, J.R., Jr. Death ligand tumor necrosis factor-related apoptosis-inducing ligand inhibits experimental autoimmune thyroiditis. Endocrinology 2005, 146, 4721–4726. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Seol, D.W.; Mi, Z.; Robbins, P.D. Intra-articular injection of recombinant TRAIL induces synovial apoptosis and reduces inflammation in a rabbit knee model of arthritis. Arthritis Res Ther 2006, 8, R16. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Hirata, S.; Fukushima, S.; Matsunaga, Y.; Ito, T.; Uchino, M.; Nishimura, Y.; Senju, S. Dual effects of TRAIL in suppression of autoimmunity: the inhibition of Th1 cells and the promotion of regulatory T cells. J Immunol 2010, 185, 5259–5267. [Google Scholar] [CrossRef] [PubMed]
- Cretney, E.; McQualter, J.L.; Kayagaki, N.; Yagita, H.; Bernard, C.C.; Grewal, I.S.; Ashkenazi, A.; Smyth, M.J. TNF-related apoptosis-inducing ligand (TRAIL)/Apo2L suppresses experimental autoimmune encephalomyelitis in mice. Immunol Cell Biol 2005, 83, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Annibaldi, A.; Walczak, H. Death Receptors and Their Ligands in Inflammatory Disease and Cancer. Cold Spring Harb Perspect Biol 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- McGrath, E.E.; Marriott, H.M.; Lawrie, A.; Francis, S.E.; Sabroe, I.; Renshaw, S.A.; Dockrell, D.H.; Whyte, M.K. TNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammation. Journal of leukocyte biology 2011, 90, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Zahn, S.; Rehkamper, C.; Ferring-Schmitt, S.; Bieber, T.; Tuting, T.; Wenzel, J. Interferon-alpha stimulates TRAIL expression in human keratinocytes and peripheral blood mononuclear cells: implications for the pathogenesis of cutaneous lupus erythematosus. Br J Dermatol 2011, 165, 1118–1123. [Google Scholar] [CrossRef]
- Nguyen, V.; Cudrici, C.; Zernetkina, V.; Niculescu, F.; Rus, H.; Drachenberg, C.; Rus, V. TRAIL, DR4 and DR5 are upregulated in kidneys from patients with lupus nephritis and exert proliferative and proinflammatory effects. Clin Immunol 2009, 132, 32–42. [Google Scholar] [CrossRef]
- Daigle, I.; Simon, H.U. Alternative functions for TRAIL receptors in eosinophils and neutrophils. Swiss Med Wkly 2001, 131, 231–237. [Google Scholar] [CrossRef]
- Robertson, N.M.; Zangrilli, J.G.; Steplewski, A.; Hastie, A.; Lindemeyer, R.G.; Planeta, M.A.; Smith, M.K.; Innocent, N.; Musani, A.; Pascual, R.; et al. Differential expression of TRAIL and TRAIL receptors in allergic asthmatics following segmental antigen challenge: evidence for a role of TRAIL in eosinophil survival. J Immunol 2002, 169, 5986–5996. [Google Scholar] [CrossRef]
- Weckmann, M.; Collison, A.; Simpson, J.L.; Kopp, M.V.; Wark, P.A.; Smyth, M.J.; Yagita, H.; Matthaei, K.I.; Hansbro, N.; Whitehead, B.; et al. Critical link between TRAIL and CCL20 for the activation of TH2 cells and the expression of allergic airway disease. Nat Med 2007, 13, 1308–1315. [Google Scholar] [CrossRef]
- Weckmann, M.; Kopp, M.V.; Heinzmann, A.; Mattes, J. Haplotypes covering the TNFSF10 gene are associated with bronchial asthma. Pediatr Allergy Immunol 2011, 22 Pt 1, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Zauli, G.; Pandolfi, A.; Gonelli, A.; Di Pietro, R.; Guarnieri, S.; Ciabattoni, G.; Rana, R.; Vitale, M.; Secchiero, P. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) sequentially upregulates nitric oxide and prostanoid production in primary human endothelial cells. Circulation research 2003, 92, 732–740. [Google Scholar] [CrossRef]
- Secchiero, P.; Gonelli, A.; Carnevale, E.; Corallini, F.; Rizzardi, C.; Zacchigna, S.; Melato, M.; Zauli, G. Evidence for a proangiogenic activity of TNF-related apoptosis-inducing ligand. Neoplasia (New York, N.Y 2004, 6, 364–373. [Google Scholar] [CrossRef]
- Cartland, S.P.; Genner, S.W.; Zahoor, A.; Kavurma, M.M. Comparative Evaluation of TRAIL, FGF-2 and VEGF-A-Induced Angiogenesis In Vitro and In Vivo. Int J Mol Sci 2016, 17, 2025. [Google Scholar] [CrossRef]
- Kavurma, M.M.; Schoppet, M.; Bobryshev, Y.V.; Khachigian, L.M.; Bennett, M.R. TRAIL stimulates proliferation of vascular smooth muscle cells via activation of NF-kappaB and induction of insulin-like growth factor-1 receptor. The Journal of biological chemistry 2008, 283, 7754–7762. [Google Scholar] [CrossRef] [PubMed]
- Na, H.J.; Hwang, J.Y.; Lee, K.S.; Choi, Y.K.; Choe, J.; Kim, J.Y.; Moon, H.E.; Kim, K.W.; Koh, G.Y.; Lee, H.; et al. TRAIL negatively regulates VEGF-induced angiogenesis via caspase-8-mediated enzymatic and non-enzymatic functions. Angiogenesis 2014, 17, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolo, B.A.; Cartland, S.P.; Prado-Lourenco, L.; Griffith, T.S.; Gentile, C.; Ravindran, J.; Azahri, N.S.; Thai, T.; Yeung, A.W.; Thomas, S.R.; Kavurma, M.M. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) Promotes Angiogenesis and Ischemia-Induced Neovascularization Via NADPH Oxidase 4 (NOX4) and Nitric Oxide-Dependent Mechanisms. J Am Heart Assoc 2015, 4. [Google Scholar] [CrossRef]
- Yen, M.L.; Tsai, H.F.; Wu, Y.Y.; Hwa, H.L.; Lee, B.H.; Hsu, P.N. TNF-related apoptosis-inducing ligand (TRAIL) induces osteoclast differentiation from monocyte/macrophage lineage precursor cells. Molecular immunology 2008, 45, 2205–2213. [Google Scholar] [CrossRef]
- Sambandam, Y.; Baird, K.L.; Stroebel, M.; Kowal, E.; Balasubramanian, S.; Reddy, S.V. Microgravity Induction of TRAIL Expression in Preosteoclast Cells Enhances Osteoclast Differentiation. Scientific reports 2016, 6, 25143. [Google Scholar] [CrossRef]
- Freer-Prokop, M.; O’Flaherty, J.; Ross, J.A.; Weyman, C.M. Non-canonical role for the TRAIL receptor DR5/FADD/caspase pathway in the regulation of MyoD expression and skeletal myoblast differentiation. Differentiation 2009, 78, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.L.; Lee, T.A.; Tsai, T.L.; Lin, W.W. TRAIL-induced keratinocyte differentiation requires caspase activation and p63 expression. J Invest Dermatol 2011, 131, 874–883. [Google Scholar] [CrossRef]
- Zoller, V.; Funcke, J.B.; Keuper, M.; Abd El Hay, M.; Debatin, K.M.; Wabitsch, M.; Fischer-Posovszky, P. TRAIL (TNF-related apoptosis-inducing ligand) inhibits human adipocyte differentiation via caspase-mediated downregulation of adipogenic transcription factors. Cell death & disease 2016, 7, e2412. [Google Scholar] [CrossRef]
- Dawson, S.H.; Arnold, N.D.; Pickworth, J.A.; Francis, S.E.; Lawrie, A. TRAIL Deficient Mice Are Protected from Sugen/Hypoxia Induced Pulmonary Arterial Hypertension. Diseases 2014, 2, 260–273. [Google Scholar] [CrossRef]
- Hameed, A.G.; Arnold, N.D.; Chamberlain, J.; Pickworth, J.A.; Paiva, C.; Dawson, S.; Cross, S.; Long, L.; Zhao, L.; Morrell, N.W.; et al. Inhibition of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) reverses experimental pulmonary hypertension. J Exp Med 2012, 209, 1919–1935. [Google Scholar] [CrossRef]
- Liu, H.; Yang, E.; Lu, X.; Zuo, C.; He, Y.; Jia, D.; Zhu, Q.; Yu, Y.; Lv, A. Serum Levels of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Correlate with the Severity of Pulmonary Hypertension. Pulm Pharmacol Ther 2015. [Google Scholar] [CrossRef] [PubMed]
- Secchiero, P.; Zerbinati, C.; Rimondi, E.; Corallini, F.; Milani, D.; Grill, V.; Forti, G.; Capitani, S.; Zauli, G. TRAIL promotes the survival, migration and proliferation of vascular smooth muscle cells. Cell Mol Life Sci 2004, 61, 1965–1974. [Google Scholar] [CrossRef]
- Secchiero, P.; Gonelli, A.; Carnevale, E.; Milani, D.; Pandolfi, A.; Zella, D.; Zauli, G. TRAIL promotes the survival and proliferation of primary human vascular endothelial cells by activating the Akt and ERK pathways. Circulation 2003, 107, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Choi, K.; Ryu, S.W.; Kang, S.W.; Choi, C. TRAIL promotes caspase-dependent pro-inflammatory responses via PKCdelta activation by vascular smooth muscle cells. Cell death & disease 2011, 2, e223. [Google Scholar] [CrossRef]
- Tanner, M.A.; Thomas, T.P.; Grisanti, L.A. Death receptor 5 contributes to cardiomyocyte hypertrophy through epidermal growth factor receptor transactivation. Journal of molecular and cellular cardiology 2019, 136, 1–14. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Hsu, J.L.; Wang, H.C.; Wu, S.J.; Hong, C.J.; Cheng, I.H. Alterations of the Neuroinflammatory Markers IL-6 and TRAIL in Alzheimer’s Disease. Dement Geriatr Cogn Dis Extra 2015, 5, 424–434. [Google Scholar] [CrossRef]
- Uberti, D.; Ferrari-Toninelli, G.; Bonini, S.A.; Sarnico, I.; Benarese, M.; Pizzi, M.; Benussi, L.; Ghidoni, R.; Binetti, G.; Spano, P.; et al. Blockade of the tumor necrosis factor-related apoptosis inducing ligand death receptor DR5 prevents beta-amyloid neurotoxicity. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 2007, 32, 872–880. [Google Scholar] [CrossRef]
- Frenkel, D. A new TRAIL in Alzheimer’s disease therapy. Brain 2015, 138 Pt 1, 8–10. [Google Scholar] [CrossRef]
- Fossati, S.; Ghiso, J.; Rostagno, A. TRAIL death receptors DR4 and DR5 mediate cerebral microvascular endothelial cell apoptosis induced by oligomeric Alzheimer’s Abeta. Cell death & disease 2012, 3, e321. [Google Scholar] [CrossRef]
- Cantarella, G.; Di Benedetto, G.; Puzzo, D.; Privitera, L.; Loreto, C.; Saccone, S.; Giunta, S.; Palmeri, A.; Bernardini, R. Neutralization of TNFSF10 ameliorates functional outcome in a murine model of Alzheimer’s disease. Brain 2015, 138 Pt 1, 203–216. [Google Scholar] [CrossRef]
- Cartland, S.P.; Harith, H.H.; Genner, S.W.; Dang, L.; Cogger, V.C.; Vellozzi, M.; Di Bartolo, B.A.; Thomas, S.R.; Adams, L.A.; Kavurma, M.M. Non-alcoholic fatty liver disease, vascular inflammation and insulin resistance are exacerbated by TRAIL deletion in mice. Scientific reports 2017, 7. [Google Scholar] [CrossRef]
- Lee, M.; Shin, E.; Bae, J.; Cho, Y.; Lee, J.Y.; Lee, Y.H.; Lee, B.W.; Kang, E.S.; Cha, B.S. Dipeptidyl peptidase-4 inhibitor protects against non-alcoholic steatohepatitis in mice by targeting TRAIL receptor-mediated lipoapoptosis via modulating hepatic dipeptidyl peptidase-4 expression. Scientific reports 2020, 10, 19429. [Google Scholar] [CrossRef] [PubMed]
- Hirsova, P.; Weng, P.; Salim, W.; Bronk, S.F.; Griffith, T.S.; Ibrahim, S.H.; Gores, G.J. TRAIL Deletion Prevents Liver, but Not Adipose Tissue, Inflammation during Murine Diet-Induced Obesity. Hepatol Commun 2017, 1, 648–662. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.J.; Wang, P.; Tsabary, G.; Chen, Y.H. Critical roles of TRAIL in hepatic cell death and hepatic inflammation. J Clin Invest 2004, 113, 58–64. [Google Scholar] [CrossRef]
- Walczak, H. Death receptor-ligand systems in cancer, cell death, and inflammation. Cold Spring Harb Perspect Biol 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.L.; Hsu, P.N.; Liao, H.J.; Lee, B.H.; Tsai, H.F. TRAF-6 dependent signaling pathway is essential for TNF-related apoptosis-inducing ligand (TRAIL) induces osteoclast differentiation. PLoS One 2012, 7, e38048. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Prado-Lourenco, L.; Khachigian, L.M.; Bennett, M.R.; Di Bartolo, B.A.; Kavurma, M.M. TRAIL promotes VSMC proliferation and neointima formation in a FGF-2-, Sp1 phosphorylation-, and NFkappaB-dependent manner. Circulation research 2010, 106, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Lluis, J.M.; Nachbur, U.; Cook, W.D.; Gentle, I.E.; Moujalled, D.; Moulin, M.; Wong, W.W.; Khan, N.; Chau, D.; Callus, B.A.; et al. TAK1 is required for survival of mouse fibroblasts treated with TRAIL, and does so by NF-kappaB dependent induction of cFLIPL. PLoS One 2010, 5, e8620. [Google Scholar] [CrossRef]
- Azijli, K.; Weyhenmeyer, B.; Peters, G.J.; de Jong, S.; Kruyt, F.A. Non-canonical kinase signaling by the death ligand TRAIL in cancer cells: discord in the death receptor family. Cell Death Differ 2013. [Google Scholar] [CrossRef]
- Chekkat, N.; Lombardo, C.M.; Seguin, C.; Lechner, M.C.; Dufour, F.; Nomine, Y.; De Giorgi, M.; Frisch, B.; Micheau, O.; Guichard, G.; et al. Relationship between the agonist activity of synthetic ligands of TRAIL-R2 and their cell surface binding modes. Oncotarget 2018, 9, 15566–15578. [Google Scholar] [CrossRef] [PubMed]
- Somasekharan, S.P.; Koc, M.; Morizot, A.; Micheau, O.; Sorensen, P.H.; Gaide, O.; Andera, L.; Martinou, J.C. TRAIL promotes membrane blebbing, detachment and migration of cells displaying a dysfunctional intrinsic pathway of apoptosis. Apoptosis 2013, 18, 324–336. [Google Scholar] [CrossRef]
- Fritsche, H.; Heilmann, T.; Tower, R.J.; Hauser, C.; von Au, A.; El-Sheikh, D.; Campbell, G.M.; Alp, G.; Schewe, D.; Hubner, S.; et al. TRAIL-R2 promotes skeletal metastasis in a breast cancer xenograft mouse model. Oncotarget 2015, 6, 9502–9516. [Google Scholar] [CrossRef]
- Vilimanovich, U.; Bumbasirevic, V. TRAIL induces proliferation of human glioma cells by c-FLIPL-mediated activation of ERK1/2. Cell Mol Life Sci 2008, 65, 814–826. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, G.; Zhang, C.; Yang, H.; Liu, J.; Hu, H.; Wu, P.; Liu, S.; Yang, L.; Chen, X.; et al. TRAIL promotes epithelial-to-mesenchymal transition by inducing PD-L1 expression in esophageal squamous cell carcinomas. Journal of experimental & clinical cancer research: CR 2021, 40, 209. [Google Scholar] [CrossRef]
- Chaudhary, P.M.; Eby, M.; Jasmin, A.; Bookwalter, A.; Murray, J.; Hood, L. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity 1997, 7, 821–830. [Google Scholar] [CrossRef]
- Jeremias, I.; Debatin, K.M. TRAIL induces apoptosis and activation of NFkappaB. Eur Cytokine Netw 1998, 9, 687–688. [Google Scholar]
- Humphreys, L.M.; Fox, J.P.; Higgins, C.A.; Majkut, J.; Sessler, T.; McLaughlin, K.; McCann, C.; Roberts, J.Z.; Crawford, N.T.; McDade, S.S.; et al. A revised model of TRAIL-R2 DISC assembly explains how FLIP(L) can inhibit or promote apoptosis. EMBO reports 2020, 21, e49254. [Google Scholar] [CrossRef]
- MacFarlane, M.; Harper, N.; Snowden, R.T.; Dyer, M.J.; Barnett, G.A.; Pringle, J.H.; Cohen, G.M. Mechanisms of resistance to TRAIL-induced apoptosis in primary B cell chronic lymphocytic leukaemia. Oncogene 2002, 21, 6809–6818. [Google Scholar] [CrossRef] [PubMed]
- Fullsack, S.; Rosenthal, A.; Wajant, H.; Siegmund, D. Redundant and receptor-specific activities of TRADD, RIPK1 and FADD in death receptor signaling. Cell death & disease 2019, 10, 122. [Google Scholar] [CrossRef]
- Chang, Z.; Dang, T.; Che, N.; Yu, H.; Chai, J.; Chen, W. Esophageal cancer cells convert the death signal from TRAIL into a stimulus for survival during acid/bile exposure. Dig Liver Dis 2020, 52, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Pobezinskaya, Y.L.; Morgan, M.J.; Liu, Z.G. The role of TRADD in TRAIL-induced apoptosis and signaling. FASEB J 2011, 25, 1353–1358. [Google Scholar] [CrossRef]
- Lafont, E.; Kantari-Mimoun, C.; Draber, P.; De Miguel, D.; Hartwig, T.; Reichert, M.; Kupka, S.; Shimizu, Y.; Taraborrelli, L.; Spit, M.; et al. The linear ubiquitin chain assembly complex regulates TRAIL-induced gene activation and cell death. EMBO J 2017, 36, 1147–1166. [Google Scholar] [CrossRef]
- Wajant, H. TRAIL- and TNF-induced signaling complexes-so similar yet so different. EMBO J 2017, 36, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Dorn, S.; Schoergenhofer, C.; Krainer, M.; Muller, M.; Jilma, B. LUBAC and ABIN-1 Modulate TRAIL-Based NF-kappaB Induction in Human Embryonic Kidney 293 Cells. Biores Open Access 2018, 7, 81–89. [Google Scholar] [CrossRef]
- Zhang, L.; Blackwell, K.; Workman, L.M.; Chen, S.; Pope, M.R.; Janz, S.; Habelhah, H. RIP1 Cleavage in the Kinase Domain Regulates TRAIL-Induced NF-kappaB Activation and Lymphoma Survival. Mol Cell Biol 2015, 35, 3324–3338. [Google Scholar] [CrossRef] [PubMed]
- Harper, N.; Farrow, S.N.; Kaptein, A.; Cohen, G.M.; MacFarlane, M. Modulation of tumor necrosis factor apoptosis-inducing ligand- induced NF-kappa B activation by inhibition of apical caspases. The Journal of biological chemistry 2001, 276, 34743–34752. [Google Scholar] [CrossRef] [PubMed]
- Grimm, S.; Stanger, B.Z.; Leder, P. RIP and FADD: two “death domain”-containing proteins can induce apoptosis by convergent, but dissociable, pathways. Proc Natl Acad Sci U S A 1996, 93, 10923–10927. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Devin, A.; Cook, A.; Keane, M.M.; Kelliher, M.; Lipkowitz, S.; Liu, Z.G. The death domain kinase RIP is essential for TRAIL (Apo2L)-induced activation of IkappaB kinase and c-Jun N-terminal kinase. Mol Cell Biol 2000, 20, 6638–6645. [Google Scholar] [CrossRef] [PubMed]
- Azijli, K.; Yuvaraj, S.; van Roosmalen, I.; Flach, K.; Giovannetti, E.; Peters, G.J.; de Jong, S.; Kruyt, F.A. MAPK p38 and JNK have opposing activities on TRAIL-induced apoptosis activation in NSCLC H460 cells that involves RIP1 and caspase-8 and is mediated by Mcl-1. Apoptosis 2013, 18, 851–860. [Google Scholar] [CrossRef]
- Tang, W.; Wang, W.; Zhang, Y.; Liu, S.; Liu, Y.; Zheng, D. Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced chemokine release in both TRAIL-resistant and TRAIL-sensitive cells via nuclear factor kappa B. FEBS J 2009, 276, 581–593. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.Y.; Kim, D.G.; Koo, G.B.; Yu, J.W.; Kim, Y.S. TRADD is critical for resistance to TRAIL-induced cell death through NF-kappaB activation. FEBS letters 2011, 585, 2144–2150. [Google Scholar] [CrossRef] [PubMed]
- Favaro, F.; Luciano-Mateo, F.; Moreno-Caceres, J.; Hernandez-Madrigal, M.; Both, D.; Montironi, C.; Puschel, F.; Nadal, E.; Eldering, E.; Munoz-Pinedo, C. TRAIL receptors promote constitutive and inducible IL-8 secretion in non-small cell lung carcinoma. Cell death & disease 2022, 13, 1046. [Google Scholar] [CrossRef]
- Oh, Y.T.; Yue, P.; Wang, D.; Tong, J.S.; Chen, Z.G.; Khuri, F.R.; Sun, S.Y. Suppression of death receptor 5 enhances cancer cell invasion and metastasis through activation of caspase-8/TRAF2-mediated signaling. Oncotarget 2015, 6, 41324–41338. [Google Scholar] [CrossRef]
- Wang, T.T.; Jeng, J. Coordinated regulation of two TRAIL-R2/KILLER/DR5 mRNA isoforms by DNA damaging agents, serum and 17beta-estradiol in human breast cancer cells. Breast Cancer Res Treat 2000, 61, 87–96. [Google Scholar] [CrossRef]
- Sun, S.Y. Understanding the Role of the Death Receptor 5/FADD/caspase-8 Death Signaling in Cancer Metastasis. Mol Cell Pharmacol 2011, 3, 31–34. [Google Scholar]
- Ganten, T.M.; Sykora, J.; Koschny, R.; Batke, E.; Aulmann, S.; Mansmann, U.; Stremmel, W.; Sinn, H.P.; Walczak, H. Prognostic significance of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor expression in patients with breast cancer. J Mol Med 2009, 87, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Macher-Goeppinger, S.; Aulmann, S.; Tagscherer, K.E.; Wagener, N.; Haferkamp, A.; Penzel, R.; Brauckhoff, A.; Hohenfellner, M.; Sykora, J.; Walczak, H.; et al. Prognostic value of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors in renal cell cancer. Clin Cancer Res 2009, 15, 650–659. [Google Scholar] [CrossRef]
- Shlyakhtina, Y.; Pavet, V.; Gronemeyer, H. Dual role of DR5 in death and survival signaling leads to TRAIL resistance in cancer cells. Cell death & disease 2017, 8, e3025. [Google Scholar] [CrossRef]
- Legler, D.F.; Micheau, O.; Doucey, M.A.; Tschopp, J.; Bron, C. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFalpha-mediated NF-kappaB activation. Immunity 2003, 18, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Yang, C.; Liu, Y.; Xiong, J.; Zhang, J.; Zhong, Y.; Zhang, G.; Zhou, F.; Zhou, Y.; Xie, C. Redistribution of DR4 and DR5 in lipid rafts accounts for the sensitivity to TRAIL in NSCLC cells. Int J Oncol 2011, 39, 1577–1586. [Google Scholar] [CrossRef]
- Marconi, M.; Ascione, B.; Ciarlo, L.; Vona, R.; Garofalo, T.; Sorice, M.; Gianni, A.M.; Locatelli, S.L.; Carlo-Stella, C.; Malorni, W.; Matarrese, P. Constitutive localization of DR4 in lipid rafts is mandatory for TRAIL-induced apoptosis in B-cell hematologic malignancies. Cell death & disease 2013, 4, e863. [Google Scholar] [CrossRef]
- Bellail, A.C.; Tse, M.C.; Song, J.H.; Phuphanich, S.; Olson, J.J.; Sun, S.Y.; Hao, C. DR5-mediated DISC controls caspase-8 cleavage and initiation of apoptosis in human glioblastomas. J Cell Mol Med 2010, 14, 1303–1317. [Google Scholar] [CrossRef]
- Lim, S.C.; Duong, H.Q.; Choi, J.E.; Lee, T.B.; Kang, J.H.; Oh, S.H.; Han, S.I. Lipid raft-dependent death receptor 5 (DR5) expression and activation are critical for ursodeoxycholic acid-induced apoptosis in gastric cancer cells. Carcinogenesis 2011, 32, 723–731. [Google Scholar] [CrossRef]
- Delmas, D.; Rébé, C.; Micheau, O.; Athias, A.; Gambert, P.; Grazide, S.; laurent, G.; Latruffe, N.; Solary, E. Redistribution of CD95, DR4 and DR5 in rafts accounts for the synergistic toxicity of resveratrol and death receptor ligands in colon carcinoma cells. Oncogene 2004, 23, 8979–8986. [Google Scholar] [CrossRef]
- Song, J.H.; Tse, M.C.; Bellail, A.; Phuphanich, S.; Khuri, F.; Kneteman, N.M.; Hao, C. Lipid rafts and nonrafts mediate tumor necrosis factor related apoptosis-inducing ligand induced apoptotic and nonapoptotic signals in non small cell lung carcinoma cells. Cancer research 2007, 67, 6946–6955. [Google Scholar] [CrossRef]
- Dickens, L.S.; Boyd, R.S.; Jukes-Jones, R.; Hughes, M.A.; Robinson, G.L.; Fairall, L.; Schwabe, J.W.; Cain, K.; Macfarlane, M. A death effector domain chain DISC model reveals a crucial role for caspase-8 chain assembly in mediating apoptotic cell death. Molecular cell 2012, 47, 291–305, Research Support, Non-U.S. 110 Gov’t. [Google Scholar] [CrossRef]
- Guegan, J.P.; Ginestier, C.; Charafe-Jauffret, E.; Ducret, T.; Quignard, J.F.; Vacher, P.; Legembre, P. CD95/Fas and metastatic disease: What does not kill you makes you stronger. Seminars in cancer biology 2020, 60, 121–131. [Google Scholar] [CrossRef]
- Muppidi, J.R.; Siegel, R.M. Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death. Nat Immunol 2004, 5, 182–189. [Google Scholar] [CrossRef]
- Muppidi, J.R.; Tschopp, J.; Siegel, R.M. Life and death decisions: secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity 2004, 21, 461–465. [Google Scholar] [CrossRef]
- Siegel, R.M.; Muppidi, J.R.; Sarker, M.; Lobito, A.; Jen, M.; Martin, D.; Straus, S.E.; Lenardo, M.J. SPOTS: signaling protein oligomeric transduction structures are early mediators of death receptor-induced apoptosis at the plasma membrane. J Cell Biol 2004, 167, 735–744. [Google Scholar] [CrossRef]
- Ponton, A.; Clement, M.V.; Stamenkovic, I. The CD95 (APO-1/Fas) receptor activates NF-kappaB independently of its cytotoxic function. The Journal of biological chemistry 1996, 271, 8991–8995. [Google Scholar] [CrossRef]
- Tauzin, S.; Chaigne-Delalande, B.; Selva, E.; Khadra, N.; Daburon, S.; Contin-Bordes, C.; Blanco, P.; Le Seyec, J.; Ducret, T.; Counillon, L.; et al. The naturally processed CD95L elicits a c-yes/calcium/PI3K-driven cell migration pathway. PLoS biology 2011, 9. [Google Scholar] [CrossRef]
- Malleter, M.; Tauzin, S.; Bessede, A.; Castellano, R.; Goubard, A.; Godey, F.; Leveque, J.; Jezequel, P.; Campion, L.; Campone, M.; et al. CD95L cell surface cleavage triggers a prometastatic signaling pathway in triple-negative breast cancer. Cancer research 2013, 73, 6711–6721. [Google Scholar] [CrossRef]
- Monet, M.; Poet, M.; Tauzin, S.; Fouque, A.; Cophignon, A.; Lagadic-Gossmann, D.; Vacher, P.; Legembre, P.; Counillon, L. The cleaved FAS ligand activates the Na(+)/H(+) exchanger NHE1 through Akt/ROCK1 to stimulate cell motility. Scientific reports 2016, 6, 28008. [Google Scholar] [CrossRef]
- Park, D.R.; Thomsen, A.R.; Frevert, C.W.; Pham, U.; Skerrett, S.J.; Kiener, P.A.; Liles, W.C. Fas (CD95) induces proinflammatory cytokine responses by human monocytes and monocyte-derived macrophages. J Immunol 2003, 170, 6209–6216. [Google Scholar] [CrossRef]
- Rescigno, M.; Piguet, V.; Valzasina, B.; Lens, S.; Zubler, R.; French, L.; Kindler, V.; Tschopp, J.; Ricciardi-Castagnoli, P. Fas engagement induces the maturation of dendritic cells (DCs), the release of interleukin (IL)-1beta, and the production of interferon gamma in the absence of IL-12 during DC-T cell cognate interaction: a new role for Fas ligand in inflammatory responses. J Exp Med 2000, 192, 1661–1668. [Google Scholar] [CrossRef]
- Trauzold, A.; Roder, C.; Sipos, B.; Karsten, K.; Arlt, A.; Jiang, P.; Martin-Subero, J.I.; Siegmund, D.; Muerkoster, S.; Pagerols-Raluy, L.; et al. CD95 and TRAF2 promote invasiveness of pancreatic cancer cells. FASEB J 2005, 19, 620–622. [Google Scholar] [CrossRef]
- Steller, E.J.; Ritsma, L.; Raats, D.A.; Hoogwater, F.J.; Emmink, B.L.; Govaert, K.M.; Laoukili, J.; Rinkes, I.H.; van Rheenen, J.; Kranenburg, O. The death receptor CD95 activates the cofilin pathway to stimulate tumour cell invasion. EMBO reports 2011, 12, 931–937. [Google Scholar] [CrossRef]
- Ruan, W.; Lee, C.T.; Desbarats, J. A novel juxtamembrane domain in tumor necrosis factor receptor superfamily molecules activates Rac1 and controls neurite growth. Mol Biol Cell 2008, 19, 3192–3202. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Qu, X.; Che, X.; Guo, T.; Li, C.; Ma, R.; Fan, Y.; Ma, Y.; Hou, K.; et al. DR5-Cbl-b/c-Cbl-TRAF2 complex inhibits TRAIL-induced apoptosis by promoting TRAF2-mediated polyubiquitination of caspase-8 in gastric cancer cells. Molecular oncology 2017, 11, 1733–1751. [Google Scholar] [CrossRef]
- Gonzalvez, F.; Lawrence, D.; Yang, B.; Yee, S.; Pitti, R.; Marsters, S.; Pham, V.C.; Stephan, J.P.; Lill, J.; Ashkenazi, A. TRAF2 Sets a threshold for extrinsic apoptosis by tagging caspase-8 with a ubiquitin shutoff timer. Molecular cell 2012, 48, 888–899. [Google Scholar] [CrossRef]
- He, W.; Wang, Q.; Xu, J.; Xu, X.; Padilla, M.T.; Ren, G.; Gou, X.; Lin, Y. Attenuation of TNFSF10/TRAIL-induced apoptosis by an autophagic survival pathway involving TRAF2- and RIPK1/RIP1-mediated MAPK8/JNK activation. Autophagy 2012, 8, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Haas, T.L.; Emmerich, C.H.; Gerlach, B.; Schmukle, A.C.; Cordier, S.M.; Rieser, E.; Feltham, R.; Vince, J.; Warnken, U.; Wenger, T.; et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Molecular cell 2009, 36, 831–844. [Google Scholar] [CrossRef]
- Kataoka, T.; Budd, R.C.; Holler, N.; Thome, M.; Martinon, F.; Irmler, M.; Burns, K.; Hahne, M.; Kennedy, N.; Kovacsovics, M.; Tschopp, J. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr Biol 2000, 10, 640–648. [Google Scholar] [CrossRef]
- Kataoka, T.; Tschopp, J. N-terminal fragment of c-FLIP(L) processed by caspase 8 specifically interacts with TRAF2 and induces activation of the NF-kappaB signaling pathway. Mol Cell Biol 2004, 24, 2627–2636. [Google Scholar] [CrossRef]
- Alvarez, S.E.; Harikumar, K.B.; Hait, N.C.; Allegood, J.; Strub, G.M.; Kim, E.Y.; Maceyka, M.; Jiang, H.; Luo, C.; Kordula, T.; et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 2010, 465, 1084–1088. [Google Scholar] [CrossRef]
- Nagahashi, M.; Yamada, A.; Katsuta, E.; Aoyagi, T.; Huang, W.C.; Terracina, K.P.; Hait, N.C.; Allegood, J.C.; Tsuchida, J.; Yuza, K.; et al. Targeting the SphK1/S1P/S1PR1 Axis That Links Obesity, Chronic Inflammation, and Breast Cancer Metastasis. Cancer research 2018, 78, 1713–1725. [Google Scholar] [CrossRef]
- Noujarede, J.; Carrie, L.; Garcia, V.; Grimont, M.; Eberhardt, A.; Mucher, E.; Genais, M.; Schreuder, A.; Carpentier, S.; Segui, B.; et al. Sphingolipid paracrine signaling impairs keratinocyte adhesion to promote melanoma invasion. Cell Rep 2023, 42, 113586. [Google Scholar] [CrossRef]
- Oh, Y.T.; Yue, P.; Sun, S.Y. DR5 suppression induces sphingosine-1-phosphate-dependent TRAF2 polyubiquitination, leading to activation of JNK/AP-1 and promotion of cancer cell invasion. Cell Commun Signal 2017, 15, 18. [Google Scholar] [CrossRef]
- Wei, W.; Wang, D.; Shi, J.; Xiang, Y.; Zhang, Y.; Liu, S.; Liu, Y.; Zheng, D. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induces chemotactic migration of monocytes via a death receptor 4-mediated RhoGTPase pathway. Molecular immunology 2010, 47, 2475–2484. [Google Scholar] [CrossRef]
- Park, K.J.; Lee, C.H.; Kim, A.; Jeong, K.J.; Kim, C.H.; Kim, Y.S. Death receptors 4 and 5 activate Nox1 NADPH oxidase through riboflavin kinase to induce reactive oxygen species-mediated apoptotic cell death. The Journal of biological chemistry 2012, 287, 3313–3325. [Google Scholar] [CrossRef]
- Hartwig, T.; Montinaro, A.; von Karstedt, S.; Sevko, A.; Surinova, S.; Chakravarthy, A.; Taraborrelli, L.; Draber, P.; Lafont, E.; Arce Vargas, F.; et al. The TRAIL-Induced Cancer Secretome Promotes a Tumor-Supportive Immune Microenvironment via CCR2. Molecular cell 2017, 65, 730–742.e735. [Google Scholar] [CrossRef]
- Salhia, B.; Rutten, F.; Nakada, M.; Beaudry, C.; Berens, M.; Kwan, A.; Rutka, J.T. Inhibition of Rho-kinase affects astrocytoma morphology, motility, and invasion through activation of Rac1. Cancer research 2005, 65, 8792–8800. [Google Scholar] [CrossRef]
- Bustelo, X.R.; Ojeda, V.; Barreira, M.; Sauzeau, V.; Castro-Castro, A. Rac-ing to the plasma membrane: the long and complex work commute of Rac1 during cell signaling. Small GTPases 2012, 3, 60–66. [Google Scholar] [CrossRef]
- Miloszewska, J.; Janik, P.; Ostrowski, J. The effect of tumor necrosis factor (TNF-alpha) on calcium (Ca2+) level. Arch Immunol Ther Exp (Warsz) 1991, 39, 99–102. [Google Scholar]
- Boehning, D.; van Rossum, D.B.; Patterson, R.L.; Snyder, S.H. A peptide inhibitor of cytochrome c/inositol 1,4,5-trisphosphate receptor binding blocks intrinsic and extrinsic cell death pathways. Proc Natl Acad Sci U S A 2005, 102, 1466–1471. [Google Scholar] [CrossRef]
- Wozniak, A.L.; Wang, X.; Stieren, E.S.; Scarbrough, S.G.; Elferink, C.J.; Boehning, D. Requirement of biphasic calcium release from the endoplasmic reticulum for Fas-mediated apoptosis. J Cell Biol 2006, 175, 709–714. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Ouadid-Ahidouch, H.; Skryma, R.; Shuba, Y. Remodelling of Ca2+ transport in cancer: how it contributes to cancer hallmarks? Philosophical transactions of the Royal Society of London. Series B, Biological sciences 2014, 369, 20130097. [Google Scholar] [CrossRef]
- Xie, T.; Chen, S.; Hao, J.; Wu, P.; Gu, X.; Wei, H.; Li, Z.; Xiao, J. Roles of calcium signaling in cancer metastasis to bone. Explor Target Antitumor Ther 2022, 3, 445–462. [Google Scholar] [CrossRef]
- Khadra, N.; Bresson-Bepoldin, L.; Penna, A.; Chaigne-Delalande, B.; Segui, B.; Levade, T.; Vacher, A.M.; Reiffers, J.; Ducret, T.; Moreau, J.F.; et al. CD95 triggers Orai1-mediated localized Ca2+ entry, regulates recruitment of protein kinase C (PKC) beta2, and prevents death-inducing signaling complex formation. Proc Natl Acad Sci U S A 2011, 108, 19072–19077. [Google Scholar] [CrossRef]
- Siegmund, D.; Lang, I.; Wajant, H. Cell death-independent activities of the death receptors CD95, TRAILR1, and TRAILR2. FEBS J 2017, 284, 1131–1159. [Google Scholar] [CrossRef]
- Reis, C.R.; Chen, P.H.; Bendris, N.; Schmid, S.L. TRAIL-death receptor endocytosis and apoptosis are selectively regulated by dynamin-1 activation. Proc Natl Acad Sci U S A 2017, 114, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Airiau, K.; Vacher, P.; Micheau, O.; Prouzet-Mauleon, V.; Kroemer, G.; Moosavi, M.A.; Djavaheri-Mergny, M. TRAIL Triggers CRAC-Dependent Calcium Influx and Apoptosis through the Recruitment of Autophagy Proteins to Death-Inducing Signaling Complex. Cells 2021, 11, 57. [Google Scholar] [CrossRef]
- Ahn, E.Y.; Lim, S.T.; Cook, W.J.; McDonald, J.M. Calmodulin binding to the Fas death domain. Regulation by Fas activation. The Journal of biological chemistry 2004, 279, 5661–5666. [Google Scholar] [CrossRef]
- Chen, J.J.; Sun, Y.; Nabel, G.J. Regulation of the proinflammatory effects of Fas ligand (CD95L). Science 1998, 282, 1714–1717. [Google Scholar] [CrossRef]
- Yuan, K.; Jing, G.; Chen, J.; Liu, H.; Zhang, K.; Li, Y.; Wu, H.; McDonald, J.M.; Chen, Y. Calmodulin mediates Fas-induced FADD-independent survival signaling in pancreatic cancer cells via activation of Src-extracellular signal-regulated kinase (ERK). The Journal of biological chemistry 2011, 286, 24776–24784. [Google Scholar] [CrossRef]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca(2+) homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem Biophys Res Commun 2015, 460, 114–121. [Google Scholar] [CrossRef]
- Gong, K.; Chen, C.; Zhan, Y.; Chen, Y.; Huang, Z.; Li, W. Autophagy-related gene 7 (ATG7) and reactive oxygen species/extracellular signal-regulated kinase regulate tetrandrine-induced autophagy in human hepatocellular carcinoma. The Journal of biological chemistry 2012, 287, 35576–35588. [Google Scholar] [CrossRef]
- Nihira, K.; Miki, Y.; Ono, K.; Suzuki, T.; Sasano, H. An inhibition of p62/SQSTM1 caused autophagic cell death of several human carcinoma cells. Cancer Sci 2014, 105, 568–575. [Google Scholar] [CrossRef]
- Fancy, R.M.; Wang, L.; Schmid, T.; Zeng, Q.; Wang, H.; Zhou, T.; Buchsbaum, D.J.; Song, Y. Characterization of the interactions between calmodulin and death receptor 5 in triple-negative and estrogen receptor-positive breast cancer cells. AN INTEGRATED EXPERIMENTAL AND COMPUTATIONAL STUDY. The Journal of biological chemistry 2016, 291, 23489. [Google Scholar] [CrossRef]
- Fancy, R.M.; Kim, H.; Zhou, T.; Zinn, K.R.; Buchsbaum, D.J.; Song, Y. Calmodulin Binding to Death Receptor 5-mediated Death-Inducing Signaling Complex in Breast Cancer Cells. J Cell Biochem 2017, 118, 2285–2294. [Google Scholar] [CrossRef]
- Chin, D.; Means, A.R. Calmodulin: a prototypical calcium sensor. Trends Cell Biol 2000, 10, 322–328. [Google Scholar] [CrossRef]
- Villalobo, A. The multifunctional role of phospho-calmodulin in pathophysiological processes. Biochem J 2018, 475, 4011–4023. [Google Scholar] [CrossRef]
- Yuan, K.; Yong, S.; Xu, F.; Zhou, T.; McDonald, J.M.; Chen, Y. Calmodulin antagonists promote TRA-8 therapy of resistant pancreatic cancer. Oncotarget 2015, 6, 25308–25319. [Google Scholar] [CrossRef]
- Kaminskyy, V.O.; Surova, O.V.; Piskunova, T.; Zborovskaya, I.B.; Tchevkina, E.M.; Andera, L.; Zhivotovsky, B. Upregulation of c-FLIP-short in response to TRAIL promotes survival of NSCLC cells, which could be suppressed by inhibition of Ca2+/calmodulin signaling. Cell death & disease 2013, 4, e522. [Google Scholar] [CrossRef]
- Cursi, S.; Rufini, A.; Stagni, V.; Condo, I.; Matafora, V.; Bachi, A.; Bonifazi, A.P.; Coppola, L.; Superti-Furga, G.; Testi, R.; Barila, D. Src kinase phosphorylates Caspase-8 on Tyr380: a novel mechanism of apoptosis suppression. EMBO J 2006, 25, 1895–1905. [Google Scholar] [CrossRef]
- Stateva, S.R.; Salas, V.; Anguita, E.; Benaim, G.; Villalobo, A. Ca2+/Calmodulin and Apo-Calmodulin Both Bind to and Enhance the Tyrosine Kinase Activity of c-Src. PLoS One 2015, 10, e0128783. [Google Scholar] [CrossRef]
- Chen, Y.; Pawar, P.; Pan, G.; Ma, L.; Liu, H.; McDonald, J.M. Calmodulin binding to the Fas-mediated death-inducing signaling complex in cholangiocarcinoma cells. J Cell Biochem 2008, 103, 788–799. [Google Scholar] [CrossRef]
- Fernandez, T.F.; Samal, A.B.; Bedwell, G.J.; Chen, Y.; Saad, J.S. Structural and biophysical characterization of the interactions between the death domain of Fas receptor and calmodulin. The Journal of biological chemistry 2013, 288, 21898–21908. [Google Scholar] [CrossRef]
- Barbero, S.; Mielgo, A.; Torres, V.; Teitz, T.; Shields, D.J.; Mikolon, D.; Bogyo, M.; Barila, D.; Lahti, J.M.; Schlaepfer, D.; Stupack, D.G. Caspase-8 association with the focal adhesion complex promotes tumor cell migration and metastasis. Cancer research 2009, 69, 3755–3763. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.; Huangfu, L.; Du, H.; Ji, X.; Xing, X.; Ji, J. Death receptor 5 promotes tumor progression in gastric cancer. FEBS Open Bio 2023, 13, 2375–2388. [Google Scholar] [CrossRef]
- Leithner, K.; Stacher, E.; Wurm, R.; Ploner, F.; Quehenberger, F.; Wohlkoenig, C.; Balint, Z.; Polachova, J.; Olschewski, A.; Samonigg, H.; et al. Nuclear and cytoplasmic death receptor 5 as prognostic factors in patients with non-small cell lung cancer treated with chemotherapy. Lung Cancer 2009, 65, 98–104, Research Support, Non-U.S. Gov’t. [Google Scholar] [CrossRef]
- Bertsch, U.; Roder, C.; Kalthoff, H.; Trauzold, A. Compartmentalization of TNF-related apoptosis-inducing ligand (TRAIL) death receptor functions: emerging role of nuclear TRAIL-R2. Cell death & disease 2014, 5, e1390. [Google Scholar] [CrossRef]
- Kojima, Y.; Nakayama, M.; Nishina, T.; Nakano, H.; Koyanagi, M.; Takeda, K.; Okumura, K.; Yagita, H. Importin beta1 protein-mediated nuclear localization of death receptor 5 (DR5) limits DR5/tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-induced cell death of human tumor cells. The Journal of biological chemistry 2011, 286, 43383–43393. [Google Scholar] [CrossRef]
- Mert, U.; Adawy, A.; Scharff, E.; Teichmann, P.; Willms, A.; Haselmann, V.; Colmorgen, C.; Lemke, J.; von Karstedt, S.; Fritsch, J.; Trauzold, A. TRAIL Induces Nuclear Translocation and Chromatin Localization of TRAIL Death Receptors. Cancers (Basel) 2019, 11, 1167. [Google Scholar] [CrossRef] [PubMed]
- Haselmann, V.; Kurz, A.; Bertsch, U.; Hubner, S.; Olempska-Muller, M.; Fritsch, J.; Hasler, R.; Pickl, A.; Fritsche, H.; Annewanter, F.; et al. Nuclear death receptor TRAIL-R2 inhibits maturation of let-7 and promotes proliferation of pancreatic and other tumor cells. Gastroenterology 2014, 146, 278–290. [Google Scholar] [CrossRef]
- Chen, J.J.; Shen, H.C.; Rivera Rosado, L.A.; Zhang, Y.; Di, X.; Zhang, B. Mislocalization of death receptors correlates with cellular resistance to their cognate ligands in human breast cancer cells. Oncotarget 2012, 3, 833–842. [Google Scholar] [CrossRef]
- Alam, M.; Ahmad, R.; Rajabi, H.; Kufe, D. MUC1-C Induces the LIN28B-->LET-7-->HMGA2 Axis to Regulate Self-Renewal in NSCLC. Mol Cancer Res 2015, 13, 449–460. [Google Scholar] [CrossRef]
- Song, H.; Xu, W.; Song, J.; Liang, Y.; Fu, W.; Zhu, X.C.; Li, C.; Peng, J.S.; Zheng, J.N. Overexpression of Lin28 inhibits the proliferation, migration and cell cycle progression and induces apoptosis of BGC-823 gastric cancer cells. Oncology reports 2015, 33, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Unachukwu, U.; Chada, K.; D’Armiento, J. High Mobility Group AT-Hook 2 (HMGA2) Oncogenicity in Mesenchymal and Epithelial Neoplasia. Int J Mol Sci 2020, 21, 3151. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.R.; Powers, J.T.; Einhorn, W.; Hoshida, Y.; Ng, T.L.; Toffanin, S.; O’Sullivan, M.; Lu, J.; Phillips, L.A.; Lockhart, V.L.; et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nature genetics 2009, 41, 843–848. [Google Scholar] [CrossRef]
- Park, S.M.; Shell, S.; Radjabi, A.R.; Schickel, R.; Feig, C.; Boyerinas, B.; Dinulescu, D.M.; Lengyel, E.; Peter, M.E. Let-7 prevents early cancer progression by suppressing expression of the embryonic gene HMGA2. Cell cycle (Georgetown, Tex 2007, 6, 2585–2590. [Google Scholar] [CrossRef]
- Mbalaviele, G.; Dunstan, C.R.; Sasaki, A.; Williams, P.J.; Mundy, G.R.; Yoneda, T. E-cadherin expression in human breast cancer cells suppresses the development of osteolytic bone metastases in an experimental metastasis model. Cancer research 1996, 56, 4063–4070. [Google Scholar]
- Chatterjee, S.; Behnam Azad, B.; Nimmagadda, S. The intricate role of CXCR4 in cancer. Adv Cancer Res 2014, 124, 31–82. [Google Scholar] [CrossRef]
- Zhang, Z.; Ni, C.; Chen, W.; Wu, P.; Wang, Z.; Yin, J.; Huang, J.; Qiu, F. Expression of CXCR4 and breast cancer prognosis: a systematic review and meta-analysis. BMC Cancer 2014, 14, 49. [Google Scholar] [CrossRef]
- Yun, J.; Frankenberger, C.A.; Kuo, W.L.; Boelens, M.C.; Eves, E.M.; Cheng, N.; Liang, H.; Li, W.H.; Ishwaran, H.; Minn, A.J.; Rosner, M.R. Signalling pathway for RKIP and Let-7 regulates and predicts metastatic breast cancer. EMBO J 2011, 30, 4500–4514. [Google Scholar] [CrossRef]
- Li, C.; Egloff, A.M.; Sen, M.; Grandis, J.R.; Johnson, D.E. Caspase-8 mutations in head and neck cancer confer resistance to death receptor-mediated apoptosis and enhance migration, invasion, and tumor growth. Molecular oncology 2014, 8, 1220–1230. [Google Scholar] [CrossRef]
- Graf, R.P.; Keller, N.; Barbero, S.; Stupack, D. Caspase-8 as a regulator of tumor cell motility. Current molecular medicine 2014, 14, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.; Ozmadenci, D.; Ichim, G.; Stupack, D. Caspase-8 function, and phosphorylation, in cell migration. Semin Cell Dev Biol 2018, 82, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Barbero, S.; Barila, D.; Mielgo, A.; Stagni, V.; Clair, K.; Stupack, D. Identification of a critical tyrosine residue in caspase 8 that promotes cell migration. The Journal of biological chemistry 2008, 283, 13031–13034. [Google Scholar] [CrossRef] [PubMed]
- Contadini, C.; Ferri, A.; Di Martile, M.; Cirotti, C.; Del Bufalo, D.; De Nicola, F.; Pallocca, M.; Fanciulli, M.; Sacco, F.; Donninelli, G.; et al. Caspase-8 as a novel mediator linking Src kinase signaling to enhanced glioblastoma malignancy. Cell Death Differ 2023, 30, 417–428. [Google Scholar] [CrossRef]
- Senft, J.; Helfer, B.; Frisch, S.M. Caspase-8 interacts with the p85 subunit of phosphatidylinositol 3-kinase to regulate cell adhesion and motility. Cancer research 2007, 67, 11505–11509. [Google Scholar] [CrossRef] [PubMed]
- Rossman, K.L.; Der, C.J.; Sondek, J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 2005, 6, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Helfer, B.; Boswell, B.C.; Finlay, D.; Cipres, A.; Vuori, K.; Bong Kang, T.; Wallach, D.; Dorfleutner, A.; Lahti, J.M.; Flynn, D.C.; Frisch, S.M. Caspase-8 promotes cell motility and calpain activity under nonapoptotic conditions. Cancer research 2006, 66, 4273–4278. [Google Scholar] [CrossRef]
- Mishra, Y.G.; Manavathi, B. Focal adhesion dynamics in cellular function and disease. Cell Signal 2021, 85, 110046. [Google Scholar] [CrossRef] [PubMed]
- Sulzmaier, F.J.; Jean, C.; Schlaepfer, D.D. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 2014, 14, 598–610. [Google Scholar] [CrossRef]
- Mandal, R.; Barron, J.C.; Kostova, I.; Becker, S.; Strebhardt, K. Caspase-8: The double-edged sword. Biochim Biophys Acta Rev Cancer 2020, 1873, 188357. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, H.; Huang, X.; Zhu, B.; Guan, S.; Cheng, W.; Lai, Y.; Zhang, X.; Hua, Z.C. MiR-7a is an important mediator in Fas-associated protein with death domain (FADD)-regulated expression of focal adhesion kinase (FAK). Oncotarget 2016, 7, 51393–51407. [Google Scholar] [CrossRef]
- Murphy, J.M.; Rodriguez, Y.A.R.; Jeong, K.; Ahn, E.E.; Lim, S.S. Targeting focal adhesion kinase in cancer cells and the tumor microenvironment. Experimental & molecular medicine 2020, 52, 877–886. [Google Scholar] [CrossRef]
- Torres, V.A.; Mielgo, A.; Barbero, S.; Hsiao, R.; Wilkins, J.A.; Stupack, D.G. Rab5 mediates caspase-8-promoted cell motility and metastasis. Mol Biol Cell 2010, 21, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.A.; Mielgo, A.; Barila, D.; Anderson, D.H.; Stupack, D. Caspase 8 promotes peripheral localization and activation of Rab5. The Journal of biological chemistry 2008, 283, 36280–36289. [Google Scholar] [CrossRef]
- Ansalone, C.; Ainsworth, R.I.; Nygaard, G.; Ai, R.; Prideaux, E.B.; Hammaker, D.; Perumal, N.B.; Weichert, K.; Tung, F.; Kodandapani, L.; et al. Caspase-8 Variant G Regulates Rheumatoid Arthritis Fibroblast-Like Synoviocyte Aggressive Behavior. ACR Open Rheumatol 2022, 4, 288–299. [Google Scholar] [CrossRef]
- Mauro, C.D.; Pesapane, A.; Formisano, L.; Rosa, R.; D’Amato, V.; Ciciola, P.; Servetto, A.; Marciano, R.; Orsini, R.C.; Monteleone, F.; et al. Urokinase-type plasminogen activator receptor (uPAR) expression enhances invasion and metastasis in RAS mutated tumors. Scientific reports 2017, 7, 9388. [Google Scholar] [CrossRef]
- de Vries, T.J.; van Muijen, G.N.; Ruiter, D.J. The plasminogen activation system in tumour invasion and metastasis. Pathology, research and practice 1996, 192, 718–733. [Google Scholar] [CrossRef]
- Chabot, V.; Dromard, C.; Rico, A.; Langonne, A.; Gaillard, J.; Guilloton, F.; Casteilla, L.; Sensebe, L. Urokinase-type plasminogen activator receptor interaction with beta1 integrin is required for platelet-derived growth factor-AB-induced human mesenchymal stem/stromal cell migration. Stem Cell Res Ther 2015, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Tarui, T.; Mazar, A.P.; Cines, D.B.; Takada, Y. Urokinase-type plasminogen activator receptor (CD87) is a ligand for integrins and mediates cell-cell interaction. The Journal of biological chemistry 2001, 276, 3983–3990. [Google Scholar] [CrossRef] [PubMed]
- Kreiling, J.L.; Byrd, J.C.; Deisz, R.J.; Mizukami, I.F.; Todd, R.F., 3rd; MacDonald, R.G. Binding of urokinase-type plasminogen activator receptor (uPAR) to the mannose 6-phosphate/insulin-like growth factor II receptor: contrasting interactions of full-length and soluble forms of uPAR. The Journal of biological chemistry 2003, 278, 20628–20637. [Google Scholar] [CrossRef] [PubMed]
- Gondi, C.S.; Kandhukuri, N.; Kondraganti, S.; Gujrati, M.; Olivero, W.C.; Dinh, D.H.; Rao, J.S. RNA interference-mediated simultaneous down-regulation of urokinase-type plasminogen activator receptor and cathepsin B induces caspase-8-mediated apoptosis in SNB19 human glioma cells. Mol Cancer Ther 2006, 5, 3197–3208. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qiu, F.; Liu, Z.; Lan, Y.; Wang, K.; Zhou, P.K.; Wang, Y.; Hua, Z.C. Urokinase-type plasminogen activator receptor regulates apoptotic sensitivity of colon cancer HCT116 cell line to TRAIL via JNK-p53 pathway. Apoptosis 2014, 19, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, B.; Darnay, B.; Aggarwal, B.; Dinh, D.H.; Kouraklis, G.; Olivero, W.C.; Gujrati, M.; Rao, J.S. Glioma cells deficient in urokinase plaminogen activator receptor expression are susceptible to tumor necrosis factor-alpha-related apoptosis-inducing ligand-induced apoptosis. Clin Cancer Res 2001, 7, 4195–4201. [Google Scholar] [PubMed]
- Li, X.; Wu, B.; Chen, L.; Ju, Y.; Li, C.; Meng, S. Urokinase-type plasminogen activator receptor inhibits apoptosis in triple-negative breast cancer through miR-17/20a suppression of death receptors 4 and 5. Oncotarget 2017, 8, 88645–88657. [Google Scholar] [CrossRef] [PubMed]
- Pavet, V.; Shlyakhtina, Y.; He, T.; Ceschin, D.G.; Kohonen, P.; Perala, M.; Kallioniemi, O.; Gronemeyer, H. Plasminogen activator urokinase expression reveals TRAIL responsiveness and supports fractional survival of cancer cells. Cell death & disease 2014, 5, e1043. [Google Scholar] [CrossRef]
- Mahmood, N.; Mihalcioiu, C.; Rabbani, S.A. Multifaceted Role of the Urokinase-Type Plasminogen Activator (uPA) and Its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic Applications. Front Oncol 2018, 8, 24. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, J.H.; Song, J.J. c-Cbl shRNA-expressing adenovirus sensitizes TRAIL-induced apoptosis in prostate cancer DU-145 through increases of DR4/5. Cancer Gene Ther 2013, 20, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Szczepanski, M.J.; Kim, S.Y.; Kim, J.H.; An, J.Y.; Kwon, Y.T.; Alcala, M.A., Jr.; Bartlett, D.L.; Lee, Y.J. c-Cbl-mediated degradation of TRAIL receptors is responsible for the development of the early phase of TRAIL resistance. Cell Signal 2010, 22, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Min, K.J.; Choi, K.S.; Kubatka, P.; Kruzliak, P.; Kim, D.E.; Kwon, T.K. Chloroquine enhances TRAIL-mediated apoptosis through up-regulation of DR5 by stabilization of mRNA and protein in cancer cells. Scientific reports 2016, 6, 22921. [Google Scholar] [CrossRef]
- Kundu, M.; Pathak, S.K.; Kumawat, K.; Basu, S.; Chatterjee, G.; Pathak, S.; Noguchi, T.; Takeda, K.; Ichijo, H.; Thien, C.B.; et al. A TNF- and c-Cbl-dependent FLIP(S)-degradation pathway and its function in Mycobacterium tuberculosis-induced macrophage apoptosis. Nat Immunol 2009, 10, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Kim, J.H.; Sun, B.K.; Alcala, M.A., Jr.; Bartlett, D.L.; Lee, Y.J. c-Cbl acts as a mediator of Src-induced activation of the PI3K-Akt signal transduction pathway during TRAIL treatment. Cell Signal 2010, 22, 377–385. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Liu, J.; Qu, J.; Hu, X.; Zhang, F.; Zheng, H.; Qu, X.; Liu, Y. TRAIL-activated EGFR by Cbl-b-regulated EGFR redistribution in lipid rafts antagonises TRAIL-induced apoptosis in gastric cancer cells. Eur J Cancer 2012, 48, 3288–3299. [Google Scholar] [CrossRef]
- Kim, J.; Kang, D.; Sun, B.K.; Kim, J.H.; Song, J.J. TRAIL/MEKK4/p38/HSP27/Akt survival network is biphasically modulated by the Src/CIN85/c-Cbl complex. Cell Signal 2013, 25, 372–379. [Google Scholar] [CrossRef]
- Xu, L.; Qu, X.; Zhang, Y.; Hu, X.; Yang, X.; Hou, K.; Teng, Y.; Zhang, J.; Sada, K.; Liu, Y. Oxaliplatin enhances TRAIL-induced apoptosis in gastric cancer cells by CBL-regulated death receptor redistribution in lipid rafts. FEBS letters 2009, 583, 943–948. [Google Scholar] [CrossRef] [PubMed]
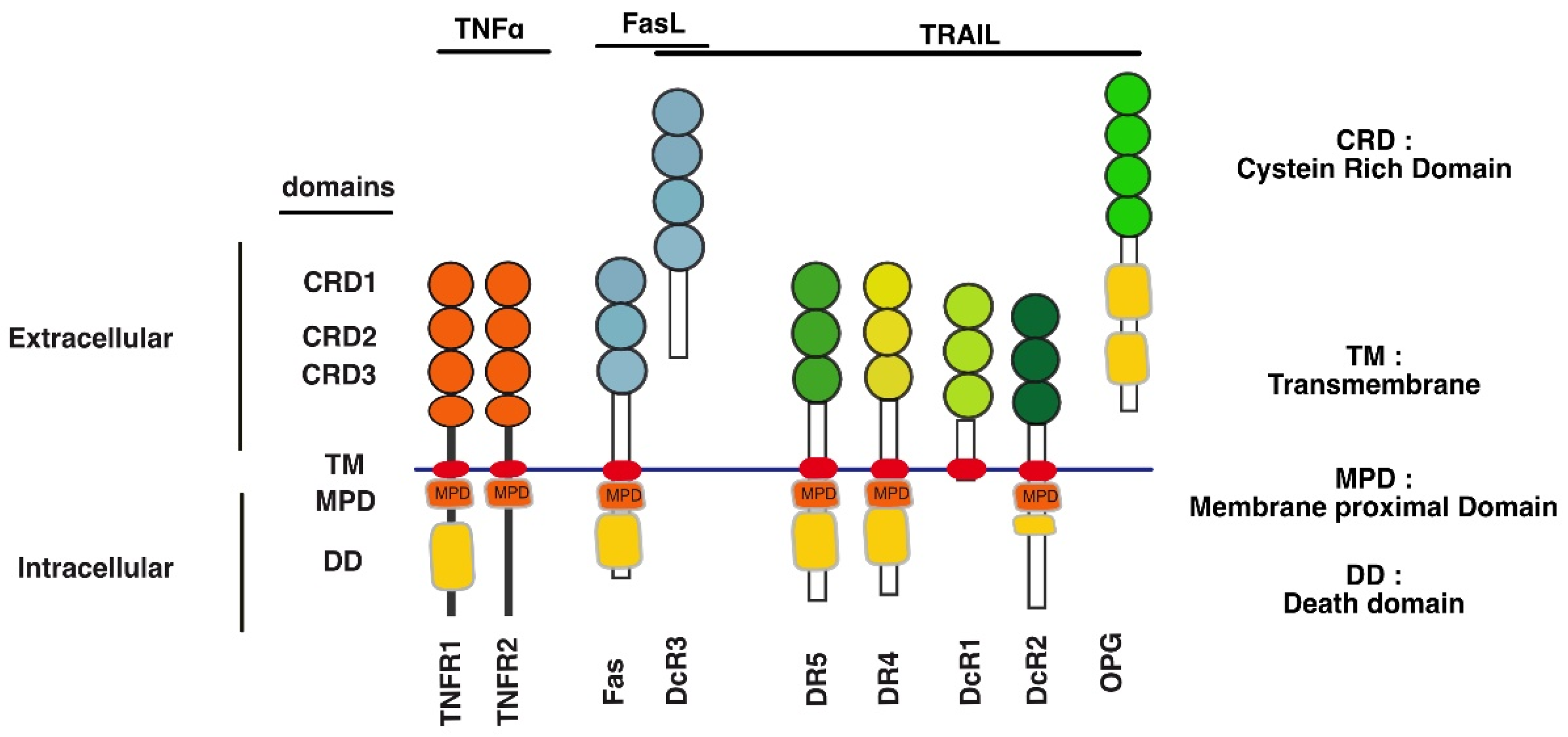
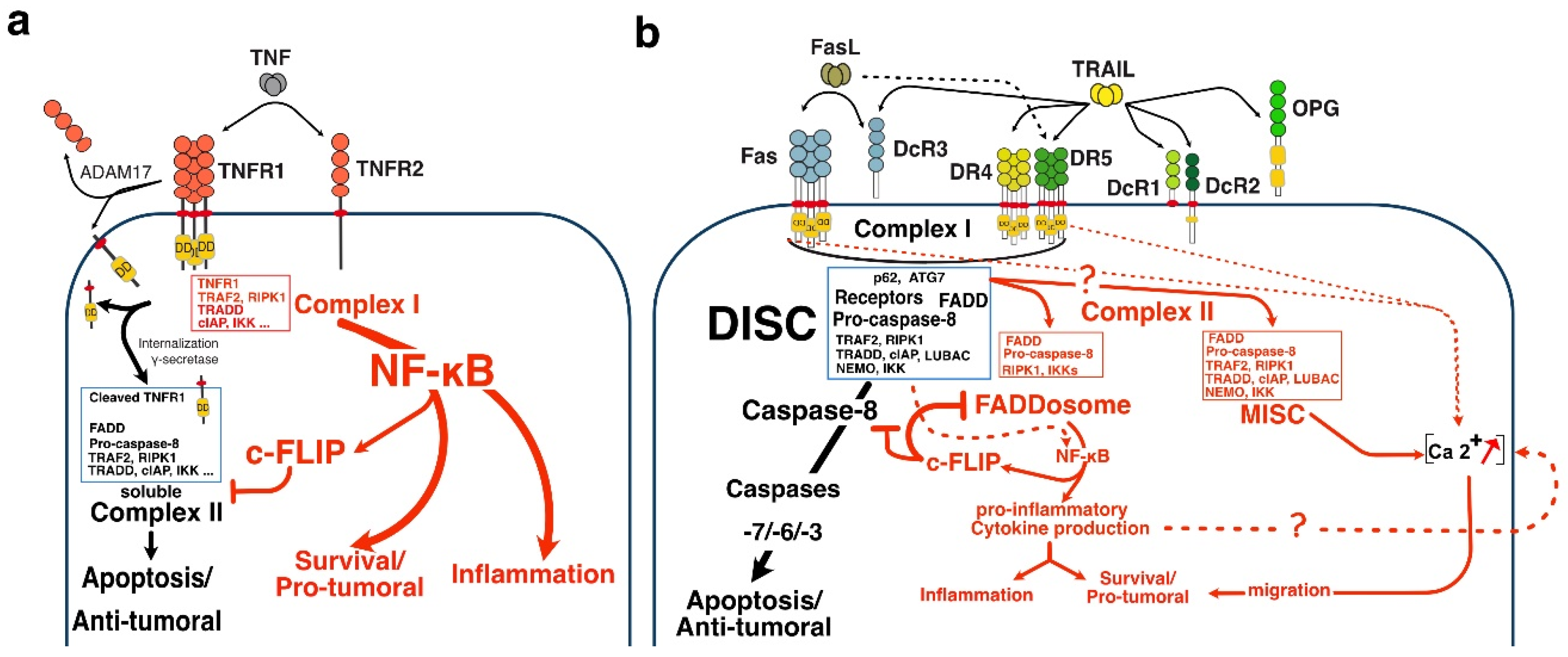
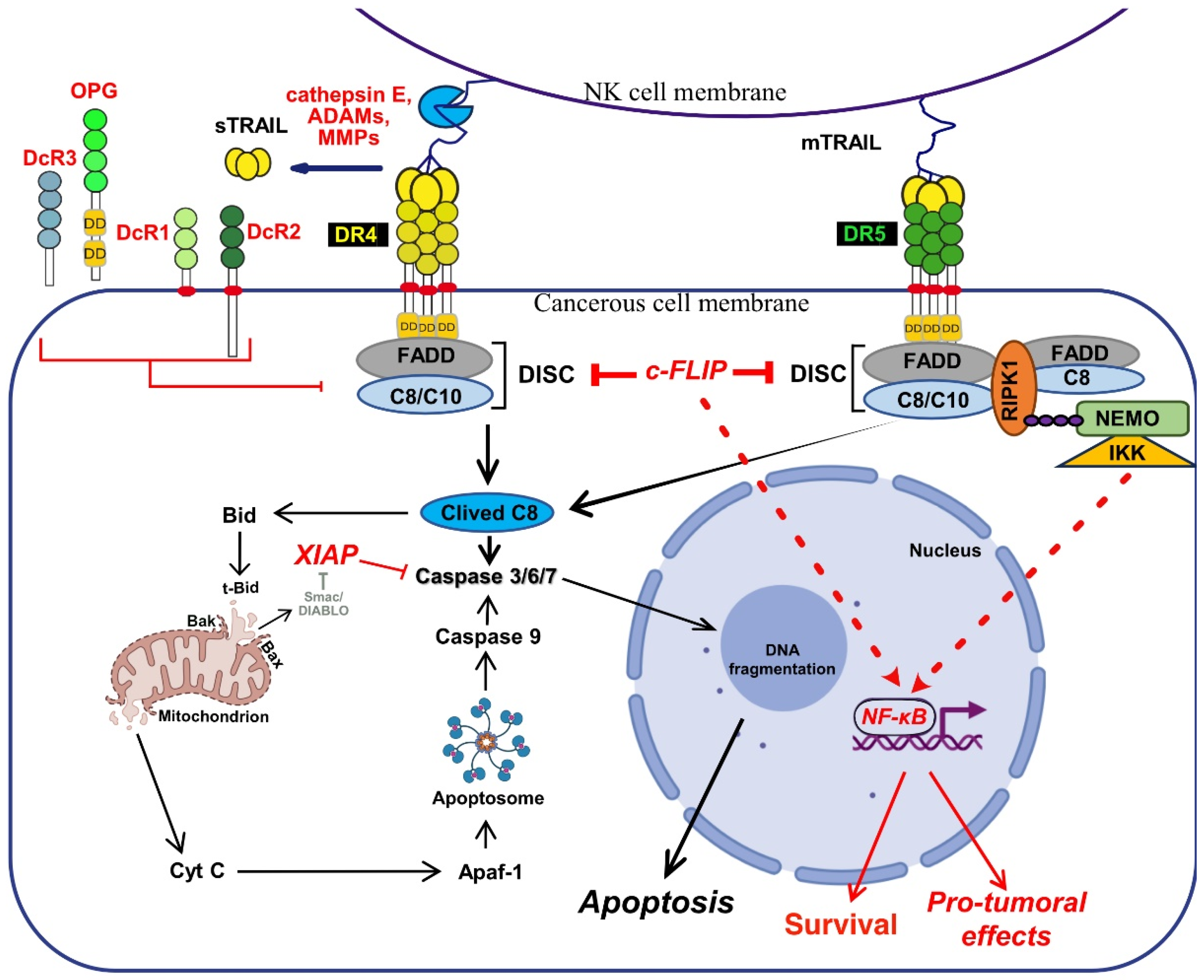
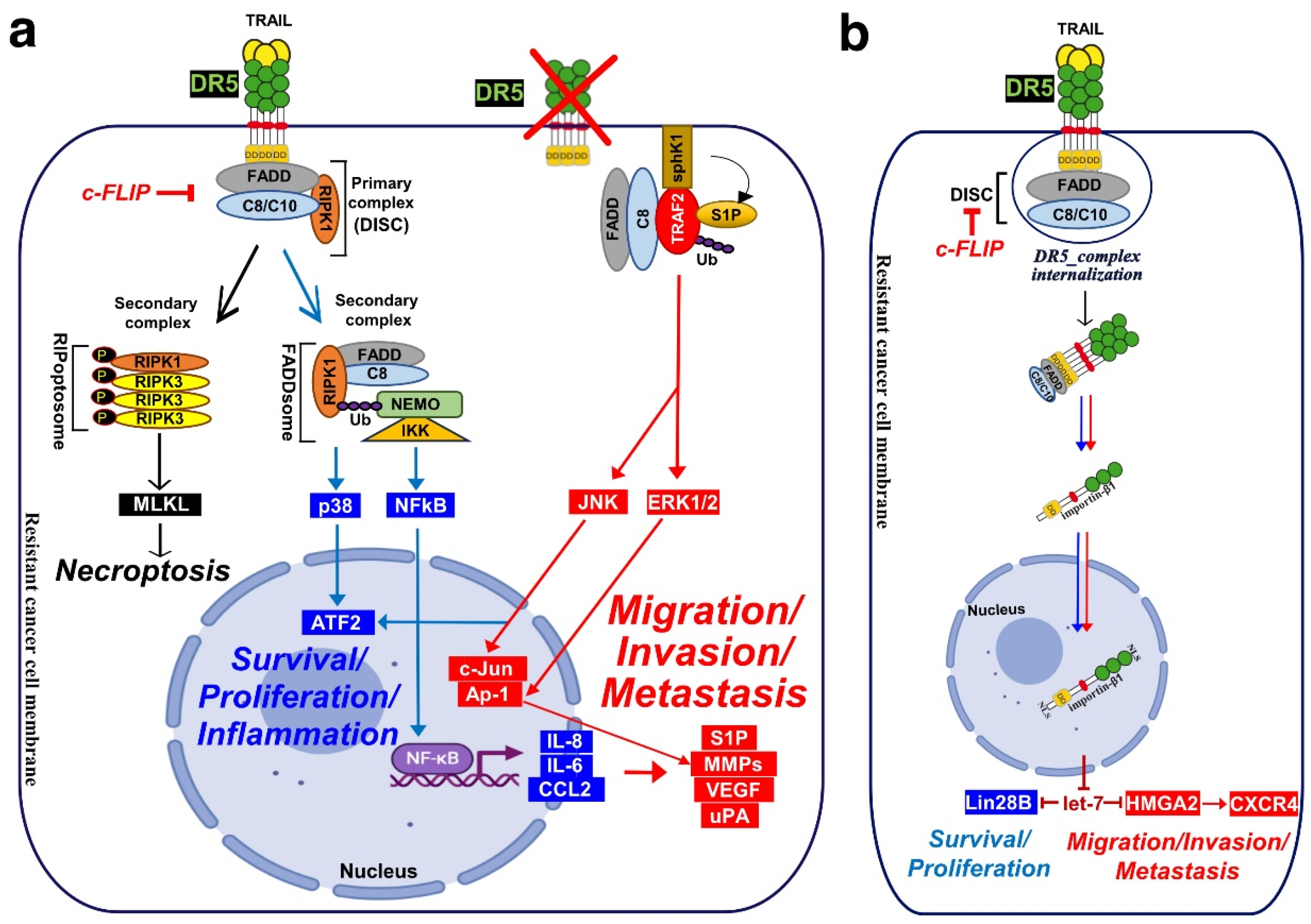
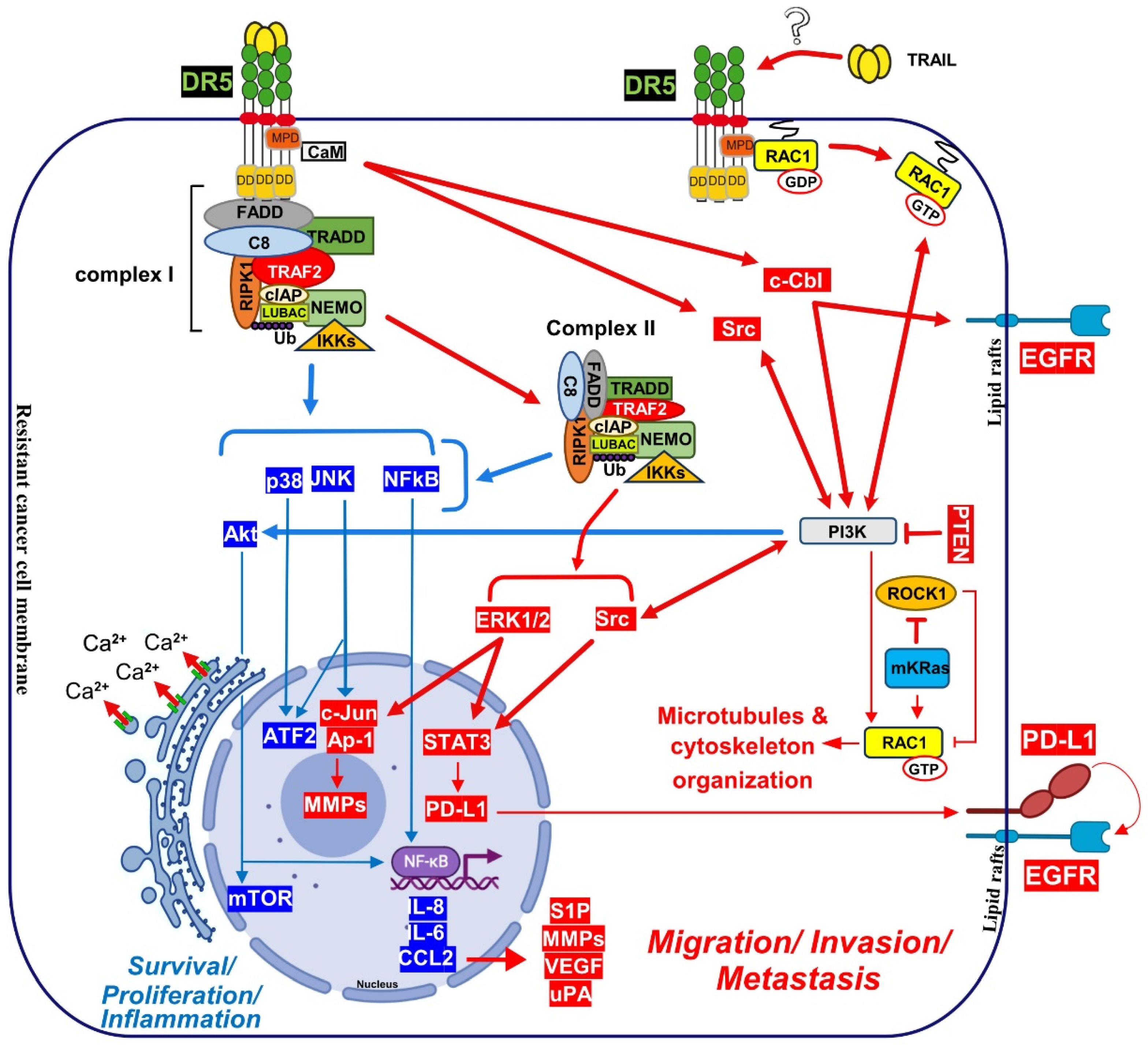
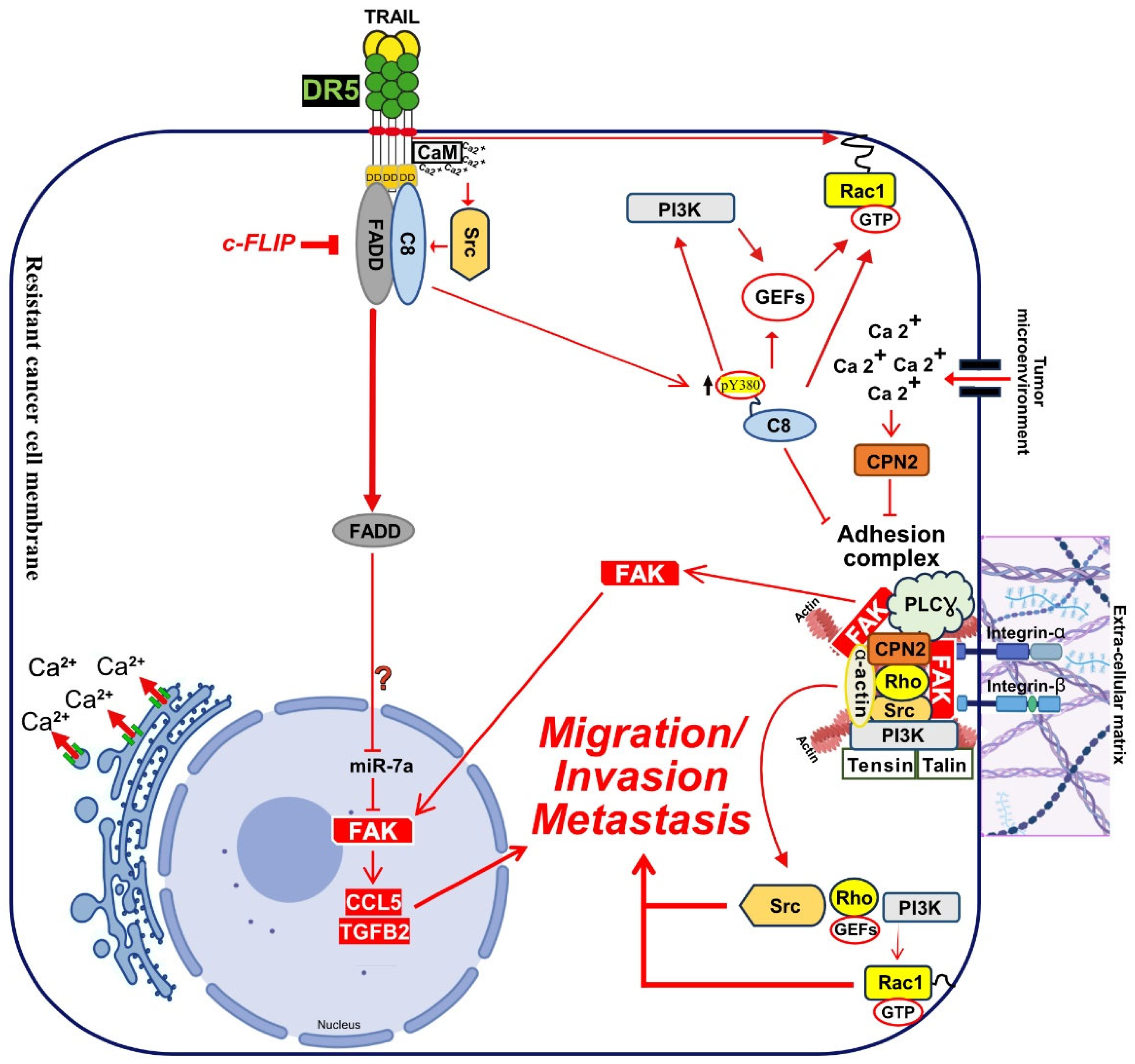
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




