Submitted:
06 February 2024
Posted:
07 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
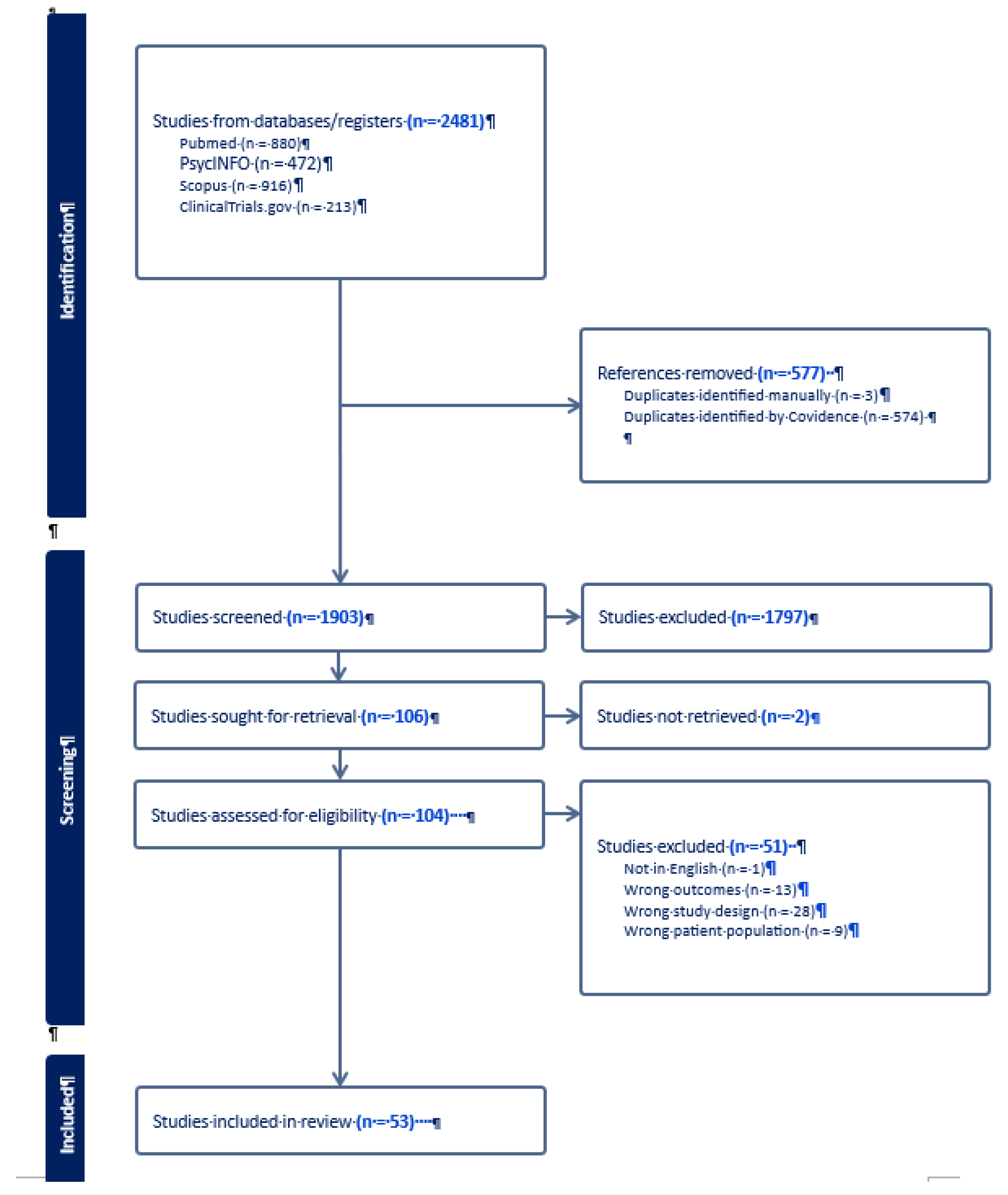
2.1. Search Strategy
2.2. Study Selection
2.3. Quality Assessment
2.4. Data Synthesis
3. Results
3.1. Pharmacological and Neuromodulatory Interventions
3.2. Psychosocial and Behavioral Interventions
4. Discussion
5. Conclusions
Author Contributions
Funding
Disclosure
References
- Connor, J.P.; Stjepanović, D.; Le Foll, B.; Hoch, E.; Budney, A.J.; Hall, W.D. Cannabis use and cannabis use disorder. Nat. Rev. Dis. Prim. 2021, 7, 1–24. [Google Scholar] [CrossRef]
- Wu, L.-T.; Zhu, H.; Mannelli, P.; Swartz, M.S. Prevalence and correlates of treatment utilization among adults with cannabis use disorder in the United States. Drug Alcohol Depend. 2017, 177, 153–162. [Google Scholar] [CrossRef]
- Compton, W.M.; Han, B.; Jones, C.M.; Blanco, C. Cannabis use disorders among adults in the United States during a time of increasing use of cannabis. Drug Alcohol Depend. 2019, 204, 107468. [Google Scholar] [CrossRef]
- Subramaniam, G.A. and N.D. Volkow, Substance misuse among adolescents: to screen or not to screen? JAMA Pediatr, 2014. 168, p. 798-9. [CrossRef]
- Chen, K.; Sheth, A.J.; Elliott, D.K.; Yeager, A. Prevalence and correlates of past-year substance use, abuse, and dependence in a suburban community sample of high-school students. Addict. Behav. 2003, 29, 413–423. [Google Scholar] [CrossRef]
- Baandrup, L. Managing the hazards of cannabis use. Acta Psychiatr. Scand. 2022, 145, 231–233. [Google Scholar] [CrossRef]
- Leung, J.; Chan, G.C.; Hides, L.; Hall, W.D. What is the prevalence and risk of cannabis use disorders among people who use cannabis? a systematic review and meta-analysis. Addict. Behav. 2020, 109, 106479. [Google Scholar] [CrossRef]
- Chawla, D., et al., Past-month cannabis use among U.S. individuals from 2002-2015: An age-period-cohort analysis. Drug Alcohol Depend, 2018. 193: p. 177-182. [CrossRef]
- Lees, R.; Hines, L.A.; D'Souza, D.C.; Stothart, G.; Di Forti, M.; Hoch, E.; Freeman, T.P. Psychosocial and pharmacological treatments for cannabis use disorder and mental health comorbidities: a narrative review. Psychol. Med. 2021, 51, 353–364. [Google Scholar] [CrossRef]
- Minerbi, A.; Häuser, W.; Fitzcharles, M.-A. Medical Cannabis for Older Patients. Drugs Aging 2018, 36, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J., et al., The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed), 2021. 74, p. 790-799. [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- de Fonseca, F.R., et al., The endocannabinoid system: Physiology and pharmacology. Alcohol and Alcoholism, 2005. 40, p. 2-14. [CrossRef]
- Todd, S.M. and J.C. Arnold, Neural correlates of interactions between cannabidiol and Δ(9) -tetrahydrocannabinol in mice: implications for medical cannabis. British journal of pharmacology, 2016. 173, p. 53-65.
- Mecha, M.; Torrao, A.S.; Mestre, L.; Carrillo-Salinas, F.J.; Mechoulam, R.; Guaza, C. Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress. Cell Death Dis. 2012, 3, e331–e331. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G., et al., Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in beta-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-kappaB involvement. Neuroscience letters, 2006. 399(1-2): p. 91-5. [CrossRef]
- Esposito, G. , et al., Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. British journal of pharmacology, 2007. 151, p. 1272-9. [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Horváth, B.; Bátkai, S.; Park, O.; Tanchian, G.; Gao, R.Y.; Patel, V.; Wink, D.A.; Liaudet, L.; et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free. Radic. Biol. Med. 2011, 50, 1368–1381. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol Attenuates Cardiac Dysfunction, Oxidative Stress, Fibrosis, and Inflammatory and Cell Death Signaling Pathways in Diabetic Cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef]
- Kumar, R.N., W. A. Chambers, and R.G. Pertwee, Pharmacological actions and therapeutic uses of cannabis and cannabinoids, in Anaesthesia. 2001. p. 1059-1068. [CrossRef]
- Pertwee, R.G. Endocannabinoids and Their Pharmacological Actions. In Endocannabinoids; Pertwee, R.G., Ed.; Springer: Berlin, Germany, 2015; Volume 231, pp. 1–37. [Google Scholar] [CrossRef]
- Volkow, N.D.; Baler, R.D.; Compton, W.M.; Weiss, S.R. Adverse Health Effects of Marijuana Use. New Engl. J. Med. 2014, 370, 2219–2227. [Google Scholar] [CrossRef]
- Harkany, T.; Keimpema, E.; Barabás, K.; Mulder, J. Endocannabinoid functions controlling neuronal specification during brain development. Mol. Cell. Endocrinol. 2008, 286, S84–S90. [Google Scholar] [CrossRef] [PubMed]
- Arseneault, L.; Cannon, M.; Witton, J.; Murray, R.M. Causal association between cannabis and psychosis: examination of the evidence. Br. J. Psychiatry 2004, 184, 110–117. [Google Scholar] [CrossRef]
- Tan, W.C.; Lo, C.; Jong, A.; Xing, L.; FitzGerald, M.J.; Vollmer, W.M.; Buist, S.A.; Sin, D.D. ; for the Vancouver Burden of Obstructive Lung Disease (BOLD) Research Group Marijuana and chronic obstructive lung disease: a population-based study. Can. Med Assoc. J. 2009, 180, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M., A. Joshi, and T. Bartter, Marijuana and lung diseases. Current Opinion in Pulmonary Medicine, 2014. 20, p. 173-179. [CrossRef]
- American Psychiatric, A. , Diagnostic and statistical manual of mental disorders : DSM-5. American Psychiatric Association. DSM. 2013.
- A Brezing, C.; Levin, F.R. The Current State of Pharmacological Treatments for Cannabis Use Disorder and Withdrawal. Neuropsychopharmacology 2017, 43, 173–194. [Google Scholar] [CrossRef]
- Trigo, J.M.; Soliman, A.; Quilty, L.C.; Fischer, B.; Rehm, J.; Selby, P.; Barnes, A.J.; Huestis, M.A.; George, T.P.; Streiner, D.L.; et al. Nabiximols combined with motivational enhancement/cognitive behavioral therapy for the treatment of cannabis dependence: A pilot randomized clinical trial. PLOS ONE 2018, 13, e0190768. [Google Scholar] [CrossRef]
- Mills, L.; Dunlop, A.; Montebello, M.; Copeland, J.; Bruno, R.; Jefferies, M.; Mcgregor, I.; Lintzeris, N. Correlates of treatment engagement and client outcomes: results of a randomised controlled trial of nabiximols for the treatment of cannabis use disorder. Subst. Abus. Treat. Prev. Policy 2022, 17, 1–12. [Google Scholar] [CrossRef]
- Lintzeris, N. , et al., Nabiximols for the Treatment of Cannabis Dependence: A Randomized Clinical Trial. JAMA Intern Med, 2019. 179, p. 1242-1253. [CrossRef]
- Lintzeris, N.; Mills, L.; Dunlop, A.; Copeland, J.; Mcgregor, I.; Bruno, R.; Kirby, A.; Montebello, M.; Hall, M.; Jefferies, M.; et al. Cannabis use in patients 3 months after ceasing nabiximols for the treatment of cannabis dependence: Results from a placebo-controlled randomised trial. Drug Alcohol Depend. 2020, 215, 108220. [Google Scholar] [CrossRef]
- Allsop, D.J. , et al., Nabiximols as an agonist replacement therapy during cannabis withdrawal: A randomized clinical trial. JAMA Psychiatry, 2014..71, p. pp. [CrossRef]
- Levin, F.R.; Mariani, J.J.; Pavlicova, M.; Brooks, D.; Glass, A.; Mahony, A.; Nunes, E.V.; Bisaga, A.; Dakwar, E.; Carpenter, K.M.; et al. Dronabinol and lofexidine for cannabis use disorder: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2015, 159, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Levin, F.R.; Mariani, J.J.; Choi, C.J.; Basaraba, C.; Brooks, D.J.; Brezing, C.A.; Pavlicova, M. Non-abstinent treatment outcomes for cannabis use disorder. Drug Alcohol Depend. 2021, 225, 108765–108765. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.P.; Hindocha, C.; Baio, G.; Shaban, N.D.C.; Thomas, E.M.; Astbury, D.; Freeman, A.M.; Lees, R.; Craft, S.; Morrison, P.D.; et al. Cannabidiol for the treatment of cannabis use disorder: a phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry 2020, 7, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Mariani, J.J.; Pavlicova, M.; Choi, C.J.; Basaraba, C.; Carpenter, K.M.; Mahony, A.L.; Brooks, D.J.; Bisaga, A.; Naqvi, N.; Nunes, E.V.; et al. Quetiapine treatment for cannabis use disorder. Drug Alcohol Depend. 2020, 218, 108366. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, A.M.; Miller, H.; Bluvstein, I.; Rapoport, E.; Schreiber, S.; Bar-Hamburger, R.; Bloch, M. Treatment of cannabis dependence using escitalopram in combination with cognitive-behavior therapy: a double-blind placebo-controlled study. Am. J. Drug Alcohol Abus. 2013, 40, 16–22. [Google Scholar] [CrossRef]
- McRae-Clark, A.L.; Baker, N.L.; Gray, K.M.; Killeen, T.K.; Wagner, A.M.; Brady, K.T.; DeVane, C.L.; Norton, J. Buspirone treatment of cannabis dependence: A randomized, placebo-controlled trial. Drug Alcohol Depend. 2015, 156, 29–37. [Google Scholar] [CrossRef]
- McRae-Clark, A.L.; Baker, N.L.; Gray, K.M.; Killeen, T.; Hartwell, K.J.; Simonian, S.J. Vilazodone for cannabis dependence: A randomized, controlled pilot trial. Am. J. Addict. 2015, 25, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.; Lintzeris, N.; Allsop, D.J.; Suraev, A.; Booth, J.; Carson, D.S.; Helliwell, D.; Winstock, A.; McGregor, I.S. Lithium carbonate in the management of cannabis withdrawal: a randomized placebo-controlled trial in an inpatient setting. Psychopharmacol. 2014, 231, 4623–4636. [Google Scholar] [CrossRef]
- Sherman, B.J.; Baker, N.L.; McRae-Clark, A.L. Effect of oxytocin pretreatment on cannabis outcomes in a brief motivational intervention. Psychiatry Res. 2017, 249, 318–320. [Google Scholar] [CrossRef]
- Gray, K.M.; Sonne, S.C.; McClure, E.A.; Ghitza, U.E.; Matthews, A.G.; McRae-Clark, A.L.; Carroll, K.M.; Potter, J.S.; Wiest, K.; Mooney, L.J.; et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Depend. 2017, 177, 249–257. [Google Scholar] [CrossRef]
- Tomko, R.L.; Baker, N.L.; Hood, C.O.; Gilmore, A.K.; McClure, E.A.; Squeglia, L.M.; McRae-Clark, A.L.; Sonne, S.C.; Gray, K.M. Depressive symptoms and cannabis use in a placebo-controlled trial of N-Acetylcysteine for adult cannabis use disorder. Psychopharmacol. 2019, 237, 479–490. [Google Scholar] [CrossRef]
- Meisel, S.N.; Padovano, H.T.; Miranda, R. Combined pharmacotherapy and evidence-based psychosocial Cannabis treatment for youth and selection of cannabis-using friends. Drug Alcohol Depend. 2021, 225, 108747–108747. [Google Scholar] [CrossRef]
- Adams, T.R.; Arnsten, J.H.; Ning, Y.; Nahvi, S. Feasibility and Preliminary Effectiveness of Varenicline for Treating Co-Occurring Cannabis and Tobacco Use. J. Psychoact. Drugs 2017, 50, 12–18. [Google Scholar] [CrossRef] [PubMed]
- McRae-Clark, A.L.; Gray, K.M.; Baker, N.L.; Sherman, B.J.; Squeglia, L.; Sahlem, G.L.; Wagner, A.; Tomko, R. Varenicline as a treatment for cannabis use disorder: A placebo-controlled pilot trial. Drug Alcohol Depend. 2021, 229, 109111–109111. [Google Scholar] [CrossRef]
- Haney, M. , et al., Naltrexone Maintenance Decreases Cannabis Self-Administration and Subjective Effects in Daily Cannabis Smokers. Neuropsychopharmacology, 2015. 40, p. 2489-98. [CrossRef]
- Sahlem, G.L.; Baker, N.L.; George, M.S.; Malcolm, R.J.; McRae-Clark, A.L. Repetitive transcranial magnetic stimulation (rTMS) administration to heavy cannabis users. Am. J. Drug Alcohol Abus. 2017, 44, 47–55. [Google Scholar] [CrossRef]
- Carroll, K.M.; Onken, L.S.; Hong, K.-I.A.; Lacadie, C.M.; Fulbright, R.K.; Tuit, K.L.; Sinha, R.; Ball, S.A.; Martino, S.; Nich, M.C.; et al. Behavioral Therapies for Drug Abuse. Am. J. Psychiatry 2005, 162, 1452–1460. [Google Scholar] [CrossRef]
- McHugh, R.K.; Hearon, B.A.; Otto, M.W. Cognitive Behavioral Therapy for Substance Use Disorders. Psychiatr. Clin. North Am. 2010, 33, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Witkiewitz, K., G. A. Marlatt, and D. Walker, Mindfulness-Based Relapse Prevention for Alcohol and Substance Use Disorders. Journal of Cognitive Psychotherapy, 2005. 19, p. 211-228. [CrossRef]
- Witkiewitz, K.; Bowen, S.; Douglas, H.; Hsu, S.H. Mindfulness-based relapse prevention for substance craving. Addict. Behav. 2013, 38, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Smedslund, G. , et al., Motivational interviewing for substance abuse. Cochrane database of systematic reviews (Online), 2011, p. CD008063-CD008063. [CrossRef]
- Prendergast, M.; Podus, D.; Finney, J.; Greenwell, L.; Roll, J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction 2006, 101, 1546–1560. [Google Scholar] [CrossRef] [PubMed]
- Meyers, R.J., H. G. Roozen, and J.E. Smith, The community Reinforcement approach an update of the evidence. Alcohol Research and Health, 2010.
- Roozen, H.G.; Boulogne, J.J.; van Tulder, M.W.; Brink, W.v.D.; De Jong, C.A.; Kerkhof, A.J. A systematic review of the effectiveness of the community reinforcement approach in alcohol, cocaine and opioid addiction. Drug Alcohol Depend. 2004, 74, 1–13. [Google Scholar] [CrossRef]
- Roozen, H.G., R. De Waart, and P. Van Der Kroft, Community reinforcement and family training: An effective option to engage treatment-resistant substance-abusing individuals in treatment, in Addiction. 2010. [CrossRef]
- Rawson, R. and M. McCann, The matrix model of intensive outpatient treatment. Behav Health Recovery Manag, 2005: p. 1-37.
- Rawson, R.A.; Shoptaw, S.J.; Obert, J.L.; McCann, M.J.; Hasson, A.L.; Marinelli-Casey, P.J.; Brethen, P.R.; Ling, W. An intensive outpatient approach for cocaine abuse treatment: The matrix model. J. Subst. Abus. Treat. 1995, 12, 117–127. [Google Scholar] [CrossRef]
- Shoptaw, S.; A Rawson, R.; McCann, M.J.; Obert, J. The Matrix Model of Outpatient Stimulant Abuse Treatment. J. Addict. Dis. 1995, 13, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.F.; Magill, M.; Stout, R.L. How do people recover from alcohol dependence? A systematic review of the research on mechanisms of behavior change in Alcoholics Anonymous. Addict. Res. Theory 2009, 17, 236–259. [Google Scholar] [CrossRef]
- Kakoschke, N.; Kemps, E.; Tiggemann, M. Approach bias modification training and consumption: A review of the literature. Addict. Behav. 2017, 64, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Martino, S.; Ball, S.A.; Nich, C.; Frankforter, T.L.; Carroll, K.M. Informal discussions in substance abuse treatment sessions. J. Subst. Abus. Treat. 2009, 36, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Kiluk, B.D. , et al., Technology-Delivered Cognitive-Behavioral Interventions for Alcohol Use: A Meta-Analysis. Alcoholism, clinical and experimental research, 2019. 43, p. 2285-2295. [CrossRef]
- Aggarwal, S.K.; Carter, G.T.; Sullivan, M.D.; ZumBrunnen, C.; Morrill, R.; Mayer, J.D. Medicinal use of cannabis in the United States: Historical perspectives, current trends, and future directions. J. Opioid Manag. 2009, 5, 153–168. [Google Scholar] [CrossRef]
- Moss, H.B.; Chen, C.M.; Yi, H.-Y. Measures of Substance Consumption Among Substance Users, DSM-IV Abusers, and Those With DSM-IV Dependence Disorders in a Nationally Representative Sample. J. Stud. Alcohol Drugs 2012, 73, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Colliver, J.; Compton, W.; Gfroerer, J.; Condon, T. Projecting Drug Use Among Aging Baby Boomers in 2020. Ann. Epidemiology 2006, 16, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Gfroerer, J. Substance abuse treatment need among older adults in 2020: the impact of the aging baby-boom cohort. Drug Alcohol Depend. 2003, 69, 127–135. [Google Scholar] [CrossRef]
- Han, B.; Gfroerer, J.C.; Colliver, J.D.; Penne, M.A. Substance use disorder among older adults in the United States in 2020. Addiction 2008, 104, 88–96. [Google Scholar] [CrossRef]
- Campanelli, C.M. , Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults: The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. Journal of the American Geriatrics Society, 2012. 60, p. 616-631.
- Campanelli, C.M. , American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults: The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. Journal of the American Geriatrics Society, 2012. 60, p. 616-31. [CrossRef]
- Schultz, S.K.; Arndt, S.; Liesveld, J. Locations of facilities with special programs for older substance abuse clients in the US. Int. J. Geriatr. Psychiatry 2003, 18, 839–843. [Google Scholar] [CrossRef] [PubMed]
- McLellan, A.T. , et al., Drug dependence, a chronic medical illness implications for treatment, insurance, and outcomes evaluation. Journal of the American Medical Association, 2000. [CrossRef]
- Oslin, D.W.; Pettinati, H.; Volpicelli, J.R. Alcoholism Treatment Adherence: Older Age Predicts Better Adherence and Drinking Outcomes. Am. J. Geriatr. Psychiatry 2002, 10, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Naegle, M.A. , Alcohol Use Screening and Assessment for Older Adults. Best Practices in Nursing Care to Older Adults, 2018.
- Naegle, M.A. , Screening for alcohol use and misuse in older adults: using the Short Michigan Alcoholism Screening Test--Geriatric Version [corrected] [published erratum appears in AM J NURS 2009 Mar;109,13]. American Journal of Nursing, 2008. 108, p. 50-59.
- Babor, T.F. , et al., The Alcohol Use Disorders Identification Test Guidelines for Use in Primary Care, in World Health Organization. 2001. p. pp1-40.
- Babor, T.F. , et al., Screening, Brief Intervention, and Referral to Treatment (SBIRT). Substance Abuse, 2007. 28, p. 7-30. [CrossRef]
- Kaminer, Y.; Burleson, J.A.; Burke, R.; Litt, M.D. The Efficacy of Contingency Management for Adolescent Cannabis Use Disorder: A Controlled Study. Subst. Abus. 2014, 35, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Stanger, C.; Ryan, S.R.; Scherer, E.A.; Norton, G.E.; Budney, A.J. Clinic- and Home-Based Contingency Management Plus Parent Training for Adolescent Cannabis Use Disorders. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Lascaux, M.I., S. Phan, O., Effectiveness of formalised therapy for adolescents with cannabis dependence: A randomised trial. Drugs: Education, Prevention and Policy, 2016. 23, 2016, p. 404-409.
- Mason, M.J.; Sabo, R.; Zaharakis, N.M. Peer Network Counseling as Brief Treatment for Urban Adolescent Heavy Cannabis Users. J. Stud. Alcohol Drugs 2017, 78, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Kaminer, Y.; Ohannessian, C.M.; Burke, R.H. Adolescents with cannabis use disorders: Adaptive treatment for poor responders. Addict. Behav. 2017, 70, 102–106. [Google Scholar] [CrossRef] [PubMed]
- de Gee, E.A. , et al., A randomized controlled trial of a brief motivational enhancement for non-treatment-seeking adolescent cannabis users. J Subst Abuse Treat, 2014. 47, p. 181-8.
- Stewart, D.G.; Felleman, B.I.; Arger, C.A. Effectiveness of Motivational Incentives for Adolescent Marijuana Users in a School-Based Intervention. J. Subst. Abus. Treat. 2015, 58, 43–50. [Google Scholar] [CrossRef]
- Mason, M.J.; Zaharakis, N.M.; Russell, M.; Childress, V. A pilot trial of text-delivered peer network counseling to treat young adults with cannabis use disorder. J. Subst. Abus. Treat. 2018, 89, 1–10. [Google Scholar] [CrossRef]
- Wolitzky-Taylor, K.; Glasner, S.; Tanner, A.; Ghahremani, D.G.; London, E.D. Targeting maladaptive reactivity to negative affect in emerging adults with cannabis use disorder: A preliminary test and proof of concept. Behav. Res. Ther. 2022, 150, 104032. [Google Scholar] [CrossRef]
- Fischer, B.; Dawe, M.; McGuire, F.; Shuper, P.A.; Capler, R.; Bilsker, D.; Jones, W.; Taylor, B.; Rudzinski, K.; Rehm, J. Feasibility and impact of brief interventions for frequent cannabis users in Canada. J. Subst. Abus. Treat. 2013, 44, 132–138. [Google Scholar] [CrossRef]
- Rigter, H.; Henderson, C.E.; Pelc, I.; Tossmann, P.; Phan, O.; Hendriks, V.; Schaub, M.; Rowe, C.L. Multidimensional family therapy lowers the rate of cannabis dependence in adolescents: A randomised controlled trial in Western European outpatient settings. Drug Alcohol Depend. 2013, 130, 85–93. [Google Scholar] [CrossRef]
- Mason, M.J.; Zaharakis, N.M.; Moore, M.; Brown, A.; Garcia, C.; Seibers, A.; Stephens, C. Who responds best to text-delivered cannabis use disorder treatment? A randomized clinical trial with young adults. Psychol. Addict. Behav. 2018, 32, 699–709. [Google Scholar] [CrossRef]
- Riggs, N.R.; Conner, B.T.; Parnes, J.E.; Prince, M.A.; Shillington, A.M.; George, M.W. Marijuana eCHECKUPTO GO: Effects of a personalized feedback plus protective behavioral strategies intervention for heavy marijuana-using college students. Drug Alcohol Depend. 2018, 190, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Walukevich-Dienst, K.; Neighbors, C.; Buckner, J.D. Online personalized feedback intervention for cannabis-using college students reduces cannabis-related problems among women. Addict. Behav. 2019, 98, 106040. [Google Scholar] [CrossRef]
- Bonar, E.E.; Goldstick, J.E.; Chapman, L.; Bauermeister, J.A.; Young, S.D.; McAfee, J.; Walton, M.A. A social media intervention for cannabis use among emerging adults: Randomized controlled trial. Drug Alcohol Depend. 2022, 232, 109345–109345. [Google Scholar] [CrossRef]
- Macatee, R.J.; Albanese, B.J.; Okey, S.A.; Afshar, K.; Carr, M.; Rosenthal, M.Z.; Schmidt, N.B.; Cougle, J.R. Impact of a computerized intervention for high distress intolerance on cannabis use outcomes: A randomized controlled trial. J. Subst. Abus. Treat. 2020, 121, 108194–108194. [Google Scholar] [CrossRef]
- Levin, F.R.; Mariani, J.J.; Pavlicova, M.; Brooks, D.; Glass, A.; Mahony, A.; Nunes, E.V.; Bisaga, A.; Dakwar, E.; Carpenter, K.M.; et al. Dronabinol and lofexidine for cannabis use disorder: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2015, 159, 53–60. [Google Scholar] [CrossRef]
- Sherman, B.J.; Baker, N.L.; Squeglia, L.M.; McRae-Clark, A.L. Approach bias modification for cannabis use disorder: A proof-of-principle study. J. Subst. Abus. Treat. 2018, 87, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Litt, M.D. , et al., Individualized assessment and treatment program (IATP) for cannabis use disorder: Randomized controlled trial with and without contingency management. Psychol Addict Behav, 2020. 34, p. 40-51. [CrossRef]
- Shekhawat, A.S.; Mathur, R.; Sarkar, S.; Kaloiya, G.S.; Balhara, Y.P.S. A randomized controlled trial of brief intervention for patients with cannabis use disorder. J. Neurosci. Rural. Pr. 2023, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rooke, S.; Copeland, J.; Norberg, M.; Hine, D.; McCambridge, J. Effectiveness of a Self-Guided Web-Based Cannabis Treatment Program: Randomized Controlled Trial. J. Med Internet Res. 2013, 15, e26. [Google Scholar] [CrossRef]
- Litt, M.D.; Kadden, R.M.; Petry, N.M. Behavioral treatment for marijuana dependence: Randomized trial of contingency management and self-efficacy enhancement. Addict. Behav. 2012, 38, 1764–1775. [Google Scholar] [CrossRef]
- Hoch, E.; Bühringer, G.; Pixa, A.; Dittmer, K.; Henker, J.; Seifert, A.; Wittchen, H. CANDIS treatment program for cannabis use disorders: Findings from a randomized multi-site translational trial. Drug Alcohol Depend. 2013, 134, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Allsop, D.J. , et al., Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry, 2014. 71, p. 281-91. [CrossRef]
- Rooke, S.E.; Gates, P.J.; Norberg, M.M.; Copeland, J. Applying technology to the treatment of cannabis use disorder: Comparing telephone versus Internet delivery using data from two completed trials. J. Subst. Abus. Treat. 2014, 46, 78–84. [Google Scholar] [CrossRef]
- Budney, A.J.; Stanger, C.; Tilford, J.M.; Scherer, E.B.; Brown, P.C.; Li, Z.; Li, Z.; Walker, D.D. Computer-assisted behavioral therapy and contingency management for cannabis use disorder. Psychol. Addict. Behav. 2015, 29, 501–511. [Google Scholar] [CrossRef]
- D'Souza, D.C.; Cortes-Briones, J.; Creatura, G.; Bluez, G.; Thurnauer, H.; Deaso, E.; Bielen, K.; Surti, T.; Radhakrishnan, R.; Gupta, A.; et al. Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: a double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry 2018, 6, 35–45. [Google Scholar] [CrossRef]
- Sinadinovic, K.; Johansson, M.; Johansson, A.-S.; Lundqvist, T.; Lindner, P.; Hermansson, U. Guided web-based treatment program for reducing cannabis use: a randomized controlled trial. Addict. Sci. Clin. Pr. 2020, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stephens, R.S.; Walker, R.; DeMarce, J.; Lozano, B.E.; Rowland, J.; Walker, D.; Roffman, R.A. Treating cannabis use disorder: Exploring a treatment as needed model with 34-month follow-up. J. Subst. Abus. Treat. 2020, 117, 108088–108088. [Google Scholar] [CrossRef] [PubMed]
- Østergård, O.K.; del Palacio-Gonzalez, A.; Nilsson, K.K.; Pedersen, M.U. The Partners for Change Outcome Management System in the psychotherapeutic treatment of cannabis use: a pilot effectiveness randomized clinical trial. Nord. J. Psychiatry 2021, 75, 633–640. [Google Scholar] [CrossRef]
- Davoudi, M.; Allame, Z.; Foroughi, A.; Taheri, A.A. A pilot randomized controlled trial of dialectical behavior therapy (DBT) for reducing craving and achieving cessation in patients with marijuana use disorder: feasibility, acceptability, and appropriateness. Trends Psychiatry Psychother. 2021, 43, 302–310. [Google Scholar] [CrossRef]
- Olthof, M.I.A.; Goudriaan, A.E.; van Laar, M.W.; Blankers, M. A guided digital intervention to reduce cannabis use: The ICan randomized controlled trial. Addiction 2023, 118, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.D.; Stephens, R.S.; Towe, S.; Banes, K.; Roffman, R. Maintenance Check-ups Following Treatment for Cannabis Dependence. J. Subst. Abus. Treat. 2015, 56, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Fuster, D.; Cheng, D.M.; Wang, N.; Bernstein, J.A.; Palfai, T.P.; Alford, D.P.; Samet, J.H.; Saitz, R. Brief Intervention for Daily Marijuana Users Identified by Screening in Primary Care: A Subgroup Analysis of the ASPIRE Randomized Clinical Trial. Subst. Abus. 2016, 37, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, J.; van Hemel-Ruiter, M.E.; Huisman, M.; Ostafin, B.D.; Wiers, R.W.; MacLeod, C.; DeFuentes-Merillas, L.; Fledderus, M.; Markus, W.; de Jong, P.J. Effectiveness of attentional bias modification training as add-on to regular treatment in alcohol and cannabis use disorder: A multicenter randomized control trial. PLOS ONE 2021, 16, e0252494. [Google Scholar] [CrossRef]
| Study ID | Participants | Intervention group | Control group | Duration | Outcomes | Overall Risk of Bias |
|---|---|---|---|---|---|---|
| Adolescent (12-18) | ||||||
| Kaminer 2014 [80] | CUD (DSM) | CBT and VBRT (n=29) | CBT and rewards(n=30) | 10 weeks | No significant difference between groups in linear change in cannabis use from sessions 1-10 or end of treatment to 3 month follow up. Self-efficacy and coping response also did not improve during treatment. | High Risk |
| Stanger 2015 [81] | CUD (DSM) | MET/CBT+CM(clinical and home based) (n=153) | MET/CBT (n=50) | 14 weeks + 12 month follow-up | METACBT+CM had significantly longer periods of abstinence than MET/CBT (OR=1.16, 95% CI=1.02,1.32, p<. 05) and greater than MET/CBT+CM+PT (OR=0.85, 95% CI=0.75,0.95, p<.01). No significant differences between groups in cannabis use frequency during or after treatment. Retention rates were similar between groups | High Risk |
| Lascaux 2016 [82] | CUD (DSM) | Formalized therapy (TAUe) (n=38) | Treatment as usual (TAU)(N=35) | 6-12 months | At 6 months, TAUe group had significantly greater reduction in days of cannabis use compared to TAU group (p=0.032). At 12 months, difference remained significant (p=0.016). | High Risk |
| Mason 2017 [83] | Heavy users | Peer Network Counseling (PNC) (n=18) | Control session (n=28) | 6 months | At 6 months, the PNC group had a 35.9% probability of cannabis abstinence compared to 13.2% in the control group. The PNC group had a 16.6% probability of using cannabis 10 times per month versus 38.1% in the control group(p=0.0034). | High Risk |
| Kaminer 2017 [84] | CUD (DSM) | Poor responders to MET/CBT randomized to Enhanced CBT or ACRA(n=80) | Good responders (no additional intervention) (n=81) | 17 weeks | 37% of poor responders completed adaptive treatment phase, 27% achieved abstinence. No significant difference between CBT and ACRA groups. At week 17, significantly more poor responders continued drug use (91% vs 71%) and failed to complete treatment (46% vs 22%) compared to good responders. | High Risk |
| deGee 2014 [85] | Heavy users | Weed-Check intervention (n=58) | Information session (n=61) | 3 months | No significant differences between groups on outcomes. But heavier users receiving the Weed-Check reduced their quantity of cannabis use more than heavier users in the control group (mean reduction of 6.1 vs 3.3 joints per week, p=0.05). | Low Risk |
| Stewart 2015 [86] | Problematic users | MI + CM (n=68) | MI alone (n=68) | 8 weeks + 16 week follow-up | MI + CM group had greater reduction in marijuana use frequency at end of treatment (Cohen’s d=-0.82) compared to MI alone (Cohen’s d=-0.33), but differences were not significant at 16 week follow-up. MI + CM group had lower marijuana-related consequences, higher use of coping strategies, and increased likelihood of attending additional treatment. | High Risk |
| Young adults (18-25) | ||||||
| McRae-Clark 2016 [39] | CUD (DSM) | Vilazodone (n=41) | Placebo tablets (n=35) | 8 weeks | No significant difference between vilazodone and placebo groups on cannabis use outcomes. Vilazodone did not provide advantage over placebo in reducing cannabis use and craving score. | Some Concerns |
| Mason 2018 [87] | CUD (DSM) | PNC-txt (n=15) | Waitlist control (n=15) | 4 weeks + 1, 2, 3 months post-baseline | The PNC-txt group had significantly greater reductions in cannabis problems (p=0.04) and cravings (p<0.05) compared to controls. More PNC-txt participants had negative urine screens for cannabis at follow-up (p=0.03).No significant difference in past 30-day cannabis use frequency |
High Risk |
| Wolitzky-Taylor 2022 [88] | CUD (DSM) | Affect Management Treatment (AMT) (n=26) | CBT (n=26) | 12 weeks | AMT had greater reductions in negative affect (p<.01) and constructs representing maladaptive reactivity to negative affect (p<.05) compared to CBT. Non-significant differences between groups in cannabis use outcomes, though AMT showed somewhat greater reductions. No significant differences between groups in number of sessions completed or rates of assessment completion. |
High Risk |
| Fischer 2013 [89] | Heavy users | C-O: Oral cannabis BI (n=25) or C-W: Written cannabis BI (n=47) | H-O: Oral health BI (n=25) or H-W: Written health BI (n=37) | 3 months | Decrease in mean number of cannabis use days from 23.79 to 22.41 in total sample (p=0.024) Reduced deep inhalation/breathholding from 77.78% to 51.61% in combined intervention groups (p=0.001) Reduced driving after cannabis use from 44.44% to 30.65% in combined intervention groups (p=0.02) |
High Risk |
| Rigter 2013 [90] | CUD (DSM) | MDFT (n=212) | IP (n=238) | 12 months | 90% MDFT cases vs 48% IP cases completed therapy (p<0.001). Mean number of cannabises use days reduced from 59.8 at baseline to 34.0 at 12 months for MDFT and from 61.5 to 42.3 for IP (difference not statistically significant, p=0.07). 18% MDFT cases had no cannabis use disorder at 12 months vs 15% IP cases (non-significant difference). Prevalence of cannabis dependence diagnosis dropped from 82% at baseline to 38% at 12 months for MDFT and from 82% to 52% for IP (slope coefficient 0.9, p=0.015) |
High Risk |
| Mason 2018 [91] | CUD (DSM) | PNC-txt (n=51) | Assessment only control (n=50) | 30 days | PNC-txt group reduced heavy cannabis use days (p=.005). PNC-txt group reduced relationship problems due to cannabis use (p=.011). No significant differences in past 30-day cannabis use | High Risk |
| Riggs 2018 [92] | Heavy users | Marijuana eCheckupToGo (Personalized feedback) (n=144) | Health stress management (n=154) | 6 weeks | The Marijuana eCHECKUP TO GO group reported: Decreases in estimated use prevalence/descriptive norms (P<0.01). Decreases in hours high per week (P<0.05). Decreases in days high per week (P<0.01). Decreases in weeks high per month (P<0.01). Decreases in periods high per week (P<0.05). | High Risk |
| Walukevich-Dienst [93] | Problematic users | PNF plus additional feedback (n=102) | PNF-only (n=102) | ~1 month | No significant differences between groups on cannabis use frequency. Women in the PFI group reported significantly fewer problems than women in the control group at follow-up. No significant differences between men in the intervention and control groups. |
|
| Meisel 2021 [45] | Heavy / Problematic users | MET-CBT + Topiramate (n=39) | MET-CBT + Placebo (n=26) | 6 weeks | Topiramate group had lower grams of cannabis use on use days but frequency was not reduced compared to placebo. Cravings were significantly blunted in topiramate group. significantly lower participants (48.72%) completed study in topiramate group versus 76.92% in placebo group. | Some Concerns |
| Bonar 2022 [94] | Heavy users | Motivational interviewing and CBT (n=76) | Attention-placebo control (n=73) | 8 weeks | At 6 months, the intervention group reduced cannabis frequency by 30.1% vs increase of 6.8% in control group (non-significant difference in adjusted model). Reduced cannabis use days by 19.2% in intervention vs 5.1% reduction for control (non-significant). Only significant difference was greater reduction in vaping days for intervention (-43.5%) vs increase in control (+16.7%) group (Cohen’s D = 0.40, p=0.020). |
Some Concerns |
| Macatee 2021 [95] | CUD (DSM) | DTI (psychoeducation and imaginal emotional exposure) (n=30) | Psychoeducation on healthy lifestyle topics (n=30) | ~4 months | Reduction in proportion of cannabis use days from pre-treatment to post-treatment: 12.2% in DTI group vs. 3% in HVC group (p=.02) No significant differences between groups on other outcomes |
Low Risk |
| Transitioning adults (between young and older adults) | ||||||
| Levin 2016 [96] | CUD (DSM) | Dronabinol + Lofexidine (n=61) | Placebo (n=61) | 11 weeks | No significant difference between groups in proportion achieving ≥21 days abstinence (27.9% intervention vs. 29.5% control). No significant differences between groups on other outcomes like abstinence in last 2 weeks, withdrawal scores, retention. | Low Risk |
| Sherman 2018 [97] | CUD (DSM) | Active Approach Bias Modification (n=33) | Sham ApBM (n=24) | 2 weeks | There was no significant difference between the active ApBM group and the sham ApBM group in terms of abstinence, frequency or quantity of cannabis use, withdrawal, or retention. However, the active ApBM group had significantly lower cannabis craving scores compared to the sham ApBM group (OR=0.28, p=0.03, 95% CI=0.09-0.91). | Low Risk |
| Litt 2019 [98] | CUD (DSM) | IATP (n=98) or IATP-CM (n=50) | MET-CBT (n=100) or MET + CBT + CM (n=51) | 12 weeks | At 14 months, probability of abstinence was 43% for IATP conditions versus less than 25% for MET-CBT conditions. IATP also led to significantly higher coping strategy use and self-efficacy. The addition of contingency management did not significantly improve outcomes. |
High Risk |
| Lintzeris 2019 [31] | Treatment seeking users | Nabiximols (n=61) | Placebo (n=67) | 12 weeks | Nabiximols group used illicit cannabis on fewer days than placebo group (estimated mean difference 18.6 days over 12 weeks, p=0.02). Higher proportion reduced use by 50% with nabiximols (54.1%) than placebo (28.9%), OR 0.35, p=0.03. No significant differences in other outcomes. | Some Concerns |
| Shekhawat 2023 [99] | CUD (DSM) | Brief intervention (n=50) | Simple advice (n=50) | 12 weeks | Significantly lesser number of days of cannabis use in past month in intervention group compared to control at 4 weeks (P<0.001), 8 weeks (P=0.002) and 12 weeks (P=0.049). No significant difference between groups in SDS cannabis withdrawal scores. | High Risk |
| Rooke 2013 [100] | Treatment seeking users | Reduce Your Use web-based intervention (n=119) | 6 modules of web-based educational information (n=111) | Up to 6 weeks | At 6 weeks, the intervention group had significantly fewer days of cannabis use (mean 12.05 vs 14.11 days, P=.02) and lower quantity consumed (mean 39.25 vs 46.16 standard cannabis units, P=.01) compared to controls. At 3 months, frequency of use remained lower (mean 12.90 vs 14.87 days, P=.02). Rates of past 30-day abstinence were not statistically significant between two groups(P>.05). |
High Risk |
| Litt 2013 [101] | CUD (DSM) | MET+CBT+CMHomework (n=71) | Case Management (CaseM) (n=71) | 9 weeks | No significant differences between groups in proportion abstinent or proportion days abstinent. Marijuana Problem Scale Scores declined in all groups, no significant differences.Latent Class Growth Model (LCGM) analysis identified subgroup of “Long-Term Abstainers” (19.5% of sample) that was more likely to be in MET+CBT+CMAbstinence group. |
High Risk |
| Hoch 2014 [102] | CUD (ICD-10) | individual psychotherapy combining CBT, MET, and problem-solving (n=149) | Delayed treatment (n=130) | Up to 6 month follow up | Self-reported abstinence at post-treatment: 53.3% for intervention group vs. 22% for control group (p < 0.001) Negative drug screens for abstinence at post-treatment: 46.3% for intervention group vs 17.7% for control group (p < 0.001). Significant difference between groups in Reduced frequency of cannabis use, severity of dependence, number of dependence symptoms and cannabis-related problems in AT group. |
High Risk |
| Weinstein 2014 [38] | CUD (DSM) | Escitalopram 10 mg/day (n=26) | Identical looking placebo (n=26) | 9 weeks | No significant difference in abstinence rates between escitalopram (11%) and placebo (27%) groups. No significant difference in withdrawal symptoms, anxiety or depression scores between groups. | Low Risk |
| Allsop 2014 [103] | CUD (DSM) | Nabiximols oral spray (n=27) | Matched placebo spray (n=24) | Outcomes measured over 9 days | Nabiximols reduced overall withdrawal severity compared to placebo (p=.01). Nabiximols reduced cravings (p=.04) and irritability (p=.01) more than placebo. Participants on nabiximols had 3.66 times higher odds of remaining in treatment (p=.02) | Low Risk |
| Rooke 2014 [104] | Treatment seeking users | Web-based intervention(n=225) | Waitlist control group (n=160) | Timing not specified. | The telephone intervention yielded larger treatment effects of reducing frequency of cannabis use (d=0.60, p<.001) compared to the web intervention (d=0.31, p=.02). The telephone intervention had lower dropout rate of 38% compared to 46% in the web intervention group (p<.01) | Some Concerns |
| Haney 2015 [48] | Heavy users | 50 mg naltrexone capsule daily (n=23) | Placebo capsule daily (n=28) | 16 days | Naltrexone group had 7.6 times lower odds of self-administering active cannabis compared to placebo group (OR = 7.6, p = 0.04). Naltrexone group gave significantly lower “Good Effect” ratings for active cannabis compared to placebo (p = 0.03) | Low Risk |
| McRae-Clark 2015 [39] | CUD (DSM) | Up to 60 mg/day of buspirone (n=88) | Identical placebo tablets (n=87) | 12 weeks | No significant difference in proportion of negative UCTs between groups over 12 weeks [OR 1.09 (0.45-2.61), p=0.86]. No significant differences in retention or craving. | Low Risk |
| Budney 2015 [105] | CUD (DSM) | MET/CBT plus abstinence-based contingency management (n=59) | Brief MET intervention (n=16) | 12 weeks with 3 and 9 months follow up | Longest duration of abstinence: significantly longer for both MET/CBT/CM groups compared to control during treatment (p<.05); no difference between computer and therapist groups. End of treatment abstinence rates: 45-47% for MET/CBT/CM groups vs 13% for control (p<.05); no difference between computer and therapist groups. Relapse rates over follow up period: no significant differences between any groups | High Risk |
| Sherman 2017 [42] | CUD (DSM) | Oxytocin (n=8) | Placebo (n=8) | 4 weeks | Both groups showed decreased use over time (p=0.006). Oxytocin group had significant reduction in daily use (p=0.022) while placebo group reduction was not significant (p=0.075).No significant difference in mean daily cannabis use between groups (p=0.412). The oxytocin group showed a significant reduction in the amount of cannabis used per day (p<0.05) compared to the placebo group. | High Risk |
| Gray 2017 [43] | CUD (DSM) | NAC 1200 mg twice daily (n=153) | Matched placebo capsules twice daily (n=149) | 12 weeks | Abstinence: No statistically significant difference between NAC and placebo groups in average odds of cannabis abstinence over time based on urine cannabinoid tests (p=0.984). Other: No significant differences in end-of-treatment or post-treatment abstinence rates. | Low Risk |
| Trigo 2018 [29] | CUD (DSM) | Nabiximols self-titrated up to max dose (n=20) | Placebo self-titrated up to max dose (n=20) | 12 weeks | No statistically significant difference between NAC and placebo groups in average odds of cannabis abstinence over time based on urine cannabinoid tests (OR=1.00, 95% CI: 0.63-1.59, p=0.984). 22.3% of urine tests negative in NAC group, 22.4% negative in placebo group. No significant differences in end-of-treatment or post-treatment abstinence rates | Low Risk |
| Sahlem 2018 [49] | CUD (DSM) | Active rTMS (n=18) | Sham rTMS (n=18) | Single session rTMS (about 30-40 minutes) | No significant reduction in total craving score between active and sham rTMS. 89% retention rate ( did not compare retention between groups.). All participants tolerated full treatment dose. | Some Concerns |
| D’Souza 2019 [106] | CUD (DSM) | PF-04457845 at 4 mg/day (n=46) | Matching placebo at the same dose (n=24) | 5-8 days inpatient plus 3 weeks outpatient treatment (4 weeks total) | Reduced withdrawal symptoms days 0-1 (p<0.05); Reduced self-reported cannabis use at week 4 (0.40 vs 1.27 joints/day; p=0.0003); Reduced urine THC-COOH at week 4 (265.55 vs 657.92 ng/mL; p=0.009) | Low Risk |
| Tomko 2020 [44] | CUD (DSM) | 2400 mg of NAC daily for 12 weeks (n=153) | Matched placebo for 12 weeks (n=149) | 12 weeks | No significant difference in abstinence rates between NAC and placebo groups. Higher baseline depression scores were associated with lower probability of abstinence during treatment (Adjusted RR=0.76, p=.007). |
Some Concerns |
| Sinadinovic 2020 [107] | Treatment seeking users | 13-module web-based treatment program (n=151) | Waitlist control group (n=152) | 3 months | No significant time x group effects were found in the intention-to-treat analyses. In the per-protocol analysis reductions were found in secondary outcomes of grams of cannabis consumed, number of dependence criteria, and CAST score. | Some Concerns |
| Stephens 2020 [108] | CUD (DSM) | PRN condition (MET/CBT) (n=43) | Fixed-dose condition (MET/CBT) (n=44) | Up to 34 months | No significant differences between groups in percentage days of cannabis use or problems at any follow-up. Fixed-dose had higher abstinence rate at 4 months (37% vs 15%, p<0.05).Significant reductions in cannabis use and associated problems at each follow-up compared to baseline in both groups (p < .001) | High Risk |
| Freeman 2020 [36] | CUD (DSM) | Oral CBD capsules (200mg, 400mg, 800mg) for 4 weeks (n=48) | Identical oral placebo capsules for 4 weeks (n=23) | 4 weeks | 400mg CBD significantly reduced urinary THC-COOH creatinine by 94.21 ng/ml and increased days abstinent from cannabis by 0.48 days/week compared to placebo. 800mg CBD significantly reduced urinary THC-COOH creatinine by 72.02 ng/ml and increased days abstinent by 0.27 days/week. | Low Risk |
| Mariani 2021 [37] | CUD (DSM) | Quetiapine, up to 300 mg daily (n=66) | Identical appearing inert placebo (n=64) | 12 weeks treatment | Odds of moderate cannabis use vs heavy use increased significantly more over time in quetiapine group (OR=1.17 per week, p<0.0001) compared to placebo (OR=1.05, p=0.16). Cannabis withdrawal decreased significantly more over time in quetiapine group (10.4% per week) compared to placebo (6.5% per week). No significant differences between groups over time in abstinence, craving scores or retention | Low Risk |
| Ostergard 2021 [109] | Treatment seeking users | PCOMS intervention (n=51) | Treatment as usual (n=49) | 8 weekly therapy and 6 months follow up | No significant differences found between groups on: Rate of attendance to sessions, dropout rates and drug days use outcomes including cannabis abstinence rates | High Risk |
| McRae-Clark 2021 [47] | CUD (DSM) | Varenicline up to 2mg/day (n=35) | Matching placebo tablets (n=37) | 6 weeks | Significantly greater reduction in urine cannabinoid levels from baseline with varenicline (-1.7 ng/mg) compared to increase with placebo (+1.9 ng/mg), RR=3.5 (0.1, 6.9). Numerically greater reduction in percentage of cannabis use days and use sessions per day with varenicline compared to placebo. No significant difference in cannabis withdrawal or craving scores between groups. Numerically but not statistically greater rates of self-reported abstinence with varenicline at end of treatment (17.1%) compared to placebo (5.4%), RR=3.2 (0.7, 14.7) | Low Risk |
| Davoudi 2021 [110] | CUD (DSM) | 16 weekly 90-minute sessions of DBT (n=30) | 8 sessions of psychoeducation (n=31) | 16-week intervention, and 2-month follow-up | DBT group had higher retention rates at post-treatment (96% vs 77%) and follow-up (96% vs 64.5%) (p < 0.05). DBT group had greater reduction in emotionality subscale of craving questionnaire (p < 0.05) but no significant difference in overall craving score between groups (p<0.05)DBT group had higher rates of abstinence at post-treatment (46% vs 16%) and follow-up (40% vs 9.5%) (p < 0.05). Among those who lapsed, DBT group had fewer consumption days among those who lapsed (p < 0.05) | Low Risk |
| Olthof 2023 [111] | Heavy users | ICan intervention (n=188) | Non-interactive educational modules (n=190) | 6 months | At 3 months, significantly larger reduction in number of grams of cannabis used in ICan group (effect size d=0.15, p=0.009). At 6 months, this difference was no longer significant (p=0.30). also, no significant difference between groups in number of cannabises use days at 6 months. No significant differences in cannabis use problems or attitudes towards seeking help. | High Risk |
| Mills 2022 [30] | CUD (ICD-10) | Nabiximols up to 32 sprays daily (n=61) | Placebo sprays up to 32 daily (n=67) | 12 weeks | Nabiximols group used illicit cannabis on 18.6 (95% CI: 3.5, 33.7) fewer days during the 12-week trial compared to placebo. Nabiximols group had greater odds of reducing cannabis use by 50% compared to placebo. also, significant greater odds of retention in intervention group. | Some Concerns |
| Older adults (25-65) | ||||||
| Johnston 2014 [41] | CUD (DSM) | Lithium carbonate 500 mg (n=16) | Identical placebo capsules(n=22) | 7 days | No significant differences between groups in total cannabis withdrawal scale scores, retention rates, rates of completion, abstinence rates or use of rescue medications. Lithium significantly reduced individual withdrawal symptoms of loss of appetite, stomach aches, and nightmares/strange dreams. | Low Risk |
| Walker 2015 [112] | CUD (DSM) | MET/CBT plus MCU sessions (n=37) | Only MET/CBT (n=37) | 9 months | MCU had significantly greater abstinent rates at 3 months (36% vs 13%; p < .05) and 9 months (26% vs 7%; p < .06). MCU used cannabis on fewer days at 3 months (25.52 vs 50.37 days; p < .05) but difference was not significant at 9 months. | High Risk |
| Fuster 2016 [113] | Heavy users | Brief Negotiated Interview (BNI) (n=59) | No intervention (n=55) | 6 months | No significant difference in days of marijuana use at 6 weeks (IRR 0.94, 95% CI 0.79-1.12, p=0.77) or 6 months (IRR 0.95, 95% CI 0.79-1.15, p=0.82) between BNI group and control. No significant difference in SIP-D drug problem scores at 6 weeks (IRR 1.37, 95% CI 0.84-2.22, p=0.20) or 6 months (IRR 1.12, 95% CI 0.69-1.82, p=0.66). | High Risk |
| Lintzeris 2020 [32] | Treatment seeking users | Nabiximols plus psychosocial interventions (n=61) | Placebo spray and PI (n=67) | 12w treatment/12w follow-up | Nabiximols group used cannabis on 6.7 fewer days at week 24 follow-up than placebo group (p=0.006). 23% of nabiximols group were abstinent at week 24 compared to 9% of placebo group (OR 3.0, p=0.035). | Some Concerns |
| Levin 2021 [34] | CUD (DSM) | Dronabinol (up to 20 mg BID) (n=79) | Placebo in dronabinol trial (n=77) | 8 weeks | No significant overall time-by-treatment effect for frequency/quantity across trials (no significant differences in the longitudinal pattern of use over time between treatment groups, while adjusted by other covariates) .Starting around midpoint, treatment groups had higher odds of moderate (2-4 days/week) versus heavy (5-7 days/week) cannabis use compared to placebo. No consistent differences between groups for odds of light (0-1 days/week) versus heavy use | Some Concerns |
| Heitmann 2021 [114] | CUD (DSM) | Treatment as usual (CBT-based outpatient treatment) + ABM (n=42) | Subgroup 1: Placebo training + TAU (n=19) Subgroup 2: TAU only (n=17) | 6- and 12-month follow-ups | No significant differences were found between the ABM intervention group and control groups on any of the primary outcomes - substance use, craving, relapse rates. The groups showed similar reductions in use from baseline to post-treatment, but relapse by 6–12-month follow-ups. | Some Concerns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





