1. Introduction
Human rotavirus (HRV) is the leading cause of childhood viral gastroenteritis, leading to significant morbidity and mortality, primarily in low- and middle-income countries (LMICs) [
1,
2]. The inclusion of live-attenuated oral vaccines in national vaccination programs has been recommended by the World Health Organization (WHO) since 2006, but their efficacy in LMICs has remained low [
2]. Several factors are thought to be responsible, including circulating maternal antibodies, concurrent use of other oral vaccines, concurrent enteric viral infection, gut dysbiosis, and chronic malnutrition [
1,
2]. Along with these factors, oral HRV vaccines come with a history of increased risk of intussusception, a rare but potentially fatal bowel blockage [
2]. To circumvent these factors that are mainly associated with the gastrointestinal (GI) tract, other routes of administration have continued to be pursued [
2]. Intramuscular (IM) non-replicating rotavirus vaccines (NRRV) may offer a solution. The non-replicating nature of these vaccines makes them a safer alternative to live-attenuated vaccines regarding HRV vaccine-specific intussusception and live-attenuated vaccination risks in general [
2,
3].
To address the obvious need for improved HRV vaccines in LMICs, CureVac has developed an mRNA-based RNActive
® NRRV for intramuscular administration. mRNA as a therapeutic molecule has been studied for decades, but until recently, there were no licensed mRNA-based vaccines available[
4]. mRNA-based vaccines have become widely known in the context of the COVID-19 pandemic, and two are now FDA-licensed for use in the United States [
5].
Vaccines employed in this study consist of chemically unmodified, sequence-engineered mRNA featuring optimized non-coding regions previously described in the context of SARS-CoV-2 vaccine development [
6,
7,
8]. mRNAs are encapsulated in lipid nanoparticles (LNPs) and encode for VP8*, a proteolytic cleavage product of the rotavirus spike protein VP4 that contains neutralizing epitopes and defines the P genotype [
9,
10,
11]. Both mRNA vaccine designs employed here are trivalent and encode for VP8* derived from P[4], P[6] and P[8] genotypes. Two different mRNA designs, described in more detail in Roier
et al., [
12] were tested. Firstly, P2-VP8*, an mRNA that features the universal T cell epitope P2 fused to VP8* and mirrors a clinically tested protein vaccine [
10,
13,
14]. Secondly, LS-P2-VP8*, in which P2-VP8* is fused to a multimerization domain derived from
Aquifex aeolicus lumazine synthase (LS) [
15]. Expression of VP8* in this context leads to nanoparticle formation aimed to support the formation of 60-mer nanoparticles that mimic the natural display of VP8* on the viral surface [
16]. The inclusion of LS in other vaccines has shown to facilitate more efficient and natural antigen presentation and B cell activation [
17,
18,
19,
20]
The objective of this study was to evaluate the immunogenicity and protective efficacy of these mRNA vaccine candidates in the gnotobiotic (Gn) pig model of HRV infection and diarrhea using the trivalent alum-adjuvanted P2-VP8* protein vaccine, kindly provided by PATH, as a comparator. The G1P[8] Wa HRV Gn pig model is a well-established model that has been used in HRV vaccine evaluation for more than 27 years [
21,
22]. It is the only laboratory animal that develops HRV infection, including mimicking human intestinal pathology, and reliably shows clinical signs of disease (diarrhea) [
21,
22]. Along with this, the Gn pig mirrors human immunity to HRV and vaccines, with serum IgA, IgG, intestinal IgA, and HRV-specific IFN-γ producing T cells correlating to protection from HRV infection and diarrhea [
3,
22,
23,
24,
25]. In general, pigs are physiologically and immunologically similar to humans, making results using swine directly translatable to humans [
21,
22,
26]. Furthermore, the Gn pig lacks any microbiota and the porcine placenta prevents the passive transfer of maternal antibodies. Therefore, Gn piglets are immunologically naïve but immunocompetent at birth [
22]. This prevents immune interference from commensal bacteria and maternal antibodies but allows for normal immunological development in response to vaccines [
22]. Through this study, we addressed two major questions: 1) Are mRNA vaccines able to confer protection in the Gn pig model of HRV infection and how does their efficacy compare to a protein-based comparator? 2) which of the two mRNA vaccine candidates, P2-VP8* or LS-P2-VP8*, confers better protection and immunogenicity in this model?
2. Materials and Methods
2.1. Rotaviruses for Challenge and Immunoassays
The virulent Wa HRV (G1P[8]) inoculum consisted of a pool composed of small intestinal contents collected from the 28
th or 29
th passage of the virus in Gn pigs. A dose of 10
5 focus-forming units (FFU) diluted in 5 mL of Diluent #5 (minimal essential media [ThermoFisher Scientific]; 100 IU of penicillin per mL, 0.1 mg of dihydrostreptomycin per mL; and 1% HEPES) was used for the oral challenge of Gn pigs. The median infectious and diarrhea doses of Wa HRV were determined previously to be approximately 1 FFU [
21]. The supernatant of Wa HRV-infected African green monkey kidney MA104 cells (ATCC CRL-2378.1™) were semi-purified by centrifugation through 40% (wt/vol) sucrose cushion and used as a positive control in antigen enzyme-linked immunosorbent assay (ELISA) and cell culture immunofluorescence (CCIF) [
27]. Recombinant P genotype-specific P2-VP8* proteins were used as detector antigens in ELISA and ELISpot and as stimulating antigens in flow cytometry analysis of T cell responses.
2.2. mRNA and Protein Vaccines
The trivalent lipid nanoparticle (LNP)-formulated mRNA vaccines P2-VP8* and LS-P2-VP8* were recently described by Roier
et al. [
16]. The mRNA vaccines are based on CureVac’s RNActive
® platform and do not contain chemically modified nucleotides. They comprise a 5′ cap1 structure, a 5′ untranslated region (UTR) from the human hydroxysteroid 17-beta dehydrogenase 4 gene (
HSD17B4), a GC-enriched open reading frame, a 3′ UTR from the human proteasome 20S subunit beta 3 gene (
PSMB3), a histone stem-loop, and a poly(A) tail. LNP encapsulation of mRNA was performed using LNP technology from Acuitas Therapeutics (Vancouver, Canada). LNPs are composed of an ionizable amino lipid, phospholipid, cholesterol, and a PEGylated lipid. The P2-VP8* mRNA vaccine design encodes a universal CD4+ T cell epitope (P2) derived from tetanus toxin [
14] (aa residues 830-844; NCBI reference sequence: WP_011100836.1) N-terminally linked to a P genotype-specific rotavirus VP8* subunit protein (aa 65-223 of VP4) by a GSGSG linker. The LS-P2-VP8* mRNA vaccine design encodes full-length lumazine synthase (NCBI reference sequence: WP_010880027.1) from
Aquifex aeolicus containing two point mutations (C37A to remove an unpaired cysteine and N102D to remove a glycosylation site [
17,
28]) N-terminally linked via (GGS)
4-GGG to P2-VP8*, which is designed as described above, except that a longer version of VP8* (aa 41-223 of VP4) is encoded. The P genotype-specific VP8* sequences always derive from the following human rotavirus A strains: BE1128 for P[8] VP4 genotype (GenBank accession number: JN849135.1), BE1322/F01322 for P[6] VP4 genotype (GenBank accession number: JF460826.1), and BE1058 for P[4] VP4 genotype (GenBank accession number: JN849123.1). The trivalent mRNA vaccines encoding genotypes P[8], P[6] and P[4] of VP8* were formulated by mixing the respective mRNAs in a 1:1:1 weight ratio prior to LNP encapsulation. An LNP-formulated mRNA encoding rabies virus glycoprotein (RABV-G) served as an irrelevant mRNA vaccine and thus as a negative control in the present study [
29,
30].
The recombinant fusion proteins P2-VP8* P[8], P2-VP8* P[6], and P2-VP8* P[4] expressed in
Escherichia coli were kindly provided by PATH (Seattle, WA, USA) and consist of tetanus toxin T cell epitope P2 linked to aa 65-223 (for P[8]) or aa 64-223 (for P[6] and P[4]) of VP4 from rotavirus strain Wa (P[8]), 1076 (P[6]), or DS-1 (P[4]), respectively, using a GSGSG linker as previously described [
9,
10,
13,
31,
32,
33]. The protein vaccines were alum-adjuvanted by adsorption of the recombinant fusion proteins to aluminum hydroxide (Alhydrogel
®; Croda, Frederikssund, Denmark) essentially as previously described [
32,
34]. Briefly, a two-fold working stock containing 720 µg/mL of trivalent P2-VP8* antigens (1:1:1 weight ratio; 240 µg/mL per antigen) in 0.5 mM PO
4 in 0.9% saline was combined with an equal volume of an Alhydrogel
® suspension (4.48 mg Al/mL diluted in 0.5 mM PO
4 in 0.9% saline) using gentle mixing for 20-24 hours to achieve a formulation with 360 µg/mL total trivalent P2-VP8* antigen containing aluminum hydroxide at 2.24 mg Al/mL in 0.5 mM phosphate buffer in 0.9% saline (pH 6.9). Trivalent P2-VP8* antigen adsorption to Alhydrogel
® was analyzed in a previous study and reported to be >90% for formulations with 360 µg/mL antigen containing aluminum hydroxide at 2.24 mg Al/mL in 0.5 mM phosphate buffer [
35].
2.3. Vaccine Inoculation, Virus Challenge, and Sample Collection of Gn Pigs
Gnotobiotic pigs used in this study were derived via hysterectomy of near-term sows and maintained in germ-free isolators for the duration of the study [
36]. Sterility was verified by weekly rectal swabs (RS) on blood agar and in thioglycolate broth. Pigs were fed ultra-high temperature (UHT) treated sterile whole cow’s milk (The Hershey Company, Hershey, PA, USA) for the duration of the study. On each vaccination day, pigs were injected IM into
M. brachiocephalicus (PID 0) or left (PID 14) or right (PID 28)
M. biceps femoris with 30 µg of irrelevant mRNA vaccine (100 µL), with 90 µg (560 µg Al) of P2-VP8* protein vaccine (250 µL), with 30 µg of P2-VP8* mRNA vaccine (100 µL), or with 30 µg or 12 µg of LS-P2-VP8* mRNA vaccine (100 µL each). The first dose of the respective vaccine was administered on post-partum day (PPD) 5 (also referred to as post-inoculation day [PID] 0) followed by two more doses at 14-day intervals for a total of three vaccinations. Serum was collected 14 h after prime vaccination on PID 1, for evaluation of vaccine-induced IFN-α levels. Serum was also collected before each vaccination at PID 14 and 28, before challenge (PID 35 or post-challenge day [PCD] 0) and at euthanasia (PCD 7) to evaluate P2-VP8*-specific IgG and IgA by direct ELISA and virus neutralizing (VN) titers by virus neutralization assay. Whole blood was taken on PID 32 for extraction of mononuclear cells (MNCs) for detection of P2-VP8*-specific IFN-γ-producing CD4+ and CD8+ T cells by flow cytometry.
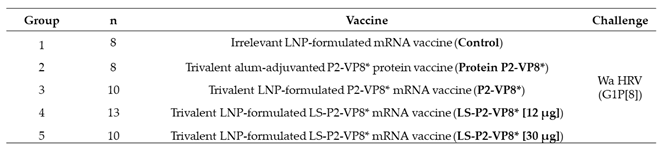
A total of 49 pigs were assigned randomly to five groups (
Table 1). Pigs were orally challenged on PID 35 (PCD 0) with 1 x 10
5 FFU of virulent Wa HRV and monitored for diarrhea and virus shedding via daily RS from PCD 0-7; RS were processed as described previously [
37]. All pigs were fed 200 mM sodium bicarbonate 10 minutes prior to challenge to neutralize stomach acidity. Pigs were euthanized at PCD 7. At euthanasia, small intestinal contents (SIC) and large intestinal contents (LIC) were collected and processed as previously described, for the detection of intestinal antibody responses and antigen presence by direct and sandwich ELISA, respectively, as well as infectious virus particle counting by CCIF [
38]. Spleen, ileum, and whole blood were collected for extraction of MNCs to be used in the detection of VP8*-specific IgG and IgA ASC responses by ELISpot and P2-VP8*-specific IFN-γ-producing CD4+ and CD8+ T cells by flow cytometry [
25,
39,
40].
2.4. Assessment of Diarrhea and Detection of Fecal Virus Shedding by Antigen ELISA and CCIF
To assess severity of diarrhea, fecal consistency was recorded, using the RS taken from PCD 0 to 7, as 0: solid, 1: pasty, 2: semi-liquid, and 3: liquid, with any score ≥2 being considered diarrhea [
21,
41,
42]. Antigen ELISA was used for detection of HRV VP6 and CCIF for detection of infectious virus particles, as previously described [
41,
42,
43,
44].
2.5. Detection of Interferon-α Induction after Prime Immunization
Serum was taken from all pigs 14 h after the prime immunization (PID 1) and interferon-α (IFN-α) levels were evaluated using a commercial ELISA kit (Thermo Fisher cat#ES7RB) according to manufacturer’s instructions. The IFN-α concentrations were calculated based on the standards in the kit and expressed as pg/mL.
2.6. Detection of P2-VP8*-specific Serum and Intestinal IgA and IgG Antibody by ELISA
Serotype-specific anti-P2-VP8* IgA and IgG antibody titers in serum (collected at PID 0, 14, 28 and PID 35/PCD 0 and PCD 7) and intestinal contents (collected at PCD 7) were measured by using a serotype-specific antibody ELISA [
27,
38,
45]. Recombinant P[8], P[6], or P[4] P2-VP8* proteins were used as detector antigens with the coating concentration of 0.5 µg/mL. Negative control wells were coated with coating buffer only (0.05 M carbonate buffer, pH 9.6). Coated plates were incubated for 1 h at 37°C, washed five times with TBST, and blocked with TBS + 5% BSA overnight at 4°C. Plates are washed five times with TBST and incubated with four-fold serial dilutions of each serum sample (starting from 1:4 to 1:65536) overnight at 4°C. For detection, plates were incubated with goat anti-pig IgA antibody horseradish peroxidase (HRP)-conjugated and goat anti-pig IgG-Fc antibody HRP-conjugated (Bethyl Laboratories, Inc. USA), 1:3000 diluted in TBST with 5% BSA, and developed with ABTS peroxidase substrate (1:1 ratio of KPL ABTS
® peroxidase substrate solution A and KPL peroxidase substrate solution B from Seracare Life Sciences, Inc. USA). The reaction was stopped with ABST peroxidase stop solution diluted 1:5 in ddH
2O. Optical density was read at 405 nm. IgA and IgG titers were expressed as the reciprocal of the highest sample dilution in which the mean A
405 of P2-VP8*-coated wells was greater than the mean A
405 of negative controls plus 3-fold SD. Titers of <4 were assigned a value of 2 for calculation of geometric mean titer (GMT).
2.7. Flow Cytometry for Detection of IFN-γ-producing CD3+CD4+ and CD3+CD8+ T Cells
Mononuclear cells isolated from peripheral blood (PCD 0 and 7) were diluted to a concentration of 1 × 10
6 cells/mL and seeded into 12-well plates [
39]. Cells were re-stimulated for 17 h at 37°C in 5% CO
2 with one of six stimulants: (1) medium (negative control), (2) PHA (10 µg/mL; positive control), (3) P[4] P2-VP8* protein (12 µg/mL), (4) P[6] P2-VP8* protein (12 µg/mL), (5) P[8] P2-VP8* protein (12 µg/mL), and (6) semi-purified attenuated Wa HRV antigen (12 µg/mL). Anti-CD49d mAb (0.5 µg/mL) was added to all samples before incubation for co-stimulation. After 12 h, Brefeldin A (5 µg/mL) was added for 5 h at 37°C in 5% CO
2. After the total 17 h incubation, cells were washed with 2 mL of commercial stain buffer and transferred to 5 mL Falcon round-bottom polypropylene tubes for 8 min centrifugation at 800 x g at 4°C, followed by discarding of the supernatant. Primary antibodies used for staining were mixed in a cocktail that included FITC-conjugated mouse (IgG2b) anti-pig CD4a, SPRD-conjugated mouse (IgG2a) anti-pig CD8a, and mouse (IgG1) anti-pig CD3ε in 100µL of stain buffer per sample. Samples were incubated with the cocktail for 15 mins at 4⁰C and subsequently washed with 500 µL of wash buffer, followed by 8 min centrifugation at 800 x g at 4°C. Then, secondary antibody APC-conjugated rat (IgG1) anti-mouse for CD3ε diluted in 100 µL of stain buffer was added and incubated for another 15 mins at 4⁰C. The washing and centrifugation steps were repeated, then, 100 µL of Permeabilizing/Fixation solution was added into each sample and incubated for 30 mins at 4⁰C. Washing and centrifugation were then repeated. PE-conjugated mouse (IgG1) anti-pig IFN-γ diluted in 100 µL of stain buffer was added and incubated for another 30 mins at 4⁰C. A last washing step with 2 mL of stain buffer was performed followed by centrifugation. Cells were resuspended in 250 µL of stain buffer and stored away from light at 4°C until delivery to Virginia Tech’s flow cytometry core for acquisition on a BD FACSArial
TM II flow cytometer.
2.8. Detection of P2-VP8*-specific Antibody-Secreting Cells by ELISpot Assay
Plates (96-well) were coated with 50 µL P[8] P2-VP8* (1.41 mg/mL), P[6] P2-VP8* (1.16 mg/mL) or P[4] P2-VP8* (1.04 mg/mL) proteins diluted to the working concentration of 5 µg/mL in 50 mM carbonate coating buffer (pH 9.6). Plates were incubated at 37⁰C for 30 min, then stored at 4⁰C overnight. Plates were washed twice with PBST (pH 7.4, with 0.05% Tween 20), blocked with 100 µL/well 4% BSA in PBS (pH 7.4) and incubated at 37⁰C for 1 h. Plates were washed three times with ddH20. Separately, extracted MNCs were diluted to a single-cell suspension at a concentration of 5 x 106 cells/mL with E-RPMI and 100 µL of each diluted cell suspension was added to duplicate wells. Plates were then centrifuged at 500 rpm for 5 min at RT, followed by incubation at 37⁰C for 12 h. Plates were then washed five times with PBST, and 100 µL/well of biotinylated goat anti-porcine IgA diluted 1:20,000 (Bethyl A100-102B, 1 mg/mL) or IgG (Bethyl A100-104B, 1 mg/mL), diluted 1:20,000 in PBST was added to each well. Plats were incubated at RT for 2 h and washed five times with PBST. To ensure MNCs were removed completely, the plates were knocked vigorously on paper towels between each wash. HRP-conjugated streptavidin diluted 1:30,000 in PBS was added (100 µL/well) and the plates were incubated at RT for 1 h. Plates were washed five times with PBST, and 50 µL/well KPL TrueBlue HRP substrate (ready-to-use substrate, VWR catalog number 95095-168) was added to each well. Plates were then incubated for 1-2 h at RT until spots become dark blue. Spots were counted with ELISpot analyzer S5 and reported as IgA or IgG ASC per 5 x105 MNC.
2.9. Virus Neutralization Assay
The rotaviruses Wa (G1P[8]), 1076 (G2P[6]) and DS-1 (G2P[4]) were used in this assay. These viruses were originally obtained from the National Institutes of Health and a stock was grown for use in the assay. The rotavirus-neutralizing antibody assay was performed by titrating respective serum samples and incubating them with a constant amount of a specific strain of rotavirus to allow neutralization of the virus. The rotavirus/serum mix was then incubated on MA104 cells in 96-well plates for 1 hour to allow adsorption of the virus. The plates were washed to remove serum and then overlaid with media. Control wells on each 96-well plate received either diluted virus without sera (virus control) or no virus or sera (cell controls). After an overnight incubation allowing for rotavirus growth, the cells were frozen and thawed to lyse the cells to release the viruses, and then the lysates were resuspended. The relative amount of non-neutralized rotavirus was determined using an antigen capture ELISA to detect rotavirus. The ELISA plates were coated with rabbit anti-rotavirus IgG antibodies. The resuspended lysates from the neutralization part of the assay were added and incubated. Following blocking with nonfat milk, guinea pig anti-rotavirus antiserum was added to the wells to detect captured rotavirus. Horseradish peroxidase (HRP) conjugated rabbit anti-guinea pig IgG (Jackson ImmunoResearch, West Grove, PA, USA) was added to detect the guinea pig anti-rotavirus antibody. The wells were developed with o-Phenylenediamine (Sigma-Aldrich), a peroxidase-sensitive colorimetric substrate. Optical density of each well was read as absorbance at 492 nm. The amount of rotavirus present in the resuspended lysate from each well is inversely related to the amount of neutralizing antibody present in the serum. Each serum dilution series was modeled using a logistic regression function. For each fitted curve the reciprocal serum dilution which corresponds to a 40% response (ED
40), compared to the virus controls, was determined and reported as the titer. The ED
40 represents the titer of the serum against a given virus, which represents a 60% reduction in the amount of virus [
46].
2.10. Statistical Analysis
Pigs were randomly assigned to treatment groups by animal care staff upon derivation, regardless of sex or size. Proportions of affected animals (infected and with diarrhea) among treatment groups were compared using Fisher’s exact test.
Daily mean fecal consistency (diarrhea) scores from PCD 0 to PCD 7 among treatment groups were analyzed using a two-way ANOVA of repeated measures through time followed by Holm-Šídák’s multiple comparisons test. Daily mean CCIF and ELISA titers of virus shedding as independent variables were also analyzed by a two-way ANOVA of repeated measures through time followed by Tukey’s multiple comparisons test.
CCIF titers and fecal consistency scores were summarized over time for each animal into six efficacy endpoints: mean peak titer (maximum CCIF titer), the area under the curve over time (AUC) of virus shedding, AUC of fecal scores, cumulative fecal consistency score, the onset and duration of viral shedding (total days of detectable titers), and the onset and duration of diarrhea (total days with fecal consistency scores > 1). The area under the curve (AUC) of diarrhea score and virus shedding were calculated in RStudio using the AUC command of the DescTools package and the spline approach. Peak titer, AUC of virus shedding, AUC of diarrhea, and cumulative fecal score were analyzed using a fitted linear mixed effects model to assess the vaccine group effect on each endpoint with litter random intercept followed by pairwise t-tests using the Kenward-Roger method [
47] and exact one-step multiplicity adjustment. Duration of viral shedding and diarrhea were analyzed using the Prentice rank sum test with litter specified as a block variable followed by Wilcoxon rank-sum tests with Holm’s multiplicity correction. For comparing intestinal ELISA antibody and serum VN antibody titers, the titers were log transformed before statistical analysis. The mean antibody titers among groups were analyzed by a general mixed model of repeated measures through time. Total numbers and mean frequencies of T cell subsets were analyzed using ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. Mean numbers of ASCs in post-mortem tissues were analyzed using the Kruskal-Wallis test followed by Dunn’s multiple comparisons test. P-type specific serum IgG and IgA responses were analyzed using the Prentice rank sum test with litter specified as a block variable followed by Wilcoxon rank-sum tests with Holm’s multiplicity correction. IFN-α levels were analyzed using a mixed effects model with litter random intercept followed by pairwise t-tests using the Kenward-Roger method and exact one-step multiplicity adjustment. Spearman correlations were tested within efficacy endpoints and between efficacy and immunogenicity endpoints.
A sensitivity analysis was also performed to determine the influence of litter variation on the efficacy results. For the efficacy endpoints analyzed via the mixed models, two variance components were estimated: the intra-experiment variability (i.e., litter to litter variation via the litter random effect) and the residual variance (i.e., the measurement error). For duration of viral shedding and diarrhea, these variance components were extracted from a one-way ANOVA with litter as the independent variable. Using these components, the intraclass correlation coefficient (ICC) for litter variation was computed: the ratio of litter variance over the total variance. The ICC ranges from 0% to 100% where 0% indicates that the experimental variability is entirely attributable to residual variation (i.e., there is no meaningful litter to litter variation) and 100% indicates that the experimental variability is entirely attributable to litter variation. The ICC for all six endpoints was minimal (< 8%) suggesting that the litter variability explained little result variation between groups.
Statistical analyses were performed using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA) and R (R Core Team, Vienna, Austria). A p value lower than 0.05 was considered as statistically significant. In cases where unadjusted p-values were statistically significant but the adjusted p-values were not, the unadjusted significance was reported.
3. Results
3.1. mRNA Vaccines induce IFN-α in Serum of Vaccinated Animals 14 h post Immunization
Serum collected 14 h post-prime immunization was evaluated for systemic induction of IFN-α. As expected, the lowest levels of IFN-α with a mean value of 167 pg/ml were detected in animals vaccinated with P2-VP8* protein vaccine. All mRNA vaccines induced IFN-α with lowest mean levels of 237 pg/ml detectable for LS-P2-VP8* at a dose of 12 µg. 30 µg of P2-VP8* and LS-P2-VP8* induced approx. 2-fold higher levels with mean IFN-α values of 557 and 442 pg/ml, respectively. (Supplementary Figure 1).
3.2. Both mRNA Vaccine Candidates Conferred Significant Reduction of Severity and Duration of Diarrhea, and Elicited Significant Protection against Infectious Virus Shedding
Gn pigs were challenged with virulent Wa HRV at post-inoculation day (PID) 35/post-challenge day (PCD) 0 and clinical signs and virus shedding was monitored via daily rectal swabs (RS). These data are summarized in Table 2. The percentage of animals with diarrhea and the mean days to onset remained largely unchanged compared to negative control animals for all mRNA as well as the protein comparator groups. Pigs who received mRNA P2-VP8* (30 µg ) and LS-P2-VP8* (30 µg) had a significantly shortened duration of diarrhea compared to the negative control pigs (3.1 days and 2.5 days versus 4.6 days, respectively) (Table 2). All pigs who received mRNA vaccines had a significantly decreased AUC of diarrhea that was 2.2-2.6 lower than control pigs (Table 2). Indeed, negative control pigs experienced a double peak of diarrhea at PCDs 3 and 6, while pigs vaccinated with LS-P2-VP8* (12 µg and 30 µg) only experienced one peak at PCD 2 and PCD 3, respectively, which then tapered off to consistently lower scores for the rest of the study (Figure 1). mRNA P2-VP8* (30 µg)-vaccinated pigs experienced a slight peak at PCD 3, and a larger peak, comparable to control pigs, at PCD 6 (Figure 1). Thoughout the observation period, mean diarrhea score values were overall lower in all mRNA groups compared to the negative control with the exception of PCD 6 for the P2-VP8* group, where scores were comparable. The reduced severity of diarrhea was also reflected by significantly decreased cumulative fecal scores that were 2.1-2.4 lower in the mRNA vaccine groups compared to the controls (Table 2). Protein P2-VP8*-vaccinated pigs experienced overall similar trends to the mRNA groups. However, there was a trend towards improved values for LS-P2-VP8* mRNA vaccine compared to protein P2-VP8* in terms of mean duration days (30 µg only), mean cumulative fecal score and AUC of diarrhea (both dose groups). In addition, mean diarrhea scores after the peak at PCD 3 trended higher in protein than in mRNA vaccinated groups for the duration of the study. An exception was PCD 6, where mean diarrhea scores in the protein P2-VP8* group were comparable to mRNA LS-P2-VP8* vaccines but lower than in mRNA P2-VP8*-vaccianted pigs. (Figure 1). Overall, a trend towards improved clinical signs elicited by LS-P2-VP8* compared to mRNA P2-VP8* and protein P2-VP8* was detected.
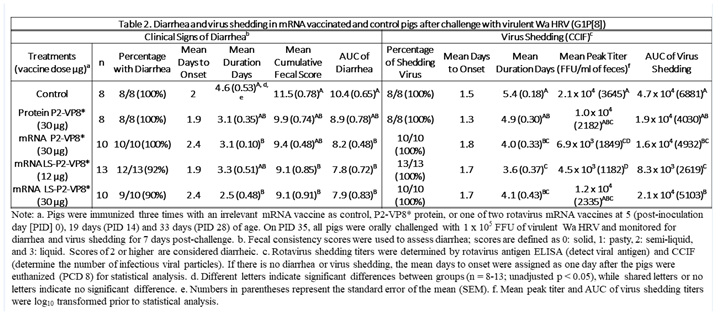
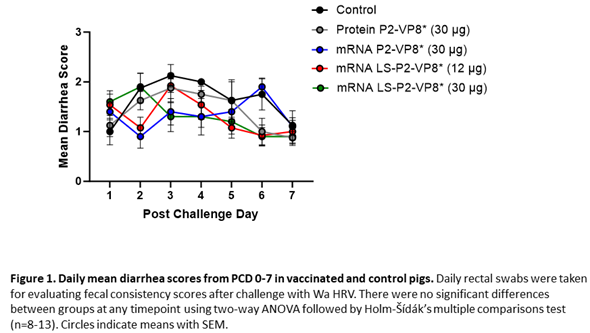
The mean duration days of virus shedding was significantly reduced (to 3.6-4.1 days) in all pigs who received mRNA vaccines compared to the control group (5.4 days) (
Table 2). The AUC of virus shedding was also significantly reduced in all pigs who received mRNA vaccines, ranging from 2.2-fold to 5.7-fold lower than the control (
Table 2). Similar to the diarrhea data, when looking at mean daily titers, negative control and protein P2-VP8*-vaccinated pigs experienced a classic double peak of daily mean CCIF titers at PCDs 2 and 5 (
Figure 2), although this effect was more pronounced in control pigs [
21,
22,
48,
49]. Pigs vaccinated with mRNA P2-VP8* shed significantly lower virus than negative control pigs on PCDs 4-5 and displayed a double peak that was comparable to the P2-VP8* protein groups (
Figure 2A). In contrast, LS-P2-VP8* (12 µg)-vaccinated pigs shed at significantly lower titers compared to negative control pigs on PCDs 2-5 and to P2-VP8* protein at PCD 2 (
Figure 2B). Titers remained low throughout the observation period and displayed a single low peak on PCDs 4-5 (
Figure 2B). When comparing LS-P2-VP8* (12 µg)-vaccinated pigs to protein-P2-VP8*-vaccinated pigs, this group shed significantly lower titers on PCD 2 (
Figure 2B). Results for LS-P2-VP8* (30 µg)-vaccinated pigs were not as pronounced; however, the group’s mean titer around the time of the typical second shedding peak (PCD 5) was significantly lower than the negative control-vaccinated pigs (
Figure 2C). This is reflected by the previously mentioned significantly shortened duration of virus shedding in this group (
Table 2). For peak viral titers, mRNA P2-VP8* and LS-P2-VP8* (12 µg)-vaccinated pigs exhibited significantly reduced (3.0-fold and 4.7-fold lower, respectively) titers compared to negative control pigs. When evaluating mRNA-vaccinated pigs against the protein-P2-VP8* vaccine (negative control excluded in analysis), LS-P2-VP8* (12 µg) conferred significantly higher protection with 2.3-fold lower AUC of virus shedding, 2.2-fold lower peak titer and 1.3 days less shedding.

Correlations across six efficacy measures of rotavirus disease burden (AUC of virus shedding, peak titer, shedding days, AUC of diarrhea, cumulative fecal score and diarrhea days) were examined (Supplementary Figure 2). The highest Spearman correlations were observed within the virus shedding and the diarrhea measurements. Cross virus shedding and diarrhea measurement correlations were uniformly lower but all positive. The positive correlation is consistent with a link between viral titers and symptomatic disease (as measured by fecal consistency scores), but incomplete correlation indicates that worse diarrhea outcomes are not entirely explained by high viral titers.
3.3. LS-P2-VP8* Vaccine Induced Strong P-type-specific Serum IgG Antibody Responses Pre- and Post-Challenge and Primed for Stronger Serum IgA Antibody Responses Post-Challenge Compared to other groups
Serum samples were collected from all pigs before each vaccination (PID 0, 14, 28), challenge (PID 35/PCD 0), and at euthanasia (PCD 7). P-type-specific IgG and IgA antibody responses were analyzed in the serum at each time point via ELISA and results are summarized in Figure 3. Both doses of LS-P2-VP8* induced significantly stronger IgG responses to P[8], P[6] and P[4] in the serum compared to all other groups, beginning at PID 14, after only one immunization (Figure 3A). This trend continued to PCD 7, at which point both dose groups still had significantly higher titers than all other groups for all three P-types.
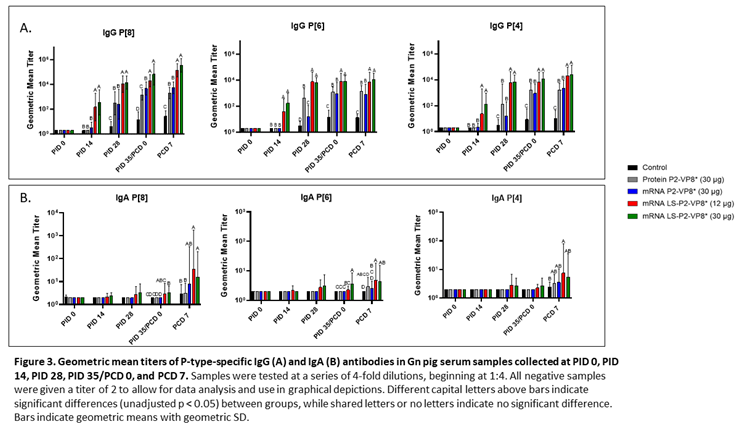
Correlations of the pre-challenge immune responses (serum IgG, serum IFN-α) with the efficacy measures of rotavirus disease burden (AUC of virus shedding, shedding days, AUC of diarrhea, diarrhea days) were assessed. For P-type-specific serum IgG responses at PID 28 and PCD 0, a consistent negative correlation was observed highlighting that higher IgG responses correlated with lower measures of disease burden (Supplementary Figure 3). At either time point, all but one correlation between IgG response titers and the virus shedding measurements were significant. While there was uniform negative correlation with the diarrhea measurements, the only significant correlation was between anti-VP8* P[8] IgG titers and AUC of diarrhea at PID 28 and PCD 0. There were no significant correlations found with serum IFN-α responses induced 14 h post-prime immunization and the efficacy measurements (Supplementary Figure 4).
Low levels of P-type specific IgA titers were detected in LS-P2-VP8*-vaccinated pigs as early as PID 14, but not to a significant degree until PID 35, when P[8]- and P[6]-specific serum IgA titers were significantly higher in the LS-P2-VP8* (30 µg) groups compared to negative control, protein P2-VP8* and mRNA P2-VP8*-vaccinated pigs (Figure 3B). Post-challenge, both doses of LS-P2-VP8* vaccine primed for significantly higher serum IgA against P[8] and P[6] VP8* compared to negative control and protein P2-VP8*-vaccinated pigs with the exception of IgA against P[6], where the increase induced by 30 µg LS-P2-VP8* was not significantly higher than in the protein P2-VP8* group. P[4]-specific IgA titers were significantly higher in LS-P2-VP8* (12 µg)-vaccinated pigs than the negative control pigs post-challenge (Figure 3B).
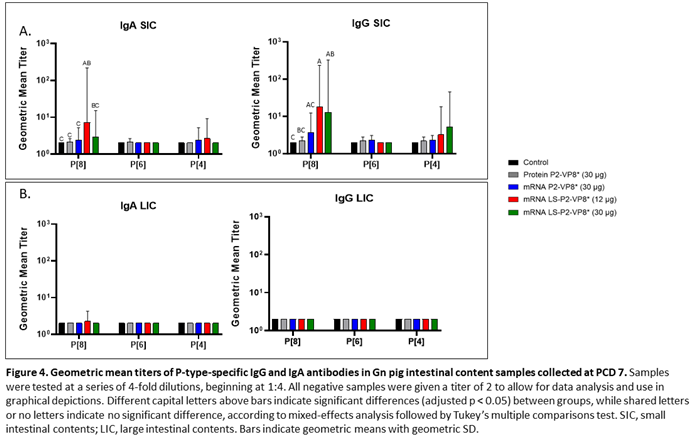
3.4. LS-P2-VP8* Vaccine Primed for Stronger P[8]-specific Small Intestinal IgA and IgG Antibody Responses Post-Challenge Compared to Other Groups
Small and large intestinal contents (SIC and LIC, respectively) were collected at euthanasia (PCD 7) for quantification of local P-type specific IgG and IgA responses induced by the vaccine candidates using the same ELISA as was used for serum samples. In the SIC, LS-P2-VP8* (12 µg) vaccine primed for significantly stronger P[8]-specific IgA and IgG responses post-challenge compared to controls and protein P2-VP8*-vaccinated pigs. (Figure 4A). There were some detectable titers of P[4]-specific IgA and IgG in the SIC of pigs vaccinated with both mRNA P2-VP8* and LS-P2-VP8* (12 µg), but not to a significant degree (Figure 4A). This is likely explained by the relatively high sequence homology between P[8] (challenge strain) and P[4] genotypes [
50]. There were no detectable IgG or IgA titers in the LIC at PCD 7, except for nominal P[8]-specific IgA titers in the LS-P2-VP8* (12 μg) group. (
Figure 4B).
3.5. LS-P2-VP8* (30 µg) Vaccine Induced Stronger HRV-specific T cell Responses in the Blood Pre-challenge and Primed for Stronger CD4+ Responses Post-challenge Compared to Other Groups
MNCs were extracted from peripheral blood pre-challenge (PCD 0) and post-challenge (PCD 7), and frequencies and total numbers of CD3+CD4+IFN-γ+ and CD3+CD8+IFN-γ+ T cells among MNCs were measured by flow cytometry and intracellular staining. There were no significant differences in the absolute numbers or frequencies among MNCs stimulated with different antigens (P[4], P[6], P[8] P2-VP8* protein or attHRV antigen). To simplify the data presentation, we calculated the means of IFN-γ+ T cell numbers or frequencies from P[4], P[6], P[8] and attHRV antigen-stimulated MNCs. The numbers or frequencies from antigen-stimulated MNCs were subtracted by the numbers or frequencies from mock-stimulated MNCs and assigned as HRV-specific IFN-γ+ T cells from each animal. Mean total numbers and frequencies of CD3+CD4+IFN-γ+ and CD3+CD8+IFN-γ+ T cells among all pigs from the five different treatment groups are presented in Figure 5 and Supplementary Figure 5.
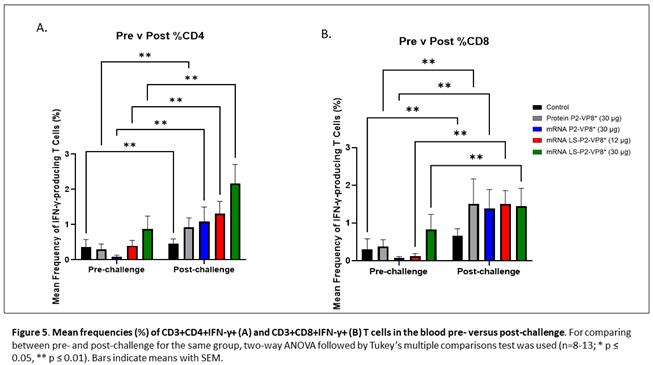
The 30 µg LS-P2-VP8* mRNA vaccine induced a greater pre-challenge T cell response that primed for a better post-challenge response of both effector and memory cells, presumably. The LS-P2-VP8* mRNA vaccine induced higher numbers (both 12 µg and 30 µg dose) and frequencies (30 µg dose only) of CD3+CD4+IFNγ+ T cell pre-challenge compared to all other groups, although they weren’t statistically significant (Supplementary Figure 5A). Mean frequencies of CD3+CD8+IFNγ+ pre-challenge in the LS-P2-VP8* (30 µg) group were higher than in all other groups, although again, they were not statistically significant (Figure 5B). All groups experienced statistically significant increases in HRV-specific mean frequencies of CD3+CD4+IFN-γ+ and CD3+CD8+IFN-γ+ T cells after challenge (Figure 5A and 5B). LS-P2-VP8* (30 µg) primed for significantly higher post-challenge mean frequencies of HRV-specific CD3+CD4+IFN-γ+ T cells compared to all other groups (Figure 5A). This trend was not detectable for CD8+ T cells, where higher mean frequencies post-challenges were induced that were comparable for all vaccinated pigs (Figure 5B).
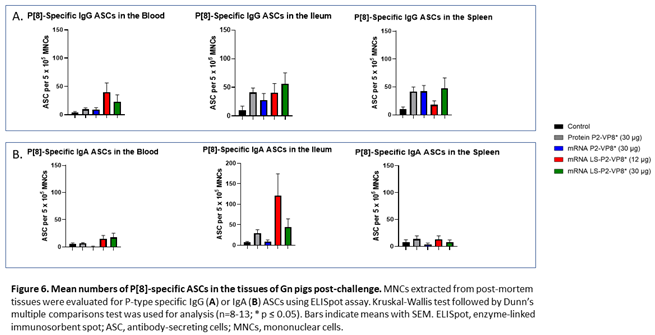
3.6. LS-P2-VP8* Vaccine Primed for Higher Blood and Intestinal ASC Responses Post-challenge Compared to the Control
MNCs extracted from blood, ileum and spleen were used to evaluate P-type-specific IgG and IgA ASC responses post-challenge by ELISpot assay. LS-P2-VP8*-vaccinated pigs had higher numbers of P[8]-specific IgG ASCs detected in the blood and exhibited a trend towards increased responses in the ileum (30 µg group) post-challenge compared to other groups although not to a significant degree. Protein P2-VP8*-vaccinated pigs had numbers comparable to mRNA P2-VP8* in the blood and spleen and to LS-P2-VP8* (12 μg)-vaccinated pigs in the ileum (Figure 6A). All vaccine groups except for the 12 µg LS-P2-VP8* group induced higher numbers of P[8]-specific IgG ASCs in the spleen compared to the negative control, indicating that vaccination was able to prime for a systemic memory B cell response upon subsequent virus exposure in the secondary lymphoid tissue (Figure 6A). Similarly, all LS-P2-VP8*-vaccinated pigs were primed for higher numbers of P[8]-specific IgA ASCs in the blood and ileum post-challenge, though these numbers were not statistically significant. (Figure 6B).
The numbers of P[6]-specific IgG ASC in ileum and spleen were similar across all groups post-challenge (Supplementary Figure 6A). A statistically not significant increase was detected for P[6]-specific IgA ASCs in the ileum upon vaccination with LS-P2-VP8* (12 μg) (Supplementary Figure 6B). Among all groups, both LS-P2-VP8* mRNA vaccine doses induced the highest numbers of P[6]- and P[4]-specific IgA ASCs in the blood post-challenge (Supplementary Figures 6B and 7B), while the 30 µg dose of LS-P2-VP8* induced the highest levels of P[4]-specific IgG ASCs in the ileum (Supplementary Figure 7A). LS-P2-VP8* (12 μg) elicited high mean numbers of P[4]-specific IgA ASCs in the ileum with high varibility (Supplementary Figure 7B).
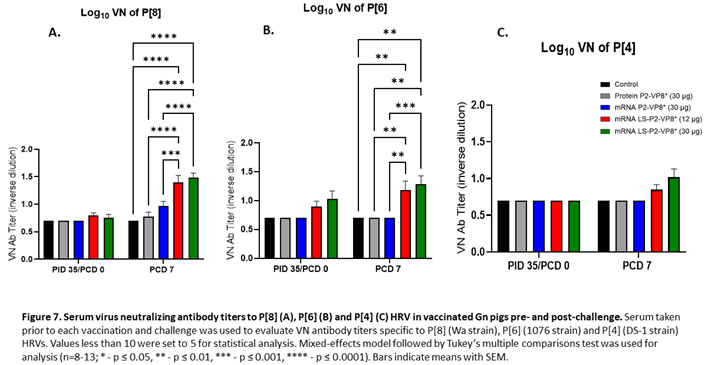
3.7. Pigs Vaccinated with LS-P2-VP8* were Primed for Significantly Higher P[8]- and P[6]-specific VN Antibody Titers Post-challenge Compared to Other Groups.
Serum samples collected pre- and post-challenge were used to evaluate P-type specific neutralizing antibody (nAb) titers in Gn pigs. None of the vaccines induced significant VN titers pre-challenge compared to the negative control group (Figure 7). However, both LS-P2-VP8* doses induced responses against P[4], P[6] and P[8] serotypes on PCD 7, while little or no responses were induced in the other groups. nAb titers against P[8]- and P[6] viruses were significantly higher in LS-P2-VP8* vaccinated animals compared to both negative control and protein P2-VP8*-vaccinated pigs (Figure 7A,B).
4. Discussion
In this study, the immunogenicity and protective efficacy of two mRNA vaccines were evaluated and compared between each other and to the protein P2-VP8* vaccine using the highly relevant Gn pig challenge model. When compared to both the protein P2-VP8* and negative control animals, the two mRNA vaccine candidates conferred improved protection from virus shedding, as evidenced by reduced duration and daily titers (AUC) measured by CCIF. Despite significant reduction in virus shedding, particularly in LS-P2-VP8*-vaccinated groups, diarrhea was still seen in almost all pigs. This is not unexpected, as it has been shown that HRV triggers the release of adenosine 5′-diphosphate (ADP) release in infected cells, resulting in dysregulation of calcium signaling in neighboring, uninfected cells [
51]. This leads to secretory diarrhea, even in the presence of minimal virus infection of the intestinal epithelial cells [
51]. Nevertheless, the LS-P2-VP8* mRNA vaccine at both doses conferred partial protection against diarrhea that was improved compared to protein P2-VP8* and negative control-vaccinated pigs upon challenge with the virulent Wa HRV. This was evidenced by the significantly reduced mean duration of diarrhea in the LS-P2-VP8* (30 µg) group, as well as the consistently lower mean daily diarrhea scores in both LS-P2-VP8* groups. Along with this, there was one pig each in both LS-P2-VP8* groups that did not experience any diarrhea at any point between PCDs 1-7. We expect with a larger sample size that there would be a greater number of LS-P2-VP8*-vaccinated pigs that experience little to no diarrhea. Both doses of the LS-P2-VP8* mRNA vaccine were also highly immunogenic and induced stronger serum IgG antibody responses specific to P[8], P[6], and P[4] HRVs both pre- and post-challenge, as well as stronger serum IgA to all three P-types post-challenge compared to all other groups. These data agree with rodent model data, which showed that the LS-P2-VP8*-based mRNA vaccines perform better in terms of immunogenicity than those encoding P2-VP8* only [
16]. It is conceivable that parenteral vaccine protection will be illustrated by markers, such as HRV-specific serum IgG and IgA [
52,
53,
54,
55] despite the historical use of intestinal IgA as a correlate of protection for live oral attenuated vaccines. Evidence supporting this idea includes the fact that hyperimmune serum has been shown to protect nonhuman primates from RV challenge, as well as the long-observed phenomenon of maternal antibody protection from natural HRV infection in the first few months of life, before vaccination can safely occur [
56,
57]. Accordingly, the correlation analyses performed in the present study indicate a link between higher serum IgG responses and lower disease burden.
Furthermore, LS-P2-VP8* vaccines were the only groups tested that were able to prime for low levels of P[8]-specific intestinal IgA and IgG responses post-challenge. Titers in this compartment were likely undetectable and low pre-and post-challenge, respectively, since inducing mucosal IgA through the use of a parenteral vaccine is generally difficult [
58]. These low responses may still contribute to protection since local mucosal IgA responses are a historically important correlate of protection in natural HRV infection as well as with the use of live oral attenuated vaccines [
52,
53,
54]. The immunogenicity seen, especially P[8]-specific IgA and P[8]-specific IgA ASCs in the intestine is consistent with the significant protection from virus shedding seen in the LS-P2-VP8* groups.
Along with B cells, CD8
+ T cells play a vital role in the total clearance of HRV infection and diarrhea in Gn pigs, even in the absence of B cells, as evidenced by a study that showed significant increases in both diarrhea and virus shedding severity that persisted past PCD 7 in CD8
+ T cell-deficient Gn pigs [
48]. Although we did see significant increases in all groups between pre- and post-challenge timepoints, there was no meaningful difference detectable between vaccinated groups. It is therefore reasonable to assume these groups’ post-challenge T cell populations were largely comprised of primary effector T cells stimulated by the virus challenge itself. Elucidating more specific markers for these populations would prove useful in future studies.
Interestingly, the lower dose (12 µg) of LS-P2-VP8* performed better than the higher dose (30 µg) in terms of protection from clinical symptoms and viral shedding. One reason why this may be is the lower stimulation of innate responses such as IFN-α level induced by LS-P2-VP8* (12 µg). Type I IFNs have been shown to have a double-edged effect in terms of mRNA vaccination. A dramatic systemic response is generally not preferable, as it may increase side effects of vaccinations such as headache, fever and fatigue as well as the possibility of stimulating a diffuse antiviral state, preventing antigen uptake and subsequent translation [
59]. However, a mild, local type I IFN response may be associated with stronger downstream adaptive responses, due to efficient recruitment of APCs to the site [
60]. It is important to note, however, that despite lower IFNα levels being induced by protein P2-VP8* vaccination, these did not correlate with increased immunogenicity as was seen in the LS-P2-VP8* (12 µg)-vaccinated pigs and there are likely other factors playing a larger role. This is further supported by the fact that we generally found no significant correlations between IFN-α responses and the efficacy measurements.
The protein vaccine P2-VP8* did not confer the same protection as reported in a previous Gn pig study [
10] that assessed a monovalent version of the protein vaccine P2-VP8* P[8] in a dose of 50 µg. This dose induced better protection from P[8] Wa HRV-induced diarrhea and virus shedding than the 90 µg dose of trivalent P2-VP8* protein vaccine employed in the present study, that only contains 30 µg of each VP8* antigen (P[8], P[6], P[4]), indicating a dose effect of the vaccine matching the challenge strain as a possible reason the discrepancy between both studies. The dose of 90 µg represents a full human dose (NCT04010448) and was chosen because its immunogenicity, safety and efficacy, i.e., ability to reduce fecal shedding of attenuated HRV in humans, were demonstrated in previous studies [
10,
13,
61]. The ongoing phase 3 clinical trial (NCT04010448) assessing safety and efficacy of the trivalent P2-VP8* protein vaccine met futility criteria at interim analysis as the protein vaccine demonstrated no superior efficacy to a licensed oral rotavirus vaccine [
62].
Although the mRNA candidates tested in this study were highly immunogenic and conferred significant protection from virus shedding and diarrhea, it is still unclear how these results of mRNA-based rotavirus vaccines will translate to humans. As shown here and as recently discussed [
12], differences in immune responses elicited by protein and mRNA vaccines support the hypothesis that mRNA encoded VP8* is able to elicit improved immune responses over protein-based vaccines and may therefor represent a viable approach to test in a clinical setting. Parenteral vaccines for enteric viruses, especially HRV, remains a subject of further research, and continue to show their status as a promising avenue of exploration for improving HRV vaccines.
5. Conclusions
Our study demonstrated the immunogenicity and partial protection induced by two trivalent mRNA vaccine candidates, P2-VP8* and LS-P2-VP8*, against HRV in comparison to P2-VP8* protein vaccine and an irrelevant mRNA vaccine, using the neonatal Gn pig model.
Both mRNA vaccines performed comparably or better (12 µg LS-P2-VP8*) than P2-VP8* protein vaccine concerning reduction of diarrhea and viral shedding. Analysis of humoral and cellular responses demonstrated increased values upon vaccination with LS-P2-VP8* compared to protein vaccine.
Trivalent LS-P2-VP8* mRNA vaccine, in which VP8* was engineered to express as a secreted nanoparticle yielded improved responses over trivalent P2-VP8* mRNA vaccine, which encodes for cytoplasmic, monomeric VP8*. While both vaccines reduced clinical symptoms and conferred significant protection from virus shedding, there was a trend towards improved responses for LS-P2-VP8*. In addition, LS-P2-VP8* mRNA was able to elicit higher levels of humoral and cellular responses than P2-VP8* mRNA vaccine. Overall, this study provides a proof of principle of an mRNA vaccine against a non-enveloped virus in a highly relevant infection model and may help to lay the ground for the development of a much-needed NRRV.
Supplementary Materials
The following supporting information can be downloaded at: Preprints.org.
Author Contributions
Conceptualization, Sandro Roier, Benjamin Petsch, Susanne Rauch and Lijuan Yuan; Methodology, Casey Hensley, Peng Zhou, Sofia Schnur, Charlotte Nyblade, Viviana Parreno, Annie Frazier, Maggie Frazier, Kelsey Kiley, Samantha O’Brien, Liang Yu and Lijuan Yuan; Validation, Sandro Roier and Lijuan Yuan; Formal analysis, Casey Hensley, Peng Zhou, Sofia Schnur, Charlotte Nyblade, Viviana Parreno, Bryan T Mayer, Ruizhe Wu and Celia Mahoney; Investigation, Casey Hensley, Peng Zhou, Sofia Schnur, Charlotte Nyblade, Viviana Parreno, Annie Frazier, Maggie Frazier, Kelsey Kiley, Samantha O’Brien, Liang Yu and Monica M McNeal; Resources, Sandro Roier, Benjamin Petsch, Susanne Rauch and Lijuan Yuan; Data curation, Casey Hensley, Peng Zhou, Sofia Schnur, Charlotte Nyblade, Viviana Parreno, Annie Frazier, Maggie Frazier, Kelsey Kiley, Samantha O’Brien and Liang Yu; Writing – original draft, Casey Hensley and Lijuan Yuan; Writing – review & editing, Casey Hensley, Sandro Roier, Peng Zhou, Sofia Schnur, Charlotte Nyblade, Viviana Parreno, Annie Frazier, Maggie Frazier, Kelsey Kiley, Samantha O’Brien, Liang Yu, Bryan T Mayer, Ruizhe Wu, Monica M McNeal, Benjamin Petsch, Susanne Rauch and Lijuan Yuan; Visualization, Casey Hensley, Sandro Roier, Charlotte Nyblade, Susanne Rauch and Lijuan Yuan; Supervision, Sandro Roier, Benjamin Petsch, Susanne Rauch and Lijuan Yuan; Project administration, Casey Hensley, Sandro Roier, Susanne Rauch and Lijuan Yuan; Funding acquisition, Benjamin Petsch, Susanne Rauch and Lijuan Yuan.
Funding
Funding for this study was provided by the Bill & Melinda Gates Foundation (INV-020846 and INV-027499).
Institutional Review Board Statement
All Gn pig experiments were conducted in accordance with protocols approved by Virginia Tech’s Institutional Animal Care and Use Committee (protocol #19-235 CVM).
Data Availability Statement
The authors declare that all relevant data supporting the findings of this study are available within the paper and its supplementary information files. Additional information and underlying data are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Drs. Jamie Stewart, Kevin Pelzer, Sherrie Clark-Deener, Calvin Lau and Amy Rizzo for veterinary services; and Karen Hall, Rachel McNeill and Kim Skroupa for animal care (Virginia Tech). A very special thanks goes to Holger Kanzler and Carl Kirkwood (Bill & Melinda Gates Foundation) for their expert advice and excellent support throughout the project. Thanks to Stanley Cryz, Jessica White, Kyle Lakatos and Marcus Estrada (PATH) for providing the P2-VP8* proteins and guidance on alum-adjuvantation. We acknowledge Nicole Meyer (Cincinnati Children’s Hospital Medical Center) for her assistance in performing the rotavirus neutralization assays. Thanks to Andreas Theß, Moritz Thran and Wolfgang Große (CureVac) for their support with generating mRNA constructs, Susanne Braeuer and Aniela Wochner (CureVac) for mRNA production, Alba Martinez Munuera, Annachiara Greco and Marius Busche (CureVac) for all their excellent work on project planning and communication.
Conflicts of Interest
S.Ro., B.P., and S.Ra. are or were employees of CureVac SE, Tübingen, Germany, a publicly listed company developing mRNA-based vaccines and immunotherapeutics and are inventors on several patents on mRNA vaccination and use thereof. S.Ro. and S.Ra hold shares in the company. The other authors declare no competing interests.
References
- Donato, C.M.; Bines, J.E. Rotaviruses and Rotavirus Vaccines. Pathogens 2021, 10, 959. [Google Scholar] [CrossRef]
- Cárcamo-Calvo, R.; Muñoz, C.; Buesa, J.; Rodríguez-Díaz, J.; Gozalbo-Rovira, R. The Rotavirus Vaccine Landscape, an Update. Pathogens 2021, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Mao, J.; Lei, S.; Twitchell, E.; Shiraz, A.; Jiang, X.; Tan, M.; Yuan, L. Parenterally Administered P24-VP8* Nanoparticle Vaccine Conferred Strong Protection against Rotavirus Diarrhea and Virus Shedding in Gnotobiotic Pigs. Vaccines 2019, 7, 177. [Google Scholar] [CrossRef]
- Schlake, T. Developing mRNA-vaccine technologies. RNA Biol 2012, 9, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum Vaccin Immunother 2022, 18, 2002083. [Google Scholar] [CrossRef] [PubMed]
- Gebre, M.S.; Rauch, S.; Roth, N.; Yu, J.; Chandrashekar, A.; Mercado, N.B.; He, X.; Liu, J.; McMahan, K.; Martinot, A.; et al. Optimization of non-coding regions for a non-modified mRNA COVID-19 vaccine. Nature 2022, 601, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.; et al. CVnCoV and CV2CoV protect human ACE2 transgenic mice from ancestral B BavPat1 and emerging B.1.351 SARS-CoV-2. Nat Commun 2021, 12, 4048. [Google Scholar] [CrossRef] [PubMed]
- Roth, N.; Schön, J.; Hoffmann, D.; Thran, M.; Thess, A.; Mueller, S.O.; Petsch, B.; Rauch, S. Optimised Non-Coding Regions of mRNA SARS-CoV-2 Vaccine CV2CoV Improves Homologous and Heterologous Neutralising Antibody Responses. Vaccines 2022, 10, 1251. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Cao, D.; Jones, R.W.; Li, J.; Szu, S.; Hoshino, Y. Construction and characterization of human rotavirus recombinant VP8* subunit parenteral vaccine candidates. Vaccine 2012, 30, 6121–6126. [Google Scholar] [CrossRef] [PubMed]
- Wen, X. Inclusion of a universal tetanus toxoid CD4(+) T cell epitope P2 significantly enhanced the immunogenicity of recombinant rotavirus DeltaVP8* subunit parenteral vaccines. Vaccine 2014, 32, 4420–4427. [Google Scholar] [CrossRef]
- Crawford, S.E.; et al. Rotavirus infection. Nat Rev Dis Primers 2017, 3, 17083. [Google Scholar] [CrossRef] [PubMed]
- Roier, S.; et al. mRNA-based VP8* nanoparticle vaccines against rotavirus are highly immunogenic in rodents. NPJ Vaccines 2023, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Groome, M.J.; Fairlie, L.; Morrison, J.; Fix, A.; Koen, A.; Masenya, M.; Jose, L.; A Madhi, S.; Page, N.; McNeal, M.; et al. Safety and immunogenicity of a parenteral trivalent P2-VP8 subunit rotavirus vaccine: A multisite, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2020, 20, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Kovacs-Nolan, J.; Mine, Y. Tandem copies of a human rotavirus VP8 epitope can induce specific neutralizing antibodies in BALB/c mice. Biochim Biophys Acta 2006, 1760, 1884–1893. [Google Scholar] [CrossRef]
- Wei, Y. Biomedical Applications of Lumazine Synthase. J Pharm Sci 2018, 107, 2283–2296. [Google Scholar] [CrossRef] [PubMed]
- Roier, S.; et al. Novel mRNA-based VP8* vaccines against rotavirus are highly immunogenic in rodents. bioRxiv, 2023, 2023.03.29.534747.
- Jardine, J.; Julien, J.-P.; Menis, S.; Ota, T.; Kalyuzhniy, O.; McGuire, A.; Sok, D.; Huang, P.-S.; MacPherson, S.; Jones, M.; et al. Rational HIV immunogen design to target specific germline B cell receptors. Science 2013, 340, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Azuma, Y.; Edwardson, T.G.W.; Hilvert, D. Tailoring lumazine synthase assemblies for bionanotechnology. Chem Soc Rev 2018, 47, 3543–3557. [Google Scholar] [CrossRef] [PubMed]
- Ladenstein, R.; Morgunova, E. Second career of a biosynthetic enzyme: Lumazine synthase as a virus-like nanoparticle in vaccine development. Biotechnol Rep (Amst) 2020, 27, e00494. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; et al. Novel virus-like nanoparticle vaccine effectively protects animal model from SARS-CoV-2 infection. PLoS Pathog 2021, 17, e1009897. [Google Scholar] [CrossRef]
- Ward, L.A.; et al. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol 1996, 77, 1431–1441. [Google Scholar] [CrossRef]
- Yuan, L. Vaccine Efficacy Evaluation: The Gnotobiotic Pig Model, 1st ed.; CRC Press/Taylor and Francis Group: 2022; pp. 1–205.
- Desselberger, U.; Huppertz, H.I. Immune responses to rotavirus infection and vaccination and associated correlates of protection. J Infect Dis 2011, 203, 188–195. [Google Scholar] [CrossRef]
- Wen, X.; Cao, D.; Jones, R.W.; Hoshino, Y.; Yuan, L. Tandem truncated rotavirus VP8* subunit protein with T cell epitope as non-replicating parenteral vaccine is highly immunogenic. Hum Vaccin Immunother 2015, 11, 2483–2489. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Kang, S.-Y.; Ward, L.A.; To, T.L.; Saif, L.J. Antibody-secreting cell responses and protective immunity assessed in gnotobiotic pigs inoculated orally or intramuscularly with inactivated human rotavirus. J Virol 1998, 72, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Pabst, R. The pig as a model for immunology research. Cell Tissue Res 2020, 380, 287–304. [Google Scholar] [CrossRef]
- Twitchell, E.L.; Tin, C.; Wen, K.; Zhang, H.; Becker-Dreps, S.; Azcarate-Peril, M.A.; Vilchez, S.; Li, G.; Ramesh, A.; Weiss, M.; et al. Modeling human enteric dysbiosis and rotavirus immunity in gnotobiotic pigs. Gut Pathog 2016, 8, 51. [Google Scholar] [CrossRef]
- Melo, M.; Porter, E.; Zhang, Y.; Silva, M.; Li, N.; Dobosh, B.; Liguori, A.; Skog, P.; Landais, E.; Menis, S.; et al. Immunogenicity of RNA Replicons Encoding HIV Env Immunogens Designed for Self-Assembly into Nanoparticles. Mol Ther 2019, 27, 2080–2090. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, C.; et al. Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: A phase 1 trial. Vaccine 2021, 39, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.; Lazzaro, S.; Habbeddine, M.; Schmidt, K.E.; Baumhof, P.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Heidenreich, R.; et al. Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. NPJ Vaccines 2017, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Fix, A.D.; Harro, C.; McNeal, M.; Dally, L.; Flores, J.; Robertson, G.; Boslego, J.W.; Cryz, S. Safety and immunogenicity of a parenterally administered rotavirus VP8 subunit vaccine in healthy adults. Vaccine 2015, 33, 3766–3772. [Google Scholar] [CrossRef]
- Lakatos, K.; McAdams, D.; White, J.A.; Chen, D. Formulation and preclinical studies with a trivalent rotavirus P2-VP8 subunit vaccine. Hum Vaccin Immunother 2020, 16, 1957–1968. [Google Scholar] [CrossRef]
- Agarwal, S.; Hickey, J.M.; McAdams, D.; White, J.A.; Sitrin, R.; Khandke, L.; Cryz, S.; Joshi, S.B.; Volkin, D.B. Effect of Aluminum Adjuvant and Preservatives on Structural Integrity and Physicochemical Stability Profiles of Three Recombinant Subunit Rotavirus Vaccine Antigens. J Pharm Sci 2020, 109, 476–487. [Google Scholar] [CrossRef] [PubMed]
- McAdams, D.; Lakatos, K.; Estrada, M.; Chen, D.; Plikaytis, B.; Sitrin, R.; White, J.A. Quantification of trivalent non-replicating rotavirus vaccine antigens in the presence of aluminum adjuvant. J Immunol Methods 2021, 494, 113056. [Google Scholar] [CrossRef] [PubMed]
- McAdams, D.; Estrada, M.; Holland, D.; Singh, J.; Sawant, N.; Hickey, J.M.; Kumar, P.; Plikaytis, B.; Joshi, S.B.; Volkin, D.B.; et al. Concordance of in vitro and in vivo measures of non-replicating rotavirus vaccine potency. Vaccine 2022, 40, 5069–5078. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Jobst, P.M.; Weiss, M. Gnotobiotic Pigs: From Establishing Facility to Modeling Human Infectious Diseases, in Gnotobiotics; Trenton R. Schoeb, K.A.E., Ed.; Sarah Tenney: 2017; pp. 349–364.
- Yang, X.; Twitchell, E.; Li, G.; Wen, K.; Weiss, M.; Kocher, J.; Lei, S.; Ramesh, A.; Ryan, E.P.; Yuan, L. High protective efficacy of rice bran against human rotavirus diarrhea via enhancing probiotic growth, gut barrier function, and innate immunity. Sci Rep 2015, 5, 15004. [Google Scholar] [CrossRef] [PubMed]
- Parre, O.V.; et al. Serum and intestinal isotype antibody responses to Wa human rotavirus in gnotobiotic pigs are modulated by maternal antibodies. J Gen Virol 1999, 80, 1417–1428. [Google Scholar] [CrossRef]
- Yuan, L.; et al. Virus-specific intestinal IFN-gamma producing T cell responses induced by human rotavirus infection and vaccines are correlated with protection against rotavirus diarrhea in gnotobiotic pigs. Vaccine 2008, 26, 3322–3331. [Google Scholar] [CrossRef]
- Yuan, L.J.; et al. Systemic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. Journal of Virology 1996, 70, 3075–3083. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Saif, L.J. Induction of mucosal immune responses and protection against enteric viruses: Rotavirus infection of gnotobiotic pigs as a model. Vet Immunol Immunopathol 2002, 87, 147–160. [Google Scholar] [CrossRef]
- Saif, L.J.; et al. The gnotobiotic piglet as a model for studies of disease pathogenesis and immunity to human rotaviruses. Arch Virol Suppl 1996, 12, 153–161. [Google Scholar]
- Saif, L.; et al. Comparative studies of the pathogenesis, antibody immune responses, and homologous protection to porcine and human rotaviruses in gnotobiotic piglets. Adv Exp Med Biol 1997, 412, 397–403. [Google Scholar]
- Vega, C.G.; et al. ROTADIAL: The first nanobody-based immunoassay to detect Group A Rotavirus. J Virol Methods 2021, 298, 114279. [Google Scholar] [CrossRef]
- To, T.L.; et al. Serum and intestinal isotype antibody responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Gen Virol 1998, 79, 2661–2672. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, D.R.; Spector, D.M.; Ward, R.L. Development of an improved method for measuring neutralizing antibody to rotavirus. J Virol Methods 1991, 33, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Kenward, M.G.; Roger, J.H. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997, 53, 983–997. [Google Scholar] [CrossRef]
- Wen, K.; et al. B-Cell-Deficient and CD8 T-Cell-Depleted Gnotobiotic Pigs for the Study of Human Rotavirus Vaccine-Induced Protective Immune Responses. Viral Immunol 2016, 29, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.S.; et al. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J Virol 2005, 79, 5428–5436. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; et al. Full genome-based classification of rotaviruses reveals a common origin between human Wa-Like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J Virol 2008, 82, 3204–3219. [Google Scholar] [CrossRef] [PubMed]
- Chang-Graham, A.L.; et al. Rotavirus induces intercellular calcium waves through ADP signaling. Science 2020, 370, 930. [Google Scholar] [CrossRef]
- Angel, J.; Steele, A.D.; Franco, M.A. Correlates of protection for rotavirus vaccines: Possible alternative trial endpoints, opportunities, and challenges. Hum Vaccin Immunother 2014, 10, 3659–3671. [Google Scholar] [CrossRef]
- Corthésy, B.; Benureau, Y.; Perrier, C.; Fourgeux, C.; Parez, N.; Greenberg, H.; Schwartz-Cornil, I. Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion. J Virol 2006, 80, 10692–10699. [Google Scholar] [CrossRef]
- Pollock, L.; Bennett, A.; Jere, K.C.; Mandolo, J.; Dube, Q.; Bar-Zeev, N.; Heyderman, R.S.; A Cunliffe, N.; Iturriza-Gomara, M. Plasma Rotavirus-specific IgA and Risk of Rotavirus Vaccine Failure in Infants in Malawi. Clin Infect Dis 2022, 75, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; E Tate, J.; Leon, J.; Haber, M.J.; E Pitzer, V.; A Lopman, B. Postvaccination Serum Antirotavirus Immunoglobulin A as a Correlate of Protection Against Rotavirus Gastroenteritis Across Settings. J Infect Dis 2020, 222, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Westerman, L.E.; McClure, H.M.; Jiang, B.; Almond, J.W.; Glass, R.I. Serum IgG mediates mucosal immunity against rotavirus infection. Proc Natl Acad Sci USA 2005, 102, 7268–7273. [Google Scholar] [CrossRef] [PubMed]
- Otero, C.E.; Langel, S.N.; Blasi, M.; Permar, S.R. Maternal antibody interference contributes to reduced rotavirus vaccine efficacy in developing countries. PLoS Pathog 2020, 16, e1009010. [Google Scholar] [CrossRef] [PubMed]
- Kaul, D.; Ogra, P.L. Mucosal responses to parenteral and mucosal vaccines. Dev Biol Stand 1998, 95, 141–146. [Google Scholar] [PubMed]
- Fleming, S.B. Viral Inhibition of the IFN-Induced JAK/STAT Signalling Pathway: Development of Live Attenuated Vaccines by Mutation of Viral-Encoded IFN-Antagonists. Vaccines 2016, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- De Beuckelaer, A.; Grooten, J.; De Koker, S. Type I Interferons Modulate CD8(+) T Cell Immunity to mRNA Vaccines. Trends Mol Med 2017, 23, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Groome, M.J.; Koen, A.; Fix, A.; Page, N.; Jose, L.; A Madhi, S.; McNeal, M.; Dally, L.; Cho, I.; Power, M.; et al. Safety and immunogenicity of a parenteral P2-VP8-P[8] subunit rotavirus vaccine in toddlers and infants in South Africa: A randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2017, 17, 843–853. [Google Scholar] [CrossRef]
- Chen, J.; Grow, S.; Iturriza-Gómara, M.; Hausdorff, W.P.; Fix, A.; Kirkwood, C.D. The Challenges and Opportunities of Next-Generation Rotavirus Vaccines: Summary of an Expert Meeting with Vaccine Developers. Viruses 2022, 14, 2565. [Google Scholar] [CrossRef]
Table 1.
Allocation of Gn pigs.
Table 1.
Allocation of Gn pigs.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).