Submitted:
31 January 2024
Posted:
02 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Amphiphilic Graft Copolymers, PECH-g-PS
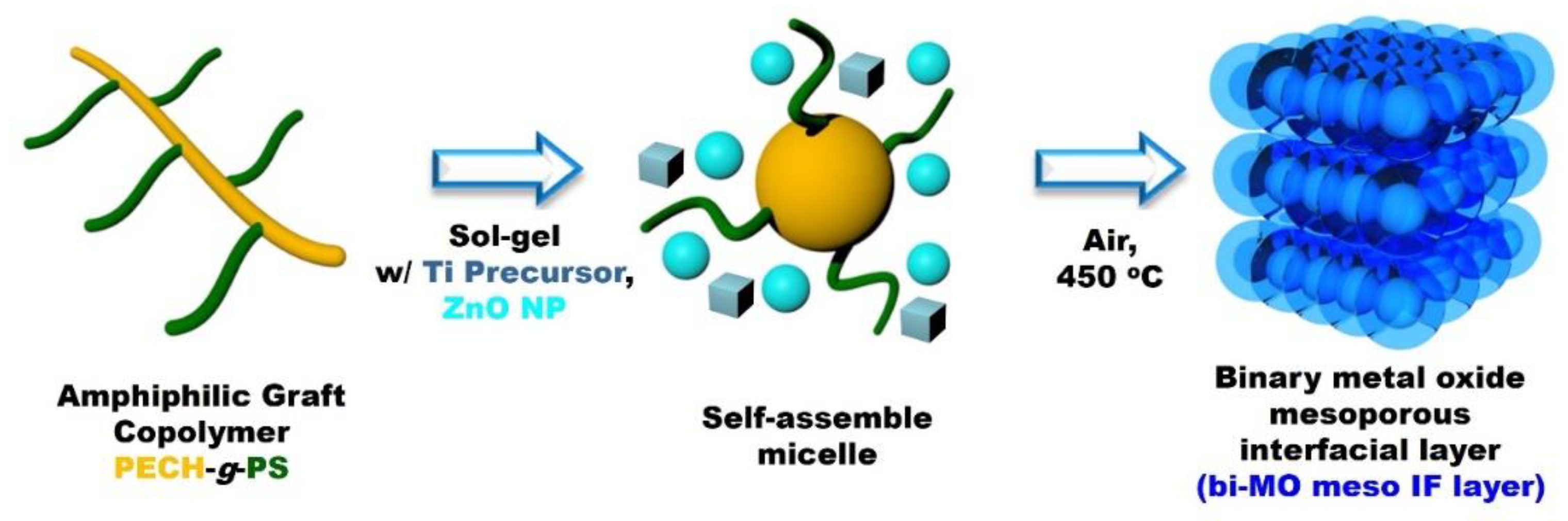
2.3. Preparation of Binary Metal Oxide Mesoporous Interfacial Layer (bi-MO Meso IF Layer)
2.4. Preparation of Photoanode
2.5. Fabrication of DSSCs
2.6. Characterization
2.7. Measurement of Dye Adsorption Value
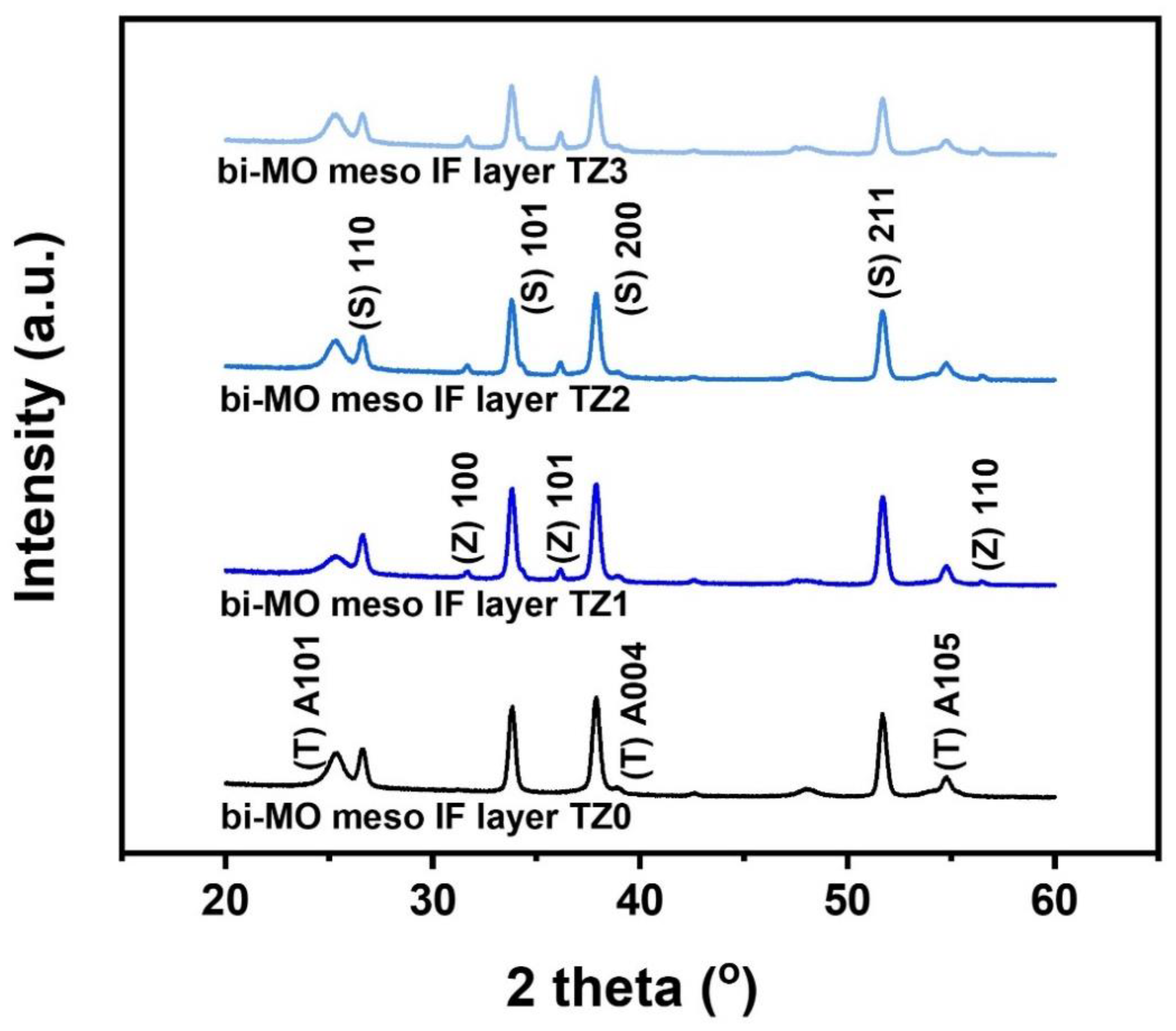
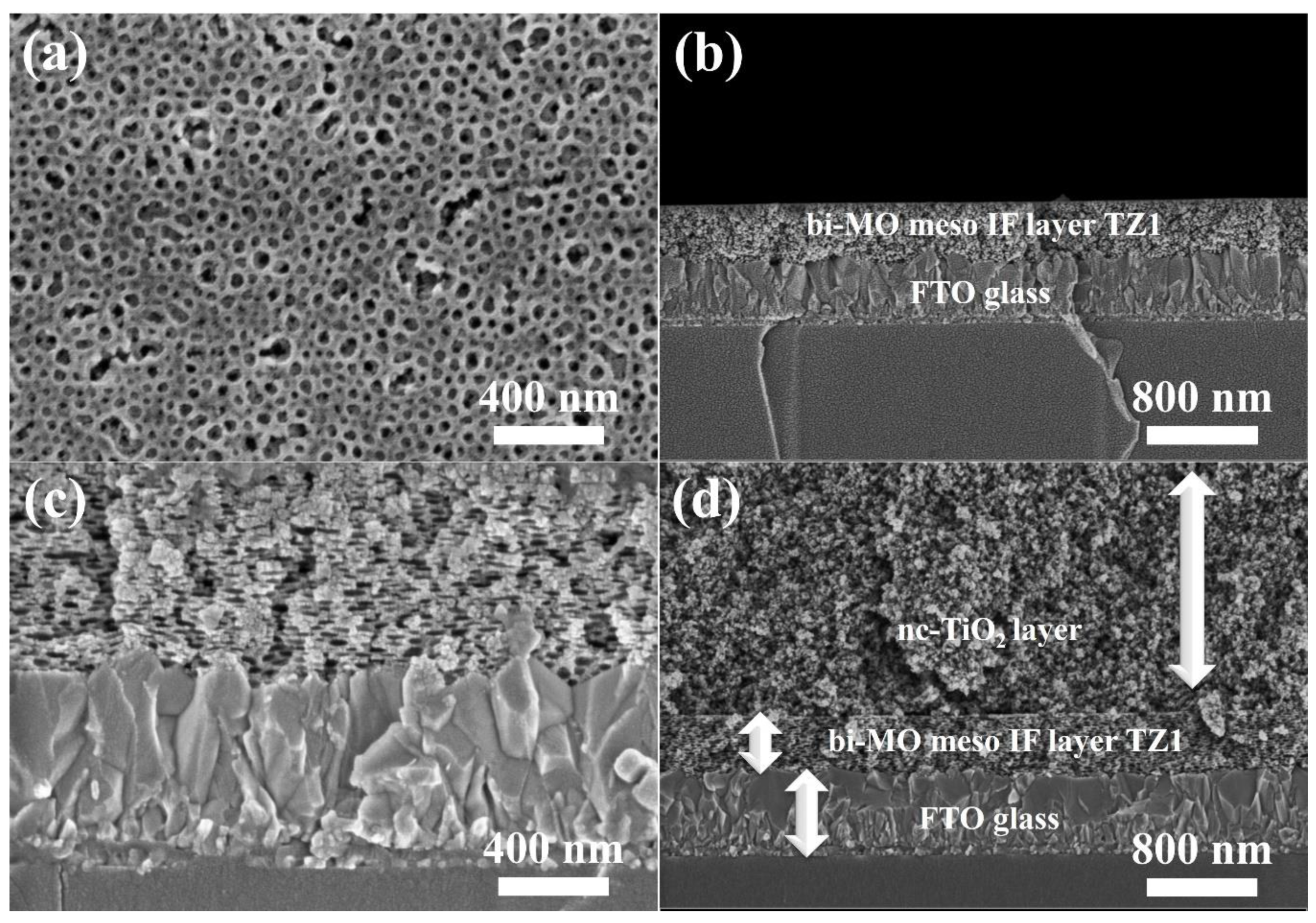
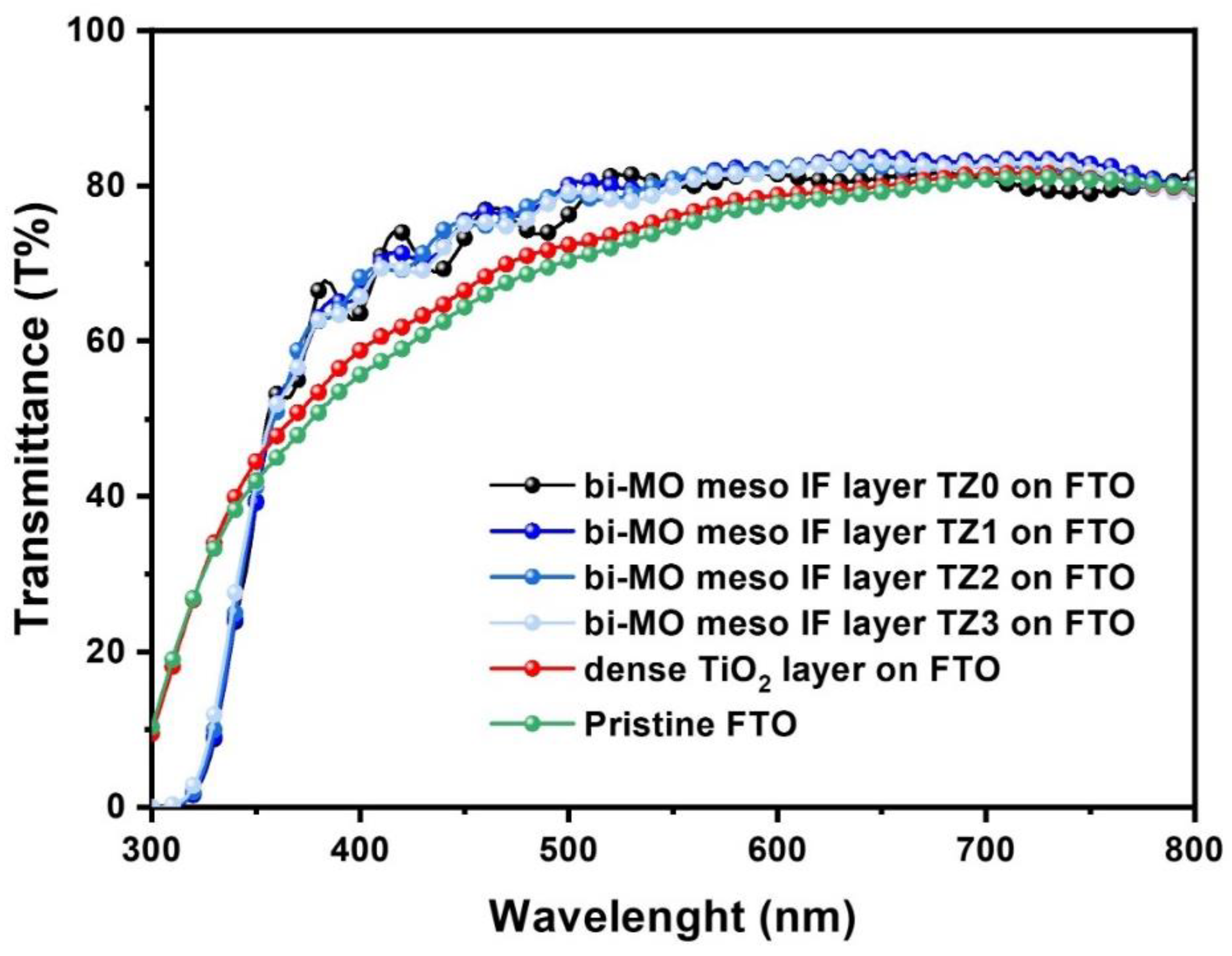
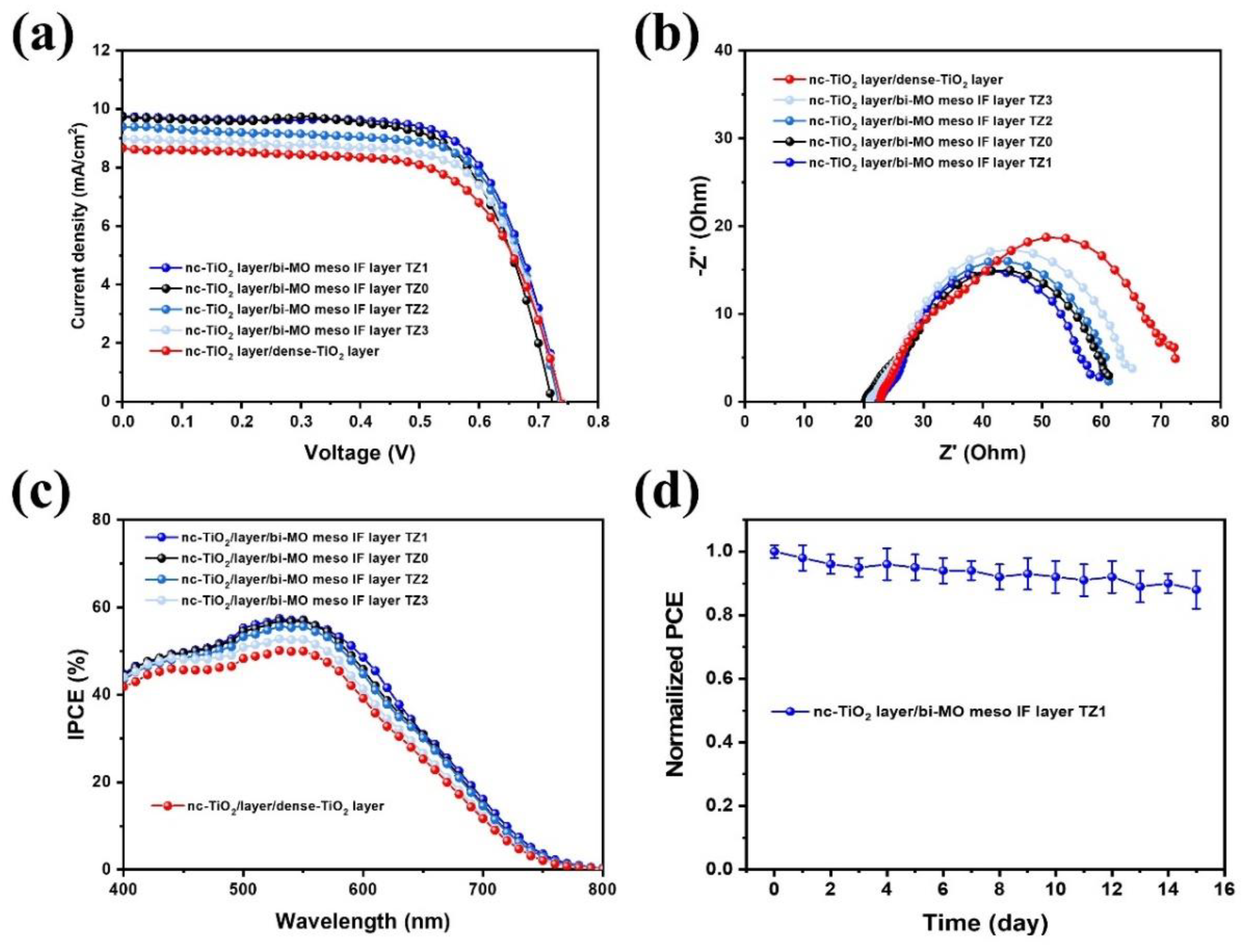
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O'regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films, Nature, 1991, 353, 737. [CrossRef]
- Ye, M.; Wen, X.; Wang, M.; Iocozzia, J.; Zhang, N.; Lin, C.; Lin, Z. Recent advances in dye-sensitized solar cells: from photoanodes, sensitizers and electrolytes to counter electrodes, Mater. Today, 2015, 18, 155. [CrossRef]
- Swami, S.K.; Kumar, N.; Radu, D.R.; Cho, S.W.; Lee, J. Lithium Incorporation into TiO2 Photoanode for Performance Enhancement of Dye-Sensitized Solar Cells, ACS Appl. Energy Mater. 2023, 6, 8599. [CrossRef]
- Yavuz, C.; Ela, S. E. Fabrication of g-C3N4-reinforced CdS nanosphere-decorated TiO2 nanotablet composite material for photocatalytic hydrogen production and dye-sensitized solar cell application, J. Alloy. Compd. 2023, 936, 168209. [CrossRef]
- Alizadeh, A.; Shariatinia, Z. Auspicious energy conversion performance of dye-sensitized solar cells based on Gd2O3-impregnated SmTiO3 perovskite/TiO2 nanocomposite photoelectrodes, Electrochim. Acta. 2023, 450, 142280. [CrossRef]
- Koo, H. J.; Kim, Y. J.; Lee, Y. H.; Lee, W. I.; Kim, K.; Park, N. G. Nano-embossed Hollow Spherical TiO2 as Bifunctional Material for High-Efficiency Dye-Sensitized Solar Cells. Adv. Mater., 2008, 20, 195. [CrossRef]
- Yang, S. C.; Yang, D. J.; Kim, J.; Hong, J. M.; Kim, H. G.; Kim, I. D.; Lee, H. Hollow TiO2 Hemispheres Obtained by Colloidal Templating for Application in Dye-Sensitized Solar Cells. Adv. Mater., 2008, 20, 1059.
- Arthi, G.; Archana, J.; Navaneethan, M.; Ponnusamy, S.; Hayakawa, Y.; Muthamizhchelvan, C.; Ramaraj, S. G. Solvothermal synthesis of 3D hierarchical rutile TiO2 nanostructures for efficient dye-sensitized solar cells, Mater. Lett., 2023, 337, 133961. [CrossRef]
- Wang, D.; Li, Y.; Ding, Y.; Jia, X.; Zhong, D.; Zhang, X.; Zhao, J.; Fang, Y. Facile Synthesis of a Multifunctional SnO2 Nanoparticles/Nanosheets Composite for Dye-Sensitized Solar Cells, ACS Omega, 2023, 8, 44578. [CrossRef]
- Vasanthapriya, R.; Neelakandeswari, N.; Uthayarani, K.; Chitra, M. Parthenium hysterophorous flower template assisted SnO2 nanostructure as photoanode for dye sensitized solar cell, Chem. Phys. Impact, 2023, 7, 100308. [CrossRef]
- Park, J.T.; Lee, C.S.; Kim, J.H. One-pot synthesis of hierarchical mesoporous SnO2 spheres using a graft copolymer: enhanced photovoltaic and photocatalytic performance, RSC Adv., 2014, 4, 31452. [CrossRef]
- Sheikh, A.; Soni, K.; Brajpuriya, R.; Lakshmi, N. Investigation of the structural and electrochemical properties of a ZnO-SnO2 composite and its electrical properties for application in dye-sensitized solar cells. New J. Chem., 2023, 47, 7346.
- Chen, W.; Qiu, Y.; Zhong, Y.; Wong, K. S.; Yang, S. High-Efficiency Dye-Sensitized Solar Cells Based on the Composite Photoanodes of SnO2 Nanoparticles/ZnO Nanotetrapods. J. Phys. Chem. A, 2009, 114, 3127. [CrossRef]
- Chae, Y.; Park, J. T.; Koh, J. K.; Kim, J.H.; Kim, E. All-solid, Flexible Solar Textiles Based on Dye-sensitized Solar Cells with ZnO Nanorod Arrays on Stainless Steel Wires, Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2013, 178, 1117. [CrossRef]
- Wang, Z.; Liu, Y.; Li, L.; Gao, S.; Zhu, D.; Yu, X.; Cheng, S.; Zheng, D.; Xiong, Y. An investigation of the effects of ZnO inverse opal pore size in the composite of ZnO nanorods/ZnO inverse opal on the performance of quantum dot-sensitized solar cells, Dalton Trans., 2023, 52, 81. [CrossRef]
- Athithya, S.; Harish, S.; Ikeda, H.; Navaneethan, M.; Archana, J. Controlled synthesis of monodispersed ZnO nanospindle decorated TiO2 mesospheres for enhanced charge transport in dye-sensitized solar cells, CrystEngComm, 2023, 25, 3198. [CrossRef]
- Han, J.; Fan, F.; Xu, C.; Lin, S.; Wei, M.; Duan, X.; Wang, Z. L. ZnO nanotube-based dye-sensitized solar cell and its application in self-powered devices, Nanotechnology, 2010, 21, 405203. [CrossRef]
- Mylsamy, S.; Govindasamy, T.; Subramanian, B. Systematic exploration of defect-rich 2D nanopetal assembled 3D ZnO nanoflowers for improved photocurrent generation and photocatalytic performance. J. Environ. Chem. Eng. 2024, 12, 111700. [CrossRef]
- Kilic, B.; Günes, T.; Besirli, I.; Sezginer, M.; Tuzemen, S. Construction of 3-dimensional ZnO-nanoflower structures for high quantum and photocurrent efficiency in dye sensitized solar cell. Appl. Surf. Sci., 2014, 318, 32.
- Mir, N.; Salavati-Niasari, M.; Davar, F. Preparation of ZnO nanoflowers and Zn glycerolate nanoplates using inorganic precursors via a convenient rout and application in dye sensitized solar cells. Chem. Eng. J., 2012, 181, 779. [CrossRef]
- Aseena, S.; Nelsa, A.; Babu, V.S.; Beena, S. Effect of Carbon Nanotube Content in ZnO/Carbon Nanotube Based Photoanode for Dye Sensitized Solar Cells, ECS J. Solid State Sci. Technol., 2022, 11, 061011. [CrossRef]
- Vijayanath, S.; Janaki, K.; Gopal, R.; Ragupathi, C.; Rangasamy, B.; Alam. M. M. Fabrication of highly efficient and cost-effective dye-sensitized solar cells using ZnO/MWCNT nanocomposite as photoanode, J. Solid State Electrochem., 2023, 27, 183. [CrossRef]
- Thate, A.G.; Pakhare, K.S.; Patil, S.S.; Bhuse, V.M. Fabrication of TiO2-ZnO nanocomposite photoanodes to enhance the dye-sensitized solar cell efficiency, Res. Chem. Intermed. 2023, 49, 147. [CrossRef]
- Yang, P.; Xiao, X.; Li, Y.; Ding, Y.; Qiang, P.; Tan, X.; Jin, H. Hydrogenated ZnO Core–Shell Nanocables for Flexible Supercapacitors and Self-Powered Systems, ACS Nano, 2013, 7, 2617. [CrossRef]
- Kumar, A.; Nayak, D.; Sahoo, P.; Nandi, B. K.; Saxena, V. K.; Thangavel, R. Fabrication of porous and visible light active ZnO nanorods and ZnO@TiO2 core-shell photocatalysts for self-cleaning applications, Phys. Chem. Chem. Phys., 2023, 25, 16423.
- Mahajan, P.; Datt, R.; Gupta, V.; Arya, S. Synthesis and characterization of ZnO@WO3 core/shell nanoparticles as counter electrode for dye-sensitized solar cell, Surf. Interfaces, 2022, 30, 101920.
- Kanmani, S. S.; Ramachandran, K. Synthesis and characterization of TiO2/ZnO core/shell nanomaterials for solar cell applications, Renew. Energy, 2012, 43, 149.
- Chandiran, A. K.; Abdi-Jalebi, M.; Nazeeruddin, M. K.; Grȧtzel, M. Analysis of Electron Transfer Properties of ZnO and TiO2 Photoanodes for Dye-Sensitized Solar Cells, ACS Nano, 2014, 8, 2261. [CrossRef]
- Anta, J. A.; Guillén, E.; Tena-Zaera, R. Electron Transport and Recombination in ZnO-Based Dye-Sensitized Solar Cells, J. Phys. Chem. C, 2012, 116, 11413.
- Park, J. T.; Koh, J. H.; Seo, J. A.; Roh, D. K.; Kim, J. H. Templated Formation of Silver Nanoparticles Using Amphiphilic Poly(epichlorohydrine-g-styrene) Film, Macromol. Res. 2009, 17, 301. [CrossRef]
- Park, J.T.; Moon, J.; Choi, G.H.; Lim, S.M.; Kim, J.H. Facile graft copolymer template synthesis of mesoporous polymeric metal-organic frameworks to produce mesoporous TiO2:Promising platforms for photovoltaic and photocatalytic applications, J. Ind. Eng. Chem. 2020, 84, 384. [CrossRef]
- Lim, J.M.; Park, J.; Park, J.T.; Bae, S. Preparation of quasi-solid-state electrolytes using a coal fly ash derived zeolite-X and -A for dye-sensitized solar cells, J. Ind. Eng. Chem. 2019, 71, 378.
- Lim, S.M.; Moon, J.; Baek, U.C.; Lee, J.Y.; Chae, Y.; Park, J.T. Shape-Controlled TiO2 Nanomaterials-Based Hybrid Solid-State Electrolytes for Solar Energy Conversion with a Mesoporous Carbon Electrocatalyst, Nanomaterials 2021, 11, 913. [CrossRef]
- Wang, Z. S.; Kawauchi, H.; Kashima, T.; Arakawa, H. Significant influence of TiO2 photoelectrode morphology on the energy conversion efficiency of N719 dye-sensitized solar cell, Coord. Chem. Rev., 2004, 248, 1381. [CrossRef]
- Park, J.T.; Koh, J.H.; Seo, J.A.; Cho, Y.S.; Kim, J.H. Synthesis and characterization of TiO2/Ag/polymer ternary nanoparticles via surface-initiated atom transfer radical polymerization, Appl. Surf. Sci. 2011, 257, 8301. [CrossRef]
- Park, J.T.; Ahn, S.H.; Roh, D.K.; Lee, C.S.; Kim, J.H. Multifunctional Organized Mesoporous Tin Oxide Films Templated by Graft Copolymers for Dye-Sensitized Solar Cells, ChemSusChem 2014, 7, 2037.
- Zheng, S.; Li, X.; Zhang, J.; Wang, J.; Zhao, C.; Hu, X.; Wu, Y.; He, Y. One-step preparation of MoOx/ZnS/ZnO composite and its excellent performance in piezocatalytic degradation of Rhodamine B under ultrasonic vibration, J. Environ. Sci. 2023, 125, 1.
- Nussbaumer, R. J.; Caseri, W. R.; Smith, P.; Tervoort, T. Polymer-TiO2 Nanocomposites: A Route Towards Visually Transparent Broadband UV Filters and High Refractive Index Materials, Macromol. Mater. Eng., 2003, 288, 44.
- Gumus, C.; Ozkendir, O. M.; Kavak, H.; Ufuktepe, Y. Structural and optical properties of zinc oxide thin films prepared by spray pyrolysis method, J. Optoelectron. Adv. Mater., 2006, 8, 299.
- Mohsenzadegan, N.; Nouri, E.; Mohammadi, M. R. Efficient quasi-solid-state dye-sensitized solar cells aided by mesoporous TiO2 beads and a non-volatile gel polymer electrolyte, CrystEngComm. 2023, 25, 3210. [CrossRef]
- Wen, J.; Liu, Y.; Li, T.; Liu, C.; Wang, T.; Liu, Y.; Zhou, Y.; Li, G.; Sun, Z. Low Cost and Strongly Adsorbed Melamine Formaldehyde Sponge Electrolyte for Nontraditional Quasi-Solid Dye-Sensitized Solar Cells, ACS Appl. Energy Mater. 2023, 6, 4952. [CrossRef]
- Fang, D.; Tan, Y.; Ren, Y.; Zheng, S.; Xiong, F.; Wang, A.; Chang, K.; Mi, B.; Cao, D.; Gao, Z. Simple Solution Preparation of Cs2SnI6 Films and Their Applications in Solid-State DSSCs, ACS Appl. Mater. Interfaces. 2023, 15, 32538.
- Moon, J.; Shin, W.; Park, J. T.; Jang, H. Solid-state solar energy conversion from WO3 nano and microstructures with charge transportation and light scattering characteristics, Nanomaterials 2019, 9, 1797. [CrossRef]
- Lim, S. M.; Moon, J.; Choi, G.H.; Baek, U.C.; Lim, J.H.; Park, J. T.; Kim, J. H. Surface carbon shell-functionalized ZrO2 as nanofiller in polymer gel electrolytes-based dye-sensitized solar cells, Nanomaterials 2019, 9, 1418. [CrossRef]
- Lee, J.Y.; Choi, G.H.; Moon, J.; Choi, W.S.; Park, J.T. 1D Co4S3 Nanoneedle Array with Mesoporous Carbon Derived from Double Comb Copolymer as an Efficient Solar Conversion Catalyst, Appl. Surf. Sci. 2021, 535, 147637. [CrossRef]
- Song, E.; Moon, J.; Lee, J.Y.; Lee, C.O.; Chi, W.S.; Park, J.T. High-Voltage Solar Energy Conversion Based on a ZIF-67 Derived Binary Redox-Quasi-Solid-State Electrolyte, J. Electroanal. Chem. 2021, 893, 115264. [CrossRef]
| Photoanode |
Voc (V) |
Jscd (mA/cm2) |
Jsce (mA/cm2) |
FF |
η (%) |
dye adsorption value (nmol/cm2) |
|---|---|---|---|---|---|---|
| nc-TiO2 layer /bi-MO meso IF layer TZ0 |
0.72±0.04 | 9.7±0.5 | 9.3 | 0.68±0.02 | 4.7±0.15 | 68 |
| nc-TiO2 layer /bi-MO meso IF layer TZ1 |
0.74±0.04 | 9.8±0.5 | 9.5 | 0.69±0.02 | 5.0±0.15 | 70 |
| nc-TiO2 layer /bi-MO meso IF layer TZ2 |
0.73±0.05 | 9.4±0.6 | 9.2 | 0.69±0.03 | 4.8±0.20 | 69 |
| nc-TiO2 layer /bi-MO meso IF layer TZ3 |
0.73±0.05 | 9.0±0.7 | 8.9 | 0.68±0.02 | 4.6±0.20 | 64 |
| nc-TiO2 layer /dense-TiO2 layer |
0.72±0.04 | 8.7±0.7 | 8.6 | 0.67±0.03 | 4.2±0.20 | 55 |
| Photoanode | Rs (Ω) |
R1 (Ω) |
R2 (Ω) |
Ws (Ω) |
|---|---|---|---|---|
| nc-TiO2 layer /bi-MO meso IF layer TZ0 |
19.9±3.0 | 9.9±1.5 | 37±5.5 | 2.4±0.3 |
| nc-TiO2 layer /bi-MO meso IF layer TZ1 |
22.2±2.8 | 9.6±1.3 | 33±4.5 | 2.1±0.1 |
| nc-TiO2 layer /bi-MO meso IF layer TZ2 |
21.8±3.1 | 9.7±1.6 | 38±5.7 | 2.4±0.2 |
| nc-TiO2 layer /bi-MO meso IF layer TZ3 |
21.0±3.0 | 9.9±1.5 | 40±5.6 | 2.6±0.2 |
| nc-TiO2 layer /dense-TiO2 layer |
23.2±3.3 | 10.1±1.8 | 43±5.8 | 3.2±0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





