1. Introduction
We recently reported a novel canine lysosomal storage disease (LSD) with similarities to the neuronal ceroid lipidoses in two Dalmatian littermates with a homozygous nullifying single-base deletion and reading-frame shift in
CNP, the gene that encodes a protein with 2’,3’-cyclic-nucleotide 3’-phosphodiesterase enzyme activity (CNPase) and a structural role in myelin [
1]. The affected littermates developed slowly progressing neurodegenerative signs that were first noticed when they were a year and a half old. The neurodegenerative signs included behavioral changes, cognitive decline, incoordination, and visual impairment. Results of magnetic resonance imaging of both littermates when 5-years old were consistent with diffuse cerebrocortical, cerebellar, and brainstem atrophy. After reaching eight years of age or older, some relatives of the littermates that were heterozygous for the
CNP single-base deletion began showing neurologic signs including aggression, tremors, loss of appetite and weight, restlessness, behavioral changes, kyphosis, ataxia, sleep disturbance and loss of balance.
Due to the progression of neurodegenerative signs, the Dalmatian littermates with the homozygous deletion were euthanized when seven and eight years old. Fluorescence microscopic examination of unstained tissue from both littermates detected accumulations of cytoplasmic autofluorescent storage granules in the cerebellar cortex, cerebral cortex, optic nerve, and cardiac muscle. Immunohistochemical staining with antibodies raised to LAMP2 confirmed that the autofluorescence came from lysosomes. Electron microscopic examination of these same tissues found membrane-bounded cytoplasmic storage bodies with variable and complex ultrastructural appearances. In addition, the myelin sheaths surrounding the optic nerve axons exhibited abnormal ballooning between the layers of myelin membranes. Immunohistochemical staining with anti-CNPase antibodies produced pronounced staining in nerve fiber tracts of the cerebellum, cerebral cortex, optic nerve, and cardiac muscle from a control dog, but failed to detect CNPase antigen in the affected Dalmatian littermates [
1].
We here describe a similar disorder that has occurred in a different dog breed, the Weimaraner. In addition, we report that the likely cause of the Weimaraner disease was a homozygous missense mutation in CNP.
2. Materials and Methods
A 5-year, 1-month old neutered male Weimaraner (proband) was presented for neurological evaluation by a veterinary neurologist (MS) for an approximately 3-month history of pelvic limb ataxia, episodes of fecal incontinence, and lethargy. On neurologic examination, the proband was ambulatory with moderate paraparesis and proprioceptive pelvic limb ataxia characterized by a long-strided pelvic limb gait. A myelopathy was suspected. Standard MR images (1.5 Tesla instrument) of the thoracolumbar and cervical spine was performed and no significant spinal cord abnormalities were identified. Additional MR imaging of the brain was performed (T2-weighted sagittal, dorsal, and transverse images; T1-weighted post-gadolinium contrast sagittal, dorsal, and transverse images; FLAIR and T2-weighted gradient echo transverse images), which revealed pronounced cerebral parenchymal atrophy (
Figure 1). Cerebrospinal fluid analysis was unremarkable. Based on these findings a neurodegenerative disease process was suspected. Approximately 1.5 years after the initial onset of clinical signs, humane euthanasia was elected due to the progression of neurologic dysfunction that included increased falling, worsening fecal incontinence, cognitive decline, incoordination, decreased interest in food, changes in posture, and episodes of trance-like behavior.
Following euthanasia, the eyes, brain and heart ventricular wall were collected and preserved as described previously [
2]. Chemically fixed slices of the retinas, optic nerves, cerebral cortex, cerebellar cortex, and cardiac muscle were cryo-embedded and sectioned with a cryostat. The unstained cryostat sections were examined for autofluorescence with a Zeiss Axiophot microscope equipped with an Olympus DP72 color digital camera as described previously [
1]. Slices of fixed cerebral cortex, cerebellar cortex, and cardiac muscle were embedded in paraffin. Sections of the paraffin-embedded samples were immunostained using BioLegend anti-CNPase primary antibody (cat. no. 836403), Abcam anti-mitochondrial ATP synthase subunit c primary antibody (cat. no. ab180149), Abcam anti-ATPB primary antibody (cat. no. ab14730), Agilent Dako anti-GFAP primary antibody (cat. no. Z0334), and Fujifilm Wako anti-Iba1 primary antibody (cat. no. 019-19741). Immunostaining was performed as described previously [
1,
3]. Additional slices of these tissues and of the optic nerve were examined with electron microscopy as described previously [
1].
Genomic DNA was prepared from EDTA-anticoagulated blood using a previously described procedure [
4]. The proband’s DNA was submitted to the University of Missouri Genomics Technology Core Facility for library preparation and 2X150 bp paired-end sequencing on their Illumina NovaSeq 6000 sequencer. A previously described data-processing pipeline was used to align the sequence reads to a current canine reference genome assembly (Dog10K_Boxer_Tasha) and to analyze them with Ensembl annotation in conjunction with reads from 4,024 other whole genome sequences obtained from the NCBI Sequence Read Archive (SRA) [
1]. The SRA BioSample identifiers for all 4,025 whole genome sequences used in this analysis are listed in Supplementary File 1. The amino acid positions for canine CNPase were numbered according to ENSCAFT00000102206.
We used an allelic discrimination assay to genotype individual dogs for a candidate variant at position 20,355,460 on chromosome 9. For this assay, the sequences of the PCR primers were5’-CAGAGCTGCAGTTTCCTTTCCT-3′ and 5′-AGCGTCTTGCACTCTTGCA-3′. The competing probes sequences were 5′-VIC-TGGCCACCGTCTCCT-NFQ-3′ (reference allele) and 5′-FAM-TGGCCACCATCTTCCT-NFQ-3′ (variant allele).
4. Discussion
For diagnostic purposes, we received clinical records, postmortem tissues, and blood as a source of DNA from a Weimaraner (the proband) with an adult-onset, slowly progressive neurodegenerative disease. Examination of the postmortem tissues by fluorescence-, light-, and electron-microscopy, and immunohistochemistry suggested that the dog had an LSD similar to neuronal ceroid lipofuscinosis (NCL) [
13]; however, marked abnormalities in myelin structure distinguished the disease from most previously recognized LSDs. Examination of the proband’s whole genome sequence identified a candidate for causality: a rare homozygous missense mutation in
CNP, the gene that encodes CNPase. This variant was recognized as a plausible causal candidate because a different homozygous
CNP variant was known to cause a similar neurological disease of Dalmatian dogs that was also characterized by autofluorescent storage body accumulation and myelin abnormalities [
1]. The absence of immunohistochemical staining of CNPase antigen in brain sections from the proband strongly supported the homozygous
CNP missense mutation as the cause of the neurodegenerative disease.
Previous reports have described murine, human, and canine
CNP deficiency diseases [
1,
14,
15]. The
CNP deficiency disease in transgenic mice was first described in 2003.
Cnp nullizygous mice appeared normal through their first five months of life. After that, the mice developed progressive neurologic signs including
ataxia, gait abnormalities, weight loss, hind-limb paralysis, convulsions, kyphosis and reduced life span. Among the post-mortem lesions were generalized brain atrophy with marked white-matter loss, axonal spheroids containing multivesicular storage bodies, myelin abnormalities including enlarged myelin inner tongues and disorganized paranodal structures, and both an astrogliosis and a microgliosis [
15]. Subsequent examinations of the
Cnp nullizygous mice found that structural myelin abnormalities preceded the overt clinical signs. Specifically, paranodal disorganization was detected at three months of age [
16] and in the small-diameter fibers axonal degeneration, spheroid formation, redundant myelin loops, and swollen inner tongues (including some containing autophagic vacuoles) can be detected shortly after birth [
17].
A 2020 report described an infantile-onset, progressive neurodegenerative disease in a male child with a homozygous
CNP missense mutation identified by exome sequencing [
14]. This patient appeared to have developed normally until 16-months of age when he became abnormally irritable. After that, he exhibited delayed or regressive development. He developed microcephaly, episodic body stiffness and dystonia, scoliosis, progressive hypotonia and died from aspiration pneumonia at five years of age. Brain MRI showed markedly reduced white matter volume. The patient’s apparently normal parents and a female sibling were heterozygous for the
CNP missense mutation. The patient’s two earlier-born male siblings had similar clinical histories and died at seven and eight years of age. The genotypes of the latter siblings were not reported [
14].
The putative causal mutation in the affected dogs predicts a substitution of methionine for threonine at position 42 in the CNPase isoform 2 (position 22 in isoform 1). Based on the immunohistochemical findings, this single amino acid substitution appears to have resulted in lack of detectable CNPase protein in brain tissues from the proband. The similar human neurological disorder that results from a Ser82Leu substitution resulted in an almost complete absence of CNP protein in cultured cells from an affected patient relative to a healthy control subject [
14]. Together these findings suggest that both Thr42 and Ser82 are essential for maintaining the stability of the protein in both species (Supplementary File 2). In the human cells, inhibition of proteosome function did not rescue the lack of CNPase protein, indicating that the deficiency of the mutant protein was not due to accelerated degradation by proteosomes. Further research will be necessary to elucidate the mechanisms by which these amino acid substitutions destabilize the protein.
Mice that are heterozygous for a
Cnp knockout allele appear to be phenotypically normal when younger, but by 19 months of age these heterozygotes accumulated axonal spheroids and developed a more intense microgliosis and astrogliosis in comparison to age-matched wildtype mice [
18]. In addition, these aged heterozygotes exhibited abnormal behavior which may be reflective of depression and catatonia [
18]. These findings suggest that individuals of other species that are heterozygous for deleterious
CNP variants may be at risk for developing milder later-onset neurological signs. In the current study, our archived DNA samples from Weimaraners included eight from dogs that were at least 8 years old and heterozygous for the likely causal
CNP missense mutation. All eight exhibited hindleg weakness that was not associated with a variant in
SOD1 that underlies late-onset hindlimb weakness in many breeds [
9]. It is possible that this later-onset sign is related to the
CNP variant. It does not appear that the parents or other older relatives of the children with the
CNP-related disorder were evaluated for subtle neurological abnormalities that might be expected based on the mouse data. Further research will be necessary to determine whether human subjects heterozygous for deleterious
CNP variants develop late-onset neurological signs.
CNP encodes at least two forms of CNPase protein that have apparently unrelated functions: enzymatic catalysis of nucleoside 2′,3′-cyclic monophosphates hydrolysis to nucleoside 2′-monophosphates [
19,
20] and a structural role in myelin where CNPase accounts for approximately 5% of the myelin-associated protein in the central nervous system and 0.3% of the protein in the peripheral nervous system [
19,
20,
21]. Alternative first exons and alternative initiation start sites express transcripts that encode two major CNPase isoforms, sometimes referred to as CNPase1 and CNPase2, with identical amino acid sequences except that CNPase2 has an additional 20-amino-acid extension at the N-terminal end [
22,
23]. When unphosphorylated, the N-terminal extension functions as a mitochondrial targeting signal [
24]. Although it has been localized to the mitochondrial inner and outer membranes, the precise roles of CNPase in the mitochondria remain to be elucidated. However, studies indicate that is involved in regulating the functioning of the mitochondrial transition pore [
20]. Abnormal regulation of the mitochondrial transition pore can result in cell damage or death [
25,
26]. Thus, deficiency in CNP protein would be predicted to alter both myelin structure and mitochondrial function. Clearly the structure of the myelin sheaths surrounding axons is profoundly abnormal in both Dalmatians and Weimaraners that lack CNP as a result of different mutations, indicating that CNP protein is necessary for maintaining the normal organization of myelin sheaths. The mechanism by which it does so remains to be elucidated.
Based on ultrastructural analyses, it appears that some of the abnormal myelin is taken up into neurons and accumulates in storage bodies. Mitochondrial function was not assessed in either of the canine disorders or in the human subjects with a CNP deficiency. However, the disease-related accumulation of storage bodies of both the affected Dalmatians and Weimaraner occurs within the mitochondria-rich regions of the cardiac muscles suggesting that these inclusions may be derived from damaged mitochondria. Storage bodies in both cardiac muscle and nervous tissue of CNP-deficient dogs of both breeds contain subunit c protein from the mitochondrial inner membrane, consistent with a mitochondrial origin of at least some of the contents of the disease-related storage bodies. Abnormal or damaged mitochondria are likely incorporated into disease-related storage bodies via autophagocytosis.
The accumulation of subunit c-containing autofluorescent storage bodies as a result of CNPase deficiency resembles that which occurs in many of the NCL disorders [
13,
27,
28]. However, in the NCLs the storage body accumulation is usually linked to a mutation that results in a direct impairment of lysosomal function. The CNP protein has not been shown to directly influence lysosomal function, so the storage body accumulation that occurs as a result of CNP deficiency is likely to be secondary to formation of myelin- and mitochondrial-derived substrates that get incorporated into phagolysosomes and cannot be degraded efficiently. The accumulation of these storage bodies, in addition to myelin abnormalities and mitochondrial dysfunction, may contribute to the development the neurological signs associated with CNP deficiency. The slow progression of disease signs may be explained by a gradual accumulation of storage material in neurons over time and the effects of this accumulation on cell function.
Author Contributions
Conceptualization, G.S.J., and M.L.K.; methodology, M.K., G.B., G.S.J., and T.M..; software, G.B.; validation, T.M., G.S.J., and M.L.K.; formal analysis, G.B., R.S.C., and M.L.K.; investigation, S.C.K., G.S.J., T.M, J.G., S.G.P., and M.L.K.; resources, M.S., G.D.K. and G.S.J. data curation, G.B. and T.M.; writing—original draft preparation, S.C.K., G.S.J., G.B., M.S., G.D.K., and M.L.K.; writing—review and editing, S.C.K., G.S.J., G.B., T.M., M.S., S.G.P., J.G., G.D.K., and M.L.K.; supervision, G.S.J. and M.L.K.; project administration, G.S.J. and M.L.K.; funding acquisition, G.S.J. and M.L.K. All authors have read and agreed to the published version of the manuscript.
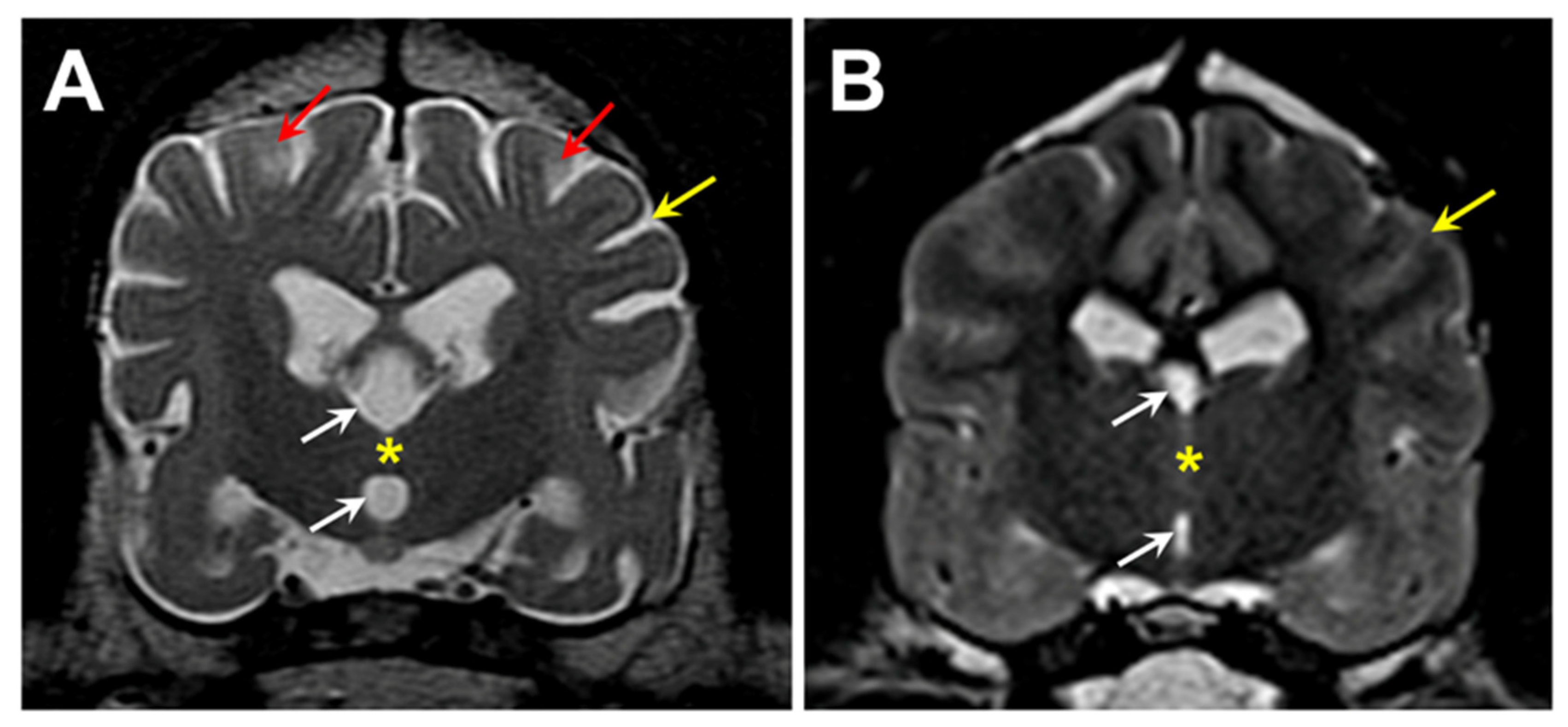
Figure 1.
T2-weighted transverse MR images of the brain from the level of the interthalamic adhesion from the proband (A) and from an age- and weight-matched control diagnosed with idiopathic epilepsy (B). The proband exhibited cerebral parenchymal atrophy characterized by an abnormally small interthalamic adhesion (yellow asterisks), an enlarged third ventricle (white arrows), and widened subarachnoid spaces (yellow arrows). Areas of increased signal intensity within the cerebral cortex parenchyma white matter were also present (red arrows).
Figure 1.
T2-weighted transverse MR images of the brain from the level of the interthalamic adhesion from the proband (A) and from an age- and weight-matched control diagnosed with idiopathic epilepsy (B). The proband exhibited cerebral parenchymal atrophy characterized by an abnormally small interthalamic adhesion (yellow asterisks), an enlarged third ventricle (white arrows), and widened subarachnoid spaces (yellow arrows). Areas of increased signal intensity within the cerebral cortex parenchyma white matter were also present (red arrows).
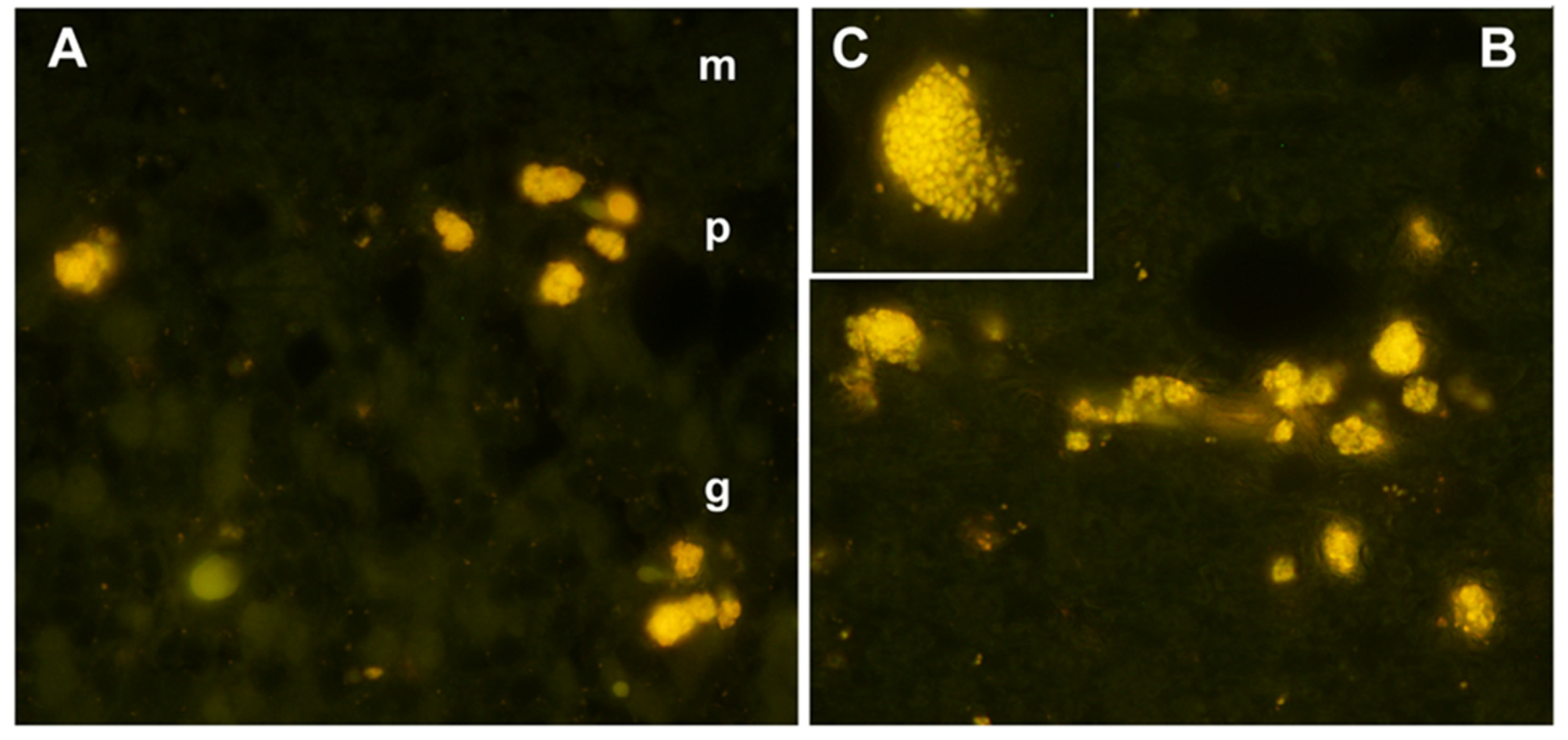
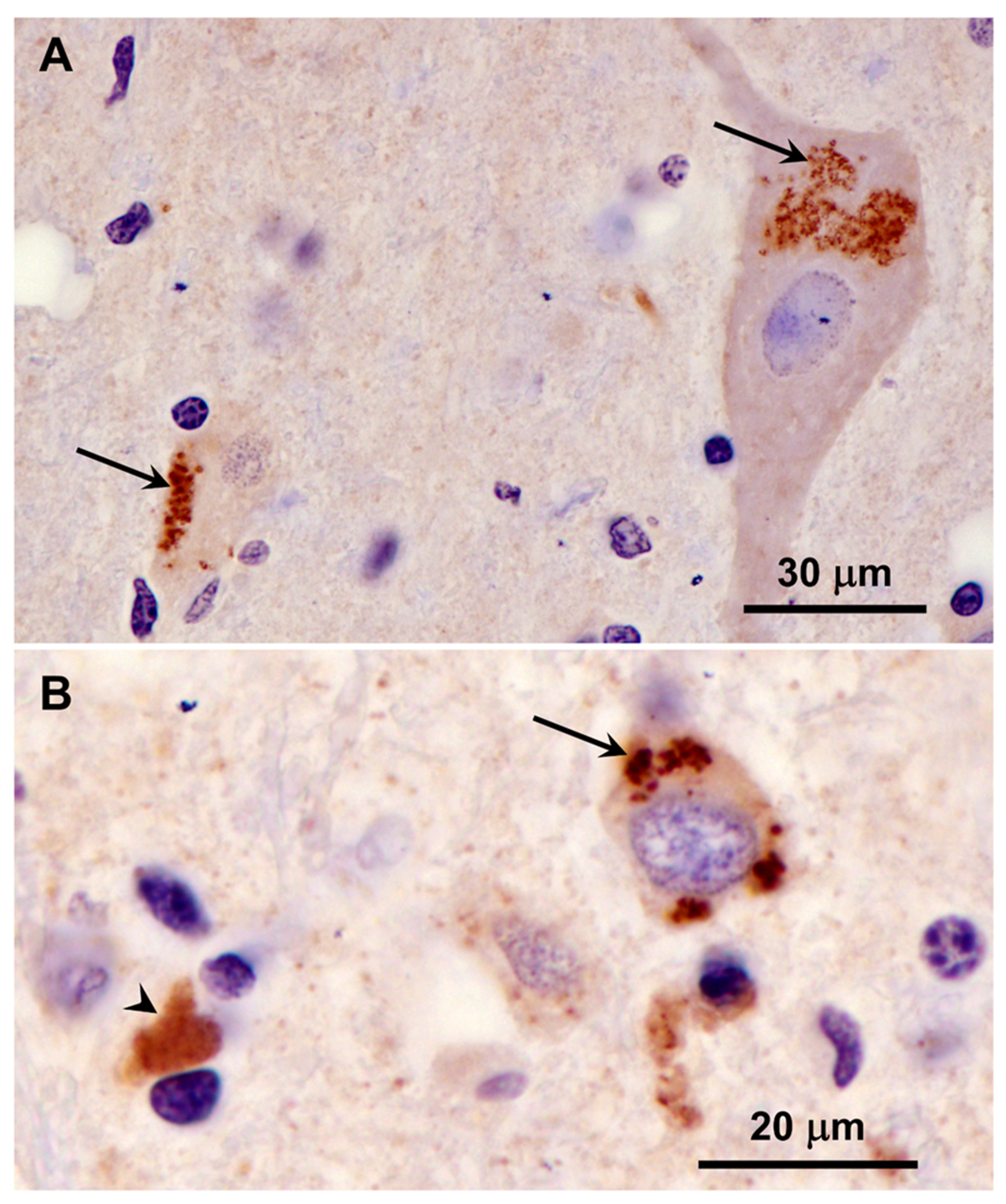
Figure 2.
Fluorescence micrographs of unstained cryosections of the cerebellar cortex (A) and cerebral cortex (B and C) of the proband showing yellow-emitting storage bodies in each tissue. In the cerebellar cortex, autofluorescent storage bodies were localized to the Purkinje cell (p) and granular (g) layers, with minimal autofluorescence in the molecular layer (m). In the cerebral cortex, cells containing the autofluorescent inclusions were distributed throughout the gray matter. In most of the affected cells, the storage bodies could be seen to consist of aggregates of autofluorescent granules (C).
Figure 2.
Fluorescence micrographs of unstained cryosections of the cerebellar cortex (A) and cerebral cortex (B and C) of the proband showing yellow-emitting storage bodies in each tissue. In the cerebellar cortex, autofluorescent storage bodies were localized to the Purkinje cell (p) and granular (g) layers, with minimal autofluorescence in the molecular layer (m). In the cerebral cortex, cells containing the autofluorescent inclusions were distributed throughout the gray matter. In most of the affected cells, the storage bodies could be seen to consist of aggregates of autofluorescent granules (C).
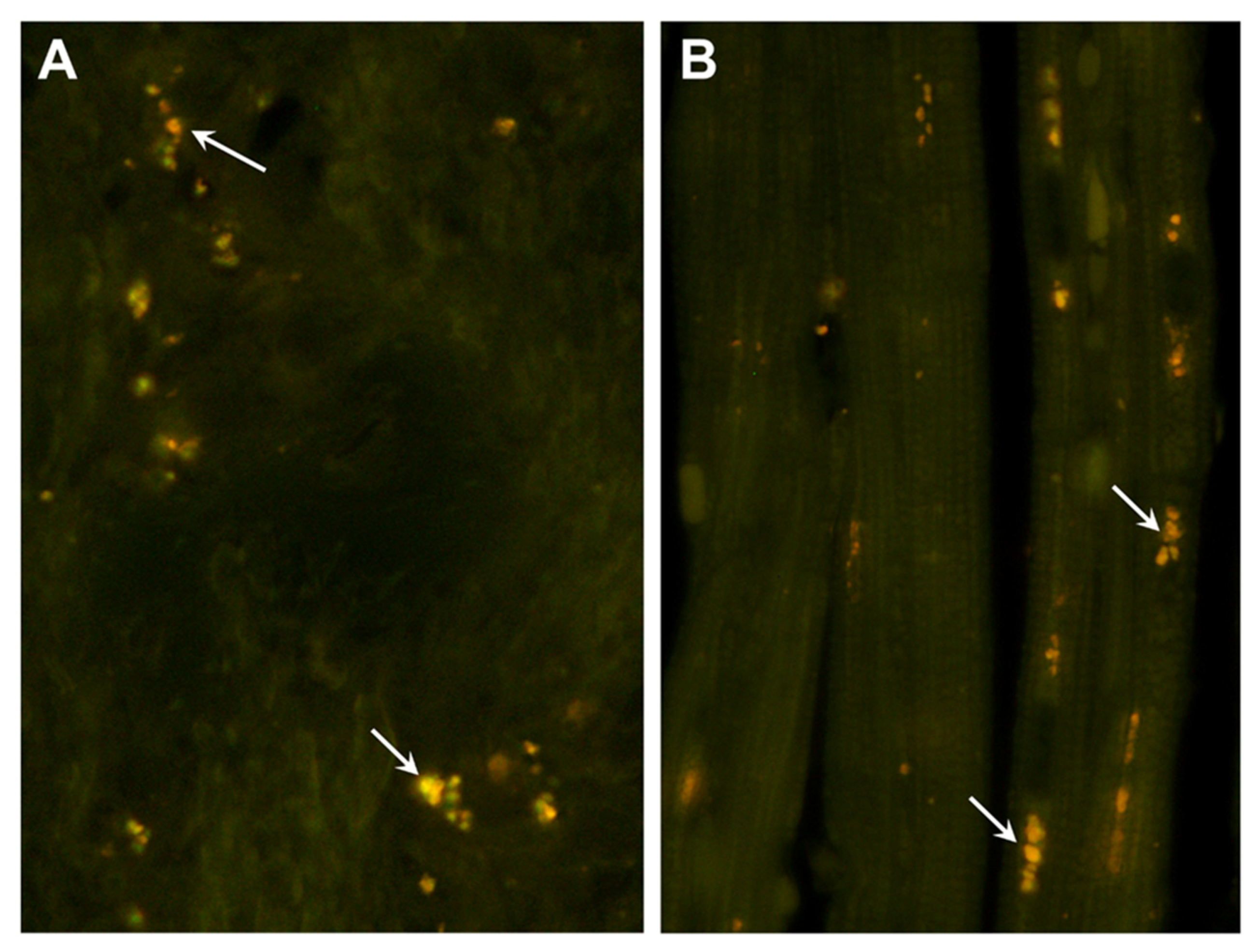
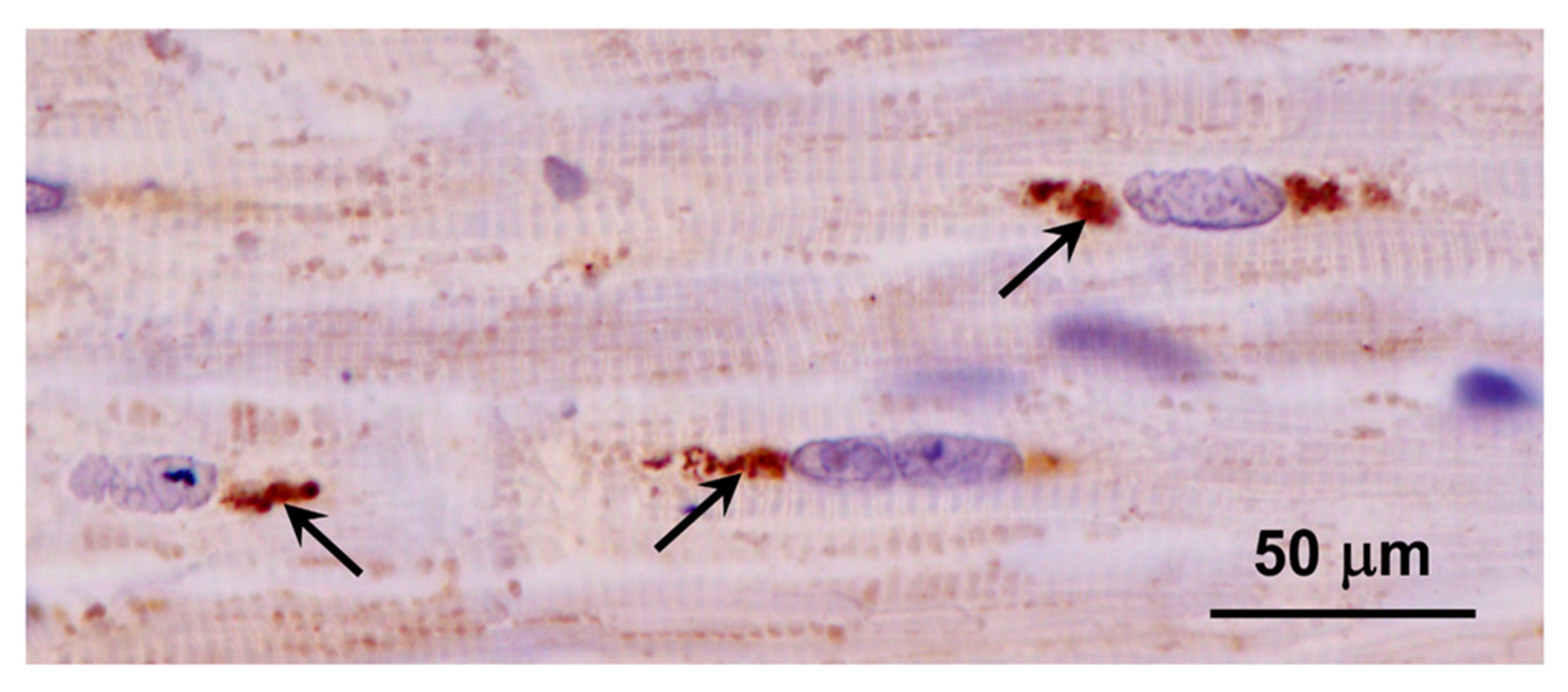
Figure 3.
Fluorescence micrographs of unstained cryosections of the optic nerve (A) and cardiac muscle (B) of the proband showing autofluorescent storage bodies in each tissue (arrows). In the optic nerve, the storage bodies consisted primarily of individual small granules that had yellow to orange fluorescence emissions. In the cardiac muscles, the autofluorescent inclusions exhibited orange emission and were arrayed in linear groupings along the long axes of the muscle fibers.
Figure 3.
Fluorescence micrographs of unstained cryosections of the optic nerve (A) and cardiac muscle (B) of the proband showing autofluorescent storage bodies in each tissue (arrows). In the optic nerve, the storage bodies consisted primarily of individual small granules that had yellow to orange fluorescence emissions. In the cardiac muscles, the autofluorescent inclusions exhibited orange emission and were arrayed in linear groupings along the long axes of the muscle fibers.
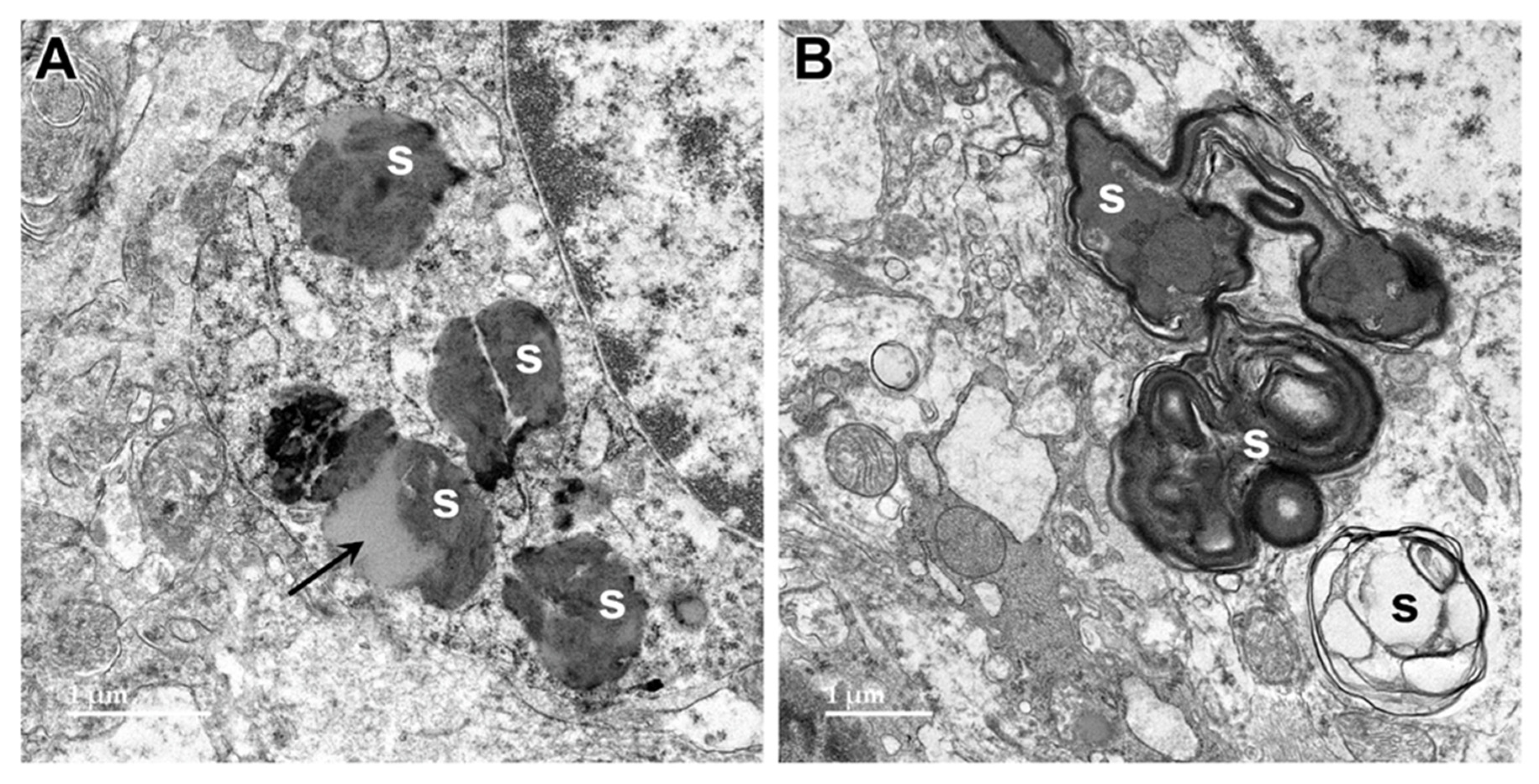
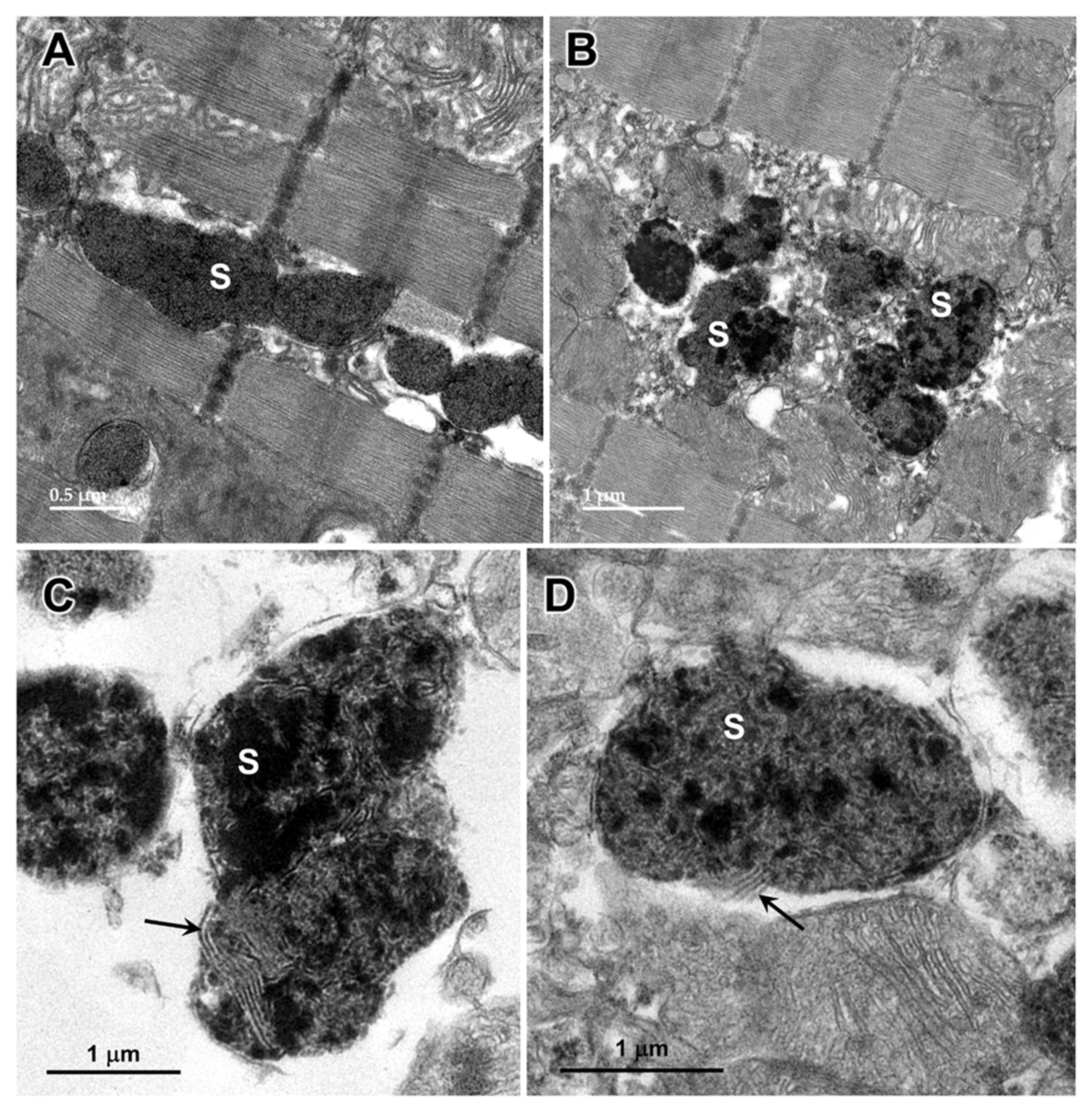
Figure 4.
Electron micrographs showing examples of storage bodies (s) in cells of the cerebellar cortex of the proband. The storage bodies were heterogenous. The contents of some storage bodies were mixtures of electron-dense and lipid-like (arrow) components (A). The contents of other storage bodies consisted primarily of layers of membrane-like materials (B).
Figure 4.
Electron micrographs showing examples of storage bodies (s) in cells of the cerebellar cortex of the proband. The storage bodies were heterogenous. The contents of some storage bodies were mixtures of electron-dense and lipid-like (arrow) components (A). The contents of other storage bodies consisted primarily of layers of membrane-like materials (B).
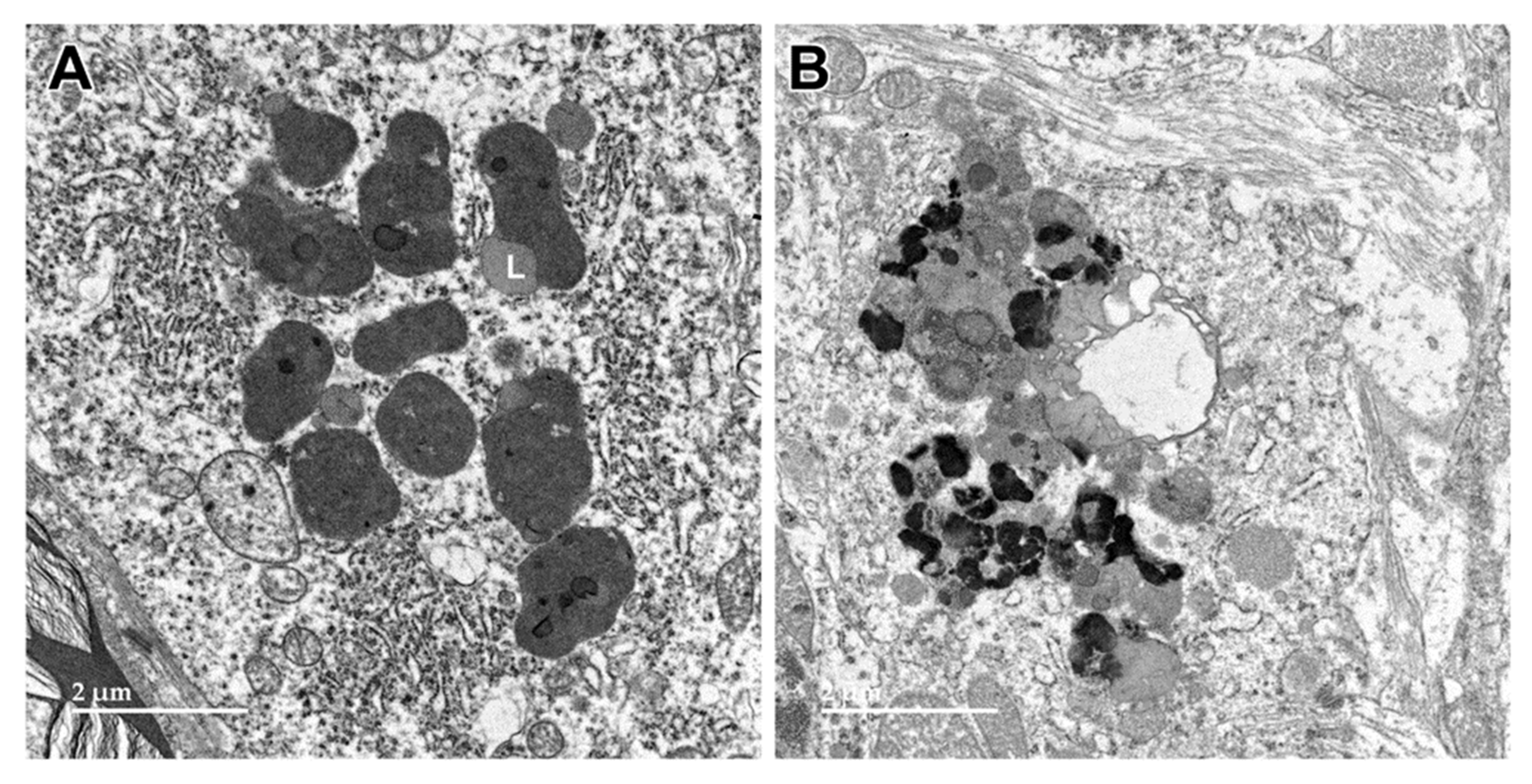
Figure 5.
Electron micrograph of a large storage body in a cell of the cerebellar cortex of the proband. This type of storage body consisted of aggregates of large numbers of smaller components, some of which were quite electron-dense (arrows), and some of which had the appearance characteristic of lipid droplets (L).
Figure 5.
Electron micrograph of a large storage body in a cell of the cerebellar cortex of the proband. This type of storage body consisted of aggregates of large numbers of smaller components, some of which were quite electron-dense (arrows), and some of which had the appearance characteristic of lipid droplets (L).
Figure 6.
Electron micrographs of storage bodies in cells of the cerebral cortex gray matter from the proband. Lipid-like components (L) were present in some of the storage bodies.
Figure 6.
Electron micrographs of storage bodies in cells of the cerebral cortex gray matter from the proband. Lipid-like components (L) were present in some of the storage bodies.
Figure 7.
Electron micrographs showing additional examples of storage bodies (s) in cells of the cerebral cortex gray matter of the proband.
Figure 7.
Electron micrographs showing additional examples of storage bodies (s) in cells of the cerebral cortex gray matter of the proband.
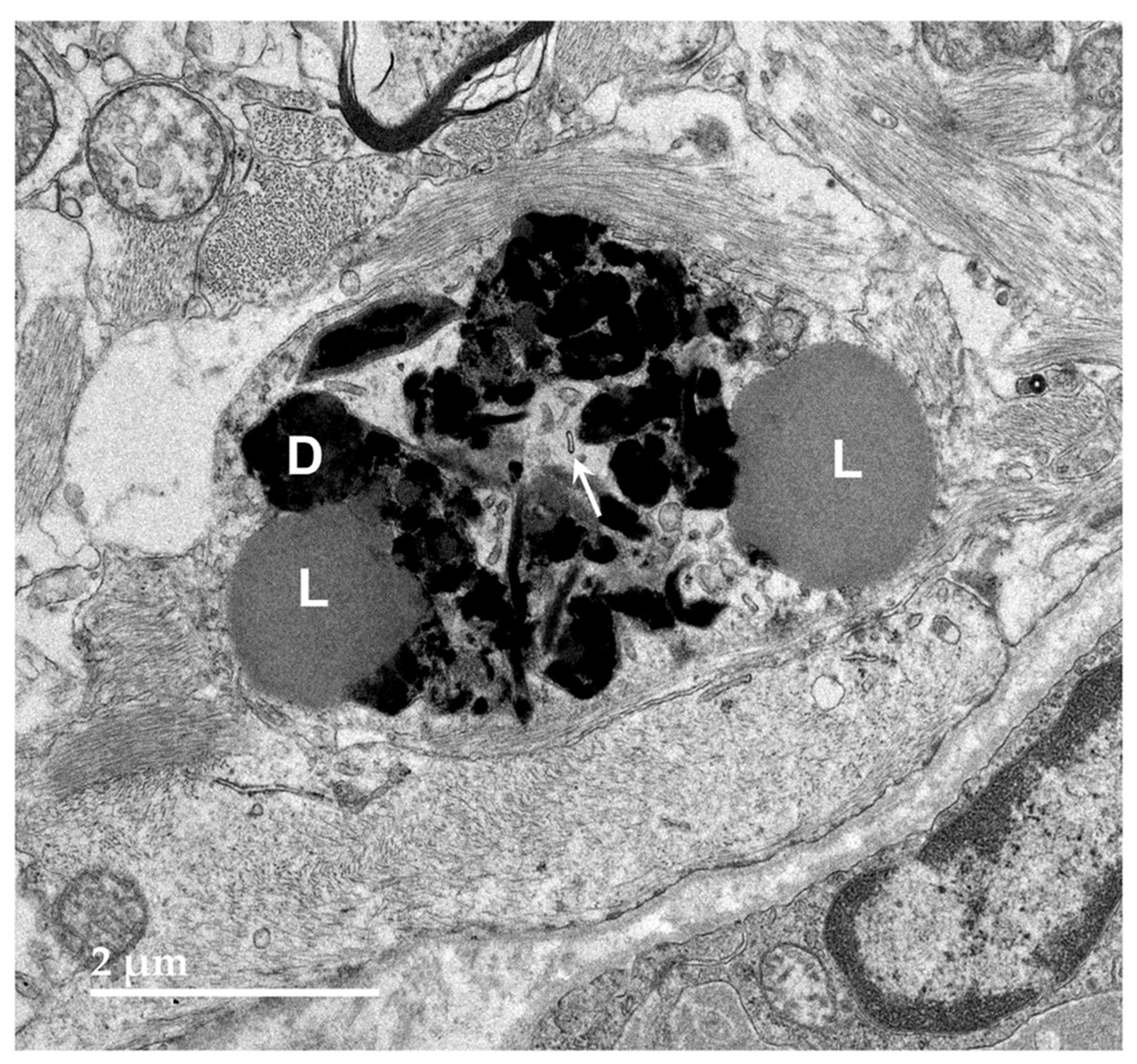
Figure 8.
Electron micrograph of an intracellular inclusion in a cell of the cerebral cortical white matter of the proband. The contents of the inclusion body were heterogenous, consisting primarily of lipid-like components (L), aggregates of very electron-dense globular structures (D), and small vesicular structures (arrow).
Figure 8.
Electron micrograph of an intracellular inclusion in a cell of the cerebral cortical white matter of the proband. The contents of the inclusion body were heterogenous, consisting primarily of lipid-like components (L), aggregates of very electron-dense globular structures (D), and small vesicular structures (arrow).
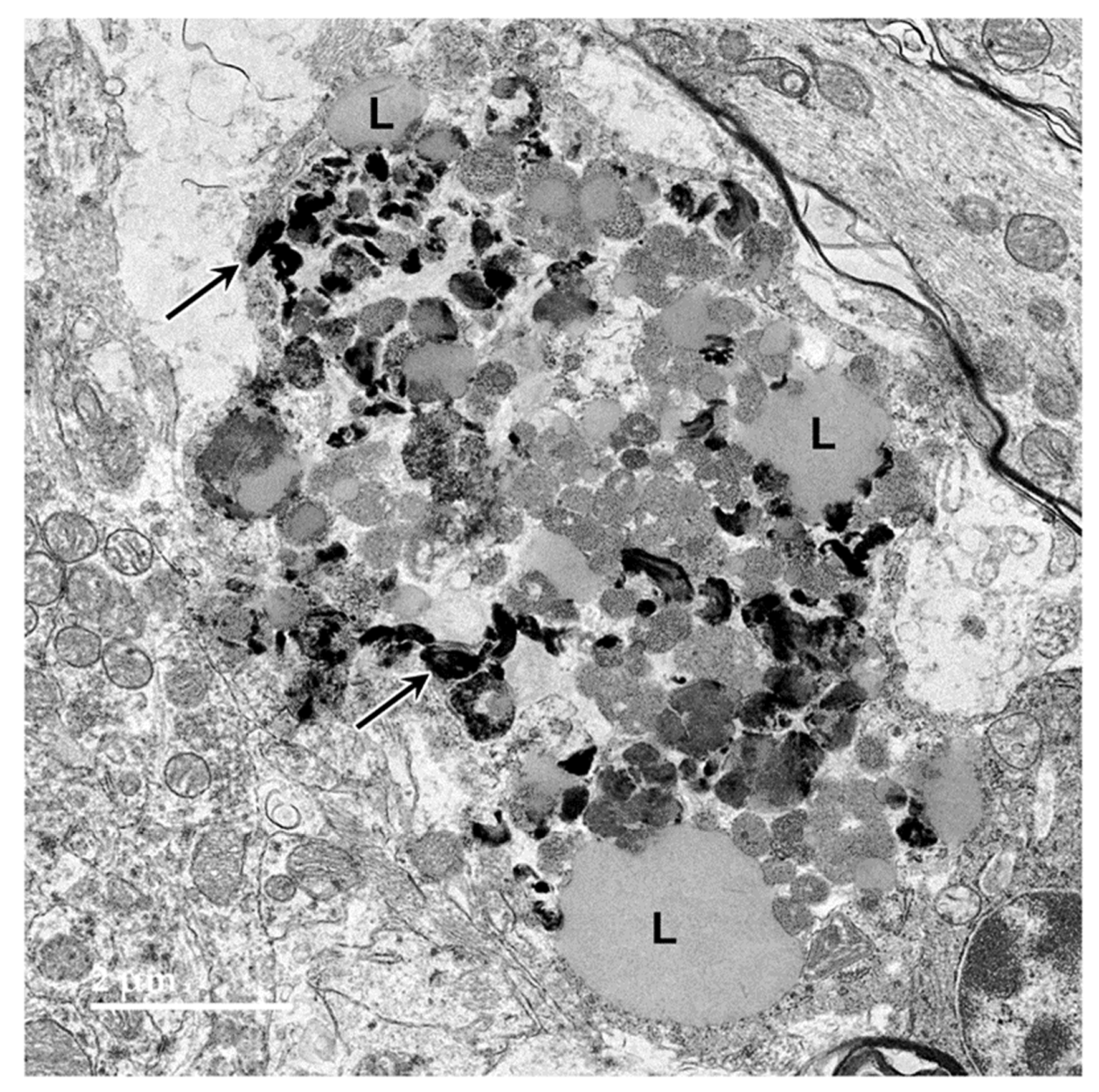
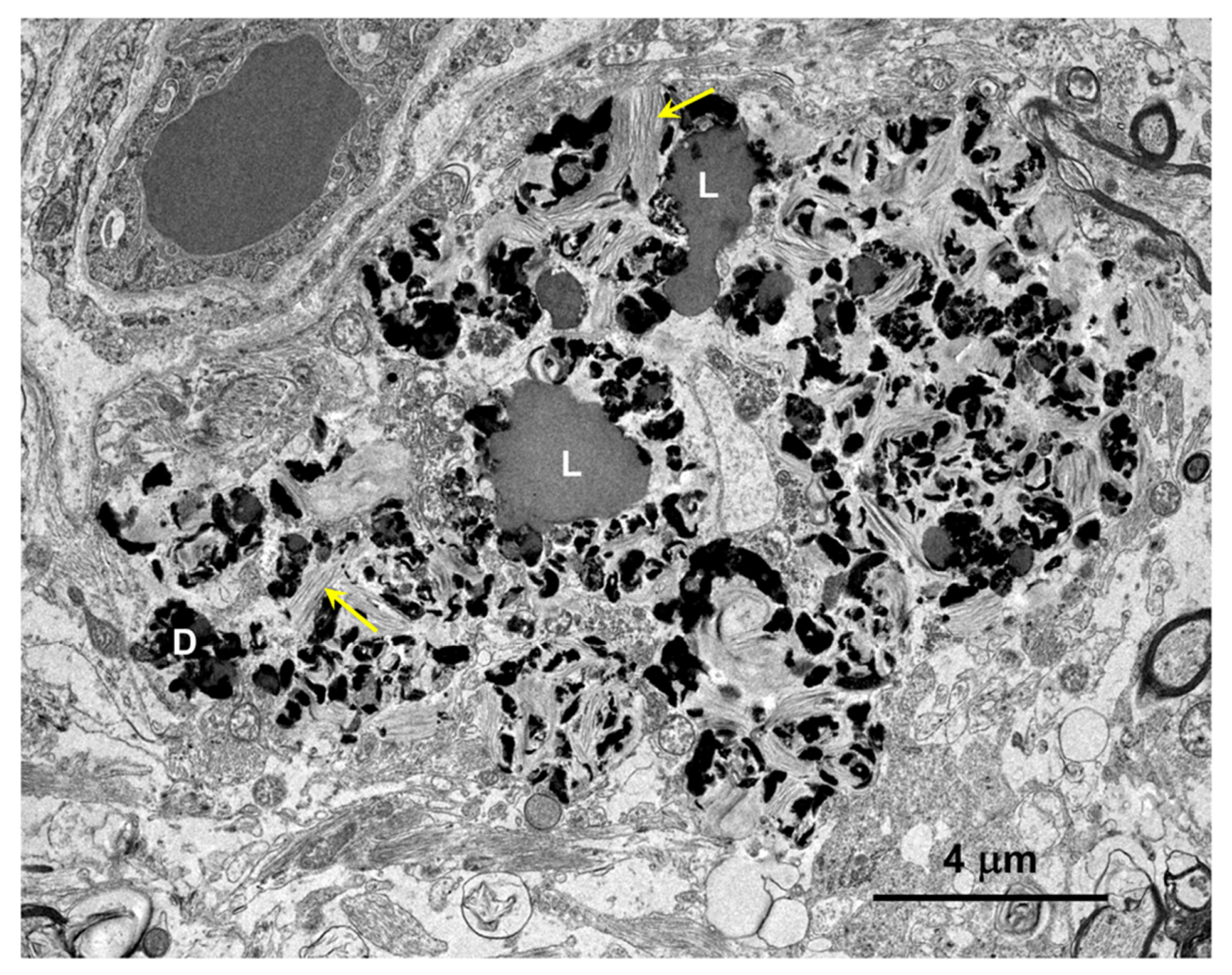
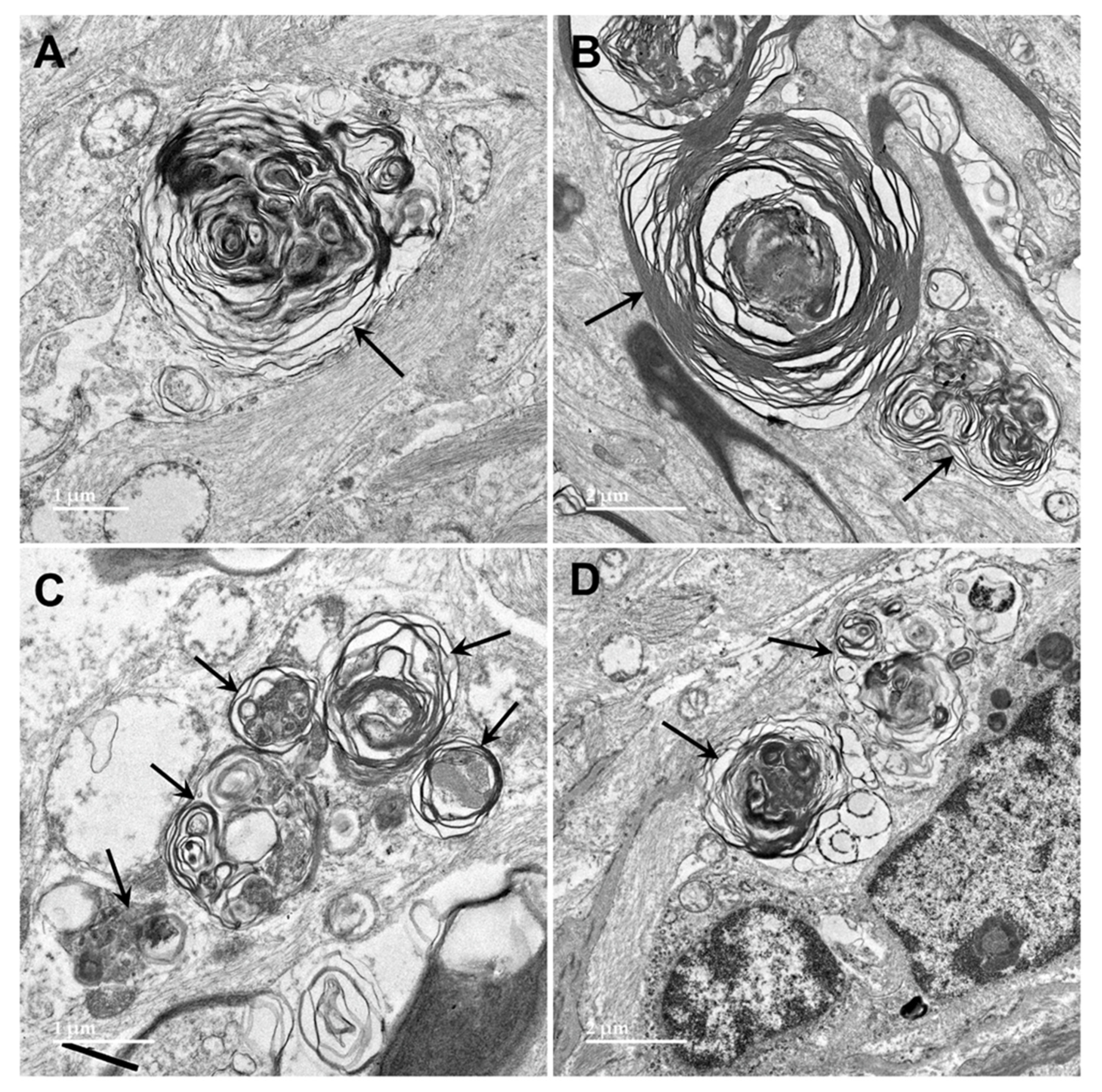
Figure 9.
Electron micrograph of a large cluster of intracellular inclusions in the cerebral cortical white matter of the proband. The contents of the inclusion bodies within the cluster were heterogenous, consisting of lipid-like components (L), aggregates of very electron-dense globular structures (D), and membrane-like components (arrows).
Figure 9.
Electron micrograph of a large cluster of intracellular inclusions in the cerebral cortical white matter of the proband. The contents of the inclusion bodies within the cluster were heterogenous, consisting of lipid-like components (L), aggregates of very electron-dense globular structures (D), and membrane-like components (arrows).
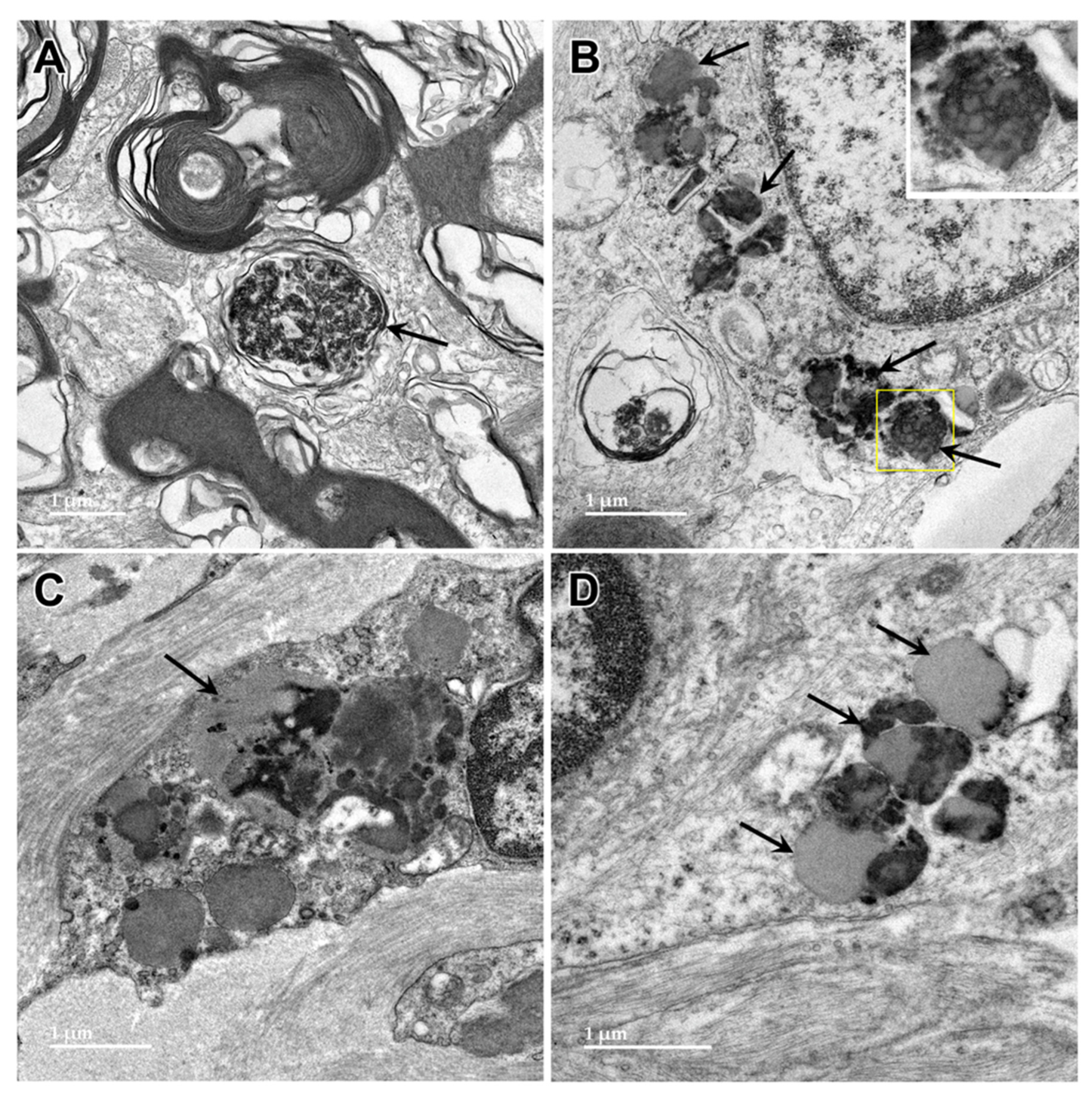
Figure 10.
Electron micrographs showing lipofuscin-like inclusions in cells of the optic nerve from the proband (arrows). The inclusion framed in yellow in (B) is shown at higher magnification in the inset. The contents of these inclusions were quite heterogeneous and included lipid-like components, vesicular structures and irregularly shaped areas of high electron density.
Figure 10.
Electron micrographs showing lipofuscin-like inclusions in cells of the optic nerve from the proband (arrows). The inclusion framed in yellow in (B) is shown at higher magnification in the inset. The contents of these inclusions were quite heterogeneous and included lipid-like components, vesicular structures and irregularly shaped areas of high electron density.
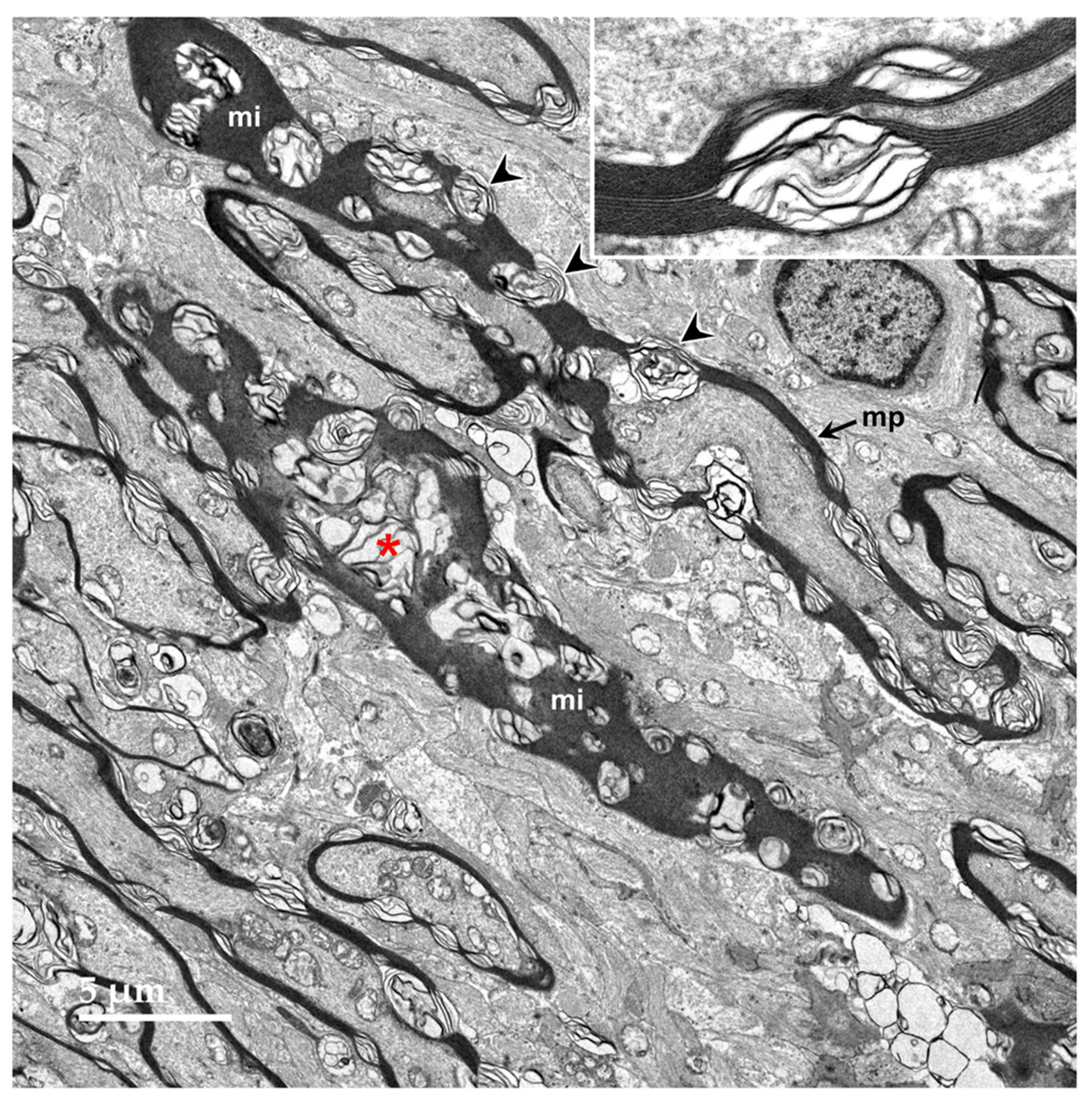
Figure 11.
Oblique longitudinal section of the optic nerve from the proband. In some areas, the myelin sheaths surrounding the axons are seen in profile where the plane of section was perpendicular to the axonal membranes (mp), and in other areas the plane of section near parallel with the myelin membranes (mi). In both orientations, areas of ballooning of the myelin sheaths could be seen (arrowheads and red asterisk). Inset shows at higher magnification where an area of tightly packed myelin membranes transitions to an area where they are ballooned apart.
Figure 11.
Oblique longitudinal section of the optic nerve from the proband. In some areas, the myelin sheaths surrounding the axons are seen in profile where the plane of section was perpendicular to the axonal membranes (mp), and in other areas the plane of section near parallel with the myelin membranes (mi). In both orientations, areas of ballooning of the myelin sheaths could be seen (arrowheads and red asterisk). Inset shows at higher magnification where an area of tightly packed myelin membranes transitions to an area where they are ballooned apart.
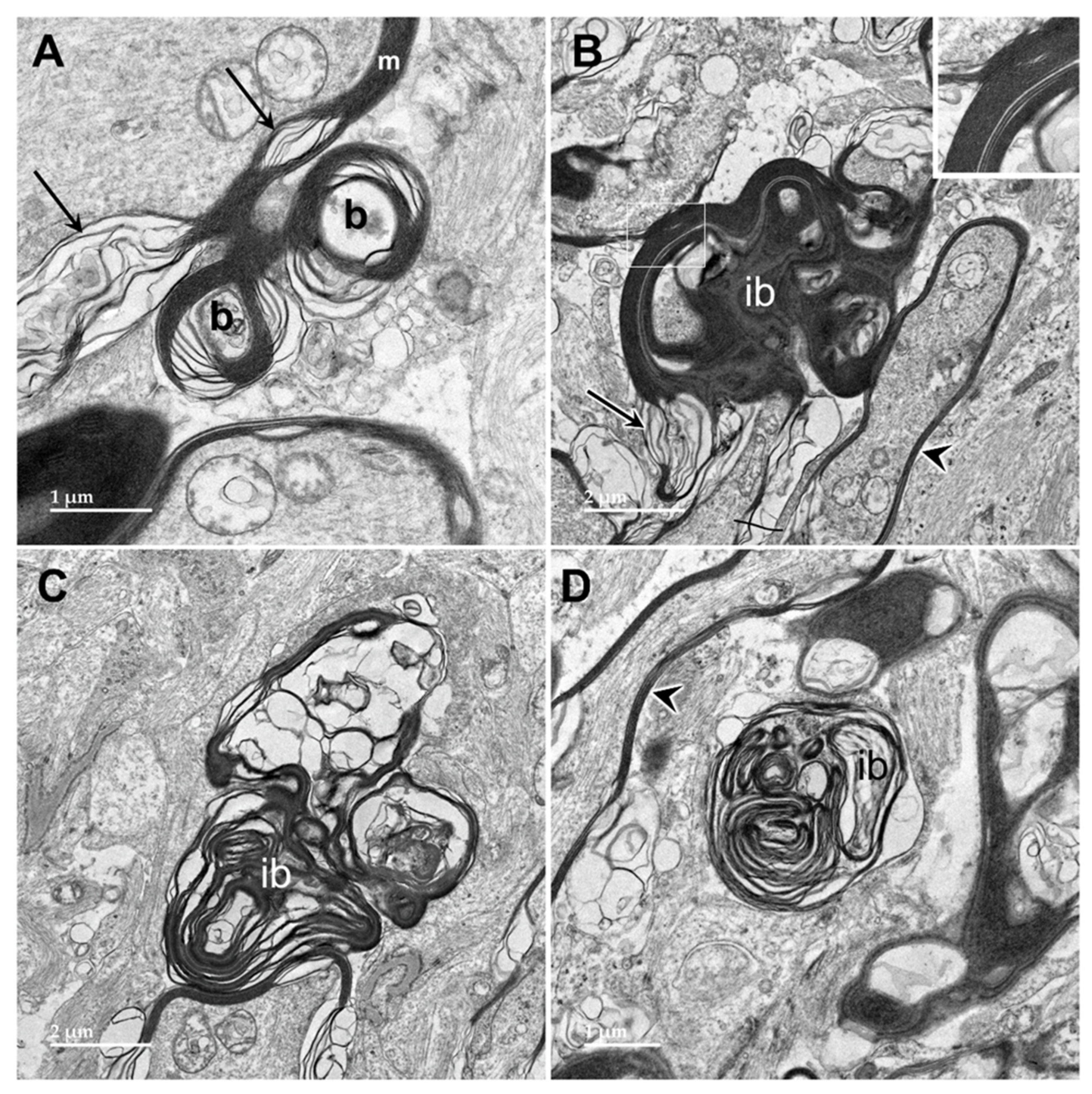
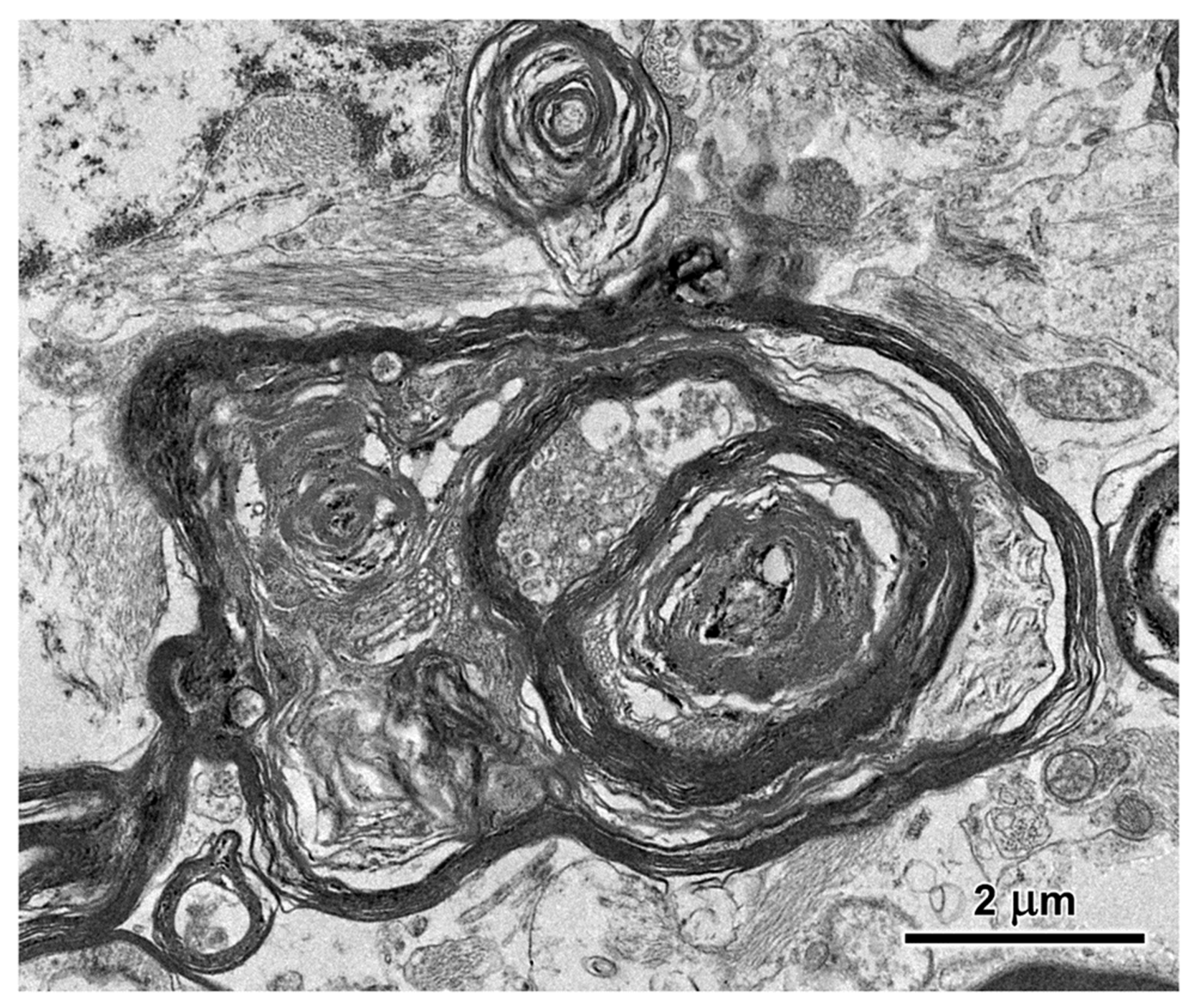
Figure 12.
Electron micrographs of longitudinal sections of the optic nerve from the proband. The myelin sheaths surrounding the axons (m in A, arrowheads in B and D) consisted of areas where the layers of membranes were tightly packed interspersed with regions of pronounced ballooning between the layers (arrows in A and B). In places, spherical buds projected out from the myelin sheaths (b in A). In other places, these buds were quite large (B) with the myelin folding back on itself to form large inclusion bodies (ib). Within these inclusions, some of the myelin-derived material retained the tight packing of normal myelin (inset in B). In other inclusion bodies, apparently derived from the myelin but separated from the sheaths, the membranous contents were more loosely packed and often formed fingerprint-like patters (C and D).
Figure 12.
Electron micrographs of longitudinal sections of the optic nerve from the proband. The myelin sheaths surrounding the axons (m in A, arrowheads in B and D) consisted of areas where the layers of membranes were tightly packed interspersed with regions of pronounced ballooning between the layers (arrows in A and B). In places, spherical buds projected out from the myelin sheaths (b in A). In other places, these buds were quite large (B) with the myelin folding back on itself to form large inclusion bodies (ib). Within these inclusions, some of the myelin-derived material retained the tight packing of normal myelin (inset in B). In other inclusion bodies, apparently derived from the myelin but separated from the sheaths, the membranous contents were more loosely packed and often formed fingerprint-like patters (C and D).
Figure 13.
Electron micrographs of longitudinal sections of the optic nerve from the proband. Additional inclusion bodies (arrows) in cells of the optic nerve had ultrastructural features suggesting that they were derived from myelin, but also other features, including amorphous and flocculent electron-dense materials.
Figure 13.
Electron micrographs of longitudinal sections of the optic nerve from the proband. Additional inclusion bodies (arrows) in cells of the optic nerve had ultrastructural features suggesting that they were derived from myelin, but also other features, including amorphous and flocculent electron-dense materials.
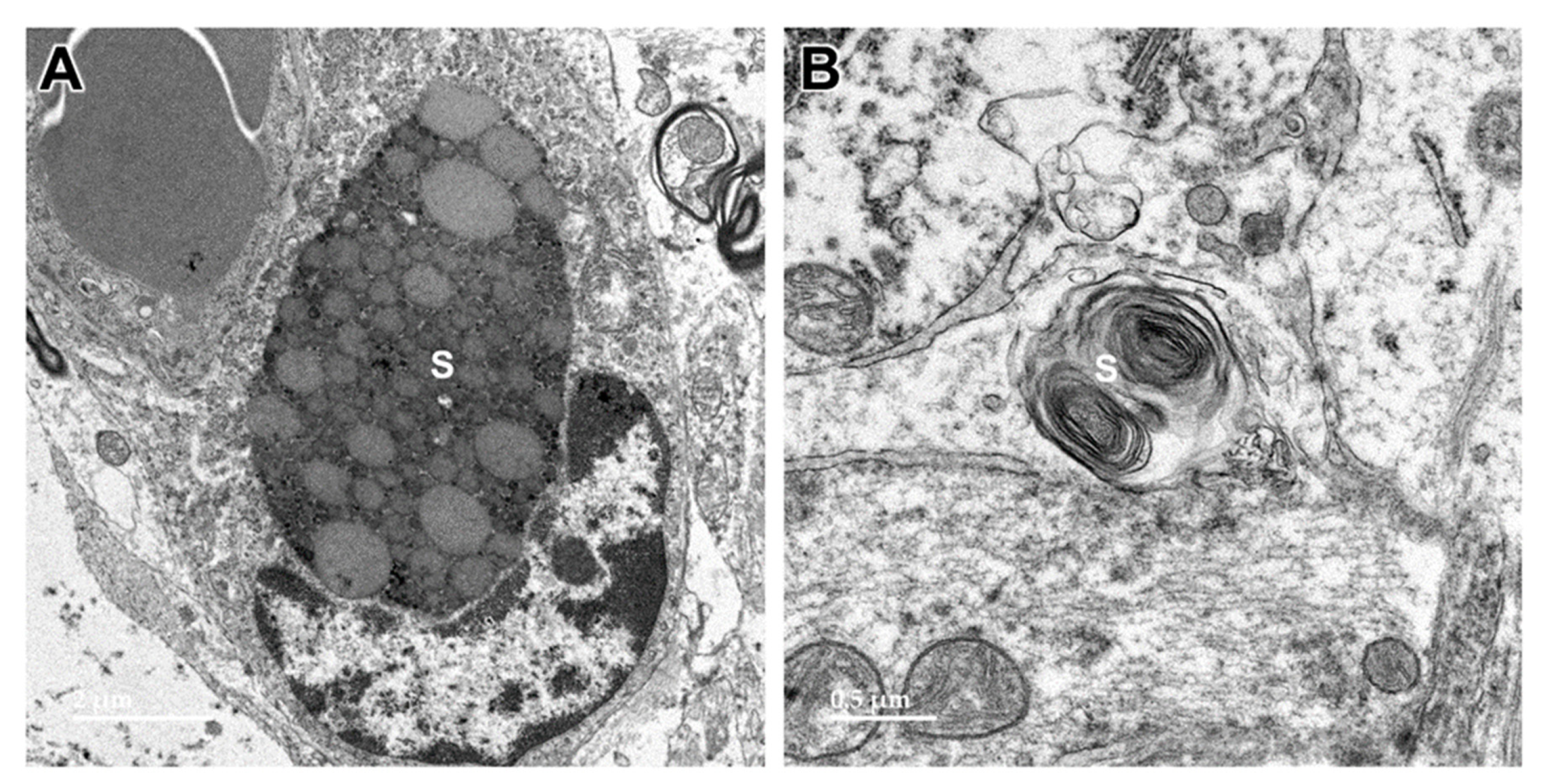
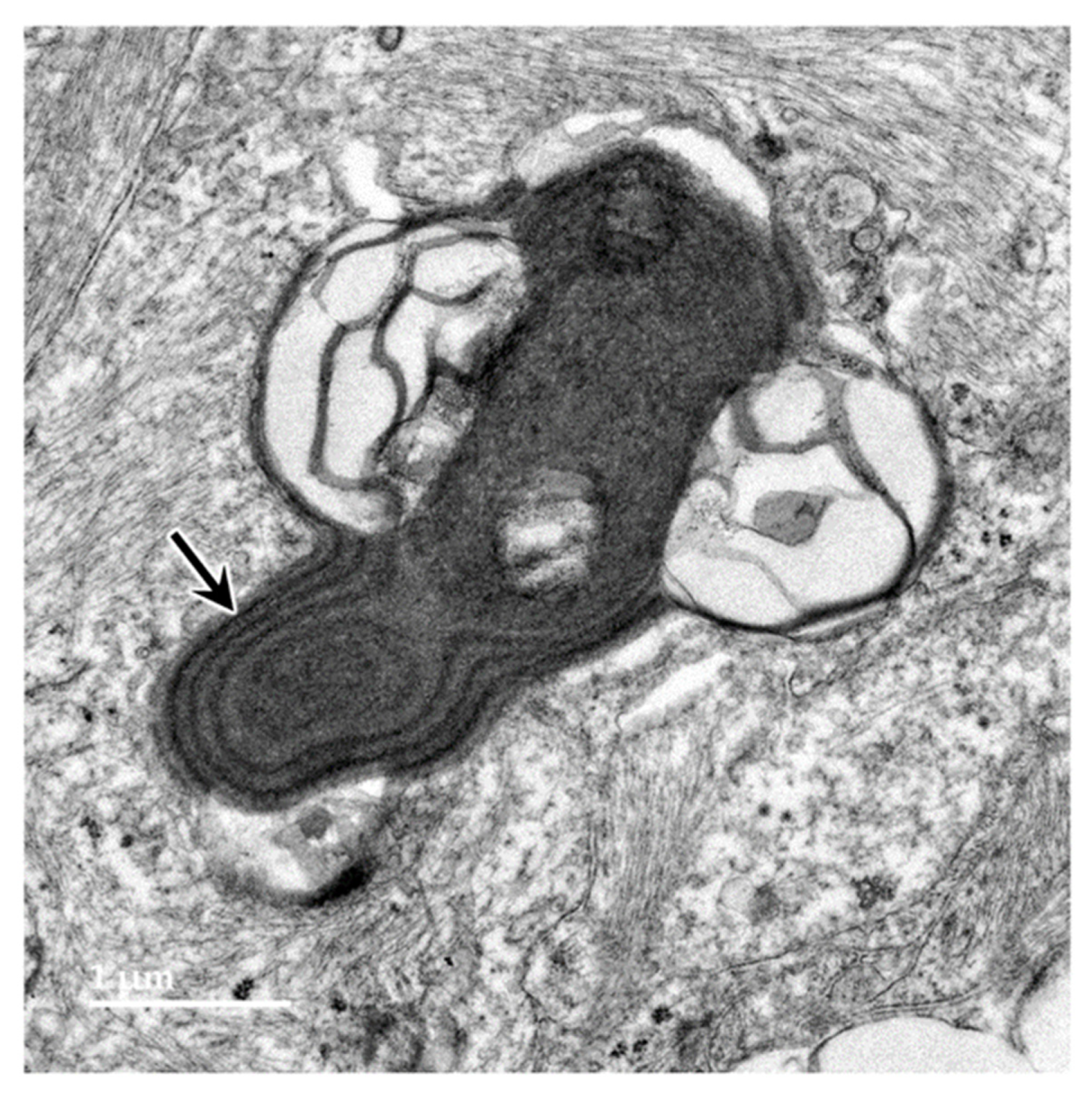
Figure 14.
Electron micrograph of an inclusion body in an optic nerve neuron showing myelin-like membranes embedded in an electron-dense amorphous matrix (arrow) extending into electron lucent areas where the membranes are more loosely packed and irregularly arranged.
Figure 14.
Electron micrograph of an inclusion body in an optic nerve neuron showing myelin-like membranes embedded in an electron-dense amorphous matrix (arrow) extending into electron lucent areas where the membranes are more loosely packed and irregularly arranged.
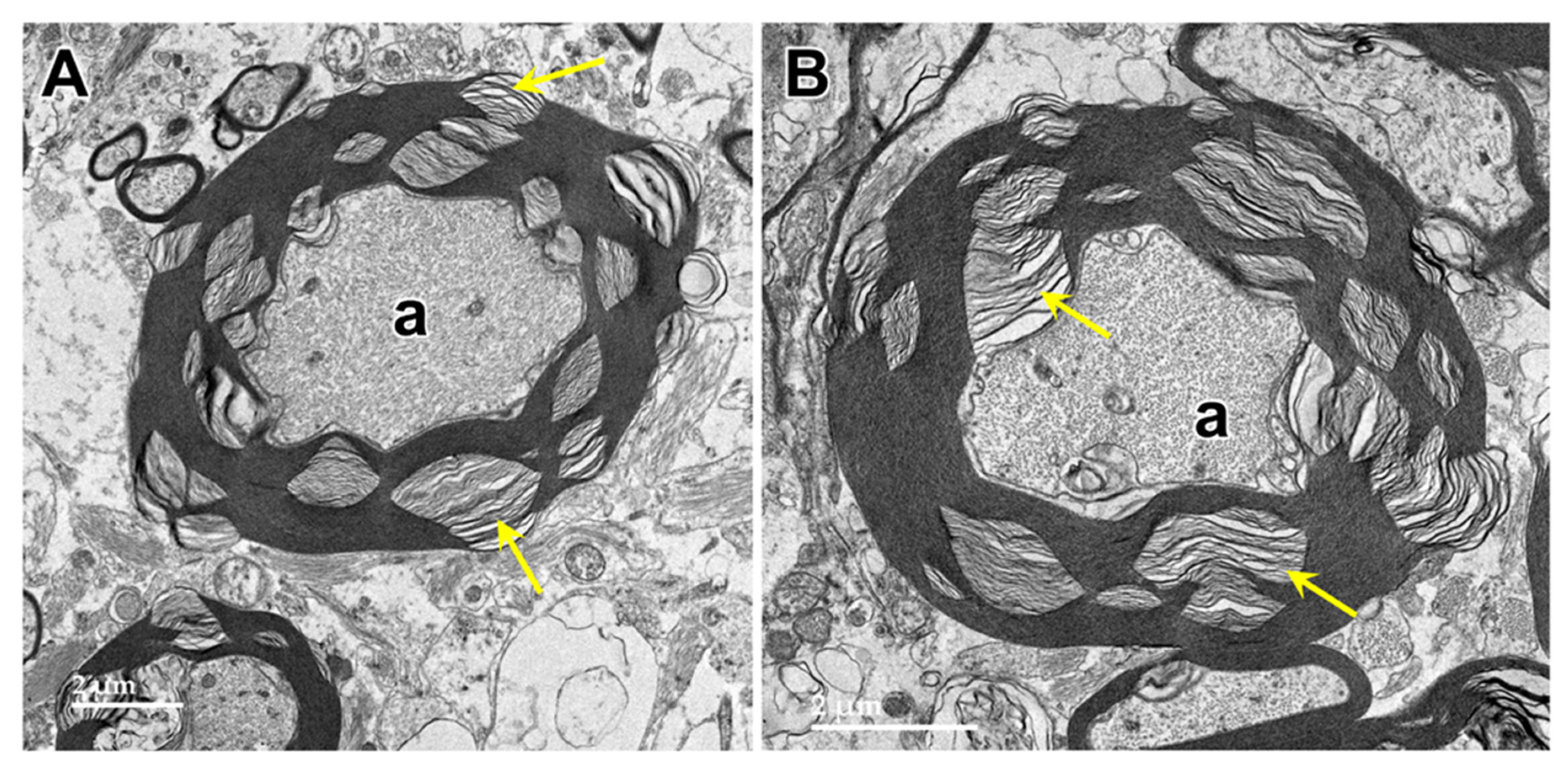
Figure 15.
Electron micrographs of cross-sections of axons (a) in the cerebrocortical white matter of the proband. The myelin sheaths surrounding almost every axon contained numerous areas where there were pronounced gaps between the individual myelin layers (arrows).
Figure 15.
Electron micrographs of cross-sections of axons (a) in the cerebrocortical white matter of the proband. The myelin sheaths surrounding almost every axon contained numerous areas where there were pronounced gaps between the individual myelin layers (arrows).
Figure 16.
Electron micrograph of cross-section of a degenerating axon in the cerbrocortical white matter of the proband. The axoplasm has been largely replaced by myelin membranes that appear to have collapsed inward into the axon.
Figure 16.
Electron micrograph of cross-section of a degenerating axon in the cerbrocortical white matter of the proband. The axoplasm has been largely replaced by myelin membranes that appear to have collapsed inward into the axon.
Figure 17.
Electron micrographs showing storage bodies (s) in cardiac muscle from the proband. The storage bodies occurred in clusters between the myofibrils (A and B). At high magnification (C and D), the contents of the storage bodies could be seen to consist of parallel arrays of membrane-like structures (arrows) and clumps of very electron-dense amorphous materials.
Figure 17.
Electron micrographs showing storage bodies (s) in cardiac muscle from the proband. The storage bodies occurred in clusters between the myofibrils (A and B). At high magnification (C and D), the contents of the storage bodies could be seen to consist of parallel arrays of membrane-like structures (arrows) and clumps of very electron-dense amorphous materials.
Figure 18.
Immunohistochemical localization of mitochondrial ATP synthase subunit c protein in sections of cerebral cortex gray matter from the proband. Aggregates of punctate immunostained inclusions were present in large neurons (arrows in A), as well as smaller cells (arrow in B). In some of the smaller cells areas of more diffuse immunostaining was observed (arrowhead in B).
Figure 18.
Immunohistochemical localization of mitochondrial ATP synthase subunit c protein in sections of cerebral cortex gray matter from the proband. Aggregates of punctate immunostained inclusions were present in large neurons (arrows in A), as well as smaller cells (arrow in B). In some of the smaller cells areas of more diffuse immunostaining was observed (arrowhead in B).
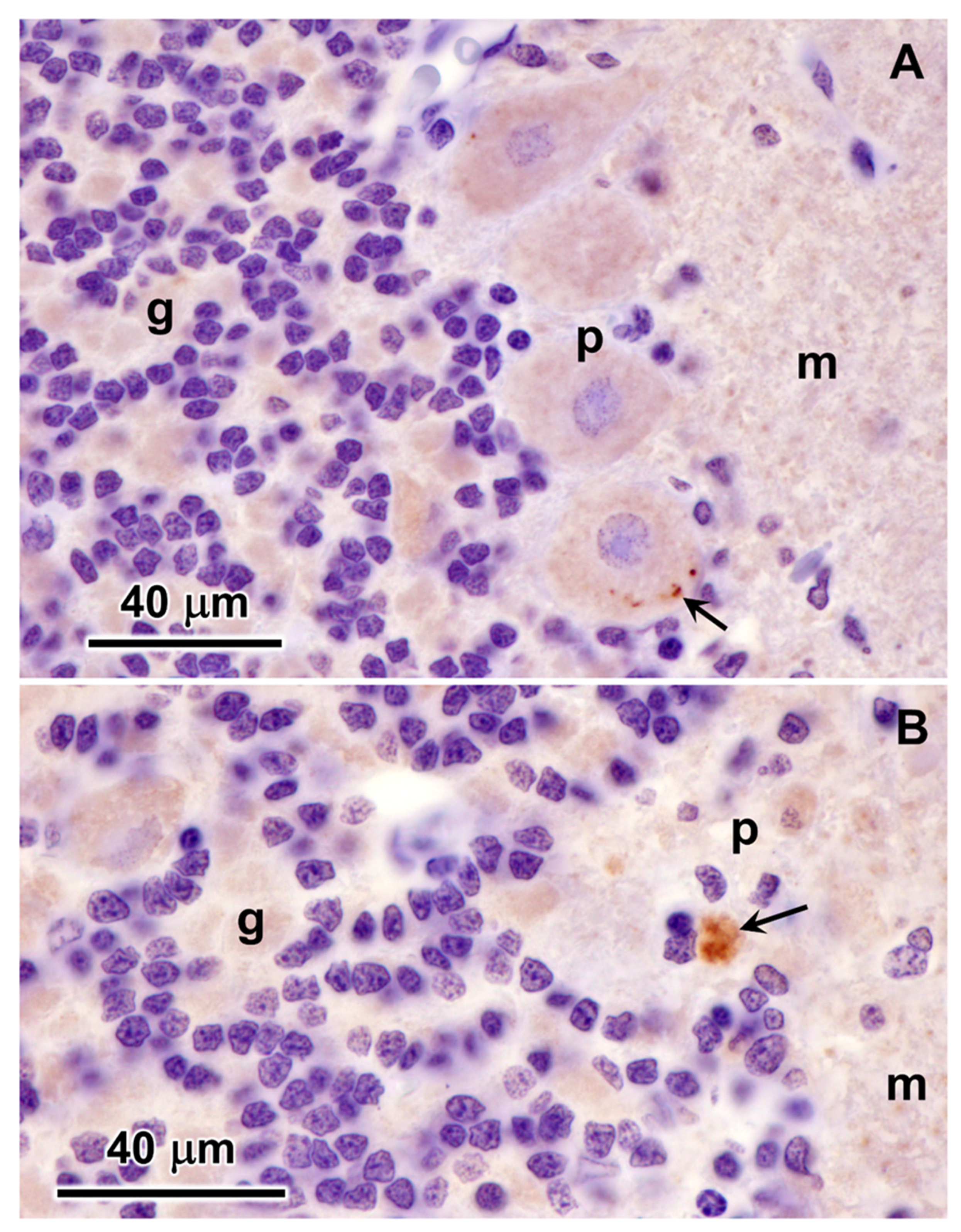
Figure 19.
Immunohistochemical localization of mitochondrial ATP synthase subunit c protein in sections of cerebellar cortex from the proband. A small subset of Purkinje cells contained a few punctate immunostained inclusions (arrow in A). In addition, some small cells at the boundary between the granule cell and the Purkinje cell layers contained immunolabled inclusions (arrow in B). These cells were relatively rare. Layers of the cerebellar cortex: granular layer (g); Purkinje cell layer (p); molecular layer (m).
Figure 19.
Immunohistochemical localization of mitochondrial ATP synthase subunit c protein in sections of cerebellar cortex from the proband. A small subset of Purkinje cells contained a few punctate immunostained inclusions (arrow in A). In addition, some small cells at the boundary between the granule cell and the Purkinje cell layers contained immunolabled inclusions (arrow in B). These cells were relatively rare. Layers of the cerebellar cortex: granular layer (g); Purkinje cell layer (p); molecular layer (m).
Figure 20.
Immunohistochemical localization of mitochondrial ATP synthase subunit c protein in sections of cardiac muscle from the proband. Aggregates of immunostained inclusions were present in the muscle fibers adjacent to the cell nuclei (arrows).
Figure 20.
Immunohistochemical localization of mitochondrial ATP synthase subunit c protein in sections of cardiac muscle from the proband. Aggregates of immunostained inclusions were present in the muscle fibers adjacent to the cell nuclei (arrows).
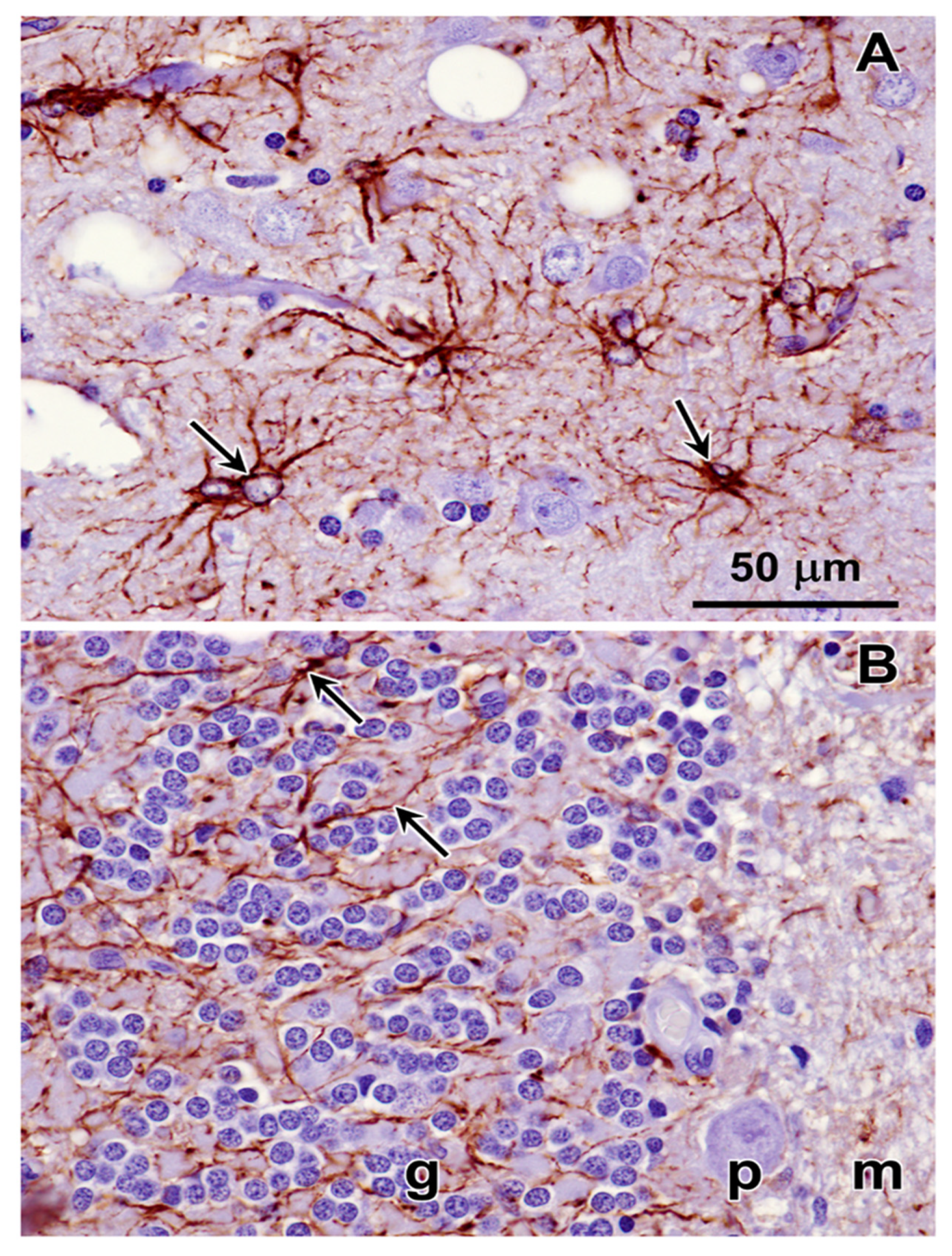
Figure 21.
Immunohistochemical localization of the activated astrocyte marker GFAP in sections of cerebral cortex (A) and cerebellum (B) from the proband. Activated astrocytes were abundant throughout the cerebral cortex gray matter (arrows in A). In the cerebellum, labeled processes of activated astrocytes were abundant primarily in the granular layer (arrows in B). Layers of the cerebellar cortex: granular layer (g); Purkinje cell layer (p); molecular layer (m).
Figure 21.
Immunohistochemical localization of the activated astrocyte marker GFAP in sections of cerebral cortex (A) and cerebellum (B) from the proband. Activated astrocytes were abundant throughout the cerebral cortex gray matter (arrows in A). In the cerebellum, labeled processes of activated astrocytes were abundant primarily in the granular layer (arrows in B). Layers of the cerebellar cortex: granular layer (g); Purkinje cell layer (p); molecular layer (m).
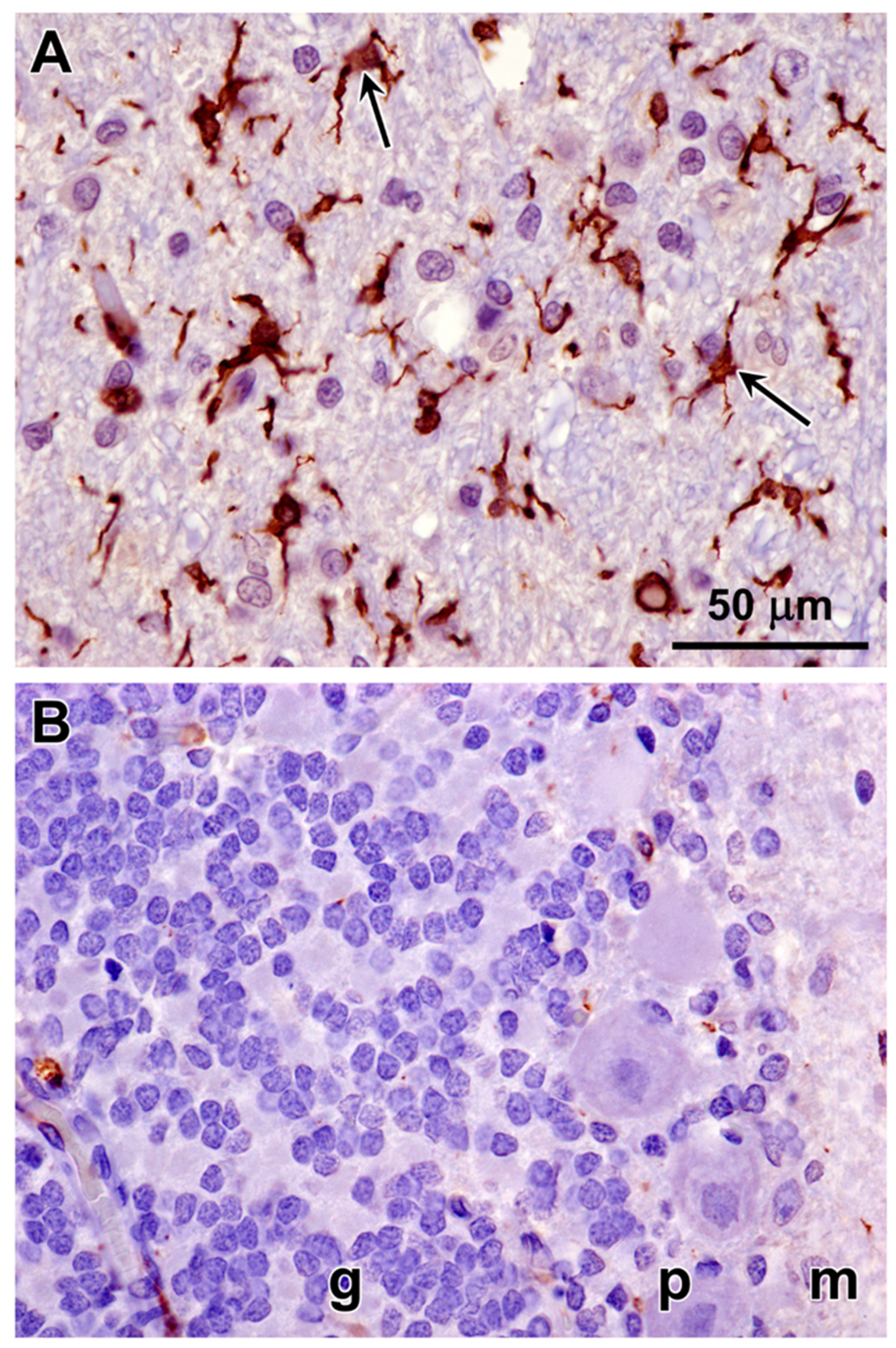
Figure 22.
Immunohistochemical localization of the microglial activation marker Iba1 in sections of cerebral cortex (A) and cerebellar cortex (B) from the proband. Activated microglia were abundant throughout the cerebral cortex gray matter (arrows in A). Very little Iba1 immunolabeling was observed in sections of the cerebellar cortex (B). Layers of the cerebellar cortex: granular layer (g); Purkinje cell layer (p); molecular layer (m).
Figure 22.
Immunohistochemical localization of the microglial activation marker Iba1 in sections of cerebral cortex (A) and cerebellar cortex (B) from the proband. Activated microglia were abundant throughout the cerebral cortex gray matter (arrows in A). Very little Iba1 immunolabeling was observed in sections of the cerebellar cortex (B). Layers of the cerebellar cortex: granular layer (g); Purkinje cell layer (p); molecular layer (m).
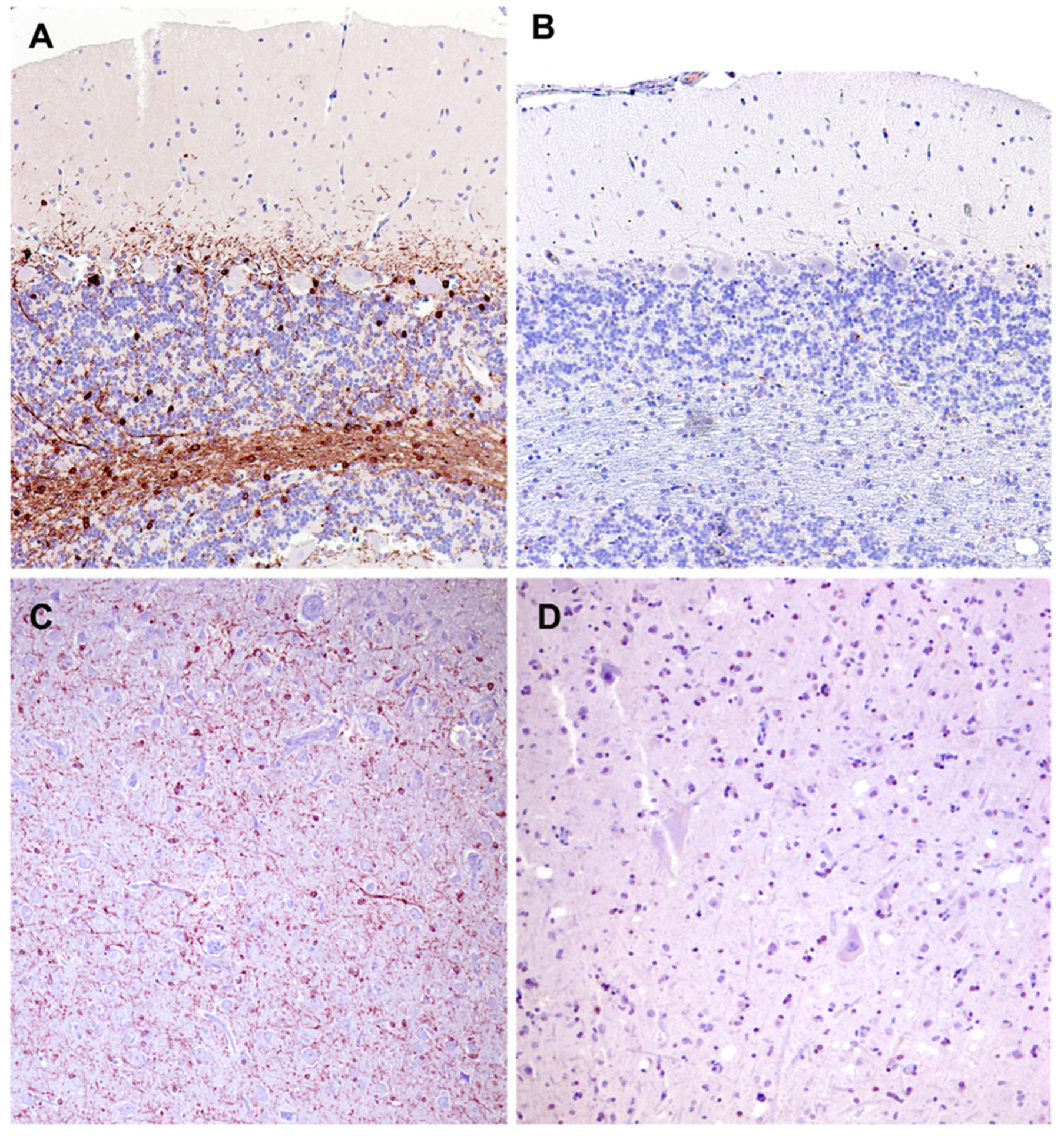
Figure 23.
Paraffin sections of cerebellar cortex (A and B) and cerebral cortex gray matter (C and D) from an approximately 2 year old mixed breed dog with no neurological disorder (A and C) and from the proband (B and C). Sections were immunostained for localization of CNPase protein (brown color). CNPase immunolabel that was present in the tissues from the normal dog was not observed in the same tissues from the proband.
Figure 23.
Paraffin sections of cerebellar cortex (A and B) and cerebral cortex gray matter (C and D) from an approximately 2 year old mixed breed dog with no neurological disorder (A and C) and from the proband (B and C). Sections were immunostained for localization of CNPase protein (brown color). CNPase immunolabel that was present in the tissues from the normal dog was not observed in the same tissues from the proband.
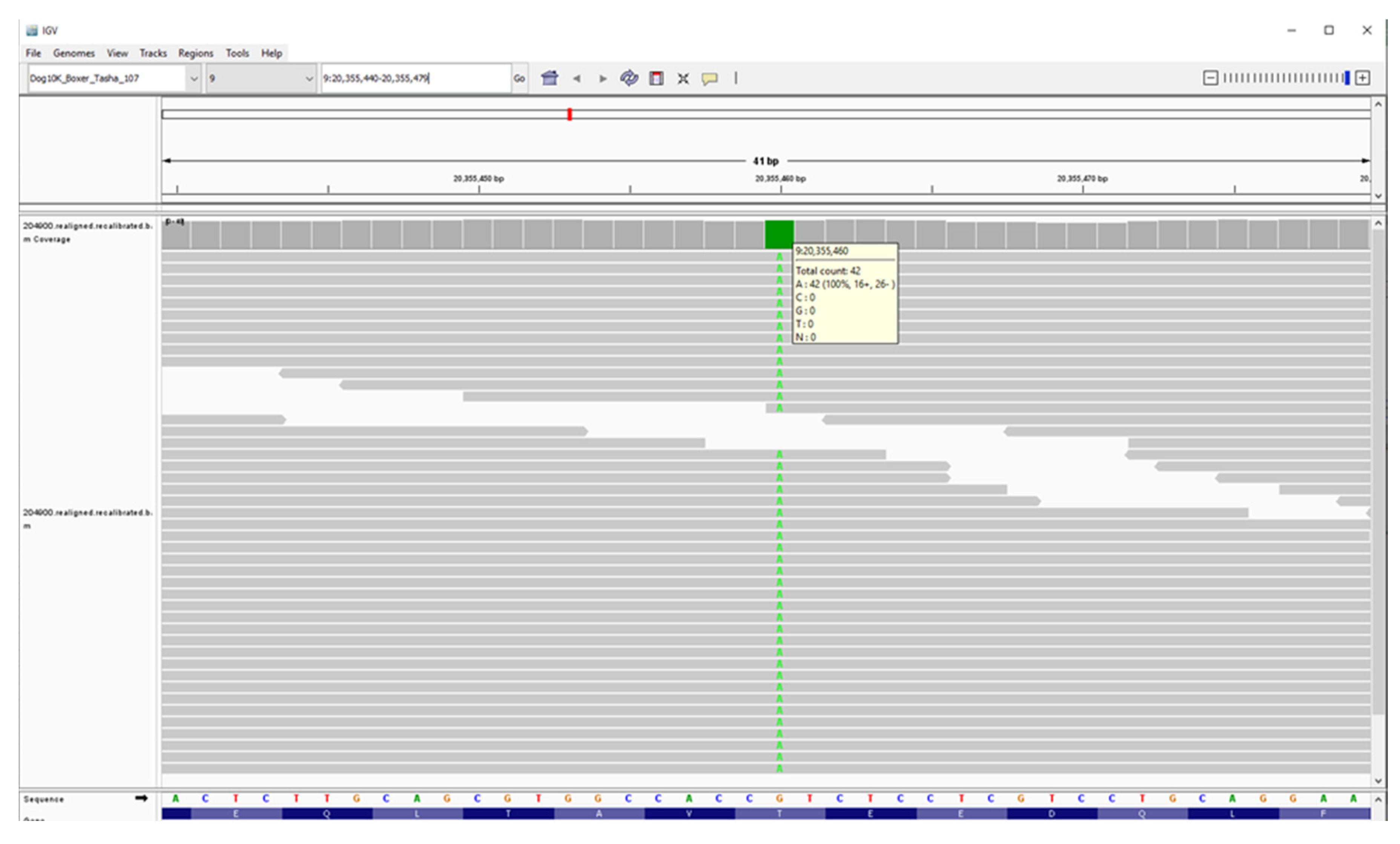
Figure 24.
Screenshot of the proband’s whole genome sequence reads aligned to the reference sequence in the vicinity of position 20,355,460 on chromosome 9, as viewed with the Integrative Genomics Viewer. The variant A is highlighted in green.
Figure 24.
Screenshot of the proband’s whole genome sequence reads aligned to the reference sequence in the vicinity of position 20,355,460 on chromosome 9, as viewed with the Integrative Genomics Viewer. The variant A is highlighted in green.
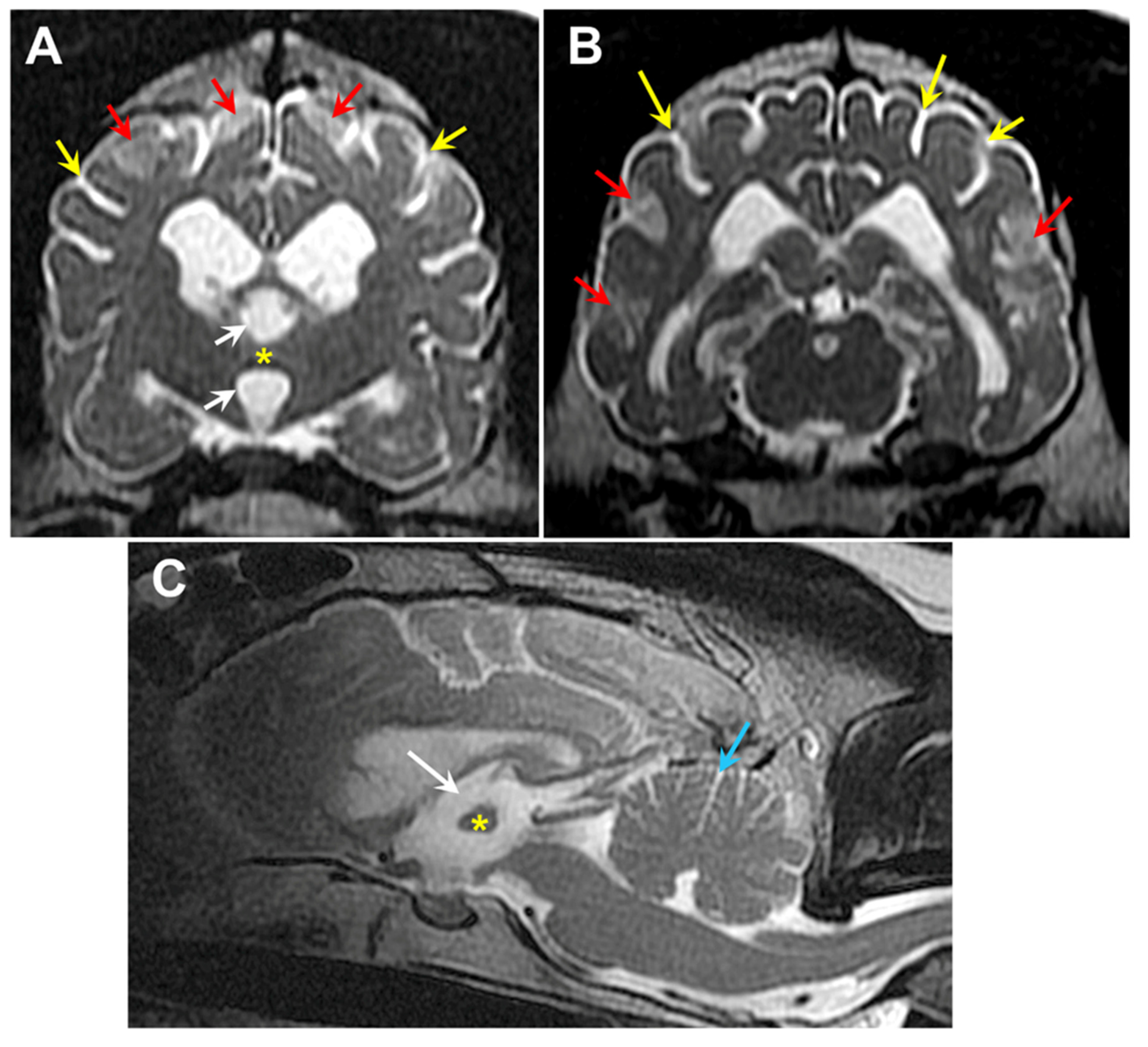
Figure 25.
T2-weighted MR images of the brain from Dog 2: transverse views at the level of the interthalamic adhesion (A) and at more caudal location (B), and a saggital view (C). The dog exhibited cerebral parenchymal atrophy characterized by an abnormally small interthalamic adhesion (yellow asterisks), an enlarged third ventricle (white arrows), and widened cerebral cortical sulci (yellow arrows). Increased CSF volume between the cerebellar folia (blue arrow) was indicative of cerebellar atrophy. The dog also exhibited multiple areas of increased signal intensity within the cerebral cortex parenchyma (red arrows).
Figure 25.
T2-weighted MR images of the brain from Dog 2: transverse views at the level of the interthalamic adhesion (A) and at more caudal location (B), and a saggital view (C). The dog exhibited cerebral parenchymal atrophy characterized by an abnormally small interthalamic adhesion (yellow asterisks), an enlarged third ventricle (white arrows), and widened cerebral cortical sulci (yellow arrows). Increased CSF volume between the cerebellar folia (blue arrow) was indicative of cerebellar atrophy. The dog also exhibited multiple areas of increased signal intensity within the cerebral cortex parenchyma (red arrows).