1. Introduction
In order to increase the share of renewable energy utilization in the global energy industry, it is necessary to continuously improve materials and devices for energy conversion and storage [
1]. One of the ways is to increase the energy density in lithium-ion batteries (LIBs). This can be achieved by using anode materials with capacitance exceeding that of graphite. Promising materials are silicon [
2,
3], germanium [
4,
5], transition metal oxides [
6,
7], as well as various compositions of the above materials with graphite. Despite the high theoretical capacity of silicon, its performance in lithiation and delithiation can be ensured only by using nanosized and submicron particles or thin films [
8].
Traditionally, obtaining silicon of the specified sizes involves a number of operations: reduction of quartz to silicon of metallurgical purity; chlorination and hydrochlorination of metallurgical silicon; reduction of chlorosilanes to silicon crystals; zone recrystallization of silicon and obtaining silicon-based anodes by plasma or laser sputtering methods [
9]. Methods of electrolytic deposition of silicon of a given morphology from molten salts are an alternative [
1,
10,
11,
12].
One of the most promising and frequently used electrolytes for silicon production are water-soluble KF–KCl melts with K
2SiF
6, SiO
2, and SiCl
4 additives [
13,
14,
15]. Nowadays, the kinetics of the cathodic process depending on the substrate material and polarization conditions has been well studied, experimental samples of silicon deposits depending on the electrolysis parameters have been obtained, and a diagram characterizing the influence of these parameters on the morphology of silicon deposits has been proposed [
15]. The regulation of silicon deposits morphology can also be realized by introducing additives into the electrolyte that influence the physicochemical properties of the electrolyte and the parameters of electrochemical nucleation. This is primarily concerned with changing the electrical conductivity and surface tension of the melt. Earlier [
16] it was proposed to use halide melts based on iodides for this purpose. As a result of studies, the possibility of obtaining thin silicon films and their microalloying was shown [
17].
The present work shows the possibility of using KI-KF-KCl-K2SiF6 melt for electrodeposition of submicron silicon fibers for their application in the anode of a lithium-ion battery.
2. Experiment
For measurements and electrodeposition we used chemically pure salts (JSC "Vekton"), which were preliminary purified from impurities by hydrofluorination (KF, K
2SiF
6) and iodination (KI), as well as preliminary potentiostatic electrolysis [
14,
16]. Silicon electrodeposition was carried out in a sealed stainless steel retort [
14] filled with electrochemically purified argon. The container for the melt was a glassy carbon crucible placed in a graphite container. A graphite cylinder was used as the working electrode, while monocrystalline silicon served as the counter electrode and quasi-reference electrode. The retort was placed in a vertical resistance furnace and heated to the operating temperature of 720°C. The melt temperature was set using an
S-type thermocouple and a USB-TC01 thermocouple module (National Instruments, USA). At the end of electrolysis, the deposits were washed from the electrolyte residue in aqueous HF solution and dried in a vacuum at 200°C.
The morphology and elemental composition of the obtained samples were investigated by using a Tescan Vega 4 scanning electron microscope (Tescan, Czech Republic) with an Xplore 30 EDS detector (Oxford, UK).
Electrochemical performance of obtained silicon fibers was investigated in a 3-electrode half-cell [
18]. An electrically conductive additive of 10 wt% graphite and 10 wt% binder (CMC in deionized water) were used in order to fabricate Si-based anode. LIBs anode half-cell fabrication was performed in an argon-filled glove box (O
2, H
2O < 0.1 ppm). Stainless steel mesh with the investigated anode material was used as the working electrode and two separate lithium strips as the counter and reference electrodes. All electrodes were divided by 2 layers of polypropylene separator and tightly placed in the cell. The cell was filled with 1 ml of electrolyte—1 M LiPF
6 in a mixture of EC/DMC/DEC (1:1:1 by volume). Electrochemical measurements and cycling experiments were performed using a P-20X8 PGSTAT (Electrochemical Instruments, Russia).
3. Results and Discussion
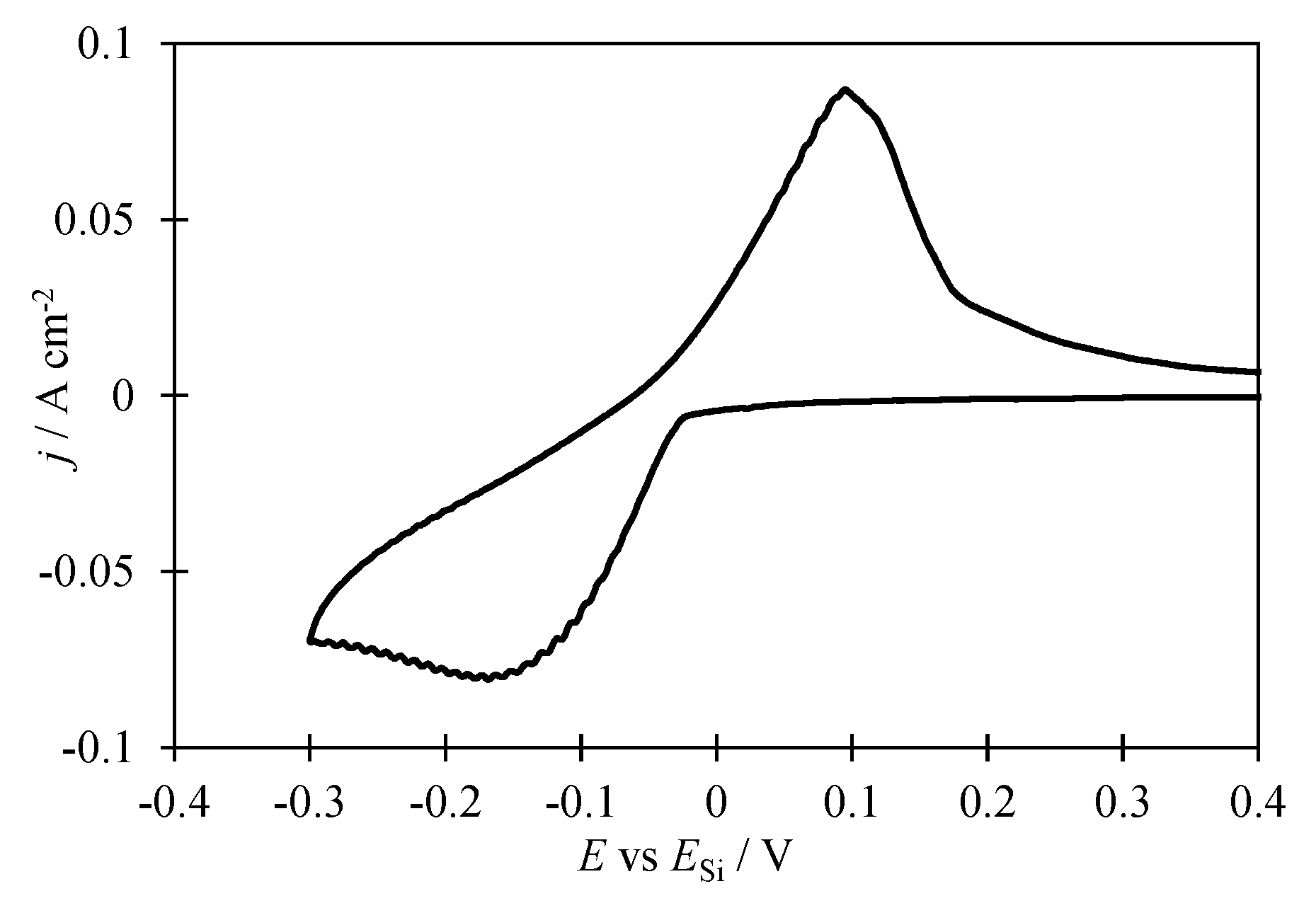
Voltammetry in KI–KF–KCl–K2SiF6 melt. To determine the parameters (potential, current density) of silicon electrodeposition on glassy carbon in KI–KF–KCl–K
2SiF
6 melt voltammetry dependence was obtained, which is shown in
Figure 1. The investigated process starts at potentials negative to 0 V relative to the silicon quasi-reference electrode with the formation of a single cathodic peak of electroreduction of silicon ions at a potential of about -0.17 V and a cathodic current density of 0.08 A cm
−2. Further shifting of the glassy carbon potential leads to potassium release and electrode destruction. When sweeping the working electrode potential into the anodic region, a single dissolution peak of electrodeposited silicon is also formed at a potential of 0.08 V and an anodic current density of 0.08 A cm
−2. The form of the voltametric dependence indicates the occurrence of the investigated process in one 4-electron stage under the experimental conditions. Similar dependences were obtained in halide melts of other compositions [
3,
12,
13,
14,
15,
16,
17]. Based on electrochemical measurements, a cathodic current density of 0.05 A cm
−2 was chosen for electrodeposition of silicon in the form of particles with a high specific surface.
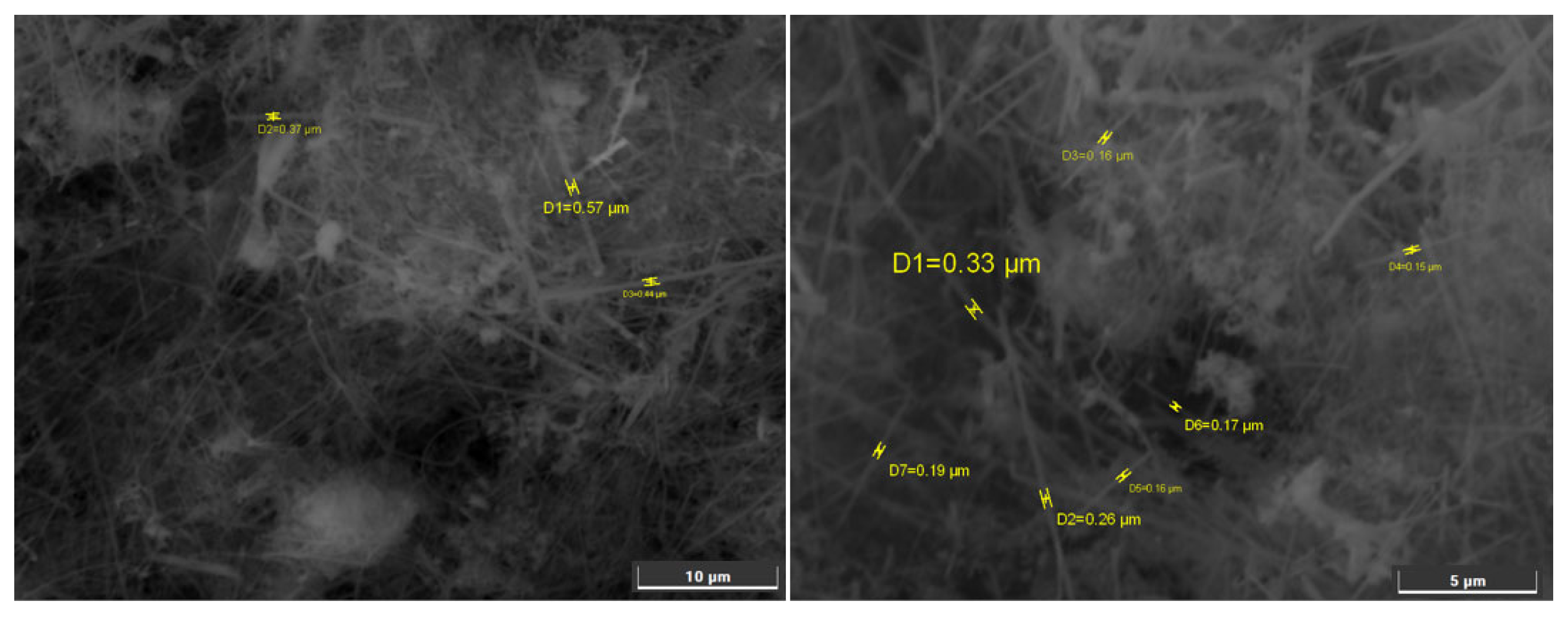
Morphology of the obtained silicon. The electrodeposition of silicon from KI–KF–KCl–K
2SiF
6 melt on glassy carbon was carried out at a cathodic current density of 0.05 A cm
−2. The cathode potential ranged from -0.1 to -0.2 V relative to the potential of the silicon quasi-reference electrode. SEM-images of the obtained silicon after washing in HF solution are shown in
Figure 3. The obtained silicon deposits were represented by orderly shaped fibers with average diameter from 0.1 to 0.3 μm and length from 5 to 20 μm. This morphology provides a high specific surface area for lithium intercalation. EDS analysis shows 99.2-99.8 wt% silicon in obtained samples with expected oxygen residual impurities.
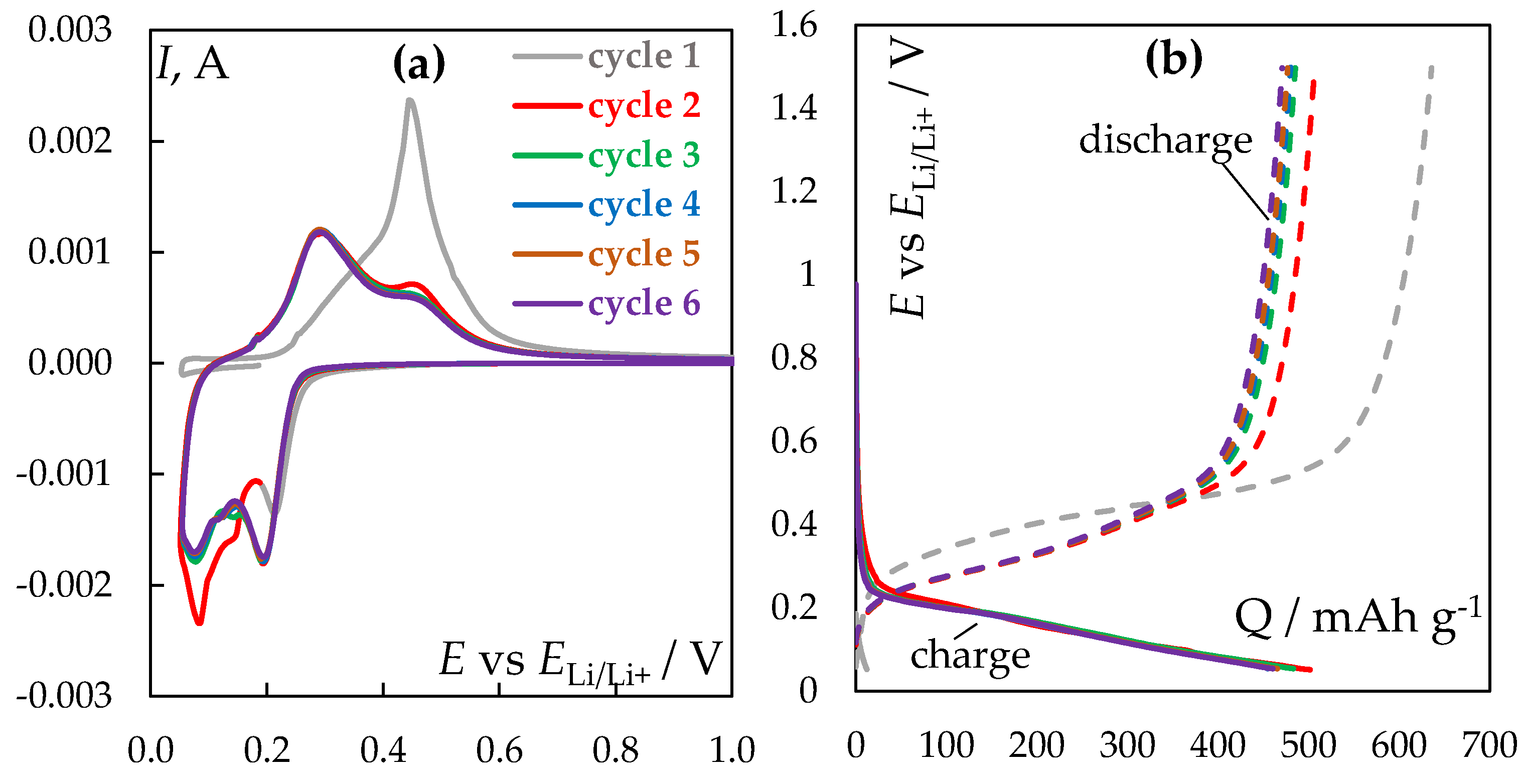
Electrochemical performance. Figure 3a shows cyclic voltametric dependences for the initial 6 charge-discharge cycles. By the 3
rd cycle, the reproducibility of the obtained dependences is observed. In the cathodic region, one can note peaks in the potential region negative to 0.2 V associated with the discharge of lithium ions and the formation of Li
xSi
y-type compounds of variable composition [
8]. To prevent the release of elemental lithium, the sweep was carried out to a potential of 0.05 V relative to the potential of the lithium electrode. In the anodic region, two distinct peaks are formed at potentials around 0.3 and 0.48 V, associated with the oxidation of lithium from the obtained Li
xSi
y compounds.
Figure 3b shows the charge-discharge dependencies corresponding to the initial 6 cycles in potential-capacity coordinates. According to the given data, the charge proceeds in the potential region from 0.2 to 0.05 V, while the discharge proceeds at the potential from 0.2 to 0.5 V. This indicates the capacitive character of the lithium reduction reaction with its subsequent intercalation into the silicon volume.
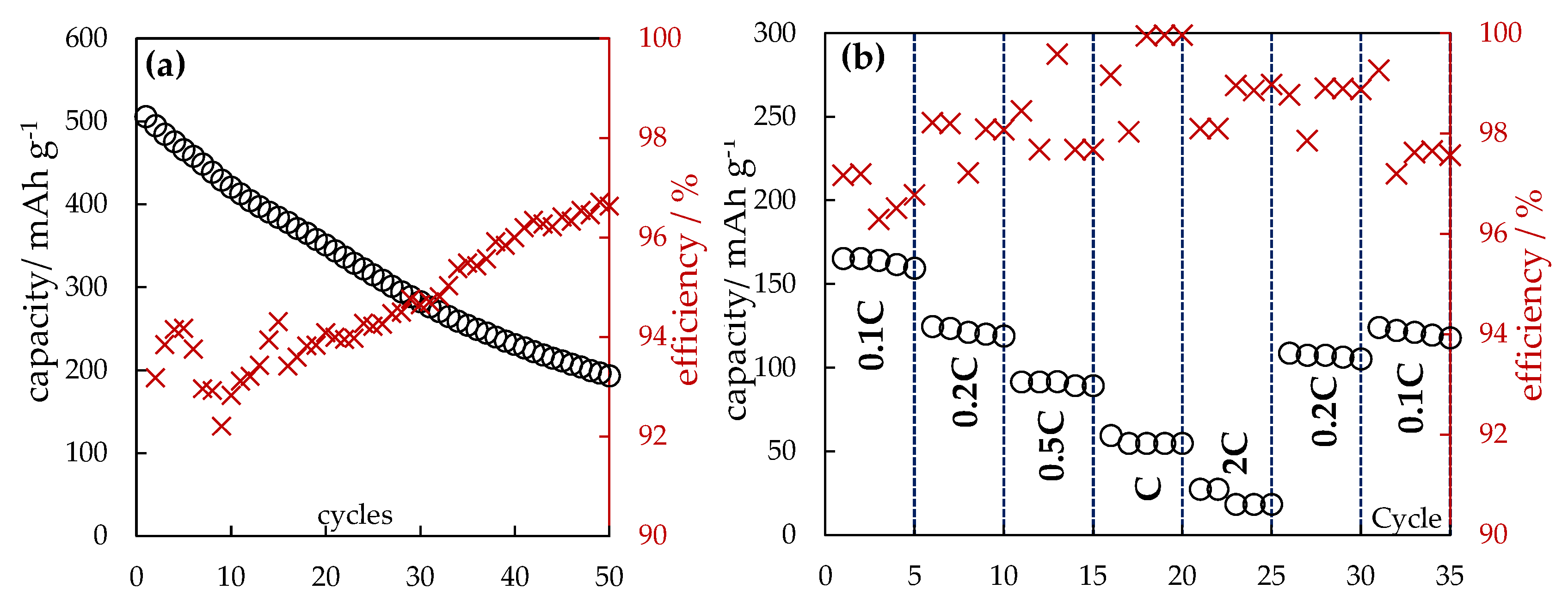
The dependences in
Figure 4 characterize the electrochemical behavior of the anode based on the obtained silicon fibers under multiple lithiation with 0.1C current (
Figure 4a) as well as with different currents (
Figure 4b). During the first 50 cycles, the discharge capacity decreased from 520 to 200 mAh g
−1, after which it stabilized at the value of 200-220 mAh g
−1.
Figure 4b shows the possibility of charging the studied samples at high currents up to 2C. Also, despite the relatively low values of the discharge capacity, the operability of the fabricated anode half-cell during a relatively high number of cycles is noted. At current C, the fabricated anode was cycled for 600 cycles. The discharge capacity at the first cycle was 41 and at the last cycle it was 9 mAh g
−1, indicating a gradual degradation of this material.
The results obtained are comparable to the results of studies on the electrochemical behavior of electrodeposited silicon fibers from other melts [
3,
16], while the relatively low capacitance and its decrease during cycling may indicate degradation of the liquid electrolyte or the substrate-anode interface. Therefore, the behavior of the obtained silicon fibers should be studied with other electrolytes and structural materials.
4. Conclusions
The cathodic process on glassy carbon in the (mol%) 75KI–16KF–8KCl–1K2SiF6 melt at 720°C has been studied by cyclic voltammetry. It is shown that the investigated process proceeds in one stage: silicon electroreduction takes place in the potential range from 0 to -0.3 V relative to the potential of the silicon quasi-reference electrode. On the basis of electrochemical measurements, the parameters of silicon electrodeposition in the form of deposits with a high specific surface were chosen: cathodic current density of 0.05 A cm−2 with control of the cathode potential. During galvanostatic electrolysis of KI–KF–KCl–K2SiF6 melt, silicon particles in the form of ordered-shaped fibers with an average diameter from 0.1 to 0.3 μm and length from 5 to 20 μm were obtained.
The obtained silicon fibers were used to fabricate a sample of anode half-cell of LIB in order to study the electrochemical behavior of the obtained silicon during lithiation/delithiation. It is shown that the charge of the anode based on the obtained silicon fibers takes place at potentials from 0.2 to 0.05 V relative to the lithium potential, which is due to the formation of lithium-silicon compounds. Accordingly, the anode discharge occurs at potentials from 0.2 to 0.5 V. A change in discharge capacity from 520 to 200 mAh g−1 during the first 50 charge/discharge cycles at a charge current of 0.1C and a Coulomb efficiency of 98-100% is shown. The possibility of charging silicon-based anode samples at charging currents up to 2C was also noted; the discharge capacity was 25 to 250 mAh g−1.
It is concluded that it is necessary to conduct further studies using alternative materials of electrolyte and current supply to the anode.
Author Contributions
Conceptualization, A.S.; methodology; L.M., A.L., N.L.; validation, L.M., A.L., N.L.; formal analysis, L.M., A.L., N.L.; investigation, L.M., A.L., N.L.; writing—original draft preparation, A.L., A.S.; writing—review and editing, A.S.; supervision, A.S.; project administration, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work is performed in the frame of the State Assignment number 075-03-2023-006 dated 16/01/2023 (the theme number FEUZ-2020-0037).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Author declares no conflict of interest.
References
- Miao, J. Review on Electrode Degradation at Fast Charging of Li-Ion and Li Metal Batteries from a Kinetic Perspective. Electrochem 2023, 4, 156–180. [Google Scholar] [CrossRef]
- Suzdaltsev, A. Silicon Electrodeposition for Microelectronics and Distributed Energy: A Mini-Review. Electrochem 2022, 3, 760–768. [Google Scholar] [CrossRef]
- Gevel, T.; Zhuk, S.; Leonova, N.; Leonova, A.; Trofimov, A.; Suzdaltsev, A.; Zaikov, Y. Electrochemical Synthesis of Nano-Sized Silicon from KCl–K2SiF6 Melts for Powerful Lithium-Ion Batteries. Appl. Sci. 2021, 11, 10927. [Google Scholar] [CrossRef]
- Stokes, K.; Flynn, G.; Geaney, H.; Bree, G.; Ryan, K.M. Axial Si–Ge Heterostructure Nanowires as Lithium-Ion Battery Anodes. Nano Lett. 2018, 18, 5569–5575. [Google Scholar] [CrossRef] [PubMed]
- Kulova, T.L. New Electrode Materials for Lithium–Ion Batteries (Review). Rus. J. Electrochem. 2013, 49, 1–25. [Google Scholar] [CrossRef]
- Wang, J.; Stenzel, D.; Azmi, R.; Najib, S.; Wang, K.; Jeong, J.; Sarkar, A.; Wang, Q.; Sukkurji, P.A.; Bergfeldt, T.; et al. Spinel to Rock-Salt Transformation in High Entropy Oxides with Li Incorporation. Electrochem 2020, 1, 60–74. [Google Scholar] [CrossRef]
- Purwanto, A.; Muzayanha, S.U.; Yudha, C.S.; Widiyandari, H.; Jumari, A.; Dyartanti, E.R.; Nizam, M.; Putra, M.I. High Performance of Salt-Modified–LTO Anode in LiFePO4 Battery. Appl. Sci. 2020, 10, 7135. [Google Scholar] [CrossRef]
- Galashev, A.Y. Molecular Dynamic Study of the Applicability of Silicene Lithium Ion Battery Anodes: A Review. Electrochem. Mat. & Techn. 2023, 2, 20232012. [Google Scholar] [CrossRef]
- Prosini, P.P.; Rufoloni, A.; Rondino, F.; Santoni, A. Silicon nanowires used as the anode of a lithium-ion battery. AIP Conf. Proc. 2015, 1667, 020008. [Google Scholar] [CrossRef]
- Padamata, S.K.; Saevarsdottir, G. Silicon Electrowinning by Molten Salts Electrolysis. Frontiers in Chem. 2023, 11, 1133990. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Slade, T.; Stolt, M.J.; Li, L.; Girard, S.N.; Mai, L.; Jin, S. Low-Temperature Molten-Salt Production of Silicon Nanowires by the Electrochemical Reduction of CaSiO3. Angew. Chem. 2017, 129, 14645–14649. [Google Scholar] [CrossRef]
- Pavlenko, O.B.; Ustinova, Y.A.; Zhuk, S.I.; Suzdaltsev, A.V.; Zaikov, Y.P. Silicon Electrodeposition from Low-Melting LiCl–KCl–CsCl Melts. Rus. Met. (Metally) 2022, 2022, 818–824. [Google Scholar] [CrossRef]
- Zaykov, Y.P.; Zhuk, S.I.; Isakov, A.V.; Grishenkova, O.V.; Isaev, V.A. Electrochemical Nucleation and Growth of Silicon in the KF–KCl–K2SiF6 Melt. J. Solid State Electrochem. 2015, 19, 1341–1345. [Google Scholar] [CrossRef]
- Zhuk, S.I.; Minchenko, L.M.; Suzdaltsev, A.V.; Isakov, A.V.; Zaikov, Y.P. Silicon Electrodeposition from the KF–KCl–K2SiF6 and KF–KCl–KI–K2SiF6 Melts. Izvestiya. Non-Ferrous Metallurgy 2023, 17. [Google Scholar] [CrossRef]
- Yasuda, K.; Kato, T.; Norikawa, Yu.; Nohira, T. Silicon Electrodeposition in a Water-Soluble KF–KCl Molten Salt: Properties of Si Films on Graphite Substrates. J. Electrochem. Soc. 2021, 168, 112502. [Google Scholar] [CrossRef]
- Abdurakhimova, R.K.; Laptev, M.V.; Leonova, N.M.; Leonova, A.M.; Schmygalev, A.S.; Suzdaltsev, A.V. Electroreduction of Silicon from the NaI–KI–K2SiF6 Melt for Lithium-Ion Power Sources. Chimica Techno Acta 2022, 9, 20229424. [Google Scholar] [CrossRef]
- Laptev, M.V.; Khudorozhkova, A.O.; Isakov, A.V.; Grishenkova, O.V.; Zhuk, S.I.; Zaikov, Y.P. Electrodeposition of Aluminum-Doped Thin Silicon Films from a KF–KCl–KI–K2SiF6–AlF3 Melt. J. Serb. Chem. Soc. 2021, 86, 1075–1087. [Google Scholar] [CrossRef]
- Leonova, A.M.; Bashirov, O.A.; Leonova, N.M.; Lebedev, A.S.; Trofimov, A.A.; Suzdaltsev, A.V. Synthesis of C/SiC Mixtures for Composite Anodes of Lithium-Ion Power Sources. Applied Sciences 2023, 13, 901. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).