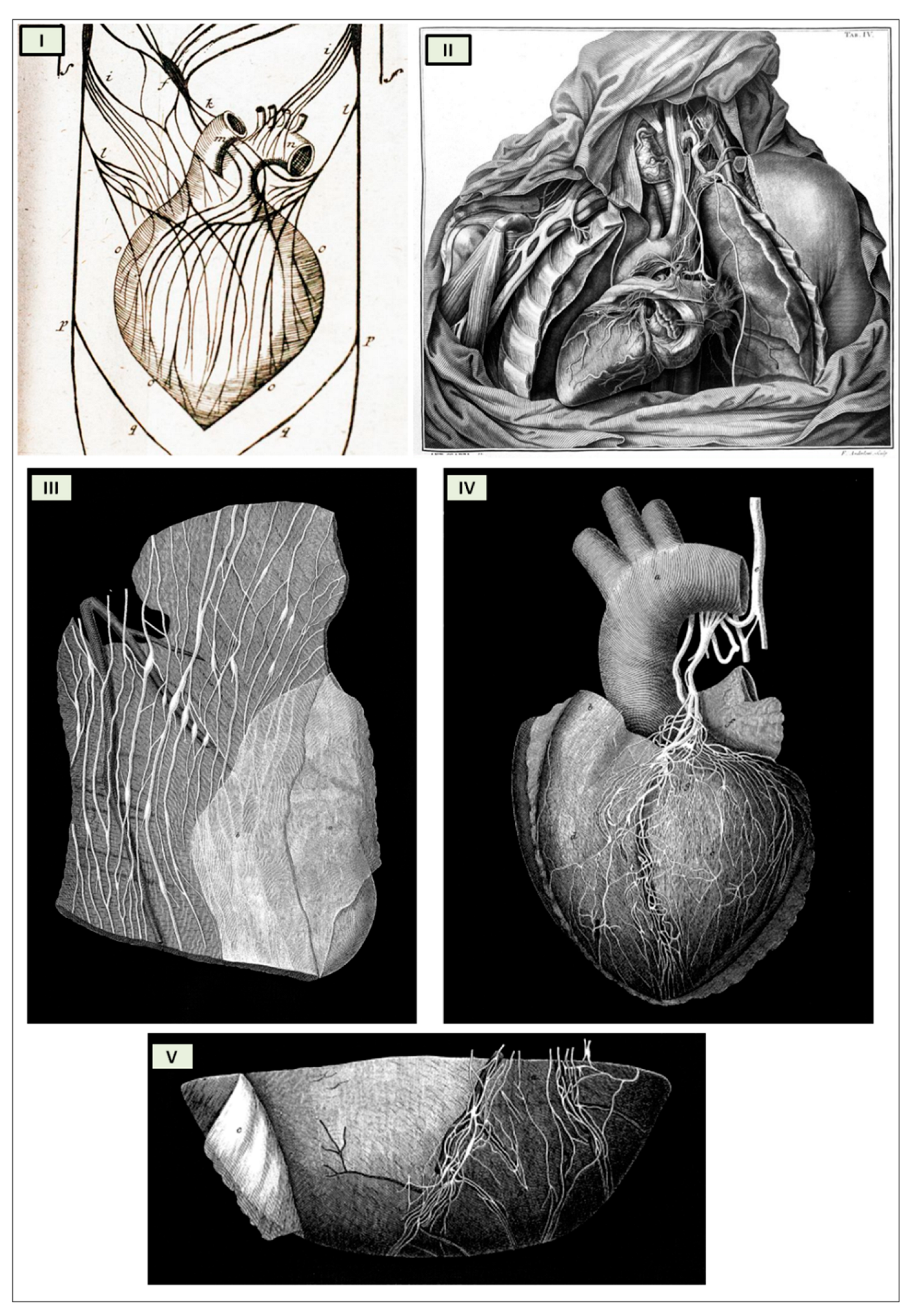
Antonio Scarpa's groundbreaking illustrations of the cardiac nerves in his 1794 work "Tabulae Nevrologicae" earned him a prominent position among the pioneers of modern neurology. His meticulous depictions and detailed anatomical observations challenged the prevailing theories of Albrecht von Haller and Johann Bernhard Jacob Behrends (1792), who maintained that the heart's rhythmic contractions were solely driven by its own intrinsic "irritability" and not influenced by neuronal activity. Scarpa's work effectively refuted these erroneous notions.
Terni's discovery of the spinal sympathetic innervation of the heart provided a critical piece of the puzzle in understanding the complex interplay between the nervous system and the cardiovascular system. His work not only challenged the traditional neurogenic theory but also paved the way for further research into the neural regulation of heart function.
THE CARDIAC NEUROMODULATION
The autonomic nervous system stands out as a distinct anatomical and functional division of the nervous system. This distinction traces back to Marie François Xavier Bichat (1771-1802).
In his seminal work "Physiological Researches upon Life and Death" (1800), Bichat defined life as the sum of those functions that resist death and observed that living organisms constantly strive to maintain themselves in a state of equilibrium despite the destructive forces that surround them. Bichat further divided life into two distinct domains: organic life ("vie organique"), also known as the vegetative system, which corresponded to the process of nourishment, and animal life, ("vie animale"), or animal system which corresponded to the process of interaction with the environment.
Bichat conceptualized organic life as the essential functions of the heart, intestines, and other internal organs, controlled by a system of ganglia (“ganglions”), clusters of independent neural centers (little indipendent brains) in the chest cavity. In contrast, animal life pertained to symmetrical organs (eyes, ears, and limbs), governed by habits and memories but primarily driven by intelligence and astuteness. This aligned with the brain's characteristic attributes, yet it would not be feasible without the heart, the cornerstone of organic life. Bichat's use of the term "animale" harkens back to the Latin noun "anima" or soul [
25]. In this manner, Bichat effectively shifts the traditional paleochristian conception of the soul's abode from the “heart” to the “brain”, a hallmark of the Enlightenment era.
In 1886, Langley employed nicotine and other medications to meticulously investigate the sympathetic and parasympathetic nervous systems, which he subsequently unified under the concept of the 'Autonomic Nervous System' [
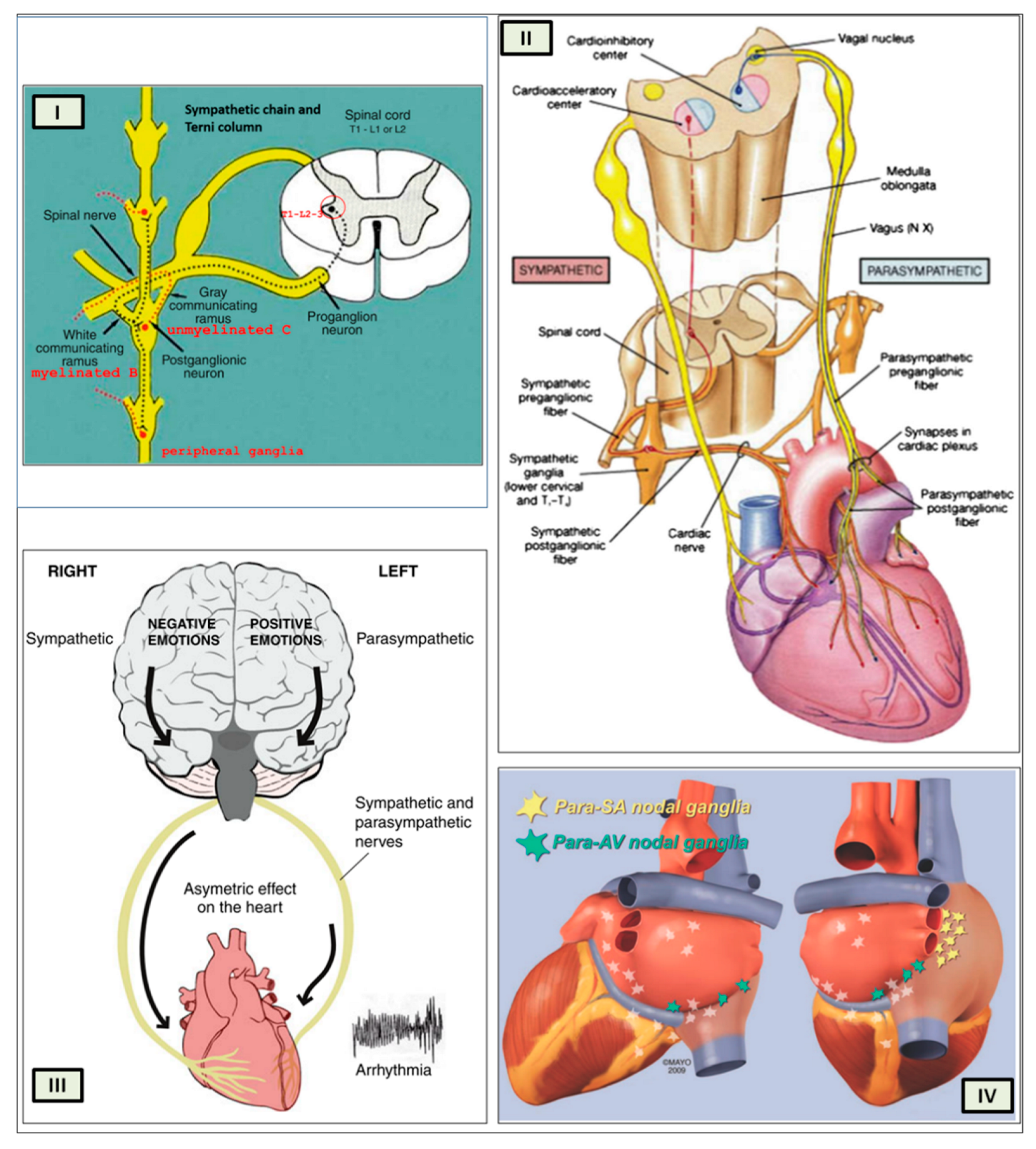
26]. Several regions of the neuraxis orchestrate the central control of orthosympathetic and parasympathetic output. This intricate network of central autonomic connections plays a pivotal role in regulating visceral functions and maintaining homeostasis by adapting to internal and external stimuli. The central autonomic system is hierarchically organized into four distinct levels: spinal, bulbopontine, pontomesencephalic, and forebrain (
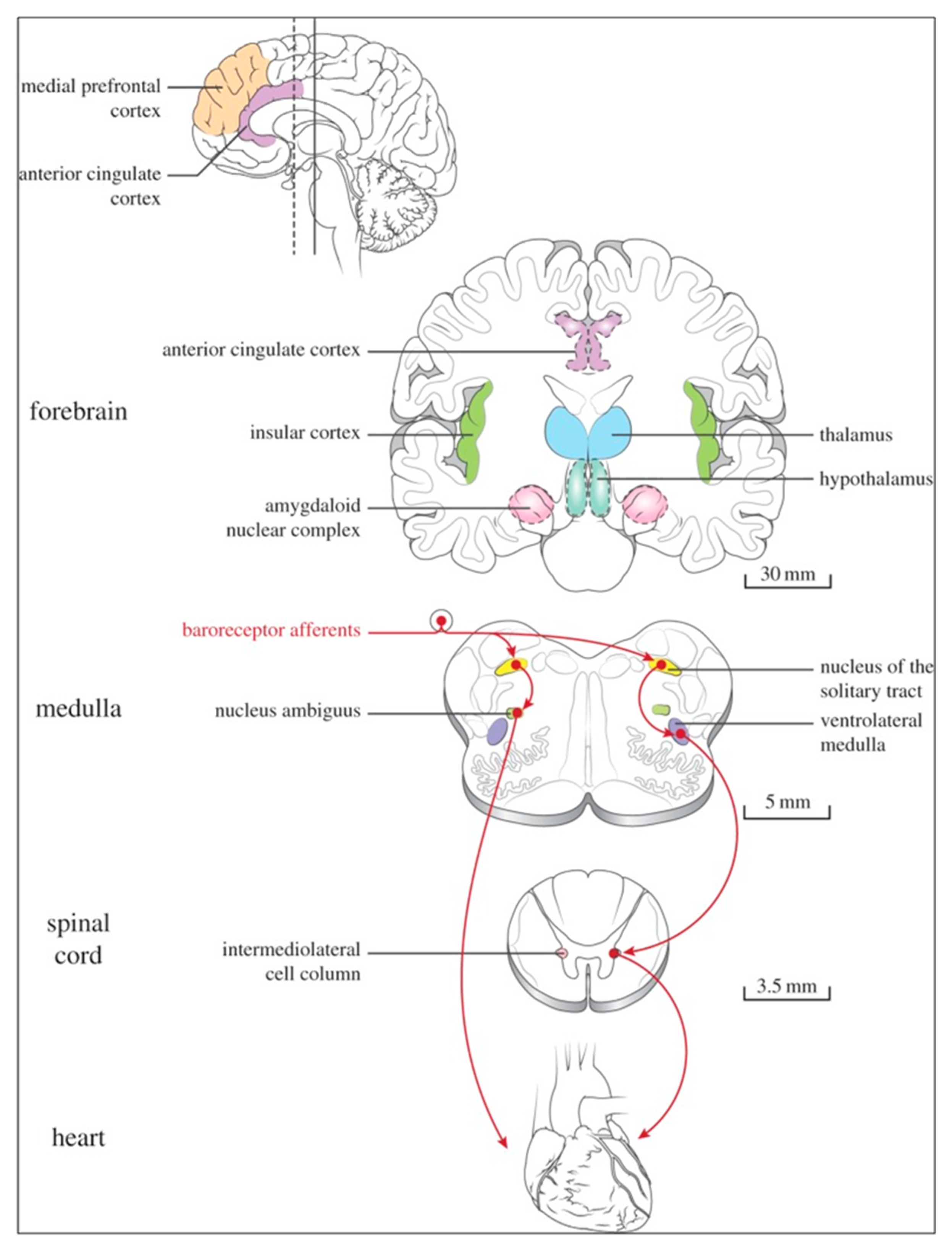
Figure 6 and
Figure 7) [27, 28, 29].
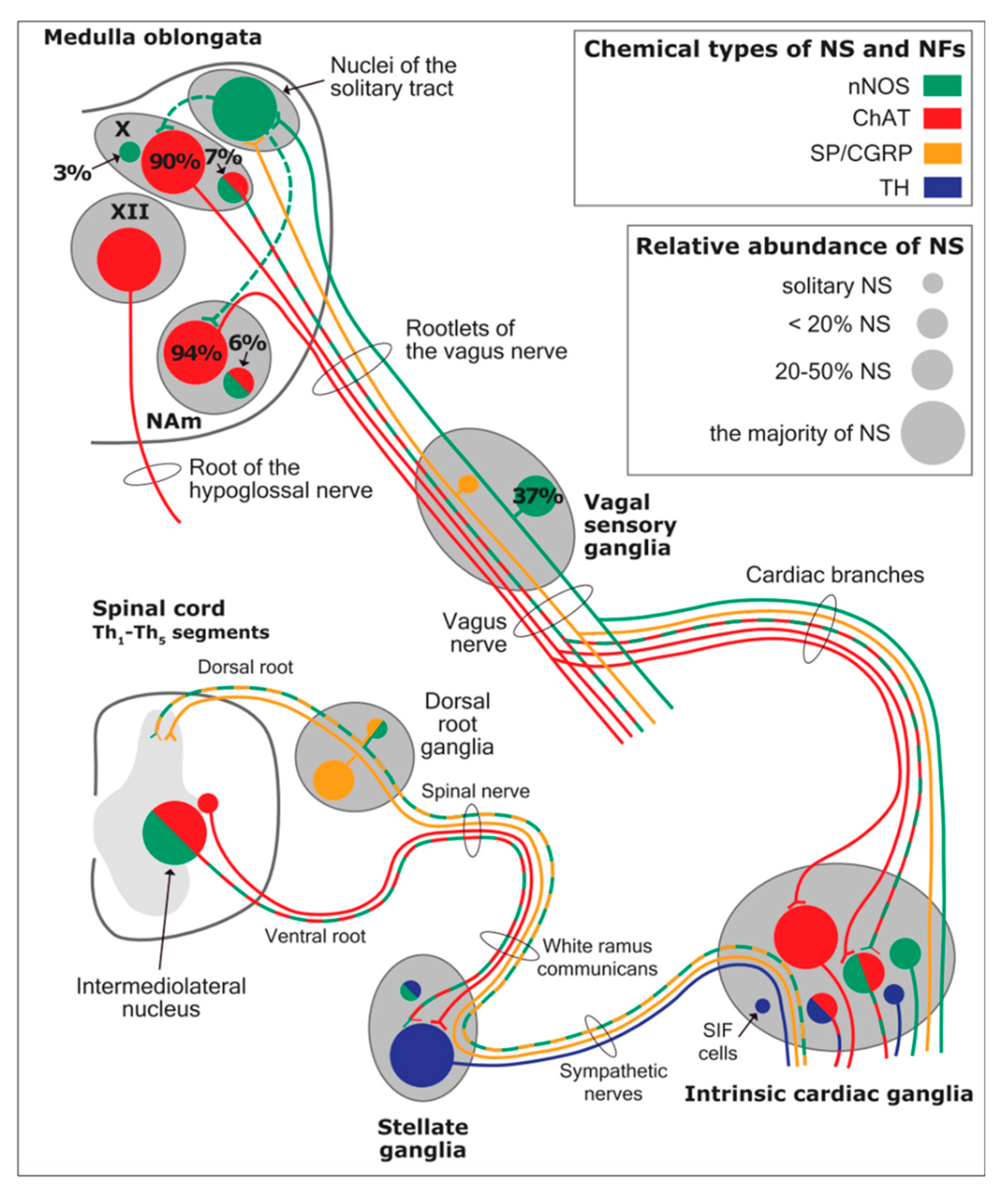
The cardiac preganglionic parasympathetic axons of the vagus nerve (in red) primarily arise from medullar neurons placed in the dorsal motor nucleus of the vagus (DMV) expanding presumably wider into the ventrolateral portion of the nucleus ambiguus (NAmb). It has been suggested that preganglionic parasympathetic axons originating from neuronal somata adjacent to NAmb are B fibres slowing rapidly the heart rate, conduction and force of myocardial contraction. A second group of cardiac parasympatethic preganglionic axons originates from the dorsal motor nucleus (DMNV) and these are the C fibers propagating slower neural impulses. Selective stimulation of the DMNV axons affects atrioventricular conduction and ventricular contraction and reduces the heart rate (HR), however, it starts off more slowly and exhibits a more varied pharmacology than what is observed after stimulating the vagus nerve's B fibers. The two groups of neurons have somewhat different discharge patterns apart from conduction speed. While the DMNV neurons show a non-uniform, non-respiratory-dependent discharge and are unresponsive to baroreceptors and chemoreceptors, the cardiac neurons next to NAmb exhibit a respiratory rhythm and receive input from both sources. These features of medullar parasympathetic neurons have implied a theory that they presumably reflect differential functions of the two groups. An experiment that targets distinct populations of intrinsic cardiac ganglionic cells by nerve terminals from the proper DMNV and those near the NAmb, each anterogradely labeled with a different fluorescent tracer, supports this theory [
29]. It is also possible that DMNV neurones innervate small, intensely fluorescent (SIF) cells in ganglia. On the contrary, Jones [
30] has hypothesized that the tonic input transmitted by the more slowly moving C fibers may interconnect with the rhythmic respiratory input carried by the faster vagal B fibers on the same cardiac ganglia. The preganglionic sympathetic innervation in blue.
At the spinal cord level, segmental sympathetic or sacral parasympathetic reflexes mediate specific responses to stimuli, albeit under the influence of higher brain regions. These reflexes play a crucial role in maintaining cardiovascular, respiratory, gastrointestinal, and micturition functions. The lower brainstem, also known as the bulbopontine level, plays a pivotal role in reflex control of core physiological processes. It regulates circulation, respiration, gastrointestinal functions, and micturition through a network of interconnected neurons. The upper brainstem, referred to as the pontomesencephalic level, engages in sophisticated pain modulation and behavioral integration in response to stress. It orchestrates physiological and psychological adaptations to challenging situations. The forebrain level encompasses the Hypothalamus, the master regulator of the autonomic nervous system. It interacts with the anterior limbic circuit, a network comprising the Insula, anterior Cingulate cortex, and Amygdala, to modulate emotional and visceral responses.
The cortex of the Insula functions as the primary interoceptive cortex and combines sensations of the viscera, pain, and temperature. The dorsal Insula has an organization that is specialized for visceral processing and acquaires inputs from gustative, visceral, muscle, and skin receptors through the thalamic region. Through their connections with limbic and neocortical association areas, the neurons of the dorsal portion of the Insula extend to the right anterior Insula, which connects these interoceptive signals with emotional and cognitive elaboration. As a result of this integration, one can consciously sense their body. Additionally, the Insula acts as a visceromotor area and controls both sympathetic and parasympathetic outputs, primarily through a relay in the lateral Hypothalamus.
The anterior Cingulated cortex is connected to the anterior portion of the Insula and is divided in ventral (emotional and default mode network) and dorsal (cognitive and frontoparietal awareness networks) regions. Subcallosal and precallosal regions make up the cortical ventral anterior part of the Cingulate gyrus, which is connected to the brainstem, Prefrontal cortex, Amygdala, Hypothalamus, and the Insula on an extensive level. Through their connections, the Anterior Cingulate cortex regulates Autonomic systems.
The Amygdala enriches the neuronal sensory afferents with an affective and/or emotional connotation through a downstream neuronal network with which it participates in the neuroendocrine and autonomic response to stress. Through its extensive connections with the Hypothalamus and Brainstem, including the Periaqueductal gray and the medullary reticular region, the central nucleus of the Amygdala (CeA) plays a key role in the coordination of stress responses, particularly alarm responses. The Hypothalamus is a center for visceromotor activity and starts selective patterns of autonomic and endocrine responses based on various stimuli such as variations in the temperature of blood, hypoglycemia, osmolarity, or outside agents of stress. The Preoptic-hypothalamic area is divided into three functional regions: Periventricular, Medial, and Lateral. The periventricular zone comprehends the the circadian pacemaker, localized in the Suprachiasmatic nucleus, and numerous regions implicated in neuroendocrine manage through the pituitary gland. The medial zone includes the Medial Preoptic area, Dorsomedial nucleus (DMH) and Paraventricular nucleus (PVN), which are involved in osmoregulation, thermoregulation, and stress reactions. The PVN, DMH, and Lateral Hypothalamic areas are the primary autonomic efferences of the hypothalamus. The PVN contains several groups of neurons that are selectively activated during stress responses, including Magnocellular neurons that release arginine-vasopressin (AVP) into the systemic circulation, neurons that release corticotropin-releasing hormone and stimulate the adrenocortical axis, and neurons that project to brainstem and spinal cord autonomic nuclei. The PVN regulates stress reactions, food and sodium intake, glucose metabolism, and cardiovascular, renal, gastrointestinal, and respiratory functions through these efferences. The DMH is involved in stress responses, thermoregulation, and cardiovascular control. Arousal, eating, and reward-driven behaviors are all regulated by hypocretin/orexin neurons in the posterior lateral Hypothalamus.
The Periaqueductal Grey matter of the midbrain (PAG), the Parabrachial nucleus (PBN) of the pons, and various medullary regions, such as the nucleus of the solitary tract (NTS), the ventrolateral reticular formation of the medulla, and the medullary raphe (
Figure 6 and
Figure 7) are all involved in autonomic output [
28]. The PAG is comprised of several longitudinal columns that coordinate the micturition reflex, participate in cardiovascular responses related to respiratory regulation, and modulate pain through their diverse spinal, brainstem, and cortical connections. The PAG integrates forebrain and lower brainstem activity in response to tasks including stress, pain control, and somatic adaptative changing. The PBN is an important location for relaying information to the Hypothalamus, Amygdala, and Thalamus from the spinal cord's converging visceral, thermoreceptive, and discomfort stimuli. The PBN in particular plays a significant role in the regulation of the digestive, cardiac, and breathing systems. The NTS has multiple subnuclei that are arranged "viscerotropically" (i.e., with a special affinity for various organs) and acts as the first relay station for flavour and visceral afferent information. Therefore, taste inputs are allowed in the rostral region of the NTS, gastrointestinal afferents are allowed in the intermediate part, and baroreceptor, cardiac, chemoreceptor, and pulmonary afferents are allowed in the caudal part. The NTS sends this neuronal input to the upper brainstem and forebrain areas, either directly or via the PBN. As a result, the NTS serves as the first central " switch"-station for all medullary reflexes controlling the function of the heart (baroreflex and cardiac reflexes), the breathing process (carotid chemoreflex and pulmonary mechanoreflexes), and digestive tract peristalsis. The Rostral Ventrolateral Medulla (RVLM), which contains the C1 group of adrenergic neurons holding epinephrine, is a crucial region for the control of arterial blood pressure. The sympathoexcitatory RVLM neurons collect and combine a wide range of inputs from the brainstem and forebrain, particularly the Hypothalamus, including the PVN. The efferent neurotransmission of Glutamate in the RVLM sends direct and rhythmic stimulation to sympathetic preganglionic neurons, which govern the cardiac function and peripheral resistance in general. The cardiopulmonary reflexes, baroreflex and chemoreflexes belong to the reflexes under the control of the RVLM. These reflexes are mediated by inhibitory GABA neurons in the distal Ventrolateral medulla, which receives inhibitory signals from the baroreceptor-sensitive neural pathway in the NTS.
GABAergic neural populations of the caudal ventrolateral medulla preserve a ritmic, constantly slow inhibitory manage on the upper ventrolateral medulla and retransmit the inhibitory signals from the NTS, producing the inhibitory element of the sympathetic arterial baroreflex. Data from neural stimulations show that the caudal medulla holds pressure-dependent region too. Furthermore, the A1 neuronal population in the distal ventrolateral medulla releases Norepinephrine to the Hypothalamus and is implicated in a reflex route that causes the production of Vasopressin or Arginine Vasopressin (AVP) in reaction to hypovolemia or hypotension.
The upper portion of the ventromedial medulla, which includes the Caudal Raphe nuclei, is important for body temperature control, pain regulation, and autonomic respiratory control. Through input to preganglionic sympathetic neurons, which cause effective cutaneous vasoconstriction and thermogenesis without shaking in brown adipose tissue, one group of medullary raphe neurons initiates adrenergic responses to cold.
The maintenance of circulation, temperature control, and displacing of localized blood flow during stress and exercise depend heavily on the sympathetic efferences. Pregangliar neurons, which are mostly found in the intermediolateral cell column of the thoracolumbar spinal cord's T1 to L2 segments, are the source of sympathetic efferences. The intermediolateral neurons are organized into segregated functional units that give the innervations to specific subgroups of sympathetic ganglion neurons and collect different separate portions of afferent inputs, triggering segmental somatic and viscerosympathetic reflexes. Premotor neurons in the brainstem and Hypothalamus recruit different pregangliar sympathetic units in an integrated manner to start particular patterns of responses to particular internal or external events that cause stress, such as exposure to extreme temperature, low blood sugar, dehydration, changes of posture, physical activity, and/or stress. The principal sources of premotor sympathetic innervation include the RVLM, medullary raphe, A5 noradrenergic neuronal population of the pons, PVN, and lateral hypothalamic region.
Opposite to the sympathetic system, which affects multiple effectors, the parasympathetic system mediates organ-specific reflexes. Most vagal preganglionic parasympathetic neurons, which are arranged viscerotropically and innervate local ganglia in the liver, pancreas, enteric nervous system (ENS), and respiratory tract, are found in the DMV. The DMV generates all vago-vagal responses controlling digestive tract movement and secretion after receiving information from the NTS. The Dorsal Motor nucleus is the source of a substantial population of cardiac vagus nerve (
Figure 6).
The NTS activates the cardiovagal neurons next to the NAmb during the baroreflex and inhibits them during inspiration, as will be covered in greater detail later. For life in a constantly changing environment and to maintain homeostasis, interactions between the heart and the brain are crucial (
Figure 8) [31, 32]. Neurons located in the lateral grey matter at the S2-S4 segments of the sacral spinal cord are the source of the sacral preganglionic output. With synchronized interactions with both lumbar sympathetic neurons at the T12-L2 levels and somatic motor neurons of the Onuf nucleus at the S2-S4 levels, which innervate the external urinary sphincter and pelvic floor, these neurons play a crucial role in normal defecation, micturition, and sexual organ function (
Figure 6) [
27].
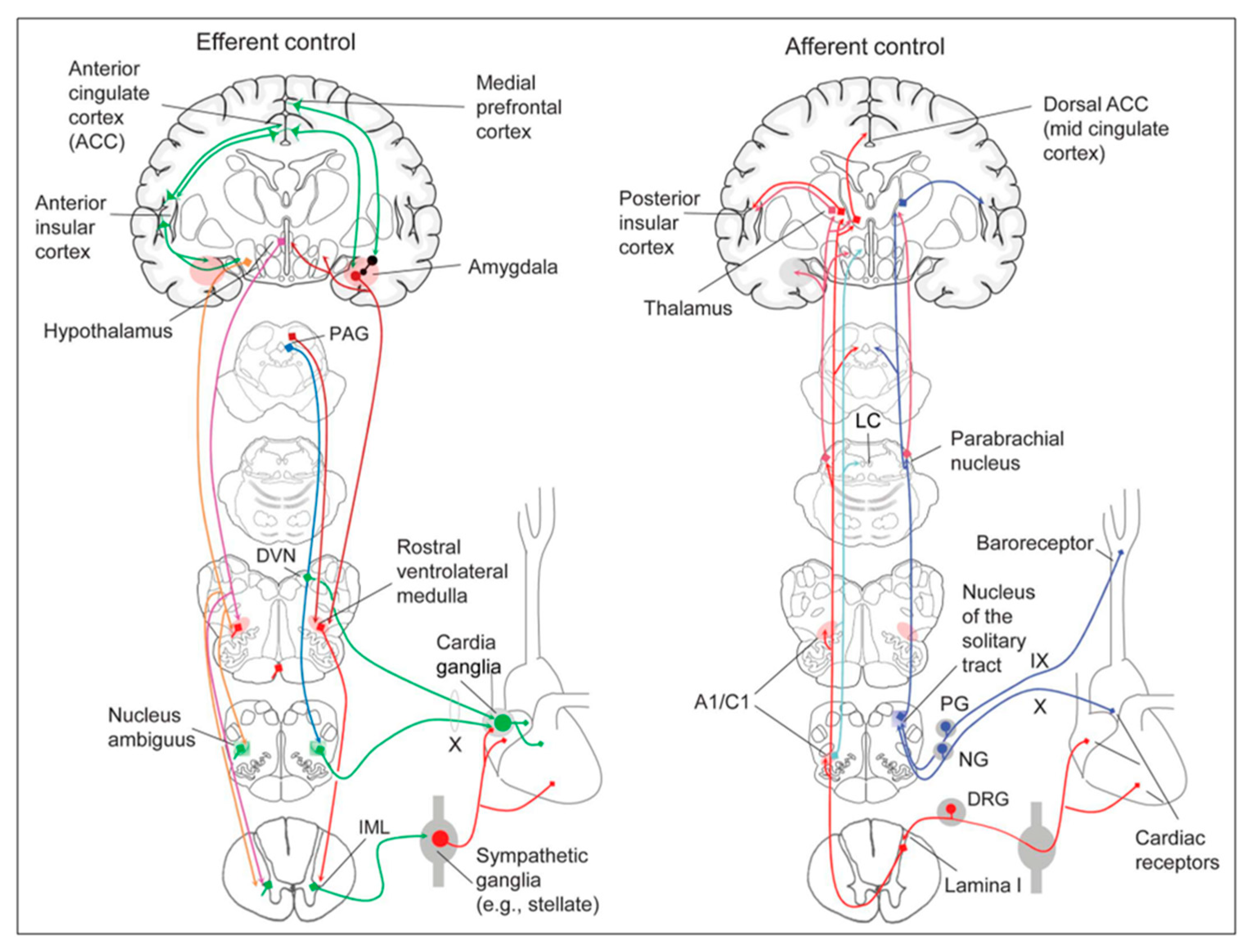
Cardiac function is controlled neurally by the anterior insular region, anterior part of the cingulate gyrus cortex (ACC), amygdala, hypothalamus, periaqueductal grey matter, parabrachial nucleus, and several medulla areas. Further, these regions are connected to homeostatic reflexes, responses to emotions, and stress reactions. By means of the sympathetic and parasympathetic neural systems, they govern and regulate heart rate (HR) and cardiac contractility (
Figure 9) [
34]. It is still unclear if this central control might be lateralized.
The heart's intrinsic electrophysiological properties arise from pacemaker activity generated by specialized cardiomyocytes within the cardiac intrinsic conduction system, comprising the sinoatrial (SA) node, atrioventricular (AV) node, bundle of His, and Purkinje fiber network. The HR, excitability, and contractile function of these cardiomyocytes depend on the interplay between their intrinsic characteristics and regulation by the vagus and sympathetic nerves via the intrinsic cardiac ganglionated plexus or intrinsic cardiac nervous system (ICNS).
The HR (chronotropism) is regulated by the SA node's spontaneous depolarization (automatism), which is controlled by a “voltage regulator”. The voltage regulator is produced by the cyclic activation and deactivation of different membrane ion channels, and a “calcium regulator” that is triggered by rhythmic release of Ca2+ from the sarcoplasmic reticulum via the ryanodine receptor 2 (RYR2). The rhythmic increase in cytosolic Ca2+ activates the Ca2+-Na+ exchanger current, leading to depolarization.
The cardiac cycle starts with depolarization spreading through connexion channels to neighboring cardiomyocytes, followed by the opening of voltage-gated Na+ (Nav1.5) channels. These channels are rapidly inactivated by depolarization, which activates both L-type calcium channels accountable for the action potential plateau, and voltage-gated K+ channels responsible for repolarization. The synchronized activity of these channels produces the excitability of the His-Purkinje system (bathmotropism), the velocity of AV conduction, or dromotropism (PR interval), and the duration of the cardiac action potential (QT interval). The systolic contraction (inotropism) occurs through excitation-contraction coupling, when calcium released from the sarcoplasmic reticulum through RYR2 binds to the troponin complex and activates the contractile machinery. Cellular relaxation during diastole (lusitropism) occurs when cytosolic Ca2+ is removed by the sarcoendoplasmic reticulum Ca2+-ATPase (SERCA) uptake pump, which is negatively regulated by the protein phospholamban (inhibitor of Ca2+-ATPase).
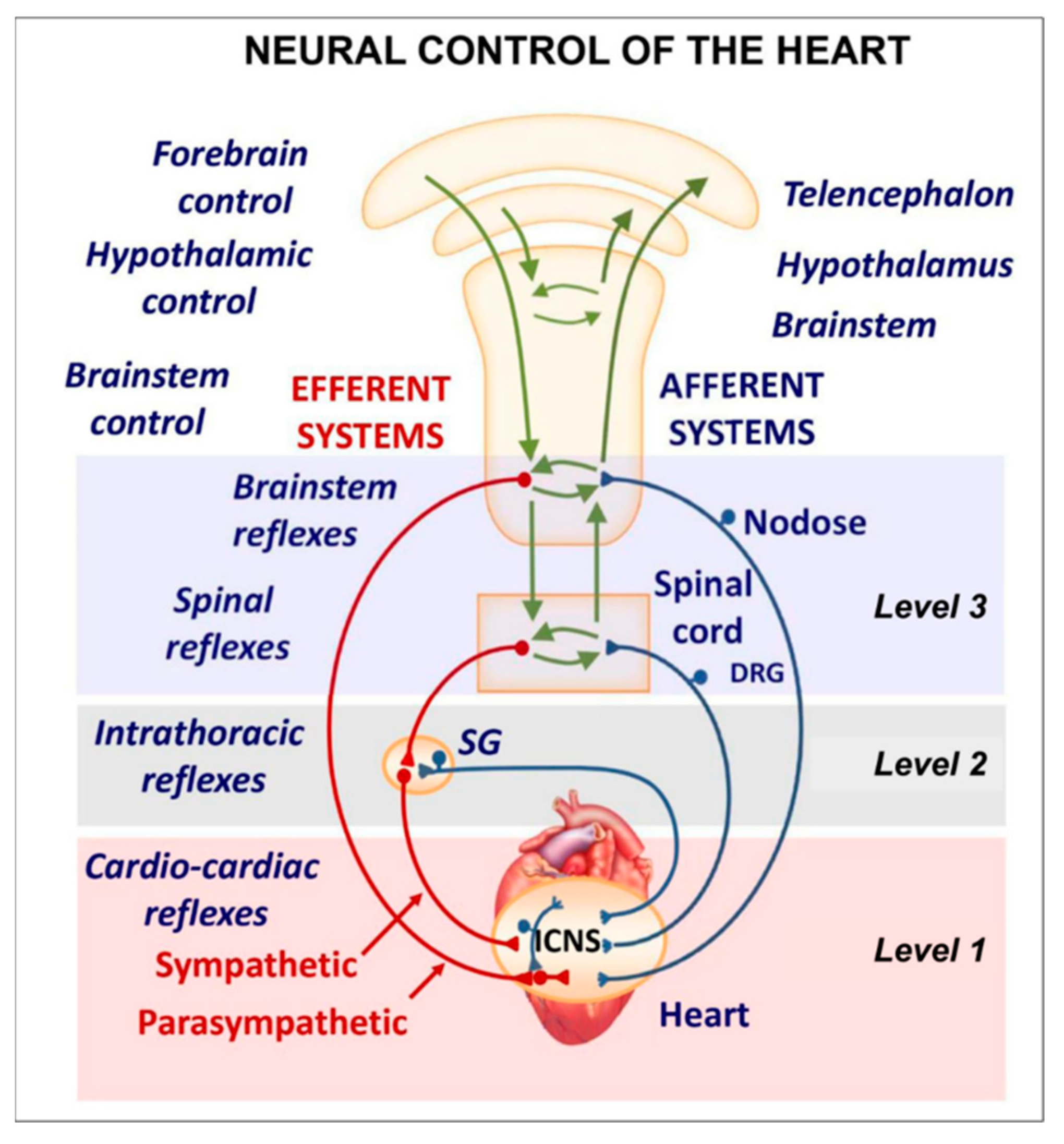
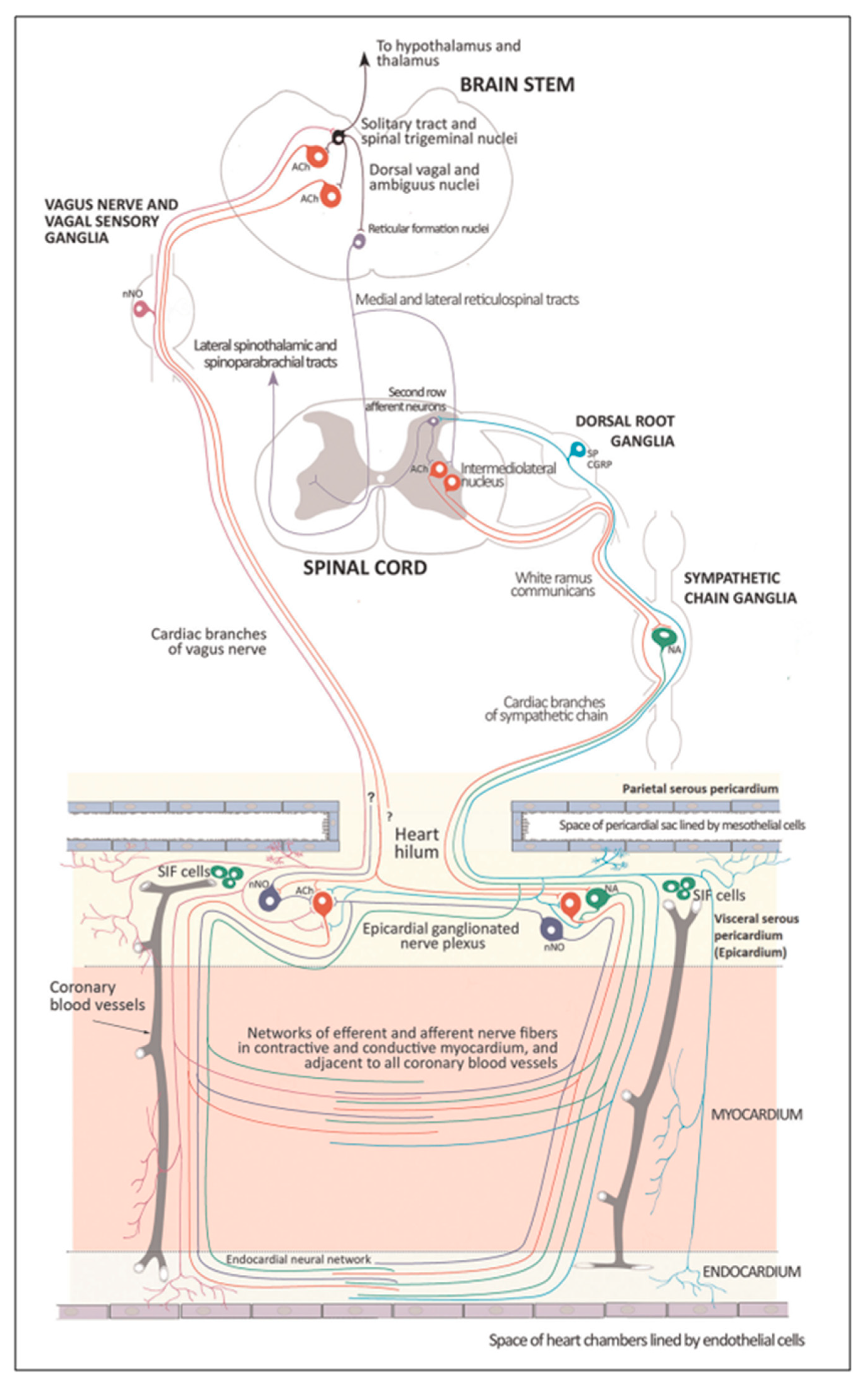
The cardiac nervous system is coupled to areas distributed throughout the neuraxis (
Figure 8 and
Figure 9) and includes intrinsic and extrinsic neural- networks. Morphologically, the ICNS is an intrinsic neural plexus with epicardial ganglia, as described by Lee in 1849 (
Figure 4, III-V). Its function is controlled by extrinsic influences mediated by the vagal and sympathetic nerves. A variety of different kinds of neurons are present in the intrinsic cardiac ganglia, most likely including the following subtypes: afferent neurons, motor (parasympathetic and sympathetic) neurons, and linking local circuit neurons (interneurons) make up the first three types of neurons. Vagal or sympathetic inputs regulate their intrinsic reactivity. The modulation of cardio-cardiac reflexes is significantly influenced by this network of ganglionic cells (
Figure 10 and
Figure 11).
When the sympathetic efferent route is activated, the parasympathetic pathway is often inhibited, and vice versa, according to the obsolete theory of an autonomic balance. The dorsal motor nucleus of the brainstem and the spinal cord, respectively, both possess preganglionic efferents of the parasympathetic and sympathetic systems that innervate the heart. Sympathetic transmission occurs in the sympathetic ganglion chain near the spinal cord from preganglionic (dashed) to postganglionic (solid). Preganglionic parasympathetic axons synapse with postganglionic parasympathetic components in the cardiac plexus, which is reached by postganglionic axons from the sympathetic trunk.
Furthermore, it is evident that certain physiological reactions can entail the simultaneous cardiac stimulation of both sympathetic and parasympathetic activation. In comparison to vagal regulation, the development of adrenergic innervation of the heart occurred relatively later in the course of evolution [it is absent in elasmobranch fish]; hence, the simultaneous activation of both autonomic pathways may be very important in expanding the heart's functional capacity to fulfill the metabolic requirements of the body within constantly changing behavioral and environmental circumstances.
Using immunohistochemical methods, it has been evidentially proven that a range of neurochemical substances are present. Epicardial ganglia, as well as intracardiac ganglia situated within the boundaries of the heart hilum, exhibit immunoreactivity (IR) towards neurotransmitters and neuromodulators such as choline acetyltransferase (ChAT), the enzyme accountable for the synthesis of acetylcholine (ACh); tyrosine hydroxylase (TH), the enzyme which catalyze the production of the sympathetic neurotransmitter noradrenaline (NA); vasoactive intestinal peptide (VIP, recognized to co-release alongside ACh); neuropeptide Y (NPY, reputed to co-liberate together with NA); neuronal nitric oxide synthase (nNOS, involved in NO synthesis by parasympathetic, sympathetic, and non-adrenergic non-cholinergic (NANC) nerves) [
37] (
Figure 12); synaptophysin (a tracer for presynaptic fibers); substance P (sub P), and calcitonin gene-related peptide (CGRP).
Most ganglion cells utilize acetylcholine as their primary transmitter.
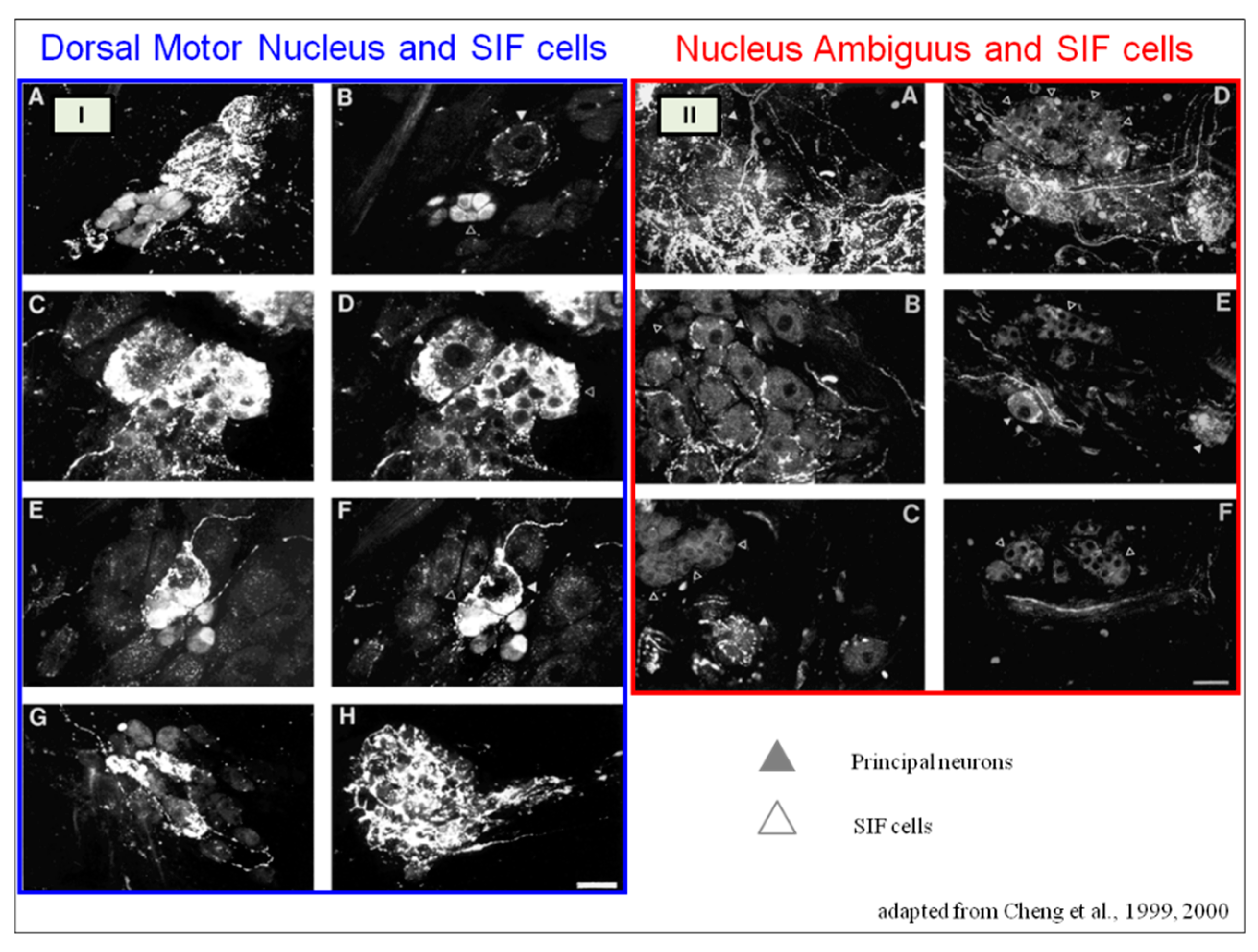
SIF cells are TH- positive and are often found inside of larger ganglia, in tiny clusters, or scattered along the atrial and ventricular walls. The exact function of SIF cells in the heart is still unclear, since the majority of them are not adjacent to axons and presumably are not under neural control, according to numerous observations by Pauza and his co-workers [41, 42; 43, 44]; Pauziene et al., [40, 45].
An intriguing feature of the cardiac ganglia is the presence of biphenotypic neurons, i.e. neurons showing both ChAT-IR and TH-IR. These neurons account for 10 to 20% of all intrinsic cardiac neurons. Cholinergic cardiac neurons may also exhibt immunoreactivity for nitric oxide, i.e. such cholinergic neurons should be considered biphenotypic nitrergic [46, 47]. This feature of producing and perhaps releasing both neurotransmitters is most interesting and needs further investigation [36; 48].
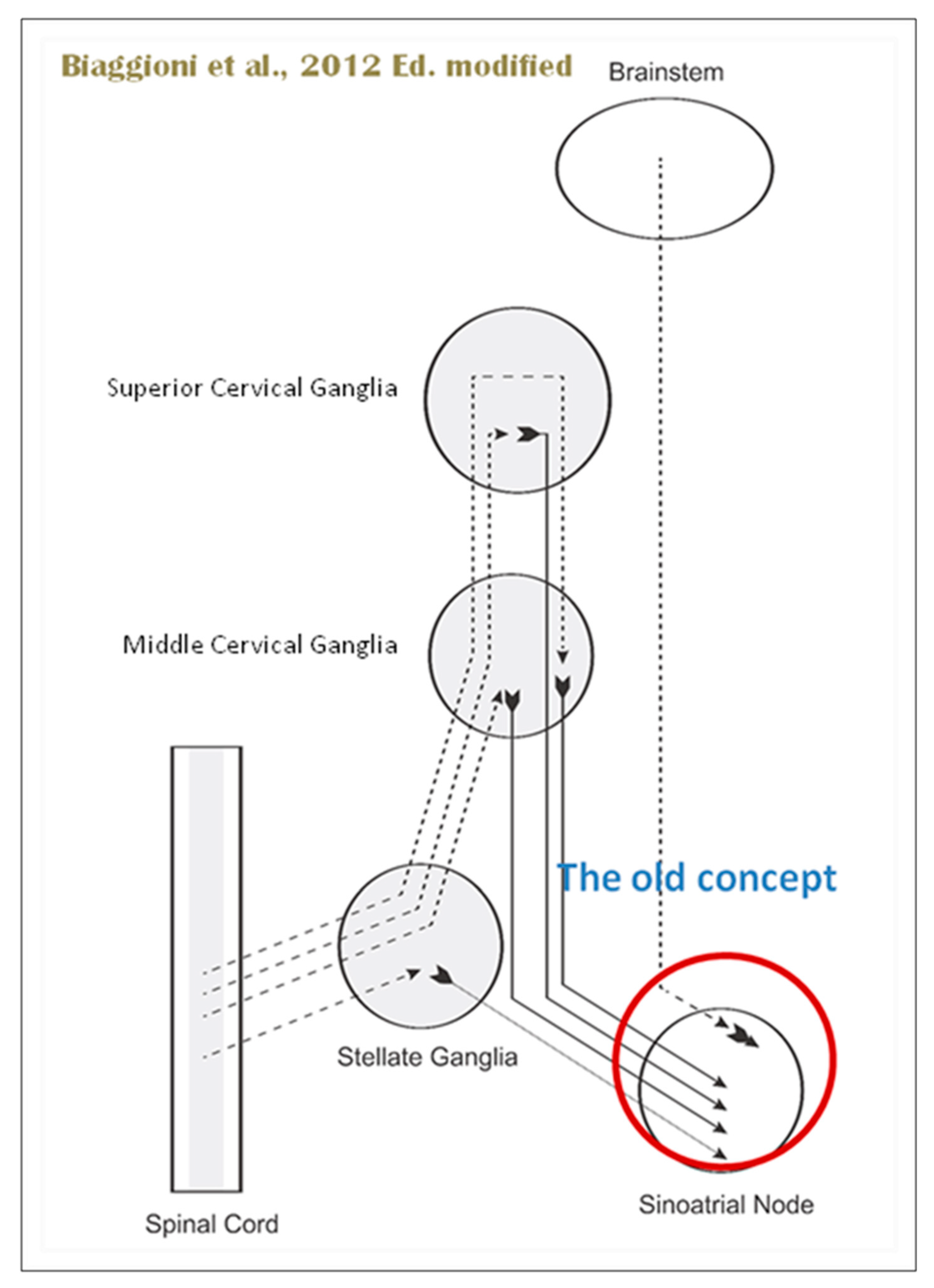
As mentioned above in respect to the extrinsic sympathetic innervation, the cardiac preganglionic axons originate from neurons of the intermediolateral (IML) nucleus of the Terni’s column of the spinal cord (
Figure 5, II). Rhythmic excitatory glutamatergic inputs from neurons of the rostral ventrolateral medulla (RVLM) are collected by IML neurons. The cardiac preganglionic sympathetic neurons are neurons liberating Acetylcholine and project to neurons with Norepinephrine release of the superior and middle cervical, cervicothoracic (stellate), and first four thoracic ganglia, which give rise to axons that innervate the heart via the superior, middle, and inferior cardiac nerves. The right-to-left map of sympathetic nerves is asymmetrical [
50] and shows differences in the expression between individuals; this may clarify their heterogeneous effect on cardiac electrophysiologic properties.
The cardiovagal innervation represents the extrinsic parasympathetic output. The preganglionic cardiovagal neurons are cholinergic and their axons extend to the cardiac ganglia through superior cervical, inferior cervical and thoracic rami which merge with adrenergic nerves of the heart to form the extrinsic cardiac plexus in the mediastinum. Vagal nerve fibres distribute within the walls atria and ventricles, sinoatrial (SA), atrioventricular (AV) nodes, [
51].
Sympathetic activation elicits an increase of inotropism and dromotropism, a faster conduction through the AV node, with an boost in the stimulation of the His-Purkinje system which determines a strenghtening of contraction force during systole, and faster relaxation of the cardiac muscle cells during diastole.
The primary impact of the vagus, on the other hand, is suppression of the pacemaker activity of the SA node (reduction in heart rate, HR), decreased AV conduction, and diminished excitability of the His-Purkinje system by cholinergic neurons of the cardiac ganglia.
The tonic vagal control of the SA node automatism prevails over that one of the sympathetic system during resting conditions. HR has a circadian pattern; it increases in the early morning because the sympathetic activity surges and then decreases during sleep, particularly during non-REM sleep, for vagal predominance. However, a phasic transient vagal interruption and sympathetic activation result in HR surges during REM sleep. The vagal activity rapidly decreases in response to orthostatic stress, hypovolemia, or exercise. In conditions with very low basal HR (e.g., athletes, during non-REM sleep, or patients with sinusal bradycardia), vagal activation could unexpectedly increase HR by reducing the interval between depolarizations of the atria. The vagal activation, particularly in the ventricles, is higher in the setting of prominent simultaneous adrenergic activation; this so-called “accentuated antagonism” depends on presynaptic inhibition of sympathetic transmission.
HR variability (HRV) results from interactions between the vagal and sympathetic influences on the SA node. HRV can be evaluated during deep breathing and during the Valsalva manoeuvre.
The cardiac preganglionic sympathetic IML neurons are tonically activated by premotor glutamatergic sympathoexcitatory neurons of RVLM, which acts as a common effector of descending and reflex pathways controlling blood pressure (BP) and cardiac function; some of these neurons also synthesize epinephrine (C1 group). Psychological stress, pain, hypoxia, hypovolemia, and hypoglycemia activate RVLM neurons both directly and via descending inputs from the forebrain. RVLM is inhibited by the baroreflex via disynaptic inhibition from the NTS, mediated by GABA neurons of the caudal portion of ventrolateral medulla (
Figure 8 and
Figure 5, II). The NAmb contains the majority of cardioinhibitory vagal motoneurons that control SA automatism and AV node conduction. These neurons are activated by glutamatergic inputs from barosensitive neurons of the NTS and inhibited by local GABAergic neurons and by GABAergic neurons of the medullary ventral respiratory group that are active during inspiration. In this way, the vagal control of the HR is modulated on a beat-to-beat basis by respiration: cardiovagal activity is lower during inspiration and higher during expiration. This physiologic condition is known as respiratory sinus arrhythmia (RSA). RSA is an important measure of cardiovagal output and health, and declines linearly with age. In addition, the Hering-Breuer reflex generated by pulmonary mechanoreceptors via the NTS may contribute to the RSA. The caudal region of the solitary nucleus gets afferents mostly from vagal and glossopharyngeal afferents from baroreceptors, cardiac receptors, chemoreceptors, and pulmonary receptors. Respectively, the vagal afferents by aortic baroreceptor and chemoreceptor, cardiac and visceral sensory receptors in most organs of the thoracic and abdominal cavities with cell bodies in the nodose ganglion (NG), together with carotid baroreceptor and chemoreceptor of glossopharyngeal afferents with cell bodies in the petrosal ganglion (PG), provide inputs to the nucleus of the solitary tract. This nucleus begins a variety of cardiovascular reflexes and also carries cardiovascular receptor inputs to the thalamus and parabrachial nucleus. Therefore, it is the primary essential station for all reflexes of medulla, inclusive of the baroreflex and cardiac reflexes controlling BP and HR.
The baroreceptor reflex (baroreflex) is a fundamental BP buffering tool and is activated by the mechanical deformation of vessel walls in the carotid sinus and aortic arch during systole. An increase in BP stimulates baroreceptor afferents of the glossopharyngeal and vagus nerves, which activate the NTS via a monosynaptic excitatory input. The barosensitive NTS neurons initiate sympathoinhibitory and cardioinhibitory responses with two different pathways. The sympathoinhibitory pathway controls total peripheral resistance via disynaptic inhibition of RVLM neurons mediated by Gamma-Aminobutyric Acid neurons of the caudal ventrolateral medulla. Through the second pathway, the cardioinhibitory signal elicits a decrease in HR via direct excitatory inputs from the NTS to cardiovagal neurons of the NAmb. Numerous cardiovascular reflexes are triggered by afferents from the heart, coronary, and pulmonary arteries. Among these are cardiac unmyelinated afferents to the thoracic dorsal root ganglia, which run along the adrenergic nerves and provide input to the dorsal horn (primarily lamina I) and intermediate grey matter of the spinal cord, as well as myelinated and unmyelinated vagal afferents with cell bodies in the nodose ganglion, which provide input to the NTS. On the other side, myelinated vagal afferents are activated by atrial distension due to an increase in blood volume, which triggers the reflex of sympathetic input to the SA node, resulting in an increase in HR, as well as suppression of renal adrenergic activity and arginine vasopressin production, favoring salt and water excretion. Furthermore, unmyelinated spinal and vagal afferents innervating the ventricles are stimulated by strong mechanical or chemical stimuli, in particular products of ischemia or inflammation such as adenosine triphosphate, serotonin, and prostanoids.
Spinothalamic projections from lamina I neurons elicit the sensation of cardiac pain; these afferents can also trigger excitatory cardiac reflexes via local interneurons projecting to the IML (the so-called “cardio-cardiac reflex”). In response to chemical stimulation of myocardial injury, unmyelinated vagal afferents in the ventricles may trigger a decrease in BP and HR (Bezold-Jarisch reflex). Stimulation of pulmonary arterial baroreceptors at physiologic pressure produces reflex vasoconstriction and respiratory stimulation. This could be implicated in cardiovascular control during exercise or in hypoxic conditions.
The cortex of Insula (IC), ACC, Amygdala central nucleus (CeA), and numerous nuclei of Hypothalamus (
Figure 8) project to medullary and spinal nuclei to control the activity of the heart. These projections are either direct or indirect via a relay in the periaqueductal grey. Afferent cardiovascular information carried by the dorsal horn (layer I) or NTS neurons reaches cortical areas through the Thalamus. Visceral afferents are also conveyed to the parabrachial nucleus of the pons, which relays the information to the Thalamus, Hypothalamus, and Amygdala, and to catecholaminergic neurons of the A1/C1 group of the ventrolateral medulla (
Figure 6). The role of these areas and the possible hemispheric lateralization of the control of cardiac function are yet poorly understood. The IC has been implicated in a wide range of functional activities as well as the underlying causes of several neurologic diseases. It is separated into two zones: dorsocaudal and rostroventral. The dorsocaudal zone contains many areas that receive thalamic-relay gustatory, viscerosensory, somatosensory, pain, and vestibular afferents. The rostroventral zone, which is connected to the ACC and the amygdala, is primarily engaged in affective neural elaboration. Electrical stimulation of the insula in patients undergoing surgical treatment for intractable epilepsy elicits a variety of visceromotor phenomena, including changes in BP and HR. Oversimplifying, stimulation of the left IC more frequently elicited a small decrease in HR and BP whereas stimulation of the right IC elicited the opposite effect. These findings, further supported by fMRI studies, suggest that the left IC primarily regulates the parasympathetic and the right IC the sympathetic influence on the heart (
Figure 5, III) [
51].
The “laterality hypothesis” has a “cardiotopic” counterpart of asymmetric autonomic nerve distribution around the heart. This complex rotation around the heart axis may be correlated to remodelling/regression of the right aortic arch during embryonic development, whereas the left-sided arch persists and/ or to the cardiac tube rotation during the formation of the four embryonic heart chambers. Typically, the right vagus regulates the activity of cardiac atria whereas the left vagus controls the activity of ventricles. HR is therefore regulated by asymmetric sympathetic and parasympathetic innervation of the sinoatrial (SA) node. In factual terms, the right stellate cardiac nerve is the primary source of sympathetic input of sinus atrial node, whereas the right vagus is the primary source of parasympathetic SA input. Contrarily, left side inputs are primarily responsible for controlling conduction time in atrioventricular fibres, which is enhanced by parasympathetic and reduced through sympathetic activity, again in both systems. However, left-side sympathetic stimulation is primarily responsible for increasing contraction of the myocardium. In conclusion, the right side primarily regulates cardiac frequency, whereas the left side mostly controls ventricular activity and pulse pressure. (
Figure 5, IV). The evaluation of asymmetric anatomical and functional autonomic nerve distribution in humans is important to understand anomalies of heart with left-right predominance and to identify new treatments [51, 52,53].
The ACC integrates autonomic responses with behavioral arousal via its wide associations with the IC, Amygdale, Prefrontal cortex, Hypothalamus, and Brainstem autonomic nuclei. Functional MRI studies show that the ventral ACC is involved, as described above, in the “default mode network”, activated in the resting state in conditions of self-monitoring. Whereas, the dorsal ACC, together with the anterior IC, is a central element of the so-called “salience network”; it is primarily recruited during tasks which demand cognitive control such as conflict resolution, and is connected with an enhance in sympathetic conduction, resulting in an HR increase. On the contrary, the subgenual ACC and adjacent ventromedial prefrontal cortex are inactivatedat the same time. Pharmacological and neuroimaging studies show that subgenual ACC stimulation depends on HRV mediated by vagus nerve, particularly in the right hemisphere.
The Amygdala provides emotive meaning to inputs from sensory systems and is engaged in fear- conditioning processes. The Amygdala includes several nuclei, among these, the basolateral nuclear complex and the CeA. The medial aspect of the CeA projects to the hypothalamus and brainstem and triggers the autonomic, endocrine, and motor manifestations of fear responses. sympathoexcitatory responses involve excitatory connections to the RVLM and inhibition of barosensitive neurons of the NTS. Functional neuroimaging studies have demonstrated a coactivation of the lateral and medial amygdala in relation to changes in HRV both at rest and during emotional tasks. The orbitofrontal and ventromedial prefrontal cortices offer an inhibition on the Amygdala via GABAergic neuronal population in the lateral CeA and in the intercalate nucleus between the basolateral Amygdala and the CeA; these prefrontal modulation are involved in mechanisms of emotional regulation, including fear extinction. In fact, in addition to promoting vagal output, these prefrontal areas tonically inhibit sympathoexcitatory responses initiated in the amygdala.
Sympathoexcitatory responses during stress are mediated by projections from the Hypothalamus. It tunes autonomic output to the heart via inputs that originate primarily from the PVN, DMH, and lateral hypothalamic area; these hypothalamic projections reach the Periaqueductal Grey Parabrachial nucleus, RVLM, NAmb, Dorsal Motor nucleus of the vagus, NTS, and IML. Hypothalamus modulates the baroreflex via NTS or NAmb connections. Experimental studies indicate that the inputs from the IC to the Hypothalamus are mostly ipsilateral.
Lower HRV and baroreflex sensitivity (BRS) are important markers of cardiovascular risk, including an increased incidence of ventricular arrhythmias in individuals with primary cardiac disease, due to the fact that they reflect deficiencies in forebrain vagal or sympathetic drive, brainstem reflexes, or vagal or sympathetic output (as occurs in diabetic or amyloid neuropathy). Reduced HRV, mainly at the expense of decreased high-frequency HF (vagal) component and sometimes associated with indices of increased sympathetic activity, has been described in patients with ischemic stroke, epilepsy, multiple sclerosis, and Parkinson’s disease.
Central autonomic abnormalities can cause a wide range of cardiac arrhythmias, some of which are life-threatening. Vagal hyperactivity, for example, causes bradyarrhythmias such as AV block, whereas sympathetic overdrive causes both supraventricular and ventricular tachycardia, and both sympathetic and vagal hyperstimulation can cause atrial fibrillation (AF). Sympathetic activity in the ventricles is proarrhythmic, whereas vagal activation is antiarrhythmic.
Seizures and cardiac dysfunction are characterized by a number of shared features, including: cardiac arrhythmias as an ictal event that can localize the seizure focus, seizures and cardiac arrhythmias as co-occurring manifestations of channelopathies, and cardiovascular dysregulation and ictal arrhythmias as a potential mechanism underlying Sudden Unexpected Death in Epilepsy (SUDEP). These features suggest that seizures and cardiac dysfunction may be linked by common underlying mechanisms. Sinus tachycardia occurs in 80% to 100% of patients before, during, or after a temporal lobe seizure; paroxysmal AF, supraventricular or ventricular tachycardia, and ventricular fibrillation may also occur. Furthermore, temporal lobe seizures may lead to ictal bradycardia and asystole. Seizures, more common in patients with left-sided seizures, can also produce alterations of cardiac repolarization as manifested by acute changes in corrected QT (QTc) interval, which may be in part due to ictal hypoxemia. Mutations in genes encoding Na+ or K+ channels are associated with the coexistence of long QT syndrome (LQTS) or Brugada syndrome and epilepsy. Autonomic cardiovascular manifestations of seizures may have a role in the pathophysiology of sudden unexpected death in epilepsy, but this causal relationship is yet to be established.
Autonomic nervous system dysfunction is a widespread occurrence that relates significant heart diseases to neurologic problems. Many neurologic diseases, such as ischemic stroke, sudden cardiac death, and epilepsy, may have higher morbidity due to these severe autonomic effects on the heart.
LIGHT- HEARTEDLY
At the end of our review, we wish to propose a point of convergence between the sacredness of psychostasis with its social and artistic expression, and the science of cardiac innervation. We believe that they converge in the biological nature of contemporary neuroscience and introduce us to the neural mechanisms of cardioprotection and resilience.
As we discussed previously, a prevailing notion posits the concept of autonomic balance, whereby the activation of sympathetic and parasympathetic efferents is mutually inhibitory. While this holds true for the baroreceptor reflex, there is evidence to suggest that certain physiological responses may elicit simultaneous activation of both systems to safeguard the heart. In fact, the regulation of heart function is often governed by the synergistic interplay of sympathetic and parasympathetic stimulation, mirroring the combined control of urine production, penile erection and the activity of secretory glands by both autonomic branches. For instance, combined stimulation of the sympathetic and vagal fibers in the heart can result in bradycardia (by peripheral chemoreceptors), tachycardia (by somatic nociceptors), or biphasic rhythms in the cardiac frequency (during the startle response). The cardiac response elicited during a dive provides a prime example of the potential physiological implications of combined sympathetic and parasympathetic activation. Provoked bradycardia is a remarkable physiological adaptation that markedly lowers cardiac oxygen consumption, effectively shielding the heart from the detrimental effects of hypoxia. Given that bradycardia significantly diminishes ventricular contractions due to non-neural mechanisms, it is plausible that augmenting the influx of noradrenergic signals to the ventricular myocardium could counterbalance the frequency-dependent contractility reduction, thereby optimizing stroke volume and emulating the conditions induced by the chemoreflex. Another method might be increased sympathetic outflow via the stimulation of beta-adrenoreceptors in coronary arterioles, which may reduce the resistance of the coronary artery in order to sustain blood flow and oxygen release to the heart. A hypothesized explanation for this combined effect involves a likely release of neuropeptides in ICNS or the activation of SIF cells in the ganglionic population of the heart. SIF cells have extensive vagal innervation in the cardiac ganglia and their fluorescent activity is confined to vesicles carrying catecholaminergic neurotrasmettitors. Therefore, the discharge of catecholamines from these vesicles may be the cause of the paradoxical vagally mediated tachycardia (54, 55, 56, 57, 58) (
Figure 13). One of our Authors remains sceptical about the theory of vagal innervation of SIF cells, as this is in sharp contrast to what seen in analogue cells of the adrenal medullas. Although in rare cases nitrergic axons are adjacent to SIF cells, Pauza has not identified parasympathetic (cholinergic) nerve fibres in close vicinity to cardiac SIF cells across many animals such as rats, mice, guinea-pigs, rabbits, pigs, sheep and humans [
59] (
Figure 14). Schematic representations of the two hypotheses are provided in
Figure 13 and
Figure 14.
Yet another plausible explanation for heart-activity-dependent tachycardia is the release of a co-transmitter from the vagus nerve, accompanied by a distinct pattern of co-vagal neuropeptide transmission under various conditions. Moreover, it can be posited that dysregulation of this cardioprotective balance system can act as both a cause and an outcome of numerous cardiac pathologies [
54].
Resilience has bestowed an additional evolutionary advantage upon the psyche-soma relationship, entailing an individual's ability to swiftly bounce back and adapt to adversity and/or stressful conditions while safeguarding psychophysiological assets. Greater resilience is correlated with faster cardiovascular recuperation following subjective emotional experiences, manifest as reduced perceived stress, enhanced recovery from illness or trauma, and improved management of chronic pain and early dementia. Compromised resilience stems from autonomic nervous system dysregulation, which can be assessed by measuring vagal regulation via RSA. As we've already mentioned, RSA is the rhythmic variation in heart rate that is linked to breathing and is brought on by the vagal nerve's activity. The heart rate increases during inhalation when vagal activity is reduced, but at some point during expiration, vagal activity is reintegrated, causing the heart rate to slow down. The activation of a branch of vagal fibers that originates from the medullary NAmb and terminates at the sino-atrial node of the heart is the primary mechanism by which RSA is induced.
As proposed by Stephen Porges's 1995 Polyvagal Theory (PVT), the NAmb vagal fibers represent novel evolutionary adaptations that have emerged to facilitate complex emotional responses and social interactions among animals. Accordingly, RSA measures are considered to offer a non-invasive window into how this relatively recent NAmb vagal system engages with emotional experiences [60, 61].
PVT postulates the existence of three distinct neural networks within the sympathetic and parasympathetic nervous systems that respond to the perception of risk (i.e., safety, danger, threat to life) in a hierarchical manner governed by an evolutionary principle. This order of activation aligns with the Jacksonian principle of dissolution, which suggests that newer adaptation networks are initially engaged, and if they prove insufficient, older systems are recruited [
62]. From this perspective, the three autonomic systems form a phylogenetically ordered hierarchy of responses [
63].
Consequently, the three polyvagal neural networks are associated with social communicative behavior, the defensive strategy of "aggressive" mobilization, and defensive immobilization. Specifically, they represent: 1. The ventral vagal complex (VVC) underpins the brain mechanisms that mediate the "social engagement system," attitudes toward others, and relationships. The NAmb, the motor component of the VVC, governs and controls the muscles of the pharynx, larynx, and possibly the bronchi and heart. 2. The fight-or-flight response, the fundamental and earliest defense mechanism employed by animals, is primarily associated with the sympathetic nervous system. This defensive strategy necessitates heightened metabolic output to facilitate locomotor behavior. When the VVC fails to quell the perception of danger, the sympathetic system assumes hierarchical precedence. Upon activation, the sympathetic nervous system elicits a cascade of physiological changes, including increased muscle tone, redistribution of blood to major muscle groups, suppression of gastrointestinal function, dilation of bronchioles, augmented heart rate and respiration, and the release of catecholamines. 3. Emerging from the DMNV, the dorsal vagal complex (DVC) delivers the primary vagal motor fibers to the subdiaphragmatic organs and innervates the cardiac ganglia. This circuit, designed to respond adaptively to overwhelming threat or fear, represents the most primitive (i.e., evolutionarily earliest) stress response. When activated, the DVC triggers a passive reaction characterized by a decrease in muscle tone, a significant reduction in cardiac output to conserve metabolic resources, and changes in intestinal and bladder function via reflexes leading to defecation and urine excretion. This visceral suppression represents an effort to minimize metabolic and oxygen demands to the bare minimum for survival, particularly in its cardioprotective role. This response often manifests as behavioral shutdown, collapse, and a "freezing" response in humans, and can be interpreted as a dissociative state that can lead to loss of consciousness.
Therefore, saying that a resilient person faces life lightly, has a physiological value because this person shows a strong aptitude to activate the new parasympathetic system (VVC) and stimulate left hemispheric predominance. (
Figure 15) [
63].
This comprehensive review has meticulously traced the historical trajectory of medical and neuroanatomical research on cardiac innervation, shedding light on the nascent biomolecular concepts of cardiac neuromodulation. This endeavor underscores the unremitting progress of anatomical science, poised to be further enriched and corroborated by genetics and genomics studies in the foreseeable future. The recent identification of neurocardiac genes has emerged as a paradigm-shifting discovery. These genes typically encode ion channel proteins, simultaneously expressed in both the autonomic nervous system and the myocardium. Alterations in these genes now appear to be implicated in diverse instances of sudden cardiac and neurological demise [
65]. As such, this line of inquiry warrants vigorous pursuit. The neurocardiac axis stands as the cornerstone of the nascent scientific understanding of psychostasis. Recently, the polyvagal theory has been leveraged to illuminate the significance of the neurocardiac axis and its interplay with the COVID-19 pandemic [66, 67] and other respiratory infections, culminating in autonomic nervous system dysregulation.
In summation, further research is imperative to unravel the intricate implications of the neurocardiac axis for psychostasis, long COVID, and long non-COVID-19 ARIs.