Submitted:
25 January 2024
Posted:
25 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Bispecific T Cell Engager
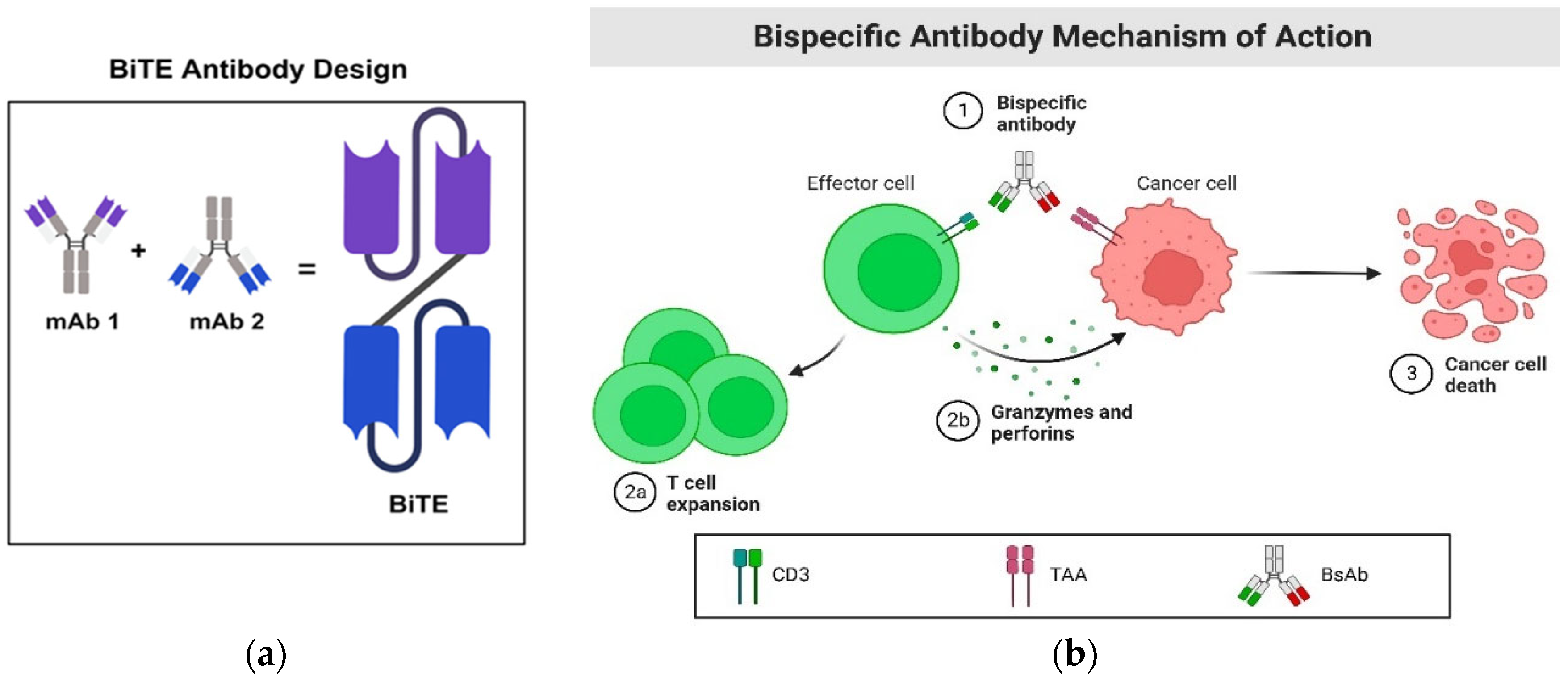
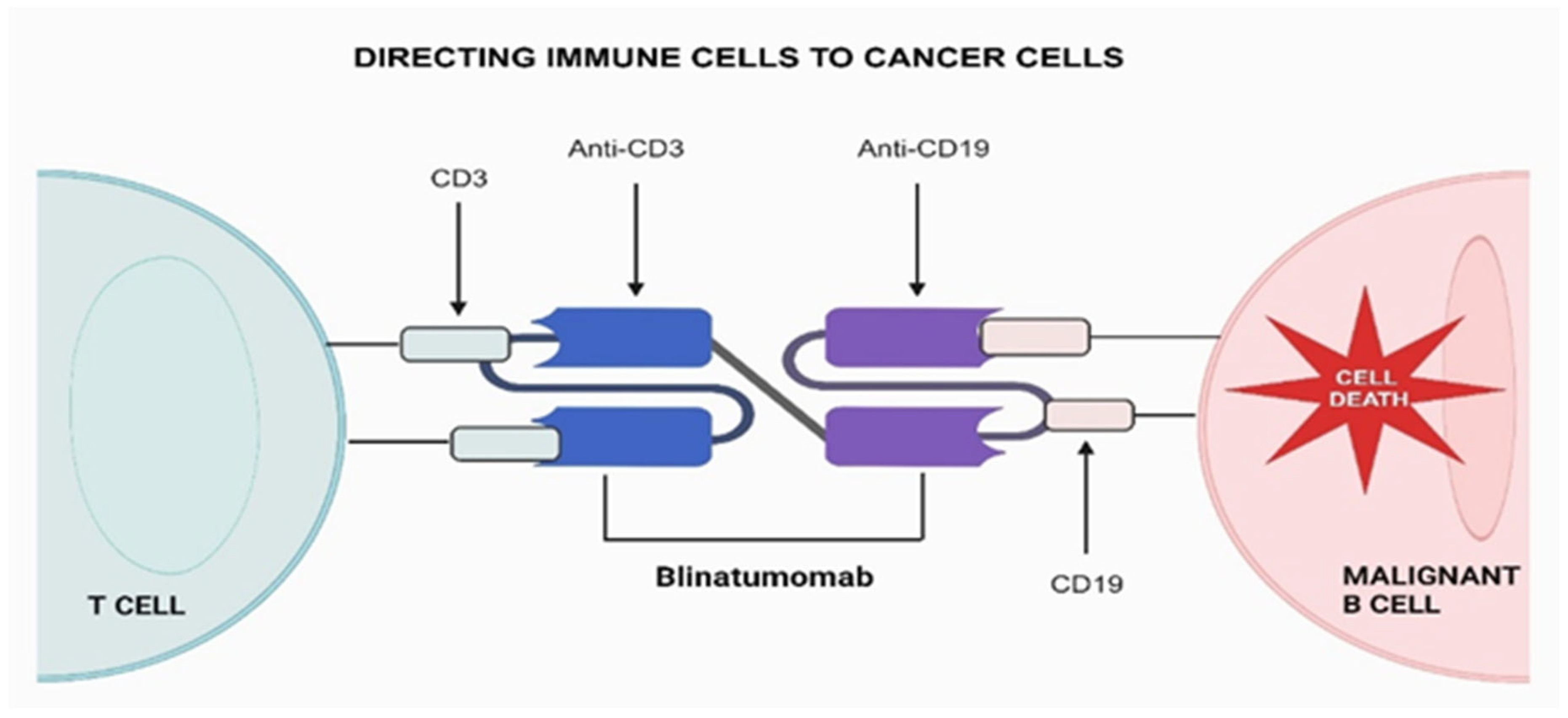
Blinatumomab, the First FDA Approved BiTE Construct
Immune Checkpoint Bispecific Antibodies
| Target | Name | Condition | Status | Phase | NCT ID |
| PD-L1 and TGF-β | SHR-1701 | Advanced solid tumors | Unknown | Phase I | NCT03710265 |
| CTLA-4×PD-L1 | KN064 | Advanced Solid Tumors | Completed | Phase 1 | NCT03733951 |
| PD-1 and CTLA-4 | MEDI5752 | Advanced solid tumors | Recruiting | Phase I | NCT03530397 |
| MGD019 | Advanced solid tumors | Active, not recruiting | Phase 1 | NCT03761017 | |
| AK104 | Hepatocellular carcinoma | Recruiting | Phase I/II | NCT04444167 | |
| COMPASSION-03 | Advanced solid tumors | Active, not recruiting | Phase I/II | NCT03852251 | |
| LAG-3 × PD-L1 | ABL501 | Advanced solid tumors | Recruiting | Phase I | NCT05101109 |
| FS118 | Advanced solid tumors | Active, not recruiting | Phase I/II | NCT03440437 | |
| AK104 | NSCLC | Active, not recruiting | Phase I/II | NCT04646330 | |
| LAG-3 × PD-1 | MGD013 | Advanced liver cancer | Terminated | Phase I/II | NCT04212221 |
| RG6139 | Advanced solid tumors | Recruiting | Phase I/II | NCT04140500 | |
| Not Given | Advanced solid tumors | Recruiting | Phase I | NCT05577182 | |
| TIM-3 × PD-L1 | LY3415244 | Advanced solid tumors | Terminated | Phase I | NCT03752177 |
| ABL501 | Advanced solid tumors | Recruiting | Phase I | NCT05101109 | |
| TIGIT×PD-L1 | HLX301 | Advanced solid tumors | Recruiting | Phase I/II | NCT05102214 |
| TIGIT×PD-1 | ARTEMIDE-01 | Advanced NSCLC | Recruiting | Phase I/II | NCT04995523 |
| LB1410 | Advanced Solid Tumor | Recruiting | Phase I | NCT05357651 | |
| TIM-3 × PD-1 | AZD7789 | Lymphoma | Recruiting | Phase I/II | NCT04931654 |
| RG7769 | Advanced Solid Cancer | Recruiting | Phase I | NCT03708328 | |
| Lomvastomig | Advanced Solid Cancer | Active, not recruiting | Phase II | NCT04785820 | |
| Tobemstomig | Non-small Cell Lung Cancer | Recruiting | Phase II | NCT05775289 | |
| 4-1BB×PD-L1 | ABL503 | Advanced Solid Cancer | Recruiting | Phase I | NCT04762641 |
| PRS-344 | Advanced Solid Cancer | Recruiting | Phase I/II | NCT05159388 | |
| GEN1046 | Advanced Solid Cancer | Recruiting | Phase I/II | NCT03917381 | |
| CD27×PD-L1 | CDX-527 | Advanced Solid Cancer | Completed | Phase I | NCT04440943 |
| PD-L1 and CD137 | MCLA-145 | Advanced Solid Cancer | Recruiting | Phase I | NCT03922204 |
| AP203 | Advanced Solid Cancer | Not yet recruiting | Phase I/II | NCT05473156 | |
| FS222 | Advanced Solid Cancer | Recruiting | Phase I | NCT04740424 | |
| PD-L1 and VEGF | PM8002 | Advanced Solid Cancer | Recruiting | Phase II | NCT05879055 |
| HB0025 | Advanced Solid Cancer | Recruiting | Phase I | NCT04678908 | |
| IMM2510 | Advanced Solid Cancer | Recruiting | Phase I | NCT05972460 | |
| PD-1/ VEGF | AK112 | NSCLC | Recruiting | Phase II | NCT04736823 |
Future Directions
Conclusions
References
- Salvaris, R., J. Ong, and G. P. Gregory. 2021. "Bispecific Antibodies: A Review of Development, Clinical Efficacy and Toxicity in B-Cell Lymphomas." J Pers Med 11 (5). [CrossRef]
- Henricks, L. M., J. H. Schellens, A. D. Huitema, and J. H. Beijnen. 2015. "The use of combinations of monoclonal antibodies in clinical oncology." Cancer Treat Rev 41 (10): 859-67. [CrossRef]
- Han, Y., D. Liu, and L. Li. 2020a. "PD-1/PD-L1 pathway: current researches in cancer." Am J Cancer Res 10 (3): 727-742.
- Ma, J., Y. Mo, M. Tang, J. Shen, Y. Qi, W. Zhao, Y. Huang, Y. Xu, and C. Qian. 2021. "Bispecific Antibodies: From Research to Clinical Application." Front Immunol 12: 626616. [CrossRef]
- Acheampong, D. O., C. K. Adokoh, P. Ampomah, D. S. Agyirifor, I. Dadzie, F. A. Ackah, and E. A. Asiamah. 2017. "Bispecific Antibodies (bsAbs): Promising Immunotherapeutic Agents for Cancer Therapy." Protein Pept Lett 24 (5): 456-465. [CrossRef]
- Ma, Y., J. Xue, Y. Zhao, Y. Zhang, Y. Huang, Y. Yang, W. Fang, Y. Guo, Q. Li, X. Ge, J. Sun, B. Zhang, J. Xiao, L. Zhang, and H. Zhao. 2023. "Phase I trial of KN046, a novel bispecific antibody targeting PD-L1 and CTLA-4 in patients with advanced solid tumors." J Immunother Cancer 11 (6). [CrossRef]
- Wu, Y., M. Yi, S. Zhu, H. Wang, and K. Wu. 2021. "Recent advances and challenges of bispecific antibodies in solid tumors." Exp Hematol Oncol 10 (1): 56. [CrossRef]
- Brinkmann, U., and R. E. Kontermann. 2021. "Bispecific antibodies." Science 372 (6545): 916-917. [CrossRef]
- Moreau, P., A. L. Garfall, N. W. C.J van de Donk, H. Nahi, J. F. San-Miguel, A. Oriol, A. K. Nooka, T. Martin, L. Rosinol, A. Chari, L. Karlin, L. Benboubker, M. V. Mateos, N. Bahlis, R. Popat, B. Besemer, J. Martínez-López, S. Sidana, M. Delforge, L. Pei, D. Trancucci, R. Verona, S. Girgis, S. X. W. Lin, Y. Olyslager, M. Jaffe, C. Uhlar, T. Stephenson, R. Van Rampelbergh, A. Banerjee, J. D. Goldberg, R. Kobos, A. Krishnan, and S. Z. Usmani. 2022. "Teclistamab in Relapsed or Refractory Multiple Myeloma." N Engl J Med 387 (6): 495-505. [CrossRef]
- Lesokhin, A. M., M. H. Tomasson, B. Arnulf, N. J. Bahlis, H. Miles Prince, R. Niesvizky, P. Rodrίguez-Otero, J. Martinez-Lopez, G. Koehne, C. Touzeau, Y. Jethava, H. Quach, J. Depaus, H. Yokoyama, A. E. Gabayan, D. A. Stevens, A. K. Nooka, S. Manier, N. Raje, S. Iida, M. S. Raab, E. Searle, E. Leip, S. T. Sullivan, U. Conte, M. Elmeliegy, A. Czibere, A. Viqueira, and M. Mohty. 2023. "Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results." Nat Med 29 (9): 2259-2267. [CrossRef]
- Keam, S. J. 2023. "Talquetamab: First Approval." Drugs 83 (15): 1439-1445. [CrossRef]
- Scott, L. J., and E. S. Kim. 2018. "Emicizumab-kxwh: First Global Approval." Drugs 78 (2): 269-274. [CrossRef]
- Shirley, M. 2022. "Faricimab: First Approval." Drugs 82 (7): 825-830. [CrossRef]
- Wu, B., R. Jug, C. Luedke, P. Su, C. Rehder, C. McCall, A. S. Lagoo, and E. Wang. 2017. "Lineage Switch Between B-Lymphoblastic Leukemia and Acute Myeloid Leukemia Intermediated by "Occult" Myelodysplastic Neoplasm: Two Cases of Adult Patients With Evidence of Genomic Instability and Clonal Selection by Chemotherapy." Am J Clin Pathol 148 (2): 136-147. [CrossRef]
- Li, H., P. Er Saw, and E. Song. 2020. "Challenges and strategies for next-generation bispecific antibody-based antitumor therapeutics." Cell Mol Immunol 17 (5): 451-461. [CrossRef]
- Underwood, D. J., J. Bettencourt, and Z. Jawad. 2022. "The manufacturing considerations of bispecific antibodies." Expert Opin Biol Ther 22 (8): 1043-1065. [CrossRef]
- Zhang, T., Y. Lin, and Q. Gao. 2023. "Bispecific antibodies targeting immunomodulatory checkpoints for cancer therapy." Cancer Biol Med 20 (3): 181-95. [CrossRef]
- Yu, G. H., A. M. Li, X. Li, Z. Yang, and H. Peng. 2017. "Bispecific antibody suppresses osteosarcoma aggressiveness through regulation of NF-κB signaling pathway." Tumour Biol 39 (6): 1010428317705572. [CrossRef]
- Zhong, Z., M. Zhang, Y. Ning, G. Mao, X. Li, Q. Deng, X. Chen, D. Zuo, X. Zhao, E. Xie, H. Wang, L. Guo, B. Li, K. Xiao, and X. He. 2022. "Development of a bispecific antibody targeting PD-L1 and TIGIT with optimal cytotoxicity." Sci Rep 12 (1): 18011. [CrossRef]
- Esfandiari, A., S. Cassidy, and R. M. Webster. 2022. "Bispecific antibodies in oncology." Nat Rev Drug Discov 21 (6): 411-412. [CrossRef]
- Löffler, A., P. Kufer, R. Lutterbüse, F. Zettl, P. T. Daniel, J. M. Schwenkenbecher, G. Riethmüller, B. Dörken, and R. C. Bargou. 2000. "A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes." Blood 95 (6): 2098-103.
- Emmanuel Owusu Ansah, Andy Baah, Emmanuel Boateng Agyenim, "Vaccine Boosting CAR-T Cell Therapy: Current and Future Strategies", Advances in Cell and Gene Therapy, vol. 2023, Article ID 8030440, 9 pages, 2023. [CrossRef]
- Viardot, A., M. E. Goebeler, G. Hess, S. Neumann, M. Pfreundschuh, N. Adrian, F. Zettl, M. Libicher, C. Sayehli, J. Stieglmaier, A. Zhang, D. Nagorsen, and R. C. Bargou. 2016. "Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma." Blood 127 (11): 1410-6. https://www.ncbi.nlm.nih.gov/pubmed/26755709. [CrossRef]
- Simão, D. C., K. K. Zarrabi, J. L. Mendes, R. Luz, J. A. Garcia, W. K. Kelly, and P. C. Barata. 2023. "Bispecific T-Cell Engagers Therapies in Solid Tumors: Focusing on Prostate Cancer." Cancers (Basel) 15 (5). [CrossRef]
- Subklewe, M. 2021. "BiTEs better than CAR T cells." Blood Adv 5 (2): 607-612. [CrossRef]
- Buzzetti, M., and M. Gerlinger. 2023. "Assessing the toxicity of bispecific antibodies." Nat Biomed Eng. [CrossRef]
- Wang, D. R., X. L. Wu, and Y. L. Sun. 2022. "Therapeutic targets and biomarkers of tumor immunotherapy: response versus non-response." Signal Transduct Target Ther 7 (1): 331. https://www.ncbi.nlm.nih.gov/pubmed/36123348.
- Li, J., R. Piskol, R. Ybarra, Y. J. Chen, D. Slaga, M. Hristopoulos, R. Clark, Z. Modrusan, K. Totpal, M. R. Junttila, and T. T. Junttila. 2019. "CD3 bispecific antibody-induced cytokine release is dispensable for cytotoxic T cell activity." Sci Transl Med 11 (508). [CrossRef]
- Wang, K., G. Wei, and D. Liu. 2012. "CD19: a biomarker for B cell development, lymphoma diagnosis and therapy." Exp Hematol Oncol 1 (1): 36. [CrossRef]
- Shimabukuro-Vornhagen, A., P. Gödel, M. Subklewe, H. J. Stemmler, H. A. Schlößer, M. Schlaak, M. Kochanek, B. Böll, and M. S. von Bergwelt-Baildon. 2018. "Cytokine release syndrome." J Immunother Cancer 6 (1): 56. [CrossRef]
- Slaga, D., D. Ellerman, T. N. Lombana, R. Vij, J. Li, M. Hristopoulos, R. Clark, J. Johnston, A. Shelton, E. Mai, K. Gadkar, A. A. Lo, J. T. Koerber, K. Totpal, R. Prell, G. Lee, C. Spiess, and T. T. Junttila. 2018. "Avidity-based binding to HER2 results in selective killing of HER2-overexpressing cells by anti-HER2/CD3." Sci Transl Med 10 (463). [CrossRef]
- Singh, A., S. Dees, and I. S. Grewal. 2021. "Overcoming the challenges associated with CD3+ T-cell redirection in cancer." Br J Cancer 124 (6): 1037-1048. [CrossRef]
- Bacac, M., T. Fauti, J. Sam, S. Colombetti, T. Weinzierl, D. Ouaret, W. Bodmer, S. Lehmann, T. Hofer, R. J. Hosse, E. Moessner, O. Ast, P. Bruenker, S. Grau-Richards, T. Schaller, A. Seidl, C. Gerdes, M. Perro, V. Nicolini, N. Steinhoff, S. Dudal, S. Neumann, T. von Hirschheydt, C. Jaeger, J. Saro, V. Karanikas, C. Klein, and P. Umaña. 2016a. "A Novel Carcinoembryonic Antigen T-Cell Bispecific Antibody (CEA TCB) for the Treatment of Solid Tumors." Clin Cancer Res 22 (13): 3286-97. [CrossRef]
- Zhu, X. Y., Q. X. Li, Y. Kong, K. K. Huang, G. Wang, Y. J. Wang, J. Lu, G. Q. Hua, Y. L. Wu, and T. L. Ying. 2023. "A novel human single-domain antibody-drug conjugate targeting CEACAM5 exhibits potent in vitro and in vivo antitumor activity." Acta Pharmacol Sin. [CrossRef]
- Simão, D. C., K. K. Zarrabi, J. L. Mendes, R. Luz, J. A. Garcia, W. K. Kelly, and P. C. Barata. 2023a. "Bispecific T-Cell Engagers Therapies in Solid Tumors: Focusing on Prostate Cancer." Cancers (Basel) 15 (5). [CrossRef]
- Oates, J., N. J. Hassan, and B. K. Jakobsen. 2015. "ImmTACs for targeted cancer therapy: Why, what, how, and which." Mol Immunol 67 (2 Pt A): 67-74. [CrossRef]
- Howlett, S., T. J. Carter, H. M. Shaw, and P. D. Nathan. 2023. "Tebentafusp: a first-in-class treatment for metastatic uveal melanoma." Ther Adv Med Oncol 15: 17588359231160140. [CrossRef]
- Pulte, E. D., J. Vallejo, D. Przepiorka, L. Nie, A. T. Farrell, K. B. Goldberg, A. E. McKee, and R. Pazdur. 2018a. "FDA Supplemental Approval: Blinatumomab for Treatment of Relapsed and Refractory Precursor B-Cell Acute Lymphoblastic Leukemia." Oncologist 23 (11): 1366-1371. https://www.ncbi.nlm.nih.gov/pubmed/30018129. [CrossRef]
- Mocquot, P., Y. Mossazadeh, L. Lapierre, F. Pineau, and F. Despas. 2022. "The pharmacology of blinatumomab: state of the art on pharmacodynamics, pharmacokinetics, adverse drug reactions and evaluation in clinical trials." J Clin Pharm Ther 47 (9): 1337-1351. [CrossRef]
- Boissel, N., S. Chiaretti, C. Papayannidis, J. M. Ribera, R. Bassan, A. N. Sokolov, N. Alam, A. Brescianini, I. Pezzani, G. Kreuzbauer, G. Zugmaier, R. Foà, and A. Rambaldi. 2023. "Real-world use of blinatumomab in adult patients with B-cell acute lymphoblastic leukemia in clinical practice: results from the NEUF study." Blood Cancer J 13 (1): 2. [CrossRef]
- Burt, R., D. Warcel, and A. K. Fielding. 2019. "Blinatumomab, a bispecific B-cell and T-cell engaging antibody, in the treatment of B-cell malignancies." Hum Vaccin Immunother 15 (3): 594-602. [CrossRef]
- Zhou, S., M. Liu, F. Ren, X. Meng, and J. Yu. 2021. "The landscape of bispecific T cell engager in cancer treatment." Biomark Res 9 (1): 38. [CrossRef]
- Jabbour, E., and H. Kantarjian. 2016. "Chemoimmunotherapy as a new standard of care for Burkitt leukaemia/lymphoma." Lancet 387 (10036): 2360-1. [CrossRef]
- Zhao, Y., I. Aldoss, C. Qu, J. C. Crawford, Z. Gu, E. K. Allen, A. E. Zamora, T. B. Alexander, J. Wang, H. Goto, T. Imamura, K. Akahane, G. Marcucci, A. S. Stein, R. Bhatia, P. G. Thomas, S. J. Forman, C. G. Mullighan, and K. G. Roberts. 2021a. "Tumor-intrinsic and -extrinsic determinants of response to blinatumomab in adults with B-ALL." Blood 137 (4): 471-484. https://www.ncbi.nlm.nih.gov/pubmed/32881995. [CrossRef]
- Li, Y., T. Moriyama, S. Yoshimura, X. Zhao, Z. Li, X. Yang, E. Paietta, M. R. Litzow, M. Konopleva, J. Yu, H. Inaba, R. C. Ribeiro, C. H. Pui, and J. J. Yang. 2022. "PAX5 epigenetically orchestrates CD58 transcription and modulates blinatumomab response in acute lymphoblastic leukemia." Sci Adv 8 (50): eadd6403. [CrossRef]
- Haddox, C. L., A. A. Mangaonkar, D. Chen, M. Shi, R. He, J. L. Oliveira, M. R. Litzow, A. Al-Kali, W. J. Hogan, and M. A. Elliott. 2017. "Blinatumomab-induced lineage switch of B-ALL with t(4:11)(q21;q23) KMT2A/AFF1 into an aggressive AML: pre- and post-switch phenotypic, cytogenetic and molecular analysis." Blood Cancer J 7 (9): e607. [CrossRef]
- Rayes, A., R. L. McMasters, and M. M. O'Brien. 2016. "Lineage Switch in MLL-Rearranged Infant Leukemia Following CD19-Directed Therapy." Pediatr Blood Cancer 63 (6): 1113-5. [CrossRef]
- Jacoby, E., S. M. Nguyen, T. J. Fountaine, K. Welp, B. Gryder, H. Qin, Y. Yang, C. D. Chien, A. E. Seif, H. Lei, Y. K. Song, J. Khan, D. W. Lee, C. L. Mackall, R. A. Gardner, M. C. Jensen, J. F. Shern, and T. J. Fry. 2016. "CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity." Nat Commun 7: 12320. [CrossRef]
- Stass, S., J. Mirro, S. Melvin, C. H. Pui, S. B. Murphy, and D. Williams. 1984. "Lineage switch in acute leukemia." Blood 64 (3): 701-6.
- Perna, F., and M. Sadelain. 2016. "Myeloid leukemia switch as immune escape from CD19 chimeric antigen receptor (CAR) therapy." Transl Cancer Res 5 (Suppl 2): S221-S225. [CrossRef]
- Aldulescu, M., K. Leuer, L. J. Jennings, K. L. Yap, and S. Gong. 2023. "Lineage switch from acute myeloid leukemia to B-lymphoblastic lymphoma with an acquired PIK3R1 loss-of-function mutation." Am J Hematol 98 (1): E1-E3. [CrossRef]
- Dorantes-Acosta, E., and R. Pelayo. 2012a. "Lineage switching in acute leukemias: a consequence of stem cell plasticity?" Bone Marrow Res 2012: 406796. [CrossRef]
- Yang, W., S. Xie, Y. Li, J. Wang, J. Xiao, K. Huang, X. Wang, Y. Wu, L. Ma, and D. Nie. 2022. "Lineage switch from lymphoma to myeloid neoplasms: First case series from a single institution." Open Med (Wars) 17 (1): 1466-1472. [CrossRef]
- Pui, C. H., S. C. Raimondi, F. G. Behm, J. Ochs, W. L. Furman, N. J. Bunin, R. C. Ribeiro, P. A. Tinsley, and J. Mirro. 1986. "Shifts in blast cell phenotype and karyotype at relapse of childhood lymphoblastic leukemia." Blood 68 (6): 1306-10.
- Zuna, J., H. Cavé, C. Eckert, T. Szczepanski, C. Meyer, E. Mejstrikova, E. Fronkova, K. Muzikova, E. Clappier, D. Mendelova, P. Boutard, A. Schrauder, J. Sterba, R. Marschalek, J. J. van Dongen, O. Hrusak, J. Stary, and J. Trka. 2007. "Childhood secondary ALL after ALL treatment." Leukemia 21 (7): 1431-5. [CrossRef]
- Li, L. Z., Q. Sun, Y. Fang, L. J. Yang, Z. Y. Xu, J. H. Hu, L. Cao, J. Y. Huang, M. Hong, J. Y. Li, and S. X. Qian. 2020. "A report on Lineage switch at relapse of CD19 CAR-T therapy for Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia." Chin Med J (Engl) 133 (16): 2001-2003. [CrossRef]
- Rayes, A., R. L. McMasters, and M. M. O'Brien. 2016. "Lineage Switch in MLL-Rearranged Infant Leukemia Following CD19-Directed Therapy." Pediatr Blood Cancer 63 (6): 1113-5. [CrossRef]
- Wölfl, M., M. Rasche, M. Eyrich, R. Schmid, D. Reinhardt, and P. G. Schlegel. 2018. "Spontaneous reversion of a lineage switch following an initial blinatumomab-induced ALL-to-AML switch in." Blood Adv 2 (12): 1382-1385. [CrossRef]
- Iacobucci, I., and C. G. Mullighan. 2022. "KMT2A-rearranged leukemia: the shapeshifter." Blood 140 (17): 1833-1835. [CrossRef]
- Shimony, S., and M. R. Luskin. 2023. "Unraveling KMT2A-rearranged ALL." Blood 142 (21): 1764-1766. [CrossRef]
- Meyer, C., P. Larghero, B. Almeida Lopes, T. Burmeister, D. Gröger, R. Sutton, N. C. Venn, G. Cazzaniga, L. Corral Abascal, G. Tsaur, L. Fechina, M. Emerenciano, M. S. Pombo-de-Oliveira, T. Lund-Aho, T. Lundán, M. Montonen, V. Juvonen, J. Zuna, J. Trka, P. Ballerini, H. Lapillonne, V. H. J. Van der Velden, E. Sonneveld, E. Delabesse, R. R. C. de Matos, M. L. M. Silva, S. Bomken, K. Katsibardi, M. Keernik, N. Grardel, J. Mason, R. Price, J. Kim, C. Eckert, L. Lo Nigro, C. Bueno, P. Menendez, U. Zur Stadt, P. Gameiro, L. Sedék, T. Szczepański, A. Bidet, V. Marcu, K. Shichrur, S. Izraeli, H. O. Madsen, B. W. Schäfer, S. Kubetzko, R. Kim, E. Clappier, H. Trautmann, M. Brüggemann, P. Archer, J. Hancock, J. Alten, A. Möricke, M. Stanulla, J. Lentes, A. K. Bergmann, S. Strehl, S. Köhrer, K. Nebral, M. N. Dworzak, O. A. Haas, C. Arfeuille, A. Caye-Eude, H. Cavé, and R. Marschalek. 2023. "The KMT2A recombinome of acute leukemias in 2023." Leukemia 37 (5): 988-1005. [CrossRef]
- He, R. R., Z. Nayer, M. Hogan, R. S. Cuevo, K. Woodward, D. Heyer, C. A. Curtis, and J. F. Peterson. 2019. "Immunotherapy- (Blinatumomab-) Related Lineage Switch of." Case Rep Hematol 2019: 7394619. [CrossRef]
- Fournier, E., L. Inchiappa, C. Delattre, J. M. Pignon, F. Danicourt, M. Bemba, C. Roche-Lestienne, A. Daudignon, G. Decool, C. Roumier, F. Dumezy, L. Fournier, N. Grardel, C. Preudhomme, and N. Duployez. 2019. "Increased risk of adverse acute myeloid leukemia after anti-CD19-targeted immunotherapies in." Leuk Lymphoma 60 (7): 1827-1830. [CrossRef]
- Nagel, I., M. Bartels, J. Duell, H. H. Oberg, S. Ussat, H. Bruckmueller, O. Ottmann, H. Pfeifer, H. Trautmann, N. Gökbuget, A. Caliebe, D. Kabelitz, M. Kneba, H. A. Horst, D. Hoelzer, M. S. Topp, I. Cascorbi, R. Siebert, and M. Brüggemann. 2017. "Hematopoietic stem cell involvement in." Blood 130 (18): 2027-2031. [CrossRef]
- Zoghbi, A., U. Zur Stadt, B. Winkler, I. Müller, and G. Escherich. 2017. "Lineage switch under blinatumomab treatment of relapsed common acute lymphoblastic leukemia without MLL rearrangement." Pediatr Blood Cancer 64 (11). [CrossRef]
- Du, J., K. M. Chisholm, K. Tsuchiya, K. Leger, B. M. Lee, J. C. Rutledge, C. R. Paschal, C. Summers, and M. Xu. 2021. "Lineage Switch in an Infant B-Lymphoblastic Leukemia With t(1;11)(p32;q23);." Pediatr Dev Pathol 24 (4): 378-382. [CrossRef]
- Meyer, C., J. Hofmann, T. Burmeister, D. Gröger, T. S. Park, M. Emerenciano, M. Pombo de Oliveira, A. Renneville, P. Villarese, E. Macintyre, H. Cavé, E. Clappier, K. Mass-Malo, J. Zuna, J. Trka, E. De Braekeleer, M. De Braekeleer, S. H. Oh, G. Tsaur, L. Fechina, V. H. van der Velden, J. J. van Dongen, E. Delabesse, R. Binato, M. L. Silva, A. Kustanovich, O. Aleinikova, M. H. Harris, T. Lund-Aho, V. Juvonen, O. Heidenreich, J. Vormoor, W. W. Choi, M. Jarosova, A. Kolenova, C. Bueno, P. Menendez, S. Wehner, C. Eckert, P. Talmant, S. Tondeur, E. Lippert, E. Launay, C. Henry, P. Ballerini, H. Lapillone, M. B. Callanan, J. M. Cayuela, C. Herbaux, G. Cazzaniga, P. M. Kakadiya, S. Bohlander, M. Ahlmann, J. R. Choi, P. Gameiro, D. S. Lee, J. Krauter, P. Cornillet-Lefebvre, G. Te Kronnie, B. W. Schäfer, S. Kubetzko, C. N. Alonso, U. zur Stadt, R. Sutton, N. C. Venn, S. Izraeli, L. Trakhtenbrot, H. O. Madsen, P. Archer, J. Hancock, N. Cerveira, M. R. Teixeira, L. Lo Nigro, A. Möricke, M. Stanulla, M. Schrappe, L. Sedék, T. Szczepański, C. M. Zwaan, E. A. Coenen, M. M. van den Heuvel-Eibrink, S. Strehl, M. Dworzak, R. Panzer-Grümayer, T. Dingermann, T. Klingebiel, and R. Marschalek. 2013. "The MLL recombinome of acute leukemias in 2013." Leukemia 27 (11): 2165-76. [CrossRef]
- Piciocchi, A., M. Messina, L. Elia, A. Vitale, S. Soddu, A. M. Testi, S. Chiaretti, M. Mancini, F. Albano, A. Spadano, M. Krampera, M. Bonifacio, R. Cairoli, C. Vetro, F. Colella, F. Ferrara, G. Cimino, R. Bassan, P. Fazi, and M. Vignetti. 2021. "Prognostic impact of KMT2A-AFF1-positivity in 926 BCR-ABL1-negative B-lineage acute lymphoblastic leukemia patients treated in GIMEMA clinical trials since 1996." Am J Hematol 96 (9): E334-E338. [CrossRef]
- Richard-Carpentier, G., H. M. Kantarjian, G. Tang, C. C. Yin, J. D. Khoury, G. C. Issa, F. Haddad, N. Jain, F. Ravandi, N. J. Short, C. D. DiNardo, K. Takahashi, M. Y. Konopleva, N. G. Daver, T. Kadia, G. Garcia-Manero, R. Garris, S. O'Brien, and E. Jabbour. 2021. "Outcomes of acute lymphoblastic leukemia with KMT2A (MLL) rearrangement: the MD Anderson experience." Blood Adv 5 (23): 5415-5419. [CrossRef]
- van der Sluis, I. M., P. de Lorenzo, R. S. Kotecha, A. Attarbaschi, G. Escherich, K. Nysom, J. Stary, A. Ferster, B. Brethon, F. Locatelli, M. Schrappe, P. E. Scholte-van Houtem, M. G. Valsecchi, and R. Pieters. 2023. "Blinatumomab Added to Chemotherapy in Infant Lymphoblastic Leukemia." N Engl J Med 388 (17): 1572-1581. [CrossRef]
- Qi, Y., H. Liu, X. Li, Y. Shi, J. Mu, J. Li, Y. Wang, and Q. Deng. 2023. "Blinatumomab as salvage therapy in patients with relapsed/refractory B-ALL who have failed/progressed after anti-CD19-CAR T therapy." Ann Med 55 (1): 2230888. [CrossRef]
- Shah, B. D., M. R. Bishop, O. O. Oluwole, A. C. Logan, M. R. Baer, W. B. Donnellan, K. M. O'Dwyer, H. Holmes, M. L. Arellano, A. Ghobadi, J. M. Pagel, Y. Lin, R. D. Cassaday, J. H. Park, M. Abedi, J. E. Castro, D. J. DeAngelo, A. K. Malone, R. Mawad, G. J. Schiller, J. M. Rossi, A. Bot, T. Shen, L. Goyal, R. K. Jain, R. Vezan, and W. G. Wierda. 2021. "KTE-X19 anti-CD19 CAR T-cell therapy in adult relapsed/refractory acute lymphoblastic leukemia: ZUMA-3 phase 1 results." Blood 138 (1): 11-22. [CrossRef]
- Lee, J. B., H. R. Kim, and S. J. Ha. 2022. "Immune Checkpoint Inhibitors in 10 Years: Contribution of Basic Research and Clinical Application in Cancer Immunotherapy." Immune Netw 22 (1): e2. [CrossRef]
- Razaghi, A., M. Durand-Dubief, N. Brusselaers, and M. Björnstedt. 2023. "Combining PD-1/PD-L1 blockade with type I interferon in cancer therapy." Front Immunol 14: 1249330. [CrossRef]
- Meybodi, S. M., B. Farasati Far, A. Pourmolaei, F. Baradarbarjastehbaf, M. Safaei, N. Mohammadkhani, and A. A. Samadani. 2023. "Immune checkpoint inhibitors promising role in cancer therapy: clinical evidence and immune-related adverse events." Med Oncol 40 (8): 243. [CrossRef]
- Wang, Y., S. Yang, L. Wan, W. Ling, H. Chen, and J. Wang. 2023. "New developments in the mechanism and application of immune checkpoint inhibitors in cancer therapy (Review)." Int J Oncol 63 (1). [CrossRef]
- Bonaventura, P., T. Shekarian, V. Alcazer, J. Valladeau-Guilemond, S. Valsesia-Wittmann, S. Amigorena, C. Caux, and S. Depil. 2019. "Cold Tumors: A Therapeutic Challenge for Immunotherapy." Front Immunol 10: 168. [CrossRef]
- Wang, L., H. Geng, Y. Liu, L. Liu, Y. Chen, F. Wu, Z. Liu, S. Ling, Y. Wang, and L. Zhou. 2023. "Hot and cold tumors: Immunological features and the therapeutic strategies." MedComm (2020) 4 (5): e343. [CrossRef]
- Blanco, B., C. Domínguez-Alonso, and L. Alvarez-Vallina. 2021. "Bispecific Immunomodulatory Antibodies for Cancer Immunotherapy." Clin Cancer Res 27 (20): 5457-5464. [CrossRef]
- Wang, Y., J. Du, Z. Gao, H. Sun, M. Mei, Y. Ren, and X. Zhou. 2023. "Evolving landscape of PD-L2: bring new light to checkpoint immunotherapy." Br J Cancer 128 (7): 1196-1207. [CrossRef]
- Takehara, T., E. Wakamatsu, H. Machiyama, W. Nishi, K. Emoto, M. Azuma, K. Soejima, K. Fukunaga, and T. Yokosuka. 2021. "PD-L2 suppresses T cell signaling via coinhibitory microcluster formation and SHP2 phosphatase recruitment." Commun Biol 4 (1): 581. [CrossRef]
- Kotanides, H., Y. Li, M. Malabunga, C. Carpenito, S. W. Eastman, Y. Shen, G. Wang, I. Inigo, D. Surguladze, A. L. Pennello, K. Persaud, S. Hindi, M. Topper, X. Chen, Y. Zhang, D. K. Bulaon, T. Bailey, Y. Lao, B. Han, S. Torgerson, D. Chin, A. Sonyi, J. N. Haidar, R. D. Novosiadly, C. M. Moxham, G. D. Plowman, D. L. Ludwig, and M. Kalos. 2020. "Bispecific Targeting of PD-1 and PD-L1 Enhances T-cell Activation and Antitumor Immunity." Cancer Immunol Res 8 (10): 1300-1310. [CrossRef]
- Yearley, J. H., C. Gibson, N. Yu, C. Moon, E. Murphy, J. Juco, J. Lunceford, J. Cheng, L. Q. M. Chow, T. Y. Seiwert, M. Handa, J. E. Tomassini, and T. McClanahan. 2017. "PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer." Clin Cancer Res 23 (12): 3158-3167. [CrossRef]
- Wei, J., W. Montalvo-Ortiz, L. Yu, A. Krasco, K. Olson, S. Rizvi, N. Fiaschi, S. Coetzee, F. Wang, E. Ullman, H. S. Ahmed, E. Herlihy, K. Lee, L. Havel, T. Potocky, S. Ebstein, D. Frleta, A. Khatri, S. Godin, S. Hamon, J. Brouwer-Visser, T. Gorenc, D. MacDonald, A. Hermann, A. Chaudhry, A. Sirulnik, W. Olson, J. Lin, G. Thurston, I. Lowy, A. J. Murphy, E. Smith, V. Jankovic, M. A. Sleeman, and D. Skokos. 2022. "CD22-targeted CD28 bispecific antibody enhances antitumor efficacy of odronextamab in refractory diffuse large B cell lymphoma models." Sci Transl Med 14 (670): eabn1082. [CrossRef]
- Ke, H., F. Zhang, J. Wang, L. Xiong, X. An, X. Tu, C. Chen, Y. Wang, B. Mao, S. Guo, C. Ju, X. He, R. Sun, L. Zhang, O. A. O'Connor, and Q. X. Li. 2023. "HX009, a novel BsAb dual targeting PD1 x CD47, demonstrates potent anti-lymphoma activity in preclinical models." Sci Rep 13 (1): 5419. [CrossRef]
- Dovedi, S. J., M. J. Elder, C. Yang, S. I. Sitnikova, L. Irving, A. Hansen, J. Hair, D. C. Jones, S. Hasani, B. Wang, S. A. Im, B. Tran, D. S. Subramaniam, S. D. Gainer, K. Vashisht, A. Lewis, X. Jin, S. Kentner, K. Mulgrew, Y. Wang, M. G. Overstreet, J. Dodgson, Y. Wu, A. Palazon, M. Morrow, G. J. Rainey, G. J. Browne, F. Neal, T. V. Murray, A. D. Toloczko, W. Dall'Acqua, I. Achour, D. J. Freeman, R. W. Wilkinson, and Y. Mazor. 2021. "Design and Efficacy of a Monovalent Bispecific PD-1/CTLA4 Antibody That Enhances CTLA4 Blockade on PD-1." Cancer Discov 11 (5): 1100-1117. [CrossRef]
- Geuijen, C., P. Tacken, L. C. Wang, R. Klooster, P. F. van Loo, J. Zhou, A. Mondal, Y. B. Liu, A. Kramer, T. Condamine, A. Volgina, L. J. A. Hendriks, H. van der Maaden, E. Rovers, S. Engels, F. Fransen, R. den Blanken-Smit, V. Zondag-van der Zande, A. Basmeleh, W. Bartelink, A. Kulkarni, W. Marissen, C. Y. Huang, L. Hall, S. Harvey, S. Kim, M. Martinez, S. O'Brien, E. Moon, S. Albelda, C. Kanellopoulou, S. Stewart, H. Nastri, A. B. H. Bakker, P. Scherle, T. Logtenberg, G. Hollis, J. de Kruif, R. Huber, P. A. Mayes, and M. Throsby. 2021. "A human CD137×PD-L1 bispecific antibody promotes anti-tumor immunity via context-dependent T cell costimulation and checkpoint blockade." Nat Commun 12 (1): 4445. [CrossRef]
- Lin, W., Y. Zhang, Y. Yang, B. Lin, M. Zhu, J. Xu, Y. Chen, W. Wu, B. Chen, X. Chen, J. Liu, H. Wang, F. Teng, X. Yu, J. Lu, Q. Zhou, and L. Teng. 2023. "Anti-PD-1/Her2 Bispecific Antibody IBI315 Enhances the Treatment Effect of Her2-Positive Gastric Cancer through Gasdermin B-Cleavage Induced Pyroptosis." Adv Sci (Weinh) 10 (30): e2303908. [CrossRef]
- Perez-Santos, M. 2020a. "Bispecific anti-PD-1/CTLA-4 antibody for advanced solid tumors." Pharm Pat Anal 9 (5): 149-154. [CrossRef]
| Drug (Company) |
Trade name | Target antigen | Approved Countries | Year Approved | Approved indications |
| Blinatumomab (Amgen) | Blincyto | CD3/CD19 | FDA | 2014 | adults and children with B-cell precursor acute lymphoblastic leukemia (ALL) in first or second complete remission with minimal residual disease (MRD) greater than or equal to 0.1%. |
| Emacizumab-kxwh (Genentech) |
Hemlibra | FIXa/ FX | FDA | 2017 | the treatment is recommended for adult and pediatric patients, including newborns, with hemophilia A. This includes individuals with congenital factor VIII deficiency, whether or not they have developed factor VIII (FVIII) inhibitors |
| Amivantamab-vmjw(Janssen Biotech) | Rybrevant | EGFR/c-Met | FDA/EMA | 2021 | adult patients with locally advanced or metastatic non-small cell lung cancer who have EGFR exon 20 insertion mutations and have previously received platinum-based chemotherapy |
| Tebentafusp-tebn (Immunocore) |
Kimmtrak* | CD3/ gp100 | FDA | 2022 | for the treatment of adult patients with unresectable or metastatic uveal melanoma who are HLA-A*02:01-positive. |
| Faricimab-svoa (Roche) | Vabysmo | VEGF-A/Ang-2 | FDA | 2022 | To treat neovascular (wet) age-related macular degenerated and diabetic macular edema |
| Mosunetuzumab-axgb (Genentech) | Lunsumio | CD3/CD20 | EMA/FDA | 2022 | Patients with advanced non-small cell lung cancer (NSCLC), harboring EGFR exon 20 insertion mutations, facing disease progression after platinum-based chemotherapy, |
| Cadonilimab (Akeso) |
Kaitanni | PD-1/CTLA-4 | CFDA | 2022 | For patients with relapsed or metastatic cervical cancer (r/mCC) who have experienced disease progression following platinum-based chemotherapy |
| Teclistamab-cqyv (Janssen Biotech) |
Tecvavli | CD3/BCMA | EMA/FDA | 2022 | adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody |
| Epcoritamab-bysp (Genmab) |
Epkinly | CD3/CD20 | FDA/EMA | 2023 | adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), including cases arising from indolent lymphoma and high-grade B-cell lymphoma after two or more lines of systemic therapy |
| Glofitamab-gxbm (Genentech) | Columvi | CD3/CD20 | FDA | 2023 | For adult with relapsed or refractory diffuse large B-cell lymphoma (DLBCL, NOS) or large B-cell lymphoma (LBCL) arising from follicular lymphoma, after two or more lines of systemic therapy. |
| Talquetamab-tgvs (Janssen Biotech) | Talvey | GPRC5D/ CD3 | EMA/FDA | 2023 | adults with relapsed or refractory multiple myeloma who have undergone at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody. |
| Elranatamab (Pfizer) | Elrexfio | BCMA/CD3 | FDA/EMA | 2023 | for adults with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody. |
| Odronextamab* | Regeneron | CD20/CD3 | FDA | FDA decision is on March 31, 2024. | adult patients with relapsed/refractory (R/R) follicular lymphoma (FL) or R/R diffuse large B-cell lymphoma (DLBCL) who have progressed after at least two prior systemic therapies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





