1. Introduction
Fibromyalgia (FM) is the third most common cause of musculoskeletal pain, next to low back pain and osteoarthritis in terms of prevalence [
1]. FM prevalence is around 2.7% globally when using the 2010 modified classification criteria of the American College of Rheumatology (ACR) [
2]. The total health care costs are estimated to be three times higher for fibromyalgia patients than in other populations [
3]. Furthermore, fibromyalgia can cause inability to work in patients and productivity loss in society [
4]. FM is a complex, chronic pain condition that primarily involves the musculoskeletal system [
5]. FM pain control cannot be achieved by medication alone [
6]. This unsatisfactory pharmacologic response may be related to the complex mechanism of FM pain. Current evidence suggests that immune cells, glia, and neurons form an integrated network, which coordinates immune responses and modulates the excitability of pain pathways and results in central sensitization [
7]. A human functional imaging study demonstrated higher neuroinflammation in fibromyalgia patients compared to healthy controls across several brain areas (such as primary somatosensory cortex, medial frontal gyrus) [
8]. In 2008, a Japanese team published a mouse model of fibromyalgia [
9]. They used intermittent cold stress (ICS) to induce abnormal pain, mechanical allodynia, and hyperalgesia, which lasts for more than two weeks. This model exhibits sex hormone-independent female predominance of chronic pain, which is similar to clinical fibromyalgia patients. Our previous research showed aggravated neuroinflammation in this ICS FM model [
10,
11].
Toll-like receptors (TLRs) recognize microbial components and initiates signal transduction pathways to induce gene expression. These gene products control innate immune responses and the direct the development of antigen-specific acquired immunity [
12]. TLRs can be classified based on their location in the cell. TLR1, TLR2, TLR4, TLR5, and TLR6 are located on the cell surface. TLR3, TLR7, TLR8, and TLR9 are found within endosomes [
13]. TLR4 is a type I transmembrane protein that can be activated by bacterial lipopolysaccharide (LPS) and can induce the production of pro-inflammatory cytokines and interferons [
14,
15,
16]. TLR4 pathway is expressed primarily in microglia and is crucial for regulating inflammatory responses [
17,
18,
19,
20]. During inflammation in the CNS, the innate immune system, including microglia, is activated by TLR4. Activated TLR4 then triggers intracellular signaling in two distinct pathways including MyD88/NF-kB and TRIF/IRF3 (15). TLR4 signaling pathway was reported to be involved in major depressive disorders (21) and peripheral neuropathic pain [
22]. Loss-of-function mutation in the Tlr4 gene reliably reduced microglial activation and led to lower levels of inflammatory mediators [
23]. Antagonism of TLR4 attenuated NF-κB pathways and thus decreased the inflammatory mediators secreted by astrocytes [
24]. Furthermore, TLR4 plays a crucial role in central neuroinflammation including IL-1β upregulation [
25].
Acupuncture is a traditional Chinese medicine technique, representing an ancient physiological system that believes health to be the result of harmony among bodily functions as well as between body and nature [
26]. Acupuncture was first developed around 3000 years ago and has been shown to provide therapeutic effects in many diseases [
27], especially pain management. Compared to conventional treatment, several potential advantages are associated with acupuncture, including its low cost, low number of complications [
28], the ability to offer a personalized treatment program, and fewer adverse effects [
29]. Clinically, two meta-analyses summarized the efficacy of acupuncture in relieving stiffness and pain in FM patients [
30,
31]. Regarding its mechanism, our previous article showed electroacupuncture can reduce cold stress pain through downregulation of interleukins, TNF-α, and IFN-γ in mouse plasma [
11]. Electroacupuncture can reduce mechanical and thermal hyperalgesia in the same ICS mouse model of FM by attenuating brain TRPV1 signaling [
10].
In this study, we aimed to determine the role of inflammatory mediators and TLR4 signaling pathway in ICS-induced FM pain mouse model. We hypothesized that inflammation underlies FM in mice through the activation of receptors such as TLR4. Furthermore, the therapeutic effect and detailed mechanisms of EA in this model are still unknown. We tested whether EA can relieve FM by regulating inflammatory mediators and related mechanisms. We also aimed to provide new evidence for the roles of EA and TLR4 in central sensitization. Chemogenetics precisely block somatosensory cortex (SSC) activity displayed an analgesic effect through TLR4 pathway. Our data provide novel evidence to support the clinical use of EA in treating FM.
2. Materials and Methods
2.1. Animals
There are totally 40 female C57BL/6 mice, aged 8-12 weeks, were used in this study. After arriving, the mice were kept in a 12h light-dark cycle with food and water ad libitum. A sample size of ten animals per group was calculated as the number required for an alpha of 0.05 and a power of 80%. In addition, the number of animals used here and their suffering were minimized. The laboratory workers were blind to treatment allocation during the experiments and analysis. The use of these animals was approved by the Institute of Animal Care and Use Committee of China Medical University (Permit no. CMUIACUC-2021-317), Taiwan, following the Guide for the use of Laboratory Animals (National Academy Press). Mice were subdivided into four groups: Normal group (Group 1: Normal); ICS-induced FM group (Group 2: FM); 2Hz Electroacupuncture group (Group 3: 2Hz EA), and Sham EA group (Group 4: Sham EA).
2.2. FM Model and Bio-Plex ELISA
All mice were host at room temperature, 24 ± 1°C, before experiments. In the ICS-induced FM model, not in normal group, 2 mice were caged in a plexiglass cage (13 × 18.8 × 29.5 cm) covered with a stainless-steel mesh. On the first day (Day 0), the mice were kept in a cold room at 4 °C overnight (from 4:00 pm-10am). The mice were next moved to 24 °C for 30 min at 10 am. After 30 min, mice were then moved back to the cold room at 4 °C for 30 min. This process was repeated for till 4:00 pm. The mice were then placed in the 4 °C cold room overnight. Normal mice were kept at room temperature from Day 0 to 7 of the experiment, with no interventions applied. Mice plasma was collected and analyzed on Bio-Plex cytokine assays (BIO-RAD, CA, USA).
2.3. EA Treatments
The mice were anaesthetized with 5% isoflurane for induction, and then maintained in 1% isoflurane. Under anesthesia, a pair of stainless-steel acupuncture needles (1” inch, 36G, YU KUANG, Taiwan) were bilaterally inserted at a depth of 3-4 mm into the murine equivalent of the human ZuSanLi (ST36) acupoints. The murine ST36 is located approximately 4 mm below and 1–2 mm lateral to the midpoint of the knee in mice. A bilateral subcutaneous transverse insertion of acupuncture needles into the scapular region was considered to be the sham acupoint 10. In both the EA and Sham EA groups, electrical stimuli were delivered by Trio 300 stimulator (Ito, Japan) at an intensity of 1mA for 20 minutes at 2Hz with a pulse width of 100 μs. The EA and Sham EA treatment caused slight visible muscle twitching around the area of insertion. The EA and Sham EA stimulation was applied thrice from day 5 to 7, following the FM protocol.
2.4. Pain Behavior Test
The mechanical and thermal pain behaviors were determined 3 times from day 5 to 7 throughout the experiment after the induction of the FM model. All mice were moved to the behavior analysis room, and were adapted to the environment for at least 30 min before behavior tests. All experiments were performed at room temperature and the stimuli were applied only when the animals were calm but not sleeping or grooming. First, the von Frey filament test was conducted. Mechanical sensitivity was measured by testing the force of responses to stimulation with 3 applications of the electronic, calibrated von Frey filament (IITC Life Science Inc., USA). Mice were placed onto a metal mesh (75x25x45 cm) and covered with a plexiglass cage (10x6x11 cm). Subjects were then mechanically stimulated by the tip of the filament at the plantar region of the right hind paw. The filament gram counts were recorded when the stimulation caused the subject to withdraw its hind paw. Second, the Hargreaves’ assessment was used to measure thermal pain behavior by testing the time of response to thermal stimulation with 3 applications using Hargreaves' test IITC analgesiometer (IITC Life Sciences, SERIES8, Model 390G). The mice were placed in a plexiglass cage on top of a glass sheet. The thermal stimulator was positioned under the glass sheet and the focus of the projection bulb was aimed exactly at the middle of the plantar surface of the right hind paw. A cut-off time of 20s was set to prevent tissue damage. In the thermal paw withdrawal test, the nociception threshold was assessed using the latency of paw withdrawal upon stimulus, and was recorded when the constant applied heat stimulation caused the subject to withdraw its hind paw.
2.5. Western Blot Analysis
The mice were anaesthetized with 1% isoflurane and cervical dislocation. The thalamus, medial frontal cortex (mPFC), SSC, and amygdala tissues were immediately excised to extract proteins. Tissues were initially placed on ice and later stored at -80°C, pending protein extraction. Total proteins were homogenized in cold radioimmunoprecipitation (RIPA) lysis buffer containing 50 mM Tris-HCl pH 7.4, 250 mM NaCl, 1% NP-40, 5 mM EDTA, 50 mM NaF, 1 mM Na3VO4, 0.02% NaN3, and 1× protease inhibitor cocktail (AMRESCO). The extracted proteins were subjected to 8% SDS-Tris glycine gel electrophoresis and transferred to a PVDF membrane. The membrane was blocked with 5% non-fat milk in TBS-T buffer (10 mM Tris pH 7.5, 100 mM NaCl, 0.1% Tween 20), incubated with a primary antibody in TBS-T with 1% bovine serum albumin (BSA) for 1 hour at room temperature antibody against TLR4 (∼35 kDa, 1 : 1000, Millipore, USA), MyD88 (∼35 kDa, 1 : 1000, Millipore, USA), TRAF6 (∼87 kDa, 1 : 1000, Millipore, USA), pERK1/2 (∼42–44 kDa, 1 : 1000, Millipore, USA), pp38 (∼41 kDa, 1 : 1000, Millipore, USA), pJNK (∼42 kDa, 1 : 1000, Millipore, USA), and pNF𝜅B (∼65 kDa, 1 : 1000, Millipore, USA), in TBS-T with 1% bovine serum albumin. Peroxidase-conjugated anti-rabbit antibody, anti-mouse antibody or anti-goat antibody (1: 5000) was used as the appropriate secondary antibody. The bands were visualized by an enhanced chemiluminescent substrate kit (PIERCE) with LAS-3000 Fujifilm (Fuji Photo Film Co., Ltd.). Where applicable, the image intensities of specific bands were quantified with NIH ImageJ software (Bethesda, MD, USA).
2.6. Immunofluorescence
Mice were euthanized with a 5% isoflurane via inhalation and intracardially perfused with normal saline followed by 4% paraformaldehyde. The brain was immediately dissected and post fixed with 4% paraformaldehyde at 4 ºC for 3 days. The tissues were placed in 30% sucrose for cryoprotection overnight at 4 ºC. The brain was embedded in an Optimal cutting temperature (OCT) compound and rapidly frozen using liquid nitrogen before storing the tissues at -80 ºC. Frozen segments were cut at 20-μm width on a cryostat then instantaneously placed on glass slides. The samples were fixed with 4% paraformaldehyde, then incubated with a blocking solution, consisting of 3% BSA, 0.1% Triton X-100, and 0.02% sodium azide, for 1 h at room temperature. After blocking, the samples were incubated with the primary antibody (1:200, Alomone), TLR4 and Iba1, prepared in 1% bovine serum albumin solution at 4ºC overnight. The samples were then incubated with the secondary antibody (1:500), 488-conjugated AffiniPure donkey anti-rabbit IgG (H + L), 594-conjugated AffiniPure donkey anti-goat IgG (H + L) and Peroxidase-conjugated AffiniPure donkey anti-mouse IgG (H + L) for 2 h at room temperature before being fixed with cover slips for immunofluorescence visualization. The samples were observed by an epi-fluorescent microscope (Olympus, BX-51, Japan) with 20 x numerical aperture (NA = 1.4) objective. The images were analyzed by NIH ImageJ software (Bethesda, MD, USA).
2.7. Chemogenetics Operation
All mice were anesthetized with isoflurane and fixed in a stereotaxic device, a 23-gauge, 2 mm stainless cannula was inserted into SSC, 0.5 mm posterior and 1.5 mm lateral of bregma at 175 μm below the cortical superficial and fixed to the skull with dental glue. The inoculation cannula was inserted and connected with the Hamilton needle through a PE duct to inject 0.3 μL of viral solution more than 3 min through the pump (KD Scientific). After injection, the cannula was well-maintained at SSC for additional 2 min to permit fluid to diffuse. Next, 0.3 μL of hM4iD DREADD (designer receptors exclusively activated by designer drugs: AAV8-hSyn-hM4D(Gi)-mCherry; Addgene Plasmid #50477) were injected into the SSC over two weeks. Clozapine N-oxide (CNO; Sigma C0832) was injected to active the DREADD. CNO was thawed in 5% dimethyl sulfoxide (DMSO; Sigma D2650) and diluted with normal saline before intraperitoneal injection of 1 mg/kg at day 0.
2.8. Statistical Analysis
Statistical analysis was performed using the SPSS statistic program. All statistic data are presented as the mean ± standard error (SEM). Statistical significance among all groups was tested using the one-way ANOVA test, followed by a post hoc Tukey’s test. Values of p < 0.05 were considered statistically significant.
4. Discussion
We used the classical intermittent cold stress mice model to induce abnormal pain, mechanical allodynia and hyperalgesia, with gender hormone-independent female predominance of chronic pain, which was similar to clinical fibromyalgia patients. The behavior test confirmed the mechanical and thermal hyperalgesia in the FM mice. And the EA but not sham EA treatment reversed this hyperalgesia. The serum analysis showed increase pro-inflammatory cytokines: IL-1β, IL-2, IL-5, IL-6, IL-9, IL-12, IL-17A, TNF-α, IFN-γ, MCP-1 in FM mice. Similarly, EA but not sham EA treatment could reduce these pro-inflammatory cytokines. Furthermore, the molecular analysis found increased Iba1 and TLR4 related molecules in FM mice brain, indicating neuroinflammation. And EA treatment could reduce this neuroinflammation in FM mice.
Clinically, fibromyalgia is complex and difficult to treat. It was known as "nociplastic pain" [
33], that chronic pain is maintained in part by central sensitization, a phenomenon of synaptic plasticity, and increased neuronal responsiveness in central pain pathways after a stimulus [
34]. Fibromyalgia has features of central sensitization [
35], hyperalgesia, allodynia [
36], and hypersensitivity to various external stimuli such as sounds or lights [
37,
38]. A meta-analysis summarized 37 fibromyalgia syndrome papers, including 1264 subjects, and found consistent results of differences between FM patients and controls in pain-related brain areas [
39]. So, in our study, we focused on four mice brain areas: thalamus, prefrontal cortex, somatosensory cortex, and amygdala. The thalamus and somatosensory cortex are well-known pain-related brain region. Where prefrontal cortex receives projections from the mediodorsal nucleus of the thalamus [
40]. And the amygdala plays a crucial rule in emotional processing of pain. The pain modulatory role of amygdala is based on its projections to descending pain regions in the brain [
41]. We found increase expression of microglia and TLR4 pathway in these four brain regions.
Central sensitization is an important phenomenon and maintains the chronicity of pain. Accumulating evidence suggests that central sensitization is driven by neuroinflammation in the peripheral and central nervous system [
34]. A characteristic feature of neuroinflammation is the activation of glial cells, such as microglia and astrocytes, in the spinal cord and brain, leading to the release of proinflammatory cytokines and chemokines. Currently some researchers used proton magnetic resonance spectroscopy (MRS) to evaluate the brain neuroinflammation in patients with chronic pain [
42]. Another study used 18kDa translocator protein (TSPO) as a marker of CNS inflammation, used radioligand [11C]PBR28 bonded to TPSO. Then they could visualize the CNS inflammation by PET and MRI imaging. Most importantly, they found the signal of neuroinflammation decreased after local steroid injection, consistent with clinical pain release [
43]. Furthermore, a Japanese team created human-induced microglia-like (iMG) cells from human peripheral blood monocytes [
44]. They found higher expression of tumor necrosis factor (TNF)-α at mRNA and protein levels in ATP-stimulated iMG cells from patients with fibromyalgia compared to cells from healthy individuals. Interestingly, there was a moderate correlation between ATP-induced upregulation of TNF-α expression and clinical parameters of subjective pain and other mental manifestations of patients with fibromyalgia. Their findings suggest that microglia in patients with fibromyalgia are hypersensitive to ATP. And TNF-α from microglia may be a key factor underlying the complex pathology of fibromyalgia. According to above research, we used the classical central sensitization mice model of fibromyalgia to investigate the neuroinflammation in mice brain, focused on the microglia.
Toll-like receptors (TLRs) are pivotal components of the innate immune response, and shaping the adaptive immune response. However, TLR signaling pathways must be tightly regulated because undue TLR stimulation may disrupt the fine balance between pro- and anti-inflammatory responses [
45]. TLR4 had the role in chronic pain [
46] and neuropathic pain [
47], but not specific linking to fibromyalgia. Our previous research of fibromyalgia found alteration of TLR4 by electroacupuncture treatment [
6].
Complementary and alternative medicine (CAM) is commonly used to treat patients with fibromyalgia [
48]. About 91% of fibromyalgia patients have been identified as ever users of CAM [
49]. One of the most commonly used forms of CAM is acupuncture [
50]. For example, in Japan, the annual acupuncture utilization percentage ranges from 4.8 to 6.7%, with lifetime experiences of acupuncture ranging 19.4%–26.7% [
51]. A recent meta-analysis summarized 15 neuroimaging studies of musculoskeletal pain, including fibromyalgia, and revealed activated clusters in multiple cortical and subcortical brain structures in response to acupuncture, including the thalamus, insula, caudate, claustrum, and lentiform nucleus [
52]. However, controlling placebo effect is extremely important in acupuncture studies. In 1995, Beecher’s published an article “The powerful placebo,” which started the discussion of placebo effect [
53]. In patients with fibromyalgia, an interesting review including 124 studies found that placebo treatment affected pain and other patient-centered outcomes such as sleep, fatigue, function, and overall quality of life [
54]. Therefore, we compared true electroacupuncture and sham electroacupuncture even in our study. Moreover, we selected the acupoint ST36 (Zusanli) because of its well-recognized analgesic effect in mouse pain models [
55].
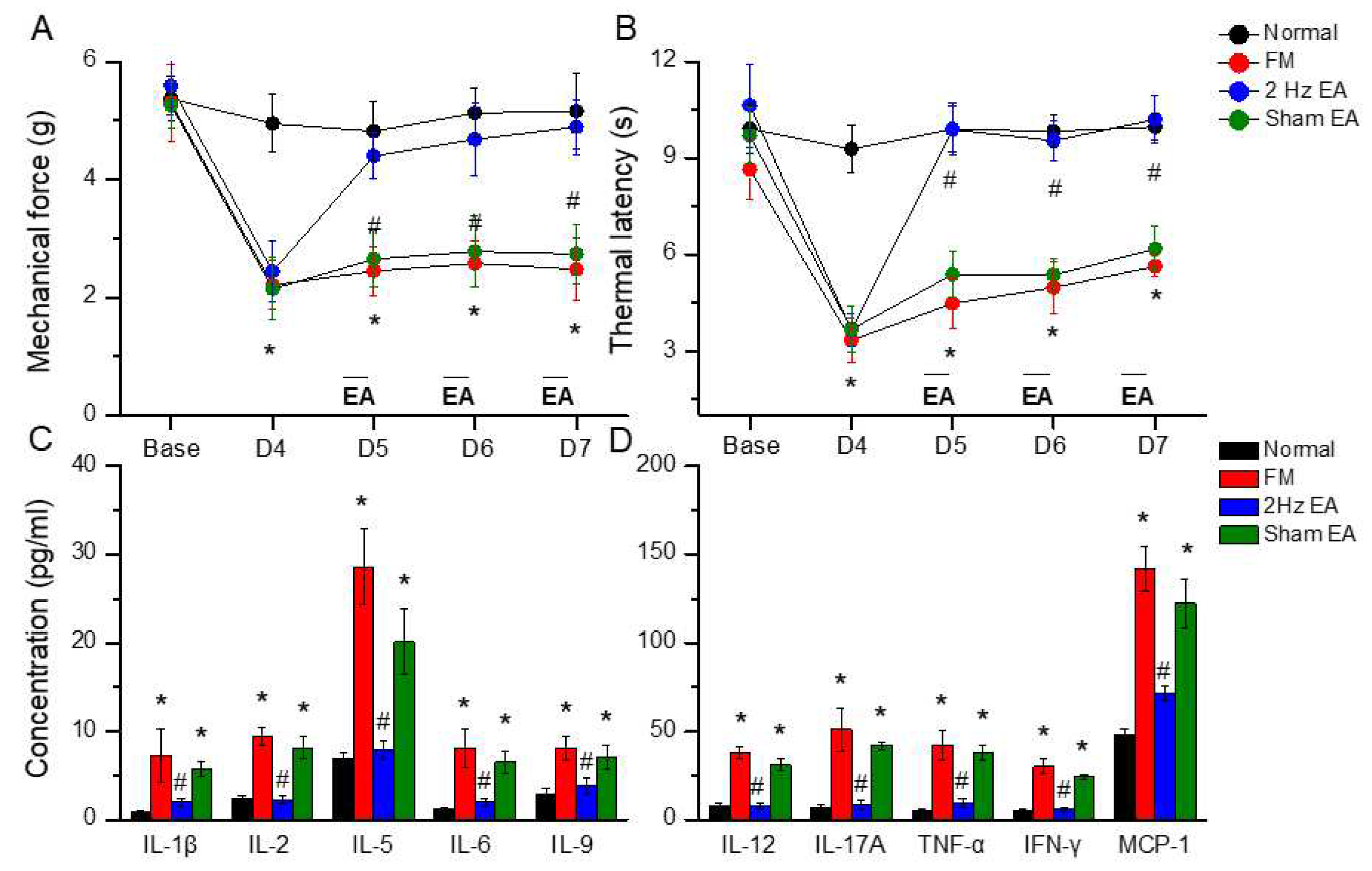
Figure 1.
Mechanical withdrawal, thermal latency, and the concentration of inflammatory mediators in normal, FM, EA, and sham EA mice. (A) Mechanical threshold from the von Frey tests. (B) Thermal latency from the Hargreaves’ test. (C) IL-1β, IL-2, IL-5, IL-6, and IL-9 (D) IL-12, IL-17A, TNF-α, IFN-γ, and MCP-1 in the mice plasma. *means statistical difference when compared to the normal mice. #indicates statistical significance when compared to the FM groups. IL= Interleukin; IFN= Interferon; TNF= Tumor necrosis factor; MCP= Monocyte Chemoattractant Protein. n=6 in all groups.
Figure 1.
Mechanical withdrawal, thermal latency, and the concentration of inflammatory mediators in normal, FM, EA, and sham EA mice. (A) Mechanical threshold from the von Frey tests. (B) Thermal latency from the Hargreaves’ test. (C) IL-1β, IL-2, IL-5, IL-6, and IL-9 (D) IL-12, IL-17A, TNF-α, IFN-γ, and MCP-1 in the mice plasma. *means statistical difference when compared to the normal mice. #indicates statistical significance when compared to the FM groups. IL= Interleukin; IFN= Interferon; TNF= Tumor necrosis factor; MCP= Monocyte Chemoattractant Protein. n=6 in all groups.
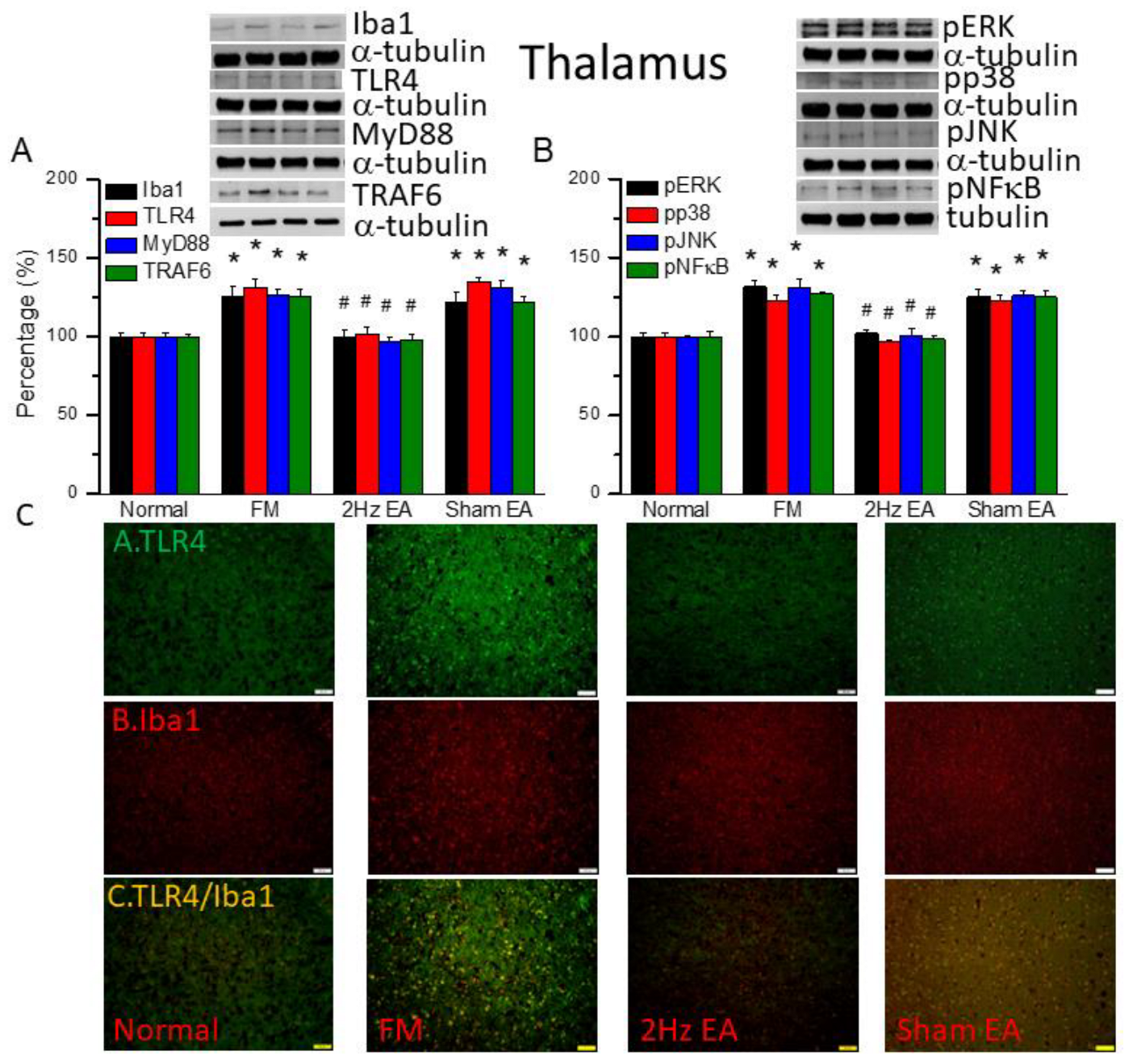
Figure 2.
The levels of Iba1 and TLR4 related molecules in the mice thalamus. The western blot bands contain four lanes of protein expression in order of Normal, FM, 2Hz EA, and sham EA. (A) Iba1, TLR4, MyD88, TRAF6. (B) pERK, pp38, pJNK, and pNFκB. *indicates statistical significance when compared with the normal group. #indicates statistical significance when compared to the FM group. n = 6 in all groups. (C) Immunofluorescence staining of TLR4, Iba1, and double staining protein expression in the mice thalamus. Scale bar means 100 μm. n = 4 in all groups.
Figure 2.
The levels of Iba1 and TLR4 related molecules in the mice thalamus. The western blot bands contain four lanes of protein expression in order of Normal, FM, 2Hz EA, and sham EA. (A) Iba1, TLR4, MyD88, TRAF6. (B) pERK, pp38, pJNK, and pNFκB. *indicates statistical significance when compared with the normal group. #indicates statistical significance when compared to the FM group. n = 6 in all groups. (C) Immunofluorescence staining of TLR4, Iba1, and double staining protein expression in the mice thalamus. Scale bar means 100 μm. n = 4 in all groups.
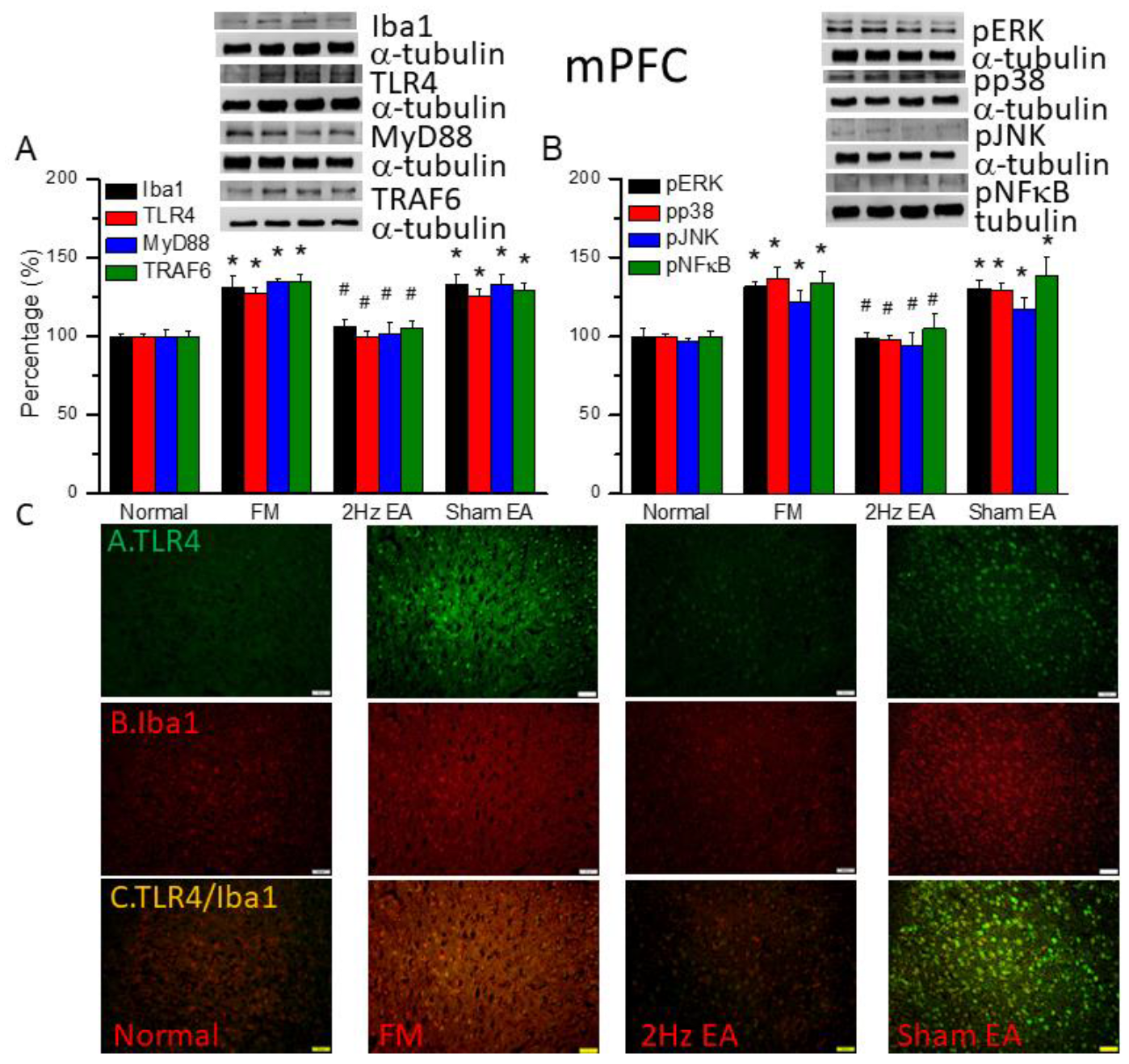
Figure 3.
The levels of Iba1 and TLR4 related molecules in the mice mPFC. The western blot bands contain four lanes of protein expression in order of Normal, FM, 2Hz EA, and sham EA. (A) Iba1, TLR4, MyD88, TRAF6. (B) pERK, pp38, pJNK, and pNFκB. *indicates statistical significance when compared with the normal group. #indicates statistical significance when compared to the FM group. n = 6 in all groups. (C) Immunofluorescence staining of TLR4, Iba1, and double staining protein expression in the mice mPFC. Scale bar means 100 μm. n = 4 in all groups.
Figure 3.
The levels of Iba1 and TLR4 related molecules in the mice mPFC. The western blot bands contain four lanes of protein expression in order of Normal, FM, 2Hz EA, and sham EA. (A) Iba1, TLR4, MyD88, TRAF6. (B) pERK, pp38, pJNK, and pNFκB. *indicates statistical significance when compared with the normal group. #indicates statistical significance when compared to the FM group. n = 6 in all groups. (C) Immunofluorescence staining of TLR4, Iba1, and double staining protein expression in the mice mPFC. Scale bar means 100 μm. n = 4 in all groups.
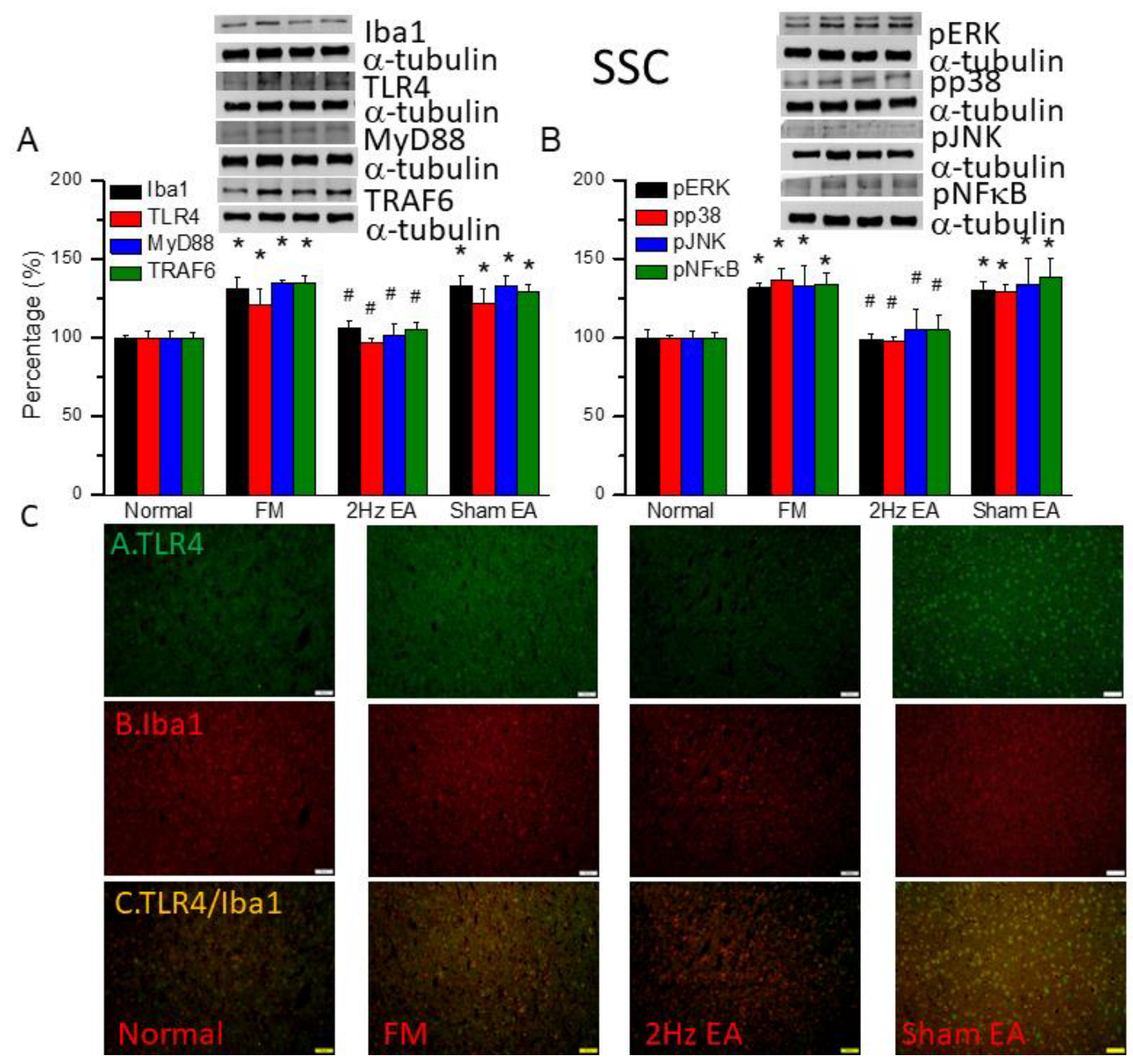
Figure 4.
The levels of Iba1 and TLR4 related molecules in the mice SSC. The western blot bands contain four lanes of protein expression in order of Normal, FM, 2Hz EA, and sham EA. (A) Iba1, TLR4, MyD88, TRAF6. (B) pERK, pp38, pJNK, and pNFκB. *indicates statistical significance when compared with the normal group. #indicates statistical significance when compared to the FM group. n = 6 in all groups. (C) Immunofluorescence staining of TLR4, Iba1, and double staining protein expression in the mice SSC. Scale bar means 100 μm. n = 4 in all groups.
Figure 4.
The levels of Iba1 and TLR4 related molecules in the mice SSC. The western blot bands contain four lanes of protein expression in order of Normal, FM, 2Hz EA, and sham EA. (A) Iba1, TLR4, MyD88, TRAF6. (B) pERK, pp38, pJNK, and pNFκB. *indicates statistical significance when compared with the normal group. #indicates statistical significance when compared to the FM group. n = 6 in all groups. (C) Immunofluorescence staining of TLR4, Iba1, and double staining protein expression in the mice SSC. Scale bar means 100 μm. n = 4 in all groups.
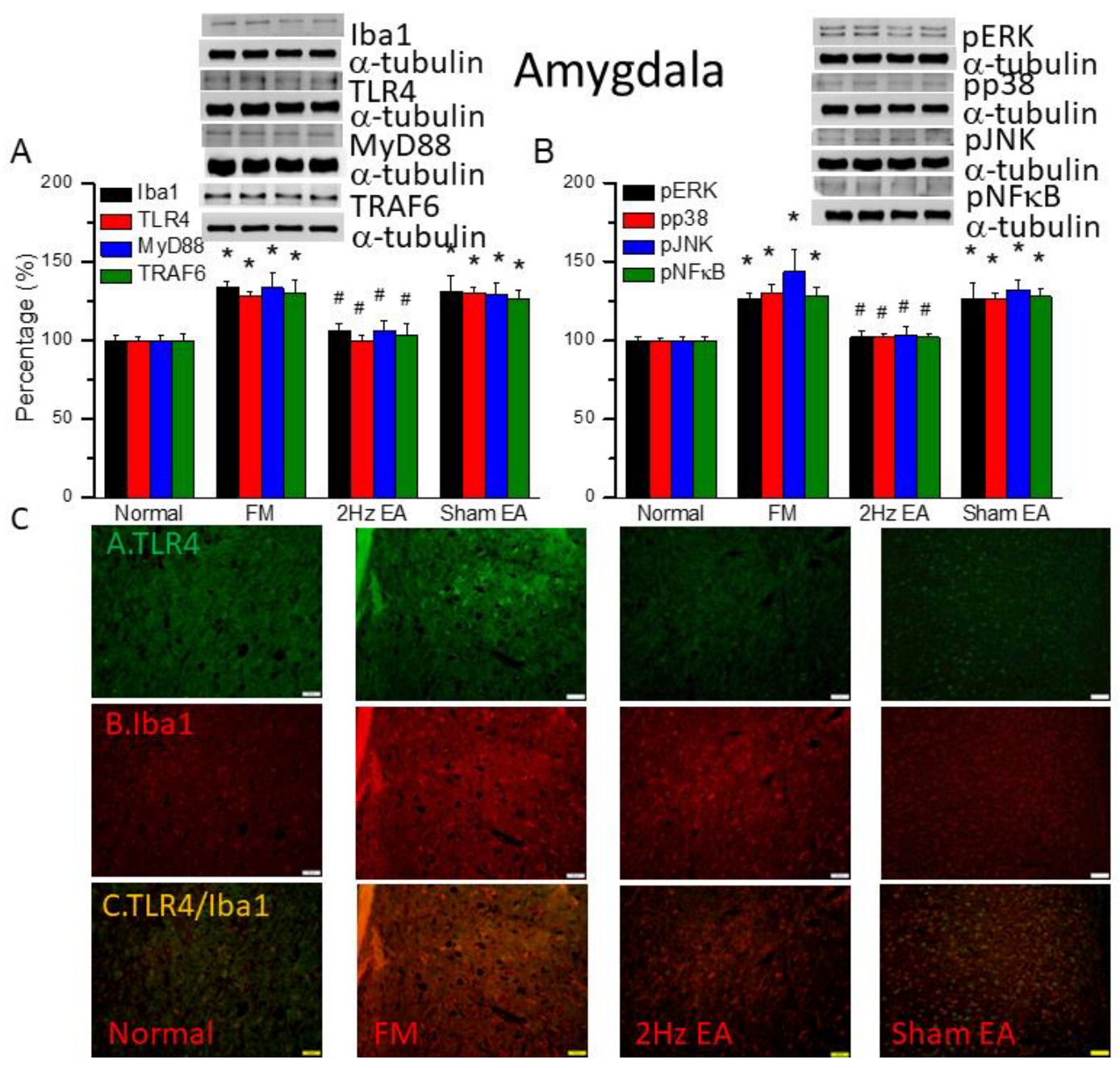
Figure 5.
The levels of Iba1 and TLR4 related molecules in the mice amygdala. The western blot bands contain four lanes of protein expression in order of Normal, FM, 2Hz EA, and sham EA. (A) Iba1, TLR4, MyD88, TRAF6. (B) pERK, pp38, pJNK, and pNFκB. *indicates statistical significance when compared with the normal group. #indicates statistical significance when compared to the FM group. n = 6 in all groups. (C) Immunofluorescence staining of TLR4, Iba1, and double staining protein expression in the mice amygdala. Scale bar means 100 μm. n = 4 in all groups.
Figure 5.
The levels of Iba1 and TLR4 related molecules in the mice amygdala. The western blot bands contain four lanes of protein expression in order of Normal, FM, 2Hz EA, and sham EA. (A) Iba1, TLR4, MyD88, TRAF6. (B) pERK, pp38, pJNK, and pNFκB. *indicates statistical significance when compared with the normal group. #indicates statistical significance when compared to the FM group. n = 6 in all groups. (C) Immunofluorescence staining of TLR4, Iba1, and double staining protein expression in the mice amygdala. Scale bar means 100 μm. n = 4 in all groups.
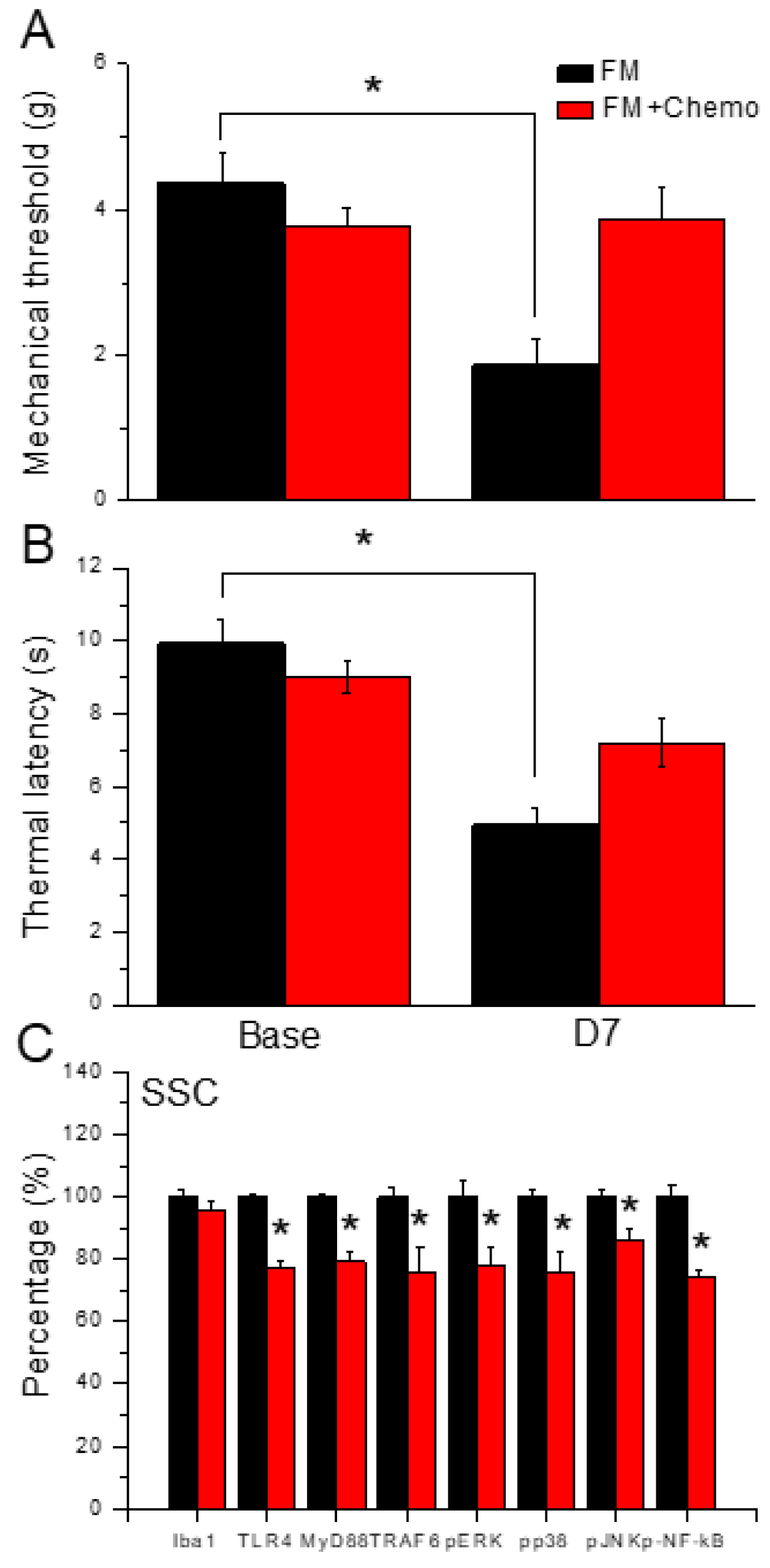
Figure 6.
Pain behavior of FM and FM mice treated with the chemogenetics technique. Black column: FM group, red column: FM treated with chemogenetics. * P < 0.05 compared with the FM group. (A) Mechanical hyperalgesia (von Frey test). (B) Thermal hyperalgesia (Hargreaves’ test). (C) Protein levels of Iba1, TLR4, MyD88, TRAF6, pERK, pJNK, pp38, and pNFkB were measured in mice SSC.
Figure 6.
Pain behavior of FM and FM mice treated with the chemogenetics technique. Black column: FM group, red column: FM treated with chemogenetics. * P < 0.05 compared with the FM group. (A) Mechanical hyperalgesia (von Frey test). (B) Thermal hyperalgesia (Hargreaves’ test). (C) Protein levels of Iba1, TLR4, MyD88, TRAF6, pERK, pJNK, pp38, and pNFkB were measured in mice SSC.
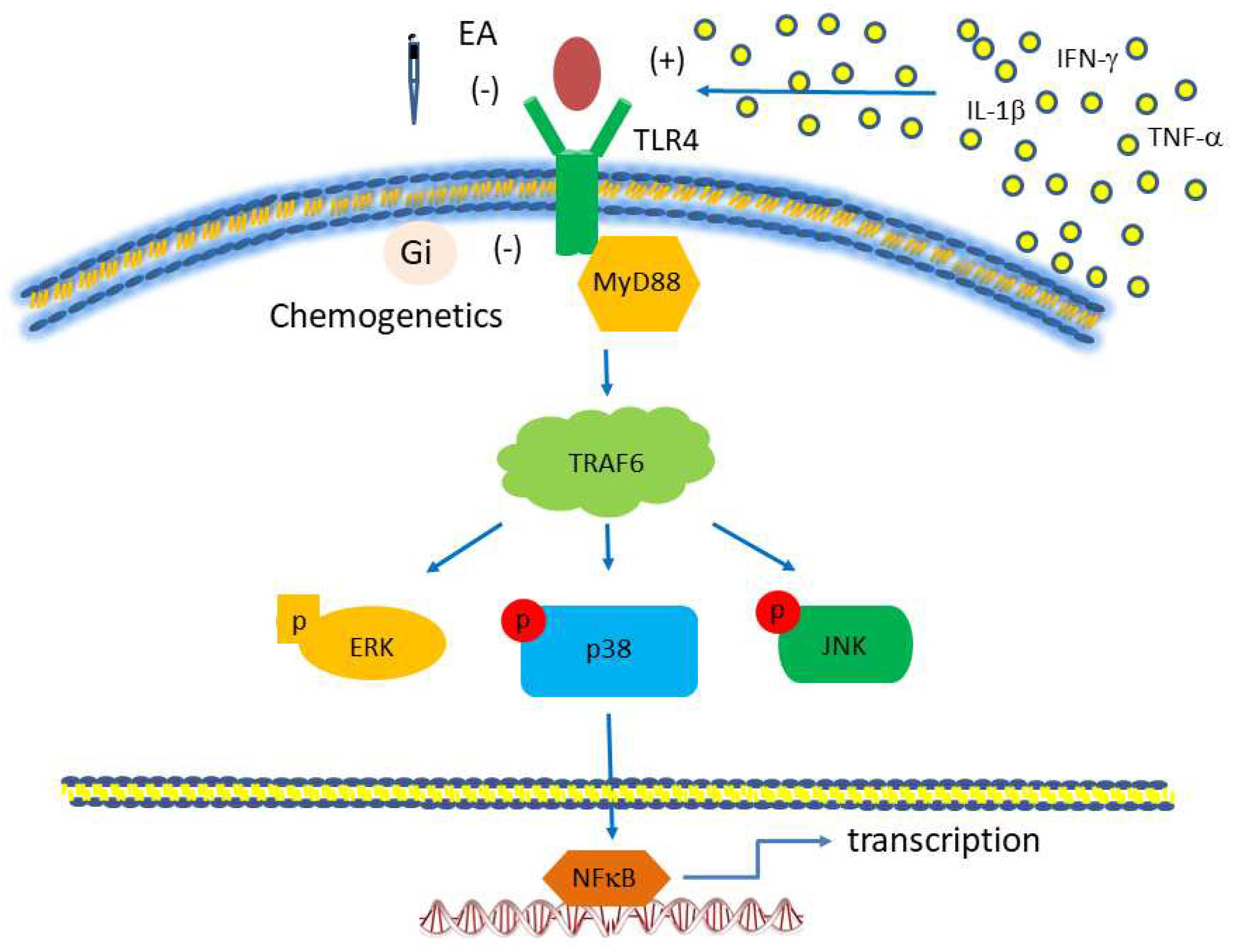
Figure 7.
Toll-like receptor (TLR) signaling pathway, focusing on TLR4. The red boxes are the proteins we analyzed in this study. Abbreviations: ERK= extracellular signal-regulated kinase; IFN= interferon; JNK= c-Jun N-terminal kinase; MyD88= myeloid differentiation primary-response protein 88; TLR, Toll-like receptors; TRAF= tumor necrosis factor receptor-associated factor.
Figure 7.
Toll-like receptor (TLR) signaling pathway, focusing on TLR4. The red boxes are the proteins we analyzed in this study. Abbreviations: ERK= extracellular signal-regulated kinase; IFN= interferon; JNK= c-Jun N-terminal kinase; MyD88= myeloid differentiation primary-response protein 88; TLR, Toll-like receptors; TRAF= tumor necrosis factor receptor-associated factor.