Submitted:
23 January 2024
Posted:
23 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
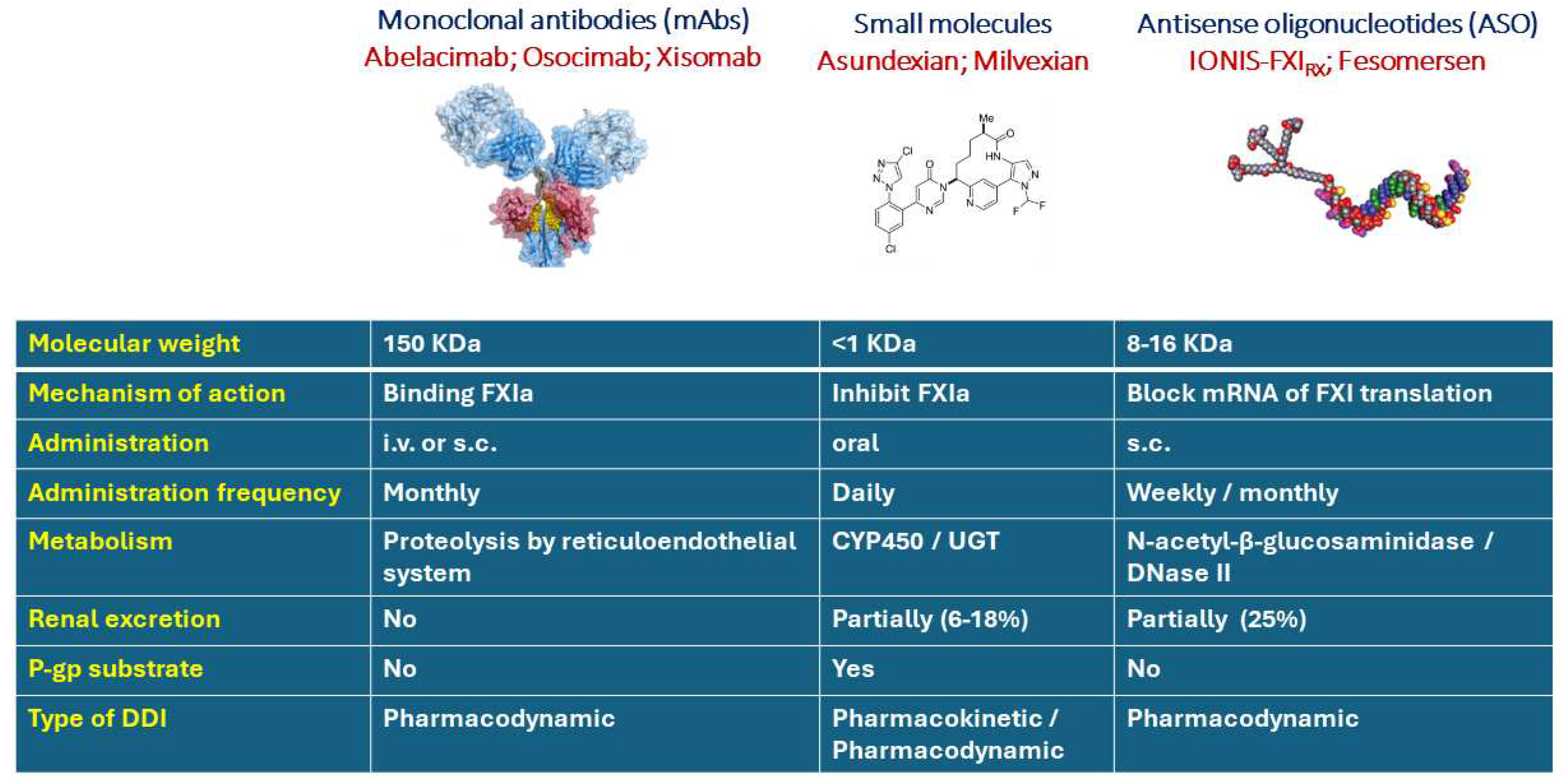
2. Pharmacological considerations of drug-drug interaction with mAbs and ASO
3. DOAC and small drug molecules anti FXIa: potential differences on DDIs
4. Clinical evidence of DDI with milvexian and asundexian
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Ferri, N.; Colombo, E.; Tenconi, M.; Baldessin, L.; Corsini, A. Drug-Drug Interactions of Direct Oral Anticoagulants (DOACs): From Pharmacological to Clinical Practice. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef]
- Chan, N.C.; Weitz, J.I. New Therapeutic Targets for the Prevention and Treatment of Venous Thromboembolism With a Focus on Factor XI Inhibitors. Arterioscler Thromb Vasc Biol 2023, 43, 1755–1763. [Google Scholar] [CrossRef]
- Duga, S.; Salomon, O. Congenital factor XI deficiency: an update. Seminars in thrombosis and hemostasis 2013, 39, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.bayer.com/media/en-us/oceanic-af-study-stopped-early-due-to-lack-of-efficacy/.
- Koch, A.W.; Schiering, N.; Melkko, S.; Ewert, S.; Salter, J.; Zhang, Y.; McCormack, P.; Yu, J.; Huang, X.; Chiu, Y.H.; et al. MAA868, a novel FXI antibody with a unique binding mode, shows durable effects on markers of anticoagulation in humans. Blood 2019, 133, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Campello, E.; Simioni, P.; Prandoni, P.; Ferri, N. Clinical Pharmacology of Factor XI Inhibitors: New Therapeutic Approaches for Prevention of Venous and Arterial Thrombotic Disorders. Journal of clinical medicine 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.F.; Baker, B.F.; Pham, N.; Swayze, E.; Geary, R.S. Pharmacology of Antisense Drugs. Annu Rev Pharmacol Toxicol 2017, 57, 81–105. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Bethune, C.; Smyth, A.; Tyrwhitt, J.; Jung, S.W.; Yu, R.Z.; Wang, Y.; Geary, R.S.; Weitz, J.; Bhanot, S.; et al. Phase 2 Study of the Factor XI Antisense Inhibitor IONIS-FXIRx in Patients With ESRD. Kidney Int Rep 2022, 7, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, R.Z.; Henry, S.; Geary, R.S. Pharmacokinetics and Clinical Pharmacology Considerations of GalNAc(3)-Conjugated Antisense Oligonucleotides. Expert opinion on drug metabolism & toxicology 2019, 15, 475–485. [Google Scholar] [CrossRef]
- Keizer, R.J.; Huitema, A.D.; Schellens, J.H.; Beijnen, J.H. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clinical pharmacokinetics 2010, 49, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, M.A.; Tseng, C.M.; Roskos, L.K. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today 2006, 11, 81–88. [Google Scholar] [CrossRef]
- Marathe, A.; Peterson, M.C.; Mager, D.E. Integrated cellular bone homeostasis model for denosumab pharmacodynamics in multiple myeloma patients. The Journal of pharmacology and experimental therapeutics 2008, 326, 555–562. [Google Scholar] [CrossRef]
- Ridker, P.M.; Tardif, J.C.; Amarenco, P.; Duggan, W.; Glynn, R.J.; Jukema, J.W.; Kastelein, J.J.P.; Kim, A.M.; Koenig, W.; Nissen, S.; et al. Lipid-Reduction Variability and Antidrug-Antibody Formation with Bococizumab. N Engl J Med 2017, 376, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Roskos, L.K.; Kellermann, S.; Foon, K.A. Human Antiglobulin Responses. Measuring Immunity. Basic Biology and Clinical Assessment 2005, Chapter 13, 172–186. [Google Scholar]
- Clark, M. Antibody humanization: a case of the ’Emperor’s new clothes’? Immunol Today 2000, 21, 397–402. [Google Scholar] [CrossRef]
- Ferri, N.; Corsini, A.; Sirtori, C.R.; Ruscica, M. Bococizumab for the treatment of hypercholesterolaemia. Expert opinion on biological therapy 2017, 17, 909–910. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.A.; Freedholm, D.; Widener, N.; Wang, X.; Simard, E.; Cullen, C.; Al-Saady, N.M.; Lepor, N.E.; Coulter, S.; Lovern, M.; et al. Pharmacokinetics and pharmacodynamics of Abelacimab (MAA868), a novel dual inhibitor of Factor XI and Factor XIa. Journal of thrombosis and haemostasis : JTH 2022, 20, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, B.; Thomas, D.; Schwers, S.; Wiegmann, S.; Prange, W.; Yassen, A.; Boxnick, S. First randomized evaluation of safety, pharmacodynamics, and pharmacokinetics of BAY 1831865, an antibody targeting coagulation factor XI and factor XIa, in healthy men. Journal of thrombosis and haemostasis : JTH 2022, 20, 1684–1695. [Google Scholar] [CrossRef]
- Lorentz, C.U.; Verbout, N.G.; Wallisch, M.; Hagen, M.W.; Shatzel, J.J.; Olson, S.R.; Puy, C.; Hinds, M.T.; McCarty, O.J.T.; Gailani, D.; et al. Contact Activation Inhibitor and Factor XI Antibody, AB023, Produces Safe, Dose-Dependent Anticoagulation in a Phase 1 First-In-Human Trial. Arteriosclerosis, thrombosis, and vascular biology 2019, 39, 799–809. [Google Scholar] [CrossRef]
- Maini, R.N.; Breedveld, F.C.; Kalden, J.R.; Smolen, J.S.; Davis, D.; Macfarlane, J.D.; Antoni, C.; Leeb, B.; Elliott, M.J.; Woody, J.N.; et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis and rheumatism 1998, 41, 1552–1563. [Google Scholar] [CrossRef]
- Seitz, K.; Zhou, H. Pharmacokinetic drug-drug interaction potentials for therapeutic monoclonal antibodies: reality check. Journal of clinical pharmacology 2007, 47, 1104–1118. [Google Scholar] [CrossRef]
- Gunawan, P.I.; Idarto, A.; Saharso, D. Acanthamoeba Infection in a Drowning Child. Ethiop J Health Sci 2016, 26, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.Z.; Graham, M.J.; Post, N.; Riney, S.; Zanardi, T.; Hall, S.; Burkey, J.; Shemesh, C.S.; Prakash, T.P.; Seth, P.P.; et al. Disposition and Pharmacology of a GalNAc3-conjugated ASO Targeting Human Lipoprotein (a) in Mice. Mol Ther Nucleic Acids 2016, 5, e317. [Google Scholar] [CrossRef]
- Donner, A.J.; Wancewicz, E.V.; Murray, H.M.; Greenlee, S.; Post, N.; Bell, M.; Lima, W.F.; Swayze, E.E.; Seth, P.P. Co-Administration of an Excipient Oligonucleotide Helps Delineate Pathways of Productive and Nonproductive Uptake of Phosphorothioate Antisense Oligonucleotides in the Liver. Nucleic Acid Ther 2017, 27, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Geary, R.S.; Wancewicz, E.; Matson, J.; Pearce, M.; Siwkowski, A.; Swayze, E.; Bennett, F. Effect of dose and plasma concentration on liver uptake and pharmacologic activity of a 2’-methoxyethyl modified chimeric antisense oligonucleotide targeting PTEN. Biochem Pharmacol 2009, 78, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Geary, R.S.; Yu, R.Z.; Watanabe, T.; Henry, S.P.; Hardee, G.E.; Chappell, A.; Matson, J.; Sasmor, H.; Cummins, L.; Levin, A.A. Pharmacokinetics of a tumor necrosis factor-alpha phosphorothioate 2’-O-(2-methoxyethyl) modified antisense oligonucleotide: comparison across species. Drug metabolism and disposition: the biological fate of chemicals 2003, 31, 1419–1428. [Google Scholar] [CrossRef]
- Shemesh, C.S.; Yu, R.Z.; Gaus, H.J.; Greenlee, S.; Post, N.; Schmidt, K.; Migawa, M.T.; Seth, P.P.; Zanardi, T.A.; Prakash, T.P.; et al. Elucidation of the Biotransformation Pathways of a Galnac3-conjugated Antisense Oligonucleotide in Rats and Monkeys. Mol Ther Nucleic Acids 2016, 5, e319. [Google Scholar] [CrossRef]
- Shemesh, C.S.; Yu, R.Z.; Warren, M.S.; Liu, M.; Jahic, M.; Nichols, B.; Post, N.; Lin, S.; Norris, D.A.; Hurh, E.; et al. Assessment of the Drug Interaction Potential of Unconjugated and GalNAc(3)-Conjugated 2’-MOE-ASOs. Mol Ther Nucleic Acids 2017, 9, 34–47. [Google Scholar] [CrossRef]
- Yu, R.Z.; Warren, M.S.; Watanabe, T.; Nichols, B.; Jahic, M.; Huang, J.; Burkey, J.; Geary, R.S.; Henry, S.P.; Wang, Y. Lack of Interactions Between an Antisense Oligonucleotide with 2’-O-(2-Methoxyethyl) Modifications and Major Drug Transporters. Nucleic Acid Ther 2016, 26, 111–117. [Google Scholar] [CrossRef]
- Willmann, S.; Marostica, E.; Snelder, N.; Solms, A.; Jensen, M.; Lobmeyer, M.; Lensing, A.W.A.; Bethune, C.; Morgan, E.; Yu, R.Z.; et al. PK/PD modeling of FXI antisense oligonucleotides to bridge the dose-FXI activity relation from healthy volunteers to end-stage renal disease patients. CPT Pharmacometrics Syst Pharmacol 2021, 10, 890–901. [Google Scholar] [CrossRef]
- Verhamme, P.; Yi, B.A.; Segers, A.; Salter, J.; Bloomfield, D.; Buller, H.R.; Raskob, G.E.; Weitz, J.I.; Investigators, A.-T. Abelacimab for Prevention of Venous Thromboembolism. The New England journal of medicine 2021, 385, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.I.; Bauersachs, R.; Becker, B.; Berkowitz, S.D.; Freitas, M.C.S.; Lassen, M.R.; Metzig, C.; Raskob, G.E. Effect of Osocimab in Preventing Venous Thromboembolism Among Patients Undergoing Knee Arthroplasty: The FOXTROT Randomized Clinical Trial. Jama 2020, 323, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Dilger, A.K.; Pabbisetty, K.B.; Corte, J.R.; De Lucca, I.; Fang, T.; Yang, W.; Pinto, D.J.P.; Wang, Y.; Zhu, Y.; Mathur, A.; et al. Discovery of Milvexian, a High-Affinity, Orally Bioavailable Inhibitor of Factor XIa in Clinical Studies for Antithrombotic Therapy. Journal of medicinal chemistry 2022, 65, 1770–1785. [Google Scholar] [CrossRef]
- Perera, V.; Wang, Z.; Luettgen, J.; Li, D.; DeSouza, M.; Cerra, M.; Seiffert, D. First-in-human study of milvexian, an oral, direct, small molecule factor XIa inhibitor. Clin Transl Sci 2022, 15, 330–342. [Google Scholar] [CrossRef]
- Perera, V.; Abelian, G.; Li, D.; Wang, Z.; Zhang, L.; Lubin, S.; Bello, A.; Murthy, B. Single-Dose Pharmacokinetics of Milvexian in Participants with Normal Renal Function and Participants with Moderate or Severe Renal Impairment. Clinical pharmacokinetics 2022, 61, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Piel, I.; Engelen, A.; Lang, D.; Schulz, S.I.; Gerisch, M.; Brase, C.; Janssen, W.; Fiebig, L.; Heitmeier, S.; Kanefendt, F. Metabolism and Disposition of the Novel Oral Factor XIa Inhibitor Asundexian in Rats and in Humans. European journal of drug metabolism and pharmacokinetics 2023, 48, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Kanefendt, F.; Brase, C.; Jungmann, N.; Fricke, R.; Engelen, A.; Schmitz, S. Pharmacokinetics of asundexian with combined CYP3A and P-gp inhibitors and an inducer: Target in vitro and in vivo studies. British journal of clinical pharmacology 2023. [Google Scholar] [CrossRef]
- Roehrig, S.; Ackerstaff, J.; Jimenez Nunez, E.; Teller, H.; Ellerbrock, P.; Meier, K.; Heitmeier, S.; Tersteegen, A.; Stampfuss, J.; Lang, D.; et al. Design and Preclinical Characterization Program toward Asundexian (BAY 2433334), an Oral Factor XIa Inhibitor for the Prevention and Treatment of Thromboembolic Disorders. Journal of medicinal chemistry 2023, 66, 12203–12224. [Google Scholar] [CrossRef]
- Gnoth, M.J.; Buetehorn, U.; Muenster, U.; Schwarz, T.; Sandmann, S. In vitro and in vivo P-glycoprotein transport characteristics of rivaroxaban. The Journal of pharmacology and experimental therapeutics 2011, 338, 372–380. [Google Scholar] [CrossRef]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Corsini, A.; Ferri, N.; Proietti, M.; Boriani, G. Edoxaban and the Issue of Drug-Drug Interactions: From Pharmacology to Clinical Practice. Drugs 2020, 80, 1065–1083. [Google Scholar] [CrossRef] [PubMed]
- Kubitza, D.; Heckmann, M.; Distler, J.; Koechel, A.; Schwers, S.; Kanefendt, F. Pharmacokinetics, pharmacodynamics and safety of BAY 2433334, a novel activated factor XI inhibitor, in healthy volunteers: A randomized phase 1 multiple-dose study. British journal of clinical pharmacology 2022, 88, 3447–3462. [Google Scholar] [CrossRef] [PubMed]
- Kanefendt, F.; Brase, C.; Unger, S.; Kubitza, D. Effects of Tablet Formulation, Food, or Gastric pH on the Bioavailability of Asundexian. Clinical pharmacology in drug development 2023, 12, 219–230. [Google Scholar] [CrossRef]
- Perera, V.; Wang, Z.; Lubin, S.; Christopher, L.J.; Chen, W.; Xu, S.; Seiffert, D.; DeSouza, M.; Murthy, B. Effects of Itraconazole and Diltiazem on the Pharmacokinetics and Pharmacodynamics of Milvexian, A Factor XIa Inhibitor. Cardiol Ther 2022, 11, 407–419. [Google Scholar] [CrossRef]
- Perera, V.; Wang, Z.; Lubin, S.; Christopher, L.J.; Chen, W.; Xu, S.; Seiffert, D.; DeSouza, M.; Murthy, B. Effects of rifampin on the pharmacokinetics and pharmacodynamics of milvexian, a potent, selective, oral small molecule factor XIa inhibitor. Sci Rep 2022, 12, 22239. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Guidance for industry. Drug interaction studies—design, data analysis, implications for dosing, and labeling instructions. 2018. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM292362.pdf (accessed on 6 June 2018).
| Drug | Dabigatran | Rivaroxaban | Apixaban | Edoxaban | Milvexian | Asundexian |
|---|---|---|---|---|---|---|
| Target | Thrombin | FXa | FXa | FXa | FXIa | FXIa |
| Ki (nmol/L) | 4.5 | 0.4 | 0.08 | 0.56 | 0.11 | 1.00 |
| Bioavailability | 6.5% | 80% | 50% | 60% | ND | 94% |
| Effect of food | Prolonged but not reduced | Increased (20 mg) |
None | None | Partially increased | Partially reduced |
| Vd | 60-70 L | 50 L | 21 L | >300L | 347L | NA |
| Proteins bound | 35% | >90% | 87% | 40-59% | 92% | 94% |
| Prodrug | Yes | No | No | No | No | No |
| Tmax (h) | 1-3 | 2-4 | 3-4 | 2 | 3-4 | 2.5-3 |
| Half lifetime (h) | 12-17 | 5-9 (sani) | 8-15 | 8-11 | 9-10 | 14-17 |
| Metabolism (CYP) | Conjugation | 3A4 (18%), 2J2, Independent from CYP |
CYP3A4 (25%), CYP1A2, CYP2J2, CYP2C8, CYP2C9, CYP2C19 | 3A4 (<4%) | 3A4 | 3A4 Modest 2C8, 2C9, 1A1 and 2D6 inhibitor Modest CYP3A4 inducer |
| Substrate P-gp | Yes (only prodrug) | Yes | Yes | Yes | Yes | Yes |
| Substrate of other transporters | NA | BCRP/ABCG2 | BCRP/ABCG2 | NA | No OATP | NA |
| Renal excretion | 80% | 65% | 27% | 35% | 7-18% | 6% |
| Posology | BID | OD | BID | OD | BID/OD | BID/OD |
| P-gp Inhibitor | Non-P-gp inhibitor | P-gp Inducer | |
|---|---|---|---|
| Strong CYP3A inhibitor | itraconazole, ketoconazole, clarithromycin, lopinavir, indinavir, ritonavir, telaprevir | voriconazole, fluconazole | |
| Moderate CYP3A inhibitor | erythromycin, verapamil, diltiazem, dronedarone | not identified | doxorubicin |
| Weak CYP3A inhibitor | lapatinib, quinidine, cyclosporine, felodipine, azithromycin, ranolazine, ticagrelor, chloroquine, hydroxychloroquine | cimetidine | vinblastine |
| CYP3A Inducers | carbamazepine, phenytoin, phenobarbital, rifampin, dexamethasone, tocilizumab, St. John’s Wort |
| Treatment | Analyte | AUC ratio day 1/day 1 (90% CI) |
AUC ratio day 10/day 1 (90% CI) |
|---|---|---|---|
| Asundexian 25 mg OD + midazolam | Midazolam | 1.04 (0.97-1.12) | 1.06 (0.99-1.14) |
| α-Hydroxymidazolam | 1.07 (0.97-1.17) | 1.06 (0.96-1.16) | |
| Asundexian 75 mg OD + midazolam | Midazolam | 1.04 (0.98-1.12) | 1.17 (1.10-1.26) * |
| α-Hydroxymidazolam | 0.99 (0.92-1.06) | 0.99 (0.92-1.06) |
| Parameter | asundexian | asundexian + Itraconazole | Ratio asundexian +Itraconazole / asundexian | ||
| AUC μg h L−1 | 6920 | 14000 | 2.02 | ||
| Cmax μg L−1 | 377 | 387 | 1.03 | ||
| tmax | 3.48 | 2.49 | 0.71 | ||
| t1/2 | 16.2 | 28.9 | 1.78 | ||
| CL/F | 3.61 | 1.78 | 0.49 | ||
| CLR | 0.307 | 0.180 | 0.59 | ||
| Parameter | asundexian | asundexian + verapamil | Ratio asundexian+ verapamil / asundexian | asundexian + fluconazole | Ratio asundexian+ fluconazole / asundexian |
| AUC μg h L−1 | 6360 | 11200 | 1.76 | 7430 | 1.168 |
| Cmax μg L−1 | 347 | 396 | 1.14 | 359 | 1.035 |
| tmax | 3.47 | 3.00 | 0.87 | 3.45 | 0.994 |
| t1/2 | 21.8 | 22.6 | 1.04 | 15.5 | 0.711 |
| CL/F | 3.93 | 2.24 | 0.57 | 3.37 | 0.858 |
| CLR | 0.297 | 0.215 | 0.72 | 0.313 | 1.054 |
| Parameter | asundexian | asundexian + carbamazepine | Ratio asundexian+ carbamazepine / asundexian | ||
| AUC μg h L−1 | 11500 | 6380 | 0.55 | ||
| Cmax μg L−1 | 623 | 509 | 0.82 | ||
| tmax | 2.00 | 3.00 | 1.50 | ||
| t1/2 | 14.4 | 11.0 | 0.76 | ||
| CL/F | 4.36 | 7.84 | 1.80 | ||
| CLR | 0.265 | 0.377 | 1.42 | ||
| Parameter | milvexian |
milvexian following repeated doses of rifampicin |
Ratio milvexian+ rifampicin / milvexian |
| Cmax, ng/mL | 599 | 132 | 0.22 |
| AUC, ng·h/mL | 6153 | 923 | 0.15 |
| Tmax, h | 3.5 | 4.0 | 1.14 |
| T1/2, h | 13.21 | 8.85 | 0.67 |
| Parameter | milvexian | milvexian / itraconazole | Ratio milvexian+ itraconazole / milvexian |
| Cmax, ng/mL | 229 | 293 | 1.28 |
| AUC, ng·h/mL | 2144 | 5342 | 2.49 |
| Tmax, h | 3.0 | 4.0 | 1.33 |
| T1/2, h | 11.6 | 17.1 | 1.47 |
| Parameter | milvexian | milvexian / diltiazem | Ratio milvexian+ diltiazem / milvexian |
| Cmax, ng/mL | 248 | 272 | 1.10 |
| AUC, ng·h/mL | 2220 | 3059 | 1.38 |
| Tmax, h | 3.0 | 4.0 | 1.33 |
| T1/2, h | 12.3 | 13.6 | 1.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





