Submitted:
22 January 2024
Posted:
23 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study design and data collection
2.2. Genomic analysis
2.3. Statistical analysis
3. Results
3.1. Baseline characteristics
3.2. Genomic variants
3.3. Changes in clinical features after DMARD treatment
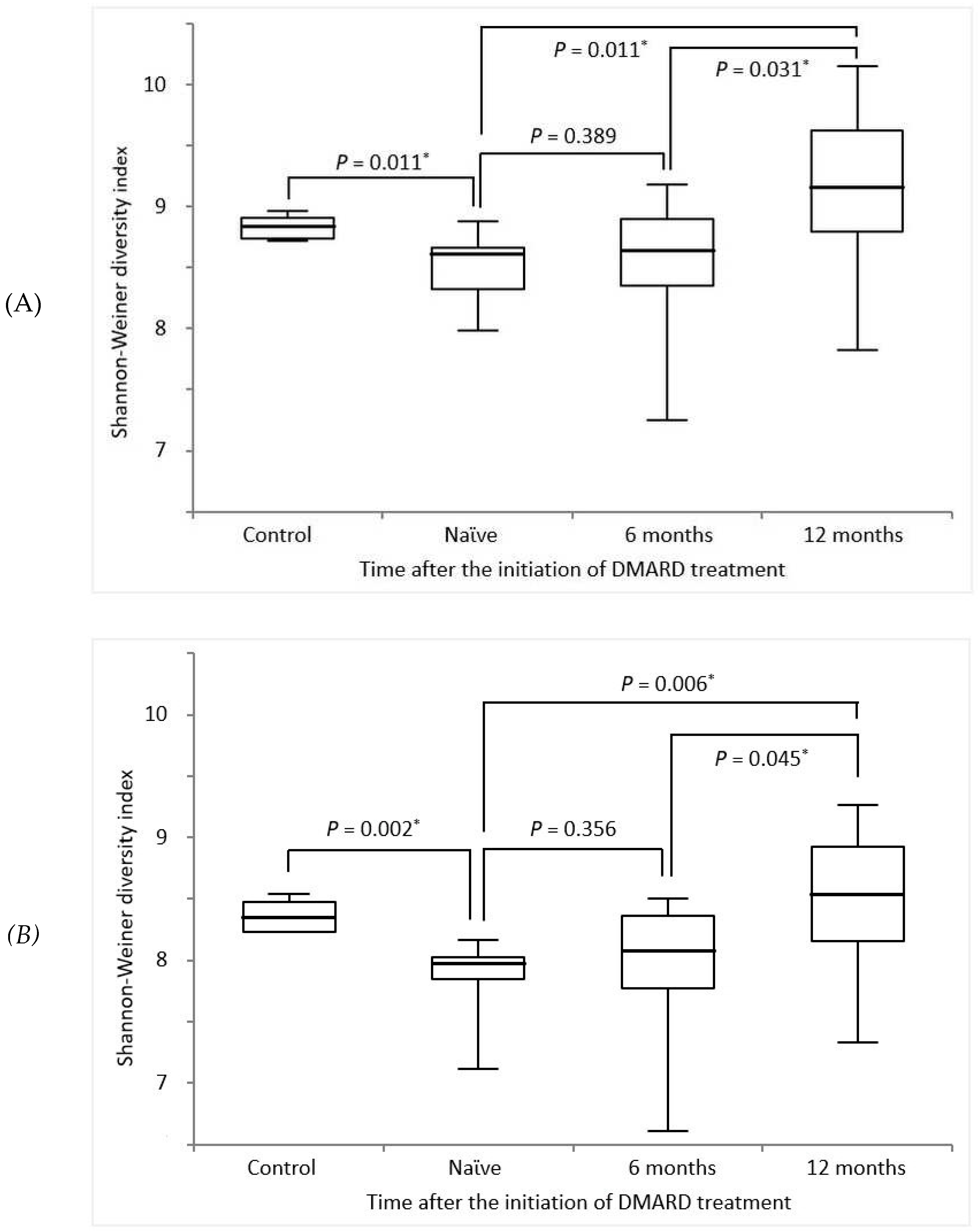
3.4. Changes in TCR diversity after DMARD treatment
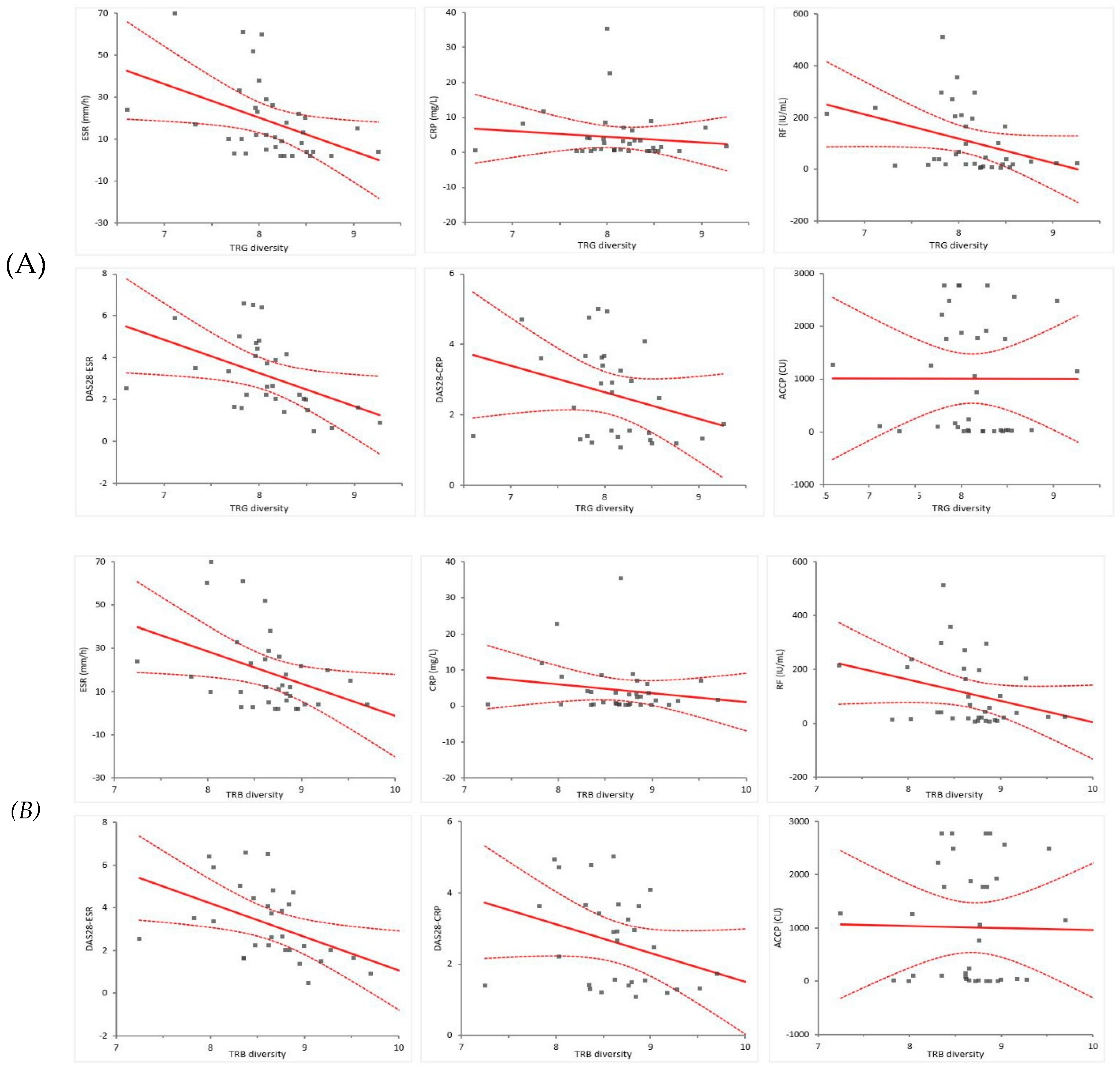
3.5. Relationship between TCR diversity and RA-related factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gravallese, E.M.; Firestein, G.S. Rheumatoid arthritis - common origins, divergent mechanisms. N Engl J Med 2023, 388, 529–542. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Silman, A.J.; Pearson, J.E. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res 2002, 4 Suppl 3, S265–272. [Google Scholar] [CrossRef]
- Klareskog, L.; Catrina, A.I.; Paget, S. Rheumatoid arthritis. Lancet 2009, 373, 659–672. [Google Scholar] [CrossRef]
- Seldin, M.F.; Amos, C.I.; Ward, R.; Gregersen, P.K. The genetics revolution and the assault on rheumatoid arthritis. Arthritis Rheum 1999, 42, 1071–1079. [Google Scholar] [CrossRef]
- MacGregor, A.J.; Snieder, H.; Rigby, A.S.; Koskenvuo, M.; Kaprio, J.; Aho, K.; Silman, A.J. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum 2000, 43, 30–37. [Google Scholar] [CrossRef]
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef]
- Kurreeman, F.A.; Padyukov, L.; Marques, R.B.; Schrodi, S.J.; Seddighzadeh, M.; Stoeken-Rijsbergen, G.; van der Helm-van Mil, A.H.; Allaart, C.F.; Verduyn, W.; Houwing-Duistermaat, J.; et al. A candidate gene approach identifies the traf1/c5 region as a risk factor for rheumatoid arthritis. PLoS Med 2007, 4, e278. [Google Scholar] [CrossRef]
- Oliver, J.E.; Worthington, J.; Silman, A.J. Genetic epidemiology of rheumatoid arthritis. Curr Opin Rheumatol 2006, 18, 141–146. [Google Scholar] [CrossRef]
- Prokunina, L.; Padyukov, L.; Bennet, A.; de Faire, U.; Wiman, B.; Prince, J.; Alfredsson, L.; Klareskog, L.; Alarcón-Riquelme, M. Association of the pd-1.3a allele of the pdcd1 gene in patients with rheumatoid arthritis negative for rheumatoid factor and the shared epitope. Arthritis Rheum 2004, 50, 1770–1773. [Google Scholar] [CrossRef]
- Sandigursky, S.; Silverman, G.J.; Mor, A. Targeting the programmed cell death-1 pathway in rheumatoid arthritis. Autoimmun Rev 2017, 16, 767–773. [Google Scholar] [CrossRef]
- Johnson, D.B.; Frampton, G.M.; Rioth, M.J.; Yusko, E.; Xu, Y.; Guo, X.; Ennis, R.C.; Fabrizio, D.; Chalmers, Z.R.; Greenbowe, J.; et al. Targeted next generation sequencing identifies markers of response to pd-1 blockade. Cancer Immunol Res 2016, 4, 959–967. [Google Scholar] [CrossRef]
- Saad, M.N.; Mabrouk, M.S.; Eldeib, A.M.; Shaker, O.G. Genetic case-control study for eight polymorphisms associated with rheumatoid arthritis. PLoS One 2015, 10, e0131960. [Google Scholar] [CrossRef]
- Stahl, E.A.; Raychaudhuri, S.; Remmers, E.F.; Xie, G.; Eyre, S.; Thomson, B.P.; Li, Y.; Kurreeman, F.A.; Zhernakova, A.; Hinks, A.; et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet 2010, 42, 508–514. [Google Scholar] [CrossRef]
- Eyre, S.; Bowes, J.; Diogo, D.; Lee, A.; Barton, A.; Martin, P.; Zhernakova, A.; Stahl, E.; Viatte, S.; McAllister, K.; et al. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet 2012, 44, 1336–1340. [Google Scholar] [CrossRef]
- Zhernakova, A.; Stahl, E.A.; Trynka, G.; Raychaudhuri, S.; Festen, E.A.; Franke, L.; Westra, H.J.; Fehrmann, R.S.; Kurreeman, F.A.; Thomson, B.; et al. Meta-analysis of genome-wide association studies in celiac disease and rheumatoid arthritis identifies fourteen non-hla shared loci. PLoS Genet 2011, 7, e1002004. [Google Scholar] [CrossRef]
- Kurreeman, F.A.; Stahl, E.A.; Okada, Y.; Liao, K.; Diogo, D.; Raychaudhuri, S.; Freudenberg, J.; Kochi, Y.; Patsopoulos, N.A.; Gupta, N.; et al. Use of a multiethnic approach to identify rheumatoid- arthritis-susceptibility loci, 1p36 and 17q12. Am J Hum Genet 2012, 90, 524–532. [Google Scholar] [CrossRef]
- Wiley, G.B.; Kelly, J.A.; Gaffney, P.M. Use of next-generation DNA sequencing to analyze genetic variants in rheumatic disease. Arthritis Res Ther 2014, 16, 490. [Google Scholar] [CrossRef]
- Turcinov, S.; Af Klint, E.; Van Schoubroeck, B.; Kouwenhoven, A.; Mia, S.; Chemin, K.; Wils, H.; Van Hove, C.; De Bondt, A.; Keustermans, K.; et al. Diversity and clonality of t cell receptor repertoire and antigen specificities in small joints of early rheumatoid arthritis. Arthritis Rheumatol 2023, 75, 673–684. [Google Scholar] [CrossRef]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The american rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An american college of rheumatology/european league against rheumatism collaborative initiative. Arthritis Rheum 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Fleischmann, R.; van der Heijde, D.; Koenig, A.S.; Pedersen, R.; Szumski, A.; Marshall, L.; Bananis, E. How much does disease activity score in 28 joints esr and crp calculations underestimate disease activity compared with the simplified disease activity index? Ann Rheum Dis 2015, 74, 1132–1137. [Google Scholar] [CrossRef]
- Yang, P.; He, Y.; Qing, P.; Xu, W.; Xie, D.; Cazier, J.B.; Liu, X.; Varnai, C.; Zhou, Y.; Zhao, Y.; et al. Application of t-cell receptor repertoire as a novel monitor in dynamic tracking and assessment: A cohort-study based on ra patients. J Cell Mol Med 2022, 26, 6042–6055. [Google Scholar] [CrossRef]
- Viljakainen, J.; Raju, S.C.; Viljakainen, H.; Figueiredo, R.A.O.; Roos, E.; Weiderpass, E.; Rounge, T.B. Meal regularity plays a role in shaping the saliva microbiota. Front Microbiol 2020, 11, 757. [Google Scholar] [CrossRef]
- Imboden, J.B. The immunopathogenesis of rheumatoid arthritis. Annu Rev Pathol 2009, 4, 417–434. [Google Scholar] [CrossRef]
- Stahl, E.A.; Wegmann, D.; Trynka, G.; Gutierrez-Achury, J.; Do, R.; Voight, B.F.; Kraft, P.; Chen, R.; Kallberg, H.J.; Kurreeman, F.A.; et al. Bayesian inference analyses of the polygenic architecture of rheumatoid arthritis. Nat Genet 2012, 44, 483–489. [Google Scholar] [CrossRef]
- Schumacher, R.F.; Mella, P.; Badolato, R.; Fiorini, M.; Savoldi, G.; Giliani, S.; Villa, A.; Candotti, F.; Tampalini, A.; O'Shea, J.J.; et al. Complete genomic organization of the human jak3 gene and mutation analysis in severe combined immunodeficiency by single-strand conformation polymorphism. Hum Genet 2000, 106, 73–79. [Google Scholar] [CrossRef]
- Angelini, J.; Talotta, R.; Roncato, R.; Fornasier, G.; Barbiero, G.; Dal Cin, L.; Brancati, S.; Scaglione, F. Jak-inhibitors for the treatment of rheumatoid arthritis: A focus on the present and an outlook on the future. Biomolecules 2020, 10. [Google Scholar] [CrossRef]
- Tamiya, G.; Shinya, M.; Imanishi, T.; Ikuta, T.; Makino, S.; Okamoto, K.; Furugaki, K.; Matsumoto, T.; Mano, S.; Ando, S.; et al. Whole genome association study of rheumatoid arthritis using 27 039 microsatellites. Hum Mol Genet 2005, 14, 2305–2321. [Google Scholar] [CrossRef]
- Croft, M.; Siegel, R.M. Beyond tnf: Tnf superfamily cytokines as targets for the treatment of rheumatic diseases. Nat Rev Rheumatol 2017, 13, 217–233. [Google Scholar] [CrossRef]
- Raychaudhuri, S.; Remmers, E.F.; Lee, A.T.; Hackett, R.; Guiducci, C.; Burtt, N.P.; Gianniny, L.; Korman, B.D.; Padyukov, L.; Kurreeman, F.A.; et al. Common variants at cd40 and other loci confer risk of rheumatoid arthritis. Nat Genet 2008, 40, 1216–1223. [Google Scholar] [CrossRef]
- Okada, Y.; Terao, C.; Ikari, K.; Kochi, Y.; Ohmura, K.; Suzuki, A.; Kawaguchi, T.; Stahl, E.A.; Kurreeman, F.A.; Nishida, N.; et al. Meta-analysis identifies nine new loci associated with rheumatoid arthritis in the japanese population. Nat Genet 2012, 44, 511–516. [Google Scholar] [CrossRef]
- Plenge, R.M.; Scolnick, E.M.; Altshuler, D. Validating therapeutic targets through human genetics. Nat Rev Drug Discov 2013, 12, 581–594. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, S.; Zhou, C.; Wu, J.; Jiao, Y.; Lin, L.; Lu, X.; Yang, B.; Zhang, W.; Xiao, X.; et al. Comprehensive tcr repertoire analysis of cd4(+) t-cell subsets in rheumatoid arthritis. J Autoimmun 2020, 109, 102432. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Zhao, M.; Fu, L.; Liu, L.; Wu, J.; Luo, S.; Wang, L.; Wang, Z.; Lin, L.; et al. T cell receptor β repertoires as novel diagnostic markers for systemic lupus erythematosus and rheumatoid arthritis. Ann Rheum Dis 2019, 78, 1070–1078. [Google Scholar] [CrossRef]
- Ria, F.; Penitente, R.; De Santis, M.; Nicolò, C.; Di Sante, G.; Orsini, M.; Arzani, D.; Fattorossi, A.; Battaglia, A.; Ferraccioli, G.F. Collagen-specific t-cell repertoire in blood and synovial fluid varies with disease activity in early rheumatoid arthritis. Arthritis Res Ther 2008, 10, R135. [Google Scholar] [CrossRef]
| Baseline variables | Unit | Feature | Patients withRA (n=14) | HCs(n=5) | p-Value |
| Age | Years | Median (IQR) | 57 (45–69) | 38 (31–48) | 0.034* |
| Female sex | No. (%) | 12 (85.7) | 3 (60.0) | 0.046* | |
| Medical history of | |||||
| Hypertension | No. (%) | 3 (21.4) | 1 (20.0) | 0.946 | |
| Diabetes mellitus | No. (%) | 3 (21.4) | 0 (0.0) | 0.259 | |
| Obesity (BMI > 25 kg/m2) | No. (%) | 4 (28.6) | 1 (20.0) | 0.709 | |
| Duration before the first visit | Months | Median (IQR) | 8 (3-12) | - | - |
| RA criteria fulfillment | |||||
| ACR 1987 | No. (%) | 10 (71.4) | - | - | |
| ACR/EULAR 2010 | No. (%) | 14 (100.0) | - | - | |
| Disease-related variants | No. (%) | 4 (28.6) | - | - | |
| Laboratory findings | |||||
| ANA-positive | No. (%) | 12 (85.7) | 0 (0.0) | <0.001* | |
| RF-positive | No. (%) | 13 (92.9) | 0 (0.0) | <0.001* | |
| ACCP-positive | No. (%) | 13 (92.9) | 0 (0.0) | <0.001* | |
| ESR | mm/h | Median (IQR) | 29 (12–53) | 5 (2–8) | <0.001* |
| CRP | mg/L | Median (IQR) | 3.7 (2.2–8.2) | 1.3 (0.3–2.8) | 0.019* |
| Patient | Gene | DNA change | AA change | Zygosity | Class |
| 1 | JAK3 | 1333C>T | Arg445Ter | Hetero | PV |
| PADI4 | 1861G>C | Glu621Gln | Hetero | VUS | |
| TNFSF18 | 167T>C | Met56Th | Hetero | VUS | |
| TRAF1 | 385C>T | Arg129Trp | Hetero | VUS | |
| 2 | NFKB1 | 2708A>G | His903Arg | Hetero | VUS |
| 3 | TNFSF18 | 93G>A | Met31Ile | Hetero | VUS |
| 4 | TNFSF18 | 94C>T | Pro32Ser | Hetero | VUS |
| Variables | Unit | Feature | Baseline | Time after DMARD treatment | p-Value | ||
| 6 months | 12 months | Baseline vs6 months | Baseline vs12 months | ||||
| Laboratory findings | |||||||
| ESR | mm/h | Median (IQR) | 29 (12-53) | 11 (3-22) | 10 (5-20) | 0.006* | 0.005* |
| CRP | mg/L | Median (IQR) | 3.7 (2.2-8.2) | 1.6 (0.7-7.0) | 0.9 (0.4-3.5) | 0.148 | 0.029* |
| RF titer | IU/mL | Median (IQR) | 148.5 (55.7-239.8) | 42.0 (19.0-107.3) | 33.5 (22.8-99.1) | 0.051 | 0.033* |
| ACCP titer | CU | Median (IQR) | 1266.2 (37.8-1970.2) | 947.8 (36.6-1831.8) | 801.4 (150.6-1905.1) | 0.963 | 0.748 |
| Joint counts | |||||||
| TJC44 | no. | Median (IQR) | 4 (3-9) | 1 (0-3) | 1 (0-2) | 0.003* | <0.001* |
| SJC44 | no. | Median (IQR) | 3 (2-5) | 0 (0-1) | 0 (0-1) | <0.001* | <0.001* |
| TJC28 | no. | Median (IQR) | 3 (2-5) | 0 (0-1) | 0 (0-1) | <0.001* | <0.001* |
| SJC28 | no. | Median (IQR) | 2 (1-3) | 0 (0-1) | 0 (0-1) | <0.001* | <0.001* |
| Disease measures | |||||||
| DAS28-ESR | Mean ± SD | 4.75 ± 1.26 | 2.52 ± 0.89 | 2.16 ± 1.21 | <0.001* | <0.001* | |
| DAS28-CRP | Mean ± SD | 3.68 ± 0.89 | 2.03 ± 0.90 | 1.98 ± 0.85 | <0.001* | <0.001* | |
| HAQ score | Mean ± SD | 1.18 ± 0.65 | 0.64 ± 0.32 | 0.53 ± 0.35 | 0.013* | 0.008* | |
| Pain VAS score | Mean ± SD | 6.71 ± 1.98 | 2.14 ± 1.17 | 1.89 ± 1.18 | <0.001* | <0.001* | |
| SDAI | Mean ± SD | 16.54 ± 8.22 | 5.43 ± 3.62 | 3.68 ± 2.46 | <0.001* | <0.001* | |
| CDAI | Mean ± SD | 16.43 ± 8.20 | 5.23 ± 3.47 | 4.76 ± 4.25 | <0.001* | <0.001* | |
| TRB | TRG | ||||
| Spearman's r(95% CI) | p-Value | Spearman's r (95% CI) | p-Value | ||
| Laboratory findings | |||||
| ESR | -0.435 (-0.689 to -0.084) | 0.015* | -0.378 (-0.652 to -0.017) | 0.036* | |
| CRP | -0.163 (-0.489 to 0.213) | 0.380 | -0.063 (-0.417 to 0.308) | 0.737 | |
| RF titer | -0.267 (-0.575 to 0.107) | 0.146 | -0.277 (-0.583 to 0.096) | 0.131 | |
| ACCP titer | 0.076 (-0.488 to 0.226) | 0.684 | -0.019 (-0.380 to 0.347) | 0.919 | |
| Disease measures | |||||
| DAS28-ESR | -0.580 (-0.780 to -0.274) | <0.001* | -0.575 (-0.777 to -0.268) | <0.001* | |
| DAS28-CRP | -0.389 (-0.660 to -0.029) | 0.031* | -0.358 (-0.638 to -0.007) | 0.048* | |
| HAQ score | -0.382 (-0.655 to -0.020) | 0.034* | -0.337 (-0.624 to 0.031) | 0.064 | |
| Pain VAS score | -0.498 (-0.729 to -0.163) | 0.004* | -0.481 (-0.719 to -0.143) | 0.006* | |
| SDAI | -0.552 (-0.763 to -0.236) | 0.001* | -0.576 (-0.777 to -0.268) | <0.001* | |
| CDAI | -0.561 (-0.768 to -0.248) | 0.001* | -0.587 (-0.784 to -0.284) | <0.001* | |
| Slope | (95% CI) | Intercept | (95% CI) | ||
| TRB | Laboratory findings | ||||
| ESR (mm/h) | -14.89 | (-25.71 to -4.08) | 147.79 | (53.54 to 242.00) | |
| CRP (mg/L) | -2.52 | (-7.04 to 2.01) | 26.20 | (-13.22 to 65.62) | |
| RF (IU/mL) | -78.84 | (-156.60 to -1.04) | 793.42 | (115.55 to 1471.28) | |
| ACCP (CU) | -41.09 | (-756.07 to 673.89) | 1368.08 | (-4861.37 to 7597.43) | |
| Disease measures | |||||
| DAS28-ESR | -1.57 | (-2.59 to -0.55) | 16.78 | (7.89 to 25.66) | |
| DAS28-CRP | -0.81 | (-1.62 to 0.01) | 9.58 | (2.50 to 16.66) | |
| HAQ score | -0.64 | (-1.14 to -0.13) | 6.39 | (1.99 to 10.80) | |
| Pain VAS score | -1.99 | (-3.84 to -0.16) | 20.99 | (4.99 to 36.99) | |
| SDAI | -6.42 | (-11.85 to -0.98) | 64.68 | (17.54 to 111.93) | |
| CDAI | -6.40 | (-11.81 to -0.99) | 64.42 | (17.39 to 111.45) | |
| TRG | Laboratory findings | ||||
| ESR (mm/h) | -16.06 | (-27.64 to -4.48) | 148.62 | (54.42 to 242.82) | |
| CRP (mg/L) | -1.62 | (-6.52 to 3.29) | 17.43 | (-22.50 to 57.35) | |
| RF (IU/mL) | -94.32 | (-176.45 to -12.20) | 873.51 | (205.61 to 1541.40) | |
| ACCP (CU) | -2.10 | (-768.76 to 764.55) | 1027.77 | (-5207.48 to 7263.02) | |
| Disease measures | |||||
| DAS28-ESR | -1.60 | (-2.74 to -0.46) | 16.07 | (6.84 to 25.30) | |
| DAS28-CRP | -0.75 | (-1.66 to 0.15) | 8.67 | (1.34 to 16.00) | |
| HAQ score | -0.68 | (-1.23 to -0.12) | 0.64 | (1.85 to 10.85) | |
| Pain VAS score | -2.12 | (-4.14 to -0.10) | 20.79 | (4.45 to 37.14) | |
| SDAI | -6.15 | (-12.22 to -0.08) | 58.68 | (9.55 to 107.81) | |
| CDAI | -6.15 | (-12.19 to -0.11) | 58.54 | (9.65 to 107.43) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





