Introduction
Wound care is a significant healthcare expense that poses a medical challenge requiring a multidisciplinary approach. Current therapies involve supportive care measures, but a paradigm shift is needed to improve outcomes. Successful wound repair requires several coordinated steps that must be monitored carefully. Wound care entails a skilled workforce utilising cutting-edge technologies and best practices. Policymakers, healthcare providers, and stakeholders must focus on developing innovative solutions to optimise wound repair outcomes and minimise costs (Lin et al., 2017). Wound healing is a complex process that involves multiple phases and is resolved after the healing process. However, chronic wounds can form if this healing process deviates from the norm leading to unresolved wound healing negatively impacting patients' quality of life. Managing chronic wounds requires specialised treatment such as nanotherapeutics, stem cell therapy, bioengineered skin grafts, and 3D bioprinting-based strategies (Kolimi et al., 2022). Wound healing is a natural and dynamic physiological process that is essential for tissue restoration after injury. The repair of damaged tissue involves a complex interaction between various cell types releasing cytokines and mediators, and the vascular system (Wallace et al, 2023). Skin is part of the integumentary system and is the human body's largest organ, covering an average surface area of 1.85 m2. It serves as a physical barrier that protects against the external environment and helps to regulate body functions (Praveen et al, 2022). Wounds to the skin can be group into two main types: acute and chronic wounds. Acute wounds are caused by injuries or trauma to the skin and underlying tissues, such as cuts, burns, and surgical incisions. The healing process involves a sequence of molecular events, eventually leading to the restoration of structural integrity (Kamila et al., 2021). The body undergoes four stages of healing after an injury. Firstly, haemostasis to stop bleeding. Secondly, the inflammatory phase to fight infection by attracting immune cells. Thirdly, proliferation to form new granulation tissue and blood vessels. Lastly, remodelling of the granulation tissue often leading to scar formation (Kamila et al., 2021). Acute wound healing is a complex process that involves different kind of immune cells such as neutrophils, monocyte, macrophages, and T cells releasing cytokines, chemokines and growth factor. Understanding their orchestrated interplay is crucial for effective wound treatments in order to promote optimal outcomes (Kamila et al., 2021; Luis et al., 2019). In cases where the immune response is disrupted during the wound-healing process, a medical condition known as chronic wounds may develop. Chronic wounds can cause significant complications and can be extremely challenging to manage, thereby necessitating a comprehensive understanding of the underlying pathophysiology. Hence, it is imperative to identify and address any factor that might compromise the efficacy of the wound-healing process to ensure a favourable patient outcome (Maria et al., 2020). Any failure in the normal wound healing process results in abnormal scar formation and a chronic state, which is more susceptible to infections (Kolimi et al., 2022). Chronic wounds require specialised treatment due to their unresolved inflammatory process and lack of healing which creates a significant impact on patients’ quality of life. Neovascularisation is essential for wound closure and improves chronic wound healing, such as in the diabetic microenvironment. A self-adaptive, multifunctional, and injectable hydrogel containing the pro-angiogenic drug DFO@G has been developed to reduce oxidative stress and accelerate angiogenesis for rapid wound closure. This hydrogel can potentially treat diabetic wounds (Shao et al., 2023). Novel skin regeneration techniques employing molecular scaffoldings activated with growth factors, bioactive molecules, and genetically modified cells are being explored to overcome the limitations of current wound-healing technologies. Nano-drug delivery systems (nano-DDSs) have proved promising in skin regeneration by speeding up wound healing and improving the quality of the healing process. They are non-toxic and compatible with the skin and can activate specific transport mechanisms to enhance drug retention. Nano-DDSs have immense potential for future research and development in this area (Sandhiya et al., 2009).
These advanced approaches aim to facilitate personalised therapy design and provide more effective treatment options for patients. By integrating cutting-edge biological therapies and engineering principles, these regenerative strategies have the potential to significantly enhance the healing process and improve patient outcomes (Tottoli et al., 2020).
Growth factors are endogenous signalling molecules that play a crucial role in regulating cellular responses essential for wound healing processes. These processes include but are not limited to migration, proliferation, and differentiation. As such, growth factors are considered key players in the regulation of tissue repair and regeneration. The clinical effectiveness of exogenously applied growth factors is limited due to various factors, including their low in-vivo stability, restricted absorption through the skin surrounding wound lesions, elimination by exudation before reaching the wound area, and the presence of unwanted side effects (Park et al., 2017). A study scrutinised the role of four distinct growth factors, namely granulocyte-macrophage colony-stimulating factor (GM-CSF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF). External growth factors and cytokines have shown promising results in improving wound healing with GM-CSF, PDGF, bFGF, and VEGF being the four growth factors with the most potential. However, current studies are limited and larger randomized controlled trials are needed to confirm efficacy, side effects and long-term outcomes (Barrientos et al., 2014).
Colostrum, a biological substance produced by all female mammals prior to the production of milk, is a rich source of growth factors that play a vital role in wound healing, regulation of dermis and epidermis growth, and new blood vessel development. In the first few days of life, prior to nursing, infants absorb colostrum which provides immunological and biological triggers to the new organism. The primary function of colostrum is to activate the immune system, stimulate enzyme production, and provide important epithelial growth factors. Colostrum also contains prostaglandins that safeguard the mucosa of the digestive apparatus, regulate gastric acidity, and aid in enzyme release (Kim et al., 2022). Given its biological benefits, GF20®, a purified and standardised colostrum product derived from bovine animals, is of great interest to the scientific community. The present study describes the development of a novel formula known as Rigemed D, which contains 200 mg of 20 growth factors (GF20®) and 20 billion exosomes extracted from bovine colostrum (Milk-exo) (Kim et al., 2022).
The present study aimed to investigate the potential therapeutic applications of a novel formulation for wound healing. Specifically, Rigemed D was utilised at different doses to treat HaCat cells and fibroblasts, with the objective of assessing cell proliferation and migration, as well as the levels of DNA damage and cell viability. Moreover, immunohistochemistry was conducted on fibroblasts before and after treatments with varying oxidative stress levels. Finally, the level of angiogenesis was investigated after treatment with Rigemed D. Hydrogels represents a promising class of materials that have gained significant commercial traction owing to their impressive performance characteristics. This material type has demonstrated success in a variety of applications, making it an attractive option for a range of industries. Given their unique properties, such as high water content and biocompatibility, hydrogels are particularly well-suited for use in biomedical applications such as drug delivery, tissue engineering, and wound healing (Luneva et al., 2022). The purpose of this research study was to investigate the appropriateness of a specific formulation in the development of a 3D bio-printed membrane utilising hydrogel. The study aims to identify the suitability of the proposed formulation in achieving the desired outcome.
Materials and Methods
Cell lines
The human dermal fibroblasts (HDF) (106-05A, Merck) and immortalised human keratinocytes (HaCaT cells) (T0020001, Caltag Medsm) were grown in Dulbecco’s Modified Eagle Medium (DMEM) with both low and high glucose variants provided by Thermo-Fisher. Both media were supplemented with 10% foetal bovine serum. Human umbilical vein endothelial cells (HUVEC) (C0035C, Fisher) were grown in endothelial cell growth supplements from bovine neural tissue (Merck). The medium was replaced every 2 days, starting when the cells reached 80% confluence. All cultures were maintained at 37°C in a humidified environment containing 5% CO
2. Rigemed D was sourced from the Italian company Dermaroma (
https://dermoaroma.com). The exosomes were purchased from a company called Medrik, with cat number Med-SeExo (
https://medrik-dynamic.com/).
In vitro cell viability assay
The present study utilised the Cell Counting Kit-8 (CCK-8) assay, obtained from Abcam (ab228554), to evaluate cell viability after treatment with Rigemed D. A total of 3,000 cells were initially seeded into each well of a 96-well plate, comprising a negative vehicle control , a positive control (50 µM hydrogen peroxide), and groups subjected to varying concentrations (1, 1.5, and 2% v/v) of the novel wound treatment mixture Rigemed D. The cells were incubated for three distinct periods of 1, 24 , and 48 hours. After each incubation period, 10 μl CCK-8 reagent was added to the cell suspension and left to incubate for 1-2 hours at 37°C. The absorbance (OD value) was subsequently measured at a wavelength of 450 nm to determine the cell viability. The CCK-8 assay is a reliable and commonly used method for determining cell viability. Measuring the amount of formazan dye produced by the metabolic activity of living cells provides a rapid and sensitive means of assessing cell viability. In this study, the assay was successfully employed to evaluate the impact of Rigemed D on cell viability, with results indicating a concentration and time-dependent effect on cell survival.
Scratch-wound assay
Cells were grown in DMEM medium containing 10% FBS in 6-well plates at 37°C with 5% CO2 until they reached 80% confluence. Then, mitomycin C (5 µg/mL) was applied to each well and incubated at the same conditions for 2 hours. After incubation, the medium containing mitomycin C (MMC) was removed, 1x PBS was added, and a scratch was made manually on the cell monolayer using a 200-µL pipette tip. Images were captured at 0, 1, and 24 hours after scratching HFB and HaCaT cell layers.
Immunohistochemistry (IHC)
The fibroblast marker Vimentin (ab254015) from Abcam and S100A4 primary antibodies (ab197896) were used in this study. Fibroblast cells were cultured in an 8-well cell culture chamber until they reached 80% confluency. Following this, they were treated under various conditions for 24 hours. After the 24-hour treatment, cells were rinsed with PBS, fixed with 4% Paraformaldehyde, washed again in PBS, and exposed to 0.2% TritonX-100 in PBS. Following this, the cells were incubated in 10% normal blocking serum (donkey serum) in PBS for 90 minutes. The blocking serum was replaced with the primary Vimentin cytoskeleton marker antibody, diluted in 1% normal serum (donkey serum), and left for 24 hours at 4℃. After a PBS wash, cells were incubated with a fluorescent-conjugated secondary antibody (Alexa) for one hour at room temperature. Finally, slides were mounted using a Vectashield mounting medium containing DAPI (4′,6-diamidino-2-phenylindole), phalloidin-conjugate working solution for 20 minutes and imaged using the Invitrogen EVOS M5000 microscope imaging system.
Angiogenesis
The angiogenesis experiment followed Sigma Aldrich's kit protocol. HUVECs were gathered, suspended in media, and placed on ECMatrixTM at 5 x 104 cells per well. In each well of a 96-well plate, 3,000 cells were initially seeded. The cells were then exposed to various treatments involving Rigemed D, which included the following conditions: a negative control, a positive control with 50 µM of hydrogen peroxide, and groups with different concentrations (1, 1.5, and 2% v/v) of Rigemed D. Treatments were introduced within a sterile, ventilated fume hood. The gel and cells on the plate were incubated at 37°C for 6 hours. After incubation, cell observation occurred using an inverted light microscope at 40X magnification.
Results
CCK-8
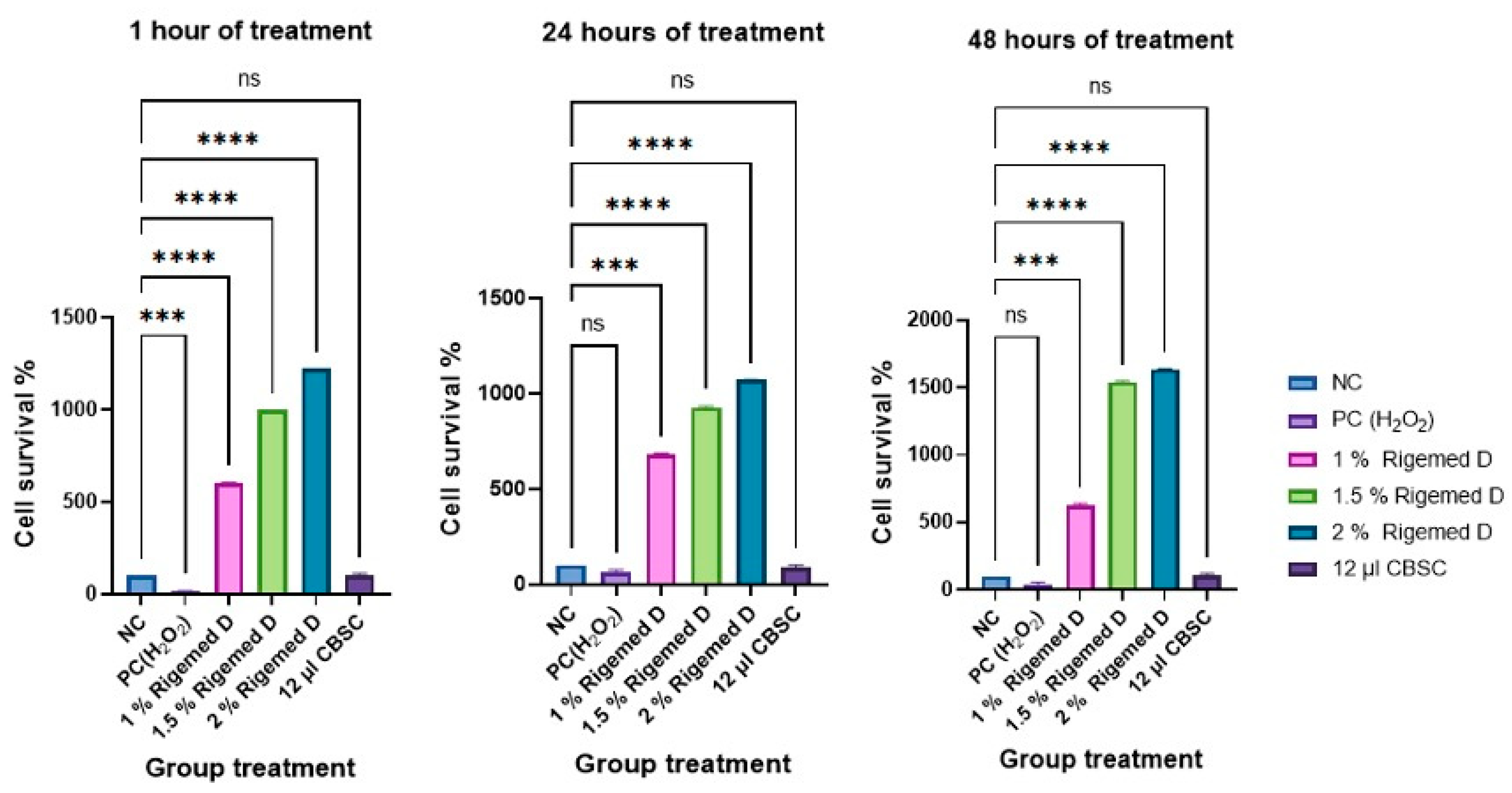
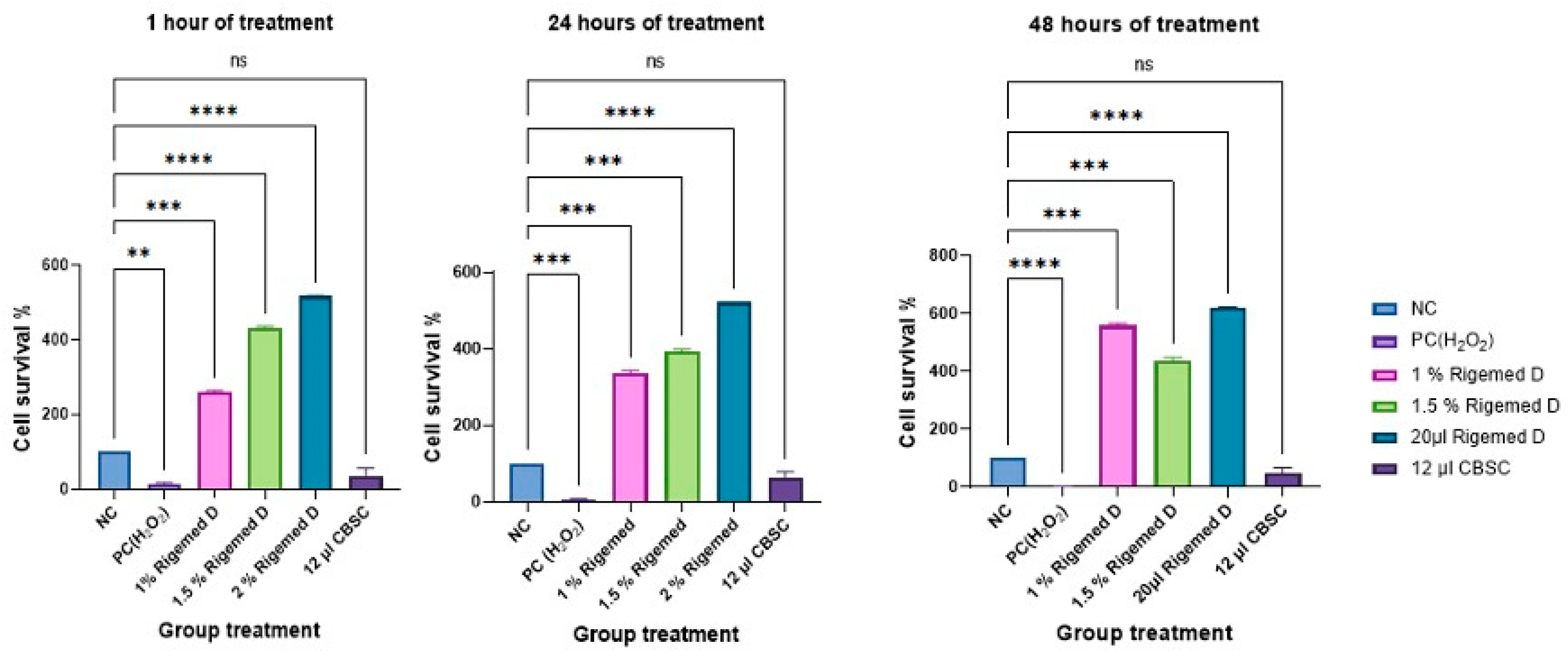
The CCK-8 assay was conducted for both, human dermal fibroblasts and the HaCaT cells, and the results are presented in
Figure 1 and
Figure 2, respectively. The results showed a statistically significant increase in the level of cell viability after treatment with RD at different doses (p < 0.0001) compared to without treatment and PC.
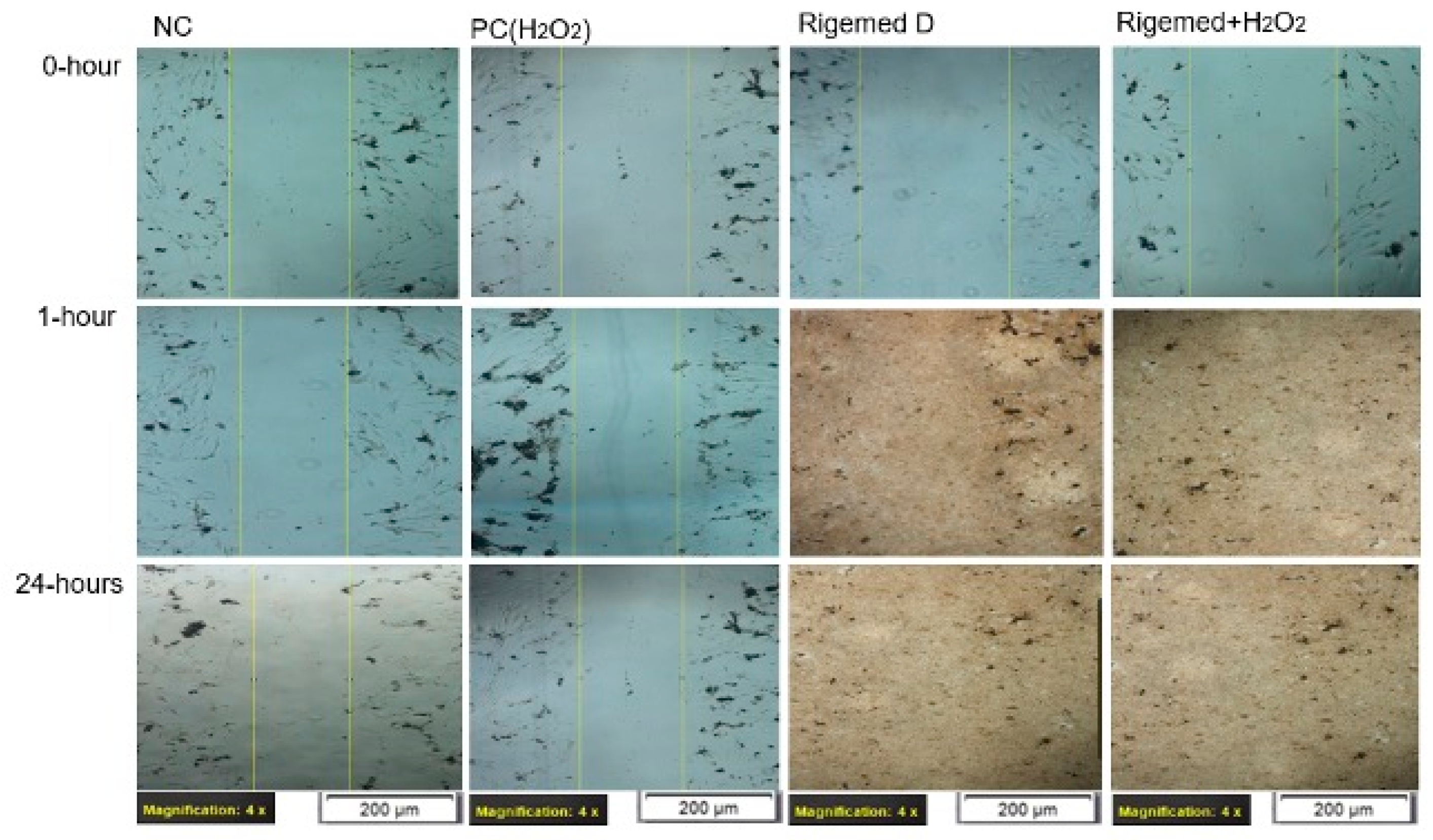
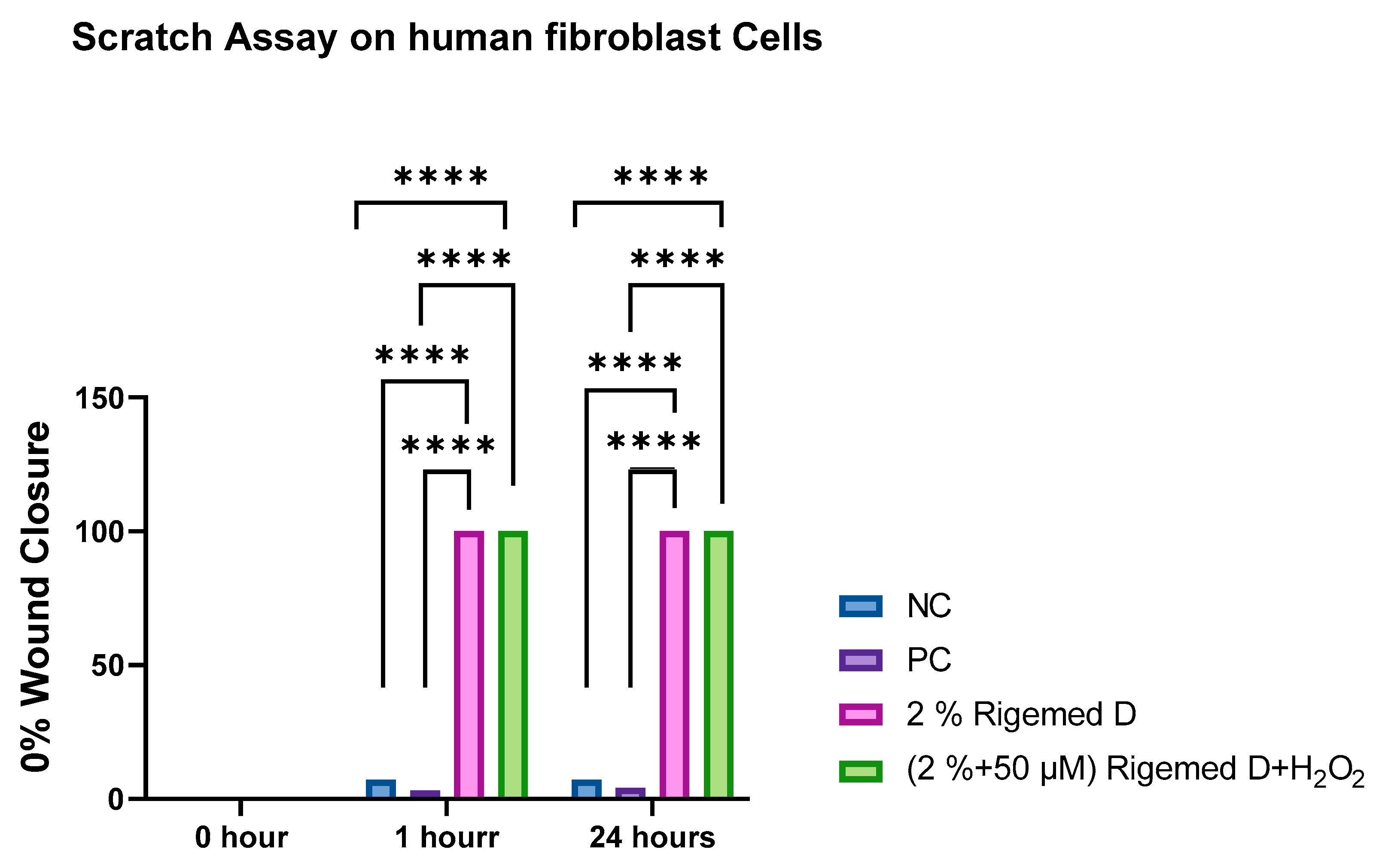
Scratch-wound assay
The assay was performed with both human dermal fibroblasts (HFB) and the HaCaT cell lines (Wallace et al., 2023). Subsequently, the cells were exposed to different treatments: 0% RD as a negative control, 50 µM of hydrogen peroxide as a positive control, 2% RD, and 2% RD supplemented with 50 µM hydrogen peroxide as an oxidative stress inducer. Following the creation of the scratch on the confluent HFB and HaCaT cell lines, images were captured at three time points: 0, 1, and 24 hours. The results of these observations are presented in
Figure 3,
Figure 4,
Figure 5 and
Figure 6, respectively.
The experimental results demonstrated a significant increase in the level of cell proliferation following a 1-hour and 24-hour treatment with RD, despite the presence of H2O2 (p < 0.0001), respectively.
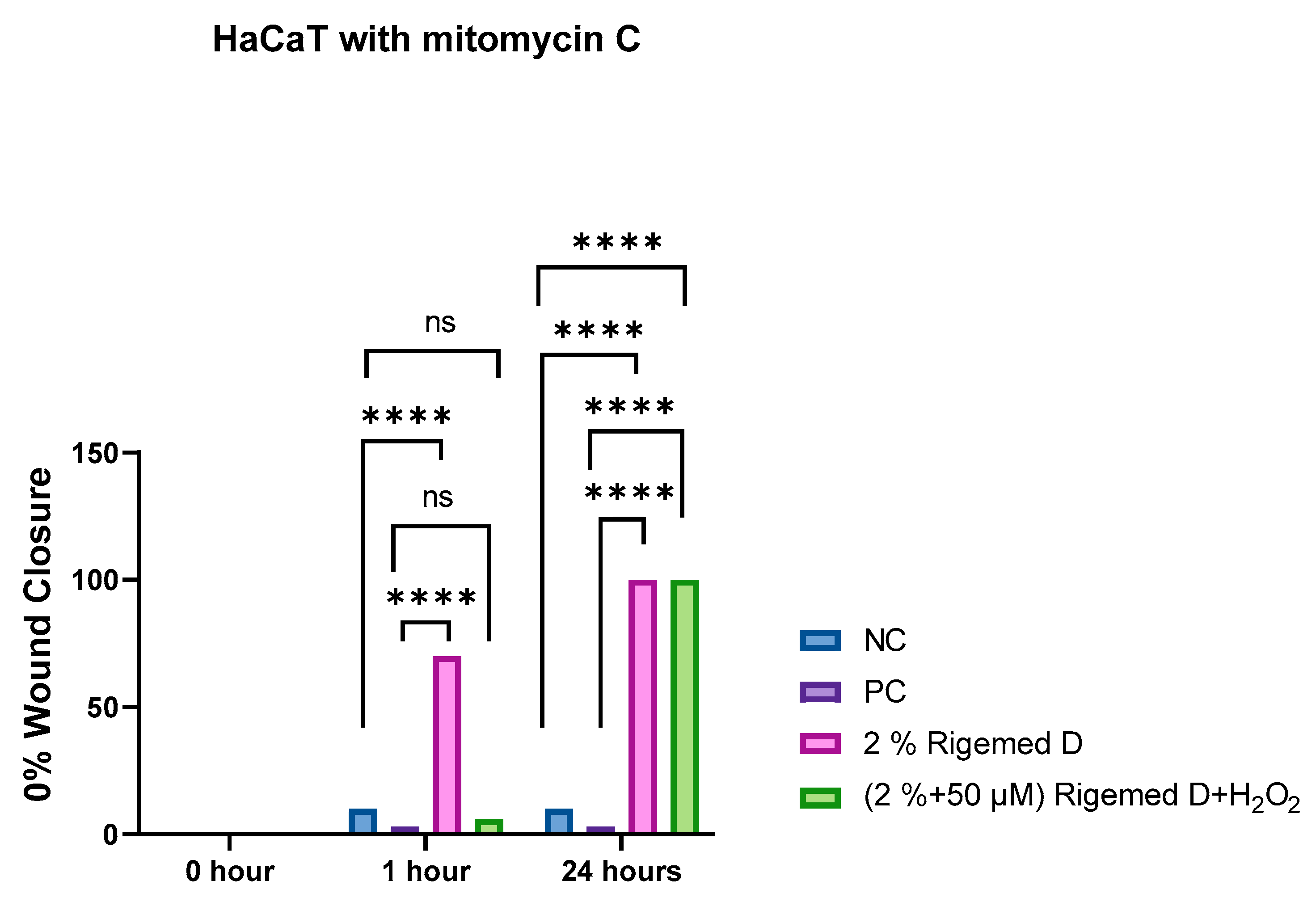
In order to evaluate the degree of improvement in cell migration, cultured cells underwent treatment with 5 µg/mL of mitomycin C, followed by incubation for a period of 2 hours at a temperature of 37°C with 5% CO
2. Subsequently, the medium containing mitomycin C was removed from the cells, and the scratch assay was performed. The cells were exposed to different treatment conditions, including 0% RD as a negative control, 50 µM of hydrogen peroxide as a positive control, 2% RD, and 2% RD supplemented with 50 µM of hydrogen peroxide as an oxidative stress inducer. Following the scratch assay on the confluent HFB and HaCaT cell lines, images were captured at three distinct time points: 0, 1, and 24 hours. The results obtained from this experiment indicated a significant increase in cell proliferation subsequent to treatment with Rigemed D at both 1 hour and 24 hours of observation, as illustrated in
Figure 7,
Figure 8,
Figure 9 and
Figure 10, respectively (p < 0.0001).
Immunohistochemistry assay (IHC)
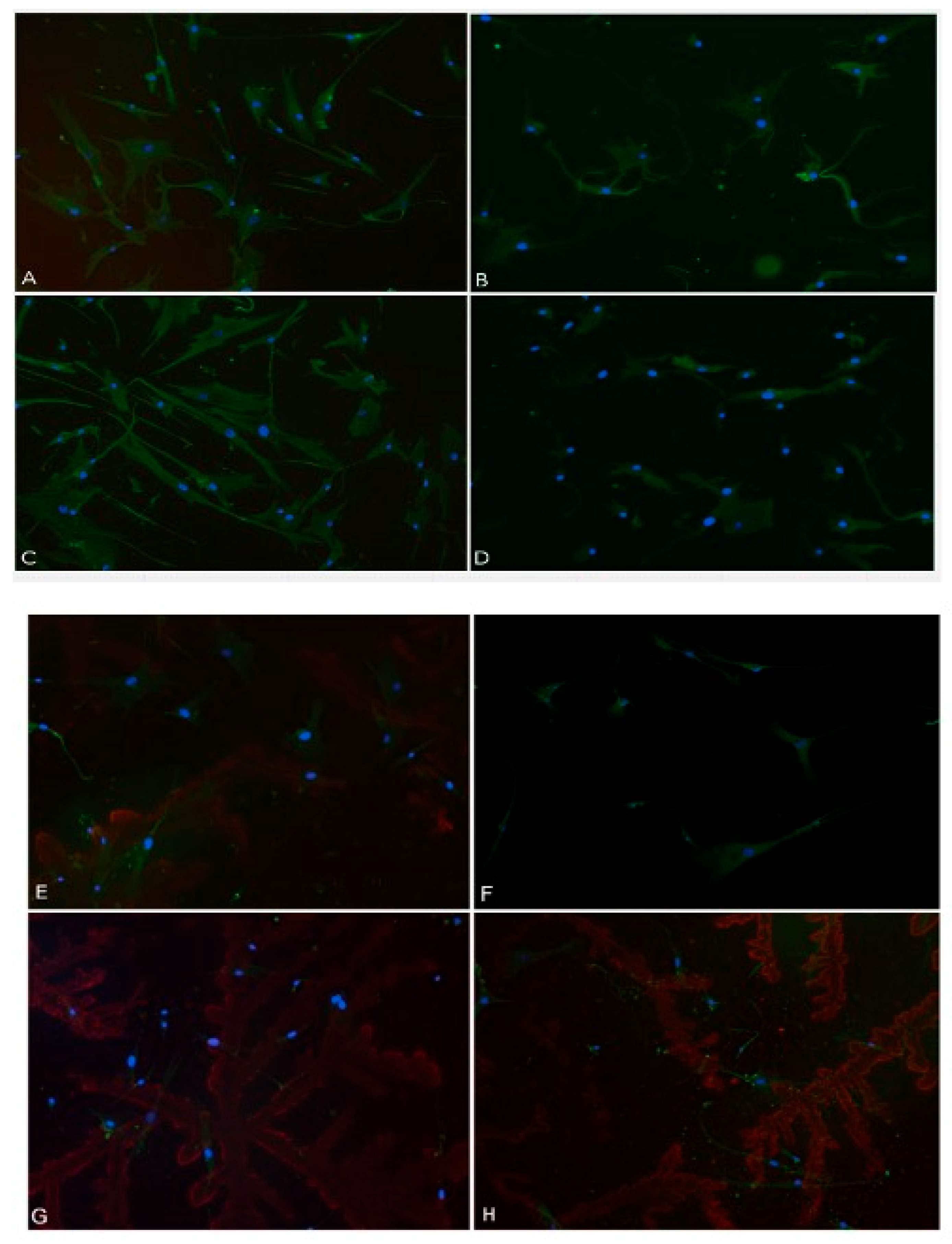
The present investigation aimed to study the impact of Rigemed D compound on a human fibroblast cell line via the employment of an immunohistochemistry (IHC) assay. The cultured fibroblast cells were subjected to various treatments, including 2% RD, a negative control without any compound, positive control (50 µM hydrogen peroxide), and a combination of 2% RD supplemented with 50 µM hydrogen peroxide to induce oxidative stress. The Invitrogen EVOS microscope imaging system was utilized to capture images of the slides. The results of the study demonstrated a statistically significant increase in cell growth, the number of nuclei, and fibro-skeletal structures (
Figure 12 and
Figure 13).
Angiogenesis
The objective of the study was to evaluate the impact of Rigemed D compound on Human Umbilical Vein Endothelial Cells (HUVEC) in relation to angiogenesis, which is the process of new blood vessel development from pre-existing ones.
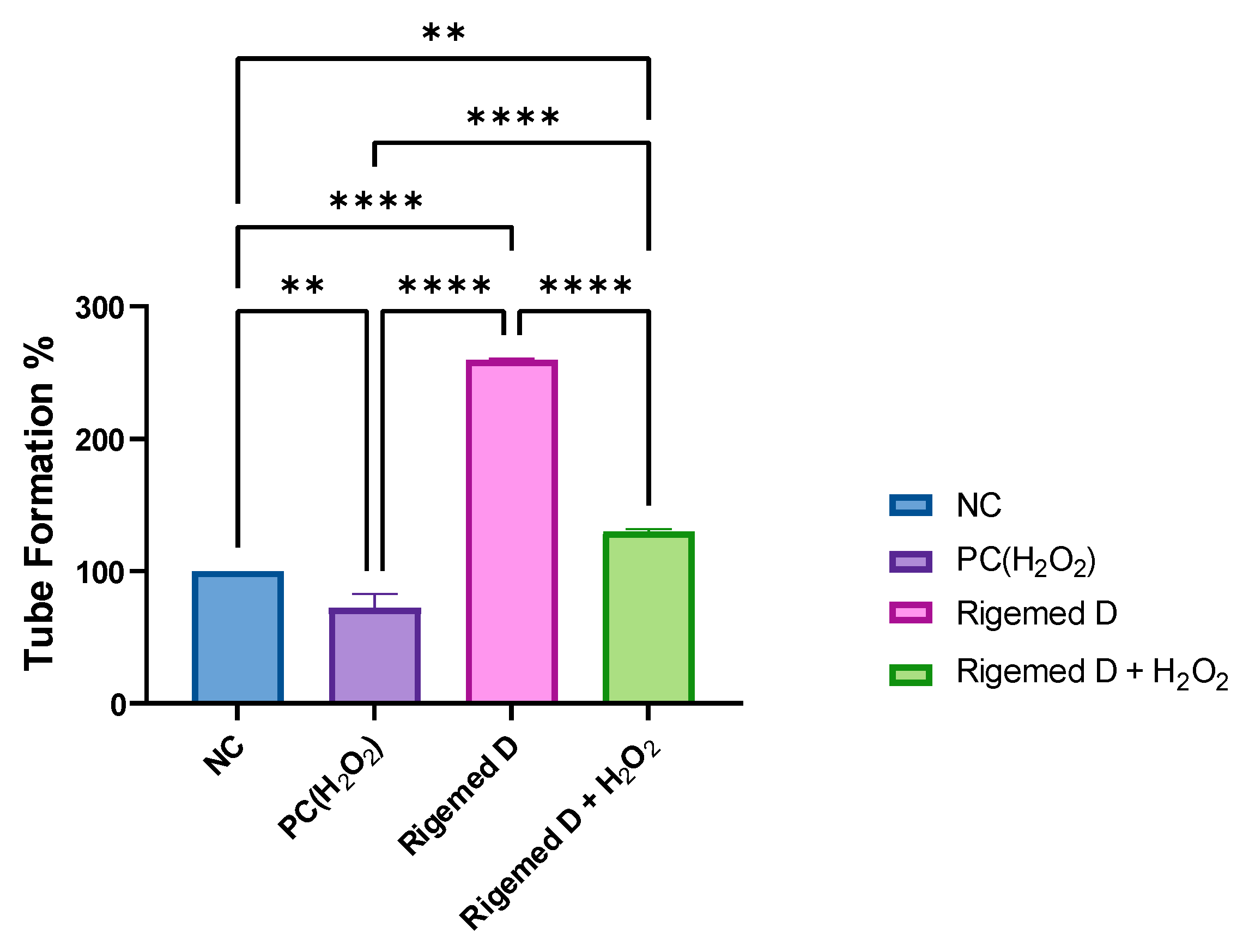
The HUVECs were harvested, suspended in media, and seeded onto the polymerized ECMatrixTM at a density of 5 x 104 cells per well. The cells were subjected to various treatments, including 2% RD, 50 µM hydrogen peroxide (as a positive control), a combination of 2% RD and 50 µM hydrogen peroxide, and a negative control with no treatment. The findings of this study reveal that the Rigemed D mixture produced a significant increase in the level of tube formation and angiogenesis, almost three times compared to the control group without treatment, even in the presence of hydrogen peroxide, the increase was doubled (p < 0.001 and p < 0.01, respectively).
Discussion
To fully understand the causes of chronic wounds such as venous, arterial, diabetic, and pressure ulcers, it is necessary to compare the normal healing process with the abnormal inflammatory response that is typical of chronic wounds. The key factors contributing to chronic wound development are ageing, hypoxia, ischaemia, reperfusion injury, and bacterial colonisation (Zhao et al., 2016). Wound healing is a complex process that involves multiple stages, with haemostasis, inflammation, proliferation, and tissue regeneration being the most critical steps (Fallah et al., 2021). The focus of this study was to examine a new mixture called Rigemed D (RD) extracted from bovine colostrum. RD contains growth factors and exosomes. This study aimed to evaluate the efficacy of RD on two different types of cell lines, namely fibroblasts and HaCat cells, and its potential to promote wound healing and to support angiogenesis; in particular, highlighting the role of RD in the different stages of wound healing, including haemostasis, inflammation, proliferation, and tissue regeneration. Tissue injury and the subsequent inflammation are often accompanied by an increase in the production of reactive oxygen species (ROS) and gap junction-mediated cell-to-cell contact causing damage to various biomolecules, leading to protein dysfunction or cellular death, especially in chronic wounds and in particular diabetic ulcers. However, forming hydrogen peroxide (H2O2) after tissue injury is a crucial aspect of the subsequent wound-healing process. H2O2 acts as an early damage signal and helps regulate several critical phases of the wound-healing response (van der Vliet and Janssen-Heininger, 2014, Jessop et al., 2021, Li et al., 2020, Nelson et al., 2012, Wang and Shi, 2020).
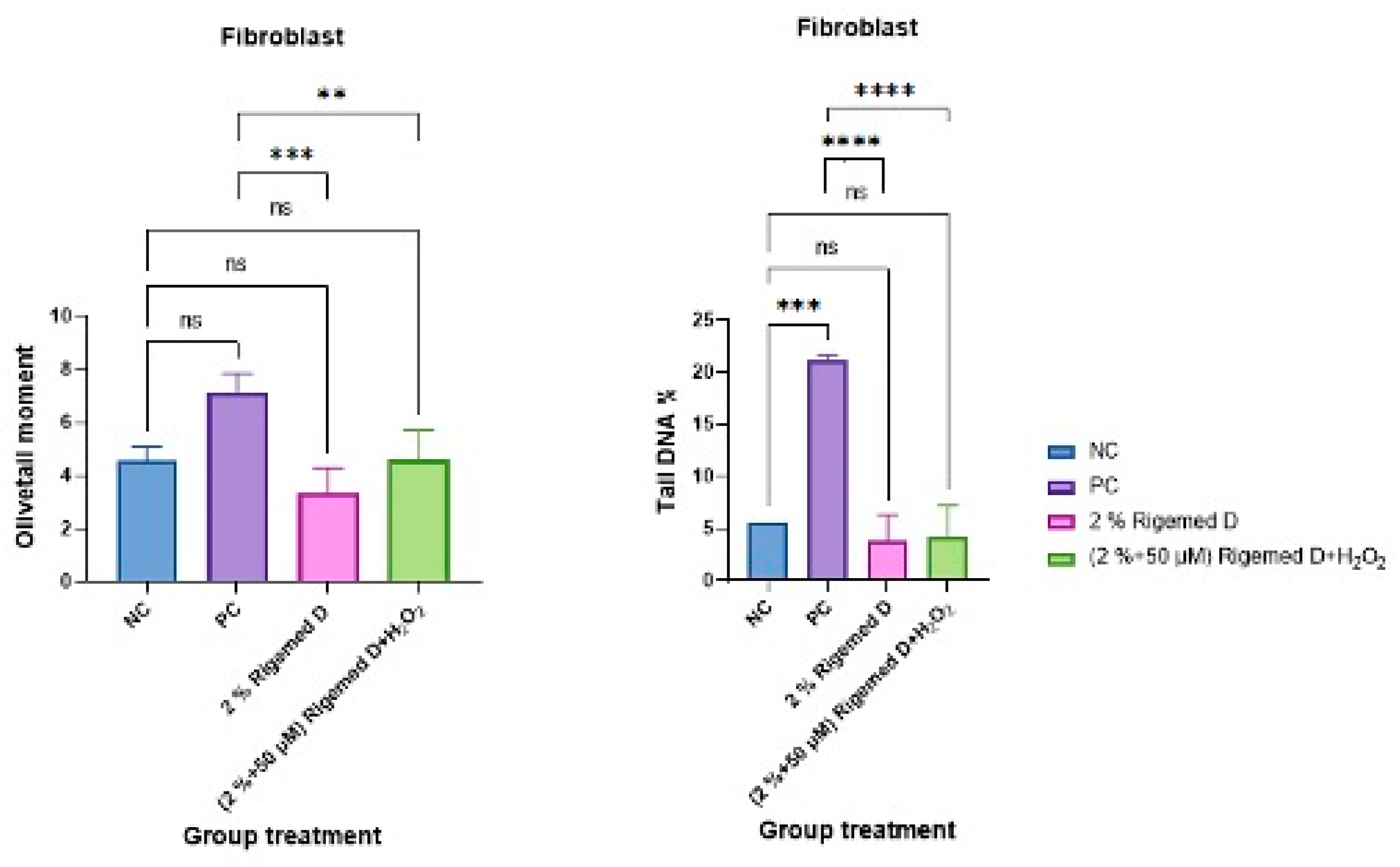
To evaluated the efficacy of Rigemed D in protecting cells against oxidative stress the Comet assay was used to investigate RD’s potential to reduce DNA damage induced by ROS (
Figure 11). Fibroblasts were treated with RD in the presence of H
2O
2 to simulate an environment with high levels of oxidative stress. The results showed that RD significantly reduced DNA damage induced by 50 μM H
2O
2 compared to the positive control (p < 0.0001) (
Figure 11). These findings demonstrate that Rigemed D has antioxidative properties protecting cells and their cellular constituents from oxidative damage. Providing a protective mechanism against free radicals, especially for oxidative chronic diabetic wounds, will be crucial for treatment and treatment time as it may significantly contribute to resolving the interrupted healing of chronic wounds (Spampinato et al., 2020).
In skin regeneration, the proliferation of fibroblasts and keratinocytes is an important vital step. These cells are primarily involved in the inflammatory response alongside the immune cells, one of the most critical stages of the epithelial regeneration process. As such, any disruption of this process can lead to impaired wound healing and may result in chronic status (Wojtowicz et al., 2014, Zhang et al., 2022). The RD mixture contains besides various growth factors also colostrum exosomes, which have been identified as a potential activator of the Wnt/β-catenin signalling pathway. This pathway plays a crucial role in promoting the proliferation of human hair follicle dermal papilla (DP) cells, leading to hair growth (Kim et al., 2022). The effect of RD treatment with varying concentrations (1-2% v/v) on the cell viability of fibroblasts and HaCat cells was assessed using the CCK-8 assay. This allowed to investigate the ability of colostrum growth factors and exosomes to promote cell proliferation. The results of our study revealed that the presence of RD significantly increased the viability and survival of fibroblasts and HaCat cells at 1, 24, and 48 hours post-treatment and even compared to CBSC-derived exosomes with p < 0.001 and p < 0.0001, respectively (
Figure 1 and
Figure 2). Fibroblasts have the ability to adopt a myofibroblast phenotype, which is influenced by keratinocytes. This process, vital for wound closure, depends on a delicate balance between pro-inflammatory factors and a transforming growth factor (TGF)-β-dominated environment. Fibroblasts produce several types of collagens, the primary structural proteins in the skin (Werner et al., 2007). Type I is the predominant form in adults, while collagen type III is most abundant during gestational development (Diller and Tabor, 2022). Bovine colostrum contains a diverse range of beneficial substances like growth factors and exosomes, which are the main constituents of RD. Colostrum is also rich in antibodies, cytokines, micronutrients, oligosaccharides, and other proteins. Besides growth factors and exosomes, a significant proportion of proteins in GF20
® constitutes immunoglobulins IgG and IgA and several other immune-related factors such as cytokines (Kim et al., 2022).
Fibroblast growth factor (FGF), the principal growth factor being present in GF20
®, plays a pivotal role in the growth and differentiation of cells of mesodermal or neuroectodermal origin (Xiong et al., 2022, Liu et al., 2021). The current study revealed a substantial increase in the proliferation of fibroblasts and Hacat cells upon treatment with RD, both after 1 hour and 24 hours by conducting the scratch assay. In addition, the cells showed a notable increase in proliferation even in the presence of oxidative stress by H
2O
2. The results were statistically significant (p < 0.01 and p < 0.0001, respectively). As shown in
Figure 3 and
Figure 4, these findings suggest that RD enhanced proliferation of fibroblasts and Hacat cells
in vitro (
Figure 5 and
Figure 6), suggesting that it can potentially be used to treat wounds and speed up healing. Transforming growth factor beta 11 (TGF- β1) is another important factor that promotes proliferation, differentiation, adhesion, migration, and other functions in various cell types (Lichtman et al., 2016, Kim et al., 2018). When using mitomycin C-treated cells in the scratch assay to inhibit cellular proliferation and migration, 2% PD was able to significantly stimulated proliferation and migration of fibroblasts and HaCat cells at 1 hour (
Figure 7,
Figure 8,
Figure 9 and
Figure 10). Furthermore, this effect was sustained for up to 24 hours, even in the presence of H
2O
2, and was statistically significant with p values of less than 0.01 and 0.0001 at the respective time points. These findings suggest that adding RD to MMC-treated cells improved cell migration even in the presence of free radicals (
Figure 7 and
Figure 8). The insulin-like growth factor 1 (IGF-1) is also present in RD and is similar in structure and function to insulin. IGF-1 mediates growth and development, improves wound healing and proliferation processes and sustains cell differentiation under inflammatory stimuli in the cells (Stuard et al., 2020, Emmerson et al., 2012, Reckenbeil et al., 2017).
The degree of angiogenesis in wounds shows a correlation with the inflammatory response since inflammatory cells generate a multitude of proangiogenic mediators. It has been suggested that the selective reduction of inflammation and angiogenesis could be a means of improving scarring during the tissue remodelling. These concepts establish a link between excessive inflammation and the production of a dense but poorly perfused capillary network, leading to suboptimal healing outcomes (DiPietro, 2016). The composition of bovine colostrum exosomes within the RD is rich in immunoglobulins and lactoferrins. As a result, these natural compounds play a significant role in fortifying the immune response and protecting against bacterial proliferation and growth. The presence of these essential proteins in bovine colostrum exosomes can help to enhance the body's natural ability to defend against harmful pathogens, making it a valuable resource in the field of immunology (Yadav et al., 2016). The closure of a wound is a complex process that requires cell proliferation, adequate blood perfusion, and interaction between inflammatory cells, growth factors, and extracellular matrix molecules. The efficiency of wound closure is thus greatly influenced by the orchestration of these elements. A comprehensive understanding of these mechanisms is crucial for developing effective therapies and treatment strategies for wound healing (Rodrigues et al., 2019).
In addition to the above-mentioned growth factors, the nerve growth factor NGF is present in RD and stimulates nerve growth activity and regulates the growth and differentiation of sympathetic and certain sensory neurons. Platelet-derived growth factor PDGF is another factor that exhibits the potential to stimulate the growth or differentiation of cells of mesodermal origin. During wound healing, in particular during the proliferation stage, where granulation tissue is generated, vascular endothelial growth factor VEGF plays a crucial role in angiogenesis by stimulating endothelial cells of nearby capillaries to proliferate and migrate, ultimately forming new vessels. The results of our study reveal an amplified level of angiogenesis even in the presence of the oxidative stress inducer, 80 μM H
2O
2, as demonstrated in
Figure 14 and
Figure 15 (p < 0.0001).
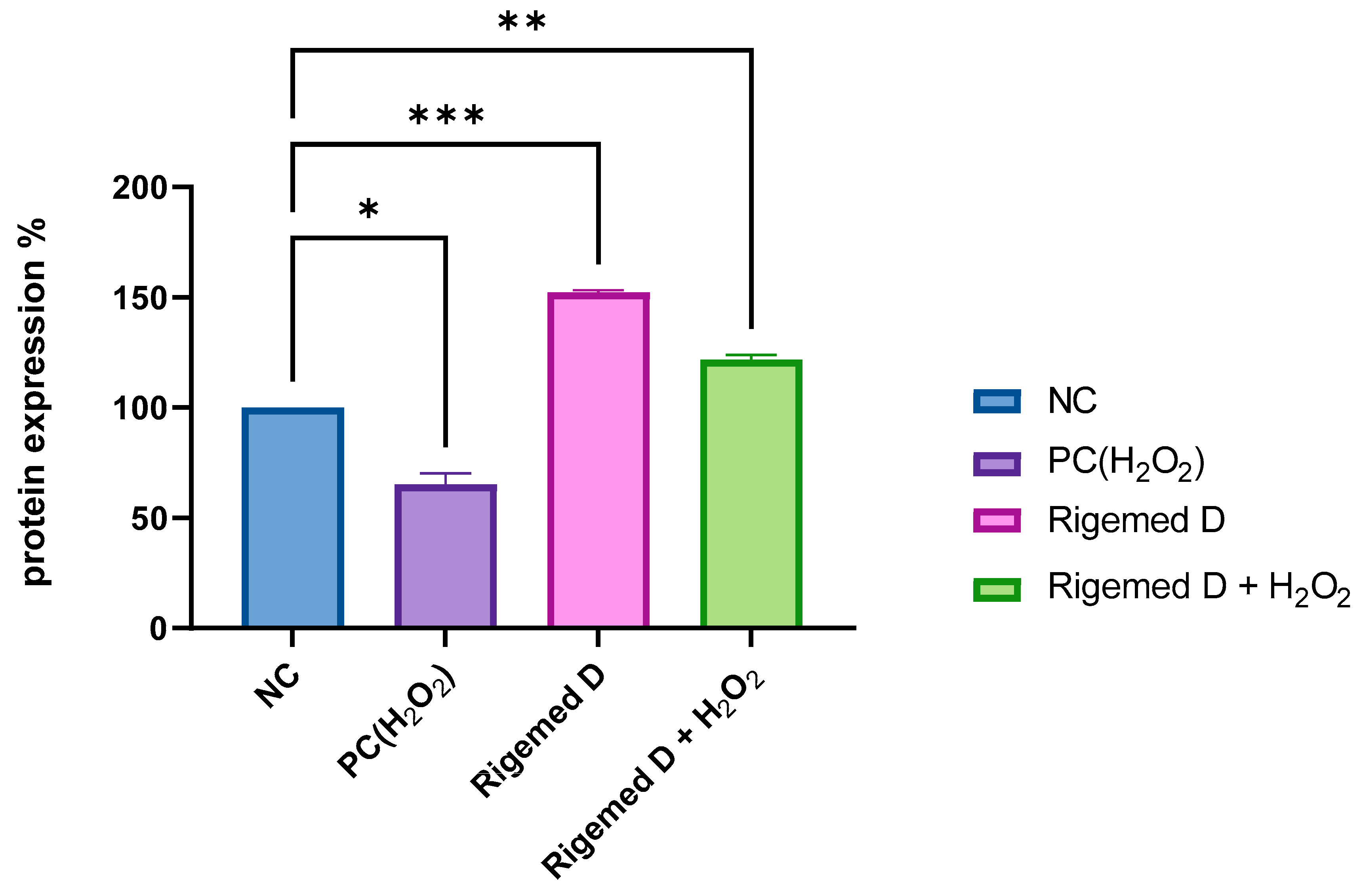
Furthermore, RD contains epidermal growth factor EGF, which plays an essential role in the development epidermis. Bone morphogenetic protein 2 (BMP2) is another crucial factor that contributes to the development of bone and cartilage, as well as cardiac cell differentiation. The present study reveals a significant impact of the RD on fibroblast proliferation, both prior to and following DNA damage induced by H
2O
2 (p < 0.0001) and investigated by using immunohistochemistry staining cells with DAPI (nuclear stain) and fluorochrome-conjugated Vimentin cytoskeleton marker antibodies (
Figure 12 and
Figure 13). The findings suggest that RD may play a crucial role in regulating cellular proliferation, especially in conditions where DNA damage is prevalent. These results may have implications for the development of novel therapeutic interventions aimed at reducing the adverse effects of DNA damage on cellular growth and proliferation, which is of particular relevance in the context of cancer research.
The findings of this study might have a significant impact on future research in the field of wound healing. In particular, the potential clinical benefits of Rigemed D are obvious, making it a promising candidate for accelerating wound healing. Our results on Rigemed D will help to develop more effective treatments for wound healing in the future providing insights into its promising therapeutic potential in developing the 3D Bio Print membrane.
Data Accessibility Statement
The datasets used and/or analysed in this study are available from the corresponding author upon reasonable request.
Acknowledgments
The research team extends its sincere gratitude to Dermoaroma/BioTech for generously providing the Rigemed D and related consumables that facilitated the successful completion of this research project. We would like to express our gratitude to Mr Emtiaz Aziz, our diligent laboratory technician, for his unwavering commitment to this project.
Conflicts of Interest Statement
The authors declare that there is no conflict of interest.
References
- Barrientos, S.; Brem, H.; Stojadinovic, O.; Tomic-Canic, M. Clinical Application Of Growth Factors And Cytokines In Wound Healing. Wound Repair Regen 2014, 22, 569–578. [Google Scholar] [CrossRef]
- Diller, R.B.; Tabor, A.J. The Role Of The Extracellular Matrix (Ecm) In Wound Healing: A Review. Biomimetics (Basel) 2022, 7, 87. [Google Scholar] [CrossRef]
- Dipietro, L.A. Angiogenesis And Wound Repair: When Enough Is Enough. J Leukoc Biol 2016, 100, 979–984. [Google Scholar] [CrossRef]
- Emmerson, E.; Campbell, L.; Davies, F.C.; Ross, N.L.; Ashcroft, G.S.; Krust, A.; Chambon, P.; Hardman, M.J. Insulin-Like Growth Factor-1 Promotes Wound Healing In Estrogen-Deprived Mice: New Insights Into Cutaneous Igf-1r/Eralpha Cross Talk. J Invest Dermatol 2012, 132, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Fallah, N.; Rasouli, M.; Amini, M.R. The Current And Advanced Therapeutic Modalities For Wound Healing Management. J Diabetes Metab Disord 2021, 20, 1883–1899. [Google Scholar] [CrossRef] [PubMed]
- Jessop, Z.M.; Garcia-Gareta, E.; Zhang, Y.; Jovic, T.H.; Badiei, N.; Sharma, V.; Whitaker, I.S.; Kang, N. Role Of Hydrogen Peroxide In Intra-Operative Wound Preparation Based On An In Vitro Fibrin Clot Degradation Model. Jpras Open 2021, 29, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, Y.; Kim, E.H.; Jang, H.; Cho, H.; Han, G.; Song, H.K.; Kim, S.H.; Yang, Y. Potential Of Colostrum-Derived Exosomes For Promoting Hair Regeneration Through The Transition From Telogen To Anagen Phase. Front Cell Dev Biol 2022, 10, 815205. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Sheppard, D.; Chapman, H.A. Tgf-Beta1 Signaling And Tissue Fibrosis. Cold Spring Harb Perspect Biol 2018, 10, a022293. [Google Scholar] [CrossRef] [PubMed]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies To Accelerate Wound Healing: Trajectory And Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, J.; Zhang, H.; Lam, C.W.K.; Luo, W.; Zhou, H.; Zhang, W. Protective Effect Of Thymidine On Dna Damage Induced By Hydrogen Peroxide In Human Hepatocellular Cancer Cells. Acs Omega 2020, 5, 21796–21804. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, M.K.; Otero-Vinas, M.; Falanga, V. Transforming Growth Factor Beta (Tgf-Beta) Isoforms In Wound Healing And Fibrosis. Wound Repair Regen 2016, 24, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc In Wound Healing Modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Deng, J.; Li, W.; Nie, X. Fibroblast Growth Factor In Diabetic Foot Ulcer: Progress And Therapeutic Prospects. Front Endocrinol (Lausanne) 2021, 12, 744868. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Wordsworth, J.; Wang, C.; Jurk, D.; Lawless, C.; Martin-Ruiz, C.; Von Zglinicki, T. A Senescent Cell Bystander Effect: Senescence-Induced Senescence. Aging Cell 2012, 11, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Hwang, S.R.; Yoon, I.S. Advanced Growth Factor Delivery Systems In Wound Management And Skin Regeneration. Molecules 2017, 22, 1259. [Google Scholar] [CrossRef] [PubMed]
- Reckenbeil, J.; Kraus, D.; Stark, H.; Rath-Deschner, B.; Jager, A.; Wenghoefer, M.; Winter, J.; Gotz, W. Insulin-Like Growth Factor 1 (Igf1) Affects Proliferation And Differentiation And Wound Healing Processes In An Inflammatory Environment With P38 Controlling Early Osteoblast Differentiation In Periodontal Ligament Cells. Arch Oral Biol 2017, 73, 142–150. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol Rev 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Sandhiya, S.; Dkhar, S.A.; Surendiran, A. Emerging Trends Of Nanomedicine--An Overview. Fundam Clin Pharmacol 2009, 23, 263–269. [Google Scholar] [CrossRef]
- Shao, Z.; Yin, T.; Jiang, J.; He, Y.; Xiang, T.; Zhou, S. Wound Microenvironment Self-Adaptive Hydrogel With Efficient Angiogenesis For Promoting Diabetic Wound Healing. Bioact Mater 2023, 20, 561–573. [Google Scholar] [CrossRef]
- Stuard, W.L.; Titone, R.; Robertson, D.M. The Igf/Insulin-Igfbp Axis In Corneal Development, Wound Healing, And Disease. Front Endocrinol (Lausanne) 2020, 11, 24. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process And New Emerging Technologies For Skin Wound Care And Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Van Der Vliet, A.; Janssen-Heininger, Y.M. Hydrogen Peroxide As A Damage Signal In Tissue Injury And Inflammation: Murderer, Mediator, Or Messenger? J Cell Biochem 2014, 115, 427–435. [Google Scholar] [CrossRef]
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases. Statpearls. Treasure Island (Fl) Ineligible Companies. Disclosure: Brandon Basehore Declares No Relevant Financial Relationships With Ineligible Companies. Disclosure: Patrick Zito Declares No Relevant Financial Relationships With Ineligible Companies. 2023. [Google Scholar]
- Wang, Z.; Shi, C. Cellular Senescence Is A Promising Target For Chronic Wounds: A Comprehensive Review. Burns Trauma 2020, 8, Tkaa021. [Google Scholar] [CrossRef]
- Wojtowicz, A.M.; Oliveira, S.; Carlson, M.W.; Zawadzka, A.; Rousseau, C.F.; Baksh, D. The Importance Of Both Fibroblasts And Keratinocytes In A Bilayered Living Cellular Construct Used In Wound Healing. Wound Repair Regen 2014, 22, 246–255. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, L.; Liu, P.; Yu, T.; Lin, C.; Yan, C.; Hu, Y.; Zhou, W.; Sun, Y.; Panayi, A.C.; Cao, F.; Xue, H.; Hu, L.; Lin, Z.; Xie, X.; Xiao, X.; Feng, Q.; Mi, B.; Liu, G. All-In-One: Multifunctional Hydrogel Accelerates Oxidative Diabetic Wound Healing Through Timed-Release Of Exosome And Fibroblast Growth Factor. Small 2022, 18, E2104229. [Google Scholar] [CrossRef]
- Yadav, R.; Angolkar, T.; Kaur, G.; Buttar, H.S. Antibacterial And Antiinflammatory Properties Of Bovine Colostrum. Recent Pat Inflamm Allergy Drug Discov 2016, 10, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, W.; Ren, Z.; Lu, Y.; Hamushan, M.; Cheng, P.; Xu, Z.; Shen, H.; Zhao, C.; Han, P.; Zhong, W. Chiral Supramolecular Hydrogel Loaded With Dimethyloxalyglycine To Accelerate Chronic Diabetic Wound Healing By Promoting Cell Proliferation And Angiogenesis. Gels 2022, 8, 437. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation In Chronic Wounds. Int J Mol Sci 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
The cell survival of human fibroblast cells at 1h, 24h and 48h following treatment with Rigemed (1, 1.5, 2 %), NC (negative control, cells without treatment); PC (positive control, 80 µM H2O2); CBSC (Cord Blood Stem Cells 12 µl); ns stands for non-significant. The number of asterisks denotes the degree of significance between results: * p < 0.05; p<0.05, ** p<0.01, *** p<0.001. Errors bars represent the standard error of the mean (SEM) (The experiment was repeated 3 times).
Figure 1.
The cell survival of human fibroblast cells at 1h, 24h and 48h following treatment with Rigemed (1, 1.5, 2 %), NC (negative control, cells without treatment); PC (positive control, 80 µM H2O2); CBSC (Cord Blood Stem Cells 12 µl); ns stands for non-significant. The number of asterisks denotes the degree of significance between results: * p < 0.05; p<0.05, ** p<0.01, *** p<0.001. Errors bars represent the standard error of the mean (SEM) (The experiment was repeated 3 times).
Figure 2.
The cell survival of HaCaT cells at 1h, 24h and 48h following treatment with Rigemed (1, 1.5, 2 %), NC (negative control, cells without treatment); PC (positive control, 50 µM H2O2); CBSC (Cord Blood Stem Cells 12 µl); ns stands for non-significant. The number of asterisks denotes the degree of significance between results: * p < 0.05; p<0.05, ** p<0.01, *** p<0.001. Errors bars represent the standard error of the mean (SEM) (The experiment was repeated 3 times).
Figure 2.
The cell survival of HaCaT cells at 1h, 24h and 48h following treatment with Rigemed (1, 1.5, 2 %), NC (negative control, cells without treatment); PC (positive control, 50 µM H2O2); CBSC (Cord Blood Stem Cells 12 µl); ns stands for non-significant. The number of asterisks denotes the degree of significance between results: * p < 0.05; p<0.05, ** p<0.01, *** p<0.001. Errors bars represent the standard error of the mean (SEM) (The experiment was repeated 3 times).
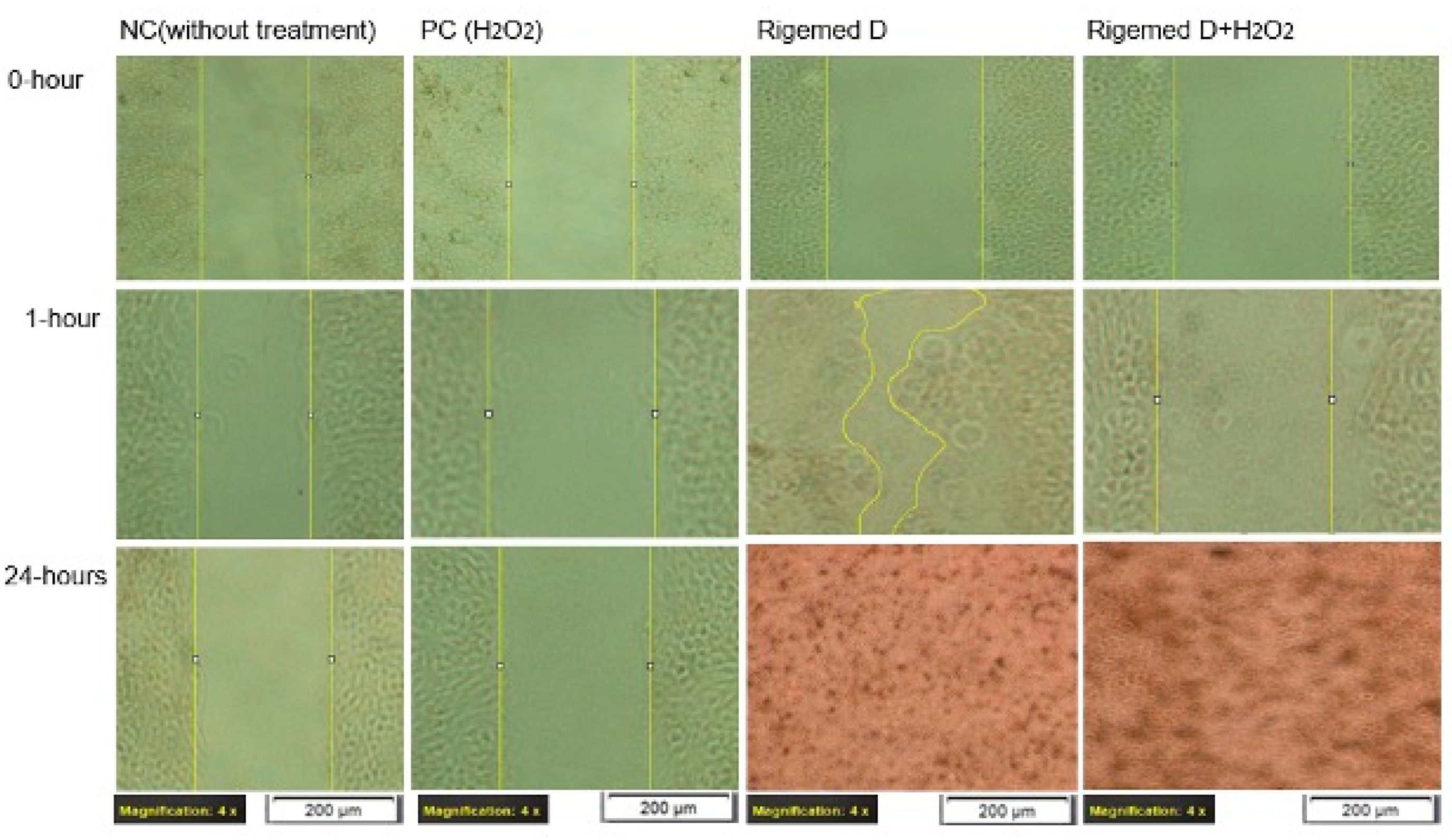
Figure 3.
Scratch assay on human fibroblast cell line. The images were captured with a magnification of 40X in each case.
Figure 3.
Scratch assay on human fibroblast cell line. The images were captured with a magnification of 40X in each case.
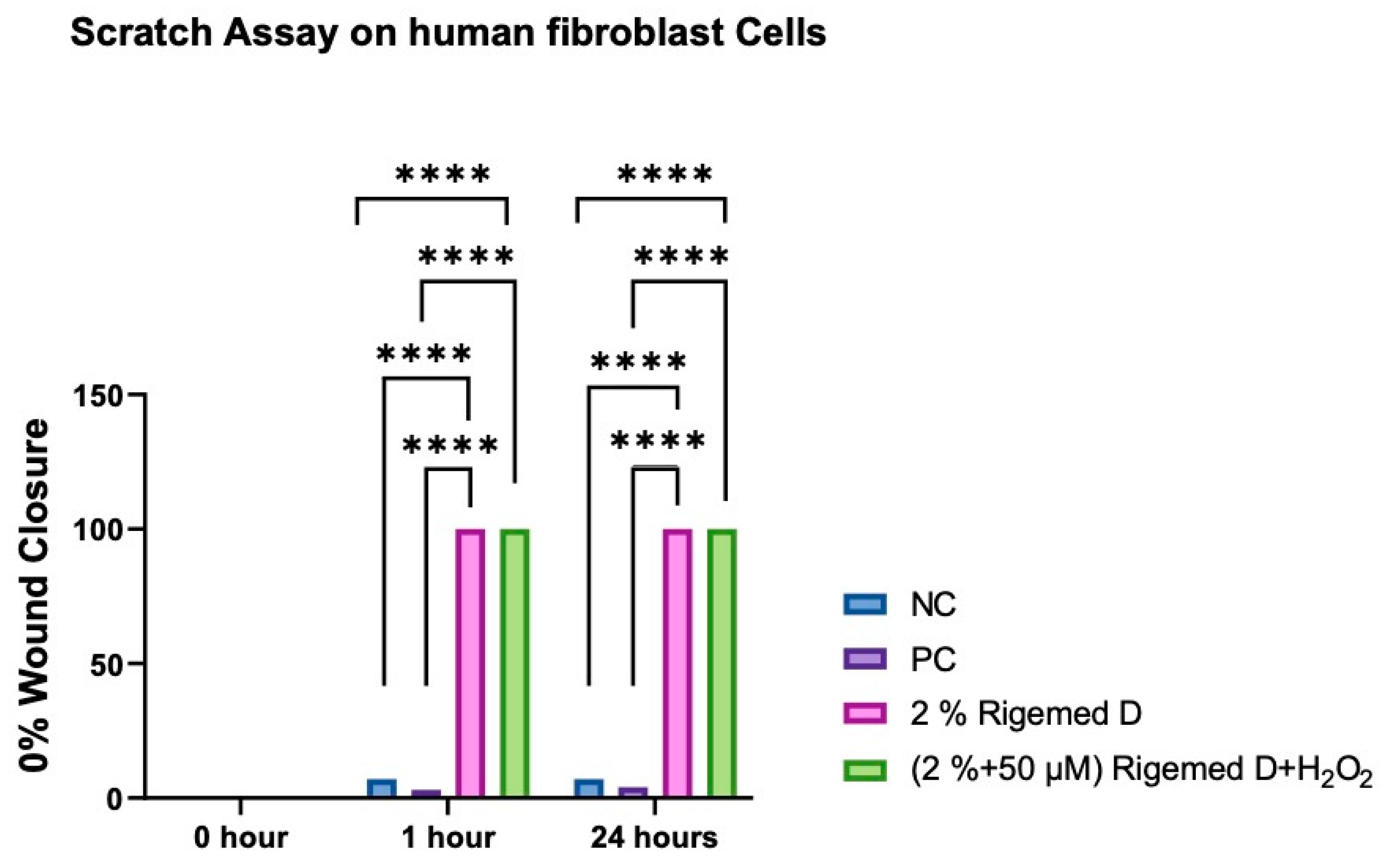
Figure 4.
Scratch assay on fibroblast Cells. The results represent the size of the wound in percentage (%), with 0% indicating the original wound size and 100% representing the complete absence of the wound. The number of asterisks denotes the degree of significance between results: * p < 0.05; ** p < 0.0014; *** p < 0.0001; **** p < 0.0001. The experiment was repeated 3 times.
Figure 4.
Scratch assay on fibroblast Cells. The results represent the size of the wound in percentage (%), with 0% indicating the original wound size and 100% representing the complete absence of the wound. The number of asterisks denotes the degree of significance between results: * p < 0.05; ** p < 0.0014; *** p < 0.0001; **** p < 0.0001. The experiment was repeated 3 times.
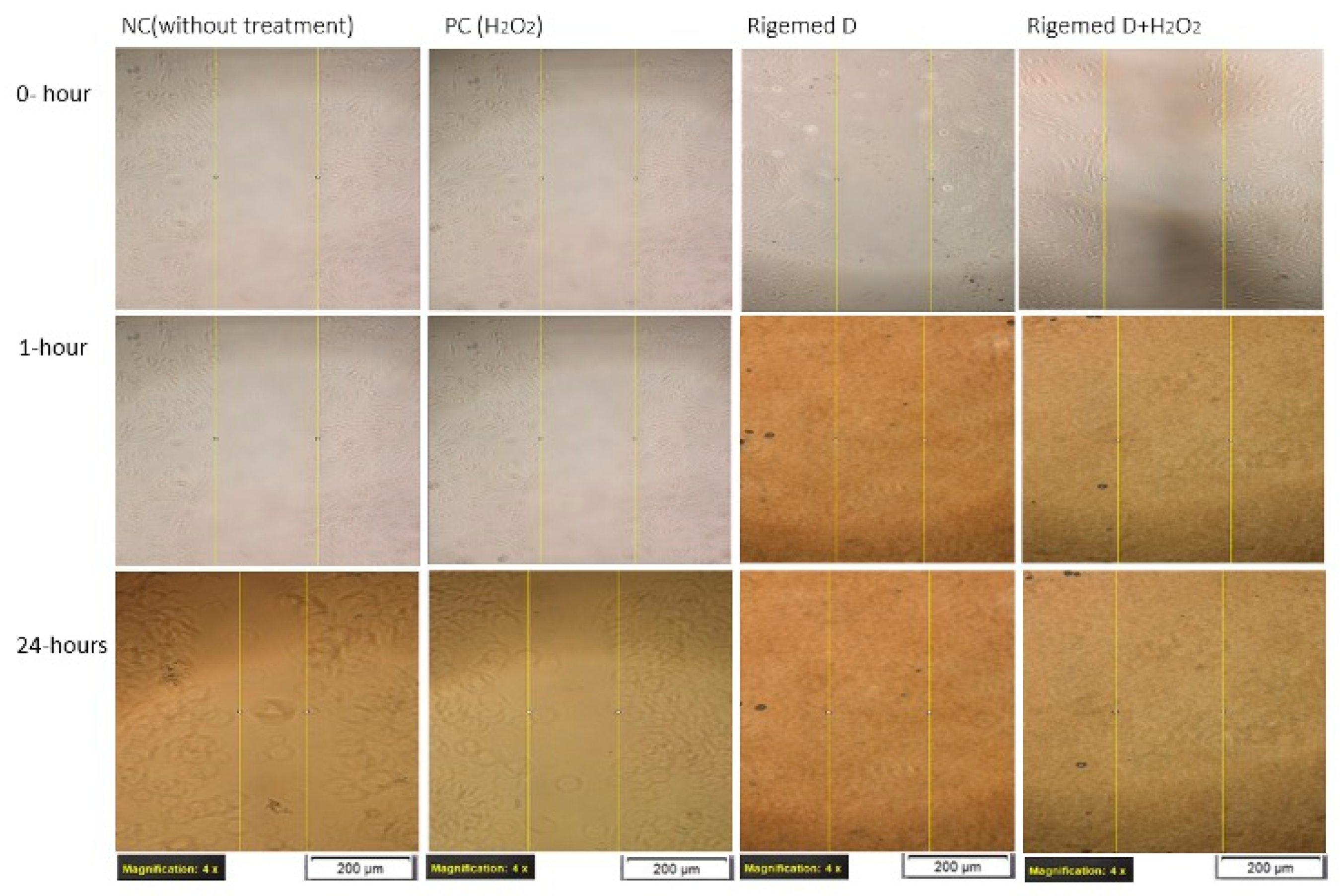
Figure 5.
Scratch assay on HaCaT cell line. The images were captured with a magnification of 40X in each case.
Figure 5.
Scratch assay on HaCaT cell line. The images were captured with a magnification of 40X in each case.
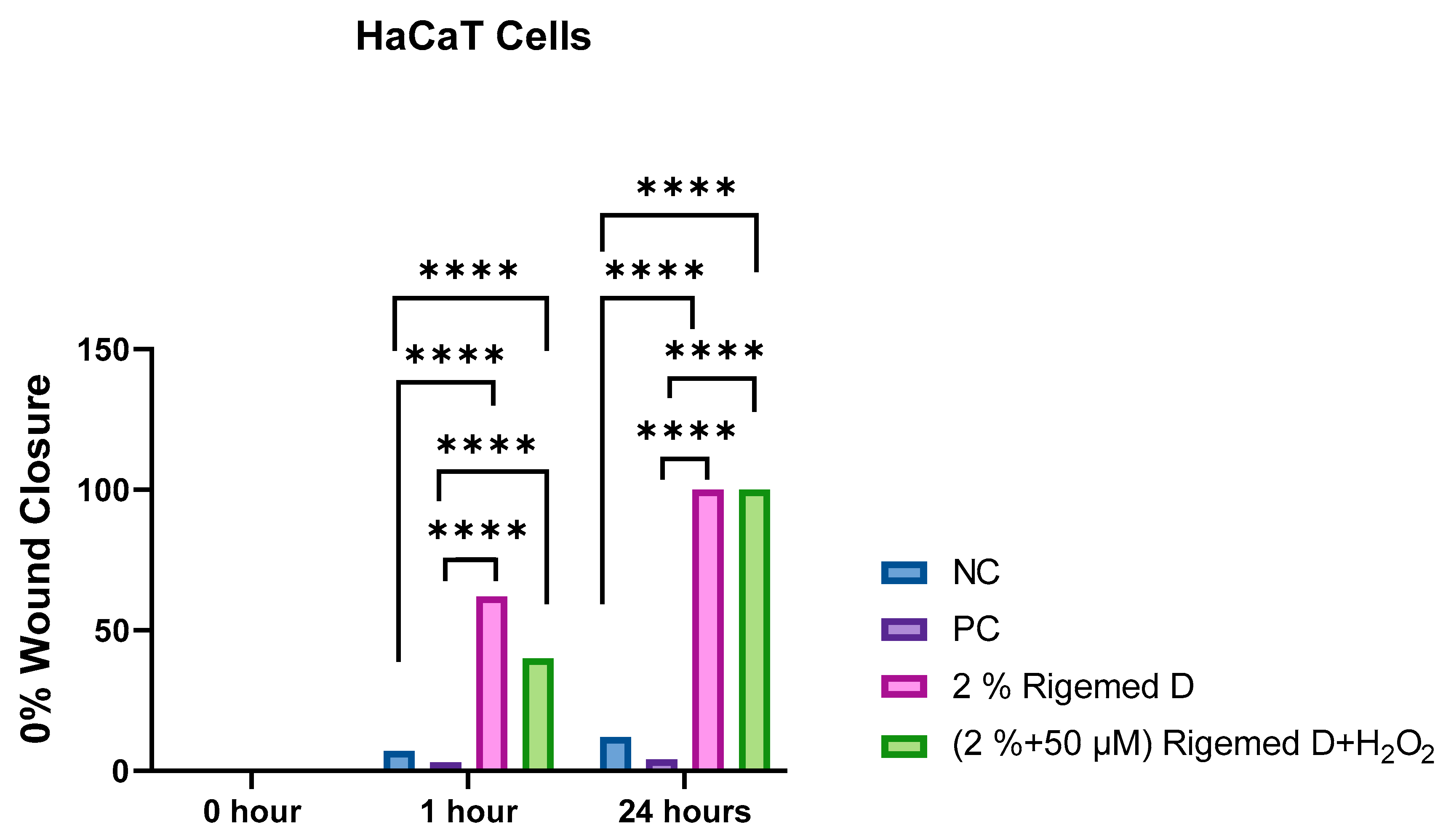
Figure 6.
Scratch assay on HaCaT Cells. The results represent the size of the wound in percentage (%), with 0% indicating the original wound size and 100% representing the complete absence of the wound. The number of asterisks denotes the degree of significance between results: * p < 0.05; ** p < 0.0014; *** p < 0.0001; **** p < 0.0001.
Figure 6.
Scratch assay on HaCaT Cells. The results represent the size of the wound in percentage (%), with 0% indicating the original wound size and 100% representing the complete absence of the wound. The number of asterisks denotes the degree of significance between results: * p < 0.05; ** p < 0.0014; *** p < 0.0001; **** p < 0.0001.
Figure 7.
Scratch assay with mitomycin C (MMC) on human fibroblast cell line. The images were captured with a magnification of 40X in each case.
Figure 7.
Scratch assay with mitomycin C (MMC) on human fibroblast cell line. The images were captured with a magnification of 40X in each case.
Figure 8.
Scratch assay on fibroblast Cells. The results represent the size of the wound in percentage (%), with 0% indicating the original wound size and 100% representing the complete absence of the wound. The number of asterisks denotes the degree of significance between results: * p < 0.05; ** p < 0.0014; *** p < 0.0001; **** p < 0.0001.
Figure 8.
Scratch assay on fibroblast Cells. The results represent the size of the wound in percentage (%), with 0% indicating the original wound size and 100% representing the complete absence of the wound. The number of asterisks denotes the degree of significance between results: * p < 0.05; ** p < 0.0014; *** p < 0.0001; **** p < 0.0001.
Figure 9.
Scratch assay with mitomycin C on HaCaT cell line. The images were captured with a magnification of 40X in each case.
Figure 9.
Scratch assay with mitomycin C on HaCaT cell line. The images were captured with a magnification of 40X in each case.
Figure 10.
Scratch assay on HaCaT Cells with mitomycin C. The results represent the size of the wound in percentage (%), with 0% indicating the original wound size and 100% representing the complete absence of the wound. The number of asterisks denotes the degree of significance between results: * p < 0.05; ** p < 0.0014; *** p < 0.0001; **** p < 0.0001.
Figure 10.
Scratch assay on HaCaT Cells with mitomycin C. The results represent the size of the wound in percentage (%), with 0% indicating the original wound size and 100% representing the complete absence of the wound. The number of asterisks denotes the degree of significance between results: * p < 0.05; ** p < 0.0014; *** p < 0.0001; **** p < 0.0001.
Figure 11.
Effect of novel Rigemed D on Human Fibroblast Cells as measured using OTM in the comet assay. NC (negative control without treatment); PC (positive control 50 µM H2O2); Rigemed D (2% ); Rigemed D and hydrogen peroxide (2% + 50 µMM); ns stands for non-significant. The number of asterisks denote the degree of significance between results: (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; ns = non-significant) errors bars represent the standard error of the mean (SEM).
Figure 11.
Effect of novel Rigemed D on Human Fibroblast Cells as measured using OTM in the comet assay. NC (negative control without treatment); PC (positive control 50 µM H2O2); Rigemed D (2% ); Rigemed D and hydrogen peroxide (2% + 50 µMM); ns stands for non-significant. The number of asterisks denote the degree of significance between results: (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; ns = non-significant) errors bars represent the standard error of the mean (SEM).
Figure 12.
Immunohistochemistry (IHC) conducted on fibroblast cells using Vimentin cytoskeleton marker antibody, S100A4 Primary antibody, and DAPI. (A, E) cells without treatment; (B, F) Cells treated with 50µM hydrogen peroxide; (C, G) Cells treated with 2% RD; (D, H) Cells treated with 2% RD and 50 µM hydrogen peroxide. Standard error of the mean (SEM).
Figure 12.
Immunohistochemistry (IHC) conducted on fibroblast cells using Vimentin cytoskeleton marker antibody, S100A4 Primary antibody, and DAPI. (A, E) cells without treatment; (B, F) Cells treated with 50µM hydrogen peroxide; (C, G) Cells treated with 2% RD; (D, H) Cells treated with 2% RD and 50 µM hydrogen peroxide. Standard error of the mean (SEM).
Figure 13.
Immunohistochemistry (IHC) conducted on fibroblast cells using Vimentin cytoskeleton marker antibody. The graphical representation illustrating the quantity of protein expression in fibroblast cells. NC (negative control without treatment); PC (positive control 50 µM H2O2); 2% RD; 2% RD and 50 µM hydrogen peroxide; ns stands for non-significant. The number of asterisks denote the degree of significance between results: (* p <0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; ns = non-significant). Errors bars represent the standard error of the mean (SEM).
Figure 13.
Immunohistochemistry (IHC) conducted on fibroblast cells using Vimentin cytoskeleton marker antibody. The graphical representation illustrating the quantity of protein expression in fibroblast cells. NC (negative control without treatment); PC (positive control 50 µM H2O2); 2% RD; 2% RD and 50 µM hydrogen peroxide; ns stands for non-significant. The number of asterisks denote the degree of significance between results: (* p <0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; ns = non-significant). Errors bars represent the standard error of the mean (SEM).
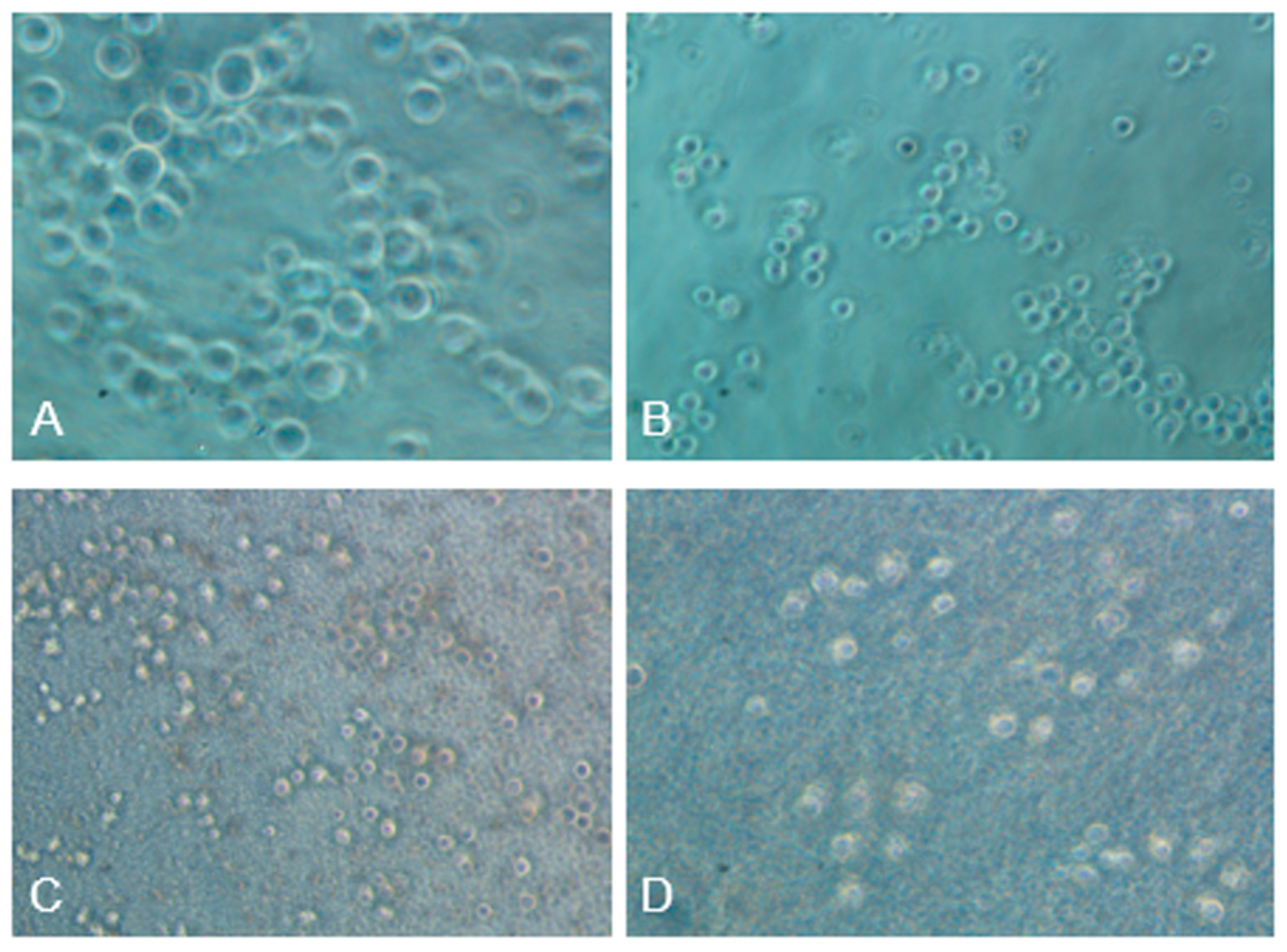
Figure 14.
Angiogenesis assay conducted on Human Umbilical Vein Endothelial Cells (HUVEC) (A) cells without treatment; (B) Cells treated with 50µM hydrogen peroxide, (C) Cells treated with 2% RD; (D) Cells treated with 2% RD and 50 µM hydrogen peroxide.
Figure 14.
Angiogenesis assay conducted on Human Umbilical Vein Endothelial Cells (HUVEC) (A) cells without treatment; (B) Cells treated with 50µM hydrogen peroxide, (C) Cells treated with 2% RD; (D) Cells treated with 2% RD and 50 µM hydrogen peroxide.
Figure 15.
Angiogenesis assay conducted on Human Umbilical Vein Endothelial Cells (HUVEC). Graph displaying the percentage of formed tubes in angiogenesis.NC (negative control without treatment); PC (positive control 50 µM H2O2); 2% RD; 2% RD and 50 µM hydrogen peroxide; ns stands for non-significant. The number of asterisks denotes the degree of significance between results: (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; ns = non-significant). Errors bars represent the standard error of the mean (SEM).
Figure 15.
Angiogenesis assay conducted on Human Umbilical Vein Endothelial Cells (HUVEC). Graph displaying the percentage of formed tubes in angiogenesis.NC (negative control without treatment); PC (positive control 50 µM H2O2); 2% RD; 2% RD and 50 µM hydrogen peroxide; ns stands for non-significant. The number of asterisks denotes the degree of significance between results: (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; ns = non-significant). Errors bars represent the standard error of the mean (SEM).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).