1. Introduction
Tight junctions (TJs) are the main intercellular junctions that act as permeability barriers and confer polarity to epithelial cells. Claudins (CLDN)s in turn are key proteins that make up the tight junction stands [
1]. These TJs are important for the endothelial and epithelial barriers, which not only protect the internal organs, but also acts as a selective barrier between the body and the external environment [
2]. CLDNs are a family of 27 proteins, functioning not only as a barrier to enforce the integrity of TJ complexes but also as a pore-forming channel to maintain the cell permeability [
3].
However, the molecular mechanisms and patterns of CLDNs expression in cancers are still unclear and need further investigation. Advanced research showed that CLDNs also regulate cell signal transduction, proliferation, dedifferentiation, and distant metastasis in cancer biology. Therefore, CLDNs have become a promising target in antineoplastic therapy.
2. Tight Junctions and the Structure of the Claudins Proteins
The neighboring cells in the epithelial sheets are connected by various types of cell-cell junction; which can be categorized as TJs, adherens junctions and desmosomes [
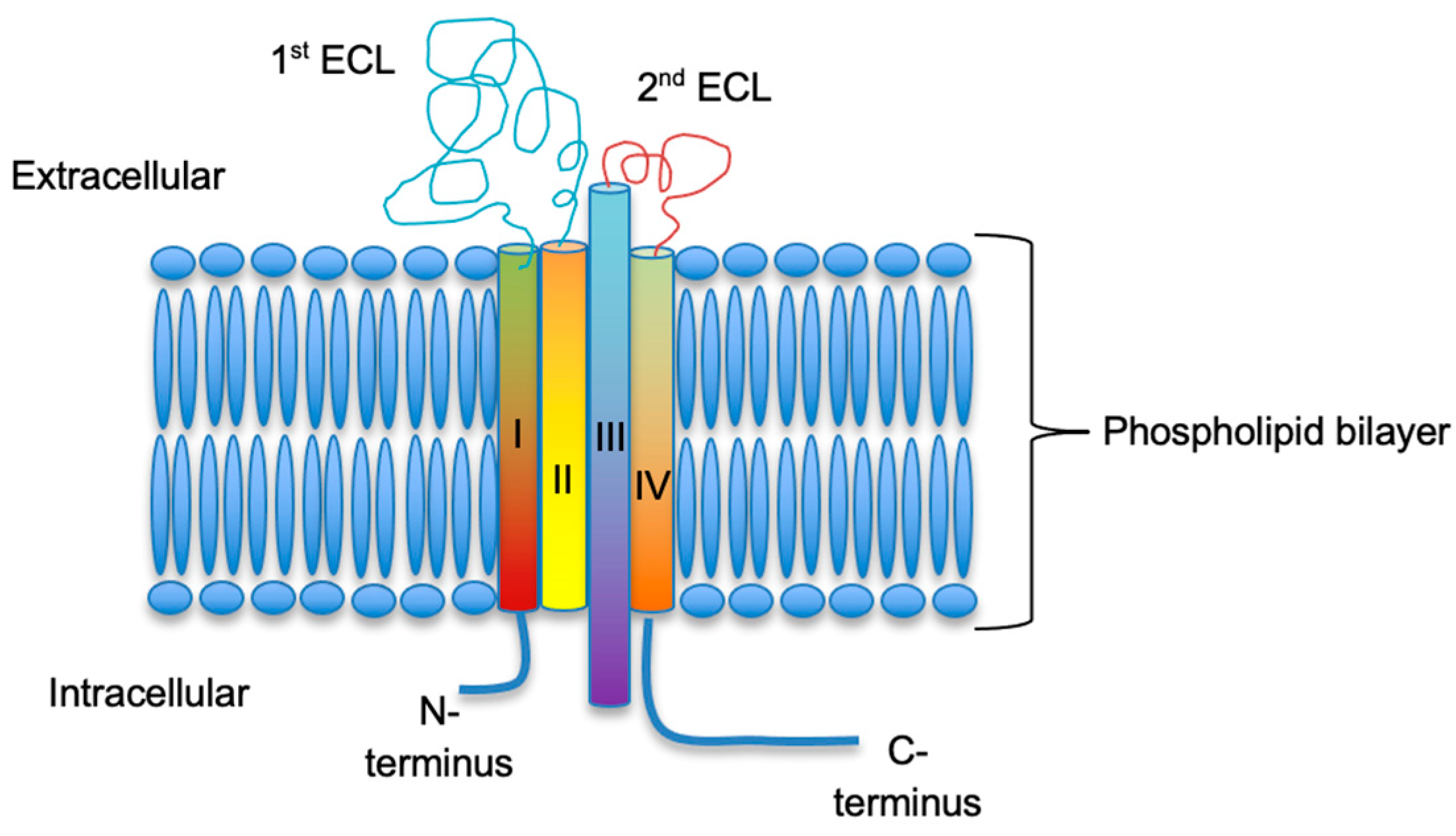
4]. Overall, CLDNs constitute a highly related family of proteins, that share a structural topology of four transmembrane segments, a large extracellular loop containing a consensus sequence motif, a second shorter extracellular loop, an internal C-terminus, and a very short internal N-terminal region [
5]. The two extracellular loops are crucial for the formation of paracellular barriers and pores for solutes and are thought to determine the permeability characteristics of TJs (
Figure 1). CLDNs mostly have a similar structure especially in the membrane-spanning regions. The exceptions are CLDN16, which contains a 66 aa extension at the N-terminus, CLDN18, which has an extension in the second extracellular loop, and CLDN23 with a longer C-terminal tail. CLDNs 6 and 9 are the most similar, followed by CLDNs 3 and 4, and CLDNs 1 and 7 [
3,
6].
CLDNs and their tightness and pore functions are highly dynamic, as indicated by the short half-life of, e.g., CLDN4 with about 4 h [
7].
3. Physiological Functions of Claudins
The distinct tightness properties of a given tissue and a given claudin seem to be largely dependent on the combination of the claudins that are expressed and on the manner in which they copolymerize [
8].
The different CLDNs exert different cellular functions, not only that they have a role in TJs, they also play a role in cations permeability via the formation of cation pores e.g., CLDNs 2, 7, 10, 15 and 16 increase paracellular cation permeability, [
9,
10] while CLDNs 4, 5, 8, 11, 14, and 19 decrease the paracellular cation permeability [
11,
12,
13].
The role of CLDNs in anions exchange has been more controversial, e.g., CLDN 7 knock down in some studies have been reported to decrease chloride permeation, while in other studies its overexpression was linked to the same outcome [
14,
15].
CLDNs are also found in cellular projections and have a role in cell mobility as shown in a study of CLDN-4, where knockdown of CLDN4 resulted in decreased cell migration. This was noted in the cultured normal and tumor cells [
16]. this function is through the interaction of the CLDN’s second extracellular domain with the extracellular matrix.
CLDNs also have a role in the epithelial-to-mesenchymal transition; one of the most important functions of CLDN proteins in disease progression [
17].
4. Claudins Expression in Different Tissues
Mammalian CLDNs are divided into classic and non-classic CLDNs. Classic CLDNs include 1–10, 14, 15, 17, and 19. Non-classic CLDNs comprise 11–13, 16, 18, and 20–24. The non-classic CLDNs have a longer C-terminus [
18].
Each CLDN protein has a different function and unique expression pattern based on the cell or tissue type resulting in tissue specific barrier characteristics [
8]. For example, CLDN1 is expressed in brain, kidney, liver, testis, fetal lung alveolar epithelial (HFL) cells, bronchiolar epitheliums, and pancreatic ducts and exocrine glands [
3]. CLDN3 is expressed in pancreatic ducts and exocrine glands [
19]. CLDN3 is present in colorectal, thyroid, salivary gland, pancreas, prostate cancer, liver and kidney tissues [
3]. CLDN4 is expressed in Breast, ovary, prostate, bladder, gastrointestinal mucosa, bile duct, HFL cells, type II alveolar epitheliums, bronchiolar epitheliums, and pancreatic ducts and exocrine glands [
3]. CLDN5 is expressed in HFL cells, type II alveolar epitheliums, and vascular endothelial cells and has a role in the formation of the blood brain barrier [
20]. CLDN10a is expressed in the renal tissue and had a role in anion channels formation [
21]. CLDN18.2 is expressed in Gastric mucosal epithelial cells and has a role in regulating the formation of gastric mucosal barrier [
22] and the permeability of H
+ between the gastric mucosal epithelial cells [
23].
5. Claudins Expression and Regulation in Cancer
Downregulation of CLDN-1 and CLDN-7 was observed in breast, esophageal, and prostate cancers but upregulation of CLDN-1 has been reported in colon, nasopharyngeal, ovarian and oral squamous cell cancers [
3,
24,
25,
26]. Overexpression of CLDN-3 and CLDN-4 are reported in ovarian, breast, gastric, pancreatic, prostate, and uterine cancers [
27,
28].
5.1. Genetic Alterations (Amplification)
The expression of CLDNs is regulated at different levels. E.g., CLDN1 expression is inhibited at a protein and mRNA levels by overexpression of the Slug or Snail transcription factors. An inverse correlation in the levels of CLDN1 and Slug transcripts were observed in invasive breast cancer [
29]. CLDN1 expression was also suppressed by the transcription factor RUNX3 which is a tumor suppressor in gastric cancer [
30].
5.2. Epigenetic Modifications
DNA methylation has been shown to alter the expression of CLDNs. DNA promoter hypermethylation is associated with downregulation of CLDN1 and CLDN7 in breast cancer, and CLDN11 in gastric cancer cells [
31].
In addition, loss of repressive histone methylations, including H3K27me3 and H4K20me3, is also associated with the overexpression of CLDN3 and CLDN4 in ovarian cancer [
32].
Decreased CLDN2 levels have been reported with the use of azacitidine, a DNA methylation inhibitor, and trichostatin A, and sodium butyrate, histone deacetylase (HDAC) inhibitors, giving these chemicals the potential to have an anti-cancer effect [
33]. CLDN1 expression was also shown to be regulated through the modulation of mRNA stability in colon cancer cells in a HDAC dependent mechanism [
34].
6. Role of Claudins in Cancer
6.1. Tumor Suppressor Effect
TJ proteins are believed to work as tumor suppressor because they are the hallmark of epithelial cells and their expression decrease in parallel with progression of cancer [
35]. In vitro and vivo studies of pancreatic cancer showed that overexpression of CLDN4 enhanced the cell-to-cell adhesion and prevented cancer cells from invasion and distant metastasis via the transforming growth factor beta and Ras/Raf/extracellular signal-regulated kinase pathways [
36].
Genetic analysis from human breast tumor cells showed that a significant number of triple negative breast cancer (TNBC) tumors have low expression of CLDNs genes (i.e., CLDN3, -4, -7, and E-cadherin) [
37]. Phenotypically, they behave like mammary stem cells or epithelial precursor cells [
38] and have a poor prognosis [
39] associated with early onset of cancer, high histology grade, large tumor size, lymphocytic infiltration, and low local recurrence rate [
40]. Further study in vivo mice models demonstrated that activation of the RAS system in the luminal epithelial cells could be the origin for development of basal-like, CLDN low mammary breast cancer [
41].
A recent study showed that CLDN6 was found to have an inhibitory function in breast cancer metastasis by upregulating the WIP expression then the WIP regulates the actin cytoskeleton autophagy pathways in vivo and vitro studies. This finding could explain why low levels of CLDN6 expression were found in metastasis breast cancer [
42].
CLDN7 is also reported as tumor suppressor protein in colorectal cancer. CLDN7 knockout mice model and colorectal cell lines showed significant tumor growth, tumor cells migration and inhibition in apoptosis via SOX-9 mediated Wnt/β-catenin signaling pathway [
43]. Moreover, CLDN7 was shown to have a similar tumor inhibitory effect in oral squamous cell carcinoma [
44].
Another in vitro study investigated the role of CLDN17 in head and neck cancer cells and CLDN17 gene expression profiles in oral cancer tissues were analyzed. There was an association with lower CLDN17 expression and higher tumor staging, poorer tumor histological grading and worse clinical prognosis [
45]. Therefore, CLDN17 was believed to have a tumor suppressor effect in oral cancer by inhibiting epithelial-mesenchymal transformation, the tumor invasion and migration.
6.2. Tumor Promoter Effect
Although CLDNs are believed to function as tumor suppressors due to their sealing effect at TJs, they are also found to have oncogenic properties such as cell growth, proliferation, invasion, migration and metastasis. Recent study showed that CLDNs 3 and 4 are highly expressed in ovarian cancer cell-lines which increased cell survival and promoted the cancer metastasis by enhancing matrix metalloproteinase-2 (MMP-2) activity [
46]. CLDN4 is also a tumor promoter gene in urothelial bladder cancer. Hypomethylation of CLDN4 promoter region was associated with cancer metastasis and hypermethylation of CLDN4 would be a new potential targeted therapy for bladder cancer [
47].
Similarly, CLDN1 promotes oral squamous carcinoma cell invasion by activating membrane type MMP (MT1-MMP) and MMP-2 [
48]. In human colorectal cancer there is an increase in CLDN 2 expression, indicating a potential role in the pathogenesis of colorectal cancer [
1].This was again noted by Wei et al. who demonstrated that, in colorectal cancer, CLDN2 is upregulated and is associated with poor prognosis. CLDN2 suppression promoted N-myc downstream regulated gene 1 (NDRG1) transcription which prohibited the tumor progression and metastasis in vitro and in vivo models [
49].
Moreover, antibodies which block the CLDN18, could reduce the cell proliferation in bile duct carcinoma and tumor promoter effects of CLDN18 went through epidermal growth factor, RAS, and extracellular signal-related kinase (ERK) 1/2 pathways in vitro studies [
50].
CLDN6 is an oncofetal antigen that is typically silent in normal tissues, but reactivated in germline tumors like testicular, ovarian, and uterine cancer. This implies or suggests that CLDN6 may have a potential as a diagnostic marker or even a therapeutic target in these cancer types [
1]. The similar tumor promoter effect of CLDN6 was demonstrated in human hepatocellular carcinoma (hHCC). High expression of CLDN6 was associated with tumor’s differentiation of hHCC according to the cancer genome atlas (TCGA) database. In vitro study, silencing of CLDN6 gene resulted in decreased tumor proliferation, migration and invasion with upregulated E-cadherin and down regulated N-cadherin and Vimentin [
51].
6.3. Tumor Markers
Transmembrane types of CLDN proteins are highly expressed in precancerous and cancerous cells in certain types of cancers, and they have shown a potential as tumor markers.
CLDN1 is shown to have an inhibitory effect on cancer metastasis in lung adenocarcinoma as the upregulation of CLDN1 suppressed ERK1/2 signaling pathway. Moreover, CLDN1 also enhances the efficacy of chemotherapy so CLDN1 is not only a potential prognosis marker but also the predictive marker for chemotherapy benefits in metastatic cancer [
52].
In the immunohistochemistry study of CLDN4 in pleural and peritoneal fluid or tissue biopsies, the CLDN4 stain was strongly positive in primary carcinoma and metastatic lesions but not in mesothelioma [
53]. CLDN4 can be used as a tumor marker to differentiate neoplastic metastases versus mesothelioma. The sensitivity and specificity of CLDN4 was evaluated by immunocytochemistry in cell blocks which including non-conclusive encompassing atypia of undetermined significance (AUS), suspicious for malignancy (SFM) and benign cases. Interestingly, CLDN4 is positive for 100% of adenocarcinoma cases and negative for 100% of mesothelial and mesothelioma effusions. Overall, sensitivity, specificity, positive predictive and negative predictive values for CLDN4 in metastatic adenocarcinoma are 85%, 100%, 100%, 75% respectively [
54].
In addition, a recent study proved that CLDN15 can be used as a novel tumor marker for malignant pleural mesotheliomas [
55]. Further studies of lung biopsy tissue, showed that the adenocarcinoma tumor has the highest staining of CLDN4 and atypical adenomatous hyperplasia cells have higher scores compared to the normal alveolar epithelium which indicates that CLDN4 is involved in the early tumorigenesis process [
56].
In gastrointestinal cancer, membrane bound CLDN7 and CLDN18 were proved as a reliable immunohistochemical markers to diagnosis pancreatic ductal neoplasia [
57] and CLDN18 has high sensitivity and specificity to diagnose biliary tract adenocarcinoma or intraepithelial neoplasia [
58]. CLDN3, CLDN7 and CLDN1 are highly expressed in colorectal adenocarcinoma and CLDN4 staining were strong in colorectal and pancreatic cancer tissue samples [
59].
CLDN6 is expressed at elevated levels in multiple human cancers including ovarian and endometrial malignancies, with little or no detectable expression in normal adult tissue. This expression profile makes CLDN6 an ideal target for development of a potential therapeutic antibody-drug conjugate (ADC) [
60].
6.4. Role of Claudins in Cancer Metastasis
According to the epithelial-mesenchymal transition (EMT) hypothesis, the epithelial cell transforms to mesenchymal cells by losing epithelial cell marker (e.g., E-Cadherin) and by gaining mesenchymal cell marker (e.g., N-cadherin) this allows the epithelial cells to acquire mesenchymal characteristics such as increased migration rate. This phenomenon is one of the established phenomena of cancer progression [
36]. This depends on the CLDN and the type of cancer; high expression of CLDN1 in colorectal cancer, HCC, and lung cancer might enhance cancer cell aggressiveness through promotion of EMT [
18]. Nevertheless, CLDN3 could inhibit EMT in HCC [
61] and lung cancer [
62]. CLDN6 promotes EMT in gastric cancer [
63] while its downregulation in breast cancer promoted cancer invasiveness and progression [
64].
In colon cancer, nuclear localization of CLDN1 was noted frequently, and manipulation of CLDN1 expression significantly affected the EMT marker changes and distant metastasis in vitro and vivo studies. [
65] CLDN1 expression in hepatocellular carcinoma (HCC) promote EMT via the c-Abl/Raf/Ras/ERK signaling pathway. [
66] Therefore, the CLDN-1 targeted therapy would be a novel antineoplastic therapy in future.
In vitro study of melanoma cells showed that cytoplasmic CLDN-1 promoted metastatic ability and it can be blocked by regulating phosphorylation pathway via protein kinase activity. [
67] Similar finding was reported in the follicular thyroid carcinoma cell lines in which tumor invasion and migration were promoted by CLDN-1 localized in the nucleus [
68].
6.5. Claudins and Chemoresistant Tumor
CLDNs have overall EMT, tumor invasion and tumor stemness capacities which are fundamental factors to develop chemoresistance in cancer. CLDN6 is known as a marker for pluripotent stem cells because it is highly expressed in undifferentiated cells but not in normal tissue [
69]. In the TNBC cell line, CLDN6 promotes adriamycin resistant cancer clones via afadin (AF-6)/ERKs pathway [
70]. CLDN6 also became an interesting target for cancer therapy and CLDN-6 targeting chimeric antigen receptor (CAR)-T cell therapy has positive outcomes in vitro and mice models [
71,
72].
Suppression of CLDN3 in non-small cell lung cancer decreased cancer stemness and improved the chemosensitivity [
73]. CLDN1 causes chemoresistance in CRC via upregulating of ephrin type-A receptor 2 (EPHA2) tyrosine kinase which enhances downstream AKT signaling pathway and CD44 expression which promotes cancer stemness and chemoresistance [
74]. It causes 5-FU resistance in colon cancer cell lines [
75], cisplatin resistance [
76] or doxorubicin resistance [
77] in lung cancer and drug resistance in liver cancer. On the other hand, CLDN18 regulates cancer stem cells in lung cancer and CLDN2 promote self-proliferation of colorectal cancer cells [
78,
79]. CLDN3 and CLDN4 also regulate the cisplatin sensitivity in ovarian cancer cells via copper transporter (CTR1) [
80]. Interestingly, CLDN7 enhance cisplatin sensitivity in lung cancer cells via caspase pathway [
81]. Therefore, CLDNs induce not only tumorigenesis but also treatment resistance in cancer cells.
7. Investigational Role of CLDNs in Early Detection of Cancer
In a study of radiolabeled anti-CLDN4 monoclonal antibodies, connected to 125I, when injected into mice with severe combined immunodeficiency (SCID) bearing the PNAC-1 xenografts, the highest uptake was noted in the liver (4.5%) followed by the PANC-1 tumors (4%) and the spleen (3.5%). Similarly, increased uptake primarily in the tumor was seen in SCID mice bearing Colo357 cell xenograft tumors (originating from pancreatic carcinoma).
When examined with SPECT-CT, SCID mice bearing L3.6PL cell pancreatic cancer xenografts (a highly metastatic and aggressive subclone of Colo357 cells), the highest uptake was seen in the tumor tissue, liver and spleen, with the tumor uptake being 2.5 times that of the spleen and 2 times that of the liver. The investigators, however, concluded that 125I-labeled anti-CLDN4 antibody can be used for SPECT-CT to detect pancreatic cancer, however, due to the low-energy gamma photon emission, this could only be used for imaging of small animals, with a sensitive gamma camera [
82].
On the other hand, a meta-analysis of the use of CLDN-3 for evaluation of prostate cancer, showed that CLDN3 is indeed one of the strongest 2 markers overexpressed in cancer, when compared to prostate specific antigen. However, it was deemed not superior to PSMA scan because its expression is not drastically different in normal and cancerous tissues [
83]
8. Therapeutic Targeting of Claudins
An effective cancer therapy molecule should meet two criteria: first, restricted expression in specific tissues to avoid side effects, and second, positive expression with exposed epitopes in cancerous tissues for targeted treatment. CLDNs have been identified as meeting both criteria, which make them promising targets for cancer therapy [
84].
CLDNs, typically located within the TJ complex in normal tissues, are known to become more accessible in malignant tissues due to extra junctional mislocalization. This is a unique expression profile that makes CLDNs theoretical attractive targets for selective drug delivery with minimal adverse effects. Several approaches, such as Clostridium perfringens enterotoxins (CPE), monoclonal antibodies (mAbs), C-CPE, mAb-drug conjugates, bispecific T cell engagers (BiTEs), and chimeric antigen receptor (CAR) T cells, continued to be explored for targeting CLDNs in cancer patients. Ongoing phase I to phase III clinical studies indicate the potential significance of CLDNs-targeted agents [
84].
8.1. Monoclonal Antibodies (mAbs)
The clinical success in the usage of CLDNs as targets was demonstrated in the prevention and cure of Hep. C virus in 2005 by Hofner et al. who subsequently started exploring CLDNs as targets for antibody-based cancer therapies [
84]. Suzuki et al. succeded in generating a mAb (KM3900 (IgG2a)) that targets CLDN4. KM3900 was found to bind to CLDN4 on pancreatic and ovarian cancer cells but not normal cells causing dose-dependent antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) in vitro, as well as in-vivo tumor growth inhibition in mice models [
85].
Zolbetuximab (IMAB362, Claudiximab) is a chimeric IgG1 antibody, highly specific for CLDN18.2. Its binding to CLDN18.2 induced also ADCC and CDC. When combined with chemotherapy, zolbetuximab enhances T-cell infiltration and induce pro-inflammatory cytokines [
86]. Its safety was investigated in the phase I/II trial [
87]. (
Table 1).
Zolbetuximab combined with Interleukin-2 and zoledronic acid was investigated in patients with gastroesphageal junction (GEJ) cancer that failed multiple lines of therapy, 11 out of 20 patients had disease control [
88].
In the SPOTLIGHT randomized, double blind, placebo controlled, phase III trial, zolbetuximab combined with mFOLFOX6 was investigated as a first line treatment for HER2 negative, CLDN18.2 positive, locally advanced unresectable or metastatic GEJ adenocarcinoma. The study showed a significantly improved overall survival being 18.23 months compared to 15.53 months. The results of SPOTLIGHT are promising, as they support Zolbetuximab-based therapy for patients with high expression of CLDN18.2 biomarker. The most common treatment adverse events were nausea, vomiting and decreased appetite, which are consistent with previous phase 1 and phase 2 studies. This is encouraging as there are no new safety warnings to take into consideration. This study demonstrated clinically significant benefits for patients with CLDN 18.2 positive, HER-2 negative disease and most likely Zolbetuzimab is going to be considered as a first-line treatment option in combination with chemotherapy [
89].
Monoclonal antibodies targeting CLDNs, particularly CLDN1 and CLDN4 hold promise as therapeutic agents in the treatment of various cancers, with potential synergistic effects when combined with known anti-cancer agents like 5-fluorouracil and anti-EGFR antibodies. Additionally, antibody drug conjugates have shown promise in inhibiting tumor growth and metastasis in specific cancer types for example pancreatic and gastric tumors [
84].
8.2. Clostridium Perfringens Enterotoxins (CPE)
Clostridium perfringens enterotoxins (CPE) and its C-terminus domain recognizes specific amino acid sequences in the extracellular loops of CLDNs 4, and 3. This recognition leads to the disruption of tight junctions and perforation of the plasma membrane, which ultimately causes cell death. This cytotoxic effect has been observed in various cancer types, for example non-small cell lung cancer, prostate cancer, gastric and ovarian cancer. The impairment of tight junctions by CPE disrupts tumor microenvironment barrier, this enhances drug delivery to cancer cells, making them more susceptible to anti-cancer drugs and even suppressing metastasis. Conjugation of CPE and anti-cancer drugs turns into a carrier for targeted delivery to cancer cells expressing CLDN4. However, the clinical use of CPE may be limited by immunogenicity and potential toxicity, similar to how clostridium perfringens causes mucosal epithelial damage, food poisoning and even CPE induced shock [
90].
8.3. Chimeric Antigen Receptor T-Cell (CAR-T) Cell Therapy
CAR-T cell therapy has been effective in treating B cell malignancies but faces challenges in solid tumors. Although it has shown promise in targeting CLDNs expressed on solid tumor cells as demonstrated in preclinical models with engineered CAR-T cells with high specificity for CLDN6 and CLDN18.2. For example, the development of CARvac, an RNA vaccine that enhances CAR-T cell engraftment, is a novel strategy to improve effectiveness of CAR-T therapy. Another alternative includes BiTES, a method to target CLDN18.2, potentially enhancing the immune response against cancer cells. Ongoing phase I clinical trials recruiting patients with advanced tumors positive for CLDN18.2 suggests active research and a promising avenue for treating solid tumors [
1,
84].
Currently phase I clinical trial are undergoing to investigate CLDN18.2 targeted CAR-T in patients with unresectable, locally advanced, or metastatic gastric, GEJ, esophageal, or pancreatic adenocarcinoma (NCT05539430) [
91].
8.4. Calcium and Vitamin D Supplementation
In a large multicenter randomized. Placebo-controlled, partial 2x2 factorial chemoprevention clinical trial, testing the efficacy of calcium and vitamin D supplementation on rectal adenocarcinoma recurrence, after supplementation for 3-5 years, patients with removed colorectal adenomas did not have a significantly lower risk of rectal adenomas [
92]. Despite of those findings, subjects from that study were selected to participate in an adjunct trial, where they were randomized to 4 treatment groups: 1,200 mg/d calcium supplementation, 1,000 IU/d vitamin D3 supplementation, combination of both, and placebo. Then biopsies from the normal mucosa were collected at baseline, and year 1 of follow up to examine the expression of the tight junctions’ proteins. It was noted that CLDN1, occludins and mucin-12 expression increased by 14% (P=0.17), 23% (P=0.11), and 22% (P=0.07) in the calcium group compared to the no calcium group [
93]. A finding that raises the question about the clinical potential for calcium and vitamin D supplementation in colorectal carcinogenesis, and metastasis.
Table 1.
Clinical trials targeting various Claudins.
Table 1.
Clinical trials targeting various Claudins.
| |
| Clinical trial ID |
Phase |
Drug |
Target |
Cancer |
Results |
| NCT03874897 [94]. |
I |
CLDN18.2 CAR-T |
CLDN18.2 |
Previously treated Gastrointestinal cancer |
ORR 48.6%/*-DCR 73.0% |
| FAST trial /*-NCT01630083 [95]. |
II. |
epirubicin + oxaliplatin + capecitabine (EOC)+ Zolbetuximab Vs EOC + Placebo |
CLDN18.2 |
G/GEJ and esophageal adenocarcinomas |
DCR 76.2% |
| SPOTLIGHT trial /*-NCT03504397 [89]. |
III |
mFOLFOX6 + Zolbetuximab Vs mFOLFOX6 + placebo |
CLDN18.2 |
Locally advanced or metastatic HER-2 negative G/GEJ adenocarcinoma |
PFS 10.61 Vs 8.67 months in Rx Vs placebo group. /*-HR 0·75, p=0·0066 |
| GLOW trial /*-NCT03653507 [96]. |
III |
CAPOX + Zolbetuximab Vs CAPOX + placebo/*- |
CLDN18.2 |
Locally advanced or metastatic HER-2 negative G/GEJ adenocarcinoma |
PFS 8.21 Vs 6.80 months in Rx Vs placebo group. /*-HR 0.687, p=0·0007 |
| MONO trial/*-NCT01197885 [97]. |
IIa |
zolbetuximab as a single agent |
CLDN18.2 |
Advanced relapsed or refractory G/GEJ or esophageal adenocarcinomas |
PR= 9% |
| Lordick et al., NCT01671774 [98]. |
I |
zolbetuximab alone or in combination with ZA or with ZA plus IL-2 |
CLDN18.2 |
Relapsed or refractory G/GEJ or esophageal adenocarcinomas |
PFS 37.3 weeks with zolbetuximab aloneVs 7.1 to 12.7 weeks in other Rx arms. /*-OS 60.9 Weeks in Zolbetuximab + ZA + IL-2 arm, numerically higher than other arms. |
| ILUSTRO trial. [99] |
II |
Zolbetuximab monotherapy (in ≥ third line) Vs Zolbetuximab + mFOLFOX6 (in first line) Vs Zolbetuximab + Pembrolizumab (in ≥ third line) |
CLDN18.2 |
Advanced /metastatic G/GEJ adenocarcinoma |
ORR 71.4% in Zolbetuximab + mFOLFOX group, but )% in the other 2 cohorts. |
| McDermott et al. [100]. |
Preclinical trial |
Humanized anti-CLDN6 monoclonal antibody coupled to monomethyl auristatin E (MMAE) via a cleavable linker |
CLDN6 |
CLDN6-high expressing cell line and xenograft models |
|
| BNT211-01 trial NCT04503278 [101]. |
I/II |
(CAR) T with or without a CAR-T cell-amplifying RNA vaccine (CARVac) |
CLDN6 |
Relapsed/refractory CLDN6-positive solid tumors |
Unconfirmed ORR 33% /*-(57% inpatient with germ cell tumors) |
| Adra et al. NCT03760081 [102]. |
II |
ASP1650: a chimeric-mouse/human-IgG1 antibody |
CLDN6 |
Testicular germ cell tumors with average 3 prior lines of therapy |
The study was stopped at the end of Simon Stage-I due to lack of efficacy |
| ORR: overall response rate; DCR: disease control rate; G/GEJ: Gastric/Gastro-esophageal junction; PFS: progression free survival; FOLFOX: 5-fluorouracil + folic acid + oxaliplatin; CAPOX: capecitabine + oxaliplatin; PR: partial response; ZA: zoledronic acid; |
9. Conclusions
CLDNs are important key proteins of the tight junctions, which have an important role in epithelial cell polarity and the formation of the permeability barriers. There are 27 subtypes of CLDNs proteins, that have a differential presentation in the different tissues, to assist in their variable functions. The formation of TJs have always been examined as a barrier for cancer formation and metastasis.
In addition, mutation of some CLDNs has been causally associated with human diseases, and CLDNs have been found to be deregulated in various cancers. Depending on the type of the CLDN, they have been found to have tumor suppressor, or tumor promoter effects. They also have a role in cancer metastasis as well development of cancer chemoresistance.
Due to their restricted expression in tissues but positive expression with exposed epitopes in cancerous tissues, CLDNs have been studies as a therapeutic target for cancer treatment; with the most robust data derived from clinical trials being for targeting CLDN18.2 in gastric and gastroesophageal cancer. The monoclonal antibody Zolbetuximab, have been shown to improve the ORR in phase 3 trials when combined with chemotherapy. Other therapeutic strategies include targeting CLDN18.2 with CAR-T cellular therapy. Another CLDN investigated as a therapeutic target for cancer is CLDN6. Data available from phase I/II trial have not shown much improvement when targeting CLDN6 with monoclonal antibodies, nor with CAR-T. This is, however, a great area for future research and developmental therapeutics.
Author Contributions
Caroline Hana, contributed to original draft preparation, graph creation, review and editing. Nyein Nyein Thaw Dar, contributed to original draft preparation, review and editing. Michael Galo Venegas, contributed to original draft preparation, review and editing. Michel Vulfovich, contributed to article review, editing and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, J., Context-Dependent Roles of Claudins in Tumorigenesis. Front Oncol, 2021. 11: p. 676781.
- Schneeberger, E.E. and R.D. Lynch, The tight junction: a multifunctional complex. Am J Physiol Cell Physiol, 2004. 286(6): p. C1213-28.
- Hewitt, K.J., R. Agarwal, and P.J. Morin, The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer, 2006. 6: p. 186.
- Niessen, C.M., Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol, 2007. 127(11): p. 2525-32.
- Furuse, M., K. Fujita, T. Hiiragi, et al., Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol, 1998. 141(7): p. 1539-50.
- Günzel, D. and A.S. Yu, Claudins and the modulation of tight junction permeability. Physiol Rev, 2013. 93(2): p. 525-69.
- Van Itallie, C.M., O.R. Colegio, and J.M. Anderson, The Cytoplasmic Tails of Claudins Can Influence Tight Junction Barrier Properties through Effects on Protein Stability. The Journal of Membrane Biology, 2004. 199(1): p. 29-38.
- Furuse, M. and S. Tsukita, Claudins in occluding junctions of humans and flies. Trends in Cell Biology, 2006. 16(4): p. 181-188.
- Amasheh, S., N. Meiri, A.H. Gitter, et al., Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. Journal of cell science, 2002. 115(24): p. 4969-4976.
- Van Itallie, C.M., A.S. Fanning, and J.M. Anderson, Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. American Journal of Physiology-Renal Physiology, 2003. 285(6): p. F1078-F1084.
- Van Itallie, C., C. Rahner, and J.M. Anderson, Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. The Journal of clinical investigation, 2001. 107(10): p. 1319-1327.
- Wen, H., D.D. Watry, M.C.G. Marcondes, et al., Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Molecular and cellular biology, 2004. 24(19): p. 8408-8417.
- Yu, A.S., A.H. Enck, W.L. Lencer, et al., MEMBRANE TRANSPORT, STRUCTURE, FUNCTION, AND BIOGENESIS-Claudin-8 Expression in Madin-Darby Canine Kidney Cells Augments the Paracellular Barrier to Cation Permeation. Journal of Biological Chemistry, 2003. 278(19): p. 17350-17359.
- Alexandre, M.D., Q. Lu, and Y.-H. Chen, Overexpression of claudin-7 decreases the paracellular Cl–conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. Journal of cell science, 2005. 118(12): p. 2683-2693.
- Alexandre, M.D., B.G. Jeansonne, R.H. Renegar, et al., The first extracellular domain of claudin-7 affects paracellular Cl− permeability. Biochemical and biophysical research communications, 2007. 357(1): p. 87-91.
- Webb, P.G., M.A. Spillman, and H.K. Baumgartner, Claudins play a role in normal and tumor cell motility. BMC Cell Biology, 2013. 14(1): p. 19.
- Wang, D.W., W.H. Zhang, G. Danil, et al., The role and mechanism of claudins in cancer. Front Oncol, 2022. 12: p. 1051497.
- Tao, D., B. Guan, H. Li, et al., Expression patterns of claudins in cancer. Heliyon, 2023. 9(11): p. e21338.
- Kyuno, D., H. Yamaguchi, T. Ito, et al., Targeting tight junctions during epithelial to mesenchymal transition in human pancreatic cancer. World J Gastroenterol, 2014. 20(31): p. 10813-24.
- Greene, C., N. Hanley, and M. Campbell, Claudin-5: gatekeeper of neurological function. Fluids and Barriers of the CNS, 2019. 16(1): p. 1-15.
- Van Itallie, C.M., S. Rogan, A. Yu, et al., Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. American Journal of Physiology-Renal Physiology, 2006. 291(6): p. F1288-F1299.
- Tamura, A., Y. Yamazaki, D. Hayashi, et al., Claudin-based paracellular proton barrier in the stomach. Annals of the New York Academy of Sciences, 2012. 1258(1): p. 108-114.
- Hayashi, D., A. Tamura, H. Tanaka, et al., Deficiency of claudin-18 causes paracellular H+ leakage, up-regulation of interleukin-1β, and atrophic gastritis in mice. Gastroenterology, 2012. 142(2): p. 292-304.
- Zhou, B., A. Moodie, A.A. Blanchard, et al., Claudin 1 in Breast Cancer: New Insights. J Clin Med, 2015. 4(12): p. 1960-76.
- Usami, Y., H. Chiba, F. Nakayama, et al., Reduced expression of claudin-7 correlates with invasion and metastasis in squamous cell carcinoma of the esophagus. Hum Pathol, 2006. 37(5): p. 569-77.
- Miyamoto, K., T. Kusumi, F. Sato, et al., Decreased expression of claudin-1 is correlated with recurrence status in esophageal squamous cell carcinoma. Biomed Res, 2008. 29(2): p. 71-6.
- Honda, H., M.J. Pazin, H. Ji, et al., Crucial roles of Sp1 and epigenetic modifications in the regulation of the CLDN4 promoter in ovarian cancer cells. J Biol Chem, 2006. 281(30): p. 21433-21444.
- Honda, H., M.J. Pazin, T. D’Souza, et al., Regulation of the CLDN3 gene in ovarian cancer cells. Cancer Biol Ther, 2007. 6(11): p. 1733-42.
- Martínez-Estrada, O.M., A. Cullerés, F.X. Soriano, et al., The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. Biochem J, 2006. 394(Pt 2): p. 449-57.
- Chang, T.L., K. Ito, T.K. Ko, et al., Claudin-1 has tumor suppressive activity and is a direct target of RUNX3 in gastric epithelial cells. Gastroenterology, 2010. 138(1): p. 255-265. e3.
- Di Cello, F., L. Cope, H. Li, et al., Methylation of the claudin 1 promoter is associated with loss of expression in estrogen receptor positive breast cancer. PLoS One, 2013. 8(7): p. e68630.
- Kwon, M.J., Emerging roles of claudins in human cancer. Int J Mol Sci, 2013. 14(9): p. 18148-80.
- Hichino, A., M. Okamoto, S. Taga, et al., Down-regulation of Claudin-2 Expression and Proliferation by Epigenetic Inhibitors in Human Lung Adenocarcinoma A549 Cells. J Biol Chem, 2017. 292(6): p. 2411-2421.
- Krishnan, M., A.B. Singh, J.J. Smith, et al., HDAC inhibitors regulate claudin-1 expression in colon cancer cells through modulation of mRNA stability. Oncogene, 2010. 29(2): p. 305-12.
- Tobioka, H., H. Isomura, Y. Kokai, et al., Occludin expression decreases with the progression of human endometrial carcinoma. Hum Pathol, 2004. 35(2): p. 159-64.
- Michl, P., C. Barth, M. Buchholz, et al., Claudin-4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer Res, 2003. 63(19): p. 6265-71.
- Herschkowitz, J.I., K. Simin, V.J. Weigman, et al., Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol, 2007. 8(5): p. R76.
- Hennessy, B.T., A.M. Gonzalez-Angulo, K. Stemke-Hale, et al., Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res, 2009. 69(10): p. 4116-24.
- Prat, A., J.S. Parker, O. Karginova, et al., Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res, 2010. 12(5): p. R68.
- Dias, K., A. Dvorkin-Gheva, R.M. Hallett, et al., Claudin-Low Breast Cancer; Clinical & Pathological Characteristics. PLoS One, 2017. 12(1): p. e0168669.
- Rädler, P.D., B.L. Wehde, A.A. Triplett, et al., Highly metastatic claudin-low mammary cancers can originate from luminal epithelial cells. Nat Commun, 2021. 12(1): p. 3742.
- Dong, Y., Q. Jin, M. Sun, et al., CLDN6 inhibits breast cancer metastasis through WIP-dependent actin cytoskeleton-mediated autophagy. J Exp Clin Cancer Res, 2023. 42(1): p. 68.
- Xu, C., Y.H. Ding, K. Wang, et al., Claudin-7 deficiency promotes stemness properties in colorectal cancer through Sox9-mediated Wnt/β-catenin signalling. J Transl Med, 2021. 19(1): p. 311.
- Li, X. and W. Yang, IRF2-induced Claudin-7 suppresses cell proliferation, invasion and migration of oral squamous cell carcinoma. Exp Ther Med, 2022. 23(1): p. 7.
- Xu, Y.N., M.S. Deng, Y.F. Liu, et al., Tight junction protein CLDN17 serves as a tumor suppressor to reduce the invasion and migration of oral cancer cells by inhibiting epithelial-mesenchymal transition. Arch Oral Biol, 2022. 133: p. 105301.
- Agarwal, R., T. D’Souza, and P.J. Morin, Claudin-3 and claudin-4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase-2 activity. Cancer Res, 2005. 65(16): p. 7378-85.
- Maesaka, F., M. Kuwada, S. Horii, et al., Hypomethylation of CLDN4 Gene Promoter Is Associated with Malignant Phenotype in Urinary Bladder Cancer. Int J Mol Sci, 2022. 23(12).
- Oku, N., E. Sasabe, E. Ueta, et al., Tight junction protein claudin-1 enhances the invasive activity of oral squamous cell carcinoma cells by promoting cleavage of laminin-5 gamma2 chain via matrix metalloproteinase (MMP)-2 and membrane-type MMP-1. Cancer Res, 2006. 66(10): p. 5251-7.
- Wei, M., Y. Zhang, X. Yang, et al., Claudin-2 promotes colorectal cancer growth and metastasis by suppressing NDRG1 transcription. Clin Transl Med, 2021. 11(12): p. e667.
- Takasawa, K., A. Takasawa, M. Osanai, et al., Claudin-18 coupled with EGFR/ERK signaling contributes to the malignant potentials of bile duct cancer. Cancer Lett, 2017. 403: p. 66-73.
- Lu, Y., Q. Dang, Y. Bo, et al., The Expression of CLDN6 in Hepatocellular Carcinoma Tissue and the Effects of CLDN6 on Biological Phenotypes of Hepatocellular Carcinoma Cells. J Cancer, 2021. 12(18): p. 5454-5463.
- Wu, J.E., Y.Y. Wu, C.H. Tung, et al., DNA methylation maintains the CLDN1-EPHB6-SLUG axis to enhance chemotherapeutic efficacy and inhibit lung cancer progression. Theranostics, 2020. 10(19): p. 8903-8923.
- Facchetti, F., S. Lonardi, F. Gentili, et al., Claudin 4 identifies a wide spectrum of epithelial neoplasms and represents a very useful marker for carcinoma versus mesothelioma diagnosis in pleural and peritoneal biopsies and effusions. Virchows Arch, 2007. 451(3): p. 669-80.
- Hruaii, V., B. Thirunavukkarasu, V. Prabha, et al., Claudin-4 immunocytochemistry is specific and sensitive for the diagnosis of malignant carcinomatous effusions: Results from a pilot study. Diagn Cytopathol, 2024. 52(1): p. 30-41.
- Watanabe, M., T. Higashi, K. Ozeki, et al., CLDN15 is a novel diagnostic marker for malignant pleural mesothelioma. Scientific Reports, 2021. 11(1): p. 12554.
- Yamada, G., M. Murata, A. Takasawa, et al., Increased expressions of claudin 4 and 7 in atypical adenomatous hyperplasia and adenocarcinoma of the lung. Med Mol Morphol, 2016. 49(3): p. 163-9.
- Soini, Y., A. Takasawa, M. Eskelinen, et al., Expression of claudins 7 and 18 in pancreatic ductal adenocarcinoma: association with features of differentiation. J Clin Pathol, 2012. 65(5): p. 431-6.
- Keira, Y., A. Takasawa, M. Murata, et al., An immunohistochemical marker panel including claudin-18, maspin, and p53 improves diagnostic accuracy of bile duct neoplasms in surgical and presurgical biopsy specimens. Virchows Arch, 2015. 466(3): p. 265-77.
- Holczbauer, Á., B. Gyöngyösi, G. Lotz, et al., Distinct claudin expression profiles of hepatocellular carcinoma and metastatic colorectal and pancreatic carcinomas. J Histochem Cytochem, 2013. 61(4): p. 294-305.
- Tsukita, S., H. Tanaka, and A. Tamura, The Claudins: From Tight Junctions to Biological Systems. Trends Biochem Sci, 2019. 44(2): p. 141-152.
- Jiang, L., Y.-D. Yang, L. Fu, et al., CLDN3 inhibits cancer aggressiveness via Wnt-EMT signaling and is a potential prognostic biomarker for hepatocellular carcinoma. Oncotarget, 2014. 5(17): p. 7663.
- Che, J., D. Yue, B. Zhang, et al., Claudin-3 inhibits lung squamous cell carcinoma cell epithelial-mesenchymal transition and invasion via suppression of the Wnt/β-catenin signaling pathway. International journal of medical sciences, 2018. 15(4): p. 339.
- Yu, S., Y. Zhang, Q. Li, et al., CLDN6 promotes tumor progression through the YAP1-snail1 axis in gastric cancer. Cell death & disease, 2019. 10(12): p. 949.
- Ren, Y., Q. Wu, Y. Liu, et al., Gene silencing of claudin-6 enhances cell proliferation and migration accompanied with increased MMP-2 activity via p38 MAPK signaling pathway in human breast epithelium cell line HBL-100. Mol Med Rep, 2013. 8(5): p. 1505-10.
- Dhawan, P., A.B. Singh, N.G. Deane, et al., Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest, 2005. 115(7): p. 1765-76.
- Stebbing, J., A. Filipović, and G. Giamas, Claudin-1 as a promoter of EMT in hepatocellular carcinoma. Oncogene, 2013. 32(41): p. 4871-2.
- French, A.D., J.L. Fiori, T.C. Camilli, et al., PKC and PKA phosphorylation affect the subcellular localization of claudin-1 in melanoma cells. Int J Med Sci, 2009. 6(2): p. 93-101.
- Zwanziger, D., J. Badziong, S. Ting, et al., The impact of CLAUDIN-1 on follicular thyroid carcinoma aggressiveness. Endocr Relat Cancer, 2015. 22(5): p. 819-30.
- Ben-David, U., N. Nudel, and N. Benvenisty, Immunologic and chemical targeting of the tight-junction protein Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nature Communications, 2013. 4(1): p. 1992.
- Yang, M., Y. Li, Y. Ruan, et al., CLDN6 enhances chemoresistance to ADM via AF-6/ERKs pathway in TNBC cell line MDAMB231. Mol Cell Biochem, 2018. 443(1-2): p. 169-180.
- Reinhard, K., B. Rengstl, P. Oehm, et al., An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science, 2020. 367(6476): p. 446-453.
- Stadler, C.R., H. Bähr-Mahmud, L.M. Plum, et al., Characterization of the first-in-class T-cell-engaging bispecific single-chain antibody for targeted immunotherapy of solid tumors expressing the oncofetal protein claudin 6. Oncoimmunology, 2016. 5(3): p. e1091555.
- Ma, L., W. Yin, H. Ma, et al., Targeting claudin-3 suppresses stem cell-like phenotype in nonsquamous non-small-cell lung carcinoma. Lung Cancer Manag, 2019. 8(1): p. Lmt04.
- Primeaux, M., X. Liu, S. Gowrikumar, et al., Claudin-1 interacts with EPHA2 to promote cancer stemness and chemoresistance in colorectal cancer. Cancer Lett, 2023. 579: p. 216479.
- Gowrikumar, S., M. Primeaux, K. Pravoverov, et al., A Claudin-Based Molecular Signature Identifies High-Risk, Chemoresistant Colorectal Cancer Patients. Cells, 2021. 10(9).
- Zhao, Z., J. Li, Y. Jiang, et al., CLDN1 Increases Drug Resistance of Non-Small Cell Lung Cancer by Activating Autophagy via Up-Regulation of ULK1 Phosphorylation. Med Sci Monit, 2017. 23: p. 2906-2916.
- Akizuki, R., R. Maruhashi, H. Eguchi, et al., Decrease in paracellular permeability and chemosensitivity to doxorubicin by claudin-1 in spheroid culture models of human lung adenocarcinoma A549 cells. Biochim Biophys Acta Mol Cell Res, 2018. 1865(5): p. 769-780.
- Zhou, B., P. Flodby, J. Luo, et al., Claudin-18-mediated YAP activity regulates lung stem and progenitor cell homeostasis and tumorigenesis. J Clin Invest, 2018. 128(3): p. 970-984.
- Paquet-Fifield, S., S.L. Koh, L. Cheng, et al., Tight Junction Protein Claudin-2 Promotes Self-Renewal of Human Colorectal Cancer Stem-like Cells. Cancer Res, 2018. 78(11): p. 2925-2938.
- Shang, X., X. Lin, G. Manorek, et al., Claudin-3 and claudin-4 regulate sensitivity to cisplatin by controlling expression of the copper and cisplatin influx transporter CTR1. Mol Pharmacol, 2013. 83(1): p. 85-94.
- Hoggard, J., J. Fan, Z. Lu, et al., Claudin-7 increases chemosensitivity to cisplatin through the upregulation of caspase pathway in human NCI-H522 lung cancer cells. Cancer Sci, 2013. 104(5): p. 611-8.
- Team, T.M.R., [125I]Anti-claudin 4 monoclonal antibody. 2007.
- Amaro, A., A.I. Esposito, A. Gallina, et al., Validation of proposed prostate cancer biomarkers with gene expression data: a long road to travel. Cancer Metastasis Rev, 2014. 33(2-3): p. 657-71.
- Li, J., Targeting claudins in cancer: diagnosis, prognosis and therapy. Am J Cancer Res, 2021. 11(7): p. 3406-3424.
- Suzuki, M., M. Kato-Nakano, S. Kawamoto, et al., Therapeutic antitumor efficacy of monoclonal antibody against Claudin-4 for pancreatic and ovarian cancers. Cancer Sci, 2009. 100(9): p. 1623-30.
- Singh, P., S. Toom, and Y. Huang, Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. Journal of Hematology & Oncology, 2017. 10(1): p. 105.
- Schuler, M.H., Z. Zvirbule, F. Lordick, et al., Safety, tolerability, and efficacy of the first-in-class antibody IMAB362 targeting claudin 18.2 in patients with metastatic gastroesophageal adenocarcinomas. 2013, American Society of Clinical Oncology.
- Sahin, U., S.-E. Al-Batran, W. Hozaeel, et al., IMAB362 plus zoledronic acid (ZA) and interleukin-2 (IL-2) in patients (pts) with advanced gastroesophageal cancer (GEC): Clinical activity and safety data from the PILOT phase I trial. 2015, American Society of Clinical Oncology.
- Shitara, K., F. Lordick, Y.J. Bang, et al., Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet, 2023. 401(10389): p. 1655-1668.
- Fujiwara-Tani, R., S. Mori, R. Ogata, et al., Claudin-4: A New Molecular Target for Epithelial Cancer Therapy. Int J Mol Sci, 2023. 24(6).
- Zhen, D.B., R. Thota, C. del Corral, et al., A phase 1, open-label, dose escalation and expansion, multicenter study of claudin 18.2-targeted chimeric antigen receptor T-cells in patients with unresectable, locally advanced, or metastatic gastric, gastroesophageal junction, esophageal, or pancreatic adenocarcinoma. Journal of Clinical Oncology, 2023. 41(4_suppl): p. TPS480-TPS480.
- Baron, J.A., E.L. Barry, L.A. Mott, et al., A Trial of Calcium and Vitamin D for the Prevention of Colorectal Adenomas. N Engl J Med, 2015. 373(16): p. 1519-30.
- Mandle, H.B., F.A. Jahan, R.M. Bostick, et al., Effects of supplemental calcium and vitamin D on tight-junction proteins and mucin-12 expression in the normal rectal mucosa of colorectal adenoma patients. Mol Carcinog, 2019. 58(7): p. 1279-1290.
- Qi, C., J. Gong, J. Li, et al., Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat Med, 2022. 28(6): p. 1189-1198.
- Sahin, U., Ö. Türeci, G. Manikhas, et al., FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol, 2021. 32(5): p. 609-619.
- Shah, M.A., K. Shitara, J.A. Ajani, et al., Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med, 2023. 29(8): p. 2133-2141.
- Türeci, O., U. Sahin, H. Schulze-Bergkamen, et al., A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Ann Oncol, 2019. 30(9): p. 1487-1495.
- Lordick, F., P. Thuss-Patience, M. Bitzer, et al., Immunological effects and activity of multiple doses of zolbetuximab in combination with zoledronic acid and interleukin-2 in a phase 1 study in patients with advanced gastric and gastroesophageal junction cancer. J Cancer Res Clin Oncol, 2023. 149(9): p. 5937-5950.
- Klempner, S.J., K.W. Lee, K. Shitara, et al., ILUSTRO: Phase II Multicohort Trial of Zolbetuximab in Patients with Advanced or Metastatic Claudin 18.2-Positive Gastric or Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res, 2023. 29(19): p. 3882-3891.
- McDermott, M.S.J., N.A. O’Brien, B. Hoffstrom, et al., Preclinical Efficacy of the Antibody-Drug Conjugate CLDN6-23-ADC for the Treatment of CLDN6-Positive Solid Tumors. Clin Cancer Res, 2023. 29(11): p. 2131-2143.
- Mackensen, A., J. Haanen, C. Koenecke, et al., CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumors: the phase 1 BNT211-01 trial. Nat Med, 2023. 29(11): p. 2844-2853.
- Adra, N., D.J. Vaughn, L.H. Einhorn, et al., A phase II study assessing the safety and efficacy of ASP1650 in male patients with relapsed refractory germ cell tumors. Invest New Drugs, 2022. 40(5): p. 1087-1094.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).