1. Introduction
People who inject drugs (PWID) are responsible for half of the 1,7 million new cases of hepatitis C virus (HCV) transmissions occurring worldwide every year [
1]. While this population represents a relevant reservoir of HCV infection and related morbidity [
2], it is also susceptible to stigma, socioeconomic problems, and poor access to healthcare. Altogether, these constraints and the high risk of reinfection after HCV cure, make HCV elimination a difficult goal to achieve in this population even in the era of effective and user-friendly direct acting antivirals (DAA) against HCV [
3].
The Cascade of Care (CoC) has emerged as an efficient system to evaluate programs for the diagnosis, linkage to care, and treatment of HCV [
4]. By dividing HCV treatment into a series of well-defined steps necessary for achieving a sustained virologic response (SVR), the care cascade allows for consistent monitoring of global progress toward public health goals and facilitates the identification of critical areas of care delivery systems that may need improvement.
Population-based estimates of the care cascade for HCV among PWIDs show very low levels of treatment engagement with only 8%–17% beginning DAA therapy and 7% completing their course [
5,
6,
7].
To overcome this problem, international guidelines recommend that HCV treatment in drug users should be integrated with treatment for drug addiction [
8]. Implementing HCV treatment in institutions outside of the traditional confines of the healthcare system, lowering barriers to healthcare access, and reducing stigma may facilitate successful progression through the cascade of care [
9]. These objectives can be reached through two major approaches to drug addiction treatment, harm reduction and recovery-oriented programs.
Harm reduction does not directly address addiction, but aims to reduce health, social, and legal consequences of substance use disorders (SUD), including infections such as HCV, drug overdoses, crime, prostitution, and social marginalization [
10]. The tools of this Public Health approach are opioid agonist treatment (OAT), needle exchange programs (NEP), supervised injection sites, low threshold services, mobile outreach programs, etc. In the harm reduction community-based setting, the rate of successful HCV cure (SVR) in active drug users results to be improved (42% among those eligible for treatment and 89.6% among those completing DAA treatment) [
11]. However, the main limitation of this approach is that the problem of addiction is not addressed as a priority, and there is still a risk of HCV reinfection [
12].
On the other hand, recovery-oriented treatments pursue the aim of recovery, defined as “a voluntarily maintained lifestyle characterized by abstinence, personal health, and citizenship” [
13]. Among recovery models there are different approaches: outpatient organizations such as “Narcotics Anonymous”, short-term residential treatment (“Rehab”), and long-term residential treatment (Therapeutic Communities, TCs). In this study, we will discuss HCV micro-elimination in a therapeutic community treatment setting. The major drawbacks of therapeutic communities, especially those which do not treat with opioid agonists, is the low rate of retention in treatment (9% to 56%) [
14], due to the length of treatment, the intensity of the program, and the need for long-lasting motivation to fully engage with the program. Long-term recovery from addiction is directly related to the length of stay in residential treatment [
15,
16], however, despite a long research tradition in TCs [
17,
18], the evidence base for the effectiveness of TCs is limited according to the prevailing Cochrane hierarchy of scientific evidence [
19].
To our knowledge, no published data exist regarding HCV therapy in people with non-pharmacological SUD treatment, living in therapeutic communities. There are studies on the micro-elimination of HCV in prisons [
20,
21,
22], but this context is different from that of a TC for several reasons, particularly the risk of dropout.
Our study describes the cascade of care of HCV treatment in the Therapeutic Community of San Patrignano, the largest TC in Europe [
23].
The main objective was to verify the feasibility, effectiveness, and any strengths and weaknesses in the different phases of the cascade of care. A second objective was to assess the risk of reinfection in a population in which the treatment of hepatitis C is supplemented with SUD treatment aimed at recovery.
2. Materials and Methods
2.1. Study Setting
The study was conducted in the community of ‘‘San Patrignano’’ (SPTC) a private residential community for the rehabilitation of people affected by SUDs, located in Northern Italy [
24]. The property, which extends for 1,000 acres, consists of several residential buildings and extensive pastoral and agricultural lands. San Patrignano has welcomed in the past 42 years over 26,000 people, offering them a home, health and legal assistance, the opportunity to study, learn a job and ultimately change their life [
23].
The treatment, which lasts about 30 months, is free of charge. Opioid agonist treatment (OAT) is used only in the detoxification phase upon entry into San Patrignano.
Individuals can have access to SPTC through: (a) Public Health Services (SerD), (b) from a network of non-profit associations all over Italy and other European countries, or (c) through the justice system frequently as alternative sentencing.
2.2. Study population
Among roughly 2400 subjects already residing at SPTC at the start of the study (January 1, 2018) or admitted thereafter (until March 31, 2022), we considered in this study cohort only PWIDs currently in treatment for addiction who were 18 years or older.
Baseline information was recorded for each individual on admission, including demographic data, date of admission to SPTC and duration of stay, substance use history (age at first use of injectable substances and duration of addiction, substances abused, history of OAT), history of incarceration and history of HCV-testing and treatment.
Serologic testing for HCV and HIV is routinely offered to residents, and clinical and laboratory data are routinely collected by treating physicians. A Medical Center, including outpatient facilities and a 50-bed ward, provides care and treatment to all residents [
25,
26,
27]. This study was approved by the ethics committee of the local health authority (Area Vasta Romagna, CEROM ref n. 3373/2019).
2.3. Assessment of HCV infection
HCV testing was performed using standard laboratory procedures at Rimini Hospital. Briefly, blood samples were collected from each participant at the time of enrolment (at baseline). All PWIDs found to be seropositive for HCV were tested for the presence of HCV-RNA. Viraemic subjects with viremia were eligible for treatment with DAA. Anti-HCV seropositive persons but HCV-RNA negative, were classified as having spontaneously cleared the infection or been cured with previous HCV-treatment, depending on availability of treatment history. Individuals with chronic HCV-infection were assessed for liver fibrosis using a FibroScan procedure and staged according to the METAVIR fibrosis scoring system, and classified as absence of fibrosis (F0), mild fibrosis (F1 – portal fibrosis), significant fibrosis (F2 – periportal fibrosis), severe fibrosis (F3 – bridging fibrosis) or presence of cirrhosis (F4) [
28].
Data on anti-HCV antibodies, ribonucleic acid testing, treatment and its outcome were used to populate the HCV care cascade.
For the Cascade of Care (CoC), we assessed transition through the HCV care cascade by extending consecutive cascade milestones previously used [
29]. In the current study, the stages of the HCV care cascade were defined as: 1. Testing for HCV antibodies; 2. Reactivity to the HCV antibody test; 3. Access to HCV RNA testing; 4. HCV-RNA confirmation; 5. DAA treatment initiation; 6. Treatment completion and post-treatment assessment for HCV cure. All the stages of HCV care were carried out inside the SPTC Medical Center.
DAA treatment was administered according to the standard Italian guidelines (8 or 12 weeks of treatment with DAA). The effect of HCV treatment was assessed at the end of treatment and virological response (HCV-RNA negative) at this time point was defined as End of Treatment Response (EoTR). HCV cure was defined as a sustained virological response (SVR) - being negative for HCV RNA at 12 weeks after the end of treatment.
PWIDs leaving SPTC at the end of their treatment for addiction were considered as discharged, while those who voluntarily quit before completing their addiction treatment period were considered as dropouts.
The follow-up for HCV reinfection was done (a) in SPTC Medical Center, for those still in treatment or followed as outpatients; (b) contacting the person directly for those discharged from SPTC; and (c) through the above-mentioned network of non-profit Associations. Follow-up was completed on December 31, 2022.
2.4. Statistical analysis
Statistical analysis involved descriptive statistics, by reporting frequencies and percentages for qualitative variables while median and interquartile range (IQR) for describing quantitative variables.
A comparison of study participants’ characteristics was performed using Chi-square for trend, Chi-square test or Fisher exact tests for categorical variables and Mann-Whitney test for numeric variables as appropriate.
Milestones of HCV CoC achievement, as defined above, were reported as counts and relative percentages.
CoC from study enrolment to DAA response was reported using a flow chart, which included reasons for not treatment or treatment interruption, or incomplete assessments.
Moreover, a Poisson regression analysis was performed to identify factors associated with DAA treatment initiation in chronically HCV-infected individuals not already treated with DAA, present at SPTC on January 1, 2018 or entered thereafter. This analysis included computing Incidence Rate Ratios (IRRs) and corresponding 95% confidence intervals (CI), both in univariable and multivariable regression (then calculating adjusted IRR, aIRR) [
30]. Person-days (PDs) at risk for DAA treatment initiation were calculated from January 1, 2018 or from the date of entry at SPTC (for those entered after January 1, 2018) to date of DAA initiation, last follow-up visit, date of death, or the end date of the study (March 31, 2022), whichever came first.
All statistical analyses were performed using SPSS Statistical Software ver. 28.0 (IBM SPSS Statistics, IBM Corp., Armonk, NY, USA) or STATA ver. 17 (StataCorp LLC, College Station, TX, USA) and a p-value <0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
A total of 811 PWIDs admitted at SPTC during the study period were included and evaluated. There were 625 males (77.1%) with a median age of 32 years at entry (IQR: 26-39) who were mostly Italians (92.6%) (
Table 1). Median age at initiation of intravenous drug use was 20 years (IQR: 18-24) with a median period of drug use of 7 years (IQR: 3-16). The vast majority of PWIDs reported to be addicted to heroin (97.2%) with or without cocaine or other substances. In addition, 84.1% PWID had been on OAT treatment (maintenance or tapering) before SPTC entry.
Almost a quarter of PWIDs had a history of incarceration (26.6%) and 55 (6.8%) tested positive for HIV. Regarding history of previous HCV-testing, 642 (79.2%) individuals reported prior HCV-testing before SPTC-entry, of which 377 (58.7%) reported a previous HCV-positive test result and subsequent treatment with Interferon or DAA.
3.2. HCV-positivity assessment and HCV Cascade of Care
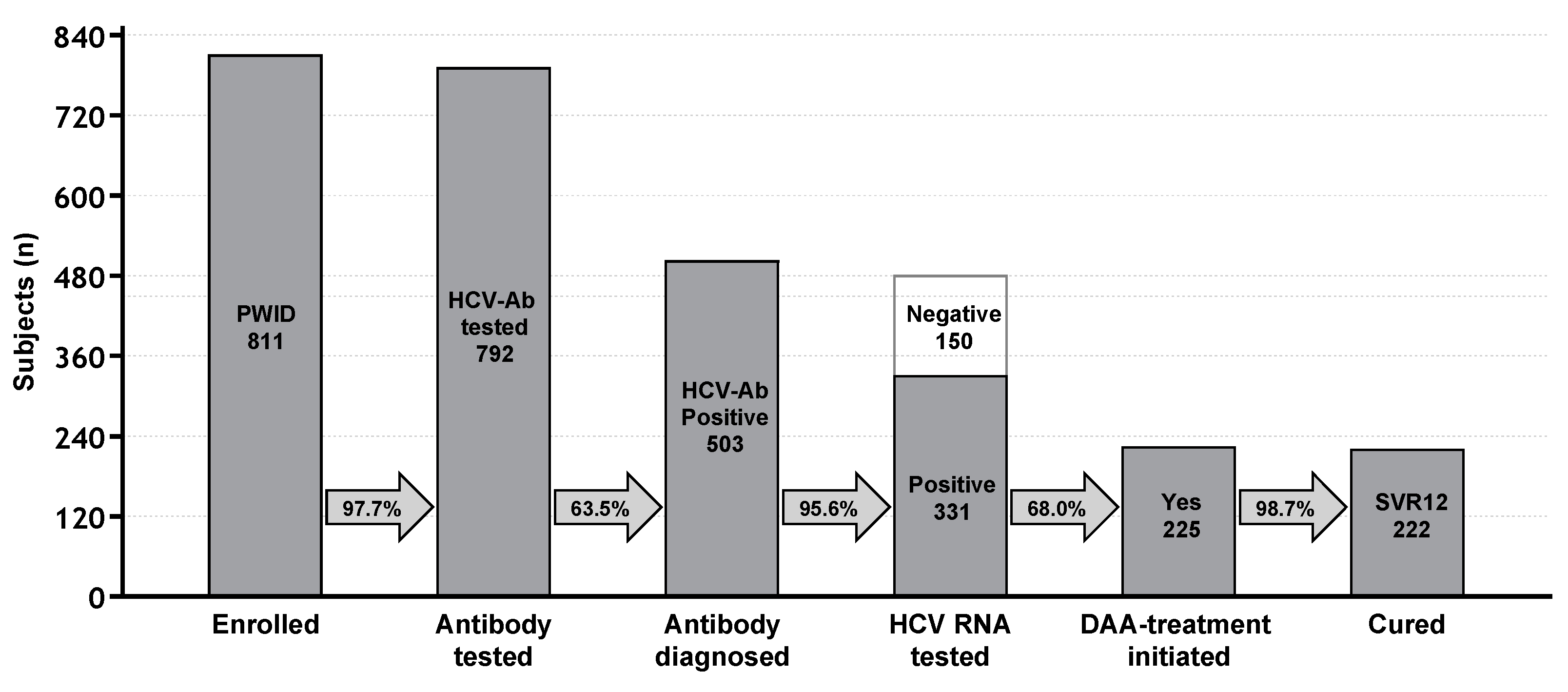
A vast majority of participants (792, 97.7%) were screened for HCV infection at entry, while only a small fraction of PWIDs (n=19) were not tested due to early dropping-out from SPTC.
A total of 503 PWIDs (63.5%) were found to be anti-HCV seropositive of which 377 were aware of their infection status (74.9%). Out of 503 PWIDs found HCV-positive, 35 had a history of a most recent HCV-negative test and 91 were never tested before for HCV; this latter group corresponded to a seroprevalence of 58.3% among those never tested for HCV before entry (n=156).
Table 2 reports all characteristics of PWID tested for HCV-Ab categorized according to HCV-positivity.
HCV-seropositivity was associated with older age compared to HCV-free individuals (median age 35 years vs. 29, p<0.001). HCV-seropositivity was also associated with a longer period of drug addiction (11 vs 4 years, p<0.001, data not shown on table) and a younger age at initiation of intravenous drug use (20 vs 21 years, p<0.001), compared to HCV-free individuals. HCV-seropositive PWIDs were more likely to be co-infected with HIV (p<0.001) and have a history of incarceration (p<0.001). No association with gender or nationality was observed.
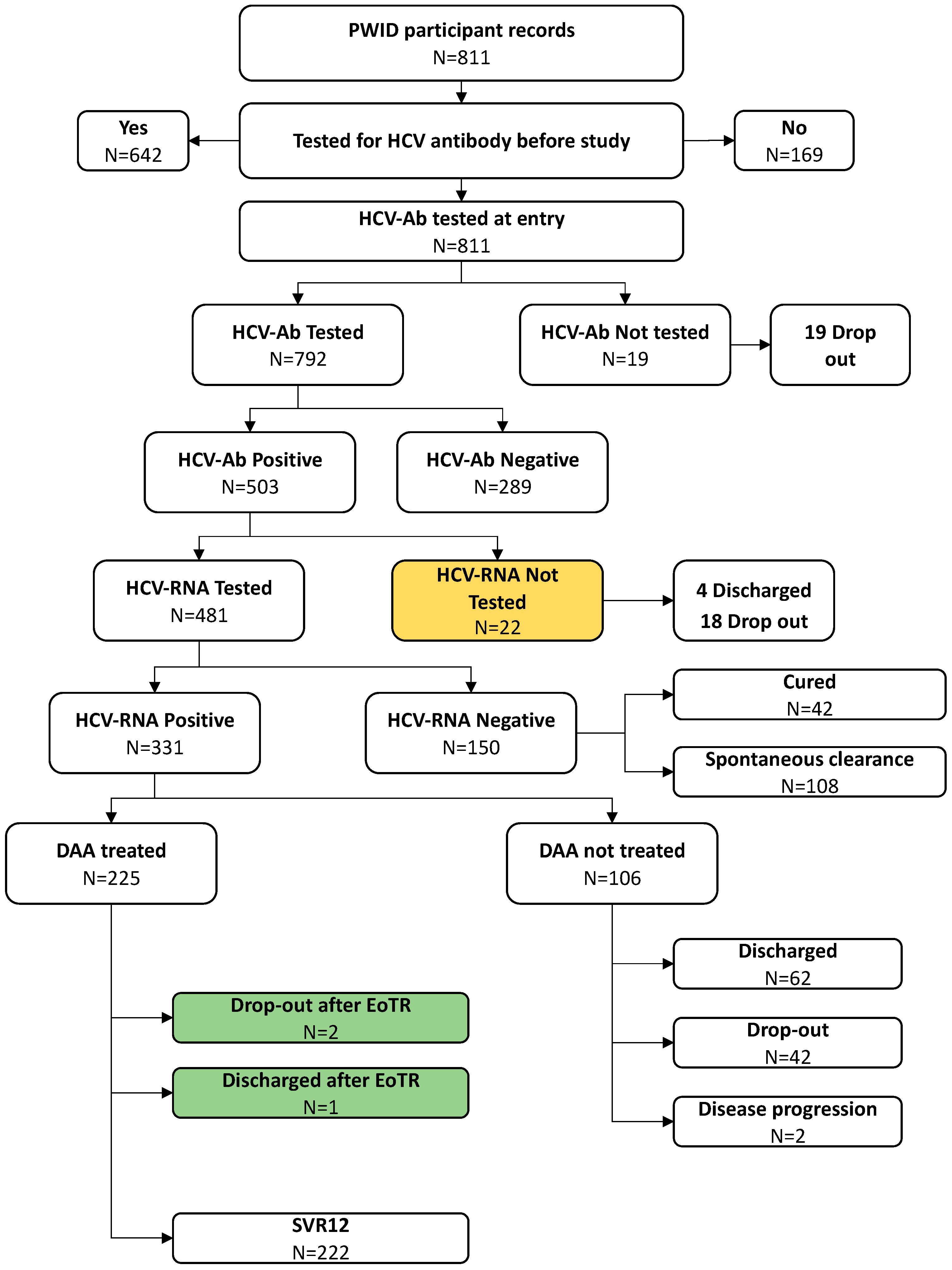
Among those found to be seropositive for HCV, HCV-RNA testing was completed among 481/503 individuals (95.6%); twenty-two subjects were not tested for HCV-RNA because of being discharged early (n=4) or dropped-out (n=18) (
Figure 1).
The proportion of those found to be HCV-RNA positive was 68.8% (331/481) and all of them were considered and proposed for DAA treatment.
Overall, during the study period, DAA was prescribed to 225 (68.0%) chronically infected subjects, while 106 (32.0%) HCV-RNA positive PWIDs did not receive DAA therapy due to either drop-out (n=42 - 39.6%) or being discharged from SPTC before the start of DAA treatment (n=62 - 58.5%); moreover, two enlisted subjects were not treated with DAA due to worsening health conditions, not related to HCV infection, that precluded them from DAA initiation.
Figure 1 schematically reports the path of all PWIDs included in the study to calculate the final CoC.
The results of the analysis conducted to identify factors associated with DAA initiation are showed in
Table 3.
In the univariable analysis, a diagnosis of severe fibrosis or cirrhosis (Metavir score F3-F4), HIV positivity, being Italian and having a late SPTC admission were associated with a faster DAA initiation, while no association was found according to age, gender, years of drug addiction or history of incarceration (data not shown).
In the final model, factors significantly associated with DAA initiation were liver disease severity by Fibroscan (aIRR=2.43 for those with F3-F4 and aIRR=1.58 for those with F2 vs. those with F0-F1, p<0.001), being HIV-positive (aIRR=2.39, p<0.001) and a late period of last entry to the SPTC (aIRR=4.25 for those last admitted in 2020 or later and aIRR=1.48 for those admitted within 2018-2019 vs. those admitted before 2018, p<0.001); moreover, to be noted that non-Italians were least likely to be treated (aIRR=0.60, p<0.080) while on the contrary HIV-positive persons were more likely to be treated (aIRR=2.39).
In terms of result of treatment, among 225 PWIDs who started treatment with DAA, three subjects (1.3%) completed DAA treatment and reached EoTR but left SPTC before SVR assessment (two drop-out and one subject discharged before assessment), whereas 222 (98.7%) PWID were completely assessed and considered cured (SVR12).
Figure 2 summarizes the achievement of milestone of CoC as above defined.
3.3. Outcome and HCV reinfection
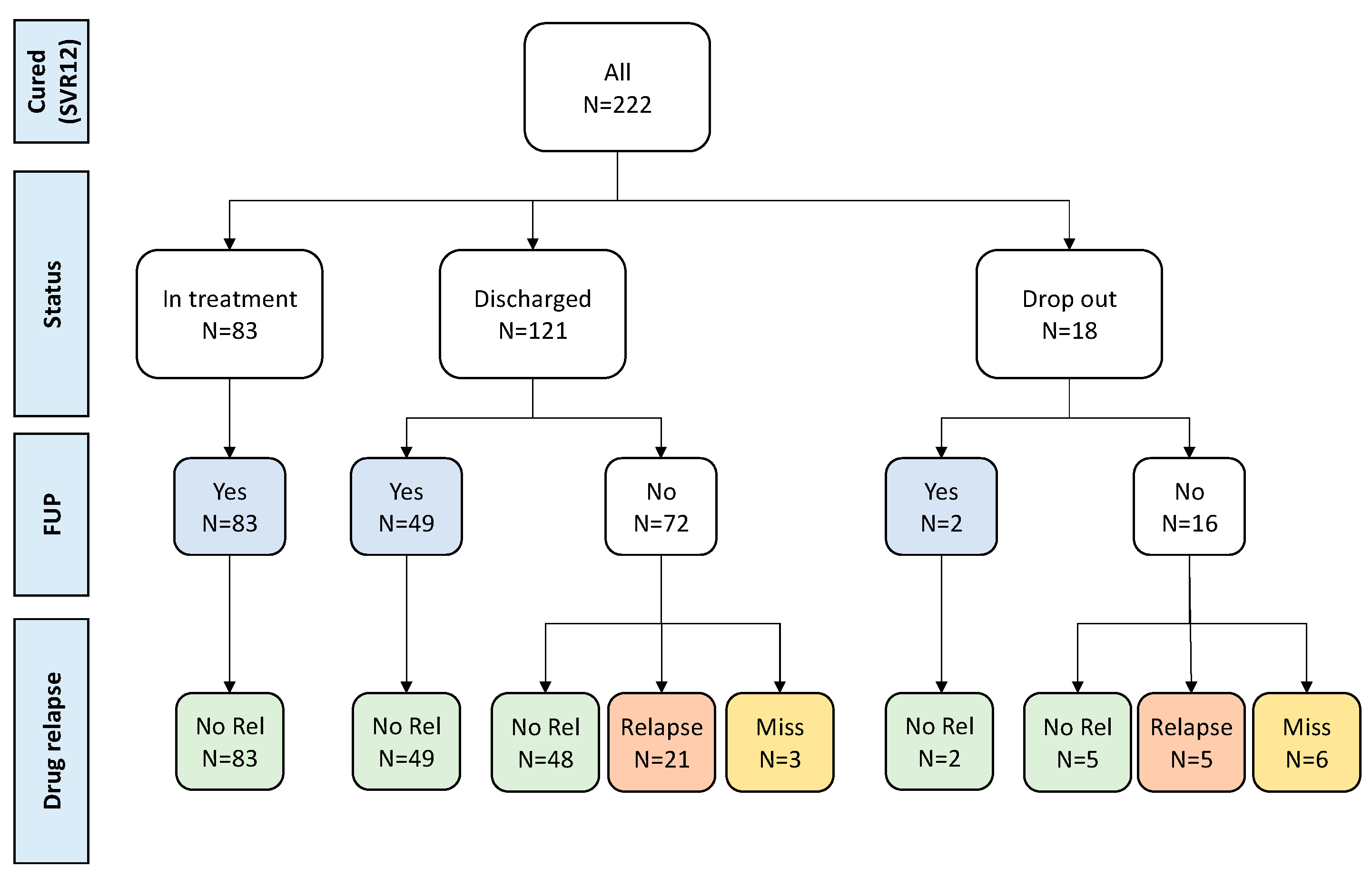
Follow-up data were actively collected in all 222 patients successfully treated with a median of 2.73 years (IQR: 2.42-3.52 years) post SVR ascertainment. 134 (60.4%) had follow-up testing for HCV-RNA (83 still at SPTC) and no cases of HCV-RNA infection were observed (
Figure 3).
No virological follow up data was available for the remaining 88 PWID (72 regularly discharged and 16 who dropped out). Of these 88 PWIDs, 53 subjects who were contacted at follow-up remained abstinent from drugs, and we can assume they were unlikely to be HCV reinfected. Twenty-six PWIDs relapsed on drugs (21 regularly discharged and 5 dropped out), while for the remaining 9 (three discharged and 6 dropped out), data on eventual relapse on drugs was missing.
Overall, among 222 successfully treated PWID, 187 (84.2%) can be considered not reinfected during follow-up because they were found to be HCV-RNA negative at follow-up re-testing (134/187=71.7%), or because they remained abstinent from drugs (53/187, 28.3%). Potentially, HCV-reinfection could have occurred in up to 35 subjects who relapsed on drugs or for which information on HCV-RNA testing or drug relapse is missing.
4. Discussion
Despite the availability of effective and user friendly direct-acting antiviral drugs (DAAs), the eradication of HCV in PWID population is a difficult goal to achieve due to various factors, including stigma, limited access to care, and the risk of reinfection after effective treatment.
It is a shared opinion that the treatment of HCV infection should be combined with the treatment of SUD, and that one of the most effective strategies is to design micro-elimination interventions. These interventions differ depending on the varying contexts in which they operate, but are united with the goal of bringing health care "on the spot" by concentrating all the phases that lead to an improved access to care (“cascade of care”) in a single area (“point of care”), rendering it easier to meet patients [
31,
32]. This avoids the need for patients to access other healthcare facilities, which may sometimes be a place of stigma, deprivation of privacy, or bureaucratic delays.
This study describes an experience of the micro-elimination of hepatitis C in a population that, as far as we know, has never been demonstrated in people recovering from drug addiction in a residential TC (San Patrignano, Italy).
TCs are one of the therapeutic responses to SUD; the first of these types of communities were born in the US during the 1960s and then further developed throughout the world, differentiating themselves significantly according to their cultural contexts. These TCs aim at Recovery, defined as “a voluntarily maintained lifestyle characterized by abstinence, personal health and citizenship” that can be reached with or without (as in San Patrignano TC) the use of agonist medications. [
13].
TCs are almost always born from private initiatives and are more often connected to spiritual movements than to scientific research centers. Historically, there has not been a culture of data collection and publication within these TCs. Therefore, their treatment models were not considered “evidence based”.
There is little literature on their effectiveness in terms of retention in treatment (which highlights rather negative data), and there is almost nothing in relation to the outcome (recovery) of individuals who complete treatment.
However, people with SUD in residential treatment are still numerous (13,671 in Italy, 10.1% compared to those in treatment in Public Health Services [
33] and constitute a particular population, with characteristics that can favour the treatment of many comorbidities (including HCV infection) and promote a strong motivation to change and prioritization of health in general.
The major potential drawback of TCs, also for the HCV micro-elimination perspective, is the high risk of drop out due to drug craving or lack of motivation (“early drop out”), in addition to the emergence of deeper psychological or psychiatric problems of the person if not properly understood and treated (“late drop out”).
The experience described, relating to a TC scan that hosts an average of 1,000 people with SUD (decreased to 800 in the two years of the Covid-19 pandemic), highlights how a residential, drug-free care context is also optimal for the micro-elimination of HCV, an objective achieved in the present study.
Overall, 68% of eligible people started DAA treatment, 222 completed it, and the SVR rate was obtained as 100% in people for whom it could be evaluated; the remaining three subjects who were not evaluated at the standard 12 weeks after the end of treatment were assessed as being negative at the end of the treatment protocol (EoTR). Given the almost complete efficacy of cure in our population, all 225 subjects who completed the DAA treatment can be considered cured.
In analyzing reasons why not all patients with HCV infection were able to receive treatment, two main causes can be identified: (a) treatment dropouts and/or regular discharges, and (b) bureaucratic-administrative problems.
Bureaucratic-administrative problems had, in our opinion, a much more consistent impact. Initially, antivirals could be prescribed only to patients with more advanced liver fibrosis (F3-F4). Nonetheless, after overcoming these limitations and extending the treatment to all chronically HCV-infected subjects irrespective of liver-disease prioritization, the waiting times for the availability of drugs were still very long. Recently, the waiting times have shortened and a rapid cascade of care has been possible, significantly decreasing the number of untreated patients: 100 in the first two years, and 6 in the recent years (2020-2022). Still, the bureaucracy needed to start DAA-treatment after subject enlistment requires 3-4 months.
This study was not designed to assess the outcome of the therapeutic program for addiction, but through the network of non-profit, private association and/or direct contacts between discharged PWID with their educators in SPTC, we have reliable information about what happens in their daily life, including eventual drug use relapses.
Assuming that reinfection risk is practically absent in those individuals who achieve both HCV eradication and drug addiction recovery, we can claim that 187 PWID (84.2%) did not experienced HCV reinfection because they remained abstinent from drugs; for 134 (71.7%) of them we also have the virological confirmation of persistent HCV elimination.
We cannot exclude that HCV reinfection could have occurred in those 26 PWIDs who relapsed on drug use, as well as in the remaining 9 PWIDs lost to follow up after leaving the therapeutic program.
In conclusion, residential TCs stand as an optimal point of care for the treatment of HCV infection in PWIDs. The risk of losing patients for HCV treatment could be greatly reduced by speeding up bureaucratic processes, whereas integrating HCV and addiction treatment stands as the best prevention of reinfection with HCV.
Author Contributions
conceptualized and designed the study supervised the study and contributed to data interpretation, P.P., A.B. and E.G.; contribute to funding acquisition, A.B.; wrote the first draft of the manuscript and referenced the appropriate literature, P.P. and A.B.; data curation, formal analysis and also contributed to the article drafting, A.N., C.C. and G.S.; participated to the investigation and clinical management of patients and reviewed the manuscript, A.B., R.G. and C.S.; collaborate to the investigation and data curation, R.P. and S.D. All authors have read and agreed to the published version of the manuscript.
Funding
The study was financed by an unrestricted grant from GILEAD Sciences and by Line 3 Ricerca Corrente INMI Spallanzani. This study was funded by a research grant from Gilead sciences (LEGA-C grant IN-IT-987-5396) to AB. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation or approval of the manuscript; and decision to submit the manuscript for publication.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the Area Vasta Romagna, CEROM ref n. 3373/2019 (Italy).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The dataset will be made available upon reasonable request.
Conflicts of Interest
Antonio Boschini has received institutional grants from Gilead Science. None of the other authors have any conflicts of interest to report.
References
- Hill, A.M.; Nath, S.; Simmons, B. The road to elimination of hepatitis C: analysis of cures versus new infections in 91 countries. J. Virus. Erad. 2017, 3, 117–123. [Google Scholar] [CrossRef]
- Trickey, A.; Fraser, H.; Lim, A.G.; Peacock, A.; College, S.; Walker, J.G.; et al. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: a modelling study. Lancet Gastroenterol. Hepatol. 2019, 4, 435–444. [Google Scholar] [CrossRef]
- Bourliere, M.; Pietri, O. Hepatitis C virus therapy: No one will be left behind. International Journal of Antimicrobial Agents 2019, 53, 755–760. [Google Scholar] [CrossRef]
- Safreed-Harmon, K.; Blach, S.; Aleman, S.; Bollerup, S.; Cooke, G.; Dalgard, O.; et al. The Consensus Hepatitis C Cascade of Care: standardized reporting to monitor progress toward elimination. Clin. Infect. Dis. 2019, 69, 2218–2227. [Google Scholar] [CrossRef]
- Morris, M.D.; Mirzazadeh, A.; Evans, J.L.; Briceno, A.; Coffin, P.; Hahn, J.A.; et al. Treatment cascade for hepatitis C virus in young adult people who inject drugs in San Francisco: low number treated. Drug Alcohol Depend. 2019, 198, 133–135. [Google Scholar] [CrossRef]
- Socías, ME.; Ti, L.; Wood, E.; Nosova, E.; Hull, M.; Hayashi, K.; et al. Disparities in uptake of direct-acting antiviral therapy for hepatitis C among people who inject drugs in a Canadian setting. Liver Int. 2019, 39, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Tsui, J.I.; Miller, C.M.; Scott, J.D.; Corcorran, M.A.; Dombrowski, J.C.; Glick, S.N. Hepatitis C continuum of care and utilization of healthcare and harm reduction services among persons who inject drugs in Seattle. Drug Alcohol Depend. 2019, 195, 114–120. [Google Scholar] [CrossRef]
- EASL recommendations on treatment of hepatitis C: final update of the series. J. Hepatol. 2020, 73, 1170–1218. [CrossRef]
- Socías, M.E.; Karamouzian, M.; Parent, S.; Barletta, J.; Bird, K.; Ti, L. Integrated models of care for people who inject drugs and live with hepatitis C virus: a systematic review. Int. J. Drug Policy 2019, 72, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Platt, L.; Minozzi, S.; Reed, J.; Vickerman, P.; Hagan, H.; French, C.; et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction 2018, 113, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Winetsky, D.; Burack, D.; Antoniou, P.; Garcia, B.; Gordon, P.; Scherer, M. Psychosocial Factors and the Care Cascade for Hepatitis C Treatment Colocated at a Syringe Service Program. J. Infect. Dis. 2020, 222 (Suppl 5), S392–S400. [Google Scholar] [CrossRef]
- Lens, S.; Miralpeix, A.; Gálvez, M.; Martró, E.; González, N.; Rodríguez-Tajes, S.; et al. HCV microelimination in harm reduction centres has benefits beyond HCV cure but is hampered by high reinfection rates. JHEP Rep. 2022, 4, 100580. [Google Scholar] [CrossRef]
- Betty Ford Institute Consensus Panel. What is recovery? A working definition from the Betty Ford Institute. J. Subst. Abuse Treat. 2007, 33, 221–228. [Google Scholar] [CrossRef]
- Malivert, M.; Fatséas, M.; Denis, C.; Langlois, E.; Auriacombe, M. Effectiveness of therapeutic communities a systematic review. Eur. Addict. Res. 2012, 18, 1–11. [Google Scholar] [CrossRef]
- Hubbard, R.L.; Craddock, S.G.; Anderson, J. Overview of 5-year follow up outcomes in the drug abuse treatment outcome studies (DATOS). Journal of Substance Abuse Treatment 2003, 25, 125–134. [Google Scholar] [CrossRef]
- De Leon, G. The Therapeutic Community: Theory, Model and Method; Publisher: Springer Publishing Company, 2000. [Google Scholar]
- Broekaert, E.; Vandevelde, S.; Soyez, V.; Yates, R.; Slater, A. The third generation of therapeutic communities: the early development of the TC for addictions in Europe. Eur.Addict. Res. 2006, 12, 1–11. [Google Scholar] [CrossRef]
- De Leon, G. Is the therapeutic community an evidence-based treatment? What the evidence says. International Journal of Therapeutic Communities 2010, 31, 104–128. [Google Scholar]
- Smith, L.A.; Gates, S.; Foxcroft, D. Therapeutic communities for substance related disorder. Cochrane Database Syst. Rev. 2006, (1), CD005338. [Google Scholar] [CrossRef] [PubMed]
- Fiore, V.; De Matteis, G.; Ranieri, R.; Saderi, L.; Pontali, E.; Muredda, A.; et al. HCV testing and treatment initiation in an Italian prison setting: A step-by-step model to micro-eliminate hepatitis C. Int. J. Drug. Policy 2021, 90, 103055. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Zarębska-Michaluk, D.; Ciupkeviciene, E.; Drazilova, S.; Frankova, S.; Grgurevic, I.; et al. HCV Elimination in Central Europe with Particular Emphasis on Microelimination in Prisons. Viruses 2022, 14, 482. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, L.; Sheehan, Y.; Cochrane, A.; Grebely, J.; Lloyd, AR.; Treloar, C. Reducing barriers to the hepatitis C care cascade in prison via point-of-care RNA testing: a qualitative exploration of men in prison using an integrated framework. Addiction 2023, 118, 1153–1160. [Google Scholar] [CrossRef]
- San Patrignano Therapeutic Community. Available online: www.sanpatrignano.org (accessed on 30 November 2023).
- Devlin, A.M.; Wight, D. Mechanisms and context in the San Patrignano drug recovery community, Italy: a qualitative study to inform transfer to Scotland. Drugs: Education, Prevention and Policy (Abingdon Engl) 2021, 28, 85–96. [Google Scholar] [CrossRef]
- Boschini, A.; Smacchia, C.; Di Fine, M.; Schiesari, A.; Ballarini, P.; Arlotti, M.; et al. Community-acquired pneumonia in a cohort of former injection drug users with and without human immunodeficiency virus infection: incidence, etiologies, and clinical aspects. Clin. Infect. Dis. 1996, 23, 107–113. [Google Scholar] [CrossRef]
- Vergori, A.; Boschini, A.; Notari, S.; Lorenzini, P.; Castilletti, C.; Colavita, F.; Matusali, G.; Tartaglia, E.; Gagliardini, R.; Boschi, A.; Cimini, E.; Maeurer, M.; Piselli, P.; Angeli, L.; Antinori, A.; Agrati, C.; Girardi, E. SARS-CoV-2 Specific Immune Response and Inflammatory Profile in Advanced HIV-Infected Persons during a COVID-19 Outbreak. Viruses 2022, 14, 1575. [Google Scholar] [CrossRef]
- Sala, I.; Jarach, C.M.; Bagnardi, V.; Cattaruzza, M.S.; Morri, M.; Ottogalli, P.; et al. SARS-CoV-2 Infection in San Patrignano, the Largest European Drug Rehabilitation Community. Int. J. Environ. Res. Public Health 2023, 20, 2136. [Google Scholar] [CrossRef]
- Bedossa, P.; Poynard, T. The METAVIR Cooperative Study Group. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996, 24, 289–293. [Google Scholar] [CrossRef]
- Yehia, B.R.; Herati, R.S.; Fleishman, J.A.; Gallant, J.E.; Agwu, A.L.; Berry, S.A.; et al. Hepatitis C virus testing in adults living with HIV: a need for improved screening efforts. PLoS One 2014, 9, e102766. [Google Scholar] [CrossRef]
- Breslow, N.E.; Day, N.E. Statistical methods in cancer research. The design and analysis of cohort studies, vol. 2. Lyon: IARC Press; 1987, IARC Scientific Publications No. 82.
- Schwarz, T.; Horváth, I.; Fenz, L.; Schmutterer, I.; Rosian-Schikuta, I.; Mårdh, O. Interventions to increase linkage to care and adherence to treatment for hepatitis C among people who inject drugs: A systematic review and practical considerations from an expert panel consultation. Int. J. Drug Policy 2022, 102, 103588. [Google Scholar] [CrossRef]
- Howell, J.; Traeger, M.W.; Williams, B.; Layton, C.; Doyle, J.S.; Latham, N.; et al. The impact of point-of-care hepatitis C testing in needle and syringe exchange programs on linkage to care and treatment uptake among people who inject drugs: An Australian pilot study. J. Viral Hepat. 2022, 29, 375–384. [Google Scholar] [CrossRef]
- Annual report to Parliament on the phenomenon of drug addiction in Italy year 2022 (2021 data). Department of Drug Policies. Available online: https://www.iss.it/en/-/relazione-annuale-al-parlamento-sul-fenomeno-delle-tossicodipendenze-in-italia-anno-2022-dati-2021- (accessed on 30 November 2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).