Submitted:
18 January 2024
Posted:
19 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. RESULTS
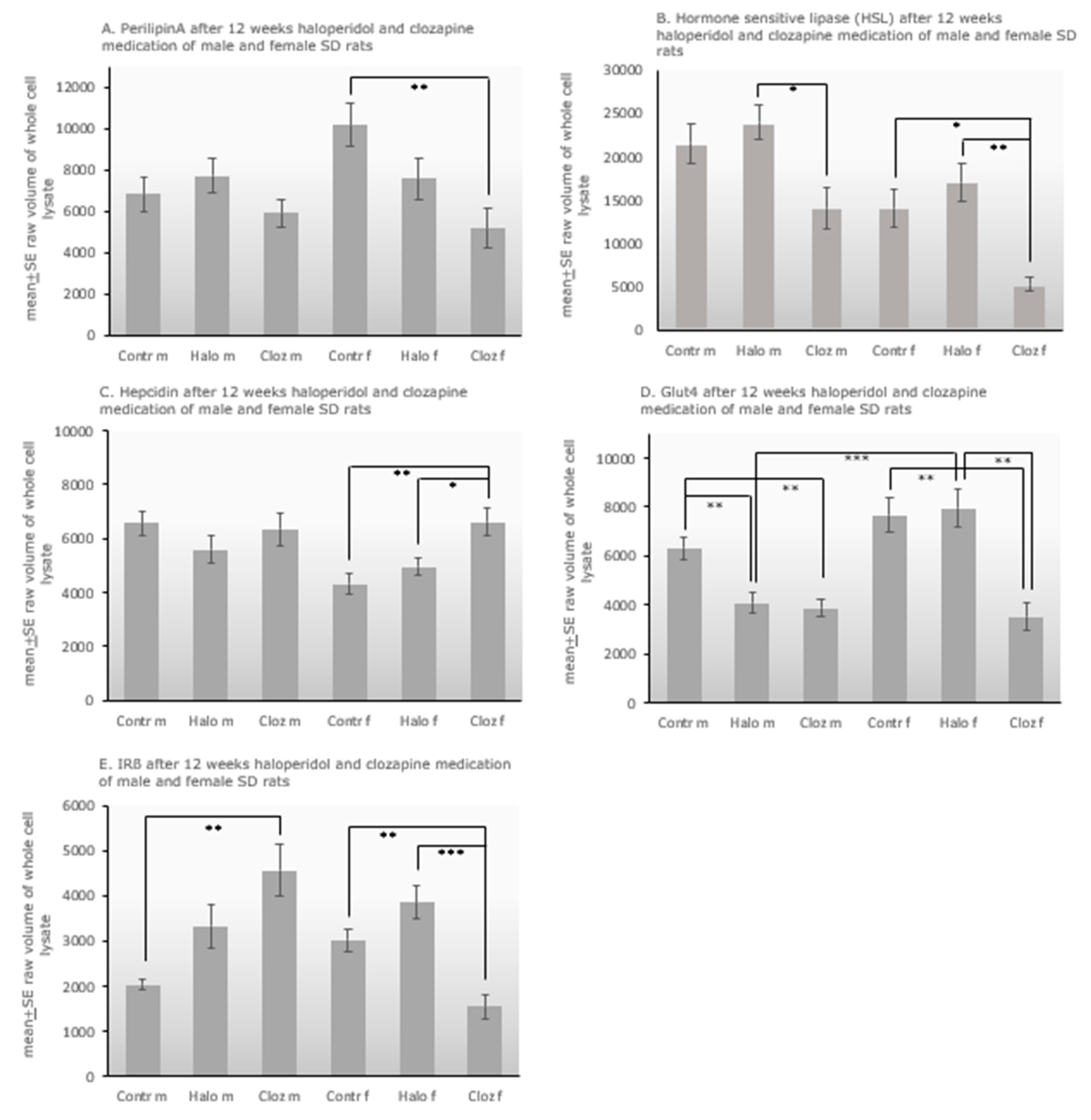
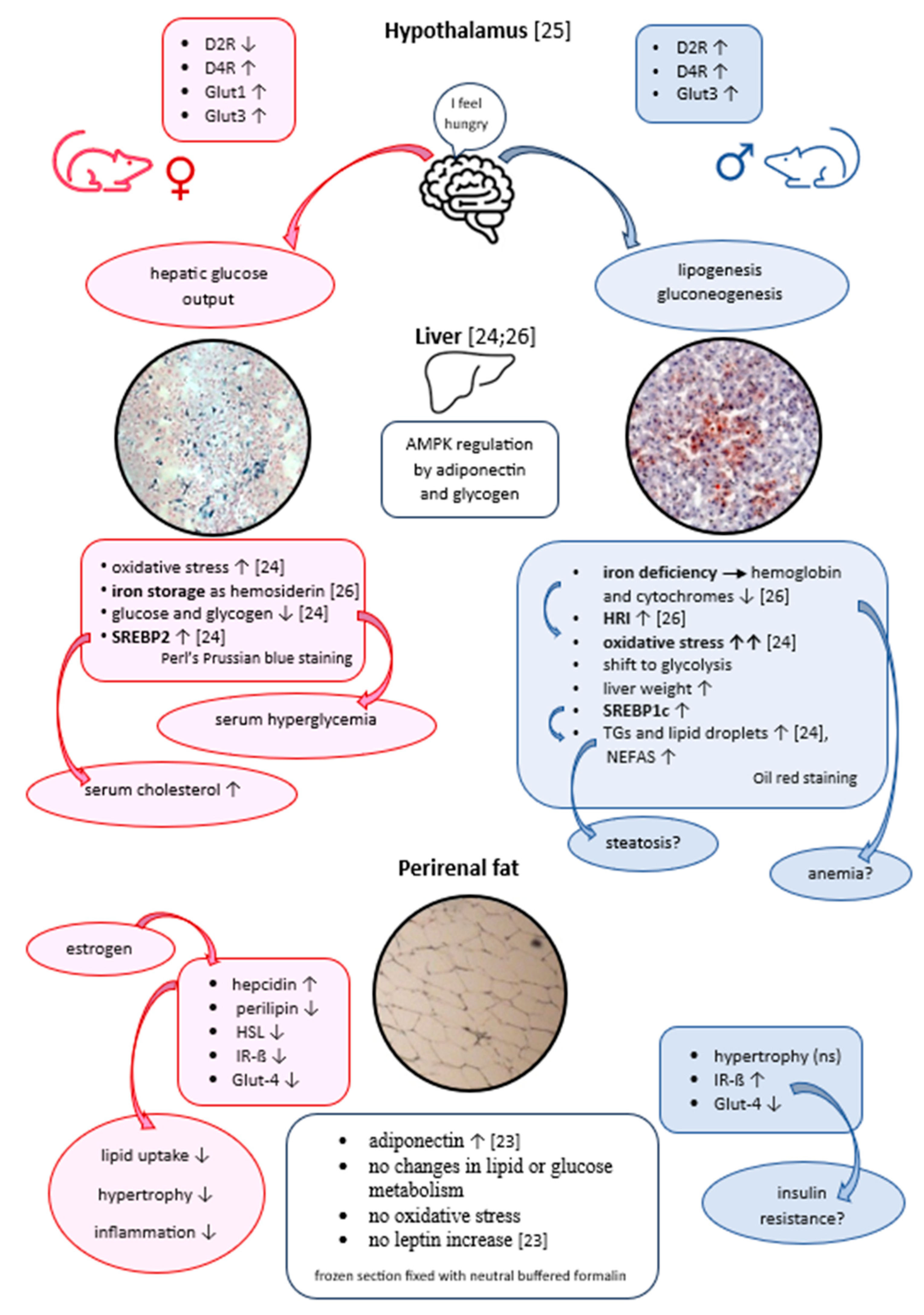
2.1. Effect of haloperidol and clozapine on percentage body weight gain after 12-week medication of clozapine and haloperidol on male and female Sprague Dawley rats.
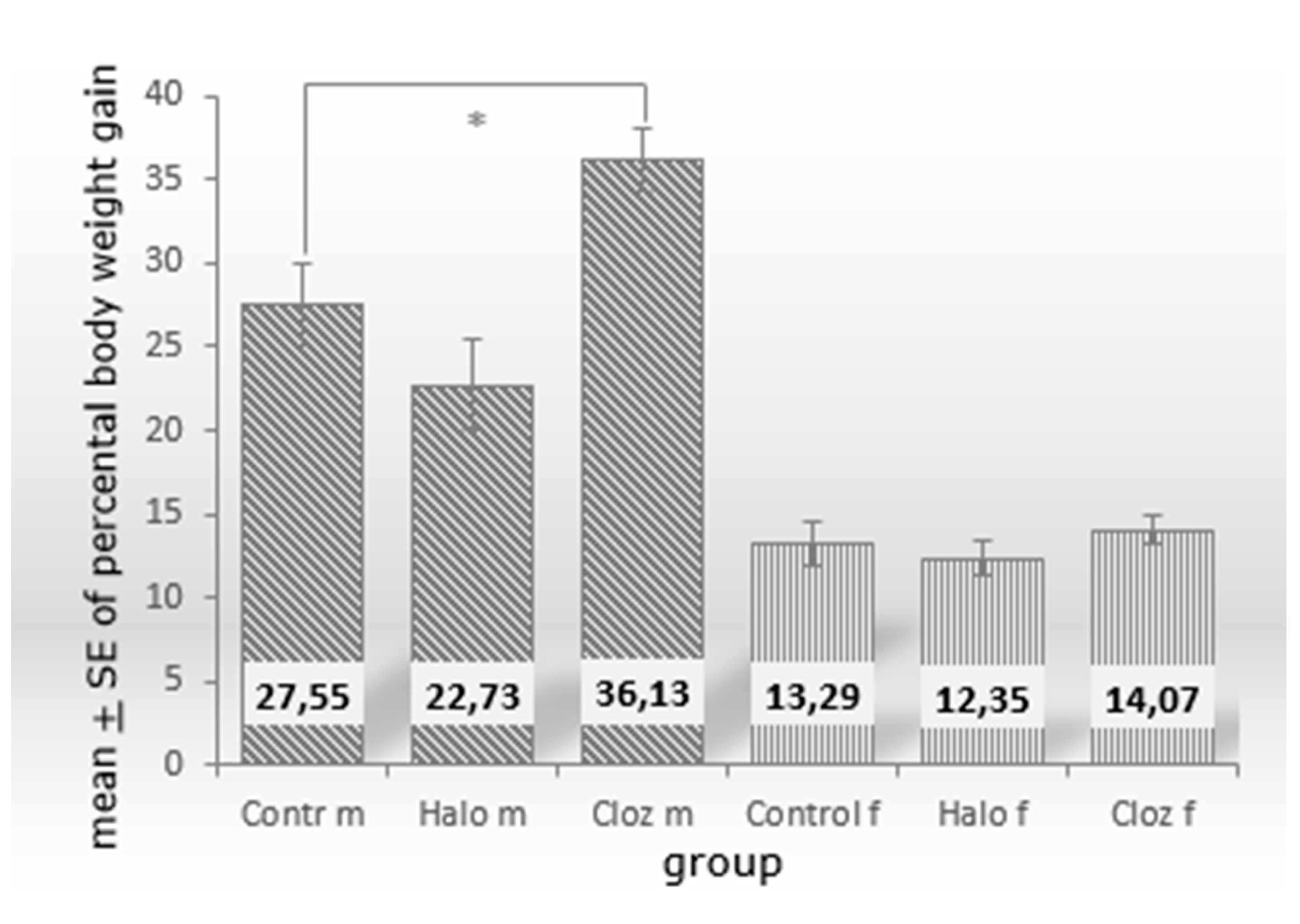
2.2. Effect of haloperidol and clozapine on adipocyte mass, neutral fat content, adipocyte area value, and ferric iron content of perirenal VAT
 |
2.3. Effect of haloperidol and clozapine on triglycerides, and NEFAs in rat perirenal adipose tissue as well as serum cholesterol/HDL ratio and LDL/HDL ratio, and hepatic Chol/HDL ratio
2.4. Effect of haloperidol and clozapine on glucose, glycogen, and lactate contents in rat perirenal VAT
2.5. Effect of haloperidol and clozapine on oxidative stress of rat perirenal VAT and on plasma estrogen level in female rats
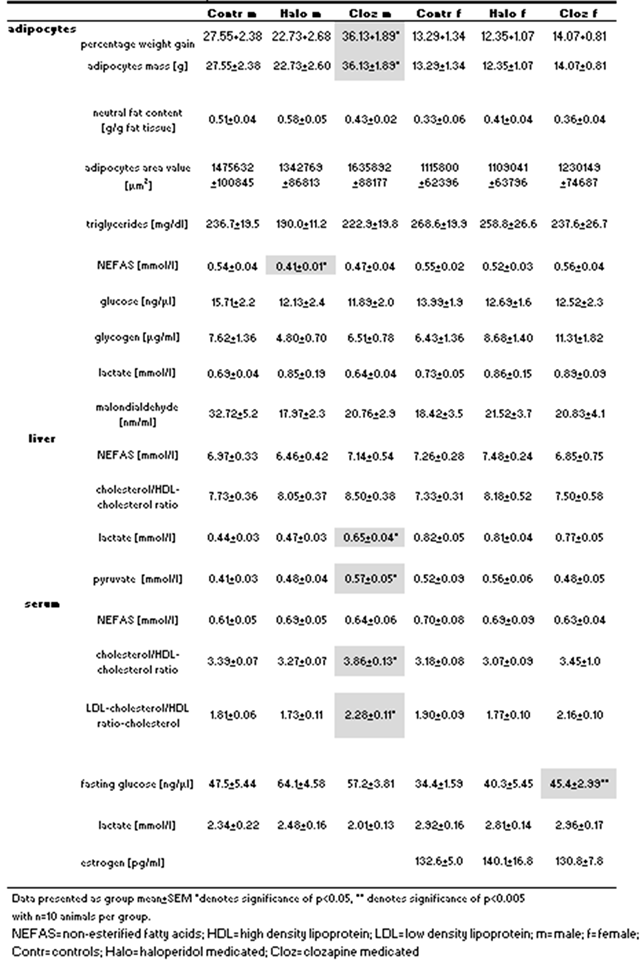
2.6. Protein expression of Perilipin-A, HSL, hepcidin, Glut-4, IR-ß in perirenal adipocytes of haloperidol- and clozapine-medicated rats.
3. DISCUSSION
4. Limitations
5. CONCLUSION
6. Methods
6.1. Ethics statement
6.2. Experimental design
6.3. Determination of percentage weight, neutral fat content and adipocyte area value
6.4. Determination of ferric iron by Perl’s Prussian blue staining of frozen fat tissue sections
6.5. Determination of triglycerides and non-esterified fatty acids (NEFAs) in adipocytes, cholesterol/HDL-cholesterol ratio in liver and serum and LDL-cholesterol/HDL-cholesterol ratio in serum
6.6. Determination of glucose, glycogen, and lactate in lysates of fat tissue
6.7. Determination of oxidative stress in perirenal fat of all rats and of estrogen levels in serum
6.8. Western blot analysis of adipocyte lysates of the drug-medicated rats and controls
6.9. Statistical analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dayabandera, M.; Hanwella, R.; Ratnatunga, S.; Seneviratne, S.; Suraweera, C.; de Silva, V.A. Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatr Dis Treat. 2017, 13,2231-2241. PMID: 28883731 PMCID: PMC5574691. [CrossRef]
- Alonso-Pedrero, L.; Bes-Rastrollo, M.; Marti, A. Effects of antidepressant and antipsychotic use on weight gain: a systematic review. Obesity Rev. 2019,20(12),1680-1690. PMID: 31524318. [CrossRef]
- Ferreira, V.; Grajales, D.; Valverde, A.M. Adipose tissue as a target for second generation (atypical) antipsychotics: a molecular review. BBA Mol Cell Biol Lipids.2020,1865(2),158534. PMID:31672575. [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomquist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; Concha, H.; Hassan, M.; Ryden, M.; Frisen, J.; Arner P. Dynamics of fat cell turnover in human. Nature. 2008,453(7196),783-787. PMID:18454136. [CrossRef]
- Vestri, H.S.; Maianu, L.; Moellering, D.R.; Garvey, W.T. Atypical antipsychotic drugs directly impair insulin action in adipocytes: effects on glucose transport, lipogenesis, and antilipolysis. Neuropsychopharmacol. 2006,32(4),765-772. PMID:16823387. [CrossRef]
- Wilson, J.L.; Enriori, P.J. A talk between fat tissue, gut, pancreas and brain to control body weight. Mol Cell Endocrinol. 2015,418(2),108-119. PMID:26316427. [CrossRef]
- Yang, A.; Mottillo, E.P. Adipocyte lipolysis: from molecular mechanisms of regulation to disease and therapeutics. Biochem J. 2020,477(5),985-1008. PMID:32168372 PMCID: PMC7187988. [CrossRef]
- Rotondo, F.; Ho-Palma, A.C.; Remesar, X.; Fernandez-Lopez, J.A.; del Mar Romero, M.; Alemany, M. Glycerol is synthesized and secreted by adipocytes to dispose of excess glucose, via glycerogenesis and increased acyl-glycerol turnover. Scientific Reports. 2017,7,8983. PMID:28827624 PMCID:PMC5567128. [CrossRef]
- Meyer, L.K.; Ciaraldi, T.P.; Henry, R.R.; Wittgrove, A.C.; Phillips, S.A. Adipose tissue depot and cell size dependency of adiponectin synthesis and secretion in human obesity. Adipocyte. 2013,2(4),217-226. PMID:24052897 PMCID:PMC3774697. [CrossRef]
- Chau, Y.Y.; Bandiera, R.; Serrels, A.; Martinez-Estrada, O.M.; Qing, W.; Lee, M.; Slight, J.; Thornburn, A.; Berry, R.; McHaffie, S.; Stimson, R.H.; Walker, B.R.; Chapuli, R.M.; Schedl, A.; Hastie, N. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014,16(4),367-375. PMID:24609269 PMCID: PMC4060514. [CrossRef]
- Huang, N.; Mao, E.W.; Hou, N.N.; Liu, Y.P.; Han, F.; Sun, X.D. Novel insight into perirenal adipose tissue: a neglected adipose depot linking cardiovascular and chronic kidney disease. World J Diabetes. 2020,11(4),115-125. PMID:32313610 PMCID:PMC7156295. [CrossRef]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010,11(1),11-18. PMID:19656312. [CrossRef]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M.T. Adipose tissue: an endocrine organ playing a role in metabolic regulation. Horm Mol Biol Clin Invest. 2016,26(1),25-42. PMID:26910750. [CrossRef]
- Friedman, J.M. Leptin and the regulation of body weight. Keio J Med. 2011,60(1),1-9. PMID:21460597. [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016,23(5),770-784. PMID:27166942 PMCID:PMC4864949. [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017,18,1321. PMID:28635626 PMCID:PMC5486142. [CrossRef]
- Ukkola, O.; Santaniemi, M. Adiponectin: a link between excess adiposity and associated comorbidities? J Mol Med. 2002,80,606-702. PMID:12436346. [CrossRef]
- Shabalala, S.C.; Dludla, P.V.; Mabasa, L.; Kappo, A.P.; Basson, A.K.; Pheiffer, C.; Johnson, R. The effect of adiponectin in the pathogenesis of non-alcoholic fatty liver desease (NAFDL) and the potential role of polyphenols in the modulation of adiponectin signaling. Biomed Pharmacotherapy. 2020,131,110785. PMID:33152943. [CrossRef]
- Gatselis, N.K.; Ntaios, G.; Makaritsis, K.; Dalekos, G.N. Adiponectin: a key playmaker adipocytokine in non-alcoholic fatty liver disease. Clin Exp Med. 2014,14(2),121-131. PMID:23292294. [CrossRef]
- Thompson, N.M.; Gill, D.A.S.; Loveridge, N.; Houstan, P.A.; Robinson, I.C.A.F.; Wells, T. Ghrelin and des-octanoyl ghrelinpromote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinol. 2004,145(1),234-242. PMID: 14551228. [CrossRef]
- Miao, H.; Pan, H.; Wang, L.; Yang, H.; Zhu, H.; Gong, F. Ghrelin promotes proliferation and inhibits differentiation of 3L3-L1 and human primary preadipocytes. Front Physiol. 2019,article10,1296. PMID:31681009 PMCID:PMC6798085. [CrossRef]
- Carvalho, E.; Schellhorn, S.E.; Zabolotny, J.M.; Martin, S.; Tozzo, E.; Peroni, O.D.; Houseknecht, K.L.; Mundt, A.; James, D.E.; Kahn, B.B. GLUT4 overexpression or deficiency in adipocytes of transgenic mice alters the composition of GLUT4 vesicles and the subcellular localization of GLUT4 and insulin-responsive aminopeptidase. J Biol Chem. 2004,279(20),21598-21605. PMID: 14985357. [CrossRef]
- von Wilmsdorff, M.; Bouvier, M.L.; Henning, U.; Schmitt, A.; Schneider-Axmann, T.; Gaebel, W. The sex-dependent impact of chronic clozapine and haloperidol treatment on characteristics of the metabolic syndrome in a rat model. Pharmacopsychiatry. 2013,46(1),1-9. PMID: 22915487. [CrossRef]
- von Wilmsdorff, M.; Bouvier, M.L.; Henning, U.; Schmitt, A.; Schneider-Axmann, T.; Gaebel, W. Sex-dependent metabolic alterations of rat liver after 12-week exposition to haloperidol or clozapine. Horm Metab Res. 2014,46(11),782-788. PMID: 25105542. [CrossRef]
- Bouvier, M.L.; Fehsel, K.; Schmitt, A.; Meisenzahl-Lechner, E.; Gaebel, W.; von Wilmsdorff, M. Sex-dependent alterations of dopamine receptor and glucose transporter density in rat hypothalamus under long-term clozapine and haloperidol medication. Brain Behav. 2020,e01694. PMID: 32525610. [CrossRef]
- Bouvier, M.L.; Fehsel, K.; Schmitt, A.; Meisenzahl-Lechner, E.; Gaebel, W., von Wilmsdorff, M. Sex-dependent effects of long-term clozapine or haloperidol medication on red blood cells and liver iron metabolism in Sprague Dawley rats as a model of metabolic syndrome. BMC Pharmacol and Toxicol. 2022,23,8. PMID: 35033194. [CrossRef]
- Manu, P.; Dima, L.; Shulman, M.; Vancampfort, D.; De Hert, M.; Correll, C.U. Weight gain and obesity in schizophrenia: epidemiology, pathobiology, and management. Acta Psychiatr Scand. 2015,132,97-108. PMID:26016380. [CrossRef]
- Guo, D.H.; Yamamoto, M.; Hernandez, C.M.; Khodadadi, H.; Baban, B.; Stranahan, A.M. Beige adipocytes mediate the neuroprotective and anti-inflammatory effects of subcutaneous fat in obese mice. Nature Communications. 2021,12,4623. PMID:34330904 PMCID:PMC8324783. [CrossRef]
- Engfeldt, P., Arner, P. Lipolysis in human adipocytes, effects of cell size, age and regional differences. Horm Metabol Res Suppl. 1988,19,26-29. PMID:3069692.
- Perez-Torres, I.; Gutierrez-Alvarez, Y.; Guarner-Lans, V.; Diaz-Diaz, E.; Pech, M.L.; Del Carmen Caballero-Chacon, S. Intra-abdominal fat adipocyte hypertrophy through progressive alteration of lipolysis and lipogenesis in metabolic syndrome rats. Nutrients. 2019,11,1529-1540. PMID:31284400 PMCID:PMC6683042. [CrossRef]
- Löffler, D.; Landgraf, K.; Körner, A.; Kratzsch, J.; Kirkby, K.C.; Himmerich, H. Modulation of triglyceride accumulation in adipocytes by psychopharmacological agents in vitro. J Psychiatric Res. 2016,72,37-42. PMID:26524413. [CrossRef]
- Sertié, A.L.; May Suzuki, A.; Sertié, R.A.L.; Andreotti, S.; Lima, F.B.; Passos-Bueno, M.R.; Gattaz, W.F. Effects of antipsychotics with different weight gain liabilities on human in vitro models of adipose tissue differentiation and metabolism. Prog Neuro-Psychopharm Biol Psych. 2011,35,1884-1890, PMID:21840366. [CrossRef]
- Westerink, J.; Olijhoek, K.; Koppen, A.; Faber, D.R.; Kalkhoven, E.; Monajemi, H.; van Asbeck, B.S.; van der Graaf, Y.; Visseren, F.L.J. The relation between body iron stores and adipose tissue function in patients with manifest vascular disease. Eur J Clin Invest. 2013,43(12),240-1249. PMID:24245570. [CrossRef]
- Britton, L.; Jaskowski, L.A.; Bridle, K.; Secondes, E.; Wallace, D.; Santrampurwala, N.; Reiling, J.; Miller, G.; Mangiafico, S.; Andrikopoulos, S.; Subramaniam, V.N.; Crawford, D. Ferroportin expression in adipocytes does not contribute to iron homeostasis or metabolic responses to a high calorie diet. Cell Mol Gastroenterol Hepatol. 2018,5(3),319-331. PMID: 29552621. [CrossRef]
- Zhang, Z.; Funcke, J.B.; Zi, Z.; Zhao, S.; Straub, L.G.; Zhu, Y.; Zhu, Q.; Crewe. C.; An, Y.; Chen, S.; Li, N.; Wang, M.; Ghaben, A.L.; Lee, C.; Gautron, L.; Engelking, L.; Raj. P.; Deng, Y.…Scherer. P.E. Adipocyte iron levels impinge on a fat-gut crosstalk to regulate intestinal lipid absorption and mediate protection from obesity. Cell Metabol. 2021,33(8),1624-1639.e9 PMID:34174197 PMCID:PMC8338877. [CrossRef]
- Ma, X.; Pham, V.T.; Mori, H.; MacDougald, O.A.; Shah, Y.M.; Bodary, P.F. Iron elevation and adipose tissue remodelling in the epididymal depot of a mouse model of polygenic obesity. PLOS One. 2017,12(6),e179889. PMID:28651003 PMCID:PMC5484604. [CrossRef]
- Lu, M.L.; Wang, T.N.; Lin, T.Y.; Shao, W.C.; Chang, S.H.; Chou, J.Y.; Ho, Y.F.; Liao, Y.T.; Chen, V.C. Differential effects of olanzapine and clozapine on plasma levels of adipocytokines and total ghrelin. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015,58,47-50. PMID:25496829. [CrossRef]
- Shimizu, H., Shimomura, Y., Hayashi, R. et al. Serum leptin concentration is associated with total body fat mass, but not abdominal fat distribution. Int J Obes 1997; 21: 536–541. PMID:22128212 PMCID:PMC3208009. [CrossRef]
- Meier, U.; Gressner, A.M. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004,50(9),1511-1525. PMID:15265818. [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin regulation and function. Compr Physiol. 2018,8(3),1031-1063. PMID: 29978896. [CrossRef]
- Liu, Z.; Cui, C.; Xu, P.; Dang, R.; Cai, H.; Liao, D.; Yang, M.; Feng, Q.; Yan, X.; Jiang, P. Curcumin activates AMPK pathway and regulates lipid metabolism in rats following prolonged clozapine exposure. Front Neurosci. 2017,11,article 558. PMID:29046626 PMCID:PMC5632657. [CrossRef]
- Canfran-Duque, A.; Casado, M.E.; Pastor, O.; Sanchez-Wandelmer, J.; de la Pena, G.; Lerma, M.; Mariscal, P.; Bracher, F.; Lasuncion, M.A.; Busto, R. Atypical antipsychotics alter cholesterol and fatty acid metabolism in vitro. J Lipid Res. 2013,52,310-324. PMID:23175778 PMCID:PMC3588861. [CrossRef]
- McBride, A.; Ghilagaber, S.; Nikolaev, A.; Hardie, D.G. The glycogen-binding domain on the AMPK beta sununit allows the kinase to act as a glycogen sensor. Cell Metab. 2009,9(1),23-34. PMID:19117544. [CrossRef]
- Izumida, Y.; Yahagi, N.; Takeuchi, Y.; Nishi, M.; Shikama, A.; Takarada, A.; Masuda, Y.; Kubota, M.; Matsuzaka, T.; Nakagawa, Y.; Iizuka, Y.; Itaka, K.; Kataoka, K.; Shioda, S.; Niijima, A.; Yamada, T.; Katagiri, H.; Nagai, R.; Yamada, N.; Kadowaki, T.; Shimano, H. Glycogen shortage during fasting triggers liver-brain-adipose neurocircuitry to facilitate fat utilization. Nature Commun. 2013,4,2316. PMID:23939267 PMCID:PMC3753545. [CrossRef]
- Idonije, O.B.; Festus, O.O.; Akpamu, U.; Ohhiai, O.; Iribhogbe, O.I.; Iyalomhe, G.B.S. A comparative study of the effects of clozapine and risperidone monotherapy on lipid profile in Nigerian patients with schizophrenia. Int J Pharmacol. 2012,8(3),169-176. [CrossRef]
- Kim, J.H.; Lee, J.O.; Lee, S.K.; Jung, J.; You, G.Y.; Park, S.H.; Park, M.; Kim, S.D.; Kim, H.S. Clozapine activates AMP-activated protein kinase (AMPK) in C2C12 myotube cells and stimulates glucose uptake. Life Sci. 2010,87(1-2),42-48. PMID:20515698. [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.; Gao, B.; Wierzbicki, M.; Verbeuren, T.; Shaw, R.; Cohen, R.; Zang, M. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin resistant mice. Cell Metab 2011; 13(4): 376-388. PMID:21459323 PMCID:PMC3086578. [CrossRef]
- Albaugh, V.L.; Vary, T.C.; Ilkayeva, O.; Wenner, B.R.; Maresca, K.P.; Joyal, J.L.; Breazeale, S.; Elich, T.D.; Lang, C.H.; Lynch, C.J. Atypical antipsychotics rapidly and inappropriately switch peripheral utilization to lipids, impairing metabolic flexibility in rodents. Schizophr Bull. 2012,38(1),153-166. PMID:0494946 PMCID:PMC3245588. [CrossRef]
- Rodriguez, A. Novel molecular aspects of ghrelin and leptin in the control of adipobiology and the cardiovascular system. Obes Facts. 2014,7,82-95. PMID:24685565 PMCID:PMC5644879. [CrossRef]
- Straub, B.K.; Stoeffel, P.; Heid, H.; Zimbelmann, R.; Schirmacher, P. Differential pattern of lipid droplet-associated proteins and de novo perilipin expression in hepatocyte steatogenesis. Hepatology. 2008,47,1936-1946. PMID:18393390. [CrossRef]
- Krycer, J.R.; Elkington, S.D.; Diaz-Vegas, A.; Cooke, K.C.; Burchfield, J.G.; Fisher-Wellman, K.H.; Cooney, G.J.; Fazakerley, D.J.; James, D.E. Mitochondrial oxidants, but not respiration, are sensitive to glucose in adipocytes. J Biol Chem. 2020,295(1),99-110. PMID: 31744882. [CrossRef]
- Murashita, M.; Kusumi, I.; Hosoda, H.; Kangawa, K.; Koyama, T. Acute administration of clozapine concurrently increases blood glucose and circulating plasma ghrelin levels in rats. Psychoneuroendocrinology. 2007,32,777-784. PMID:17629416. [CrossRef]
- Sasaki, N.; Iwase, M.; Uchizono, Y.; Nakamura, U.; Imoto, H.; Abe, S.; Iida, M. The atypical antipsychotic clozapine impairs insulin secretion by inhibiting glucose metabolism and distal steps in rat pancreatic islets. Diabetologia. 2006,49(12),2930-2938. PMID:17072584. [CrossRef]
- Smith, G.C.; Chaussade, C.; Vickers, M.; Jensen, J.; Shepherd, P.R. Atypical antipsychotic drugs induce derangements in glucose homeostasis by acutely increasing glucagon secretion and hepatic glucose output in the rat. Diabetologia. 2008,51(12),2309-2317. PMID:18843478. [CrossRef]
- Leguisamo, N.M.; Lehnen, A.M.; Machado, U.F.; Okamoto, M.M.; Markoski, M.M.; Pinto, G.H.; Schaan, B.D. Glut4 content decreases along with insulin resistance and high levels of inflammatory markers in rats with metabolic syndrome. Cardiovascular Diabetology. 2012,11,100-110. PMID:22897936 PMCID:PMC3439702. [CrossRef]
- Sarvari, A.K.; Vereb, Z.; Uray, I.P.; Fesüs, L.; Balajthy, Z. Atypical antipsychotics induce both proinflammatory and adipogenic gene expression in human adipocytes in vitro. Biochem Biophys Res Commun. 2014,450,1383-1389. PMID:25019983. [CrossRef]
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Eur J Physiol. 2020,472,1273-1298. PMID:32591906 PMCID:PMC7462924. [CrossRef]
- Sorge S. Der Einfluss des atypischen Neuroleptikums Clozapin auf das Verhalten und das Immunsystem der Ratte. Thesis, München 2003.
- Cooper, G.D.; Harrold, J.A.; Halford, J.C.G.; Goudie, A.J. Chronic clozapine treatment in female rats does not induce weight gain or metabolic abnormalities but enhances adiposity: implications for animal models of antipsychotic-induced weight gain. Prog Neuropsychopharmacol Biol Psychiatry. 2008,32(2),428-436. PMID:17933447. [CrossRef]
- Chusyd, D.E.; Wang, D.; Huffman, D.M.; Nagy, T.R. Relationships between rodent white adipose fat pads and human white adipose fat depots. Front Nutr. 2016,3,10 PMID:27148535 PMCID: PMC4835715. [CrossRef]
- Palmer, B.F.; Clegg, D.J. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015,402,113-119. PMID:25578600. [CrossRef]
- Romeis, B. Mikroskopische Technik (17. neubearbeitete und erweiterte Auflage, herausgegeben von P. Böck). München – Wien – Baltimore 1989. Urban und Schwarzenberg. ISBN: 3-541-11227-1.
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analyt Biochem. 1979,95,351-358. PMID:36810. [CrossRef]
- Romero-Calvo, I.; Ocon, B.; Martinez-Moya, P.; Suarez, M.D.; Zarzuelo, A.;Martinet-Augustin, O.; de Medina, F.S. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem. 2010, 401(2),318-320. PMID:20206115. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




