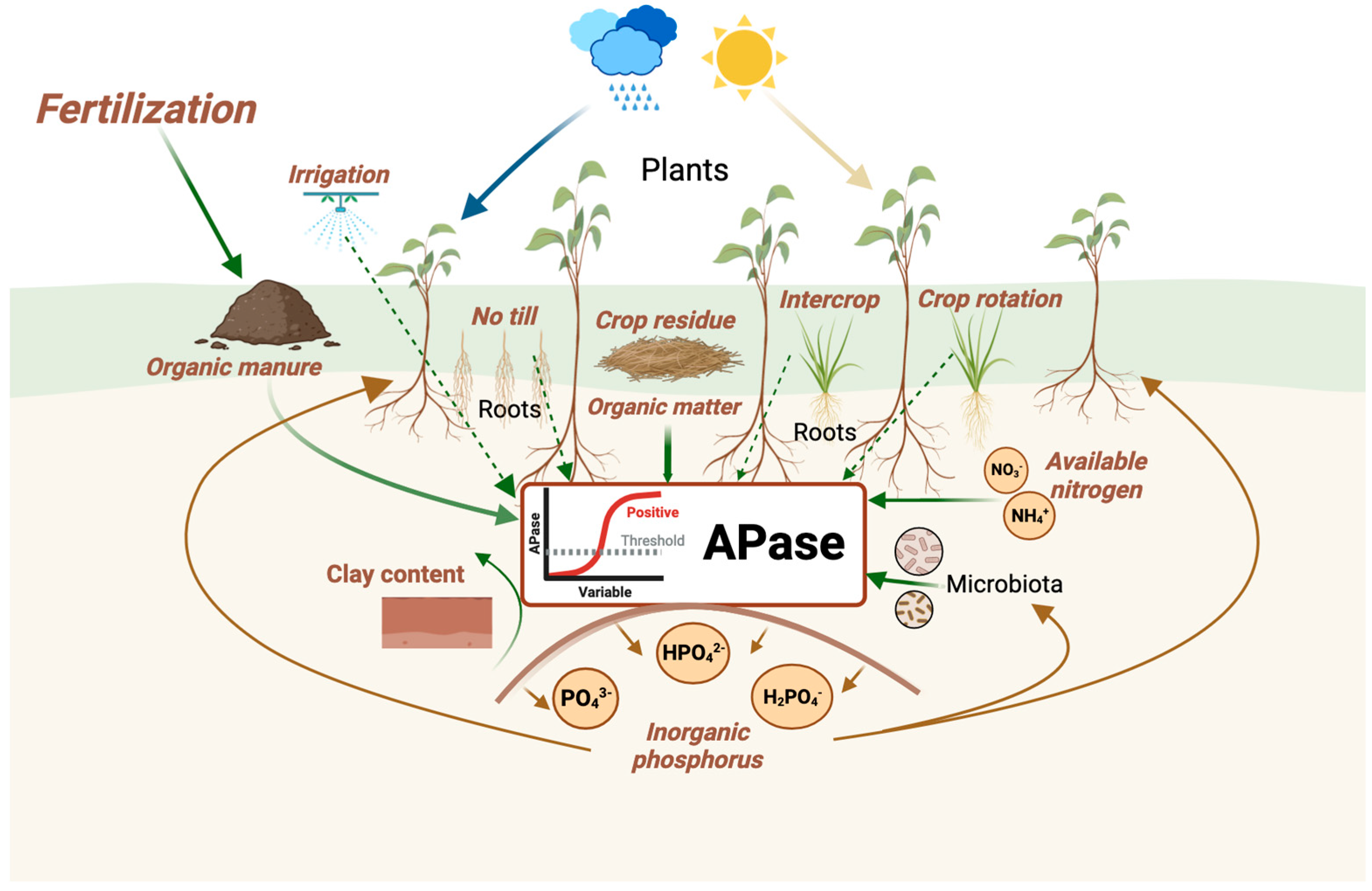
1. Introduction
Phosphorus (P) is an essential element for cell development in all living organisms [1]. As a component of nucleic acids (DNA, RNA), P is indispensable for reproduction and protein synthesis. Additionally, it plays a crucial role in energy-storing molecules like adenosine triphosphate (ATP) or cytidine triphosphate (CTP) among others, supplying the energy needed for diverse cellular endergonic processes [2]. This is why P is an important limiting nutrient for crop and plant growth in a range of natural and managed ecosystems, given that only 0.1% of the P available in soil is in the inorganic form that can be assimilated by plants [3-5]. Soil enzymes released by plant roots, soil mesofauna, and living or dead microbes [6-8] contribute to the decomposition of organic matter and allow nutrient recycling [9,10]. The mechanisms governing how the composition, timing, spatial location, and quantity of soil enzymes adapt to environmental changes have been studied elsewhere [11,12]. These studies underscore the crucial role of soil enzymes in biogeochemical cycles and ecosystem responses to drivers of global change.
In the P cycle, soil phosphatase enzymes release P contained in organic matter for reuse by living organisms [13]. This process involves the hydrolysis of various P esters (carbon-oxygen-phosphorus monoesters, carbon-oxygen-phosphorus-oxygen-carbon diesters, carbon-phosphorus phosphonates, phosphoric triester hydrolases, triphosphoric acid monoester hydrolases) into soluble phosphate ions. This process provides soil-accessible and assimilable P for plant uptake [14,15]. Extracellular phosphatase enzymes are secreted by soil microorganisms, fauna, and plant roots [16], while intracellular (endogenous) phosphatase enzymes are within the cytoplasm of proliferating microbial, animal and plant cells, restricted to the periplasmic space of gram-negative bacteria or within non-proliferating cells such as fungal spores, protozoan cysts, plant seeds and bacterial endospores [17,18]. Extracellular monoester hydrolases (APases) are included in a wide group of phosphoric monoester hydrolases (or phosphomonoesterases) [19], and its predominant forms, across a wide range of soil pH conditions, are acid phosphatase (ACP; EC 3.1.3.2) and alkaline phosphatase (ALP; EC 3.1.3.1). ACP is produced by plants in the phloem, cortex, epidermis and roots [20,21] and also by microorganisms [22], and is active in acid/neutral soils with pH ≤7. ALP is produced by microorganisms and animals and is active in basic soils with pH >7 [23-27]. The most well-studied group of ALP are encoded by different genes (i.e. phoA, phoD, phoX) [28] and the phoD gene is the form which has the highest abundance in soils [29].
Agricultural and livestock production covers approximately 5 billion hectares (38%) of the Earth's land surface, with around 66% consisting of livestock-grazed grasslands and 33% being cropland [30]. While APase activity in managed soils has been reported to be lower compared to natural ecosystems [31], its activity is in turn influenced by a combination of natural environmental conditions and anthropogenic factors, together with strong seasonal variations [32]. APase activity in agricultural soils is significantly impacted by management practices, including tillage, the crop species or crop rotation [33-35], as well as fertilization methods [36-38], in combination with various soil biophysicochemical and environmental factors [40,41]. Several quantitative studies have investigated APase response to various factors such as climatic effects [42-44], soil properties [31], fertilization [45-49], and pollution [50,51] across different ecosystems. However, a comprehensive global analysis specifically centred on APases in agricultural lands is yet to be conducted. Therefore, a preliminary qualitative analysis is needed to assess the APase response in agriculture managed soils. This should be augmented by incorporating findings from quantitative analyses published to date, thereby enhancing the comprehensiveness of this qualitative study. Such an analysis should encompass all potential factors that could either augment, diminish, or have no effect on APase activity to address the challenge of identifying patterns within agricultural systems. To achieve this goal, we i) summarize the direction of the relationships between a variety of influential factors on APase activity in agricultural lands and ii) identify gaps in knowledge. This will help to direct future quantitative studies toward specific areas, leveraging a broad and well-documented qualitative foundation.
2. Materials and Methods
Using the Web of Science and Scopus databases, a bibliographic search was carried out including research papers, reviews and meta-analyses published from 1977 to until December 2022. We carried out a search using different combinations of terms: “phosphatase* AND soil AND agriculture”, “phosphatase* AND soil AND agricultural”, “phosphatase* AND soil AND crop”, “phosphatase* AND soil AND arable”, and “phosphatase* AND grassland” in the title, abstract or keywords. We only selected papers reporting field, glasshouse, and laboratory studies using arable land and managed grassland, and where soil APase was experimentally assessed. APase must be evaluated alongside other parameters from bulk soil. Only studies that used para-nitrophenol as a substrate to measure APase activity were included [52,53,8], where ACP and potential ALP activity following this method is usually measured at pH 6.5 and pH 11.0 respectively [54]. Article search and selection process is detailed in
Figure S1.
Among all the selected studies for analysis, the response of ACP and ALP activity have been categorized according to these factors: biophysicochemical parameters, included total microbe activity, microbe abundance, microbial biomass P content, microbial biomass carbon content, microbial biomass nitrogen content, microbe diversity, phoD gene abundance and richness, earthworm abundance, soil depth, soil moisture content, clay content, sand content, microaggregate content, pH, cation exchange capacity, electrical conductivity, chlorine anion content, carbonate content, iron content, exchangeable aluminium content, grade of salinity, soil organic carbon/matter, total organic carbon, dissolved organic carbon, nitrate nitrogen form, ammonium nitrogen form, total nitrogen, soil carbon:nitrogen ratio, labile inorganic P, available P, organic P, labile organic P, soil carbon:P ratio and available potassium. Regarding the agricultural management practices’ factor, we registered any land use change, crop rotation and cover cropping, tillage practices, types of inorganic and organic fertilization and rates, weed and pest management practices, irrigation practices and livestock, grazing and mowing management. Pollution was included as soil contaminants content. Concerning climatic variables and climate-change treatments, mean annual temperature, mean annual precipitation, drought, soil water scarcity, soil water availability, seasonal variations and the impact of carbon dioxide fertilization in these studies was annotated. When available, crop yield responses were also taken.
All analyses underwent a review process involving vote counting, categorizing the direction of the effect as either positive, negative or non-existent (neutral). When the papers were meta-analyses and reviews, it was not possible to separate the results obtained by different analytical methods, therefore only those that had selected studies agreeing with our selection criteria were included (Table Sa). Consequently, our dataset comprised 675 papers, encompassing 267 individual observations of ACP activity, 218 individual observations of ALP activity, and 190 paired observations involving both ACP and ALP. Additionally, twelve meta-analyses and one review were also considered in this study, acknowledging that certain studies within these publications overlap with those selected in order to function as a qualitative complement to this analysis (Supplementary material Table Sb,
Tables S1-S18).
3. Results and discussion
3.1. Soil biophysicochemical properties
3.1.1. Soil microbes and fauna
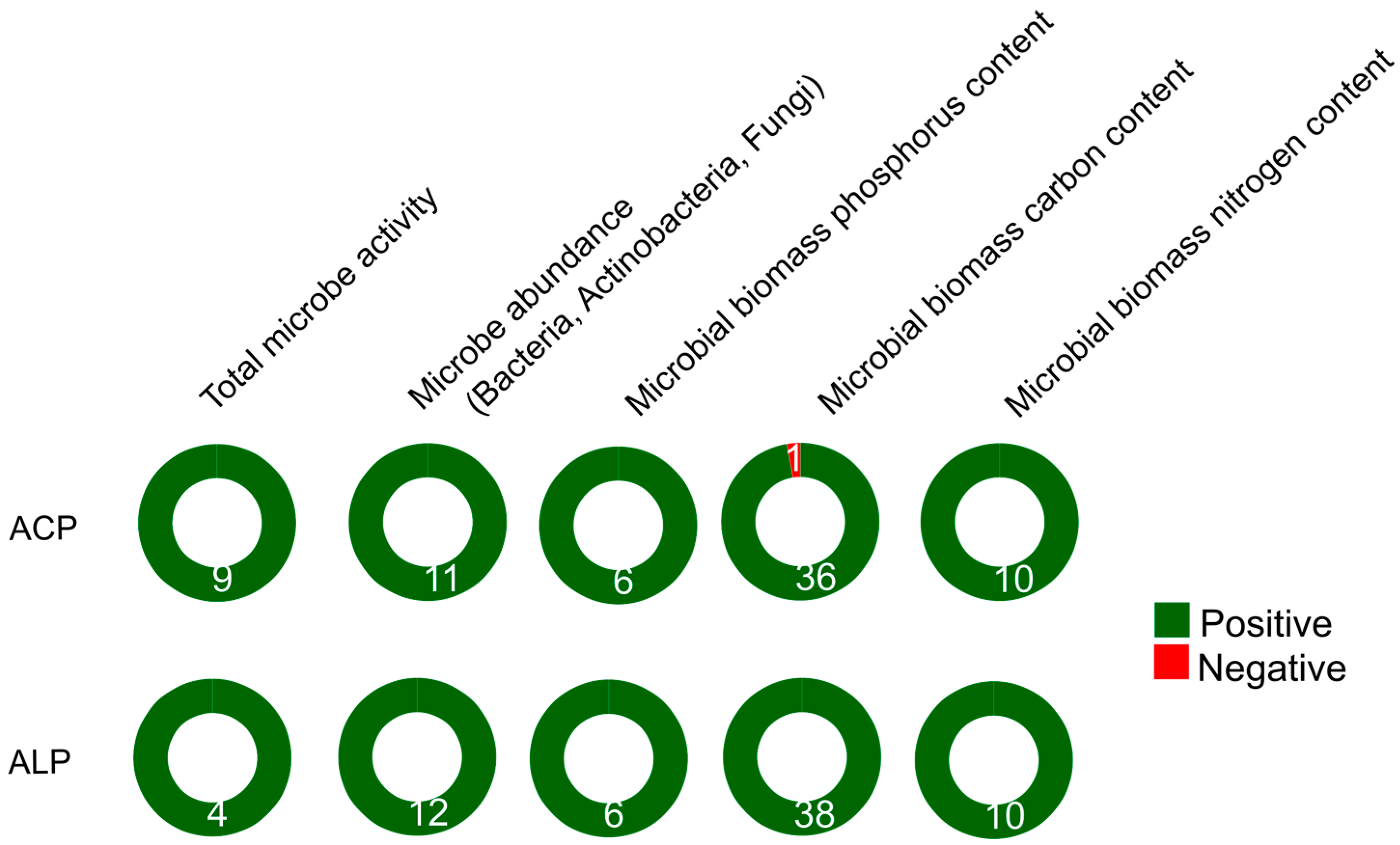
There is a positive relationship between the activity of soil microbes and APases (
Figure 1,
Table S1). This is influenced by the structure of bacterial and fungal communities [55,56], highlighting the role of microorganisms in facilitating nutrients movement within the soil [57]. Accordingly, the availability of soil P for plants is closely associated with the abundance of microorganisms and the presence of exoenzymes like APases [58]. When the activity of ACP in soil is low, microorganisms may adjust the activity of ALP in response of the nutritional needs of plants and microbes [59]. The activity of soil microorganisms varies throughout crop development, increasing in tandem with APase activity, as a response to crop growth, thereby reflecting the complex interactions between soil, plants, and the atmosphere [60]. The activities of ACP and ALP are positively linked with the biomass of fungi, bacteria in general, and specifically actinobacteria (
Figure 1,
Table S1). Additionally, ALP activity is positively associated with soil respiration [61], as well as with the activities of dehydrogenase and urease enzymes [62].
Positive relationship between microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) have been demonstrated [63], and both are indicative of microbial biomass [64]. MBC serves as a crucial nutrient pool for ecosystem nutrient cycling [65], and soil properties, such as soil organic matter (SOM) are usually positively associated with MBC [66]. Our findings provide evidence of positive associations between APase activity and MBC, but also with microbial biomass P (MBP), and MBN (
Figure 1,
Table S1). Although ALP activity has been proposed as an early indicator of change in soil biological status [65], it does not show a strong association with specific soil bacterial community composition [67], suggesting that it may be less sensitive compared to other enzymatic activities such as urease or dehydrogenase [56]. Consequently, ALP activity may not be a reliable indicator of soil microbial abundance [68], plausibly due to the diverse sources of this enzyme originating from both microorganisms and microbial plant secretions [69].
The relationship between soil bacterial diversity (measured by the Shannon diversity index), phoD gene abundance and richness and earthworm abundance and biomass with ACP and ALP activity is inconclusive (
Table S1). Microbial richness demonstrated a moderate but positive linkage with plant diversity [70] and the abundance of the bacterial phoD gene is generally positively interrelated with ALP activity. On the other hand, soil microbial activity, in turn influenced by plant root exudates, plays a more substantial role in driving APase activity compared to soil type [71]. This positive association with APases contributes to P availability in soil, potentially benefiting plant development [72,73]. The incorporation of earthworms alongside with crop residues has demonstrated an increase in ALP activity [74,75]. This effect has been linked to the mitigation of soil compaction caused by crop residues, thereby microbial conditions through improved water and oxygen supply [76,77]. Although ACP activity might also elevate with earthworm addition, it’s noteworthy that available studies combined earthworms with biochar, lacking independent analysing of the isolated effects of earthworms [78]. Moreover, soil management practices influence on earthworm metabolism and dynamic processes since enzyme activities in the casts produced in compacted soils are less stimulated [76]. Unfortunately, there is currently no available information regarding the impact of soil mesofauna groups on APase activity, despite their pivotal role in regulating organic matter decomposition and soil ecosystem functioning.
A summary of APase responses to soil biological factors is shown in
Figure 1.
3.1.2. Soil depth, moisture, texture and structure
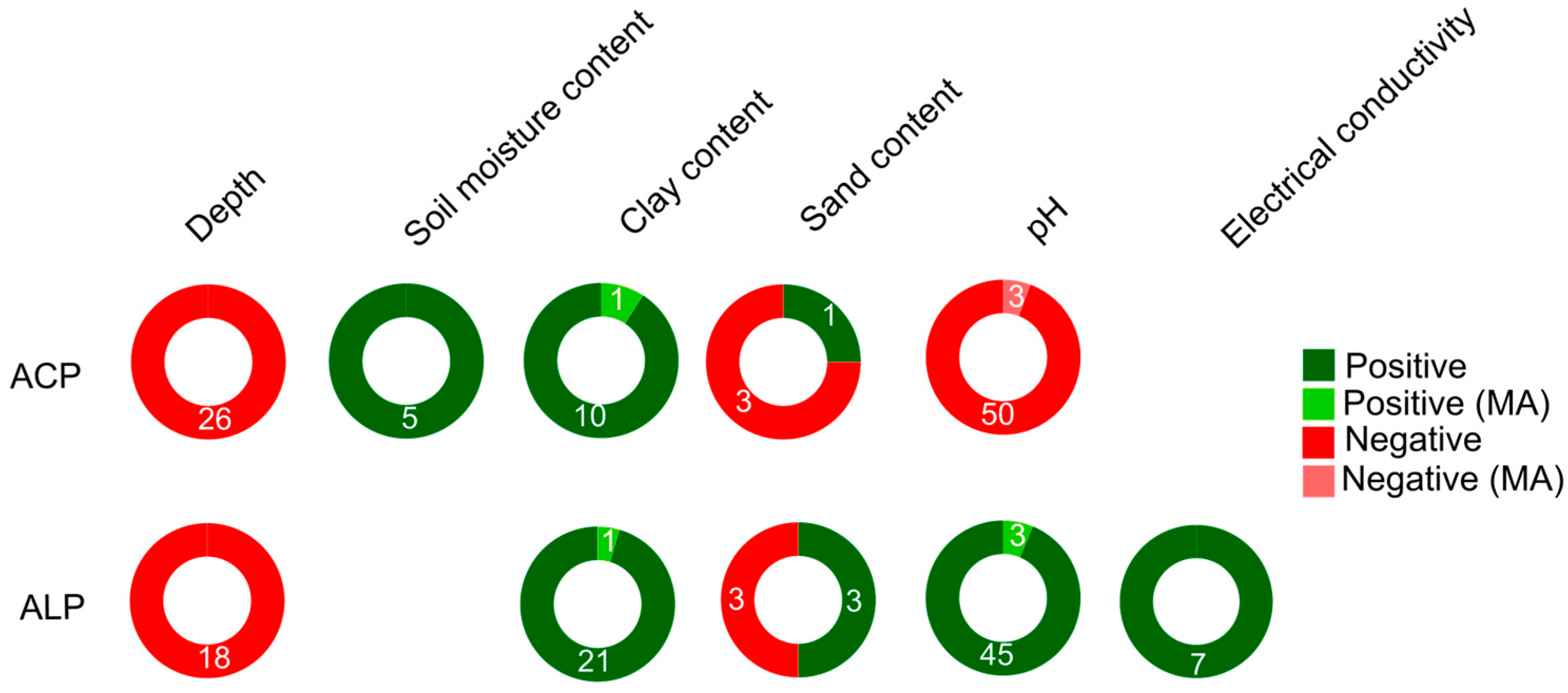
Several studies consistently demonstrated a decrease in ACP and ALP activities with increasing soil depth (
Figure 2,
Table S2). This decline aligns in root density and lower abundance of heterotrophic microorganisms (bacteria and fungi). Notably, soil moisture content has also a positive linkage with APase activity and the functional potential of soil microbial communities [79], reflecting its role in optimizing soil conditions for plant root and microbial growth [80] (
Figure 2,
Table S2). Some studies have consistently shown a positive trend between APase activity and soil structure (microporosity) and a higher clay content (
Figure 2,
Table S2), which agrees with the well-studied connection between those properties and soil microbial and biochemical properties [81,82]. More specifically, ACP activity has been positively correlated with fine soil particle fractions such as silt [83] and clay [84]. The increase in ACP and ALP activity with higher clay content is also consistent with a meta-analysis conducted by Aponte et al. [50] and is likely associated to the increase on enzyme longevity in soil caused by clay minerals while preserving their activity [68]. In contrast, sandy soils often exhibit a decrease in APase activity owing to several factors, including their diminished organic matter content, limited water-holding capacity, and reduced microbial biomass [55]. Nevertheless, some studies have suggested a positive relationship between APase activity and soil sand content, potentially attributed to increased bioaccessibility and bioavailability of nutrients such as nitrates or exchangeable cations [59,85]. Regarding soil structure, there are no conclusive results to assess whether microaggregates play a significant role in the transformation of soil P via APases thus lower concentrations of phosphate monoesters and diesters [86]. Consequently, a probable inverse relationship exists between the abundance microaggregates (particle size <0.25 mm) and the activities of ALP and ACP enzymes.
3.1.3. Soil pH and associated factors
Soil pH influences a variety of chemical and biochemical processes in soil [87]. In agricultural soil studies, the pH range typically spans from pH 5.5 to 7.5, and therefore APase assessments are often focused on ACP, due to the experimental buffer solutions that are typically adjusted to pH 6.5 followed by Tabatabai’s method [8]. Consistently, the maximum ACP activity is observed in acidic to neutral soils, while the peak potential ALP activity is found in alkaline (calcareous) soils [88-91, 55] (
Figure 2,
Table S3). This trend aligns with several meta-analyses [45, 46, 42]. Nevertheless, the activity of APases is potential and it can be increased or reduced due to agricultural practices that modify soil pH. Factors such as high precipitation, acid rain, oxidative weathering, and crop management practices can lead to a decrease in soil pH which promotes acidic activity. Conversely, weathering of silicates, aluminosilicates, or carbonate mineral compounds can increase soil pH which promotes basic activity. For instance, organic fertilizer application in maize cultivation [92] in acidic soils have demonstrated increased ALP activity due to their positive impact on soil pH. Conversely, practices like the use of rice straw biochar [93] or applying a no-till management in maize and bean cropping [94,95] have resulted in decreased ACP activity by elevating soil pH.
Microelements and organic compounds in the soil, such as carbonates (CO32-), iron (Fe), and aluminium (Al) oxides, influence the release of P from organic compounds, the size of P fractions, and P uptake, which in turn affect APase activity [96]. Specifically, soil CO32- content could be negatively associated with ACP activity and positively associated with ALP activity, likely due to its neutralizing capacity, which shifts soil pH from neutral to alkaline [97]. The soil Fe content interacts positively with both ACP and ALP activity, as its availability increases with higher organic matter content [98]. Lastly, soil exchangeable aluminium (Al3+) content has a negative connection with ACP activity due to pH increases after lime amendments, where calcium ions (Ca2+) hydrolyse and react with soluble Al3+ to form insoluble Al hydroxide compounds [99] (
Table S3).
The total cation exchange capacity (CEC) and electrical conductivity (EC) of the soil are partly related to soil pH [100,101], and available studies indicate a positive association between APase activity and CEC and EC (
Figure 2,
Table S3). Additionally, higher concentrations of chloride ions (Cl-) in the soil can decrease ACP activity by inhibiting the growth of soil microflora, thus affecting enzymatic activity [102] but there are no conclusive results directly correlated with this ion. However, high salt content in soils is a growing issue exacerbated by climate change, and it poses significant challenges to agricultural production. Salinity and sodicity, the latter referring to a high sodium (Na+) content, have detrimental effects on crop growth and the biochemical processes essential for maintaining soil quality [103]. In relation to APase activity, although the results are not significant it seems that salinity has a negative impact (
Table S4) partly due to a decrease in the activity of soil microbes and associated microbial biomass with reductions in the release of enzymes [103] and partly due to the likely direct toxic effects of some ions, particularly Cl-, on microbial growth [104] (
Table S4).
A summary of APase responses to soil depth, moisture, texture and pH-related factors is shown in
Figure 2.
3.1.4. Carbon
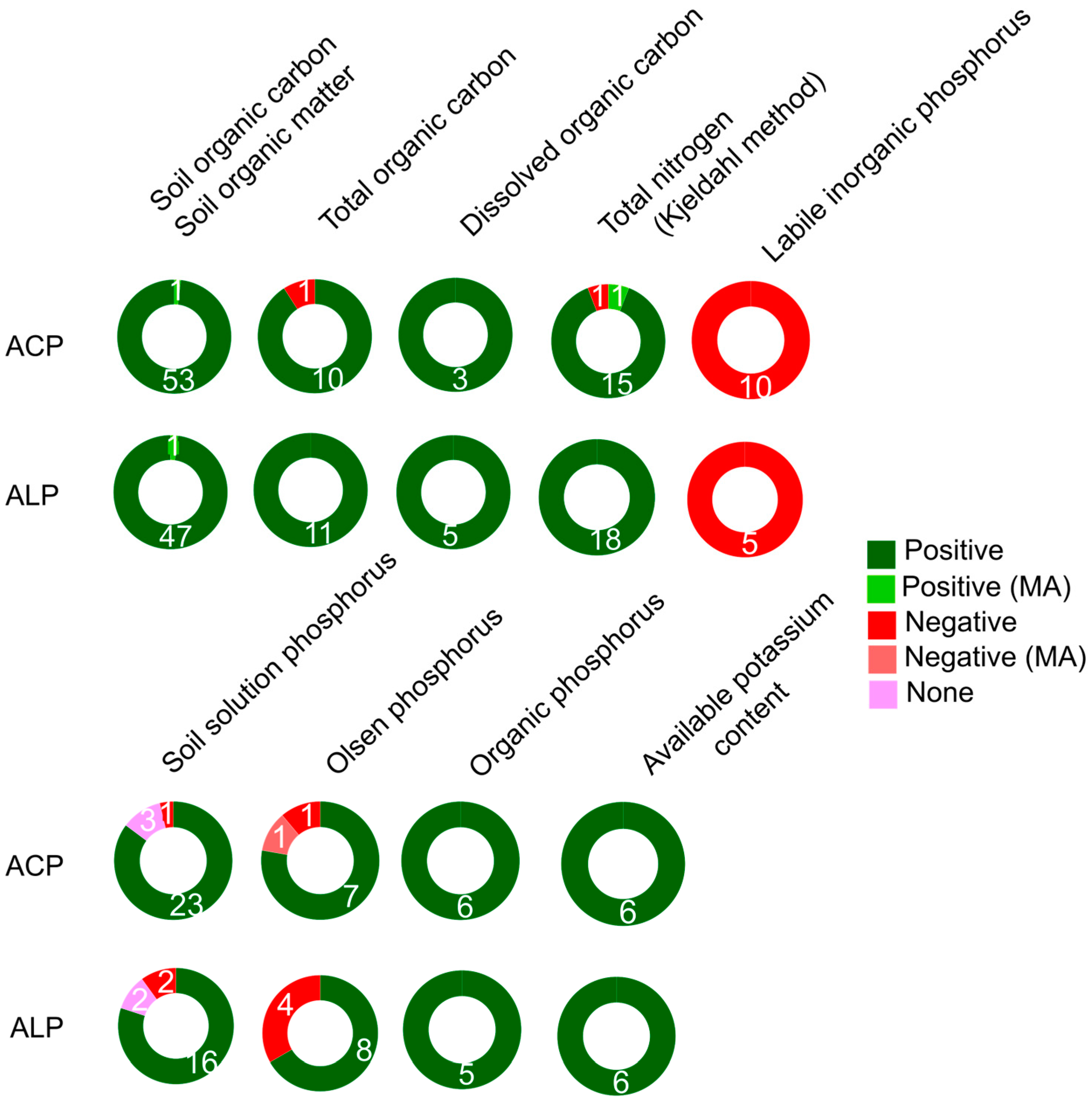
Soil organic carbon (SOC) is a crucial component of soil health and is derived from living and decomposing organic matter such as plant litter, root and microbial exudates, dead microorganisms and fauna, and faecal material [105]. Both single studies and meta-analyses have clearly demonstrated a positive linkage between indicators of soil organic matter, including SOM, SOC, total organic carbon, and dissolved organic carbon and the two APases (ACP and ALP) (
Figure 3,
Table S5). This positive association is explained because the substrate for APases, soil organic P, is linked to SOC [106]. Quantifying soil organic matter (SOM) often does not provide detailed information about the underlying soil processes that contribute to its accumulation [107]. Certain agricultural practices, such as reduced tillage and cover cropping, have been shown to increase SOM levels [108,109], through higher levels of microbial biomass that stimulate decomposition processes and enhance the stabilization of organic compounds [110].
3.1.5. Nitrogen
Nitrogen is a crucial nutrient for plant growth and is considered an indicator of soil fertility and quality. Nitrate (NO3-) and ammonium (NH4+) are the primary forms of N available for plants, and their concentrations are often positively correlated with the activity of ACP and ALP. Higher concentrations of NO3- and NH4+ can have a positive impact on the formation and persistence of microbial biomass, which in turn can influence the activity of APases [111]. However, the fact that negative effects have sometimes also been found between both (NO3--N and NH4+-N) and APases indicate that there can be interactions among specific soil, environment and management conditions leading to contrasting patterns (
Table S6). For instance, when negative effects of NO3- on ALP activity have been reported, this has been attributed to the stabilization of ALP by soil colloids formed by organic matter and clay minerals [112] as well as the influence of SOC on the structure and composition phoD-harboring bacteria and ALP activity [67]. Both a meta-analysis and multiple studies have shown a positive association between APase activity and total soil N content, often determined using the Kjeldahl method (
Figure 3). This relationship is likely due to the positive correlation between N and SOC content [113, 42], suggesting that APase activity is induced by C and N mineralization and the availability of their decomposition products [114] (
Figure 3,
Table S6). The C:N ratio of soil organic matter also influences APase activity, and a lower C:N ratio indicates rapid decomposition of organic matter, regardless of soil microbial biomass, and can result in increased APase activity. The positive connection between APases and the C:N ratio tends to be stronger than their connection with the C:P ratio [115].
3.1.6. Phosphorus
As expected, and indicated by various studies, APase activity is closely associated with soil P content (
Figure 3,
Table S7). It is important for comprehending the dynamics of soil P and for effective P management in both natural and agricultural ecosystems [116]. The bulk of the soil P exists in three general groups of compounds, namely organic P, calcium-bound inorganic P and iron or aluminium-bound inorganic P where organic P is distributed among the biomass, labile or passive fractions of soil organic matter, inorganic P and calcium compounds predominate in most alkaline soils while the iron and aluminium forms are most important in acidic soils [117]. Since most of the P in each group is of very low solubility and not readily available for plant uptake, biotic processes controlled primarily by bacterial and fungal decomposition, indirectly affect P availability for plants by influencing the form of soil minerals that chemically bind P [118]. For instance, in cropping systems with low levels of C and inorganic N, it becomes essential to supplement the soil with other mineral nutrients (e.g., potassium) and implement effective biological control strategies to ensure proper P cycling and availability for plants [119]. In terms of readily plant-available soil P content, studies considered different fractions notably labile inorganic P (Pi), soil solution P, or other P fractions. The former comprises P fractions dissolved in the soil solution, directly accessible to plants, while the latter encompasses fractionation methods for inorganic P extraction. These extraction methods often involve sodium bicarbonate-P (commonly referred to as Olsen P, detailed separately) or P solubilization using reagents such as dilute acid-fluoride, dilute hydrochloric acid, sulfuric acid, or water, among other techniques [120]. Conversely, there exist P fractions existing in organic forms, cited as organic P, that are not immediately available to plants, including labile organic P (Po). As previously mentioned, organic P denotes P bound within organic matter, while Po, like NaHCO3-Po, represents P that can be relatively easily mineralized [121].
The activity of APases in soil is influenced by the P content, and its response is dynamic depending on the availability of P to plants. A priori, high levels of available soil P content can lead to a reduction in APase activity, as plants and microbes adapt to the abundant P supply. Conversely, under P limitation, APase activity can increase to facilitate P uptake and meet or even surpass plant P demands [24] (
Table S7). This trend is confirmed by Sun et al., [42] which showed a negative correlation between both APases and Olsen P and soil solution P. In this case, the negative association has been attributed to the hydrolysis of P compounds by other APase enzymes in the NaHCO3-extractable fraction, leading to an increase in dissolved inorganic P in the soil solution. However, other studies showed a positive association between APase activity (both ACP and ALP) and Olsen P, soil solution P and organic P (
Figure 3) which means that the dynamics of P fractions, particularly Olsen P, are closely related to plant development and can be influenced by climate and intrinsic soil characteristics [122,123]. Additionally, the addition of organic P sources, which increase the soluble P content, can negatively impact APase activity, as they contribute to the pool of available inorganic P in the soil [124]. It seems that there is a relationship between these enzymes and the promotion of root growth and nutrient uptake by crops [126] which indicates that the positive relationship is directly associated with particular cases, and that management is crucial to determine their correlation. When APase activity shows a negative linkage with the content of Po in the soil (
Table S7), it suggests that APases are not the limiting factor in the utilization of organic P, but rather it is the availability of APase-hydrolysable P compounds that limits the process [24]. It is important to consider that when a wider group of phosphoric monoester hydrolases enzymes are assessed together, the high levels of inter-enzyme variation strength the relations of available P [126].
3.1.7. Potassium
Potassium (K) plays a crucial role in plant growth and soil fertility [127]. Therefore, soil content of available K decreases more in cultivated soils than in natural ecosystems during the plant growth due to erosion/runoff [62]. Studies indicate a positive linkage between the activity of ACP and ALP enzymes and the available K content in the soil (
Figure 3,
Table S8). The studies do not deeply into the relationship of K with other factors that may also affect APase activity. For this reason, further research is needed to fully understand the specific mechanisms and trade-offs associated with K and its impact on P acquisition in managed ecosystems [128].
A summary of APase responses to carbon, nitrogen, phosphorus and potassium is shown in
Figure 3.
3.2. Responses to agro-ecosystem management
3.2.1. Conversion from natural to managed ecosystems
Cropland soils experience more intensive human disturbance and receive lower inputs of plant residues, root exudates, and senescent leaves compared to soils in natural and semi-natural ecosystems [129]. This human activity negatively impacts the soil biological and biochemical properties, leading to a decline in P and C cycling [130]. Non-managed ecosystems like native forests, on the other hand, exhibit higher microbial activity due to their abundant SOM and available P content [131], which facilitates the transformation of organic P into inorganic forms [132,133]. Cropland soils generally have lower SOC and MBC compared to non-managed soils [134], and the global activity of extracellular enzymes is diminished as a result [135]. Furthermore, the activity of APases is influenced by common management practices [136], with lower intensity management systems generally exhibiting higher APase activity compared to higher intensity management systems (
Figure 4,
Table S9).
On the other hand, the conversion of intensively managed agricultural land back to grassland and forest systems, using native plant species [138, 131, 138], improves the supply of organic matter, enhancing APase activity, especially ACP [139]. Furthermore, a meta-analysis made by Margalef et al. [41] has shown that invasive plant species can also increase ACP and ALP activity compared to native species, potentially due to differences in litter quality or quantity and related effects of changes in soil chemistry on microbial communities.
3.2.2. Crop rotations and species
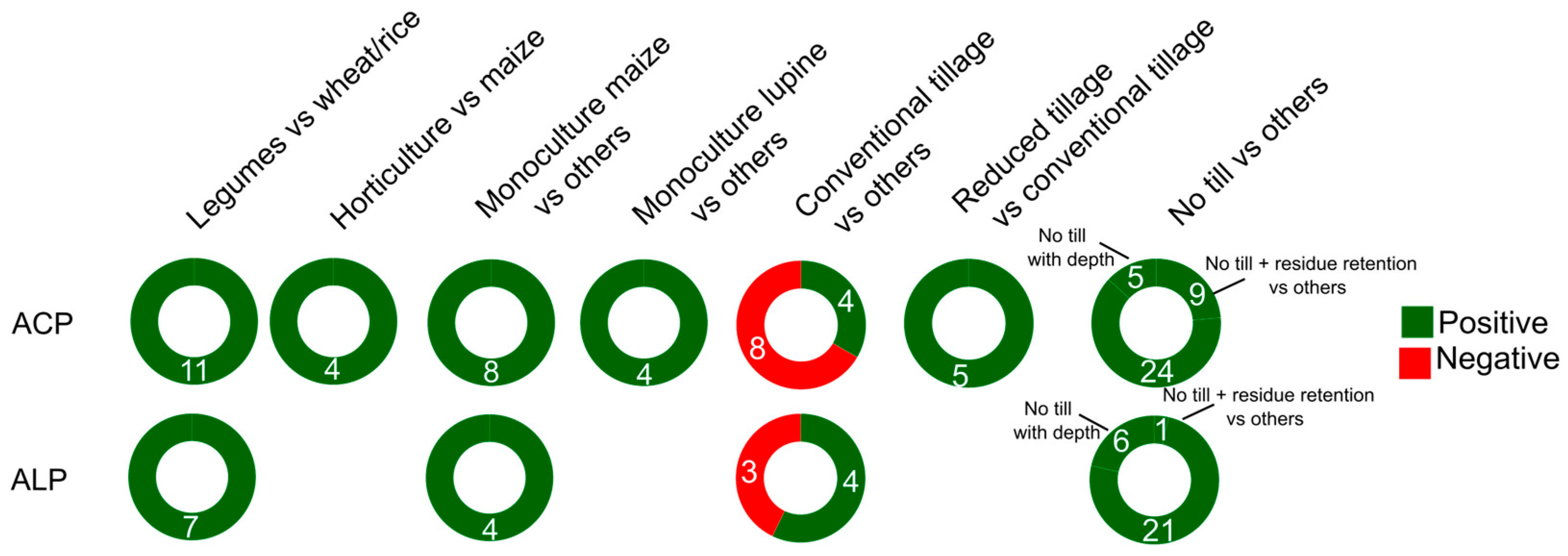
APase activity in agricultural systems is influenced by crop rotation type, the crop species concerned and also by cover and intercropping practices (
Figure 4,
Table S10). Higher levels of ACP and ALP activity are observed in crop rotations in cereal-based rotations compared to cereal-legume rotations. This positive response has been attributed to increased ionic exchange capacity, SOC, MBC, and availability of essential nutrients such as P, K, and magnesium (Mg), as well as a greater presence of earthworms in rotation systems [140, 63]. The inclusion of legumes and/or grasses in crop rotations, also as an intercrop, increases the synergism of microbial attributes (e.g., MBC, soil basal respiration, metabolic quotient, soil cultivable bacteria, fungi, actinobacteria and microorganisms with cellulolytic activity) [141] leading to higher productivity and economic profitability.
Different crop species influence soil N content, C sequestration, and P accumulation in long-term cropping systems [142], promoting efficient water, energy, and C use efficiency for crop production [143]. Maize monoculture, for example, exhibits higher soil APase activity compared to soybean, cowpea, or cotton, attributed to its deeper rooting system and this links to its growth advantage in low P availability conditions [144, 145]. Legume cultivation, especially lupine, which is the most well studied, enhances soil nutrient availability in a broad sense [146], and results in higher ACP and ALP activity compared to grain crops like wheat and rice, as legumes offer benefits on soil microbial communities, ensuring stability in intensive production systems [147]. Additionally, genetically modified crops, such as transgenic cotton, have been found to enhance ACP and ALP activity, although the effect is crop-specific and may not apply uniformly (e.g., in rice it is ACP that is enhanced). In horticultural crops like mango, kiwifruit, lettuce, potato, and tomato the activity of ACP is higher compared to cereal crops, attributed partly to intensive fertilization and irrigation management [148] (
Figure 5,
Table S10).
The use of cover crops (i.e., specific crops planted primarily to manage and could improve soil health rather than for direct harvest) has a positive impact on soil and crop health by improving pest and disease control, increasing water availability, and enhancing the abundance and activity of soil microorganisms [150]. Cover crops have been shown to promote microbe-mediated processes that enhance ACP and ALP activities, likely through the increase of labile C and moisture in soil, maintenance of high organic matter levels, and stabilization of soil temperature [150-152]. Intercropping, which involves cultivating two or more crop species within a single cropping season, results in greater ACP and ALP activities compared to monocropping. As mentioned before, the use of legumes leads to an increase in APase activity, as reflected in the study results (
Figure 4,
Table S10). This may be due to the differential secretion of root exudates by intercropped species, which might provide a higher diversity of labile C substrates, with knock-on effects on soil microorganisms, thereby increasing enzyme activity [153]. Moreover, when intercropping is associated with fertilization (section 3.2.4), APase activity is evidently enhanced.
A summary of APase responses to land use change, crop rotation, cover crops and intercropping is shown in
Figure 4.
3.2.3. Soil tillage
Conventional soil tillage, which involves mechanical soil turning, aims to improve soil structure for sowing, seedling establishment, and weed control [154,155]. However, intensive tillage practices increase the risk of soil erosion and surface runoff, particularly following heavy rainfall, leading to the loss of SOM [156]. In contrast, reduced (conservation) tillage practices minimize soil disturbance, resulting in better conservation of SOM [157], increased MBC, MBN [158], and higher availability of K and Mg. Along with the improvements in soil physical properties, soil aggregation, and reduced decomposition, reduced tillage contributes to the promotion of APase activity [159] (
Figure 5,
Table S11).
No-till practices, which involve minimal soil disturbance and surface accumulation of crop residues, have distinct advantages in soil top layers even compared to reduced tillage. No-till practices lead to even greater reductions in the decomposition of labile organic matter, resulting in increased soil moisture, C, and N levels [160, 161, 64]. These practices also have positive effects on P fractions (e.g., inorganic, organic, and available P) [162]. The increased availability of substrates for enzymes in the presence of higher residue inputs enhances the activity of enzymes such as ACP and ALP [163] (
Figure 5,
Table S11).
A summary of APase responses to crop species and tillage is shown in
Figure 5.
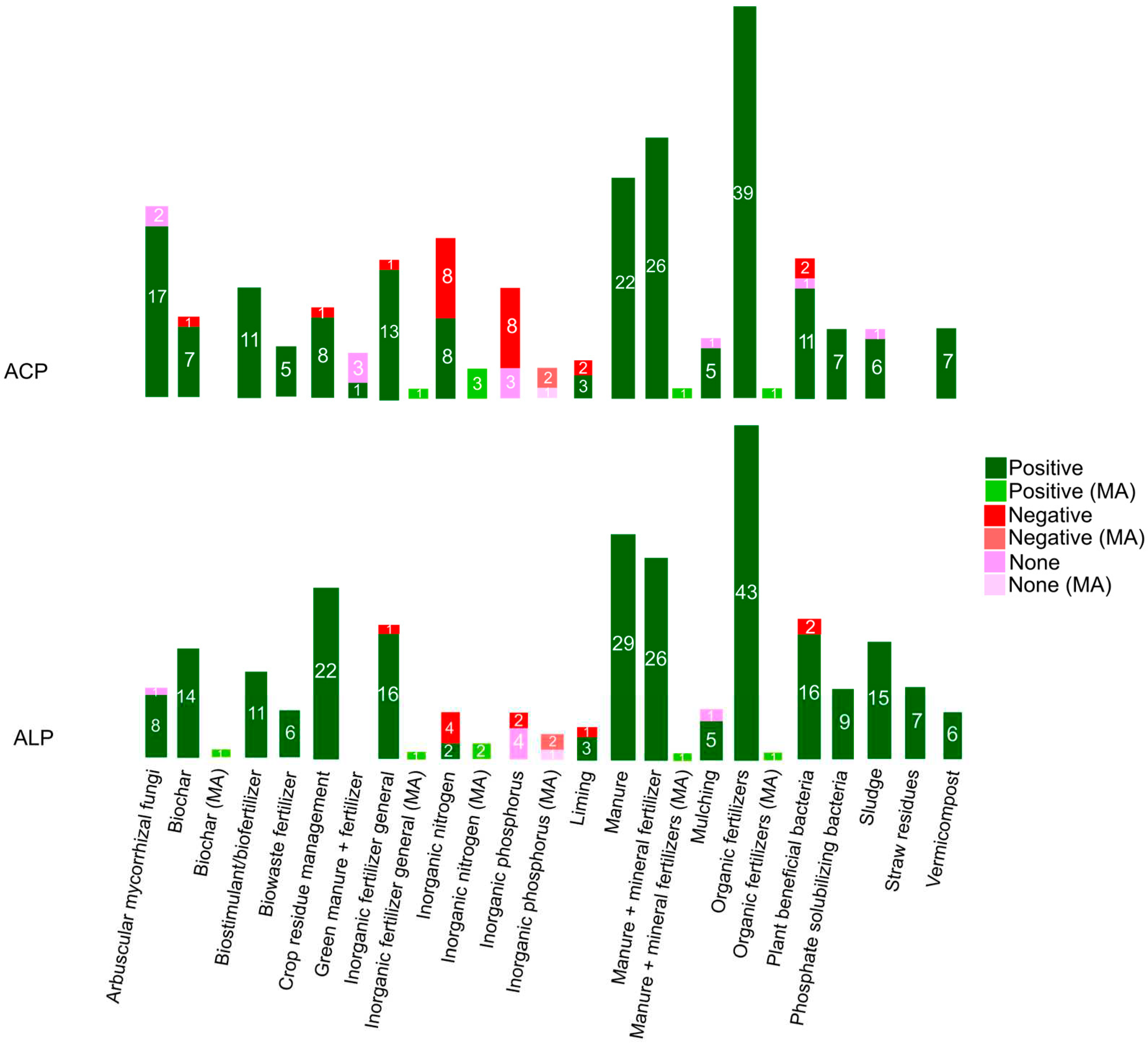
3.2.4. Soil fertilization
Fertilization of agricultural soils to increase crop yields tends to positively impact APase activity (
Figure 6,
Table S12), although concurrent factors such as fertilizer nutrient balance and type, crop species, and growth stage may determine its activity (Jiang et al., 2019).
The application of combined (NPK) chemical (inorganic) fertilizer generally promotes APase activity [47]. Nitrogen fertilization in particular tends to enhance the activities of ACP [48, 41, 49] and ALP [41,49]. This suggests a connection between APases and the cycling of N. However, there are also reports indicating that ACP and ALP activity may decrease after mineral N fertilization, which suggests that substrate availability (i.e., specific organic N or P substrates in soil suspensions and soil filtrates) are more important than P deficiency [164, 45]. Inorganic P fertilization alone tends to decrease the activity of both APases [41, 49] although there is also a meta-analysis suggesting no significant effects [45].
The long-term application of organic fertilizer, derived from plant and animal material, is an important strategy in enhancing soil quality by increasing the abundance of soil microbes and the activity of extracellular enzymes such as ACP and ALP [165,47]. Organic fertilizers have a positive association with soil pH, especially in relation to ALP activity and P content [92], leading to improved availability of soil nutrients, including labile C, N, and P through mineralization, as well as enhanced microbial biomass and abundance [166, 167]. Various soil amendments, such as vermicompost (i.e., organic material biodegraded by earthworms and microorganisms), biostimulants (e.g., humic substances, marine macro-algae, protein hydrolysates, microbial inoculants, and plant extracts), biowastes (i.e., optimal doses of organic compounds and metals), or sludge (i.e., rich in organic matter, NO3--N, copper (Cu), cadmium (Cd), and organic P), have also shown the ability to increase ACP and ALP activity [50] although do not report on their direct correlation over the very long term. The optimization of APase activity in soils without the addition of inorganic fertilizers, can improve soil conservation, P release and overall agricultural sustainability in ecosystems [168].
The co-application of inorganic and organic fertilizers in agricultural soils is a common practice due to their complementary composition and functions, resulting in increased ACP and ALP activity, thereby providing high levels of plant-available P [169, 47].
Lime application to acid soils increases pH levels, improving plant access to essential nutrients for growth [170], and has positive effects on ACP and ALP activity. However, it should be noted that when Ca based lime is applied, reductions in APase activity have been observed, indicating that Ca availability may not be a limiting factor for plant growth [171].
Combine fertilizers (organic or inorganic) with green manures (i.e., refer to non-crop plants, typically legumes, that are cultivated specifically to improve nutrient content in the soil) can have an impact on APase activity. While short-term trials combining green manure with fertilizers have not shown a significant effect on ACP activity, there is evidence for positive impacts on ACP and ALP activity in these trials when legume green manures are added with fertilizers [172, 173]. This is attributed to the increase in SOM content and the contribution of N fixed by symbiotic legume root bacteria [152].
Crop residues, whether applied on the soil surface as mulch or incorporated into the soil, can have positive effects on P transformation rates and soil P plant available pool [174]. When crop residues, such as straw, are purposefully left on the soil surface, they gradually degrade over time, providing a greater and more sustained supply of substrate for soil [175]. Mulching also increase the supply of carbohydrates and available nutrients such as N, P and K [176] having a positive impact on microbial communities [177]. This prolonged breakdown of residues contributes to an increase in SOC content (Sharma and Dhaliwal, 2019) which in turn enhances ACP and ALP activity. The increased activity of APases resulting from crop residue mulching not only improves soil quality but also has the potential to reduce the need for chemical fertilizer inputs, leading to greater economic returns [178].
Generally, biochar amendments are known to have a positive effect on both ACP and ALP activity (
Table S12). According to the findings of Pokharel et al. [46], the addition of biochar to soil increases the sensitivity of ALP to changes in pH. This heightened sensitivity results in an increased microbial demand for P and/or the potential limitation of P availability in the soil due to restricted microbial growth. However, despite these effects on ALP activity, the researchers did not observe significant impacts on ACP activity.
The practice of burning crop residues, on the other hand, releases environmental pollutants into the atmosphere (particulates carbon dioxide (CO2) and carbon monoxide) and has negative impacts on ACP and ALP activity (
Table S12). This is due to the changes it induces in soil chemical and biochemical processes, resulting in decreased soil nutrients, bacterial densities and MBC [179,180].
Plant beneficial microbes (PBMs) are increasingly used in biotechnology to reduce the reliance on agrochemicals with the aim of increasing soil nutrition, tolerance to stress, soil health and crop yields [181,182]. Phosphate solubilizing bacteria (PSB) significantly contribute to the enhance of APase activity and the availability of P to plants (
Figure 6,
Table S12). This is achieved through their possession of enzymes and metabolic mechanisms, enabling the conversion of insoluble forms of P into accessible forms for plant uptake [67]. They accomplish this through the mineralization of organic P and the solubilization of inorganic P minerals, leading to greater P uptake in plant biomass [183]. Incorporating PSB into the soil also results in faster humification of fresh organic matter and enhances mycorrhizal and endobacterial activities [184]. Likewise, soil inputs of bacteria, such as Bacillus, Pseudomonas, Aspergillus, Azospirillium, and Streptomyces can increase both ACP and ALP activity, restore soil fertility, and promote plant productivity taking into account addition parameters (e.g., EC, pH and ionic concentration) to ensure proper nutritional management of the crop [185]. The input of arbuscular mycorrhizal fungi to soils assists the plants in absorbing nutrients by hydrolysing organic P, similar to solubilizing bacteria does, enhancing APase activity. Additionally, soil acidification caused by fungi increases the availability of organic P substrates for APases, particularly ACP [54].
A summary of APase responses to agroecosystem fertilizer management practices is shown in
Figure 6.
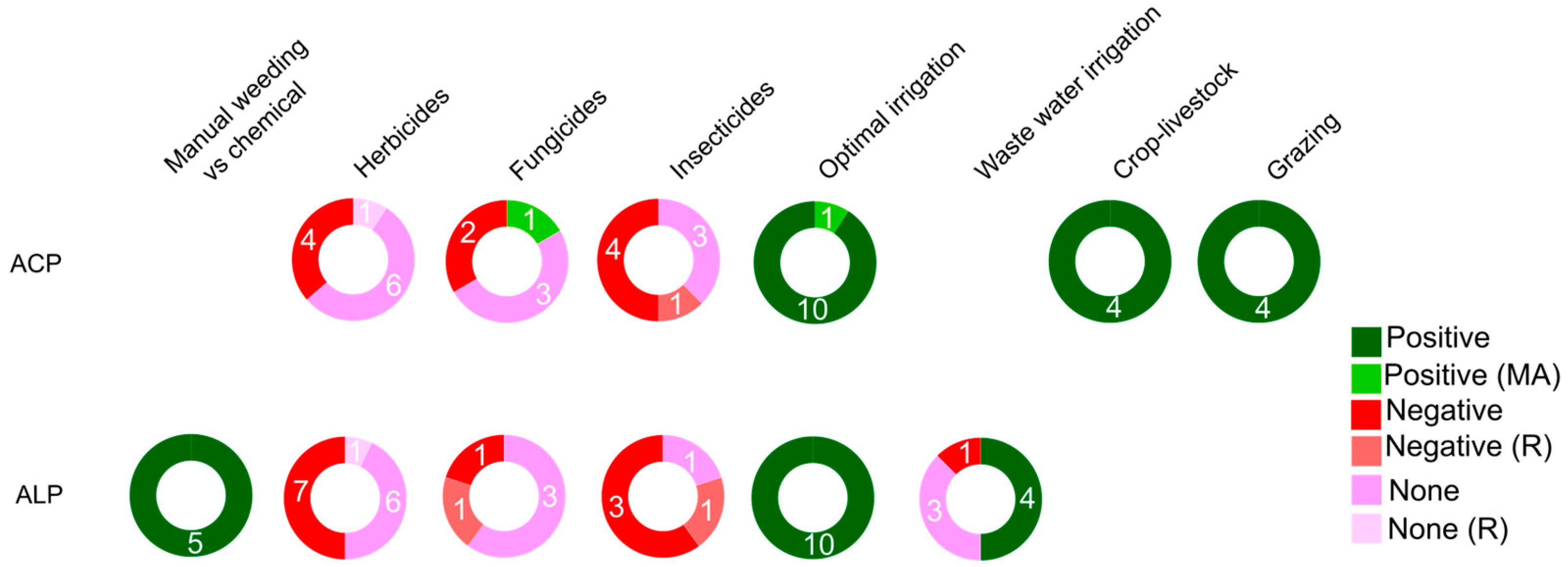
3.2.5. Pest and weed management
Plant protection products, including herbicides, fungicides, and insecticides, are widely used in agriculture to mitigate the detrimental effects of competition, disease, and herbivory on crop yields. However, their application can lead to changes in soil function and health, affecting soil respiration, biomass, and APase activity (
Figure 7,
Table S13). The impact of fungicides on APases is a topic of debate, with one meta-analysis reporting an increase in ACP activity rather than ALP activity [51], possibly due to the predominance of ACP analysis in agricultural soils. Likewise, the effects of insecticides on APases do not exhibit a clear trend. The results found suggest decreases in ACP and ALP activity, followed by recovery in ALP activity within 7 to 30 days after insecticide application [186, 51].
Weed control plays a crucial role in reducing competition for resources by minimizing non-crop plant abundance. While manual weeding tends to increase APase activity (
Figure 7,
Table S13), the use of herbicides can result in either negative or negligible impacts on ACP and ALP activity [51]. Importantly, any adverse effects from herbicide use typically do not persist beyond 30 days after application [187]. However, cultivating crops in competition with weeds comparably with weeds cultivated alone negatively impacts ACP activity, microbial activity, and inorganic P solubilization [188].
3.2.6. Irrigation
Crop irrigation is a practice that involves providing controlled amounts of fresh water or wastewater to sustain and enhance yields in water-scarce regions [189]. Optimal irrigation levels have been found to positively affect APase activity (
Figure 7,
Table S14). Irrigated soils have increased availability of soil nutrients, leading to higher demand for P by plants and microbes during plant growth [42]. Moreover, irrigation strategies can influence P availability, affecting P storage [190] and the abundance of bacteria, which may explain the observed impacts on APases. Research on wastewater irrigation has shown varying effects on ACP and ALP activity, as it affects soil microbial activity and the microbial community [191]. However, long-term use of wastewater may potentially reduce agricultural crop yield [192].
3.2.7. Livestock, grazing and mowing management
Livestock can play a significant role in enhancing agroecosystem function [141], and it can be managed within a livestock-only system (pasture) or in combination with crop production (livestock integration). In both cases, the presence of livestock contributes to an increase in soil MBC content and ACP activity (
Figure 7,
Table S15). Grazing-based pasture management has been linked to various positive effects, including higher soil pH, increased water content, and elevated levels of NO3-, NH4+, organic matter, and C:N ratios [193]. These conditions promote greater APase activity, mostly ACP (
Figure 7,
Table S15). On the other hand, mowing encourages the growth of plant species with competitive strategies [194], while the contact between cut residues (substrates) and the soil reduces the activity of ALP [195] (
Table S15).
A summary of APase responses to pest and weed management, irrigation, crop-livestock and grazing management is shown in
Figure 7.
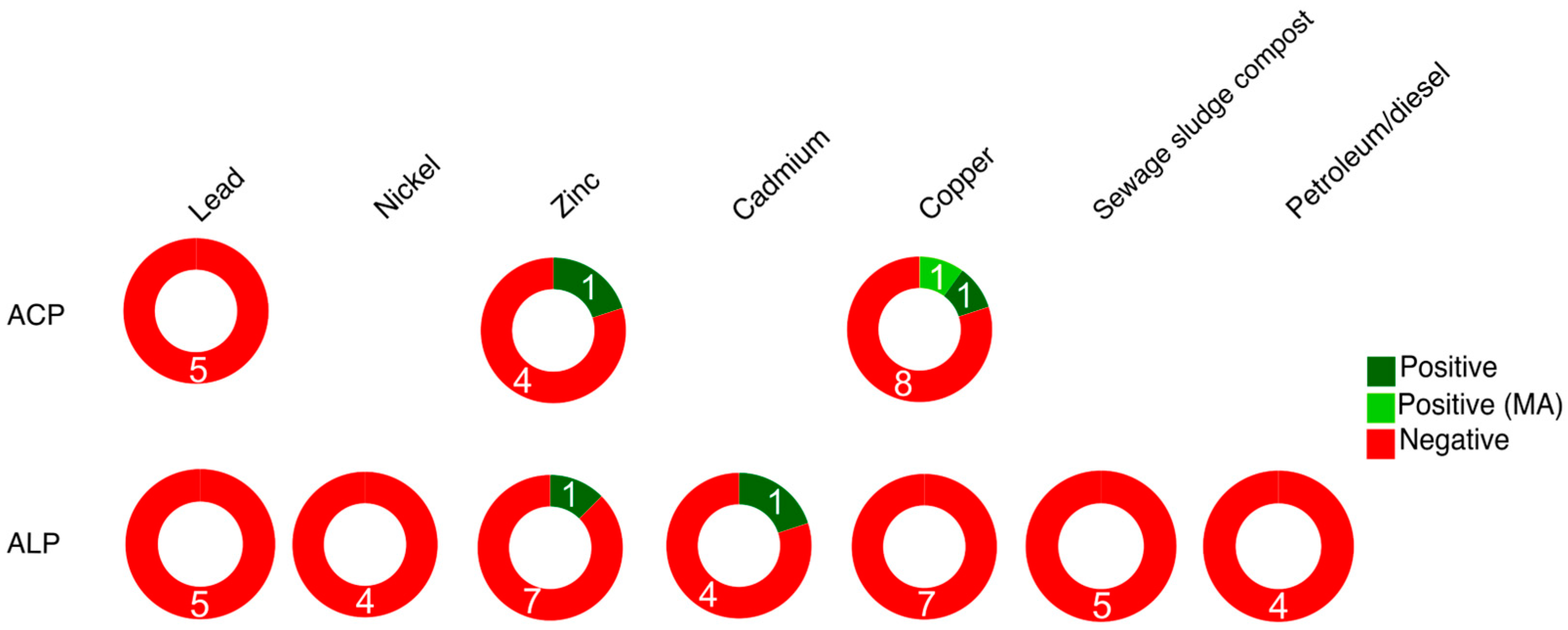
3.3. Responses to soil pollutants
Soil pollution caused by heavy metals can disrupt biochemical, physiological, and metabolic processes. These pollutants alter nutrient stoichiometry and result in slower P cycling due to an imbalance between litter, soil organic matter, and the elemental composition of microbial biomass [50]. Heavy metals have an impact on APase activity (
Figure 8,
Table S16); negative responses of APase activity due to lead (Pb), chromium (Cr), nickel (Ni), zinc (Zn), cadmium (Cd), copper (Cu), manganese (Mn), arsenic (As), and mercury (Hg) have been observed, while positive responses are reported in one meta-analysis made by Aponte et al., [50] concerning Cu and Cd. The negative APase responses are attributed to the harmful effects of heavy metals on soil microorganisms [196], while the positive responses may indicate microbial metabolic stimulation resulting from increased levels of metals acting as micronutrients, such as Cu, Mn, cobalt (Co), Zn, and Cr [197]. Although heavy metals generally inhibit APase activity, the extent of the response depends on the initial metal composition in the soil, organic matter content, and the inhibition of microbial activity [81]. In soils with high organic matter content, heavy metal impact on APases is relatively lower compared to other enzymes due to the positive association between APase and soil C abundance [198].
Negative effects on APase activity have been observed following the use of sewage sludge compost with high concentrations of heavy metals such as Pb, As, Cr, Cd, Ba, and Ag [50]. Similarly, soil pollution caused by petroleum and nanomaterials (NMs) also negatively affects APase activity, also leading to a decrease in bacterial species richness and diversity [199]. The use of NMs as biocides and plant growth promoters influences soil properties and enzyme activity and a meta-analysis made by Lin et al., [200] showed that C, Cu, and Ag NMs result in a decrease in ACP activity, whereas low soil concentrations of Fe NMs stimulate ACP activity (
Table S16).
A summary of APase responses to soil pollutants is shown in
Figure 8.
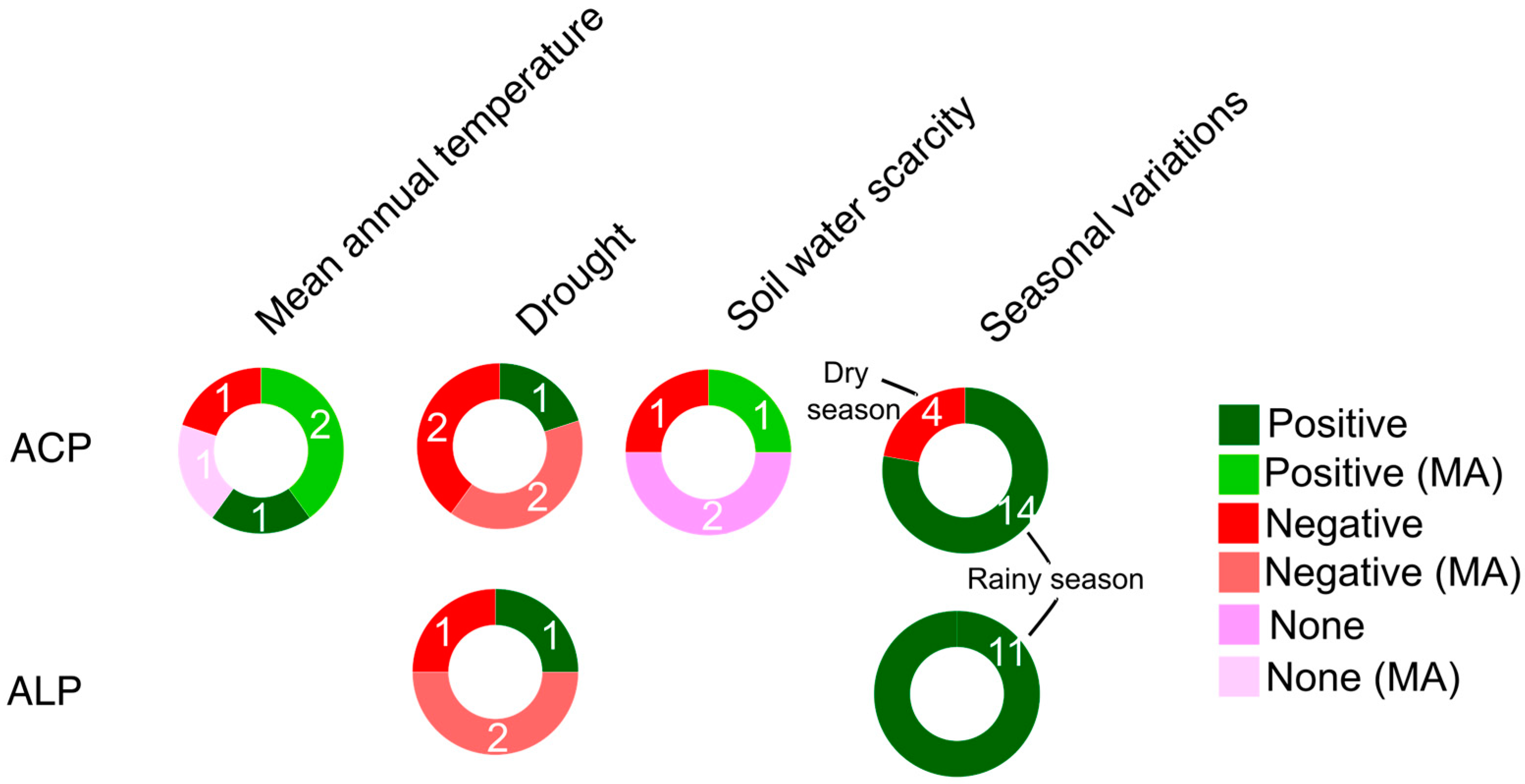
3.4. Impacts of climate change
The rapid global temperature increases, shifts in rainfall patterns, and rising atmospheric CO2 concentrations that the planet is experiencing are significantly impacting plant stoichiometry and productivity, potentially affecting APase activity (
Table S17). Existing meta-analyses have suggested that climate warming could increase ACP activity in agroecosystems and forests [42, 43], primarily due to reduced soil P content (e.g., Olsen P and total soil P) resulting from accelerated plant growth and enhanced plant P acquisition [201]. However, another meta-analysis that encompassed grasslands and other natural ecosystems found no correlation between temperature and both APases [41].
The predicted increase in rainfall intensity in some areas under ongoing climate change is likely to lead to higher topsoil nutrient losses, as high soil water availability to plants can elevate groundwater chemistry, including the dissolved content of bicarbonate, sulphate, Cl- anions, and Na+, Ca2+, Mg2+, and K+ cations [202]. Elevated mean annual precipitation (MAP) levels have been linked to increased ACP and ALP activity [42] compared to controls in models of humid grassland soils and irrigated [203,204]. Conversely, drier conditions are also expected to become more frequent under climate change in some regions, resulting in reduced demand for available P forms and associated enzyme activity [205]. APase activity tends to respond negatively to water scarcity and drought in agroecosystems (
Figure 9,
Table S17), particularly in grasslands and other natural ecosystems under Mediterranean climate conditions known for their seasonal aridity [41]. However, individual studies focused on temperate pasturelands have reported mixed responses, as changes in precipitation amounts may not significantly alter microbial biomass, allowing soil microbes to adapt to soil drying [206].
APase activity exhibits seasonal variations (
Figure 9,
Table S17), with higher activity recorded during periods of increased plant growth. In contrast, APase activity tends to be lower during drier cropping periods when human activities in agroecosystems are more pronounced [207].
The influence of anthropogenic CO2 emissions on APases are not significative but ongoing increases have been linked to enhanced ACP and ALP activities in grasslands and natural ecosystems [41], likely due to elevated microbial activity and increased soil P availability [208] (
Table S17). not sufficient to determine the reason why this trend is the way it is.
A summary of APase responses to climate change factors is shown in
Figure 9.
3.5. Relationship between APases and crop yields
Investigating the potential effects of promoting APase activity in agricultural soils on crop yields is important for addressing global goals of increasing food security and crop productivity. Studies have primarily focused on cereals, although a few other crops have also been examined (
Table S18). Positive connections have been observed between APase activity and yields of wheat, maize, barley, beet, fava bean, and lentil. However, the available literature does not show any association between APase activity and tree fruit yields (such as organic plum and orange). Interestingly, a negative relationship has been reported between rice yield and ALP activity, which can be attributed to variations in P availability from inorganic and organic sources, other P-regulating enzymes, and changes in soil pH [209]. Crop yield is influenced by various soil physicochemical parameters, including N, SOM, and high accumulation of dry matter [210,211]. Additionally, while crop yields are directly correlated with the amount of plant available P [212] and low soil available P directly affects APase release, there are limited studies that have directly associated APases with crop yield, suggesting that this link needs further research.
4. Conclusions
Due to the extensive number of studies evaluated and the results obtained, this systematic review, which is partly quantitative but predominantly qualitative, underscores the significance of APases in driving P uptake in agroecosystems and their role in the global P cycle. Observable changes in APase activity can be attributed to soil biophysicochemical properties, agricultural management practices, environmental pollutants, and climate change factors.
Firstly, microbial abundance, biomass, and activity demonstrate a positive relationship with both ACP and ALP. These enzymes are further correlated with pH levels, showing a positive association with soil texture—especially clay content—soil moisture, soil organic carbon, and available forms of nitrogen and phosphorus.
Secondly, the activity of ACP and ALP is generally enhanced by management practices promoting soil health. These practices include optimal irrigation, conservation or no-tillage techniques, crop rotation or intercropping, cover crops, and organic fertilization through the use of amendments such as organic manures, vermicompost, green manures, crop residue management, biochar, and biostimulants/biofertilizers containing beneficial bacteria and fungi (see
Figure 10).
On the other hand, factors such as soil depth, salinity, pesticide and sewage sludge use, and high concentrations of heavy metals or other pollutants in agricultural soils have a detrimental effect on APase activity. For this reason, the activity of APases is used as an indicator of soil quality in agricultural systems.
Several knowledge gaps have been identified in this review, such as the relationship between APases and crop productivity, which still remains unclear. However, there seems to be a direct relationship between cereal and legume production with the activity of APases that should be studied, especially when intercropping or crop rotations are used. Reviewing APase responses to crop management practices is problematic due to the diverse and complex nature of agronomic techniques. Thus, interrelation between P availability on one hand and the production and activity of APase on the other hand exhibits highly nuanced cause-and-effect dynamics. However, it is noteworthy that the adoption of conservative soil practices linked to non-intensive agricultural management holds promise for enhancing the response of APase activity.
The relationship between APases and P has been widely studied, but not the relationship with K, which is also important for plant growth and soil fertility. Plant uptake of P is influenced by the availability of K, which in turn depends on N and C levels. This extremely complex mechanism, involving microorganisms as well, should be experimentally studied, incorporating these strategies that increase enzymatic capacity investment and reduce competition and interference with other organisms.
Moreover, strategies to affect APase activity also involve other soil parameters altered by agricultural practices. For instance, increased CO32-, which is carried by water and mobilized between soil horizons and common in the pH range of agricultural soils, negatively affects the activity of ACP which is directly linked to plants and consequently may affect their production. Moreover, assessing APase with respect to the availability of nutrients (P or N) in relation to C (e.g., C:P, C:N) would yield valuable information to designate it as a key soil quality variable. These ratios are crucial indicators of soil fertility, microbial activity and plant nutrient uptake, influencing the overall ecosystem health and productivity. The repeated, excessive use of mineral fertilizers in agricultural soils for decades has substantially altered the microbial population adapting to this nutrient excess which directly affects ALP activity mainly released by soil microorganisms. Studies evaluating the response of APases based on soil mesofauna, as well as macrofauna, which regulate soil organic matter transformations and significantly influence nutrient dynamics, are lacking. The activity of these organisms alongside the involved species can notably change P availability in active soils and in parallel, may enhance crop yield.
Ultimately, although the selected studies are too diverse to produce a meaningful summary estimate of the effect of more than two factors, the results demonstrate that there is sufficient data to focus on combined factors that clearly enhance APase activity. The information obtained will enable us to manage agricultural systems to promote the capabilities of plants and associated microorganisms to assimilate nutrients more effectively and rapidly, and at the same time enhance our understanding of microbial-mediated processes and the dynamics of soil health. The results obtained could guide professional practice on one hand, and future research on the other. This approach could achieve a cost-benefit ratio where APases, among other enzymes, would play a determining role.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org., Figure S1: title; Table S1: title; Video S1: title.
Author Contributions
Conceptualization, P.C.R, X.D, C.P and J.P; methodology, P.C.R.; data collection and formal analysis, P.C.R; writing—original draft preparation, P.C.R.; writing—review and editing, P.C.R, X.D, C.P, J.P; funding acquisition, X.D, J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JP’s research was supported by the TED2021-132627B-I00 grant, funded by MCIN and the European Union NextGeneration EU/PRTR.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Acknowledgments
Giovanni Peratoner at Laimburg Research Center (Italy) for help in statistical understanding of field responses of APases.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wrage, N., Chapuis-Lardy, L., Isselstein, J. Phosphorus, Plant Biodiversity and Climate Change. In: Lichtfouse, E. (eds) Sociology, Organic Farming, Climate Change and Soil Science. Sust. Agric. Rev., 2010, vol 3. Springer, Dordrecht. [CrossRef]
- Malhotra, H., Vandana, Sharma, S., Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In: Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B. (eds) Plant Nutrients and Abiotic Stress Tolerance, 2018. Springer, Singapore. [CrossRef]
- Ghosh, P., Rathinasabapathi, B., Ma, L. Q. Phosphorus solubilization and plant growth enhancement by arsenic-resistant bacteria. Chemosphere, 2015, 134, 1-6. [CrossRef]
- Zhu, J., Li, M., Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sc. Total Environ., 2018, 612. [CrossRef]
- Peñuelas, J., Poulter, B., Sardans, J., Ciais, P., van der Velde, M., Bopp, L., Boucher, O., Godderis Y., Hinsinger, P., Llusia, J., Nardin, E., Vicca, S., Obersteiner, M., Janssens, I.A. Human-Induced Nitrogen–Phosphorus Imbalances Alter Natural and Managed Ecosystems across the Globe. Nat. Commun., 2013, 4(1):2934. [CrossRef]
- Bandick, A.K., Dick, R.P. Field management effects on enzyme activities. Soil Biol. Biochem., 1999, 31:1471-1479.
- Dick, W.A. Influence of long-term tillage and crop rotation combinations on soil enzyme activities. Soil Sci. Soc. Am. J., 1984, 48(3), 569–574. [CrossRef]
- Tabatabai M.A. Methods of soil analysis, Part 2. Microbiological and Biochemical properties. Chapter 37: Soil Enzymes. Soil Sci. Soc. Am. J., 1994, 677 S. Segoe Rd. Madison, WI 53711, USA.
- Burns, R.G. Soil enzymes. Academic Press, 1978, London. c.9b01784.
- Kiss, S., Pasca, D., Drägan-Bularda, M. Enzymology of disturbed soils. Dev. Soil Sc., 1998, 26. Elsevier, Amsterdam.
- Allison, S. D., Weintraub, M. N., Gartner, T. B., Waldrop, M. P. 2010. Evolutionary-economic principles as regulators of soil enzyme production and ecosystem function. In G. Shukla & A. Varma (Eds.), Soil Enzym. Soil Biol., 2010, Vol. 22. Springer. https://doi. org/10.1007/978-3-642-14225-3_12.
- Zuccarini, P., Sardans, J., Asensio, L., Peñuelas, J. Altered activities of extracellular soil enzymes by the interacting global environmental changes. Glob. Change Biol., 2023, 29, 2067–2091. [CrossRef]
- Burns, R.G. Dick, R.P. Enzymes in the environament. Act., Ecol. Appl., 2002, Marcel Dekker, New York.
- Schmidt, G., Laskowski, M.Sr. Phosphate ester cleavage (Survey), p. 3.35. In P.D. Boyer et al. (ed.) The enzymes. 2nd ed., 1961, Academic Press, New York.
- Acosta-Martínez, V., Lascano, R., Calderón, F., Booker, J. D., Zobeck, T.M., Upchurch, D.R. Dryland Cropping Systems Influence the Microbial Biomass and Enzyme Activities in a Semiarid Sandy Soil. Biol. Fert. Soils, 2011, 47(6):655–67. [CrossRef]
- Cawley, G.C. Leave-one-out cross-validation based model selection criteria for weighted LS-SVMs, in the 2006 IEEE International Joint Conference on Neural Network Proceedings (Vancouver, BC), 2006, 1661–1668. [CrossRef]
- Burns, R.G. Enzyme activity in soil: location and a possible role in microbial ecology. Soil Biol. Biochem., 1982, 4(5):423-427. [CrossRef]
- Joner, E.J., van Aarle, I.M., Vosatka, M. Phosphatase activity of extra-radical arbuscular mycorrhizal hyphae: A review. Plant Soil, 2000, 226: 199-210. [CrossRef]
- Park, Y., Solhtalab, M., Thongsomboon, W., Aristilde, L. Strategies of organic phosphorus recycling by soil bacteria: acquisition, metabolism, and regulation. Environ. Micro. R., 2022, 14(1): 3-24. [CrossRef]
- McLean, J., and Gahan P.B. The Distribution of Acid Phosphatases and Esterases in Differentiating Roots of Vicia Faba. Histochemie, 1970, 24(1):41–49. [CrossRef]
- Juma, N.G., Tabatabai, M.A. Phosphatase activity in corn and soybean roots: Conditions for assay and effects of metals. Plant Soil, 1988, 107: 39-47.
- Carricondo-Martínez, I., Falcone, D., Berti, F., Orsini, F., Salas-Sanjuan, M.D.C. Use of Agro-Waste as a Source of Crop Nutrients in Intensive Horticulture System. Agron., 2022, 12, 1–12. [CrossRef]
- Alef, K., Nannipieri, P. (Eds.) 7 - Enzyme activities, in: Methods in Applied Soil Microbiology and Biochemistry. Academic Press, London, 1995, pp. 311–373. [CrossRef]
- Tarafdar, J.C., Claassen, N. Organic Phosphorus Compounds as a Phosphorus Source for Higher Plants through the Activity of Phosphatases Produced by Plant Roots and Microorganisms. Biol. Fert. Soils, 1988, 5(4):308–12. [CrossRef]
- Juma, N.G., Tabatabai, M.A. Effects of trace elements on phosphatase activity in soils. Soil Sci. Soc. Am. J., 1977, 41: 343-346.
- Juma, N.G., Tabatabai, M.A. Distribution of phosphomonoesterases in soils. Soil Sci., 1978, 126:101-108.
- Tabatai M.A., Methods of soil analysis, Part 2. Microbiological and Biochemical properties. Chapter 37: Soil Enzymes. Soil Science Society of America, 1994, 677 S. Segoe Rd. Madison, WI 53711, USA.
- Neal, A.L., Blackwell, M., Akkari, E., Guyomar, C., Clark, I., and Hirsch, P.R. Phylogenetic distribution, biogeography and the effects of land management upon bacte- rial non-specific acid phosphatase gene diversity and abundance. Plant Soil, 2018, 427: 175–189.
- Ragot, S.A., Kertesz, M.A., Mészaros, E., Frossard, E., Bünemann, E.K. Soil phoD and phoX alkaline phosphatase gene diversity responds to multiple environmental factors. FEMS Microbiol Ecol 93: 1–15.10.1093/femsec/fiw212.Rao, M.A., Scelza, R., Gianfreda, L., 2014. Soil enzymes. In Enzymes in Agricultural Sciences, 2017, Eds.; OMICS Group eBooks: Foster City, CA, 2014; pp 10−43.
- Faostat. Comparar datos (2021, 17 September.). Organización de las Naciones Unidas para la alimentación y la Agricultura http://www.fao.org/faostat/es/#compare.
- Margalef, O., Sardans, J., Fernández-Martínez, M., Molowny-Horas, R., Janssens, A., Ciais, P., Goll, D., Richter, A., Obersteiner, M., Asensio, D., Peñuelas, J. Global patterns of phosphatase activity in natural soils. Sc. R., 2017, 7:1337. [CrossRef]
- Arora, R., Sharma, V., Sharma, S., Maini, A., Dhaliwal, S. S. Temporal Changes in Soil Biochemical Properties with Seasons under Rainfed Land Use Systems in Shiwalik Foothills of Northwest India. Agrof. Syst., 2021, 95(8):1479–91. [CrossRef]
- Choudhary, M., Jat, H.S., Datta, A., Yadav, A.K., Sapkota, T.B., Mondal, S., Meena, R.P., Sharma, P.C., Jat, M.L. Sustainable Intensification Influences Soil Quality, Biota, and Productivity in Cereal-Based Agroecosystems. Ap. Soil Ecol., 2018, 126:189–98. [CrossRef]
- Dick, R.P., Rasmussen, P.E., Kerle, E.A. Influence of Long-Term Residue Management on Soil Enzyme Activities in Relation to Soil Chemical Properties of a Wheat-Fallow System. Biol. Fert. Soils, 1988, 6(2):159–64. [CrossRef]
- Eichler-Löbermann, B., Zicker, T., Kavka, M., Busch, S., Brandt, C., Stahn, P., Miegel, K. Mixed Cropping of Maize or Sorghum with Legumes as Affected by Long-Term Phosphorus Management. Field Crops Res., 2021, 265. [CrossRef]
- Chen, S., Cade-Menun, B.J., Bainard, L.K., Luce, M. St., Hu, Y., Chen, Q. The Influence of Long-Term N and P Fertilization on Soil P Forms and Cycling in a Wheat/Fallow Cropping System. Geoderma, 2021, 404:115274. [CrossRef]
- Dutta, D., Meena, A.L., Chethan Kumar, G., Mishra, R.P., Ghasal, P.C., Kumar, A., Chaudhary, J., Bhanu, C., Kumar, V., Kumar, A., Tewari, R.B., Panwar, A.S. Long Term Effect of Organic, Inorganic and Integrated Nutrient Management on Phosphorous Dynamics under Different Cropping Systems of Typic Ustochrept Soil of India. Com. Soil Sci. Plant Anal., 2020, 51(21):2746–63. [CrossRef]
- Singh, S. R., Kundu, D.K., Dey, P., Singh, P., Mahapatra, B.S. Effect of Balanced Fertilizers on Soil Quality and Lentil Yield in Gangetic Alluvial Soils of India. J. Agric. Sci., 2018a, 156(2):225–40. [CrossRef]
- Grafe, M., Kurth, J.K., Panten, K., Raj, A.D., Baum, C., Zimmer, D., Leinweber, P., Schloter, M., Schulz, S. Effects of Different Innovative Bone Char Based P Fertilizers on Bacteria Catalyzing P Turnover in Agricultural Soils. Agric., Ecos. Environ., 2021, 314(February):107419. [CrossRef]
- Monkiedje, A., Spiteller, M., Fotio, D., Sukul, P. The Effect of Land Use on Soil Health Indicators in Peri-Urban Agriculture in the Humid Forest Zone of Southern Cameroon. J. Environ. Quality, 2006, 35(6):2402–9. [CrossRef]
- Margalef, O., Sardans, J., Maspons, J., Molowny-Horas, R., Fernández-Martínez, M., Janssens, I.A., Richter, A., Ciais, P., Obersteiner, M., Peñuelas, J. The effect of global change on soil phosphatase activity. Glob. Chang. Biol., 2021, 27, 5989–6003. [CrossRef]
- Sun Y., Goll, D.S., Ciais, P., Peng, S., Margalef, O., Asensio, D., Sardans, J., Peñuelas, J. Spatial Pattern and Environmental Drivers of Acid Phosphatase Activity in Europe. Fron. Big Data, 2020, 3: 51. [CrossRef]
- Meng, C., Dashuan T., Hui Z., Zhaolei L., Han Y., Chen H., Niu, S. Global Meta-Analysis on the Responses of Soil Extracellular Enzyme Activities to Warming. Sci. Total Environ., 2020, 705:135992. [CrossRef]
- Gao, D., Bai, E., Li, M., Zhao, C., Yu, K., Hagedorn, F. Responses of Soil Nitrogen and Phosphorus Cycling to Drying and Rewetting Cycles: A Meta-Analysis. Soil Biol. Biochem., 148, 2020, 107896. [CrossRef]
- Janes-Bassett, V., Blackwell, M.S.A., Blair, G., Davies, J., Haygarth, P.M., Mezeli, M.M., Stewart, G. A meta-analysis of phosphatase activity in agricultural settings in response to phosphorus deficiency. Soil Biol. Biochem., 2022 ,165 : 108537. [CrossRef]
- Pokharel, P., Ma, Z., Chang, S.X. Biochar increases soil microbial biomass with changes in extra- and intracellular enzyme activities: a global meta-analysis. Biochar, 2020, 2: 65–79 . [CrossRef]
- Miao, F., Li, Y., Cui, S., Jagadamma, S., Yang, G., Zhang, Q. Soil extracellular enzyme activities under long-term fertilization management in the croplands of China: a meta- analysis. Nut. Cyc. Agroecosyst., 2019,114, pages 125–138. [CrossRef]
- Jian, S., Li, J., Chen, J., Wang, G., Mayes, M.A., Dzantor, K.E., Hui, D., Luo, Y. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem., 2016,101: 32e43. [CrossRef]
- Marklein, A.R., Houlton, B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phyt., 2012, 193 (3): 696-704. [CrossRef]
- Aponte, H., Meli, P., Butler, B., Paolini, J., Matus, F., Merino, C., Cornejo, P., Kuzyakov, Y. Meta-analysis of heavy metal effects on soil enzyme activities. Sci. Total Environ., 2020, 737 : 139744. [CrossRef]
- Riah, W., Laval, K., Laroche-Ajzenberg, E., Mougin, C., Latour, X., Trinsoutrot-Gattin, I. Effects of pesticides on soil enzymes: a review. Environ. Chem. Let., 2014,12:257-273. [CrossRef]
- Tabatabai M.A., Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem., 1969, 1: 301-307.
- Eivazi, F., Tabatabai, M.A. Phosphatases in Soils. Soil Biol. Biochem., 1977, 9, 167-172. [CrossRef]
- Wang, F., Jiang, R., Kertesz, M.A., Zhang, F., Feng, G. Arbuscular mycorrhizal fungal hyphae mediating acidification can promote phytate mineralization in the hyphosphere of maize (Zea mays L.). Soil Biol. Biochem., 2013, 65:69-74. [CrossRef]
- Gesolmino, A., Azzellino, A. Multivariate Analysis of Soils: Microbial Biomass, Metabolic Activity, and Bacterial-community Structure and Their Relationships with Soil Depth and Type. J. Plant Nutr. Soil Sci., 2011, 174(3):381–94. [CrossRef]
- Chowdhury, N., Rasid, M.M. Evaluation of brick kiln operation impact on soil microbial biomass and enzyme activity. Soil Sci. An., 2021, 72: 132232-.10.37501/soilsa/132232.
- Sharma, P., Singh, G., Singh, R.P. Conservation Tillage and Optimal Water Supply Enhance Microbial Enzyme (Glucosidase, Urease and Phosphatase) Activities in Fields under Wheat Cultivation during Various Nitrogen Management Practices. Arch. Agron. Soil Sci., 2013, 59(7):911–28. [CrossRef]
- Scaramal da Silva, A., Colozzi Filho, A., Shigueyoshi Nakatani, A., José Alves, S., Souza de Andrade, D., Guimarães, MdF. Atributos Microbiológicos Do Solo Em Sistema de Integração. R. Bras. Cien. Do Solo, 2015, 39(1):40–48. [CrossRef]
- Woźniak, M., Gałazką, A., Siebielec, G., Frąc, M. Can the Biological Activity of Abandoned Soils Be Changed by the Growth of Paulownia Elongata × Paulownia Fortunei- Preliminary Study on a Young Tree Plantation. Agric. (Switzerland), 2022, 12(2). [CrossRef]
- Dubey, A.N., Chattopadhyaya, N., Mandal, N. Variation in Soil Microbial Population and Soil Enzymatic Activities Under Zincated Nanoclay Polymer Composites (ZNCPCs), Nano-ZnO and Zn Solubilizers in Rice Rhizosphere. Agric. Res., 2021, 10(1):21–31. [CrossRef]
- Antolín, M. C., Pascual, I., García, C., Polo, A., Sánchez-Díaz, M. Growth, Yield and Solute Content of Barley in Soils Treated with Sewage Sludge under Semiarid Mediterranean Conditions. Field Crops Res., 2005, 94(2–3):224–37. [CrossRef]
- Maini, A., Sharma, V., Sharma, S. Assessment of Soil Biochemical Properties and Soil Quality Index under Rainfed Land Use Systems in Submontane Punjab, India. Indian J. Biochem. Biophys., 2022, 59(3):357–67. [CrossRef]
- Borase, D.N., Nath, C.P., Hazra, K.K., Senthilkumar, M., Singh, S.S., Praharaj, C.S., Singh, U., Kumar, N. Long-Term Impact of Diversified Crop Rotations and Nutrient Management Practices on Soil Microbial Functions and Soil Enzymes Activity. Ecol. Ind., 2020, 114(March):106322. [CrossRef]
- Hatti, V., Ramachandrappa, B.K., Mudalagiriyappa, S.A., Thimmegowda, M.N. Soil properties and productivity of rainfed finger millet under conservation tillage and nutrient management in Eastern dry zone of Karnataka. J. Environ. Biol., 2018, 19: 612-624. http://doi.org/10.22438/jeb/39/5/MRN-724.
- Angers, D. A., Bissonnette, N., Legere, A., Samson, N. Microbial and Biochemical Changes Induced by Rotation and Tillage in a Soil under Barley Production. Canadian J. Soil Sci., 1993, 73(1):39–50. [CrossRef]
- Sepat, S., Behera, U.K., Sharma, A.R., Das, T.K., Bhattacharyya, R. Productivity, Organic Carbon and Residual Soil Fertility of Pigeonpea-Wheat Cropping System under Varying Tillage and Residue Management. Proceedings of the National Academy of Sciences India Section B., Biol. Sci., 2014, 84(3):561–71. [CrossRef]
- Wang, M., Wu, Y., Zhao, J., Liu, Y., Chen, Z., Tang, Z., Tian, W., Xi, Y., Zhang, J. Long-Term Fertilization Lowers the Alkaline Phosphatase Activity by Impacting the PhoD-Harboring Bacterial Community in Rice-Winter Wheat Rotation System. Sci. Total Environ., 2022, 821. [CrossRef]
- Banerjee, M.R., Burton, D.L., Grant, C.A. Influence of Urea Fertilization and Urease Inhibitor on the Size and Activity of the Soil Microbial Biomass under Conventional and Zero Tillage at Two Sites. Can. J. Soil Sci., 1999, 79(2):255–63. [CrossRef]
- Al-Taweel, J.L.S. and Al-Jubouri, G.A.A. Effect of Agricultural Exploitation on the Activity of Alkaline Phosphatase and Its Kinetic Properties in Some Soils. Al-Qadisiyah J. Agric. Sci., 2019, (QJAS) (P-ISSN: 2077-5822, E-ISSN: 2617-1479) 9(1):120–35. [CrossRef]
- Liu, L., Zhu, K., Wurzburger, N., Zhang, J. Relationships between plant diversity and soil microbial diversity vary across taxonomic groups and spatial scales. Ecosphere, 2020, 11(1):e02999. 10.1002/ecs2.2999.
- Furtak, K., Gawryjołek, K., Gajda, A.M., Gałązka, A. Effects of Maize and Winter Wheat Grown under Different Cultivation Techniques on Biological Activity of Soil. Plant Soil Environ., 2017, 63(10):449–54. [CrossRef]
- Mandal, N., Datta, S.C., Dwivedi, B.S., Manjaiah, K.M., Meena, M.C., Bhowmik, A. Zincated Nanoclay Polymer Composite (ZNCPC): Effect on DTPA-Zn, Olsen-P and Soil Enzymatic Activities in Rice Rhizosphere. Com. Soil Sci Plant Anal., 2021, 52(17):2032–44. [CrossRef]
- Wu, F., Wan, J.H.C., Wu, S., Wong, M. Effects of Earthworms and Plant Growth-Promoting Rhizobacteria (PGPR) on Availability of Nitrogen, Phosphorus, and Potassium in Soil. J. Plant Nut. Soil Sci., 2012, 175(3):423–33. [CrossRef]
- Tao, J., Griffiths, B., Zhang, S., Chen, X., Liu, M., Hu, F., Li, H. Effects of Earthworms on Soil Enzyme Activity in an Organic Residue Amended Rice-Wheat Rotation Agro-Ecosystem. Ap. Soil Ecol., 2019, 42(3):221–26. [CrossRef]
- Balachandar, R., Biruntha, M., Yuvaraj, A., Thangaraj, R., Subbaiya, R., Govarthanan, M., Kumar, P., Karmegam, N. Earthworm Intervened Nutrient Recovery and Greener Production of Vermicompost from Ipomoea Staphylina. An Invasive Weed with Emerging Environmental Challenges. Chemosphere, 2021, 263. [CrossRef]
- Buck, C., Langmaack, M., Schrader, S. Influence of Mulch and Soil Compaction on Earthworm Cast Properties. Ap. Soil Ecol., 2000, 14(3):223–29. [CrossRef]
- Soane, B.D., van Ouwerkerk, C. Chapter 1 - Soil Compaction Problems in World Agriculture’. Pp. 1–21 in Developments in Agricultural Engineering. 1994, Vol. 11, edited by B. D. Soane and C. van Ouwerkerk. Elsevier. [CrossRef]
- Noronha, F.R., Manikandan, S.K., Nair, V. Role of Coconut Shell Biochar and Earthworm (Eudrilus Euginea) in Bioremediation and Palak Spinach (Spinacia Oleracea L.) Growth in Cadmium-Contaminated Soil. J. ¡Environ. Manag., 2022, 302(PA):114057. [CrossRef]
- Brockett, B.F. T., Prescott C.E., Grayston, S.J. Soil Moisture Is the Major Factor Influencing Microbial Community Structure and Enzyme Activities across Seven Biogeoclimatic Zones in Western Canada. Soil Biol. Biochem., 2012, 44(1):9–20. [CrossRef]
- Ojeda, G., Patrício J., Navajas H., Comellas L., Alcañiz J.M., Ortiz O., Marks E., Natal-da-Luz T., Sousa J.P. Effects of Nonylphenols on Soil Microbial Activity and Water Retention. Ap. Soil Ecol., 2013, 64:77–83. [CrossRef]
- Calvarro, L.M., de Santiago-Martín, A., Quirós Gómez, J., González-Huecas, C., Quintana, J.R., Vázquez, A., Lafuente, A.L., Rodríguez Fernández, T.M., Ramírez Vera, R. Biological Activity in Metal-Contaminated Calcareous Agricultural Soils: The Role of the Organic Matter Composition and the Particle Size Distribution. Environ. Sci. Pol. Res., 2014, 21(9):6176–87. [CrossRef]
- Gispert, M., Emran, M., Pardini, G., Doni, S., Ceccanti, B. The Impact of Land Management and Abandonment on Soil Enzymatic Activity, Glomalin Content and Aggregate Stability. Geoderma, 2013, 202–203:51–61. [CrossRef]
- Garg, S., Bahl, G.S. Phosphorus Availability to Maize as Influenced by Organic Manures and Fertilizer P Associated Phosphatase Activity in Soils. Biores. Tech., 2008, 99(13):5773–77. [CrossRef]
- Nedyalkova, K., Donkova, R., Malinov, I. Acid Phosphatase Activity under the Impact of Erosion Level in Agricultural Soils of Different Type and Land Use. Bulgarian J. Agric. Sci., 2020, 26(6):1217–22.
- Bergstrom, D. W., Monreal, C. M., King, D. J. Sensitivity of Soil Enzyme Activities to Conservation Practices. Soil Sci. Soc. Am. J., 1998, 62(5):1286–95. [CrossRef]
- Wei, K., Chen, Z., Zhu, A., Zhang, J., Chen, L. Application of 31P NMR Spectroscopy in Determining Phosphatase Activities and P Composition in Soil Aggregates Influenced by Tillage and Residue Management Practices. Soil Till. Res., 2014,138:35–43. [CrossRef]
- Odutola O. S. Introductory Chapter: Relevance of Soil pH to Agriculture. Soil pH for Nutrient Availability and Crop Performance. IntechOpen, 2019, DOI: 10.5772/intechopen.82551.
- Mandal, A., Thakur, J.K., Sahu, A., Manna, M.C., Rao, A.S., Sarkar, B., Patra, A.K. Effects of Bt-Cotton on Biological Properties of Vertisols in Central India. Arch. Agron. Soil Sci., 2018, 65(5):670–85. [CrossRef]
- Ortiz, J., Faggioli, V.S., Ghio, H., Boccolini, M.F., Ioele, J.P., Tamburrini, P., Garcia, F.O., Gudelj, V. Long-Term Impact of Fertilization on the Structure and Functionality of Microbial Soil Community | Impacto a Largo Plazo de La Fertilización Sobre La Estructura y Funcionalidad de La Comunidad Microbiana Del Suelo. Cien. Suelo, 2020, 38(1):45–55.
- Truu, M., Truu, J., Ivask, M. Soil Microbiological and Biochemical Properties for Assessing the Effect of Agricultural Management Practices in Estonian Cultivated Soils. Euro. J. Soil Biol., 2008, 44(2):231–37. [CrossRef]
- Laxminarayana, K. Effect of Mycorrhiza, Organic Sources, Lime, Secondary and Micro-Nutrients on Soil Microbial Activities and Yield Performance of Yam Bean (Pachyrhizus Erosus L.) in Alfisols. Com. Soil Sci. Plant Anal., 2017, 48(2):186–200. [CrossRef]
- Durrer, A., Gumiere, T., Rumenos Guidetti Zagatto, M., Petry Feiler, H., Miranda Silva, A.M., Longaresi, R.H., Homma, S.K., Cardoso, E.J.B.N. Organic Farming Practices Change the Soil Bacteria Community, Improving Soil Quality and Maize Crop Yields. PeerJ, 2021, 9:1–24. [CrossRef]
- Yang, L., Zhao, F., Chang, Q., Li, T., Li, F. Effects of Vermicomposts on Tomato Yield and Quality and Soil Fertility in Greenhouse under Different Soil Water Regimes. Agric. Water Manag., 2015, 160:98–105. [CrossRef]
- Roldán, A., Salinas-García, J.R., Alguacil, M.M.M., Caravaca, F. Soil Sustainability Indicators Following Conservation Tillage Practices under Subtropical Maize and Bean Crops. Soil Till. Res., 2007, 93(2):273–82. [CrossRef]
- Swedrzyńska D, Małecka I, Blecharczyk A, Swedrzyński A, Starzyk J. Effects of various long-term tillage systems on some chemical and biological properties of soil. Pol. J. Environ. Stud., 2013, 22(6):1835–44.
- Mahmood, M., Xu, T., Ahmed, W., Yang, J., Li, J., Mehmood, S., Liu, W., Weng, J., Li, W. Variability in Soil Parent Materials at Different Development Stages Controlled Phosphorus Fractions and Its Uptake by Maize Crop. Sustain. (Switzerland), 2022, 14(9). [CrossRef]
- Siddaramappa, R., Wright, R.J., Codling, E.E., Gao, G., McCarty, G.W. Evaluation of Coal Combustion Byproducts as Soil Liming Materials: Their Influence on Soil PH and Enzyme Activities. Biol. Fert. Soils, 1994, 17(3):167–72. [CrossRef]
- Yu, S., He, Z.L., Stoffella, P.J., Calvert, D.V., Yang, X.E., Banks, D.J., Baligar, V.C. Surface Runoff Phosphorus (P) Loss in Relation to Phosphatase Activity and Soil P Fractions in Florida Sandy Soils under Citrus Production. Soil Biol. Biochem., 2006, 38(3):619–28. [CrossRef]
- Meena, H.M., Prakasha, H.C. The Impact of Biochar, Lime and Fertilizer on Soil Acidity and Microbiological Properties and Their Relationship with Yield of Rice and Cowpea in an Acidic Soil of Southern India. J. Plant Nutr., 2021, 45(3):358–68. [CrossRef]
- Purnamasari, L., Rostaman, T., Widowati, L. R. Anggria, L. Comparison of Appropriate Cation Exchange Capacity (CEC) Extraction Methods for Soils from Several Regions of Indonesia. IOP Conference Series: Earth Environ. Sci., 2021, 648(1):012209. [CrossRef]
- Smith, J.L., Doran. J.W. Measurement and Use of pH and Electrical Conductivity for Soil Quality Analysis. Pp. 169–85 in Methods for Assessing Soil Quality, SSSA Special Publications, 1996. [CrossRef]
- Dinesh, R., Ramanathan, G., Singh, H. Influence of Chloride and Sulphate Ions on Soil Enzymes. J. Agron. Crop Sci., 1995, 175(2):129–33. [CrossRef]
- Rietz, D.N., Haynes, R.J. Effects of Irrigation-Induced Salinity and Sodicity on Soil Microbial Activity. Soil Biol. Biochem., 2003, 35(6):845–54. [CrossRef]
- Garcia, C., Hernandez, T. Influence of Salinity on the Biological and Biochemical Activity of a Calciorthird Soil. Plant and Soil, 1996, 178(2):255–63. [CrossRef]
- European Commission - DG ENV. Soil biodiversity: functions, threats and tools for policy makers. Technical report 2010-049. [CrossRef]
- Tipping, E., Somerville, C.J., Luster, J. The C:N:P:S stoichiometry of soil organic matter. Biogeochem., 2016, 130, 117–131. [CrossRef]
- Bergstrom, D. W., Monreal, C. M., King, D. J., Sensitivity of Soil Enzyme Activities to Conservation Practices. Soil Sci. Soc. Am. J., 1998, 62(5):1286–95. [CrossRef]
- Kooch, Y., Ehsani, S., Akbarinia, M. Stoichiometry of Microbial Indicators Shows Clearly More Soil Responses to Land Cover Changes than Absolute Microbial Act.. Ecol. Eng., 2019, 131:99–106. [CrossRef]
- Lungmuana, Singh, S.B.B., Choudhury, B.U.U., Vanthawmliana, Saha, S., Hnamte, V. Transforming Jhum to Plantations: Effect on Soil Microbiological and Biochemical Properties in the Foot Hills of North Eastern Himalayas, India. Catena, 2019, 177:84–91. [CrossRef]
- de Jesus Franco, A., Valadares da Silva, A.P., Silva Souza, A.B., Loverde Oliveira, R., Rodrigues Batista, E., Damacena de Souza, E., Oliveira Silva, A., Carbone Carneiro, M.A. Plant diversity in integrated crop-livestock systems increases the soil enzymatic activity in the short term. Pesq. Agropec. Trop., Goiânia, 2020, 50: 1-11. [CrossRef]
- Monkiedje, A., Spiteller, M., Fotio, D., Sukul, P. The Effect of Land Use on Soil Health Indicators in Peri-Urban Agriculture in the Humid Forest Zone of Southern Cameroon. J. Environ. Qual., 2006, 35(6):2402–9. [CrossRef]
- Adrover, M., Moyà, G., Vadell, J. Seasonal and Depth Variation of Soil Chemical and Biological Properties in Alfalfa Crops Irrigated with Treated Wastewater and Saline Groundwater. Geoderma, 2017, 286:54–63. [CrossRef]
- Palmer, J., Thorburn, P.J., Biggs, J.S., Dominati, E.J., Probert, M.E., Meier, E.A., Huth, I.N, Dodd, M., Snow, V., Larsen, J.R., Parton, W.J. Nitrogen Cycling from Increased Soil Organic Carbon Contributes Both Positively and Negatively to Ecosystem Services in Wheat Agro-Ecosystems. Front. Plant Sci., 2017, 8:731. [CrossRef]
- Cattaneo, F., Di Gennaro, P., Barbanti, L., Giovannini, C., Labra, M., Moreno, B., Benitez, E., Marzadori, C. Perennial Energy Cropping Systems Affect Soil Enzyme Activities and Bacterial Community Structure in a South European Agricultural Area. Ap. Soil Ecol., 2014, 84:213–22. [CrossRef]
- Singh, A., Ghoshal, N. Impact of Herbicide and Various Soil Amendments on Soil Enzymes Activities in a Tropical Rainfed Agroecosystem. Euro. J. Soil Biol., 2013, 54:56–62. [CrossRef]
- Sigua, G.C., Stone, K.C., Bauer, P.J., Szogi, A.A. Phosphorus Dynamics and Phosphatase Activity of Soils under Corn Production with Supplemental Irrigation in Humid Coastal Plain Region, USA. Nutr. Cycl. Agroecosyst., 2017 109(3):249–67. [CrossRef]
- Weil, R.R., Brady, N.C.The Nature and Properties of Soils. 15th Edition, 2017, Pearson, New York.
- Cross, A.F., Schlesinger, W.H. A literature review and evaluation of the Hedley fractionation: applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma, 1995, 64, 197–214. [CrossRef]
- Zibilske, L. M., Bradford, J.M. Tillage Effects on Phosphorus Mineralization and Microbial Activity. Soil Sci., 2003, 168(10):677–85. [CrossRef]
- Olsen, S.R., Sommers, L.E. Phosphorus. In: Page, A.L., Ed., Methods of Soil Analysis Part 2 Chemical and Microbiological Properties, American Society of Agronomy, Soil Science Society of America, 1982, Madison, 403-430.
- Li F.R., Liu L.L., Liu J.L., Yang K. Abiotic and biotic controls on dynamics of labile phosphorus fractions in calcareous soils under agricultural cultivation. Sci. Total Environ., 2019, 2019;681:163–74. Available from: https://doi.org/10.1016/j.scitotenv.2019.05.091.
- Koper, J., Lemanowicz, J. Effect of Varied Mineral Nitrogen Fertilization on Changes in the Content of Phosphorus in Soil and in Plant and the Activity of Soil Phosphatases. Ecol. Chem. Eng., 2008, S 15(4):465–71.
- Atoloye, I.A., Jacobson, A., Creech, E., Reeve, J. Variable Impact of Compost on Phosphorus Dynamics in Organic Dryland Soils Following a One-Time Application. Soil Sci. Soc. Am. J., 2021, 85(4):1122–38. [CrossRef]
- Madejón, E., Burgos, P., López, R., Cabrera, F. Agricultural Use of Three Organic Residues: Effect on Orange Production and on Properties of a Soil of the “Comarca Costa de Huelva” (SW Spain). Nutr. Cyc. Agroecosyst., 2003, 65(3):281–88. [CrossRef]
- Li, Q., Chen, J., Wu, L., Luo, X., Li, Na, Arafat, Y., Lin, S., Lin, W. Belowground Interactions Impact the Soil Bacterial Community, Soil Fertility, and Crop Yield in Maize/Peanut Intercropping Systems. Inte. J. Molec. Sci., 2018, 19: 622. 10.3390/ijms19020622.
- Waldrop, M.P., Balser, T.C., Firestone, M.K. Linking Microbial Community Composition to Function in a Tropical Soil. Soil Biol. Biochem., 2000, 32(13):1837–46. [CrossRef]
- Khan, S.A., Mulvaney, R.L., Ellsworth, T.R. The Potassium Paradox: Implications for Soil Fertility, Crop Production and Human Health. Ren. Agric. Food Syst., 2014, 29(1):3–27. [CrossRef]
- Honvault, N., Houben, D., Nobile, C., Firmin, S., Lambers, H., Faucon, M.P. Tradeoffs among Phosphorus-Acquisition Root Traits of Crop Species for Agroecological Intensification. Plant and Soil, 2020, 461(1–2):137–50. [CrossRef]
- Riffaldi, R., Saviozzi, A., Levi-Minzi, R., Cardelli, R. Biochemical Properties of a Mediterranean Soil as Affected by Long-Term Crop Management Systems. Soil Till. Res., 2022, 67(1):109–14. [CrossRef]
- Cui, Y., Fang, L., Guo, X., Wang, X., Wang, Y., Zhang, Y., Zhang, X. Responses of Soil Bacterial Communities, Enzyme Activities, and Nutrients to Agricultural-to-Natural Ecosystem Conversion in the Loess Plateau, China. J. Soils Sed., 2019, 19(3). [CrossRef]
- Li, C., Veum, K.S., Goyne, K.W., Nunes, M.R., Acosta-Martinez, V.A. Chronosequence of soil health under tallgrass prairie reconstruction: Ap. Soil Ecol., 2021, 164: 103939-.10.1016/j.apsoil.2021.103939.
- Da Cunha, J.R., de Cassia de Freitas, R., de Almeida Taveres Souza, D.J., Santana Gualberto, A.V., Antunes de Souza, H., Fernando Carvalho Leite, L. Soil Biological Attributes in Monoculture and Integrated Systems in the Cerrado Region of Piaui State, Brazil. Acta Scientiarum-Agronomy, 2021 43. [CrossRef]
- Balota, E., L., Machineski, O., Truber, P.V. Soil Enzyme Activities under Pig Slurry Addition and Different Tillage Systems. Agron., 2011, 33(4):729–37. [CrossRef]
- Barcelos Martins, L.N., de Aguiar Santiago, F.L., Montecchia, M.S., Correa, O.S., Saggin Junior, O.J., Damacena de Souza, E., Barbosa Paulino, H., Carbone Carneiro, M.A. Biochemical and Biological Properties of Soil from Murundus Wetlands Converted into Agricultural Systems. R. Bras. Cienc. Solo, 2019, 43 :e0180183. [CrossRef]
- Carlos, F.S., Schaffer, N., Mariot, R.F., Schmitt Fernandes, R., Luiz Boechat, C., Fernando Wurdig Roesch, L., de Oliveira Camargo, F.A. Soybean Crop Incorporation in Irrigated Rice Cultivation Improves Nitrogen Availability, Soil Microbial Diversity and Activity, and Growth of Ryegrass. Ap. Soil Ecol., 2022, 170. [CrossRef]
- Katsalirou, E., Deng, S., Gerakis, A., Nofziger, D.L. Long-Term Management Effects on Soil P, Microbial Biomass P, and Phosphatase Activities in Prairie Soils. European J. Soil Biol., 2016, 76:61–69. [CrossRef]
- Sciubba, L., Mazzon, M., Cavani, L., Baldi, E., Toselli, M., Ciavatta, C., Marzadori, C. Soil Response to Agricultural Land Abandonment: A Case Study of a Vineyard in Northern Italy. Agronomy, 2021, 11(9). [CrossRef]
- Garcia, C., Roldan, A., Hernandez, T. Changes in Microbial Activity after Abandonment of Cultivation in a Semiarid Mediterranean Environment. J. Environ. Qual., 1997, 26(1):285–91. [CrossRef]
- Paz-Ferreiro, J., Trasar-Cepeda, C., Leiros, M.C., Seoane, S., Gil-Sotres, F. Biochemical Properties in Managed Grassland Soils in a Temperate Humid Zone: Modifications of Soil Quality as a Consequence of Intensive Grassland Use. Biol., Fert. Soils, 2009, 45(7):711–22. [CrossRef]
- Woźniak, A., and Kawecka-Radomska, M. Crop Management Effect on Chemical and Biological Properties of Soil. Inter. J. Plant Prod., 2016, 10(3):391–402.
- Martins Sousa, H., Ribeiro Correa, A., de Motta Silva, B., da Silva Oliveira, S., da Silva Campos, D.T., Wruck, F.J. Dynamics of soil microbiological attributes in integrated crop-livestock systems in the cerrado-amozonônia ecotone. R. Catinga, 2020, 33(1):9–20. [CrossRef]
- Dou, F., Wright, A.L., Mylavaparu, R.S., Jiang, X., Matocha, J.E. Soil Enzyme Activities and Organic Matter Composition Affected by 26 Years of Continuous Cropping. Pedosphere, 2016, 26(5):618–25. [CrossRef]
- Ansari, M. A., Saha, S., Das, A., Lal, R., Das, B., Choudhury, B. U., Roy, S. S., Sharma, S. K., Singh, I. M., Meitei, Ch.B., et al. Energy and Carbon Budgeting of Traditional Land Use Change with Groundnut Based Cropping System for Environmental Quality, Resilient Soil Health and Farmers Income in Eastern Indian Himalayas. J. Environ. Manag., 2021, 293: 112892. [CrossRef]
- Gao, Y., Zhou, P., Mao, L., Zhi, Y., Zhang, C., Shi, W. Effects of Plant Species Coexistence on Soil Enzyme Activities and Soil Microbial Community Structure under Cd and Pb Combined Pollution. J. Environ. Sci., 2010, 22(7):1040–48. [CrossRef]
- Wang, X., Deng, X., Pu, T., Song, C., Yong, T., Yang, F., Sun, X., Liu, W., Yan, Y., Du, J., Su, K., Yang W. Contribution of Interspecific Interactions and Phosphorus Application to Increasing Soil Phosphorus Availability in Relay Intercropping Systems. Field Crops Res., 2017, 204:12–22. [CrossRef]
- Saad, R.F., Kobaissi, A., Echevarria, G., Kidd, P., Calusinska, M., Goux, X., Benizri, E. Influence of New Agromining Cropping Systems on Soil Bacterial Diversity and the Physico-Chemical Characteristics of an Ultramafic Soil. Sci. Total Environ., 2018, 645:380–92. [CrossRef]
- Aparna, K., Rao, D. L. N., Balachandar, D. Microbial Populations, Activity and Gene Abundance in Tropical Vertisols Under Intensive Chemical Farming. Pedosphere, 2016, 26(5):725–32. [CrossRef]
- Lago, M. del C.F., Gallego, P.P., Briones, M.J.I. Intensive cultivation of kiwifruit alters the detrital foodweb and accelerates soil C and N losses. Front. Microbiol., 2019, 10, 1–10. [CrossRef]
- Feng, H., Sekaran, U., Wang, T., Kumar, S. On-Farm Assessment of Cover Cropping Effects on Soil C and N Pools, Enzyme Activities, and Microbial Community Structure. J. Agric. Sci., 2021, 159(3–4):216–26. [CrossRef]
- Adetunji, A.T., Ncube, B., Meyer, A.H., Olatunji, O.S., Mulidzi, R., Lewu, F.B. Soil PH, Nitrogen, Phosphatase and Urease Activities in Response to Cover Crop Species, Termination Stage and Termination Method. Heliyon, 2021, 7(1):e05980. [CrossRef]
- Yadav, D., Shivay, Y.S., Singh, Y.V., Sharma, V.K., Bhatia, A. Water Use and Soil Fertility under Rice–Wheat Cropping System in Response to Green Manuring and Zinc Nutrition. Com. Soil Sci. Plant Analys., 2019, 50(22):2836–47. [CrossRef]
- Balota, E. L., Chaves, D., César, J. Enzymatic activity and mineralization of carbon and nitrogen in soil cultivated with coffee and green manures. Rev. Bras. Ciênc. Solo., 2010, 34(5):1573-83. DOI: 10.1590/S0100-06832010000500010.
- Wang, M., Wu, C., Cheng, Z., Meng, H., Zhang, M. Soil Chemical Property Changes in Eggplant/Garlic Relay Intercropping Systems under Continuous Cropping. Plos one, 2014, 9(10): e111040. [CrossRef]
- Kroulík, M., Kumhála, F., Hůla, J., Honzík, I. The Evaluation of Agricultural Machines Field Trafficking Intensity for Different Soil Tillage Technologies’. Soil Till. Res., 2009, 105(1):171–75. [CrossRef]
- Buhler, D.D. Weed management. Pp. 323–28 in Encyclopedia of Soils in the Environment, 2005, edited by D. Hillel. Oxford: Elsevier.
- Swedrzynska D., Małecka I., Blecharczyk A., Swędrzyński A., Starzyk J. Effects of Various Long-Term Tillage Systems on Some Chemical and Biological Properties of Soil. Pol. J. Environ.l Stud., 2013, 22:1835–44.
- Melero, S., López-Bellido, R.J., López-Bellido, L., Muñoz-Romero, V., Moreno, F., Murillo, J.M. Long-Term Effect of Tillage, Rotation and Nitrogen Fertiliser on Soil Quality in a Mediterranean Vertisol. Soil Till. Res., 2011, 114(2):97–107. [CrossRef]
- Gajda, A.M., and Przewłoka, B. Soil Biological Activity as Affected by Tillage Intensity. Inter. Agrophys., 2012, 26(1):15–23. [CrossRef]
- Parihar, C.M., Yadav, M.R., Jat, S.L., Singh, A.K., Kumar, B., Pradhan, S., Chakraborty, D., Jat, M.L., Jat, R.K., Saharawat, Y.S., Saharawat, Y.S., Yadav, O.P. Long Term Effect of Conservation Agriculture in Maize Rotations on Total Organic Carbon, Physical and Biological Properties of a Sandy Loam Soil in North-Western Indo-Gangetic Plains. Soil Till. Res., 2016, 161:116–28. [CrossRef]
- Doran, J.W. Soil microbial and biochemical changes associated with reduced tillage.. Soil Sci. Soc. Am. J., 1980, 44, 765-771.
- Redel, Y.D., Rubio, R., Rouanet, J.L., Borie, F. Phosphorus Bioavailability Affected by Tillage and Crop Rotation on a Chilean Volcanic Derived Ultisol. Geoderma, 2007, 139(3–4):388–96. [CrossRef]
- Yang, X., Bao, X., Yang, Y., Zhao, Y., Liang, C., Xie, H. Comparison of Soil Phosphorus and Phosphatase Activity under Long-Term No-Tillage and Maize Residue Management. Plant, Soil Environ., 2019, 65(8):408–15. [CrossRef]
- Ahmed, W., Qaswar, M., Jing, H., Wenjun, D., Geng, S., Kailou, L., Ying, M., Ao, T., Mei, S., Chao, L., Khan, M. N., Huimin, Z. Tillage Practices Improve Rice Yield and Soil Phosphorus Fractions in Two Typical Paddy Soils. J. Soils Sed., 2019, 20(2):850–61. [CrossRef]
- Jarosch, K.A., Kandeler, E., Frossard, E., Bünemann, E.K. Is the Enzymatic Hydrolysis of Soil Organic Phosphorus Compounds Limited by Enzyme or Substrate Availability? Soil Biol. Biochem., 2019, 139:107628. [CrossRef]
- Igalavithana, A.D., Lee, S.S., Niazi, N.K., Lee, Y.H., Kim, K.H., Park, J.H., Moon, D.H., Ok, Y.S. Assessment of Soil Health in Urban Agriculture: Soil Enzymes and Microbial Properties. Sustainability (Switzerland), 2017, 9(2). [CrossRef]
- Chatterjee, D., Nayak, A.K., Mishra, A., Swain, C.K., Kumar, U., Bhaduri, D., Panneerselvam, P., Lal, B., Gautam, P., Pathak, H. Effect of Long-Term Organic Fertilization in Flooded Rice Soil on Phosphorus Transformation and Phosphate Solubilizing Microorganisms. J. Soil Sci. Plant Nutr., 2021, 21(2):1368–81. [CrossRef]
- Dhanker, R., Chaudhary, S., Goyal, S., Kumar, R. Soil Microbial Properties and Functional Diversity in Response to Sewage Sludge Amendments. Archives of Agron. Soil Sci., 2021, 68(6):809–22. [CrossRef]
- Adetunji, A.T., Lewu, F.B., Mulidzi, R., Ncube, B. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: a review. J. Soil Sci. Plant Nutr., 2017, 7 (3):794-807. http://dx.doi.org/10.4067/S0718-95162017000300018.
- Nobile, C., D. Houben, E. Michel, S. Firmin, H. Lambers, E. Kandeler, and Faucon, M.P. Phosphorus-Acquisition Strategies of Canola, Wheat and Barley in Soil Amended with Sewage Sludges. Sci. Rep., 2019, 9(1):1–11. [CrossRef]
- Leirós, M.C., Trasar-Cepeda, C., García-Fernández, F., Gil-Sotres, F. Defining the Validity of a Biochemical Index of Soil Quality. Biol. Fert. Soils, 1999, 30(1–2):140–46. [CrossRef]
- Makoi, J.H.J.R., Bambara, S., Ndakidemi, P.A. Rhizosphere Phosphatase Enzyme Activities and Secondary Metabolites in Plants as Affected by the Supply of Rhizobium, Lime and Molybdenum in Phaseolus Vulgaris L., Aus. J. Soil Sci., 2010, Vol. 4. https://www.cropj.com/ndakidemi_4_8_2010_590_597.pdf.
- Bolton, H. Jr., Elliott, L.F., Papendick, R.I., Bezdicek, D.F. Soil microbial biomass and selected soil enzyme activities: effect of fertilization and cropping practices. Soil Biol. Biochem., 1985, 17, 297-302. http://dx.doi.org/10.1007/BF00257821.
- Dhull, S., Goyal, S., Kapoor, K., Mundra, M. Microbial Biomass Carbon and Microbial Activities of Soils Receiving Chemical Fertilizers and Organic Amendments. International J. Phytoremed., 2004, 21(1):641–47. [CrossRef]
- Singh, G., Bhattacharyya, R., Das, T.K., Sharma, A.R., Ghosh, A., Das, S., Jha, P. Crop Rotation and Residue Management Effects on Soil Enzyme Activities, Glomalin and Aggregate Stability under Zero Tillage in the Indo-Gangetic Plains. Soil Till. Res., 2018b, 184. [CrossRef]
- Chellappa, J., Laxmisagara Sagar, K., Sekaran, U., Kumar, S., Sharma, P., 2021. Soil Organic Carbon, Aggregate Stability and Biochemical Activity under Tilled and No-Tilled Agroecosystems. J. Agric. Food Res., 2021, 4:100139. [CrossRef]
- Tu, C., Ristaino, J.B, Hu, S. Soil Microbial Biomass and Activity in Organic Tomato Farming Systems: Effects of Organic Inputs and Straw Mulching. Soil Biol. Biochem., 2006, 38(2):247–55. [CrossRef]
- Wyszkowska, J. Effect of Soil Contamination with Treflan 480 EC on Biochemical Properties of Soil. Pol. J. Environ. Stud., 2002, 11(1):71–77.
- Gaind, S., Nain, L. Chemical and Biological Properties of Wheat Soil in Response to Paddy Straw Incorporation and Its Biodegradation by Fungal Inoculants. Biodegradation, 2017, 18(4):495–503. [CrossRef]
- Hoyle, F.C., Murphy, D.V., Seasonal Changes in Microbial Function and Diversity Associated with Stubble Retention versus Burning. Austr. J. Soil Res., 2006, 44(4):407–23. [CrossRef]
- Trujillo-Narcía, A., Rivera-Cruz. M.C., Magaña-Aquino, M., Trujillo-Rivera, E.A. The Burning of Sugarcane Plantation in the Tropics Modifies the Microbial and Enzymatic Processes in Soil and Rhizosphere. J. Soil Sci. Plant Nutr., 2019, 19(4):906–19. [CrossRef]
- Emami, S., Alikhani, H.A., Pourbabaee, A.A., Etesami, H., Sarmadian, F., Motesharezadeh, B., Taghizadeh–Mehrjardi, R. Performance Evaluation of Phosphate-Solubilizing Fluorescent Pseudomonads in Minimizing Phosphorus Fertilizer Use and Improving Wheat Productivity: A Two-Year Field Study. J. Soil Sci. Plant Nutr., 2022, 22(2):1224–37. [CrossRef]
- Parnell, J.J., Berka, R., Young, H.A., Sturino, J.M., Kang, Y., Barnhart, D. M., DiLeo, M.V. From the Lab to the Farm: An Industrial Perspective of Plant Beneficial Microorganisms. Front. Plant Sci., 2016, 7. [CrossRef]
- Tian, J., Ge, F., Zhang, D., Deng, S., Liu, X. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Biology, 2021, 10(2). [CrossRef]
- Valarini, P.J., Alvarez, M.C.D., Gasco, J.M., Guerrero, F., Tokeshi, H. Assessment of Soil Properties by Organic Matter and EM-Microorganism Incorporation. Rev. Bras. Cienc. Solo, 2003, 27(3):519–25.
- Ruiz, J.L., and Salas, MdC. Evaluation of Organic Substrates and Microorganisms as Bio-Fertilisation Tool in Container Crop Production. Agronomy, 2019, 9(11). [CrossRef]
- Mahapatra, B., Adak, T., Patil N.K.B., Pandi G. G. P., Gowda G. B., Jambhulkar N. N., Yadav M. K., Panneerselvam P., Kumar U., Munda S., Munda S., Jena M. Imidacloprid Application Changes Microbial Dynamics and Enzymes in Rice Soil. Ecotox. Environ. Saf., 2017, 144:123–30. [CrossRef]
- Meher, S., Saha, S., Tiwari, N., Panneerselvam, P., Munda, S., Mahapatra, A., Jangde, H.K. Herbicide-Mediated Effects on Soil Microbes, Enzymes and Yield in Direct Sown Rice. Agric. Res., 2021. [CrossRef]
- Fialho, C.M.T., Silva, A.A., Melo, C.A.D., Costa, M.D., Souza, M.WR., Reis, L.A.C. Weed Interference in Soybean Crop Affects Soil Microbial Activity and Biomass. Planta Daninha, 2020, 38. [CrossRef]
- Romero-Trigueros, C., Díaz-López, M., Vivaldi, G.A., Camposeo, S., Nicolás, E., Bastida, F. Plant and Soil Microbial Community Responses to Different Water Management Strategies in an Almond Crop. Sci. Total Environ., 2021, 778. [CrossRef]
- Zhang, Y., Wang X., Xu, F., Song, T., Du, H., Gui, Y., Xu. M., Cao, Y., Dang, X., Rensing, C., Zhang, J., Xu, W. Combining Irrigation Scheme and Phosphorous Application Levels for Grain Yield and Their Impacts on Rhizosphere Microbial Communities of Two Rice Varieties in a Field Trial. J. Agric. Food Chem., 2019, 67(38):10577–86. [CrossRef]
- García-Orenes, F., Caravaca, F., Morugán-Coronado, A., Roldán, A. Prolonged Irrigation with Municipal Wastewater Promotes a Persistent and Active Soil Microbial Community in a Semiarid Agroecosystem. Agric. Water Manag., 2015,149:115–22. [CrossRef]
- Kayikcioglu, H.H. Can Treated Wastewater Be Used as an Alternative Water Resource for Agricultural Irrigation? Changes in Soil and Plant Health after Three Years of Maize Cultivation in Western Anatolia, Turkey. Ap. Ecol. Environ. Res., 2018, 16(6):8131–61. [CrossRef]
- Galindo, F.S., Delate, K., Heins, B., Phillips, H., Smith, A., Pagliari, P.H. Cropping System and Rotational Grazing Effects on Soil Fertility and Enzymatic Activity in an Integrated Organic Crop-Livestock System. Agronomy, 2020, 10(6):1–18. [CrossRef]
- Catorci, A., Ottaviani, G., Ballelli, S., Cesaretti, S. Functional differentiation of central apennine grasslands under mowing and grazing disturbance regimes. Pol. J. Ecol., 2011, 59 (1): 115-128.
- Zibilske, L.M., Makus, D.J. Black Oat Cover Crop Management Effects on Soil Temperature and Biological Properties on a Mollisol in Texas, USA. Geoderma, 2009, 149(3–4):379–85. [CrossRef]
- Kunito, T., Saeki, K., Goto, S., Hayashi, H., Oyaizu, H., Matsumoto, S. Copper and Zinc Fractions Affecting Microorganisms in Long-Term Sludge-Amended Soils. Biores. Tech., 2001, 79(2):135–46. [CrossRef]
- Mandal, N., Datta, S.C., Dwivedi, B.S., Manjaiah, K.M., Meena, M.C., Bhowmik, A. Zincated Nanoclay Polymer Composite (ZNCPC): Effect on DTPA-Zn, Olsen-P and Soil Enzymatic Activities in Rice Rhizosphere. Com. Soil Sci. Plant Anal., 2021, 52(17):2032–44. [CrossRef]
- De Santiago-Martín, A., Cheviron, N., Quintana, J.R., González, C., Lafuente, A.L., Mougin, C. Metal Contamination Disturbs Biochemical and Microbial Properties of Calcareous Agricultural Soils of the Mediterranean Area. Arch. Environ. Cont. Toxicol., 2013, 64(3):388–98. [CrossRef]
- Mitter, E.K., Germida, J.J. and de Freitas, J.R. Impact of diesel and biodiesel contamination on soil microbial community activity and structure. Sci. Rep., 2021, 11, 10856. [CrossRef]
- Lin, J., Ma, K., Chen, H., Chen, Z., Xing, B. Influence of Different Types of Nanomaterials on Soil Enzyme Activity: A Global Meta-Analysis. Nano Today, 2021, 42:101345. [CrossRef]
- Sardans, J., Peñuelas, J., Estiarte, M. Warming and drought alter soil phosphatase activity and soil P availability in a Mediterranean shrubland. Plant Soil, 2006, 289: 227–238. [CrossRef]
- Yao, Y., Dai, Q., Gao, R., Gan, Y., Yi, X. Effects of rainfall intensity on runoff and nutrient loss of gently sloping farmland in a karst area of SW China. PLoS One, 2021, 16(3):e0246505. [CrossRef]
- Ghiloufi, W., Chaieb, M. Environmental Factors Controlling Vegetation Attributes, Soil Nutrients and Hydrolases in South Mediterranean Arid Grasslands. Ecol. Eng., 2021, 161:106155. [CrossRef]
- Morugán-Coronado, A., García-Orenes, F., McMillan, M., Pereg, L. The effect of moisture on soil microbial properties and nitrogen cyclers in Mediterranean sweet orange orchards under organic and inorganic fertilization. Sci. Total Environ., 2019, 655, 158–167. [CrossRef]
- Sardans, J., Peñuelas, J. Increasing drought decreases phosphorus availability in an evergreen Mediterranean forest. Plant Soil, 2004, 267.1: 367-377. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.709.5500&rep=rep1&type=pdf.
- Landesman, W.J., Dighton, J. Response of Soil Microbial Communities and the Production of Plant-Available Nitrogen to a Two-Year Rainfall Manipulation in the New Jersey Pinelands. Soil Biol. Biochem., 2010, 42(10):1751–58. [CrossRef]
- Jaskulska, R. The Level of Luvisols Biochemical Activity in Midfield Shelterbelt and Winter Triticale (xTriticosecale Wittm. ex A. Camus) Cultivation. Agronomy, 2020, 10: 1644. [CrossRef]
- Dey, S. K., Chakrabarti, B., Purakayastha, T.J., Prasanna, R., Mittal, R., Singh, S.D., Pathak, H. Interplay of Phosphorus Doses, Cyanobacterial Inoculation, and Elevated Carbon Dioxide on Yield and Phosphorus Dynamics in Cowpea. Environ. Monit. Assess., 2019, 191(4). [CrossRef]
- Basak, N., Mandal, B., Datta, A., Mitran, T., Biswas, S., Dhar, D., Badole, S., Saha, B., Hazra, G.C. Impact of Long-Term Application of Organics, Biological, and Inorganic Fertilizers on Microbial Activities in Rice-Based Cropping System. Com. Soil Sci. Plant Anal., 2017, 48(20):2390–2401. [CrossRef]
- de Castro Lopes, A., Gomes de Sousa, D.M., Chaer, G.M., Bueno dos Reis Junior, F., Goedert, W.J., de Carvalho Mendes, I. Interpretation of Microbial Soil Indicators as a Function of Crop Yield and Organic Carbon. Soil Sci. Soc. Am. J., 2013, 77, 461. http://dx.doi.org/10.2136/sssaj2012.0191.
- Tarafdar, J.C., Rao, A.V. Contribution of Aspergillus Strains to Acquisition of Phosphorus by Wheat (Triticum Aestivum L.) and Chick Pea (Cicer Arietinum Linn.) Grown in a Loamy Sand Soil. Ap. Soil Ecol., 1996, 3(2):109–14. [CrossRef]
- Moharana, P.C., Biswas, D.R. Phosphorus Delivery Potential in Soil Amended with Rock Phosphate Enriched Composts of Variable Crop Residues under Wheat–Green Gram Cropping Sequence. Com. Soil Sci. Plant Anal., 2022, 53(8):1000–1017. [CrossRef]
Figure 1.
Number of single studies reporting direction of responses of ACP and ALP activities to soil biological factors. Factors with fewer or equal than three entries in ACP or ALP response are excluded from the figure as they are considered unrepresentative (e.g., microbe diversity (Shannon diversity index), phoD gene abundance and richness and earthworm abundance).
Figure 1.
Number of single studies reporting direction of responses of ACP and ALP activities to soil biological factors. Factors with fewer or equal than three entries in ACP or ALP response are excluded from the figure as they are considered unrepresentative (e.g., microbe diversity (Shannon diversity index), phoD gene abundance and richness and earthworm abundance).
Figure 2.
Number of single and meta-analysis studies reporting direction of responses of ACP and ALP to soil depth, moisture, texture and pH-related factors. Factors with fewer or equal than three entries in ACP or ALP response are excluded from the figure as they are considered unrepresentative (e.g., microaggregate content, cation exchange capacity, chlorine anion content, carbonate content, iron content and exchangeable aluminium content). The number of meta-analyses (MA) has been counted in order to complement the qualitative analysis based on vote counting.
Figure 2.
Number of single and meta-analysis studies reporting direction of responses of ACP and ALP to soil depth, moisture, texture and pH-related factors. Factors with fewer or equal than three entries in ACP or ALP response are excluded from the figure as they are considered unrepresentative (e.g., microaggregate content, cation exchange capacity, chlorine anion content, carbonate content, iron content and exchangeable aluminium content). The number of meta-analyses (MA) has been counted in order to complement the qualitative analysis based on vote counting.
Figure 3.
Number of single and meta-analysis studies reporting direction of responses of ACP and ALP to carbon, nitrogen, phosphorus and potassium. Factors with fewer than three entries in ACP and ALP response are excluded from the figure as they are considered unrepresentative (e.g., salt content, nitrate-N, ammonium-N, labile organic P and soil carbon:P ratio). The number of meta-analyses (MA) has been counted in order to complement the qualitative analysis based on vote counting.
Figure 3.
Number of single and meta-analysis studies reporting direction of responses of ACP and ALP to carbon, nitrogen, phosphorus and potassium. Factors with fewer than three entries in ACP and ALP response are excluded from the figure as they are considered unrepresentative (e.g., salt content, nitrate-N, ammonium-N, labile organic P and soil carbon:P ratio). The number of meta-analyses (MA) has been counted in order to complement the qualitative analysis based on vote counting.
Figure 4.
Number of single studies reporting direction of responses of ACP and ALP to land use change, crop rotation, cover crops and intercropping. Factors with fewer or equal than three entries in ACP or ALP response are excluded from the figure as they are considered unrepresentative (e.g., revegetation, plant invasion and forest clearance for cropland).
Figure 4.
Number of single studies reporting direction of responses of ACP and ALP to land use change, crop rotation, cover crops and intercropping. Factors with fewer or equal than three entries in ACP or ALP response are excluded from the figure as they are considered unrepresentative (e.g., revegetation, plant invasion and forest clearance for cropland).
Figure 5.
Number of single studies reporting direction of responses of ACP and ALP to crop species and tillage. Factors with fewer or equal than three entries in ACP and ALP response are excluded from the figure as they are considered unrepresentative (e.g. comparison between wheat and maize/rice, barley vs horticulture, monoculture sorghum vs others, monoculture transgenic cotton vs cotton, monoculture transgenic rice vs rice, no till with residue retention vs others and no till with depth vs others).
Figure 5.
Number of single studies reporting direction of responses of ACP and ALP to crop species and tillage. Factors with fewer or equal than three entries in ACP and ALP response are excluded from the figure as they are considered unrepresentative (e.g. comparison between wheat and maize/rice, barley vs horticulture, monoculture sorghum vs others, monoculture transgenic cotton vs cotton, monoculture transgenic rice vs rice, no till with residue retention vs others and no till with depth vs others).
Figure 6.
Number of single and meta-analysis studies reporting direction of ACP and ALP responses to agroecosystem fertilizer management practices. Factors with fewer or equal than three entries in ACP and ALP response are excluded from the figure as they are considered unrepresentative (e.g., green manure alone). The number of meta-analyses (MA) has been counted in order to complement the qualitative analysis based on vote counting.
Figure 6.
Number of single and meta-analysis studies reporting direction of ACP and ALP responses to agroecosystem fertilizer management practices. Factors with fewer or equal than three entries in ACP and ALP response are excluded from the figure as they are considered unrepresentative (e.g., green manure alone). The number of meta-analyses (MA) has been counted in order to complement the qualitative analysis based on vote counting.
Figure 7.
Number of single and meta-analysis/review studies reporting direction of responses of ACP and ALP pest and weed management, irrigation, crop-livestock and grazing management. Factors with fewer or equal than three entries in ACP and ALP response are excluded from the figure as they are considered unrepresentative (e.g., mowing). The number of meta-analyses (MA) and reviews (R) has been counted in order to complement the qualitative analysis based on vote counting.
Figure 7.
Number of single and meta-analysis/review studies reporting direction of responses of ACP and ALP pest and weed management, irrigation, crop-livestock and grazing management. Factors with fewer or equal than three entries in ACP and ALP response are excluded from the figure as they are considered unrepresentative (e.g., mowing). The number of meta-analyses (MA) and reviews (R) has been counted in order to complement the qualitative analysis based on vote counting.
Figure 8.
Number of single and meta-analysis studies reporting direction of responses of ACP and ALP to soil pollutants. Factors with fewer or equal than three entries in ACP and ALP response are excluded from the figure as they are considered unrepresentative (e.g., chromium, manganese, arsenic, mercury and nanomaterials). The number of meta-analyses (MA) has been counted in order to complement the qualitative analysis based on vote counting.
Figure 8.
Number of single and meta-analysis studies reporting direction of responses of ACP and ALP to soil pollutants. Factors with fewer or equal than three entries in ACP and ALP response are excluded from the figure as they are considered unrepresentative (e.g., chromium, manganese, arsenic, mercury and nanomaterials). The number of meta-analyses (MA) has been counted in order to complement the qualitative analysis based on vote counting.
Figure 9.
Number of single and meta-analysis studies reporting direction of responses of ACP and ALP to climate change factors. Factors with fewer or equal than three entries in ACP and ALP response are excluded from the figure as they are considered unrepresentative (e.g., mean annual precipitation, soil water availability, CO2 fertilization). The number of meta-analyses (MA) has been counted in order to complement the qualitative analysis based on vote counting.
Figure 9.
Number of single and meta-analysis studies reporting direction of responses of ACP and ALP to climate change factors. Factors with fewer or equal than three entries in ACP and ALP response are excluded from the figure as they are considered unrepresentative (e.g., mean annual precipitation, soil water availability, CO2 fertilization). The number of meta-analyses (MA) has been counted in order to complement the qualitative analysis based on vote counting.
Figure 10.
The factors influencing APase activity in belowground environments can be summarized through colour-coded lines and names. Green lines represent the most positive influential factors. The solid lines refer to internal soil processes, the dashed lines correspond to crop management on the soil. When the concentration of inorganic P in the soil is low, plants, roots, and microbiota release APase. The brown lines represent the role of APase activity in providing assimilable P for plant and microbiota uptake. Physicochemical properties, such as soil organic matter, available N, clay content, and management practices like organic manure fertilization, no-till, crop residue utilization, intercropping, and crop rotation, are also depicted in brown as they enhance APase activity. Additionally, climate factors that increase APase activity, including optimal water levels, rainfall (indicated with a cloud) and temperature (indicated with a sun), are also shown.
Figure 10.
The factors influencing APase activity in belowground environments can be summarized through colour-coded lines and names. Green lines represent the most positive influential factors. The solid lines refer to internal soil processes, the dashed lines correspond to crop management on the soil. When the concentration of inorganic P in the soil is low, plants, roots, and microbiota release APase. The brown lines represent the role of APase activity in providing assimilable P for plant and microbiota uptake. Physicochemical properties, such as soil organic matter, available N, clay content, and management practices like organic manure fertilization, no-till, crop residue utilization, intercropping, and crop rotation, are also depicted in brown as they enhance APase activity. Additionally, climate factors that increase APase activity, including optimal water levels, rainfall (indicated with a cloud) and temperature (indicated with a sun), are also shown.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).