1. Introduction
Immune response to beta-coronavirus (SARS-CoV-2; severe acute respiratory syndrome coronavirus 2) does not always lead to the development of a clinical syndrome called COVID-19 (coronavirus disease 2019) among others due to the low response of antibodies in the cancer population [
1,
2]. The prevalence of asymptomatic SARS-CoV-2 infection in patients with cancer is similar to the general population, while at the same time, patients with cancer may be at increased risk of severe course of COVID-19 [
3]. The phenomenon of asymptomatic infection is believed to include, among others, the inflammation that accompanies every cancer disease, involving components of innate and acquired immunity [
4]. In the literature, we find data that only every third patient with diagnosed cancer undergoing anticancer therapy responds to the complete 2-dose vaccination by producing antibodies [
5]. The booster effect (third dose) equalizes the chances between patients' subpopulations during oncological treatment [
6,
7].
It is worth noting that during a 12-month observation, it was proven that chemotherapy or radiotherapy increases the pool of immunoglobulin class G (IgG) antibodies against SARS-CoV-2 in cancer patients [
8]. It has also been proven that documenting SARS-CoV-2 infection with a molecular test is insufficient if it does not correlate with the patient's clinical condition [
9]. Solodky et al. show that oncological treatment one month after identifying the virus makes it undetectable in tests [
10]. Hence, an essential message for cancer patients is to get vaccinated, regardless of the test results, which may be false negative or positive.
As it has been shown, the imperfection of diagnostic tests, primarily their sensitivity, influences the virus to remain below the detection value in the laboratory; the clinical characteristics of such a situation are called long-COVID-19, a disease in which the shedding of the virus is also prolonged as the disease drags on.
Oncology patients suffer from a chronic inflammatory process caused by the presence of cancer cells. This process involves innate and acquired immunity, gradually depleting the body's defence reserves. Therefore, only chemotherapy and radiotherapy, which deprive the body of the source or cause of chronic inflammation, can stimulate CD8+ T cells, B lymphocytes and plasma cells, and immune memory cells to an appropriate humoral response [
8].
Only the third dose of the vaccine against SARS-CoV-2 infection works similarly in patients with soft tissue tumours [
11]. This phenomenon has not been observed in oncohematological patients suffering from B-cell leukaemias or lymphomas, where, as a result of permanent myelosuppression, the humoral response is impaired [
8]. The exception is patients suffering from Hodgkin's lymphoma, where antibody-dependent SARS-CoV-2 induction of anti-tumor response [
12], and patients with multiple myeloma [
9]. In the case of non-haematological cancers, such as breast cancer in women and prostate cancer, studies conducted on small groups of patients showed an increased humoral response, which was associated with chronic inflammation and immunosuppressive treatment [
11].
This study aimed to answer the question about the impact of the cancer process on the humoral response in oncological patients vaccinated against SARS-CoV-2 infection and in patients after COVID-19. In the conducted studies, attention was paid to the potential impact of the anti-infective response, in this case, the antiviral response, on the potential of the anticancer response. This is important because a post-infectious or post-vaccination increase in the concentration of IgG antibodies, secondary response antibodies, provides a chance to "strengthen" the anti-infective humoral response for many months.
2. Materials and Methods
Our study materials were blood specimens collected through venipuncture sampling. The concentration of antibodies was evaluated 4 h after blood collection. If an immediate assessment was not possible, the serum was collected and stored at −80 ◦C.
All 1668 participants were in the study. The study was conducted when patients visited a physician at an appointment in hospital. All of them were referred for antibody tests voluntarily due to their history of diseases, lack of immunity, and the possibility of receiving vaccinations during oncological treatment. Some of them had antibodies ordered several times. Medical staff were required to be fully vaccinated against COVID-19 during the pandemic. Because the government regulated vaccine administration in 2021, the first group was medical personnel, followed by people over 60, and only then cancer patients. The first administration of the vaccine was mainly with Pfizer (BNT162b2; Comirnaty), AstraZeneca (AZD1222; Vaxzevria), Moderna (MRNA-1273; Spikevax) and Johnson&Johnson (Ad26.COV2.S). Booster doses (2021/2022) were often the Pfizer or Moderna vaccine.
During 2-years observation two class antibodies was detected (n=5082). They had elevated levels of IgM and IgG antibodies (n=2541 each), oriented specifically towards SARS-CoV-2, were detected by chemiluminescent immunoassay (CLIA; MAGLUMI, Snibe Diagnostic, Shenzhen, China). Results greater than or equal to 0.2 AU/mL SARS-CoV-2 IgG and 1.0 AU/mL IgM, were considered indicative of a reaction and regarded as positive, according to the manufacturer’s protocol.
Of the study samples, 82% population was vaccinated, including 416 samples from cancer persons who received only the Pfizer-BioNTech BNT162b2 vaccine (Comirnaty). BNT162b2 is 95% effective in preventing COVID-19, and similar vaccine efficacy is observed across subgroups defined by age, sex, race, and ethnicity. The Bioethics Commission of Medical University approved our research.
A group of people with a histopathologically confirmed diagnosis of oncological disease (Cancer), people undergoing oncological diagnosis (N-Cancer) and a group of control people, hospital employees tested before and after vaccination (Non-Oncology), were distinguished.
Table 1 contains the characteristics of the study, and
Table 2 is supplemented with units of diseases examined and the share of vaccinated people. Additionally, a group was distinguished with diagnoses of autoimmune diseases (Autoimmunology) and specific diseases (O-Cancer, H-Cancer). Because the wave of SARS-CoV-2 infections repeatedly affected our population, we did not keep statistics on flu-like illnesses; only vaccination statistics (groups S1-S5 were specified) were kept. COVID-19 molecular and antigen tests were performed routinely when severe infections were suspected.
Statistical Analysis
Nonparametric statistical methods were applied for the case–control analyses because many of the considered variables were not normally distributed. The Mann–Whitney test was adopted to compare two groups to each other, whereas the Kruskal–Wallis, with the post-hoc test, was applied to assess more than two groups. Results were considered statistically significant when p < 0.05. We used PQStat Software 2021 (PQStat Software, Poznan, Poland) and Tibco Statistics 13.3 (TIBCO Software Inc., Palo Alto, CA, USA) for statistical analyses.
3. Results
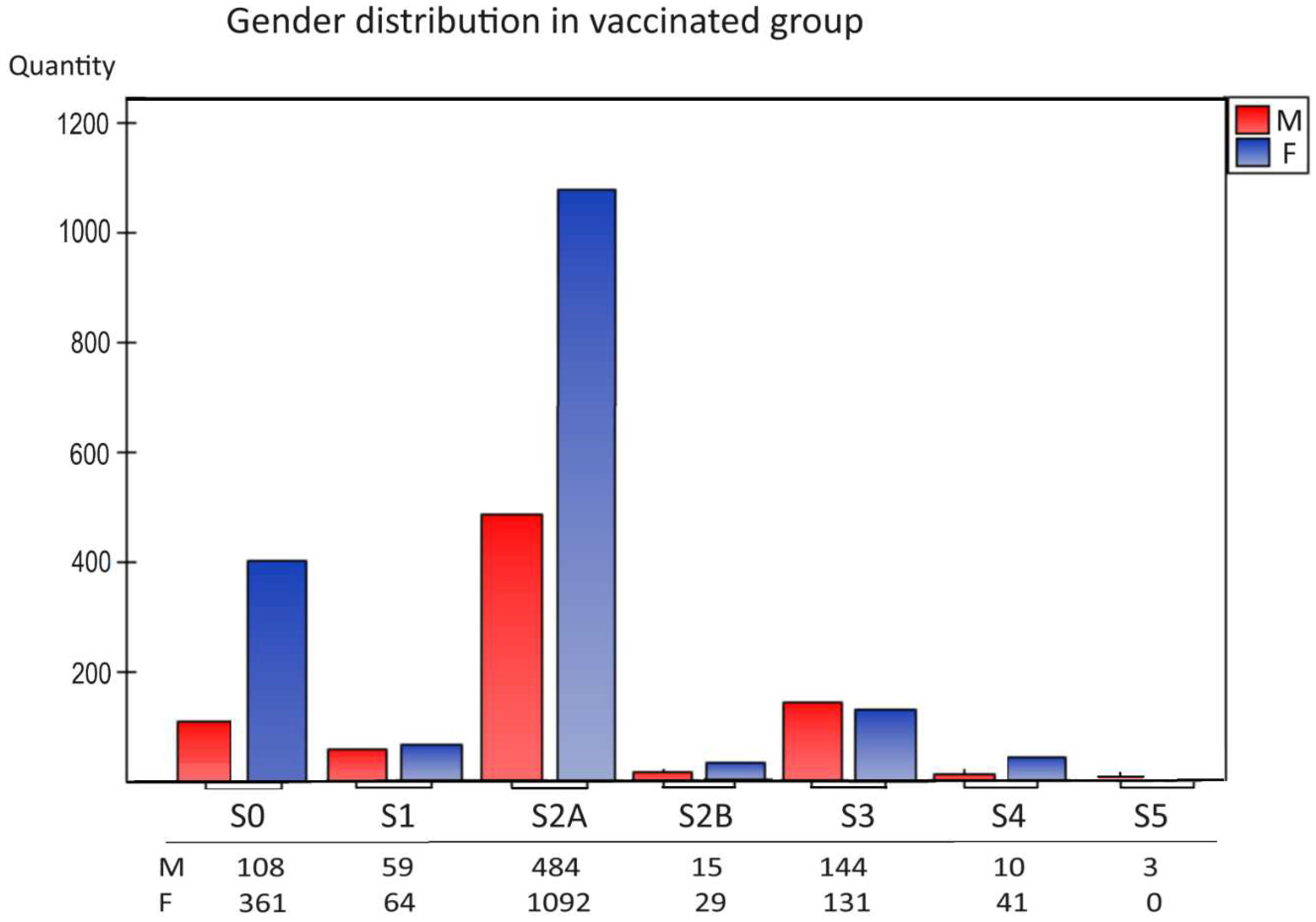
The analysis of the distribution of vaccinations used (S1-S5) in our study showed that the most significant population were people fully vaccinated with BNT162b2, 2-dose series mRNA vaccines dedicated to hospital workers and oncology patients 2021 (
Figure 1,
group S2A). The next group consisted of people vaccinated with three doses (
Figure 1,
group S3). Young people chose 1-dose vaccination (S1), while few people were vaccinated with other preparations (mRNA-1273 vaccine (Moderna), Ad26.COV2.S vaccine (Johnson&Johnson) and AZD1222 vaccine (AstraZeneca), 2-full dose BNT162b2 (S2A), 2-full dose currently available vaccines (S2B), and received the fourth (S4) and fifth doses (S5)
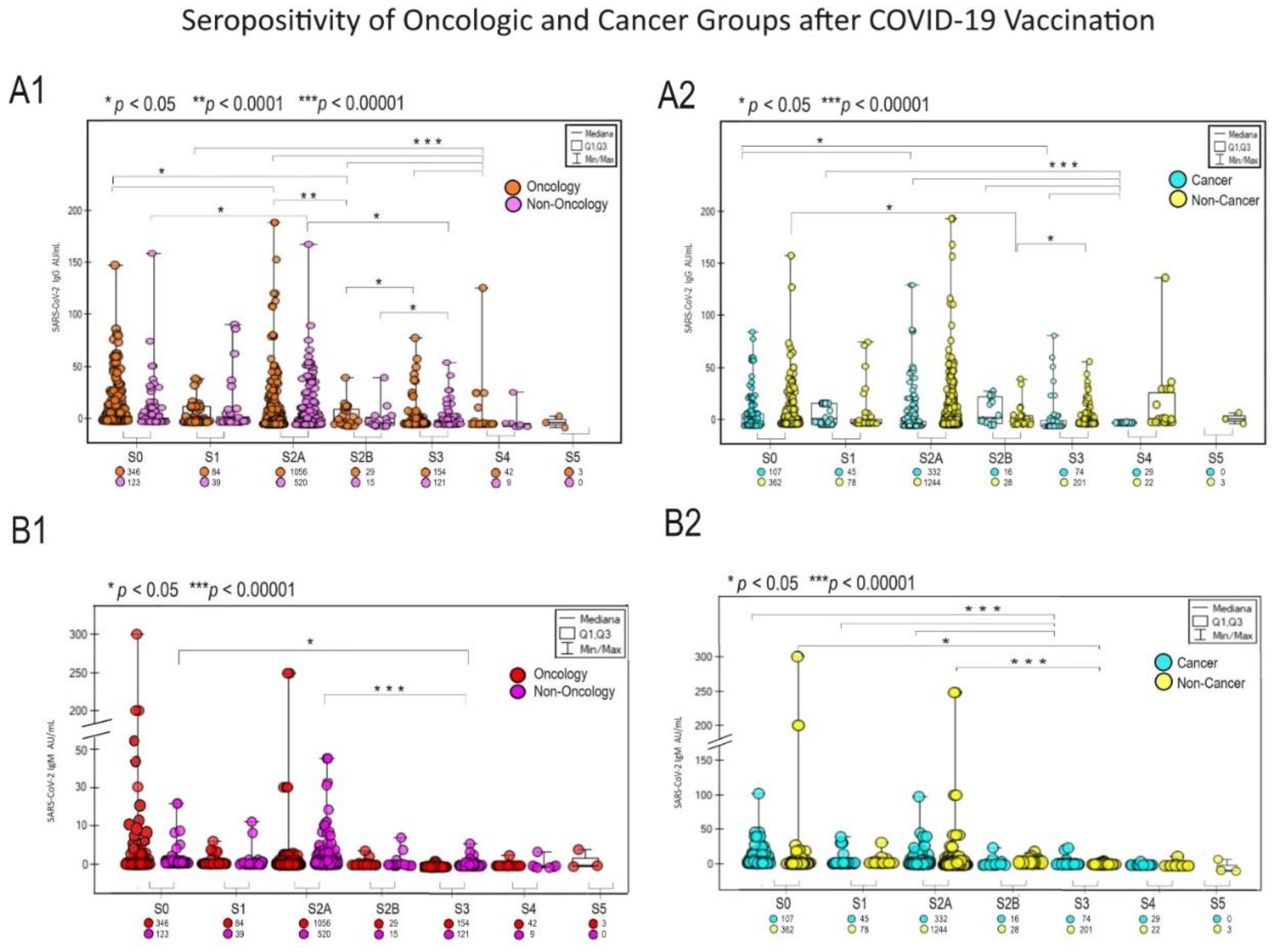
A significant response to vaccination was observed in the patient population oncology and cancer among unvaccinated people (
Figure 2 graphs A and B, for IgG and IgM appropriately). The highest IgG response occurred in the S3 group vaccinated oncology and cancer patients (p<0.05) and S4 non-oncological and non-cancer (p<0.00001)
Figure 2 A1 and A2.
A detailed breakdown of the cancer group into the most common types (
Table 2) revealed differences between breast, lung, colon, kidney and testicular cancer populations from other cancer and disease states in the COVID-19 pandemic (p-value significant).
3.1. Breast cancer
In breast cancer (
Figure 3), the response to vaccination was more noticeable by IgG levels for unvaccinated individuals (
Figure 3 d, e, f), despite the effect of their treatment oncology (chemotherapy, radiotherapy, surgery) (
Figure 3 k) and the type and time vaccine administration (
Figure 3 g, h). A higher share of antibodies was noticed in the population < 60 years old—breast cancer in vaccinated persons (
Figure 3 l). BNT162b2 vaccine and other vaccines significantly (collectively as non-BNT162b2 due to small sample size) affected IgG antibody levels (
Figure 3 i, j).
A noted natural feature of breast tissue is sensitivity to SARS-CoV-2 levels IgG for "non-inflammatory" breast (
Figure 3 a) and "post-inflammatory" breast with cysts (
Figure 3 b) during monthly observation before the development of the cancer process (
Figure 3 c), and then its response to vaccination in 2021/2022 (
Figure 3 d).
3.2. Lung and colon cancer
The humoral IgG response to vaccination in the lung cancer population (
Figure 4) is other than for breast cancer. As you can see, the reaction from the healthy tissue (
Figure 4 a) is different from cancer's (
Figure 4 b). A similar response phenomenon again draws attention higher in unvaccinated patients (in subsequent years) than in vaccinated patients cancer patients (
Figure 4 c, e, f) and in oncological patients (
Figure 4 d). The lowest share of antibodies was noticed in the population >60 years old—lung cancer in vaccinated persons (
Figure 4 g).
Colon cancer (
Figure 4 j), the only one from the gastrointestinal tract showed a more excellent IgG response than the other conditions (
Figure 4 h, i) and the rest observed cancer (oesophagus, stomach, small intestine, rectum). Even though the trend was there similar to lung cancer (higher share of non-vaccinated cancer antibodies patients), no statistically significant relationship was observed for colon cancer, both for those treated, vaccinated, and in age groups.
3.3. Other cancers
Additionally, we observed a similar significant response in a group of vaccinated patients treated for kidney and testicular cancer (
Table 2). In the entire group of vaccinated cancers, the response was different, but the strongest response was for breast cancer (
Figure 3), colon and lung cancer (
Figure 4).
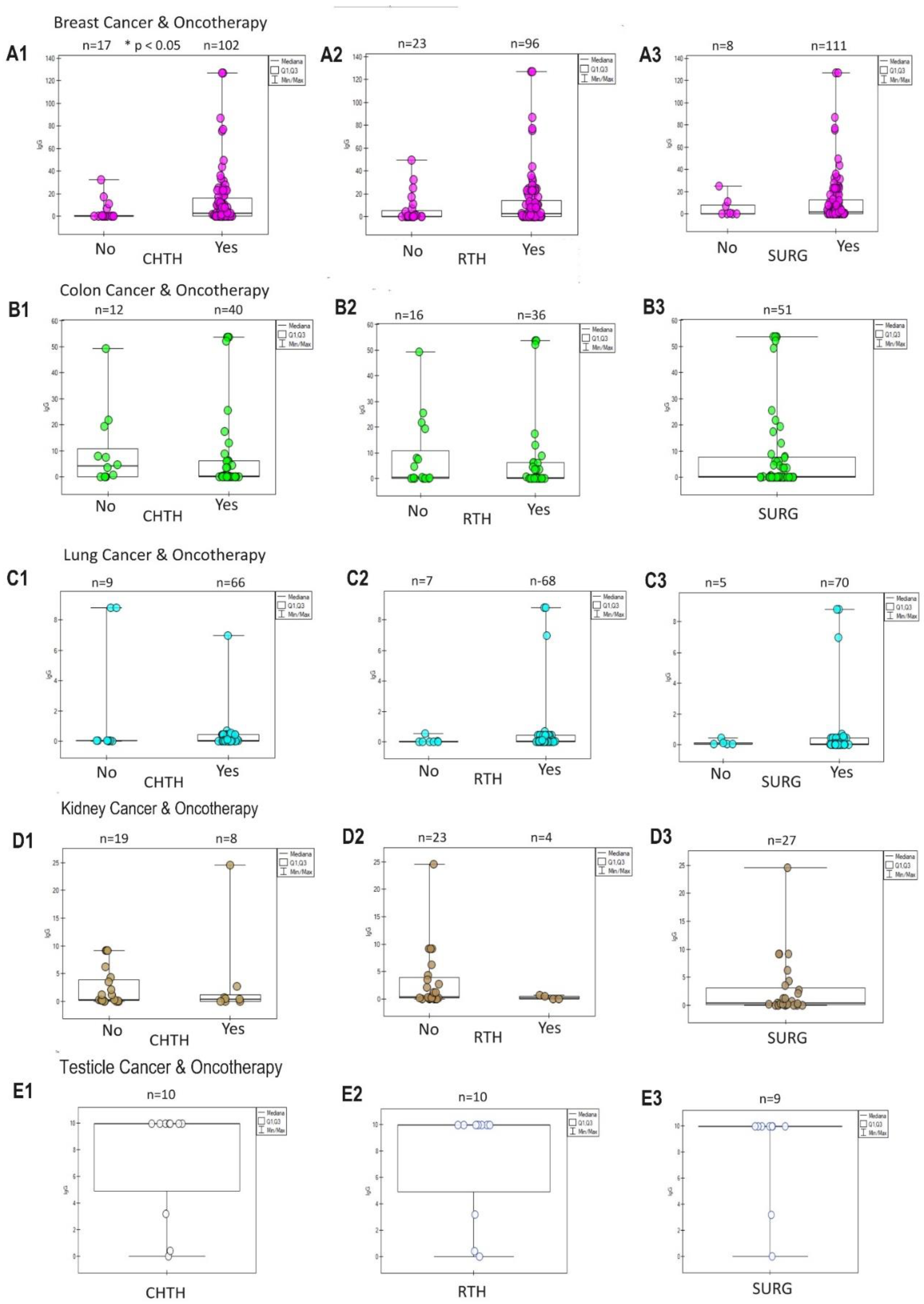
The assessment of the response of tumours to oncological treatment is visible in
Figure 5. A typical positive response (increase in IgG) in the group of patients treated oncologically was observed in breast cancer (
Figure 5 A1, A2, A3), intestinal cancer (
Figure 5 B1, B2, B3) and lungs (
Figure 5 C1, C2, C3). A negative response (decrease in IgG) was visible only in the group treated with CHTH (
Figure 5 D1) and RTH (
Figure 5 D2) of kidney cancer. The interpretation of treatment in small group kidney and testicular cancer is incomplete due to the lack of a control group (
Figure 5 D3, E1, E2, E3).
We also see the COVID-19-sensitive potential (positive) in non-vaccinated patients' non-oncological diseases, such as rheumatoid arthritis, Graves` disease, and fibroma (
Table 2.).
The humoral response measured by the SARS-CoV-2 IgG and IgM antibody concentration naturally increased during the pandemic and after vaccination. It was higher among people <60 years of age, which may be explained by the population's ageing of the immune system.
4. Discussion
This study aimed to answer the question about the impact of the cancer process on the humoral response in oncological patients vaccinated against SARS-CoV-2 infection and in patients after COVID-19. The studies also drew attention to the potential impact of the anti-infective response, in this case, the antiviral response, on the potentiation of the anticancer response. These observations are important because a post-infectious or post-vaccination increase in the concentration of IgG antibodies, secondary response antibodies, provides a potential opportunity for many months of "strengthening" the humoral response, which is expected to have a significant impact on the course of anticancer therapy.
The type of cancer, its histopathological characteristics and its stage of advancement generate differently intense and targeted immune responses [
13]. The question, therefore, arises whether some cancers do not naturally generate a more excellent humoral response directed against the SARS-CoV-2. Recognized contraindications to the implementation of systemic cancer treatment include coexisting inflammation, systemic reinfections, herpes zoster and herpes, leading to immunosuppression. On the other hand, a parallel viral infection, e.g. with the SARS-CoV-2 in oncological patients, due to the existing immune deficiency, may lead to the development and progression of an often life-threatening infectious process. In the context of the data cited, the question arises whether, despite these "alarm" signals, we should vaccinate oncology patients, including those undergoing anticancer therapy, with the virus.
The results contained in our previous work, which assessed the post-vaccination response to BNT162b2 (Pfizer: Comirnaty) and the reaction after COVID-19, based on a 2-year analysis of 5 cases of differentiated humoral response, showed surprising conclusions [
14]. In the summary of the work, administering at least three doses of mRNA vaccine should serve as the basis for immunization, and a three-month interval may be the best alternative to the vaccination schedule for non-immunocompromised (healthy) people. Our observations were confirmed in the works of other authors [
6].
Given the above, the advisability of vaccinating people with the so-called risk groups, i.e. patients over 65 years of age with immunological deficiencies related to the ageing process, people with risk factors for the severe course of COVID-19, such as obesity, arterial hypertension or circulatory failure.
Should we also vaccinate people with clinical signs of infection with SARS-CoV-2 despite the lack of confirmation of the presence of the virus by PCR tests, which, as we mentioned above, may be responsible for the low sensitivity of the tests used [
15]? The magnitude of the post-vaccination response measured by the concentration of IgM and IgG antibodies varies individually; in the case of oncology patients, it is influenced by the nature of the tumour, its histopathological type, stage of advancement, and the therapy used [
16]. In the case of breast cancer therapy based on CDK 4/6 inhibitors, which inhibit the cycle and division of cancer cells, it has a specific effect by inhibiting the effect of physiological estrogens on cancer cells [
17]. The conducted research proved the safety of early immunization, i.e. two weeks after the end of COVID-19, in the population of women with breast cancer [
18].
In the case of breast cancer chemotherapy, despite a rapid antibody response, a decrease in mainly S-RBD antibodies is usually observed [
19]. In Canada, where COVID-19 vaccination coverage reached over 70% of the population, the incidence of breast and intestinal cancer decreased by almost half after the SARS-CoV-2 pandemic [
19]. It is suggested that 10-20% of the general population may not show an antibody response for various reasons, while almost 80% of oncology patients experience infections asymptomatically or with few symptoms [
20].
5. Summary
Preliminary results of the studies that are the subject of this study show significant variability of the humoral response in patients followed for two years. In our opinion, the most sensitive and correlating with the clinical condition of the assessed patients are specific SARS-CoV-2 in the IgG class, the significant increase of which was observed in the case of reinforcement with "subsequent" doses of the vaccine [
21]. We used the criteria for diagnosing virus activation in asymptomatic people proposed in the study of post-vaccination immunity of our patients in 2021, adopting a cut-off for SARS-CoV-2 at the level of IgG >0.2 AU/ml and IgM >1.0 AU/ml [
22], which significantly improved detection of seroconversion of the SARS-CoV-2 in the population patients undergoing oncological treatment. The observations contained in this study prove that vaccination of oncological patients against SARS-CoV-2 infection may be a potential target in multifactorial therapy of cancer diseases [23, 24].
6. Conclusions
We observed a much greater COVID-19-dependent response (concentrations IgG and IgM SARS-CoV-2) in patients with cancer: breast, lung and colon independent of oncological therapy and vaccination (
Figure 3,
Figure 4).
An argument for re-vaccination in this group of people immunocompetent, with an apparent deficit of hemopoiesis after chemotherapy or radiotherapy, is that their early (primary) immune response is naturally weak by a small amount of the first specific SARS-CoV-2 IgG antibodies determined in research.
Additionally, we observed an ageing effect on the immune system in people 60+ of both sexes, despite the population increase in humoral response in groups aged during two years of observation.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
P.K. and A.S.-B. analyzed the data and drafted the manuscript. R.M. participated in data analysis and B.M., A.H., A.G. extensively reviewed the manuscript. Other authors: D.E.K., M.B., and S.S. contributed to clinical and laboratory data acquisition and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive specific funding but was performed as part of the work of the authors at Maria Sklodowska-Curie Bialystok Oncology Centre, Poland.
Institutional Review Board Statement
The Bioethics Commission of Medical University approved our research. Approval code: APK.002.267.2021; approval date: 29 April 2021.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data are available on request.
Acknowledgments
We thank all health-care workers involved in examinations in Maria Sklodowska-Curie Bialystok Oncology Centre in Bialystok, Poland. All the individuals included in this work have consented to the acknowledgement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang H, Han H, He T, Labbe KE, Hernandez AV, Chen H, Velcheti V, Stebbing J, Wong KK. Clinical Characteristics and Outcomes of COVID-19-Infected Cancer Patients: A Systematic Review and Meta-Analysis. J Natl Cancer Inst. 2021;113(4):371-80. Epub 2020/11/03. PubMed PMID: 33136163; PMCID: PMC7665647. [CrossRef]
- Liu T, Zeng G, Tao H, Shi Y, Group C-iCPR, Wang T, Liu T, Guo F, Zhou F, Wang X. Low prevalence of IgG antibodies to SARS-CoV-2 in cancer patients with COVID-19. Int J Cancer. 2020;147(11):3267-9. Epub 2020/06/12. PubMed PMID: 32525566; PMCID: PMC730085. [CrossRef]
- Ibrahim M, Natarajan V, Murthy P, Meghal T, Xu Y, Wiesel O. The prevalence of asymptomatic COVID-19 infection in cancer patients. A cross-sectional study at a tertiary cancer center in New York City. Cancer Treat Res Commun. 2021;27:100346. Epub 2021/03/24. PubMed PMID: 33756172; PMCID: PMC7908877. [CrossRef]
- Sievers BL, Cheng MTK, Csiba K, Meng B, Gupta RK. SARS-CoV-2 and innate immunity: the good, the bad, and the "goldilocks". Cell Mol Immunol. 2023. Epub 2023/11/21. PubMed PMID: 37985854. [CrossRef]
- Mahase E. Covid-19: Just a third of blood cancer patients had antibodies against delta variant after two vaccine doses, study finds. BMJ. 2021;375:n2623. Epub 2021/10/29. PubMed PMID: 34706860. [CrossRef]
- Overheu O, Lendowski S, Quast DR, Kuhn D, Vidal Blanco E, Kraeft AL, Steinmann E, Kourti E, Lugnier C, Steinmann J, Reinacher-Schick A, Pfaender S. Longitudinal data on humoral response and neutralizing antibodies against SARS-CoV-2 Omicron BA.1 and subvariants BA.4/5 and BQ.1.1 after COVID-19 vaccination in cancer patients. J Cancer Res Clin Oncol. 2023;149(12)10633-44. Epub 2023/06/10 23:42. PubMed PMID: 37300723; PMCID: PMC10257184. [CrossRef]
- Lee LYW, Ionescu MC, Starkey T, Little M, Tilby M, Tripathy AR, McKenzie HS, Al-Hajji Y, Appanna N, Barnard M, Benny L, Burnett A, Cattell EL, Clark JJ, Khan S, Ghafoor Q, Panneerselvam H, Illsley G, Harper-Wynne C, Hattersley RJ, Lee AJ, Lomas O, Liu JK, McCauley A, Pang M, Pascoe JS, Platt JR, Patel G, Patel V, Potter VA, Randle A, Rigg AS, Robinson TM, Roques TW, Roux RL, Rozmanowski S, Taylor H, Tuthill MH, Watts I, Williams S, Beggs A, Iveson T, Lee SM, Middleton G, Middleton M, Protheroe A, Fittall MW, Fowler T, Johnson P, Programme UKCC. COVID-19: Third dose booster vaccine effectiveness against breakthrough coronavirus infection, hospitalisations and death in patients with cancer: A population-based study. Eur J Cancer. 2022;175:1-10. Epub 2022/09/10. PubMed PMID: 36084618; PMCID: PMC9276646. [CrossRef]
- Huang Y, Yu J, Li D, He K, Liu W, Wang L, Chen Y, Xie C, Wu X. Durable tracking anti-SARS-CoV-2 antibodies in cancer patients recovered from COVID-19. Sci Rep. 2021;11(1):17381. Epub 2021/09/01. PubMed PMID: 34462453; PMCID: PMC8405618. [CrossRef]
- Fong D, San Nicolo KO, Alber M, Mitterer M. Evaluating the longitudinal effectiveness of preventive measures against COVID- and seroprevalence of IgG antibodies to SARS-CoV-2 in cancer outpatients and healthcare workers. Wien Klin Wochenschr. 2021;133(7-8):359-63. Epub 2021/01/28. PubMed PMID: 33502609; PMCID: PMC7838655. [CrossRef]
- Solodky ML, Galvez C, Russias B, Detourbet P, N'Guyen-Bonin V, Herr AL, Zrounba P, Blay JY. Lower detection rates of SARS-COV2 antibodies in cancer patients versus health care workers after symptomatic COVID-19. Ann Oncol. 2020;31(8):1087-8. Epub 2020/05/04. PubMed PMID: 32360743; PMCID: PMC7252166. [CrossRef]
- Fendler A, Shepherd STC, Au L, Wu M, Harvey R, Schmitt AM, Tippu Z, Shum B, Farag S, Rogiers A, Carlyle E, Edmonds K, Del Rosario L, Lingard K, Mangwende M, Holt L, Ahmod H, Korteweg J, Foley T, Barber T, Emslie-Henry A, Caulfield-Lynch N, Byrne F, Deng D, Kjaer S, Song OR, Queval C, Kavanagh C, Wall EC, Carr EJ, Caidan S, Gavrielides M, MacRae JI, Kelly G, Peat KKelly D, Murra A, Kelly K, O'Flaherty M, Shea RL, Gardner G, Murray D, Yousaf N, Jhanji S, Tatham K, Cunningham D, Van As N, Young K, Furness AJS, Pickering L, Beale R, Swanton C, Gandhi S, Gamblin S, Bauer DLV, Kassiotis G, Howell M, Nicholson E, Walker S, Larkin J, Turajlic S, consortium C. Omicron neutralising antibodies after third COVID-19 vaccine dose in patients with cancer. Lancet. 2022;399(10328):905-7. Epub 2022/01/30. PubMed PMID: 35090602; PMCID: PMC8789238. [CrossRef]
- Challenor S, Tucker D. SARS-CoV-2-induced remission of Hodgkin lymphoma. Br J Haematol. 2021;192(3):415. Epub 2021/01/03. PubMed PMID: 33386647. [CrossRef]
- Kim J, Chang E, Park SY, Lee DW, Kang CK, Choe PG, Kim NJ, Oh MD, Park WB, Lee KH, Im SA. Evaluation of Seropositivity After Standard Doses of Vaccination Against SARS-CoV-2 in Patients With Early Breast Cancer Receiving Adjuvant Treatment. Oncologist. 2022;27(12):e931-e7. Epub 2022/10/12. PubMed PMID: 36218350; PMCID: PMC9732225. [CrossRef]
- Kosiorek P, Stroz S, Hryniewicz A, Kazberuk DE, Milewski R, Bartoszewicz K, Borkowska MJ, Stasiak-Barmuta A. A new set-up of vanishing antibodies: A biennial follow-up of five different clients' humoral responses against SARS-CoV-2 aftersystemic vaccination in an oncology hospital in Poland. Health Sci Rep. 2023;6(4):e1172. Epub 20230330. PubMed PMID: 37008810; PMCID: PMC10064024. [CrossRef]
- Mungmunpuntipantip R, Wiwanitkit V. SARS-COV-2 IgG and IgM Antibodies in Cancer Patients. Asian Pac J Cancer Prev. 2021;22(8):2311. Epub 2021/08/29. PubMed PMID: 34452540; PMCID: PMC8629452. [CrossRef]
- Almehmadi M, Salih MM, Shafie A, Alsharif A, Alsiwiehr N, El-Askary A, Alzahrani K, Al-Hazmi A, Aljuaid A, Abdulazziz O, Almalki AA, Allahyani M, Eed E, Alharbi AM, Halawi M, Allam HH, Abutawil H, Alosimi E, Gharib AF. Seroprevalence of IgM and IgG Against SARS-CoV-2 after Two Doses of Pfizer-BioNTech COVID-19 Vaccine in Women with Breast Cancer. Clin Lab. 2022;68(11). Epub 2022/11/16. PubMed PMID: 36378000. [CrossRef]
- Nelli F, Fabbri A, Botticelli A, Giannarelli D, Marrucci E, Fiore C, Virtuoso A, Berrios JRG, Scagnoli S, Pisegna S, Cirillo A, Panichi V, Massari A, Silvestri MA, Ruggeri EM. Immune responses and clinical outcomes following the third dose of SARS-CoV-2 mRNA-BNT162b2 vaccine in advanced breast cancer patients receiving targeted therapies: a prospective study. Front Oncol. 2023;13:1280416. Epub 2023/11/29. PubMed PMID: 38023235; PMCID: PMC10662103. [CrossRef]
- Halpern N, Boursi B, Shacham-Shmueli E, Gal-Yam EN, Margalit O, Golan T, Beller T, Strauss G, Yahav D, Leshem E. Early Systemic Anti-neoplastic Treatment Post SARS-CoV-2 Infection in Patients with Breast Cancer. Oncol Ther. 2023;11(4):513-9. Epub 20231021. PubMed PMID: 37864026; PMCID: PMC10673789. [CrossRef]
- Decker KM, Feely A, Bucher O, Czaykowski P, Hebbard P, Kim JO, Pitz M, Singh H, Thiessen M, Lambert P. New Cancer Diagnoses Before and During the COVID-19 Pandemic. JAMA Netw Open. 2023;6(9):e2332363. Epub 20230905. PubMed PMID: 37669049; PMCID: PMC10481240. [CrossRef]
- Titova E, Kan VW, Ip A, Lozy T, Shier K, Prakash VP, Starolis M, Ansari S, Goldgirsh K, Kim S, Pelliccia MC, Mccutchen A, Megalla M, Gunning TS, Kaufman HW, Meyer WA, Perlin DS. Immunocompetent and immunocompromised cancer populations: Post-vaccination humoral and cellular immune responses against SARS-CoV-2. Journal of Clinical Oncology. 2023;41(16_suppl):e18779-e. [CrossRef]
- Gupta S, Chauhan N, Kumari P, Bansal M, Chahar A. To study the pattern of seroconversion for SARS-CoV-2 IgG antibodies in COVID-infected cancer patients and to correlate it clinically-A cross-sectional study. J Cancer Res Ther. 2023;19(Supplement):S404-S8. Epub 2023/05/06. PubMed PMID: 37148008. [CrossRef]
- Kosiorek P, Kazberuk DE, Hryniewicz A, Milewski R, Stroz S, Stasiak-Barmuta A. Systemic COVID-19 Vaccination Enhances the Humoral Immune Response after SARS-CoV-2 Infection: A Population Study from a Hospital in Poland Criteria for COVID-19 Reimmunizaticines (Basel). 2022;10(2). Epub 20220219. PubMed PMID: 35214792; PMCID: PMC8875391. [CrossRef]
- Cazeau N, Palazzo M, Savani M, Shroff RT. COVID-19 Vaccines and Immunosuppressed Patients With Cancer: Critical Considerations. Clin J Oncol Nurs. 2022;26(4):367-73. PubMed PMID: 35939727; PMCID: PMC9713690. [CrossRef]
- Costanzo M, De Giglio MAR, Roviello GN. Deciphering the Relationship between SARS-CoV-2 and Cancer. Int J Mol Sci. 2023;24(9). Epub 2023/05/13. PubMed PMID: 37175509; PMCID: PMC10178366. [CrossRef]
Figure 1.
Characteristics of gender distribution in the vaccinated group (S1-S5). The number of sample representatives of each group is below the group symbol. There were no differences between sexes in groups—the S0-control group. S0 (no vaccination), S1 (single vaccine: Johnson&Johnson(J&J), AstraZeneca(AZ), Moderna, Pfizer(COM; Comirnaty), S2A (full two-dose one vaccination) and S2B (two-dose vaccination Pfizer and Moderna), S3, S4, S5-additional vaccination.
Figure 1.
Characteristics of gender distribution in the vaccinated group (S1-S5). The number of sample representatives of each group is below the group symbol. There were no differences between sexes in groups—the S0-control group. S0 (no vaccination), S1 (single vaccine: Johnson&Johnson(J&J), AstraZeneca(AZ), Moderna, Pfizer(COM; Comirnaty), S2A (full two-dose one vaccination) and S2B (two-dose vaccination Pfizer and Moderna), S3, S4, S5-additional vaccination.
Figure 2.
The diagrams present the significance of different SARS-CoV-2 IgG (A) and IgM (B) responses to COVID-19 vaccination between the Oncologic vs. Non-Oncologic group (A1, B1) and the Cancer vs.Non-Cancer Group (A2, B2). Mediana and correlation (p-value) between groups presented. Below the group symbol are the amounts of representatives of each group. The Vaccinated Group: S0 (no vaccination), S1 (single vaccine: Johnson&Johnson, AstraZeneca, Moderna, Pfizer), S2A (full two-dose one vaccination) and S2B (two-dose vaccination Pfizer and Moderna), S3, S4, S5 booster mostly Pfizer and Moderna. Antibody concentrations are measured in artificial units per mL (AU/mL). P-values represent comparison with groups (*) p<0.05, (**) p<0.001, (***) p<0.0001.
Figure 2.
The diagrams present the significance of different SARS-CoV-2 IgG (A) and IgM (B) responses to COVID-19 vaccination between the Oncologic vs. Non-Oncologic group (A1, B1) and the Cancer vs.Non-Cancer Group (A2, B2). Mediana and correlation (p-value) between groups presented. Below the group symbol are the amounts of representatives of each group. The Vaccinated Group: S0 (no vaccination), S1 (single vaccine: Johnson&Johnson, AstraZeneca, Moderna, Pfizer), S2A (full two-dose one vaccination) and S2B (two-dose vaccination Pfizer and Moderna), S3, S4, S5 booster mostly Pfizer and Moderna. Antibody concentrations are measured in artificial units per mL (AU/mL). P-values represent comparison with groups (*) p<0.05, (**) p<0.001, (***) p<0.0001.
Figure 3.
Specific humoral response of particular IgG to SARS-CoV-2 infection and COVID-19 vaccination in oncological patients with breast disease over two years (monthly). Breast cancer (c) stands out from breast diseases (a,b) in oncological patients. In breast cancer, the statistical significance of the IgG response (d) results from the difference in the post-vaccination response separately for those vaccinated with Pfizer (BNT162b2) (g) and the administration of other vaccines (i). All of them are working. Surprisingly, the response is more significant in unvaccinated patients (f) than in vaccinated patients (e) for breast cancer. In this group, the humoral response is significant for all three doses (h, i) of the vaccine used (S1, S2, S3) regardless of the type (and combination of vaccines each other) (j). It was observed (k), regardless of vaccination (non-vaccinated group), a more significant increase in the humoral response in treated patients (radiotherapy, chemotherapy, surgery) and a weaker post-vaccination response in groups of cancer patients over 60 years of age (l). All of this contributes to the description of the breast cancer anti-infectious response to the COVID-19 pandemic, which may help to understand SARS-CoV-2 fight cancer. S0 (no vaccination), S1 (single vaccine: Johnson&Johnson(J&J), AstraZeneca (AZ), Moderna, Pfizer(COM; Comirnaty), S2A (full two-dose one vaccination) and S2B (two-dose vaccination Pfizer and Moderna), S3, S4, S5-additional vaccination. CHTH-chemotherapy; RTH-radiotherapy; SURG-surgery; n-amount of study samples. P-values represent comparison with groups (*) p<0.05, (**) p<0.001, (***) p<0.0001.
Figure 3.
Specific humoral response of particular IgG to SARS-CoV-2 infection and COVID-19 vaccination in oncological patients with breast disease over two years (monthly). Breast cancer (c) stands out from breast diseases (a,b) in oncological patients. In breast cancer, the statistical significance of the IgG response (d) results from the difference in the post-vaccination response separately for those vaccinated with Pfizer (BNT162b2) (g) and the administration of other vaccines (i). All of them are working. Surprisingly, the response is more significant in unvaccinated patients (f) than in vaccinated patients (e) for breast cancer. In this group, the humoral response is significant for all three doses (h, i) of the vaccine used (S1, S2, S3) regardless of the type (and combination of vaccines each other) (j). It was observed (k), regardless of vaccination (non-vaccinated group), a more significant increase in the humoral response in treated patients (radiotherapy, chemotherapy, surgery) and a weaker post-vaccination response in groups of cancer patients over 60 years of age (l). All of this contributes to the description of the breast cancer anti-infectious response to the COVID-19 pandemic, which may help to understand SARS-CoV-2 fight cancer. S0 (no vaccination), S1 (single vaccine: Johnson&Johnson(J&J), AstraZeneca (AZ), Moderna, Pfizer(COM; Comirnaty), S2A (full two-dose one vaccination) and S2B (two-dose vaccination Pfizer and Moderna), S3, S4, S5-additional vaccination. CHTH-chemotherapy; RTH-radiotherapy; SURG-surgery; n-amount of study samples. P-values represent comparison with groups (*) p<0.05, (**) p<0.001, (***) p<0.0001.
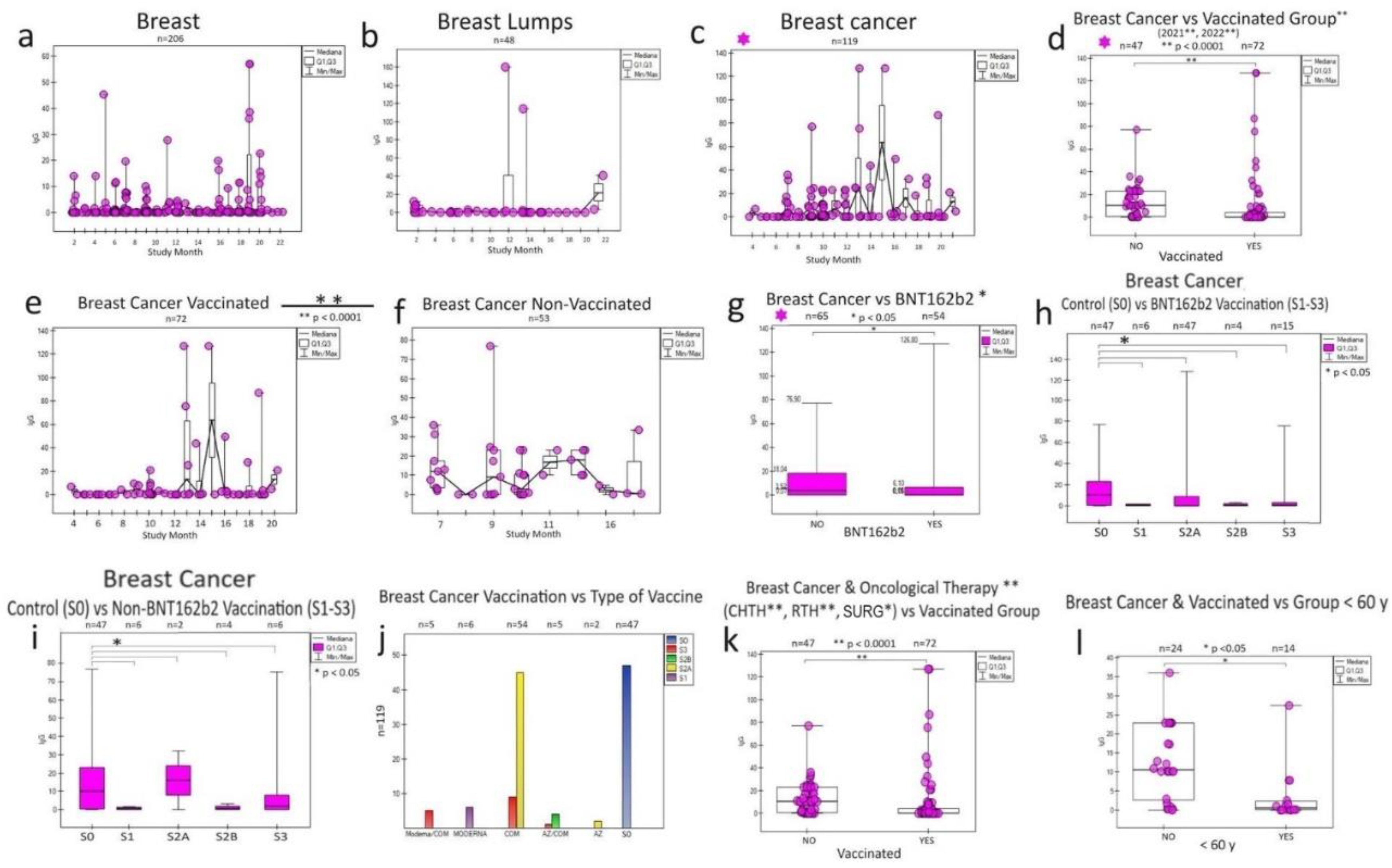
Figure 4.
The graphs show differences in the humoral response of specific SARS-CoV-2 IgG in lung and colon cancer in response to COVID-19 vaccinations during a two-year (monthly) follow-up. Both in the lungs (a) and in the intestine (h, i), there are pre-oncological states that overlap with the tumour humoral response for the lungs (b) and large intestine (j). Statistical correlations were found between the vaccinated group (c,e,f) and in the groups for oncological treatment (d) and the group of older people > 60 years (g) only in the case of lung cancer, but not in colon cancer (k,l,m,n,o). In both cancers, there are no comparison groups to demonstrate the effects of the type of vaccines. It is noteworthy that colon cancer is significant despite similar relationships and the predominance of group unvaccinated cancer patients (as in breast cancer and lung cancer) over vaccinated ones (c,k),which also accounts for the specific humoral response of this cancer to SARS-CoV-2 infection. S0 (no vaccination), S1 (single vaccine: Johnson&Johnson(J&J), AstraZeneca (AZ), Moderna, Pfizer(COM; Comirnaty), S2A (full two-dose one vaccination) and S2B (two-dose vaccination Pfizer and Moderna), S3, S4, S5-additional vaccination. CHTH-chemotherapy; RTH-radiotherapy; SURG-surgery; n-amount of study samples. P-values represent comparison with groups (*) p<0.0001.
Figure 4.
The graphs show differences in the humoral response of specific SARS-CoV-2 IgG in lung and colon cancer in response to COVID-19 vaccinations during a two-year (monthly) follow-up. Both in the lungs (a) and in the intestine (h, i), there are pre-oncological states that overlap with the tumour humoral response for the lungs (b) and large intestine (j). Statistical correlations were found between the vaccinated group (c,e,f) and in the groups for oncological treatment (d) and the group of older people > 60 years (g) only in the case of lung cancer, but not in colon cancer (k,l,m,n,o). In both cancers, there are no comparison groups to demonstrate the effects of the type of vaccines. It is noteworthy that colon cancer is significant despite similar relationships and the predominance of group unvaccinated cancer patients (as in breast cancer and lung cancer) over vaccinated ones (c,k),which also accounts for the specific humoral response of this cancer to SARS-CoV-2 infection. S0 (no vaccination), S1 (single vaccine: Johnson&Johnson(J&J), AstraZeneca (AZ), Moderna, Pfizer(COM; Comirnaty), S2A (full two-dose one vaccination) and S2B (two-dose vaccination Pfizer and Moderna), S3, S4, S5-additional vaccination. CHTH-chemotherapy; RTH-radiotherapy; SURG-surgery; n-amount of study samples. P-values represent comparison with groups (*) p<0.0001.
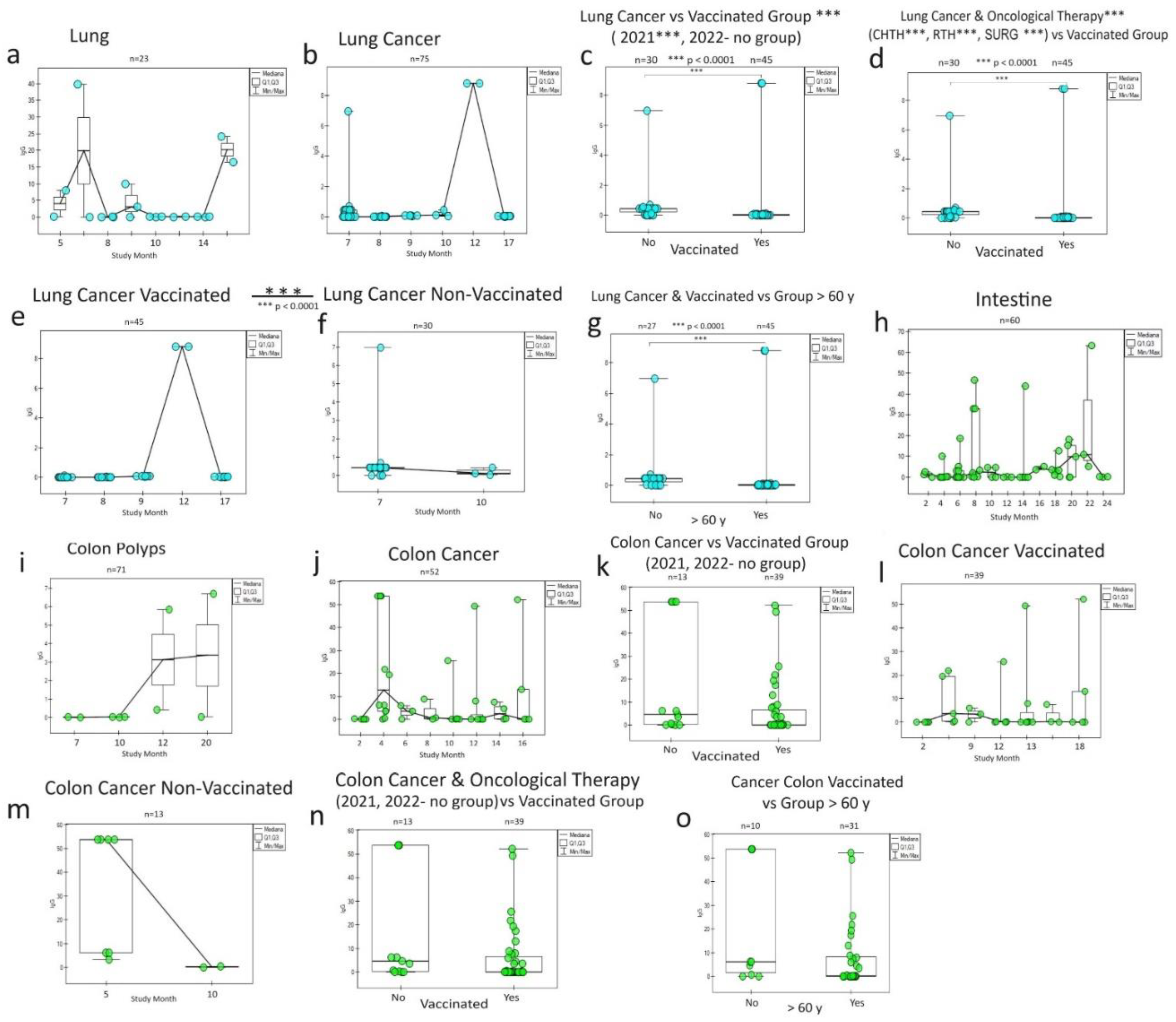
Figure 5.
The diagrams show the humoral response of SARS-CoV-2 IgG antibodies to oncological therapy: chemotherapy (CHTH), radiotherapy (RTH), surgery (SURG) in oncological patients with breast cancer
(A1, A2, A3), colon cancer
(B1, B2, B3), lungs
(C1, C2, C3), kidneys
(D1, D2, D3), testicles
(E1, E2, E3), states in which statistical significance was observed in the vaccinated and unvaccinated groups (
Table 2). CHTH response is the strongest. Here, we observe a dual response to RTH: positive (rise IgG) in the breast, colon, and lung, and negative (fall IgG) in the kidney, and SURG response: positive (rise) in the breast, colon, and lung. When there is only one group in the study (testicle cancer and surgery in kidney cancer), the effect of oncotherapy is unknown. * The p-value is presented only in the CHTH breast cancer group—n-amount of study samples.
Figure 5.
The diagrams show the humoral response of SARS-CoV-2 IgG antibodies to oncological therapy: chemotherapy (CHTH), radiotherapy (RTH), surgery (SURG) in oncological patients with breast cancer
(A1, A2, A3), colon cancer
(B1, B2, B3), lungs
(C1, C2, C3), kidneys
(D1, D2, D3), testicles
(E1, E2, E3), states in which statistical significance was observed in the vaccinated and unvaccinated groups (
Table 2). CHTH response is the strongest. Here, we observe a dual response to RTH: positive (rise IgG) in the breast, colon, and lung, and negative (fall IgG) in the kidney, and SURG response: positive (rise) in the breast, colon, and lung. When there is only one group in the study (testicle cancer and surgery in kidney cancer), the effect of oncotherapy is unknown. * The p-value is presented only in the CHTH breast cancer group—n-amount of study samples.
Table 1.
Characteristics of study.
Table 1.
Characteristics of study.
| n(%) of total antibodies masurements* |
Quantity (all) |
Vaccinated |
2020 |
2021 |
2022 |
| Quantity |
Quantity |
Quantity |
| All |
2541 (100%)* |
2073 (100%) |
606 |
1361 |
574 |
| Man |
823 (32.39) |
716 (34.54) |
124 |
498 |
201 |
| Woman |
1718 (67.61) |
1357 (65.46) |
482 |
863 |
373 |
| Clinical division |
|
|
|
|
|
| Oncology group |
1714 (67.44) |
1369 (66.04) |
400 |
1007 |
307 |
| Cancer group |
604 (23.77) |
498 (24.02) |
65 |
455 |
84 |
| Cancer |
566 (22.27) |
465 (22.43) |
63 |
430 |
73 |
| O-Cancer |
29 (1.14) |
24 (1.16) |
2 |
18 |
9 |
| H-Cancer |
9 (0.35) |
9 (0.43) |
0 |
7 |
2 |
| Non-Cancer group |
|
|
|
|
|
| Autoimmunology |
104 (4.09) |
73 (3.52) |
33 |
50 |
21 |
| Non-Cancer |
1006 (39.59) |
798 (38.49) |
302 |
502 |
202 |
| Non-Oncology group |
|
|
|
|
|
| |
827 (32.56) |
704 (33.96) |
206 |
354 |
267 |
| Treatment division |
599 (23.58) |
494 (23.83) |
62 |
453 |
84 |
| Radiotherapy |
376 (22.54) |
281 (13.56) |
38 |
287 |
51 |
| +chemotherapy |
327 (12.87) |
232 (11.19) |
37 |
261 |
29 |
| Chemotherapy |
435 (26.08) |
316 (15.24) |
42 |
344 |
49 |
| +surgery |
305 (12.00) |
204 (9.84) |
41 |
232 |
32 |
| Surgery |
446 (26.74) |
326 (15.72) |
59 |
337 |
50 |
| +radiotherapy |
283 (11.14) |
194 (9.36) |
39 |
213 |
31 |
| Non-treatment |
|
|
|
|
|
| |
1942 (76.47) |
1579 (81.31) |
544 |
908 |
490 |
| |
|
Cancer |
2020 |
2021 |
2022 |
| Vaccinated division |
2073 (100%) |
566 (100%) |
499 |
1089 |
484 |
| Pfizer |
1835 (88.52) |
416 (73.50) |
486 |
985 |
364 |
| Moderna |
67 (3.23) |
25 (4.42) |
4 |
45 |
18 |
| AstraZeneca |
98 (4.72) |
31 (5.48) |
6 |
39 |
53 |
| Johnson&Johnson |
75 (3.62) |
25 (4.42) |
5 |
21 |
49 |
| Non-Vaccinated |
|
|
|
|
|
| |
469 |
107 |
107 |
272 |
90 |
| Sex |
median y (min-max) |
Cancer 566 (100%) |
median y (min-max) |
median y (min-max) |
median y (min-max) |
| Man |
51 (16-97) |
292 (51.59) |
51(21-86) |
57 (16-97) |
44 (21-84) |
| Woman |
50 (18-91) |
274 (48.41) |
48 (21-80) |
53 (18-91) |
48 (22-88) |
| Age range |
|
|
|
|
|
| <40 |
34 (16-39) |
28 (4.95) |
34(21-39) |
34(16-39) |
35(21-39) |
| 40-60 |
50 (41-59) |
135 (23.85) |
52(41-59) |
50(41-59) |
49(41-59) |
| >60 |
70 (61-97) |
364 (64.31) |
65(61-86) |
70.5(61-97) |
68(61-88) |
Table 2.
Detailed division of the conducted research (disease entity, (n) number of tests (%), number of samples from vaccinated people (Vacc).
Table 2.
Detailed division of the conducted research (disease entity, (n) number of tests (%), number of samples from vaccinated people (Vacc).
| Cancer |
n=566 (%) |
Vacc n=429 |
p-Value |
N-Cancer |
n=1006 (%) |
Vacc n=818 |
p-Value |
|
|
| Breast |
119 (21.03) |
72 |
p=0.009* |
Breast |
259 (25.75) |
206 |
p=0.149 |
|
|
| Prostate |
88 (15.55) |
79 |
p=0.101 |
Skin |
110 (10.93) |
81 |
p=0.983 |
|
|
| Lung |
75 (13.25) |
45 |
P=0.0001* |
Polyps intestine |
71 (7.06) |
56 |
p=0.191 |
|
|
| Colon |
52 (9.20) |
39 |
p=0.068 |
Spine |
63 (6.26) |
56 |
p=0.392 |
|
|
| Tongue |
39 (6.89) |
37 |
|
Intestine |
60 (5.96) |
58 |
p=0.167 |
|
|
| Rectal |
33 (5.83) |
28 |
p=0.761 |
Breast lumps |
54 (5.37) |
48 |
p=0.679 |
|
|
| Skin |
28 (4.95) |
27 |
|
Thyroid nodule |
32 (3.18) |
25 |
|
|
|
| Kidney |
27 (4.77) |
20 |
p=0.007* |
Thyroid |
31 (3.08) |
21 |
p=0.359 |
|
|
| Uterine |
25 (4.41) |
17 |
p=0.169 |
Gastritis |
30 (2.98) |
20 |
p=0.38 |
|
|
| Brain |
19 (3.36) |
19 |
|
Hypothyroidism |
29 (2.88) |
22 |
|
|
|
| Testical |
10 (1.77) |
7 |
p=0.005* |
Ovarian |
23 (2.29) |
21 |
p=0.155 |
|
|
| Stomach |
9 (1.59) |
7 |
|
Lung |
23 (2.29) |
21 |
p=0.411 |
|
|
| Esophageal |
7 (1.24) |
7 |
|
Naevus |
22 (2.19) |
17 |
p=0.968 |
|
|
| Thyroid |
2 (1.06) |
4 |
|
Stomach |
20 (1.99) |
18 |
p=0.377 |
|
|
| Pancreas |
5 (0.88) |
2 |
p=0.386 |
Ovarian cyst |
20 (1.99) |
13 |
p=0.342 |
|
|
| Glioblastoma |
3 (0.53) |
3 |
|
Prostate hyperplasia |
18 (1.79) |
12 |
p=0.882 |
|
|
| Laryngeal |
3 (0.53) |
3 |
|
Diverticulitis |
15 (1.49) |
13 |
p=0.923 |
|
|
| Gallblader |
3 (0.53) |
3 |
|
Naevus pigmentosus |
14 (1.39) |
12 |
p=0.059 |
|
|
| Bladder |
3 (0.53) |
3 |
|
Lipoma |
13 (1.29) |
4 |
p=0.392 |
|
|
| Sarcoma |
2 (0.35) |
2 |
|
Nephrolithiasis |
12 (1.19) |
11 |
|
|
|
| Vulvar |
2 (0.35) |
2 |
|
Seborrheic papilloma |
10 (1.00) |
5 |
|
|
|
| Thymus |
2 (0.35) |
2 |
|
Uterine |
8 (0.80) |
6 |
p=0.092 |
|
|
| Liver |
2 (0.35) |
2 |
|
Struma nodosa |
7 (0.70) |
6 |
|
|
|
| Bone |
2 (0.35) |
2 |
|
Fibroma |
7 (0.70) |
4 |
p=0.05* |
|
|
| Pleural mesothelioma |
1 (0.18) |
1 |
|
Endometriosis |
7 (0.70) |
4 |
p=0.582 |
|
|
| Carcinoid tumor |
1 (0.18) |
1 |
|
Uterine polyps |
5 (0.50) |
3 |
p=0.767 |
|
|
| Salivary gland |
1 (0.18) |
1 |
|
Pancreatic cyst |
4 (0.40) |
3 |
|
|
|
| Ovarian |
1 (0.18) |
1 |
|
Hernia |
4 (0.40) |
2 |
|
|
|
| Cervical |
1 (0.18) |
1 |
|
Common warts |
3 (0.30) |
2 |
|
|
|
| Astrocytoma |
1 (0.18) |
1 |
|
Sciatica |
3 (0.30) |
1 |
|
|
|
| |
|
Vacc n=23 |
|
Prostatitis |
3 (0.30) |
1 |
|
|
|
| O-Cancer |
n=29 (%) |
p-Value |
Uterine fibrosis |
3 (0.30) |
0 |
|
|
|
| Cervical dysplasia |
11 (37.93) |
11 |
|
Cervical |
3 (0.30) |
0 |
|
|
|
| Melanocytoma |
7 (24.14) |
6 |
|
Abscesses |
3 (0.30) |
3 |
|
|
|
| Myeloma |
4 (13.79) |
1 |
|
Gastric polyps |
2 (0.20) |
22 |
|
|
|
| Acustic neuroma |
2 (6.90) |
0 |
|
Nevus flammeus |
2 (0.20) |
10 |
|
|
|
| Lymphoma |
2 (6.90) |
2 |
|
Neuralgia |
2 (0.20) |
1 |
|
|
|
| Adenomyosis uterus |
1 (3.45) |
1 |
|
Kidney |
2 (0.20) |
2 |
|
|
|
| Thrombocythemia |
1 (3.45) |
1 |
|
Tinea corporis |
1(0.10) |
1 |
|
|
|
| Myeloid leukemia |
1 (3.45) |
1 |
|
Testicular nodule |
1 (0.10) |
0 |
p=0.304 |
|
|
| |
|
Vacc n=72 |
|
Pancreatic |
1 (0.10) |
1 |
|
|
|
| Autoimmunology |
n=104 (%) |
p-Value |
Ostitis |
1 (0.10) |
1 |
|
|
|
| Allergic disease |
27 (25.96) |
15 |
p=0.286 |
Nose nodule |
1 (0.10) |
1 |
|
|
|
| Rheumatoid arthritis |
26 (25.00) |
16 |
p=0.006* |
Liver |
1 (0.10) |
1 |
|
|
|
| Hashimoto`s disease |
19 (18.27) |
16 |
p=0.240 |
Herpes simplex |
1 (0.10) |
1 |
|
|
|
| Graves` disease |
11 (10.58) |
5 |
p=0.004* |
COPD # |
1 (0.10) |
1 |
|
|
|
| Hyperthyroidism |
5 (4.81) |
5 |
|
Fatty liver |
1 (0.10) |
1 |
|
|
|
| Sclerodermia |
4 (3.85) |
4 |
|
|
|
|
|
|
|
| Psoriasis |
2 (1.92) |
2 |
|
H-Cancer |
n=9 (%) |
Vacc |
p-Value |
|
|
| Lyme disease |
2 (1.92) |
2 |
|
Hodgkin`s limphoma |
9 (100) |
9 |
|
|
|
| Liver hemangiomas |
2 (1.92) |
2 |
|
|
|
|
|
|
|
| Ulcerative colitis |
2 (1.92) |
2 |
|
Non-Oncology (Control) |
|
|
|
|
|
| Autoimmune thyroiditis |
2 (1.92) |
2 |
|
n=827(%) |
Vacc |
p-Value |
|
|
| Crohn`s disease |
1 (0.96) |
1 |
|
|
827 (100) |
692 |
|
|
|
| Celiac disease |
1 (0.96) |
1 |
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).