Submitted:
17 January 2024
Posted:
17 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
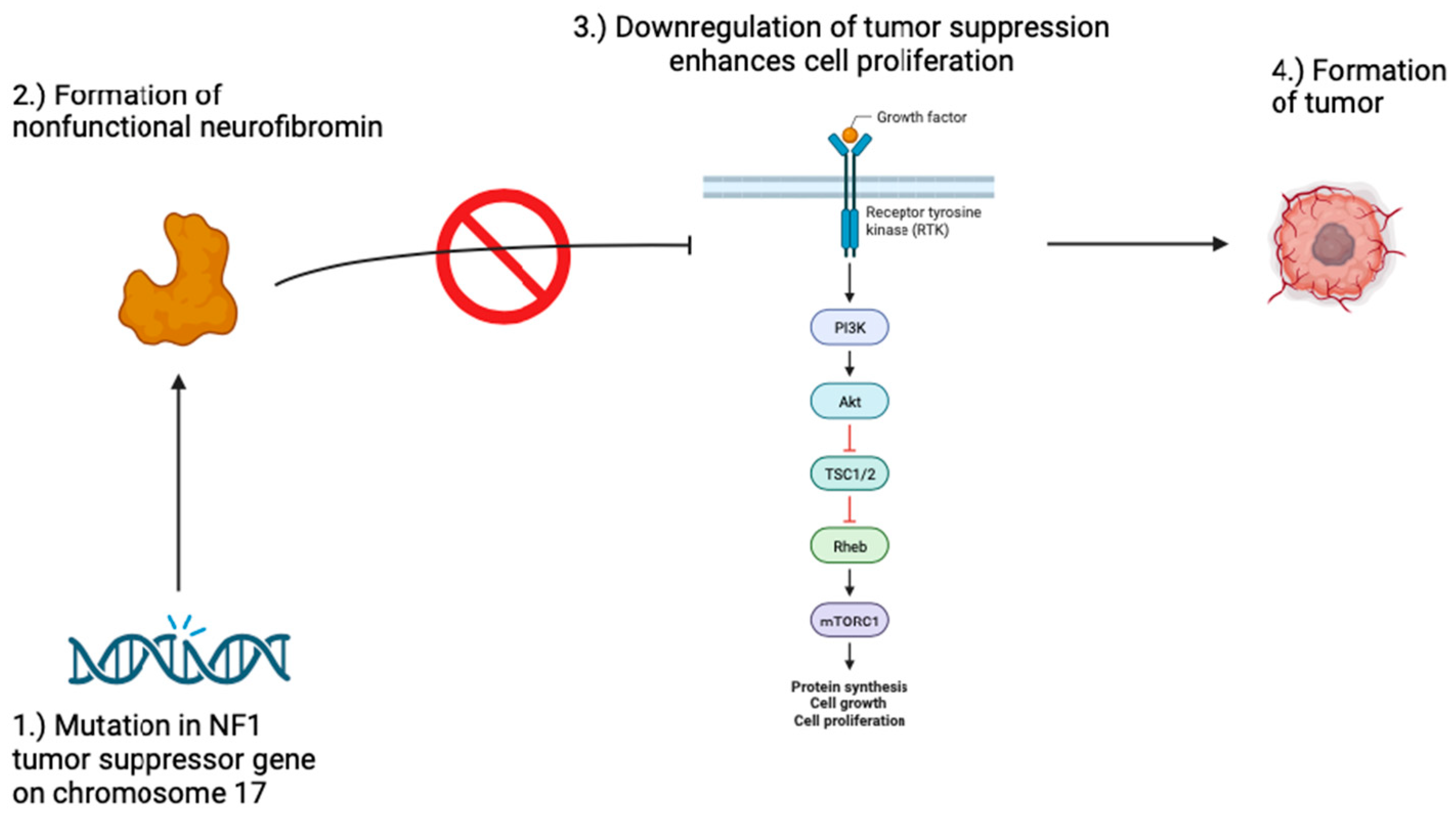
2. Neurofibromatosis 1 & 2
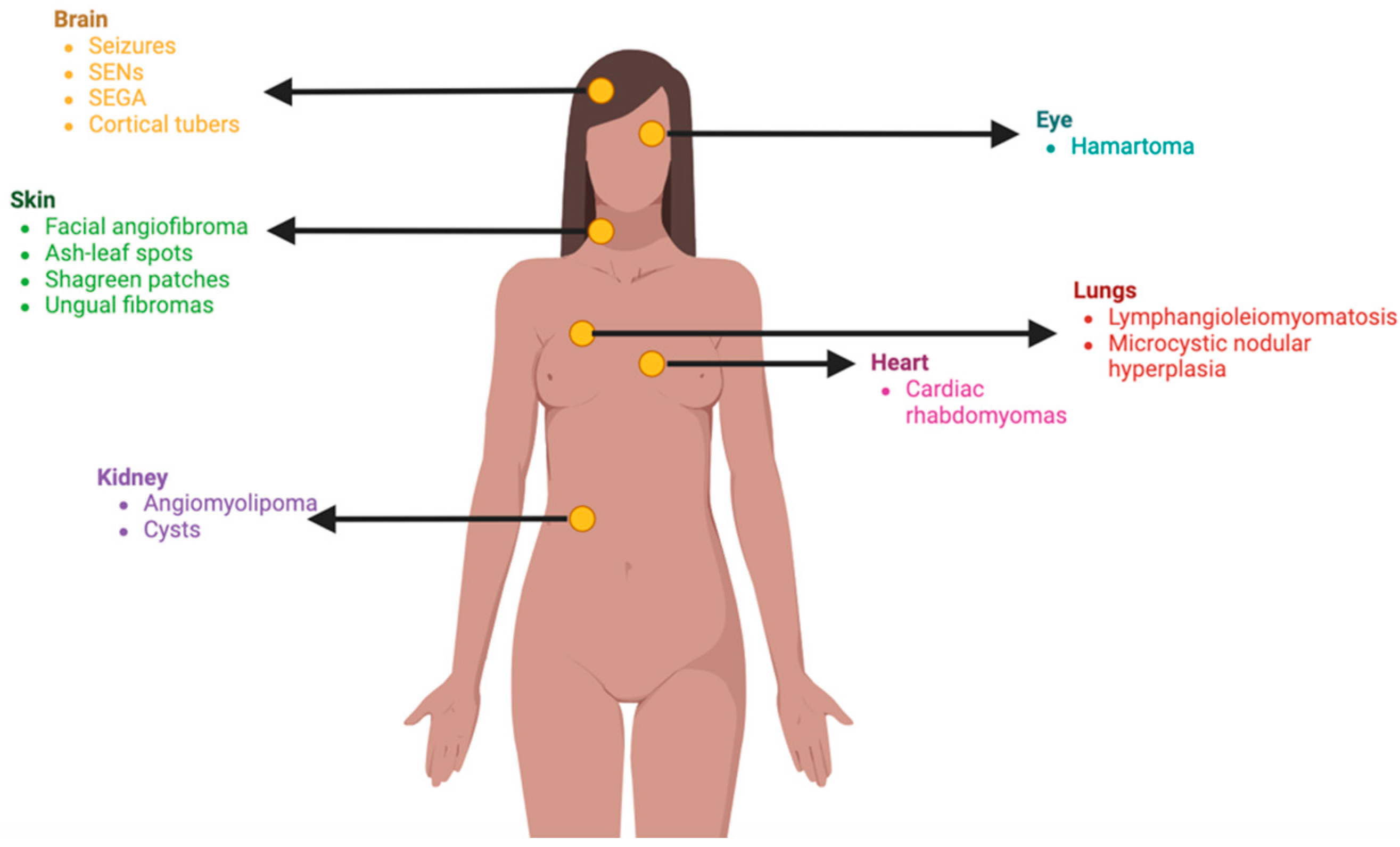
3. Tuberous Sclerosis
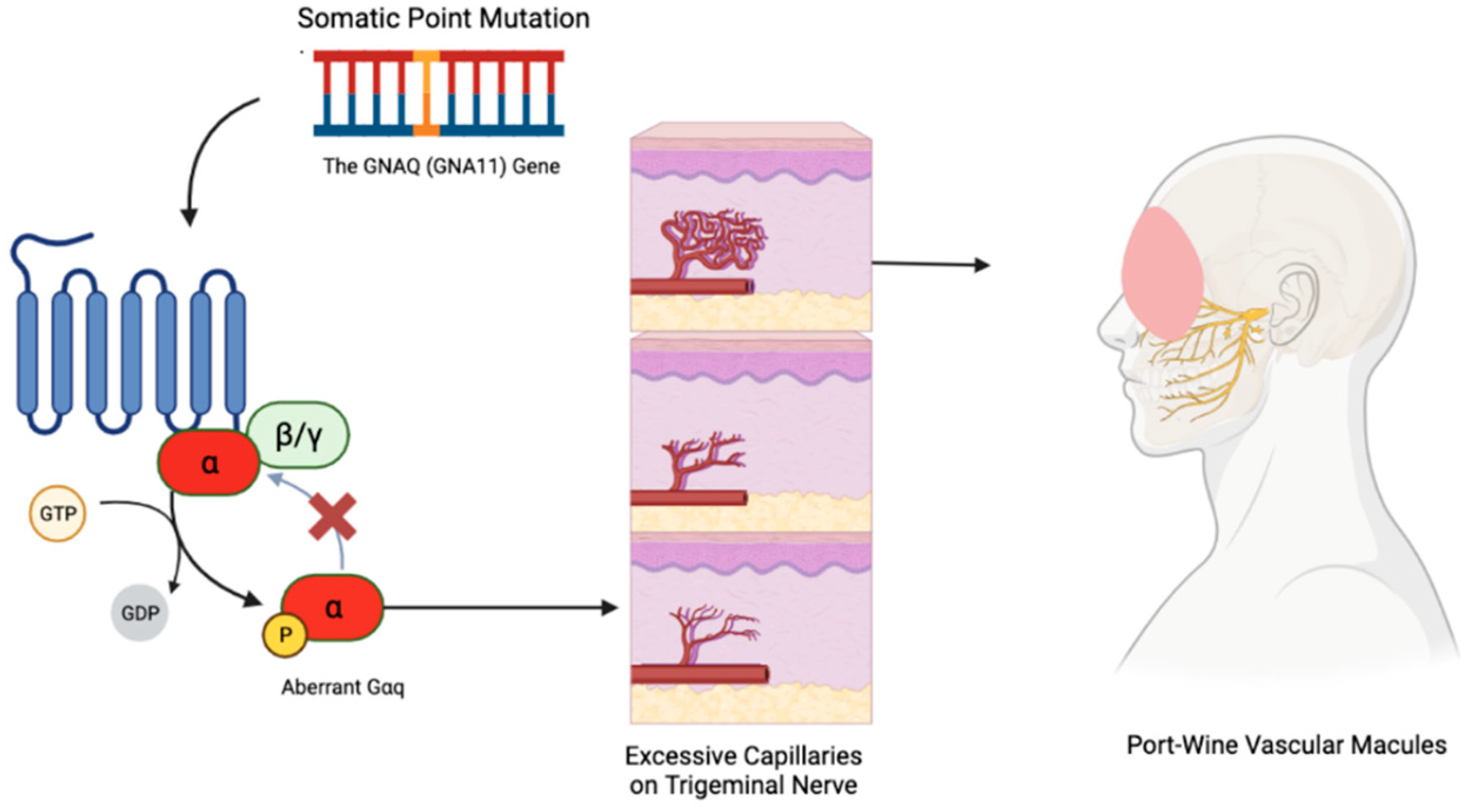
4. Sturge-Weber-Syndrome
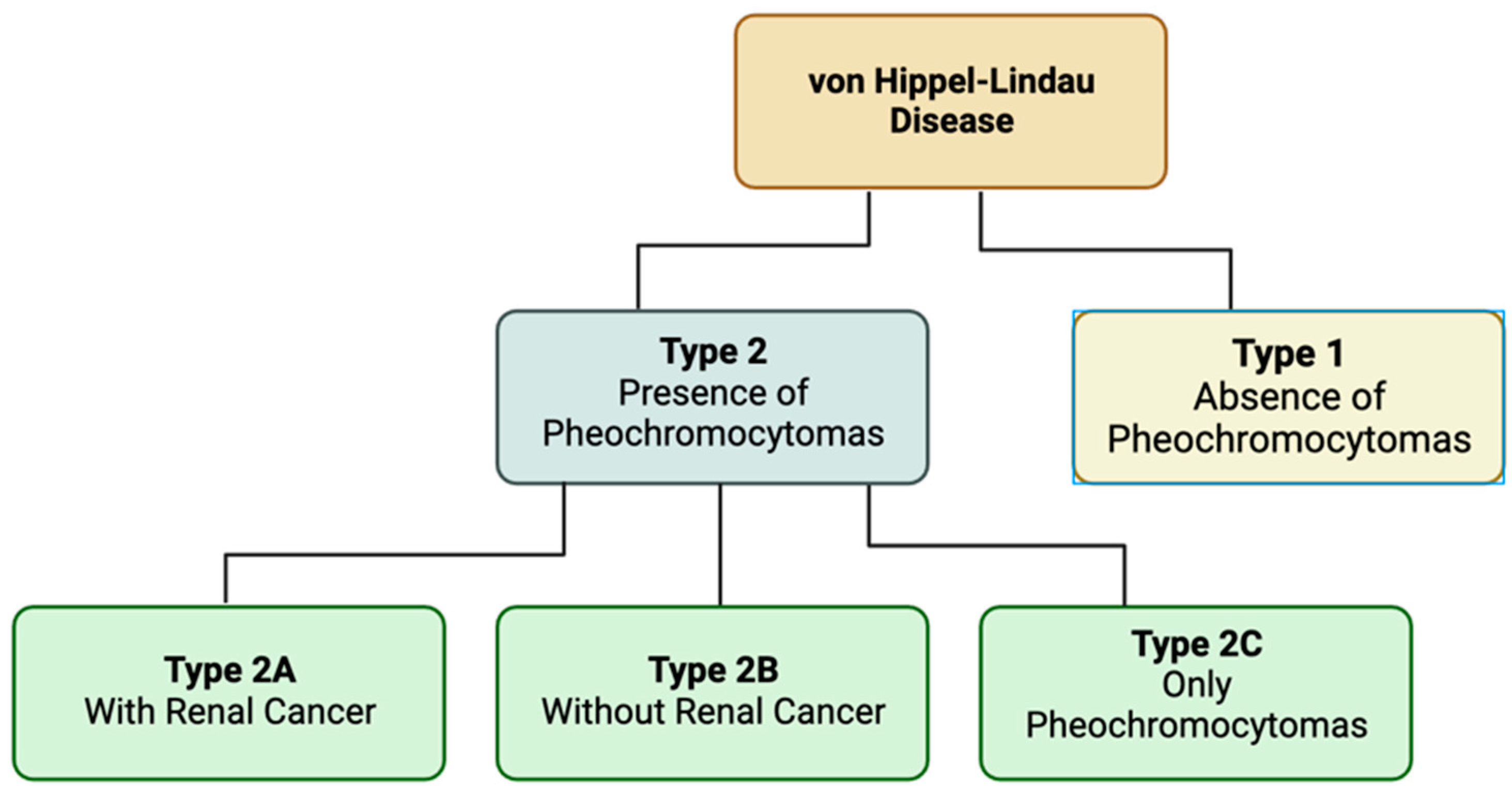
5. Von-Hippel-Lindau Disease
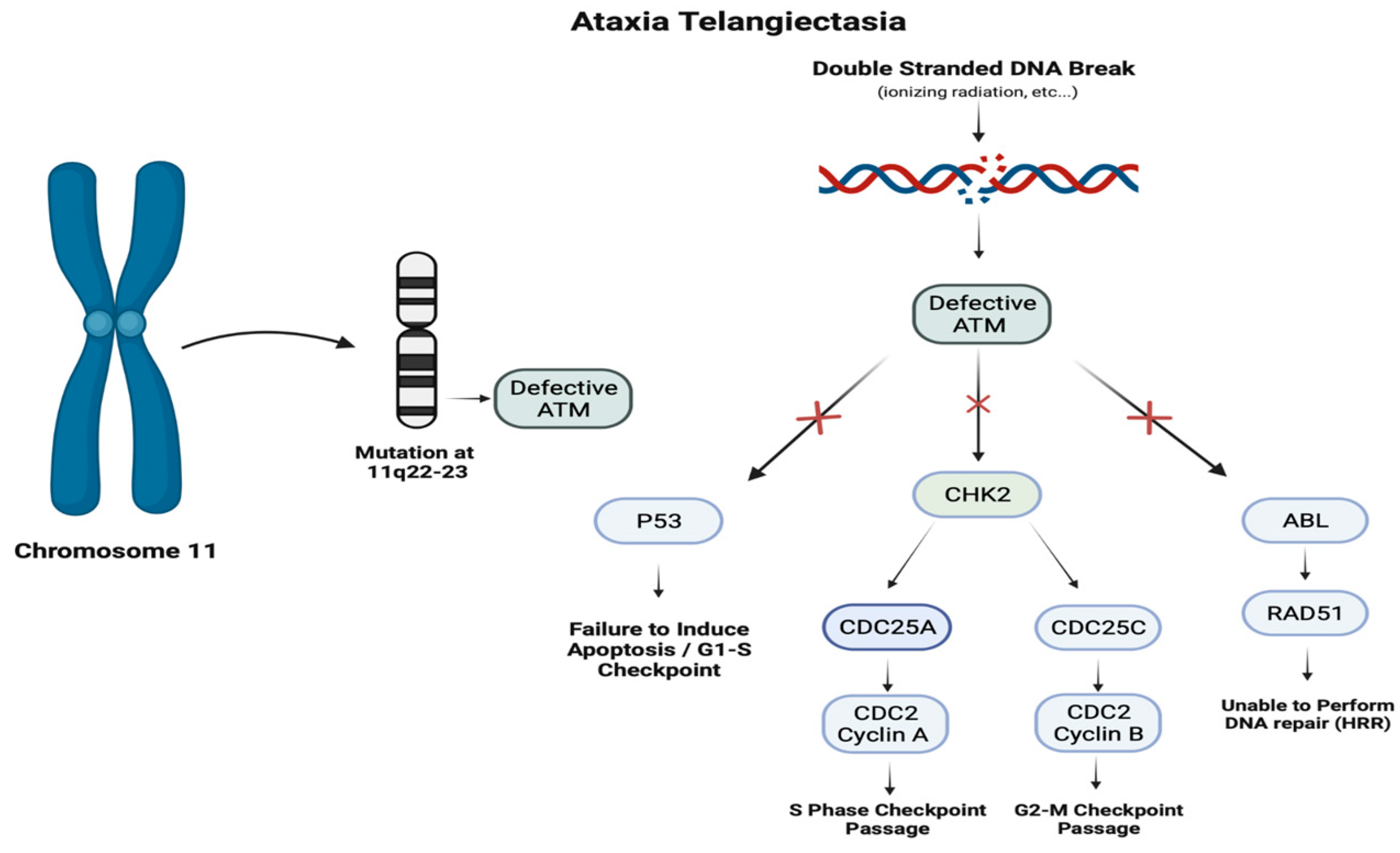
6. Ataxia-Telangiectasia
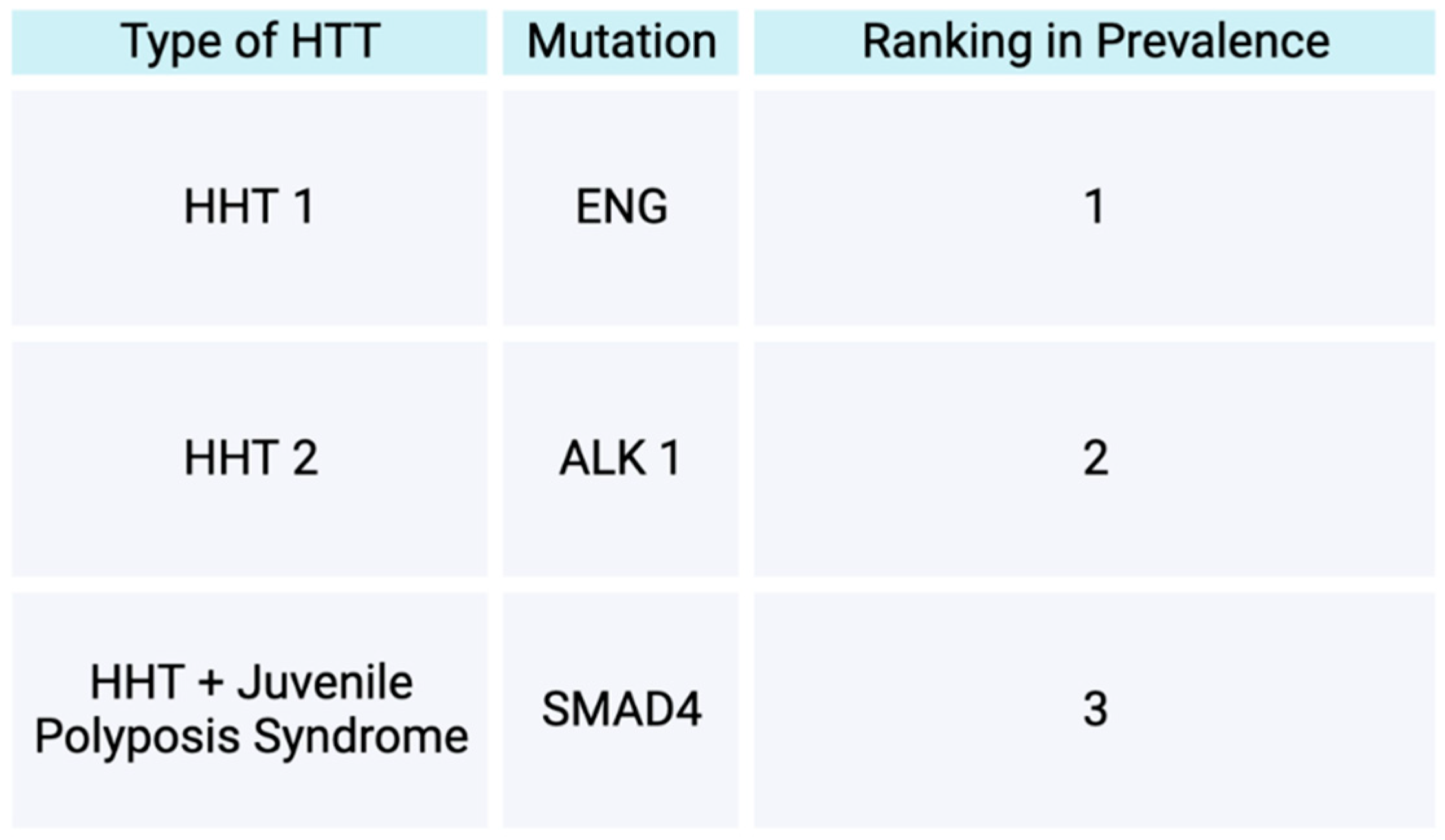
7. Osler-Weber-Rendu
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Klar, N.; Cohen, B.; Lin, D.D.M. Neurocutaneous Syndromes. Handb. Clin. Neurol. 2016, 135, 565–589. [Google Scholar] [CrossRef] [PubMed]
- Le, C.; Bedocs, P.M. Neurofibromatosis. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- Gutmann, D.H.; Ferner, R.E.; Listernick, R.H.; Korf, B.R.; Wolters, P.L.; Johnson, K.J. Neurofibromatosis Type 1. Nat. Rev. Dis. Primers 2017, 3, 17004. [Google Scholar] [CrossRef] [PubMed]
- Giraud, J.-S.; Bièche, I.; Pasmant, É.; Tlemsani, C. NF1 Alterations in Cancers: Therapeutic Implications in Precision Medicine. Expert. Opin. Investig. Drugs 2023, 32, 941–957. [Google Scholar] [CrossRef]
- Bergoug, M.; Doudeau, M.; Godin, F.; Mosrin, C.; Vallée, B.; Bénédetti, H. Neurofibromin Structure, Functions and Regulation. Cells 2020, 9, 2365. [Google Scholar] [CrossRef] [PubMed]
- Legius, E.; Brems, H. Genetic Basis of Neurofibromatosis Type 1 and Related Conditions, Including Mosaicism. Childs Nerv. Syst. 2020, 36, 2285–2295. [Google Scholar] [CrossRef] [PubMed]
- Bikowska-Opalach, B.; Jackowska, T. [Neurofibromatosis type 1 - description of clinical features and molecular mechanism of the disease]. Med. Wieku Rozwoj 2013, 17, 334–340. [Google Scholar] [PubMed]
- Nasi, L.; Alexopoulos, A.; Kokkinou, E.; Roka, K.; Tzetis, M.; Tsipi, M.; Kakourou, T.; Kanaka-Gantenbein, C.; Chrousos, G.; Kattamis, A.; et al. Characteristics of Café-Au-Lait Macules and Their Association with the Neurofibromatosis Type I Genotype in a Cohort of Greek Children. Acta Derm. Venereol. 2023, 103, adv5758. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y. Neurofibromatosis 1 (von Recklinghausen Disease). Keio J. Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- Hida, T.; Idogawa, M.; Okura, M.; Sugita, S.; Sugawara, T.; Sasaki, Y.; Tokino, T.; Yamashita, T.; Uhara, H. Genetic Analyses of Mosaic Neurofibromatosis Type 1 with Giant Café-Au-Lait Macule, Plexiform Neurofibroma and Multiple Melanocytic Nevi. J. Dermatol. 2020, 47, 658–662. [Google Scholar] [CrossRef]
- Walaszek, Z.; Hanausek-Walaszek, M.; Webb, T.E. Dietary Glucarate-Mediated Reduction of Sensitivity of Murine Strains to Chemical Carcinogenesis. Cancer Lett. 1986, 33, 25–32. [Google Scholar] [CrossRef]
- Taal, W.; van Dijk, S.A.; Noordhoek, C.; Broen, M.P.G.; Gijtenbeek, J.M.M.A.; Oostenbrink, R. [Symptomatic tumors in neurofibromatosis type 1: a diagnostic challenge]. Ned. Tijdschr. Geneeskd. 2023, 167, D7864. [Google Scholar] [PubMed]
- Khatri, N.; Raza, M.L.; Aijaz, A.; Ramesh, R.; Gianchand, N.; Khan, F.A.A. Neurofibroma: Case Series with Clinical Features and Recommendations. Acta Neurol. Taiwan. 2024, 33(3), 112–121. [Google Scholar]
- Magwood, G.S.; Ellis, C.; Buie, J.N.J.; Slan, S.; Bonilha, L.; Adams, R.J. High Tech and High Touch: Recruitment Strategies for Enrolling African American Stroke Survivors in Community Based Intervention under Nurse Guidance after Stroke (CINGS) Trial. Contemp. Clin. Trials Commun. 2021, 24, 100844. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.M.; Wolters, P.L.; Dombi, E.; Baldwin, A.; Whitcomb, P.; Fisher, M.J.; Weiss, B.; Kim, A.; Bornhorst, M.; Shah, A.C.; et al. Selumetinib in Children with Inoperable Plexiform Neurofibromas. N. Engl. J. Med. 2020, 382, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Espírito Santo, V.; Passos, J.; Nzwalo, H.; Carvalho, I.; Santos, F.; Martins, C.; Salgado, L.; Silva, C.E.; Vinhais, S.; Vilares, M.; et al. Selumetinib for Plexiform Neurofibromas in Neurofibromatosis Type 1: A Single-Institution Experience. J. Neurooncol 2020, 147, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Ardern-Holmes, S.; Fisher, G.; North, K. Neurofibromatosis Type 2. J. Child. Neurol. 2017, 32, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, A.M.; Fernández-Valle, C. Role of Merlin/NF2 Inactivation in Tumor Biology. Oncogene 2016, 35, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Freeman, S.; Gokhale, C.; Wallace, A.; Lloyd, S.K.; Axon, P.; Ward, C.L.; Rutherford, S.; King, A.; Huson, S.M.; et al. Bilateral Vestibular Schwannomas in Older Patients: NF2 or Chance? J. Med. Genet. 2015, 52, 422–424. [Google Scholar] [CrossRef]
- Halliday, D.; Emmanouil, B.; Pretorius, P.; MacKeith, S.; Painter, S.; Tomkins, H.; Evans, D.G.; Parry, A. Genetic Severity Score Predicts Clinical Phenotype in NF2. J. Med. Genet. 2017, 54, 657–664. [Google Scholar] [CrossRef]
- Gregory, G.E.; Jones, A.P.; Haley, M.J.; Hoyle, C.; Zeef, L.A.H.; Lin, I.-H.; Coope, D.J.; King, A.T.; Evans, D.G.; Paszek, P.; et al. The Comparable Tumour Microenvironment in Sporadic and NF2-Related Schwannomatosis Vestibular Schwannoma. Brain Commun. 2023, 5, fcad197. [Google Scholar] [CrossRef]
- Lloyd, S.K.W.; Evans, D.G.R. Neurofibromatosis Type 2 (NF2): Diagnosis and Management. Handb. Clin. Neurol. 2013, 115, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.R.; Messiaen, L.; Legius, E.; Pancza, P.; Avery, R.A.; Blakeley, J.O.; Babovic-Vuksanovic, D.; Ferner, R.; Fisher, M.J.; Friedman, J.M.; et al. Updated Diagnostic Criteria and Nomenclature for Neurofibromatosis Type 2 and Schwannomatosis: An International Consensus Recommendation. Genet. Med. 2022, 24, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, E.C.; Rhodes, S.D.; Yates, C.W. Advances in Targeted Therapy for Neurofibromatosis Type 2 (NF2)-Associated Vestibular Schwannomas. Curr. Oncol. Rep. 2023, 25, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, S.; Akagami, R. Prospective Comparison of Quality of Life before and after Observation, Radiation, or Surgery for Vestibular Schwannomas. J. Neurosurg. 2009, 111, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Chari, D.A.; Vasilijic, S.; Welling, D.B.; Stankovic, K.M. New Developments in Neurofibromatosis Type 2 and Vestibular Schwannoma. Neurooncol Adv. 2021, 3, vdaa153. [Google Scholar] [CrossRef] [PubMed]
- Hiruta, R.; Saito, K.; Bakhit, M.; Fujii, M. Current Progress in Genomics and Targeted Therapies for Neurofibromatosis Type 2. Fukushima J. Med. Sci. 2023, 69, 95–103. [Google Scholar] [CrossRef]
- Northrup, H.; Krueger, D.A. International Tuberous Sclerosis Complex Consensus Group Tuberous Sclerosis Complex Diagnostic Criteria Update: Recommendations of the 2012 Iinternational Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 2013, 49, 243–254. [Google Scholar] [CrossRef]
- Roach, E.S. Applying the Lessons of Tuberous Sclerosis: The 2015 Hower Award Lecture. Pediatr Neurol 2016, 63, 6–22. [Google Scholar] [CrossRef]
- Jones, A.C.; Shyamsundar, M.M.; Thomas, M.W.; Maynard, J.; Idziaszczyk, S.; Tomkins, S.; Sampson, J.R.; Cheadle, J.P. Comprehensive Mutation Analysis of TSC1 and TSC2-and Phenotypic Correlations in 150 Families with Tuberous Sclerosis. Am. J. Hum. Genet. 1999, 64, 1305–1315. [Google Scholar] [CrossRef]
- Au, K.S.; Williams, A.T.; Roach, E.S.; Batchelor, L.; Sparagana, S.P.; Delgado, M.R.; Wheless, J.W.; Baumgartner, J.E.; Roa, B.B.; Wilson, C.M.; et al. Genotype/Phenotype Correlation in 325 Individuals Referred for a Diagnosis of Tuberous Sclerosis Complex in the United States. Genet. Med. 2007, 9, 88–100. [Google Scholar] [CrossRef]
- van Slegtenhorst, M.; de Hoogt, R.; Hermans, C.; Nellist, M.; Janssen, B.; Verhoef, S.; Lindhout, D.; van den Ouweland, A.; Halley, D.; Young, J.; et al. Identification of the Tuberous Sclerosis Gene TSC1 on Chromosome 9q34. Science 1997, 277, 805–808. [Google Scholar] [CrossRef] [PubMed]
- European Chromosome 16 Tuberous Sclerosis Consortium Identification and Characterization of the Tuberous Sclerosis Gene on Chromosome 16. Cell 1993, 75, 1305–1315. [CrossRef] [PubMed]
- Caban, C.; Khan, N.; Hasbani, D.M.; Crino, P.B. Genetics of Tuberous Sclerosis Complex: Implications for Clinical Practice. Appl. Clin. Genet. 2017, 10, 1–8. [Google Scholar] [CrossRef]
- Feliciano, D.M. The Neurodevelopmental Pathogenesis of Tuberous Sclerosis Complex (TSC). Front. Neuroanat. 2020, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.; Chakraborty, R.; Mahajan, Z.; Sharma, A.; Sethi, S.K.; Raina, R. Renal Manifestations of Tuberous Sclerosis Complex. J. Kidney Cancer VHL 2020, 7, 5–19. [Google Scholar] [CrossRef]
- Teng, J.M.C.; Cowen, E.W.; Wataya-Kaneda, M.; Gosnell, E.S.; Witman, P.M.; Hebert, A.A.; Mlynarczyk, G.; Soltani, K.; Darling, T.N. Dermatologic and Dental Aspects of the 2012 International Tuberous Sclerosis Complex Consensus Statements. JAMA Dermatol 2014, 150, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Jacks, S.K.; Witman, P.M. Tuberous Sclerosis Complex: An Update for Dermatologists. Pediatr. Dermatol. 2015, 32, 563–570. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Kanish, B.; Bhatia, A. Tuberous Sclerosis. Indian. Dermatol. Online J. 2015, 6, 142–143. [Google Scholar] [CrossRef]
- Cardis, M.A.; DeKlotz, C.M.C. Cutaneous Manifestations of Tuberous Sclerosis Complex and the Paediatrician’s Role. Arch. Dis. Child. 2017, 102, 858–863. [Google Scholar] [CrossRef]
- Dao, D.-P.D.; Pixley, J.N.; Akkurt, Z.M.; Feldman, S.R. A Review of Topical Sirolimus for the Treatment of Facial Angiofibromas in Tuberous Sclerosis Complex. Ann. Pharmacother. 2023, 10600280231182421. [Google Scholar] [CrossRef]
- Borzęcki, A.; Chyl-Surdacka, K.; Turska, M. Spectacular Effect of Massive Facial Angiofibromas Removal With a Carbon Dioxide Laser as a Manifestation of a Tuberous Sclerosis Complex. J. Lasers Med. Sci. 2021, 12, e24. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M.D.; Tang, H.; Gallione, C.J.; Baugher, J.D.; Frelin, L.P.; Cohen, B.; North, P.E.; Marchuk, D.A.; Comi, A.M.; Pevsner, J. Sturge-Weber Syndrome and Port-Wine Stains Caused by Somatic Mutation in GNAQ. N. Engl. J. Med. 2013, 368, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Happle, R. Phacomatosis Pigmentovascularis Revisited and Reclassified. Arch. Dermatol. 2005, 141, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Fois, A.; Calistri, L.; Balestri, P.; Vivarelli, R.; Bartalini, G.; Mancini, L.; Berardi, A.; Vanni, M. Relationship between Café-Au-Lait Spots as the Only Symptom and Peripheral Neurofibromatosis (NF1): A Follow-up Study. Eur. J. Pediatr. 1993, 152, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Espino, L.F.; Ivars, M.; Antoñanzas, J.; Baselga, E. Sturge-Weber Syndrome: A Review of Pathophysiology, Genetics, Clinical Features, and Current Management Approache. Appl. Clin. Genet. 2023, 16, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Poliner, A.; Fernandez Faith, E.; Blieden, L.; Kelly, K.M.; Metry, D. Port-Wine Birthmarks: Update on Diagnosis, Risk Assessment for Sturge-Weber Syndrome, and Management. Pediatr. Rev. 2022, 43, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Wetzel-Strong, S.E.; Galeffi, F.; Benavides, C.; Patrucco, M.; Bullock, J.L.; Gallione, C.J.; Lee, H.K.; Marchuk, D.A. Developmental Expression of the Sturge-Weber Syndrome-Associated Genetic Mutation in Gnaq: A Formal Test of Happle’s Paradominant Inheritance Hypothesis. Genetics 2023, 224, iyad077. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, J.; Frelin, L.P.; McCann, M.; Pardo, C.A.; Cohen, B.A.; Comi, A.M.; Pevsner, J. Identification of a Mosaic Activating Mutation in GNA11 in Atypical Sturge-Weber Syndrome. J. Invest. Dermatol. 2021, 141, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.C.; Zeng, Z.; Rivière, J.-B.; O’Shaughnessy, R.; Al-Olabi, L.; St-Onge, J.; Atherton, D.J.; Aubert, H.; Bagazgoitia, L.; Barbarot, S.; et al. Mosaic Activating Mutations in GNA11 and GNAQ Are Associated with Phakomatosis Pigmentovascularis and Extensive Dermal Melanocytosis. J. Invest. Dermatol. 2016, 136, 770–778. [Google Scholar] [CrossRef]
- Waelchli, R.; Aylett, S.E.; Robinson, K.; Chong, W.K.; Martinez, A.E.; Kinsler, V.A. New Vascular Classification of Port-Wine Stains: Improving Prediction of Sturge-Weber Risk. Br. J. Dermatol. 2014, 171, 861–867. [Google Scholar] [CrossRef]
- Dompmartin, A.; van der Vleuten, C.J.M.; Dekeuleneer, V.; Duprez, T.; Revencu, N.; Désir, J.; Te Loo, D.M.W.M.; Flucke, U.; Eijkelenboom, A.; Schultze Kool, L.; et al. GNA11-Mutated Sturge-Weber Syndrome Has Distinct Neurological and Dermatological Features. Eur. J. Neurol. 2022, 29, 3061–3070. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, J.; Mehta, S.; Mulye, S. Paroxysmal Vascular Events in Sturge-Weber Syndrome: Role of Aspirin. J. Pediatr. Neurosci. 2014, 9, 39–41. [Google Scholar] [CrossRef]
- Yeom, S.; Comi, A.M. Updates on Sturge-Weber Syndrome. Stroke 2022, 53, 3769–3779. [Google Scholar] [CrossRef] [PubMed]
- Comi, A.M. Sturge-Weber Syndrome and Epilepsy: An Argument for Aggressive Seizure Management in These Patients. Expert. Rev. Neurother. 2007, 7, 951–956. [Google Scholar] [CrossRef]
- Day, A.M.; McCulloch, C.E.; Hammill, A.M.; Juhász, C.; Lo, W.D.; Pinto, A.L.; Miles, D.K.; Fisher, B.J.; Ball, K.L.; Wilfong, A.A.; et al. Physical and Family History Variables Associated With Neurological and Cognitive Development in Sturge-Weber Syndrome. Pediatr. Neurol. 2019, 96, 30–36. [Google Scholar] [CrossRef]
- Koenraads, Y.; van Egmond-Ebbeling, M.B.; de Boer, J.H.; Imhof, S.M.; Braun, K.P.J.; Porro, G.L. SWS study group Visual Outcome in Sturge-Weber Syndrome: A Systematic Review and Dutch Multicentre Cohort. Acta Ophthalmol. 2016, 94, 638–645. [Google Scholar] [CrossRef]
- Sujansky, E.; Conradi, S. Sturge-Weber Syndrome: Age of Onset of Seizures and Glaucoma and the Prognosis for Affected Children. J. Child. Neurol. 1995, 10, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Gittins, S.; Steel, D.; Brunklaus, A.; Newsom-Davis, I.; Hawkins, C.; Aylett, S.E. Autism Spectrum Disorder, Social Communication Difficulties, and Developmental Comorbidities in Sturge-Weber Syndrome. Epilepsy Behav. 2018, 88, 1–4. [Google Scholar] [CrossRef]
- Chapieski, L.; Friedman, A.; Lachar, D. Psychological Functioning in Children and Adolescents with Sturge-Weber Syndrome. J. Child. Neurol. 2000, 15, 660–665. [Google Scholar] [CrossRef]
- Léauté-Labréze, C.; Boralevi, F.; Pedespan, J.-M.; Meymat, Y.; Taïeb, A. Pulsed Dye Laser for Sturge-Weber Syndrome. Arch. Dis. Child. 2002, 87, 434–435. [Google Scholar] [CrossRef]
- Oakes, W.J. The Natural History of Patients with the Sturge-Weber Syndrome. Pediatr. Neurosurg. 1992, 18, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Parsa, C.F. Sturge-Weber Syndrome: A Unified Pathophysiologic Mechanism. Curr. Treat. Options Neurol. 2008, 10, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Miyajima, M.; Sugano, H.; Iimura, Y.; Kato, M.; Tsurusaki, Y.; Miyake, N.; Saitsu, H.; Arai, H.; Matsumoto, N. The Somatic GNAQ Mutation c.548G>A (p.R183Q) Is Consistently Found in Sturge-Weber Syndrome. J. Hum. Genet. 2014, 59, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Sabeti, S.; Ball, K.L.; Bhattacharya, S.K.; Bitrian, E.; Blieden, L.S.; Brandt, J.D.; Burkhart, C.; Chugani, H.T.; Falchek, S.J.; Jain, B.G.; et al. Consensus Statement for the Management and Treatment of Sturge-Weber Syndrome: Neurology, Neuroimaging, and Ophthalmology Recommendations. Pediatr. Neurol. 2021, 121, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Smegal, L.F.; Sebold, A.J.; Hammill, A.M.; Juhász, C.; Lo, W.D.; Miles, D.K.; Wilfong, A.A.; Levin, A.V.; Fisher, B.; Ball, K.L.; et al. Multicenter Research Data of Epilepsy Management in Patients With Sturge-Weber Syndrome. Pediatr. Neurol. 2021, 119, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, B.; Yang, J.; Bi, M.; Bi, L.; Fan, W. Efficacy of Hemoporfin-Mediated Photodynamic Therapy in Treating Sturge-Weber Syndrome Associated Port-Wine Stains: A Retrospective Study. Indian. J. Dermatol. Venereol. Leprol. 2023, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, A.J.; Luat, A.F.; Juhász, C.; Ho, M.L.; Argersinger, D.P.; Cavuoto, K.M.; Enriquez-Algeciras, M.; Tikkanen, S.; North, P.; Burkhart, C.N.; et al. A Multidisciplinary Consensus for Clinical Care and Research Needs for Sturge-Weber Syndrome. Pediatr. Neurol. 2018, 84, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, M.I.; Singh, A.K. Von Hippel-Lindau Syndrome. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- Schmidt, L.S.; Linehan, W.M. Genetic Predisposition to Kidney Cancer. Semin. Oncol. 2016, 43, 566–574. [Google Scholar] [CrossRef]
- Klingler, J.-H.; Gläsker, S.; Bausch, B.; Urbach, H.; Krauss, T.; Jilg, C.A.; Steiert, C.; Puzik, A.; Neumann-Haefelin, E.; Kotsis, F.; et al. Hemangioblastoma and von Hippel-Lindau Disease: Genetic Background, Spectrum of Disease, and Neurosurgical Treatment. Childs Nerv. Syst. 2020, 36, 2537–2552. [Google Scholar] [CrossRef]
- Reisch, N.; Peczkowska, M.; Januszewicz, A.; Neumann, H.P.H. Pheochromocytoma: Presentation, Diagnosis and Treatment. J. Hypertens. 2006, 24, 2331–2339. [Google Scholar] [CrossRef]
- Gläsker, S.; Neumann, H.P.H.; Koch, C.A.; Vortmeyer, A.; Von Hippel-Lindau, Disease. Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth (MA), 2000. [Google Scholar]
- Chittiboina, P.; Lonser, R.R. Von Hippel-Lindau Disease. Handb. Clin. Neurol. 2015, 132, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Sgambati, M.T.; Stolle, C.; Choyke, P.L.; Walther, M.M.; Zbar, B.; Linehan, W.M.; Glenn, G.M. Mosaicism in von Hippel-Lindau Disease: Lessons from Kindreds with Germline Mutations Identified in Offspring with Mosaic Parents. Am. J. Hum. Genet. 2000, 66, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G. Molecular Basis of the VHL Hereditary Cancer Syndrome. Nat. Rev. Cancer 2002, 2, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Chappell, J.C.; Payne, L.B.; Rathmell, W.K. Hypoxia, Angiogenesis, and Metabolism in the Hereditary Kidney Cancers. J. Clin. Invest. 2019, 129, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Ferzli, P.G.; Millett, C.R.; Newman, M.D.; Heymann, W.R. The Dermatologist’s Guide to Hereditary Syndromes with Renal Tumors. Cutis 2008, 81, 41–48. [Google Scholar] [PubMed]
- Singh, A.D.; Shields, C.L.; Shields, J.A. Von Hippel-Lindau Disease. Surv. Ophthalmol. 2001, 46, 117–142. [Google Scholar] [CrossRef]
- Ahmad, W.A.W.; Khanom, M.; Yaakob, Z.H. Heart Failure in Pregnancy: An Overview. Int. J. Clin. Pract. 2011, 65, 848–851. [Google Scholar] [CrossRef]
- Damsky, W.E.; Bosenberg, M. Melanocytic Nevi and Melanoma: Unraveling a Complex Relationship. Oncogene 2017, 36, 5771–5792. [Google Scholar] [CrossRef]
- Jha, S.K.; Mendez, M.D. Cafe Au Lait Macules. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- van Leeuwaarde, R.S.; Ahmad, S.; van Nesselrooij, B.; Zandee, W.; Giles, R.H. Von Hippel-Lindau Syndrome. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington, Seattle: Seattle (WA), 1993. [Google Scholar]
- Wolters, W.P.G.; Dreijerink, K.M.A.; Giles, R.H.; van der Horst-Schrivers, A.N.A.; van Nesselrooij, B.; Zandee, W.T.; Timmers, H.J.L.M.; Seute, T.; de Herder, W.W.; Verrijn Stuart, A.A.; et al. Multidisciplinary Integrated Care Pathway for von Hippel-Lindau Disease. Cancer 2022, 128, 2871–2879. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kakutani, S.; Sato, Y.; Hanashi, A.; Kinoshita, Y.; Ishikawa, A. Drug Review: Pazopanib. Jpn. J. Clin. Oncol. 2018, 48, 503–513. [Google Scholar] [CrossRef]
- Jonasch, E.; Donskov, F.; Iliopoulos, O.; Rathmell, W.K.; Narayan, V.K.; Maughan, B.L.; Oudard, S.; Else, T.; Maranchie, J.K.; Welsh, S.J.; et al. Belzutifan for Renal Cell Carcinoma in von Hippel-Lindau Disease. N. Engl. J. Med. 2021, 385, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.W.-S.; Lenton, J.; Smith, J.; Jagdev, S.; Ralph, C.; Vasudev, N.; Bhattarai, S.; Lewington, A.; Kimuli, M.; Cartledge, J.; et al. Multimodal Image-Guided Ablation on Management of Renal Cancer in Von-Hippel-Lindau Syndrome Patients from 2004 to 2021 at a Specialist Centre: A Longitudinal Observational Study. Eur. J. Surg. Oncol. 2022, 48, 672–679. [Google Scholar] [CrossRef]
- Gläsker, S.; Krüger, M.T.; Klingler, J.-H.; Wlodarski, M.; Klompen, J.; Schatlo, B.; Hippchen, B.; Neumann, H.P.H.; Van Velthoven, V. Hemangioblastomas and Neurogenic Polyglobulia. Neurosurgery 2013, 72, 930–935; discussion 935. [Google Scholar] [CrossRef]
- Krüger, M.T.; Steiert, C.; Gläsker, S.; Klingler, J.-H. Minimally Invasive Resection of Spinal Hemangioblastoma: Feasibility and Clinical Results in a Series of 18 Patients. J. Neurosurg. Spine 2019, 1–10. [Google Scholar] [CrossRef]
- Greenberger, S.; Berkun, Y.; Ben-Zeev, B.; Levi, Y.B.; Barziliai, A.; Nissenkorn, A. Dermatologic Manifestations of Ataxia-Telangiectasia Syndrome. J. Am. Acad. Dermatol. 2013, 68, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Shaikh, A.G. Past and Present of Eye Movement Abnormalities in Ataxia-Telangiectasia. Cerebellum 2019, 18, 556–564. [Google Scholar] [CrossRef]
- van Os, N.J.H.; van Deuren, M.; Weemaes, C.M.R.; van Gaalen, J.; Hijdra, H.; Taylor, A.M.R.; van de Warrenburg, B.P.C.; Willemsen, M.A.A.P. Classic Ataxia-Telangiectasia: The Phenotype of Long-Term Survivors. J. Neurol. 2020, 267, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Sirajwala, A.A.; Khan, S.; Rathod, V.M.; Gevariya, V.C.; Jansari, J.R.; Patel, Y.M. Ataxia-Telangiectasia: A Case Report and a Brief Review. Cureus 2023, 15, e39346. [Google Scholar] [CrossRef]
- Riboldi, G.M.; Samanta, D.; Frucht, S. Ataxia Telangiectasia. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- Jiang, Y.; Chen, H.-C.; Su, X.; Thompson, P.A.; Liu, X.; Do, K.-A.; Wierda, W.; Keating, M.J.; Plunkett, W. ATM Function and Its Relationship with ATM Gene Mutations in Chronic Lymphocytic Leukemia with the Recurrent Deletion (11q22.3-23.2). Blood Cancer J. 2016, 6, e465. [Google Scholar] [CrossRef]
- Amirifar, P.; Ranjouri, M.R.; Yazdani, R.; Abolhassani, H.; Aghamohammadi, A. Ataxia-Telangiectasia: A Review of Clinical Features and Molecular Pathology. Pediatr. Allergy Immunol. 2019, 30, 277–288. [Google Scholar] [CrossRef]
- Yildiz, A.; Kaya, Y.; Tanriverdi, O. Effect of the Interaction Between Selenium and Zinc on DNA Repair in Association With Cancer Prevention. J. Cancer Prev. 2019, 24, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Zhang, J.; He, K.; Zhang, J. Ataxia Telangiectasia and Rad3-Related Inhibitors and Cancer Therapy: Where We Stand. J. Hematol. Oncol. 2019, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Rothblum-Oviatt, C.; Wright, J.; Lefton-Greif, M.A.; McGrath-Morrow, S.A.; Crawford, T.O.; Lederman, H.M. Ataxia Telangiectasia: A Review. Orphanet J. Rare Dis. 2016, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Cavone, F.; Cappelli, S.; Bonuccelli, A.; D’Elios, S.; Costagliola, G.; Peroni, D.; Orsini, A.; Consolini, R. Ataxia Telangiectasia Arising as Immunodeficiency: The Intriguing Differential Diagnosis. J. Clin. Med. 2023, 12, 6041. [Google Scholar] [CrossRef]
- Kumar, N.; Aggarwal, P.; Dev, N.; Kumar, G. Ataxia Telangiectasia: Learning from Previous Mistakes. BMJ Case Rep. 2012, 2012, bcr2012007246. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi, R.; Britschgi, C.; Joset, P.; Oneda, B.; Schindler, D.; Meier, U.R.; Rauch, A. Severe Reaction to Radiotherapy Provoked by Hypomorphic Germline Mutations in ATM (Ataxia-Telangiectasia Mutated Gene). Mol. Genet. Genomic Med. 2020, 8, e1409. [Google Scholar] [CrossRef]
- Moeini Shad, T.; Yazdani, R.; Amirifar, P.; Delavari, S.; Heidarzadeh Arani, M.; Mahdaviani, S.A.; Sadeghi-Shabestari, M.; Aghamohammadi, A.; Rezaei, N.; Abolhassani, H. Atypical Ataxia Presentation in Variant Ataxia Telangiectasia: Iranian Case-Series and Review of the Literature. Front. Immunol. 2021, 12, 779502. [Google Scholar] [CrossRef] [PubMed]
- Veenhuis, S.; van Os, N.; Weemaes, C.; Kamsteeg, E.-J.; Willemsen, M. Ataxia-Telangiectasia. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington, Seattle: Seattle (WA), 1993. [Google Scholar]
- Perlman, S.; Becker-Catania, S.; Gatti, R.A. Ataxia-Telangiectasia: Diagnosis and Treatment. Semin. Pediatr. Neurol. 2003, 10, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Gatti, R.A.; Berkel, I.; Boder, E.; Braedt, G.; Charmley, P.; Concannon, P.; Ersoy, F.; Foroud, T.; Jaspers, N.G.; Lange, K. Localization of an Ataxia-Telangiectasia Gene to Chromosome 11q22-23. Nature 1988, 336, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Paucar, M.; Taylor, A.M.R.; Hadjivassiliou, M.; Fogel, B.L.; Svenningsson, P. Progressive Ataxia with Elevated Alpha-Fetoprotein: Diagnostic Issues and Review of the Literature. Tremor Other Hyperkinet Mov. (N. Y) 2019, 9. [Google Scholar] [CrossRef]
- Chopra, C.; Davies, G.; Taylor, M.; Anderson, M.; Bainbridge, S.; Tighe, P.; McDermott, E.M. Immune Deficiency in Ataxia-Telangiectasia: A Longitudinal Study of 44 Patients. Clin. Exp. Immunol. 2014, 176, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Long, S.S. Parents Can Accurately Observe the Severity of Respiratory Illness in Their Infants Born Term and Preterm. J. Pediatr. 2019, 214, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Giardino, G.; Fusco, A.; Romano, R.; Gallo, V.; Maio, F.; Esposito, T.; Palamaro, L.; Parenti, G.; Salerno, M.C.; Vajro, P.; et al. Betamethasone Therapy in Ataxia Telangiectasia: Unraveling the Rationale of This Serendipitous Observation on the Basis of the Pathogenesis. Eur. J. Neurol. 2013, 20, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Hirch, T.; Brander, N.; Schenk, F.; Pöllmann, S.J.; Reichenbach, J.; Schubert, R.; Modlich, U. Expression of a Large Coding Sequence: Gene Therapy Vectors for Ataxia Telangiectasia. Sci. Rep. 2023, 13, 19386. [Google Scholar] [CrossRef] [PubMed]
- McGrath-Morrow, S.A.; Rothblum-Oviatt, C.C.; Wright, J.; Schlechter, H.; Lefton-Greif, M.A.; Natale, V.A.; Crawford, T.O.; Lederman, H.M. Multidisciplinary Management of Ataxia Telangiectasia: Current Perspectives. J. Multidiscip. Healthc. 2021, 14, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Unes, S.; Tuncdemir, M.; Eroglu-Ertugrul, N.G.; Kerem Gunel, M. Effectiveness of Physical Therapy on Ataxia-Telangiectasia: A Case Report. Pediatr. Phys. Ther. 2021, 33, E103–E107. [Google Scholar] [CrossRef] [PubMed]
- Zannolli, R.; Buoni, S.; Betti, G.; Salvucci, S.; Plebani, A.; Soresina, A.; Pietrogrande, M.C.; Martino, S.; Leuzzi, V.; Finocchi, A.; et al. A Randomized Trial of Oral Betamethasone to Reduce Ataxia Symptoms in Ataxia Telangiectasia. Mov. Disord. 2012, 27, 1312–1316. [Google Scholar] [CrossRef]
- Kwei, K.T.; Kuo, S.-H. An Overview of the Current State and the Future of Ataxia Treatments. Neurol. Clin. 2020, 38, 449–467. [Google Scholar] [CrossRef]
- Sabino Pinho de Oliveira, B.; Putti, S.; Naro, F.; Pellegrini, M. Bone Marrow Transplantation as Therapy for Ataxia-Telangiectasia: A Systematic Review. Cancers (Basel) 2020, 12, 3207. [Google Scholar] [CrossRef]
- Kim, J.; Woo, S.; de Gusmao, C.M.; Zhao, B.; Chin, D.H.; DiDonato, R.L.; Nguyen, M.A.; Nakayama, T.; Hu, C.A.; Soucy, A.; et al. A Framework for Individualized Splice-Switching Oligonucleotide Therapy. Nature 2023, 619, 828–836. [Google Scholar] [CrossRef]
- Macri, A.; Wilson, A.M.; Shafaat, O.; Sharma, S. Osler-Weber-Rendu Disease. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- Hammill, A.M.; Wusik, K.; Kasthuri, R.S. Hereditary Hemorrhagic Telangiectasia (HHT): A Practical Guide to Management. Hematology Am. Soc. Hematol. Educ. Program. 2021, 2021, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.A.; Turcios, N.L. Pulmonary Manifestations of Skin Disorders in Children. Pediatr. Clin. North. Am. 2021, 68, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Hyldahl, S.J.; El-Jaji, M.Q.; Schuster, A.; Kjeldsen, A.D. Skin and Mucosal Telangiectatic Lesions in Hereditary Hemorrhagic Telangiectasia Patients. Int. J. Dermatol. 2022, 61, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Mylavarapu, C.; Lu, A.J.; Burns, E.A.; Samorajski, J.; Gotur, D.; Baker, K. Diffuse Cerebral Edema and Impending Herniation Complicating Hepatic Encephalopathy in Hereditary Hemorrhagic Telangiectasia. Case Rep. Med. 2022, 2022, 2612544. [Google Scholar] [CrossRef] [PubMed]
- Alkhalid, Y.; Darji, Z.; Shenkar, R.; Clancy, M.; Dyamenahalli, U.; Awad, I.A. the multidisciplinary faculty of the HHT Center of Excellence at University of Chicago Medicine Multidisciplinary Coordinated Care of Hereditary Hemorrhagic Telangiectasia (Osler-Weber-Rendu Disease). Vasc. Med. 2023, 28, 153–165. [Google Scholar] [CrossRef]
- Al-Samkari, H. Hereditary Hemorrhagic Telangiectasia: Systemic Therapies, Guidelines, and an Evolving Standard of Care. Blood 2021, 137, 888–895. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




