Submitted:
15 January 2024
Posted:
16 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Study design and population
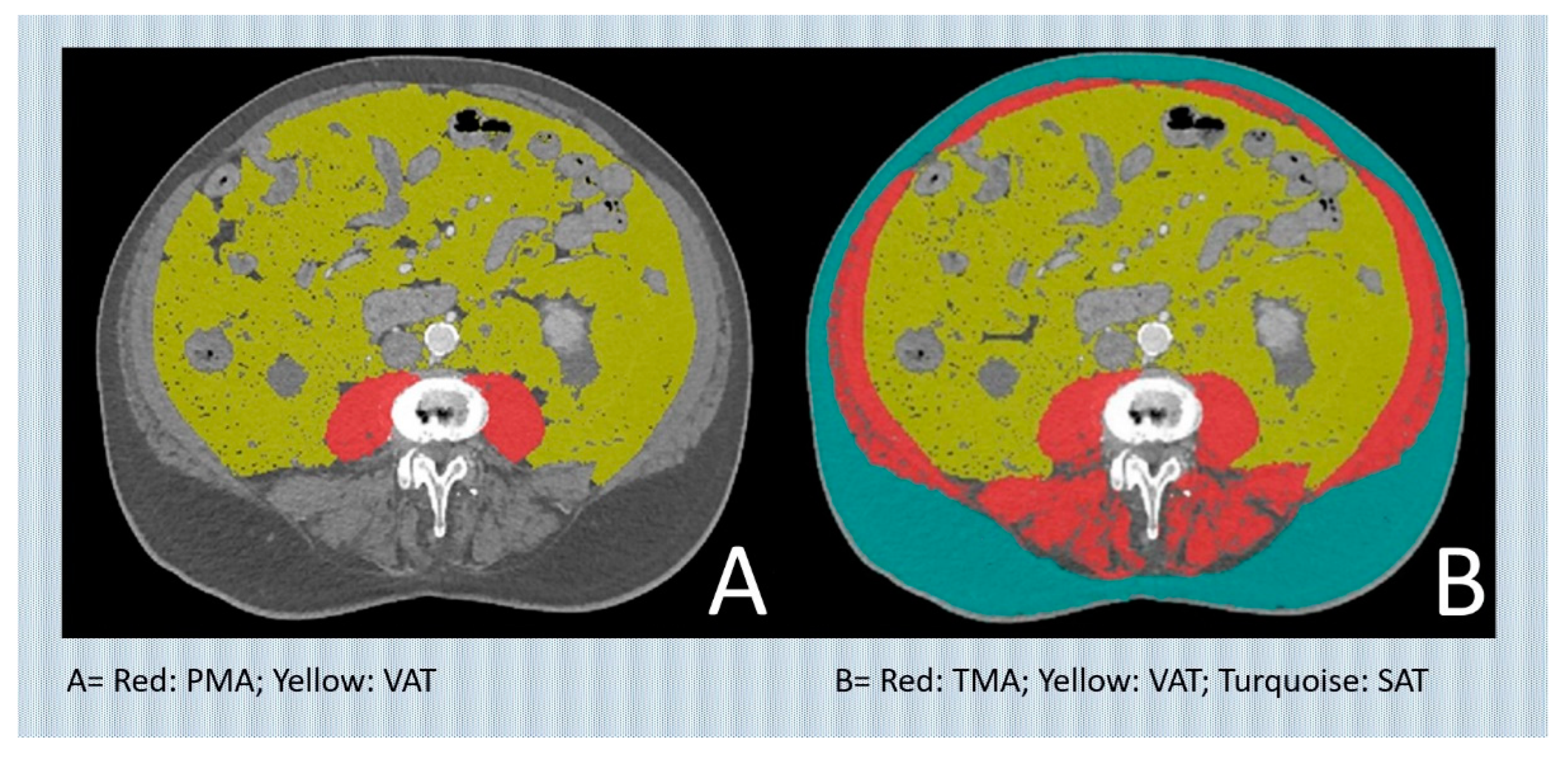
CT assessment
Data collection
Endpoints
Statistical analysis
Results
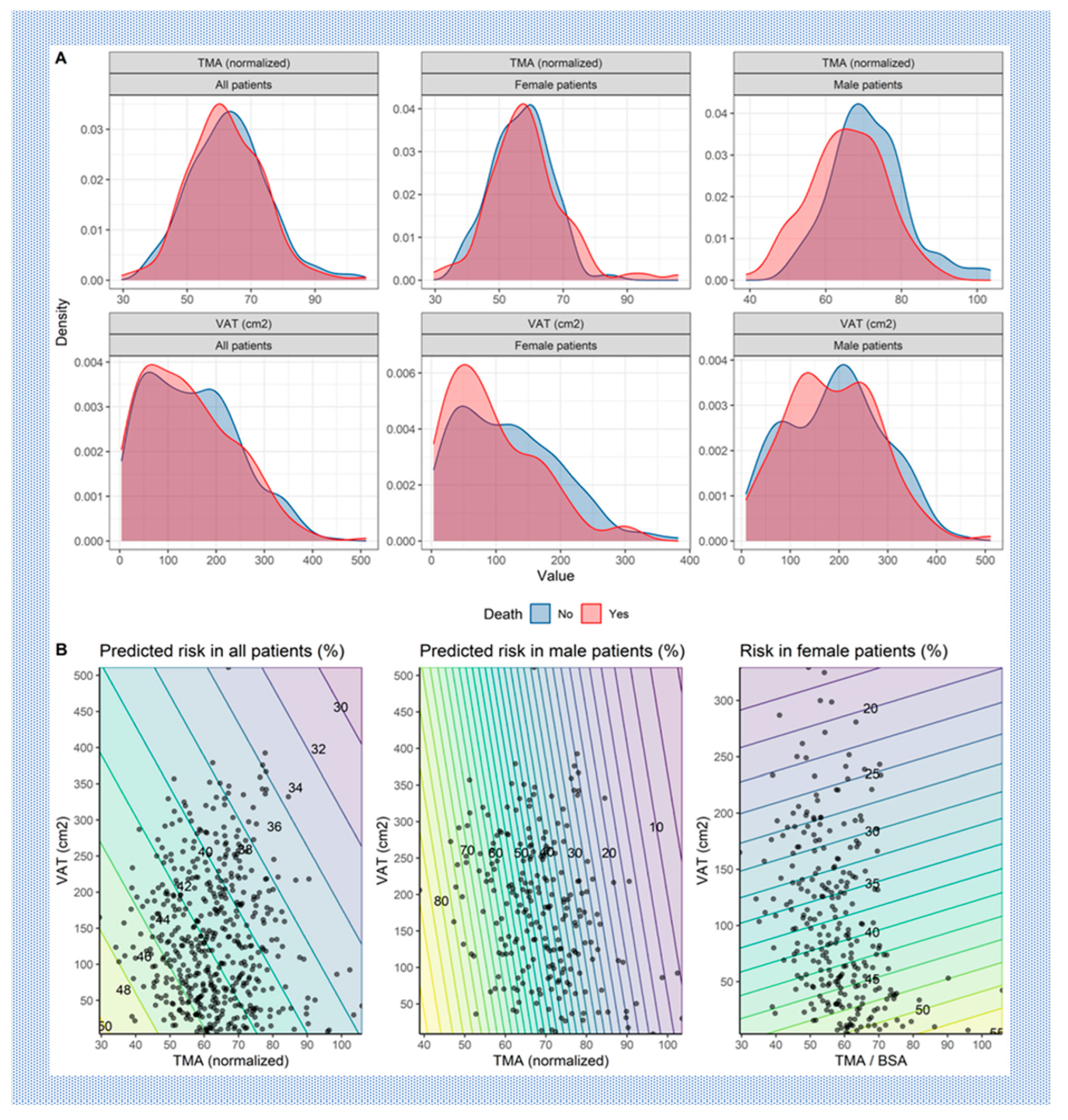
- I) Muscle mass:
- II) Fat mass:
- III) Interaction of Muscle and Fat mass:
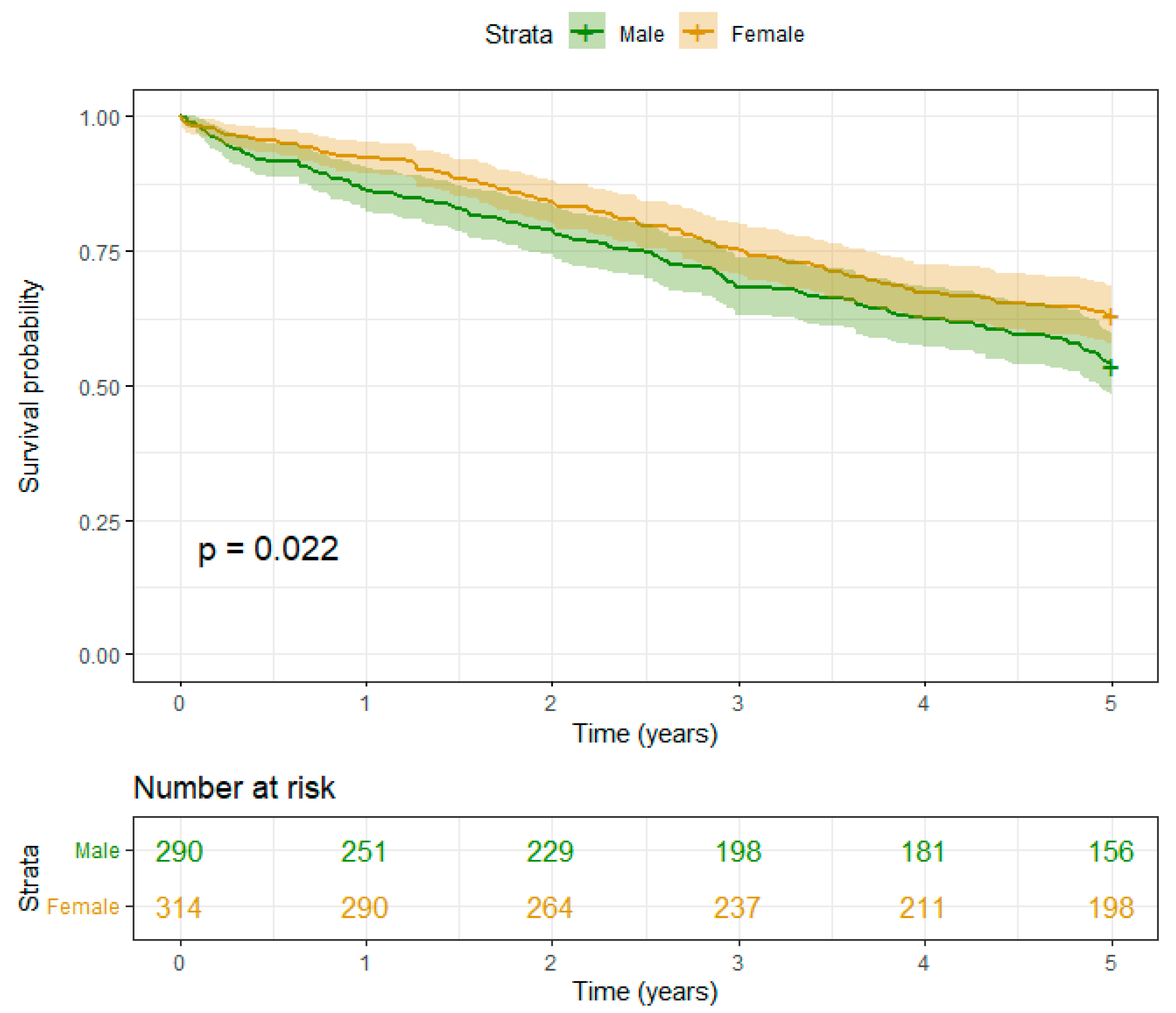
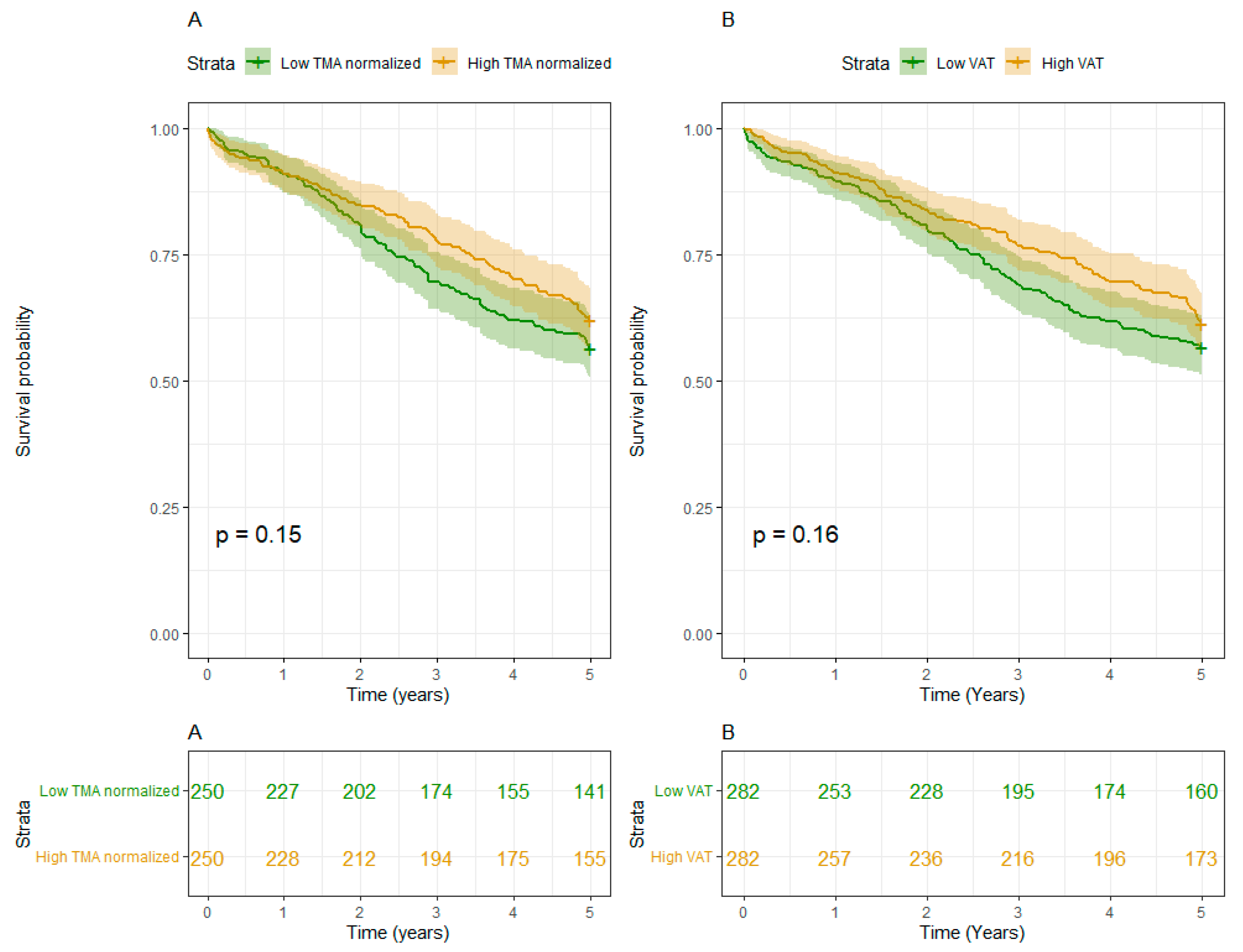
Discussion
Study Limitations
Data Availability Statements
Acknowledgments
Disclosures
Abbreviations
| TAVR | Transcatheter aortic valve replacement |
| AS | Aortic valve stenosis |
| TMA | Total muscle area |
| nTMA | normalized total muscle area |
| PMA | Psoas muscle area |
| VAT | Visceral adipose tissue |
References
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Dowling, C.; Kondapally Seshasai, S.R.; Firoozi, S.; Brecker, S.J. Transcatheter aortic valve replacement versus surgery for symptomatic severe aortic stenosis: A reconstructed individual patient data meta-analysis. Catheter. Cardiovasc. Interv. 2020, 96, 158–166. [Google Scholar] [CrossRef]
- Makkar, R.R.; Thourani, V.H.; Mack, M.J.; Kodali, S.K.; Kapadia, S.; Webb, J.G.; Yoon, S.H.; Trento, A.; Svensson, L.G.; Herrmann, H.C.; et al. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 799–809. [Google Scholar] [CrossRef]
- Grossman, Y.; Barbash, I.M.; Fefer, P.; Goldenberg, I.; Berkovitch, A.; Regev, E.; Fink, N.; Ben-Zekry, S.; Brodov, Y.; Kogan, A.; et al. Addition of albumin to Traditional Risk Score Improved Prediction of Mortality in Individuals Undergoing Transcatheter Aortic Valve Replacement. J. Am. Geriatr. Soc. 2017, 65, 2413–2417. [Google Scholar] [CrossRef]
- Afilalo, J.; Lauck, S.; Kim, D.H.; Lefèvre, T.; Piazza, N.; Lachapelle, K.; Martucci, G.; Lamy, A.; Labinaz, M.; Peterson, M.D.; et al. Frailty in Older Adults Undergoing Aortic Valve Replacement: The FRAILTY-AVR Study. J. Am. Coll. Cardiol. 2017, 70, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, L.; Kirk, B.H.; Jørgensen, T.H. Frailty: An Important Measure in Patients Considered for Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2018, 11, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Ilic, I.; Faron, A.; Heimann, M.; Potthoff, A.L.; Schäfer, N.; Bode, C.; Borger, V.; Eichhorn, L.; Giordano, F.A.; Güresir, E.; et al. Combined Assessment of Preoperative Frailty and Sarcopenia Allows the Prediction of Overall Survival in Patients with Lung Cancer (NSCLC) and Surgically Treated Brain Metastasis. Cancers 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Bentov, I.; Kaplan, S.J.; Pham, T.N.; Reed, M.J. Frailty assessment: from clinical to radiological tools. Br. J. Anaesth. 2019, 123, 37–50. [Google Scholar] [CrossRef]
- Flexman, A.M.; Street, J.; Charest-Morin, R. The impact of frailty and sarcopenia on patient outcomes after complex spine surgery. Curr. Opin. Anaesthesiol. 2019, 32, 609–615. [Google Scholar] [CrossRef]
- Kołodziejska, K.; Witowski, J.; Tylec, P.; Grochowska, A.; Przytuła, N.; Lis, M.; Pędziwiatr, M.; Rubinkiewicz, M. Radiological Features for Frailty Assessment in Patients Requiring Emergency Laparotomy. J. Clin. Med. 2022, 11. [Google Scholar] [CrossRef]
- de Bree, R.; Meerkerk, C.D.A.; Halmos, G.B.; Mäkitie, A.A.; Homma, A.; Rodrigo, J.P.; López, F.; Takes, R.P.; Vermorken, J.B.; Ferlito, A. Measurement of Sarcopenia in Head and Neck Cancer Patients and Its Association With Frailty. Front. Oncol. 2022, 12, 884988. [Google Scholar] [CrossRef]
- Okamura, H.; Kimura, N.; Mieno, M.; Yuri, K.; Yamaguchi, A. Preoperative sarcopenia is associated with late mortality after off-pump coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 2020, 58, 121–129. [Google Scholar] [CrossRef]
- Canales, C.; Mazor, E.; Coy, H.; Grogan, T.R.; Duval, V.; Raman, S.; Cannesson, M.; Singh, S.P. Preoperative Point-of-Care Ultrasound to Identify Frailty and Predict Postoperative Outcomes: A Diagnostic Accuracy Study. Anesthesiology 2022, 136, 268–278. [Google Scholar] [CrossRef]
- Meng, N.H.; Li, C.I.; Liu, C.S.; Lin, W.Y.; Lin, C.H.; Chang, C.K.; Li, T.C.; Lin, C.C. Sarcopenia Defined by Combining Height- and Weight-Adjusted Skeletal Muscle Indices is Closely Associated With Poor Physical Performance. J. Aging Phys. Act. 2015, 23, 597–606. [Google Scholar] [CrossRef]
- McIsaac, D.I. Preoperative Frailty Assessment: An Opportunity to Add Value to Perioperative Care. Anesthesiology 2022, 136, 255–257. [Google Scholar] [CrossRef]
- Mamane, S.; Mullie, L.; Piazza, N.; Martucci, G.; Morais, J.; Vigano, A.; Levental, M.; Nelson, K.; Lange, R.; Afilalo, J. Psoas Muscle Area and All-Cause Mortality After Transcatheter Aortic Valve Replacement: The Montreal-Munich Study. Can. J. Cardiol. 2016, 32, 177–182. [Google Scholar] [CrossRef]
- Saji, M.; Lim, D.S.; Ragosta, M.; LaPar, D.J.; Downs, E.; Ghanta, R.K.; Kern, J.A.; Dent, J.M.; Ailawadi, G. Usefulness of Psoas Muscle Area to Predict Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. Am. J. Cardiol. 2016, 118, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Mok, M.; Allende, R.; Leipsic, J.; Altisent, O.A.; Del Trigo, M.; Campelo-Parada, F.; DeLarochellière, R.; Dumont, E.; Doyle, D.; Côté, M.; et al. Prognostic Value of Fat Mass and Skeletal Muscle Mass Determined by Computed Tomography in Patients Who Underwent Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2016, 117, 828–833. [Google Scholar] [CrossRef]
- Okuno, T.; Koseki, K.; Nakanishi, T.; Ninomiya, K.; Tomii, D.; Tanaka, T.; Sato, Y.; Osanai, A.; Sato, K.; Koike, H.; et al. Prognostic Impact of Computed Tomography-Derived Abdominal Fat Area on Transcatheter Aortic Valve Implantation. Circ. J. 2018, 82, 3082–3089. [Google Scholar] [CrossRef] [PubMed]
- Stortecky, S.; Franzone, A.; Heg, D.; Tueller, D.; Noble, S.; Pilgrim, T.; Jeger, R.; Toggweiler, S.; Ferrari, E.; Nietlispach, F.; et al. Temporal trends in adoption and outcomes of transcatheter aortic valve implantation: a SwissTAVI Registry analysis. European heart journal. Qual. Care Clin. Outcomes 2019, 5, 242–251. [Google Scholar] [CrossRef]
- Tomii, D.; Okuno, T.; Heg, D.; Lanz, J.; Praz, F.; Stortecky, S.; Windecker, S.; Pilgrim, T. Validation of the VARC-3 Technical Success Definition in Patients Undergoing TAVR. JACC Cardiovasc. Interv. 2022, 15, 353–364. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R. Foundation for Statistical; Computing: Vienna, Austria,, 2020; https://www.R-project.org/.
- Rodríguez, A.J.; Scott, D.; Hodge, A.; English, D.R.; Giles, G.G.; Ebeling, P.R. Associations between hip bone mineral density, aortic calcification and cardiac workload in community-dwelling older Australians. Osteoporos. Int. 2017, 28, 2239–2245. [Google Scholar] [CrossRef]
- Shi, L.; Yu, X.; Pang, Q.; Chen, X.; Wang, C. The associations between bone mineral density and long-term risks of cardiovascular disease, cancer, and all-cause mortality. Front. Endocrinol. 2022, 13, 938399. [Google Scholar] [CrossRef]
- Shibata, K.; Yamamoto, M.; Yamada, S.; Kobayashi, T.; Morita, S.; Kagase, A.; Tokuda, T.; Shimura, T.; Tsunaki, T.; Tada, N.; et al. Clinical Outcomes of Subcutaneous and Visceral Adipose Tissue Characteristics Assessed in Patients Underwent Transcatheter Aortic Valve Replacement. CJC Open 2021, 3, 142–151. [Google Scholar] [CrossRef]
- Bocca, G.; Mastoridis, S.; Yeung, T.; James, D.R.C.; Cunningham, C. Visceral-to-subcutaneous fat ratio exhibits strongest association with early post-operative outcomes in patients undergoing surgery for advanced rectal cancer. Int. J. Colorectal Dis. 2022, 37, 1893–1900. [Google Scholar] [CrossRef]
- He, A.Q.; Li, C.Q.; Zhang, Q.; Liu, T.; Liu, J.; Liu, G. Visceral-to-Subcutaneous Fat Ratio Is a Potential Predictor of Postoperative Complications in Colorectal Cancer. Med. Sci. Monit. 2021, 27, e930329. [Google Scholar] [CrossRef]
- Pernik, M.N.; Hicks, W.H.; Akbik, O.S.; Nguyen, M.L.; Luu, I.; Traylor, J.I.; Deme, P.R.; Dosselman, L.J.; Hall, K.; Wingfield, S.A.; et al. Psoas Muscle Index as a Predictor of Perioperative Outcomes in Geriatric Patients Undergoing Spine Surgery. Global Spine J. 2023, 13, 2016–2024. [Google Scholar] [CrossRef]
- Miao, S.L.; Ye, X.N.; Lin, T.T.; Qiu, Y.H.; Huang, J.Y.; Zheng, X.W.; Chen, F.F. The psoas muscle density as a predictor of postoperative complications and 30-day mortality for acute mesenteric ischemia patients. Abdom. Radiol. 2022, 47, 1644–1653. [Google Scholar] [CrossRef]
- Batista, A.F.R.; Petty, D.; Fairhurst, C.; Davies, S. Psoas muscle mass index as a predictor of long-term mortality and severity of complications after major intra-abdominal colorectal surgery - A retrospective analysis. J. Clin. Anesth. 2023, 84, 110995. [Google Scholar] [CrossRef]
- Balsam, L.B. Psoas muscle area: a new standard for frailty assessment in cardiac surgery? J. Thorac. Dis. 2018, 10 (Suppl. 33), S3846–S3849. [Google Scholar] [CrossRef]
- Paknikar, R.; Friedman, J.; Cron, D.; Deeb, G.M.; Chetcuti, S.; Grossman, P.M.; Wang, S.; Englesbe, M.; Patel, H.J. Psoas muscle size as a frailty measure for open and transcatheter aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2016, 151, 745–751. [Google Scholar] [CrossRef]
- Zuckerman, J.; Ades, M.; Mullie, L.; Trnkus, A.; Morin, J.F.; Langlois, Y.; Ma, F.; Levental, M.; Morais, J.A.; Afilalo, J. Psoas Muscle Area and Length of Stay in Older Adults Undergoing Cardiac Operations. Ann. Thorac. Surg. 2017, 103, 1498–1504. [Google Scholar] [CrossRef]
- Hawkins, R.B.; Mehaffey, J.H.; Charles, E.J.; Kern, J.A.; Lim, D.S.; Teman, N.R.; Ailawadi, G. Psoas Muscle Size Predicts Risk-Adjusted Outcomes After Surgical Aortic Valve Replacement. Ann. Thorac. Surg. 2018, 106, 39–45. [Google Scholar] [CrossRef]
| All patients N=584 | |
|---|---|
| Gender: | |
| Male | 280 (47.9%) |
| Female | 304 (52.1%) |
| Age (years) | 82.8 [79.0;86.5] |
| Height (cm) | 165 [158;172] |
| Weight (kg) | 71.0 [62.0;83.0] |
| Body mass index (kg/m2) | 25.6 [22.7;29.7] |
| Diabetes mellitus [Yes] | 156 (26.7%) |
| Arterial hypertension [Yes] | 506 (86.6%) |
| Dyslipidemia [Yes] | 391 (67.0%) |
| Chronic obstructive pulmonary disease [Yes] | 61 (10.4%) |
| History of cerebrovascular accident [Yes] | 71 (12.2%) |
| Transient ischemic attack [Yes] | 31 (5.31%) |
| Coronary artery disease [Yes] | 346 (59.2%) |
| History of myocardial infarction [Yes] | 87 (14.9%) |
| Atrial fibrillation [Yes] | 203 (34.8%) |
| Peripheral artery disease [Yes] | 69 (11.8%) |
| History of cardiac surgery [Yes] | 59 (10.1%) |
| Dyspnea [Yes] | 578 (99.1%) |
| Body surface area (m2, Haycock) | 1.81 [1.67;1.99] |
| Visceral adipose tissue (cm2) | 134 [68.5;216] |
| Total muscle area (cm2) | 110 [93.4;131] |
| Total muscle Area normalized by body surface area (-) | 62.3 [54.5;70.1] |
| Subcutaneous adipose tissue (cm2) | 148 [106;207] |
| Creatinine (µmol/l) | 95.5 [77.0;120] |
| Brain natriuretic peptide (pg/ml) | 257 [108;625] |
| Albumin (g/L) | 34.0 [32.0;36.0] |
| Mean Gradient (mmHg) | 39.0 [28.0;47.0] |
| Peak Gradient (mmHg) | 63.0 [45.0;78.0] |
| Aortic Valve Area (cm2) | 0.70 [0.60;0.90] |
| Indexed Aortic Valve Area (cm2) | 0.27 [0.21;0.32] |
| Left ventricular ejection fractionLVEF (%) | 60.0 [50.0;65.0] |
| Logistic Euro Score | 9.18 [5.83;17.7] |
| Linear Euro Score | 8.00 [6.00;10.0] |
| Euro Score II | 3.67 [2.24;6.66] |
| STS predicted risk of mortality | 4.12 [2.90;6.31] |
| Cox regression | |||
|---|---|---|---|
| Characteristic | HR | 95% CI | p |
| Age (years) | 1.023 | 0.998, 1.049 | 0.069 |
| BMI (kg/m2) | 1.005 | 0.97, 1.046 | 0.8 |
| Sex: | |||
| Female | 0.036 | 0.003, 0.396 | 0.007 |
| TMA (normalized) | 0.96 | 0.927, 0.997 | 0.033 |
| VAT (cm2) | 1.002 | 0.99, 1.015 | 0.7 |
| Sex * TMA (normalized) | |||
| Female * TMA (normalized) | 1.048 | 1.013, 1.084 | 0.007 |
| Sex * VAT | |||
| Female * VAT (cm2) | 0.997 | 0.99, 1.001 | 0.2 |
| TMA (normalized) * VAT (cm2) | 0.9999 | 0.9998, 1.00011 | 0.5 |
| Female patients | Male patients | |||||
|---|---|---|---|---|---|---|
| Characteristic | HR1 | 95% CI1 | p | HR1 | 95% CI1 | p |
| Age (years) | 1.029 | 0.99, 1.070 | 0.14 | 1.018 | 0.99, 1.052 | 0.3 |
| BMI (kg/m2) | 1.003 | 0.948, 1.061 | >0.9 | 1.006 | 0.95, 1.065 | 0.8 |
| TMA (normalized) | 1.009 | 0.98, 1.038 | 0.6 | 0.96 | 0.917, 0.999 | 0.044 |
| VAT (cm2) | 1.001 | 0.98, 1.019 | >0.9 | 1.001 | 0.99, 1.017 | 0.9 |
| TMA (normalized) * VAT (cm2) | 0.9999 | 0.9996, 1.00022 | 0.6 | 0.99995 | 0.9997, 1.00017 | 0.7 |
| 1HR = Hazard Ratio, CI = Confidence Interval | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





