1. Introduction
The COVID-19 pandemic, which broke out in late 2019 has been responsible for several million fatalities and multifaceted disease-associated chronic illnesses [
1,
2,
3,
4]. Since then, the sequence, structure and host cell entry mechanisms of SARS-CoV-2, as well as the innate and adaptive immune responses after infection have been studied in great detail [
5,
6,
7]. The binding of SARS-CoV-2 via its receptor-binding domain (RBD) to its cognate receptor ACE2 on human cells has been revealed as a critical target for active and passive immunization strategies and anti-viral treatment regimens [
8,
9,
10]. Accordingly, treatments focusing on the ACE2-RBD interaction can be studied by virus-neutralization tests and by molecular interaction assays (MIAs) [
11,
12].
During the initial evolution of SARS-CoV-2 from the original strain towards other variants (e.g. Alpha to Delta), the sequence and structure of RBD had remained highly conserved so that vaccines and therapeutic antibodies developed against the original strain retained their effectiveness [
13,
14,
15].
However, at the end of 2021 a novel variant, termed Omicron, emerged which differed from all previous variants substantially in the sequence of the spike protein S and especially in its RBD [
15,
16]. It turned out that the available vaccines and therapeutic antibodies showed a reduced efficacy for Omicron [
13,
15]. Although Omicron seemed to cause milder forms of COVID-19 in the general population [
17,
18], the decreased effects of available active and passive immunizations [
13,
15,
19,
20] became a major concern, especially for vulnerable persons. In particular, elderly subjects [
21], patients suffering from malignant diseases under therapy [
22], immunocompromised patients [
23] and patients with immunodeficiency [
24] showed a strongly reduced adaptive immunity to Omicron and continue to be at risk for developing severe COVID-19.
Although Omicron and its subvariants have established themselves worldwide for more than 2 years, there are still relatively few Omicron-specific treatment and prevention strategies. Regarding treatment, only a limited number of drugs which are effective for Omicron are available, such as for example antiviral drugs targeting the viral RNA-dependent RNA polymerase (i.e., remdesivir) [
25], virus proteases (i.e., Paxlovid) [
25] and virus replication (i.e., the siRNA approach-based MIR19) [26,27, clinicaltrials.gov: NCT05783206]. Likewise, relatively few Omicron-specific vaccines have become available [
https://covid19.trackvaccines.org/] and there is still a need for Omicron-neutralizing antibodies.
We have previously found that only vaccines including structurally preserved and folded RBD, but not unfolded RBD, can induce SARS-CoV-2-neutralizing antibody responses [
28]. Based on this knowledge we had generated a SARS-CoV-2 vaccine which was based on two RBDs from the original Wuhan-hu1 (wild-type) strain fused to the hepatitis B virus PreS antigen [
29]. This vaccine antigen termed PreS-RBD was expressed as recombinant folded fusion protein and, upon immunization, induced a potent neutralizing antibody response against the SARS-CoV-2 wild-type strain. PreS-RBD–induced antibodies reacted not only with wild-type RBD but showed also strong cross-reactivity with a variety of SARS-CoV-2 variants, including Omicron [
29].
The goal of this study was to refine the PreS-RBD vaccine for Omicron. For this purpose we have developed and compared two subunit vaccines which are strain-specific (Wuhan hu-1 wild-type: W-PreS-W; Omicron: O-PreS-O) a bivalent vaccine based on a mix of W-PreS-W and O-PreS-O, and a chimeric vaccine combining RBDs from Wuhan hu-1 wild-type and Omicron in a single fusion protein (W-PreS-O).
We report the biochemical and biophysical characterization of the vaccine antigens, the comparison of their immunogenicity in a murine model and their abilities to induce Omicron-neutralizing antibodies by virus-neutralization.
2. Materials and Methods
Expression and purification of recombinant proteins
Three recombinant PreS-RBD fusions were based on synthetic genes containing a cDNA coding for HBV-derived PreS [
30], which was flanked at the 5´ and 3´end by DNA sequences coding for a N-terminal and C-terminal SARS-CoV-2 RBD. The synthetic genes were codon optimized for HEK293 cell expression, contained a 5´DNA coding for an IL-2 signal peptide and a 3´ DNA coding for a hexahistidine tag and were cloned into the BamHI and EcoRI sites of plasmid pcDNA3.1(+) (Genscript, Leiden, Netherlands). Expression in HEK293F cells and subsequent purification via Ni-NTA agarose was performed as previously described [
29].
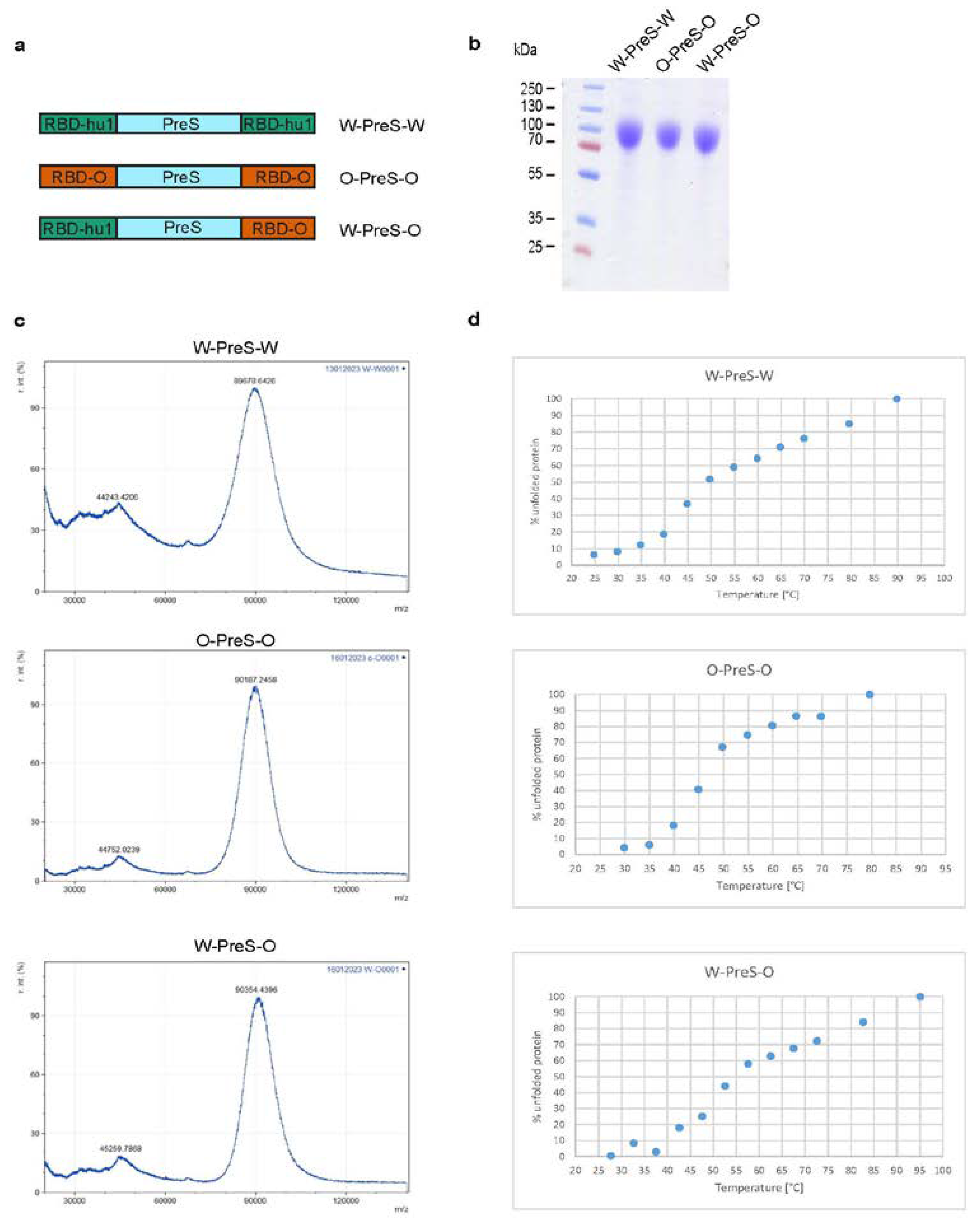
Figure 1a provides an overview of the corresponding recombinant fusion proteins. For the W-PreS-W fusion, the amino acid sequences of RBD derived from SARS-CoV-2 wild-type strain hu-1 (GenBank accession Nr.: QHD43416.1) was used as described previously [
29]. For O-PreS-O, the RBD-encoding sequence from SARS-CoV-2 Omicron BA.1 (Pango B.1.1.529) was used and for W-PreS-O the PreS was flanked by a N-terminal RBD-hu1 and a C-terminal RBD from Omicron BA.1. Recombinant proteins were analyzed for purity by SDS-PAGE and Coomassie blue staining (
Figure 1b).
Matrix-assisted laser desorption and ionization-time-of flight mass spectrometry
Laser desorption mass spectra of the recombinant proteins were acquired with an Axima Confidence matrix-assisted laser desorption and ionization instrument (Shimadzu Biotech, Japan). Purified PreS-RBD fusion proteins were mixed 1:1 with saturated sinapinic acid as matrix and applied to the target (Kratos Analytical, Manchester, UK) by pre-coated dried droplet technique. Measurements were performed in linear mode with laser power 120. Calibration was performed with standard proteins (Cytochrome C, Carboxyanhydrase, BSA). Results are given as relative intensity to mass-to-charge ratio (m/z) and were obtained by mMass Software (Open Source Mass Spectrometry Tool:
http://www.mmass.org/) (
Figure 1c).
Thermal denaturation, renaturation and determination of the melting temperature by circular dichroism (CD)
Far UV circular dichroism (CD) spectra of PreS-fusion proteins were collected on a Jasco J-1500 CD Spectrometer (Japan Spectroscopic Co., Tokyo, Japan) using a 1 mm path length quartz cuvette at protein concentrations of 0.5 mg/ml. Spectra were measured from 260 to 190 nm and recorded by increasing the temperature from 20°C to 95°C at a heating rate of 1°C/min. Results were expressed as the mean residue ellipticity [θ] at a given wavelength. At 230 nm % folded and unfolded protein was calculated with θ at 20°C as 0% unfolded and θ at 95°C as 100% unfolded. The melting temperature was determined by non-linear least-squares data fitting [
31] (
Figure 1d).
Immunization of mice, blood sampling and manipulations
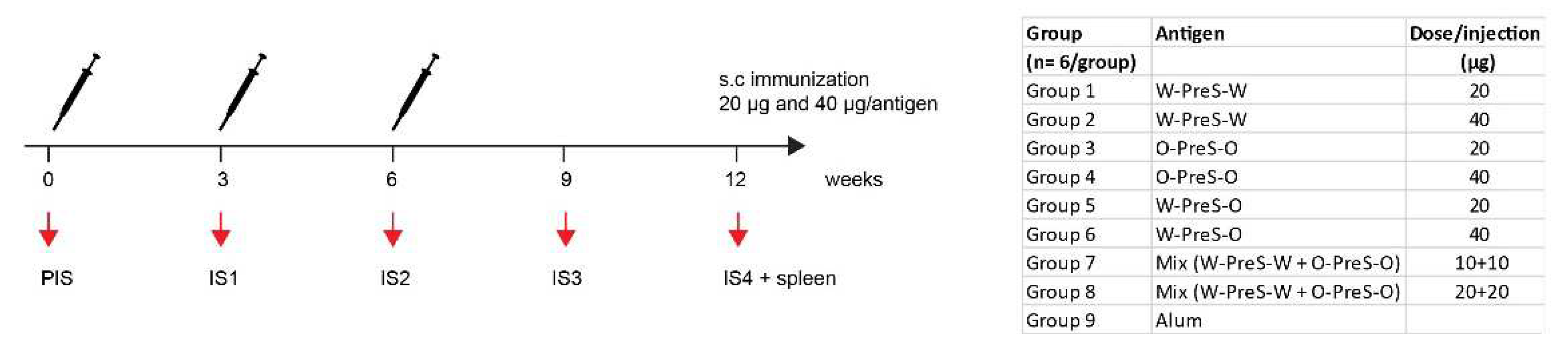
BALB/c mice (female, age: 6 to 8 weeks) were purchased from Charles River (Kisslegg, Germany) and experimental procedures were approved by the Animal Ethics Committee of the Medical University of Vienna and the Austrian Federal Ministry of Science, Research and Economy (2022-0.301.523). Groups of mice (n=6) were immunized subcutaneously three times in three-weekly intervals with 20 µg or 40 µg PreS-RBD fusion proteins (
Figure 2) adsorbed to aluminum hydroxide (Alu-Gel-S, SERVA, Heidelberg, Germany) with a final volume of 150 µl (0.39 mg aluminum hydroxide/ml, 10 mM NaH
2PO
4, 0.9% NaCl, pH 7.2). Serum samples were obtained from tail veins before the first immunization (PIS) and in three-weekl intervals before the second and third immunization (IS1, IS2) as well as 3 weeks (IS3) and 6 weeks (IS4) after the third immunization (
Figure 2). Thereafter mice were sacrificed and spleens were removed. For six mice, immunized with 40 µg PreS-RBD fusion protein mix (
Figure 2) and three non-immunized control mice a histopathological examination was performed.
Measurements of specific antibodies
IgG
1 and IgG
2a antibody levels specific for HEK cell-expressed RBD-hu1 [
29] and RBD-Omicron (BA.1) (Genscript) were measured by ELISA in mouse serum samples. Recombinant proteins were coated overnight (2 µg/ml) at 4 °C onto NUNC Maxisorp 96 well plates (Thermofisher, Waltham, MA, USA). After blocking with 2% BSA/PBST mouse serum samples were added (1: 500 to 1:8000 dilutions as indicated in figure legends) and incubated overnight at 4 °C. After washing three times with PBST, 1:1000 diluted purified rat anti-mouse IgG
1 or IgG
2a (both BD Pharmingen, NJ, USA) were added and incubated for two hours. Afterwards, plates were washed three times and incubated for one hour with 1:2000 diluted HRP-labeled goat anti-rat antibodies (GE Healthcare UK Limited, Chalfont St Giles, United Kingdom). Bound antibodies were detected with ABTS and optical density (OD) was measured at 405/492 nm with the Infinite F50 ELISA reader after 10 minutes (Tecan, Männedorf, Switzerland). In order to allow a comparison of OD levels, reference sera were included on each plates for standardization. Thus, semi-quantitative OD levels obtained in the experiments can be directly compared. All measurements were performed in duplicates with <5% difference and results are given as averages of duplicates.
Virus neutralization assays
Neutralization of SARS-CoV-2 Omicron BA.1 was determined by measuring 50% virus neutralization titers of mouse serum samples obtained six weeks after three immunizations (IS4) as previously described [
29,
32]. Triplicate determinations for each serum sample (n=6 per group, except for W-PreS-W 20 µg, due to lack of sera n=5) were performed. The 50% virus neutralization titer (VNT50) was reported as the interpolated reciprocal of the dilution yielding a 50% reduction in the anti-SARS-CoV-2 nucleocapsid protein staining.
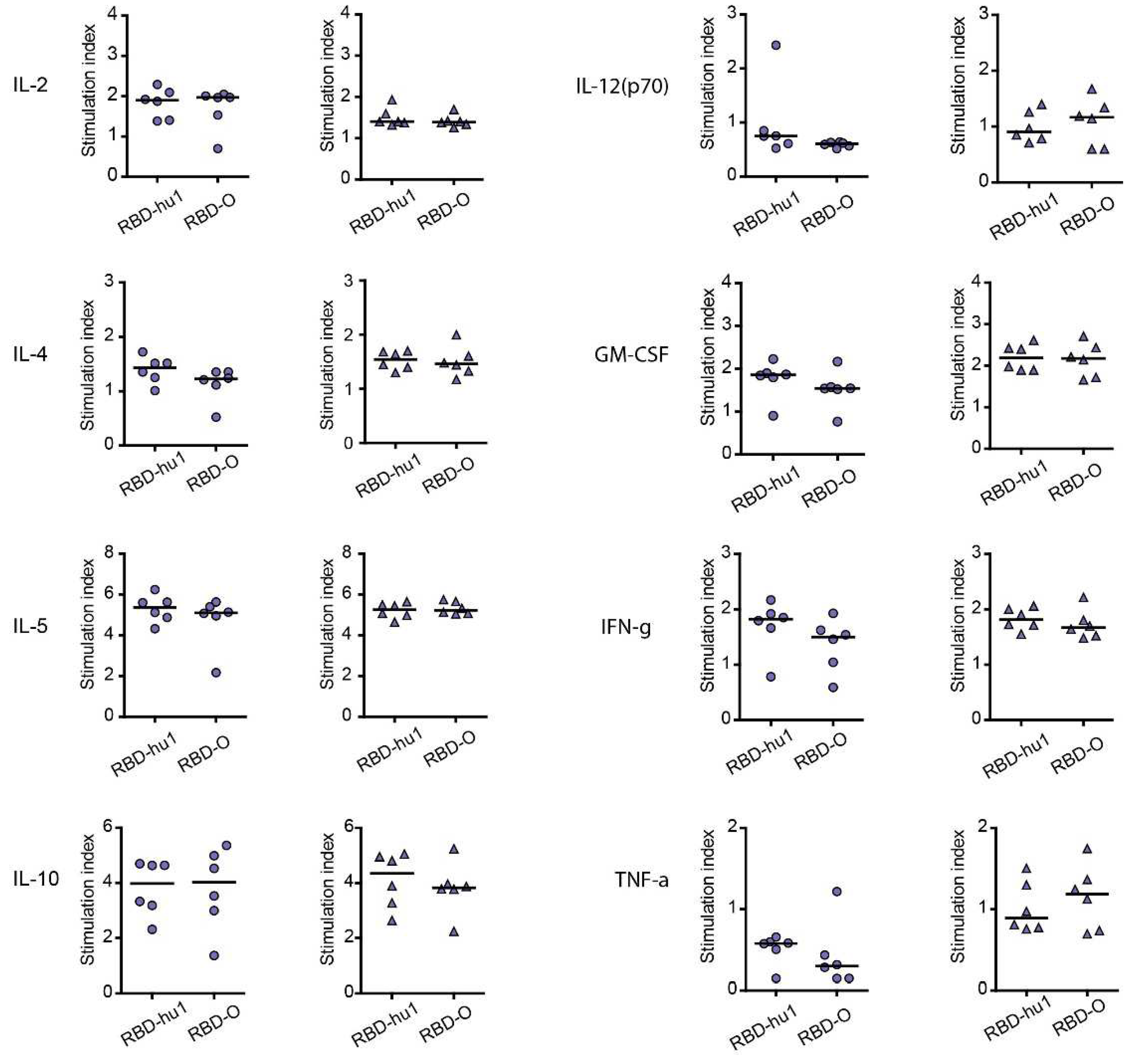
Measurement of specific cytokine production
Spleens of immunized mice were removed under aseptic conditions six weeks after the third immunization (
Figure 2) and splenocytes were isolated and stimulated with 5 µg/ml RBD-hu1, RBD-Omicron BA.1 or medium for 5 days as previously described [
33]. Thereafter, supernatants were analyzed regarding their specific mouse IL-2, IL-4, IL-5, IL-10, IL-12(p70), GM-CSF, IFN-γ and TNF-α concentrations with Bio-Plex Pro Mouse Cytokine Th1/Th2 panel (Bio-Rad Laboratories, CA, USA) following the manufacturer’s instructions. All cellular stimulations and cytokine measurements were performed in triplicates and calculated as average values for each individual mouse. Specific cytokine production upon stimulation with antigen is shown as stimulation index and was calculated as the average measurement of cytokine divided by the average of triplicate of unstimulated cells (medium only).
Histological examination
A full necropsy was performed in nine animals (highest dose, i.e., 40 µg mix n=6, no treatment n=3) and samples of liver, spleen, kidneys, lungs, and heart were fixed in 4% buffered formalin and embedded in paraffin for histological evaluation. HE-stained sections were evaluated in a descriptive manner by a pathologist blinded to group assignments.
Statistics
For group size calculations of mice the GINGER tool of the Institute of Clinical Biometry, Medical University of Vienna (
https://clinicalbiometrics.shinyapps.io/GINGER) was used. With a two-sided significance level of 0.05 pairwise post-hoc comparisons using Tukey's HSD correction between 9 groups with 6 subjects in each group have 80% power to detect a mean difference in the primary outcome variable IgG of 0.5 OD if the within-group standard deviation is 0.2 OD, corresponding to an effect size of 2.5.
Differences in immunoglobulin reactivity and VNT50 titers and specific cytokines shown as stimulation index were determined using two-tailed Mann-Whitney U-test with 95% confidence interval and correlations were assessed by Spearman´s rank correlation by using GraphPad Prism Version 5.00 (La Jolla, CA, USA). p values of <0.05 were considered as significant.
3. Results
3.1. Recombinant Vaccine Antigens Represent Defined, Folded and Stable Proteins
For the last two years, Omicron and its subvariants have been the major cause of COVID-19. Accordingly, it was our goal to refine our previously described subunit vaccine, which was based on a recombinant PreS-RBD fusion protein containing two SARS-CoV-2 wild-type RBDs for vaccination against Omicron. For this purpose, we compared the earlier described wild-type-derived (W-PreS-W), with a new construct containing two RBDs from Omicron (O-PreS-O) and a chimeric “all-in-one” protein with one RBD from wild-type and another from Omicron (W-PreS-O) (
Figure 1a). The three proteins were expressed in HEK cells and subsequently purified to homogeneity by Nickel affinity chromatography. The three glycoproteins were visualized as bands of approximately 90 kDa by SDS-PAGE (
Figure 1b). Mass spectrometry revealed prominent peaks at 89678 Da, 90187 Da and 90354 Da for W-PreS-W, O-PreS-O, and W-PreS-O, respectively (
Figure 1c). The smaller peaks at 44243 Da (W-PreS-W), 44752 Da (O-PreS-O) and 45259 Da (W-PreS-O) corresponded to the doubly charged forms of the proteins (
Figure 1c). Thus, the results obtained for the three recombinant glycoproteins by SDS-PAGE and mass spectrometry are in good agreement. Since it has been reported, that the ability of the RBD and PreS-RBD proteins to induce neutralizing antibodies depends on the intact fold of the respective proteins we were interested to examine the thermal stability of the three antigens. For this purpose, the percentage of folded protein was analyzed from +20°C to nearly +100°C by circular dichroism spectroscopy (
Figure 1d). The recombinant proteins seemed to be quite temperature stable with melting points for W-PreS-W of 53.1°C, O-PreS-O of 44.9°C and for W-PreS-O of 57.2°C (
Figure 1d). More than 80% of each of the proteins remained folded up to a temperature of 40°C.
Figure 1.
(a) Scheme of the recombinant PreS-RBD fusion proteins. RBD domains from hu-1 (green) and from Omicron (orange) were fused to the N- and C- terminus of PreS (blue) as indicated. (b) Coomassie blue-stained SDS-PAGE of HEK cell-expressed and purified PreS-RBD fusion proteins. Molecular weights are indicated in kDa on the left margins. (c) MALDI analyses of fusion proteins. Y-axes: relative intensity as percentage of most abundant signal intensity. X-axes: mass/charge ratio. (d) Melting curve of PreS-RBD fusion proteins. Shown are the melting curves of PreS-RBD fusion proteins as percent unfolded protein (y-axes) at increasing temperatures in °C (x-axes).
Figure 1.
(a) Scheme of the recombinant PreS-RBD fusion proteins. RBD domains from hu-1 (green) and from Omicron (orange) were fused to the N- and C- terminus of PreS (blue) as indicated. (b) Coomassie blue-stained SDS-PAGE of HEK cell-expressed and purified PreS-RBD fusion proteins. Molecular weights are indicated in kDa on the left margins. (c) MALDI analyses of fusion proteins. Y-axes: relative intensity as percentage of most abundant signal intensity. X-axes: mass/charge ratio. (d) Melting curve of PreS-RBD fusion proteins. Shown are the melting curves of PreS-RBD fusion proteins as percent unfolded protein (y-axes) at increasing temperatures in °C (x-axes).
3.2. Formulation of the Subunit Vaccines and Immunization Schedule
We previously developed allergen-specific immunotherapy vaccines based on fusion proteins consisting of PreS and allergen-derived peptides which were formulated by adsorption to aluminum hydroxide [
34,
35]. These vaccines were shown to be safe in clinical immunotherapy trials and induced antibodies blocking the allergen-IgE interaction. Accordingly, we formulated PreS-RBD fusion protein vaccines by adsorbing different amounts (20 μg, 40 μg) of PreS-RBD fusion proteins (W-PreS-W, O-PreS-O, W-PreS-O) or equimolar mixes of them (W-PreS-W + O-PreS-O) (i.e., 10+10 μg or 20+20 μg) (
Figure 2). Aluminum hydroxide alone was used as a negative control. Three subcutaneous immunizations were performed in three-week time intervals (
Figure 2). For a preliminary analysis of safety, the following parameters were assessed. No significant differences of weight and weight gain between groups immunized with PreS-RBD fusion constructs or alum were detected during weekly weight monitoring (
Figure S1). Additionally, we performed a weekly comprehensive assessment of the health status of the mice from the different groups as described [
36]. This assessment showed no relevant abnormalities. For six representative mice immunized three times with the highest dose (40 µg) of the mix of PreS-RBD and three non-treated mice, a histopathological examination of organs (i.e., liver, spleen, kidney, lung and hearts) was performed. As exemplified by the representative animals, there were no significant differences detectable between immunized and non-treated groups by necropsy and histological evaluation was observed (
Figure S2).
Figure 2.
Scheme of immunizations and treatments. Mice (n=6/group) were immunized subcutaneously three times in three-weekly intervals with 20 µg or 40 µg of PreS-RBD fusion proteins or alum as control. Time points of blood sampling (PIS, IS1, IS2, IS3, IS4) and splenectomy (IS4) are indicated.
Figure 2.
Scheme of immunizations and treatments. Mice (n=6/group) were immunized subcutaneously three times in three-weekly intervals with 20 µg or 40 µg of PreS-RBD fusion proteins or alum as control. Time points of blood sampling (PIS, IS1, IS2, IS3, IS4) and splenectomy (IS4) are indicated.
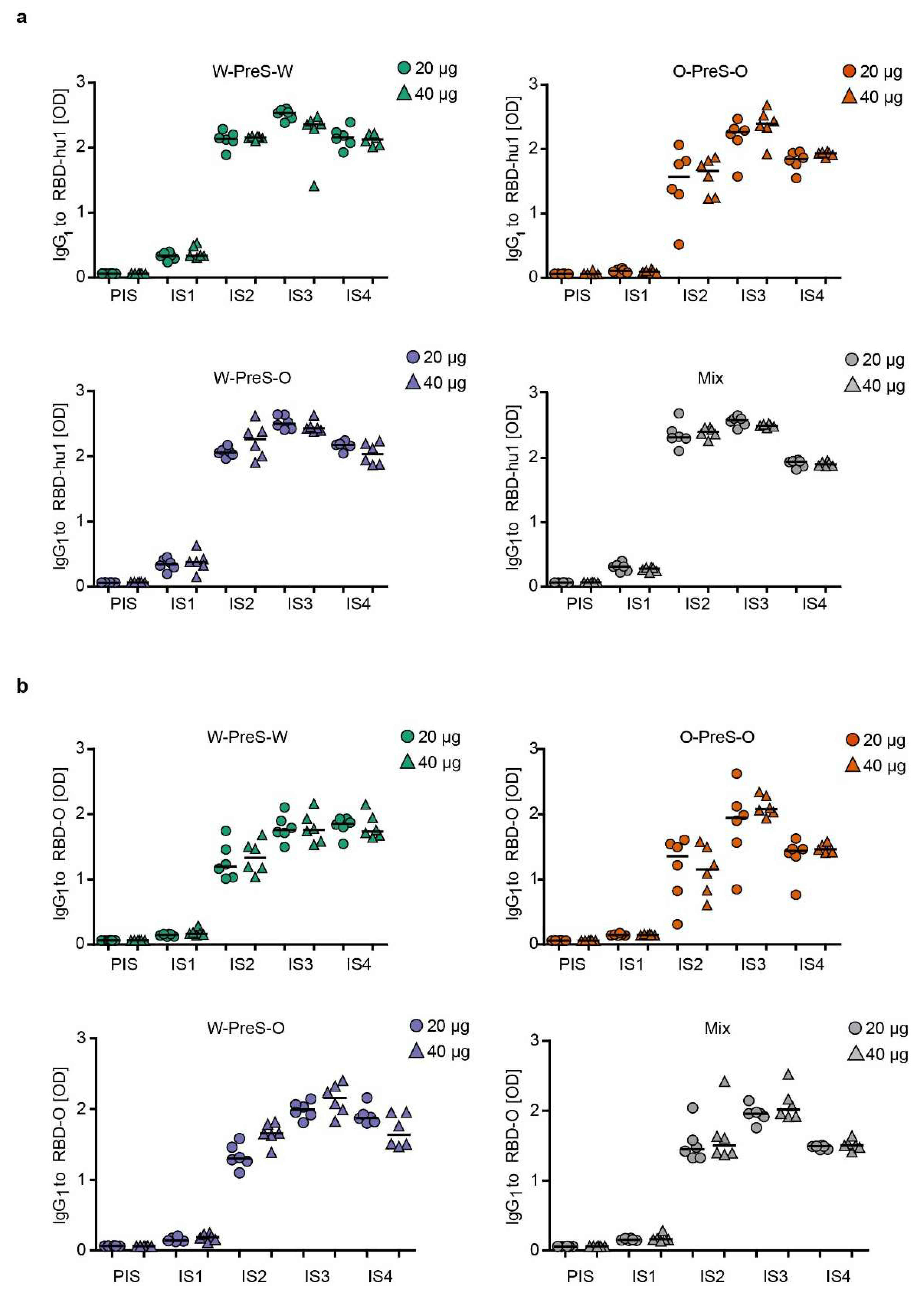
3.3. Immunization with W-PreS-O Induces High IgG Antibody Levels Specific for Wild-Type- and Omicron RBD
Figure 3 shows the time-dependent kinetics of IgG
1 antibody levels specific for wild-type RBD (
Figure 3a) and Omicron RBD (
Figure 3b) in mice immunized with different doses of the different vaccines. The first RBD-specific antibodies became detectable already three weeks after the first immunization and antibody levels strongly increased 3 weeks after the second and third immunization (
Figure 3). For each of the tested vaccines (W-PreS-W, O-PreS-O, W-PreS-O, mix of W-PreS-W and O-PreS-O), Omicron RBD-specific IgG
1 levels were somewhat lower than wild-type RBD-specific antibody levels at the corresponding time points but these differences were not statistically significant (
Figure 3a,b). Vaccines including wild-type RBD induced higher wild-type RBD-specific IgG
1 levels than the only Omicron RBD-based vaccine (
Figure 3a). Again, this difference was not significant.
However, the trend that vaccines containing strain-specific RBDs induced higher IgG
1 levels to the corresponding RBD was not observed for Omicron (
Figure 3b). W-PreS-W, the mix and especially W-PreS-O induced robust Omicron-specific IgG
1 levels which were comparable or even higher than Omicron RBD-specific IgG
1 levels induced by O-PreS-O. Of note, lower levels of Omicron RBD-specific IgG
1 levels in the O-PreS-O (median OD IgG
1 IS3 20 µg: 1.94; 40 µg: 2,07; IS4 20 µg: 1.43; 40 µg: 1.46) and in the mix groups (median OD IgG
1: IS3 = 20 µg: 1.95; 40 µg: 2,01; IS4 = 20 µg: 1.48; 40 µg: 1.50), compared to the W-PreS-W (median OD IgG
1: IS3 = 20 µg: 1.75; 40 µg: 1.75; IS4 = 20 µg: 1.84; 40 µg: 1.73) and W-PreS-O (median OD IgG
1: IS3 = 20 µg: 1.99; 40 µg: 2.15; IS4 = 20 µg: 1.87; 40 µg: 1.63) groups were observed (
Figure 2a). However, also these results were not significantly different from each other. Further, we noted, that RBD-specific IgG
1 levels induced by O-PreS-O in the individual mice were more heterogeneous than those induced by the other vaccines. With the exception of W-PreS-W-induced Omicron-specific IgG
1 levels, we observed a small but distinct decline of RBD-specific IgG
1 levels at time-point IS4 (i.e., six weeks after the last immunization) (
Figure 3a,b).
Figure 3.
Time course of RBD-specific IgG1 responses in immunized mice. Shown are (a) RBD-hu1- and (b) RBD-Omicron-specific IgG1 responses in serum samples from mice (1:500 dilution), immunized with 20 µg (circles) or 40 µg (triangles) of PreS-RBD fusion protein vaccines at indicated time points (x-axes). OD405/492 nm values corresponding to IgG1 antibody levels are shown (y-axes). Horizontal bars represent median values for each group.
Figure 3.
Time course of RBD-specific IgG1 responses in immunized mice. Shown are (a) RBD-hu1- and (b) RBD-Omicron-specific IgG1 responses in serum samples from mice (1:500 dilution), immunized with 20 µg (circles) or 40 µg (triangles) of PreS-RBD fusion protein vaccines at indicated time points (x-axes). OD405/492 nm values corresponding to IgG1 antibody levels are shown (y-axes). Horizontal bars represent median values for each group.
3.4. The Aluminum-Hydroxide Adsorbed W-PreS-O Vaccines Induces a Mixed Th1/Th2 Response Specific for RBD
Cytokine responses to RBD-wild-type and RBD-Omicron were measured in supernatants of cultured splenocytes obtained at time point IS4 from mice immunized with the different PreS-RBD vaccines.
Figure 4 shows the RBD-specific production of cytokines in mice immunized with 20 µg or 40 µg W-PreS-O. We found comparable effects of the PreS-RBD vaccines on cytokine responses for both doses of vaccines and for RBD from wild-type and Omicron SARS-CoV-2 (
Figure 4). An induction of RBD-specific Th2 cytokines (i.e., IL-4, IL-5) but also RBD-specific Th1 cytokines (IFN-γ, GM-CSF) and the tolerogenic cytokine IL-10 was observed. An increase of IL-2 indicative of T cell stimulation was noted, whereas the inflammatory cytokines (TNF-α, IL-12) were not increased. A balanced Th1/Th2 ratio (IFN-γ/IL-4) of 0.91 for RBD-hu-1, 0.88 for RBD-Omicron was noted suggesting that the alumn-adjuvanted PreS-RBD-based vaccine induced a balanced RBD-specific Th1/Th2 response.
In the murine system the balanced production of specific IgG
1 and IgG
2a antibodies is indicative of a mixed Th1/Th2 immune response [
37]. We therefore measured also RBD-specific IgG
2a levels in sera from immunized mice using the very same serum dilution, which had been used for measuring specific IgG
1 levels (
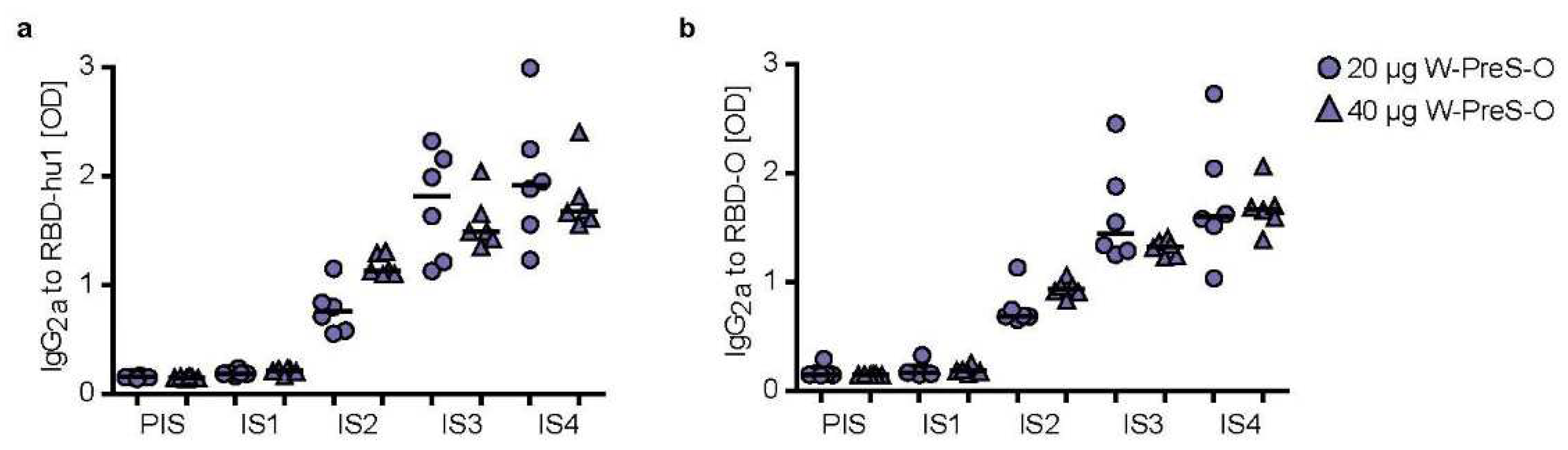
Figure 5).
Figure 5 shows the kinetics and levels of IgG
2a towards wild-type (
Figure 5a) and Omicron RBD (
Figure 5b) in mice immunized with 20 μg or 40 μg of W-PreS-O. A robust induction of IgG
2a specific for RBD from both SARS-CoV-2 strains was observed with specific levels of IgG
2a corresponding to RBD-specific IgG
1 levels (
Figure 3 and
Figure 5). There was no statistically significant difference regarding the induction of RBD-specific IgG
1 and IgG
2a antibody levels for the two doses (i.e., 20 μg or 40 μg of W-PreS-O). However, we noted a difference regarding the kinetics of antibody production when comparing the development and duration of RBD-specific IgG
1 and IgG
2a antibodies. RBD-specific IgG
1 increased earlier than RBD-specific IgG
2a (
Figure 3 and
Figure 5). Importantly, RBD-specific IgG2
a levels did not show a decrease at the time point IS4 when compared to IS3 whereas IgG
1 decreased (
Figure 3 and
Figure 5). In detail, the following results were obtained: for 20 µg median OD hu-1-sIgG
2a IS3: 1.81, IS4: 1.91; OD Omicron-sIgG
2a IS3: 1.44, IS4: 1.56 and for 40 µg (OD hu-1-sIgG
2a IS3: 1.48, IS4: 1.67; OD Omicron-sIgG
2a IS3: 1.32 , IS4: 1.67) doses from three weeks (IS3) to six weeks after the third immunization (IS4) (
Figure 5a,b).
3.5. W-PreS-O-Induced the Highest Virus Neutralization Titers against Omicron
It has been previously reported that high levels of RBD-specific IgG are correlated with high VNTs towards SARS-CoV-2 wild-type strain [
28,
38] and that hu-1-induced antibodies partly cross-react with variants of concern [
15,
28,
39]. However, functional assays, such as virus neutralization assays or molecular interaction assays are especially useful for the measurement of antibodies protecting against virus infection [
12,
40,
41]. Therefore, serum samples obtained from mice six weeks after the third immunization with the PreS-RBD fusion constructs were analyzed regarding their capacity to neutralize SARS-CoV-2 Omicron (
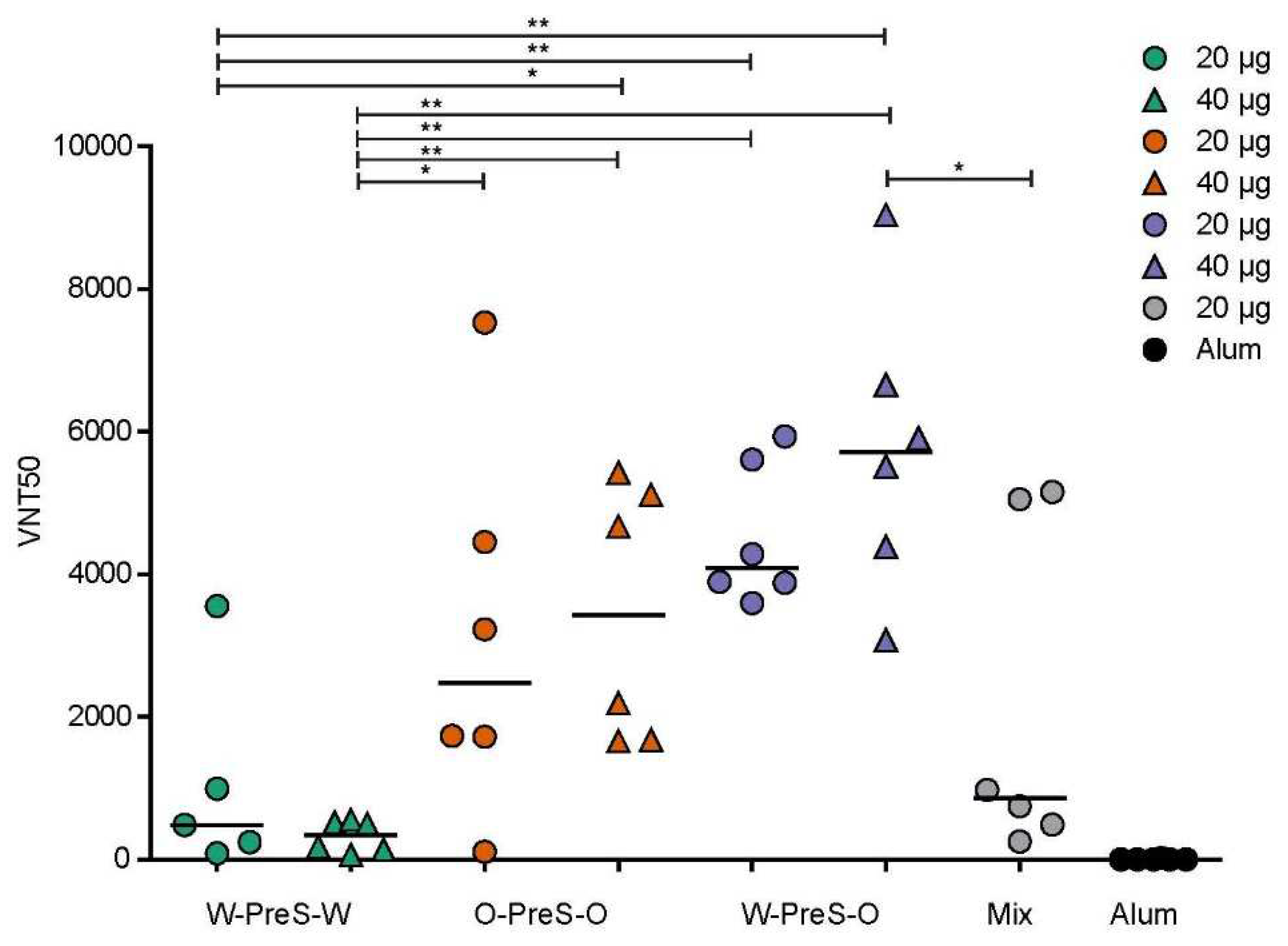
Figure 6).
We found that W-PreS-O-induced antibodies had the highest SARS-CoV-2 Omicron virus neutralization titers (20 µg VNT50 min: 3595, max: 4938, median: 4088; 40 µg VNT50 min: 3076, max: 9042, median: 5711). By contrast, the virus neutralizing capacity of W-PreS-W induced antibodies was modest (20 µg VNT50 min: 87, max: 3554, median: 482; 40 µg VNT50 min: 66, max: 552, median: 338) compared to W-PreS-O and O-PreS-O (
Figure 5). Interestingly, immunization with the bivalent mix comprising the two strain-specific antigens, W-PreS-W and O-PreS-O, led to much lower VNT50 titers (20 µg mix VNT50: min: 254, max: 5155, median: 860) than the corresponding W-PreS-O vaccine (i.e., 20 µg) (median VNT50: 4088). However, even more interesting was the fact that the median virus neutralization titers against Omicron induced by the O-PreS-O vaccine were considerably lower (20 µg VNT50 min: 109, max: 7530, median: 2480; 40 µg VNT50 min: 1658, max: 5429, median: 3430) than those induced by the W-PreS-O vaccine (20 µg VNT50 median: 4088; 40 µg VNT50 median: 5711). However, these differences were statistically not significant.
Figure 6.
SARS-CoV-2 Omicron neutralization by antibodies induced with the PreS-RBD vaccines. VNT50 titers (y-axis) obtained with serum samples (IS4) of mice immunized with PreS-RBD fusion proteins or alum alone as indicated (x-axis) are shown as median values of triplicate determinations for each serum. Median VNT50 titers are shown by horizontal bars. Significant differences between each of the PreS-RBD-vaccinated groups determined by two-tailed Mann-Whitney U-test, p values: (** < 0.001, * < 0.01) are indicated.
Figure 6.
SARS-CoV-2 Omicron neutralization by antibodies induced with the PreS-RBD vaccines. VNT50 titers (y-axis) obtained with serum samples (IS4) of mice immunized with PreS-RBD fusion proteins or alum alone as indicated (x-axis) are shown as median values of triplicate determinations for each serum. Median VNT50 titers are shown by horizontal bars. Significant differences between each of the PreS-RBD-vaccinated groups determined by two-tailed Mann-Whitney U-test, p values: (** < 0.001, * < 0.01) are indicated.
3.6. Lack of Association of W-PreS-O-Induced Omicron-Specific IgG1 with IgG2a Antibody Levels and Omicron-Specific Antibody Levels with Neutralization of Omicron
When analyzing the kinetics of RBD Omicron-specific IgG
1 and IgG
2a in mice immunized with W-PreS-O, we found different kinetics of antibody responses. Omicron-specific IgG
1 increased and declined earlier than Omicron-specific IgG
2a (
Figure 3 and
Figure 5). In order to obtain information regarding a possible association of RBD Omicron-specific IgG
1 and IgG
2a responses and thus between synchronization of subclass responses and/or epitope-specificity, we performed a correlation analysis of both subclass responses (
Figure S3, S4). There was a weak correlation between RBD wild-type-specific IgG
1 and IgG
2a antibody levels at time points IS2 and IS3, which disappeared completely at time point IS4 (
Figure S4a). No correlation between RBD Omicron-specific IgG
1 and IgG
2a antibody levels was found at any of the three time points i.e., IS2, IS3 and especially not at time point IS4 when Omicron-specific virus neutralization was studied (
Figure 5).
Next, we investigated if there is any association between RBD Omicron-specific IgG
1 or IgG
2a levels induced by immunization with W-PreS-O at time point IS4. In fact,
Figure S5 shows that there was no correlation of RBD wild-type- or RBD Omicron-specific IgG
1 levels with Omicron-specific virus neutralization titers even when tested with different serum dilutions ranging from 1:500 to 1:8000 (
Figure S5). Likewise, we studied at time point IS4 if RBD Omicron-specific IgG
2a levels induced by immunization with W-PreS-O correlated with Omicron-specific virus neutralization titers. However, neither for mice which had been immunized with 20 µg nor with 40 µg any correlation of antibody levels and virus neutralization titers was observed (
Figure S6). Even when mice immunized with 20 μg or 40 μg were analyzed together, no correlation was found (
Figure S6).
4. Discussion
Although Omicron and Omicron subvariants have accounted for the majority of SARS-CoV-2 infections for the last two years, only relatively few Omicron-specific SARS-CoV-2 vaccines are currently available [covid19.trackvaccines.org]. We have previously reported the construction,
in vitro and
in vivo characterization of a SARS-CoV-2 subunit vaccine, which is based on a recombinant fusion protein consisting of HBV-derived PreS and two SARS-CoV-2 wild-type RBDs, termed PreS-RBD [
29]. The recombinant PreS-RBD fusion protein could be produced by expression in HEK cells as a soluble, folded protein in large amounts. HBV-derived PreS was included into PreS-RBD as an immunological carrier protein to ensure additional PreS-derived T cell help [
42]. Accordingly, we observed that all rabbits immunized with PreS-RBD developed RBD-specific neutralizing antibodies. This could not be achieved with a vaccine based only on RBD without carrier protein indicating that the PreS-RBD vaccine may indeed overcome immunological non-responsiveness due to the integration of PreS as immunological carrier.
The aim of this study was to improve the previously described PreS-RBD vaccine regarding the induction of Omicron-neutralizing antibodies. For this purpose we constructed a fusion protein which was identical to the previous PreS-RBD (W-PreS-W) except that Wuhan RBD was replaced by Omicron RBD (O-PreS-O), and another construct which contained one Wuhan RBD and one Omicron RBD (W-PreS-O). We were able to express and purify all three vaccine antigens (i.e., W-PreS-W, O-PreS-O, W-PreS-O) as soluble and folded proteins and easily formulate them by adsorption to aluminum hydroxide. The three vaccines based on single recombinant fusion proteins were then compared to each other and to an equimolar mix of W-PreS-W and O-PreS-O (i.e., a bivalent vaccine) regarding their abilities to induce wild-type RBD- and Omicron RBD-specific antibodies. All four vaccines were well tolerated by the vaccinated mice as indicated by clinical symptom scores, gain of body weight and histopathological examination, suggesting that they have a favorable safety profile.
Furthermore, all tested vaccines induced comparable IgG1 antibody responses specific for wild-type RBD. Omicron RBD-specific IgG1 antibody responses were slightly higher in W-PreS-O immunized mice compared to mice immunized with W-PreS-W, O-PreS-O or the bivalent mix of W-PreS-W and O-PreS-O but the differences were not statistically significant. The levels of Omicron RBD-specific IgG1 antibodies were comparable to those of Omicron RBD-specific IgG2a levels. It thus seemed that the aluminum hydroxide-adsorbed vaccines induced a mixed Th1/Th2 phenotype. This was confirmed by the analysis of cytokine responses, detected in supernatants of cultured splenocytes obtained from the immunized mice after stimulation with wild-type- and Omicron-RBD. In fact, we found a parallel and equivalent induction of Th2 cytokines (i.e., IL-4, IL-5) as well as of Th1 cytokines (IFN-γ, GM-CSF) whereas there was no increase of inflammatory cytokines (TNF-α, IL-12). Thus, the aluminum hydroxide-formulated vaccines seemed to induce a balanced immune response.
Regarding antibody responses, we found a profound difference regarding the kinetics of RBD-specific IgG1 and IgG2a responses. RBD-specific IgG1 levels increased more quickly than RBD-specific IgG2a but decreased much earlier than RBD-specific IgG2a levels.
Thus, it seems that specific IgG
1 responses dominate in the early phase of protection whereas IgG
2a antibodies are more relevant for sustained protection, at least in the murine model. One possible, but not exclusive explanation for the different kinetics of specific IgG
1 and IgG
2a responses may be that a sequential class switch has occurred in the immunized mice, because in the mouse the Cγ1 constant region gene is located within the
Igh locus upstream of the Cγ2a gene, but downstream of the Cμ gene [
43]. We therefore investigated whether there is a correlation of the levels of RBD-specific IgG
1 and IgG
2a. However, there was no significant correlation between RBD-specific IgG
1 and IgG
2a levels and it is therefore possible that both, a sequential but also direct class switch to IgG
1 and IgG
2a most likely to a varying degree has occurred in the individual animals. Alternatively, it is possible that IgG
1 and IgG
2 subclass responses originate from different B cell clones following an independent class-switch program involving a Th1 and Th2 pathway. The latter possibility would be in accordance with the lack and/or poor of correlation of RBD-specific antibodies.
The most important finding of our study came from the assessment of the Omicron-neutralizing antibody titers. Although there were only minor differences regarding RBD-specific IgG1 and IgG2a antibody levels, we found that the W-PreS-O induced antibodies had the highest SARS-CoV-2 Omicron virus neutralization capacities, which exceeded by far those induced by the W-PreS-W-based vaccine. The W-PreS-O neutralizing antibody titers were also higher than those induced by the bivalent W-PreS-W and O-PreS-O mix and, surprisingly, also higher than the Omicron-specific VNTs induced by the O-PreS-O-based vaccine. The finding that the median VNT induced by the W-PreS-O-based vaccine was approximately twice as high as that of the O-PreS-O vaccine may be explained by the fact that the W-PreS-O-based vaccine is able to activate a broader repertoire of RBD-specific T cells and B cells resulting in a broader T cell and antibody response.
In summary, our study indicates that the vaccine based on W-PreS-O is superior to the vaccines based on W-PreS-W, O-PreS-O and the bivalent mix of W-PreS-W and O-PreS-O regarding the induction of Omicron-neutralizing antibodies. Furthermore, the W-PreS-O-based vaccine has the advantage that only one recombinant fusion protein is sufficient for the production of a vaccine conveying broadly neutralizing antibodies against strains related both to Wuhan and to Omicron. Should further studies performed in the Syrian hamster model and in human clinical trials confirm the so far obtained promising results, W-PreS-O may represent a useful SARS-CoV-2 subunit vaccine suitable for repeated pre-seasonal booster immunizations to achieve sustained protection against a variety of SARS-CoV-2 strains and especially against currently prevailing Omicron strains.
5. Patents
P.G., B.K., W.F.P and R.V. are authors on a patent application regarding the vaccine.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Figure S1: Weight development of mice immunized with (a) alum alone (negative control) or with 20 µg or 40 µg of the PreS-RBD fusion proteins (b-e) as measured weekly (x-axes) as gram body weight (y-axes). Figure S2: Representative hematoxylin–eosin stains of liver, spleen, kidney, heart and lung sections obtained from a non-immunized mouse (upper panel) or a mouse immunized with 40 µg of the mixture of W-PreS-W and O-PreS-O (high dose of PreS-RBD, lower panel). Figure S3: IgG
1 levels specific for (a) RBD-hu1 and (b) RBD-Omicron of mice immunized with 20 µg (circles) or 40 µg (triangles) of the PreS-RBD fusion proteins. Figure S4: Association of IgG
1 and IgG
2a antibody levels specific for (a) RBD-hu1 and (b) RBD-Omicron of mice immunized with 20 µg or 40 µg of W-PreS-O at time points IS2, IS3 and IS4. Figure S5: Lack of correlation of virus neutralization titers (VNT50, x-axes) in sera of mice (IS4) immunized with W-PreS-O (W-O) with levels of IgG antibodies (OD values, y-axis) to folded RBD-hu1 (left) and RBD-Omicron (right) measured at dilutions (a) 1:500, (b) 1:1000, (c) 1:2000, (d) 1:4000 and (e)1:8000. Figure S6: Lack of correlation of virus neutralization titers (VNT50, x-axes) in sera of mice (IS4) immunized with W-PreS-O with levels of IgG antibodies (OD values, y-axis) to folded RBD-Omicron measured at dilutions 1:500 of (a) both groups, (b) mice immunized with 20 µg and (c) mice immunized with 20 µg W-PreS-O.
Author Contributions
Conceptualization: P.G. and R.V.; methodology: P.G., B.K., A.O.R., L.G., G.T., A.N.A.S., M.F.T., M.S., V.F., S.H., LP; visualization: P.G.; writing—original draft preparation: P.G. and R.V.; writing—review and editing: W.K., W.F.P., A.O.R., L.G., H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Austrian Science Fund, Grant number P34253-B and in part by the Danube Allergy Research Cluster program funded by the Country of Lower Austria and the Medical University of Vienna. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Ethics Committee of the Medical University of Vienna and the Austrian Federal Ministry of Science, Research and Economy (2022-0.301.523).
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Acknowledgments
We thank Andrea Vlasaty for animal care talking and assistance with handling of animals and Karin Stiasny (Medical University of Vienna, Center for Virology) for kindly providing Omicron BA.1 strain.
Conflicts of Interest
Rudolf Valenta has received research grants from HVD Life-Sciences, Vienna, Austria, WORG Pharmaceuticals, Hangzhou, China and from Viravaxx AG, Vienna, Austria. He serves as consultant for Viravaxx AG. Rudolf Valenta, Pia Gattinger, Bernhard Kratzer and Winfried Pickl are authors on a patent application regarding the vaccine. The other authors have no conflict of interest to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The authors with Russian affiliation declare that they have prepared the article in their “personal capacity” and/or that they are employed at an academic/research institution where research or education is the primary function of the entity.
References
- Davis:, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Roncon, L.; Zuin, M.; Barco, S.; Valerio, L.; Zuliani, G.; Zonzin, P.; Konstantinides, S.V. Incidence of acute pulmonary embolism in COVID-19 patients: Systematic review and meta-analysis. Eur J Intern Med 2020, 82, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chen, T.; Zhang, X.; Hu, B.; Shi, H. Causal relationship between COVID-19 and myocarditis or pericarditis risk: a bidirectional Mendelian randomization study. Front Cardiovasc Med 2023, 10, 1271959. [Google Scholar] [CrossRef] [PubMed]
- Khoshdel-Rad, N.; Zahmatkesh, E.; Shpichka, A.; Timashev, P.; Vosough, M. Outbreak of chronic renal failure: will this be a delayed heritage of COVID-19? J Nephrol 2021, 34, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Brüggen, M.C.; O'Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Xu, Y.; Wang, T.; Xie, F. Innate and adaptive immune response in SARS-CoV-2 infection-Current perspectives. Front Immunol 2022, 13, 1053437. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines 2021, 6, 104. [Google Scholar] [CrossRef]
- Taylor, P.C.; Adams, A.C.; Hufford, M.M.; de la Torre, I.; Winthrop, K.; Gottlieb, R.L. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol 2021, 21, 382–393. [Google Scholar] [CrossRef]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado Del Pozo, C.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.P.C.; Quadros, H.C.; Fernandes, A.M.S.; Gonçalves, L.P.; Badaró, R.; Soares, M.B.P.; Machado, B.A.S. An Overview of the Conventional and Novel Methods Employed for SARS-CoV-2 Neutralizing Antibody Measurement. Viruses 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Gattinger, P.; Ohradanova-Repic, A.; Valenta, R. Importance, Applications and Features of Assays Measuring SARS-CoV-2 Neutralizing Antibodies. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Rössler, A.; Riepler, L.; Bante, D.; von Laer, D.; Kimpel, J. SARS-CoV-2 Omicron Variant Neutralization in Serum from Vaccinated and Convalescent Persons. N Engl J Med 2022, 386, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Cicchitto, G.; Cardillo, L.; de Martinis, C.; Sabatini, P.; Marchitiello, R.; Abate, G.; Rovetti, A.; Cavallera, A.; Apuzzo, C.; Ferrigno, F.; et al. Effects of Casirivimab/Imdevimab Monoclonal Antibody Treatment among Vaccinated Patients Infected by SARS-CoV-2 Delta Variant. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Gattinger, P.; Tulaeva, I.; Borochova, K.; Kratzer, B.; Trapin, D.; Kropfmüller, A.; Pickl, W.F.; Valenta, R. Omicron: A SARS-CoV-2 variant of real concern. Allergy 2022, 77, 1616–1620. [Google Scholar] [CrossRef]
- Shah, M.; Woo, H.G. Omicron: A Heavily Mutated SARS-CoV-2 Variant Exhibits Stronger Binding to ACE2 and Potently Escapes Approved COVID-19 Therapeutic Antibodies. Front Immunol 2021, 12, 830527. [Google Scholar] [CrossRef]
- Wolter, N.; Jassat, W.; Walaza, S.; Welch, R.; Moultrie, H.; Groome, M.; Amoako, D.G.; Everatt, J.; Bhiman, J.N.; Scheepers, C.; et al. Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa: a data linkage study. Lancet 2022, 399, 437–446. [Google Scholar] [CrossRef]
- Menni, C.; Valdes, A.M.; Polidori, L.; Antonelli, M.; Penamakuri, S.; Nogal, A.; Louca, P.; May, A.; Figueiredo, J.C.; Hu, C.; et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of Omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet 2022, 399, 1618–1624. [Google Scholar] [CrossRef]
- Focosi, D.; Casadevall, A. A Critical Analysis of the Use of Cilgavimab plus Tixagevimab Monoclonal Antibody Cocktail (Evusheld™) for COVID-19 Prophylaxis and Treatment. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Zhou, H.; Dcosta, B.M.; Landau, N.R.; Tada, T. Resistance of SARS-CoV-2 Omicron BA.1 and BA.2 Variants to Vaccine-Elicited Sera and Therapeutic Monoclonal Antibodies. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Breznik, J.A.; Rahim, A.; Zhang, A.; Ang, J.; Stacey, H.D.; Bhakta, H.; Clare, R.; Liu, L.M.; Kennedy, A.; Hagerman, M.; et al. Early Omicron infection is associated with increased reinfection risk in older adults in long-term care and retirement facilities. EClinicalMedicine 2023, 63, 102148. [Google Scholar] [CrossRef] [PubMed]
- Mair, M.J.; Mitterer, M.; Gattinger, P.; Berger, J.M.; Valenta, R.; Fong, D.; Preusser, M. Inhibition of SARS-CoV-2 Omicron BA.1 and BA.4 Variants After Fourth Vaccination or Tixagevimab and Cilgavimab Administration in Patients With Cancer. JAMA Oncol 2022, 8, 1694–1696. [Google Scholar] [CrossRef] [PubMed]
- Nevejan, L.; Ombelet, S.; Laenen, L.; Keyaerts, E.; Demuyser, T.; Seyler, L.; Soetens, O.; Van Nedervelde, E.; Naesens, R.; Geysels, D.; et al. Severity of COVID-19 among Hospitalized Patients: Omicron Remains a Severe Threat for Immunocompromised Hosts. Viruses 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Nadesalingam, A.; Cantoni, D.; Aguinam, E.T.; Chan, A.C.; Paloniemi, M.; Ohlendorf, L.; George, C.; Carnell, G.; Lyall, J.; Ferrari, M.; et al. Vaccination and protective immunity to SARS-CoV-2 Omicron variants in people with immunodeficiencies. Lancet Microbe 2023, 4, e58–e59. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.I.; Correia, I.; Seagal, J.; DeGoey, D.A.; Schrimpf, M.R.; Hardee, D.J.; Noey, E.L.; Kati, W.M. Antiviral Drug Discovery for the Treatment of COVID-19 Infections. Viruses 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Khaitov, M.; Nikonova, A.; Shilovskiy, I.; Kozhikhova, K.; Kofiadi, I.; Vishnyakova, L.; Nikolskii, A.; Gattinger, P.; Kovchina, V.; Barvinskaia, E.; et al. Silencing of SARS-CoV-2 with modified siRNA-peptide dendrimer formulation. Allergy 2021, 76, 2840–2854. [Google Scholar] [CrossRef]
- Khaitov, M.; Nikonova, A.; Kofiadi, I.; Shilovskiy, I.; Smirnov, V.; Elisytina, O.; Maerle, A.; Shatilov, A.; Shatilova, A.; Andreev, S.; et al. Treatment of COVID-19 patients with a SARS-CoV-2-specific siRNA-peptide dendrimer formulation. Allergy 2023, 78, 1639–1653. [Google Scholar] [CrossRef]
- Gattinger, P.; Niespodziana, K.; Stiasny, K.; Sahanic, S.; Tulaeva, I.; Borochova, K.; Dorofeeva, Y.; Schlederer, T.; Sonnweber, T.; Hofer, G.; et al. Neutralization of SARS-CoV-2 requires antibodies against conformational receptor-binding domain epitopes. Allergy 2022, 77, 230–242. [Google Scholar] [CrossRef]
- Gattinger, P.; Kratzer, B.; Tulaeva, I.; Niespodziana, K.; Ohradanova-Repic, A.; Gebetsberger, L.; Borochova, K.; Garner-Spitzer, E.; Trapin, D.; Hofer, G.; et al. Vaccine based on folded receptor binding domain-PreS fusion protein with potential to induce sterilizing immunity to SARS-CoV-2 variants. Allergy 2022, 77, 2431–2445. [Google Scholar] [CrossRef] [PubMed]
- Niespodziana, K.; Focke-Tejkl, M.; Linhart, B.; Civaj, V.; Blatt, K.; Valent, P.; van Hage, M.; Grönlund, H.; Valenta, R. A hypoallergenic cat vaccine based on Fel d 1-derived peptides fused to hepatitis B PreS. J Allergy Clin Immunol 2011, 127, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Kemmer, G.; Keller, S. Nonlinear least-squares data fitting in Excel spreadsheets. Nat Protoc 2010, 5, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Ohradanova-Repic, A.; Gebetsberger, L.; Tajti, G.; Kundi, M.; Stockinger, H.; Wiedermann, U.; Grabmeier-Pfistershammer, K. Full seroconversion in initial non-responders with higher antibody levels after heterologous COVID-19 vaccination schedule. Immunol Lett 2022, 250, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Akinfenwa, O.; Huang, H.J.; Linhart, B.; Focke-Tejkl, M.; Vrtala, S.; Poroshina, A.; Nikonova, A.; Khaitov, M.; Campion, N.J.; Eckl-Dorna, J.; et al. Preventive Administration of Non-Allergenic Bet v 1 Peptides Reduces Allergic Sensitization to Major Birch Pollen Allergen, Bet v 1. Front Immunol 2021, 12, 744544. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, V.; Neubauer, A.; Gevaert, P.; Zidarn, M.; Worm, M.; Aberer, W.; Malling, H.J.; Pfaar, O.; Klimek, L.; Pfützner, W.; et al. Safety and efficacy of immunotherapy with the recombinant B-cell epitope-based grass pollen vaccine BM32. J Allergy Clin Immunol 2018, 142, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Zieglmayer, P.; Focke-Tejkl, M.; Schmutz, R.; Lemell, P.; Zieglmayer, R.; Weber, M.; Kiss, R.; Blatt, K.; Valent, P.; Stolz, F.; et al. Mechanisms, safety and efficacy of a B cell epitope-based vaccine for immunotherapy of grass pollen allergy. EBioMedicine 2016, 11, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Ullman-Culleré, M.H.; Foltz, C.J. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci 1999, 49, 319–323. [Google Scholar]
- Stevens, T.L.; Bossie, A.; Sanders, V.M.; Fernandez-Botran, R.; Coffman, R.L.; Mosmann, T.R.; Vitetta, E.S. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 1988, 334, 255–258. [Google Scholar] [CrossRef]
- Souiri, A.; Lemriss, S.; El Maliki, B.; Falahi, H.; El Fahime, E.; El Kabbaj, S. SARS-CoV-2-Neutralizing Antibody Response and Correlation of Two Serological Assays with Microneutralization. Vaccines (Basel) 2023, 11, 590. [Google Scholar] [CrossRef]
- Schwarze, M.; Krizsan, A.; Brakel, A.; Pohl, F.; Volke, D.; Hoffmann, R. Cross-Reactivity of IgG Antibodies and Virus Neutralization in mRNA-Vaccinated People Against Wild-Type SARS-CoV-2 and the Five Most Common SARS-CoV-2 Variants of Concern. Front Immunol 2022, 13, 915034. [Google Scholar] [CrossRef]
- Hueda-Zavaleta, M.; Gómez de la Torre, J.C.; Cáceres-DelAguila, J.A.; Muro-Rojo, C.; De La Cruz-Escurra, N.; Copaja-Corzo, C.; Aragón-Ayala, C.J.; Benítes-Zapata, V.A. Neutralizing Antibodies as Predictors of Vaccine Breakthrough Infection in Healthcare Workers Vaccinated with or without a Heterologous Booster Dose: A Cohort Study during the Third COVID-19 Wave in Peru. Vaccines (Basel) 2023, 11, 447. [Google Scholar] [CrossRef]
- Seekircher, L.; Bánki, Z.; Kimpel, J.; Rössler, A.; Schäfer, H.; Falkensammer, B.; Bante, D.; Forer, L.; Schönherr, S.; Harthaller, T.; et al. Immune response after two doses of the BNT162b2 COVID-19 vaccine and risk of SARS-CoV-2 breakthrough infection in Tyrol, Austria: an open-label, observational phase 4 trial. Lancet Microbe 2023, 4, e612–e621. [Google Scholar] [CrossRef] [PubMed]
- Siskind, G.W.; Paul, W.E.; Benacerraf, B. Studies on the effect of the carrier molecule on antihapten antibody synthesis. I. Effect of carrier on the nature of the antibody synthesized. J Exp Med 1966, 123, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Alt, F.W.; Ferrier, P.; Malynn, B.; Lutzker, S.; Rothman, P.; Berman, J.; Blackwell, K.; Mellis, S.; Pollock, R.; Furley, A.; et al. Control of recombination events during lymphocyte differentiation. Heavy chain variable region gene assembly and heavy chain class switching. Ann N Y Acad Sci 1988, 546, 9–24. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).