Submitted:
15 January 2024
Posted:
16 January 2024
You are already at the latest version
Abstract
Keywords:
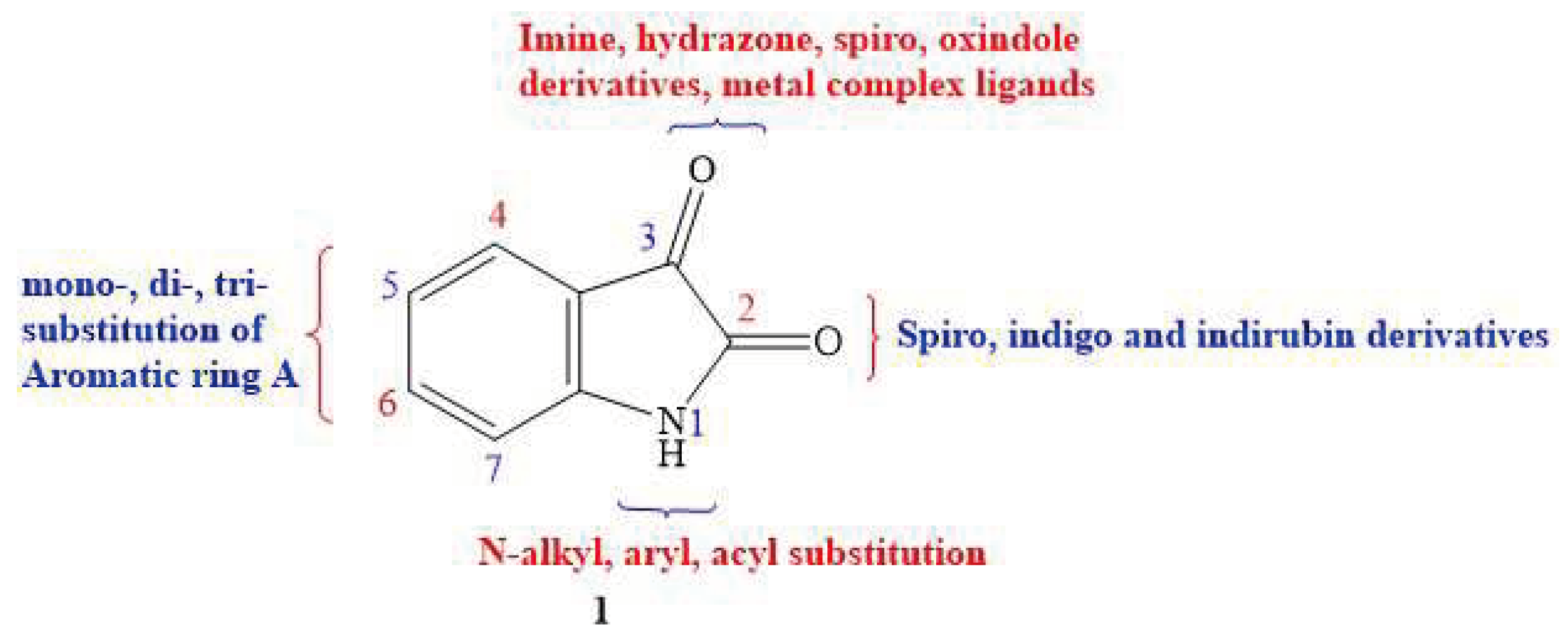
1. Introduction
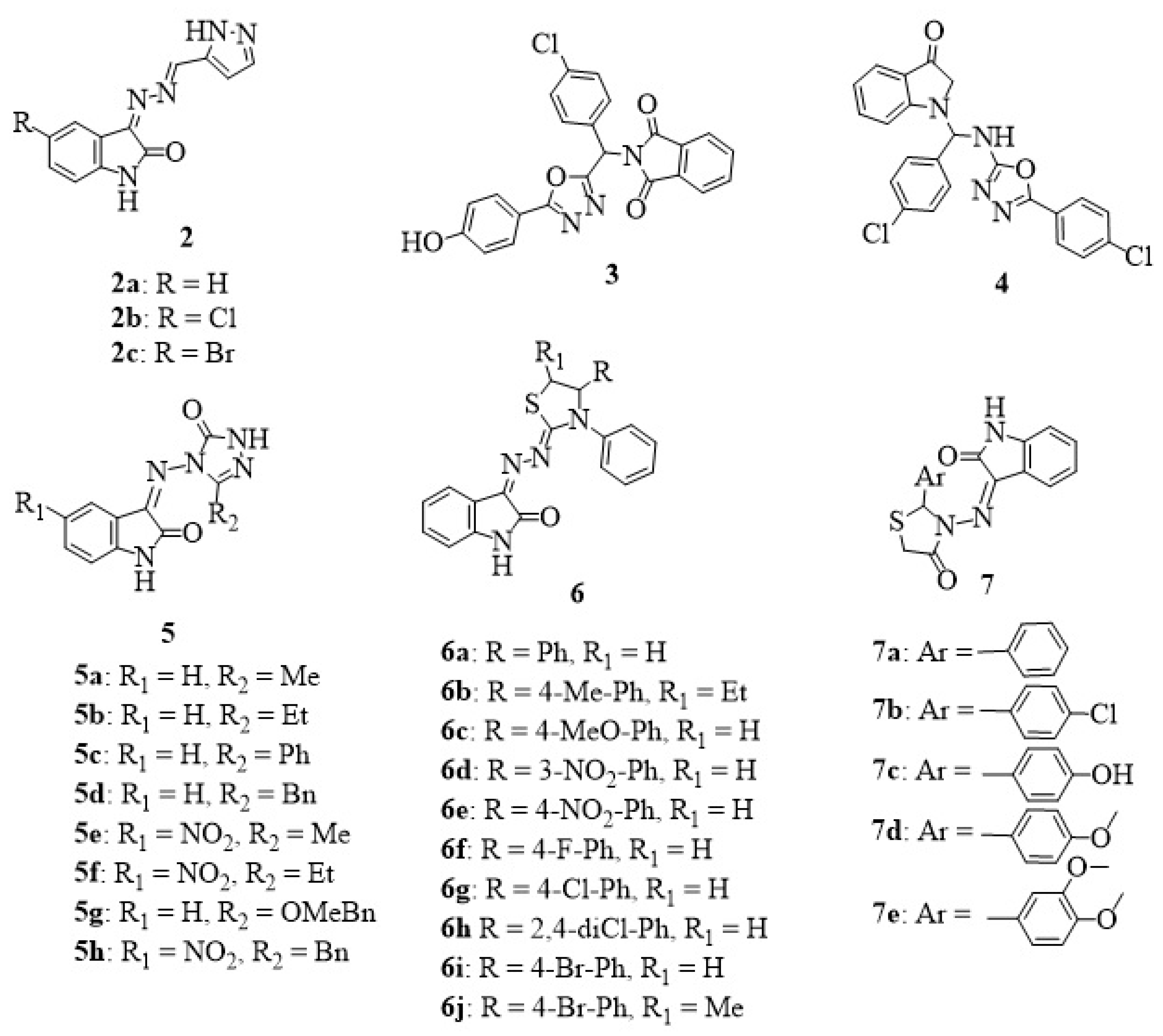
2. Isatin-Azole Hybrids
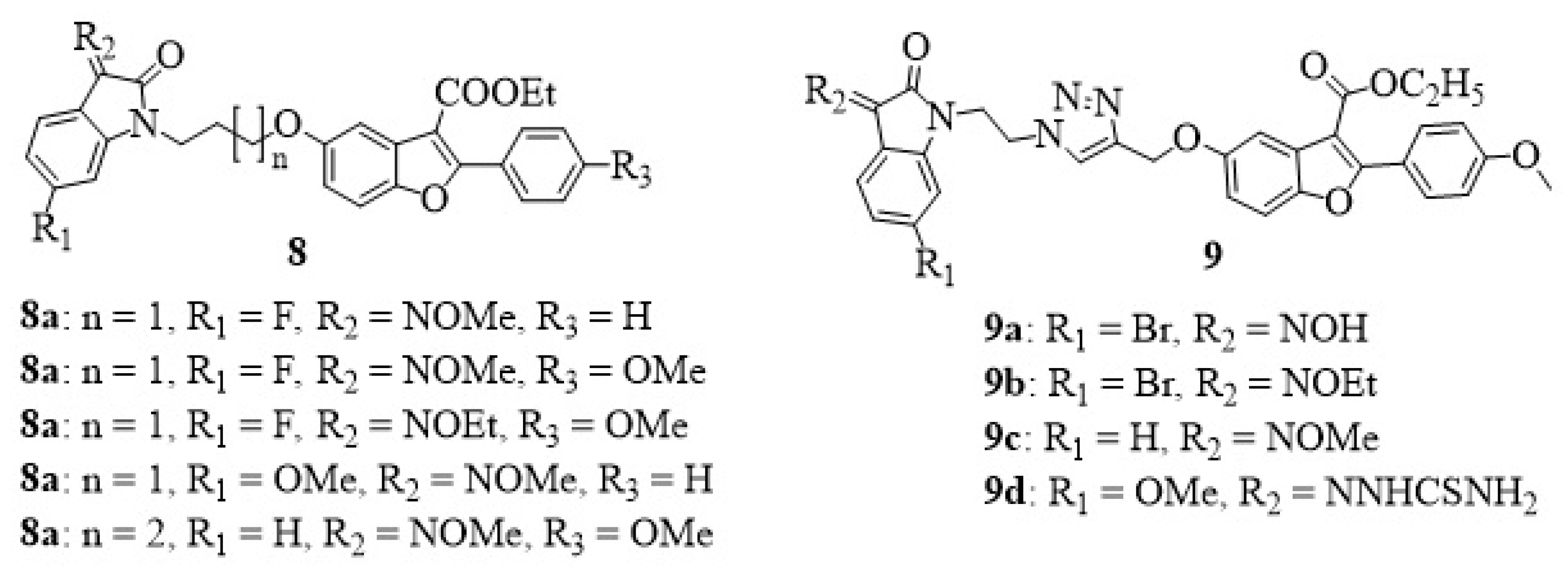
3. Isatin-Furan Hybrids
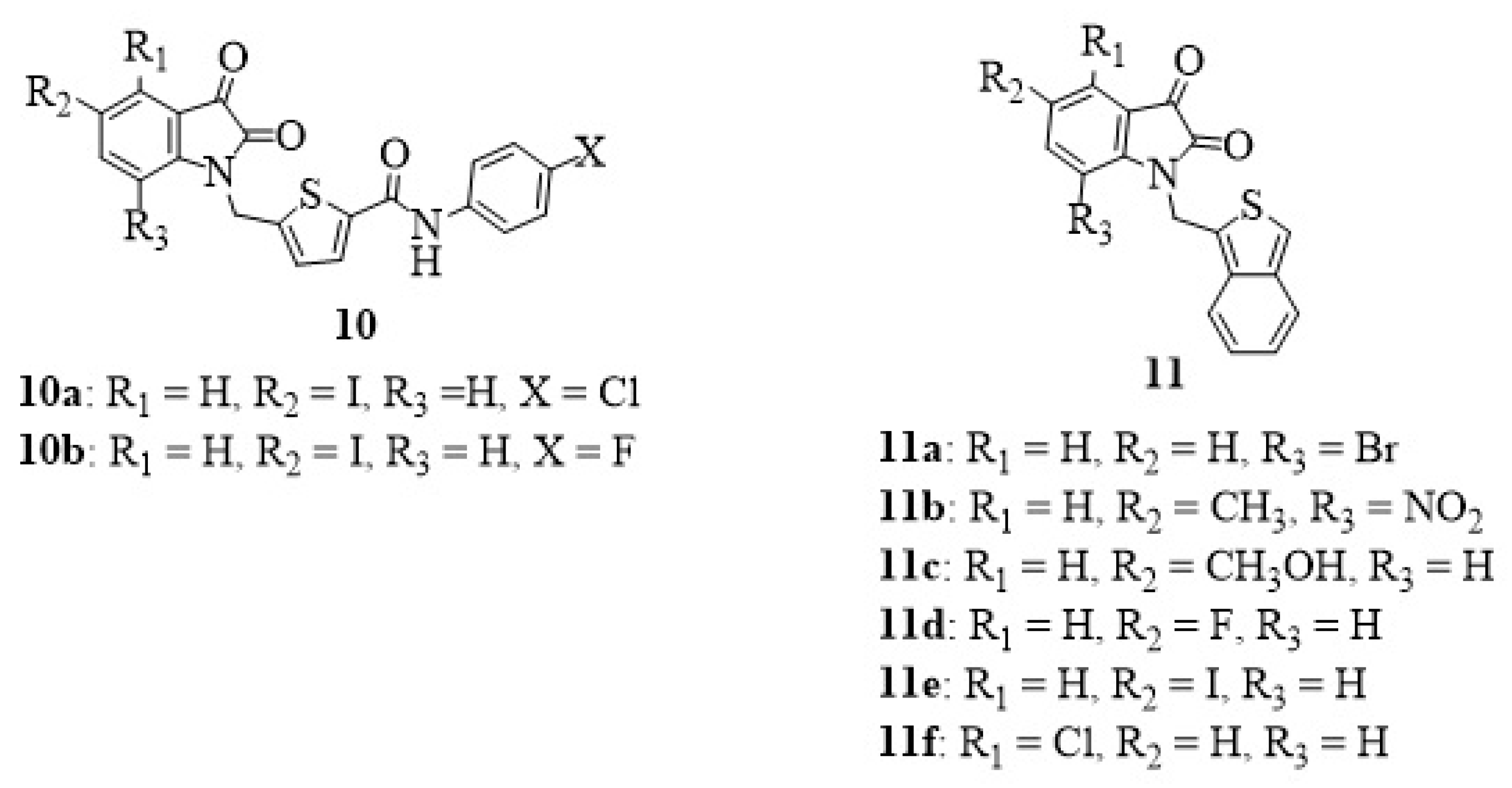
4. Isatin-Thiophene Hybrids
5. Isatin-Indole Hybrids
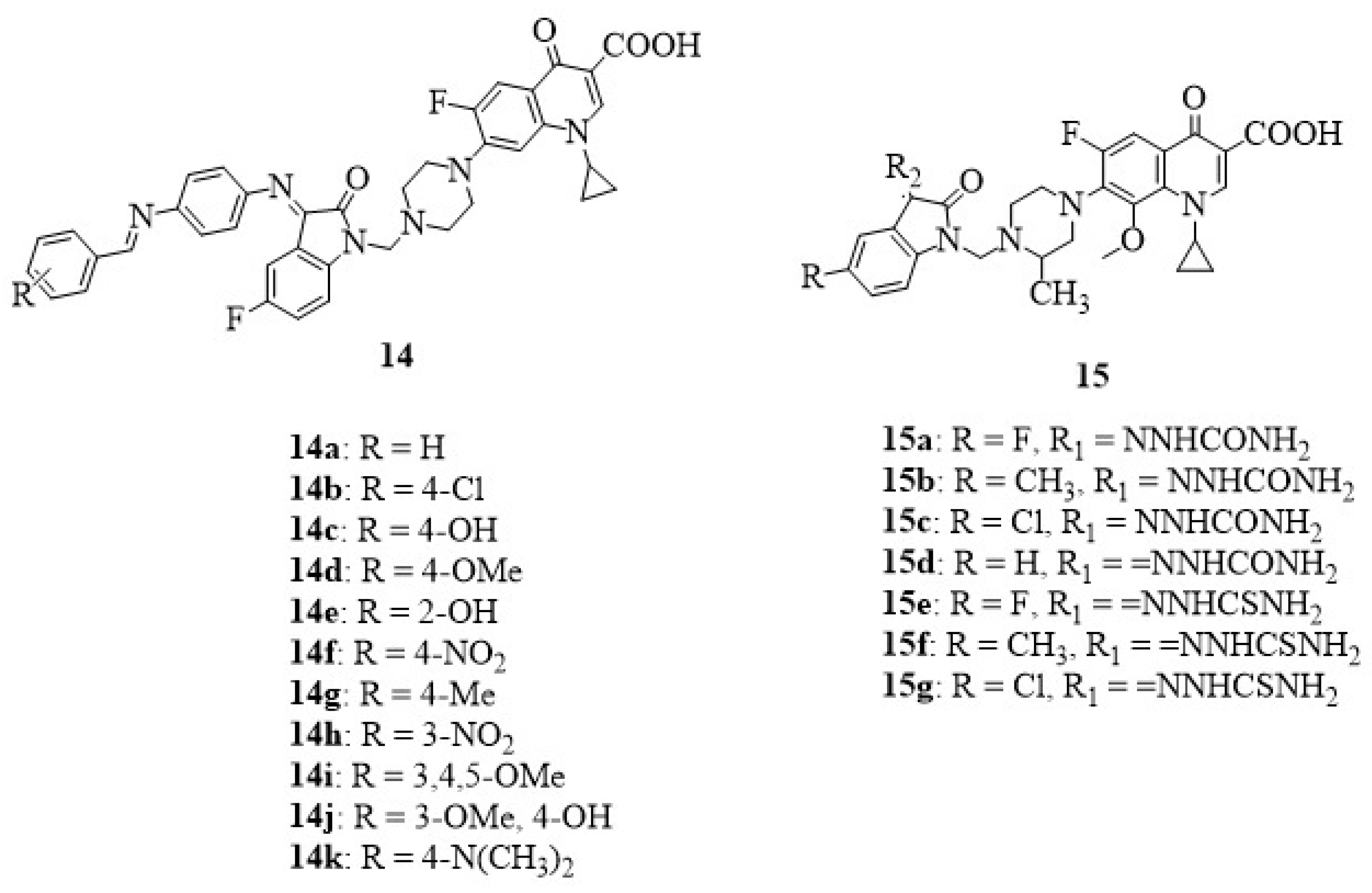
6. Isatin-Fluoroquinolone Hybrids
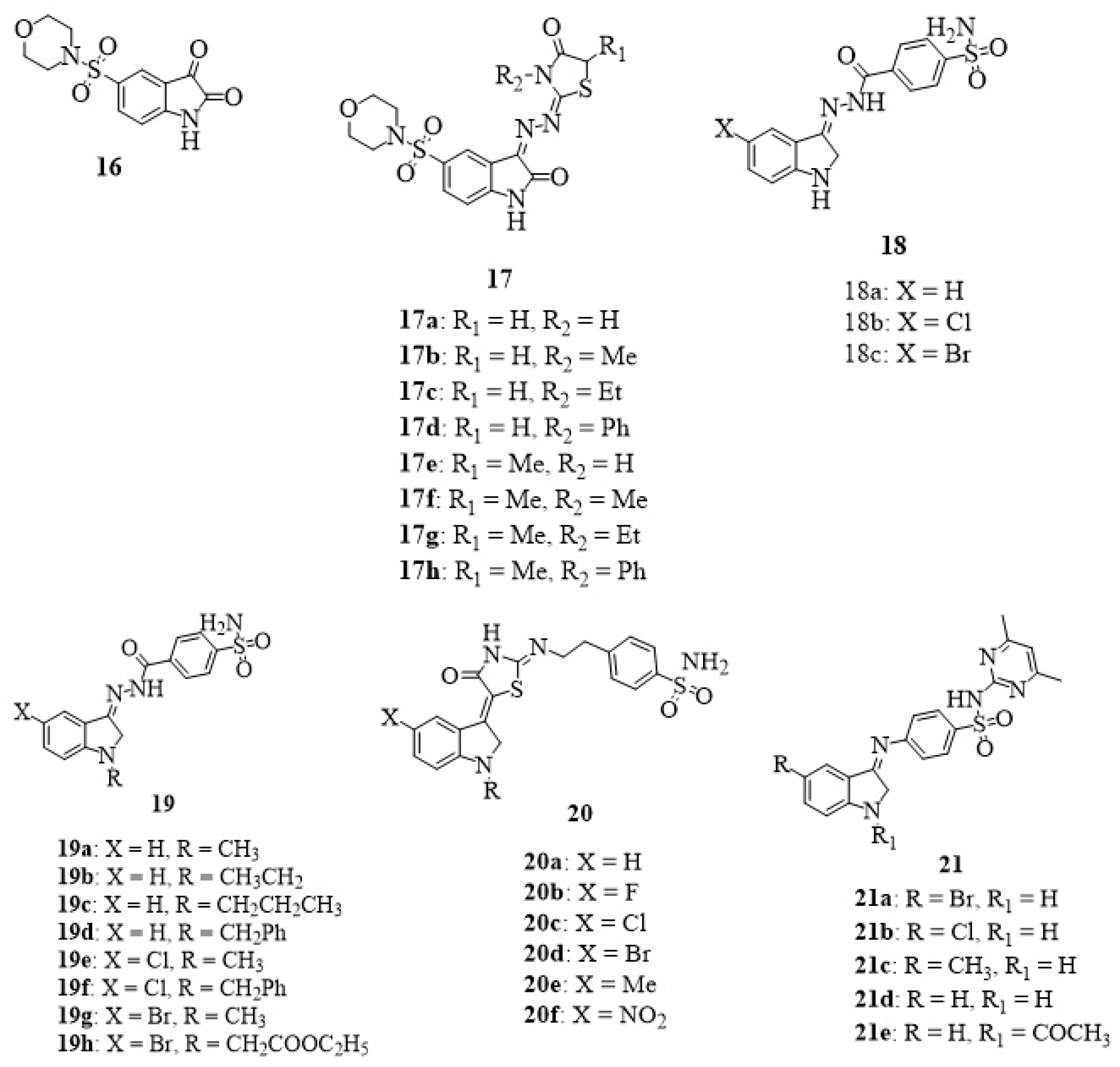
7. Isatin-Sulfonamide Hybrids
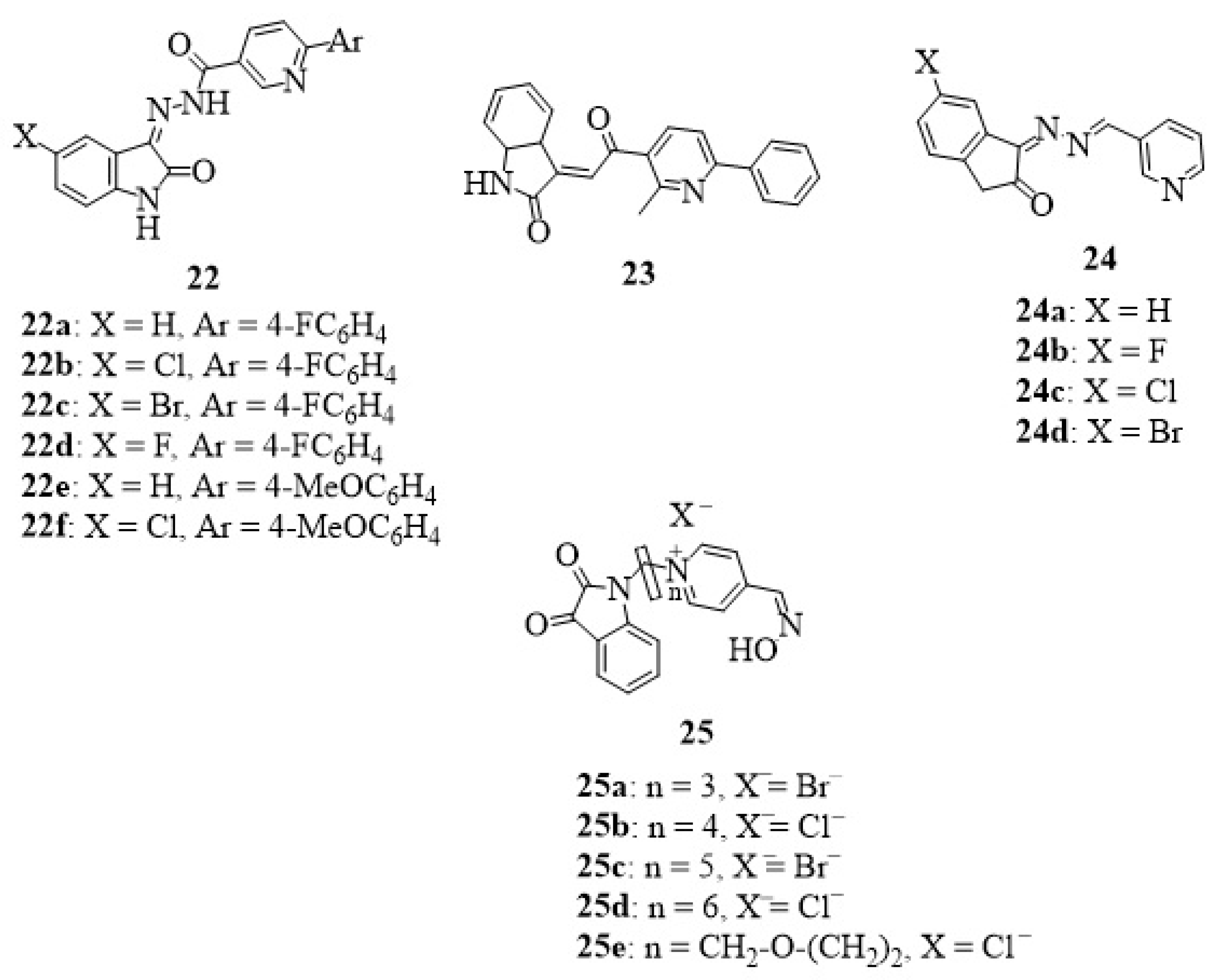
8. Isatin-Pyridine Hybrids
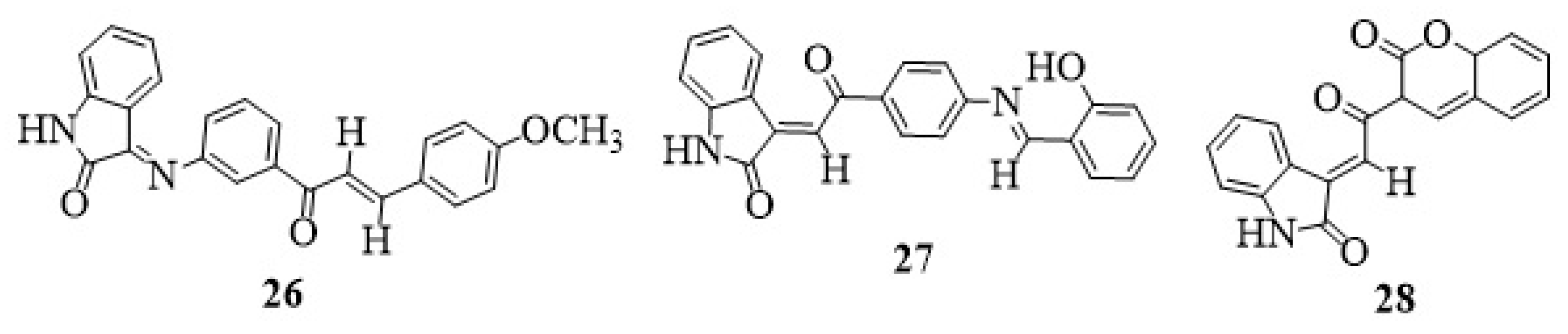
9. Isatin-Chalcone Hybrids
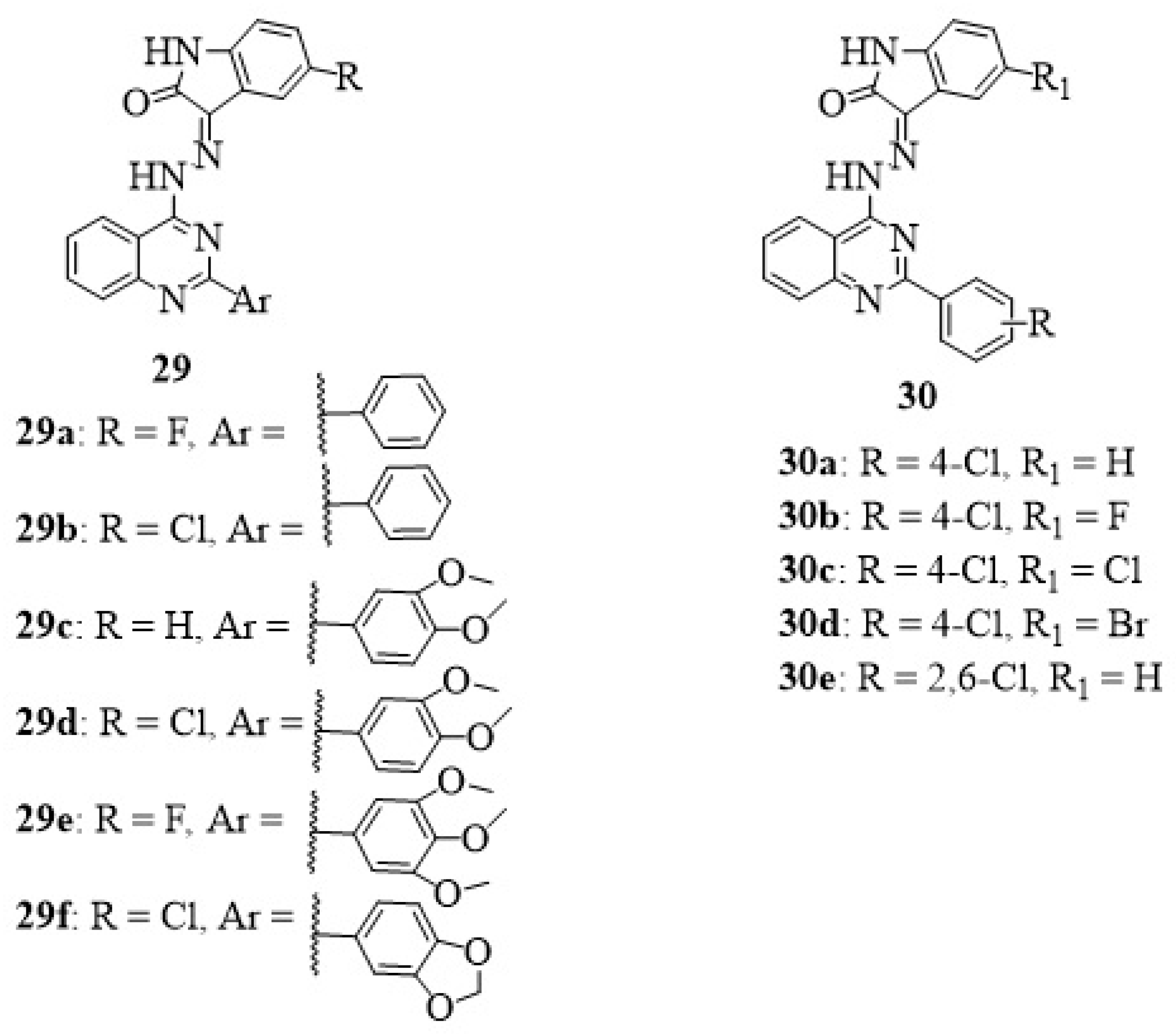
10. Isatin-Quinazoline Hybrids
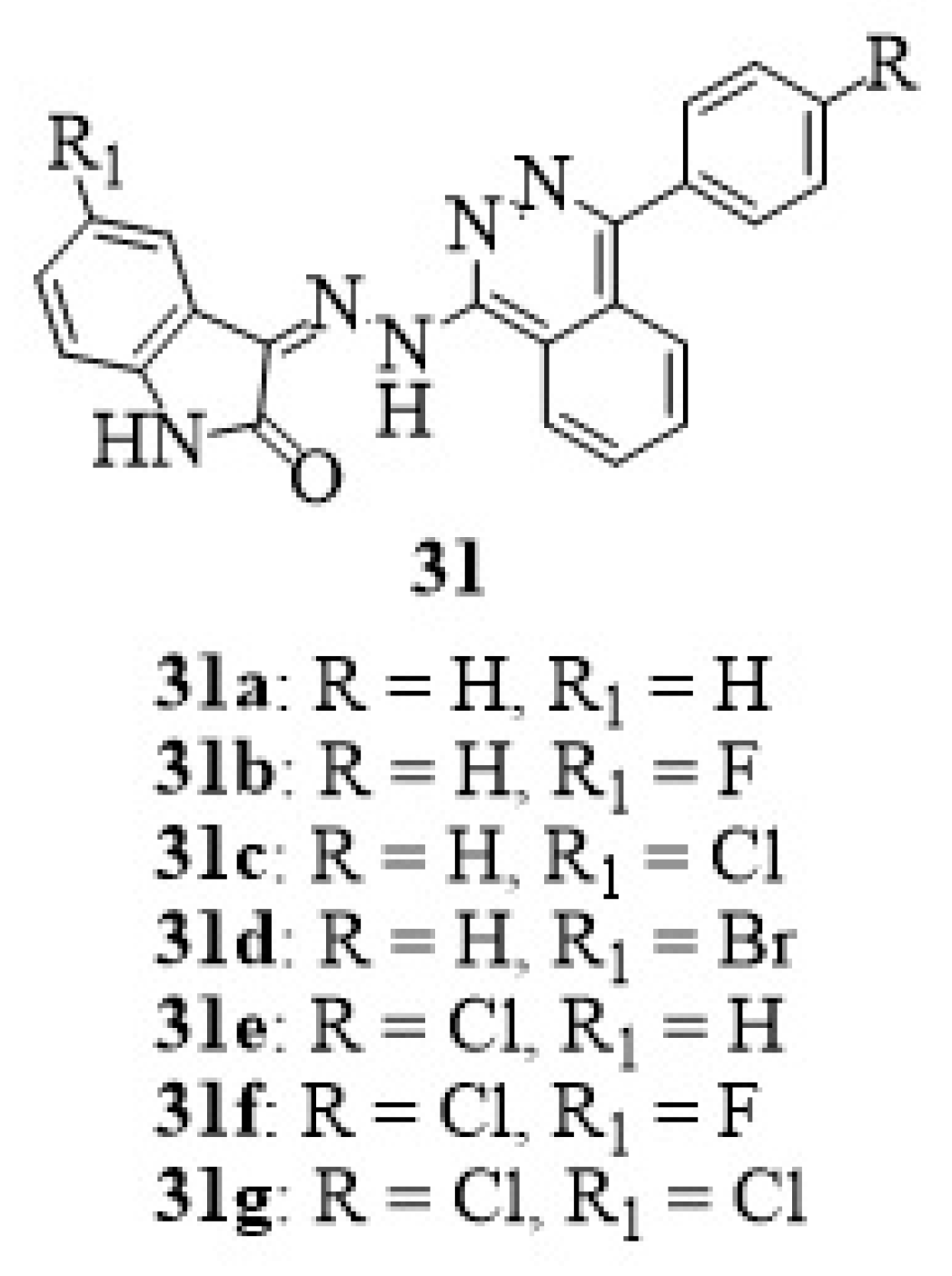
11. Isatin-Pthalazine Hybrids
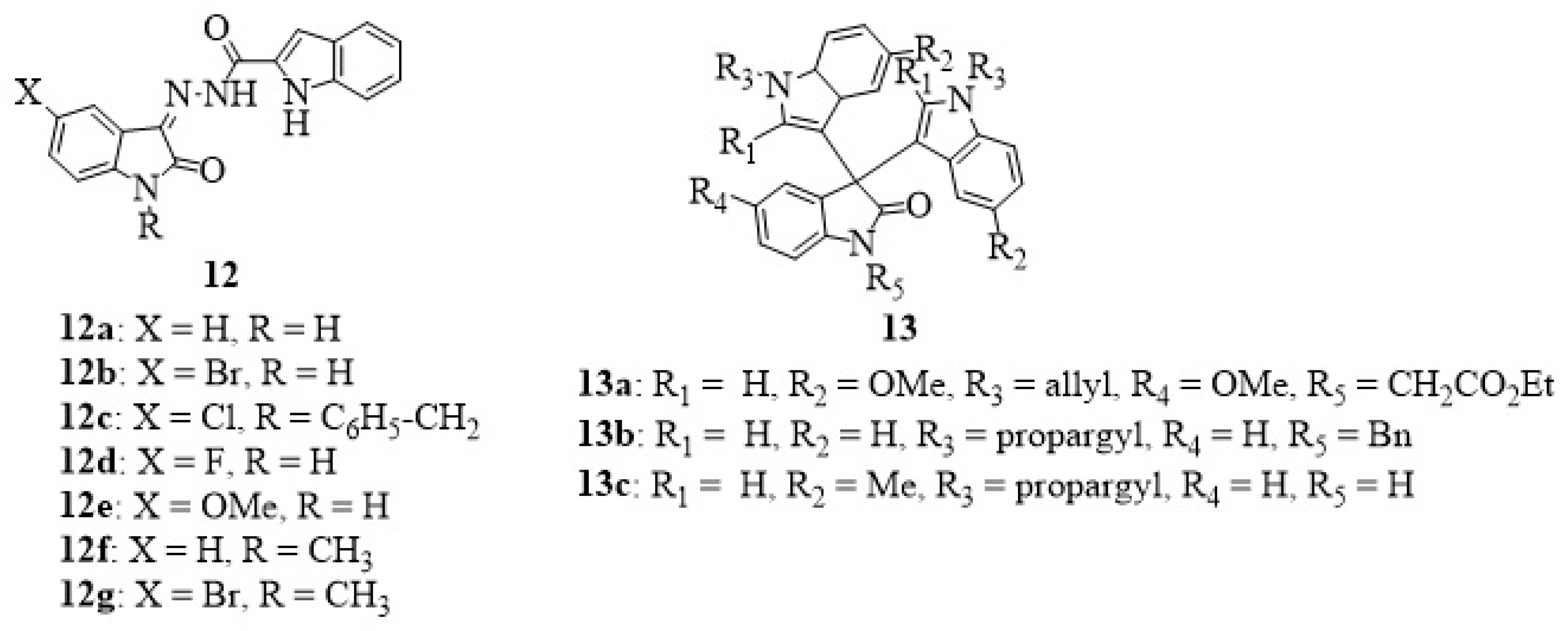
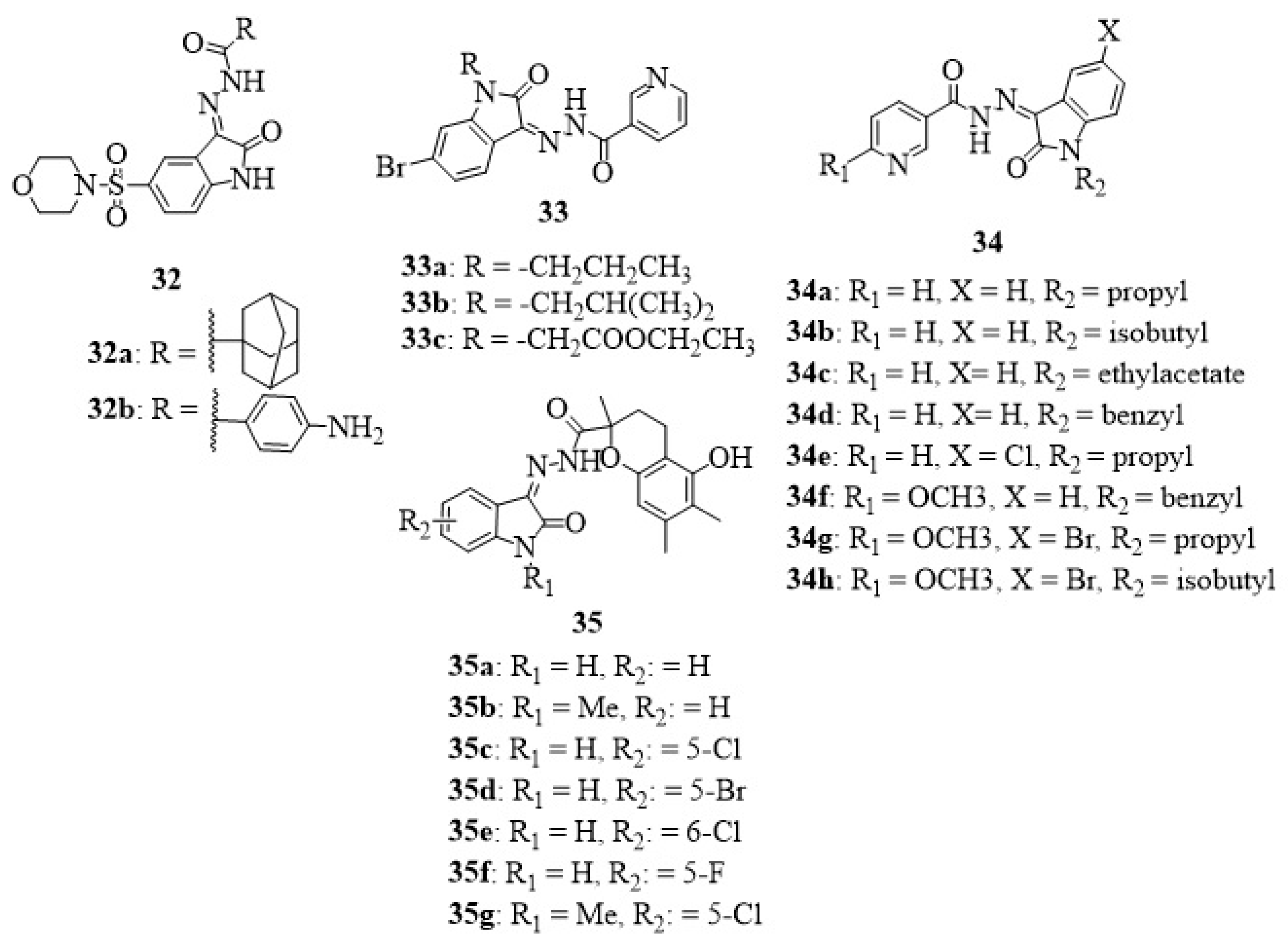
12. Isatin-Hydrazide Hybrids
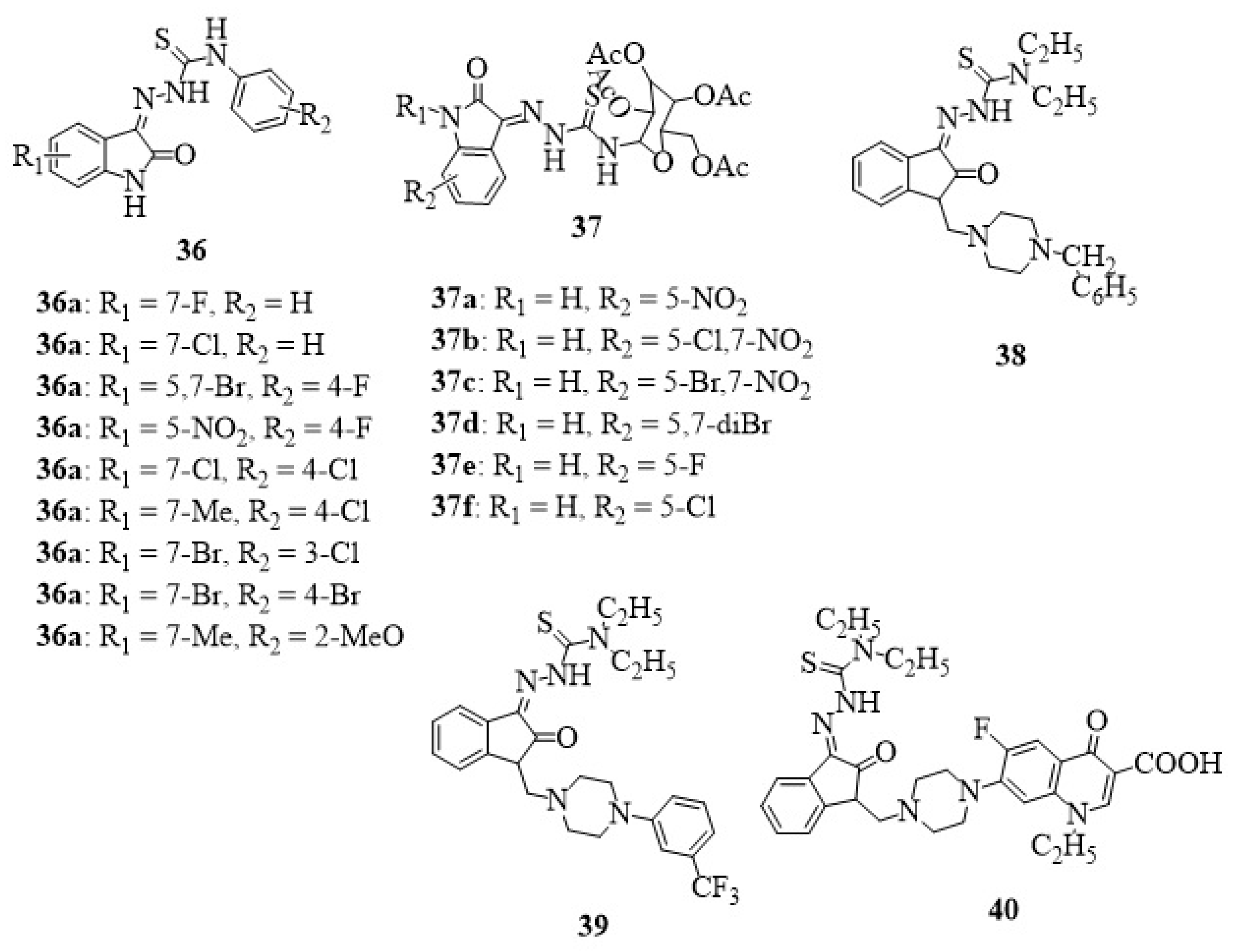
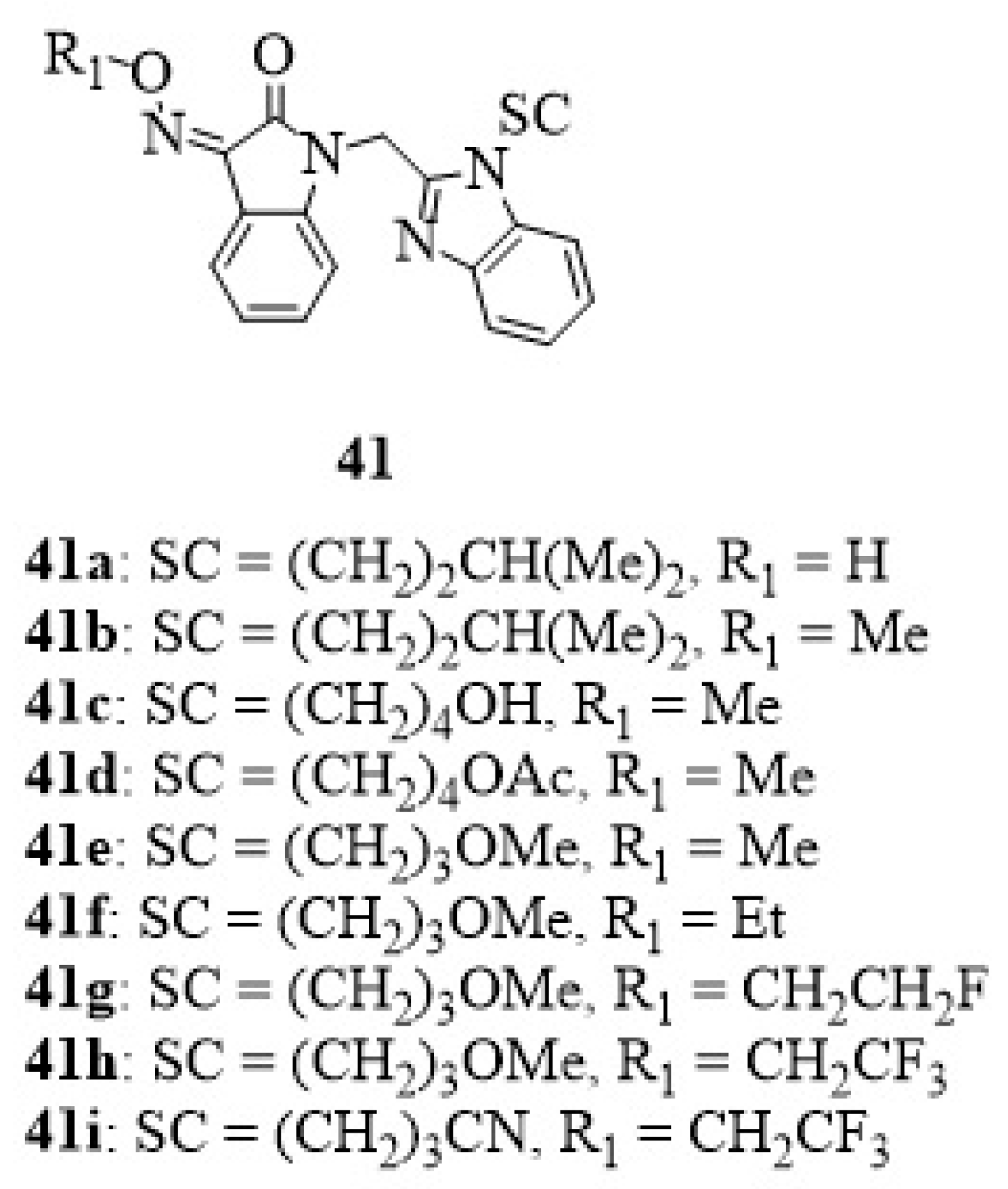
13. Isatin-Thiosemicarbazone Hybrids
14. Isatin-Oxime Hybrids
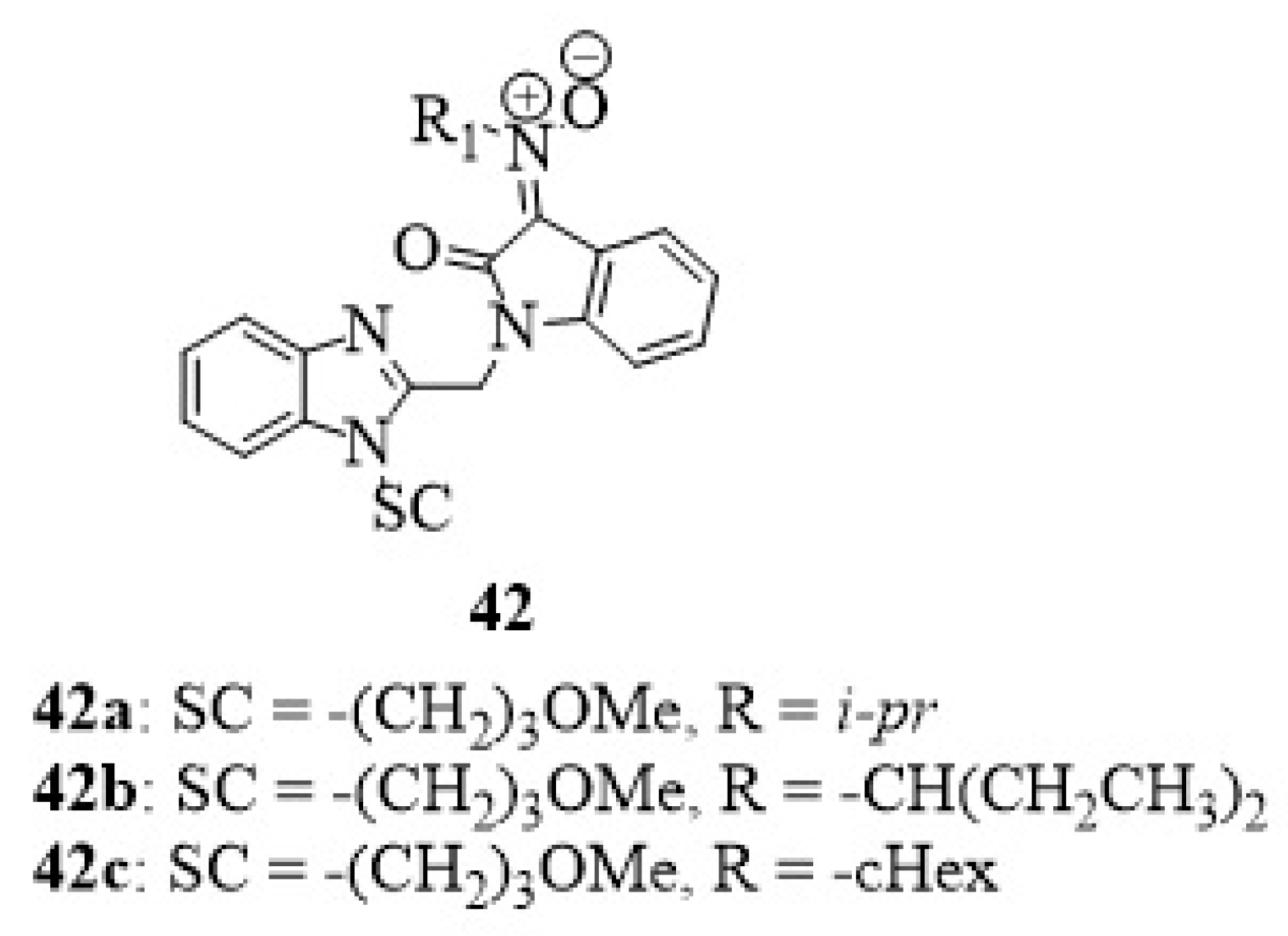
15. Isatin-Nitrone Hybrids
16. Isatin-Piperazine Hybrids
17. Isatin-Uracil Hybrids
18. Isatin-Coumarin Hybrids
19. Isatin-Thiolactone Hybrids
20. Isatin-Pyrimidine Hybrids
21. Isatin-Quinoline Hybrids
22. Isatin-Thioacetazone Hybrids
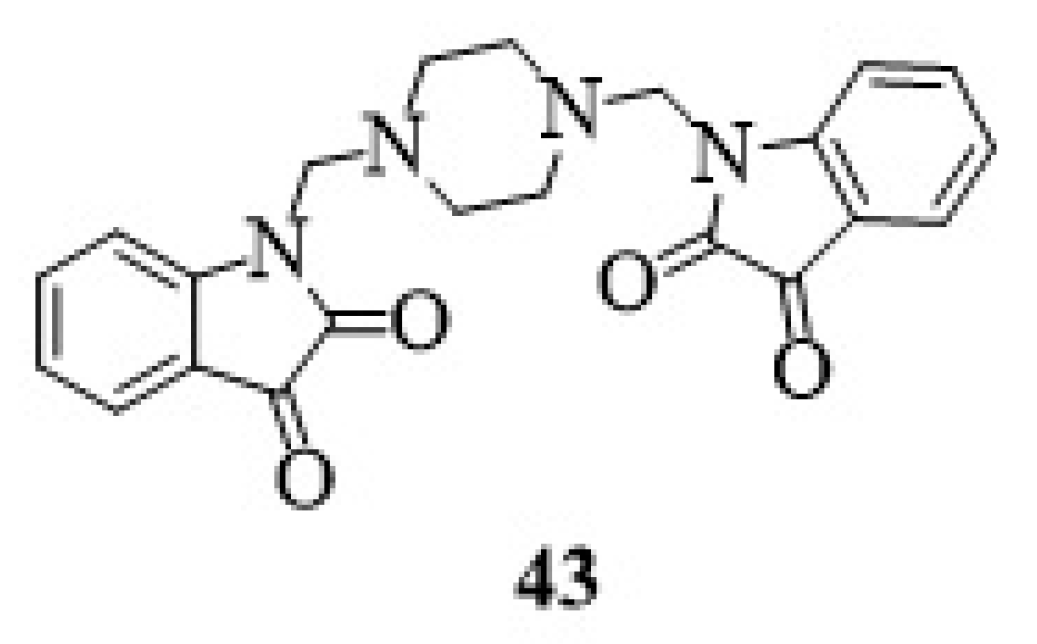
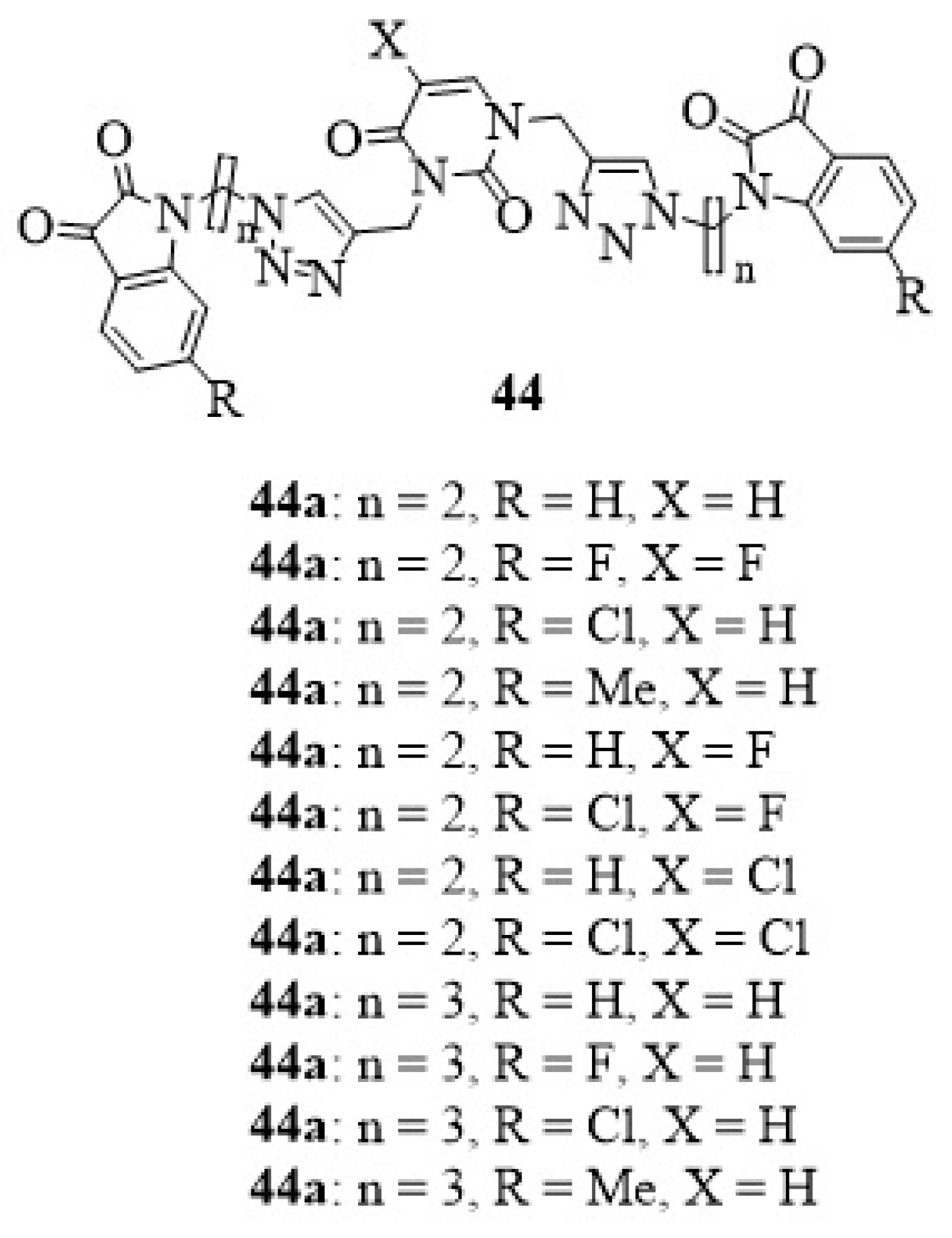
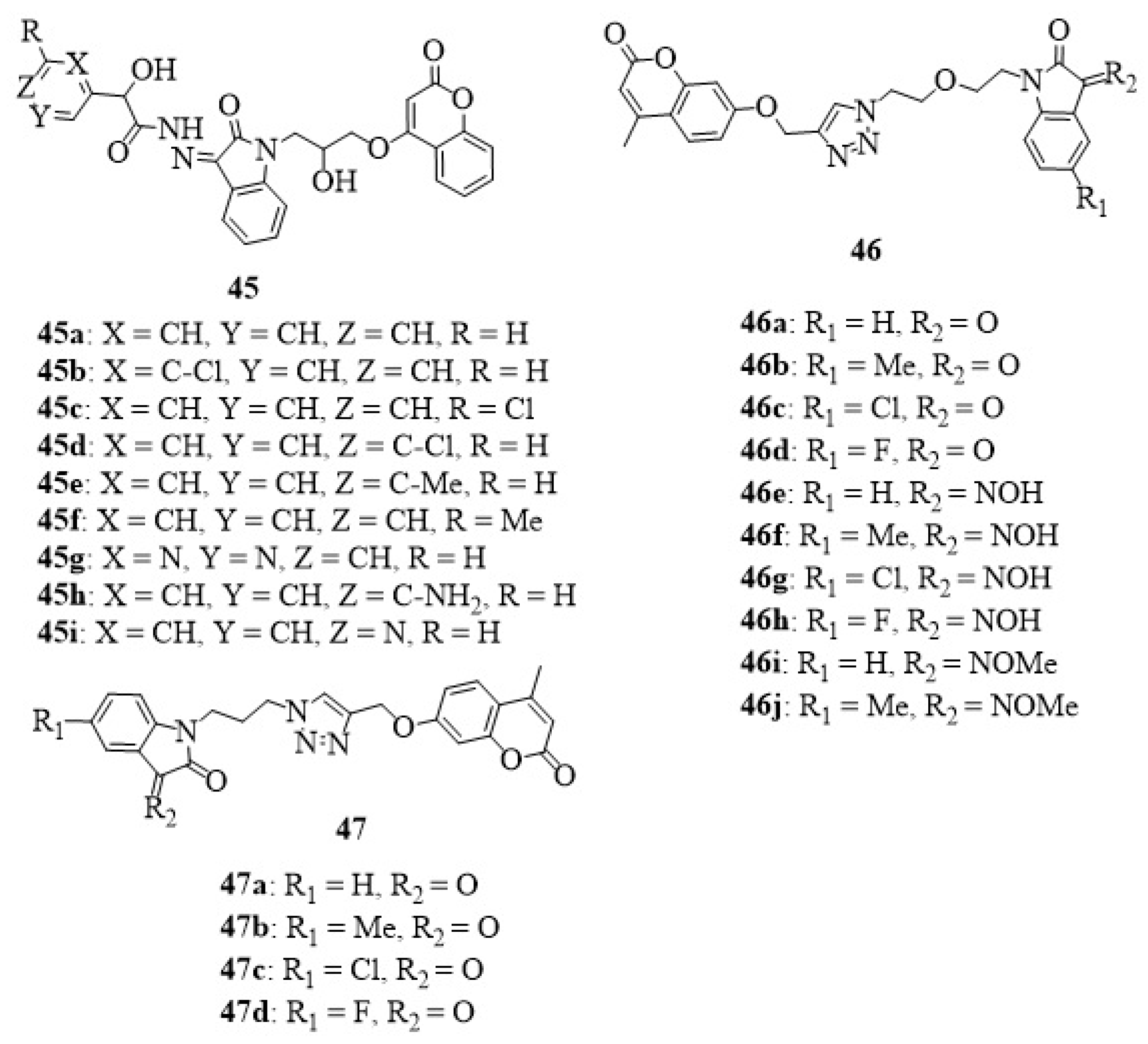
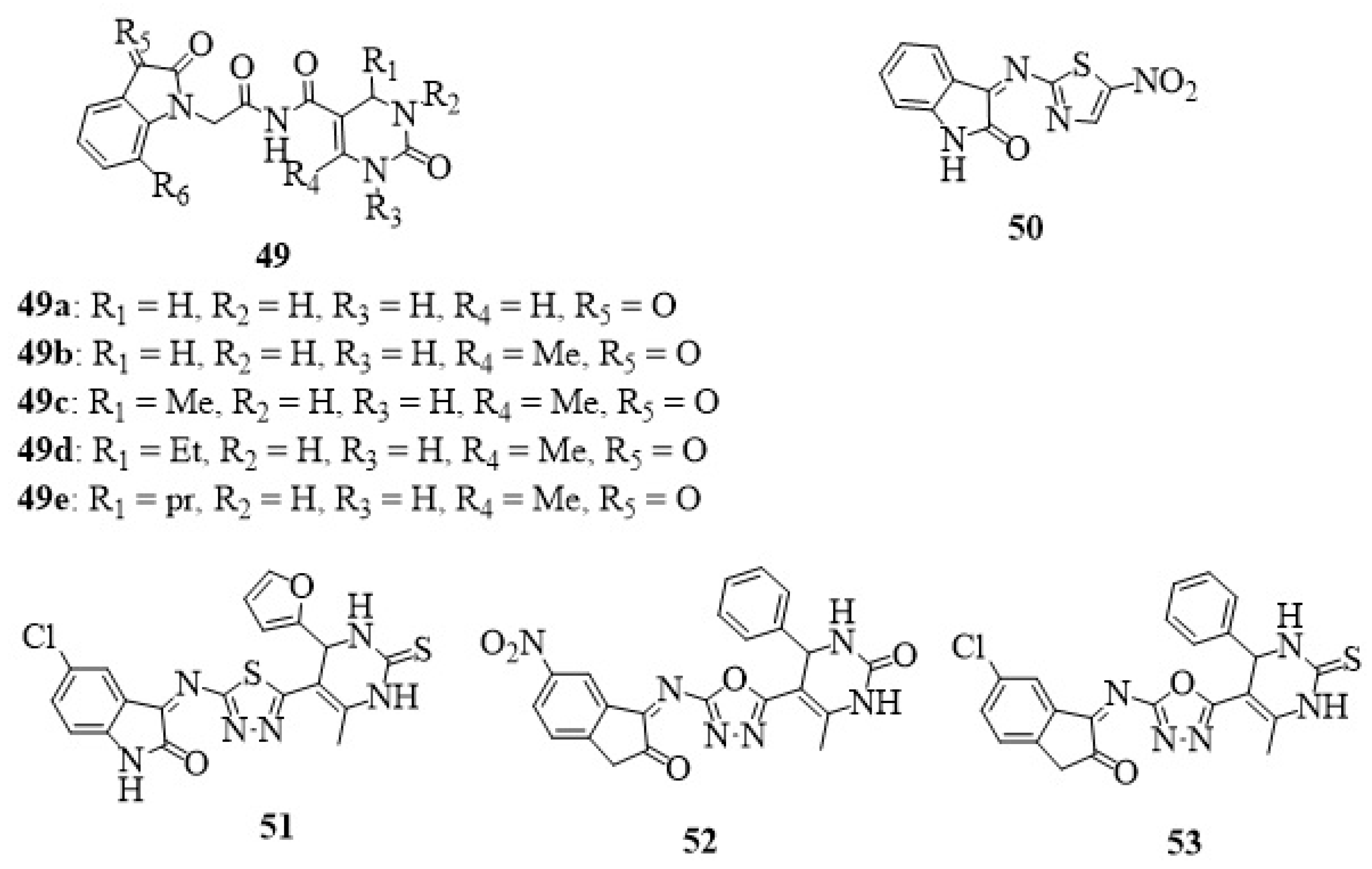
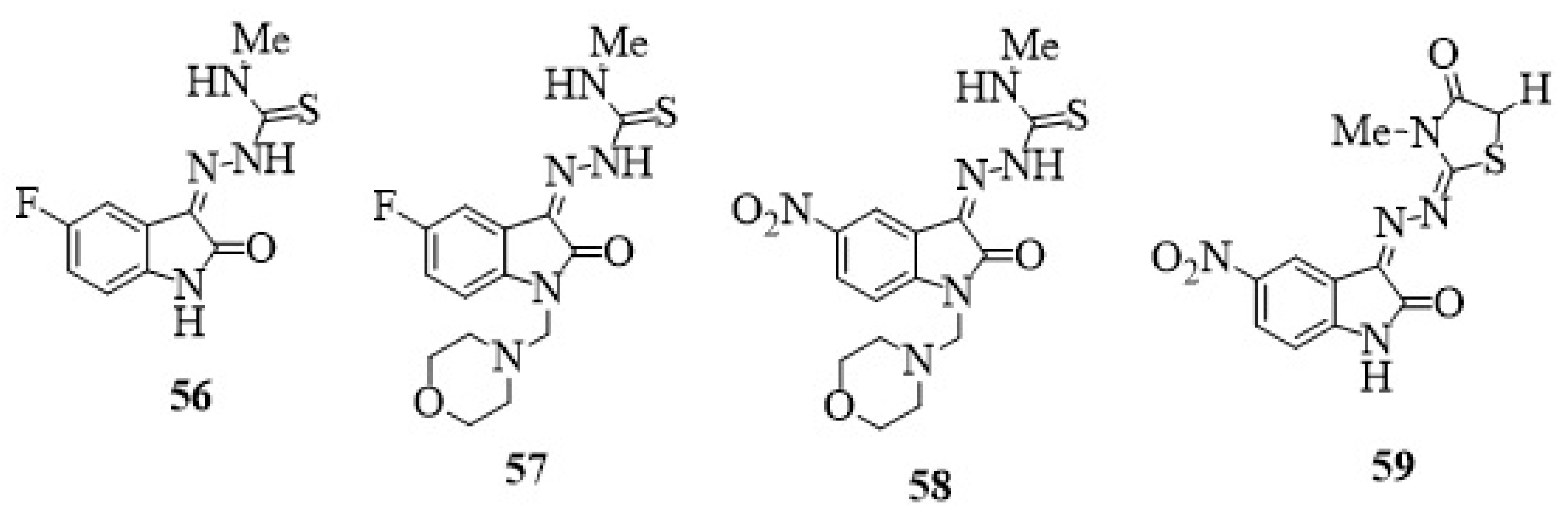
23. Other Isatin Hybrids
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Medvedev, A.; Buneeva, O.; Gnedenko, O.; Ershov, P.; Ivanov, A. Isatin, an endogenous nonpeptide biofactor: A review of its molecular targets, mechanisms of actions, and their biomedical implications. BioFactors 2018, 44, 95–108. [Google Scholar] [CrossRef]
- Shalini, S.C.; Arora, A.; Kumar, V. A mini review on isatin, an anticancer scaffold with potential activities against neglected tropical diseases (NTDs). Pharmaceuticals 2022, 15, 536. [Google Scholar] [CrossRef]
- Sagnou, M.; Mavroidi, B.; Kaminari, A.; Boukos, N.; Pelecanou, M. Novel isatin thiosemicarbazone derivatives as potent inhibitors of β-amyloid peptide aggregation and toxicity. ACS Chemical Neuroscience 2020, 11, 2266–2276. [Google Scholar] [CrossRef]
- Rezki, N.; Almehmadi, M.A.; Ihmaid, S.; Shehata, A.M.; Omar, A.M.; Ahmed, H.E.A.; Aouad, M.A. Novel scaffold hopping of potent benzothiazole and isatin analogues linked to 1,2,3-triazole fragment that mimic quinazoline epidermal growth factor receptor inhibitors: Synthesis, antitumor and mechanistic analyses. Bioorganic Chemistry 2020, 103, 104133. [Google Scholar] [CrossRef]
- Pakravan, P.; Kashanian, S.; Khodaei, M.M.; Harding, F.A. Biochemical and pharmacological characterization of isatin and its derivatives: From structure to activity. Pharmacological Reports 2013, 65, 313–335. [Google Scholar] [CrossRef]
- Chauhan, G.; Pathak, D. P.; Ali, F.; Bhutani, R.; Kapoor, G.; Khasimbi, S. Advances in synthesis, derivatization and bioactivity of isatin: A review. Current Organic Synthesis 2021, 18, 37–74. [Google Scholar] [CrossRef]
- Ding, Z.; Zhou, M.; Zeng, C. Recent advances in isatin hybrids as potential anticancer agents. Archiv der Pharmazie 2020, 353, e1900367. [Google Scholar] [CrossRef]
- Hou, Y.; Shang, C.; Wang, H.; Yun, J. Isatin–azole hybrids and their anticancer activities. Archiv der Pharmazie 2020, 353, e1900272. [Google Scholar] [CrossRef]
- Guo, H. Isatin derivatives and their anti-bacterial activities. European Journal of Medicinal Chemistry 2019, 164, 678–688. [Google Scholar] [CrossRef]
- Nikalje, A.P.; Ansari, A.; Bari, S.; Ugale, V. Synthesis, biological activity, and docking study of novel isatin coupled thiazolidin-4-one derivatives as anticonvulsants. Archiv der Pharmazie 2015, 348, 433–445. [Google Scholar] [CrossRef]
- Smitha, S.; Pandeya, S.N.; Stables, J.P.; Ganapathy, S. Anticonvulsant and sedative-hypnotic activities of n-acetyl / methyl isatin derivatives. Scientia Pharmaceutica 2008, 76, 621–636. [Google Scholar] [CrossRef]
- Chahal, V.; Nirwan, S.; kakkar, R. Isatin and its derivatives: A survey of recent syntheses, reactions, and applications. Medicinal Chemistry Communications 2019, 10, 351. [Google Scholar] [CrossRef]
- Motiwale, M.; Yadav, N.S.; Kumar, S.; Kushwaha, T.; Choudhir, G.; Sharma, S.; Singour, P.K. Finding potent inhibitors for COVID-19 main protease (Mpro): An in silico approach using SARSCoV-3CL protease inhibitors for combating CORONA. Journal of Biomolecular Structure and Dynamics 2022, 40, 1534–1545. [Google Scholar] [CrossRef]
- Badavath, V.N.; Kumar, A.; Samanta, P.K.; Maji, S.; Das, A.; Blum, G.; Jha, A.; Sen, A. Determination of potential inhibitors based on isatin derivatives against SARS-CoV-2 main protease (Mpro): A molecular docking, molecular dynamics and structure-activity relationship studies. Journal of Biomolecular Structure and Dynamics 2022, 40, 3110–3128. [Google Scholar] [CrossRef]
- Bal, T.R.; Anand, B.; Yogeeswari, P.; Sriram, D. Synthesis and evaluation of anti-HIV activity of isatin beta-thiosemicarbazone derivatives. Bioorganic & Medicinal Chemistry Letters 2005, 15, 4451–4455. [Google Scholar] [CrossRef]
- Sriram, D.; Yogeeswari, P.; Meena, K. Synthesis, anti-HIV and antitubercular activities of isatin derivatives. Pharmazie 2006, 61, 274–277. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, S.; Gao, C.; Fan, J.; Zhao, F.; Lv, Z.; Feng, L. Isatin hybrids and their anti-tuberculosis activity. Chinese Chemical Letters 2017, 28, 159–167. [Google Scholar] [CrossRef]
- Gao, F.; Ye, L.; Wang, Y.; Kong, F.; Zhao, S.; Xiao, J.; Huang, G. Benzofuran-isatin hybrids and their in vitro anti-mycobacterial activities against multi-drug resistant Mycobacterium tuberculosis. European Journal of Medicinal Chemistry 2019, 183, 111678. [Google Scholar] [CrossRef]
- Gao, F.; Chen, Z.; Ma, L.; Fan, Y.; Chen, L.; Lu, G. Synthesis and biological evaluation of moxifloxacin-acetyl-1,2,3-1Htriazole-methylene-isatin hybrids as potential anti-tubercular agents against both drug-susceptible and drug-resistant Mycobacterium tuberculosis strains. European Journal of Medicinal Chemistry 2019, 180, 648–655. [Google Scholar] [CrossRef]
- Thanh, N.D.; Giang, N.T.K.; Quyen, T.H.; Huong, D.T.; Toan, V.N. Synthesis and evaluation of in vivo antioxidant, in vitro antibacterial, MRSA and antifungal activity of novel substituted isatin N-(2,3,4,6-tetra-O-acetyl-beta-D-glucopyranosyl)thiosemicarbazones. European Journal of Medicinal Chemistry 2016, 123, 532–543. [Google Scholar] [CrossRef]
- Akdemir, A.; Güzel-Akdemir, Ö.; Karali, N.; Supuran, C.T. Isatin analogs as novel inhibitors of Candida spp. beta-carbonic anhydrase enzymes. Bioorganic & Medicinal Chemistry 2016, 24, 1648–1652. [Google Scholar] [CrossRef]
- Susithra, E.; Rajkumar, S.; Pansare, S.K.W.; Praveena, S.; Arun, P.V.P.S. Design, synthesis, antimicrobial and anticancer activity of some novel benzoxazole-isatin conjugates. Biointerface Research in Applied Chemistry 2022, 12, 2392–2403. [Google Scholar] [CrossRef]
- Tangadanchu, V.K.R.; Sui, Y.; Zhou, C. Isatin-derived azoles as new potential antimicrobial agents: Design, synthesis and biological evaluation. Bioorganic & Medicinal Chemistry Letters 2021, 41, 128030. [Google Scholar] [CrossRef]
- Muglu, H.; Cavus, M.S.; Bakir, T.; Yakan, H. Synthesis, characterization, quantum chemical calculations and antioxidant activity of new bis-isatin carbohydrazone and thiocarbohydrazone derivatives. Journal of Molecular Structure 2019, 1196, 819–827. [Google Scholar] [CrossRef]
- Wakchaure, N.D. Review on common methods to synthesize substituted 1H-indole-2,3-dione (isatin) derivatives and their medicinal significance. American Journal of Pharmtech Research 2012, v, 289–310. [Google Scholar]
- Nisha; Gut, J.; Rosenthal, P.J.; Kumar, V. β-amino-alcohol tethered 4-aminoquinoline-isatin conjugates: Synthesis and antimalarial evaluation. European Journal of Medicinal Chemistry 2014, 24, 566–573. [Google Scholar] [CrossRef]
- Raj, R.; Gut, J.; Rosenthal, P.J.; Kumar, V. 1H-1,2,3-Triazole-tethered isatin-7-chloroquinoline and 3-hydroxy-indole-7-chloroquinoline conjugates: Synthesis and antimalarial evaluation. Bioinorganic and Medicinal Chemistry Letters 2014, 24, 756–759. [Google Scholar] [CrossRef]
- Sharma, P.K.; Balwani, S.; Mathur, D.; Malhotra, S.; Singh, B.K.; Prasad, A.K.; Len, C.; Eycken, E.V.V.; Ghosh, B.; Richards, N.G.; Parmar, V.S. Synthesis and anti-inflammatory activity evaluation of novel triazolyl-isatin hybrids. Journal of Enzyme Inhibition and Medicinal Chemistry 2016, 31, 1520–1526. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Elsaman, T.; Al-Nour, M.Y. Synthesis, anti-inflammatory activity, and in silico study of novel diclofenac and isatin conjugates. International Journal of Medicinal Chemistry 2018, 2018, 9139786. [Google Scholar] [CrossRef]
- Abdulrahmana, S.H.; Al-healya, F.M.; Ali, W.K. A review on computational study of tribulin compound and its derivatives: QSAR studies. Annals of Romanian Society for Cell Biology 2021, 25, 8725–8735. [Google Scholar]
- Ma, T.; Chen, R.; Xue, H.; Miao, Z.; Chen, L.; Zhang, H.; Shi, X. Di-isatin heteronuclear compounds and their antibacterial activity. Journal of Heterocyclic Chemistry 2019, 57, 503–509. [Google Scholar] [CrossRef]
- Xu, J.-H.; Fan, Y.L.; Zhou, J. Quinolone–triazole hybrids and their biological activities. Journal of Heterocyclic Chemistry 2018, 55, 1854–1862. [Google Scholar] [CrossRef]
- Song, F.; Li, Z.; Bian, Y.; Huo, X.; Fang, J.; Shao, L.; Zhou, M. Indole/isatin-containing hybrids as potential antibacterial agents. Archiv der Pharmazie 2020, 353, e2000143. [Google Scholar] [CrossRef]
- M, R.K.; Gideon, D.A.; Mariadasse, R.; Nirusimhan, V.; A, S.R.; Edward, J.C.; Jeyaraman, J.; Dhayabaran, V. In silico evaluation of isatin-based derivatives with RNA-dependent RNA polymerase of the novel coronavirus SARS-CoV-2. Journal of Biomolecular Structure and Dynamics 2022, 40, 6710–6724. [Google Scholar] [CrossRef]
- Afroz, M.; Vasanthi, R.; Fathima, A. A review on medicinal importance of isatin scaffolds with anti-mycobacterial activity. Journal of Cardiovascular Disease Research 2021, 12, 1155–1170. [Google Scholar]
- Freitas, L.A.B.; Santos, A.C.S.; Silva, G.D.C.; Albuquerque, F.N.N.; Silva, E.D.; Simone, C.A.; Pereira, V.R.A.; Alves, L.C.; Gomes, P.A.T.M. Structural improvement of new thiazolyl-isatin derivatives produces potent and selective trypanocidal and leishmanicidal compounds. Chemico-Biological Interactions 2021, 345, 109561. [Google Scholar] [CrossRef]
- Babita, A.; Khan, N.S.; Khan, P.; Queen, A.; Hussain, A.; Rehman, M.T.; Alajmi, M.F.; El-seedi, H.; Ali, S.; Hassan, M.I.; Abid, M. Design and development of Isatin-triazole hydrazones as potential inhibitors of microtubule affinity-regulating kinase 4 for the therapeutic management of cell proliferation and metastasis. European Journal of Medicinal Chemistry 2019, 163, 840–852. [Google Scholar] [CrossRef]
- Jiang, Y.; Qian, A.; Li, Y. 1H-1,2,3-Triazole tethered isatin-moxifloxacin: Design, synthesis and in vitro anti-mycobacterial evaluation. Archiv der Pharmazie 2019, 352, 1900040. [Google Scholar] [CrossRef]
- Gao, F.; Wang, T.; Gao, M.; Zhang, X.; Liu, Z.; Zhao, S.; Lv, Z.; Xiao, J. Benzofuran-isatin-imine hybrids tethered via different length alkyl linkers: Design, synthesis and in vitro evaluation of anti-tubercular and anti-bacterial activities as well as cytotoxicity. European Journal of Medicinal Chemistry 2019, 165, 323–331. [Google Scholar] [CrossRef]
- Liang, P.-H. Characterization and inhibition of SARS-coronavirus main protease. Current Topics in Medicinal Chemistry 2006, 6, 361–376. [Google Scholar] [CrossRef]
- Al-Wabli, R.I.; Almomen, A.A.; Almutari, M.S.; Keeton, A.B.; Piazza, G.A.; Attia, MI. New isatin–indole conjugates: Synthesis, characterization, and a plausible mechanism of their in vitro antiproliferative activity. Drug Design, Development and Therapy 2020, 14, 483–495. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.; Lv, Z.; Gao, F.; Wang, Y.; Zhang, F.; Bai, L.; Deng, J. Fluoroquinolone-isatin hybrids and their biological activities. European Journal of Medicinal Chemistry 2019, 162, 396–406. [Google Scholar] [CrossRef]
- Limpachayaporn, P.; Wagner, S.; Kopka, K.; Schober, M.S.; Haufe, G. Synthesis of 7-halogenated isatin sulfonamides: Nonradioactive counterparts of caspase-3/-7 inhibitor-based potential radiopharmaceuticals for molecular imaging of apoptosis. Journal of Medicinal Chemistry 2014, 57, 9383–9395. [Google Scholar] [CrossRef]
- Selvam, P.; Chandramohan, M.; Hurst, B.; Smee, D.F. Activity of isatine-sulfadimidine derivatives against 2009 pandemic H1N1 influenza virus in cell culture. Antiviral Chemistry & Chemotherapy 2010, 20, 143–145. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Altoukhy, A.; Mahrous, H.; Abdel-Aziz, H.A. Design, synthesis and QSAR study of certain isatin-pyridine hybrids as potential antiproliferative agents. European Journal of Medicinal Chemistry 2015, 90, 684–694. [Google Scholar] [CrossRef]
- Kitagawa, D.A.S.; Rodrigues, R.F.; Silva, T.N.; Santos, W.V.; Rocha, V.C.V.; Almeida, J.S.F.D.; Bernardo, L.B.; Carvalho-Silva, T.; Nepovimova, E.; Kuca, K.; Franca, T.C.C.; Cavalcante, S.F.A. Design, synthesis, in silico studies and in vitro evaluation of isatin-pyridine oximes hybrids as novel acetylcholinesterase reactivators. Journal of Enzyme Inhibition and Medicinal Chemistry 2021, 36, 1370–1377. [Google Scholar] [CrossRef]
- Devale, T.L.; Parikh, J.; Miniyar, P.; Sharma, P.; Shrivastava, B.; Murumkar, P. Dihydropyrimidinone-isatin hybrids as novel non-nucleoside HIV-1 reverse transcriptase inhibitors. Bioorganic Chemistry 2017, 70, 256–266. [Google Scholar] [CrossRef]
- Elsayed, Z.M.; Eldehna, W.M.; Abdel-Aziz, M.; Hassab, M.A.E.; Elkaeed, E.B.; Al-Warhi, T.; Abdel-Aziz, H.; Abou-seri, S.; Mohammed, E.R. Development of novel isatin–nicotinohydrazide hybrids with potent activity against susceptible/ resistant Mycobacterium tuberculosis and bronchitis causing–bacteria. Journal of Enzyme Inhibition and Medicinal Chemistry 2021, 36, 384–393. [Google Scholar] [CrossRef]
- Fayed, E.A.; Eldin, R.R.E.; Mehany, A.B.M.; Bayoumi, A.H.; Ammar, Y.A. Isatin-Schiff’s base and chalcone hybrids as chemically apoptotic inducers and EGFR inhibitors; design, synthesis, anti-proliferative activities and in silico evaluation. Journal of Molecular Structure 2021, 1234, 130159. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Almahli, H.; Al-Ansary, G.H.; Ghabbour, H.A.; Aly, M.H.; Ismael, O.E.; Al-Dhfyan, A.; Abdel-Aziz, H.A. Synthesis and in vitro anti-proliferative activity of some novel isatins conjugated with quinazoline/phthalazine hydrazines against triple-negative breast cancer MDA-MB-231 cells as apoptosisinducing agents. Journal of Enzyme Inhibition and Medicinal Chemistry 2017, 32, 600–613. [Google Scholar] [CrossRef]
- Fares, M.; Eldehna, W.M.; Abou-Seri, S.M.; Abdel-Aziz, H.A.; Aly, M.H.; Tolba, M.F. Design, synthesis and in vitro antiproliferative activity of novel isatin-quinazoline hybrids. Archiv der Pharmazie 2015, 348, 144–154. [Google Scholar] [CrossRef]
- Sin, N.; Venables, B.L.; Combrink, K.D.; Gulgeze, H.B.; Yu, K.; Civiello, R.L.; Thuring, J.; Wang, X.A.; Yang, Z.; Zadjura, L.; Marino, A.; Kadow, K.F.; Cianci, C.W.; Clarke, J.; Genovesi, E.V.; Medina, I.; Lamb, L.; Krystal, M.; Meanwell, N.A. Respiratory syncytial virus fusion inhibitors. Part 7: Structure–activity relationships associated with a series of isatin oximes that demonstrate antiviral activity in vivo. Bioorganic & Medicinal Chemistry Letters 2009, 19, 4857–4862. [Google Scholar] [CrossRef]
- Wang, J.; Yun, D.; Yao, J.; Fu, W.; Huang, F.; Chen, L.; Wei, T.; Yu, C.; Xu, H.; Zhou, X.; Huang, Y.; Wu, J.; Qui, P.; Li, W. Design, synthesis and QSAR study of novel isatin analogues inspired Michael acceptor as potential anticancer compounds. European Journal of Medicinal Chemistry 2018, 144, 493–503. [Google Scholar] [CrossRef]
- Omar, A.Z.; Mosa, T.W.; El-Sadany, S.K.; Hamed, E.A.; El-Atawy, M. Novel piperazine based compounds as potential inhibitors for SARS-CoV-2 Protease Enzyme: Synthesis and molecular docking study. Journal of Molecular Structure 2021, 1245, 131020. [Google Scholar] [CrossRef]
- Kumar, K.; Sagar, S.; Esau, L.; Kaur, M.; Kumar, V. Synthesis of novel 1H-1,2,3-triazole tethered C-5 substituted uracile isatin conjugates and their cytotoxic evaluation. European Journal of Medicinal Chemistry 2012, 58, 153–159. [Google Scholar] [CrossRef]
- Khatoon, S.; Aroosh, A.; Islam, A.; Kalsoom, S.; Ahmad, F.; Hameed, S.; Abbasi, S.W.; Yasinzai, M.; Naseer, M.M. Novel coumarin-isatin hybrids as potent antileishmanial agents: Synthesis, in silico and in vitro evaluations. Bioorganic Chemistry 2021, 110, 104816. [Google Scholar] [CrossRef]
- Hans, R.H.; Wiid, I.J.F.; Helden, P.D.V.; Wan, B.; Franzblau, S.G.; Gut, J.; Rosenthal, P.J.; Chibale, K. Novel thiolactone–isatin hybrids as potential antimalarial and antitubercular agents. Bioorganic & Medicinal Chemistry Letters 2011, 21, 2055–2058. [Google Scholar] [CrossRef]
- Bozorova, K.; Zhaoa, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorganic & Medicinal Chemistry 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Bennani, F.E.; Doudach, L.; Cherrah, Y.; Ramli, Y.; Karrouchi, K.; Ansar, M.; Faouzi, M.E.A. Overview of recent developments of pyrazole derivatives as an anticancer agent in different cell line. Bioorganic Chemistry 2020, 97, 103470. [Google Scholar] [CrossRef]
- Rostom, S.A.F.; Ashour, H.M.A.; Razik, H.A.A.E.; Fattah, A.E.F.H.A.E.; El-Din, N.N. Azole antimicrobial pharmacophore-based tetrazoles: Synthesis and biological evaluation as potential antimicrobial and anticonvulsant agents. Bioorganic & Medicinal Chemistry 2009, 17, 2410–2422. [Google Scholar] [CrossRef]
- Gao, F.; Wang, T.; Xiao, J.; Huang, G. Antibacterial activity study of 1,2,4-triazole derivatives. European Journal of Medicinal Chemistry 2019, 173, 274–281. [Google Scholar] [CrossRef]
- Shu Zhang, Z.X.; Xu, Z.; Gao, C.; Ren, Q.; Lv, L.C.Z.; Feng, L. Triazole derivatives and their anti-tubercular activity. European Journal of Medicinal Chemistry 2017, 138, 501–513. [Google Scholar] [CrossRef]
- Ding, Z.; Hou, P.; Liu, B. Gatifloxacin-1,2,3-triazole-isatin hybrids and their antimycobacterial activities. Archiv der Pharmazie 2019, 352, e1900135. [Google Scholar] [CrossRef]
- Solomon, V.R.; Hu, C.; Lee, H. Hybrid pharmacophore design and synthesis of isatin–benzothiazole analogs for their anti-breast cancer activity. Bioorganic & Medicinal Chemistry 2009, 17, 7585–7592. [Google Scholar] [CrossRef]
- Javida, M.T.; Rahim, F.; Taha, M.; Nawaz, M.; Wadood, A.; Ali, M.; Mosaddik, A.; Shah, S.A.A.; Farooq, R.K. Synthesis, SAR elucidations and molecular docking study of newly designed isatin based oxadiazole analogs as potent inhibitors of thymidine phosphorylase. Bioorganic Chemistry 2018, 79, 323–333. [Google Scholar] [CrossRef]
- Ibrahim, H.S.; Abou-seri, S.M.; Tanc, M.; Elaasser, M.M.; Abdel-Aziz, H.A.; Supuran, C.T. Isatin-pyrazole benzenesulfonamide hybrids potently inhibit tumorassociated carbonic anhydrase isoforms IX and XII. European Journal of Medicinal Chemistry 2015, 103, 583–593. [Google Scholar] [CrossRef]
- El-Naggar, M.; Eldehna, W.M.; Almahli, H.; Elgez, A.; Fares, M.; Elaasser, M.M.; Abdel-Aziz, H.A. Novel thiazolidinone/thiazolo[3,2-a] benzimidazolone-isatin conjugates as apoptotic anti-proliferative agents towards breast cancer: One-pot synthesis and in vitro biological evaluation. Molecules 2018, 23, 1420. [Google Scholar] [CrossRef]
- Eldehna, W.M.; El Hassab, M.A.; Abo-Ashour, M.F.; Al-Warhi, T.; Elaasser, M.M.; Safwat, N.A.; Suliman, H.; Ahmed, M.; Al-Rashood, S.T.; Abdel-Aziz, H.A.; El-Haggar, R. Development of isatin-thiazolo[3,2-a]benzimidazole hybrids as novel CDK2 inhibitors with potent in vitro apoptotic anti-proliferative activity: Synthesis, biological and molecular dynamics investigations. Bioorganic Chemistry 2021, 110, 104748. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Al-Wabli, R.I.; Almutairi, M.S.; Keeton, A.B.; Piazza, G.A.; Abdel-Aziz, H.A.; Attia, M.I. Synthesis and biological evaluation of certain hydrazonoindolin-2-one derivatives as new potent anti-proliferative agents. Journal of Enzyme Inhibition and Medicinal Chemistry 2018, 33, 867–878. [Google Scholar] [CrossRef]
- Özil, M.; Menteşe, E.; Yılmaz, F.; İslamoğlu, F.; Kahveci, B. Synthesis of novel triazol compounds containing isatin as potential antibacterial and antifungal agents by microwave and conventional methods. Journal of Chemical Research 2011, 35, 268–271. [Google Scholar] [CrossRef]
- Xiang, P.; Cao, Q.; Dong, Q.; Yang, X.; Tang, J.; Bai, H. Furan-site transformations of obacunone as potent insecticidal agents. Heliyon 2018, 4, e01064. [Google Scholar] [CrossRef]
- Ansari, M.F.; Siddiqui, S.M.; Ahmad, K.; Avecilla, F.; Dharavath, S.; Gourinath, S.; Azam, A. Synthesis, antiamoebic and molecular docking studies of furan-thiazolidinone hybrids. European Journal of Medicinal Chemistry 2016, 124, 393–406. [Google Scholar] [CrossRef]
- Gao, F.; Yang, H.; Lu, T.; Chen, Z.; Ma, L.; Xu, Z.; Schaffer, P.; Lu, G. Design, synthesis and anti-mycobacterial activity evaluation of benzofuran-isatin hybrids. European Journal of Medicinal Chemistry 2018, 159, 277–281. [Google Scholar] [CrossRef]
- Archna; Pathania, S.; Chawla, P.A. Thiophene-based derivatives as anticancer agents: An overview on decade’s work. Bioorganic Chemistry 2020, 101, 104026. [Google Scholar] [CrossRef]
- Schaper, K.; Müller, T.J.J. thiophene syntheses by ring forming multicomponent reactions. Topics in Current Chemistry 2018, 376, 38. [Google Scholar] [CrossRef]
- Chen, L.-R.; Wang, Y.-C.; Lin, Y.W.; Chou, S.-Y.; Chen, S.-F.; Liu, L.T.; Wu, Y.-T.; Kuo, C.-J.; Chen, T.S.-S.; Juang, S.-H. Synthesis and evaluation of isatin derivatives as effective SARS coronavirus 3CL protease inhibitors. Bioorganic & Medicinal Chemistry Letters 2005, 15, 3058–3062. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, R.K. Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives. Bioorganic Chemistry 2019, 89, 103021. [Google Scholar] [CrossRef]
- Han, Y.; Dong, W.; Guo, Q.; Li, X.; Huang, L. The importance of indole and azaindole scaffold in the development of antitumor agents. European Journal of Medicinal Chemistry 2020, 203, 112506. [Google Scholar] [CrossRef]
- Patil, R.; Patil, S.A.; Beaman, K.D.; Patil, S.A. Indole molecules as inhibitors of tubulin polymerization: Potential new anticancer agents, an update (2013-2015). Future Medicinal Chemistry 2016, 8, 1291–1316. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, P.; Pathak, D. Biological importance of the indole nucleus in recent years: A comprehensive review. Journal of Heterocyclic Chemistry 2010, 47, 491–502. [Google Scholar] [CrossRef]
- Praveen, C.; Ayyanar, A.; Perumal, P.T. Practical synthesis, anticonvulsant, and antimicrobial activity of N-allyl and N-propargyl di(indolyl)indolin-2-ones. Bioorganic & Medicinal Chemistry Letters 2011, 21, 4072–4077. [Google Scholar] [CrossRef]
- Mohammed, A.A.M.; Suaifan, G.A.R.Y.; Shehadeh, M.B.; Okechukwu, P.N. Design, synthesis and antimicrobial evaluation of novel glycosylated-fluoroquinolones derivatives. European Journal of Medicinal Chemistry 2020, 202, 112513. [Google Scholar] [CrossRef]
- Patel, M.M.; Patel, L.J. Design, synthesis, molecular docking, and antibacterial evaluation of some novel flouroquinolone derivatives as potent antibacterial agent. ScientificWorld Journal 2014, 2014, 897187. [Google Scholar] [CrossRef]
- Vu, T.H.; Ha-Duong, N.T.; Aubry, A.; Capton, E.; Fechter, P.; Plésiat, P.; Verbeke, P.; Serradji, N. In vitro activities of a new fluoroquinolone derivative highly active against Chlamydia trachomatis. Bioorganic Chemistry 2019, 83, 180–185. [Google Scholar] [CrossRef]
- Prakash, C. R.; Raja, S. Synthesis, characterization and in vitro antimicrobial activity of some novel 5-substituted Schiff and Mannich base of isatin derivatives. Journal of Saudi Chemical Society 2013, 17, 337–344. [Google Scholar] [CrossRef]
- Sriram, D.; Aubry, A.; Yogeeswari, P.; Fisher, L. M. Gatifloxacin derivatives: Synthesis, antimycobacterial activities, and inhibition of Mycobacterium tuberculosis DNA gyrase. Bioorganic & Medicinal Chemistry Letters 2006, 16, 2982–2985. [Google Scholar] [CrossRef]
- Kumar, V.S.; Verma, R.; Xue, F.; Kumar, T.P.; Girish, Y.R.; Rakesh, K.P. Antibacterial activities of sulfonyl or sulfonamide containing heterocyclic derivatives and its structure-activity relationships (SAR) studies: A critical review. Bioorganic Chemistry 2020, 105, 104400. [Google Scholar] [CrossRef]
- Wan, Y.; Fang, G.; Chen, H.; Deng, X.; Tang, Z. Sulfonamide derivatives as potential anti-cancer agents and their SARs elucidation. European Journal of Medicinal Chemistry 2021, 226, 113837. [Google Scholar] [CrossRef]
- Farag, A.A. Synthesis and antimicrobial activity of 5-(morpholinosulfonyl)isatin derivatives incorporating a thiazole moiety. Drug Research 2014, 65, 373–379. [Google Scholar] [CrossRef]
- Abo-Ashour, M.F.; Eldehna, W.M.; Nocentini, A.; Bonardi, A.; Bua, S.; Ibrahim, H.S.; Elaasser, M.M.; Kryštof, V.; Jorda, R.; Gratteri, P.; Abou-Seri, S.M.; Supuran, C.T. 3-Hydrazinoisatin-based benzenesulfonamides as novel carbonic anhydrase inhibitors endowed with anticancer activity: Synthesis, in vitro biological evaluation and in silico insights. European Journal of Medicinal Chemistry 2019, 184, 111768. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Nocentini, A.; Al-Rashood, S.T.; Hassan, G.S.; Alkahtani, H.M.; Almehizia, A.A.; Reda, A.M.; Abdel-Aziz, H.A.; Supuran, C.T. Tumor-associated carbonic anhydrase isoform IX and XII inhibitory properties of certain isatin-bearing sulfonamides endowed with in vitro anticancer activity towards colon cancer. Bioorganic Chemistry 2018, 81, 425–432. [Google Scholar] [CrossRef]
- Selvam, P.; Chandramohan, M.; Hurst, B.L.; Smee, D.F. Activity of isatine-sulfadimidine derivatives against 2009 pandemic H1N1 influenza virus in cell culture. Antiviral Chemistry and Chemotherapy 2010, 20, 143–146. [Google Scholar] [CrossRef]
- Albratty, M.; Alhazmi, H. A. Novel pyridine and pyrimidine derivatives as promising anticancer agents: A review. Arabian Journal of Chemistry 2022, 15, 103846. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A privileged structure in medicinal chemistry. Chemical Reviews 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone derivatives: Role in anticancer therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Bharti, D.S.K.; Asati, V. Chalcone Scaffolds as Anti-infective Agents: Structural and Molecular Target Perspectives. European Journal of Medicinal Chemistry 2015, 101, 496–524. [Google Scholar] [CrossRef]
- Sahu, N.K.; Balbhadra, S.S.; Choudhary, J.; Kohli, D.V. Exploring pharmacological significance of chalcone scaffold: A review. Current Medicinal Chemistry 2012, 19, 209–225. [Google Scholar] [CrossRef]
- Hameeda, A.; Al-Rashida, M.; Uroos, M.; Ali, S.A.; Arshia; Ishtiaq, M.; Khan, K.M. Quinazoline and quinazolinone as important medicinal scaffolds: A comparative patent review. Expert Opinion on Therapeutic Patents 2018, 28, 281–297. [Google Scholar] [CrossRef]
- Khan, I.; Ibrar, A.; Abbas, N.; Saeed, A. Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds: Synthetic approaches and multifarious applications. European Journal of Medicinal Chemistry 2014, 76, 193–244. [Google Scholar] [CrossRef]
- Bayoumi, W.A.; Barghash, A.M.; Gineinah, M.M.; Massoud, M.A.; Abdelal, A.M. Design, synthesis and antioxidant evaluation of certain new phthalazine derivatives. Der Pharma Chemica 2014, 3, 89–102. [Google Scholar]
- Popiołek, Ł. Hydrazide–hydrazones as potential antimicrobial agents: Overview of the literature since 2010. Medicinal Chemistry Research 2017, 26, 287–301. [Google Scholar] [CrossRef]
- Angelova, V.T.; Valcheva, V.; Vassilev, N.G.; Buyukliev, R.; Momekov, G.; Dimitri, I.; Saso, L.; Djukic, M.; Shivachev, B. Antimycobacterial activity of novel hydrazide-hydrazone derivatives with 2H chromene and coumarin scaffold. Bioinorganic and Medicinal Chemistry Letters 2017, 27, 223–227. [Google Scholar] [CrossRef]
- Salem, M. A.; Ragab, A.; El-Khalafawy, A.; Makhlouf, A. H.; Askar, A. A.; Ammar, Y. A. Design, synthesis, in vitro antimicrobial evaluation and molecular docking studies of indol-2-one tagged with morpholinosulfonyl moiety as DNA gyrase inhibitors. Bioorganic Chemistry 2020, 96, 103619. [Google Scholar] [CrossRef]
- Rawat, P.; Verma, S.M. Synthesis and pharmacological evaluation of 6-hydroxy-2,5,7,8-tetramethylN′-(2-oxoindolin-3-ylidene)chroman-2-carbohydrazide derivatives as antimicrobial agents. Journal of Chemical and Pharmaceutical Research 2016, 8, 149–154. [Google Scholar]
- Kalinowski, D. S.; Quach, P.; Richardson, D. R. Thiosemicarbazones: The new wave in cancer treatment. Future Medicinal Chemistry 2009, 1, 1143–1151. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Guo, H.; Li, Z.S.; Song, F.-H.; Wang, W.-M.; Dai, H.-Q.; Wang, J.-G. Synthesis and evaluation of isatin-β-thiosemicarbazones as novel agents against antibiotic-resistant Gram-positive bacterial species. European Journal of Medicinal Chemistry 2015, 101, 419–430. [Google Scholar] [CrossRef]
- Yang, Y.; Pannecouque, C.; Clercq, E.D.; Zhuang, C.; Chen, F. Privileged scaffold inspired design of novel oxime-biphenyl-DAPYs in treatment of HIV-1. Bioinorganic Chemistry 2020, 99, 103825. [Google Scholar] [CrossRef]
- Moodie, L.W.K.; Cervin, G.; Trepos, R.; Labriere, C.; Hellio, C.; Pavia, H.; Svenson, J. Design and biological evaluation of antifouling dihydrostilbene oxime hybrids. Marine Biotechnology 2018, 20, 257–267. [Google Scholar] [CrossRef]
- Gornostaev, L.M.; Tsvetkov, V.B.; Markova, A.A.; Lavrikova, T.I.; Khalyavina, Y.G.; Kuznetsova, A.; Kaluzhny, D.N.; Shunayev, A.V.; Tsvetkova, M.V.; Glazunova, V.A.; Chernyshev, V.; Shtil, A.A. The oxime derivatives of 1-R-1H-naphtho[2,3-d][1,2,3]triazole-4,9-dione 2-oxides: Synthesis and properties. Anti-cancer Agents in Medicinal Chemistry 2017, 17, 1814–1823. [Google Scholar] [CrossRef]
- Ma, C.-M.; Nakamura, N.; Hattori, M.; Kawahata, T.; Otake, T. Inhibitory effects of triterpene-azidothymidine conjugates on proliferation of human immunodeficiency virus type 1 and its protease. Chemical & Pharmaceutical Bulletin 2002, 50, 877–880. [Google Scholar] [CrossRef]
- Floyd, R.A.; Kopke, R.D.; Choi, C.-H.; Foster, S.B.; Doblas, S.; Towner, R.A. Nitrones as therapeutics. Free Radical Biology & Medicine 2008, 45, 1361–1374. [Google Scholar] [CrossRef]
- Rosselin, M.; Poeggeler, B.; Durand, G. Nitrone derivatives as therapeutics: From chemical modification to specific-targeting. Current Topics in Medicinal Chemistry 2017, 17, 2006–2022. [Google Scholar] [CrossRef]
- Firuzi, O.; Miri, R.; Tavakkoli, M.; Saso, L. Antioxidant therapy: Current status and future prospects. Current Medicinal Chemistry 2011, 18, 3871–3888. [Google Scholar] [CrossRef]
- Marco-Contelles, J. Recent advances on nitrones design for stroke treatment. Journal of Medicinal Chemistry 2020, 63, 13413–13427. [Google Scholar] [CrossRef]
- Kant, R.; Maji, S. Recent advances in synthesis of piperazine based ligands, metal complexes and their applications. Dalton Transactions 2021, 50, 785–800. [Google Scholar] [CrossRef]
- Jain, A.; Chaudhary, J.; Khaira, H.; Chopra, B.; Dhingra, A. Piperazine: A promising scaffold with analgesic and anti-inflammatory potential. Drug research 2021, 71, 62–72. [Google Scholar] [CrossRef]
- Shaquiquzzaman, M.; Verma, G.; Marella, A.; Akhter, M.; Akhtar, W.; Khan, M.F.; Tasneem, S.; Alam, M.M. Piperazine scaffold: A remarkable tool in generation of diverse pharmacological agents. European Journal of Medicinal Chemistry 2015, 102, 487–529. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. Coumarin-containing hybrids and their anticancer activities. European Journal of Medicinal Chemistry 2019, 181, 111587. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, A.; Yang, Y.; Yang, P. Coumarin-containing hybrids and their antibacterial activities. Archiv der Pharmazie 2020, 353, e1900380. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Katerinopoulos, H.E. The coumarin moiety as chromophore of fluorescent ion indicators in biological systems. Current Pharmaceutical Design 2004, 10, 3835–3851. [Google Scholar] [CrossRef]
- Diao, Q.-P.; Guo, H.; Wang, G.-Q. Design, synthesis, and in vitro anticancer activities of diethylene glycol tethered isatin-1,2,3-triazole-coumarin Hybrids. Journal of Heterocyclic Chemistry 2019, 5, 1667–1671. [Google Scholar] [CrossRef]
- Huang, G.-C.; Xu, Y.; Xu, Z.; Lv, Z.S.; Zhang, J.; Guo, H.-Y.; Hu, Y.-Q.; Liu, M.-L.; Guan, J.; Lu, Y. Propylene-1H-1,2,3-triazole-4-methylene-tethered isatin-coumarin hybrids: Design, synthesis, and in vitro anti-tubercular evaluation. Journal of Heterocyclic Chemistry 2018, 55, 830–835. [Google Scholar] [CrossRef]
- Beck, B.; Khoury, K.K.; Herdtweck, E.; Domling, A. One-pot multicomponent synthesis of two novel thiolactone scaffolds. Molecular Diversity 2010, 14, 479–491. [Google Scholar] [CrossRef]
- Kumar, S.; Deep, A.; Narasimhan, B. Pyrimidine derivatives as potential agents acting on central nervous system. Central Nervous System Agents in Medicinal Chemistry 2015, 15, 5–10. [Google Scholar] [CrossRef]
- He, Z. X.; Zhao, T.Q.; Gong, Y.P.; Zhang, X.; Ma, L.Y.; Liu, H.M. Pyrimidine: A promising scaffold for optimization to develop the inhibitors of ABC transporters. European Journal of Medicinal Chemistry 2020, 200, 112458. [Google Scholar] [CrossRef]
- Akhaja, T.N.; Raval, J.P. Design, synthesis, in vitro evaluation of tetrahydropyrimidine-isatin hybrids as potential antibacterial, antifungal and anti-tubercular agents. Chinese Chemrical Letters 2012, 23, 446–449. [Google Scholar] [CrossRef]
- Raj, R.; Biot, C.; Carrere-Kremer, S.; Kremer, L.; Guerardel, Y.; Gut, J.; Rosenthal, P.J.; Forge, D.; Kumar, V. 7-Chloroquinoline–isatin conjugates: Antimalarial, antitubercular, and cytotoxic evaluation. Chemical Biology and Drug Design 2014, 83, 622–629. [Google Scholar] [CrossRef]
- Alahari, A.; Trivelli, X.; Guérardel, Y.; Dover, L.G.; Besra, G.S.; Sacchettini, J.C.; Reynolds, R.C.; Coxon, G.D.; Kremer. L. Thiacetazone, an antitubercular drug that inhibits cyclopropanation of cell wall mycolic acids in mycobacteria. PLoS ONE 2007, 2, e1343. [Google Scholar] [CrossRef]
- Debnath, B.; Ganguly, S. Synthesis, biological evaluation, in silico docking and virtual ADME studies of novel isatin analogs as promising antimicrobial agents. Anti-Infective Agents 2015, 13, 139–153. [Google Scholar] [CrossRef]
- Xu, Y.; Guan, J.; Xu, Z.; Zhao, S. Design, synthesis and in vitro anti-mycobacterial activities of homonuclear and heteronuclear bis-isatin derivatives. Fitoterapia 2018, 127, 383–386. [Google Scholar] [CrossRef]
- Teng, Y.O.; Zhao, H.-Y.; Wang, J.; Liu, H.; Gao, M.-L.; Zhou, Y.; Han, K.L.; Fan, Z.-C.; Zhang, Y.-M.; Sun, H.; Yu, P. Synthesis and anti-cancer activity evaluation of 5-(2-carboxyethenyl)-isatin derivatives. European Journal of Medicinal Chemistry 2016, 112, 145–156. [Google Scholar] [CrossRef]
- Raj, R.; Singh, P.; Haberkern, N.T.; Faucher, R.M.; Patel, N.; Land, K.M.; Kumar, V. Synthesis of 1H-1,2,3-triazole linked beta-lactameisatin bi-functional hybrids and preliminary analysis of in vitro activity against the protozoal parasite Trichomonas vaginalis. European Journal of Medicinal Chemistry 2013, 63, 897–906. [Google Scholar] [CrossRef]
- Nagarsenkar, A.; Guntuku, L.; Guggilapu, S. D.; Bai, K. D.; Srinivasulu, G.; Naidu, V.G.M.; Babu, B.N. Synthesis and apoptosis inducing studies of triazole linked 3-benzylidene isatin derivatives. European Journal of Medicinal Chemistry 2016, 124, 782–793. [Google Scholar] [CrossRef]
- Abo-Ashour, M.F.; Eldehna, W.M.; Nocentini, A.; Ibrahim, H.S.; Bua, S.; Abou-Seri, S.M.; Supuran, C.T. Novel hydrazido benzenesulfonamides-isatin conjugates: Synthesis, carbonic anhydrase inhibitory activity and molecular modeling studies. European Journal of Medicinal Chemistry 2018, 157, 28–36. [Google Scholar] [CrossRef]
- Guo, H. Design, Synthesis, and In Vitro Anti-mycobacterial Activities of propylene tethered benzofuran–isatin hybrids. Journal of Heterocyclic Chemistry 2018, 58, 338–342. [Google Scholar] [CrossRef]
- Kumar, S.; Saha, S.T.; Gu, L.; Palma, G.; Perumal, S.; Singh-Pillay, A.; Singh, P.; Anand, A.; Kaur, M.; Kumar, V. 1H-1,2,3-Triazole tethered nitroimidazole−isatin conjugates: Synthesis, docking, and anti-proliferative evaluation against breast cancer. ACS Omega 2018, 3, 12106–12113. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



