Submitted:
08 January 2024
Posted:
09 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Generation and Characterization of iPSCs
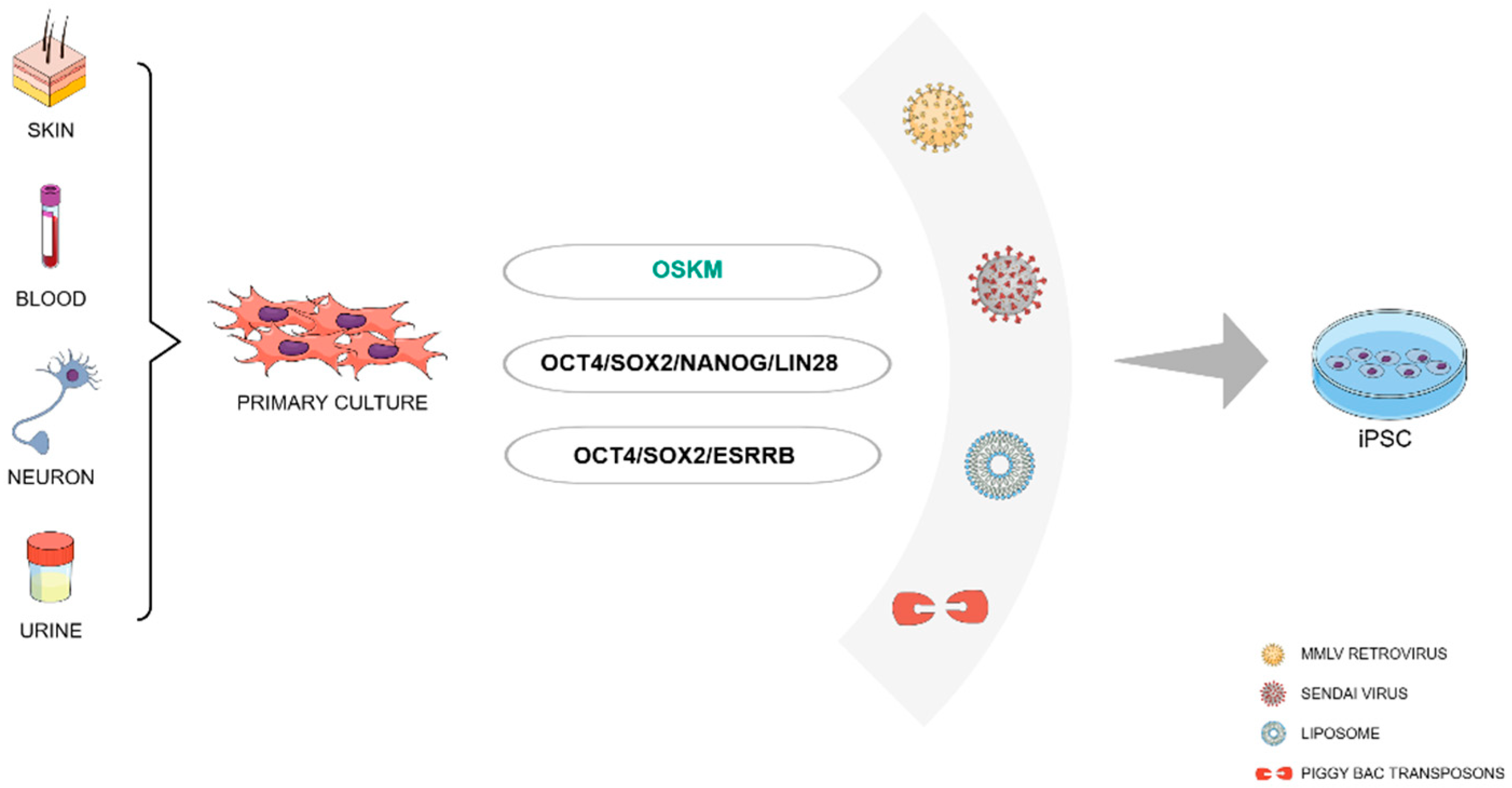
2.1. Who Can Be Reprogrammed?
2.2. The Reprogramming Recipe
2.3. Reprogramming Factors Delivery Systems
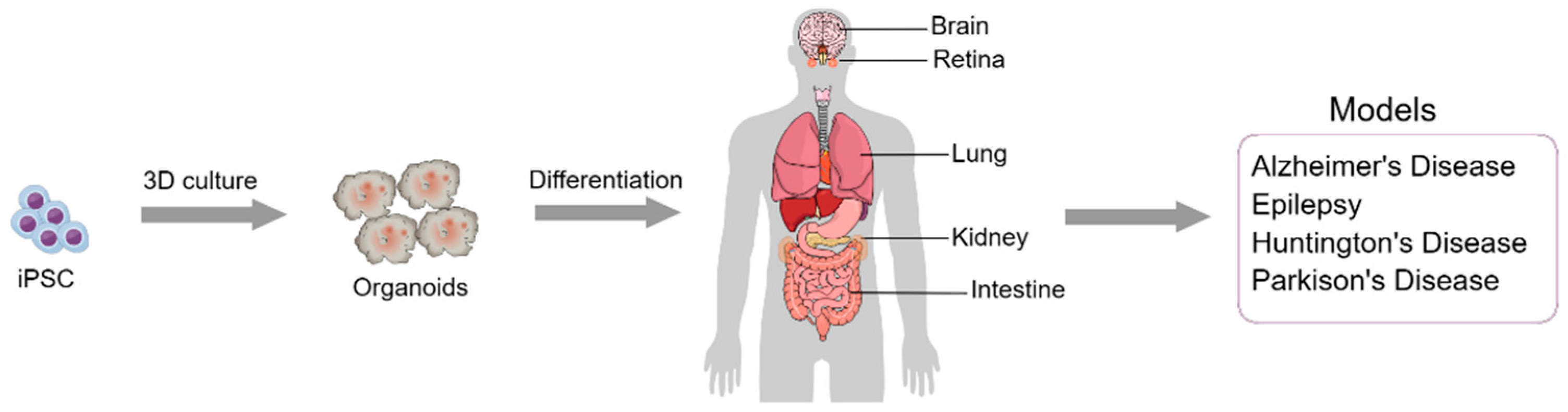
3. Organoid Models:
3.1. What They Are, How They Work, and Applications
3.2. Applications of Organoid Models in Neurological Diseases
3.2.1. Alzheimer's Disease
3.2.2. Epilepsy
3.2.3. Huntington’s Disease
3.2.4. Parkinson's Disease
3.3. Limitations in the Use of Organoid Models
4. iPSCs-Based Therapies for Neurological Diseases
5. Conclusion and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Trejo-Lopez, J.A.; Yachnis, A.T.; Prokop, S. Neuropathology of Alzheimer’s Disease. Neurotherapeutics 2022, 19, 173–185. [CrossRef]
- Curatolo, P.; Specchio, N.; Aronica, E. Advances in the Genetics and Neuropathology of Tuberous Sclerosis Complex: Edging Closer to Targeted Therapy. Lancet Neurol. 2022, 21, 843–856. [CrossRef]
- Chang, B.-L.; Chang, K.-H. Stem Cell Therapy in Treating Epilepsy. Front. Neurosci. 2022, 16, 934507. [CrossRef]
- Toman, N.G.; Grande, A.W.; Low, W.C. Neural Repair in Stroke. Cell Transplant. 2019, 28, 1123–1126. [CrossRef]
- Ribeiro, B.F.; da Cruz, B.C.; de Sousa, B.M.; Correia, P.D.; David, N.; Rocha, C.; Almeida, R.D.; Ribeiro da Cunha, M.; Marques Baptista, A.A.; Vieira, S.I. Cell Therapies for Spinal Cord Injury: A Review of the Clinical Trials and Cell-Type Therapeutic Potential. Brain J. Neurol. 2023, 146, 2672–2693. [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [CrossRef]
- Huang, Y.; Tan, S. Direct Lineage Conversion of Astrocytes to Induced Neural Stem Cells or Neurons. Neurosci. Bull. 2015, 31, 357–367. [CrossRef]
- McKinney, C.E. Using Induced Pluripotent Stem Cells Derived Neurons to Model Brain Diseases. Neural Regen. Res. 2017, 12, 1062–1067. [CrossRef]
- Paredes-Espinosa, M.B.; Paluh, J.L. Human Stem Cell-Derived Neurons and Neural Circuitry Therapeutics: Next Frontier in Spinal Cord Injury Repair. Exp. Biol. Med. Maywood NJ 2022, 247, 2142–2151. [CrossRef]
- Evans, M.J.; Kaufman, M.H. Establishment in Culture of Pluripotential Cells from Mouse Embryos. Nature 1981, 292, 154–156. [CrossRef]
- Martello, G.; Smith, A. The Nature of Embryonic Stem Cells. Annu. Rev. Cell Dev. Biol. 2014, 30, 647–675. [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [CrossRef]
- Madrid, M.; Sumen, C.; Aivio, S.; Saklayen, N. Autologous Induced Pluripotent Stem Cell-Based Cell Therapies: Promise, Progress, and Challenges. Curr. Protoc. 2021, 1, e88. [CrossRef]
- Ho, B.X.; Pek, N.M.Q.; Soh, B.-S. Disease Modeling Using 3D Organoids Derived from Human Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2018, 19, 936. [CrossRef]
- Hong, L.; Zhang, M.; Ly, O.T.; Chen, H.; Sridhar, A.; Lambers, E.; Chalazan, B.; Youn, S.-W.; Maienschein-Cline, M.; Feferman, L.; et al. Human Induced Pluripotent Stem Cell-Derived Atrial Cardiomyocytes Carrying an SCN5A Mutation Identify Nitric Oxide Signaling as a Mediator of Atrial Fibrillation. Stem Cell Rep. 2021, 16, 1542–1554. [CrossRef]
- Pasteuning-Vuhman, S.; de Jongh, R.; Timmers, A.; Pasterkamp, R.J. Towards Advanced iPSC-Based Drug Development for Neurodegenerative Disease. Trends Mol. Med. 2021, 27, 263–279. [CrossRef]
- Liu, G.; David, B.T.; Trawczynski, M.; Fessler, R.G. Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Rev. Rep. 2020, 16, 3–32. [CrossRef]
- Giorgetti, A.; Montserrat, N.; Aasen, T.; Gonzalez, F.; Rodríguez-Pizà, I.; Vassena, R.; Raya, A.; Boué, S.; Barrero, M.J.; Corbella, B.A.; et al. Generation of Induced Pluripotent Stem Cells from Human Cord Blood Using OCT4 and SOX2. Cell Stem Cell 2009, 5, 353–357. [CrossRef]
- Aasen, T.; Raya, A.; Barrero, M.J.; Garreta, E.; Consiglio, A.; Gonzalez, F.; Vassena, R.; Bilić, J.; Pekarik, V.; Tiscornia, G.; et al. Efficient and Rapid Generation of Induced Pluripotent Stem Cells from Human Keratinocytes. Nat. Biotechnol. 2008, 26, 1276–1284. [CrossRef]
- Aasen, T.; Belmonte, J.C.I. Isolation and Cultivation of Human Keratinocytes from Skin or Plucked Hair for the Generation of Induced Pluripotent Stem Cells. Nat. Protoc. 2010, 5, 371–382. [CrossRef]
- Zhou, T.; Benda, C.; Dunzinger, S.; Huang, Y.; Ho, J.C.; Yang, J.; Wang, Y.; Zhang, Y.; Zhuang, Q.; Li, Y.; et al. Generation of Human Induced Pluripotent Stem Cells from Urine Samples. Nat. Protoc. 2012, 7, 2080–2089. [CrossRef]
- Xue, Y.; Cai, X.; Wang, L.; Liao, B.; Zhang, H.; Shan, Y.; Chen, Q.; Zhou, T.; Li, X.; Hou, J.; et al. Generating a Non-Integrating Human Induced Pluripotent Stem Cell Bank from Urine-Derived Cells. PLOS ONE 2013, 8, e70573. [CrossRef]
- Jiang, Y.-F.; Chen, M.; Zhang, N.-N.; Yang, H.-J.; Rui, Q.; Zhou, Y.-F. In Vitro and in Vivo Differentiation of Induced Pluripotent Stem Cells Generated from Urine-Derived Cells into Cardiomyocytes. Biol. Open 2018, 7, bio029157. [CrossRef]
- Scalise, M.; Marino, F.; Salerno, L.; Cianflone, E.; Molinaro, C.; Salerno, N.; De Angelis, A.; Viglietto, G.; Urbanek, K.; Torella, D. From Spheroids to Organoids: The Next Generation of Model Systems of Human Cardiac Regeneration in a Dish. Int. J. Mol. Sci. 2021, 22, 13180. [CrossRef]
- Su, R.-J.; Baylink, D.J.; Neises, A.; Kiroyan, J.B.; Meng, X.; Payne, K.J.; Tschudy-Seney, B.; Duan, Y.; Appleby, N.; Kearns-Jonker, M.; et al. Efficient Generation of Integration-Free Ips Cells from Human Adult Peripheral Blood Using BCL-XL Together with Yamanaka Factors. PloS One 2013, 8, e64496. [CrossRef]
- Hanna, J.; Markoulaki, S.; Schorderet, P.; Carey, B.W.; Beard, C.; Wernig, M.; Creyghton, M.P.; Steine, E.J.; Cassady, J.P.; Foreman, R.; et al. Direct Reprogramming of Terminally Differentiated Mature B Lymphocytes to Pluripotency. Cell 2008, 133, 250–264. [CrossRef]
- Nagano, S.; Maeda, T.; Ichise, H.; Kashima, S.; Ohtaka, M.; Nakanishi, M.; Kitawaki, T.; Kadowaki, N.; Takaori-Kondo, A.; Masuda, K.; et al. High Frequency Production of T Cell-Derived iPSC Clones Capable of Generating Potent Cytotoxic T Cells. Mol. Ther. Methods Clin. Dev. 2020, 16, 126–135. [CrossRef]
- Suwanpitak, S.; Promnakhon, N.; Netsrithong, R.; Wattanapanitch, M. Efficient Generation of iPSC-Derived Hematoendothelial Progenitors and Specification Toward T Cell Lineage. Methods Mol. Biol. Clifton NJ 2022, 2454, 423–442. [CrossRef]
- Serwold, T.; Hochedlinger, K.; Swindle, J.; Hedgpeth, J.; Jaenisch, R.; Weissman, I.L. T-Cell Receptor-Driven Lymphomagenesis in Mice Derived from a Reprogrammed T Cell. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 18939–18943. [CrossRef]
- Dowey, S.N.; Huang, X.; Chou, B.-K.; Ye, Z.; Cheng, L. Generation of Integration-Free Human Induced Pluripotent Stem Cells from Postnatal Blood Mononuclear Cells by Plasmid Vector Expression. Nat. Protoc. 2012, 7, 2013–2021. [CrossRef]
- Zhou, H.; Martinez, H.; Sun, B.; Li, A.; Zimmer, M.; Katsanis, N.; Davis, E.E.; Kurtzberg, J.; Lipnick, S.; Noggle, S.; et al. Rapid and Efficient Generation of Transgene-Free iPSC from a Small Volume of Cryopreserved Blood. Stem Cell Rev. Rep. 2015, 11, 652–665. [CrossRef]
- Staerk, J.; Dawlaty, M.M.; Gao, Q.; Maetzel, D.; Hanna, J.; Sommer, C.A.; Mostoslavsky, G.; Jaenisch, R. Reprogramming of Human Peripheral Blood Cells to Induced Pluripotent Stem Cells. Cell Stem Cell 2010, 7, 20–24. [CrossRef]
- González, F.; Boué, S.; Izpisúa Belmonte, J.C. Methods for Making Induced Pluripotent Stem Cells: Reprogramming à La Carte. Nat. Rev. Genet. 2011, 12, 231–242. [CrossRef]
- Eminli, S.; Foudi, A.; Stadtfeld, M.; Maherali, N.; Ahfeldt, T.; Mostoslavsky, G.; Hock, H.; Hochedlinger, K. Differentiation Stage Determines Potential of Hematopoietic Cells for Reprogramming into Induced Pluripotent Stem Cells. Nat. Genet. 2009, 41, 968–976. [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [CrossRef]
- Feng, B.; Ng, J.-H.; Heng, J.-C.D.; Ng, H.-H. Molecules That Promote or Enhance Reprogramming of Somatic Cells to Induced Pluripotent Stem Cells. Cell Stem Cell 2009, 4, 301–312. [CrossRef]
- Kim, J.B.; Zaehres, H.; Wu, G.; Gentile, L.; Ko, K.; Sebastiano, V.; Araúzo-Bravo, M.J.; Ruau, D.; Han, D.W.; Zenke, M.; et al. Pluripotent Stem Cells Induced from Adult Neural Stem Cells by Reprogramming with Two Factors. Nature 2008, 454, 646–650. [CrossRef]
- Huangfu, D.; Osafune, K.; Maehr, R.; Guo, W.; Eijkelenboom, A.; Chen, S.; Muhlestein, W.; Melton, D.A. Induction of Pluripotent Stem Cells from Primary Human Fibroblasts with Only Oct4 and Sox2. Nat. Biotechnol. 2008, 26, 1269–1275. [CrossRef]
- Kim, J.B.; Greber, B.; Araúzo-Bravo, M.J.; Meyer, J.; Park, K.I.; Zaehres, H.; Schöler, H.R. Direct Reprogramming of Human Neural Stem Cells by OCT4. Nature 2009, 461, 649–653. [CrossRef]
- Kim, J.B.; Sebastiano, V.; Wu, G.; Araúzo-Bravo, M.J.; Sasse, P.; Gentile, L.; Ko, K.; Ruau, D.; Ehrich, M.; van den Boom, D.; et al. Oct4-Induced Pluripotency in Adult Neural Stem Cells. Cell 2009, 136, 411–419. [CrossRef]
- Tsai, S.Y.; Bouwman, B.A.; Ang, Y.S.; Kim, S.J.; Lee, D.F.; Lemischka, I.R.; Rendl, M. Single Transcription Factor Reprogramming of Hair Follicle Dermal Papilla Cells to Induced Pluripotent Stem Cells. Stem Cells 2011, 29, 964–971. [CrossRef]
- Park, I.-H.; Zhao, R.; West, J.A.; Yabuuchi, A.; Huo, H.; Ince, T.A.; Lerou, P.H.; Lensch, M.W.; Daley, G.Q. Reprogramming of Human Somatic Cells to Pluripotency with Defined Factors. Nature 2008, 451, 141–146. [CrossRef]
- Lee, J.; Park, Y.-J.; Jung, H. Protein Kinases and Their Inhibitors in Pluripotent Stem Cell Fate Regulation. Stem Cells Int. 2019, 2019, e1569740. [CrossRef]
- Li, N.; Long, B.; Han, W.; Yuan, S.; Wang, K. microRNAs: Important Regulators of Stem Cells. Stem Cell Res. Ther. 2017, 8, 110. [CrossRef]
- Subramanyam, D.; Lamouille, S.; Judson, R.L.; Liu, J.Y.; Bucay, N.; Derynck, R.; Blelloch, R. Multiple Targets of miR-302 and miR-372 Promote Reprogramming of Human Fibroblasts to Induced Pluripotent Stem Cells. Nat. Biotechnol. 2011, 29, 443–448. [CrossRef]
- Çağlayan, E.S.; Güran, Ş. Importance of Myc-Related microRNAs in Induced Pluripotency. Cell Biol. Int. 2015, 39, 987–994. [CrossRef]
- Lakshmipathy, U.; Davila, J.; Hart, R.P. miRNA in Pluripotent Stem Cells. Regen. Med. 2010, 5, 545–555. [CrossRef]
- Gomes, K.M.S.; Costa, I.C.; Santos, J.F.D.; Dourado, P.M.M.; Forni, M.F.; Ferreira, J.C.B. Induced Pluripotent Stem Cells Reprogramming: Epigenetics and Applications in the Regenerative Medicine. Rev. Assoc. Medica Bras. 1992 2017, 63, 180–189. [CrossRef]
- Brix, J.; Zhou, Y.; Luo, Y. The Epigenetic Reprogramming Roadmap in Generation of iPSCs from Somatic Cells. J. Genet. Genomics Yi Chuan Xue Bao 2015, 42, 661–670. [CrossRef]
- van den Hurk, M.; Kenis, G.; Bardy, C.; van den Hove, D.L.; Gage, F.H.; Steinbusch, H.W.; Rutten, B.P. Transcriptional and Epigenetic Mechanisms of Cellular Reprogramming to Induced Pluripotency. Epigenomics 2016, 8, 1131–1149. [CrossRef]
- Papp, B.; Plath, K. Epigenetics of Reprogramming to Induced Pluripotency. Cell 2013, 152, 1324–1343. [CrossRef]
- Huangfu, D.; Maehr, R.; Guo, W.; Eijkelenboom, A.; Snitow, M.; Chen, A.E.; Melton, D.A. Induction of Pluripotent Stem Cells by Defined Factors Is Greatly Improved by Small-Molecule Compounds. Nat. Biotechnol. 2008, 26, 795–797. [CrossRef]
- Mali, P.; Chou, B.-K.; Yen, J.; Ye, Z.; Zou, J.; Dowey, S.; Brodsky, R.A.; Ohm, J.E.; Yu, W.; Baylin, S.B.; et al. Butyrate Greatly Enhances Derivation of Human Induced Pluripotent Stem Cells by Promoting Epigenetic Remodeling and the Expression of Pluripotency-Associated Genes. STEM CELLS 2010, 28, 713–720. [CrossRef]
- Kang, S.-J.; Park, Y.-I.; So, B.; Kang, H.-G. Sodium Butyrate Efficiently Converts Fully Reprogrammed Induced Pluripotent Stem Cells from Mouse Partially Reprogrammed Cells. Cell. Reprogramming 2014, 16, 345–354. [CrossRef]
- Shi, Y.; Desponts, C.; Do, J.T.; Hahm, H.S.; Schöler, H.R.; Ding, S. Induction of Pluripotent Stem Cells from Mouse Embryonic Fibroblasts by Oct4 and Klf4 with Small-Molecule Compounds. Cell Stem Cell 2008, 3, 568–574. [CrossRef]
- Kitamura, T.; Koshino, Y.; Shibata, F.; Oki, T.; Nakajima, H.; Nosaka, T.; Kumagai, H. Retrovirus-Mediated Gene Transfer and Expression Cloning: Powerful Tools in Functional Genomics. Exp. Hematol. 2003, 31, 1007–1014. [CrossRef]
- Takahashi, K.; Okita, K.; Nakagawa, M.; Yamanaka, S. Induction of Pluripotent Stem Cells from Fibroblast Cultures. Nat. Protoc. 2007, 2, 3081–3089. [CrossRef]
- Varas, F.; Stadtfeld, M.; de Andres-Aguayo, L.; Maherali, N.; di Tullio, A.; Pantano, L.; Notredame, C.; Hochedlinger, K.; Graf, T. Fibroblast-Derived Induced Pluripotent Stem Cells Show No Common Retroviral Vector Insertions. Stem Cells Dayt. Ohio 2009, 27, 300–306. [CrossRef]
- Yao, S.; Sukonnik, T.; Kean, T.; Bharadwaj, R.R.; Pasceri, P.; Ellis, J. Retrovirus Silencing, Variegation, Extinction, and Memory Are Controlled by a Dynamic Interplay of Multiple Epigenetic Modifications. Mol. Ther. J. Am. Soc. Gene Ther. 2004, 10, 27–36. [CrossRef]
- Sommer, C.A.; Stadtfeld, M.; Murphy, G.J.; Hochedlinger, K.; Kotton, D.N.; Mostoslavsky, G. Induced Pluripotent Stem Cell Generation Using a Single Lentiviral Stem Cell Cassette. Stem Cells Dayt. Ohio 2009, 27, 543–549. [CrossRef]
- Woltjen, K.; Michael, I.P.; Mohseni, P.; Desai, R.; Mileikovsky, M.; Hämäläinen, R.; Cowling, R.; Wang, W.; Liu, P.; Gertsenstein, M.; et al. piggyBac Transposition Reprograms Fibroblasts to Induced Pluripotent Stem Cells. Nature 2009, 458, 766–770. [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [CrossRef]
- Lee, C.-T.; Bendriem, R.M.; Wu, W.W.; Shen, R.-F. 3D Brain Organoids Derived from Pluripotent Stem Cells: Promising Experimental Models for Brain Development and Neurodegenerative Disorders. J. Biomed. Sci. 2017, 24, 59. [CrossRef]
- Andrews, M.G.; Kriegstein, A.R. Challenges of Organoid Research. Annu. Rev. Neurosci. 2022, 45, 23–39. [CrossRef]
- Garreta, E.; Kamm, R.D.; Chuva De Sousa Lopes, S.M.; Lancaster, M.A.; Weiss, R.; Trepat, X.; Hyun, I.; Montserrat, N. Rethinking Organoid Technology through Bioengineering. Nat. Mater. 2021, 20, 145–155. [CrossRef]
- Turhan, A.G.; Hwang, J.W.; Chaker, D.; Tasteyre, A.; Latsis, T.; Griscelli, F.; Desterke, C.; Bennaceur-Griscelli, A. iPSC-Derived Organoids as Therapeutic Models in Regenerative Medicine and Oncology. Front. Med. 2021, 8.
- Niu, W.; Parent, J.M. Modeling Genetic Epilepsies in a Dish. Dev. Dyn. 2020, 249, 56–75. [CrossRef]
- Camp, J.G.; Badsha, F.; Florio, M.; Kanton, S.; Gerber, T.; Wilsch-Bräuninger, M.; Lewitus, E.; Sykes, A.; Hevers, W.; Lancaster, M.; et al. Human Cerebral Organoids Recapitulate Gene Expression Programs of Fetal Neocortex Development. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 15672–15677. [CrossRef]
- Wray, S. Modelling Neurodegenerative Disease Using Brain Organoids. Semin. Cell Dev. Biol. 2021, 111, 60–66. [CrossRef]
- Hirose, S.; Tanaka, Y.; Shibata, M.; Kimura, Y.; Ishikawa, M.; Higurashi, N.; Yamamoto, T.; Ichise, E.; Chiyonobu, T.; Ishii, A. Application of Induced Pluripotent Stem Cells in Epilepsy. Mol. Cell. Neurosci. 2020, 108, 103535. [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral Organoids Model Human Brain Development and Microcephaly. Nature 2013, 501, 373–379. [CrossRef]
- Renner, M.; Lancaster, M.A.; Bian, S.; Choi, H.; Ku, T.; Peer, A.; Chung, K.; Knoblich, J.A. Self-organized Developmental Patterning and Differentiation in Cerebral Organoids. EMBO J. 2017, 36, 1316–1329. [CrossRef]
- 2022 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2022, 18, 700–789. [CrossRef]
- Gatz, M.; Reynolds, C.A.; Fratiglioni, L.; Johansson, B.; Mortimer, J.A.; Berg, S.; Fiske, A.; Pedersen, N.L. Role of Genes and Environments for Explaining Alzheimer Disease. Arch. Gen. Psychiatry 2006, 63, 168. [CrossRef]
- Lee, S.J. van der; Wolters, F.J.; Ikram, M.K.; Hofman, A.; Ikram, M.A.; Amin, N.; Duijn, C.M. van The Effect of APOE and Other Common Genetic Variants on the Onset of Alzheimer’s Disease and Dementia: A Community-Based Cohort Study. Lancet Neurol. 2018, 17, 434–444. [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; Van Der Flier, W.M. Alzheimer’s Disease. The Lancet 2021, 397, 1577–1590. [CrossRef]
- Ranjan, V.D.; Qiu, L.; Tan, E.K.; Zeng, L.; Zhang, Y. Modelling Alzheimer’s Disease: Insights from in Vivo to in Vitro Three-Dimensional Culture Platforms. J. Tissue Eng. Regen. Med. 2018, 12, 1944–1958. [CrossRef]
- Bi, F.-C.; Yang, X.-H.; Cheng, X.-Y.; Deng, W.-B.; Guo, X.-L.; Yang, H.; Wang, Y.; Li, J.; Yao, Y. Optimization of Cerebral Organoids: A More Qualified Model for Alzheimer’s Disease Research. Transl. Neurodegener. 2021, 10, 27. [CrossRef]
- Pavoni, S.; Jarray, R.; Nassor, F.; Guyot, A.-C.; Cottin, S.; Rontard, J.; Mikol, J.; Mabondzo, A.; Deslys, J.-P.; Yates, F. Small-Molecule Induction of Aβ-42 Peptide Production in Human Cerebral Organoids to Model Alzheimer’s Disease Associated Phenotypes. PLoS ONE 2018, 13, e0209150. [CrossRef]
- Raja, W.K.; Mungenast, A.E.; Lin, Y.-T.; Ko, T.; Abdurrob, F.; Seo, J.; Tsai, L.-H. Self-Organizing 3D Human Neural Tissue Derived from Induced Pluripotent Stem Cells Recapitulate Alzheimer’s Disease Phenotypes. PLoS ONE 2016, 11, e0161969. [CrossRef]
- Yan, Y.; Song, L.; Bejoy, J.; Zhao, J.; Kanekiyo, T.; Bu, G.; Zhou, Y.; Li, Y. Modeling Neurodegenerative Microenvironment Using Cortical Organoids Derived from Human Stem Cells. Tissue Eng. Part A 2018, 24, 1125–1137. [CrossRef]
- Lin, Y.-T.; Seo, J.; Gao, F.; Feldman, H.M.; Wen, H.-L.; Penney, J.; Cam, H.P.; Gjoneska, E.; Raja, W.K.; Cheng, J.; et al. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron 2018, 98, 1141-1154.e7. [CrossRef]
- Fisher, R.S.; Boas, W. van E.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel Jr., J. Epileptic Seizures and Epilepsy: Definitions Proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [CrossRef]
- Falco-Walter, J. Epilepsy—Definition, Classification, Pathophysiology, and Epidemiology. Semin. Neurol. 2020, 40, 617–623. [CrossRef]
- Organization, W.H. Epilepsy: a public health imperative: summary. 2019.
- MacDonald, M.E.; Ambrose, C.M.; Duyao, M.P.; Myers, R.H.; Lin, C.; Srinidhi, L.; Barnes, G.; Taylor, S.A.; James, M.; Groot, N.; et al. A Novel Gene Containing a Trinucleotide Repeat That Is Expanded and Unstable on Huntington’s Disease Chromosomes. Cell 1993, 72, 971–983. [CrossRef]
- Arrasate, M.; Finkbeiner, S. Protein Aggregates in Huntington’s Disease. Exp. Neurol. 2012, 238, 1–11. [CrossRef]
- Andhale, R.; Shrivastava, D. Huntington’s Disease: A Clinical Review. Cureus 2022, 14, e28484. [CrossRef]
- Tabrizi, S.J.; Flower, M.D.; Ross, C.A.; Wild, E.J. Huntington Disease: New Insights into Molecular Pathogenesis and Therapeutic Opportunities. Nat. Rev. Neurol. 2020, 16, 529–546. [CrossRef]
- Bates, G.P.; Dorsey, R.; Gusella, J.F.; Hayden, M.R.; Kay, C.; Leavitt, B.R.; Nance, M.; Ross, C.A.; Scahill, R.I.; Wetzel, R.; et al. Huntington Disease. Nat. Rev. Dis. Primer 2015, 1, 15005. [CrossRef]
- Ross, C.A.; Aylward, E.H.; Wild, E.J.; Langbehn, D.R.; Long, J.D.; Warner, J.H.; Scahill, R.I.; Leavitt, B.R.; Stout, J.C.; Paulsen, J.S.; et al. Huntington Disease: Natural History, Biomarkers and Prospects for Therapeutics. Nat. Rev. Neurol. 2014, 10, 204–216. [CrossRef]
- Pouladi, M.A.; Morton, A.J.; Hayden, M.R. Choosing an Animal Model for the Study of Huntington’s Disease. Nat. Rev. Neurosci. 2013, 14, 708–721. [CrossRef]
- Ramaswamy, S.; McBride, J.L.; Kordower, J.H. Animal Models of Huntington’s Disease. ILAR J. 2007, 48, 356–373. [CrossRef]
- Park, I.-H.; Lerou, P.H.; Zhao, R.; Huo, H.; Daley, G.Q. Generation of Human-Induced Pluripotent Stem Cells. Nat. Protoc. 2008, 3, 1180–1186. [CrossRef]
- Park, I.-H.; Arora, N.; Huo, H.; Maherali, N.; Ahfeldt, T.; Shimamura, A.; Lensch, M.W.; Cowan, C.; Hochedlinger, K.; Daley, G.Q. Disease-Specific Induced Pluripotent Stem Cells. Cell 2008, 134, 877–886. [CrossRef]
- Zhang, N.; An, M.C.; Montoro, D.; Ellerby, L.M. Characterization of Human Huntington’s Disease Cell Model from Induced Pluripotent Stem Cells. PLoS Curr. 2010, 2, RRN1193. [CrossRef]
- Chae, J.-I.; Kim, D.-W.; Lee, N.; Jeon, Y.-J.; Jeon, I.; Kwon, J.; Kim, J.; Soh, Y.; Lee, D.-S.; Seo, K.S.; et al. Quantitative Proteomic Analysis of Induced Pluripotent Stem Cells Derived from a Human Huntington’s Disease Patient. Biochem. J. 2012, 446, 359–371. [CrossRef]
- HD iPSC Consortium Induced Pluripotent Stem Cells from Patients with Huntington’s Disease Show CAG-Repeat-Expansion-Associated Phenotypes. Cell Stem Cell 2012, 11, 264–278. [CrossRef]
- Mehta, S.R.; Tom, C.M.; Wang, Y.; Bresee, C.; Rushton, D.; Mathkar, P.P.; Tang, J.; Mattis, V.B. Human Huntington’s Disease iPSC-Derived Cortical Neurons Display Altered Transcriptomics, Morphology, and Maturation. Cell Rep. 2018, 25, 1081-1096.e6. [CrossRef]
- Moss, D.J.H.; Pardiñas, A.F.; Langbehn, D.; Lo, K.; Leavitt, B.R.; Roos, R.; Durr, A.; Mead, S.; TRACK-HD investigators; REGISTRY investigators; et al. Identification of Genetic Variants Associated with Huntington’s Disease Progression: A Genome-Wide Association Study. Lancet Neurol. 2017, 16, 701–711. [CrossRef]
- Telenius, H.; Kremer, B.; Goldberg, Y.P.; Theilmann, J.; Andrew, S.E.; Zeisler, J.; Adam, S.; Greenberg, C.; Ives, E.J.; Clarke, L.A. Somatic and Gonadal Mosaicism of the Huntington Disease Gene CAG Repeat in Brain and Sperm. Nat. Genet. 1994, 6, 409–414. [CrossRef]
- Świtońska, K.; Szlachcic, W.J.; Handschuh, L.; Wojciechowski, P.; Marczak, Ł.; Stelmaszczuk, M.; Figlerowicz, M.; Figiel, M. Identification of Altered Developmental Pathways in Human Juvenile HD iPSC With 71Q and 109Q Using Transcriptome Profiling. Front. Cell. Neurosci. 2018, 12, 528. [CrossRef]
- Mattis, V.B.; Tom, C.; Akimov, S.; Saeedian, J.; Østergaard, M.E.; Southwell, A.L.; Doty, C.N.; Ornelas, L.; Sahabian, A.; Lenaeus, L.; et al. HD iPSC-Derived Neural Progenitors Accumulate in Culture and Are Susceptible to BDNF Withdrawal Due to Glutamate Toxicity. Hum. Mol. Genet. 2015, 24, 3257–3271. [CrossRef]
- Smith-Geater, C.; Hernandez, S.J.; Lim, R.G.; Adam, M.; Wu, J.; Stocksdale, J.T.; Wassie, B.T.; Gold, M.P.; Wang, K.Q.; Miramontes, R.; et al. Aberrant Development Corrected in Adult-Onset Huntington’s Disease iPSC-Derived Neuronal Cultures via WNT Signaling Modulation. Stem Cell Rep. 2020, 14, 406–419. [CrossRef]
- An, M.C.; Zhang, N.; Scott, G.; Montoro, D.; Wittkop, T.; Mooney, S.; Melov, S.; Ellerby, L.M. Genetic Correction of Huntington’s Disease Phenotypes in Induced Pluripotent Stem Cells. Cell Stem Cell 2012, 11, 253–263. [CrossRef]
- Ring, K.L.; An, M.C.; Zhang, N.; O’Brien, R.N.; Ramos, E.M.; Gao, F.; Atwood, R.; Bailus, B.J.; Melov, S.; Mooney, S.D.; et al. Genomic Analysis Reveals Disruption of Striatal Neuronal Development and Therapeutic Targets in Human Huntington’s Disease Neural Stem Cells. Stem Cell Rep. 2015, 5, 1023–1038. [CrossRef]
- Xu, X.; Tay, Y.; Sim, B.; Yoon, S.-I.; Huang, Y.; Ooi, J.; Utami, K.H.; Ziaei, A.; Ng, B.; Radulescu, C.; et al. Reversal of Phenotypic Abnormalities by CRISPR/Cas9-Mediated Gene Correction in Huntington Disease Patient-Derived Induced Pluripotent Stem Cells. Stem Cell Rep. 2017, 8, 619–633. [CrossRef]
- HD iPSC Consortium Developmental Alterations in Huntington’s Disease Neural Cells and Pharmacological Rescue in Cells and Mice. Nat. Neurosci. 2017, 20, 648–660. [CrossRef]
- Bose, A.; Petsko, G.A.; Studer, L. Induced Pluripotent Stem Cells: A Tool for Modeling Parkinson’s Disease. Trends Neurosci. 2022, 45, 608–620. [CrossRef]
- Marotta, N.; Kim, S.; Krainc, D. Organoid and Pluripotent Stem Cells in Parkinson’s Disease Modeling: An Expert View on Their Value to Drug Discovery. Expert Opin. Drug Discov. 2020, 15, 427–441. [CrossRef]
- Burré, J.; Sharma, M.; Südhof, T.C. Cell Biology and Pathophysiology of α-Synuclein. Cold Spring Harb. Perspect. Med. 2018, 8, a024091. [CrossRef]
- Dettmer, U.; Newman, A.J.; Soldner, F.; Luth, E.S.; Kim, N.C.; Von Saucken, V.E.; Sanderson, J.B.; Jaenisch, R.; Bartels, T.; Selkoe, D. Parkinson-Causing α-Synuclein Missense Mutations Shift Native Tetramers to Monomers as a Mechanism for Disease Initiation. Nat. Commun. 2015, 6, 7314. [CrossRef]
- Jeong, G.R.; Lee, B.D. Pathological Functions of LRRK2 in Parkinson’s Disease. Cells 2020, 9, 2565. [CrossRef]
- Seibler, P.; Graziotto, J.; Jeong, H.; Simunovic, F.; Klein, C.; Krainc, D. Mitochondrial Parkin Recruitment Is Impaired in Neurons Derived from Mutant PINK1 Induced Pluripotent Stem Cells. J. Neurosci. 2011, 31, 5970–5976. [CrossRef]
- Burbulla, L.F.; Song, P.; Mazzulli, J.R.; Zampese, E.; Wong, Y.C.; Jeon, S.; Santos, D.P.; Blanz, J.; Obermaier, C.D.; Strojny, C.; et al. Dopamine Oxidation Mediates Mitochondrial and Lysosomal Dysfunction in Parkinson’s Disease. Science 2017, 357, 1255–1261. [CrossRef]
- Chen, X.; Sun, G.; Tian, E.; Zhang, M.; Davtyan, H.; Beach, T.G.; Reiman, E.M.; Blurton-Jones, M.; Holtzman, D.M.; Shi, Y. Modeling Sporadic Alzheimer’s Disease in Human Brain Organoids under Serum Exposure. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2021, 8, e2101462. [CrossRef]
- Ghatak, S.; Dolatabadi, N.; Trudler, D.; Zhang, X.; Wu, Y.; Mohata, M.; Ambasudhan, R.; Talantova, M.; Lipton, S.A. Mechanisms of Hyperexcitability in Alzheimer’s Disease hiPSC-Derived Neurons and Cerebral Organoids vs Isogenic Controls. eLife 2019, 8, e50333. [CrossRef]
- Gonzalez, C.; Armijo, E.; Bravo-Alegria, J.; Becerra-Calixto, A.; Mays, C.E.; Soto, C. Modeling Amyloid Beta and Tau Pathology in Human Cerebral Organoids. Mol. Psychiatry 2018, 23, 2363–2374. [CrossRef]
- Arber, C.; Lovejoy, C.; Harris, L.; Willumsen, N.; Alatza, A.; Casey, J.M.; Lines, G.; Kerins, C.; Mueller, A.K.; Zetterberg, H.; et al. Familial Alzheimer’s Disease Mutations in PSEN1 Lead to Premature Human Stem Cell Neurogenesis. Cell Rep. 2021, 34, 108615. [CrossRef]
- Abud, E.M.; Ramirez, R.N.; Martinez, E.S.; Healy, L.M.; Nguyen, C.H.H.; Newman, S.A.; Yeromin, A.V.; Scarfone, V.M.; Marsh, S.E.; Fimbres, C.; et al. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron 2017, 94, 278-293.e9. [CrossRef]
- Zhao, J.; Fu, Y.; Yamazaki, Y.; Ren, Y.; Davis, M.D.; Liu, C.-C.; Lu, W.; Wang, X.; Chen, K.; Cherukuri, Y.; et al. APOE4 Exacerbates Synapse Loss and Neurodegeneration in Alzheimer’s Disease Patient iPSC-Derived Cerebral Organoids. Nat. Commun. 2020, 11, 5540. [CrossRef]
- Park, J.-C.; Jang, S.-Y.; Lee, D.; Lee, J.; Kang, U.; Chang, H.; Kim, H.J.; Han, S.-H.; Seo, J.; Choi, M.; et al. A Logical Network-Based Drug-Screening Platform for Alzheimer’s Disease Representing Pathological Features of Human Brain Organoids. Nat. Commun. 2021, 12, 280. [CrossRef]
- Pérez, M.J.; Ivanyuk, D.; Panagiotakopoulou, V.; Di Napoli, G.; Kalb, S.; Brunetti, D.; Al-Shaana, R.; Kaeser, S.A.; Fraschka, S.A.-K.; Jucker, M.; et al. Loss of Function of the Mitochondrial Peptidase PITRM1 Induces Proteotoxic Stress and Alzheimer’s Disease-like Pathology in Human Cerebral Organoids. Mol. Psychiatry 2021, 26, 5733–5750. [CrossRef]
- Zhou, L.-T.; Liu, D.; Kang, H.-C.; Lu, L.; Huang, H.-Z.; Ai, W.-Q.; Zhou, Y.; Deng, M.-F.; Li, H.; Liu, Z.-Q.; et al. Tau Pathology Epigenetically Remodels the Neuron-Glial Cross-Talk in Alzheimer’s Disease. Sci. Adv. 2023, 9, eabq7105. [CrossRef]
- Lin, Y.-T.; Seo, J.; Gao, F.; Feldman, H.M.; Wen, H.-L.; Penney, J.; Cam, H.P.; Gjoneska, E.; Raja, W.K.; Cheng, J.; et al. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron 2018, 98, 1141-1154.e7. [CrossRef]
- Jin, M.; Xu, R.; Wang, L.; Alam, M.M.; Ma, Z.; Zhu, S.; Martini, A.C.; Jadali, A.; Bernabucci, M.; Xie, P.; et al. Type-I-Interferon Signaling Drives Microglial Dysfunction and Senescence in Human iPSC Models of Down Syndrome and Alzheimer’s Disease. Cell Stem Cell 2022, 29, 1135-1153.e8. [CrossRef]
- Choi, H.; Kim, H.J.; Yang, J.; Chae, S.; Lee, W.; Chung, S.; Kim, J.; Choi, H.; Song, H.; Lee, C.K.; et al. Acetylation Changes Tau Interactome to Degrade Tau in Alzheimer’s Disease Animal and Organoid Models. Aging Cell 2020, 19, e13081. [CrossRef]
- Kim, H.; Park, H.J.; Choi, H.; Chang, Y.; Park, H.; Shin, J.; Kim, J.; Lengner, C.J.; Lee, Y.K.; Kim, J. Modeling G2019S-LRRK2 Sporadic Parkinson’s Disease in 3D Midbrain Organoids. Stem Cell Rep. 2019, 12, 518–531. [CrossRef]
- Becerra-Calixto, A.; Mukherjee, A.; Ramirez, S.; Sepulveda, S.; Sinha, T.; Al-Lahham, R.; De Gregorio, N.; Gherardelli, C.; Soto, C. Lewy Body-like Pathology and Loss of Dopaminergic Neurons in Midbrain Organoids Derived from Familial Parkinson’s Disease Patient. Cells 2023, 12, 625. [CrossRef]
- Zheng, X.; Han, D.; Liu, W.; Wang, X.; Pan, N.; Wang, Y.; Chen, Z. Human iPSC-Derived Midbrain Organoids Functionally Integrate into Striatum Circuits and Restore Motor Function in a Mouse Model of Parkinson’s Disease. Theranostics 2023, 13, 2673–2692. [CrossRef]
- Raja, W.K.; Neves, E.; Burke, C.; Jiang, X.; Xu, P.; Rhodes, K.J.; Khurana, V.; Scannevin, R.H.; Chung, C.Y. Patient-Derived Three-Dimensional Cortical Neurospheres to Model Parkinson’s Disease. PloS One 2022, 17, e0277532. [CrossRef]
- Wulansari, N.; Darsono, W.H.W.; Woo, H.-J.; Chang, M.-Y.; Kim, J.; Bae, E.-J.; Sun, W.; Lee, J.-H.; Cho, I.-J.; Shin, H.; et al. Neurodevelopmental Defects and Neurodegenerative Phenotypes in Human Brain Organoids Carrying Parkinson’s Disease-Linked DNAJC6 Mutations. Sci. Adv. 2021, 7, eabb1540. [CrossRef]
- Sabate-Soler, S.; Nickels, S.L.; Saraiva, C.; Berger, E.; Dubonyte, U.; Barmpa, K.; Lan, Y.J.; Kouno, T.; Jarazo, J.; Robertson, G.; et al. Microglia Integration into Human Midbrain Organoids Leads to Increased Neuronal Maturation and Functionality. Glia 2022, 70, 1267–1288. [CrossRef]
- Chlebanowska, P.; Tejchman, A.; Sułkowski, M.; Skrzypek, K.; Majka, M. Use of 3D Organoids as a Model to Study Idiopathic Form of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 694. [CrossRef]
- Walter, J.; Bolognin, S.; Poovathingal, S.K.; Magni, S.; Gérard, D.; Antony, P.M.A.; Nickels, S.L.; Salamanca, L.; Berger, E.; Smits, L.M.; et al. The Parkinson’s-Disease-Associated Mutation LRRK2-G2019S Alters Dopaminergic Differentiation Dynamics via NR2F1. Cell Rep. 2021, 37, 109864. [CrossRef]
- Samarasinghe, R.A.; Miranda, O.A.; Buth, J.E.; Mitchell, S.; Ferando, I.; Watanabe, M.; Allison, T.F.; Kurdian, A.; Fotion, N.N.; Gandal, M.J.; et al. Identification of Neural Oscillations and Epileptiform Changes in Human Brain Organoids. Nat. Neurosci. 2021, 24, 1488–1500. [CrossRef]
- Steinberg, D.J.; Repudi, S.; Saleem, A.; Kustanovich, I.; Viukov, S.; Abudiab, B.; Banne, E.; Mahajnah, M.; Hanna, J.H.; Stern, S.; et al. Modeling Genetic Epileptic Encephalopathies Using Brain Organoids. EMBO Mol. Med. 2021, 13, e13610. [CrossRef]
- Eichmüller, O.L.; Corsini, N.S.; Vértesy, Á.; Morassut, I.; Scholl, T.; Gruber, V.-E.; Peer, A.M.; Chu, J.; Novatchkova, M.; Hainfellner, J.A.; et al. Amplification of Human Interneuron Progenitors Promotes Brain Tumors and Neurological Defects. Science 2022, 375, eabf5546. [CrossRef]
- Lewitzky, M.; Yamanaka, S. Reprogramming Somatic Cells towards Pluripotency by Defined Factors. Curr. Opin. Biotechnol. 2007, 18, 467–473. [CrossRef]
- Yagi, T.; Ito, D.; Okada, Y.; Akamatsu, W.; Nihei, Y.; Yoshizaki, T.; Yamanaka, S.; Okano, H.; Suzuki, N. Modeling Familial Alzheimer’s Disease with Induced Pluripotent Stem Cells. Hum. Mol. Genet. 2011, 20, 4530–4539. [CrossRef]
- Yamanaka, S. A Fresh Look at iPS Cells. Cell 2009, 137, 13–17. [CrossRef]
- Okano, H.; Yamanaka, S. iPS Cell Technologies: Significance and Applications to CNS Regeneration and Disease. Mol. Brain 2014, 7, 22. [CrossRef]
- Nori, S.; Okada, Y.; Yasuda, A.; Tsuji, O.; Takahashi, Y.; Kobayashi, Y.; Fujiyoshi, K.; Koike, M.; Uchiyama, Y.; Ikeda, E.; et al. Grafted Human-Induced Pluripotent Stem-Cell-Derived Neurospheres Promote Motor Functional Recovery after Spinal Cord Injury in Mice. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 16825–16830. [CrossRef]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [CrossRef]
- Kwokdinata, C.; Ramanujam, V.; Chen, J.; de Oliveira, P.N.; Nai, M.H.; Chooi, W.H.; Lim, C.T.; Ng, S.Y.; David, L.; Chew, S.Y. Encapsulation of Human Spinal Cord Progenitor Cells in Hyaluronan-Gelatin Hydrogel for Spinal Cord Injury Treatment. ACS Appl. Mater. Interfaces 2023. [CrossRef]
- Sieber-Blum, M. Epidermal Neural Crest Stem Cells and Their Use in Mouse Models of Spinal Cord Injury. Brain Res. Bull. 2010, 83, 189–193. [CrossRef]
- Obara, K.; Shirai, K.; Hamada, Y.; Arakawa, N.; Yamane, M.; Takaoka, N.; Aki, R.; Hoffman, R.M.; Amoh, Y. Chronic Spinal Cord Injury Functionally Repaired by Direct Implantation of Encapsulated Hair-Follicle-Associated Pluripotent (HAP) Stem Cells in a Mouse Model: Potential for Clinical Regenerative Medicine. PloS One 2022, 17, e0262755. [CrossRef]
- Polentes, J.; Jendelova, P.; Cailleret, M.; Braun, H.; Romanyuk, N.; Tropel, P.; Brenot, M.; Itier, V.; Seminatore, C.; Baldauf, K.; et al. Human Induced Pluripotent Stem Cells Improve Stroke Outcome and Reduce Secondary Degeneration in the Recipient Brain. Cell Transplant. 2012, 21, 2587–2602. [CrossRef]
- Kawai, H.; Yamashita, T.; Ohta, Y.; Deguchi, K.; Nagotani, S.; Zhang, X.; Ikeda, Y.; Matsuura, T.; Abe, K. Tridermal Tumorigenesis of Induced Pluripotent Stem Cells Transplanted in Ischemic Brain. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2010, 30, 1487–1493. [CrossRef]
- Upadhya, D.; Hattiangady, B.; Castro, O.W.; Shuai, B.; Kodali, M.; Attaluri, S.; Bates, A.; Dong, Y.; Zhang, S.-C.; Prockop, D.J.; et al. Human Induced Pluripotent Stem Cell-Derived MGE Cell Grafting after Status Epilepticus Attenuates Chronic Epilepsy and Comorbidities via Synaptic Integration. Proc. Natl. Acad. Sci. 2019, 116, 287–296. [CrossRef]
- Cunningham, M.; Cho, J.-H.; Leung, A.; Savvidis, G.; Ahn, S.; Moon, M.; Lee, P.K.J.; Han, J.J.; Azimi, N.; Kim, K.-S.; et al. hPSC-Derived Maturing GABAergic Interneurons Ameliorate Seizures and Abnormal Behavior in Epileptic Mice. Cell Stem Cell 2014, 15, 559–573. [CrossRef]
- Martin, P.; Wagh, V.; Reis, S.A.; Erdin, S.; Beauchamp, R.L.; Shaikh, G.; Talkowski, M.; Thiele, E.; Sheridan, S.D.; Haggarty, S.J.; et al. TSC Patient-Derived Isogenic Neural Progenitor Cells Reveal Altered Early Neurodevelopmental Phenotypes and Rapamycin-Induced MNK-eIF4E Signaling. Mol. Autism 2020, 11, 2. [CrossRef]
- Cai, J.; Yang, M.; Poremsky, E.; Kidd, S.; Schneider, J.S.; Iacovitti, L. Dopaminergic Neurons Derived from Human Induced Pluripotent Stem Cells Survive and Integrate into 6-OHDA-Lesioned Rats. Stem Cells Dev. 2010, 19, 1017–1023. [CrossRef]
- Meneghini, V.; Frati, G.; Sala, D.; De Cicco, S.; Luciani, M.; Cavazzin, C.; Paulis, M.; Mentzen, W.; Morena, F.; Giannelli, S.; et al. Generation of Human Induced Pluripotent Stem Cell-Derived Bona Fide Neural Stem Cells for Ex Vivo Gene Therapy of Metachromatic Leukodystrophy. Stem Cells Transl. Med. 2017, 6, 352–368. [CrossRef]
- Yoon, Y.; Kim, H.S.; Hong, C.P.; Li, E.; Jeon, I.; Park, H.J.; Lee, N.; Pei, Z.; Song, J. Neural Transplants From Human Induced Pluripotent Stem Cells Rescue the Pathology and Behavioral Defects in a Rodent Model of Huntington’s Disease. Front. Neurosci. 2020, 14, 558204. [CrossRef]
- Chang, C.-Y.; Ting, H.-C.; Liu, C.-A.; Su, H.-L.; Chiou, T.-W.; Lin, S.-Z.; Harn, H.-J.; Ho, T.-J. Induced Pluripotent Stem Cell (iPSC)-Based Neurodegenerative Disease Models for Phenotype Recapitulation and Drug Screening. Mol. Basel Switz. 2020, 25, 2000. [CrossRef]
- Aboul-Soud, M.A.M.; Alzahrani, A.J.; Mahmoud, A. Induced Pluripotent Stem Cells (iPSCs)-Roles in Regenerative Therapies, Disease Modelling and Drug Screening. Cells 2021, 10, 2319. [CrossRef]
- Kondo, T.; Asai, M.; Tsukita, K.; Kutoku, Y.; Ohsawa, Y.; Sunada, Y.; Imamura, K.; Egawa, N.; Yahata, N.; Okita, K.; et al. Modeling Alzheimer’s Disease with iPSCs Reveals Stress Phenotypes Associated with Intracellular Aβ and Differential Drug Responsiveness. Cell Stem Cell 2013, 12, 487–496. [CrossRef]
- Miura, K.; Okada, Y.; Aoi, T.; Okada, A.; Takahashi, K.; Okita, K.; Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Ohnuki, M.; et al. Variation in the Safety of Induced Pluripotent Stem Cell Lines. Nat. Biotechnol. 2009, 27, 743–745. [CrossRef]
- Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Takahashi, K.; Ichisaka, T.; Aoi, T.; Okita, K.; Mochiduki, Y.; Takizawa, N.; Yamanaka, S. Generation of Induced Pluripotent Stem Cells without Myc from Mouse and Human Fibroblasts. Nat. Biotechnol. 2008, 26, 101–106. [CrossRef]
- Okita, K.; Nakagawa, M.; Hyenjong, H.; Ichisaka, T.; Yamanaka, S. Generation of Mouse Induced Pluripotent Stem Cells without Viral Vectors. Science 2008, 322, 949–953. [CrossRef]
- Sugai, K.; Sumida, M.; Shofuda, T.; Yamaguchi, R.; Tamura, T.; Kohzuki, T.; Abe, T.; Shibata, R.; Kamata, Y.; Ito, S.; et al. First-in-Human Clinical Trial of Transplantation of iPSC-Derived NS/PCs in Subacute Complete Spinal Cord Injury: Study Protocol. Regen. Ther. 2021, 18, 321–333. [CrossRef]
- Hoveizi, E.; Mohammadi, T.; Moazedi, A.A.; Zamani, N.; Eskandary, A. Transplanted Neural-like Cells Improve Memory and Alzheimer-like Pathology in a Rat Model. Cytotherapy 2018, 20, 964–973. [CrossRef]
- Armijo, E.; Edwards, G.; Flores, A.; Vera, J.; Shahnawaz, M.; Moda, F.; Gonzalez, C.; Sanhueza, M.; Soto, C. Induced Pluripotent Stem Cell-Derived Neural Precursors Improve Memory, Synaptic and Pathological Abnormalities in a Mouse Model of Alzheimer’s Disease. Cells 2021, 10, 1802. [CrossRef]
- Yahata, N.; Asai, M.; Kitaoka, S.; Takahashi, K.; Asaka, I.; Hioki, H.; Kaneko, T.; Maruyama, K.; Saido, T.C.; Nakahata, T.; et al. Anti-Aβ Drug Screening Platform Using Human iPS Cell-Derived Neurons for the Treatment of Alzheimer’s Disease. PloS One 2011, 6, e25788. [CrossRef]
- Brownjohn, P.W.; Smith, J.; Portelius, E.; Serneels, L.; Kvartsberg, H.; De Strooper, B.; Blennow, K.; Zetterberg, H.; Livesey, F.J. Phenotypic Screening Identifies Modulators of Amyloid Precursor Protein Processing in Human Stem Cell Models of Alzheimer’s Disease. Stem Cell Rep. 2017, 8, 870–882. [CrossRef]
- Al-Gharaibeh, A.; Culver, R.; Stewart, A.N.; Srinageshwar, B.; Spelde, K.; Frollo, L.; Kolli, N.; Story, D.; Paladugu, L.; Anwar, S.; et al. Induced Pluripotent Stem Cell-Derived Neural Stem Cell Transplantations Reduced Behavioral Deficits and Ameliorated Neuropathological Changes in YAC128 Mouse Model of Huntington’s Disease. Front. Neurosci. 2017, 11, 628. [CrossRef]
- Cho, I.K.; Hunter, C.E.; Ye, S.; Pongos, A.L.; Chan, A.W.S. Combination of Stem Cell and Gene Therapy Ameliorates Symptoms in Huntington’s Disease Mice. NPJ Regen. Med. 2019, 4, 7. [CrossRef]
- Park, H.J.; Han, A.; Kim, J.Y.; Choi, J.; Bae, H.S.; Cho, G.-B.; Shin, H.; Shin, E.J.; Lee, K.-I.; Kim, S.; et al. SUPT4H1-Edited Stem Cell Therapy Rescues Neuronal Dysfunction in a Mouse Model for Huntington’s Disease. NPJ Regen. Med. 2022, 7, 8. [CrossRef]
- Doerr, J.; Böckenhoff, A.; Ewald, B.; Ladewig, J.; Eckhardt, M.; Gieselmann, V.; Matzner, U.; Brüstle, O.; Koch, P. Arylsulfatase A Overexpressing Human iPSC-Derived Neural Cells Reduce CNS Sulfatide Storage in a Mouse Model of Metachromatic Leukodystrophy. Mol. Ther. J. Am. Soc. Gene Ther. 2015, 23, 1519–1531. [CrossRef]
- Alekseenko, Z.; Dias, J.M.; Adler, A.F.; Kozhevnikova, M.; van Lunteren, J.A.; Nolbrant, S.; Jeggari, A.; Vasylovska, S.; Yoshitake, T.; Kehr, J.; et al. Robust Derivation of Transplantable Dopamine Neurons from Human Pluripotent Stem Cells by Timed Retinoic Acid Delivery. Nat. Commun. 2022, 13, 3046. [CrossRef]
- Brot, S.; Thamrin, N.P.; Bonnet, M.-L.; Francheteau, M.; Patrigeon, M.; Belnoue, L.; Gaillard, A. Long-Term Evaluation of Intranigral Transplantation of Human iPSC-Derived Dopamine Neurons in a Parkinson’s Disease Mouse Model. Cells 2022, 11, 1596. [CrossRef]
- Guo, Y.; Guan, Y.; Zhu, H.; Sun, T.; Wang, Y.; Huang, Y.; Ma, C.; Emery, R.; Guan, W.; Wang, C.; et al. Therapeutic Function of iPSCs-Derived Primitive Neuroepithelial Cells in a Rat Model of Parkinson’s Disease. Neurochem. Int. 2022, 155, 105324. [CrossRef]
- Hiller, B.M.; Marmion, D.J.; Thompson, C.A.; Elliott, N.A.; Federoff, H.; Brundin, P.; Mattis, V.B.; McMahon, C.W.; Kordower, J.H. Optimizing Maturity and Dose of iPSC-Derived Dopamine Progenitor Cell Therapy for Parkinson’s Disease. NPJ Regen. Med. 2022, 7, 24. [CrossRef]
- Grinand, L.; Takahashi, J. Automated Measurement of Fluorescence Signals Reveals a Significant Increase of the Graft-Derived Neurite Extension in Neonates Compared to Aged Rats. Regen. Ther. 2022, 19, 97–106. [CrossRef]
- Nakamura, R.; Nonaka, R.; Oyama, G.; Jo, T.; Kamo, H.; Nuermaimaiti, M.; Akamatsu, W.; Ishikawa, K.-I.; Hattori, N. A Defined Method for Differentiating Human iPSCs into Midbrain Dopaminergic Progenitors That Safely Restore Motor Deficits in Parkinson’s Disease. Front. Neurosci. 2023, 17, 1202027. [CrossRef]
- Guo, Y.; Zhu, H.; Wang, Y.; Sun, T.; Xu, J.; Wang, T.; Guan, W.; Wang, C.; Liu, C.; Ma, C. Miniature-Swine iPSC-Derived GABA Progenitor Cells Function in a Rat Parkinson’s Disease Model. Cell Tissue Res. 2023, 391, 425–440. [CrossRef]
- Zheng, X.; Han, D.; Liu, W.; Wang, X.; Pan, N.; Wang, Y.; Chen, Z. Human iPSC-Derived Midbrain Organoids Functionally Integrate into Striatum Circuits and Restore Motor Function in a Mouse Model of Parkinson’s Disease. Theranostics 2023, 13, 2673–2692. [CrossRef]
- Lavoie, N.S.; Truong, V.; Malone, D.; Pengo, T.; Patil, N.; Dutton, J.R.; Parr, A.M. Human Induced Pluripotent Stem Cells Integrate, Create Synapses and Extend Long Axons after Spinal Cord Injury. J. Cell. Mol. Med. 2022, 26, 1932–1942. [CrossRef]
- Kitagawa, T.; Nagoshi, N.; Kamata, Y.; Kawai, M.; Ago, K.; Kajikawa, K.; Shibata, R.; Sato, Y.; Imaizumi, K.; Shindo, T.; et al. Modulation by DREADD Reveals the Therapeutic Effect of Human iPSC-Derived Neuronal Activity on Functional Recovery after Spinal Cord Injury. Stem Cell Rep. 2022, 17, 127–142. [CrossRef]
- Shibata, T.; Tashiro, S.; Shibata, S.; Shinozaki, M.; Shindo, T.; Hashimoto, S.; Kawai, M.; Kitagawa, T.; Ago, K.; Matsumoto, M.; et al. Rehabilitative Training Enhances Therapeutic Effect of Human iPSC-Derived Neural Stem/Progenitor Cells Transplantation in Chronic Spinal Cord Injury. Stem Cells Transl. Med. 2023, 12, 83–96. [CrossRef]
- Zheng, Y.; Gallegos, C.M.; Xue, H.; Li, S.; Kim, D.H.; Zhou, H.; Xia, X.; Liu, Y.; Cao, Q. Transplantation of Human Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Promotes Forelimb Functional Recovery after Cervical Spinal Cord Injury. Cells 2022, 11, 2765. [CrossRef]
- Deng, P.; Wang, L.; Zhang, Q.; Chen, S.; Zhang, Y.; Xu, H.; Chen, H.; Xu, Y.; He, W.; Zhang, J.; et al. Therapeutic Potential of a Combination of Electroacupuncture and Human iPSC-Derived Small Extracellular Vesicles for Ischemic Stroke. Cells 2022, 11, 820. [CrossRef]
- Kaiser, E.E.; Waters, E.S.; Yang, X.; Fagan, M.M.; Scheulin, K.M.; Sneed, S.E.; Cheek, S.R.; Jeon, J.H.; Shin, S.K.; Kinder, H.A.; et al. Tanshinone IIA-Loaded Nanoparticle and Neural Stem Cell Therapy Enhances Recovery in a Pig Ischemic Stroke Model. Stem Cells Transl. Med. 2022, 11, 1061–1071. [CrossRef]
- Chen, Y.; Song, F.; Tu, M.; Wu, S.; He, X.; Liu, H.; Xu, C.; Zhang, K.; Zhu, Y.; Zhou, R.; et al. Quantitative Proteomics Revealed Extensive Microenvironmental Changes after Stem Cell Transplantation in Ischemic Stroke. Front. Med. 2022, 16, 429–441. [CrossRef]
- Arakawa, M.; Sakamoto, Y.; Miyagawa, Y.; Nito, C.; Takahashi, S.; Nitahara-Kasahara, Y.; Suda, S.; Yamazaki, Y.; Sakai, M.; Kimura, K.; et al. iPSC-Derived Mesenchymal Stem Cells Attenuate Cerebral Ischemia-Reperfusion Injury by Inhibiting Inflammatory Signaling and Oxidative Stress. Mol. Ther. Methods Clin. Dev. 2023, 30, 333–349. [CrossRef]
- Cao, S.-Y.; Tao, M.-D.; Lou, S.-N.; Yang, D.; Lin, Y.-H.; Wu, H.-Y.; Chang, L.; Luo, C.-X.; Xu, Y.; Liu, Y.; et al. Functional Reconstruction of the Impaired Cortex and Motor Function by hMGEOs Transplantation in Stroke. Biochem. Biophys. Res. Commun. 2023, 671, 87–95. [CrossRef]
- Martinez-Curiel, R.; Jansson, L.; Tsupykov, O.; Avaliani, N.; Aretio-Medina, C.; Hidalgo, I.; Monni, E.; Bengzon, J.; Skibo, G.; Lindvall, O.; et al. Oligodendrocytes in Human Induced Pluripotent Stem Cell-Derived Cortical Grafts Remyelinate Adult Rat and Human Cortical Neurons. Stem Cell Rep. 2023, 18, 1643–1656. [CrossRef]
- Hulme, A.J.; Maksour, S.; St-Clair Glover, M.; Miellet, S.; Dottori, M. Making Neurons, Made Easy: The Use of Neurogenin-2 in Neuronal Differentiation. Stem Cell Rep. 2022, 17, 14–34. [CrossRef]
- Barak, M.; Fedorova, V.; Pospisilova, V.; Raska, J.; Vochyanova, S.; Sedmik, J.; Hribkova, H.; Klimova, H.; Vanova, T.; Bohaciakova, D. Human iPSC-Derived Neural Models for Studying Alzheimer’s Disease: From Neural Stem Cells to Cerebral Organoids. Stem Cell Rev. Rep. 2022, 18, 792–820. [CrossRef]
- Andrews, P., Cavagnaro, J., Deans, R. et al. Harmonizing standards for producing clinical-grade therapies from pluripotent stem cells. Nat Biotechnol, 2014, 32, 724–726.
- Okano H, Nakamura M, Yoshida K, Okada Y, Tsuji O, Nori S, Ikeda E, Yamanaka S, Miura K. Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res. 2013, 112, 523-533.
| Organoid Type | Disease | Cell Type | Result | Reference |
|---|---|---|---|---|
| Cerebral Organoid | AD | iPSC | Modeling Sporadic Alzheimer’s Disease in Human Brain Organoids under Serum Exposure | [116] |
| Cerebral Organoid | AD | hiPSC | Mechanisms of hyperexcitability in Alzheimer’s disease hiPSC-derived neurons and cerebral organoids vs isogenic controls | [117] |
| Cerebral Organoid | AD | iPSC | Modeling amyloid beta and tau pathology in human cerebral organoids | [118] |
| Disease Stem Cell | AD | iPSC | Familial Alzheimer’s Disease Mutations in PSEN1 Lead to Premature Human Stem Cell Neurogenesis | [119] |
| Disease Stem Cell | AD | iPSC and hiPSC | iPSC-derived human microglia-like cells to study neurological diseases | [120] |
| Cerebral Organoid | AD | iPSC | APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer’s disease patient iPSC-derived cerebral organoids | [121] |
| Cerebral Organoid | AD | iPSC | A logical network-based drug-screening platform for Alzheimer’s disease representing pathological features of human brain organoids | [122] |
| Cerebral Organoid | AD | iPSC | Loss of function of the mitochondrial peptidase PITRM1 induces proteotoxic stress and Alzheimer’s disease-like pathology in human cerebral organoids | [123] |
| Cerebral Organoid | AD | iPSC | Tau pathology epigenetically remodels the neuron-glial cross-talk in Alzheimer’s disease | [124] |
| Disease Stem Cell | AD | iPSC | APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types | [125] |
| Disease Stem Cell | AD | iPSC | Type I Interferon Signaling Drives Microglial Dysfunction and Senescence in Human iPSC Models of Down Syndrome and Alzheimer’s Disease | [126] |
| Cerebral Organoid | AD | iPSC | Acetylation changes tau interactome to degrade tau in Alzheimer’s disease animal and organoid models | [127] |
| Cerebral Organoid | PD | hiPSC | Modeling G2019S-LRRK2 Sporadic Parkinson’s Disease in 3D Midbrain Organoids | [128] |
| Cerebral Organoid | PD | hiPSC | Lewy Body-like Pathology and Loss of Dopaminergic Neurons in Midbrain Organoids Derived from Familial Parkinson’s Disease Patient | [129] |
| Midbrain Organoid | PD | hiPSC | Human iPSC-derived midbrain organoids functionally integrate into striatum circuits and restore motor function in a mouse model of Parkinson’s disease | [130] |
| Neurospheres | PD | hiPSC and iPSC | Patient-derived three-dimensional cortical neurospheres to model Parkinson’s disease | [131] |
| Midbrain Organoid | PD | hiPSC and iPSC | Neurodevelopmental defects and neurodegenerative phenotypes in human brain organoids carrying Parkinson’s disease linked DNAJC6 mutations | [132] |
| Midbrain Organoid | PD | iPSC | Microglia integration into human midbrain organoids leads to increased neuronal maturation and functionality | [133] |
| Cerebral Organoid | PD | iPSC | Use of 3D Organoids as a Model to Study Idiopathic Form of Parkinson’s Disease | [134] |
| Cerebral Organoid | PD | iPSC | The Parkinson’s-disease-associated mutation LRRK2-G2019S alters dopaminergic differentiation dynamics via NR2F1 | [135] |
| Cerebral Organoid | Rett syndrome | hiPSC | Identification of neural oscillations and epileptiform changes in human brain organoids | [136] |
| Cerebral Organoid | TLE | iPSC | Modeling genetic epileptic encephalopathies using brain organoids | [137] |
| Cerebral Organoid | TSC | hiPSC | Amplification of human interneuron progenitors promotes brain tumors and neurological defects | [138] |
| Trial Type | Disease | Target | Result | Reference |
|---|---|---|---|---|
| Cell Therapy | AD | Rat | The transplanted rats rescued Alzheimer's cognition. | [163] |
| Cell Therapy | AD | Mouse | Grafted mice showed improved memory, synaptic plasticity, and reduced AD brain pathology, including a reduction of amyloid and tangles deposits. | [164] |
| Drug Screening | AD | hiPSC | β-secretase inhibitor IV (BSI) and γ-secretase inhibitor XXI/Compound E (GSI) showed similar effects as screening in other models. | [165] |
| Drug Screening | AD | hiPSC | Docosahexaenoic acid (DHA) treatment alleviated the stress responses in the AD neural cells. | [158] |
| Drug Screening | AD | hiPSC | The anthelminthic avermectins increases the relative production of short forms of Aβ and reduces the relative production of longer Aβ fragments in human cortical neurons. | [166] |
| Cell Therapy | HD | Mice | iPSCs survived and differentiated into region-specific neurons in both mice groups without tumor formation. | [167] |
| Cell Therapy | HD | Mice | Grafted mice showed a significant increase in lifespan. In iPSCs groups, animals showed significant improvement in motor functions and grip strength. | [168] |
| Cell Therapy | HD | Rat | Grafted rats showed significant behavioral improvements for up to 12 weeks. iPSCs enhanced endogenous neurogenesis and reconstituted the damaged neuronal connections. | [155] |
| Cell Therapy | HD | Mice | Improved neuronal dysfunction by SUPT4H1-edited iPSCs grafts. | [169] |
| Cell Therapy | MLD | Mice | Transplantation of ARSA-overexpressing precursors into ARSA-deficient mice significantly reduced sulfatide storage up to 300 µm from grafted cells. | [170] |
| Cell Therapy | MLD | Mice | Grafts of iPSCs into neonatal and adult immunodeficient MLD mice stably restored arylsulfatase A (ARSA) activity in the whole central nervous system and a significant decrease of sulfatide storage when ARSA-overexpressing cells were used. | [154] |
| Cell Therapy | PD | Rat | iPSC graft differentiated into mature mDA neurons that survive over long term and restored motor function. | [171] |
| Cell Therapy | PD | Mice | hiPSCs differentiated into mDA neurons and there was long-term motor functional recovery after transplantation. | [172] |
| Cell Therapy | PD | Rat | Grafted iPSCs could survive in Parkinsonian rat brain for at least 150 days, and many of them differentiated into tyrosine hydroxylase (TH)-positive cells. | [173] |
| Cell Therapy | PD | Rat | Intranigral engraftment to the ventral midbrain demonstrated that mDA progenitors cryopreserved on day 17, cells had a greater capacity than immature mDA neuron cells to innervate over long distances to forebrain structures. | [174] |
| Cell Therapy | PD | Rat | hiPSCs-derived dopaminergic progenitor cells integrate better into the striatum of neonates than older rats. | [175] |
| Cell Therapy | PD | Mice | More than 90% of the engrafted cells differentiated into the lineage of mDA neurons, and approximately 15% developed into mature mDA neurons without tumor formation. | [176] |
| Cell Therapy | PD | Rat | There was a neural remodel of basal ganglia circuitry and no tumorigenicity. | [177] |
| Cell Therapy | PD | Mice | iPSCs matured into mDA neurons and reverse motor function and establishment of bidirectional connections with natural brain target regions, without tumor formation. | [178] |
| Cell Therapy | SCI | Rat | Transplanted cells displayed robust integration properties including synapse formation and myelination by host. | [179] |
| Cell Therapy | SCI | Mice | Due to DREADD expression, it was shown a significant decrease in locomotor dysfunction in SCI-grafted mice, which was exclusively observed following the neurons maturation. | [180] |
| Cell Therapy | SCI | Mice | The combination of iPSCs graft and rehabilitative training therapy significantly improved motor functions. | [181] |
| Cell Therapy | SCI | Rat | Neuro pluripotent cells derived from iPSC were able to survive and differentiate into both neurons and astrocytes and, improved forelimb locomotor function. | [182] |
| Cell Therapy | Stroke | Mice | Combination of electroacupuncture and iPSC-derived extracellular vesicles treatment ameliorated neurological impairments and reduced the infarct volume and neuronal apoptosis in MCAO mice. | [183] |
| Cell Therapy | Stroke | Pig | Tanshinone IIA nanoparticles increased iPSCs engraftment, enhanced cellular and tissue recovery, and improved neurological function in a translational pig stroke model. | [184] |
| Cell Therapy | Stroke | Rat | Increased glucose metabolism and neurofunctional in iPSCs-transplanted rats. | [185] |
| Cell Therapy | Stroke | Rat | Graft of iPSCs inhibited microglial activation and expression of proinflammatory cytokines and suppressed oxidative stress and neuronal death in the cerebral cortex at the ischemic border zone. | [186] |
| Cell Therapy | Stroke | Mice | Graft survived well and primarily differentiated into GABAergic interneurons and significantly restored the sensorimotor deficits of stroke mice for a long time. | [187] |
| Cell Therapy | Stroke | Rat | Generated oligodendrocytes survived and formed myelin-ensheathing human axons in the host tissue after grafting onto adult human cortical organotypic cultures. | [188] |
| Cell Therapy | TLE | Mice | A much-reduced frequency of spontaneous recurrent seizures in grafted animals. | [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





