1. Introduction
Amplification and/or overexpression of the human epidermal growth factor receptor 2 (HER2) is a well-established prognostic marker for aggressive disease progression in patients with breast cancer [
1]. The approval of trastuzumab, a monoclonal antibody targeting HER2, in 1998, marked a significant advancement in the therapeutic landscape for HER2-positive breast cancer [
2]. Subsequently, there has been a substantial increase in the clinical development of innovative therapies aimed at this specific cancer subtype [
3]. The strategic incorporation of monoclonal antibodies and tyrosine kinase inhibitors, along with chemotherapy, endocrine therapy, and immunotherapy, has profoundly transformed the clinical outcomes for individuals diagnosed with HER2-positive breast cancer [
4]. This paradigm shift has been evidenced at both the early and advanced stages of the disease [
5].
Antibody-drug conjugates (ADCs) are an advanced modality in oncological treatment, integrating the specificity of monoclonal antibodies with the cytotoxic power of potent drugs [
6]. As targeted therapeutics, ADCs are designed to selectively home in on and neutralize cancer cells expressing antigens, with a notable focus on HER2. This targeted approach amplifies treatment efficacy while minimizing the widespread adverse effects typically associated with conventional chemotherapy. The realm of ADCs has experienced exponential progress, characterized by substantial advancements in research and development, culminating in regulatory approvals that highlight their transformative role in medical oncology. To date, two ADCs directed against HER2 - trastuzumab emtansine (T-DM1, Kadcyla) [
7] and trastuzumab deruxtecan (T-DXd, DS-8201a, Enhertu) [
8]- have been approved by the Food and Drug Administration (FDA) and other regulatory agencies throughout the world for the management of HER2-positive breast cancer. In addition, T-DXd was approved for patients with HER2-Low metastatic breast cancer [
9]. The DESTINY-Breast06 trial is testing the efficacy of T-DXd in patients with HER2-Ultra-Low breast cancer (IHC score 0, with 1-10% cells staining weakly).
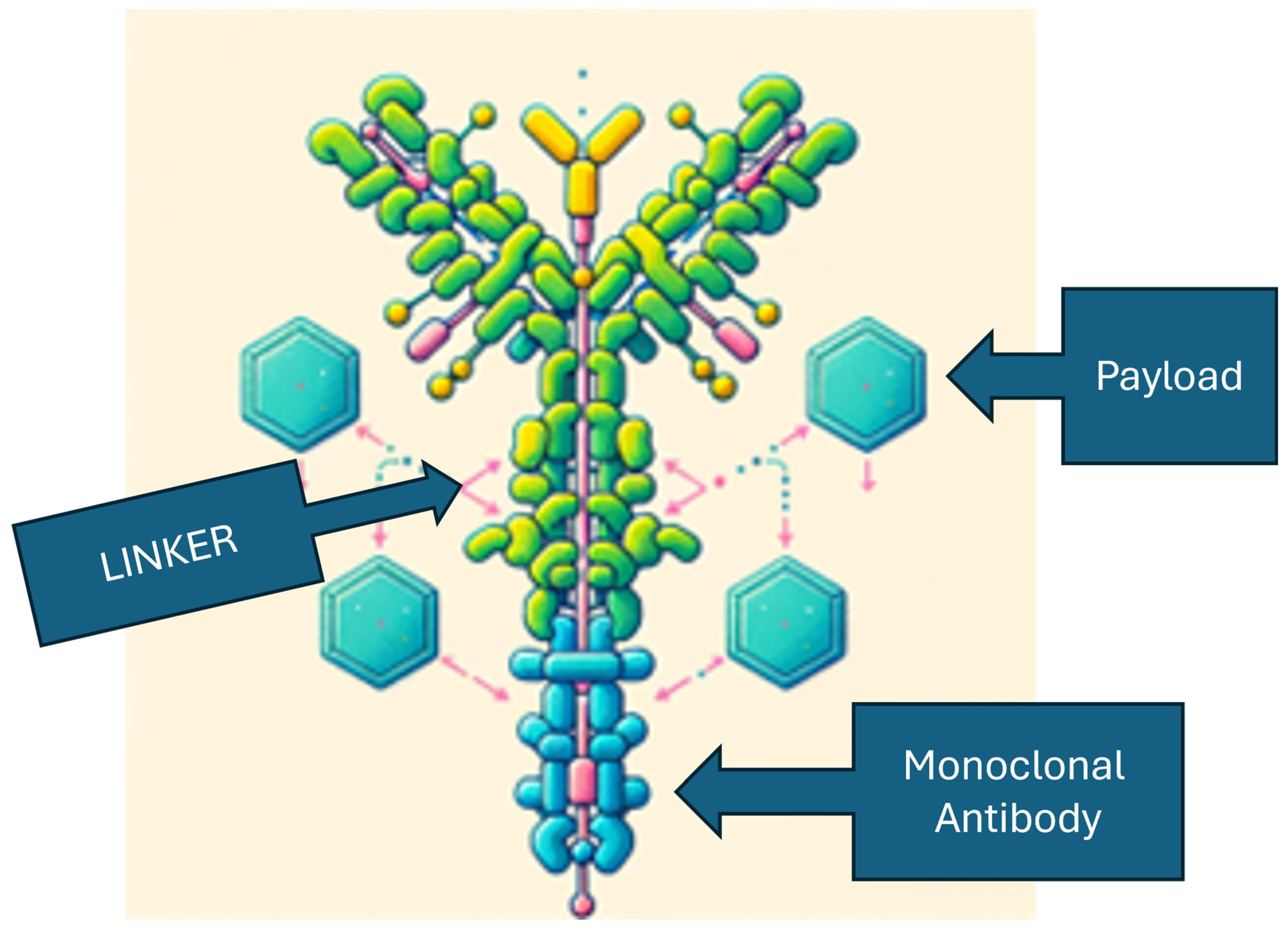
The intricate action of ADCs is orchestrated by three integral elements: a monoclonal antibody, a chemical linker with stability or cleavability properties, and a cytotoxic agent [
10]. The engineered monoclonal antibody is fine-tuned to detect and bind to an antigen prevalently expressed on the surface of cancer cells (e.g., trastuzumab). Following attachment, the antibody-drug complex is internalized via receptor-mediated endocytosis, a selective ingress contingent on distinctive cellular receptors [
11]. While pinocytosis may also facilitate ADC uptake in the absence of the target antigen, the conjugated antibody's considerable size and hydrophilic character significantly mitigate nonspecific absorption, thereby augmenting the specificity and safety of ADCs.
Upon cellular entry, the ADC is trafficked to endosomes and lysosomes, where enzymatic cleavage of the linker ensues, culminating in the release of the cytotoxic payload. This release enables the drug to unleash its cell-killing potential. The therapeutic agents employed in ADCs are diverse, ranging from microtubule disruptors to topoisomerase inhibitors. A pivotal feature of ADCs is the 'bystander effect,' wherein the liberated toxins can permeate and exterminate neighboring tumor cells that may not express the target antigen, thereby facilitating a more thorough elimination of malignant cells [
12].
ADCs targeting HER2 have emerged as a vanguard in the therapeutic arsenal against breast cancer. HER2 is a vital receptor implicated in cellular proliferation and growth regulation. ADCs targeting HER2 typically utilize trastuzumab as the antibody constituent, which specifically antagonizes the HER2 receptor, thus melding the precision of targeted therapy with the destructive force of chemotherapeutic agents (
Table 1).
2. Trastuzumab Emtansine (T-DM1)
Trastuzumab emtansine (T-DM1) represents a seminal advancement in the treatment of breast cancer, acting as the archetype ADC approved for this indication, both in the metastatic and adjuvant settings.
Maytansine is a highly potent cytotoxic agent derived from the Ethiopian plant Maytenus serrata. Due to its high toxicity, it's not used directly as a cancer treatment but rather as a part of a targeted therapy. Emtansine (also known as DM1) is a derivative of maytansine that has been chemically modified to be less toxic and more stable in the bloodstream. It is used as the cytotoxic component of T-DM1, where it is attached to the antibody trastuzumab through a stable linker. T-DM1 retains trastuzumab's inhibitory functions—especially the blockade of the PI3K/AKT pathway. In addition, trastuzumab facilitates T-DM1’s internalization and subsequent disintegration to unleash the potent microtubule-inhibitory action of the MCC-DM1 complex [
13].
The EMILIA trial showcased the preferential outcomes of T-DM1, demonstrating its ability to improve progression-free survival (PFS) rates compared to a regimen that combined lapatinib and capecitabine in patients with HER2-positive metastatic breast cancer who had received a prior taxane [
7]. The KATHERINE trial showed improvement in disease-free survival (DFS) rates in patients with early-stage HER2-positive breast cancer with residual disease following neoadjuvant trastuzumab-based treatment [
14]. This randomized trial validated the role of T-DM1 in this setting, compared to continued trastuzumab therapy.
In the KRISTINE trial, T-DM1 therapy was compared to sequential anthracycline-based chemotherapy followed by taxane in combination with trastuzumab and pertuzumab, or the TCHP (docetaxel, carboplatin, trastuzumab, pertuzumab) regimen in the neoadjuvant setting. In this study, T-DM1 demonstrated a reduced pathologic complete response rate compared to the other regimens [
15]. Nevertheless, the KRISTINE trial linked it to a more favorable safety profile, achieving pathologic complete responses in 44% of patients without conventional chemotherapy.
The most prevalent adverse effects associated with T-DM1 treatment are thrombocytopenia and liver enzyme elevation, highlighting the necessity for vigilant laboratory monitoring throughout the therapy [
7,
14,
15].
2.1. Mechanisms of Resistance to T-DM1
The immune-related mechanisms of action of T-DM1 is mediated by a complex interplay involving the IgG1 framework, which prompts antibody-dependent cellular cytotoxicity (ADCC), and HER2 binding that impedes PI3K/AKT signaling. The cytotoxicity of the drug is delivered by emtansine, which disrupts microtubules following its release into the cytosol. Resistance may be precipitated by defective binding, as well as impaired intracellular processing and metabolism, potentially augmenting the cellular expulsion of T-DM1 or altering its lysosomal degradation [
16].
Key factors contributing to resistance encompass HER2 expression downregulation, intracellular routing changes, lysosomal function debilitation, drug removal through efflux pumps, and activation of compensatory signaling pathways. Genetic mutations, such as those in PIK3CA or PTEN loss, can activate the PI3K-AKT-mTOR pathway, furthering resistance [
17]. Additionally, diminished colocalization with caveolin-1 (CAV1), essential for endocytic transport, might impair drug binding and sensitivity [
17,
18]. Finally, tumor cells might evade immunological detection, resisting T-DM1 therapy [
19]. Immunomodulatory strategies are under investigation to counteract this resistance [
20].
Given the patient-specific nature of resistance mechanisms, a spectrum of these factors may concurrently influence treatment response. Addressing T-DM1 resistance remains a formidable challenge in treating HER2-positive breast cancer.
2.2. T-DM1 Combination Therapies
Current research strives to devise approaches to surmount these mechanisms to improve patient prognosis. Personalized medicine strategies, including targeted and immune-based therapies, are under exploration to effectively counter resistance. In efforts to surmount resistance to Trastuzumab-DM1 (T-DM1) in treating HER2-positive breast cancer, the integration of T-DM1 with diverse therapeutic agents has been extensively investigated. This strategy targets specific resistance mechanisms, proposing alternate modalities to boost treatment efficacy.
A multitude of agents have been assessed for their potential synergistic effects in tandem with T-DM1, including monoclonal antibodies (e.g., pertuzumab), tyrosine kinase inhibitors (e.g., lapatinib, neratinib, tucatinib), PI3K Pathway Inhibitors (e.g., alpelisib), PD1/PDL1 checkpoint inhibitors (e.g., pembrolizumab, atezolizumab) as well as CDK4/6 inhibitors (e.g., palbociclib, ribociclib, abemaciclib) [
21,
22,
23,
24,
25].
The logic behind these combinations is to mount a diversified onslaught on HER2-positive breast cancer cells, targeting various pathways and resistance mechanisms. Utilizing T-DM1 with these agents is anticipated to ameliorate treatment results and confront the hurdles posed by drug resistance. Future clinical studies will shed light on the success of these combination treatments, propelling advancements in care for patients with HER2-positive breast cancer.
3. Trastuzumab Deruxtecan (T-DXd)
Trastuzumab deruxtecan (T-DXd) stands as a novel ADC in the treatment arsenal against breast cancer, comprising an anti-HER2 antibody conjugated to a cytotoxic payload. T-DXd distinguishes itself by utilizing a topoisomerase I inhibitor derivative (i.e., deruxtecan) as its payload, connected via a cleavable tetrapeptide-based linker [
26]. This cleavable linker is selectively severed within tumor cells, reducing off-target release and associated toxicities. T-DXd possesses a notably higher drug-to-antibody ratio (DAR) relative to T-DM1, an attribute that contributes to its potent efficacy [
27].
Clinical investigations, including the pivotal DESTINY-Breast01 and DESTINY-Breast03 trials, have demonstrated T-DXd’s efficacy in significantly prolonging progression-free survival (PFS) over T-DM1, leading to its endorsement by the FDA as a second-line therapy for HER2-positive metastatic breast cancer [
8,
28]. Furthermore, T-DXd’s approval for HER2-low breast cancer signals the recognition of a new subset within the breast cancer spectrum, expanding the therapeutic landscape [
29].
While T-DXd has shown promising clinical efficacy in HER2-positive breast cancer, its use is accompanied by potential adverse effects which require vigilant monitoring. Paramount among these is the risk of drug-related interstitial lung disease (ILD) or pneumonitis, inflammatory conditions that may progress to severe impairment of lung function and significant respiratory compromise. Other toxicities include gastrointestinal symptoms, hematological abnormalities, and rare cardiac toxicities. Regular monitoring and appropriate supportive treatments are essential for managing these side effects [
8].
3.1. T-DXd: Mechanisms of Resistance
Trastuzumab deruxtecan (T-DXd) has emerged as a significant therapeutic agent in the treatment of HER2-positive and HER2-Low metastatic breast cancer, exhibiting considerable clinical efficacy. Nonetheless, resistance to T-DXd poses a significant obstacle in clinical settings. A thorough comprehension of the resistance mechanisms is crucial to devising informed therapeutic strategies to improve patient outcomes.
HER2 Receptor Modifications: Modifications of the HER2 receptor are a primary resistance mechanism, including mutations, gene amplification, or structural alterations, which can diminish the receptor’s affinity for T-DXd. These modifications may reduce the drug’s efficacy by impairing target binding, necessitating the investigation of methods to overcome these changes in HER2 [
30].
ADC Internalization and Intracellular Trafficking: The internalization and intracellular trafficking of T-DXd are critical to its cytotoxic action. Resistance may develop from disruptions in these processes, impeding the delivery of the cytotoxic payload. Enhancing T-DXd internalization and trafficking could be a strategic approach to bypass this resistance mechanism [
31].
Drug Efflux Transporters: The expression of efflux transporters, such as P-glycoprotein (P-gp), which expel the payload (deruxtecan) from cells, can decrease its intracellular concentration and cytotoxic impact. Inhibition or circumvention of these transporters is under investigation to enhance deruxtecan’s intracellular retention [
32].
Tumor Microenvironment and Stromal Factors: The tumor microenvironment, including stromal cell-secreted factors, can confer survival advantages to cancer cells, fostering resistance to T-DXd. Targeting the tumor microenvironment through combination therapies or immune-modulating agents may address this resistance mechanism [
33].
Alternative Signaling Pathways: The activation of alternative signaling pathways, such as the PI3K/AKT/mTOR pathway, can provide survival advantages to cancer cells, undermining T-DXd’s effectiveness. Co-targeting HER2 and these alternative pathways may be essential to counteract resistance [
3,
34,
35,
36].
Tumor Heterogeneity: The intrinsic heterogeneity of tumors, both among patients and within a single tumor, can result in cancer cell populations with varying sensitivities to T-DXd, contributing to the complex nature of resistance. Personalized treatments, considering the unique molecular and phenotypic profiles of tumors, may be promising in overcoming resistance [
37].
In summary, resistance to Trastuzumab deruxtecan in breast cancer is complex and involves diverse interactions between the tumor cells and their surrounding microenvironment. Current research is focused on elucidating these mechanisms in detail and developing targeted strategies to counter resistance. Anticipated advancements in T-DXd-based treatments for HER2-positive and HER2-Low breast cancer include personalized medicine, combination therapies, and an enhanced understanding of tumor biology.
Therapeutic combinations are being rigorously evaluated to surmount resistance to Trastuzumab deruxtecan (T-DXd) in HER2-positive breast cancer therapy. These research efforts aim to refine treatment regimens and enhance patient prognoses. A range of combinations are in various stages of clinical trials, following the same paradigm as T-DM1 above. The objectives of these clinical trials are to establish the safety profiles, efficacy, and appropriate dosing regimens for these combination treatments. The forthcoming results are expected to yield critical insights into the most efficacious combinations and patient demographics best suited for these therapies. The overarching aim is to personalize treatment approaches, thereby advancing the care and outcomes for patients with HER2-positive breast cancer who exhibit resistance to T-DXd.
3.2. T-DXd in HER2-Low Breast Cancer
In the realm of breast cancer treatment, the categorization of tumors as HER2-positive or negative has been traditionally binary. However, a subset of tumors exhibit low levels of HER2 (“HER2-Low”), which is found in 45-60% of cases without HER2 amplification or overexpression [
38]. These HER2-low tumors are identified by an immunohistochemical (IHC) score of 1+ or a score of 2+ accompanied by a negative in situ hybridization (ISH) result. In the pivotal DESTINY-Breast 04 trial, Trastuzumab Deruxtecan (T-DXd) showed significant effectiveness in treating HER2-low metastatic breast cancer, achieving a 52.6% objective response rate among patients who had undergone one or two prior lines of therapy [
9].
While HER2-0 breast cancers are often less amenable to monoclonal antibody therapy, a subset known as HER2-ultra-low has been recognized, characterized by minimal HER2 protein expression. Ongoing studies are exploring the use of ADCs for this group. For example, the DESTINY-Breast06 trial is investigating the efficacy of T-DXd in patients with HER2-ultra-low metastatic breast cancer. Additionally, certain genetic mutations, like the V777L ERBB2 mutation, and MutL deficiency — related to mismatch repair system changes — suggest potential responsiveness to anti-HER2 therapies, even in HER2-negative breast cancers.
Ongoing research is assessing T-DXd against chemotherapy in hormone receptor-positive HER2-low metastatic breast cancer and exploring its combination with immune checkpoint inhibitors. Early data indicate favorable safety and efficacy, with high response rates [
39]. The combination of T-DXd with immune therapies such as PD-L1 and PD-1 inhibitors is being evaluated, showing promising activity, although questions about the incremental benefit over T-DXd alone persist. These studies underscore the potential of T-DXd as a key therapeutic for HER2-low breast cancer, offering hope for improved outcomes in this diverse patient population.
4. New HER2-Targeting ADCs on the Horizon
Emerging ADCs are being developed to enhance the therapeutic landscape of HER2-positive breast cancer treatments. The advent of such ADCs has been revolutionizing the field, particularly by expanding the potential applications beyond traditional HER2-positive cancers to include tumors with lower expression levels of HER2 or with ERBB2 mutations. These novel ADCs are characterized by their refined pharmaceutical properties, including the use of antibodies with greater affinity and specificity to the HER2 protein and potent cytotoxic payloads capable of effectively targeting cancer cells.
The innovative ADCs are designed with modifications that optimize the delivery and release of cytotoxic agents within tumor cells, aiming to leverage the tumor microenvironment to enhance anti-tumor activity. Advancements in ADC technology are not only improving the effectiveness of these therapies but also aiming to reduce resistance and adverse effects. These improvements are the result of meticulous engineering that includes the selection of high-affinity antibodies, development of potent payloads, and the creation of stable linkers that bind the payload until it reaches the tumor site (
Figure 1). The mechanism of ADCs involves the targeted delivery of these components to cells expressing specific cancer antigens, followed by the release of a cytotoxic payload to eliminate the cancer cells [
40].
The research and development of HER2 ADCs are expected to continue improving the therapeutic index, offering a broader spectrum of treatment options for patients with HER2-positive and HER2-low breast cancer. These advancements signal a shift towards precision medicine, where treatment strategies can be personalized for better clinical outcomes and an enhanced quality of life for patients with breast cancer (
Table 2).
4.1. Disitamab Vedotin (RC48)
Disitamab Vedotin (DV), known as RC48, is a novel ADC directed against HER2-expressing cancer cells. The architecture of DV is characterized by a humanized anti-HER2 monoclonal antibody linked to the cytotoxic agent monomethyl auristatin E (MMAE) through a cleavable linker [
41]. This design ensures that the monoclonal antibody binds selectively to the HER2 epitope, facilitating the internalization of MMAE, which then mediates cell death. DV's binding affinity for HER2, which is higher than that of other therapeutic agents like trastuzumab, potentially increases its therapeutic impact. Furthermore, DV can induce cytotoxicity in adjacent cells—a phenomenon known as the bystander effect. Early clinical evaluations of DV have shown promising efficacy, particularly in patients with HER2 2+/ISH-negative status, indicating a potential for tailored therapy based on HER2 expression levels [
42].
Disitamab Vedotin and similar next-generation ADCs represent a significant advance in cancer therapeutics, combining precise tumor targeting with innovative payload mechanisms and improved linker technologies. These developments contribute to ADCs with variable DARs, refined safety profiles, and expanded potential for treating diverse cancer types and disease stages. It should be noted that DV is currently approved in China for gastric cancers [
43].
4.2. ARX788
ARX788 represents an innovative ADC comprising an anti-HER2 monoclonal antibody, a non-cleavable linker, and a modified monomethyl auristatin F (MMAF), known as Amberstatin 269 (AS269) [
44]. This ADC exhibits a DAR of 1.9. Preclinical studies, including those reported by Barok et al. in 2020, demonstrate ARX788's superior efficacy over T-DM1 in trastuzumab-resistant breast cancer xenograft models [
45]. A phase I trial showed ARX788
is well tolerated and has promising anti-tumor activity in patients with HER2-positive advanced gastric adenocarcinoma (ChinaDrugTrials.org.cn: CTR20190639) [
46]. Current phase II clinical trials are evaluating ARX788's efficacy in various HER2-positive breast cancer contexts. Trial NCT05018676 is investigating its impact on HER2-low breast cancers, while NCT05018702 focuses on patients with HER2-positive cancers that have metastasized to the brain. Additionally, NCT04829604, known as ACE-Breast-03, aims to determine the effectiveness of ARX-788 in patients with HER2-positive metastatic breast cancer who have previously undergone treatment with T-DXd [
43].
4.3. SYD985
Trastuzumab duocarmazine (SYD 985) is a novel ADC that combines trastuzumab with a cleavable valine-citrulline linker and a duocarmycin derivative, seco-DUBA, which is activated by proteases to cause DNA alkylation and cell death. It exhibits a bystander effect and has a DAR of 2.7 [
47]. It showed efficacy in HER2-low breast cancer models, surpassing T-DM1. In a phase 1 trial, it demonstrated antitumor activity with ORRs of 28% for HR+ and 40% for HR− MBC patients . Ocular side effects were noted [
43].
The Phase III TULIP® study compared SYD985 with physicians' choice of treatment in participants with HER2-positive locally advanced or metastatic breast cancer. The trial met its primary endpoint, showing a statistically significant improvement in progression-free survival (PFS) for SYD985 over the physicians' choice. PFS is the duration from randomization to disease progression or death from any cause. Additionally, the study reported preliminary supportive results for overall survival (OS) [
48].
4.4. BL-M0701
BL-M07D1 is a new ADC targeting HER2 with a structure comprising the humanized antibody trastuzumab, a cathepsin B cleavable linker, and Ed-04, a camptothecin-derived topoisomerase I inhibitor. This inhibitor impedes the cell cycle in the S phase, inducing apoptosis. BL-M07D1 has a DAR of 8:1, akin to T-DXd, but with a more stable linker.
Preclinical evaluations using xenograft models revealed BL-M07D1's superior tumor inhibition, outperforming T-DXd in low HER2-expressing models and both T-DM1 and T-DXd in HER2-positive models. Notably, BL-M07D1 demonstrated potent bystander effects, suggesting an enhanced efficacy against mixed HER2-positive/negative tumors [
49]. These findings posit BL-M07D1 as a promising candidate in the treatment of a wider spectrum of breast cancers, surpassing the current HER2-targeting ADCs. A Phase I trial is ongoing in patients with metastatic breast cancer.
4.5. Zanidatamab zovodotin
Zanidatamab zovodotin, also referred to as ZW49, is an innovative bispecific ADC aimed at treating HER2-expressing or HER2-amplified cancers, including breast cancer. It is currently undergoing clinical evaluation to determine its efficacy and safety profile. The first-in-human phase I trial was designed to determine the maximum tolerated dose, characterize its safety and tolerability, and evaluate its anti-tumor activity as monotherapy [
50]. Among eight patients with breast cancer, Zanidatamab zovodotin achieved a confirmed overall response rate (ORR) of 13%, which included a partial response (PR) rate of 13% and a stable disease (SD) rate of 38%. This indicates a promising activity profile, although the response rates suggest there is a need for continued development and investigation to fully understand the potential of this ADC.
The emergence of Zanidatamab zovodotin represents a significant step forward in the treatment of HER2-positive cancers, potentially offering a new therapeutic option for patients with advanced disease who have limited treatment choices. As clinical trials progress, the oncology community eagerly anticipates further data to evaluate the full potential of this novel ADC in the treatment landscape of breast cancer [
51].
4.6 . Other ADCs in Clinical Trials
There are many other ADCs in clinical trials, including ALT-P7, SHR-A1811, TQB2102, XMT-1522 (
Table 2). The therapeutic benefit of these molecules will be determined by the results of these trials and additional future clinical trials.
5. Future Directions
HER2-targeted ADCs have emerged as a promising strategy for breast cancer treatment, with a particular focus on enhancing outcomes for both patients with HER2-positive and HER2-low breast cancer. The field of ADC research continues to advance. Ongoing clinical trials are exploring novel ADCs to further improve breast cancer therapy. Furthermore, one of the exciting frontiers in this field is the exploration of novel therapeutic sequences or combinations involving ADCs.
The benefit of sequencing HER2-targeted ADCs upon disease progression is an area of active investigation. It is possible ADCs may continue to be effective by delivering a cytotoxic payload to tumor cells through a targeted antibody, even if the antibody remains the same (e.g., trastuzumab).
By combining ADCs with other targeted agents, such as tyrosine kinase inhibitors or immune checkpoint inhibitors, multiple pathways involved in tumor growth and immune response can be simultaneously targeted. This synergistic approach aims to overcome drug resistance and maximize treatment efficacy.
The concept of precision medicine becomes even more potent with combination therapies. By identifying specific patient subgroups that may benefit from certain combinations, we can tailor treatments to individual patients based on their molecular and genetic profiles. Next generation sequencing remains an important tool in understanding the biology of each patient’s individual tumor.
In the coming years, it will be crucial to understand the benefits and potential toxicities of each HER2 ADC in clinical development. Tailored monitoring and treatment adjustments will be key to minimizing risks and enhancing the therapeutic outcome for patients with HER2-positive breast cancer treated with a specific ADC or sequence of ADCs.
6. Conclusions
The exploration of novel combinations with ADCs represents a promising frontier in cancer therapy. These combinations have the potential to expand the therapeutic reach, overcome resistance mechanisms, modulate the immune system, and offer tailored treatments to specific patient subgroups. Ongoing clinical trials and research efforts are essential in advancing our understanding of these combinations and their potential to improve patient outcomes in the complex landscape of cancer treatment.
Author Contributions
Conceptualization, B.Z. and F.J.E.; literature review, B.Z. and F.J.E.; writing—original draft preparation, review and editing, B.Z. and F.J.E. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable
Data Availability Statement
Not applicable
Conflicts of Interest
F.J.E. has served as consultant for AstraZeneca, Genentech and Novartis for activities unrelated to this manuscript. B.S.Z. has no conflicts of interest to disclose.
References
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177-182. [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001, 344, 783-792. [CrossRef]
- Esteva, F.J.; Yu, D.; Hung, M.C.; Hortobagyi, G.N. Molecular predictors of response to trastuzumab and lapatinib in breast cancer. Nat Rev Clin Oncol 2010, 7, 98-107. [CrossRef]
- Esteva, F.J.; Hubbard-Lucey, V.M.; Tang, J.; Pusztai, L. Immunotherapy and targeted therapy combinations in metastatic breast cancer. Lancet Oncol 2019, 20, e175-e186. [CrossRef]
- He, X.; Yeung, S.J.; Esteva, F.J. A new paradigm for classifying and treating HER2-positive breast cancer. Cancer Rep (Hoboken) 2023, 6, e1841. [CrossRef]
- Esteva, F.J.; Miller, K.D.; Teicher, B.A. What Can We Learn about Antibody-Drug Conjugates from the T-DM1 Experience? Am Soc Clin Oncol Educ Book 2015, e117-125. [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Dieras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012, 367, 1783-1791. [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med 2020, 382, 610-621. [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med 2022, 387, 9-20. [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin Cancer Res 2016, 22, 5097-5108. [CrossRef]
- de Goeij, B.E.; Peipp, M.; de Haij, S.; van den Brink, E.N.; Kellner, C.; Riedl, T.; de Jong, R.; Vink, T.; Strumane, K.; Bleeker, W.K.; et al. HER2 monoclonal antibodies that do not interfere with receptor heterodimerization-mediated signaling induce effective internalization and represent valuable components for rational antibody-drug conjugate design. MAbs 2014, 6, 392-402. [CrossRef]
- Xu, Z.; Guo, D.; Jiang, Z.; Tong, R.; Jiang, P.; Bai, L.; Chen, L.; Zhu, Y.; Guo, C.; Shi, J.; et al. Novel HER2-Targeting Antibody-Drug Conjugates of Trastuzumab Beyond T-DM1 in Breast Cancer: Trastuzumab Deruxtecan(DS-8201a) and (Vic-)Trastuzumab Duocarmazine (SYD985). Eur J Med Chem 2019, 183, 111682. [CrossRef]
- Krop, I.E.; Beeram, M.; Modi, S.; Jones, S.F.; Holden, S.N.; Yu, W.; Girish, S.; Tibbitts, J.; Yi, J.H.; Sliwkowski, M.X.; et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol 2010, 28, 2698-2704. [CrossRef]
- von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med 2019, 380, 617-628. [CrossRef]
- Hurvitz, S.A.; Martin, M.; Jung, K.H.; Huang, C.S.; Harbeck, N.; Valero, V.; Stroyakovskiy, D.; Wildiers, H.; Campone, M.; Boileau, J.F.; et al. Neoadjuvant Trastuzumab Emtansine and Pertuzumab in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Three-Year Outcomes From the Phase III KRISTINE Study. J Clin Oncol 2019, 37, 2206-2216. [CrossRef]
- Junttila, T.T.; Li, G.; Parsons, K.; Phillips, G.L.; Sliwkowski, M.X. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat 2011, 128, 347-356. [CrossRef]
- Li, G.; Guo, J.; Shen, B.Q.; Yadav, D.B.; Sliwkowski, M.X.; Crocker, L.M.; Lacap, J.A.; Phillips, G.D.L. Mechanisms of Acquired Resistance to Trastuzumab Emtansine in Breast Cancer Cells. Mol Cancer Ther 2018, 17, 1441-1453. [CrossRef]
- Islam, S.S.; Uddin, M.; Noman, A.S.M.; Akter, H.; Dity, N.J.; Basiruzzman, M.; Uddin, F.; Ahsan, J.; Annoor, S.; Alaiya, A.A.; et al. Antibody-drug conjugate T-DM1 treatment for HER2+ breast cancer induces ROR1 and confers resistance through activation of Hippo transcriptional coactivator YAP1. EBioMedicine 2019, 43, 211-224. [CrossRef]
- Krop, I.; Winer, E.P. Trastuzumab emtansine: a novel antibody-drug conjugate for HER2-positive breast cancer. Clin Cancer Res 2014, 20, 15-20. [CrossRef]
- Emens, L.A.; Esteva, F.J.; Beresford, M.; Saura, C.; De Laurentiis, M.; Kim, S.B.; Im, S.A.; Wang, Y.; Salgado, R.; Mani, A.; et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol 2020, 21, 1283-1295. [CrossRef]
- Phillips, G.D.; Fields, C.T.; Li, G.; Dowbenko, D.; Schaefer, G.; Miller, K.; Andre, F.; Burris, H.A., 3rd; Albain, K.S.; Harbeck, N.; et al. Dual targeting of HER2-positive cancer with trastuzumab emtansine and pertuzumab: critical role for neuregulin blockade in antitumor response to combination therapy. Clin Cancer Res 2014, 20, 456-468. [CrossRef]
- Jain, S.; Shah, A.N.; Santa-Maria, C.A.; Siziopikou, K.; Rademaker, A.; Helenowski, I.; Cristofanilli, M.; Gradishar, W.J. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast Cancer Res Treat 2018, 171, 371-381. [CrossRef]
- Cortes, J.; Dieras, V.; Lorenzen, S.; Montemurro, F.; Riera-Knorrenschild, J.; Thuss-Patience, P.; Allegrini, G.; De Laurentiis, M.; Lohrisch, C.; Oravcova, E.; et al. Efficacy and Safety of Trastuzumab Emtansine Plus Capecitabine vs Trastuzumab Emtansine Alone in Patients With Previously Treated ERBB2 (HER2)-Positive Metastatic Breast Cancer: A Phase 1 and Randomized Phase 2 Trial. JAMA Oncol 2020, 6, 1203-1209. [CrossRef]
- Sartore-Bianchi, A.; Lonardi, S.; Martino, C.; Fenocchio, E.; Tosi, F.; Ghezzi, S.; Leone, F.; Bergamo, F.; Zagonel, V.; Ciardiello, F.; et al. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: the phase II HERACLES-B trial. ESMO Open 2020, 5, e000911. [CrossRef]
- Moinard-Butot, F.; Saint-Martin, C.; Pflumio, C.; Carton, M.; Jacot, W.; Cottu, P.H.; Dieras, V.; Dalenc, F.; Goncalves, A.; Debled, M.; et al. Efficacy of trastuzumab emtansine (T-DM1) and lapatinib after dual HER2 inhibition with trastuzumab and pertuzumab in patient with metastatic breast cancer: Retrospective data from a French multicenter real-life cohort. Breast 2022, 63, 54-60. [CrossRef]
- Doi, T.; Shitara, K.; Naito, Y.; Shimomura, A.; Fujiwara, Y.; Yonemori, K.; Shimizu, C.; Shimoi, T.; Kuboki, Y.; Matsubara, N.; et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol 2017, 18, 1512-1522. [CrossRef]
- Iwata, T.N.; Ishii, C.; Ishida, S.; Ogitani, Y.; Wada, T.; Agatsuma, T. A HER2-Targeting Antibody-Drug Conjugate, Trastuzumab Deruxtecan (DS-8201a), Enhances Antitumor Immunity in a Mouse Model. Mol Cancer Ther 2018, 17, 1494-1503. [CrossRef]
- Cortes, J.; Kim, S.B.; Chung, W.P.; Im, S.A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.M.; Petry, V.; Chung, C.F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N Engl J Med 2022, 386, 1143-1154. [CrossRef]
- Narayan, P.; Osgood, C.L.; Singh, H.; Chiu, H.J.; Ricks, T.K.; Chiu Yuen Chow, E.; Qiu, J.; Song, P.; Yu, J.; Namuswe, F.; et al. FDA Approval Summary: Fam-Trastuzumab Deruxtecan-Nxki for the Treatment of Unresectable or Metastatic HER2-Positive Breast Cancer. Clin Cancer Res 2021, 27, 4478-4485. [CrossRef]
- Mukohara, T.; Hosono, A.; Mimaki, S.; Nakayama, A.; Kusuhara, S.; Funasaka, C.; Nakao, T.; Fukasawa, Y.; Kondoh, C.; Harano, K.; et al. Effects of Ado-Trastuzumab Emtansine and Fam-Trastuzumab Deruxtecan on Metastatic Breast Cancer Harboring HER2 Amplification and the L755S Mutation. Oncologist 2021, 26, 635-639. [CrossRef]
- Li, B.T.; Michelini, F.; Misale, S.; Cocco, E.; Baldino, L.; Cai, Y.; Shifman, S.; Tu, H.Y.; Myers, M.L.; Xu, C.; et al. HER2-Mediated Internalization of Cytotoxic Agents in ERBB2 Amplified or Mutant Lung Cancers. Cancer Discov 2020, 10, 674-687. [CrossRef]
- Takegawa, N.; Nonagase, Y.; Yonesaka, K.; Sakai, K.; Maenishi, O.; Ogitani, Y.; Tamura, T.; Nishio, K.; Nakagawa, K.; Tsurutani, J. DS-8201a, a new HER2-targeting antibody-drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int J Cancer 2017, 141, 1682-1689. [CrossRef]
- Hanker, A.B.; Garrett, J.T.; Estrada, M.V.; Moore, P.D.; Ericsson, P.G.; Koch, J.P.; Langley, E.; Singh, S.; Kim, P.S.; Frampton, G.M.; et al. HER2-Overexpressing Breast Cancers Amplify FGFR Signaling upon Acquisition of Resistance to Dual Therapeutic Blockade of HER2. Clin Cancer Res 2017, 23, 4323-4334. [CrossRef]
- Nagata, Y.; Lan, K.H.; Zhou, X.; Tan, M.; Esteva, F.J.; Sahin, A.A.; Klos, K.S.; Li, P.; Monia, B.P.; Nguyen, N.T.; et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 2004, 6, 117-127. [CrossRef]
- Junttila, T.T.; Akita, R.W.; Parsons, K.; Fields, C.; Lewis Phillips, G.D.; Friedman, L.S.; Sampath, D.; Sliwkowski, M.X. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell 2009, 15, 429-440. [CrossRef]
- Esteva, F.J.; Guo, H.; Zhang, S.; Santa-Maria, C.; Stone, S.; Lanchbury, J.S.; Sahin, A.A.; Hortobagyi, G.N.; Yu, D. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol 2010, 177, 1647-1656. [CrossRef]
- Prat, A.; Carey, L.A.; Adamo, B.; Vidal, M.; Tabernero, J.; Cortes, J.; Parker, J.S.; Perou, C.M.; Baselga, J. Molecular features and survival outcomes of the intrinsic subtypes within HER2-positive breast cancer. J Natl Cancer Inst 2014, 106. [CrossRef]
- Esteva, F.J., Jaffer, S. HER2-low metastatic breast cancer: molecular insights and therapeutic strategies. J Cancer Metastasis Treat 2023, 2023;9:39.
- Tarantino, P.; Tolaney, S.M.; Curigliano, G. Trastuzumab deruxtecan (T-DXd) in HER2-low metastatic breast cancer treatment. Ann Oncol 2023. [CrossRef]
- Abelman, R.O.; Medford, A.; Spring, L.; Bardia, A. Antibody-Drug Conjugates in Breast Cancer: Spotlight on HER2. Cancer J 2022, 28, 423-428. [CrossRef]
- Chen, Z.; Yuan, J.; Xu, Y.; Zhang, C.; Li, Z.; Gong, J.; Li, Y.; Shen, L.; Gao, J. From AVATAR Mice to Patients: RC48-ADC Exerted Promising Efficacy in Advanced Gastric Cancer With HER2 Expression. Front Pharmacol 2021, 12, 757994. [CrossRef]
- Sheng, X.; Yan, X.; Wang, L.; Shi, Y.; Yao, X.; Luo, H.; Shi, B.; Liu, J.; He, Z.; Yu, G.; et al. Open-label, Multicenter, Phase II Study of RC48-ADC, a HER2-Targeting Antibody-Drug Conjugate, in Patients with Locally Advanced or Metastatic Urothelial Carcinoma. Clin Cancer Res 2021, 27, 43-51. [CrossRef]
- Yu, J.; Fang, T.; Yun, C.; Liu, X.; Cai, X. Antibody-Drug Conjugates Targeting the Human Epidermal Growth Factor Receptor Family in Cancers. Front Mol Biosci 2022, 9, 847835. [CrossRef]
- Skidmore, L.; Sakamuri, S.; Knudsen, N.A.; Hewet, A.G.; Milutinovic, S.; Barkho, W.; Biroc, S.L.; Kirtley, J.; Marsden, R.; Storey, K.; et al. ARX788, a Site-specific Anti-HER2 Antibody-Drug Conjugate, Demonstrates Potent and Selective Activity in HER2-low and T-DM1-resistant Breast and Gastric Cancers. Mol Cancer Ther 2020, 19, 1833-1843. [CrossRef]
- Barok, M.; Le Joncour, V.; Martins, A.; Isola, J.; Salmikangas, M.; Laakkonen, P.; Joensuu, H. ARX788, a novel anti-HER2 antibody-drug conjugate, shows anti-tumor effects in preclinical models of trastuzumab emtansine-resistant HER2-positive breast cancer and gastric cancer. Cancer Lett 2020, 473, 156-163. [CrossRef]
- Zhang, Y.; Qiu, M.Z.; Wang, J.F.; Zhang, Y.Q.; Shen, A.; Yuan, X.L.; Zhang, T.; Wei, X.L.; Zhao, H.Y.; Wang, D.S.; et al. Phase 1 multicenter, dose-expansion study of ARX788 as monotherapy in HER2-positive advanced gastric and gastroesophageal junction adenocarcinoma. Cell Rep Med 2022, 3, 100814. [CrossRef]
- Dokter, W.; Ubink, R.; van der Lee, M.; van der Vleuten, M.; van Achterberg, T.; Jacobs, D.; Loosveld, E.; van den Dobbelsteen, D.; Egging, D.; Mattaar, E.; et al. Preclinical profile of the HER2-targeting ADC SYD983/SYD985: introduction of a new duocarmycin-based linker-drug platform. Mol Cancer Ther 2014, 13, 2618-2629. [CrossRef]
- Manich, C.S., et al. Primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann Oncol 2021.
- Wan, W., et al. BL-M07D1, a novel HER2-targeting ADC, demonstrates potent anti-tumor efficacy in preclinical pharmacodynamic models. Proceedings of the American Association for Cancer Research Annual Meeting 2023; Part 1 (Regular and Invited Abstracts); 2023 Apr 14-19; Orlando, FL. Philadelphia (PA): AACR; Cancer Res 2023;83(7_Suppl):Abstract nr 2643. 2023.
- Meric-Bernstam, F.; Beeram, M.; Hamilton, E.; Oh, D.Y.; Hanna, D.L.; Kang, Y.K.; Elimova, E.; Chaves, J.; Goodwin, R.; Lee, J.; et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: a phase 1, dose-escalation and expansion study. Lancet Oncol 2022, 23, 1558-1570. [CrossRef]
- Harding, J.J.; Fan, J.; Oh, D.Y.; Choi, H.J.; Kim, J.W.; Chang, H.M.; Bao, L.; Sun, H.C.; Macarulla, T.; Xie, F.; et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. Lancet Oncol 2023, 24, 772-782. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).