Introduction
In flight operations with fighter jets and in space crafts, inspired levels of hypoxic and hyperoxic air for pilots and astronauts vary largely due to instability of the oxygen delivery system and pressure losses in the flight cabin. This can have influence on blood flow and oxygenation of the brain and on cognitive performance (1). The change of oxygenation of arterial blood can also change peripheral arterial blood pressure and blood flow (2). The rapid changes in oxygenation of neuronal cells and the oxidative stress can lead to the production of inflammatory neuronal cell markers (3). Hyperoxia is under suspicion to cause brain damage and possible white matter densities, proven at least in animal research (4,5). But also in humans, hyperoxic flight operations for space flights showed microstructural changes in neuronal tissues as predisposition for white matter densities (6).
And, in humans repeated hypobaric hyperoxia increases inflammatory markers and might lead to symptoms of chronic fatigue (7).
Previous investigations with different FiO2 levels of supplemental oxygen in simulated flight operations showed a dose dependent response to higher FiO2 levels concerning cerebral blood flow and brain oxygenation, a statistical difference in response could be seen at FiO2 levels from 60% on (8). Despite this reduction in blood flow, it seems that a higher oxygenation enhances cognitive function and high-density EEG activity. For inflight performance of flight-crews the supplemental oxygen and high FiO2 could be an advantage (9). This is the main reason why not only in military flight operations supplemental oxygen is standard but also the Federal Aviation Administration (FAA) recommends supplemental oxygen in non-pressurized civilian airplanes at altitudes from 3750m altitude on and makes it mandatory from altitudes of 4200m on (10). Even in pressurized airplanes supplemental oxygen must be ready for the pilots and flight crews (11).
Magnetic Resonance Imaging (MRI) scans have shown in studies with hypoxic conditions in humans a reduction of cerebral blood flow in several brain regions, although one might from a physiologic standpoint assume that hypoxia increases blood flow and hyperoxia decreases blood flow (12).
Hypoxia related accidents in military flight operations are recorded and published since long (13–15), but they affect also civilian flight operations and led according to post accident investigations to major crashes like the one with famous golfer Payne Stewart on board and to a Greek airplane crash with 121 fatalities (16,17). Therefore, supplemental oxygen for flight operations makes sense not only in military flights but also for all civilian flight operations. Some researchers even lobby a reduction of hypoxia in civilian flights for crew and passengers from an altitude level of 2400m in the pressurized cabin down to 1500m equivalent altitude (18).
Supplemental oxygen in civilian flight operations for flight crews is usually delivered at FiO2 levels of 35-60% via face masks. The question is if supplemental oxygen and the achievement of a hyperoxic arterial blood gas level leads to a for neuronal cells possibly harmful level of oxidative stress.
Therefore, we aimed to investigate in a cross over physiology study with a flight simulation with healthy humans in a hypobaric chamber at most common in cabin altitude levels in civilian flight operations if delivery of supplemental oxygen leads to a significant increase of the oxidative stress markers malondialdehyde low density lipoprotein (M-LDL) and glutathione peroxidase (GPX) in arterial blood. We hypothesized that supplemental oxygen with an FiO2 level of 60% would lead to a significant increase of the two oxidative stress markers versus baseline.
Methods
Subjects
Subjects were recruited via social media and blackboard announcement. Inclusion criteria were an age between 20 and 55yrs, an overall completely healthy physical status without any need for medications, non-smoking, non-pregnacy and a covid 19 vaccination status with three completed covid-19 vaccinations.
The from this recruitment initial cohort consisted of 50 volunteers of who 12 males and 12 females were willing to participate and being scheduled for the study. All 24 underwent an extensive clinical exam by an internal medicine physician including a lung function test (NDD Easy One, NDD Zuerich, Switzerland) to exclude any ongoing respiratory disease or actual infection. All subjects hat a FVC and FEV1 > 100% of normal value based on their age and body size.
Via coin tossing keeping an equal sex distribution the 24 subjects were divided in two groups. Group one performed the flight simulation with radial catheter and group two parallel without radial catheter for blood sampling, standing by as reserve in case of problems with the puncture of the arteria radialis ad placing the radial catheter in a subject of group one. Mean age of the 12 subjects from group one was 35.7yrs (SD 12,35yrs).
The study protocol according to the standards of the Declaration of Helsinki including amendments and the subject information were reviewed and accepted by the ethical review board of the province of Bozen/South Tyrol (106-2020) and in addition by the ethical review board of the University of Innsbruck, Austria (42-2019).
Study Site and Flight Simulation
Terra X Cube is one of the world’s largest civilian hypobaric chambers with a size of 137m² and a room height of 6m (822 cubic meter volume). It is located in Bozen, Italy at an altitude of 322m above sea level and incorporated in Eurac Research. Maximal reachable altitude is 9000m, maximal rise in altitude is normally 6m/sec. For the Hypoxiflight study we stretched the rising speed to 10m/sec and used a speed of 50m/sec for the decrease of altitude. Room temperature was kept at a comfortable 22°Celsius at all times. One subject from group one and one subject from standby group two were in the chamber at the same time for three hours. The study was performed on six consecutive days with two shifts per day. One from 9-12am (9.00-12.00) and one from 2-5pm (14.00-17.00).
The radial catheter (BD Flow-Switch, Becton Dickinson, Heidelberg, Germany) was placed in the arteria radialis of the non-prominent arm of the subject in supine position under ultrasound control by an anesthesiologist before start of the trial and connected to a patient monitor (Phillips IntelliVue, Phillips Healthcare, Amsterdam, Netherlands). Arterial blood pressure, oxygen saturation (SaO2) and pulse were constantly recorded from that time point on. Baseline arterial blood samples (one for blood gases, one for centrifugation to gain serum) were drawn from a three-way valve before the first venturi valve equipped face mask was put on the subject with 35% FiO2 and 6l/min O2 flow.
Since PO2 analyzed from arterial blood gases is considered the gold standard for the measurement of arterial blood oxygenation, we concentrated on this parameter for the assessment of the oxidative level of the subjects at the different steps. Blood gas analysis was performed via Roche Opti cartridge system (Opti CCA TS, Opti Medical, Atlanta, Gorgia, USA) on site inside the chamber. The analyzer was calibrated for the specific altitude each day at each step.
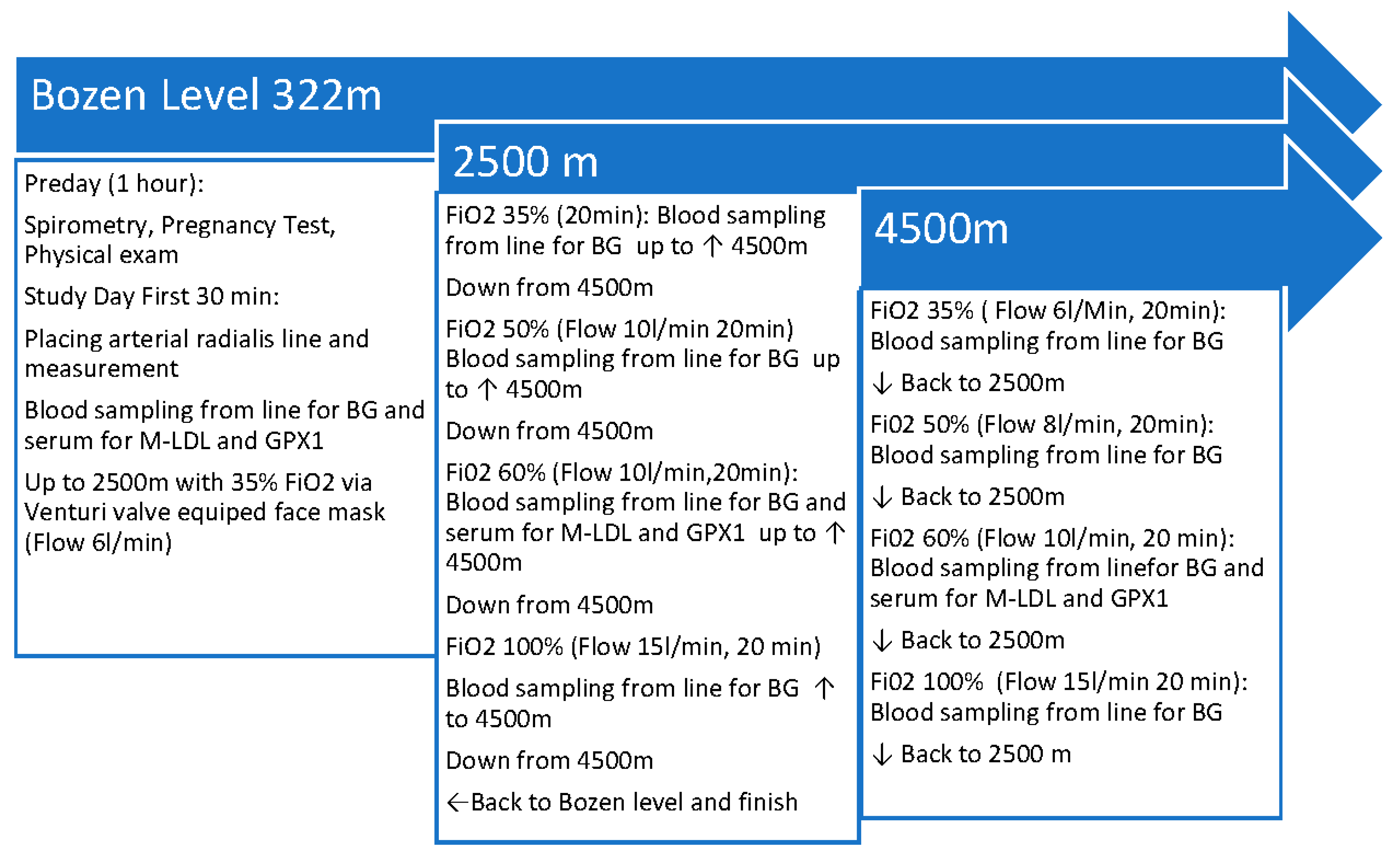
After baseline measurements an increase and decrease of altitude back and force between 2500m and 4500m, respectively the equivalent barometric pressure was performed according to a specific protocol mimicking a standard civilian flight operation for example of a flight over a mountain range like the Alps or the Rocky Mountains or after cabin pressure loss in a pressurized airline jet. The flight simulation protocol is shown in
Figure 1. After each return from 4500m to 2500m altitude, respectively the equivalent air pressure in the Terra X Cube, the face masks were changed on the subject with the specific venturi valves for the FiO2 steps of 35%, 50%, 60% and 100%. Blood gas analysis was performed at each FiO2 step and at each altitude of the protocol.
Assessment of Oxidative Stress
According to leading literature we have chosen Malondealdehyde Low Density Lipoprotein (M-LDL) and Gluthatione Peroxidase 1 as oxidative stress markers for our analysis and proof of hypothesis that there is a correlation between arterial hyperoxia and oxidative stress in serum from arterial blood in our human subjects (19–22). According to the German Ministry of Science these are also the two markers, which express oxidative stress the best on a feasible level of cost and analysis efforts (23).
Additional arterial blood for centrifugation inside the hypobaric chamber was drawn from the radial catheter at baseline and at FiO2 of 60% at both altitudes. The serum samples were transported outside the chamber via an airlock immediately after centrifugation and frozen at 21°Celsius for the further analysis at the biochemistry laboratory of the Medical University of Innsbruck. For the transport from Bozen to Innsbruck (driving time 1hr15min), after collection of all serum samples at the end of the study, the serum was kept in a Styrofoam box with dry ice. In the biochemistry laboratory the biomarkers malondialdehyde LDL and gluthationeperoxidase were analyzed via Elisa test (Abcam 242302 MDA-LDL Human and Abcam 193767 Gluthatione Peroxidase 1 Human Simple Step, Acam, Walham, Massachusetts, USA). Each test was repeated twice for accuracy and to proof reproducibility.
Statistical Work
An a priori power analysis was performed based on our hypothesis with estimated high correlation and a Pearson correlation coefficient of 0.8 between Po2 values and oxidative stress biomarkers Elisa results. At a significance of level p 0.05 (5%) and a power of 0.8 (80%) the necessary number of subjects resulted in 9 (24).
All values here are presented in means and standard deviation. Significance of difference between two values has been calculated via student -t test for paired variables, a significant difference between variables is presented as p≤ 0.05. Correlation between parameters was calculated according to Pearson and expressed with the Pearson correlation coefficient. All statistical work was performed with the Excel 2021 (Microsoft, Seattle, Washington, USA) statistics program.
Results
11 of the 12 selected subjects and the 12 standby subjects finished the flight simulation in the hypobaric chamber according to the stepwise altitude increase and decrease and supplemental oxygen increase without any problems. One subject (female, 48yrs) dropped out after baseline and 35% FiO2 supplemental oxygen at simulated 2500m due to subjectively felt stress breathing under the face mask and subsequent hyperventilation leading to dizziness. After taking the facemask off she calmed down to a completely normal respiration and disappearance of any feeling of dizziness but didn’t want to continue with the protocol.
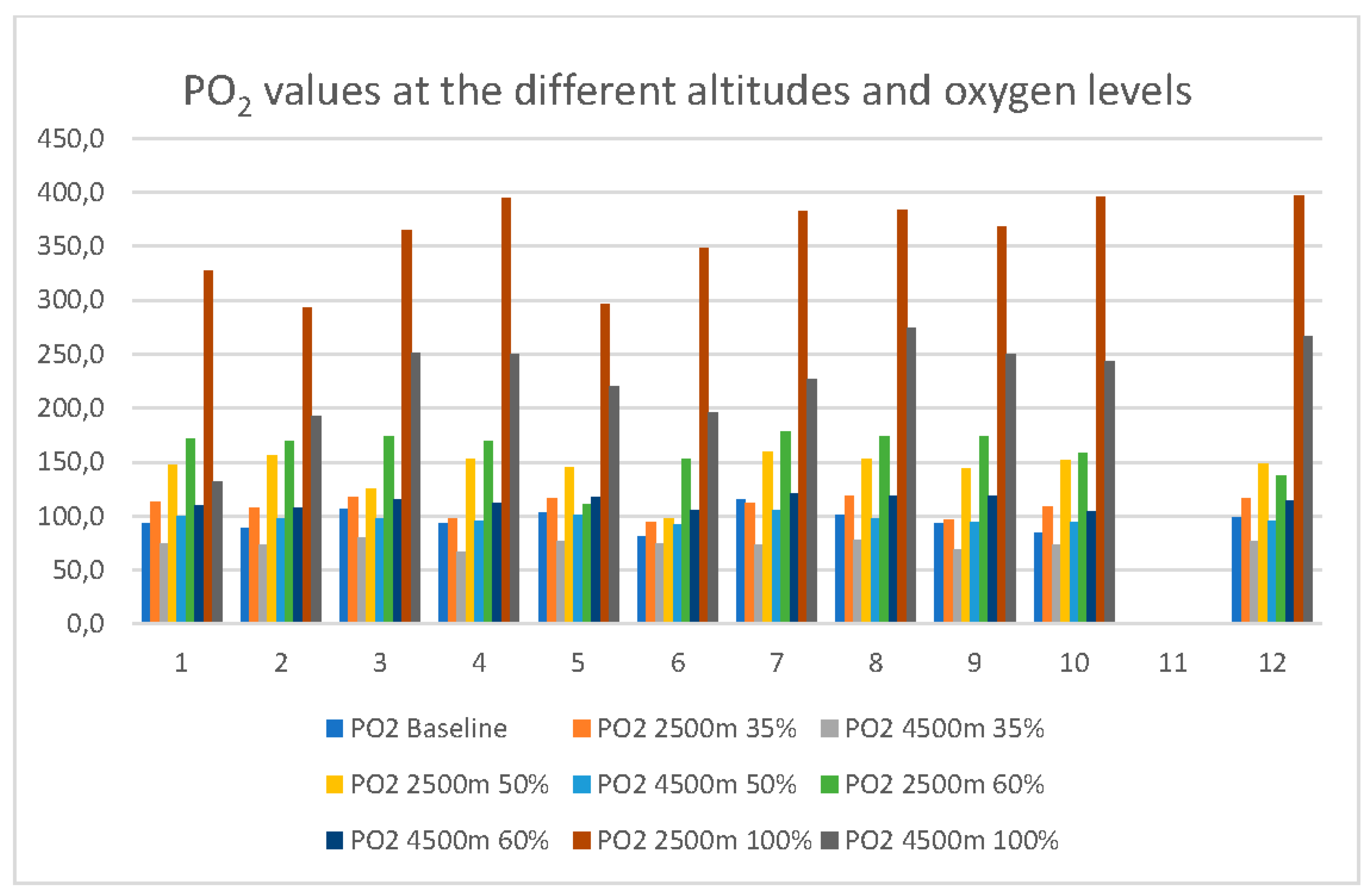
Arterial PO2 measured from the blood gases increased from baseline (322m altitude, 9761 kPa air pressure) in the mean 96.2mmHg (SD 9.9mmHg) to 160.9mmHg (mean, SD 20.5mmHg) at 2500m simulated altitude with 60% FiO2. At equivalent 4500m and 60% FiO2 mean PO2 was at 113.2mmHg (SD 5.7mmHg). All individual PO2 values from baseline to 35%,50%,60% and 100% FiO2 at 2500m and 4500m simulated altitude are presented in
Figure 2.
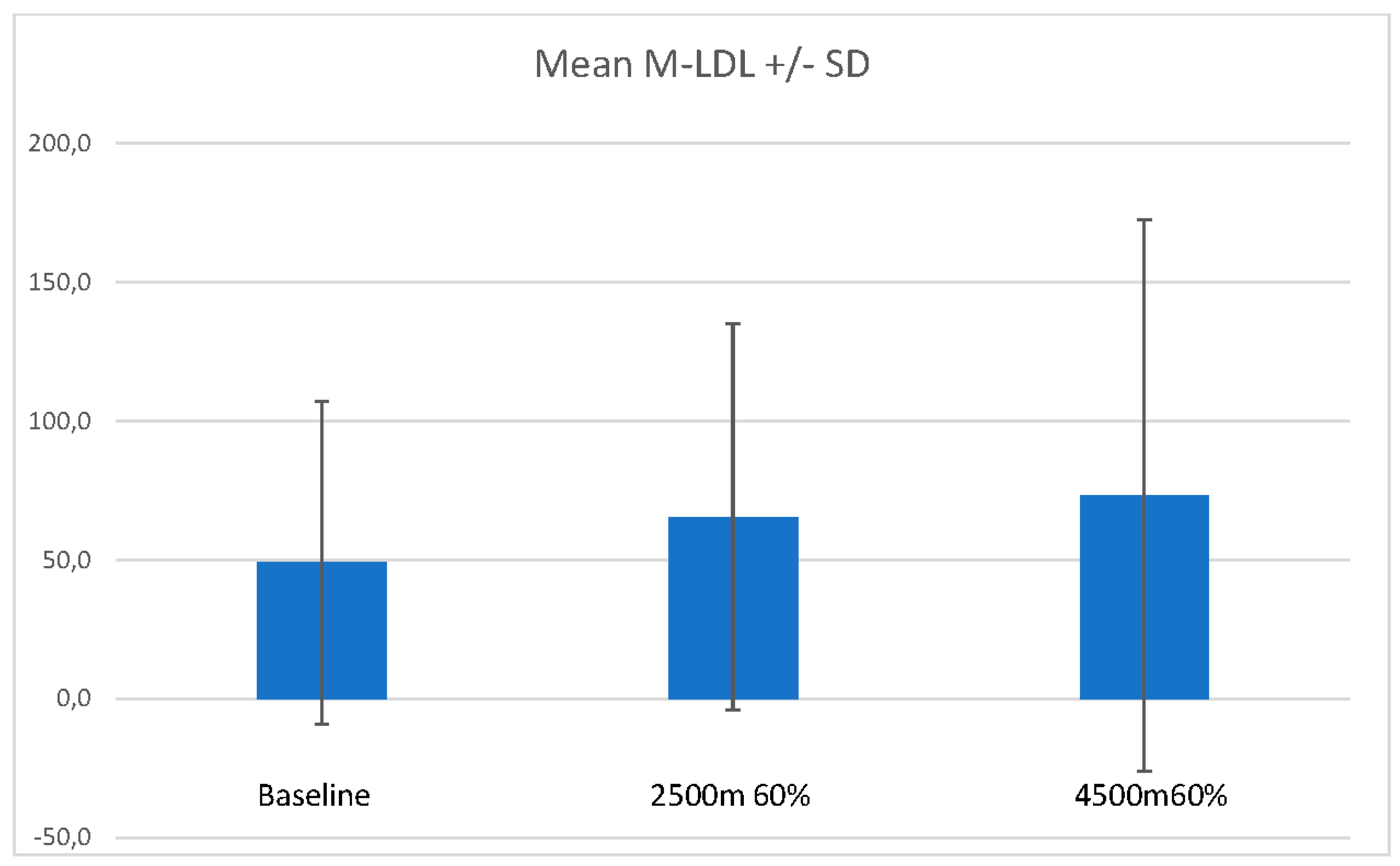
Mean malonaldehyde LDL (M-LDL) increased in the mean from 49,0ng/ml (SD 58,02ng/ml) at baseline to 65.5ng/ml (SD 69.5ng/ml) at 2500m 60% FiO2 and 73.2ng/ml (SD 99.2ng/ml) at 4500m 60% FiO2. One subject (age 38) started with a much higher base value of M-LDL (189,9 ng/ml, reason unknown, the subject had no signs of infection and a better than the suggested normal values lung function) and increased further to 222.2ng/ml at 2500m 60% FiO2 and 310.9ng/ml at 4500m 60% FiO2. If this subject had been left out from the calculation mean M-LDL for the other 10 subjects fell between 2500m 60% FiO2 and 4500m 60% FiO2 from 43.1ng/ml to 39.3ng/ml with much lower standard deviations. With and without this subject the difference between base value and value at 2500m 60%FiO2 or 4500m 60% FiO2 did not reach significance (n.s.).
Figure 3 presents M-LDL means and SD at the different protocol time points of measure.
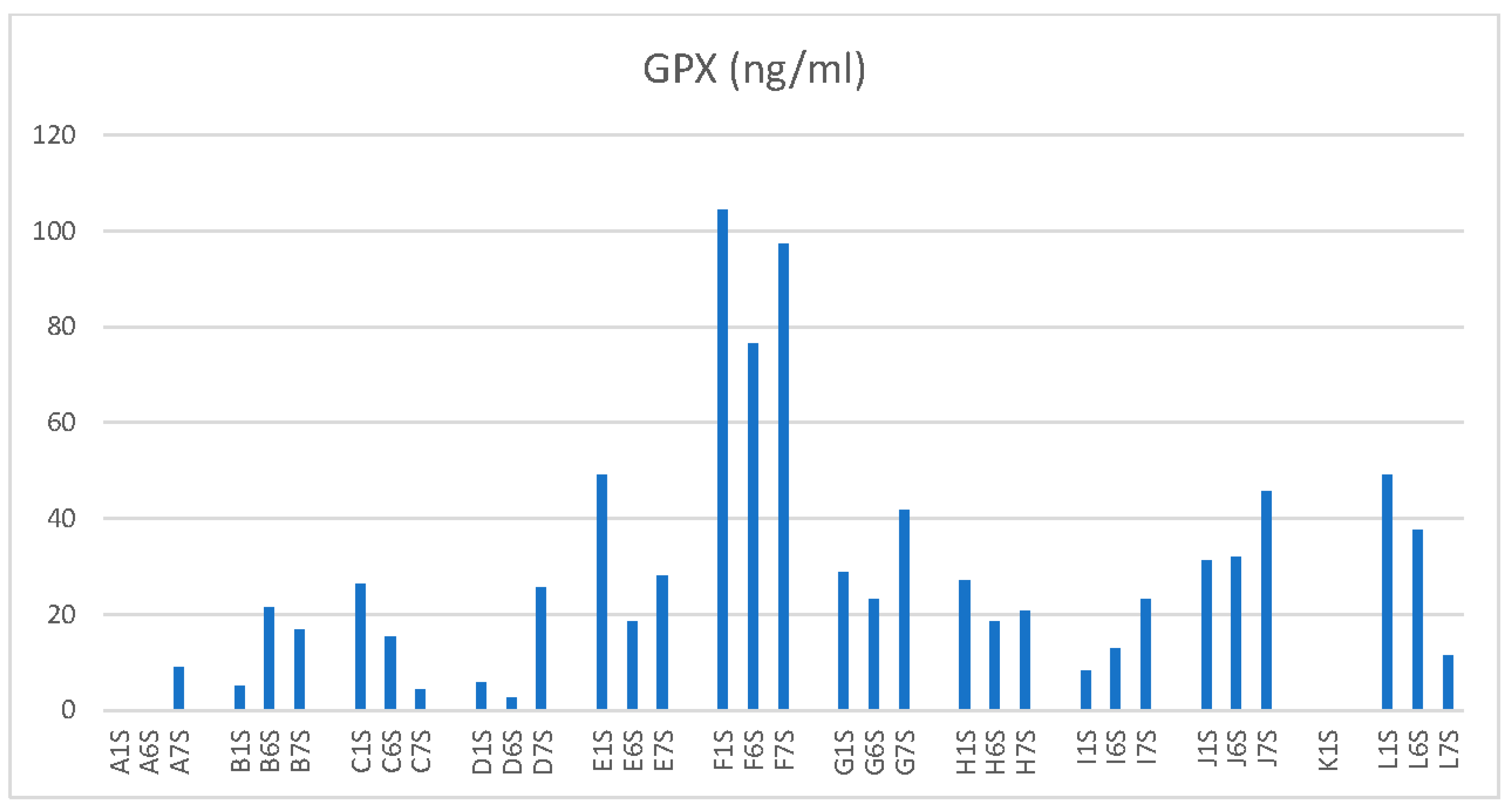
Gluthatione Peroxidase 1 (GPX 1) in the mean decreased from baseline 33.5ng/ml (SD 29.5ng/ml) to 25.9ng/ml (SD 20.3ng/ml) at 2500m 60% FiO2 and slightly also from baseline to 29.6ng/ml (SD 25.9ng/ml) at 4500m 60% FiO2. Likewise with M-LDL there was no significant difference between GPX1 at baseline and one of the altitudes with 60% supplemental O2 or the different values between altitudes.
Figure 4 shows the single GPX values for each subject.
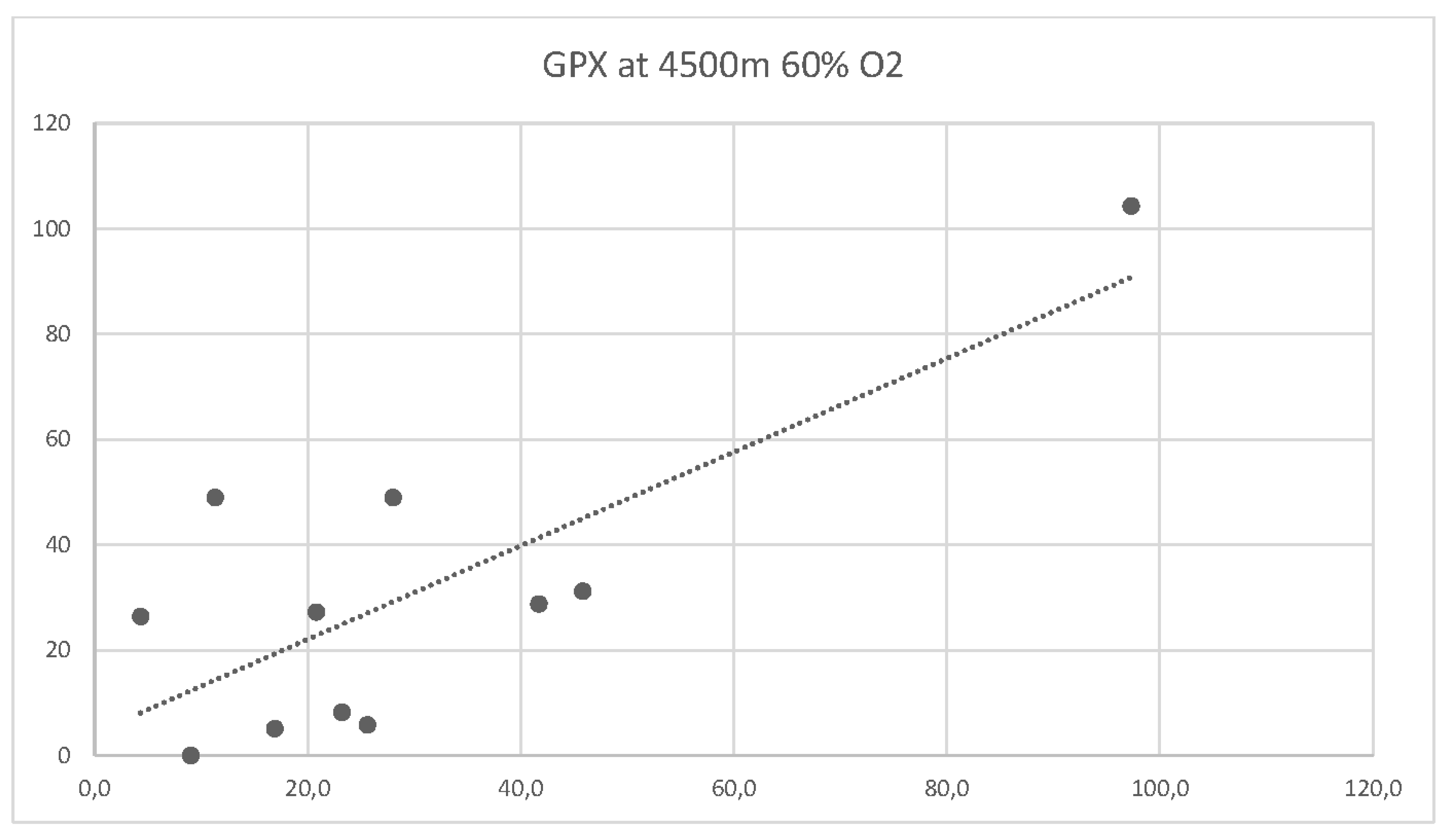
There was no correlation between M-LDL and arterial PO2 values neither at baseline, nor at one of the altitudes with supplemental 60% oxygen. For GPX1 there was a slight negative correlation to PO2 values at 2500m and 60% FiO2 (Pearson correlation coefficient -30.0 and a moderate negative correlation to PO2 values at 4500m and 60% FiO2.
Figure 5 shows the correlation curve for GPX1 and arterial PO2 at 4500m and 60% FiO2.
There is a moderate correlation between subject age and GPX1 at 4500m and 60% FiO2 with a correlation coefficient of 0.55.
Discussion
To our best knowledge this is the first hypobaric-hyperoxic flight simulation with the specific commonly occurring altitudes and recommended maximal supplemental oxygen levels for civilian airflight operations. Our results show that the most commonly recommended oxidative stress biomarkers Malondialdehyde Low Density Lipoprotein (M-LDL) and Glutathione Peroxidase 1 (GPX1) do not significantly increase in a simulated three-hour flight with changing altitudes respectively changing barometric pressure in a hypobaric chamber. This leads to the assumption that a several hour flight with supplemental oxygen through a face mask is not causing a higher level of oxidative stress and therefore is unlikely the causality for neuronal cell damage.
The baseline levels of the two measured oxidative stress biomarkers in serum from arterial blood were very individual between subjects, which led to larger standard deviations from mean values, and they did not change much in the single subjects with altitude at 60% FiO2. We do not know why the baseline levels were so individually different in our healthy subject population with clinically excluded ongoing infection or major stress factors. Age did not play a major role here, at least not concerning the baseline values. However, at 4500m and 60% supplemental oxygen there was a moderate correlation between age and GPX1 values. The higher the age the higher the level of this oxidative stress biomarker.
Our results confirm in some way the outcome of former and ongoing studies concerning military flight operations from members of our work group (7), who could show that those flight operators with higher inflammatory biomarkers have a higher tendency to develop signs of chronic fatigue, but that these higher levels of inflammatory biomarkers cannot not or only in part be causally related to number of performed flights and level of hypobaric hyperoxia.
With a non-significant increase of M-LDL and GPX1 at lower and higher altitude respectively hypobaric air pressure our results show that supplemental oxygen up to 60% FiO2 is safe under the aspect of a non-development of oxidative stress although there is a moderate correlation between arterial PO2 levels and GPX values . The positive aspects of supplemental oxygen as recommended for flight operations in airplanes without pressurized cabins or pressure loss in normally pressurized cabins by authorities seem to be senseful. Especially since most studies show a significant cognitive decline of pilots in hypoxia from 4750m on (25).
Other studies or position papers mainly from high altitude researcher John West concerning persons hiking to high altitudes, living or working at high altitudes have also shown, that the negative aspects of hypoxia like acute mountain sickness or reduced levels of cognitive function can be diluted or eliminated with supplemental oxygen (26–28).
If the oxidative stress from supplemental oxygen in flight operations is unlikely the cause for neuronal cell damage and occurring white matter densities in pilots, especially fighter pilots with crews and astronauts, the questions remain, what possible causes could be. Older and newer discussions circle around the effect of high G-forces in fighter airplane crews and in astronauts repeatedly occurring during training and flight operations (29–31). Another hypothesis is that weightlessness, as it occurs not only in space flight but repeatedly also in dog fighting in military flight operations with new fighter jets, could be the cause for neuronal damages. However, these discussed causes do extremely rarely happen in civilian flight operations with commercial airlines or private or transport flights.
An interesting side aspect of the outcome of our study is the reason of the drop out of one subject. Hyperventilation and the consequences of the alkalosis with dizziness and possible tetany (paw position) due to stressful inflight situations with the necessity of supplemental oxygen might be a bigger problem in sensible passengers or flight crew members than high concentrations of inspired oxygen or subsequently high arterial PO2 levels. Therefore flight crews and pilots should be trained in breathing techniques, which they can use for themselves but also help passengers in need.
Our study has a few limitations. One is of course the relatively low number of subjects, which is due to the large expense in such a hypobaric chamber study including and constantly during the trial placed radial line. However, although we did not proof our hypothesis with a high correlation of oxidative stress and arterial PO2 levels, we could show in the means and in the single subjects that there is no significant increase of the oxidative stress biomarkers even with these high levels of arterial PO2 in each subject in this two-to-three-hour period. Another limitation is that for feasibility and cost reasons we could only analyze two oxidative stress biomarkers at baseline and two other points of measurement. But we think that these two oxidative stress biomarkers are representative, and the level of 60% inspired oxygen is also representative of a real civilian flight situation asking for supplemental oxygen.
Conclusion
Supplemental oxygen up to 60% FiO2 via Venturi valve equipped face mask in a civilian flight simulation of three-hour duration at barometric pressure equivalent to altitudes of 2500m and 4500m does not lead to an increase in the oxidative stress markers Malondialdehyde Low Density Lipoprotein and Glutathione Peroxidase 1 in the arterial blood of healthy subjects despite high arterial PO2 levels. The recommended supplemental oxygen for flight crews in non-pressurized airplane cabins or in situations with pressure loss is not causing a high level of oxidative stress and is safe.
Funding
Expenses for the study were funded from internal sources of the Hermann Buhl Institute, Eurac Terra X Cube, Eurac Institute for Mountain Emergency Medicine and the Medical University of Innsbruck.
Conflicts of Interest
None of the authors has to declare any conflict of interest with this manuscript.
References
- Bouak, F.; Vartanian, O.; Hofer, K.; Cheung, B. Acute mild hypoxic hypoxia effects on cognitive and simulated aircraft pilot performance. Aerosp. Med. Hum. Perform. 2018, 89, 526–535. [Google Scholar] [CrossRef] [PubMed]
- West, J.B. A strategy for in-flight measurements of physiology of pilots of high-performance fighter aircraft. J. Appl. Physiol. 2013, 115, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Tamma, G.; Valenti, G.; Grossini, E.; Donnini, S.; Marino, A.; Marinelli, R.A.; Calamita, G. Aquaporin Membrane Channels in Oxidative Stress, Cell Signaling, and Aging: Recent Advances and Research Trends. Oxid. Med. Cell Longev. 2018, 2018, 1501847. [Google Scholar] [CrossRef]
- Vottier, G.; Pham, H.; Pansiot, J.; Biran, V.; Gressens, P.; Charriaut-Marlangue, C.; Baud, O. Deleterious effect of hyperoxia at birth on white matter damage in the newborn rat. Dev. Neurosci. 2011, 33, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.S.; Tenfen, L.; Joaquim, L.; Lanzzarin, E.V.R.; Bernardes, G.C.; Bonfante, S.R.; Mathias, K.; Biehl, E.; Bagio, É.; Stork, S.S.; Denicol, T.; de Oliveira, M.P.; da Silva, M.R.; Danielski, L.G.; de Quadros, R.W.; Rezin, G.T.; Terra, S.R.; Balsini, J.N.; Gava, F.F.; Petronilho, F. Hyperoxia by short-term promotes oxidative damage and mitochondrial dysfunction in rat brain. Respir. Physiol. Neurobiol. 2022, 306, 103963. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Koppelmans, V.; Riascos, R.F.; Hasan, K.M.; Pasternak, O.; Mulavara, A.P.; Bloomberg, J.J.; Seidler, R.D. Spaceflight-Associated Brain White Matter Microstructural Changes and Intracranial Fluid Redistribution. JAMA Neurol. 2019, 76, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Damato, E.G.; Fillioe, S.J.; Margevicius, S.P.; Mayes, R.S.; Somogyi, J.E.; Vannix, I.S.; Abdollahifar, A.; Turner, A.M.; Ilcus, L.S.; Decker, M.J. Increased Serum Levels of Proinflammatory Cytokines Are Accompanied by Fatigue in Military T-6A Texan II Instructor Pilots. Front. Physiol. 2022, 13, 876750. [Google Scholar] [CrossRef]
- Damato, E.G.; Fillioe, S.J.; Vannix, I.S.; Norton, L.K.; Margevicius, S.P.; Beebe, J.L.; Decker, M.J. Characterizing the Dose Response of Hyperoxia with Brain Perfusion. Aerosp. Med. Hum. Perform. 2022, 93, 493–498. [Google Scholar] [CrossRef]
- Damato, E.G.; Flak, T.A.; Mayes, R.S.; Strohl, K.P.; Ziganti, A.M.; Abdollahifar, A.; Flask, C.A.; LaManna, J.C.; Decker, M.J. Neurovascular and cortical responses to hyperoxia: Enhanced cognition and electroencephalographic activity despite reduced perfusion. J. Physiol. 2020, 598, 3941–3956. [Google Scholar] [CrossRef]
- FAR 91.211 - Supplemental oxygen, www.faa-aircraft-certification.com.
- www.ainonline.com/aviation-news/air-transport/2020-05-13/faa-amends-oxygen-mask-rule-not-without-controversy.
- Liu, J.; Li, S.; Qian, L.; Xu, X.; Zhang, Y.; Cheng, J.; Zhang, W. Effects of acute mild hypoxia on cerebral blood flow in pilots. Neurol. Sci. 2021, 42, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Saito, I.; Fujiwara, O.; Utsuki, N.; Mizumoto, C.; Arimori, T. Hypoxia-induced fatal aircraft accident revealed by voice analysis. Aviat. Space Environ. Med. 1980, 51, 402–406. [Google Scholar]
- Rayman, R.B. Sudden incapacitation in flight, 1 Jan. 1966-30 Nov. 1971. Aerosp Med. 1973, 44, 953–955. [Google Scholar] [PubMed]
- Rayman, R.B.; McNaughton, G.B. Hypoxia: USAF experience 1970-1980. Aviat Space Environ Med. 1983, 54, 357–359. [Google Scholar]
- Martin-Saint-Laurent, A.; Lavernhe, J.; Casano, G.; Simkoff, A. Clinical aspects of inflight incapacitations in commercial aviation. Aviat. Space Environ. Med. 1990, 61, 256–260. [Google Scholar] [PubMed]
- www.longislandpress.com/2023/06/06/loss-oxygen-virginia-plane-crash/.
- Aerospace Medical Association; Aviation Safety Committee; Civil Aviation Subcommittee. Cabin cruising altitudes for regular transport aircraft. Aviat. Space Environ. Med. 2008, 79, 433–439. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, 31S–38S. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Experimental Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Sies, H. Biological redox systems and oxidative stress. Cell Mol. Life Sci. 2007, 64, 2181–2188. [Google Scholar] [CrossRef]
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxid. Med. Cell Longev. 2019, 2019, 1279250. [Google Scholar] [CrossRef] [PubMed]
- Bundesgesundheitsbl - Gesundheitsforsch – Gesundheitsschutz. 2008, 51, 1464–1482. . [CrossRef]
- Hemmerich, W. StatisticGuru: Poweranalyse für Korrelationen 2018. Retrieved from https://statisticguru.deTrechner/poweranalyse-korrelationen.html.
- Shaw, D.M.; Cabre, G.; Gant, N. Hypoxic Hypoxia and Brain Function in Military Aviation: Basic Physiology and Applied Perspectives. Front. Physiol. 2021, 12, 665821. [Google Scholar] [CrossRef]
- West, J.B. Cognitive Impairment of School Children at High Altitude: The Case for Oxygen Conditioning in Schools. High. Alt. Med. Biol. 2016, 17, 203–207. [Google Scholar] [CrossRef]
- West, J.B. High-altitude medicine. Am. J. Respir. Crit. Care Med. 2012, 186, 1229–1237. [Google Scholar] [CrossRef]
- West, J.B. A new approach to very-high-altitude land travel: The train to Lhasa, Tibet. Ann. Intern. Med. 2008, 149, 898–900. [Google Scholar] [CrossRef]
- Koppelmans, V.; Mulavara, A.P.; Seidler, R.D.; De Dios, Y.E.; Bloomberg, J.J.; Wood, S.J. Cortical thickness of primary motor and vestibular brain regions predicts recovery from fall and balance directly after spaceflight. Brain Struct. Funct. 2022, 227, 2073–2086. [Google Scholar] [CrossRef]
- Li, J.S.; Sun, X.Q.; Wu, X.Y.; Rao, Z.R.; Liu, H.L.; Xie, X.P. [Influences of repeated lower +Gz exposures on high +Gz exposure induced brain injury in rats]. Space Med. Med. Eng. 2002, 15, 339–342. (In Chinese) [Google Scholar]
- Pashchenko, P.S.; Risman, B.V. Strukturnye preobrazovaniia v serom veshchestve spinnogo mozga posle vozdeĭstviia gravitatsionnykh peregruzok [Structural changes in spinal cord gray matter induced by gravitational overloads]. Morfologiia 2002, 121, 49–54. (In Russian) [Google Scholar]
- Clément, G.R.; Boyle, R.D.; George, K.A.; Nelson, G.A.; Reschke, M.F.; Williams, T.J.; Paloski, W.H. Challenges to the central nervous system during human spaceflight missions to Mars. J. Neurophysiol. 2020, 123, 2037–2063. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).