Submitted:
03 January 2024
Posted:
04 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Growth Conditions
2.2. Phage Stock Preparation
2.3. Formation of Biofilms
2.4. Characterization of Bacterial Capacity Of biofilm Formation
2.5. Formation and Quantification of the E. coli Biofilm on Plastic and Stainless Steel
2.6. Efficacy of the phT4A Phage in the Reduction of E. coli Biofilm on Plastic and Stainless Steel
2.7. Efficacy of phT4A Phage in the Prevention of E. coli Biofilm on Plastic
2.8. Evaluation of the Biofilm Formation Ability of Resistant and Sensitive Bacteria to phT4A Phage
2.9. Statistical Analysis
3. Results
3.1. Characterization of Bacterial Capacity of Biofilm Formation
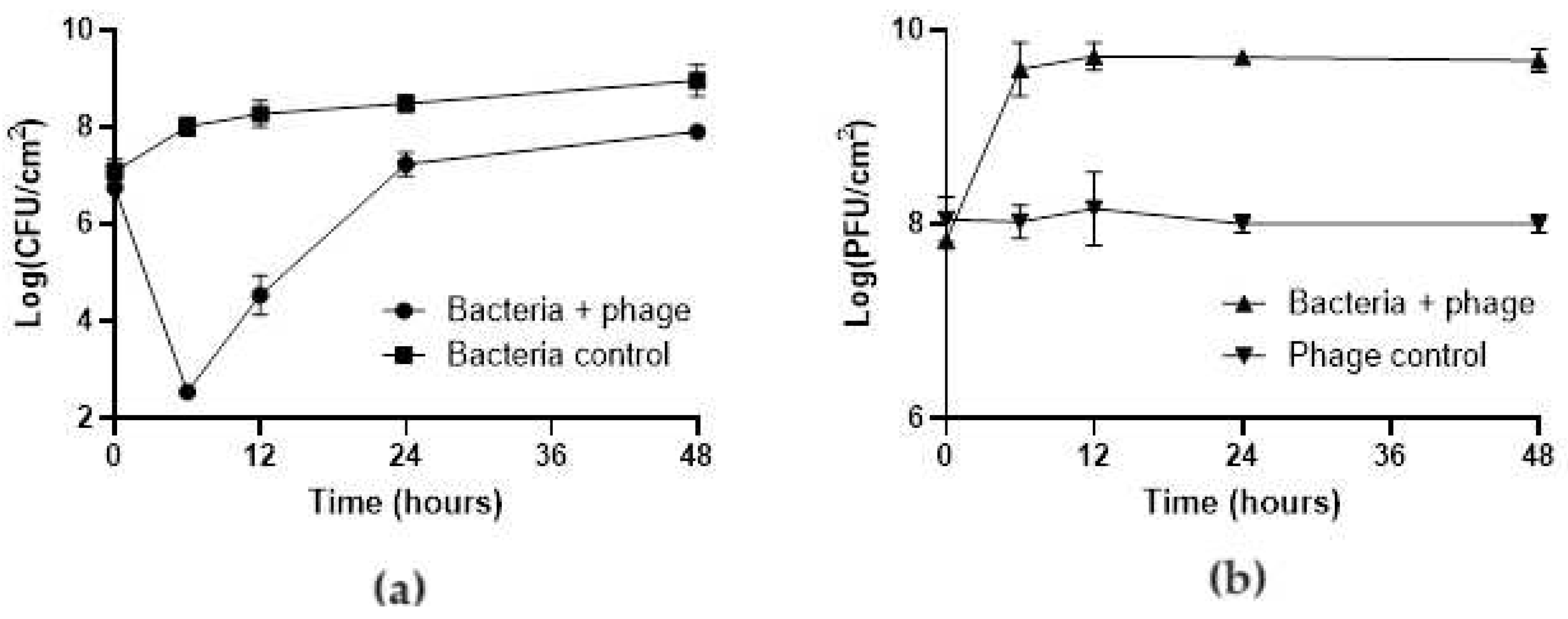
3.2. Efficacy of the phT4A Phage in the Reduction of E. coli Biofilm on Plastic and Stainless Steel
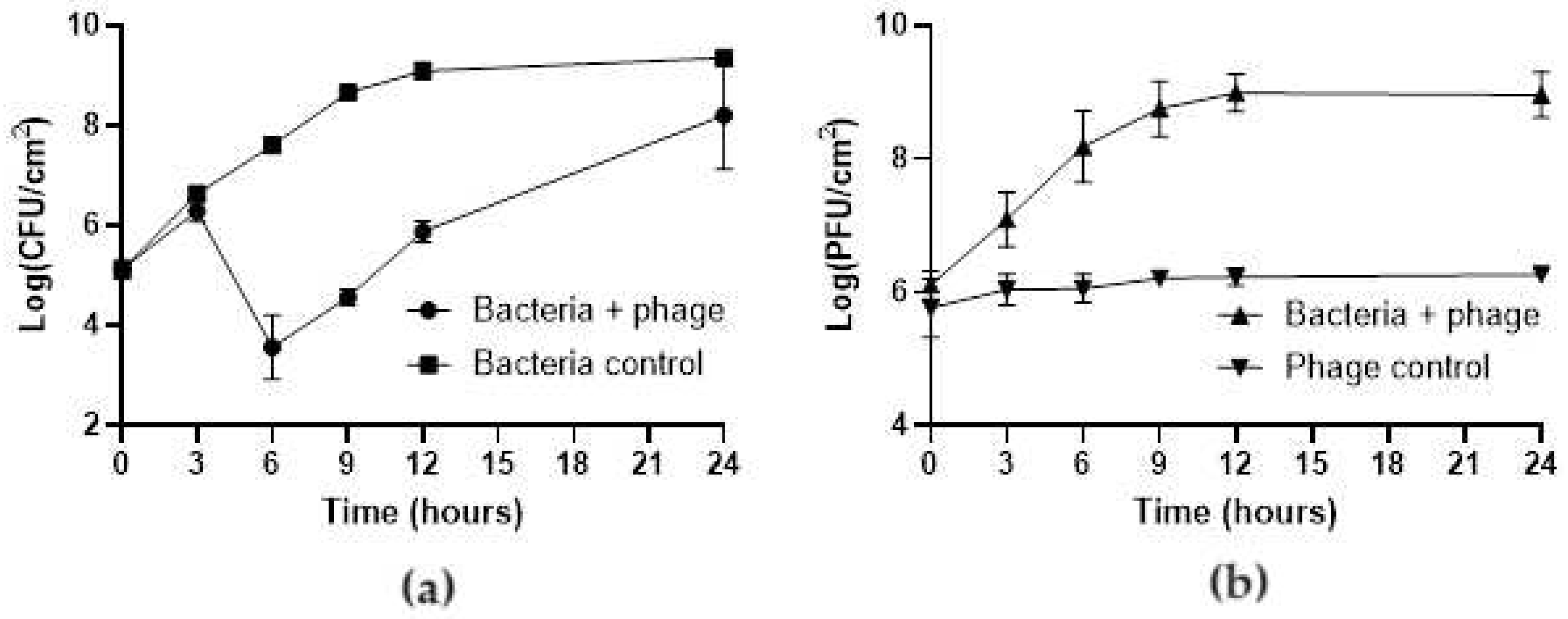
3.3. Efficacy of phT4A Phage in the Prevention of E. coli Biofilm on Plastic
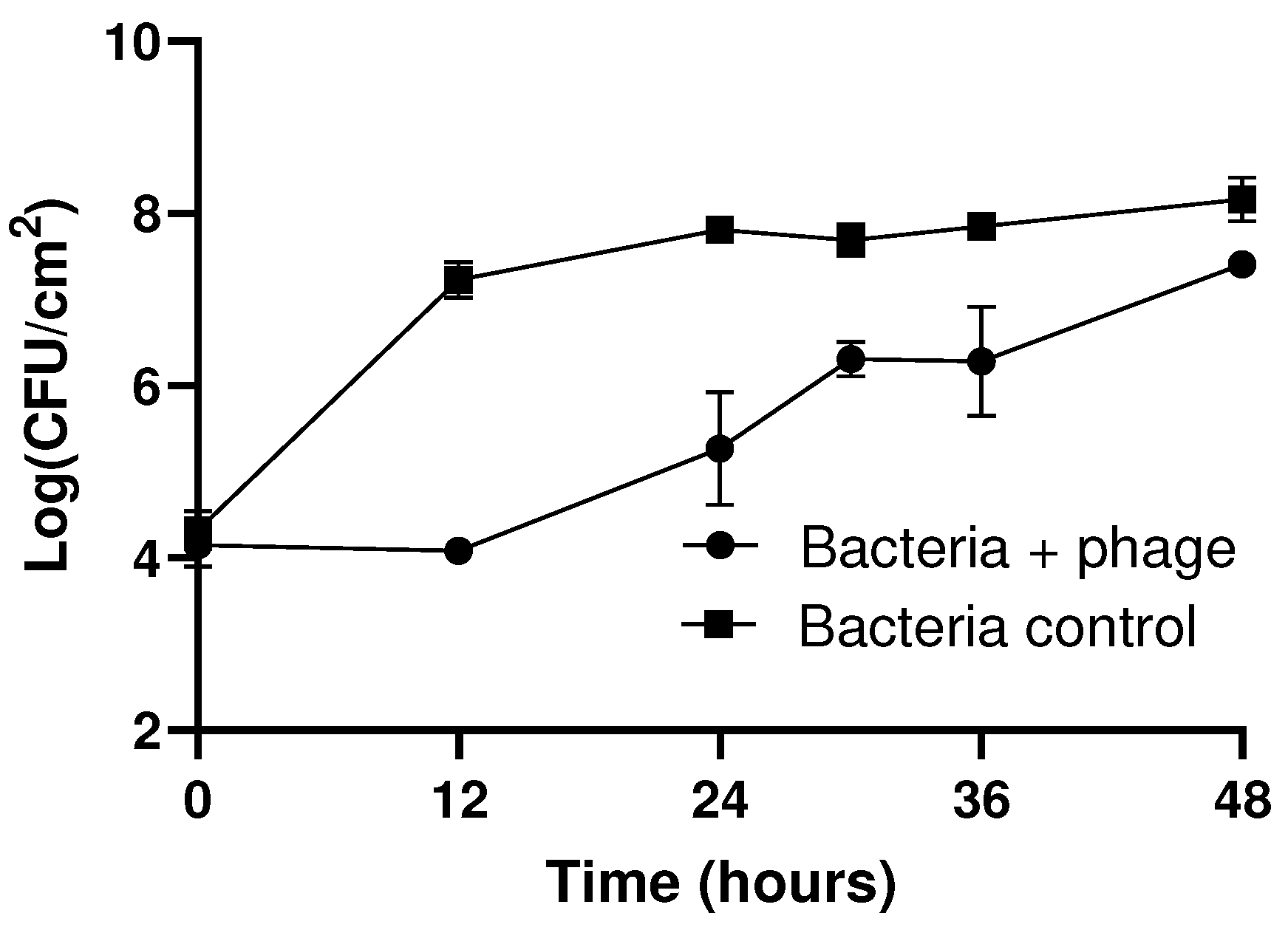
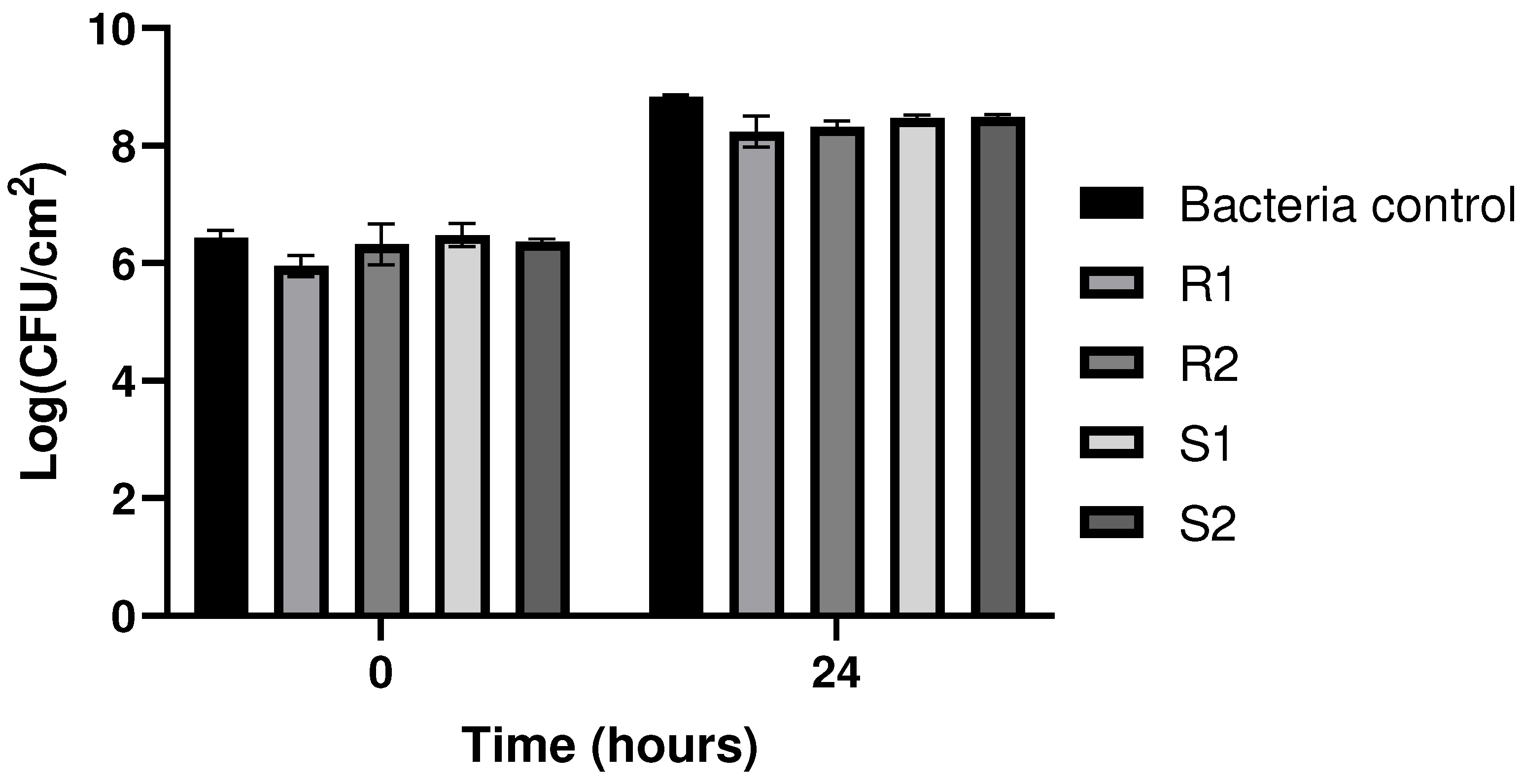
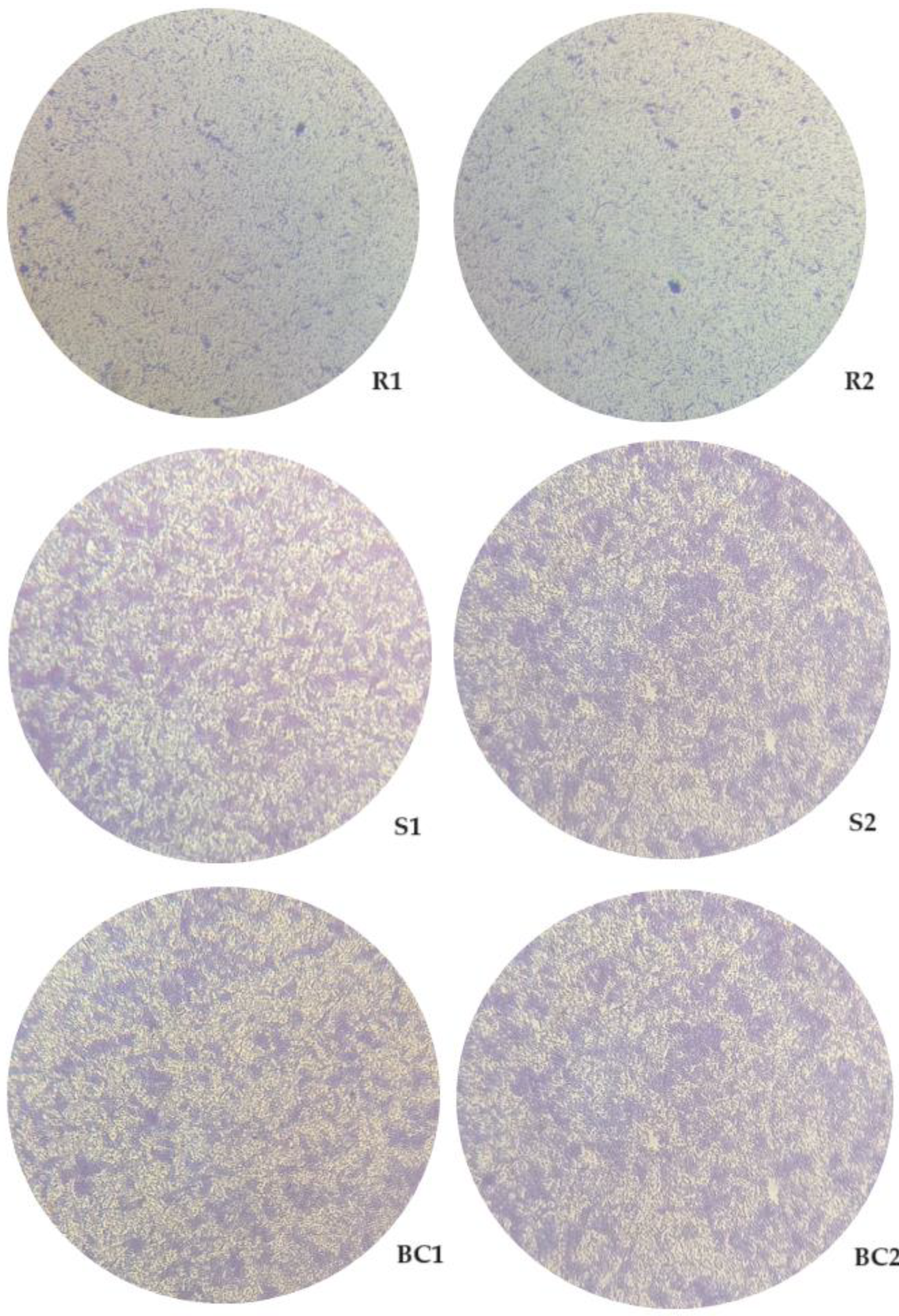
3.4. Evaluation of the Biofilm Formation Ability of Resistant and Sensitive Bacteria to phT4A Phage
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vikram, A.; Woolston, J.; Sulakvelidze, A. Phage biocontrol applications in food production and processing. Curr. Issues Mol. Biol. 2020, 40, 267–302. [Google Scholar] [CrossRef]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage applications for food production and processing. Viruses 2018, 10, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M. Bacteriophages and food production: Biocontrol and bio-preservation options for food safety. Antibiotics 2022, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gounadaki, A.S.; Skandamis, P.N.; Drosinos, E.H.; Nychas, G.J.E. Microbial ecology of food contact surfaces and products of small-scale facilities producing traditional sausages. Food Microbiol. 2008, 25, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Delgado, S.; Vázquez-Sánchez, D.; Martínez, B.; Cabo, M.L.; Rodríguez, A.; Herrera, J.J.; García, P. Incidence of Staphylococcus aureus and analysis of associated bacterial communities on food industry surfaces. Appl. Environ. Microbiol. 2012, 78, 8547–8554. [Google Scholar] [CrossRef]
- EFSA The European Union one health 2022 zoonoses report. EFSA J. 2023, 21, e8442.
- Yang, S.C.; Lin, C.H.; Aljuffali, I.A.; Fang, J.Y. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, D.; Hu, J.; Zhang, Y.; Tan, B.K.; Lin, S. Control measurements of Escherichia coli biofilm: A review. Foods 2022, 11, 1–11. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; García, P. Bacteriophages as weapons against bacterial biofilms in the food industry. Front. Microbiol. 2016, 7, 825. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, S.; Sharma, P.; Chandola, D.; Dang, S.; Gupta, S.; Gabrani, R. Escherichia coli biofilm: development and therapeutic strategies. J. Appl. Microbiol. 2016, 121, 309–319. [Google Scholar] [CrossRef]
- Capita, R.; Riesco-Peláez, F.; Alonso-Hernando, A.; Alonso-Calleja, C. Exposure of Escherichia coli ATCC 12806 to sublethal concentrations of food-grade biocides influences its ability to form biofilm, resistance to antimicrobials, and ultrastructure. Appl. Environ. Microbiol. 2014, 80, 1268–1280. [Google Scholar] [CrossRef]
- Endersen, L.; O’Mahony, J.; Hill, C.; Ross, R.P.; McAuliffe, O.; Coffey, A. Phage therapy in the food industry. Annu. Rev. Food Sci. Technol. 2014, 5, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage therapy: A renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, F.; Barr, J. Phage therapy in the postantibiotic era. Clin. Microbiol. Rev. 2019, 32, 1–25. [Google Scholar] [CrossRef]
- Lopes, A.; Pereira, C.; Almeida, A. Sequential combined effect of phages and antibiotics on the inactivation of E. coli. Microorganisms 2018, 6, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Nobrega, F.L.; Costa, A.R.; Kluskens, L.D.; Azeredo, J. Revisiting phage therapy: New applications for old resources. Trends Microbiol. 2015, 23, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Ali, Z.; Khan, M.; Bostan, N.; Naseem, S. The dawn of phage therapy. Rev. Med. Virol. 2019, 29, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.P.; Melo, L.D.R.; Vilas Boas, D.; Sillankorva, S.; Azeredo, J. Phage therapy as an alternative or complementary strategy to prevent and control biofilm-related infections. Curr. Opin. Microbiol. 2017, 39, 48–56. [Google Scholar] [CrossRef]
- Ballén, V.; Cepas, V.; Ratia, C.; Gabasa, Y.; Soto, S.M. Clinical Escherichia coli: From biofilm formation to new antibiofilm strategies. Microorganisms 2022, 10. [Google Scholar] [CrossRef]
- Crémet, L.; Corvec, S.; Batard, E.; Auger, M.; Lopez, I.; Pagniez, F.; Dauvergne, S.; Caroff, N. Comparison of three methods to study biofilm formation by clinical strains of Escherichia coli. Diagn. Microbiol. Infect. Dis. 2013, 75, 252–255. [Google Scholar] [CrossRef]
- Naves, P.; Del Prado, G.; Huelves, L.; Gracia, M.; Ruiz, V.; Blanco, J.; Rodríguez-Cerrato, V Ponte, M.; Soriano, F. Measurement of biofilm formation by clinical isolates of Escherichia coli is method-dependent. J Appl Microbiol 2008, 105, 585–590. [CrossRef]
- Pereira, C.; Moreirinha, C.; Lewicka, M.; Almeidab, P.; Clemente, C.; Romalde, J.L.; Nunes, M.L.; Almeida, A. Characterization and in vitro evaluation of new bacteriophages for the biocontrol of Escherichia coli. Virus Res. 2016, 227, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Pereira, C.; Gomes, A.; Almeida, A. Efficiency of single phage suspensions and phage cocktail in the inactivation of Escherichia coli and Salmonella Typhimurium: An in vitro preliminary study. Microorganisms 2019, 7, 94. [Google Scholar] [CrossRef]
- Adams, M.H. Bacteriophages; Interscience Publishers, John Wiley and Sons Inc: New York, USA, 1959. [Google Scholar]
- Mukane, L.; Racenis, K.; Rezevska, D.; Petersons, A.; Kroica, J. Anti-biofilm effect of bacteriophages and antibiotics against uropathogenic Escherichia coli. Antibiotics 2022, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mangieri, N.; Foschino, R.; Picozzi, C. Application of bacteriophages on Shiga Toxin-Producing Escherichia coli (STEC) biofilm. Antibiotics 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ding, Y.; Huang, C.; Wang, J.; Wang, J.; Wang, X. Genomic characterization of a novel bacteriophage STP55 revealed its prominent capacity in disrupting the dual-species biofilm formed by Salmonella Typhimurium and Escherichia coli O157: H7 strains. Arch. Microbiol. 2022, 204, 1–17. [Google Scholar] [CrossRef]
- Park, D.W.; Park, J.H. Characterization of a novel phage depolymerase specific to Escherichia coli O157:H7 and biofilm control on abiotic surfaces. J. Microbiol. 2021, 59, 1002–1009. [Google Scholar] [CrossRef]
- Filippov, A.; Sergueev, K. V.; He, Y.; Huang, X.Z.; Gnade, B.T.; Mueller, A.J.; Fernandez-Prada, C.; Nikolich, M.P. Bacteriophage-resistant mutants in Yersinia pestis: Identification of phage receptors and attenuation for mice. PLoS One 2011, 6, 1–11. [Google Scholar] [CrossRef]
- Wang, C.; Hang, H.; Zhou, S.; Niu, Y.D.; Du, H.; Stanford, K.; McAllister, T.A. Bacteriophage biocontrol of Shiga toxigenic Escherichia coli (STEC) O145 biofilms on stainless steel reduces the contamination of beef. Food Microbiol. 2020, 92, 103572. [Google Scholar] [CrossRef]
- Tabassum, R.; Shafique, M.; Khawaja, K.A.; Alvi, I.A.; Rehman, Y.; Sheik, C.S.; Abbas, Z.; Rehman, S. ur Complete genome analysis of a Siphoviridae phage TSK1 showing biofilm removal potential against Klebsiella pneumoniae. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Montso, P.K.; Mlambo, V.; Ateba, C.N. Efficacy of novel phages for control of multi-drug resistant Escherichia coli O177 on artificially contaminated beef and their potential to disrupt biofilm formation. Food Microbiol. 2021, 94, 103647. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yuan, S.; Liu, Q.; Mai, G.; Yang, J.; Deng, D.; Zhang, B.; Liu, C.; Ma, Y. In vitro design and evaluation of phage cocktails against Aeromonas salmonicida. Front. Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
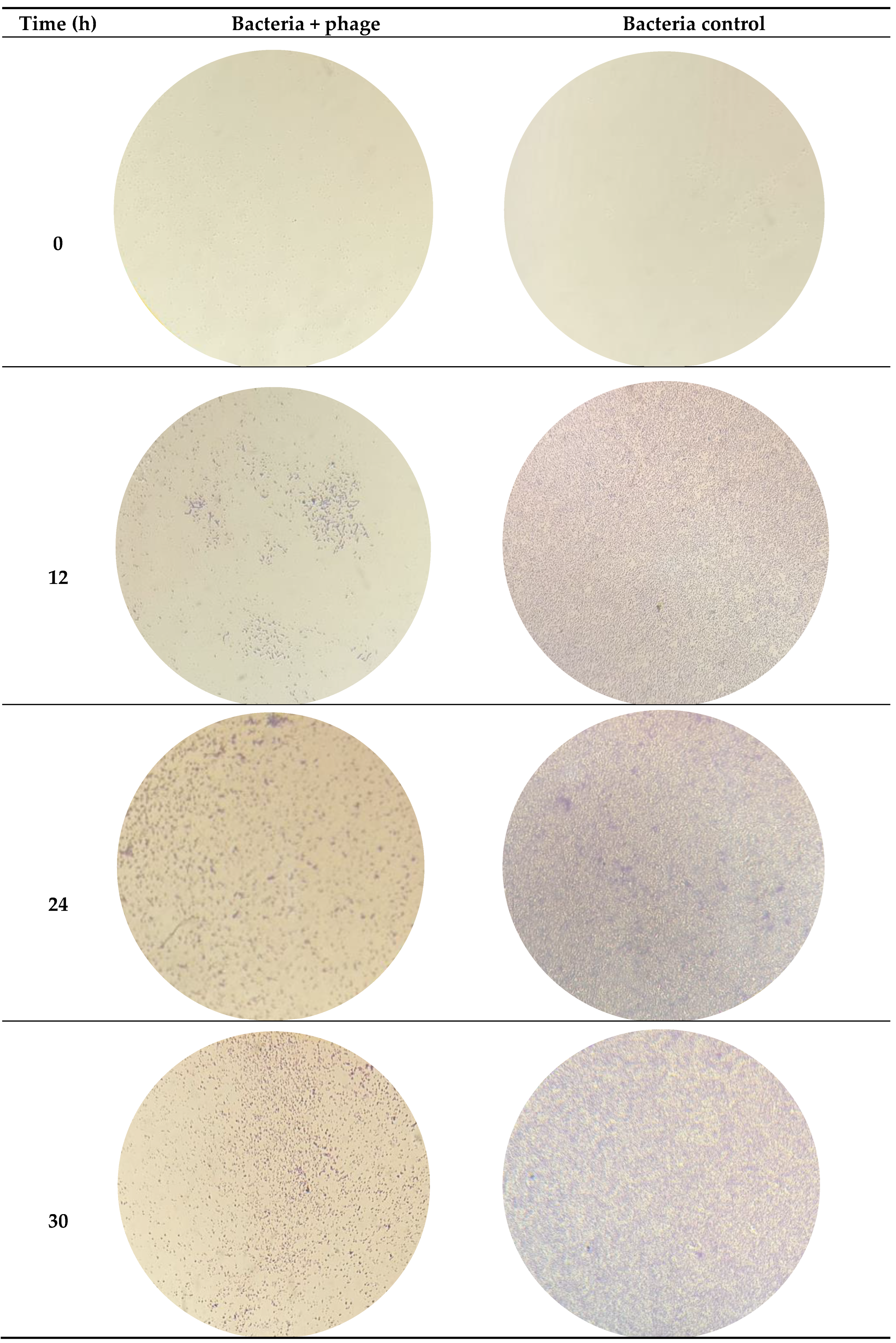
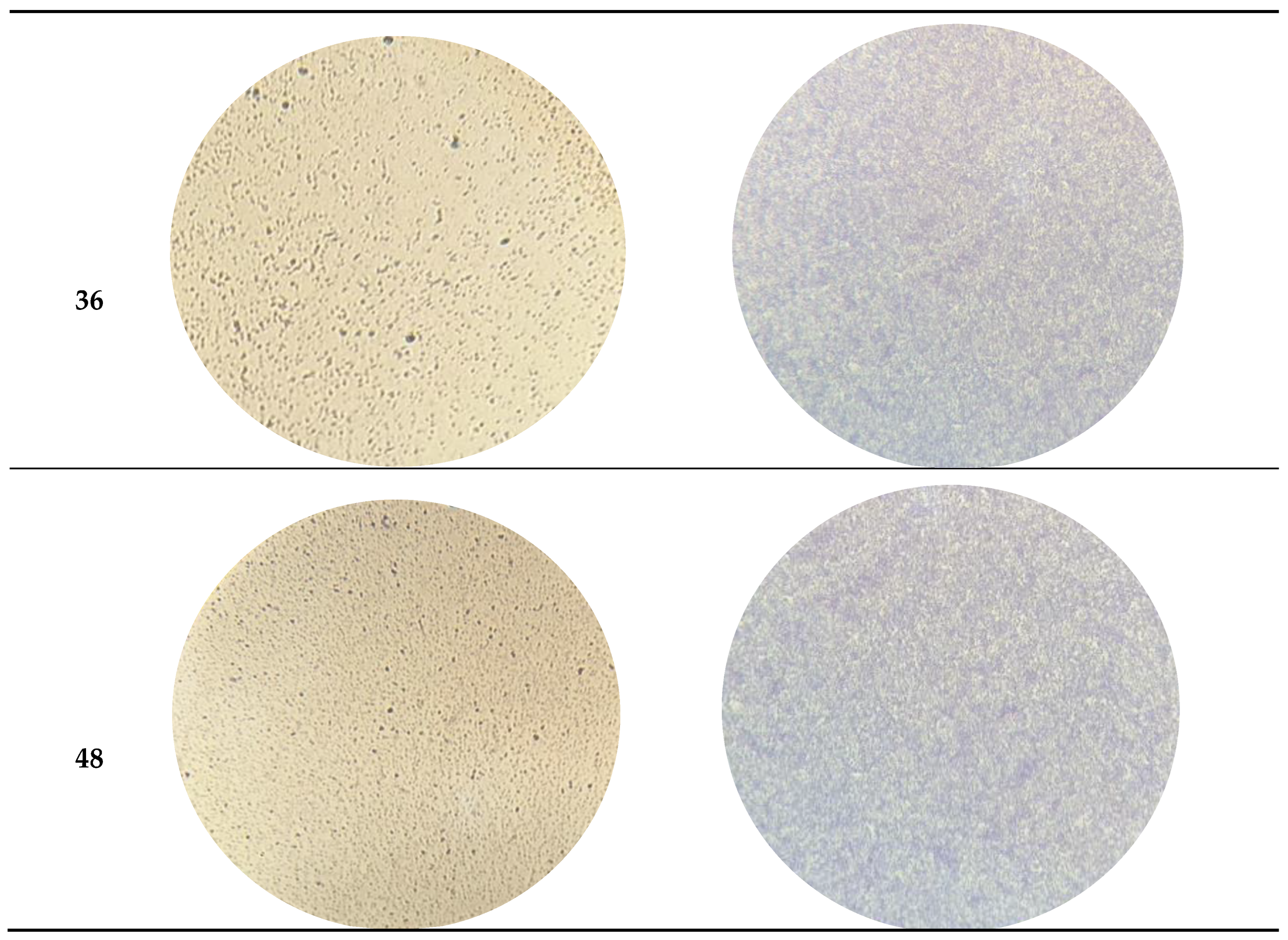
| ATCC 13706 | ATCC 25922 | |
|---|---|---|
| Stained adherent bacteria (BC or ODB) | 1.381 | 0.221 |
| Acetic acid + Crystal violet (CW) | 0.087 | |
| TSB (ODc) | 0.040 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





