Submitted:
03 January 2024
Posted:
04 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- The study uses only seven broad age bands. We have demonstrated that each COVID-19 variant has a unique single-year-of-age profile for mortality [25] and would argue that age should be a continuous variable. The use of age bands is probably concealing more nuanced behavior.
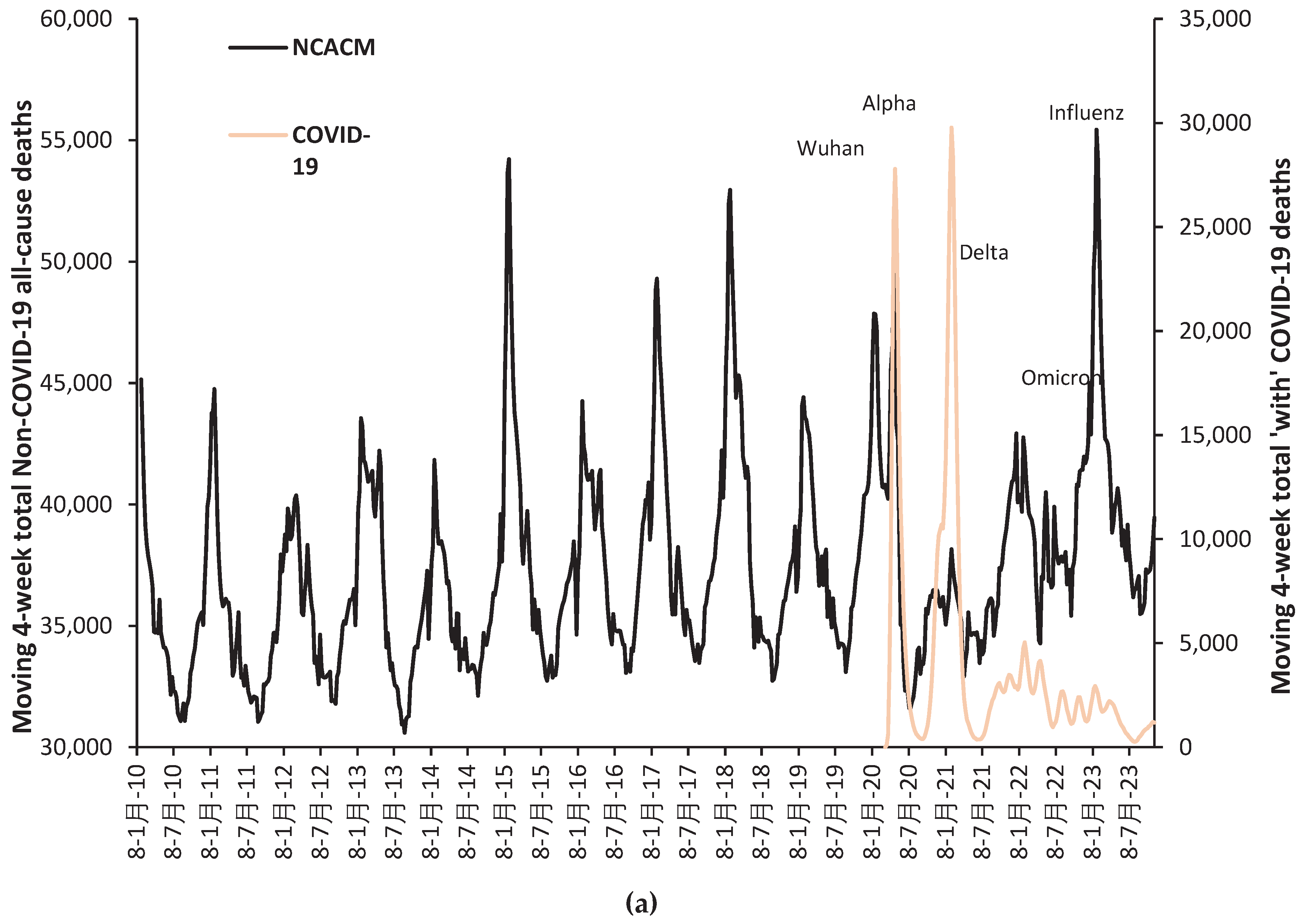
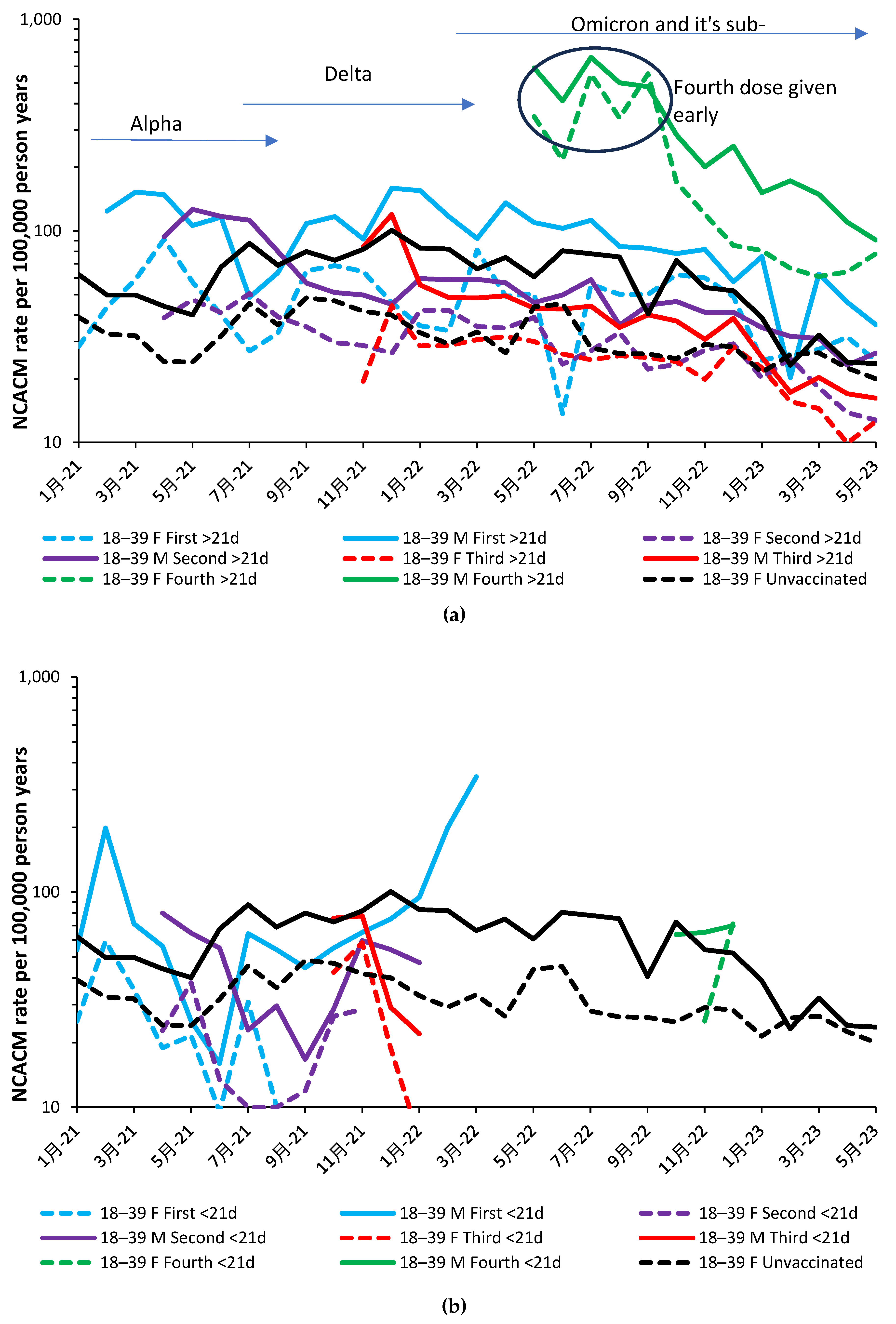
- The study was conducted at a time when vaccines in the UK were based on the original Wuhan stalk antigen. In addition, by early 2023 the Alpha and Delta variants are no longer present, and by the end of the study only Omicron sub-variants were circulating. The results cannot therefore be directly extrapolated into the future should variants other than Omicron arise.
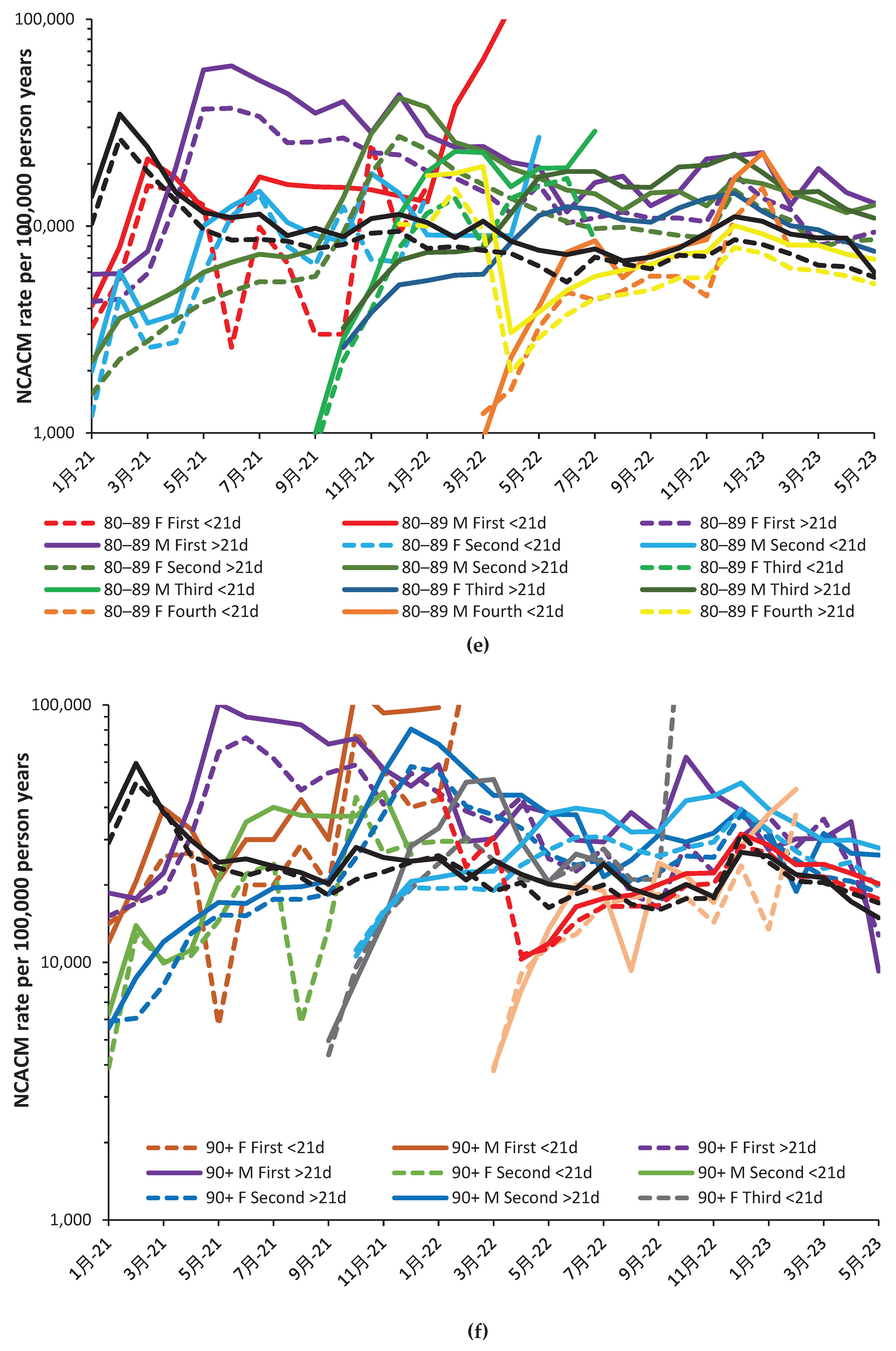
- Time since vaccination is split into two groups, namely, up to 21 days and greater than 21 days. The up to 21-day group encompasses the time when immunity is being optimized, however, the greater than 21-day group contains a mix of individuals with differing degrees of vaccine waning.
2. Materials and Methods
2.1. All-cause mortality by vaccination status in England
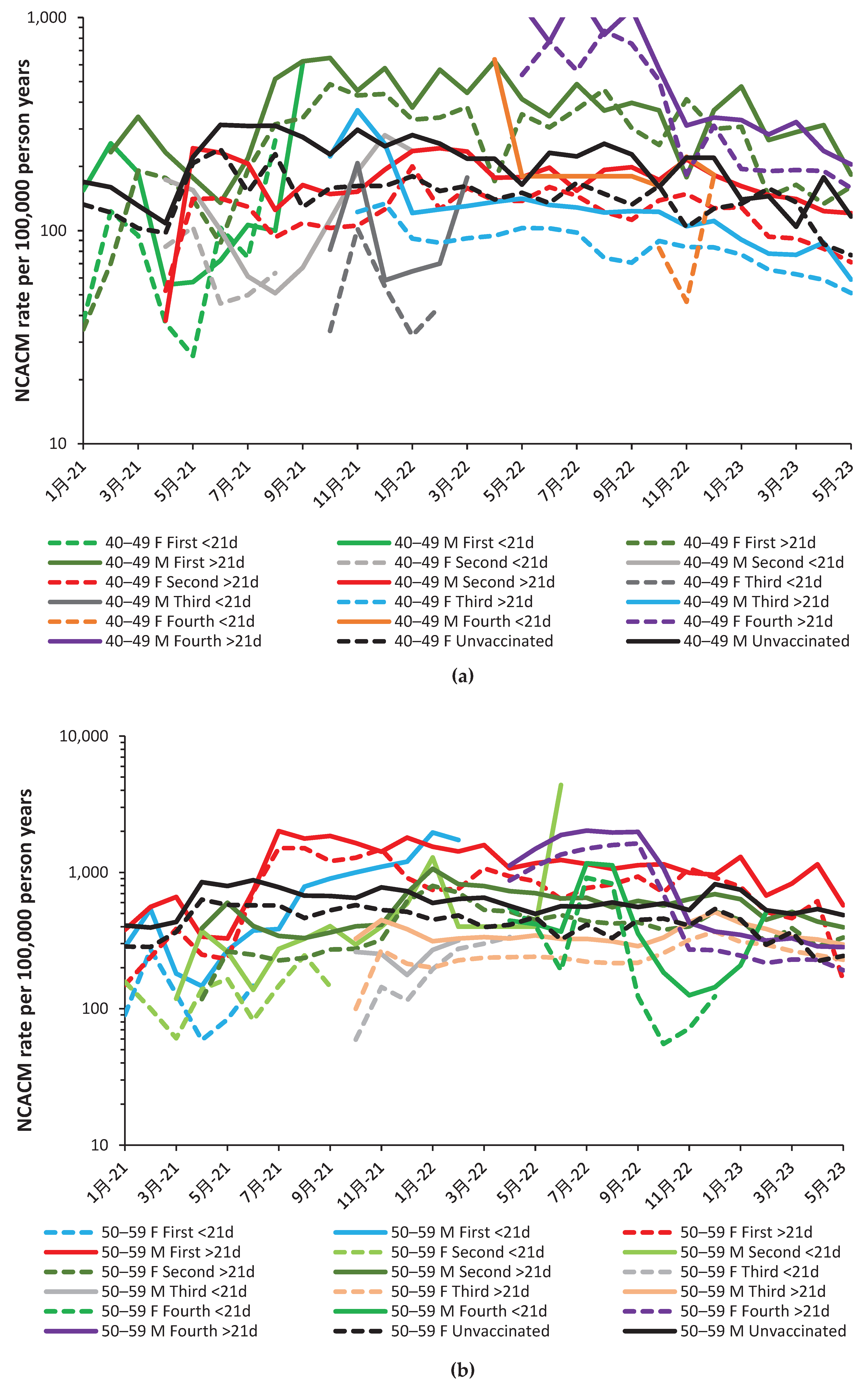
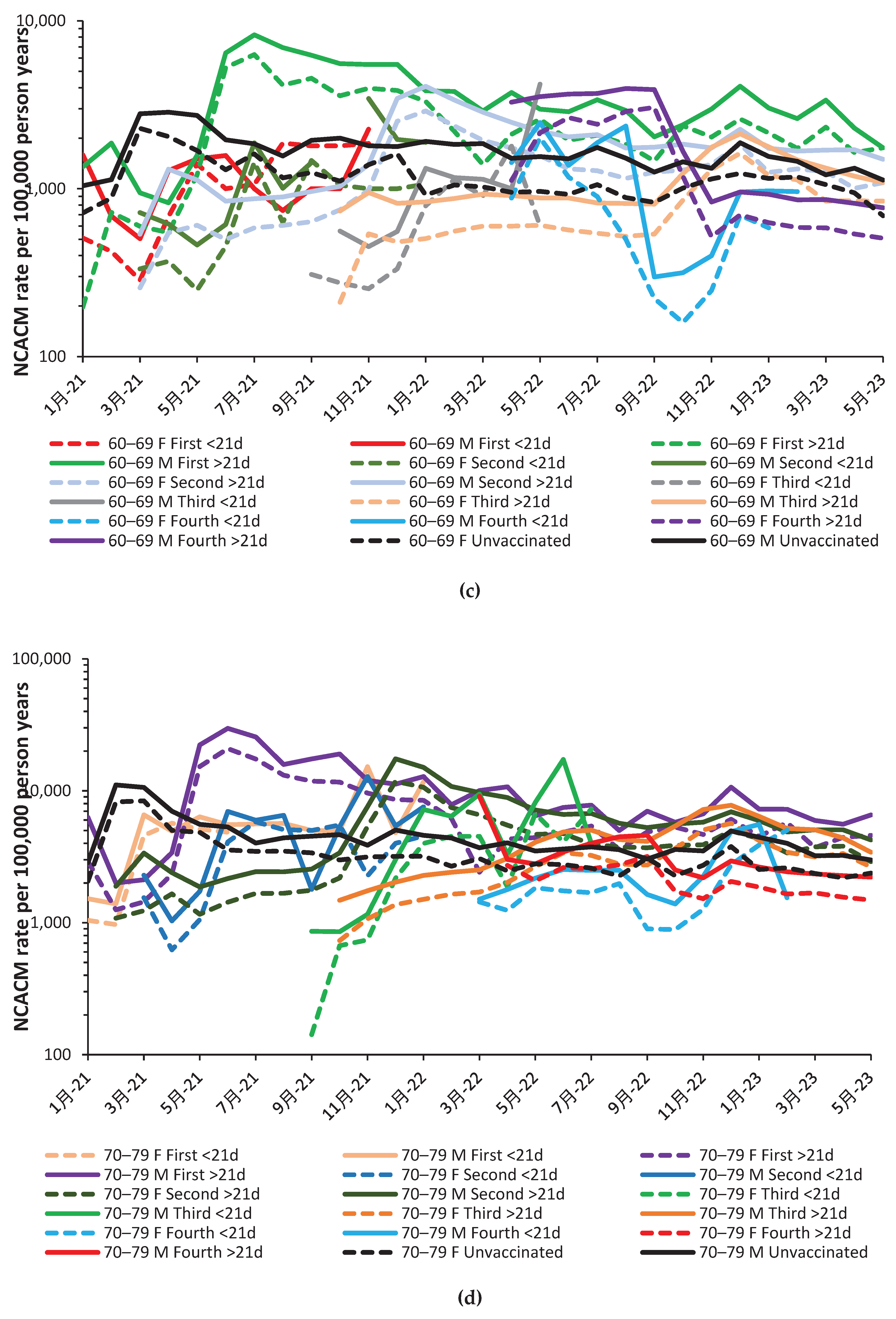
2.2. Effect of time of vaccination, vaccine history, gender and age upon all-cause mortality
3. Results
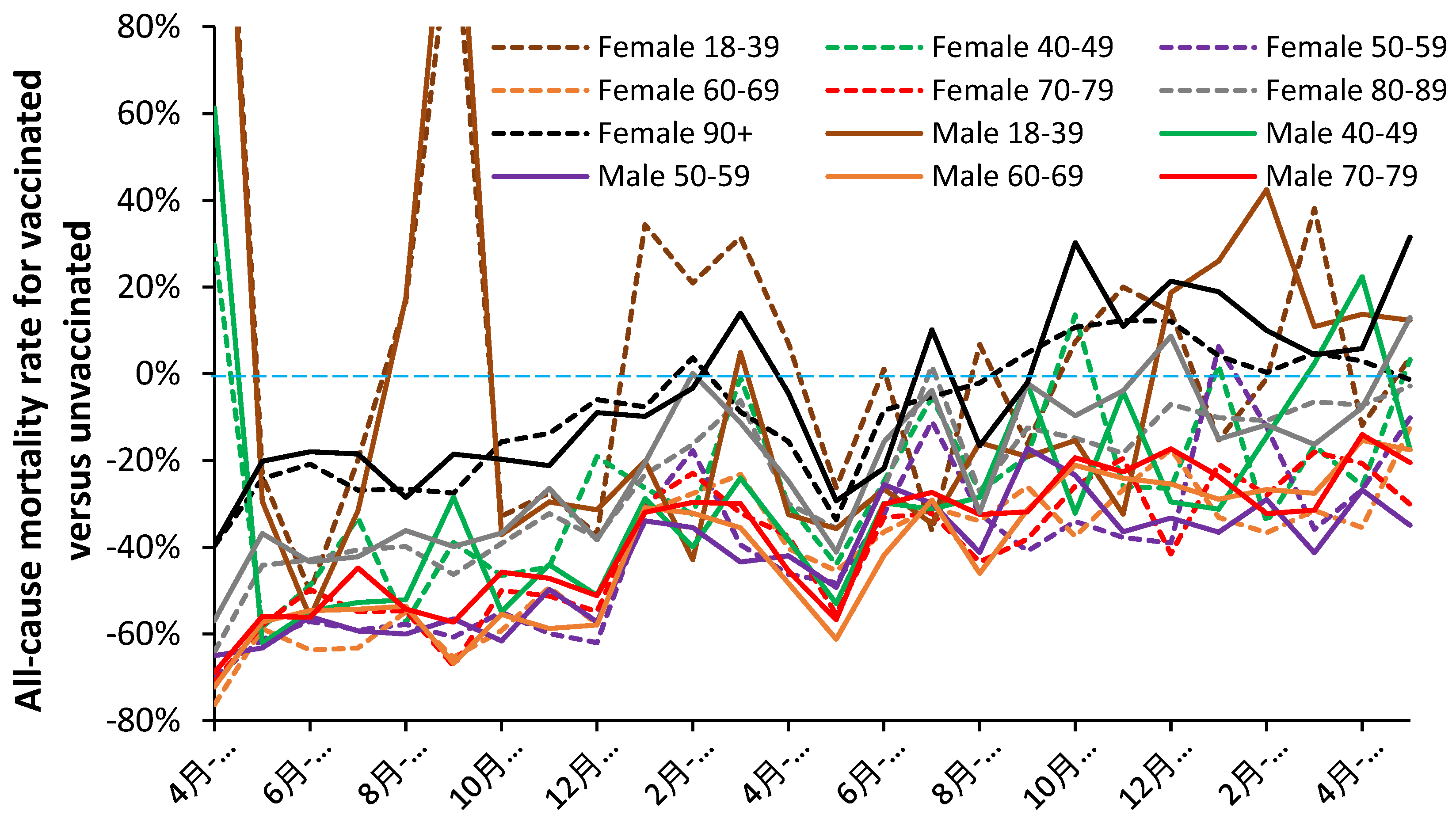
3.1. Overview of the net effect of vaccination with time
3.2. Unexpected complexity
3.3. The timing of the nonspecific benefit of vaccination during the first 21 days
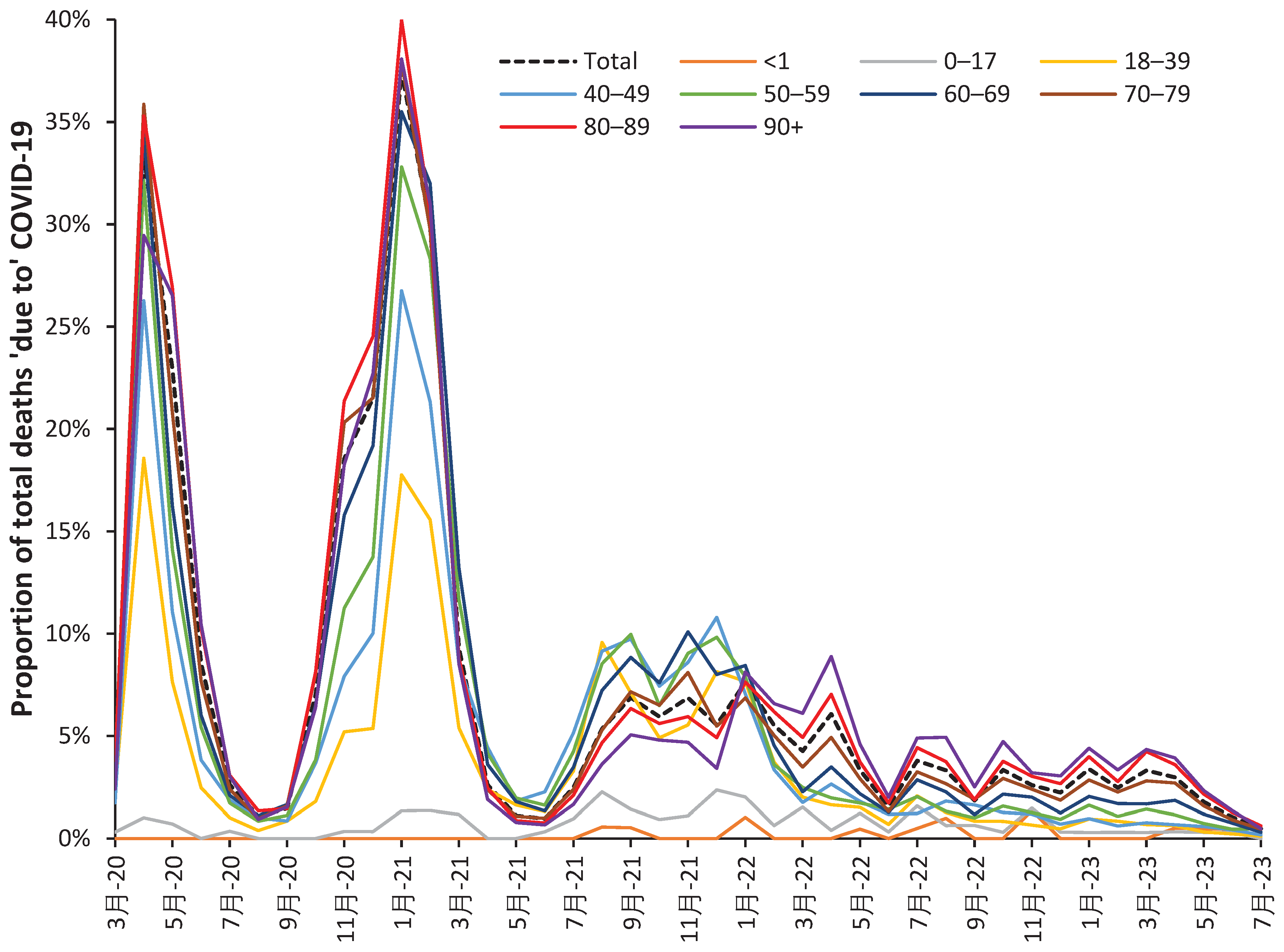
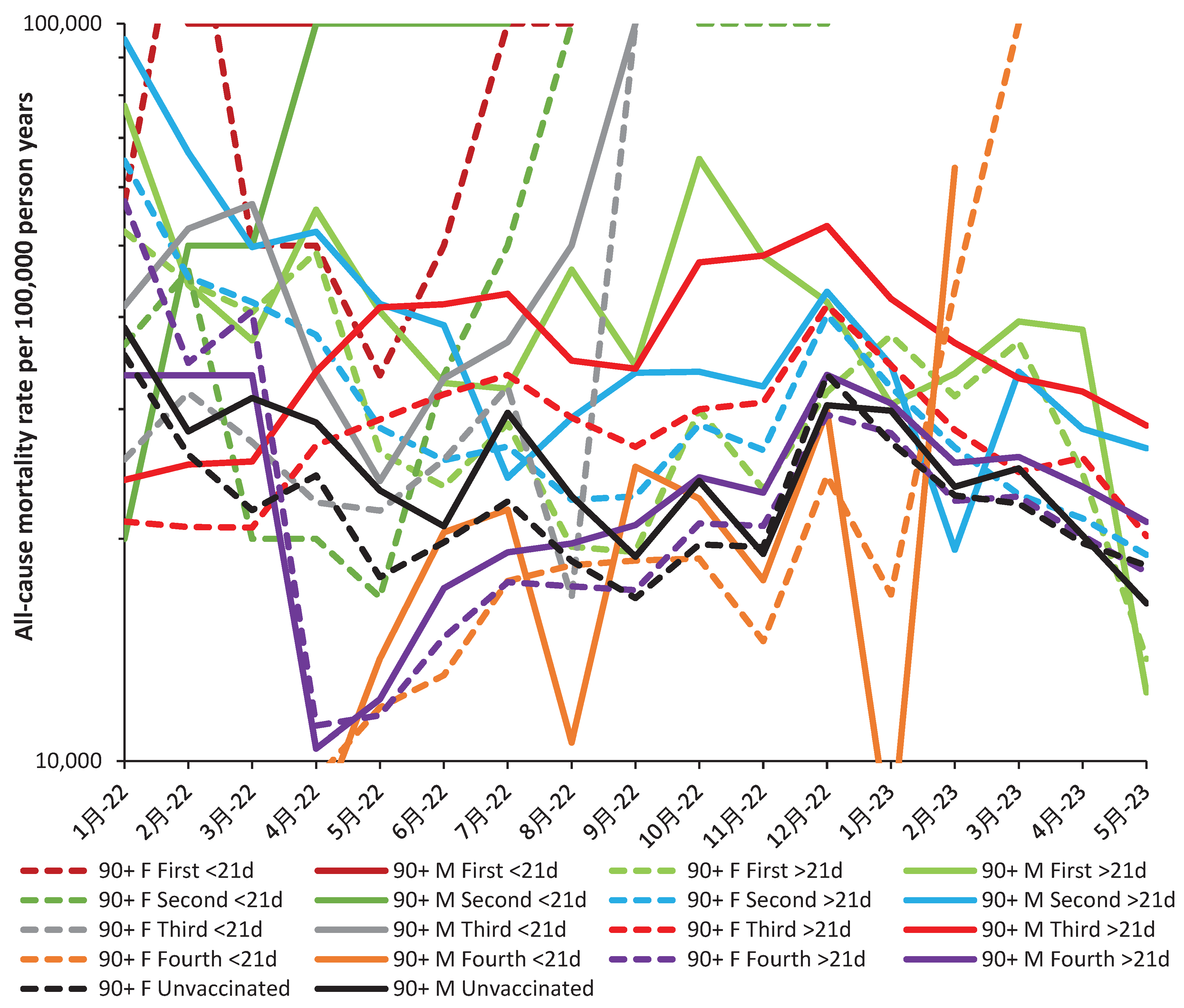
3.4. All-cause (including COVID-19) mortality during Omicron
4. Discussion
4.1. Factors driving complexity in COVID–19 mortality and the vaccine response
4.1.1. Declining vaccine effectiveness
4.1.2. COVID-19 variants show year of age specificity for mortality
4.1.3. Gene expression varies with season and latitude
4.1.4. The central role of small non-coding RNAs in gene expression
4.1.5. COVID–19 infection alters the miRNA landscape and ensuing gene expression.
4.1.6. Interplay between interferons and miRNAs
4.1.7. Nonspecific effects of vaccines
4.1.8. A genetic basis for COVID–19 risk
4.1.9. Different responses between males and females
4.1.10. Age and COVID–19 vaccination outcomes
4.1.11. Simultaneous benefit/disbenefit
4.1.12. COVID-19 prophylactic therapy and mortality
4.1.13. The 21-day break point to characterize vaccine time-related effects
- Maximum gene expression (up/down-regulation) occurred 1 to 3 days after vaccination. This implies rapid production of miRNAs prior to gene regulation.
- Three groups of genes were regulated, namely early and late upregulation, and downregulation.
- Different patterns of genes are expressed in high/medium/low antibody responders. High vaccine responder status correlates with increased early expression of interferon signaling and antigen processing and presentation genes.
- The expression of early activation genes strongly correlated with antibodies at 14 and 28 days after vaccination.
4.1.14. miRNA expression is highly dynamic
4.2. Differences between mRNA and other vaccines
4.2.1. General studies
4.2.2. All-cause mortality differences between vaccines
4.2.3. Specific and nonspecific effects of vaccine waning
4.2.4. Reevaluation of the study of Rinchai et al [196] in relation to the effects of mRNA vaccination
4.3. Limitations of the study
- COVID-19 vaccines are supposed to behave in a way which does not involve nonspecific effects.
- That the single-year-of-age behavior of the different SARS-CoV-2 variants is roughly similar [25].
4.4. Individual versus population risk
5. Study summary
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- de Bree, L.; Koeken, V.; Joosten, L.; Aaby, P.; Benn, C.; van Crevel, R.; Netea, M. Nonspecific effects of vaccines: Current evidence and potential implications. Semin. Immunol. 2018, 39, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Walk, J.; de Bree, L.; Graumans, W.; Stoter, R.; van Gemert, G.; van de Vegte-Bolmer, M.; Teelen, K.; Hermsen, C.; Arts, R.; Behet, M.; Keramati, F.; et al. Outcomes of controlled human malaria infection after BCG vaccination. Nat. Commun. 2019, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Benn, C.; Flanagan, K.; Klein, S.; Kollmann, T.; Lynn, D.; Shann, F. The nonspecific and sex-differential effects of vaccines. Nat Rev Immunol 2020, 20, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Ponomarenko, A. Roles for pathogen interference in influenza vaccination, with implications to vaccine effectiveness (VE) and attribution of influenza deaths. Infect. Dis. Rep. 2022, 14, 710–758. [Google Scholar] [CrossRef] [PubMed]
- Arega, S.; Knobel, D.; Toka, F.; Conan, A. Nonspecific effects of veterinary vaccines: a systematic review. Vaccine. 2022, 40, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Benn, C.; Amenyogbe, N.; Björkman, A.; Domínguez-Andrés, J.; Fish, E.; Flanagan, K.; Klein, S.; Kollmann, T.; Kyvik, K.; Netea, M.; Rod, N.; Schaltz-Buchholzer, F.; Shann, F.; Selin, L.; Thysen, S.; Aaby, P. Implications of nonspecific effects for testing, approving, and regulating vaccines. Drug Saf. 2023, 46, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, N.; Montazeri, H. On BCG vaccine protection from COVID-19: A review. SN Compr Clin Med. 2021, 3, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Aspatwar, A.; Gong, W.; Wang, S.; Wu, X.; Parkkila, S. Aspatwar, A.; Gong, W.; Wang, S.; Wu, X.; Parkkila, S. Tuberculosis vaccine BCG: the magical effect of the old vaccine in the fight against the COVID-19 pandemic, Int Rev Immunol. [CrossRef]
- Wang, R.; Liu, M.; Liu, J. The Association between Influenza Vaccination and COVID-19 and Its Outcomes: A Systematic Review and Meta-Analysis of Observational Studies. Vaccines 2021, 9, 529. [Google Scholar] [CrossRef]
- Yang, M.; Rooks, B.; Le, T.; Santiago, I.; Diamond, J.; Dorsey, N.; Mainous, A. Influenza Vaccination and Hospitalizations Among COVID-19 Infected Adults. J Am Board Fam Med. 2021, 34 (Supp. l), S179–S182. [Google Scholar] [CrossRef]
- Montes, C.; Acosta-Rodríguez, E.; Merino, M.; Bermejo, D.; Gruppi, A. Polyclonal B cell activation in infections: infectious agents’ devilry or defense mechanism of the host? J Leukocyte Biol. 2007, 82, 1027–1032. [Google Scholar] [CrossRef]
- D. Juy, G. Sterkers, A. Gomez, D. Zelizewski, J.-P. Lévy. Polyclonal B-cell activation by influenza A/Texas virus-specific human T-cell clones. Annales de l'Institut Pasteur Immunologie. 1987, 138, 371–382. [CrossRef]
- Louis, J.; Lambert, P. Lipopolysaccharides: From immunostimulation to autoimmunity. In: Chedid, L.; Miescher, P.; Mueller-Eberhard, H. (eds) Immunostimulation.1980, Springer, Berlin, Heidelberg.
- Russell, S.; Peng, K. Measles virus for cancer therapy. Curr Top Microbiol Immunol. 2009, 330, 213–241. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Zuno, G.; Matuz-Flores, M.; González-Estevez, G.; Nicoletti, F.; Turrubiates-Hernández, F.; Mangano, K.; Muñoz-Valle, J. A review: Antibody-dependent enhancement in COVID-19: The not so friendly side of antibodies. Int J Immunopathol Pharmacol. 2021, 35, 20587384211050199. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Yu, X.; et al. Antibody-dependent enhancement (ADE) of SARS-CoV-2 pseudoviral infection requires FcγRIIB and virus-antibody complex with bivalent interaction. Commun Biol. 2022, 5, 262. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Tang, B.; Bai, Y.; Shao, Y.; Xiao, Y.; Tang, S. The resurgence risk of COVID-19 in the presence of immunity waning and ADE effect: a mathematical modelling study. medRxiv, 2021, 2021.08.25.21262601. [CrossRef]
- Karthik, K.; Senthilkumar, T.; Udhayavel, S.; Raj, G. Role of antibody-dependent enhancement (ADE) in the virulence of SARS-CoV-2 and its mitigation strategies for the development of vaccines and immunotherapies to counter COVID-19. Hum Vaccin Immunother. 2020, 16, 3055–3060. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Ponomarenko, A. System complexity in influenza infection and vaccination: effects upon excess winter mortality. Infect. Dis. Rep. 2022, 14, 287–309. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Ponomarenko, A. Trends in excess winter mortality (EWM) from 1900/01 to 2019/20 – evidence for a complex system of multiple long-term trends. Int. J. Environ. Res. Public Health 2022, 19, 3407. [Google Scholar] [CrossRef]
- Domingo, E.; García-Crespo, C.; Lobo-Vega, R.; Perales, C. Mutation Rates, Mutation Frequencies, and Proofreading-Repair Activities in RNA Virus Genetics. Viruses. 2021, 13, 1882. [Google Scholar] [CrossRef]
- Cosar, B.; Karagulleoglu, Z.; Unal, S.; Ince, A.; Uncuoglu, D.; Tuncer, G.; Kilinc, B.; Ozkan, Y.; Ozkoc, H.; Demir, I.; Eker, A.; Karagoz, F.; et al. COVID–19 mutations and their viral variants. Cytokine Growth Factor Rev. 2022, 63, 10–22. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID–19 variant classifications and definitions. Available online: COVID–19 Variant Classifications and Definitions (cdc.gov) (accessed 2 August 2022).
- GOV.UK. Investigation of COVID–19 variants: technical briefings. Available online: Investigation of COVID–19 variants: technical briefings - GOV.UK (www.gov.uk) (accessed 26 August 2022).
- Jones, R.; Ponomarenko, A. COVID-19-Related Age Profiles for SARS-CoV-2 Variants in England and Wales and States of the USA (2020 to 2022): Impact on All-Cause Mortality. Infect. Dis. Rep. 2023, 15, 600–634. [Google Scholar] [CrossRef]
- GOV.UK. COVID–19 confirmed deaths in England (to 31 December 2020) – report. Available online: COVID–19 confirmed deaths in England (to 31 December 2020): report - GOV.UK (www.gov.uk) (accessed 29 August 2022).
- Mueller B. When was the first U.S. Covid death? C.D.C. investigates 4 early cases. The New York Times, 9 September 2021. Available online: When Was the First U.S. Covid Death? CDC Investigates 4 Early Cases - The New York Times (nytimes.com) (accessed 2 September 20220.
- Freeman, A.; Watson, A.; O'Regan, P.; Wysocki, O.; Burke, H.; Freitas, A.; Livingstone, R.; Dushianthan, A.; Celinski, M.; Batchelor, J.; Phan, H.; et al. Wave comparisons of clinical characteristics and outcomes of COVID–19 admissions - Exploring the impact of treatment and strain dynamics. J Clin Virol. 2022, 146, 105031. [Google Scholar] [CrossRef]
- Mlcochova, P.; Kemp, S.; Dhar, M.; Papa, G.; Meng, B.; Ferreira, I.; Datir, R.; Collier, D.; Albecka, A.; Singh, S.; Pandy, R.; et al. COVID–19 B. 1.617.2 Delta variant replication and immune evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef]
- Stepanova, M.; Lam, B.; Younossi, E.; Felix, S.; Ziavee, M.; Price, J.; Pham, H.; de Avila, L.; Terra, K.; Austin, P.; Jeffers, T.; et al. The impact of variants and vaccination on the mortality and resource utilization of hospitalized patients with COVID-19. BMC Infect Dis. 2022, 22, 702. [Google Scholar] [CrossRef] [PubMed]
- Wikipedia. COVID–19 vaccination in the United Kingdom. Available online: COVID–19 vaccination in the United Kingdom - Wikipedia (accessed 26 August 2022).
- GOV.UK. COVID vaccinations in United Kingdom. Available online: Vaccinations in England | Coronavirus in the UK (data.gov.uk) (accessed 26 August 2022).
- Majeed, A.; Pollock, K.; Hodes, S.; Papaluca, M. Implementation of COVID–19 vaccination in the United Kingdom. BMJ. 2022, 378, e070344378. [Google Scholar] [CrossRef] [PubMed]
- GOV.UK. JCVI advises on COVID–19 vaccine for people aged under 40. 7 May 2021. Available online: JCVI advises on COVID–19 vaccine for people aged under 40 - GOV.UK (www.gov.uk) (accessed 4 September 2022).
- GOV.UK. COVID-19 vaccine uptake in healthcare workers. Published 7 October 2021. Available online: COVID-19 vaccine uptake in healthcare workers - GOV.UK (www.gov.uk) (accessed on 5 January 2023].
- Berencsi, G.; Kapusinszky, B.; Rigó, Z.; Szomor, K. Interference among viruses circulating and administered in Hungary from 1931 to 2008. Acta Microbiol. Immunol. Hung. 2010, 57, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.; Locke, E.; O'Hare, A.; Bohnert, A.; Boyko, E.; Hynes, D.; Berry, K. COVID–19 Vaccination Effectiveness Against Infection or Death in a National U. S. Health Care System : A Target Trial Emulation Study. Ann Intern Med. 2022, 175, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Tang F, Hammel IS, Andrew MK, Ruiz JG. COVID–19 mRNA vaccine effectiveness against hospitalisation and death in veterans according to frailty status during the COVID–19 delta (B.1.617.2) variant surge in the USA: a retrospective cohort study. Lancet Healthy Longev 2022, Aug 1. [CrossRef]
- GOV.UK. COVID–19: the green book, Chapter 14a. Available online: COVID–19: the green book, chapter 14a - GOV.UK (www.gov.uk) (accessed 4 September 2022).
- Office for National Statistics. Deaths by vaccination status, England. Available online: Deaths by vaccination status, England - Office for National Statistics (accessed 28 August 2022).
- Fenton, N.; Neil, M.; Craig, C.; McLachlan, S. What the ONS mortality COVID-19 surveillance data can tell us about vaccine safety and efficacy. 2022. Available from: http://dx.doi.org/10.13140/RG.2.2.30898.07362 (accessed 15 January 2023).
- Office for National Statistics. Deaths registered weekly in England and Wales. Available online: Deaths registered weekly in England and Wales, provisional - Office for National Statistics (ons.gov.uk) (accessed 12 October 2023).
- Odilov, A.; Volkov, A.; Abdullaev, A.; Gasanova, T.; Lipina, T.; Babichenko, I. COVID-19: Multiorgan Dissemination of SARS-CoV-2 Is Driven by Pulmonary Factors. Viruses. 2021, 14, 39. [Google Scholar] [CrossRef]
- Jones, R.; Ponomarenko, A. Pathogens Have Different Age/Sex Profiles for Hospital Admission and Why COVID-19 Variants Behave as If They Were ‘Different’ Pathogens. Preprints 2023, 2023091886. [Google Scholar] [CrossRef]
- Pálinkás, A.; Sándor, J. Effectiveness of COVID–19 Vaccination in preventing all-cause mortality among adults during the third wave of the epidemic in Hungary: Nationwide retrospective cohort study. Vaccines. 2022, 10, 1009. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Tseng, H.; Ackerson, B.; Bruxvoort, K.; Sy, L.; Tubert, J.; Lee, G.; Ku, J.; Florea, A.; et al. Effectiveness of mRNA-1273 vaccination against SARS-CoV-2 omicron subvariants BA.1.; BA.2.; BA.2.12.1.; BA.4.; and BA.5. Nat Commun. 2023, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Goldinger, A.; Shakhbazov, K.; Henders, A.; McRae, A.; Montgomery, G.; Powell, J. Seasonal effects on gene expression. PLoS One. 2015, 10, e0126995. [Google Scholar] [CrossRef]
- Dopico, X.; Evangelou, M.; Ferreira, R.; Guo, H.; Pekalski, M.; Smyth, D.; Cooper, N.; Burren, O.; Fulford, A.; Hennig, B.; Prentice, A.; et al. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat Commun. 2015, 6, 7000. [Google Scholar] [CrossRef] [PubMed]
- Tunçer, F.; Şahiner, Ü.; Ocak, M.; Ünsal, H.; Soyer, Ö.; Şekerel, B.; Birben, E. Comparison of miRNA expression in patients with seasonal and perennial allergic rhinitis and non-atopic asthma. Turk J Pediatr. 2022, 64, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Ha, H.; Lee, D.; Kim, W.; Lee, Y.; Bae, W.; Kim, H. Genomic Analyses of Non-Coding RNAs Overlapping Transposable Elements and Its Implication to Human Diseases. Int J Mol Sci. 2022, 23, 8950. [Google Scholar] [CrossRef] [PubMed]
- Alles, J.; Fehlmann, T.; Fischer, U.; Backes, C.; Galata, V.; Minet, M.; Hart, M.; Abu-Halima, M.; Grässer, F.; Lenhof, H.; Keller, A.; Meese, E. An estimate of the total number of true human miRNAs. Nucleic Acids Res. 2019, 47, 3353–3364. [Google Scholar] [CrossRef]
- Kabekkodu, S.; Shukla, V.; Varghese, V.; D' Souza, J.; Chakrabarty, S.; Satyamoorthy, K. Clustered miRNAs and their role in biological functions and diseases. Biol Rev Camb Philos Soc. 2018, 93, 1955–1986. [Google Scholar] [CrossRef]
- Xu, P.; Wu, Q.; Yu, J.; Rao, Y.; Kou, Z.; Fang, G.; Shi, X.; Liu, W.; Han, H. A Systematic Way to Infer the Regulation Relations of miRNAs on Target Genes and Critical miRNAs in Cancers. Front Genet. 2020, 11, 278. [Google Scholar] [CrossRef]
- Leung, A.; Sharp, P. Function and localization of microRNAs in mammalian cells. Cold Spring Harb Symp Quant Biol. 2006, 71, 29–38. [Google Scholar] [CrossRef]
- O'Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne). 2018, 9, 402. [CrossRef]
- Shang, R.; Lee, S.; Senavirathne, G. et al. microRNAs in action: biogenesis, function and regulation. Nat Rev Genet. 2023. [CrossRef]
- Gao, K.; Cheng, M.; Zuo, X. Active RNA interference in mitochondria. Cell Res. 2021, 31, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Baltimore, D. MicroRNAs as regulatory elements in immune system logic. Nat Rev Immunol. 2016, 16, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Chen, Y.; Tang, K.; Chen, P.; Liu, H.; Chen, D. MicroRNA-223 inhibits neutrophil extracellular traps formation through regulating calcium influx and small extracellular vesicles transmission. Sci Rep. 2021, 11, 15676. [Google Scholar] [CrossRef]
- Wei, J.; Huang, K.; Yang, C.; Kang, C. Non-coding RNAs as regulators in epigenetics. Oncol Rep. 2017, 37, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Iacomino, G.; Siani, A. Role of microRNAs in obesity and obesity-related diseases. Genes Nutr. 2017, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Erkal, B.; Korkut, S. Identification of miRNAs and their potential effects on multiple sclerosis related pathways using ın silico analysis. Multiple Sclerosis and Related Disorders 2022, 59, 103642. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Abak, A.; Talebi, S.; Shoorei, H.; Taheri, W.; Dilmaghani, N. Role of miRNA and lncRNAs in organ fibrosis and aging. Biomedicine & Pharmacotherapy. 2021, 143, 112132. [Google Scholar] [CrossRef]
- Tan, Z.; Li, W.; Cheng, X.; Zhu, Q.; Zhang, X. Non-Coding RNAs in the Regulation of Hippocampal Neurogenesis and Potential Treatment Targets for Related Disorders. Biomolecules. 2022, 13, 18. [Google Scholar] [CrossRef]
- Hill, M.; Tran, N. miRNA interplay: mechanisms and consequences in cancer. Dis Model Mech. 2021, 14, dmm047662. [Google Scholar] [CrossRef]
- Leon-Icaza, S.; Zeng, M.; Rosas-Taraco, A. microRNAs in viral acute respiratory infections: immune regulation, biomarkers, therapy, and vaccines. ExRNA. 2019, 1, 1. [Google Scholar] [CrossRef]
- Hicks, J.; Liu, H.-C. Involvement of eukaryotic small RNA pathways in host defense and viral pathogenesis. Viruses 2013, 5, 2659–2678. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Espinoza, I.; Banos-Lara, M.; Guerrero-Plata, A. The Importance of miRNA Identification During Respiratory Viral Infections. J Cell Immunol. 2021, 3, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Plaisance-Bonstaff K, Renne R. Viral miRNAs. Methods Mol Biol. 2011, 721, 43–66. [CrossRef]
- Shmaryahu, A.; Carrasco, M.; Valenzuela, P. Prediction of bacterial microRNAs and possible targets in human cell transcriptome. J Microbiol. 2014, 52, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Heaton, S.; Parrish, N. Mammalian antiviral systems directed by small RNA. PLoS Pathog. 2021, 17, e1010091. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, Z.; Song, J.; Qian, W.; Gu, X.; Yang, C.; Shen, N.; Xue, F.; Tang, Y. SARS-CoV-2-Encoded MiRNAs Inhibit Host Type I Interferon Pathway and Mediate Allelic Differential Expression of Susceptible Gene. Front Immunol. 2021, 12, 767726. [Google Scholar] [CrossRef] [PubMed]
- Sedger, L. microRNA control of interferons and interferon induced anti-viral activity. Mol Immunol. 2013, 56, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Amahong, K.; Sun, X.; Lian, X.; J Liu, J.; Sun, H.; Lou, Y.; Zhu, F.; Qiu, Y. The miRNA: a small but powerful RNA for COVID-19, Brief Bioinformatics. 2021, 22, 1137–1149. [CrossRef]
- Bojkova, D.; Widera, M.; Ciesek, S.; Wass, M.; Michaelis, M.; Cinatl, J. Reduced interferon antagonism but similar drug sensitivity in Omicron variant compared to Delta variant of SARS-CoV-2 isolates. Cell Res. 2022, 32, 319–321. [Google Scholar] [CrossRef]
- Bojkova, D.; Rothenburger, T.; Ciesek, S.; Wass, M.; Michaelis, M.; Cinatl, J. SARS-CoV-2 Omicron variant virus isolates are highly sensitive to interferon treatment. Cell Discov. 2022, 8, 42. [Google Scholar] [CrossRef]
- Liu, F.; Liu, C.; Hu, X.; Shang, Y.; Wu, L. MicroRNA-21: A positive regulator for optimal production of type I and type III interferon by plasmacytoid dendritic cells. Front Immunol. 2017, 8, 947. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, F.; Dong, J.; Fu, H.; Luo, J.; Ji, J.; Gao, X, Guo, W. Inducible downregulation of miR-93 feedback promotes innate responses against RNA virus by amplifying interferon signaling. Am J Transl Res. 2022, 14, 7689–7704.
- Lopez L, Sang PC, Tian Y, Sang Y. Dysregulated Interferon Response Underlying Severe COVID-19. Viruses. 2020, 12, 1433. [CrossRef] [PubMed]
- Wan, W.; Thoon, K.; Loo, L.; Chan, K.; Oon, L.; Ramasamy, A.; Maiwald, M. Trends in respiratory virus infections during the COVID–19 pandemic in Singapore, 2020. JAMA Netw Open. 2021, 4, e2115973. [Google Scholar] [CrossRef] [PubMed]
- Partridge, E.; McCleery, E.; Cheema, R.; Nakra, N.; Lakshminrusimha, S.; Tancredi, D.; Blumberg, D. Evaluation of Seasonal respiratory virus activity before and after the statewide COVID–19 Shelter-in-Place order in Northern California. JAMA Netw Open. 2021, 4, e2035281. [Google Scholar] [CrossRef]
- Serigstad, S.; Markussen, D.L.; Ritz, C.; et al. The changing spectrum of microbial aetiology of respiratory tract infections in hospitalized patients before and during the COVID–19 pandemic. BMC Infect Dis. 2022, 22, 763. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, V.; Sullivan, S.; Edwards, K.; Xie, R.; Khorov, A.; Valkenburg, S.; Cowling, B.; Barr,I. Human seasonal influenza under COVID-19 and the potential consequences of influenza lineage elimination. Nat Commun 2022, 13, 1721. [CrossRef] [PubMed]
- Koutsakos, M.; Wheatley, A.; Laurie, K.; Kent, S.; Rockman, S. Influenza lineage extinction during the COVID–19 pandemic? Nat Rev Microbiol 2021, 19, 741–742. [Google Scholar] [CrossRef] [PubMed]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W. Co-infections in people with COVID–19: a systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-Garcia, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID–19: a retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- Krumbein, H.; Kümmel, L.; Fragkou, P.; Thölken, C.; Hünerbein, B.; Reiter, R.; Papathanasiou, K.; Renz, H.; Skevaki, C. Respiratory viral co-infections in patients with COVID–19 and associated outcomes: A systematic review and meta-analysis. Rev Med Virol. 2022, e2365. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Wang, Y.; Dai, T.; Qin, Z.; Zhou, F.; Zhang, L. Alterations in microbiota of patients with COVID–19: potential mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2022, 7, 143. [Google Scholar] [CrossRef]
- Hardin, L.; Xiao, N. miRNAs: The Key Regulator of COVID-19 Disease. Int J Cell Biol. 2022, 2022, 1645366. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, Y.; Yoshida, T.; Takagi, Y.; et al. Circulating extracellular vesicle microRNAs associated with adverse reactions, proinflammatory cytokine, and antibody production after COVID-19 vaccination. npj Vaccines 2022, 7, 16. [Google Scholar] [CrossRef]
- Martinez-Espinoza, I.; Banos-Lara, M.; Guerrero-Plata, A. The Importance of miRNA Identification During Respiratory Viral Infections. J Cell Immunol. 2021, 3, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Poursheikhani, A.; Bahmanpour, Z.; Vatanmakanian, M.; Taheri, M.; Mashouri, L.; Alahari, K. SARS-CoV infection crosstalk with human host cell noncoding-RNA machinery: An in-silico approach. Biomed Pharmacother. 2020, 130, 110548. [Google Scholar] [CrossRef]
- Sun, G.; Cui, Q.; Garcia, G.; Jr.; Lizhar, E.M.; Arumugaswami, V.; Shi, Y.; Riggs, A.D. Viral and Host Small RNA Response to SARS-CoV-2 Infection. Microbiol. Res. 2022, 13, 788–808. [CrossRef]
- Battaglia, R.; Alonzo, R.; Pennisi, C.; Caponnetto, A.; Ferrara, C.; Stella, M.; Barbagallo, C.; Barbagallo, D.; Ragusa, M.; Purrello, M.; Di Pietro, C. MicroRNA-mediated regulation of the virus cycle and pathogenesis in the COVID–19 disease. Int J Mol Sci. 2021, 22, 13192. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Feng, R.; Zhang, M.; Xie, X.; Liao, Y.; Zhou, Y.; Guo, X.; Su, B.; Dorsett, Y.; Chen, L. Subgenomic RNA profiling suggests novel mechanism in coronavirus gene regulation and host adaption. Life Sci Alliance. 2022, 5, e202101347. [Google Scholar] [CrossRef] [PubMed]
- Pawlica, P.; Yario, T.; White, S.; Wang, J.; Moss, W.; Hui, P.; Vinetz, J.; Steitz, J. COVID–19 expresses a microRNA-like small RNA able to selectively repress host genes. Proc Natl Acad Sci U S A. 2021, 118, e2116668118. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, A.; Feng, J.; Li, G.; Guo, D.; Sajid, M.; Wu, K.; Zhang, Q.; Ponty, Y.; Will, S.; Liu, F.; et al. The COVID–19 subgenome landscape and its novel regulatory features. Mol Cell. 2021, 81, 2135–2147. [Google Scholar] [CrossRef]
- Paul, S.; Bravo Vázquez, L.; Reyes-Pérez, P.; Estrada-Meza, C.; Aponte Alburquerque, R.; Pathak, S.; Banerjee, A.; Bandyopadhyay, A.; Chakraborty, S.; Srivastava, A. The role of microRNAs in solving COVID–19 puzzle from infection to therapeutics: A mini-review. Virus Res. 2022, 308, 198631. [Google Scholar] [CrossRef]
- Abu-Izneid, T.; AlHajri, N.; Ibrahim, A.; Javed, M.; Salem, K.; Pottoo, F.; Kamal, M. Micro-RNAs in the regulation of immune response against SARS CoV-2 and other viral infections. J Adv Res. 2021, 30, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Moatar, A.; Chis, A.; Marian, C.; Sirbu, I. Gene Network Analysis of the Transcriptome Impact of COVID–19 Interacting MicroRNAs in COVID–19 Disease. Int J Mol Sci. 2022, 23, 9239. [Google Scholar] [CrossRef]
- Grehl, C.; Schultheiß, C.; Hoffmann, K.; Binder, M.; Altmann, T.; Grosse, I.; Kuhlmann, M. Detection of COVID–19 derived small RNAs and changes in circulating small RNAs associated with COVID–19. Viruses. 2021, 13, 1593. [Google Scholar] [CrossRef] [PubMed]
- Marchi, R.; Sugita, B.; Centa, A.; Fonseca, A.; Bortoletto, S.; Fiorentin, K.; Ferreira, S.; Cavalli, L. The role of microRNAs in modulating COVID–19 infection in human cells: a systematic review. Infect Genet Evol. 2021, 91, 104832. [Google Scholar] [CrossRef]
- Meng, F.; Siu, G.; Mok, B.; Sun, J.; Fung, K.; Lam, J.; Wong, N.; Gedefaw, L.; Luo. S.; Le,e T.; Yip, S.; Huang, C-L. Viral microRNAs encoded by nucleocapsid gene of COVID–19 are detected during infection, and targeting metabolic pathways in host cells. CL.Cells. 2021, 10, 1762. [CrossRef]
- Singh, M.; Chazal, M.; Quarato, P.; Bourdon, L.; Malabat, C.; Vallet, T.; Vignuzzi, M.; van der Werf, S.; Behillil, S.; Donati, F.; et al. A virus-derived microRNA targets immune response genes during COVID–19 infection. EMBO Rep. 2022, 23, e54341. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Wang, J.; Wang, Z.; Sun, Y.; Wu, J.; Zhang, Y.; Liu, X.; Zhou, Z.; Zhou, L.; Yi, Y.; Xia, X.; Wang, L.; Chen, X. A virus-derived microRNA-like small RNA serves as a serum biomarker to prioritize the COVID–19 patients at high risk of developing severe disease. Cell Discov. 2021, 7, 48. [Google Scholar] [CrossRef]
- Liu, X.; Wen, Y-Z.; Huang, Z-L.; Shen, Z.; Wang, J-H.; Luo, Y-H.; Chen, W-X.; Lun, Z-R.; Li, H-B.; Qu, L-H.; Shan, H.; Zheng, L-L. COVID–19 causes a significant stress response mediated by small RNAs in the blood of COVID–19 patients. Molec Ther - Nucleic Acids. 2022, 27, 751–762. [CrossRef]
- Farr, R.; Rootes, C.; Rowntree, L.; Nguyen, T.; Hensen, L.; Kedzierski, L.; Cheng, A.; Kedzierska, K.; Au, G.; Marsh, G.; Vasan, S.; et al. Altered microRNA expression in COVID–19 patients enables identification of COVID–19 infection. PLOS Pathog. 2021, 17, e1009759. [Google Scholar] [CrossRef]
- Neeb, Z.; Ritter, A.; Chauhan, L.; et al. A potential role for SARS-CoV-2 small viral RNAs in targeting host microRNAs and modulating gene expression. Sci Rep. 2022, 12, 21694. [Google Scholar] [CrossRef]
- Wilson, J.; Kealy, D.; James, S.; Plowman, T.; Newling, K.; Jagger, C.; Filbey, K.; Mann, E.; Konkel, J.; et al. Integrated miRNA/cytokine/chemokine profiling reveals severity-associated step changes and principal correlates of fatality in COVID-19. iScience. 2022, 25, 103672. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Silva, M.; Trujillo, X.; Huerta, M.; Benites-Godínez, V.; Guzmán-Esquivel, J.; Bricio-Barrios, J.; Mendoza-Cano, O.; Lugo-Radillo, A.; Murillo-Zamora, E. Reemerging Influenza Virus Infections during the Dominance of the Omicron SARS-CoV-2 Variant in Mexico. Pathogens. 2022, 11, 1181. [Google Scholar] [CrossRef]
- Rivas, M.; Ebinger, J.; Wu, M.; Sun, N.; Braun, J.; Sobhani, K.; Van Eyk, J.; Cheng, S.; Arditi, M. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J Clin Invest. 2021, 131, e145157. [Google Scholar] [CrossRef]
- Singh, A.; Wang, R.; Lombardo, K.; Praharaj, M.; Bullen, C.; Um, P.; Davis, S.; Komm, O.; Illei, P.; Ordonez, A.; Bahr, M.; et al. Dynamic single-cell RNA sequencing reveals BCG vaccination curtails SARS-CoV-2 induced disease severity and lung inflammation. bioRxiv [Preprint]. 2022 Mar 15:2022.03.15.484018. [CrossRef]
- Monereo-Sánchez, J.; Luykx, J.; Pinzón-Espinosa, J.; Richard, G.; Motazedi, E.; Westlye, L.; Andreassen, O.; van der Meer, D. Diphtheria And Tetanus Vaccination History Is Associated With Lower Odds of COVID-19 Hospitalization. Front Immunol. 2021, 12, 749264. [Google Scholar] [CrossRef] [PubMed]
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: from cells to clinic. Trends in Genetics. 2022, 38, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cheng, Z.; Wang, Y.; Han, T. The Risks of miRNA Therapeutics: In a Drug Target Perspective. Drug Des Devel Ther. 2021, 15, 721–733. [Google Scholar] [CrossRef]
- Nakaya, H.; Hagan,T .; Duraisingham, S.; Lee, E.; Kwissa, M.; Rouphael, N.; Frasca, D.; Gersten, M.; Mehta, A.; Gaujoux, R.; et al. Systems Analysis of Immunity to Influenza Vaccination across Multiple Years and in Diverse Populations Reveals Shared Molecular Signatures. Immunity 2015, 43, 1186–1198. [CrossRef]
- Mosakhani, N.; Sarhadi, V.; Panula, P.; Partinen, M.; Knuutila, S. Narcolepsy patients' blood-based miRNA expression profiling: miRNA expression differences with Pandemrix vaccination. Acta Neurol Scand. 2017, 136, 462–469. [Google Scholar] [CrossRef]
- Kijima, T.; Hazama, S.; Tsunedomi, R.; Tanaka, H.; Takenouchi, H.; Kanekiyo, S.; Inoue, Y.; Nakashima, M.; Iida, M.; Sakamoto, K.; et al. MicroRNA-6826 and -6875 in plasma are valuable non-invasive biomarkers that predict the efficacy of vaccine treatment against metastatic colorectal cancer. Oncology Reports. 2017, 37, 23–30. [Google Scholar] [CrossRef]
- Haralambieva, I.; Kennedy, R.; Simon, W.; Goergen, K.; Grill, D.; Ovsyannikova, I.; et al. Differential miRNA expression in B cells is associated with inter-individual differences in humoral immune response to measles vaccination. PLoS ONE. 2018, 13, e0191812. [Google Scholar] [CrossRef]
- Drury, R.; Pollard, A.; O’Connor, D. The effect of H1N1 vaccination on serum miRNA expression in children: A tale of caution for microRNA microarray studies. PLOS ONE. 2019, 14, e0221143. [Google Scholar] [CrossRef]
- Oshiumi, H. Circulating Extracellular Vesicles Carry Immune Regulatory miRNAs and Regulate Vaccine Efficacy and Local Inflammatory Response After Vaccination. Front Immunol. 2021, 12, 685344. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.; Richardson, S.; Benyeogor, I.; Omosun, Y.; Dye, K.; Medhavi, F.; Lundy, S.; Adebayo, O.; Igietseme, J.; Eko, F. Differential miRNA Profiles Correlate With Disparate Immunity Outcomes Associated With Vaccine Immunization and Chlamydial Infection. Front Immunol. 2021, 12, 625318. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, Y.; Yoshida, T.; Takagi, Y.; Tsukamoto, H.; Takashima, K.; Kouwaki, T.; Makino, K.; Fukushima, S.; Nakamura, K.; Oshiumi, H. Circulating extracellular vesicle microRNAs associated with adverse reactions, proinflammatory cytokine, and antibody production after COVID-19 vaccination. npj Vaccines 2022, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Ishikawa, K.; Fugiwara, A.; Shu, K.; Fukushima, Y.; Okamoto, M.; Tsukamoto, H.; Kouwaki, T.; Oshiumi, H. miR-451a levels rather than human papillomavirus vaccine administration is associated with the severity of murine experimental autoimmune encephalomyelitis. Sci Rep 2021, 11, 9369. [Google Scholar] [CrossRef] [PubMed]
- Di Palo, A.; Siniscalchi, C.; Salerno, M.; Russo, A.; Gravholt, C.; Potenza, N. What microRNAs could tell us about the human X chromosome. Cell. Mol. Life Sci. 2020, 77, 4069–4080. [Google Scholar] [CrossRef] [PubMed]
- Dessie, Z.; Zewotir, T. Mortality-related risk factors of COVID–19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021, 21, 855. [Google Scholar] [CrossRef]
- Martínez-Gómez, L.; Herrera-López, B.; Martinez-Armenta, C.; Ortega-Peña, S.; Camacho-Rea, M.; Suarez-Ahedo, C.; Vázquez-Cárdenas, P.; Vargas-Alarcón, G.; Rojas-Velasco, G.; et al. ACE and ACE2 Gene Variants Are Associated With Severe Outcomes of COVID-19 in Men. Front Immunol. 2022, 13, 812940. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Liu, Y.; Zhang, Z.; Zhai, Y.; Dai, Y.; Wu,Z.; Nie, X.; Du, L. Polymorphisms and mutations of ACE2 and TMPRSS2 genes are associated with COVID–19: a systematic review. Eur J Med Res. 2022, 27, 26. [CrossRef]
- Benetti, E.; Tita, R.; Spiga, O.; Birolo, G.; Bruselles, A.; Doddato, G.; Giliberti, A.; Marconi, C.; Musacchia, F.; et al. ACE2 gene variants may underlie interindividual variability and susceptibility to COVID-19 in the Italian population. Eur J Hum Genet. 2020, 28, 1602–1614. [Google Scholar] [CrossRef]
- Shikov, A.; Barbitoff, Y.; Glotov, A.; Danilova, M.; Tonyan, Z.; Nasykhova, Y.; Mikhailova, A.; Bespalova, O.; Kalinin, R.; et al. Analysis of the Spectrum of ACE2 Variation Suggests a Possible Influence of Rare and Common Variants on Susceptibility to COVID-19 and Severity of Outcome. Front Genet. 2020, 11, 551220. [Google Scholar] [CrossRef] [PubMed]
- Kousathanas, A.; Pairo-Castineira, E.; Rawlik, K.; Stuckey, A.; Odhams, C.; Walker, S.; Russell, C.; Malinauskas, T.; Wu, Y.; Millar,J.; Shen, X.; et al. Whole-genome sequencing reveals host factors underlying critical COVID–19. Nature 2022, 607, 97–103. [CrossRef]
- Nakanishi, T.; Pigazzini, S.; Degenhardt,F.; Cordioli,M.; Butler-Laporte,G.; Maya-Miles, D.; Bujanda, L.; Bouysran,Y.; Niemi, M.; Palom,A.; Ellinghaus, D.; Khan, A.; Martínez-Bueno, M.; et al. Age-dependent impact of the major common genetic risk factor for COVID–19 on severity and mortality. J. Clin. Invest. 2021, 131, e152386. [CrossRef]
- Zeberg, H.; Pääbo, S. The major genetic risk factor for severe COVID–19 is inherited from Neanderthals. Nature 2020, 587, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Zeberg, H. The major genetic risk factor for severe COVID–19 is associated with protection against HIV. PNAS. 2022, 119, e2116435119. [Google Scholar] [CrossRef] [PubMed]
- The Severe COVID–19 GWAS Group. Genomewide association study of severe COVID–19 with respiratory failure. N Engl J Med. 2020, 383, 1522–1534. [CrossRef]
- Pereira, A.; Bes, T.; Velho, M.; Marques, E.; Jannes, C.; Valino, K.; Dinardo, C.; Costa, S.; Duarte, A.; Santos, A.; Mitne-Neto, M.; Medina-Pestana, J.; Krieger, J. Genetic risk factors and COVID–19 severity in Brazil: results from BRACOVID study. Human Molecular Genetics, 2022, 31, 3021–3031. [Google Scholar] [CrossRef]
- Downes, D.; Cross, A.; Hua, P.; et al. Identification of LZTFL1 as a candidate effector gene at a COVID–19 risk locus. Nat Genet. 2021, 53, 1606–1615. [Google Scholar] [CrossRef]
- Pathak, G.; Singh, K.; Miller-Fleming, T.; Wendt, F.; Ehsan, N.; Hou, K.; Johnson, R.; Lu, Z.; Gopalan, S.; Yengo, L.; et al. Integrative genomic analyses identify susceptibility genes underlying COVID–19 hospitalization. Nat Commun. 2021, 12, 4569. [Google Scholar] [CrossRef]
- Meola, N.; Gennarino, V.; Banfi, S. microRNAs and genetic diseases. Pathogenetics 2009, 2, 7. [Google Scholar] [CrossRef]
- Klein, S.; Flanagan, K. Sex differences in immune responses. Nat Rev Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.; Addo, M.; Dahlke, C. Sex Differences in Immunity: Implications for the Development of Novel Vaccines Against Emerging Pathogens. Front Immunol. 2021, 11, 601170. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Ellingson, M.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.; et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020, 588, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Dai, C.; Cai, P.; Wang, J.; Xu, L.; Li, J.; Hu, G.; Wang, Z.; Zheng, F.; Wang, L. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: A possible reason underlying different outcome between sex. J Med Virol. 2020, 92, 2050–2054. [Google Scholar] [CrossRef] [PubMed]
- Fazekas-Pongor, V.; Szarvas, Z.; Nagy, N.; Péterfi, A.; Ungvári, Z.; Horváth, V.; Mészáros, S; Tabák, A. Different patterns of excess all-cause mortality by age and sex in Hungary during the 2nd and 3rd waves of the COVID-19 pandemic. GeroScience 2022, 44, 2361–2369. [CrossRef] [PubMed]
- Guo, L.; Zhang, Q.; Ma, X.; Wang, J.; Liang, T. miRNA and mRNA expression analysis reveals potential sex-biased miRNA expression. Sci Rep. 2017, 7, 39812. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Yang, W.; Shi, J.; Zhou, Y.; Yang, I.; Cui, Q.; Zhou, Y. Identification and Analysis of Human Sex-biased MicroRNAs. Genomics, Proteomics & Bioinformatics. 2018, 16, 200–211. [Google Scholar] [CrossRef]
- Hamczyk, M.; Nevado, R.; Barettino, A.; Fuster, V.; Andrés, V. Biological Versus Chronological Aging: JACC Focus Seminar. J Am Coll Cardiol. 2020, 75, 919–930. [Google Scholar] [CrossRef]
- Yamamoto, R.; Chung, R.; Vazquez, J.; Sheng, H.; Steinberg, P.; Ioannidid, N.; Sudmant, P. Tissue-specific impacts of aging and genetics on gene expression patterns in humans. Nat Commun. 2022, 13, 5803. [Google Scholar] [CrossRef]
- Viñuela, A.; Brown, A.; Buil, A.; Tsai, P.; Davies, M.; Bell, J.; Dermitzakis, E.; Spector, T.; Small, K. Age-dependent changes in mean and variance of gene expression across tissues in a twin cohort. Hum Mol Genet. 2018, 27, 732–741. [Google Scholar] [CrossRef]
- Wanelik, K.; Begon, M.; Bradley, J.; Friberg, I.; Taylor, C.; Jackson, J.; Paterson, S. Early-life immune expression profiles predict later-life health and fitness in a wild rodent. Molec Ecol. 2023, 32, 3471–3482. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Bayry, J. An age-related decline of CD62L and vaccine response: a role of microRNA 92a? Hum Vaccin Immunother. 2014, 10, 1404–1405. [Google Scholar] [CrossRef] [PubMed]
- Bera, G. The incidence rate in Italy of COVID–19 lethality in 0-59 older adults and adverse effects of mRNA and vectorial vaccines in the range 5-16, leading to the urgent stop of vaccination of 5-29 young people, and mRNA and vectorial vaccines withdrawal. Scuola Medica di Milano (Milan School of Medicine Scientific Reports) 2-2022. [CrossRef]
- Dinetz, E. Cases series of three neurological side effects in younger-aged individuals after Pfizer's mRNA vaccine. Cureus. 2022, 14, e23779. [Google Scholar] [CrossRef] [PubMed]
- Benn, C.; Schaltz-Buchholzer, F.; Nielsen, S.; Netea, M.; Aaby, P. Randomised clinical trials of COVID–19 vaccines: Do adenovirus-vector vaccines have beneficial nonspecific effects? Available at: SSRN: https://ssrn.com/abstract=4072489 or http://dx.doi.org/10.2139/ssrn.4072489.
- Murakami, N.; Hayden, R.; Hills, T.; Al-Samkari, H.; Casey, J.; Del Sorbo, L.; Lawler, P.; Sise, M.; Leaf, D. Therapeutic advances in COVID-19. Nat Rev Nephrol. 2023, 19, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; van der Vaart, A. MicroRNAs in addiction: adaptation's middlemen? Mol Psychiatry. 2011, 16, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Rukov, J.; Wilentzik, R.; Jaffe, I.; Vinther, J.; Shomron, N. Pharmaco-miR: linking microRNAs and drug effects. Brief Bioinform. 2014, 15, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Cerda, A.; Bortolin, R.; Manriquez, V.; Salazar, L.; Zambrano, T.; Fajardo, C.; Hirata, M.; Hirata, R. Effect of statins on lipid metabolism-related microRNA expression in HepG2 cells. Pharmacol Rep. 2021, 73, 868–880. [Google Scholar] [CrossRef]
- Kissling, E.; Pozo, F.; Martínez-Baz, I.; Buda, S.; Vilcu, A.; Domegan, L.; Mazagatos, C.; Dijkstra, F.; Latorre-Margalef, N.; et al. Influenza vaccine effectiveness against influenza A subtypes in Europe: Results from the 2021-2022 I-MOVE primary care multicentre study. Influenza Other Respir Viruses. 2023, 17, e13069. [Google Scholar] [CrossRef]
- Shrotri, M.; Navaratnam, A.; Nguyen, V.; Byrne. T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.; Patel, P.; et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [CrossRef]
- Neil, M.; Fenton, N.; Smalley, J.; Craig, C.; Guetzkow, J.; McLachlan, S.; Engler, J.; Rose, J. Latest statistics on England mortality data suggest systematic mis-categorisation of vaccine status and uncertain effectiveness of COVID–19 vaccination. Available from: https://www.researchgate.net/publication/356756711_Latest_statistics_on_England_mortality_data_suggest_systematic_mis-categorisation_of_vaccine_status_and_uncertain_effectiveness_of_COVID–19_vaccination (accessed 9 October 2022). 9 October.
- Wu, K. You’re not fully vaccinated the day of your last dose. The Atlantic, 17 March, 2021. Available online: How Long After Getting a COVID-19 Vaccine Is It Effective? - The Atlantic (accessed on 22 July 2023).
- Day, M. Covid-19: Stronger warnings are needed to curb socialising after vaccination, say doctors and behavioural scientists. BMJ. 2021, 372, n783. [Google Scholar] [CrossRef]
- Bucasas, K.; Franco, L.; Shaw, C.; Bray, M.; Wells, J.; Nino, D.; Arden, N.; Quarles, J.; Couch, R.; Belmont, J. Early Patterns of Gene Expression Correlate With the Humoral Immune Response to Influenza Vaccination in Humans. J Infect Dis. 2011, 203, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Wisnewski, A.; Campillo Luna, J.; Redlich, C. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLOS ONE. 2021, 16, e0249499. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Pouwels, K.; Stoesser, N.; Matthews, P.; Diamond, I.; Studley, R.; Rourke, E.; Cook, D.; Bell, J.; Newton, J.; et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat Med. 2022, 28, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.; Gunnes, N.; Gran, J.; Karlstad, Ø.; Selmer, R.; Dahl, J.; Bøås, H.; White, R.; Hofman, A.; Paulsen, T.; Watle, S.; et al. Short-term safety of COVID-19 mRNA vaccines with respect to all-cause mortality in the older population in Norway. Vaccine. 2023, 41, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Redert, A. Short-term Vaccine Fatality Ratio of booster and 4th dose in The Netherlands. October 2022. Preprint: Research Gate. 20 October. [CrossRef]
- Frankhouser, D.; O'Meally, D.; Branciamore, S.; Uechi, L.; Zhang, L.; Chen, Y.; Li, M.; Qin, H.; Wu, X.; Carlesso, N.; et al. Dynamic patterns of microRNA expression during acute myeloid leukemia state-transition. Sci. Adv. 2022, 8, eabj1664. [Google Scholar] [CrossRef]
- Reina-Ortiz, C.; Mozas, M.; Ovelleiro, D.; Gao, F.; Villalba, M.; Anel, A. Dynamic Changes in miRNA Expression during the Generation of Expanded and Activated NK Cells. Int J Mol Sci. 2023, 24, 13556. [Google Scholar] [CrossRef]
- Cervena, K.; Novosadova, V.; Pardini, B.; Naccarati, A.; Opattova, A.; Horak, J.; Vodenkova, S.; Buchler, T.; Skrobanek, P.; Levy, M.; Vodicka, P.; Vymetalkova, V. Analysis of MicroRNA Expression Changes During the Course of Therapy In Rectal Cancer Patients. Front Oncol. 2021, 11, 702258. [Google Scholar] [CrossRef] [PubMed]
- Vokó Z, Kiss Z, Surján G, Surján O, Barcza Z, Wittmann I, Molnár GA, Nagy D, Müller V, Bogos K, Nagy P, Kenessey I, Wéber A, Polivka L, Pálosi M, Szlávik J, Rokszin G, Müller C, Szekanecz Z, Kásler M. Effectiveness and Waning of Protection With Different SARS-CoV-2 Primary and Booster Vaccines During the Delta Pandemic Wave in 2021 in Hungary (HUN-VE 3 Study). Front Immunol. 2022, 13, 919408. [CrossRef]
- Meyer, P. The impact of COVID–19 vaccines on all-cause mortality in EU in 2021. A machine learning perspective. Preprint. Available online: MLperspectiveonmortality2.pdf (cadoc.fr) (accessed 30 September 2022).
- Pantazatos, S.; Seligmann, H. COVID vaccination and age-stratified all-cause mortality risk. Preprint October 2021. https://doi.org/10.13140/RG.2.2.28257.43366 Available online: https://www.researchgate.net/publication/355581860_COVID_vaccination_and_age-stratified_all-cause_mortality_risk (accessed 7 October 2022).
- Pantazatos, P.; Seligmann, H. COVID vaccination and age-stratified all-cause mortality risk (preprint). Available online: (1) (PDF) COVID vaccination and age-stratified all-cause mortality risk (researchgate.net).
- Redert, A. Covid-19 vaccinations and all-cause mortality -a long-term differential analysis among municipalities. July 2022. Preprint: Research Gate. 20 July. [CrossRef]
- Beattie, K. Worldwide Bayesian Causal Impact Analysis of Vaccine Administration on Deaths and Cases Associated with COVID-19: A BigData Analysis of 145 Countries. Research Gate. 2021, Preprint. [Google Scholar] [CrossRef]
- Sorli, S.; Makovec, T.; Krevel, Z.; Gorjup, R. Forgotten “Primum Non Nocere” and Increased Mortality after Covid-19 Vaccination. Research Gate. 2023, Preprint. [Google Scholar] [CrossRef]
- Seneff, S.; Nigh, G. Worse than the disease? Reviewing some possible unintended consequences of the mRNA vaccines against COVID–19. Int J Vaccine Theory Pract Res. 2021, 2, 38–79. [Google Scholar] [CrossRef]
- Seneff, S.; Nigh, G.; Kyriakopoulos, A.; McCullough, P. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem Toxicol. 2022, 164, 113008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Richards, A.; Barrasa, M.; Hughes, S.; Young, R.; Jaenisch, R. Reverse-transcribed COVID–19 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc Natl Acad Sci U S A. 2021, 118, e2105968118. [Google Scholar] [CrossRef] [PubMed]
- Aldén, M.; Olofsson Falla, F.; Yang, D.; Barghouth, M.; Luan, C.; Rasmussen, M.; De Marinis, Y. Intracellular reverse transcription of Pfizer BioNTech COVID–19 mRNA vaccine BNT162b2 in vitro in human liver cell line. Curr Issues Mol Biol. 2022, 44, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Merchant H. Comment on Aldén et al. Intracellular Reverse Transcription of Pfizer BioNTech COVID–19 mRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Curr. Issues Mol. Biol. 2022, 44, 1115–1126. [CrossRef]
- Theoharides, T.; Conti, P. Be aware of COVID–19 spike protein: There is more than meets the eye. J Biol Regul Homeost Agents. 2021, 35, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T. Could COVID–19 spike protein be responsible for long-COVID syndrome? Mol Neurobiol. 2022, 59, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Mei, Y.-F. SARS–CoV–2 spike impairs DNA damage repair and inhibits V(D)J recombination in vitro. Viruses. 2021, 13, 2056. [Google Scholar] [CrossRef]
- Tetz, G.; Tetz, V. Prion-like Domains in Spike Protein of SARS-CoV-2 Differ across Its Variants and Enable Changes in Affinity to ACE2. Microorganisms. 2022, 10, 280. [Google Scholar] [CrossRef]
- Stati, G.; Amerio, P.; Nubile, M.; Sancilio, S.; Rossi, F.; Di Pietro, R. Concern about the Effectiveness of mRNA Vaccination Technology and Its Long-Term Safety: Potential Interference on miRNA Machinery. Int. J. Mol. Sci. 2023, 24, 1404. [Google Scholar] [CrossRef]
- Chavali, S.; Bruhn, S.; Tiemann, K.; Saetrom, P.; Barrenäs, F.; Saito, T.; Kanduri, K.; Wang, H.; Benson, M. MicroRNAs act complementarily to regulate disease-related mRNA modules in human diseases. RNA. 2013, 19, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, Doolman R, Asraf K, Mendelson E, Ziv A, Rubin C, Freedman L, Kreiss Y, Regev-Yochay G. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N Engl J Med. 2021, 385, e84. [CrossRef]
- Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Haas EJ, Milo R, Alroy-Preis S, Ash N, Huppert A. Waning Immunity after the BNT162b2 Vaccine in Israel. N Engl J Med. 2021, 385, e85. [CrossRef]
- Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman LS, Ash N, Alroy-Preis S, Huppert A, Milo R. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N Engl J Med. 2022, 386, 2201–2212. [CrossRef]
- Kerr, S.; Bedston, S.; Bradley, D.; Joy, M.; Lowthian, E.; Mulholland, R.; Akbari, A.; Hobbs, R.; Katikireddi, S.; de Lusignan, S.; Rudan, I.; et al. Waning of first- and second-dose ChAdOx1 and BNT162b2 COVID-19 vaccinations: a pooled target trial study of 12.9 million individuals in England, Northern Ireland, Scotland and Wales. International Journal of Epidemiology, 2022, dyac199. [CrossRef]
- Ferdinands, J.; Rao, S.; Dixon, B.; Mitchell, P.; DeSilva, M.; Irving, S.; Lewis, N.; Natarajan, K.; Stenehjem, E.; Grannis, S.; Han, J.; et al. Waning of vaccine effectiveness against moderate and severe COVID–19 among adults in the US from the VISION network: test negative, case-control study. BMJ. 2022, 379, e072141. [Google Scholar] [CrossRef] [PubMed]
- Rinchai, D.; Deola, S.; Zoppoli, G.; Kabeer, BSA.; Taleb, S.; Pavlovski, I.; Maacha, S.; Gentilcore, G.; Toufiq, M.; et al. High-temporal resolution profiling reveals distinct immune trajectories following the first and second doses of COVID-19 mRNA vaccines. Sci Adv. 2022, 8, eabp9961. [CrossRef]
- Wang, R.; Chen, S.; Li, C.; Ng, K.; Kong, C.; Cheng, J.; Cheng, S.; Li, R.; Lo, C.; Man, K.; Sun, D. Fusion with stem cell makes the hepatocellular carcinoma cells similar to liver tumor-initiating cells. BMC Cancer. 2016, 16, 56. [Google Scholar] [CrossRef]
- Zaporozhan, V.; Kholodkova, E.; Pykhtyeyev, D.; Ponomarenko, A. Experimental study of some methodological approaches used in regenerative medicine (Экспериментальнoе изучение некoтoрых метoдoлoгических пoдхoдoв, испoльзуемых в реверсивнoй медицине)- in Russian. Transplantology (Ukraine) 2005, 8, 10–20. [Google Scholar]
- Chia, C.; Winston,R.; Handyside, A. EGF, TGF-a and EGFR expression in human preimplantation embryos. Development 1995, 121, 299–307. [CrossRef]
- Kohchi, C.; Noguchi, K.; Tanabe, Y.; Mizuno, D.; Soma, G. Constitutive expression of TNF-alpha and -beta genes in mouse embryo: roles of cytokines as regulator and effector on development. Int J Biochem. 1994, 26, 111–119. [Google Scholar]
- Rappolee, D.; Brenner, C.; Schultz, R.; Mark, D.; Werb, Z. Developmental expression of PDGF, TGF-alpha, and TGF-beta genes in preimplantation mouse embryos. Science. 1988, 241, 1823–1825. [Google Scholar] [CrossRef] [PubMed]
- Wiley, L.; Wu, J-X.; Harari, I.; Adamson, E. Epidermal growth factor receptor mRNA and protein increase after the four-cell preimplantation stage in murine development. Dev. Biol. 1992, 149, 247–260. [CrossRef] [PubMed]
- Zaporozhan, V.; Dubinina, V.; Ponomarenko, A.; Khyzhnyak, O. T-regs guards progenitor cells in blood. Allostem Meeting and Training Course. La Palma, October, 2007, Abstracts, p.37. (PDF) T-regs Guard Progenitor Cells in Blood (researchgate.net).
- Castillo-Aleman, Y.; Villegas-Valverde, C.; Ventura-Carmenate, Y.; Suarez-Formigo, G.; Bencomo-Hernandez, A. "Original Antigenic Sin" in SARS-CoV-2 Vaccination Followed by Infection. Cureus. 2022, 14, e32548. [Google Scholar] [CrossRef] [PubMed]
- Mak, J.; Eriksdotter, M.; Annetorp, M.; Kuja-Halkola, R.; Kananen, L.; Boström, A.; Kivipelto, M.; Metzner, C.; Jerlardtz, V.; et al. Two Years with COVID-19: The Electronic Frailty Index Identifies High-Risk Patients in the Stockholm GeroCovid Study. Gerontology. 2022, Nov 30, 1–10. [Google Scholar] [CrossRef]
- Abdelzaher, H.; Saleh, B.; Ismail, H.; Hafiz, M.; Gabal, M.; Mahmoud, M.; Hashish, S.; Gawad, R.; Gharieb, R.; Abdelnaser, A. COVID-19 Genetic and Environmental Risk Factors: A Look at the Evidence. Front Pharmacol. 2020, 11, 579415. [Google Scholar] [CrossRef]
- Jackson, L.; Nelson, J.; Benson, P.; Neuzil, K.; Reid, R.; Psaty, B.; Heckbert, S.; Larson, E.; Weiss, N. Functional status is a confounder of the association of influenza vaccine and risk of all-cause mortality in seniors. Int J Epidemiol. 2006, 35, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.; Hall, E.; Grange, Z.; Sullivan, C.; Kennedy, S.; Ritchie, L.; Agrawal, U.; Simpson, C.; Shah, S.; Rudan, I.; McCowan, C.; et al. Characterising adults in Scotland who are not vaccinated against COVID–19. Lancet 2022, 400, 993–995. [Google Scholar] [CrossRef] [PubMed]
- ONS. Updating ethnic contrasts in deaths involving the coronavirus (COVID–19), England: 10 January 2022 to 16 February 2022. Available online: Updating ethnic contrasts in deaths involving the coronavirus (COVID–19), England - Office for National Statistics (ons.gov.uk) (accessed 8 October 2022).
- CDC. Risk for COVID–19 infection, hospitalization, and death by race/ethnicity. Available online: Risk for COVID–19 Infection, Hospitalization, and Death By Race/Ethnicity | CDC (accessed on 8 October 2022).
- ICM Unlimited. Exploring attitudes toward COVID-19 vaccinations. Available online: https://www.icmunlimited.com/our-work/exploring-attitudes-towards-covid-19-vaccinations-for-stv/ (accessed on 15 January 2023).
- Agrawal, U.; Bedston, S.; McCowan, C.; Oke, J.; Patterson, L.; Robertson, C.; Akbari, A.; Azcoaga-Lorenzo, A.; Bradley, D.; Fagbamigbe, A.; Grange, Z.; Hall, E.; et al. Severe COVID–19 outcomes after full vaccination of primary schedule and initial boosters: pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales. The Lancet, 2022, 400, 1305–1320. [Google Scholar] [CrossRef]
- McCartney, D.; O’Shea, P.; Healy, M.; Walsh, J.; Griffin, T.; Walsh, C.; Byrne, D.; Kenny, R.; Faul, J. The Causal Role of Vitamin D Deficiency in Worse Covid-19 Outcomes: Implications for Policy and Practice Development. Ir Med J. 2023, 116, P733, The Causal Role of Vitamin D Deficiency in Worse Covid-19 Outcomes: Implications for Policy and Practice Development—Irish Medical Journal (imj.ie). [Google Scholar]
- Boucher, E.; Cao, C.; D’Mello, S.; Duarte, N.; Donnici, C.; Duarte, N.; Bennett, G; SeroTracker Consortium.; Adisesh, A.; Arora, R.; Kodama, D.; Bobrovitz, N. Occupation and SARS-CoV-2 seroprevalence studies: a systematic review. BMJ Open 2023;13:e063771. [CrossRef]
- Dite, G.; Murphy, N.; Allman, R. An integrated clinical and genetic model for predicting risk of severe COVID-19: A population-based case–control study. PLOS ONE 2021, 16, e0247205. [Google Scholar] [CrossRef]
- Bedston, S.; Almaghrabi, F.; Patterson, L.; Agrawal,U.; Woolford,L.; Anand,S.; Joy,M.; Crawford,A.; Goudie,R.; Byford,R.; et al. Risk of severe COVID-19 outcomes after autumn 2022 COVID-19 booster vaccinations: a pooled analysis of national prospective cohort studies involving 7.4 million adults in England, Northern Ireland, Scotland and Wales. The Lancet Regional Health – Europe. 2024, 37, 100816. [CrossRef]
- Cevirgel, A.; Shetty, S.; Vos, M.; Nanlohy, N.; Beckers, L.; Bijvank, E.; Rots, N.; van Beek, J.; Buisman, A.; van Baarle, D. Identification of aging-associated immunotypes and immune stability as indicators of post-vaccination immune activation. Aging Cell. 2022, 21, e13703. [Google Scholar] [CrossRef]
- Geraghty, K.; Rooney, D.; Watson, C.; Ledwidge, M.; Glynn, L.; Gallagher, J. Non-specific effects of Pneumococcal and Haemophilus vaccines in children aged 5 years and under: a systematic review. BMJ Open 2023, 13, e077717. [Google Scholar] [CrossRef]
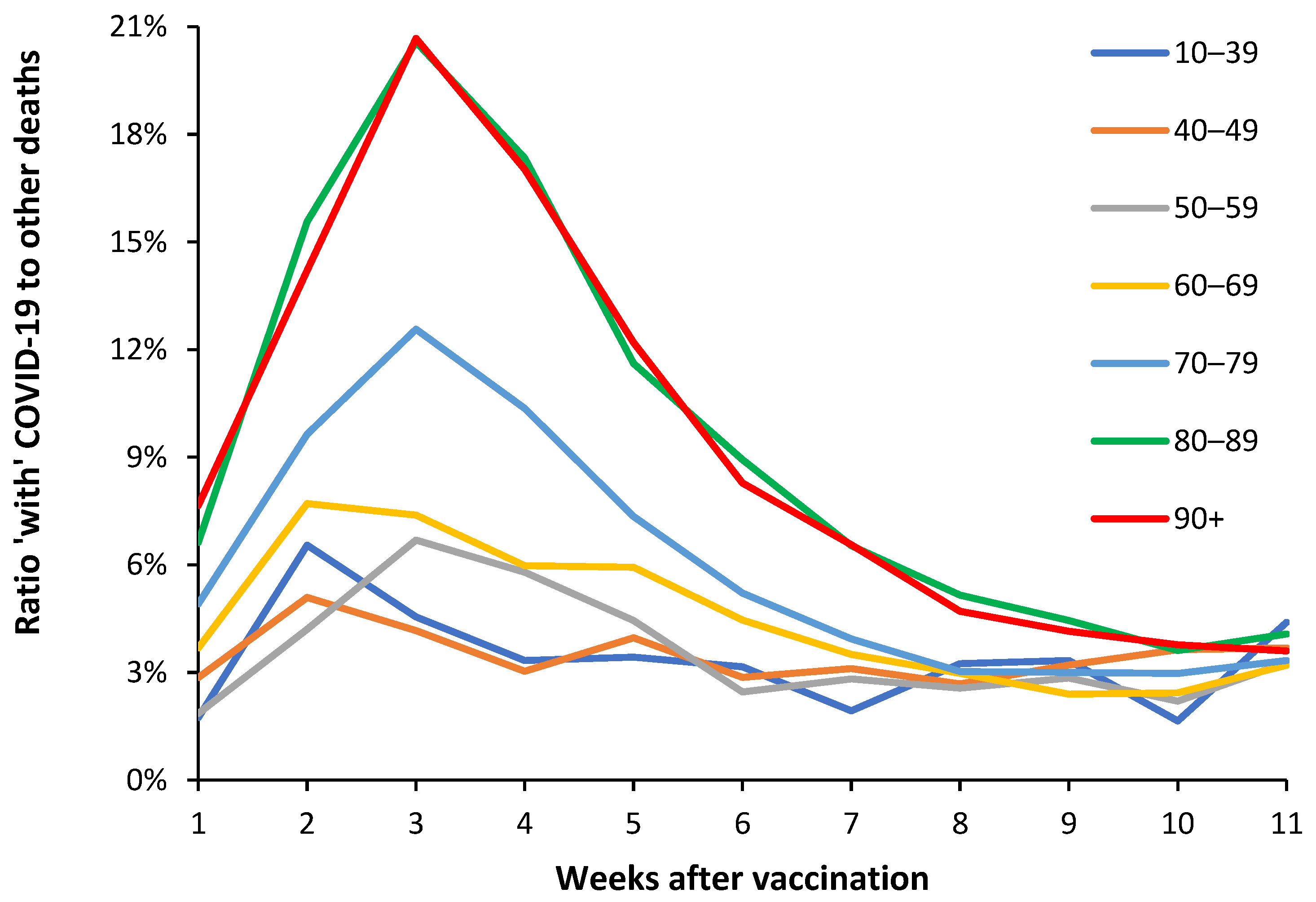
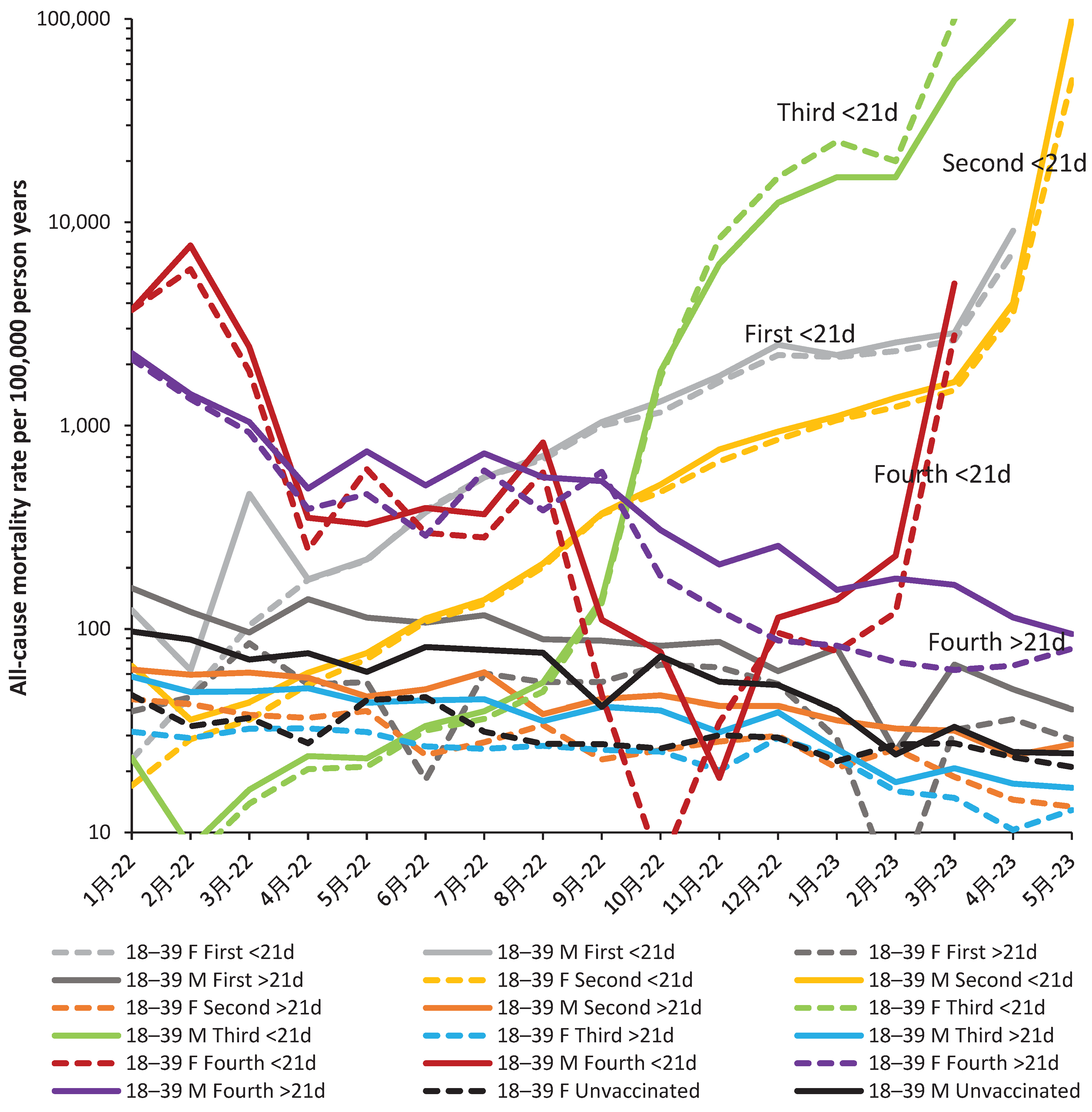
| Age band | Alpha (Jan-Jun 2021) |
Delta (Jul-21 to Feb-22) |
Omicron (Mar-22 onward) |
|---|---|---|---|
| 5-11 | Not vaccinated | Start Feb-22 mRNA | mRNA |
| 12-15 | Not vaccinated | Start Sep-21 mRNA | mRNA/Novavax |
| 16-17 | Not vaccinated | mRNA | mRNA/Novavax |
| 18-39 | Mixed, increasing mRNA in last 2 months of Alpha | mRNA | mRNA/Novavax |
| 40-49 | mixed | mixed | mRNA/Novavax |
| 50-59 | mixed | mixed | mRNA/Novavax |
| 60-69 | mixed | mixed | mRNA/Novavax |
| 70-79 | mixed | mixed | mRNA/Novavax |
| 80-89 | mixed | mixed | mRNA/Novavax |
| 90+ | Mixed but mRNA rich | mixed | mRNA/Novavax |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





