Submitted:
02 January 2024
Posted:
04 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Literature review section
- 1.
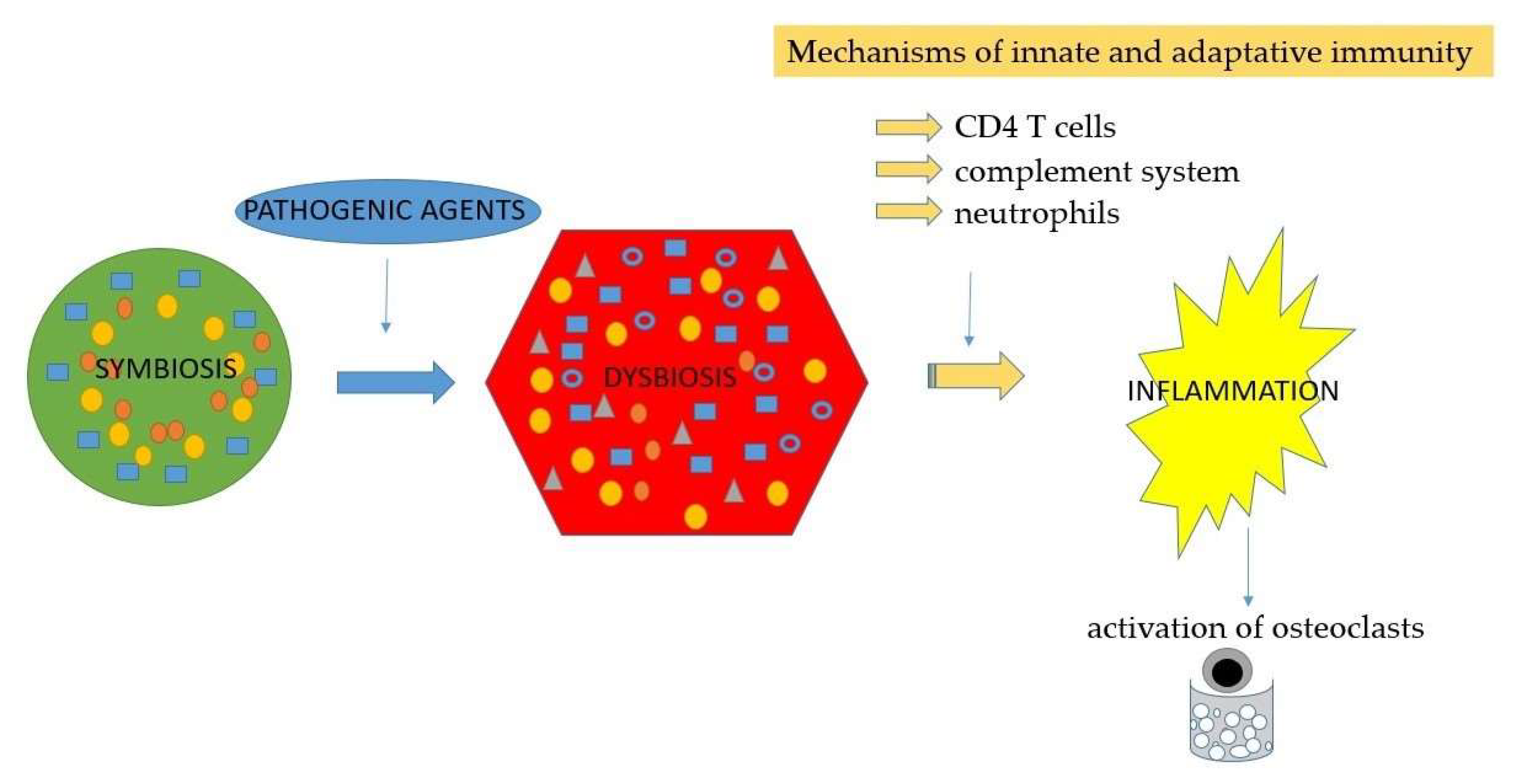
- Neutrophils – activation and involvement in periodontal disease
- 2.
- Activation of the inflammasome
2.1. Implications of the inflammasome in periodontal disease
2.2. Implications of the inflammasome in the general pathologies
- 3.
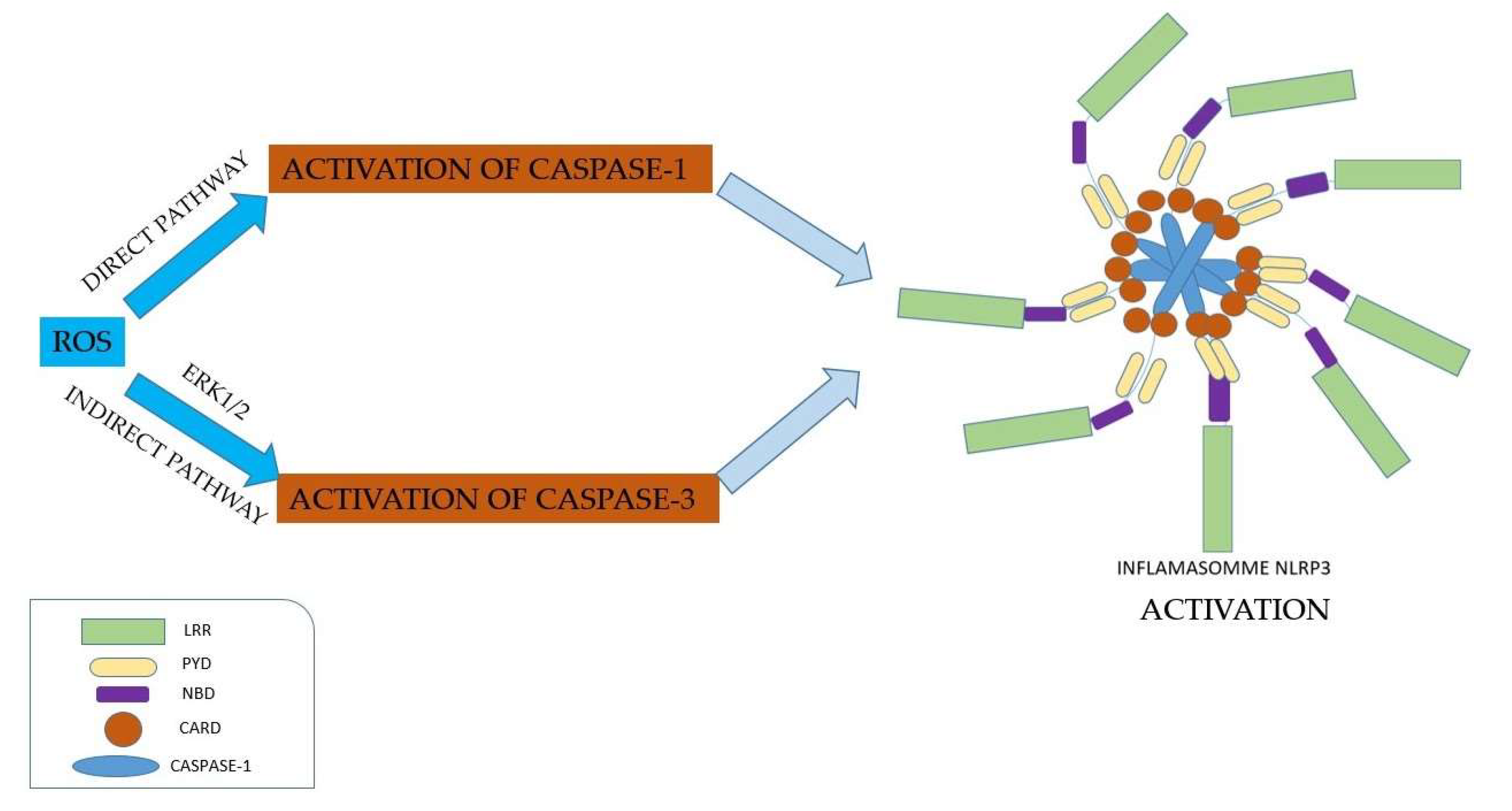
- Production of reactive oxygen species mediated by inflammasome
- 4.
- Inflammasome inhibitors
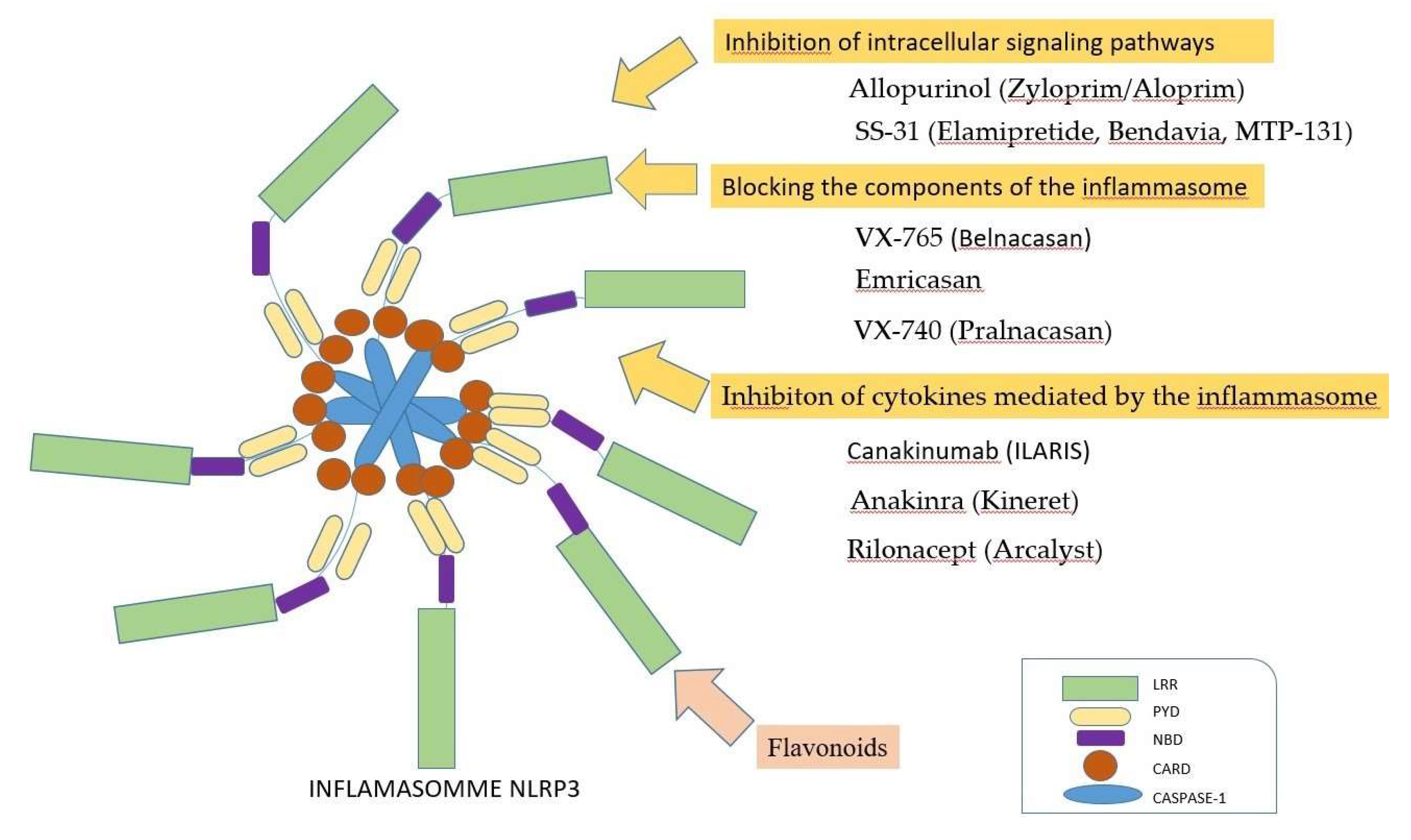
4.1. Direct inhibitors of the inflammasome
4.2. Indirect inhibitors of the inflammasome by acting on reactive oxygen species
- 5.
- Biomaterials functionalized with inflammasome inhibitors
5.1. Blocking of the inflammasome, by inhibiting intracellular signaling pathways
5.2. Inhibition of the inflammasome, by blocking its components
5.3. Blocking of the inflammasome, by inhibiting the cytokines
5.4. Inhibition of the inflammasome, by using phytochemicals
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hajishengallis, G.; Lamont, R.J. Polymicrobial communities in periodontal disease: Their quasi-organismal nature and dialogue with the host. Periodontology 2000 2021, 86, 210–230. [CrossRef]
- Williams, D.W.; Greenwell-Wild, T.; Brenchley, L.; Dutzan, N.; Overmiller, A.; Sawaya, A.P.; Webb, S.; Martin, D.; Hajishengallis, G.; Divaris, K.; et al. Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell 2021, 184, 4090–4104.e15. [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [CrossRef]
- Wang, H.; Ideguchi, H.; Kajikawa, T.; Mastellos, D.C.; Lambris, J.D.; Hajishengallis, G. Complement Is Required for Microbe-Driven Induction of Th17 and Periodontitis. J. Immunol. 2022, 209, 1370–1378. [CrossRef]
- Nauseef, W.M.; Borregaard, N. Neutrophils at work. Nat. Immunol. 2014, 15(7), 602-611. [CrossRef]
- Nussbaum, G.; Shapira, L. How has neutrophil research improved our understanding of periodontal pathogenesis? J. Clin. Periodontol. 2011, 38, 49-59. [CrossRef]
- Miralda, I.; Uriarte, S.M. Periodontal Pathogens’ strategies disarm neutrophils to promote dysregulated inflammation. Mol. Oral Microbiol. 2021, 36, 103–120. [CrossRef]
- Cowland, J.B.; Borregaard, N. Granulopoiesis and granules of human neutrophils. Immunol. Rev. 2016, 273, 11–28. [CrossRef]
- Cassatella, M.A.; Östberg, N.K.; Tamassia, N.; Soehnlein, O. Biological Roles of Neutrophil-Derived Granule Proteins and Cytokines. Trends Immunol. 2019, 40, 648–664. [CrossRef]
- Miralda, I.; Uriarte, S.M.; McLeish, K.R. Multiple Phenotypic Changes Define Neutrophil Priming. Front. Cell. Infect. Microbiol. 2017, 7, 217. [CrossRef]
- Irwandi, R.A.; Chiesa, S.T.; Hajishengallis, G.; Papayannopoulos, V.; Deanfield, J.E.; D’aiuto, F. The Roles of Neutrophils Linking Periodontitis and Atherosclerotic Cardiovascular Diseases. Front. Immunol. 2022, 13, 915081. [CrossRef]
- Elbim, C.; Rajagopalan-Levasseur, P.; Chollet-Martin, S.; Gaillard, J.-L.; Fay, M.; Hakim, J.; Fischer, A.; Casanova, J.-L.; Gougerot-Pocidalo, M.-A. Defective priming of the phagocyte oxidative burst in a child with recurrent intracellular infections. Microbes Infect. 1999, 1, 581–587. [CrossRef]
- Whitmore, L.C.; Hook, J.S.; Philiph, A.R.; Hilkin, B.M.; Bing, X.; Ahn, C.; Wong, H.R.; Ferguson, P.J.; Moreland, J.G. A Common Genetic Variant in TLR1 Enhances Human Neutrophil Priming and Impacts Length of Intensive Care Stay in Pediatric Sepsis. J. Immunol. 2016, 196, 1376–1386. [CrossRef]
- Matthews, J.B.; Wright, H.J.; Roberts, A.; Cooper, P.R.; Chapple, I.L.C. Hyperactivity and reactivity of peripheral blood neutrophils in chronic periodontitis. Clin. Exp. Immunol. 2007, 147, 255–264. [CrossRef]
- Eggleton, P.; Wang, L.; Penhallow, J.; Crawford, N.; A Brown, K. Differences in oxidative response of subpopulations of neutrophils from healthy subjects and patients with rheumatoid arthritis.. Ann. Rheum. Dis. 1995, 54, 916–923. [CrossRef]
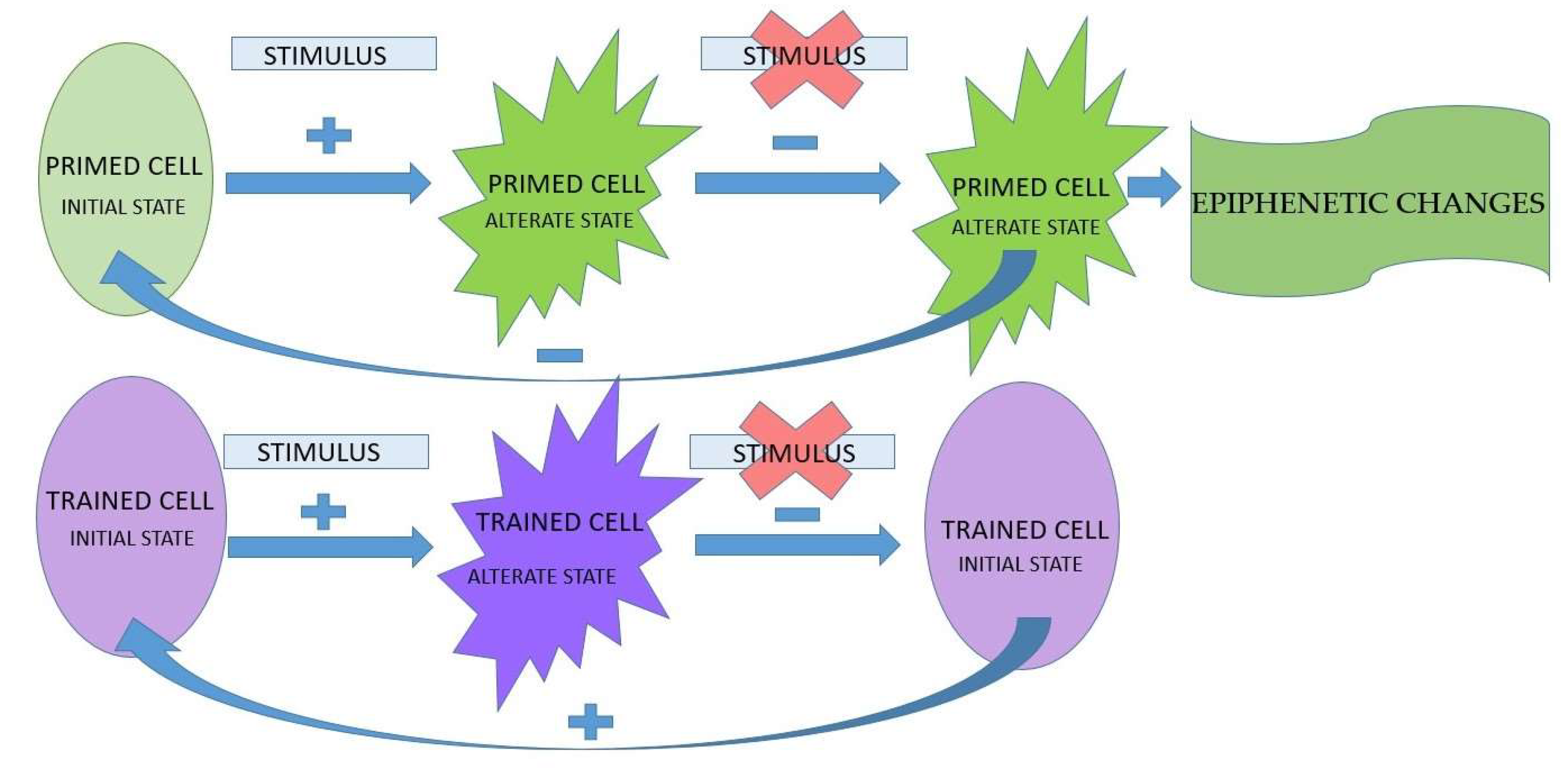
- Divangahi, M.; Aaby, P.; Khader, S.A. Trained immunity, tolerance, priming and differentiation: distinct immunological processes. Nat. Immunol. 2021, 22(1), 2-6. [CrossRef]
- Hajishengallis, G.; Diaz, P.I. Porphyromonas gingivalis: Immune Subversion Activities and Role in Periodontal Dysbiosis. Curr. Oral Heal. Rep. 2020, 7, 12–21. [CrossRef]
- Prince, L.R.; Whyte, M.K.; Sabroe, I.; Parker, L.C. The role of TLRs in neutrophil activation. Curr. Opin. Pharmacol. 2011, 11, 397–403. [CrossRef]
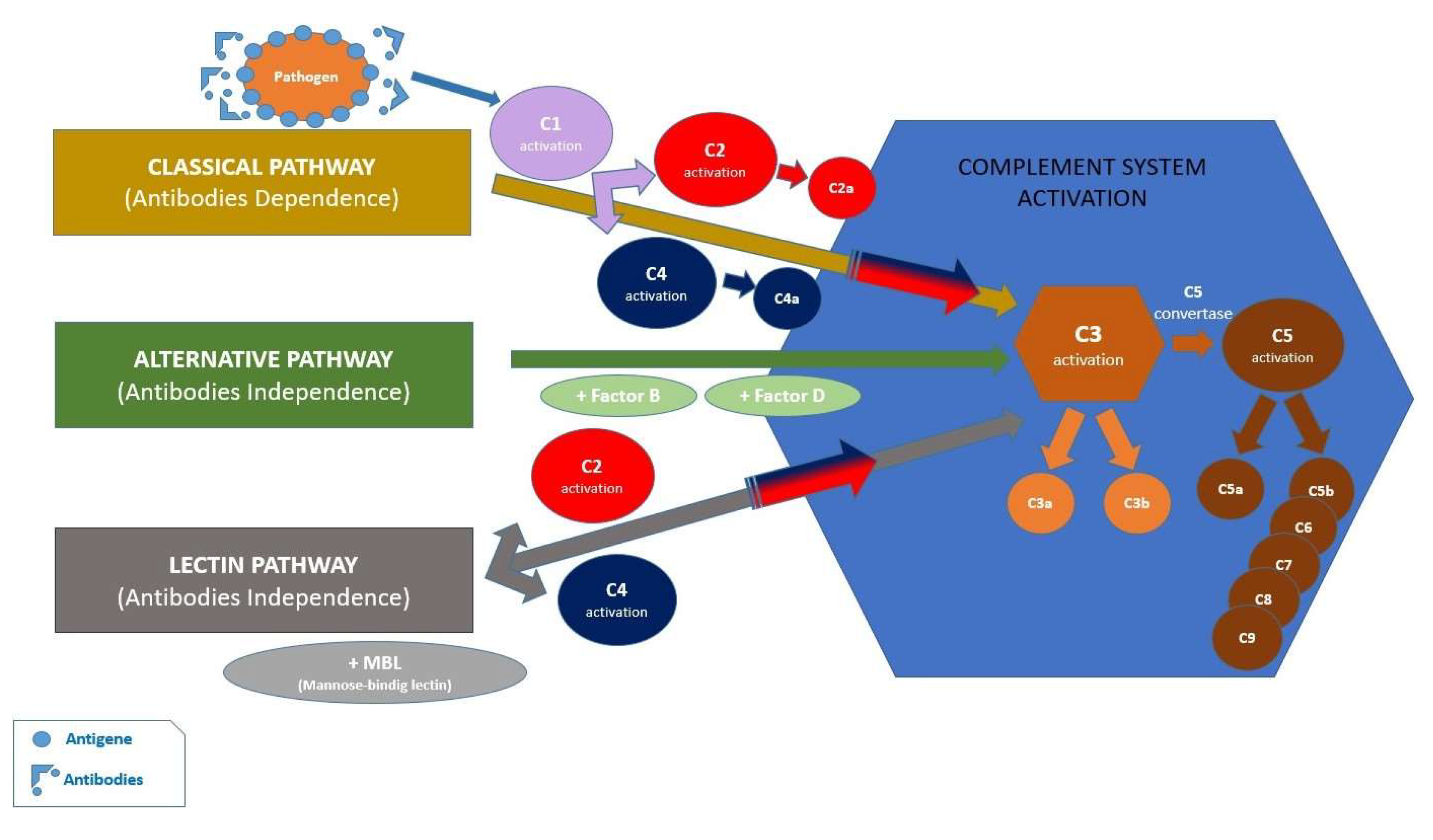
- Hajishengallis, G.; Kajikawa, T.; Hajishengallis, E.; Maekawa, T.; Reis, E.S.; Mastellos, D.C.; Yancopoulou, D.; Hasturk, H.; Lambris, J.D. Complement-Dependent Mechanisms and Interventions in Periodontal Disease. Front. Immunol. 2019, 10, 406. [CrossRef]
- Hajishengallis, G.; Reis, E.S.; Mastellos, D.C.; Ricklin, D.; Lambris, J.D. Novel mechanisms and functions of complement. Nat. Immunol. 2017, 18, 1288–1298. [CrossRef]
- Harokopakis, E.; Hajishengallis, G. Integrin activation by bacterial fimbriae through a pathway involving CD14, Toll-like receptor 2, and phosphatidylinositol-3-kinase. Eur. J. Immunol. 2005, 35, 1201–1210. [CrossRef]
- Potempa, J.; Sroka, A.; Imamura, T.; Travis, J. Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis: structure, function and assembly of multidomain protein complexes. Curr. Protein. Pept. Sci. 2003, 4(6), 397-407. [CrossRef]
- Liang, S.; Krauss, J.L.; Domon, H.; McIntosh, M.L.; Hosur, K.B.; Qu, H.; Li, F.; Tzekou, A.; Lambris, J.D.; Hajishengallis, G. The C5a Receptor Impairs IL-12–Dependent Clearance of Porphyromonas gingivalis and Is Required for Induction of Periodontal Bone Loss. J. Immunol. 2011, 186, 869–877. [CrossRef]
- Juarez-Rodriguez, M.D.; Torres-Escobar, A.; Demuth, D.R. ygiW and qseBC are co-expressed in Aggregatibacter actinomycetemcomitans and regulate biofilm growth. Microbiology 2013, 159, 989-1001. [CrossRef]
- Weigel, W.A.; Demuth, D.R.; Torres-Escobar, A.; Rodríguez, J. Aggregatibacter actinomycetemcomitans QseBC is activated by catecholamines and iron and regulates genes encoding proteins associated with anaerobic respiration and metabolism. Mol. Oral Microbiol. 2015, 30, 384–398. [CrossRef]
- Ozuna, H.; Uriarte, S.M.; Demuth, D.R. The Hunger Games: Aggregatibacter actinomycetemcomitans Exploits Human Neutrophils As an Epinephrine Source for Survival. Front. Immunol. 2021, 12. [CrossRef]
- Stark, M.A.; Huo, Y.; Burcin, T.L.; Morris, M.A.; Olson, T.S.; Ley, K. Phagocytosis of Apoptotic Neutrophils Regulates Granulopoiesis via IL-23 and IL-17. Immunity 2005, 22, 285–294. [CrossRef]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15(2), 104. [CrossRef]
- Drechsler, M.; Megens, R.; Van Zandvoort, M.; Weber, C.; Soehnlein, O. Hyperlipidemia-Triggered Neutrophilia Promotes Early Atherosclerosis. Circulation 2010, 122, 1837–1845. [CrossRef]
- Nagareddy, P.R.; Murphy, A.J.; Stirzaker, R.A.; Hu, Y.; Yu, S.; Miller, R.G.; Ramkhelawon, B.; Distel, E.; Westerterp, M.; Huang, L.-S.; et al. Hyperglycemia Promotes Myelopoiesis and Impairs the Resolution of Atherosclerosis. Cell Metab. 2013, 17, 695–708. [CrossRef]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [CrossRef]
- Kajikawa, T.; Briones, R.A.; Resuello, R.R.; Tuplano, J.V.; Reis, E.S.; Hajishengallis, E.; Garcia, C.A.; Yancopoulou, D.; Lambris, J.D.; Hajishengallis, G. Safety and Efficacy of the Complement Inhibitor AMY-101 in a Natural Model of Periodontitis in Non-human Primates. Mol. Ther. - Methods Clin. Dev. 2017, 6, 207–215. [CrossRef]
- Dahlen, G.; Basic, A.; Bylund, J. Importance of Virulence Factors for the Persistence of Oral Bacteria in the Inflamed Gingival Crevice and in the Pathogenesis of Periodontal Disease. J. Clin. Med. 2019, 8, 1339. [CrossRef]
- Bateman, G.; Hill, B.; Knight, R.; Boucher, D. Great balls of fire: activation and signalling of inflammatory caspases. Biochem. Soc. Trans. 2021, 49, 1311–1324. [CrossRef]
- Bostanci, N.; Emingil, G.; Saygan, B.; Turkoglu, O.; Atilla, G.; A Curtis, M.; Belibasakis, G.N. Expression and regulation of the NALP3 inflammasome complex in periodontal diseases. Clin. Exp. Immunol. 2009, 157, 415–422. [CrossRef]
- Marchesan, J.T.; Girnary, M.S.; Moss, K.; Monaghan, E.T.; Egnatz, G.J.; Jiao, Y.; Zhang, S.; Beck, J.; Swanson, K.V. Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics. Periodontology 2000 2020, 82, 93–114. [CrossRef]
- Harton, J.A.; Linhoff, M.W.; Zhang, J.; Ting, J.P.-Y. Cutting Edge: CATERPILLER: A Large Family of Mammalian Genes Containing CARD, Pyrin, Nucleotide-Binding, and Leucine-Rich Repeat Domains. J. Immunol. 2002, 169, 4088–4093. [CrossRef]
- Ting, J.P.; Lovering, R.C.; Alnemri, E.S.; Bertin, J.; Boss, J.M.; Davis, B.K.; Flavell, R.A.; Girardin, S.E.; Godzik, A.; Harton, J.A.; et al. The NLR Gene Family: A Standard Nomenclature. Immunity 2008, 28, 285–287. [CrossRef]
- Harijith, A.; Ebenezer, D.L.; Natarajan, V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front. Physiol. 2014, 5, 352. [CrossRef]
- Boyden, E.D.; Dietrich, W.F. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 2006, 38, 240–244. [CrossRef]
- Yilmaz, .; Sater, A.A.; Yao, L.; Koutouzis, T.; Pettengill, M.; Ojcius, D.M. ATP-dependent activation of an inflammasome in primary gingival epithelial cells infected byPorphyromonas gingivalis. Cell. Microbiol. 2010, 12, 188–198. [CrossRef]
- Belibasakis, G.N.; Johansson, A. Aggregatibacter actinomycetemcomitans targets NLRP3 and NLRP6 inflammasome expression in human mononuclear leukocytes. Cytokine 2012, 59, 124–130. [CrossRef]
- Lich, J.D.; Ting, J.P. Monarch-1/PYPAF7 and other CATERPILLER (CLR, NOD, NLR) proteins with negative regulatory functions. Microbes. Infect. 2007, 9, 672-676.
- Duncan, J.A.; Gao, X.; Huang, M.T.-H.; O'Connor, B.P.; Thomas, C.E.; Willingham, S.B.; Bergstralh, D.T.; Jarvis, G.A.; Sparling, P.F.; Ting, J.P.-Y. Neisseria gonorrhoeae Activates the Proteinase Cathepsin B to Mediate the Signaling Activities of the NLRP3 and ASC-Containing Inflammasome. J. Immunol. 2009, 182, 6460–6469. [CrossRef]
- Rathinam, V.A.K.; Vanaja, S.K.; A Fitzgerald, K. Regulation of inflammasome signaling. Nat. Immunol. 2012, 13, 333–342. [CrossRef]
- Wang, P.; Zhu, S.; Yang, L.; Cui, S.; Pan, W.; Jackson, R.; Zheng, Y.; Rongvaux, A.; Sun, Q.; Yang, G.; et al. Nlrp6 regulates intestinal antiviral innate immunity. Science 2015, 350, 826–830. [CrossRef]
- Radian, A.D.; Khare, S.; Chu, L.H.; Dorfleutner, A.; Stehlik, C. ATP binding by NLRP7 is required for inflammasome activation in response to bacterial lipopeptides. Mol. Immunol. 2015, 67, 294–302. [CrossRef]
- Williams, K.L.; Lich, J.D.; Duncan, J.A.; Reed, W.; Rallabhandi, P.; Moore, C.; Kurtz, S.; Coffield, V.M.; Accavitti-Loper, M.A.; Su, L.; et al. The CATERPILLER Protein Monarch-1 Is an Antagonist of Toll-like Receptor-, Tumor Necrosis Factor α-, and Mycobacterium tuberculosis-induced Pro-inflammatory Signals. J. Biol. Chem. 2005, 280, 39914–39924. [CrossRef]
- Gong, Y.-N.; Shao, F. Sensing bacterial infections by NAIP receptors in NLRC4 inflammasome activation. Protein Cell 2012, 3, 98–105. [CrossRef]
- Davis, B.K.; Roberts, R.A.; Huang, M.T.; Willingham, S.B.; Conti, B.J.; Brickey, W.J.; Barker, B.R.; Kwan, M.; Taxman, D.J.; Accavitti-Loper, M.-A.; et al. Cutting Edge: NLRC5-Dependent Activation of the Inflammasome. J. Immunol. 2011, 186, 1333–1337. [CrossRef]
- Wu, J.; Fernandes-Alnemri, T.; Alnemri, E. S. Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J. Clin. Immunol. 2010, 30, 693–702.
- Fernandes-Alnemri, T.; Yu, J.-W.; Datta, P.; Wu, J.; Alnemri, E.S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 2009, 458, 509–513. [CrossRef]
- Correa, R.G.; Milutinovic, S.; Reed, J.C. Roles of NOD1 (NLRC1) and NOD2 (NLRC2) in innate immunity and inflammatory diseases. Biosci. Rep. 2012, 32, 597–608. [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen Recognition by the Innate Immune System. Int. Rev. Immunol. 2011, 30, 16–34. [CrossRef]
- Sordi, M.B.; Magini, R.d.S.; Panahipour, L.; Gruber, R. Pyroptosis-Mediated Periodontal Disease. Int. J. Mol. Sci. 2022, 23, 372. [CrossRef]
- Cotranu, I.; Mareş, L. Explanatory dictionary of the Romanian language, 2nd ed. Encyclopedic Universe Gold, Romania; 2009.
- Jones, N.S.; Kshirsagar, S.; Mohanan, V.; Ramakrishnan, V.; Di Nucci, F.; Ma, L.; Mao, J.; Ding, H.; Klabunde, S.; Vucic, D.; Pan, L.; Lekkerkerker, A.N.; Chen, Y.; Rothenberg, M.E. A phase I, randomized, ascending-dose study to assess safety, pharmacokinetics, and activity of GDC-8264, a RIP1 inhibitor, in healthy volunteers. Clin. Transl. Sci. 2023, 16(10), 1997-2009. [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochem. (Moscow) 2020, 85, 1178–1190. [CrossRef]
- A Miao, E.; A Leaf, I.; Treuting, P.M.; Mao, D.P.; Dors, M.; Sarkar, A.; E Warren, S.; Wewers, M.D.; Aderem, A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010, 11, 1136–1142. [CrossRef]
- Zychlinsky, A.; Prevost, M.C.; Sansonetti, P.J. Shigella flexneri induces apoptosis in infected macrophages. Nature 1992, 358, 167–169. [CrossRef]
- Cookson, B.T.; A Brennan, M. Pro-inflammatory programmed cell death. Trends Microbiol. 2001, 9, 113–114. [CrossRef]
- Yu, C.; Zhang, C.; Kuang, Z.; Zheng, Q. The Role of NLRP3 Inflammasome Activities in Bone Diseases and Vascular Calcification. Inflammation 2021, 44, 434–449. [CrossRef]
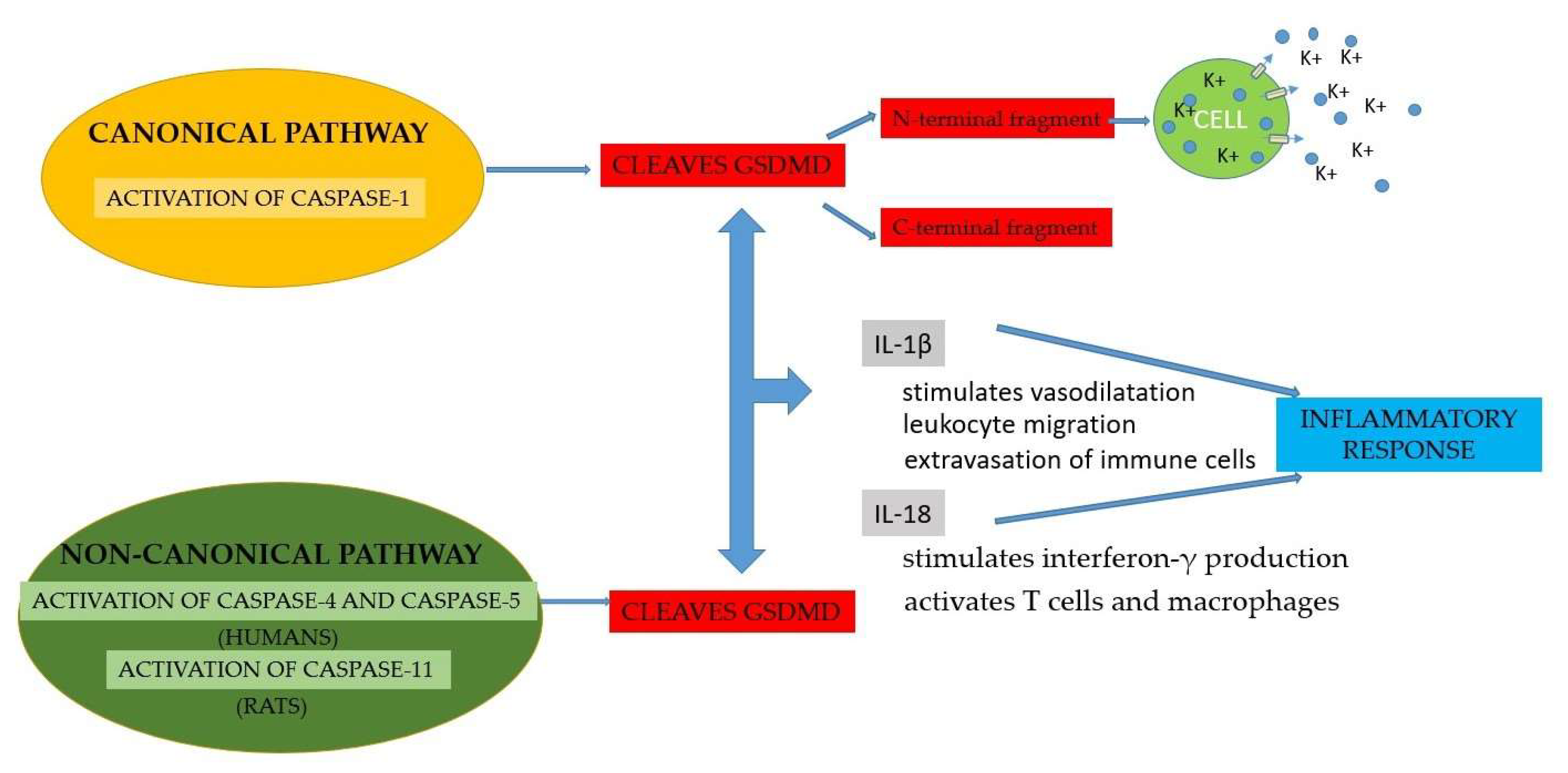
- Chen, X.; He, W.-T.; Hu, L.; Li, J.; Fang, Y.; Wang, X.; Xu, X.; Wang, Z.; Huang, K.; Han, J. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016, 26, 1007–1020. [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Inflammasomes and Their Roles in Health and Disease. Annu. Rev. Cell Dev. Biol. 2012, 28, 137–161. [CrossRef]
- Liu, W.; Liu, J.; Wang, W.; Wang, Y.; Ouyang, X. NLRP6 Induces Pyroptosis by Activation of Caspase-1 in Gingival Fibroblasts. J. Dent. Res. 2018, 97, 1391–1398. [CrossRef]
- Muñoz-Planillo, R.; Franchi, L.; Miller, L.S.; Núñez, G. A Critical Role for Hemolysins and Bacterial Lipoproteins inStaphylococcus aureus-Induced Activation of the Nlrp3 Inflammasome. J. Immunol. 2009, 183, 3942–3948. [CrossRef]
- Muñoz-Planillo, R.; Kuffa, P.; Martínez-Colón, G.; Smith, B.L.; Rajendiran, T.M.; Núñez, G. K+ Efflux Is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter. Immunity 2013, 38, 1142–1153. [CrossRef]
- Pressman, B.C. Biological Applications of Ionophores. Annu. Rev. Biochem. 1976, 45, 501–530. [CrossRef]
- Domon, H.; Takahashi, N.; Honda, T.; Nakajima, T.; Tabeta, K.; Abiko, Y.; Yamazaki, K. Up-regulation of the endoplasmic reticulum stress-response in periodontal disease. Clin. Chim. Acta 2009, 401, 134–140. [CrossRef]
- Hörauf, J.-A.; Kany, S.; Janicova, A.; Xu, B.; Vrdoljak, T.; Sturm, R.; Dunay, I.R.; Martin, L.; Relja, B. Short Exposure to Ethanol Diminishes Caspase-1 and ASC Activation in Human HepG2 Cells In Vitro. Int. J. Mol. Sci. 2020, 21, 3196. [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [CrossRef]
- Feng, Y.; Huang, X. Methodology for Comprehensive Detection of Pyroptosis. Methods Mol. Biol. 2021, 2255, 149–157. [CrossRef]
- Graves, D.; Cochran, D. The Contribution of Interleukin-1 and Tumor Necrosis Factor to Periodontal Tissue Destruction. J. Periodontol. 2003, 74, 391–401. [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Inflammasomes and Their Roles in Health and Disease. Annu. Rev. Cell Dev. Biol. 2012, 28, 137–161. [CrossRef]
- Davis, B.K.; Wen, H.; Ting, J.P.-Y. The Inflammasome NLRs in Immunity, Inflammation, and Associated Diseases. Annu. Rev. Immunol. 2011, 29, 707–735. [CrossRef]
- Ken-ichiro S. Historical aspects of studies on roles of the inflammasome in the pathogenesis of periodontal diseases. Mol. Oral Microb. 2013, 33(3), 203–211. [CrossRef]
- Schroder, K.; J. Tschopp. The inflammasomes. Cell 2010, 140, 821–832. [CrossRef]
- Martinon, F.; K. Burns; J. Tschopp. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002, 10, 417–426. [CrossRef]
- Zhong, Z.; Liang S.; Sanchez-Lopez E.; He F.; Shalapour S.; Lin X. J.; Wong J.; Ding S.; Seki E.; Schnabl B. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 2018, 560, 198–203. [CrossRef]
- Bostanci, N.; Emingil, G.; Saygan, B.; Turkoglu, O.; Atilla, G.; A Curtis, M.; Belibasakis, G.N. Expression and regulation of the NALP3 inflammasome complex in periodontal diseases. Clin. Exp. Immunol. 2009, 157, 415–422. [CrossRef]
- Xue, F.; Shu, R.; Xie, Y. The expression of NLRP3, NLRP1 and AIM2 in the gingival tissue of periodontitis patients: RT-PCR study and immunohistochemistry. Arch. Oral Biol. 2015, 60, 948–958. [CrossRef]
- Li, Y.; Li, B.; Liu, Y.; Wang, H.; He, M.; Liu, Y.; Sun, Y.; Meng, W. Porphyromonas gingivalis lipopolysaccharide affects oral epithelial connections via pyroptosis. J. Dent. Sci. 2021, 16, 1255–1263. [CrossRef]
- Shibata, K. Historical aspects of studies on roles of the inflammasome in the pathogenesis of periodontal diseases. Mol. Oral Microbiol. 2018, 33, 203–211. [CrossRef]
- Hanazawa, S.; Nakada, K.; Ohmori, Y.; Miyoshi, T.; Amano, S.; Kitano, S. Functional role of interleukin 1 in periodontal disease: induction of interleukin 1 production by Bacteroides gingivalis lipopolysaccharide in peritoneal macrophages from C3H/HeN and C3H/HeJ mice. Infect. Immun. 1985, 50, 262–270. [CrossRef]
- Jandinski, J.J.; Stashenko, P.; Feder, L.S. Localization of interleukin-1 beta in human periodontal tissue. J. Periodontol. 1991, 62(1), 36-43. [CrossRef]
- Kinane, D.; Winstanley, F.; Adonogianaki, E.; Moughal, N. Bioassay of interleukin 1 (IL-1) in human gingival crevicular fluid during experimental gingivitis. Arch. Oral Biol. 1992, 37, 153–156. [CrossRef]
- Assuma, R.; Oates, T.; Cochran, D.; Amar, S.; Graves, D.T. IL-1 and TNF Antagonists Inhibit the Inflammatory Response and Bone Loss in Experimental Periodontitis. J. Immunol. 1998, 160, 403–409. [CrossRef]
- Bostanci, N.; Meier, A.; Guggenheim, B.; Belibasakis, G.N. Regulation of NLRP3 and AIM2 inflammasome gene expression levels in gingival fibroblasts by oral biofilms. Cell. Immunol. 2011, 270, 88–93. [CrossRef]
- Guo, W.; Ye, P.; Yu, H.; Liu, Z.; Yang, P.; Hunter, N. CD24 activates the NLRP3 inflammasome through c-Src kinase activity in a model of the lining epithelium of inflamed periodontal tissues. Immun. Inflamm. Dis. 2014, 2(4), 239-253. [CrossRef]
- Xue, F.; Shu, R.; Xie, Y. The expression of NLRP3, NLRP1 and AIM2 in the gingival tissue of periodontitis patients: RT-PCR study and immunohistochemistry. Arch. Oral Biol. 2015, 60, 948–958. [CrossRef]
- Yamaguchi, Y.; Kurita-Ochiai, T.; Kobayashi, R.; Suzuki, T.; Ando, T. Activation of the NLRP3 inflammasome in Porphyromonas gingivalis-accelerated atherosclerosis. Pathog. Dis. 2015, 73. [CrossRef]
- Wang, L.; Sharif, H.; Vora, S.M.; Zheng, Y.; Wu, H. Structures and functions of the inflammasome engine. J. Allergy Clin. Immunol. 2021, 147, 2021–2029. [CrossRef]
- Bullon, P.; Navarro, J.M. Inflammasome as a Key Pathogenic Mechanism in Endometriosis. Curr. Drug Targets 2017, 18, 997–1002. [CrossRef]
- Huang, X.; Yang, X.; Ni, J.; Xie, B.; Liu, Y.; Xuan, D.; Zhang, J. Hyperglucose Contributes to Periodontitis: Involvement of the NLRP3 Pathway by Engaging the Innate Immunity of Oral Gingival Epithelium. J. Periodontol. 2015, 86, 327–335. [CrossRef]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Walle, L.V.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [CrossRef]
- Dang, E.V.; McDonald, J.G.; Russell, D.W.; Cyster, J.G. Oxysterol Restraint of Cholesterol Synthesis Prevents AIM2 Inflammasome Activation. Cell 2017, 171, 1057–1071.e11. [CrossRef]
- Warnatsch, A.; Ioannou M.; Wang Q.; Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015, 349, 316–320. [CrossRef]
- Hottz, E.D.; Lopes, J.F.; Freitas, C.; Valls-De-Souza, R.; Oliveira, M.F.; Bozza, M.T.; Da Poian, A.T.; Weyrich, A.S.; Zimmerman, G.A.; Bozza, F.A.; et al. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood 2013, 122, 3405–3414. [CrossRef]
- Segovia, J.; Sabbah, A.; Mgbemena, V.; Tsai, S.-Y.; Chang, T.-H.; Berton, M.T.; Morris, I.R.; Allen, I.C.; Ting, J.P.-Y.; Bose, S. TLR2/MyD88/NF-κB Pathway, Reactive Oxygen Species, Potassium Efflux Activates NLRP3/ASC Inflammasome during Respiratory Syncytial Virus Infection. PLOS ONE 2012, 7, e29695. [CrossRef]
- Dorhoi, A.; Nouailles, G.; Jörg, S.; Hagens, K.; Heinemann, E.; Pradl, L.; Oberbeck-Müller, D.; Duque-Correa, M.A.; Reece, S.T.; Ruland, J.; et al. Activation of the NLRP3 inflammasome by Mycobacterium tuberculosis is uncoupled from susceptibility to active tuberculosis. Eur. J. Immunol. 2012, 42, 374–384. [CrossRef]
- Franchi, L.; Núñez, G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur. J. Immunol. 2008, 38, 2085–2089. [CrossRef]
- Wen, H.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.-H.; Brickey, W.J.; Ting, J.P.-Y. Fatty acid–induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011, 12, 408–415. [CrossRef]
- Vilaysane, A.; Chun, J.; Seamone, M.E.; Wang, W.; Chin, R.; Hirota, S.; Li, Y.; Clark, S.A.; Tschopp, J.; Trpkov, K.; et al. The NLRP3 Inflammasome Promotes Renal Inflammation and Contributes to CKD. J. Am. Soc. Nephrol. 2010, 21, 1732–1744. [CrossRef]
- Zhang, W.; Cai, Y.; Xu, W.; Yin, Z.; Gao, X.; Xiong, S. AIM2 Facilitates the Apoptotic DNA-induced Systemic Lupus Erythematosus via Arbitrating Macrophage Functional Maturation. J. Clin. Immunol. 2013, 33, 925–937. [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [CrossRef]
- Gilbert, D.L. O2 and living processes: an inter-disciplinary approach. Springer, New York, 1981.
- Halliwell, B.; Gutteridge, J. Free radicals in biology and medicine, 4th ed.; Oxford Univ. Press, Oxford, 2007.
- Denev, P.; Kratchanov, C.G.; Číž, M.; Lojek, A.; Kratchanova, M.G. Bioavailability and Antioxidant Activity of Black Chokeberry (Aronia melanocarpa) Polyphenols: in vitro and in vivo Evidences and Possible Mechanisms of Action: A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 471–489. [CrossRef]
- Ellingsen, I.; Hjerkinn, E.M.; Seljeflot, I.; Arnesen, H.; Tonstad, S. Consumption of fruit and berries is inversely associated with carotid atherosclerosis in elderly men. Br. J. Nutr. 2008, 99, 674–681. [CrossRef]
- Rissanen, T.; Voutilainen, S.; Virtanen, J.; Venho, B.; Vanharanta, M.; Mursu, J.; Salonen, J. Low intake of fruits, berries and vegetables is associated with excess mortality in men: the Kuopio ischemic heart disease risk factor (KIHD) study. J. Nutr. 2003, 133, 199–204.
- Ross, J.A.; Kasum, C.M. DIETARY FLAVONOIDS: Bioavailability, Metabolic Effects, and Safety. Annu. Rev. Nutr. 2002, 22, 19–34. [CrossRef]
- Kuiper, J.J.W. Reactive Oxygen Species in Inflammasome Activation. MSc. I&I. thesis, University Medical Centre, Utrecht., 2009.
- Rahman, M.M.; Mohamed, M.R.; Kim, M.; Smallwood, S.; McFadden, G. Co-regulation of NF-kappaB and inflammasome-mediated inflammatory responses by myxoma virus pyrin domain-containing protein M013. PLoS Pathog. 2009, 5, e1000635. [CrossRef]
- Pacher, P.; Nivorozhkin, A.; Szabó, C. Therapeutic Effects of Xanthine Oxidase Inhibitors: Renaissance Half a Century after the Discovery of Allopurinol. Pharmacol. Rev. 2006, 58, 87–114. [CrossRef]
- Szeto, H.H. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br. J. Pharmacol. 2014, 171(8), 2029-2050. [CrossRef]
- Daubert, M.A.; Yow, E.; Dunn, G. Novel mitochondria-targeting peptide in heart failure treatment: a randomized, placebo-controlled trial of elamipretide. Circ. Heart Fail. 2017, 10(12), e004389. [CrossRef]
- de Torre-Minguela, C.; del Castillo, P.M.; Pelegrín, P. The NLRP3 and Pyrin Inflammasomes: Implications in the Pathophysiology of Autoinflammatory Diseases. Front. Immunol. 2017, 8, 43. [CrossRef]
- MacKenzie, S.H.; Schipper, J.L.; Clark, A.C. The potential for caspases in drug discovery. Curr. Opin. Drug Discov. Devel. 2010, 13, 568-576.
- Barreyro, F.J.; Holod, S.; Finocchietto, P.V.; Camino, A.M.; Aquino, J.B.; Avagnina, A.; Carreras, M.C.; Poderoso, J.J.; Gores, G.J. The pan-caspase inhibitor Emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis. Liver Int. 2014, 35, 953–966. [CrossRef]
- Cornelis, S.; Kersse, K.; Festjens, N.; Lamkanfi, M.; Vandenabeele, P. Inflammatory Caspases: Targets for Novel Therapies. Curr. Pharm. Des. 2007, 13, 367–385. [CrossRef]
- Kesselheim, A.S.; Solomon, D.H. Incentives for Drug Development — The Curious Case of Colchicine. New Engl. J. Med. 2010, 362, 2045–2047. [CrossRef]
- Verma, S.; Eikelboom, J.W.; Nidorf, S.M. Colchicine in cardiac dissease: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2015, 15, 96. [CrossRef]
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine—Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015, 45, 341–350. [CrossRef]
- Calabrese, L.H. Anakinra treatment of patients with rheumatoid arthritis. Ann. Pharmacother. 2002, 36(7-8), 1204-1209. [CrossRef]
- Moran, A.; Bundy, B.; Becker, D.J.; A DiMeglio, L.; E Gitelman, S.; Goland, R.; Greenbaum, C.J.; Herold, K.C.; Marks, J.B.; Raskin, P.; et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet 2013, 381, 1905–1915. [CrossRef]
- Orrock, J.E.; Ilowite, N.T. Canakinumab for the treatment of active systemic juvenile idiopathic arthritis. Expert Rev. Clin. Pharmacol. 2016, 9, 1015–1024. [CrossRef]
- Ben-Zvi, I.; Kukuy, O.; Giat, E. Anakinra for colchicine-resistant familial Mediterranean fever: a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2017, 69(4). [CrossRef]
- McKie, E.A.; Reid, J.L.; Mistry, P.C.; DeWall, S.L.; Abberley, L.; Ambery, P.D.; Gil-Extremera, B. A Study to Investigate the Efficacy and Safety of an Anti-Interleukin-18 Monoclonal Antibody in the Treatment of Type 2 Diabetes Mellitus. PLOS ONE 2016, 11, e0150018. [CrossRef]
- Curr. Top. Med. Chem. 2001, 1, 569–590. [CrossRef]
- Hertog, M.G.L.; Kromhout, D.; Aravanis, C.; Blackburn, H.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S.; et al. Flavonoid Intake and Long-term Risk of Coronary Heart Disease and Cancer in the Seven Countries Study. Arch. Intern. Med. 1995, 155, 381–386. [CrossRef]
- Oszmiański, J.; Wojdylo, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [CrossRef]
- Ohgami, K.; Ilieva, I.; Shiratori, K.; Koyama, Y.; Jin, X.-H.; Yoshida, K.; Kase, S.; Kitaichi, N.; Suzuki, Y.; Tanaka, T.; et al. Anti-inflammatory Effects of Aronia Extract on Rat Endotoxin-Induced Uveitis. Investig. Opthalmology Vis. Sci. 2005, 46, 275–281. [CrossRef]
- Tauber, A.I.; Fay, J.R.; Marletta, M.A. Flavonoid inhibition of the human neutrophil NADPH-oxidase. Biochem. Pharmacol. 1984, 33, 1367–1369. [CrossRef]
- Middleton, E. Jr. Effect of plant flavonoids on immune and inflammatory cell function. Adv. Exp. Med. Biol. 1998, 439, 175-182.
- Tordera, M.; Ferrándiz, M.L.; Alcaraz, M.J. Influence of Anti-Inflammatory Flavonoids on Degranulation and Arachidonic Acid Release in Rat Neutrophils. 1994, 49, 235–240. [CrossRef]
- Schmidt, R.L.; Lenz, L.L. Distinct Licensing of IL-18 and IL-1β Secretion in Response to NLRP3 Inflammasome Activation. PLOS ONE 2012, 7, e45186. [CrossRef]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820.
- Nicola, C. Dental Materials. Clinical and tehnological considerations. House of science books: Cluj-Napoca, Romania, 2009; pp. 16-34.
- Bernardi, S.; Macchiarelli, G.; Bianchi, S. Autologous Materials in Regenerative Dentistry: Harvested Bone, Platelet Concentrates and Dentin Derivates. Molecules 2020, 25, 5330. [CrossRef]
- Hollý, D.; Klein, M.; Mazreku, M.; Zamborský, R.; Polák, .; Danišovič, .; Csöbönyeiová, M. Stem Cells and Their Derivatives—Implications for Alveolar Bone Regeneration: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 11746. [CrossRef]
- Vouvoudi, E.C. Overviews on the Progress of Flowable Dental Polymeric Composites: Their Composition, Polymerization Process, Flowability and Radiopacity Aspects. Polymers 2022, 14, 4182. [CrossRef]
- Valandro, L.F.; Cadore-Rodrigues, A.C.; Dapieve, K.S.; Machry, R.V.; Pereira, G.K.R. A brief review on fatigue test of ceramic and some related matters in Dentistry. J. Mech. Behav. Biomed. Mater. 2023, 138, 105607. [CrossRef]
- Albeshir, E.G.; Alsahafi, R.; Albluwi, R.; Balhaddad, A.A.; Mitwalli, H.; Oates, T.W.; Hack, G.D.; Sun, J.; Weir, M.D.; Xu, H.H.K. Low-Shrinkage Resin Matrices in Restorative Dentistry-Narrative Review. Materials 2022, 15, 2951. [CrossRef]
- Zong, C.; Bronckaers, A.; Willems, G.; He, H.; de Llano-Pérula, M.C. Nanomaterials for Periodontal Tissue Regeneration: Progress, Challenges and Future Perspectives. J. Funct. Biomater. 2023, 14, 290. [CrossRef]
- Qiu, J.; Liu, X.; You, B.; Ren, N.; Liu, H. Application of Nanomaterials in Stem Cell-Based Therapeutics for Cardiac Repair and Regeneration. Small 2023, 19, e2206487. [CrossRef]
- Hu, T.; Gu, Z.; Williams, G.R.; Strimaite, M.; Zha, J.; Zhou, Z.; Zhang, X.; Tan, C.; Liang, R. Layered double hydroxide-based nanomaterials for biomedical applications. Chem. Soc. Rev. 2022, 51, 6126–6176. [CrossRef]
- Farkas, N.-I.; Marincaș, L.; Barabás, R.; Bizo, L.; Ilea, A.; Turdean, G.L.; Toșa, M.; Cadar, O.; Barbu-Tudoran, L. Preparation and Characterization of Doxycycline-Loaded Electrospun PLA/HAP Nanofibers as a Drug Delivery System. Materials 2022, 15, 2105. [CrossRef]
- Al-Enizi, A.M.; Zagho, M.M.; Elzatahry, A.A. Polymer-Based Electrospun Nanofibers for Biomedical Applications. Nanomaterials 2018, 8, 259. [CrossRef]
- Torres-Martinez, E.J.; Cornejo-Bravo, J.M.; Serrano-Medina, A.; Perez-González, G.L.; Gómez, L.J.V. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [CrossRef]
- Gao, Y.; Truong, Y.B.; Zhu, Y.; Kyratzis, I.L. Electrospun antibacterial nanofibers: Production, activity, and in vivo applications. J. Appl. Polym. Sci. 2014, 131. [CrossRef]
- Zhang, H.; Fu, Q.W.; Sun, T.W.; Chen, F.; Qi, C.; Wu, J.; Cai, Z.Y.; Qian, Q.R.; Zhu, Y.J. Amorphous calcium phosphate, hydroxyapatite and poly (d,l-lactic acid) composite nanofibers: Electrospinning preparation, mineralization and in vivo bone defect repair. Colloids Surfaces B Biointerfaces 2015, 136, 27–36.
- Liu, S.; Zheng, Y.; Liu, R.; Tian, C. Preparation and characterization of a novel polylactic acid/hydroxyapatite composite scaffold with biomimetic micro-nanofibrous porous structure. J. Mater. Sci. Mater. Med. 2020, 31, 1–11. [CrossRef]
- Hartatiek; Fahmi, N.K.; Putra, A.A.D.; Yudyanto; Nasikhudin; Utomo, J.; Ahmad, N. Effect of nano-hydroxyapatite (n-HAp)/PLA scaffold composites on porosity and microstructure. AIP Conf. Proc. 2020, 2234.
- Andrei, V.; Andrei, S.; Gal, A.F.; Rus, V.; Gherman, L.-M.; Boșca, B.A.; Niculae, M.; Barabas, R.; Cadar, O.; Dinte, E.; et al. Immunomodulatory Effect of Novel Electrospun Nanofibers Loaded with Doxycycline as an Adjuvant Treatment in Periodontitis. Pharmaceutics 2023, 15, 707. [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [CrossRef]
- Raviolo, M.; Solana, M.; Novoa, M.; Gualdesi, M.; Alba-Soto, C.; Briñón, M. Synthesis, physicochemical properties of allopurinol derivatives and their biological activity against Trypanosoma cruzi. Eur. J. Med. Chem. 2013, 69, 455–464. [CrossRef]
- Badis, K.; Merine, H.; Ramli, Y.; Larbi, O.; Memou, C.H. Effect of Polymers nature and Stirring Speeds on Physicochemical Properties and the Controlled Release of Allopurinol-loaded Microspheres. J. Mex. Chem. Soc. 2021, 66, 17–33. [CrossRef]
- Changdeo, J.S.; Kuchekar, M.V.; Shankar, B.; Rajaram, C.A. Physicochemical characterization and solubility enhancement studies of allopurinol solid dispersions. Brazilian Journal of Pharmaceutical Sciences 2011, 47. [CrossRef]
- Kandav, G.; Bhatt, D.; Jindal, D.K. FORMULATION AND EVALUATION OF ALLOPURINOL LOADED CHITOSAN NANOPARTICLES. Int. J. Appl. Pharm. 2019, 49–52. [CrossRef]
- Varrica, C.; Carvalheiro, M.; Faria-Silva, C.; Eleutério, C.; Sandri, G.; Simões, S. Topical Allopurinol-Loaded Nanostructured Lipid Carriers: A Novel Approach for Wound Healing Management. Bioengineering 2021, 8, 192. [CrossRef]
- Kandav, G.; Bhatt, D.C.; Jindal, D.K.; Singh, S.K. Formulation, Optimization, and Evaluation of Allopurinol-Loaded Bovine Serum Albumin Nanoparticles for Targeting Kidney in Management of Hyperuricemic Nephrolithiasis: Formulation, optimization, and evaluation of ABNPs for kidney targeting, AAPS. Pharm. Sci. Tech. 2020, 21, 164. [CrossRef]
- Birk, A.V.; Liu, S.; Soong, Y.; Mills, W.; Singh, P.; Warren, J.D.; Seshan, S.V.; Pardee, J.D.; Szeto, H.H. The Mitochondrial-Targeted Compound SS-31 Re-Energizes Ischemic Mitochondria by Interacting with Cardiolipin. J. Am. Soc. Nephrol. 2013, 24, 1250–1261. [CrossRef]
- Liu, D.; Jin, F.; Shu, G.; Xu, X.; Qi, J.; Kang, X.; Yu, H.; Lu, K.; Jiang, S.; Han, F.; You, J.; Du, Y.; Ji, J. Enhanced efficiency of mitochondria-targeted peptide SS-31 for acute kidney injury by pH-responsive and AKI-kidney targeted nanopolyplexes. Biomaterials 2019, 211, 57-67. [CrossRef]
- Shan, L.; Wang, F.; Zhai, D.; Meng, X.; Liu, J.; Lv, X. New Drugs for Hepatic Fibrosis. Front. Pharmacol. 2022, 13, 874408. [CrossRef]
- Harrison, S.A.; Goodman, Z.; Jabbar, A.; Vemulapalli, R.; Younes, Z.H.; Freilich, B.; Sheikh, M.Y.; Schattenberg, J.M.; Kayali, Z.; Zivony, A.; et al. A randomized, placebo-controlled trial of emricasan in patients with NASH and F1-F3 fibrosis. J. Hepatol. 2020, 72, 816–827. [CrossRef]
- Rudolphi, K.; Gerwin, N.; Verzijl, N.; van der Kraan, P.; Berg, W.v.D. Pralnacasan, an inhibitor of interleukin-1β converting enzyme, reduces joint damage in two murine models of osteoarthritis. Osteoarthr. Cartil. 2003, 11, 738–746. [CrossRef]
- Poreba, M.; Szalek, A.; Kasperkiewicz, P.; Rut, W.; Salvesen, G.S.; Drag, M. Small Molecule Active Site Directed Tools for Studying Human Caspases. Chem. Rev. 2015, 115, 12546–12629. [CrossRef]
- Alkadi, H.; Khubeiz, M.J.; Jbeily, R. Colchicine: A Review on Chemical Structure and Clinical Usage. Infect. Disord. - Drug Targets 2018, 18, 105–121. [CrossRef]
- Sonny Larsson, N.R. Reviewing Colchicaceae Alkaloids – Perspectives of Evolution on Medicinal Chemistry. Current Topics in Med. Chem. 2014, 14, 274-289.
- Ede Bodoki, D.B.; Săndulescu, R. Ab Initio study of the Na–colchicine positively charged complex. Farmacia Journal, 2015, 64.
- Gorabi, A.M.; Kiaie, N.; Reiner, .; Carbone, F.; Montecucco, F.; Sahebkar, A. The Therapeutic Potential of Nanoparticles to Reduce Inflammation in Atherosclerosis. Biomolecules 2019, 9, 416. [CrossRef]
- Ramírez, J.; Cañete, J.D. Anakinra for the treatment of rheumatoid arthritis: a safety evaluation. Expert Opin. Drug Saf. 2018, 17, 727–732. [CrossRef]
- Dubois, E.A.; Rissmann, R.; Cohen, A.F. Rilonacept and canakinumab. Br. J. Clin. Pharmacol. 2011, 71, 639–641. [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, 35. [CrossRef]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [CrossRef]
- Dobrzynska, M.; Napierala, M.; Florek, E. Flavonoid Nanoparticles: A Promising Approach for Cancer Therapy. Biomolecules 2020, 10, 1268. [CrossRef]
- Zverev, Y.F.; Rykunova, A.Y. Modern Nanocarriers as a Factor in Increasing the Bioavailability and Pharmacological Activity of Flavonoids. Appl. Biochem. Microbiol. 2022, 58, 1002–1020. [CrossRef]
- Ting, J.P.-Y.; Kastner, D.L.; Hoffman, H.M. CATERPILLERs, pyrin and hereditary immunological disorders. Nat. Rev. Immunol. 2006, 6, 183–195. [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002, 10(2), 4. [CrossRef]
- Jin, S.; Xia, X.; Huang, J.; Yuan, C.; Zuo, Y.; Li, Y.; Li, J. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. 2021, 127, 56–79. [CrossRef]
- Armenia, I.; Ayllón, C.C.; Herrero, B.T.; Bussolari, F.; Alfranca, G.; Grazú, V.; de la Fuente, J.M. Photonic and magnetic materials for on-demand local drug delivery. Adv. Drug Deliv. Rev. 2022, 191, 114584. [CrossRef]
- Zong, C.; Van Holm, W.; Bronckaers, A.; Zhao, Z.; Čokić, S.; Aktan, M.K.; Castro, A.B.; Van Meerbeek, B.; Braem, A.; Willems, G.; et al. Biomimetic Periodontal Ligament Transplantation Activated by Gold Nanoparticles Protects Alveolar Bone. Adv. Heal. Mater. 2023, 12, e2300328. [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [CrossRef]
- Ding, Q.; Cui, J.; Shen, H.; He, C.; Wang, X.; Shen, S.G.F.; Lin, K. Advances of nanomaterial applications in oral and maxillofacial tissue regeneration and disease treatment. WIREs Nanomed. Nanobiotechnology 2020, 13, e1669. [CrossRef]
- Feng, W.; Chen, Y. Chemoreactive nanomedicine. J. Mater. Chem. B 2020, 8, 6753–6764. [CrossRef]
- Lenders, V.; Koutsoumpou, X.; Sargsian, A.; Manshian, B.B. Biomedical nanomaterials for immunological applications: ongoing research and clinical trials. Nanoscale Adv. 2020, 2, 5046–5089. [CrossRef]
- Tiwari, N.; Osorio-Blanco, E.R.; Sonzogni, A.; Esporrín-Ubieto, D.; Wang, H.; Calderón, M. Nanocarriers for Skin Applications: Where Do We Stand? Angew. Chem. Int. Ed. Engl. 2022, 61, e202107960.
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: current progress and perspectives. J. Hematol. Oncol. 2021, 14, 1–27. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




