Submitted:
01 January 2024
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
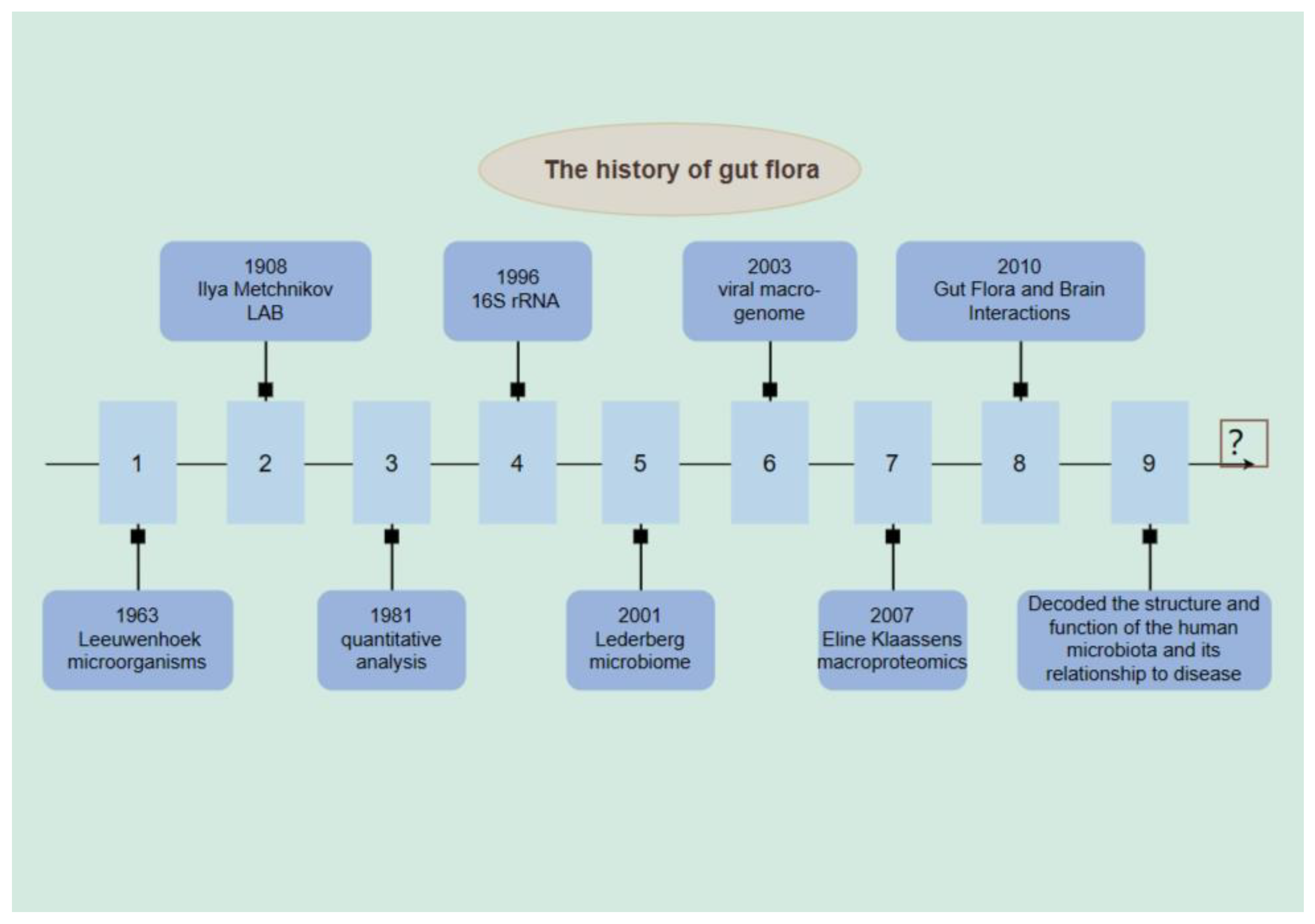
2. History of Intestinal Flora
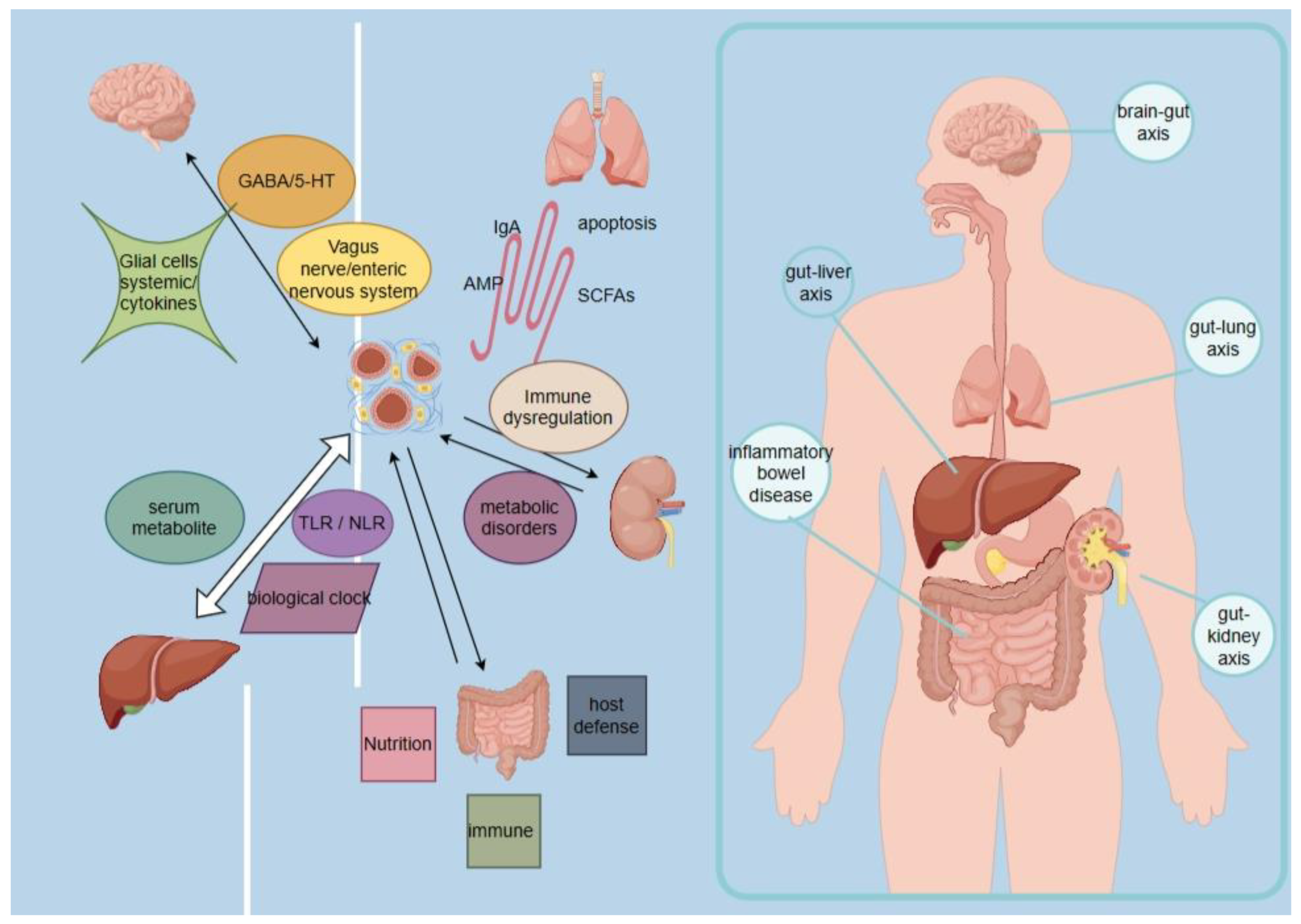
3. Mechanisms of Intestinal Flora in Disease
3.1. Gut Microbiota Regulates Brain Function through the "Gut-Brain Axis"
3.2. Intestinal Flora Regulates Liver Function through the "Intestinal-Liver Axis"
3.3. Gut flora Regulates Functions Related to Lung Diseases and Respiratory Infections through the Gut-Lung Axis (GLA)
3.4. Gut Flora Regulates Renal Function via the Gut-Renal Axis
3.5. Dysbiosis of Intestinal Flora Affects Inflammatory Bowel Disease
4. The Role of Gut Flora in Tumors
4.1. Oral Cancer (OC) and Intestinal Flora
4.2. Nasopharyngeal Carcinoma (NPC) and Intestinal flora
4.3. Thyroid Cancer (TC) and Intestinal Flora
4.4. Glioma and Intestinal Flora
5. Gut Flora in Head and Neck Tumors
6. Summary and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021, 19, 55-71. [CrossRef]
- Fujisaka S, Avila-Pacheco J, Soto M, et al. Diet, Genetics, and the Gut Microbiome Drive Dynamic Changes in Plasma Metabolites. Cell Rep. 2018, 22, 3072-3086. [CrossRef]
- Brown RL, Larkinson M, Clarke TB. Immunological design of commensal communities to treat intestinal infection and inflammation. PLoS Pathog. 2021, 17, e1009191. [CrossRef] [PubMed]
- Caballero S, Pamer EG. Microbiota-mediated inflammation and antimicrobial defense in the intestine. Annu Rev Immunol. 2015, 33: 227-56. [CrossRef]
- Fessler J, Matson V, Gajewski TF. Exploring the emerging role of the microbiome in cancer immunotherapy. J Immunother Cancer. 2019, 7, 108. [CrossRef] [PubMed]
- Hatakeyama M. Structure and function of Helicobacter pylori CagA, the first-identified bacterial protein involved in human cancer. Proc Jpn Acad Ser B Phys Biol Sci. 2017, 93, 196-219. [CrossRef]
- Goodwin AC, Destefano Shields CE, Wu S, et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc Natl Acad Sci U S A. 2011, 108, 15354-9. [CrossRef]
- Bergounioux J, Elisee R, Prunier AL, et al. Calpain activation by the Shigella flexneri effector VirA regulates key steps in the formation and life of the bacterium's epithelial niche. Cell Host Microbe. 2012, 11, 240-52. [CrossRef]
- Chaturvedi R, Asim M, Romero-Gallo J, et al. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology. 2011, 141, 1696-708.e1-2. [CrossRef]
- Kuugbee ED, Shang X, Gamallat Y, et al. Structural Change in Microbiota by a Probiotic Cocktail Enhances the Gut Barrier and Reduces Cancer via TLR2 Signaling in a Rat Model of Colon Cancer. Dig Dis Sci. 2016, 61, 2908-2920. [CrossRef]
- Johnson DB, Frampton GM, Rioth MJ, et al. Targeted Next Generation Sequencing Identifies Markers of Response to PD-1 Blockade. Cancer Immunol Res. 2016, 4, 959-967. [CrossRef]
- Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015, 350, 1084-9. [CrossRef] [PubMed]
- Porter, J.R. Antony van Leeuwenhoek: tercentenary of his discovery of bacteria. Bacteriol Rev. 1976, 40, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Majem LS, Marcos AMR, Guardia JAM, Batrina JA, Marcos A, Anta RMO. Alimentos Funcionales. Probióticos. 2002.
- Bourdelles F, Avril JL, Ghnassia JC. [Quantitative study of the faecal flora of breast- or bottle-fed neonates (author's transl)]. Archives franaises de pédiatrie. 1981, 38, 35-39.
- Ursell LK, Haiser HJ, Van Treuren W, et al. The intestinal metabolome: an intersection between microbiota and host. Gastroenterology. 2014, 146, 1470-6. [CrossRef] [PubMed]
- Brown AL, Tucker B, Baker LR, Raine AE. Seizures related to blood transfusion and erythropoietin treatment in patients undergoing dialysis. Bmj British Medical Journal. 1989, 299, 1258-1259. [CrossRef]
- Wilson KH, Blitchington RB. Human colonic biota studied by ribosomal DNA sequence analysis. Appl Environ Microbiol. 1996, 62, 2273-2278. [CrossRef]
- Lederberg, J. Infectious History. SCIENCE. 2000, 288, 287–293. [Google Scholar] [CrossRef]
- Breitbart M, Hewson I, Felts B, et al. Bacteriophages, transposons, and plasmids: metagenomic analyses of an uncultured viral community from human feces. JOURNAL OF BACTERIOLOGY. 2003, 185, 6220-6223. [CrossRef]
- Klaassens ES, De Vos WM, Vaughan EE. Metaproteomics Approach To Study the Functionality of the Microbiota in the Human Infant Gastrointestinal Tract. Applied & Environmental Microbiology. 2007, 73, 1388-92. [CrossRef]
- Heijtz RD, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences. 2011, 108, 3047-3052. [CrossRef]
- Sebastián Domingo JJ, Sánchez Sánchez C. From the intestinal flora to the microbiome. Rev Esp Enferm Dig. 2018, 110, 51-56. [CrossRef]
- Cryan JF, O'Riordan KJ, Cowan C, Sandhu KV, Dinan TG. The Microbiota-Gut-Brain Axis. PHYSIOLOGICAL REVIEWS. 2019, 99, 1877-2013.
- Fang P, Kazmi SA, Jameson KG, Hsiao EY. The Microbiome as a Modifier of Neurodegenerative Disease Risk. Cell Host & Microbe. 2020. [CrossRef]
- Morais LH, Henry IV, Mazmanian SK. The gut microbiota–brain axis in behaviour and brain disorders. NATURE REVIEWS MICROBIOLOGY. [CrossRef]
- Fluckiger A, Daillère R, Sassi M, Sixt BS, Zitvogel L. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. SCIENCE. 2020, 369, 936-942. [CrossRef]
- Abbott, A. Are infections seeding some cases of Alzheimer's disease. NATURE. 2020, 587, 22–25. [Google Scholar] [CrossRef]
- Farhangi MA, Vajdi M. Gut microbiota–associated trimethylamine N ﹐xide and increased cardiometabolic risk in adults: a systematic review and dose-response meta-analysis. NUTRITION REVIEWS. 2020. [CrossRef]
- Wang T, Rong X, Zhao C. Circadian Rhythms Coordinated With Gut Microbiota Partially Account for Individual Differences in Hepatitis B-Related Cirrhosis. Front Cell Infect Microbiol. 2022, 12: 936815. [CrossRef]
- Oikonomou T, Papatheodoridis GV, Samarkos M, Goulis I, Cholongitas E. Clinical impact of microbiome in patients with decompensated cirrhosis. WORLD JOURNAL OF GASTROENTEROLOGY. 2018. [CrossRef] [PubMed]
- Bauer KC, Littlejohn PT, Ayala V, Creus-Cuadros A, Finlay BB. Nonalcoholic Fatty Liver Disease and the Gut-Liver Axis: Exploring an Undernutrition Perspective. Gastroenterology. 2022, 162, 1858-1875.e2. [CrossRef] [PubMed]
- Lifeng L, Yunhai Y, Yunyun L, Junwei Q, Xuejing L, Gengyun Z. A Comprehensive Genome Survey Provides Novel Insights into Bile Salt Hydrolase (BSH) in Lactobacillaceae. MOLECULES. 2018, 23, 1157.
- Arab JP, Martin-Mateos RM, Shah VH. Gut–liver axis, cirrhosis and portal hypertension: the chicken and the egg. Hepatology International. 2017. [CrossRef]
- Lucie, Etienne-Mesmin, Andrew, et al. Microbiota-liver axis in hepatic disease. Hepatology Official Journal of the American Association for the Study of Liver Diseases. 2014. [CrossRef]
- John II, Behrendt CL, Ruhn KA, et al. The microbiota coordinates diurnal rhythms in innate immunity with the circadian clock. CELL. 2021, (184-16). [CrossRef]
- Mjösberg J, Rao A. Lung inflammation originating in the gut. Science. 2018, 359, 36-37. [CrossRef]
- Lai HC, Lin TL, Chen TW, et al. Gut microbiota modulates COPD pathogenesis: role of anti-inflammatory Parabacteroides goldsteinii lipopolysaccharide. Gut. 2022, 71, 309-321. [CrossRef]
- Yun KY, Zuo T, Lui CY, Zhang F, Ng SC. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. GUT. 70, 698-706.
- Gill N, Wlodarska M, Finlay BB. The future of mucosal immunology: studying an integrated system-wide organ. Nat Immunol. 2010, 11, 558-60.
- Anand S, Mande SS. Diet, Microbiota and Gut-Lung Connection. Front Microbiol. 2018, 9: 2147. [CrossRef]
- McGhee JR, Fujihashi K. Inside the mucosal immune system. PLoS Biol. 2012, 10, e1001397. [CrossRef] [PubMed]
- Dang AT, Marsland BJ. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019, 12, 843-850. [CrossRef] [PubMed]
- Perrone EE, Jung E, Breed E, et al. Mechanisms of methicillin-resistant Staphylococcus aureus pneumonia-induced intestinal epithelial apoptosis. Shock. 2012, 38, 68-75. [CrossRef]
- Yang T, Richards EM, Pepine CJ, Raizada MK. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol. 2018, 14, 442-456. [CrossRef] [PubMed]
- Wang X, Yang S, Li S, et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut. 2020, 69, 2131-2142. [CrossRef] [PubMed]
- Sirich TL, Plummer NS, Gardner CD, Hostetter TH, Meyer TW. Effect of Increasing Dietary Fiber on Plasma Levels of Colon-Derived Solutes in Hemodialysis Patients. Clinical Journal of the American Society of Nephrology. 2014, 9(9). [CrossRef]
- Plummer NS, Holmes S, Meyer TW, et al. Colonic contribution to uremic solutes. 2013. [CrossRef] [PubMed]
- Yan J, Herzog JW, Tsang K, et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U S A. 2016, 113, E7554-E7563. [CrossRef] [PubMed]
- Hahm E, Wei C, Fernandez I, et al. Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease. Nat Med. 2017, 23, 100-106. [CrossRef] [PubMed]
- Schumacher M, Wanner C, Beyersmann J. Soluble Urokinase Receptor and Chronic Kidney Disease. NEW ENGLAND JOURNAL OF MEDICINE. 2016, 374, 890-891.
- Sirich TL, Aronov PA, Plummer NS, Hostetter TH, Meyer TW. Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int. 2013, 84, 585-90. [CrossRef]
- Ito S, Yoshida M. Protein-bound uremic toxins: new culprits of cardiovascular events in chronic kidney disease patients. Toxins (Basel). 2014, 6, 665-78. [CrossRef] [PubMed]
- Koppe L, Pillon NJ, Vella RE, et al. p-Cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol. 2013, 24, 88-99. [CrossRef] [PubMed]
- Marchesi JR, Adams DH, Fava F, Hermes G, Hart A. The gut microbiota and host health. 2015. [CrossRef] [PubMed]
- LeBlanc JG, Laiño JE, del Valle MJ, et al. B-group vitamin production by lactic acid bacteria--current knowledge and potential applications. J Appl Microbiol. 2011, 111, 1297-309. [CrossRef] [PubMed]
- Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek. 2020, 113, 2019-2040. [CrossRef] [PubMed]
- Kamada N, Núñez G. Regulation of the immune system by the resident intestinal bacteria. Gastroenterology. 2014, 146, 1477-88. [CrossRef]
- Sekirov I, Russell SL, Antunes L, Finlay BB. Gut microbiota in health and disease. PHYSIOLOGICAL REVIEWS. 2010, 90, 859-904. [CrossRef]
- Kamada N, Kim YG, Sham HP, et al. Regulated Virulence Controls the Ability of a Pathogen to Compete with the Gut Microbiota. SCIENCE. 2012, 336, 1325-9. [CrossRef] [PubMed]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. NATURE. 2006, 444, 1022-1023. [CrossRef]
- Chen Y, Zhou J, Wang L. Role and Mechanism of Gut Microbiota in Human Disease. Front Cell Infect Microbiol. 2021, 11: 625913. [CrossRef]
- Bai X, Wei H, Liu W, et alBai X, Wei H, Liu W, et al. Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites. Gut. 2022, 71, 2439-2450. [CrossRef]
- Chen W, Wen L, Bao Y, et al. Gut flora disequilibrium promotes the initiation of liver cancer by modulating tryptophan metabolism and up-regulating SREBP2. Proc Natl Acad Sci U S A. 2022, 119, e2203894119. [CrossRef]
- Chen S, Gui R, Zhou XH, et al. Combined Microbiome and Metabolome Analysis Reveals a Novel Interplay Between Intestinal Flora and Serum Metabolites in Lung Cancer. Front Cell Infect Microbiol. 2022, 12: 885093. [CrossRef]
- Rodríguez-Molinero J, Migueláñez-Medrán B, Puente-Gutiérrez C, et al. Association between Oral Cancer and Diet: An Update. Nutrients. 2021, 13(4). [CrossRef]
- Sklenicka S, Gardiner S, Dierks EJ, Potter BE, Bell RB. Survival analysis and risk factors for recurrence in oral squamous cell carcinoma: does surgical salvage affect outcome. J Oral Maxillofac Surg. 2010, 68, 1270-5. [CrossRef] [PubMed]
- Perera M, Al-Hebshi NN, Speicher DJ, Perera I, Johnson NW. Emerging role of bacteria in oral carcinogenesis: a review with special reference to perio-pathogenic bacteria. J Oral Microbiol. 2016, 8: 32762. [CrossRef]
- Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021, 21, 426-440. [CrossRef] [PubMed]
- Oliva M, Schneeberger P, Rey V, et al. Transitions in oral and gut microbiome of HPV+ oropharyngeal squamous cell carcinoma following definitive chemoradiotherapy (ROMA LA-OPSCC study). Br J Cancer. 2021, 124, 1543-1551. [CrossRef] [PubMed]
- Nocini R, Muzio LL, Gibellini D, et al. Oral microbiota in oropharyngeal cancers: Friend or foe. Front Oncol. 2022, 12: 948068. [CrossRef]
- Xu AA, Hoffman K, Gurwara S, et al. Oral Health and the Altered Colonic Mucosa-Associated Gut Microbiota. Dig Dis Sci. 2021, 66, 2981-2991. [CrossRef] [PubMed]
- He YQ, Wang TM, Ji M, et al. A polygenic risk score for nasopharyngeal carcinoma shows potential for risk stratification and personalized screening. Nat Commun. 2022, 13, 1966. [CrossRef] [PubMed]
- Peters M, Meijer C, Fehrmann R, et al. Serotonin and Dopamine Receptor Expression in Solid Tumours Including Rare Cancers. Pathol Oncol Res. 2020, 26, 1539-1547. [CrossRef] [PubMed]
- Senda S, Fujiyama Y, Ushijima T, et al. Clostridium ramosum, an IgA protease-producing species and its ecology in the human intestinal tract. Microbiol Immunol. 1985, 29, 1019-28.
- Mandić AD, Woting A, Jaenicke T, et al. Clostridium ramosum regulates enterochromaffin cell development and serotonin release. Sci Rep. 2019, 9, 1177. [CrossRef]
- Jiang H, Li J, Zhang B, et al. Intestinal Flora Disruption and Novel Biomarkers Associated With Nasopharyngeal Carcinoma. Front Oncol. 2019, 9: 1346. [CrossRef]
- Guo H, Chou WC, Lai Y, et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science. 2020, 370(6516). [CrossRef]
- Yu ZK, Xie RL, You R, et al. The role of the bacterial microbiome in the treatment of cancer. BMC Cancer. 2021, 21, 934. [CrossRef]
- Gu Y, Yu Y, Ai L, et al. Association of the ATM gene polymorphisms with papillary thyroid cancer. Endocrine. 2014, 45, 454-61. [CrossRef] [PubMed]
- Lerner A, Jeremias P, Matthias T. Gut-thyroid axis and celiac disease. Endocr Connect. 2017, 6, R52-R58. [CrossRef] [PubMed]
- Vought RL, Brown FA, Sibinovic KH, McDaniel EG. Effect of changing intestinal bacterial flora on thyroid function in the rat. Horm Metab Res. 1972, 4, 43-7. [CrossRef] [PubMed]
- Yao Z, Zhao M, Gong Y, et al. Relation of Gut Microbes and L-Thyroxine Through Altered Thyroxine Metabolism in Subclinical Hypothyroidism Subjects. Front Cell Infect Microbiol. 2020, 10: 495. [CrossRef]
- DiStefano JJ 3rd, de Luze A, Nguyen TT. Binding and degradation of 3,5,3'-triiodothyronine and thyroxine by rat intestinal bacteria. Am J Physiol. 1993, 264(6 Pt 1): E966-72. [CrossRef]
- Shen CT, Zhang Y, Liu YM, et al. A distinct serum metabolic signature of distant metastatic papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2017, 87, 844-852. [CrossRef] [PubMed]
- Feng J, Zhao F, Sun J, et al. Alterations in the gut microbiota and metabolite profiles of thyroid carcinoma patients. Int J Cancer. 2019, 144, 2728-2745. [CrossRef]
- Lu G, Yu X, Jiang W, et al. Alterations of Gut Microbiome and Metabolite Profiles Associated With Anabatic Lipid Dysmetabolism in Thyroid Cancer. Front Endocrinol (Lausanne). 2022, 13: 893164. [CrossRef]
- Yano Y, Matsui T, Uno H, Hirai F, Futami K, Iwashita A. Risks and clinical features of colorectal cancer complicating Crohn's disease in Japanese patients. J Gastroenterol Hepatol. 2008, 23, 1683-8. [CrossRef] [PubMed]
- Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005, 308, 1635-8. [CrossRef] [PubMed]
- Aghajani MJ, Cooper A, McGuire H, et al. Pembrolizumab for anaplastic thyroid cancer: a case study. Cancer Immunol Immunother. 2019, 68, 1921-1934. [CrossRef] [PubMed]
- Asghari A, Umetani M. Obesity and Cancer: 27-Hydroxycholesterol, the Missing Link. Int J Mol Sci. 2020, 21(14). [CrossRef]
- Smith, PA. The tantalizing links between gut microbes and the brain. Nature. 2015, 526, 312–4. [Google Scholar] [CrossRef]
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The Central Nervous System and the Gut Microbiome. Cell. 2016, 167, 915-932. [CrossRef]
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. FRONTIERS IN NEUROENDOCRINOLOGY. 2018 : S0091302218300396. [CrossRef]
- Erny D, Hrabě de Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015, 18, 965-77. [CrossRef]
- Davis, ME. Glioblastoma: Overview of Disease and Treatment. Clin J Oncol Nurs. 2016, 20(5 Suppl): S2-8. [CrossRef]
- Routy B, Gopalakrishnan V, Daillère R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol. 2018, 15, 382-396. [CrossRef] [PubMed]
- Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018, 359, 104-108. [CrossRef] [PubMed]
- Patrizz A, Dono A, Zorofchian S, et al. Glioma and temozolomide induced alterations in gut microbiome. Sci Rep. 2020, 10, 21002. [CrossRef] [PubMed]
- Farias K, Moreli ML, Floriano VG, da Costa VG. Evidence based on a meta-analysis of human cytomegalovirus infection in glioma. Arch Virol. 2019, 164, 1249-1257. [CrossRef] [PubMed]
- Hu M, Yu B, Zhang B, et al. Human Cytomegalovirus Infection Activates Glioma Activating Transcription Factor 5 via microRNA in a Stress-Induced Manner. ACS Chem Neurosci. 2021, 12, 3947-3956. [CrossRef] [PubMed]
- Egan KM, Kim Y, Bender N, et al. Prospective investigation of polyomavirus infection and the risk of adult glioma. Sci Rep. 2021, 11, 9642. [CrossRef]
- Jiang H, Zeng W, Zhang X, Pei Y, Zhang H, Li Y. The role of gut microbiota in patients with benign and malignant brain tumors: a pilot study. Bioengineered. 2022, 13, 7847-7859. [CrossRef] [PubMed]
- Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017, 17, 271-285. [CrossRef] [PubMed]
- Lakritz JR, Poutahidis T, Levkovich T, et al. Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice. Int J Cancer. 2014, 135, 529-40. [CrossRef]
- Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015, 350, 1079-84. [CrossRef] [PubMed]
- Daillère R, Vétizou M, Waldschmitt N, et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity. 2016, 45, 931-943. [CrossRef] [PubMed]
- Yang JB, Zhu DQ, Shao M, et al. Effects of Shengmai Jianghuang San on intestinal flora in nude mice with radio resistant cells of nasopharyngeal carcinoma. Zhongguo Zhong Yao Za Zhi. 2019, 44, 553-558. [CrossRef]
- Jiang C, Wang H, Xia C, et al. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer. 2019, 125, 1081-1090. [CrossRef] [PubMed]
- Xia C, Jiang C, Li W, et al. A Phase II Randomized Clinical Trial and Mechanistic Studies Using Improved Probiotics to Prevent Oral Mucositis Induced by Concurrent Radiotherapy and Chemotherapy in Nasopharyngeal Carcinoma. Front Immunol. 2021, 12: 618150. [CrossRef]
- Zheng L, Zhang L, Tang L, et al. Gut microbiota is associated with response to (131)I therapy in patients with papillary thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2023, 50, 1453-1465. [CrossRef] [PubMed]
- Lin B, Zhao F, Liu Y, et al. Randomized Clinical Trial: Probiotics Alleviated Oral-Gut Microbiota Dysbiosis and Thyroid Hormone Withdrawal-Related Complications in Thyroid Cancer Patients Before Radioiodine Therapy Following Thyroidectomy. Front Endocrinol (Lausanne). 2022, 13: 834674. [CrossRef]
- Cheng SY, Chen NF, Kuo HM, et al. Prodigiosin stimulates endoplasmic reticulum stress and induces autophagic cell death in glioblastoma cells. Apoptosis. 2018, 23(5-6): 314-328. [CrossRef]
- Song D, Liang H, Qu B, et al. Moxidectin inhibits glioma cell viability by inducing G0/G1 cell cycle arrest and apoptosis. Oncol Rep. 2018, 40, 1348-1358. [CrossRef] [PubMed]
- Pang Z, Gu MD, Tang T. Pseudomonas aeruginosa in Cancer Therapy: Current Knowledge, Challenges and Future Perspectives. Front Oncol. 2022, 12: 891187. [CrossRef]
- Zhang M, Lu W. Enhanced glioma-targeting and stability of (L)GICP peptide coupled with stabilized peptide (D)A7R. Acta Pharm Sin B. 2018, 8, 106-115. [CrossRef]
- Al-Obaidi M, Desa M. Mechanisms of Blood Brain Barrier Disruption by Different Types of Bacteria, and Bacterial-Host Interactions Facilitate the Bacterial Pathogen Invading the Brain. Cell Mol Neurobiol. 2018, 38, 1349-1368. [CrossRef]
- Suryawanshi YR, Schulze AJ. Oncolytic Viruses for Malignant Glioma: On the Verge of Success. Viruses. 2021, 13(7). [CrossRef]
- Kim H, Roh HS, Kim JE, Park SD, Park WH, Moon JY. Compound K attenuates stromal cell-derived growth factor 1 (SDF-1)-induced migration of C6 glioma cells. Nutr Res Pract. 2016, 10, 259-64. [CrossRef]
- Hung AL, Garzon-Muvdi T, Lim M. Biomarkers and Immunotherapeutic Targets in Glioblastoma. World Neurosurg. 2017, 102: 494-506. [CrossRef]
- D'Alessandro G, Antonangeli F, Marrocco F, et al. Gut microbiota alterations affect glioma growth and innate immune cells involved in tumor immunosurveillance in mice. Eur J Immunol. 2020, 50, 705-711. [CrossRef]
- Hou X, Du H, Deng Y, et al. Gut microbiota mediated the individualized efficacy of Temozolomide via immunomodulation in glioma. J Transl Med. 2023, 21, 198. [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



