1. Introduction
Regenerative surgery is the preferred method for addressing residual intrabony periodontal defects after non-surgical periodontal therapy, as recommended by the guidelines for treating periodontitis stages 1 to 3 [
1]. In a systematic review and meta-analysis, regenerative strategies using enamel matrix derivative (EMD) or guided tissue regeneration (GTR) were found to be more effective than open flap debridement (OFD) in terms of clinical attachment level (CAL) gain and reduction of probing depth (PD). These strategies showed a significant improvement in CAL gain and PD reduction once implemented [
2]. The overall superiority was expressed by 1.27 mm greater CAL gain achieved with EMD and 1.43 mm achieved with GTR. Furthermore, this systematic review recommended the use of bone substitutes for intrabony defects with severely reduced bone walls to stabilize soft tissue and prevent collapse [
2]. Moreover, space maintenance is crucial for both, blood clot and tissue formation, according to established GTR principles [
3,
4].
A meta-analysis calculated the effect named “pocket closure” on behalf of 12 published RCTs addressing the efficacy of either GTR or EMD over OFD. The results revealed a 61.4% rate of closure looking at sites with ≤3mm residual probing depth (PD) whereas 92.1% closure rate was apparent once considering sites with a residual PD≤4mm after 12 months post-op period [
5].
In the recent years, a new agent proved sufficient in periodontal regeneration after its beneficial role in soft tissue healing has been demonstrated before. Studies conducted in vitro, pre-clinically, and as clinical case series documented the sufficient contribution of adjunctively applied hyaluronic acid to the cell and tissue reactions. In particular, the cross-linked heavy molecular weight hyaluronic acid (xHyA) showed sufficient enhancement in soft tissue healing in donor sites for the retrieval of free gingival grafts from the palate [
6]. The same formulation effectively supported soft tissue flap stabilization in recession coverage procedures carried out in an animal model as well as in patients [
7,
8]. The periodontal healing of surgically created intrabony defects was superior regarding newly formed periodontal ligament and new cementum according to histomorphometrical evaluation in a pre-clinical study by Shirakata et al. [
9]. This group repeated the experiment by creating acute furcation grade 3 defects using the same dog model and confirmed the results from the previous study for the xHyA-treated defects [
10]. A randomized clinical trial investigating three-wall intrabony defects further demonstrated non-inferiority of xHyA treated sites compared to EMD use for surgical regenerative treatment regarding CAL gain and PD reduction outcome after 24 months of follow-up [
11]. Moreover, Bozic et al. achieved >90% pocket closure rate by surgically applying xHyA with porcine particulate xenograft after 12 months of healing [
12].
Apart from clinical studies, the interaction between xHyA and fibroblasts derived from periodontal ligament was elucidated by an in vitro experiment performed on dentin discs [
13]. Another experimental study reported that the presence of xHyA on collagen substrates enhanced the gene transcription rate for bone-related proteins by osteoblast-like cells in vitro [
14]. An in vitro study showing HA impact on transcription rate of the specific mRNAs encoding for cementoblast differentiation and on their enhanced proliferation was just released [
15].
However, while the positive effects of abovementioned biologics were evident, clinical studies comparing the effects contributed by EMD or xHyA to regenerative surgical treatment of intrabony periodontal defects still need to be included. In this retrospective study, we investigated the outcomes of the two bioactive materials in combination with different adjunctive biomaterials for surgical regenerative treatment of deep intrabony defects over 12 months.
2. Materials and Methods
Patients recruited were routinely treated periodontitis patients presenting with the diagnosis of stage 3 or 4 periodontitis regardless of their grading (Caton et al., 2018). In all three centers, patients underwent a course of systematic subgingival instrumentation according to recommendations from Guidelines of the EFP concerning steps 1 and 2 before surgery schedule [
16]. The regenerative approach was favored once the re-evaluation values justified for a step 3 surgical therapy of residual pockets, i.e., with a PPD exceeding 6mm with or without BoP.
Principal investigators represented by A.B. for Center 1 (C1), M.E. for Center 2 (C2), and A.F. for Center 3 (C3) were calibrated regarding the surgical technique applied, data evaluation, and inclusion criteria for approaching the residual pockets. The modified papilla preservation incision design [
2,
17], full-flap elevation, releasing incision for coronal flap advancement as well as meticulous instrumentation of the root surface and thorough degranulation were uniformly agreed by surgical protocol. The protocol standardized neither the type of suture nor the suture technique. There were no restrictions regarding the way and type of instrumentation of the defects, i.e., ultrasonic or piezo devices were used as well as hand instruments. After completing thorough instrumentation, the defects received biomaterials considered supportive for regenerative healing. Each group was free to choose the biomaterial combination for regenerative surgery by its own preference. The Ethic Committee of Witten/Herdecke University approved the retrospective analysis of the data set from the three centers (S-203/2021, amendment from 2023).
Each operator assessed clinical parameters (PPD, CAL, BoP and GR for recession) by means of the manual periodontal probe on a regular base during supportive periodontal therapy (SPT) visits. Clinically assessed values as well as data regarding defect intrabony defect depth, defect angle, and defect wall number at baseline (prior to surgery) and after a period of 12 months were reported. The radiographical findings were assessed on periapical 2-D radiographs obtained digitally in parallel technique using a sensor holder (Sidexis, Sirona, Bensheim, Germany) at baseline and 12 months post-op at each center. The calculation of tissue alterations revealed by comparison of both radiographs is reported by each center itself.
Center 1 used the combination of xHyA (HyaDent BG, Regedent, Zürich, Switzerland) and a bovine collagen enhanced by hydroxyapatite particles (Collapat II, Symatese, Fr). Center 2 applied a combination of EMD (Emdogain, Straumann Group, IL) and an allograft containing 50% cancellous and 50% cortical Allograft bone (LifeNet Health, USA). Both centers applied the materials into the intrabony pocket closing the site by coronally repositioning soft tissue flap; both centers discarded the use of a membrane. Center 3 used xHyA alone for filling the intrabony defect component, placing a poly-lactic poly-lactid polymer membrane (Guidor matrix barrier, Sunstar, Germany) at the crest of the alveolar ridge before closing the site by soft tissue flap in the similar way as both other centers did.
The post-op regimen included pain medication, irrigation with CHX for a duration of 2 weeks, and local topical use of CHX gel for several weeks following suture removal. Each center was responsible for the choice of systemically administrated antibiotics for every case.
For metrical variables, e.g. PPD, CAL and Recession descriptive statistics including mean, standard deviation, median range and percentages were applied to summarize the sample data. Differences between groups were calculated using Analysis of Covariance (ANCOVA) or Chi-Square-Test in the case of nominal data with intra-osseous depth and defect angle and wall number as covariates. For pairwise group comparisons, Bonferoni post hoc test was used. A two tailed significance level of α=5% was applied for all analyses.
3. Results
All three centers recorded and reported uncomplicated healing. All patients were compliant with the SPT program and appeared at individual interval for re-evaluation and cleaning visits. At one-year reevaluation, all patients from three centers demonstrated significantly improved clinical parameters and positive alterations in crestal bone height followed up radiographically.
C1 enrolled 18 patients with 19 treated defects, C2 accounted for 21 patients with an equal number of treated teeth, and C3 enclosed 15 patients with 15 teeth and sites to treat, respectively. The homogeneity in patient age and defect morphology included in the three centers was confirmed by non-significant differences of defect angle, intrabony depth component, number of defect walls, initial probing depth (PD), and clinical attachment loss (CAL) loss. Furthermore, the age, gender and smoking habits were similarly distributed among the patients from each center (
Table 1). The correlation between the outcome and the radiographic defect diminution (RDD/∆Defect Fill) outcome was statistically significant only for the baseline value and the intraosseous defect component (P<0.001), not significant were the initial number of bony walls (P=0.174) or the defect angle (P=0.843). Moreover, all groups exhibited similar distribution of morphologic defect characteristics (
Table 1).
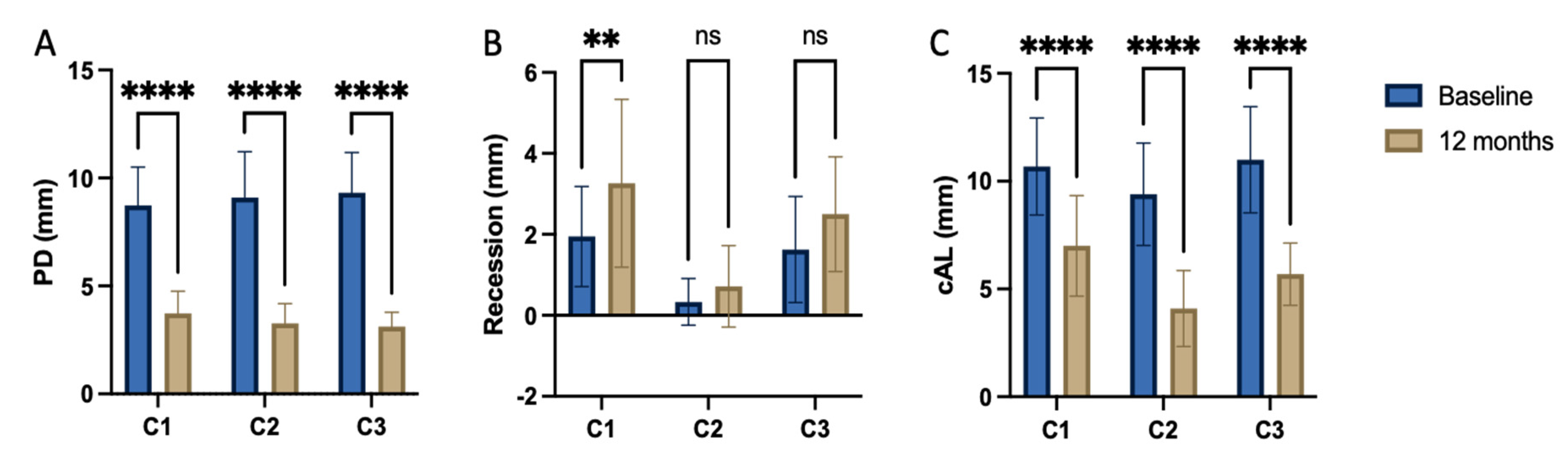
While the PD reduction was similarly effective in all three Centers (
Table 2), the inner group comparison revealed statistically significant difference in attachment level gain reported by C1, C2 and C3, (P<0.001, respectively) (
Table 2;
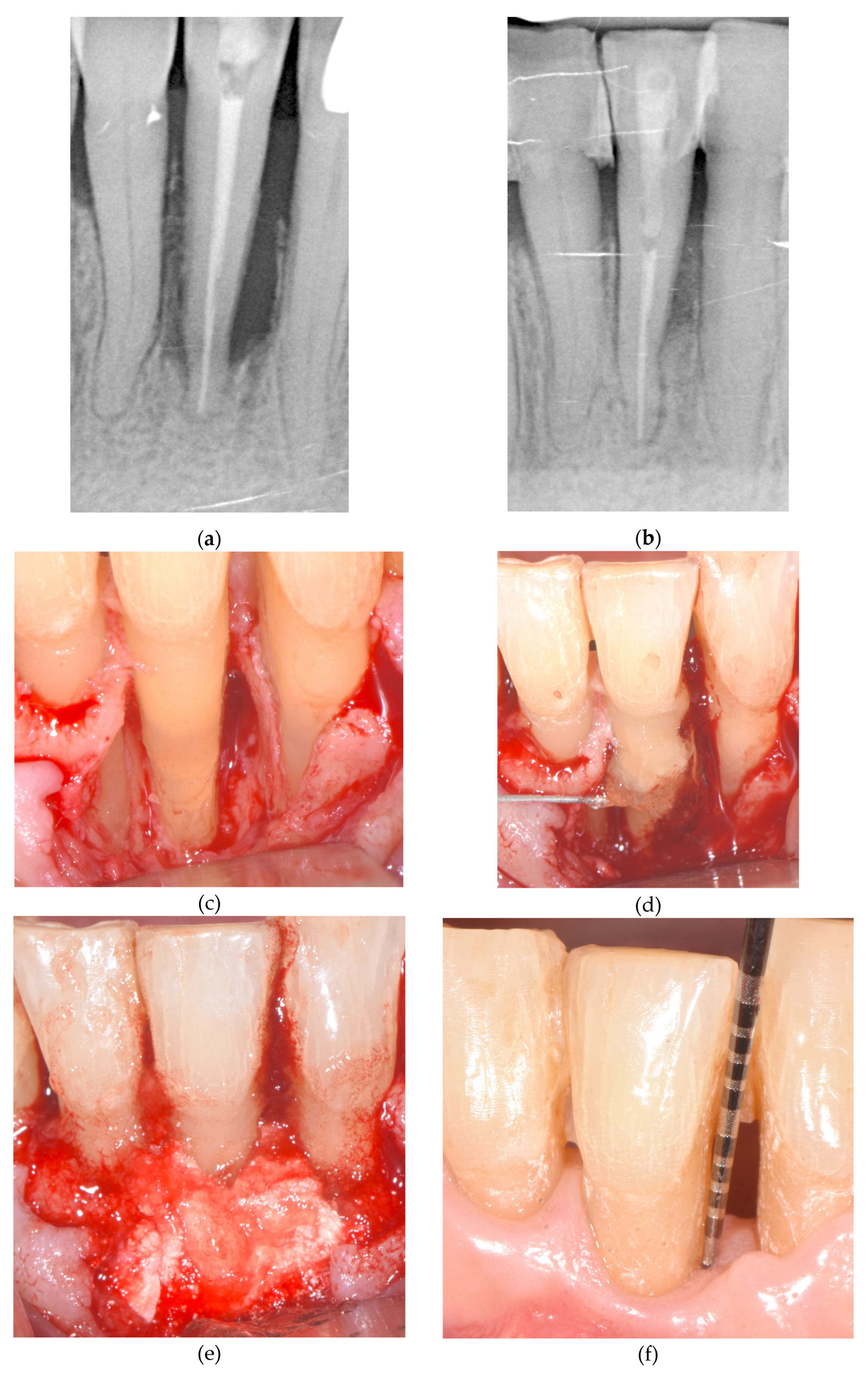
Figure 1). The
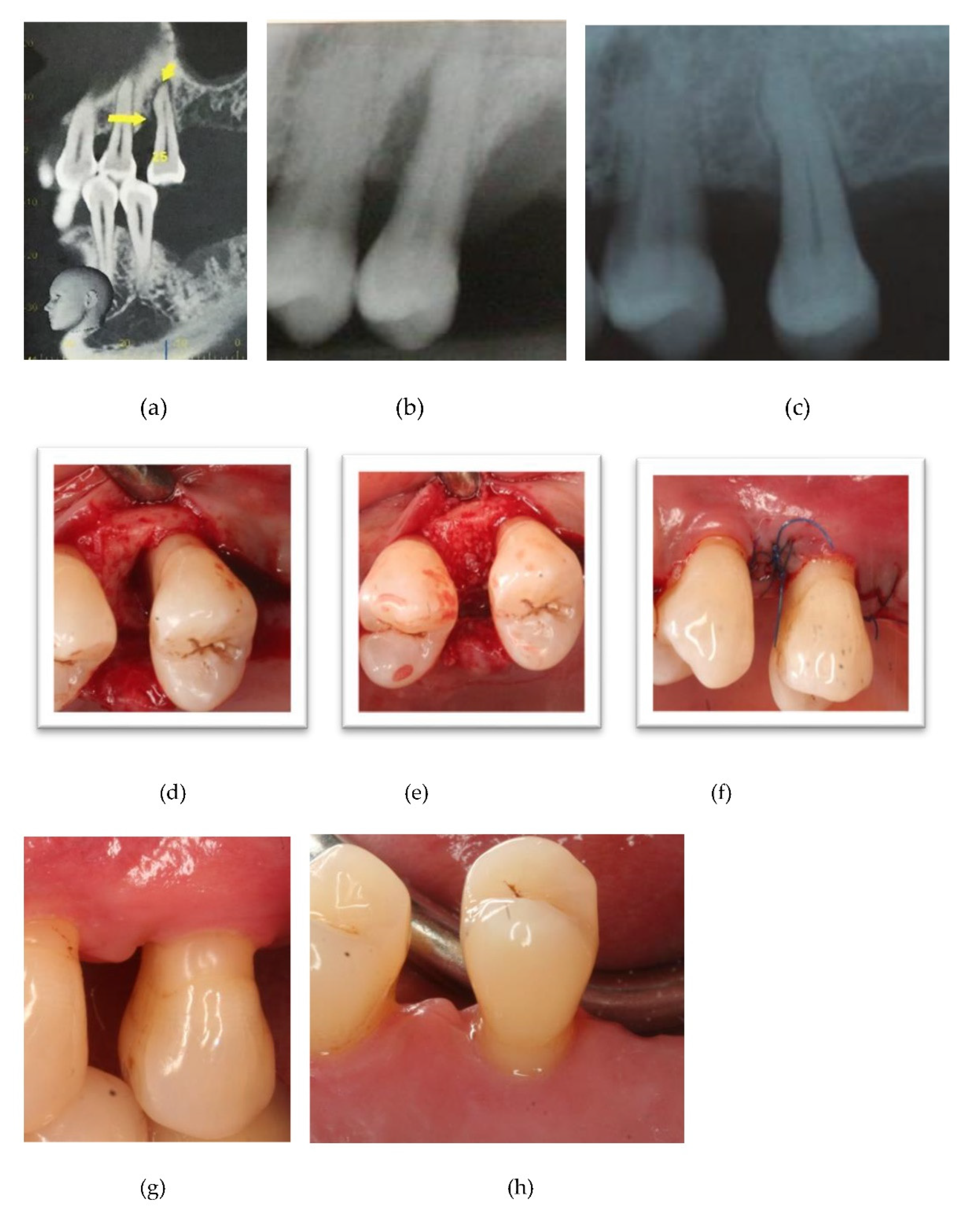
Figure 2,
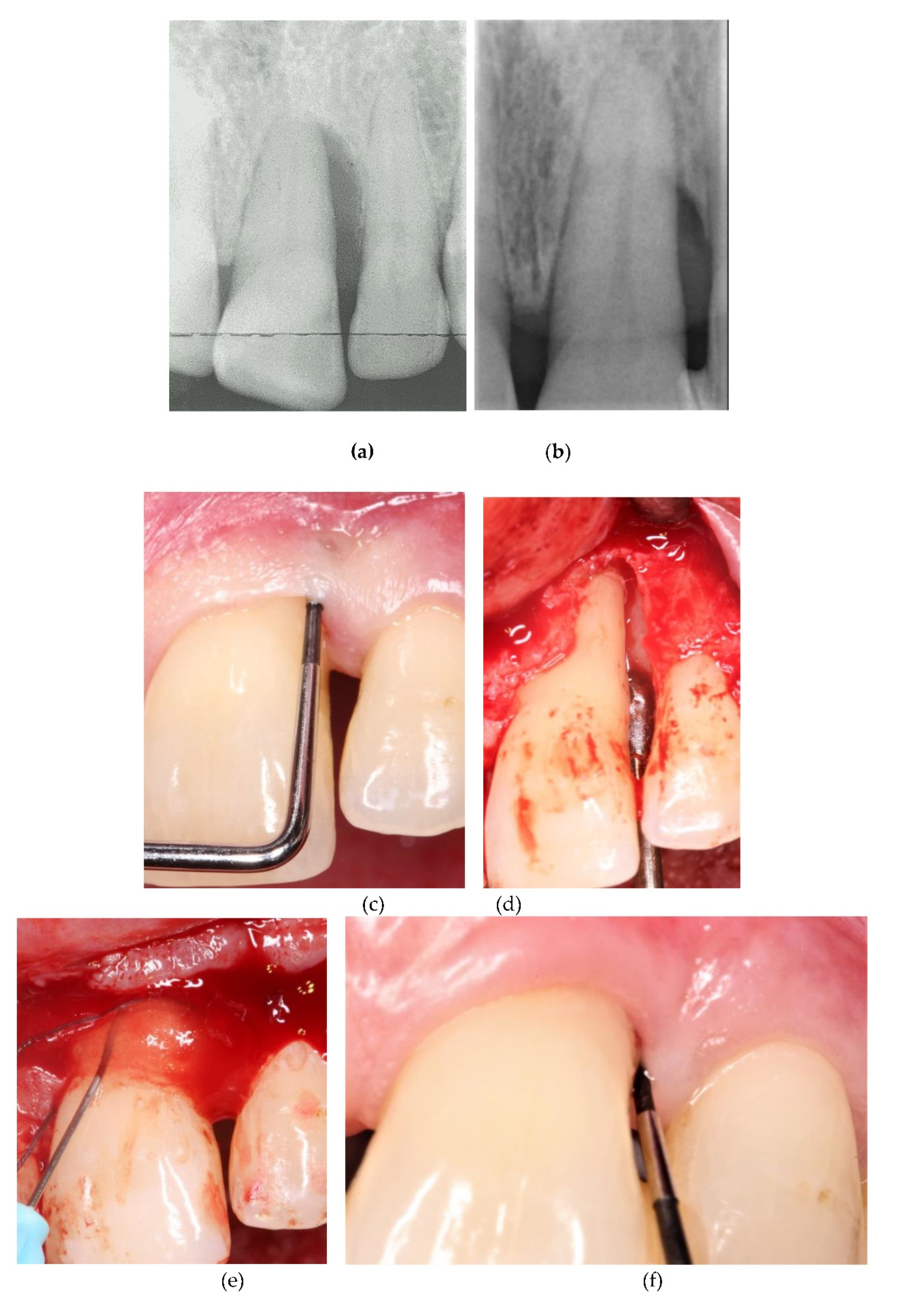
Figure 3 and
Figure 4 depict and illustrate one representative case per center including clinical images and periapical x-ray at baseline and 12-months post-op.
The ∆CAL comparison between centers favored Center 2 vs. center 1 with a P=0.006, the difference between C2 and C3 was statistically non-significant (P=0.718). The radiographic bone fill was significantly greater in patients from Centers 2 and 3 vs. Center 1 (P=.003 and =.014, respectively), (
Table 3). The difference in radiographically documented defect fill between C2 and C3 was statistically non-significant (P=1.0). The recession increased from baseline to 12 months visit at 1.2 – 1.3 mm in average for the Center 1 and 3, while Center 2 recorded a minimal recession increase of less than 0.5mm.
The rate for pocket closure was estimated at> 90% in all treated sites regardless the type of biomaterial. In detail, looking at residual PD ≤ 4mm without BoP, C1 showed 89.5%, C2 95.3% and C3 93.4% pocket closure rate. BoP appeared sufficiently reduced in all treated defects at an overall rate of 93%, without greater difference between three centers.
Among covariates the intra-osseous defect depth tested significant for the outcome in ∆PPD, ∆CAL and ∆Defect Fill (p<0.001). Other covariates such as defect angle, number of defect walls, localization or defect width (if reported) were not significantly associated with the clinical outcome (
Table 3).
4. Discussion
This retrospective data analysis displayed a sufficient improvement for the patients treated by any of the three centers. The successful treatment outcome per center documented both, the positive alteration of means in clinical parameters and expressed by pocket closure rate per center.
Each center achieved significant reduction in PPD at a clinically relevant level with pocket closure rates ranging from 90 to 94%. This change was accompanied by significant attachment level gain in each group. As corroborated by 12 months results, both, the significant clinical attachment gain and radiographic alveolar bone improvement remained constantly unaltered during the observation period (
Table 2,
Figure 1,
Figure 2 and
Figure 3).
The increased recession depth differed impressively between centers 1 and 3 compared to center 2. At both centers the recession increased by ≈ 1.3mm, whereas Center 2 reported a minimal gingiva margin level change of less than 0.5mm. The BoP tendency significantly decreased with each treatment protocol, documenting uninflamed conditions of the post-op tissues at each center after one year.
Nevertheless, the effectiveness of the chosen material composition proved to result in statistically significant differences from the baseline outcomes each Center reported. Looking at the baseline number of bony walls, which were almost alike in all three centers (P=0.137), the results from Center 3 reported for the first time a significant CAL and bone gain accompanied by a using xHyA without bone substitute in defects presenting with a diminished number of bone walls (i.e. 1.5 at average). Stabilizing the defect by a polymer-derived membrane, the pocket closure effect at the level of residual 3mm probing depth was constantly observed after 12 months (93%).
Both Centers C1 and C2 used Biologics (xHyA or EMD, respectively) for defect treatment combined with a bone substitute to increase the defect stabilization in sites, which showed similar morphology as those from C3. Although C3 patient’s defects were treated without any bone substitute, the mean values for CAL, radiographic bone fill and defect resolution, indicate greater conformity between C3 treatment approach and the EMD plus allograft treated sites from C2.
The somewhat diminished outcome at C1 may have been rooted in the choice of the substitute material. The bone substitute was a fleece with 98.9% porosity, proposed for tissue engineering [
18]. The xenograft was recommended as a hemostatic device for enclosed defects [
19]. Used in this series as a graft in an open periodontal pocket environment, even in combination with xHyA the material may have undergone more rapid degradation compared with an allograft used by C2. The high proportion of collagen in this biomaterial may have been responsible for a rapid resorption accompanied by a partial collapse of the flap into the infra-osseous defect. This healing pattern was then associated with an increasing post-operative recession and reducing the potential for regeneration of infra-osseous component. Previous studies pointed out that the type of collagen may be crucial for supporting the bone regeneration process and fast collagen degradation may be associated with limited outcome [
20].
The regenerative potential of EMD in a new attachment formation was confirmed by a plethora of RCTs and human histological studies [
21,
22,
23,
24]. The EFP guidelines recommend, however, in case of minimized number of bony walls the combined use with a particulate bone graft, which prevents the risk of tissue collapse into the defect. Thus, Center 1 and 2 combined the use of biologics (xHyA, EMD) with either a xenograft (C1) or an allograft (C2) according to the recommendations for treating defects characterized by a diminished number of walls [
25,
26]. One specific surgical technique resulted - once applied alone - in a similar CAL gain as in combination with EMD, however, the defect morphology was explicitly chosen for this clinical comparison [
27].
Center 3, however, discarded the use of any particulate material to avoid an artificial radio-opacity in the defect area, which possibly attributed to the presence of a grafting material in analyzing the radiographs. Thus, the xHyA gel applied into the intrabony defect and onto the polymer membrane was sought to enhance the soft tissue healing and to possibly support new attachment formation. Therefore, the radiographically identified bone gain within the previous extension of intrabony defect was clinically relevant and did not relate to the use of grafting material. Albeit the bone gain appears impressive, there was no sign of an ankylosed process along previously exposed roots detectable in all 15 cases. Moreover, a continuous gap between the new bone and root surface was clearly present at all post-op x-rays from this center without exception indicating that periodontal regenerative healing type occurred. These observations indicate that xHyA applied to defects with a diminished number of bone walls (mean value 1.5 walls for C3) contributed to regenerative outcome. Just published in vitro study corroborated the facilitating effect of xHyA on cementoblasts regarding the protein expression and proliferation of cells in a scratch defect model [
15]. For the C3 group, the use of the polymer barrier also attributed to the regenerative effect [
28]. Stavropoulos and Karring showed attachment level gain using the same kind of barrier in a 6–7-year observational study with PPD improvement of 3.8+/-1.1 mm and a mean CAL gain of 3.8+/-1.4 mm observed after 1 year. After 6-7 years, the corresponding values were 4.7+/-1.3 and 3.6+/-1.4 mm [
29].
The comparison of the CAL gain yielded using EMD (3.4+/-1.0 mm (p<0.001) and 2.9+/-1.4 mm (p<0.001)) vs. GTR technique (3.2+/-1.4 (p<0.001) and 2.8+/-1.2 mm (p<0.001) reported similar levels for both treatments as at 1 year evaluation as 10 years post-op, respectively [
22,
30].
The results reported by this study based on a 1-year outcome. The CAL gain reported by C1 the operator achieved without the presence of a barrier membrane and yielded within the abovementioned range. Taken together, the outcomes from C1 and C3 likely indicated that xHyA has influenced new attachment formation in patients from both centers. The C3 outcome in terms of CAL gain and radiographic defect fill was none inferior to that from C2, which used the EMD allograft combination.
The most recent meta-analysis corroborated the benefits of regenerative treatment over the OFD approach in terms of PD reduction, CAL gain and tooth retention during a 5 to 20 years post-op observation period estimating the combined treatment options as more efficacious than the monotherapy [
24]. Considering that the treatment at all three centers was completed more than 1 year ago, one argument opposing the regenerative capacity of xHyA for reconstructing periodontal tissues may address the lack of experimental data that time. Data from animal trials and the RCT study closed meanwhile the gap in the evidence for its regenerative properties histomorphometrically as well as clinically ([
9,
11].
The limitations of this retrospective data analysis are related to the nature of the study. Each center had freedom in choosing the suture technique, the suture material, and the combination of biomaterials. In so far, the surgical protocols differ, therefore the study could not represent a multi-center study with a completely standardized protocol. In a consequence, any random allocation of the patients to the groups of biomaterials was abandoned. Nevertheless, all involved surgeons were skilled, well-trained periodontists with large surgical experience, thus one can speculate that the choice of the material combination by each center reflected the most favored consideration at the time.
5. Conclusions
Within the limits of this retrospective study, we consider that xHyA used in reconstructive surgery for treating intrabony periodontal defects may contribute to the outcome at a similar range as known for EMD. However, the mechanisms behind its function are yet to be understood.
Author Contributions
Conceptualization, A.F. and D.D.; methodology, A.B., M.E. and A.F.; software, T.O.; validation, P.L.; formal analysis, P.L.; data curation, T.O.; writing—original draft preparation, A.F. and D.D.; writing—review and editing, A.B., M.E., P.L. and T.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
we included the STROBE statement to this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M. S.; Participants, E. W.; Consultants, M.; Merete Aass, A. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J Clin Periodontol 2020, 47, 4–60. [Google Scholar] [CrossRef] [PubMed]
- Nibali, L.; Koidou, V. P.; Nieri, M.; Barbato, L.; Pagliaro, U.; Cairo, F. Regenerative surgery versus access flap for the treatment of intra-bony periodontal defects: A systematic review and meta-analysis. J Clin Periodontol 2020, 47 (Suppl 22), 320–351. [Google Scholar] [CrossRef] [PubMed]
- Wikesjo, U. M.; Qahash, M.; Thomson, R. C.; Cook, A. D.; Rohrer, M. D.; Wozney, J. M.; Hardwick, W. R. Space-providing expanded polytetrafluoroethylene devices define alveolar augmentation at dental implants induced by recombinant human bone morphogenetic protein 2 in an absorbable collagen sponge carrier. Clin Implant Dent Relat Res 2003, 5, 112–23. [Google Scholar] [CrossRef] [PubMed]
- Wikesjo, U. M.; Lim, W. H.; Razi, S. S.; Sigurdsson, T. J.; Lee, M. B.; Tatakis, D. N.; Hardwick, W. R. Periodontal repair in dogs: a bioabsorbable calcium carbonate coral implant enhances space provision for alveolar bone regeneration in conjunction with guided tissue regeneration. J Periodontol 2003, 74, 957–64. [Google Scholar] [CrossRef]
- Aimetti, M.; Fratini, A.; Manavella, V.; Giraudi, M.; Citterio, F.; Ferrarotti, F.; Mariani, G. M.; Cairo, F.; Baima, G.; Romano, F. Pocket resolution in regenerative treatment of intrabony defects with papilla preservation techniques: A systematic review and meta-analysis of randomized clinical trials. J Clin Periodontol 2021, 48, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Ozener, H. O.; Dogan, B.; Kuru, B. Effect of topically applied hyaluronic acid on pain and palatal epithelial wound healing: An examiner-masked, randomized, controlled clinical trial. J Periodontol 2018, 89, 36–45. [Google Scholar] [CrossRef]
- Shirakata, Y.; Nakamura, T.; Kawakami, Y.; Imafuji, T.; Shinohara, Y.; Noguchi, K.; Sculean, A. Healing of buccal gingival recessions following treatment with coronally advanced flap alone or combined with a cross-linked hyaluronic acid gel. An experimental study in dogs. J Clin Periodontol 2021, 48, 570–580. [Google Scholar] [CrossRef]
- Pilloni, A.; Schmidlin, P. R.; Sahrmann, P.; Sculean, A.; Rojas, M. A. Correction to: Effectiveness of adjunctive hyaluronic acid application in coronally advanced flap in Miller class I single gingival recession sites: a randomized controlled clinical trial. Clin Oral Investig 2018, 22, 2961–2962. [Google Scholar] [CrossRef]
- Shirakata, Y.; Imafuji, T.; Nakamura, T.; Kawakami, Y.; Shinohara, Y.; Noguchi, K.; Pilloni, A.; Sculean, A. Periodontal wound healing/regeneration of two-wall intrabony defects following reconstructive surgery with cross-linked hyaluronic acid-gel with or without a collagen matrix: a preclinical study in dogs. Quintessence Int 2021, 0, 308–316. [Google Scholar]
- Shirakata, Y.; Imafuji, T.; Nakamura, T.; Shinohara, Y.; Iwata, M.; Setoguchi, F.; Noguchi, K.; Sculean, A. Cross-linked hyaluronic acid gel with or without a collagen matrix in the treatment of class III furcation defects: A histologic and histomorphometric study in dogs. J Clin Periodontol 2022. [CrossRef]
- Pilloni, A.; Rojas, M. A.; Marini, L.; Russo, P.; Shirakata, Y.; Sculean, A.; Iacono, R. Healing of intrabony defects following regenerative surgery by means of single-flap approach in conjunction with either hyaluronic acid or an enamel matrix derivative: a 24-month randomized controlled clinical trial. Clin Oral Investig 2021, 25, 5095–5107. [Google Scholar] [CrossRef]
- Bozic, D.; Catovic, I.; Badovinac, A.; Music, L.; Par, M.; Sculean, A. Treatment of Intrabony Defects with a Combination of Hyaluronic Acid and Deproteinized Porcine Bone Mineral. Materials (Basel) 2021, 14. [Google Scholar] [CrossRef]
- Mueller, A.; Fujioka-Kobayashi, M.; Mueller, H. D.; Lussi, A.; Sculean, A.; Schmidlin, P. R.; Miron, R. J. Effect of hyaluronic acid on morphological changes to dentin surfaces and subsequent effect on periodontal ligament cell survival, attachment, and spreading. Clin Oral Investig 2017, 21, 1013–1019. [Google Scholar] [CrossRef]
- Nobis, B.; Ostermann, T.; Weiler, J.; Dittmar, T.; Friedmann, A. Impact of Cross-Linked Hyaluronic Acid on Osteogenic Differentiation of SAOS-2 Cells in an Air-Lift Model. Materials (Basel) 2022, 15. [Google Scholar] [CrossRef]
- Hakki, S. S.; Bozkurt, S. B.; Sculean, A.; Bozic, D. Hyaluronic acid enhances cell migration, viability, and mineralized tissue-specific genes in cementoblasts. J Periodontal Res 2023. [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M. S.; Participants, E. F. P. W.; Methodological, C. , Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J Clin Periodontol 2020, 47 (Suppl 22), 4–60. [Google Scholar] [CrossRef]
- Cortellini, P.; Tonetti, M. S. Clinical concepts for regenerative therapy in intrabony defects. Periodontol 2000 2015, 68, 282–307. [Google Scholar] [CrossRef] [PubMed]
- Yunus Basha, R.; Sampath Kumar, T. S.; Doble, M. Design of biocomposite materials for bone tissue regeneration. Mater Sci Eng C Mater Biol Appl 2015, 57, 452–63. [Google Scholar] [CrossRef] [PubMed]
- Kurien, T.; Pearson, R.G.; Scammell, B.E. Bone graft substitutes currently available in orthopaedic practice: the evidence for their use. Bone Joint J 2013, 95-B, 583–597. [Google Scholar] [CrossRef]
- Friedmann, A.; Fickl, S.; Fischer, K. R.; Dalloul, M.; Goetz, W.; Kauffmann, F. Horizontal Augmentation of Chronic Mandibular Defects by the Guided Bone Regeneration Approach: A Randomized Study in Dogs. Materials 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Windisch, P.; Szendroi-Kiss, D.; Horvath, A.; Rosta, P.; Becker, J.; Gera, I.; Schwarz, F. Clinical and histologic evaluation of an enamel matrix derivative combined with a biphasic calcium phosphate for the treatment of human intrabony periodontal defects. J Periodontol 2008, 79, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Kiss, A.; Miliauskaite, A.; Schwarz, F.; Arweiler, N. B.; Hannig, M. , Ten-year results following treatment of intra-bony defects with enamel matrix proteins and guided tissue regeneration. J Clin Periodontol 2008, 35, 817–24. [Google Scholar] [CrossRef]
- Sanz, M.; Tonetti, M. S.; Zabalegui, I.; Sicilia, A.; Blanco, J.; Rebelo, H.; Rasperini, G.; Merli, M.; Cortellini, P.; Suvan, J. E. Treatment of intrabony defects with enamel matrix proteins or barrier membranes: results from a multicenter practice-based clinical trial. J Periodontol 2004, 75, 726–33. [Google Scholar] [CrossRef]
- Stavropoulos, A.; Bertl, K.; Spineli, L. M.; Sculean, A.; Cortellini, P.; Tonetti, M. Medium- and long-term clinical benefits of periodontal regenerative/reconstructive procedures in intrabony defects: Systematic review and network meta-analysis of randomized controlled clinical studies. J Clin Periodontol 2021, 48, 410–430. [Google Scholar] [CrossRef]
- Matarasso, M.; Iorio-Siciliano, V.; Blasi, A.; Ramaglia, L.; Salvi, G. E.; Sculean, A. Enamel matrix derivative and bone grafts for periodontal regeneration of intrabony defects. A systematic review and meta-analysis. Clin Oral Investig 2015, 19, 1581–1593. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Schwarz, F. Regeneration of periodontal tissues: combinations of barrier membranes and grafting materials - biological foundation and preclinical evidence: a systematic review. J Clin Periodontol 2008, 35, 106–166. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Buduneli, N.; Cortellini, P. Clinical outcomes of the entire papilla preservation technique with and without biomaterials in the treatment of isolated intrabony defects: A randomized controlled clinical trial. J Clin Periodontol 2020, 47, 470–478. [Google Scholar] [CrossRef]
- Falk, H.; Laurell, L.; Ravald, N.; Teiwik, A.; Persson, R. Guided tissue regeneration therapy of 203 consecutively treated intrabony defects using a bioabsorbable matrix barrier. Clinical and radiographic findings. J Periodontol 1997, 68, 571–581. [Google Scholar] [PubMed]
- Stavropoulos, A.; Karring, T. Long-term stability of periodontal conditions achieved following guided tissue regeneration with bioresorbable membranes: case series results after 6-7 years. J Clin Periodontol 2004, 31, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Stalpers, G.; Mollo, A.; Tonetti, M. S. Periodontal regeneration versus extraction and dental implant or prosthetic replacement of teeth severely compromised by attachment loss to the apex: A randomized controlled clinical trial reporting 10-year outcomes, survival analysis and mean cumulative cost of recurrence. J Clin Periodontol 2020, 47, 768–776. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).