Submitted:
30 December 2023
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
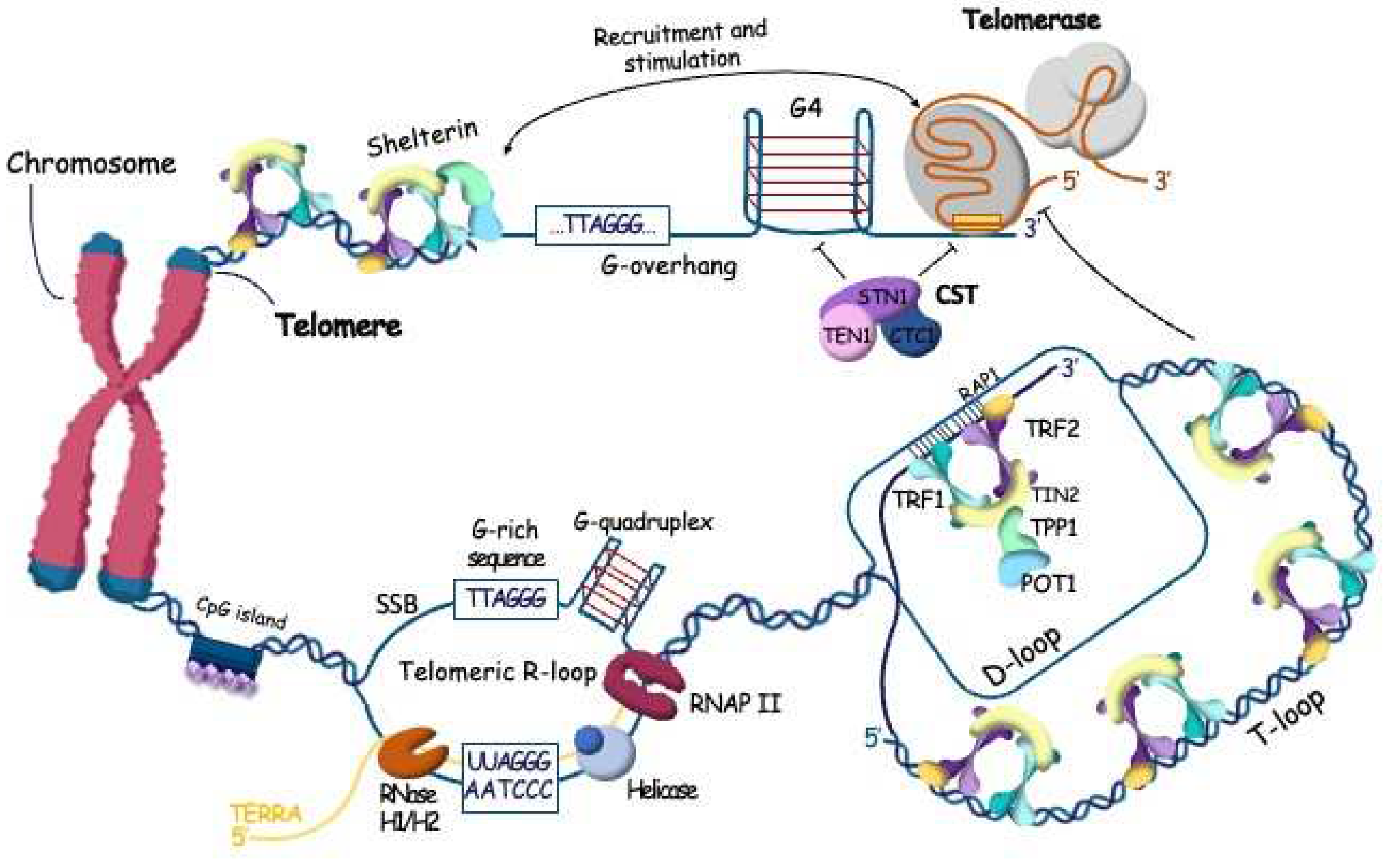
2. Telomeres: structure and regulation
3. Mechanisms of telomere length maintenance
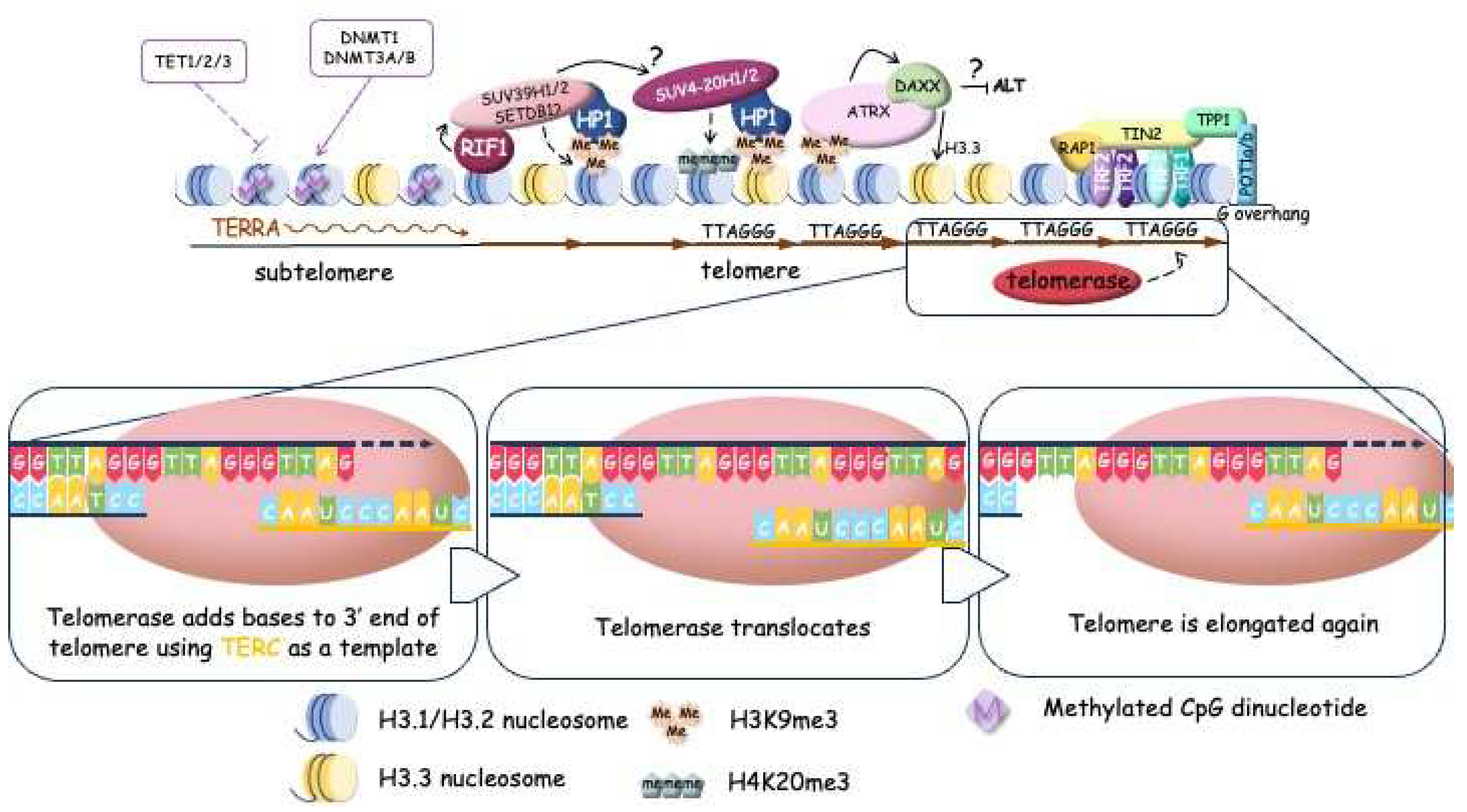
3.1. Telomere lengthening by telomerase
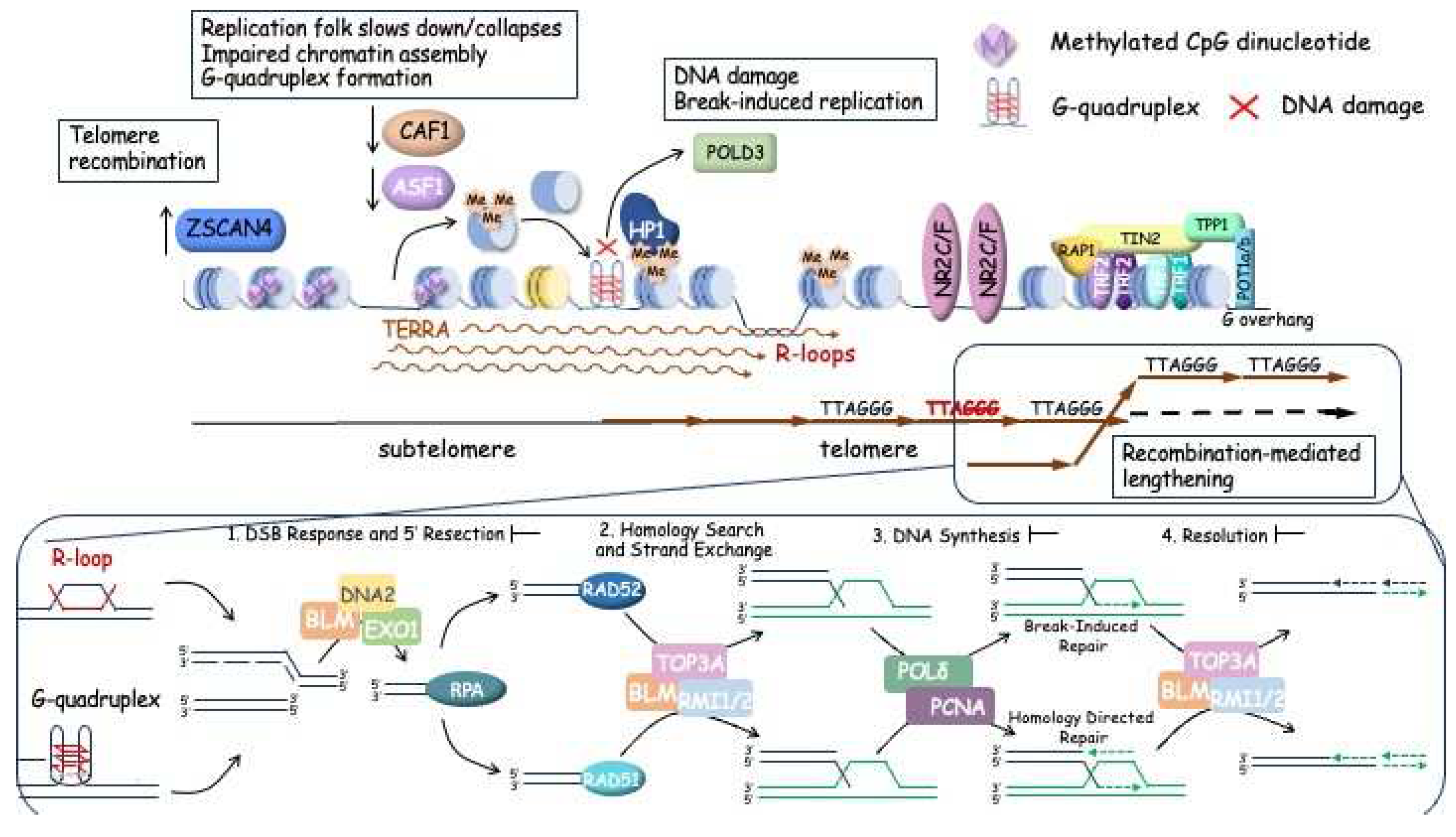
3.2. ALTernative mechanism of telomere lengthening
4. Metabolism
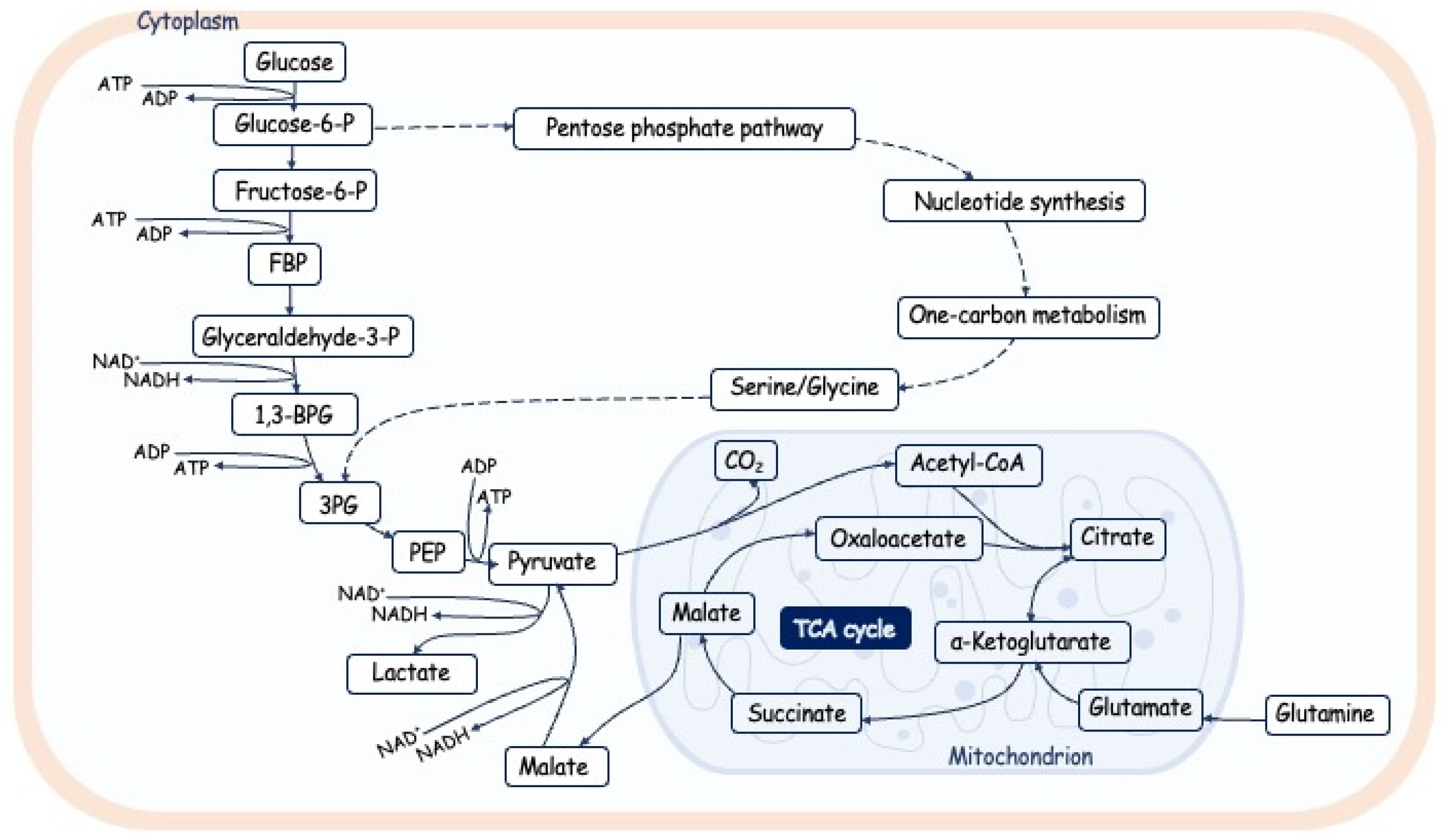
4.1. An overview of glucose metabolism
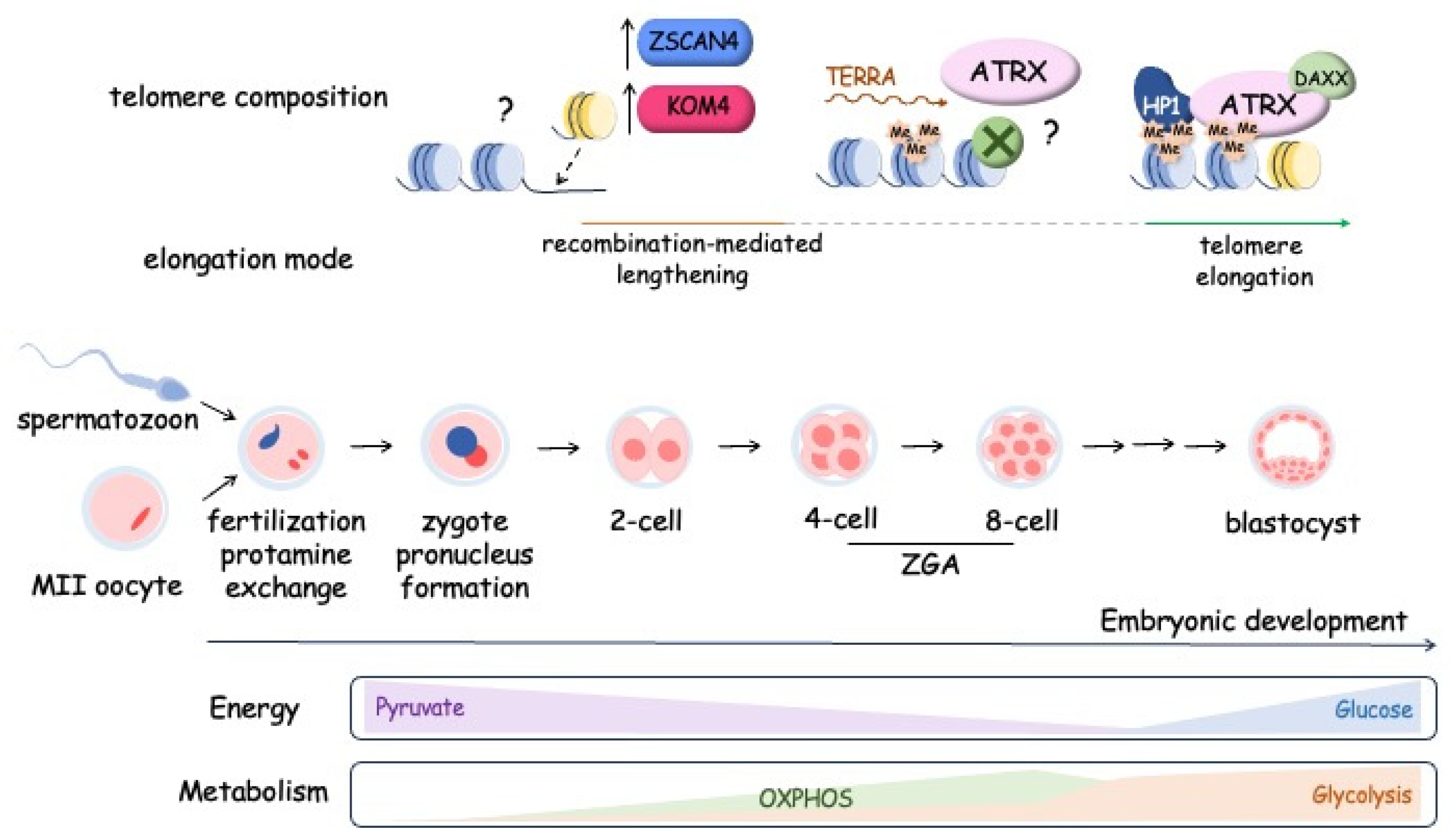
4.2. Metabolism and telomere lengthening during early development and in T-cell activation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Olovnikov, A.M. A Theory of Marginotomy. Journal of Theoretical Biology 1973, 41, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Chow, T.T.; Zhao, Y.; Mak, S.S.; Shay, J.W.; Wright, W.E. Early and Late Steps in Telomere Overhang Processing in Normal Human Cells: The Position of the Final RNA Primer Drives Telomere Shortening. Genes & Development 2012, 26, 1167–1178. [Google Scholar] [CrossRef]
- Levy, M.Z.; Allsopp, R.C.; Futcher, A.B.; Greider, C.W.; Harley, C.B. Telomere End-Replication Problem and Cell Aging. Journal of Molecular Biology 1992, 225, 951–960. [Google Scholar] [CrossRef]
- Fumagalli, M.; Rossiello, F.; Clerici, M.; Barozzi, S.; Cittaro, D.; Kaplunov, J.M.; Bucci, G.; Dobreva, M.; Matti, V.; Beausejour, C.M.; et al. Telomeric DNA Damage Is Irreparable and Causes Persistent DNA-Damage-Response Activation. Nat Cell Biol 2012, 14, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.E.; Shay, J.W. Role of Telomerase in Cellular Proliferation and Cancer. J. Cell. Physiol. 1999, 180, 10–18. [Google Scholar] [CrossRef]
- Bodnar, A.G.; Ouellette, M.; Frolkis, M.; Holt, S.E.; Chiu, C.P.; Morin, G.B.; Harley, C.B.; Shay, J.W.; Lichtsteiner, S.; Wright, W.E. Extension of Life-Span by Introduction of Telomerase into Normal Human Cells. Science 1998, 279, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Weng, N. Telomere and Adaptive Immunity. Mechanisms of Ageing and Development 2008, 129, 60–66. [Google Scholar] [CrossRef]
- Weng, N.P.; Levine, B.L.; June, C.H.; Hodes, R.J. Regulated Expression of Telomerase Activity in Human T Lymphocyte Development and Activation. J. Exp. Med. 1996, 183, 2471–2479. [Google Scholar] [CrossRef]
- Lu, W.-Y.; Forbes, S.J. Telomerase Activity Links to Regenerative Capacity of Hepatocytes. Transplantation 2018, 102, 1587–1588. [Google Scholar] [CrossRef]
- Greider, C.W.; Blackburn, E.H. A Telomeric Sequence in the RNA of Tetrahymena Telomerase Required for Telomere Repeat Synthesis. Nature 1989, 337, 331–337. [Google Scholar] [CrossRef]
- Lingner, J.; Hughes, T.R.; Shevchenko, A.; Mann, M.; Lundblad, V.; Cech, T.R. Reverse Transcriptase Motifs in the Catalytic Subunit of Telomerase. Science 1997, 276, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; Yadav, T.; Ouyang, J.; Lan, L.; Zou, L. Alternative Lengthening of Telomeres through Two Distinct Break-Induced Replication Pathways. Cell Reports 2019, 26, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Moyzis, R.K.; Buckingham, J.M.; Cram, L.S.; Dani, M.; Deaven, L.L.; Jones, M.D.; Meyne, J.; Ratliff, R.L.; Wu, J.R. A Highly Conserved Repetitive DNA Sequence, (TTAGGG)n, Present at the Telomeres of Human Chromosomes. Proc. Natl. Acad. Sci. U.S.A. 1988, 85, 6622–6626. [Google Scholar] [CrossRef] [PubMed]
- Meyne, J.; Ratliff, R.L.; Moyzis, R.K. Conservation of the Human Telomere Sequence (TTAGGG)n among Vertebrates. Proc. Natl. Acad. Sci. U.S.A. 1989, 86, 7049–7053. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.L.; Hirose, Y.; Langmore, J.P. Long G Tails at Both Ends of Human Chromosomes Suggest a C Strand Degradation Mechanism for Telomere Shortening. Cell 1997, 88, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres Shorten during Ageing of Human Fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, R.C.; Vaziri, H.; Patterson, C.; Goldstein, S.; Younglai, E.V.; Futcher, A.B.; Greider, C.W.; Harley, C.B. Telomere Length Predicts Replicative Capacity of Human Fibroblasts. Proceedings of the National Academy of Sciences 1992, 89, 10114–10118. [Google Scholar] [CrossRef]
- Morin, G.B. The Human Telomere Terminal Transferase Enzyme Is a Ribonucleoprotein That Synthesizes TTAGGG Repeats. Cell 1989, 59, 521–529. [Google Scholar] [CrossRef]
- Bryan, T.M.; Englezou, A.; Dalla-Pozza, L.; Dunham, M.A.; Reddel, R.R. Evidence for an Alternative Mechanism for Maintaining Telomere Length in Human Tumors and Tumor-Derived Cell Lines. Nat Med 1997, 3, 1271–1274. [Google Scholar] [CrossRef]
- Wright, W.E.; Piatyszek, M.A.; Rainey, W.E.; Byrd, W.; Shay, J.W. Telomerase Activity in Human Germline and Embryonic Tissues and Cells. Dev. Genet. 1996, 18, 173–179. [Google Scholar] [CrossRef]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific Association of Human Telomerase Activity with Immortal Cells and Cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- de Lange, T. Shelterin: The Protein Complex That Shapes and Safeguards Human Telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; O’Connor, M.S.; Qin, J.; Songyang, Z. Telosome, a Mammalian Telomere-Associated Complex Formed by Multiple Telomeric Proteins. Journal of Biological Chemistry 2004, 279, 51338–51342. [Google Scholar] [CrossRef] [PubMed]
- Baumann, P.; Cech, T.R. Pot1, the Putative Telomere End-Binding Protein in Fission Yeast and Humans. Science 2001, 292, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.J.; Cech, T.R. Shaping Human Telomeres: From Shelterin and CST Complexes to Telomeric Chromatin Organization. Nat Rev Mol Cell Biol 2021, 22, 283–298. [Google Scholar] [CrossRef]
- Bianchi, A. TRF1 Is a Dimer and Bends Telomeric DNA. The EMBO Journal 1997, 16, 1785–1794. [Google Scholar] [CrossRef]
- Broccoli, D.; Smogorzewska, A.; Chong, L.; de Lange, T. Human Telomeres Contain Two Distinct Myb–Related Proteins, TRF1 and TRF2. Nat Genet 1997, 17, 231–235. [Google Scholar] [CrossRef]
- van Steensel, B.; de Lange, T. Control of Telomere Length by the Human Telomeric Protein TRF1. Nature 1997, 385, 740–743. [Google Scholar] [CrossRef]
- Bilaud, T.; Brun, C.; Ancelin, K.; Koering, C.E.; Laroche, T.; Gilson, E. Telomeric Localization of TRF2, a Novel Human Telobox Protein. Nat Genet 1997, 17, 236–239. [Google Scholar] [CrossRef]
- Kim, S.; Kaminker, P.; Campisi, J. TIN2, a New Regulator of Telomere Length in Human Cells. Nat Genet 1999, 23, 405–412. [Google Scholar] [CrossRef]
- Seimiya, H.; Muramatsu, Y.; Smith, S.; Tsuruo, T. Functional Subdomain in the Ankyrin Domain of Tankyrase 1 Required for Poly(ADP-Ribosyl)Ation of TRF1 and Telomere Elongation. Molecular and Cellular Biology 2004, 24, 1944–1955. [Google Scholar] [CrossRef]
- M. Stansel, R. T-Loop Assembly in Vitro Involves Binding of TRF2 near the 3’ Telomeric Overhang. The EMBO Journal 2001, 20, 5532–5540. [Google Scholar] [CrossRef] [PubMed]
- Fouché, N.; Cesare, A.J.; Willcox, S.; Özgür, S.; Compton, S.A.; Griffith, J.D. The Basic Domain of TRF2 Directs Binding to DNA Junctions Irrespective of the Presence of TTAGGG Repeats. Journal of Biological Chemistry 2006, 281, 37486–37495. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.R.; Raghuraman, M.K.; Cech, T.R. Monovalent Cation-Induced Structure of Telomeric DNA: The G-Quartet Model. Cell 1989, 59, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Sundquist, W.I.; Klug, A. Telomeric DNA Dimerizes by Formation of Guanine Tetrads between Hairpin Loops. Nature 1989, 342, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Konishi, A.; Izumi, T.; Shimizu, S. TRF2 Protein Interacts with Core Histones to Stabilize Chromosome Ends. Journal of Biological Chemistry 2016, 291, 20798–20810. [Google Scholar] [CrossRef] [PubMed]
- Sfeir, A.; Kabir, S.; van Overbeek, M.; Celli, G.B.; de Lange, T. Loss of Rap1 Induces Telomere Recombination in the Absence of NHEJ or a DNA Damage Signal. Science 2010, 327, 1657–1661. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Chen, Y.; Lei, M.; Chang, S. TRF2-RAP1 Is Required to Protect Telomeres from Engaging in Homologous Recombination-Mediated Deletions and Fusions. Nat Commun 2016, 7, 10881. [Google Scholar] [CrossRef]
- Lototska, L.; Yue, J.; Li, J.; Giraud-Panis, M.; Songyang, Z.; Royle, N.J.; Liti, G.; Ye, J.; Gilson, E.; Mendez-Bermudez, A. Human RAP 1 Specifically Protects Telomeres of Senescent Cells from DNA Damage. EMBO Reports 2020, 21, e49076. [Google Scholar] [CrossRef]
- Ye, J.Z.-S.; Hockemeyer, D.; Krutchinsky, A.N.; Loayza, D.; Hooper, S.M.; Chait, B.T.; de Lange, T. POT1-Interacting Protein PIP1: A Telomere Length Regulator That Recruits POT1 to the TIN2/TRF1 Complex. Genes Dev. 2004, 18, 1649–1654. [Google Scholar] [CrossRef]
- O’Connor, M.S.; Safari, A.; Xin, H.; Liu, D.; Songyang, Z. A Critical Role for TPP1 and TIN2 Interaction in High-Order Telomeric Complex Assembly. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 11874–11879. [Google Scholar] [CrossRef]
- Chen, C.; Gu, P.; Wu, J.; Chen, X.; Niu, S.; Sun, H.; Wu, L.; Li, N.; Peng, J.; Shi, S.; et al. Structural Insights into POT1-TPP1 Interaction and POT1 C-Terminal Mutations in Human Cancer. Nat Commun 2017, 8, 14929. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Podell, E.R.; Cech, T.R. Structure of Human POT1 Bound to Telomeric Single-Stranded DNA Provides a Model for Chromosome End-Protection. Nat Struct Mol Biol 2004, 11, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Rai, R.; Huang, C.; Broton, C.; Long, J.; Xu, Y.; Xue, J.; Lei, M.; Chang, S.; Chen, Y. Structural and Functional Analyses of the Mammalian TIN2-TPP1-TRF2 Telomeric Complex. Cell Res 2017, 27, 1485–1502. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.; van Overbeek, M.; Donigian, J.R.; Baciu, P.; de Lange, T.; Lei, M. A Shared Docking Motif in TRF1 and TRF2 Used for Differential Recruitment of Telomeric Proteins. Science 2008, 319, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Janoušková, E.; Nečasová, I.; Pavloušková, J.; Zimmermann, M.; Hluchý, M.; Marini, V.; Nováková, M.; Hofr, C. Human Rap1 Modulates TRF2 Attraction to Telomeric DNA. Nucleic Acids Research 2015, 43, 2691–2700. [Google Scholar] [CrossRef]
- Choi, K.H.; Farrell, A.S.; Lakamp, A.S.; Ouellette, M.M. Characterization of the DNA Binding Specificity of Shelterin Complexes. Nucleic Acids Research 2011, 39, 9206–9223. [Google Scholar] [CrossRef]
- Takai, K.K.; Hooper, S.; Blackwood, S.; Gandhi, R.; de Lange, T. In Vivo Stoichiometry of Shelterin Components. Journal of Biological Chemistry 2010, 285, 1457–1467. [Google Scholar] [CrossRef]
- Pisano, S.; Marchioni, E.; Galati, A.; Mechelli, R.; Savino, M.; Cacchione, S. Telomeric Nucleosomes Are Intrinsically Mobile. Journal of Molecular Biology 2007, 369, 1153–1162. [Google Scholar] [CrossRef]
- Fajkus, J.; Trifonov, E.N. Columnar Packing of Telomeric Nucleosomes. Biochemical and Biophysical Research Communications 2001, 280, 961–963. [Google Scholar] [CrossRef]
- Soman, A.; Liew, C.W.; Teo, H.L.; Berezhnoy, N.V.; Olieric, V.; Korolev, N.; Rhodes, D.; Nordenskiöld, L. The Human Telomeric Nucleosome Displays Distinct Structural and Dynamic Properties. Nucleic Acids Research 2020, 48, 5383–5396. [Google Scholar] [CrossRef] [PubMed]
- Azzalin, C.M.; Reichenbach, P.; Khoriauli, L.; Giulotto, E.; Lingner, J. Telomeric Repeat Containing RNA and RNA Surveillance Factors at Mammalian Chromosome Ends. Science 2007, 318, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Schoeftner, S.; Blasco, M.A. Developmentally Regulated Transcription of Mammalian Telomeres by DNA-Dependent RNA Polymerase II. Nat. Cell Biol. 2008, 10, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; White, R.L.; Davis, R.W. Hybridization of RNA to Double-Stranded DNA: Formation of R-Loops. Proc. Natl. Acad. Sci. U.S.A. 1976, 73, 2294–2298. [Google Scholar] [CrossRef] [PubMed]
- White, R.L.; Hogness, D.S. R Loop Mapping of the 18S and 28S Sequences in the Long and Short Repeating Units of Drosophila Melanogaster rDNA. Cell 1977, 10, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Graf, M.; Bonetti, D.; Lockhart, A.; Serhal, K.; Kellner, V.; Maicher, A.; Jolivet, P.; Teixeira, M.T.; Luke, B. Telomere Length Determines TERRA and R-Loop Regulation through the Cell Cycle. Cell 2017, 170, 72–85. [Google Scholar] [CrossRef]
- Balk, B.; Dees, M.; Bender, K.; Luke, B. The Differential Processing of Telomeres in Response to Increased Telomeric Transcription and RNA–DNA Hybrid Accumulation. RNA Biology 2014, 11, 95–100. [Google Scholar] [CrossRef]
- Balk, B.; Maicher, A.; Dees, M.; Klermund, J.; Luke-Glaser, S.; Bender, K.; Luke, B. Telomeric RNA-DNA Hybrids Affect Telomere-Length Dynamics and Senescence. Nat. Struct. Mol. Biol. 2013, 20, 1199–1205. [Google Scholar] [CrossRef]
- Feretzaki, M.; Pospisilova, M.; Valador Fernandes, R.; Lunardi, T.; Krejci, L.; Lingner, J. RAD51-Dependent Recruitment of TERRA lncRNA to Telomeres through R-Loops. Nature 2020, 587, 303–308. [Google Scholar] [CrossRef]
- Yadav, T.; Zhang, J.-M.; Ouyang, J.; Leung, W.; Simoneau, A.; Zou, L. TERRA and RAD51AP1 Promote Alternative Lengthening of Telomeres through an R- to D-Loop Switch. Molecular Cell 2022, 82, 3985–4000. [Google Scholar] [CrossRef]
- Wang, H.; Nora, G.J.; Ghodke, H.; Opresko, P.L. Single Molecule Studies of Physiologically Relevant Telomeric Tails Reveal POT1 Mechanism for Promoting G-Quadruplex Unfolding. Journal of Biological Chemistry 2011, 286, 7479–7489. [Google Scholar] [CrossRef] [PubMed]
- Zaug, A.J.; Podell, E.R.; Cech, T.R. Human POT1 Disrupts Telomeric G-Quadruplexes Allowing Telomerase Extension in Vitro. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 10864–10869. [Google Scholar] [CrossRef] [PubMed]
- Patrick, E.M.; Slivka, J.D.; Payne, B.; Comstock, M.J.; Schmidt, J.C. Observation of Processive Telomerase Catalysis Using High-Resolution Optical Tweezers. Nat Chem Biol 2020, 16, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Latrick, C.M.; Cech, T.R. POT1–TPP1 Enhances Telomerase Processivity by Slowing Primer Dissociation and Aiding Translocation. EMBO J 2010, 29, 924–933. [Google Scholar] [CrossRef]
- Jansson, L.I.; Hentschel, J.; Parks, J.W.; Chang, T.R.; Lu, C.; Baral, R.; Bagshaw, C.R.; Stone, M.D. Telomere DNA G-Quadruplex Folding within Actively Extending Human Telomerase. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 9350–9359. [Google Scholar] [CrossRef]
- Paudel, B.P.; Moye, A.L.; Abou Assi, H.; El-Khoury, R.; Cohen, S.B.; Holien, J.K.; Birrento, M.L.; Samosorn, S.; Intharapichai, K.; Tomlinson, C.G.; et al. A Mechanism for the Extension and Unfolding of Parallel Telomeric G-Quadruplexes by Human Telomerase at Single-Molecule Resolution. eLife 2020, 9, e56428. [Google Scholar] [CrossRef]
- Wu, R.A.; Collins, K. Human Telomerase Specialization for Repeat Synthesis by Unique Handling of Primer-Template Duplex. The EMBO Journal 2014, 33, 921–935. [Google Scholar] [CrossRef]
- Wang, H. A Novel Specificity for the Primer-Template Pairing Requirement in Tetrahymena Telomerase. The EMBO Journal 1998, 17, 1152–1160. [Google Scholar] [CrossRef]
- Brown, A.F.; Podlevsky, J.D.; Qi, X.; Chen, Y.; Xie, M.; Chen, J.J.-L. A Self-Regulating Template in Human Telomerase. Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 11311–11316. [Google Scholar] [CrossRef]
- Berman, A.J.; Akiyama, B.M.; Stone, M.D.; Cech, T.R. The RNA Accordion Model for Template Positioning by Telomerase RNA during Telomeric DNA Synthesis. Nat Struct Mol Biol 2011, 18, 1371–1375. [Google Scholar] [CrossRef]
- Qi, X.; Xie, M.; Brown, A.F.; Bley, C.J.; Podlevsky, J.D.; Chen, J.J.-L. RNA/DNA Hybrid Binding Affinity Determines Telomerase Template-Translocation Efficiency. EMBO J. 2012, 31, 150–161. [Google Scholar] [CrossRef]
- Nandakumar, J.; Bell, C.F.; Weidenfeld, I.; Zaug, A.J.; Leinwand, L.A.; Cech, T.R. The TEL Patch of Telomere Protein TPP1 Mediates Telomerase Recruitment and Processivity. Nature 2012, 492, 285–289. [Google Scholar] [CrossRef]
- Zhong, F.L.; Batista, L.F.Z.; Freund, A.; Pech, M.F.; Venteicher, A.S.; Artandi, S.E. TPP1 OB-Fold Domain Controls Telomere Maintenance by Recruiting Telomerase to Chromosome Ends. Cell 2012, 150, 481–494. [Google Scholar] [CrossRef]
- Sexton, A.N.; Youmans, D.T.; Collins, K. Specificity Requirements for Human Telomere Protein Interaction with Telomerase Holoenzyme. Journal of Biological Chemistry 2012, 287, 34455–34464. [Google Scholar] [CrossRef]
- Schmidt, J.C.; Zaug, A.J.; Kufer, R.; Cech, T.R. Dynamics of Human Telomerase Recruitment Depend on Template-Telomere Base Pairing. MBoC 2018, 29, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.C.; Zaug, A.J.; Cech, T.R. Live Cell Imaging Reveals the Dynamics of Telomerase Recruitment to Telomeres. Cell 2016, 166, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Safari, A.; O’Connor, M.S.; Chan, D.W.; Laegeler, A.; Qin, J.; Songyang, Z. PTOP Interacts with POT1 and Regulates Its Localization to Telomeres. Nat Cell Biol 2004, 6, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Zaug, A.J.; Podell, E.R.; Cech, T.R. Switching Human Telomerase On and Off with hPOT1 Protein in Vitro. Journal of Biological Chemistry 2005, 280, 20449–20456. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.L.; Centore, R.C.; O’Sullivan, R.J.; Rai, R.; Tse, A.; Songyang, Z.; Chang, S.; Karlseder, J.; Zou, L. TERRA and hnRNPA1 Orchestrate an RPA-to-POT1 Switch on Telomeric Single-Stranded DNA. Nature 2011, 471, 532–536. [Google Scholar] [CrossRef]
- Rubtsova, M.P.; Skvortsov, D.A.; Petruseva, I.O.; Lavrik, O.I.; Spirin, P.V.; Prasolov, V.S.; Kisseljov, F.L.; Dontsova, O.A. Replication Protein A Modulates the Activity of Human Telomerase in Vitro. Biochemistry Mosc. 2009, 74, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Hockemeyer, D.; Sfeir, A.J.; Shay, J.W.; Wright, W.E.; de Lange, T. POT1 Protects Telomeres from a Transient DNA Damage Response and Determines How Human Chromosomes End. EMBO J 2005, 24, 2667–2678. [Google Scholar] [CrossRef]
- Denchi, E.L.; de Lange, T. Protection of Telomeres through Independent Control of ATM and ATR by TRF2 and POT1. Nature 2007, 448, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Podell, E.R.; Zaug, A.J.; Yang, Y.; Baciu, P.; Cech, T.R.; Lei, M. The POT1-TPP1 Telomere Complex Is a Telomerase Processivity Factor. Nature 2007, 445, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Redon, S.; Lingner, J. The Human CST Complex Is a Terminator of Telomerase Activity. Nature 2012, 488, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Cervantes, R.B.; Mandell, E.K.; Otero, J.H.; Lundblad, V. RPA-like Proteins Mediate Yeast Telomere Function. Nat Struct Mol Biol 2007, 14, 208–214. [Google Scholar] [CrossRef]
- Miyake, Y.; Nakamura, M.; Nabetani, A.; Shimamura, S.; Tamura, M.; Yonehara, S.; Saito, M.; Ishikawa, F. RPA-like Mammalian Ctc1-Stn1-Ten1 Complex Binds to Single-Stranded DNA and Protects Telomeres Independently of the Pot1 Pathway. Molecular Cell 2009, 36, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Wang, Y.; Diao, J.; Price, C.M. Dynamic DNA Binding, Junction Recognition and G4 Melting Activity Underlie the Telomeric and Genome-Wide Roles of Human CST. Nucleic Acids Research 2017, 45, 12311–12324. [Google Scholar] [CrossRef]
- Hom, R.A.; Wuttke, D.S. Human CST Prefers G-Rich but Not Necessarily Telomeric Sequences. Biochemistry 2017, 56, 4210–4218. [Google Scholar] [CrossRef]
- Lim, C.J.; Barbour, A.T.; Zaug, A.J.; Goodrich, K.J.; McKay, A.E.; Wuttke, D.S.; Cech, T.R. The Structure of Human CST Reveals a Decameric Assembly Bound to Telomeric DNA. Science 2020, 368, 1081–1085. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, B.; Li, T.; Liu, R.; Xiao, Y.; Geng, X.; Li, G.; Liu, Q.; Price, C.M.; Liu, Y.; et al. Mammalian CST Averts Replication Failure by Preventing G-Quadruplex Accumulation. Nucleic Acids Research 2019, 47, 5243–5259. [Google Scholar] [CrossRef]
- Casteel, D.E.; Zhuang, S.; Zeng, Y.; Perrino, F.W.; Boss, G.R.; Goulian, M.; Pilz, R.B. A DNA Polymerase-α·Primase Cofactor with Homology to Replication Protein A-32 Regulates DNA Replication in Mammalian Cells. Journal of Biological Chemistry 2009, 284, 5807–5818. [Google Scholar] [CrossRef] [PubMed]
- Ganduri, S.; Lue, N.F. STN1–POLA2 Interaction Provides a Basis for Primase-Pol α Stimulation by Human STN1. Nucleic Acids Research 2017, 45, 9455–9466. [Google Scholar] [CrossRef]
- Nakaoka, H.; Nishiyama, A.; Saito, M.; Ishikawa, F. Xenopus Laevis Ctc1-Stn1-Ten1 (xCST) Protein Complex Is Involved in Priming DNA Synthesis on Single-Stranded DNA Template in Xenopus Egg Extract. Journal of Biological Chemistry 2012, 287, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Akıncılar, S.C.; Khattar, E.; Boon, P.L.S.; Unal, B.; Fullwood, M.J.; Tergaonkar, V. Long-Range Chromatin Interactions Drive Mutant TERT Promoter Activation. Cancer Discovery 2016, 6, 1276–1291. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Lorbeer, F.K.; Shain, A.H.; McSwiggen, D.T.; Schruf, E.; Oh, A.; Ryu, J.; Darzacq, X.; Bastian, B.C.; Hockemeyer, D. Mutations in the Promoter of the Telomerase Gene TERT Contribute to Tumorigenesis by a Two-Step Mechanism. Science 2017, 357, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Peifer, M.; Hertwig, F.; Roels, F.; Dreidax, D.; Gartlgruber, M.; Menon, R.; Krämer, A.; Roncaioli, J.L.; Sand, F.; Heuckmann, J.M.; et al. Telomerase Activation by Genomic Rearrangements in High-Risk Neuroblastoma. Nature 2015, 526, 700–704. [Google Scholar] [CrossRef]
- Heaphy, C.M.; de Wilde, R.F.; Jiao, Y.; Klein, A.P.; Edil, B.H.; Shi, C.; Bettegowda, C.; Rodriguez, F.J.; Eberhart, C.G.; Hebbar, S.; et al. Altered Telomeres in Tumors with ATRX and DAXX Mutations. Science 2011, 333, 425–425. [Google Scholar] [CrossRef]
- Law, M.J.; Lower, K.M.; Voon, H.P.J.; Hughes, J.R.; Garrick, D.; Viprakasit, V.; Mitson, M.; De Gobbi, M.; Marra, M.; Morris, A.; et al. ATR-X Syndrome Protein Targets Tandem Repeats and Influences Allele-Specific Expression in a Size-Dependent Manner. Cell 2010, 143, 367–378. [Google Scholar] [CrossRef]
- Iwase, S.; Xiang, B.; Ghosh, S.; Ren, T.; Lewis, P.W.; Cochrane, J.C.; Allis, C.D.; Picketts, D.J.; Patel, D.J.; Li, H.; et al. ATRX ADD Domain Links an Atypical Histone Methylation Recognition Mechanism to Human Mental-Retardation Syndrome. Nat Struct Mol Biol 2011, 18, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Schwartzentruber, J.; Korshunov, A.; Liu, X.-Y.; Jones, D.T.W.; Pfaff, E.; Jacob, K.; Sturm, D.; Fontebasso, A.M.; Quang, D.-A.K.; Tönjes, M.; et al. Driver Mutations in Histone H3.3 and Chromatin Remodelling Genes in Paediatric Glioblastoma. Nature 2012, 482, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.; Burford, A.; Carvalho, D.; Izquierdo, E.; Fazal-Salom, J.; Taylor, K.R.; Bjerke, L.; Clarke, M.; Vinci, M.; Nandhabalan, M.; et al. Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 2017, 32, 520–537. [Google Scholar] [CrossRef]
- Mukherjee, J.; Johannessen, T.-C.; Ohba, S.; Chow, T.T.; Jones, L.; Pandita, A.; Pieper, R.O. Mutant IDH1 Cooperates with ATRX Loss to Drive the Alternative Lengthening of Telomere Phenotype in Glioma. Cancer Research 2018, 78, 2966–2977. [Google Scholar] [CrossRef]
- Sturm, D.; Witt, H.; Hovestadt, V.; Khuong-Quang, D.-A.; Jones, D.T.W.; Konermann, C.; Pfaff, E.; Tönjes, M.; Sill, M.; Bender, S.; et al. Hotspot Mutations in H3F3A and IDH1 Define Distinct Epigenetic and Biological Subgroups of Glioblastoma. Cancer Cell 2012, 22, 425–437. [Google Scholar] [CrossRef]
- Yeager, T.R.; Neumann, A.A.; Englezou, A.; Huschtscha, L.I.; Noble, J.R.; Reddel, R.R. Telomerase-Negative Immortalized Human Cells Contain a Novel Type of Promyelocytic Leukemia (PML) Body. Cancer Res 1999, 59, 4175–4179. [Google Scholar] [PubMed]
- Henson, J.D.; Cao, Y.; Huschtscha, L.I.; Chang, A.C.; Au, A.Y.M.; Pickett, H.A.; Reddel, R.R. DNA C-Circles Are Specific and Quantifiable Markers of Alternative-Lengthening-of-Telomeres Activity. Nat Biotechnol 2009, 27, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Grudic, A.; Jul-Larsen, Å.; Haring, S.J.; Wold, M.S.; Lønning, P.E.; Bjerkvig, R.; Bøe, S.O. Replication Protein A Prevents Accumulation of Single-Stranded Telomeric DNA in Cells That Use Alternative Lengthening of Telomeres. Nucleic Acids Research 2007, 35, 7267–7278. [Google Scholar] [CrossRef]
- Wu, G.; Jiang, X.; Lee, W.-H.; Chen, P.-L. Assembly of Functional ALT-Associated Promyelocytic Leukemia Bodies Requires Nijmegen Breakage Syndrome 1. Cancer Res 2003, 63, 2589–2595. [Google Scholar]
- Acharya, S.; Kaul, Z.; Gocha, A.S.; Martinez, A.R.; Harris, J.; Parvin, J.D.; Groden, J. Association of BLM and BRCA1 during Telomere Maintenance in ALT Cells. PLoS ONE 2014, 9, e103819. [Google Scholar] [CrossRef]
- Pan, X.; Drosopoulos, W.C.; Sethi, L.; Madireddy, A.; Schildkraut, C.L.; Zhang, D. FANCM, BRCA1, and BLM Cooperatively Resolve the Replication Stress at the ALT Telomeres. Proc. Natl. Acad. Sci. U.S.A. 2017, 114. [Google Scholar] [CrossRef]
- Barroso-González, J.; García-Expósito, L.; Hoang, S.M.; Lynskey, M.L.; Roncaioli, J.L.; Ghosh, A.; Wallace, C.T.; Modesti, M.; Bernstein, K.A.; Sarkar, S.N.; et al. RAD51AP1 Is an Essential Mediator of Alternative Lengthening of Telomeres. Molecular Cell 2019, 76, 217. [Google Scholar] [CrossRef] [PubMed]
- Zeman, M.K.; Cimprich, K.A. Causes and Consequences of Replication Stress. Nat Cell Biol 2014, 16, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Dilley, R.L.; Verma, P.; Cho, N.W.; Winters, H.D.; Wondisford, A.R.; Greenberg, R.A. Break-Induced Telomere Synthesis Underlies Alternative Telomere Maintenance. Nature 2016, 539, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.E.; Maréchal, A.; Flynn, R.L. SMARCAL1 Resolves Replication Stress at ALT Telomeres. Cell Reports 2016, 14, 1032–1040. [Google Scholar] [CrossRef]
- Silva, B.; Pentz, R.; Figueira, A.M.; Arora, R.; Lee, Y.W.; Hodson, C.; Wischnewski, H.; Deans, A.J.; Azzalin, C.M. FANCM Limits ALT Activity by Restricting Telomeric Replication Stress Induced by Deregulated BLM and R-Loops. Nat Commun 2019, 10, 2253. [Google Scholar] [CrossRef]
- Lu, R.; O’Rourke, J.J.; Sobinoff, A.P.; Allen, J.A.M.; Nelson, C.B.; Tomlinson, C.G.; Lee, M.; Reddel, R.R.; Deans, A.J.; Pickett, H.A. The FANCM-BLM-TOP3A-RMI Complex Suppresses Alternative Lengthening of Telomeres (ALT). Nat Commun 2019, 10, 2252. [Google Scholar] [CrossRef]
- Arora, R.; Lee, Y.; Wischnewski, H.; Brun, C.M.; Schwarz, T.; Azzalin, C.M. RNaseH1 Regulates TERRA-Telomeric DNA Hybrids and Telomere Maintenance in ALT Tumour Cells. Nat Commun 2014, 5, 5220. [Google Scholar] [CrossRef]
- Azzalin, C.M.; Lingner, J. Telomere Functions Grounding on TERRA Firma. Trends Cell Biol. 2015, 25, 29–36. [Google Scholar] [CrossRef]
- Root, H.; Larsen, A.; Komosa, M.; Al-Azri, F.; Li, R.; Bazett-Jones, D.P.; Stephen Meyn, M. FANCD2 Limits BLM-Dependent Telomere Instability in the Alternative Lengthening of Telomeres Pathway. Hum. Mol. Genet. 2016, 25, 3255–3268. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.W.; Dilley, R.L.; Lampson, M.A.; Greenberg, R.A. Interchromosomal Homology Searches Drive Directional ALT Telomere Movement and Synapsis. Cell 2014, 159, 108–121. [Google Scholar] [CrossRef]
- Potts, P.R.; Yu, H. The SMC5/6 Complex Maintains Telomere Length in ALT Cancer Cells through SUMOylation of Telomere-Binding Proteins. Nat Struct Mol Biol 2007, 14, 581–590. [Google Scholar] [CrossRef]
- Schrank, B.R.; Aparicio, T.; Li, Y.; Chang, W.; Chait, B.T.; Gundersen, G.G.; Gottesman, M.E.; Gautier, J. Nuclear ARP2/3 Drives DNA Break Clustering for Homology-Directed Repair. Nature 2018, 559, 61–66. [Google Scholar] [CrossRef]
- Garcia-Exposito, L.; Bournique, E.; Bergoglio, V.; Bose, A.; Barroso-Gonzalez, J.; Zhang, S.; Roncaioli, J.L.; Lee, M.; Wallace, C.T.; Watkins, S.C.; et al. Proteomic Profiling Reveals a Specific Role for Translesion DNA Polymerase η in the Alternative Lengthening of Telomeres. Cell Reports 2016, 17, 1858–1871. [Google Scholar] [CrossRef] [PubMed]
- Bizard, A.H.; Hickson, I.D. The Dissolution of Double Holliday Junctions. Cold Spring Harbor Perspectives in Biology 2014, 6, a016477–a016477. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Keirsey, J.; Russell, B.; Kavecansky, J.; Lillard-Wetherell, K.; Tahmaseb, K.; Turchi, J.J.; Groden, J. Telomerase-Associated Protein 1, HSP90, and Topoisomerase IIα Associate Directly with the BLM Helicase in Immortalized Cells Using ALT and Modulate Its Helicase Activity Using Telomeric DNA Substrates. Journal of Biological Chemistry 2009, 284, 14966–14977. [Google Scholar] [CrossRef] [PubMed]
- Sobinoff, A.P.; Allen, J.A.; Neumann, A.A.; Yang, S.F.; Walsh, M.E.; Henson, J.D.; Reddel, R.R.; Pickett, H.A. BLM and SLX4 Play Opposing Roles in Recombination-dependent Replication at Human Telomeres. The EMBO Journal 2017, 36, 2907–2919. [Google Scholar] [CrossRef] [PubMed]
- Hoang, S.M.; O’Sullivan, R.J. Alternative Lengthening of Telomeres: Building Bridges To Connect Chromosome Ends. Trends in Cancer 2020, 6, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Wright, W.E.; Shay, J.W. Clustered Telomeres in Phase-Separated Nuclear Condensates Engage Mitotic DNA Synthesis through BLM and RAD52. Genes Dev. 2019, 33, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Xue, C.; Kumar, S.; Crickard, J.B.; Yu, Y.; Wang, W.; Pham, N.; Li, Y.; Niu, H.; Sung, P.; et al. Rad52 Restrains Resection at DNA Double-Strand Break Ends in Yeast. Molecular Cell 2019, 76, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Malacaria, E.; Pugliese, G.M.; Honda, M.; Marabitti, V.; Aiello, F.A.; Spies, M.; Franchitto, A.; Pichierri, P. Rad52 Prevents Excessive Replication Fork Reversal and Protects from Nascent Strand Degradation. Nat Commun 2019, 10, 1412. [Google Scholar] [CrossRef]
- Verma, P.; Dilley, R.L.; Zhang, T.; Gyparaki, M.T.; Li, Y.; Greenberg, R.A. RAD52 and SLX4 Act Nonepistatically to Ensure Telomere Stability during Alternative Telomere Lengthening. Genes Dev. 2019, 33, 221–235. [Google Scholar] [CrossRef]
- West, S.C.; Blanco, M.G.; Chan, Y.W.; Matos, J.; Sarbajna, S.; Wyatt, H.D.M. Resolution of Recombination Intermediates: Mechanisms and Regulation. Cold Spring Harb Symp Quant Biol 2015, 80, 103–109. [Google Scholar] [CrossRef]
- Bhowmick, R.; Minocherhomji, S.; Hickson, I.D. RAD52 Facilitates Mitotic DNA Synthesis Following Replication Stress. Molecular Cell 2016, 64, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, S.K.; Kamileri, I.; Lugli, N.; Evangelou, K.; Da-Ré, C.; Huber, F.; Padayachy, L.; Tardy, S.; Nicati, N.L.; Barriot, S.; et al. Mammalian RAD52 Functions in Break-Induced Replication Repair of Collapsed DNA Replication Forks. Molecular Cell 2016, 64, 1127–1134. [Google Scholar] [CrossRef]
- Özer, Ö.; Bhowmick, R.; Liu, Y.; Hickson, I.D. Human Cancer Cells Utilize Mitotic DNA Synthesis to Resist Replication Stress at Telomeres Regardless of Their Telomere Maintenance Mechanism. Oncotarget 2018, 9, 15836–15846. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.T.; Cesare, A.J.; Rivera, T.; Karlseder, J. Cell Death during Crisis Is Mediated by Mitotic Telomere Deprotection. Nature 2015, 522, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Davoli, T.; Denchi, E.L.; de Lange, T. Persistent Telomere Damage Induces Bypass of Mitosis and Tetraploidy. Cell 2010, 141, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Maciejowski, J.; Li, Y.; Bosco, N.; Campbell, P.J.; de Lange, T. Chromothripsis and Kataegis Induced by Telomere Crisis. Cell 2015, 163, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Nassour, J.; Radford, R.; Correia, A.; Fusté, J.M.; Schoell, B.; Jauch, A.; Shaw, R.J.; Karlseder, J. Autophagic Cell Death Restricts Chromosomal Instability during Replicative Crisis. Nature 2019, 565, 659–663. [Google Scholar] [CrossRef]
- Bayne, S.; Liu, J.-P. Hormones and Growth Factors Regulate Telomerase Activity in Ageing and Cancer. Molecular and Cellular Endocrinology 2005, 240, 11–22. [Google Scholar] [CrossRef]
- Patrick, M.S.; Cheng, N.-L.; Kim, J.; An, J.; Dong, F.; Yang, Q.; Zou, I.; Weng, N. Human T Cell Differentiation Negatively Regulates Telomerase Expression Resulting in Reduced Activation-Induced Proliferation and Survival. Front. Immunol. 2019, 10, 1993. [Google Scholar] [CrossRef]
- Burgess, R.J.; Agathocleous, M.; Morrison, S.J. Metabolic Regulation of Stem Cell Function. J Intern Med 2014, 276, 12–24. [Google Scholar] [CrossRef]
- Almeida, L.; Lochner, M.; Berod, L.; Sparwasser, T. Metabolic Pathways in T Cell Activation and Lineage Differentiation. Seminars in Immunology 2016, 28, 514–524. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Miyazawa, H.; Aulehla, A. Revisiting the Role of Metabolism during Development. Development 2018, 145, dev131110. [Google Scholar] [CrossRef]
- Bao, Y.; Mukai, K.; Hishiki, T.; Kubo, A.; Ohmura, M.; Sugiura, Y.; Matsuura, T.; Nagahata, Y.; Hayakawa, N.; Yamamoto, T.; et al. Energy Management by Enhanced Glycolysis in G1-Phase in Human Colon Cancer Cells In Vitro and In Vivo. Molecular Cancer Research 2013, 11, 973–985. [Google Scholar] [CrossRef]
- Mitra, K.; Wunder, C.; Roysam, B.; Lin, G.; Lippincott-Schwartz, J. A Hyperfused Mitochondrial State Achieved at G 1 –S Regulates Cyclin E Buildup and Entry into S Phase. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 11960–11965. [Google Scholar] [CrossRef]
- Tudzarova, S.; Colombo, S.L.; Stoeber, K.; Carcamo, S.; Williams, G.H.; Moncada, S. Two Ubiquitin Ligases, APC/C-Cdh1 and SKP1-CUL1-F (SCF)-β-TrCP, Sequentially Regulate Glycolysis during the Cell Cycle. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 5278–5283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fan, M.; Candas, D.; Zhang, T.-Q.; Qin, L.; Eldridge, A.; Wachsmann-Hogiu, S.; Ahmed, K.M.; Chromy, B.A.; Nantajit, D.; et al. Cyclin B1/Cdk1 Coordinates Mitochondrial Respiration for Cell-Cycle G2/M Progression. Developmental Cell 2014, 29, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Sutter, B.M.; Li, B.; Tu, B.P. Acetyl-CoA Induces Cell Growth and Proliferation by Promoting the Acetylation of Histones at Growth Genes. Molecular Cell 2011, 42, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Intlekofer, A.M.; Dematteo, R.G.; Venneti, S.; Finley, L.W.S.; Lu, C.; Judkins, A.R.; Rustenburg, A.S.; Grinaway, P.B.; Chodera, J.D.; Cross, J.R.; et al. Hypoxia Induces Production of L-2-Hydroxyglutarate. Cell Metabolism 2015, 22, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, S.; Hau, M. Telomere Attrition: Metabolic Regulation and Signalling Function? Biol. Lett. 2019, 15, 20180885. [Google Scholar] [CrossRef]
- Zheng, J. Energy Metabolism of Cancer: Glycolysis versus Oxidative Phosphorylation (Review). Oncology Letters 2012, 4, 1151–1157. [Google Scholar] [CrossRef]
- Fice, H.; Robaire, B. Telomere Dynamics Throughout Spermatogenesis. Genes 2019, 10, 525. [Google Scholar] [CrossRef] [PubMed]
- Thilagavathi, J.; Mishra, S.S.; Kumar, M.; Vemprala, K.; Deka, D.; Dhadwal, V.; Dada, R. Analysis of Telomere Length in Couples Experiencing Idiopathic Recurrent Pregnancy Loss. J Assist Reprod Genet 2013, 30, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, F.; Dai, S.; Zhang, N.; Zhao, W.; Bai, R.; Sun, Y. Sperm Telomere Length Is Positively Associated with the Quality of Early Embryonic Development. Hum. Reprod. 2015, 30, 1876–1881. [Google Scholar] [CrossRef]
- Anifandis, G.; Samara, M.; Simopoulou, M.; Messini, C.I.; Chatzimeletiou, K.; Thodou, E.; Daponte, A.; Georgiou, I. Insights into the Role of Telomeres in Human Embryological Parameters. Opinions Regarding IVF. JDB 2021, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Berneau, S.C.; Shackleton, J.; Nevin, C.; Altakroni, B.; Papadopoulos, G.; Horne, G.; Brison, D.R.; Murgatroyd, C.; Povey, A.C.; Carroll, M. Associations of Sperm Telomere Length with Semen Parameters, Clinical Outcomes and Lifestyle Factors in Human Normozoospermic Samples. Andrology 2020, 8, 583–593. [Google Scholar] [CrossRef]
- Beygi, Z.; Forouhari, S.; Mahmoudi, E.; Hayat, S.M.G.; Nourimand, F. Role of Oxidative Stress and Antioxidant Supplementation in Male Fertility. CMM 2021, 21, 265–282. [Google Scholar] [CrossRef]
- Ahmed, W.; Lingner, J. PRDX1 and MTH1 Cooperate to Prevent ROS-Mediated Inhibition of Telomerase. Genes Dev. 2018, 32, 658–669. [Google Scholar] [CrossRef]
- Antunes, D.M.F.; Kalmbach, K.H.; Wang, F.; Dracxler, R.C.; Seth-Smith, M.L.; Kramer, Y.; Buldo-Licciardi, J.; Kohlrausch, F.B.; Keefe, D.L. A Single-Cell Assay for Telomere DNA Content Shows Increasing Telomere Length Heterogeneity, as Well as Increasing Mean Telomere Length in Human Spermatozoa with Advancing Age. J Assist Reprod Genet 2015, 32, 1685–1690. [Google Scholar] [CrossRef]
- De Frutos, C.; López-Cardona, A.P.; Fonseca Balvís, N.; Laguna-Barraza, R.; Rizos, D.; Gutierrez-Adán, A.; Bermejo-Álvarez, P. Spermatozoa Telomeres Determine Telomere Length in Early Embryos and Offspring. REPRODUCTION 2016, 151, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Tan, Y.; Qiu, X.; Luo, H.; Li, Y.; Li, R.; Yang, X. Sperm Telomere Length as a Novel Biomarker of Male Infertility and Embryonic Development: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2023, 13, 1079966. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bailey, S.M.; Okuka, M.; Muñoz, P.; Li, C.; Zhou, L.; Wu, C.; Czerwiec, E.; Sandler, L.; Seyfang, A.; et al. Telomere Lengthening Early in Development. Nat Cell Biol 2007, 9, 1436–1441. [Google Scholar] [CrossRef]
- Bender, H.S.; Murchison, E.P.; Pickett, H.A.; Deakin, J.E.; Strong, M.A.; Conlan, C.; McMillan, D.A.; Neumann, A.A.; Greider, C.W.; Hannon, G.J.; et al. Extreme Telomere Length Dimorphism in the Tasmanian Devil and Related Marsupials Suggests Parental Control of Telomere Length. PLoS ONE 2012, 7, e46195. [Google Scholar] [CrossRef]
- Eisenhauer, K.M.; Gerstein, R.M.; Chiu, C.-P.; Conti, M.; Hsueh, A.J.W. Telomerase Activity in Female and Male Rat Germ Cells Undergoing Meiosis and in Early Embryos1. Biology of Reproduction 1997, 56, 1120–1125. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Telomeres and Telomerase: Implications for Cancer and Aging. Radiation Research 2001, 155, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Blasco, M.A.; Trimarchi, J.R.; Keefe, D.L. An Essential Role for Functional Telomeres in Mouse Germ Cells during Fertilization and Early Development. Developmental Biology 2002, 249, 74–84. [Google Scholar] [CrossRef]
- Siderakis, M.; Tarsounas, M. Telomere Regulation and Function during Meiosis. Chromosome Res 2007, 15, 667–679. [Google Scholar] [CrossRef]
- Keefe, D.L.; Franco, S.; Liu, L.; Trimarchi, J.; Cao, B.; Weitzen, S.; Agarwal, S.; Blasco, M.A. Telomere Length Predicts Embryo Fragmentation after in Vitro Fertilization in Women—Toward a Telomere Theory of Reproductive Aging in Women. American Journal of Obstetrics and Gynecology 2005, 192, 1256–1260. [Google Scholar] [CrossRef]
- Liu, L.; Franco, S.; Spyropoulos, B.; Moens, P.B.; Blasco, M.A.; Keefe, D.L. Irregular Telomeres Impair Meiotic Synapsis and Recombination in Mice. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 6496–6501. [Google Scholar] [CrossRef]
- Treff, N.R.; Su, J.; Taylor, D.; Scott, R.T. Telomere DNA Deficiency Is Associated with Development of Human Embryonic Aneuploidy. PLoS Genet 2011, 7, e1002161. [Google Scholar] [CrossRef]
- Keefe, D.L. Telomeres and Genomic Instability during Early Development. European Journal of Medical Genetics 2020, 63, 103638. [Google Scholar] [CrossRef] [PubMed]
- Yamada-Fukunaga, T.; Yamada, M.; Hamatani, T.; Chikazawa, N.; Ogawa, S.; Akutsu, H.; Miura, T.; Miyado, K.; Tarín, J.J.; Kuji, N.; et al. Age-Associated Telomere Shortening in Mouse Oocytes. Reprod Biol Endocrinol 2013, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, M.; Ye, X.; Liu, K.; Huang, J.; Wang, L.; Ji, G.; Liu, N.; Tang, X.; Baltz, J.M.; et al. Delay in Oocyte Aging in Mice by the Antioxidant N-Acetyl-l-Cysteine (NAC). Human Reproduction 2012, 27, 1411–1420. [Google Scholar] [CrossRef]
- Kinugawa, C.; Murakami, T.; Okamura, K.; Yajima, A. Telomerase Activity in Normal Ovaries and Premature Ovarian Failure. Tohoku J. Exp. Med. 2000, 190, 231–238. [Google Scholar] [CrossRef]
- Lavranos, T.C.; Mathis, J.M.; Latham, S.E.; Kalionis, B.; Shay, J.W.; Rodgers, R.J. Evidence for Ovarian Granulosa Stem Cells: Telomerase Activity and Localization of the Telomerase Ribonucleic Acid Component in Bovine Ovarian Follicles1. Biology of Reproduction 1999, 61, 358–366. [Google Scholar] [CrossRef]
- Russo, V.; Berardinelli, P.; Martelli, A.; Giacinto, O.D.; Nardinocchi, D.; Fantasia, D.; Barboni, B. Expression of Telomerase Reverse Transcriptase Subunit (TERT) and Telomere Sizing in Pig Ovarian Follicles. J Histochem Cytochem. 2006, 54, 443–455. [Google Scholar] [CrossRef]
- Yamagata, Y.; Nakamura, Y.; Umayahara, K.; Harada, A.; Takayama, H.; Sugino, N.; Kato, H. Changes in Telomerase Activity in Experimentally Induced Atretic Follicles of Immature Rats. Endocr J 2002, 49, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Bayne, S.; Li, H.; Jones, M.E.E.; Pinto, A.R.; Van Sinderen, M.; Drummond, A.; Simpson, E.R.; Liu, J.-P. Estrogen Deficiency Reversibly Induces Telomere Shortening in Mouse Granulosa Cells and Ovarian Aging in Vivo. Protein Cell 2011, 2, 333–346. [Google Scholar] [CrossRef]
- Goto, H.; Iwata, H.; Takeo, S.; Nisinosono, K.; Murakami, S.; Monji, Y.; Kuwayama, T. Effect of Bovine Age on the Proliferative Activity, Global DNA Methylation, Relative Telomere Length and Telomerase Activity of Granulosa Cells. Zygote 2013, 21, 256–264. [Google Scholar] [CrossRef]
- Endo, M.; Kimura, K.; Kuwayama, T.; Monji, Y.; Iwata, H. Effect of Estradiol during Culture of Bovine Oocyte–Granulosa Cell Complexes on the Mitochondrial DNA Copies of Oocytes and Telomere Length of Granulosa Cells. Zygote 2014, 22, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-P.; Li, H. Telomerase in the Ovary. REPRODUCTION 2010, 140, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Butts, S.; Riethman, H.; Ratcliffe, S.; Shaunik, A.; Coutifaris, C.; Barnhart, K. Correlation of Telomere Length and Telomerase Activity with Occult Ovarian Insufficiency. The Journal of Clinical Endocrinology & Metabolism 2009, 94, 4835–4843. [Google Scholar] [CrossRef]
- Yung, Y.; Maydan, S.A.; Bart, Y.; Orvieto, R.; Aizer, A. Human Granulosa Cells of Poor Ovarian Responder Patients Display Telomeres Shortening. J Assist Reprod Genet 2023, 40, 1943–1947. [Google Scholar] [CrossRef]
- Cheng, G.; Kong, F.; Luan, Y.; Sun, C.; Wang, J.; Zhang, L.; Jiang, B.; Qi, T.; Zhao, J.; Zheng, C.; et al. Differential Shortening Rate of Telomere Length in the Development of Human Fetus. Biochemical and Biophysical Research Communications 2013, 442, 112–115. [Google Scholar] [CrossRef]
- Ozturk, S.; Sozen, B.; Demir, N. Telomere Length and Telomerase Activity during Oocyte Maturation and Early Embryo Development in Mammalian Species. Molecular Human Reproduction 2014, 20, 15–30. [Google Scholar] [CrossRef]
- Turner, S.; Wong, H.P.; Rai, J.; Hartshorne, G.M. Telomere Lengths in Human Oocytes, Cleavage Stage Embryos and Blastocysts. Molecular Human Reproduction 2010, 16, 685–694. [Google Scholar] [CrossRef]
- Le, R.; Huang, Y.; Zhang, Y.; Wang, H.; Lin, J.; Dong, Y.; Li, Z.; Guo, M.; Kou, X.; Zhao, Y.; et al. Dcaf11 Activates Zscan4-Mediated Alternative Telomere Lengthening in Early Embryos and Embryonic Stem Cells. Cell Stem Cell 2021, 28, 732–747. [Google Scholar] [CrossRef]
- Kordowitzki, P.; López De Silanes, I.; Guío-Carrión, A.; Blasco, M.A. Dynamics of Telomeric Repeat-Containing RNA Expression in Early Embryonic Cleavage Stages with Regards to Maternal Age. Aging 2020, 12, 15906–15917. [Google Scholar] [CrossRef]
- Nagaraj, R.; Sharpley, M.S.; Chi, F.; Braas, D.; Zhou, Y.; Kim, R.; Clark, A.T.; Banerjee, U. Nuclear Localization of Mitochondrial TCA Cycle Enzymes as a Critical Step in Mammalian Zygotic Genome Activation. Cell 2017, 168, 210–223. [Google Scholar] [CrossRef]
- Varela, E.; Schneider, R.P.; Ortega, S.; Blasco, M.A. Different Telomere-Length Dynamics at the Inner Cell Mass versus Established Embryonic Stem (ES) Cells. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 15207–15212. [Google Scholar] [CrossRef]
- Schaetzlein, S.; Lucas-Hahn, A.; Lemme, E.; Kues, W.A.; Dorsch, M.; Manns, M.P.; Niemann, H.; Rudolph, K.L. Telomere Length Is Reset during Early Mammalian Embryogenesis. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 8034–8038. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Kues, W.A.; Baulain, U.; Garrels, W.; Herrmann, D.; Niemann, H. Species-Specific Telomere Length Differences Between Blastocyst Cell Compartments and Ectopic Telomere Extension in Early Bovine Embryos by Human Telomerase Reverse Transcriptase. Biology of Reproduction 2011, 84, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gu, Y.; Zhou, J.; Zang, J.; Ling, X.; Li, H.; Hu, L.; Xu, B.; Zhang, B.; Qin, N.; et al. Leukocyte Telomere Length in Children Born Following Blastocyst-Stage Embryo Transfer. Nat Med 2022, 28, 2646–2653. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Li, C.; Sun, H.; Xin, Y.; Zhu, S.; Liu, Y.; Tang, S.; Han, L.; Huang, Z.; Wang, Q. Telomere Dysfunction in Oocytes and Embryos From Obese Mice. Front. Cell Dev. Biol. 2021, 9, 617225. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, K.; Liu, Y.; Fu, Y.; Gao, S.; Gong, P.; Wang, H.; Zhou, Z.; Zeng, M.; Wu, Z.; et al. Telomere Heterogeneity Linked to Metabolism and Pluripotency State Revealed by Simultaneous Analysis of Telomere Length and RNA-Seq in the Same Human Embryonic Stem Cell. BMC Biol 2017, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Tsogtbaatar, E.; Landin, C.; Minter-Dykhouse, K.; Folmes, C.D.L. Energy Metabolism Regulates Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Weng, N.; Palmer, L.D.; Levine, B.L.; Lane, H.C.; June, C.H.; Hodes, R.J. Tales of Tails: Regulation of Telomere Length and Telomerase Activity during Lymphocyte Development, Differentiation, Activation, and Aging. Immunol Rev 1997, 160, 43–54. [Google Scholar] [CrossRef]
- Quinn, K.M.; Palchaudhuri, R.; Palmer, C.S.; La Gruta, N.L. The Clock Is Ticking: The Impact of Ageing on T Cell Metabolism. Clin & Trans Imm 2019, 8, e01091. [Google Scholar] [CrossRef]
- Buck, M.D.; O’Sullivan, D.; Klein Geltink, R.I.; Curtis, J.D.; Chang, C.-H.; Sanin, D.E.; Qiu, J.; Kretz, O.; Braas, D.; van der Windt, G.J.W.; et al. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell 2016, 166, 63–76. [Google Scholar] [CrossRef]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted Role of mTOR (Mammalian Target of Rapamycin) Signaling Pathway in Human Health and Disease. Sig Transduct Target Ther 2023, 8, 375. [Google Scholar] [CrossRef]
- Shliapina, V.L.; Yurtaeva, S.V.; Rubtsova, M.P.; Dontsova, O.A. At the Crossroads: Mechanisms of Apoptosis and Autophagy in Cell Life and Death. Acta Naturae 2021, 13, 106–115. [Google Scholar] [CrossRef]
- González, A.; Hall, M.N.; Lin, S.-C.; Hardie, D.G. AMPK and TOR: The Yin and Yang of Cellular Nutrient Sensing and Growth Control. Cell Metabolism 2020, 31, 472–492. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A Nutrient and Energy Sensor That Maintains Energy Homeostasis. Nat Rev Mol Cell Biol 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat Rev Mol Cell Biol 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Rubtsova, M.; Naraykina, Y.; Vasilkova, D.; Meerson, M.; Zvereva, M.; Prassolov, V.; Lazarev, V.; Manuvera, V.; Kovalchuk, S.; Anikanov, N.; et al. Protein Encoded in Human Telomerase RNA Is Involved in Cell Protective Pathways. Nucleic Acids Research 2018. [Google Scholar] [CrossRef] [PubMed]
- Shliapina, V.; Koriagina, M.; Vasilkova, D.; Govorun, V.; Dontsova, O.; Rubtsova, M. Human Telomerase RNA Protein Encoded by Telomerase RNA Is Involved in Metabolic Responses. Front. Cell Dev. Biol. 2021, 9, 754611. [Google Scholar] [CrossRef] [PubMed]
- Rubtsova, M.; Dontsova, O. How Structural Features Define Biogenesis and Function of Human Telomerase RNA Primary Transcript. Biomedicines 2022, 10, 1650. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



