Submitted:
28 December 2023
Posted:
28 December 2023
You are already at the latest version
Abstract
Keywords:
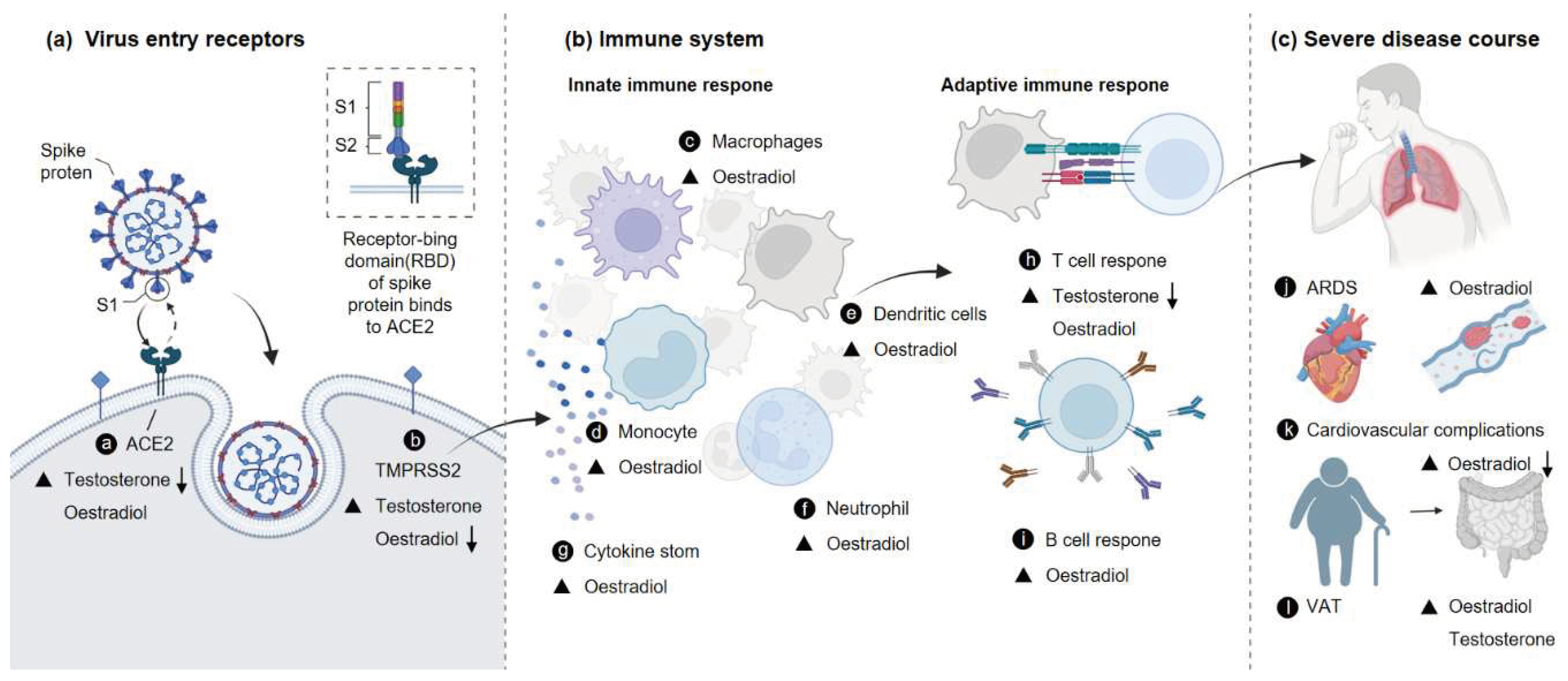
1. Introduction
2. Sex Hormones and COVID-19
2.1. Androgens and COVID-19 Severity
2.2. Estradiol and COVID-19 Severity
2.3. Progesterone and COVID-19 Severity
3. Sex Hormones and Expression of Viral Receptors
3.1. ACE2 and TMPRSS
3.2. Sex-Specific Expression of TMPRSS2 and ACE2
3.3. Regulation of TMPRSS2 and ACE2 by Sex Hormones
4. Sex Hormones and Immune Responses
4.1. Cytokine Storm
4.2. Gender Differences in Immune Responses
4.3. Sex Hormones and the Immune Response
4.4. Regulation of Disease by sex Hormones Through Immunization
5. Complications of COVID-19
5.1. Tissue Damage
5.2. Cardiovascular System
5.3. Visceral Adiposity
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team, n. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) - China, 2020. China CDC weekly 2020, 2, 113–122. [Google Scholar] [CrossRef]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nature Communications 2020, 11, 6317. [Google Scholar] [CrossRef]
- Cardoso, F.S.; Papoila, A.L.; Machado, R.S.; Fidalgo, P. Age, sex, and comorbidities predict ICU admission or mortality in cases with SARS-CoV2 infection: A population-based cohort study. Critical Care (London, England) 2020, 24, 465. [Google Scholar] [CrossRef]
- Breslin, N.; Baptiste, C.; Miller, R.; Fuchs, K.; Goffman, D.; Gyamfi-Bannerman, C.; D'Alton, M. Coronavirus disease 2019 in pregnancy: Early lessons. American journal of obstetrics & gynecology MFM 2020, 2, 100111. [Google Scholar] [CrossRef]
- Breslin, N.; Baptiste, C.; Gyamfi-Bannerman, C.; Miller, R.; Martinez, R.; Bernstein, K.; Ring, L.; Landau, R.; Purisch, S.; Friedman, A.M.; et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of New York City hospitals. American journal of obstetrics & gynecology MFM 2020, 2, 100118. [Google Scholar] [CrossRef]
- Ding, T.; Zhang, J.; Wang, T.; Cui, P.; Chen, Z.; Jiang, J.; Zhou, S.; Dai, J.; Wang, B.; Yuan, S.; et al. Potential Influence of Menstrual Status and Sex Hormones on Female Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Cross-sectional Multicenter Study in Wuhan, China. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 2021, 72, e240–e248. [Google Scholar] [CrossRef]
- Seeland, U.; Coluzzi, F.; Simmaco, M.; Mura, C.; Bourne, P.E.; Heiland, M.; Preissner, R.; Preissner, S. Evidence for treatment with estradiol for women with SARS-CoV-2 infection. BMC medicine 2020, 18, 369. [Google Scholar] [CrossRef] [PubMed]
- Samuel, R.M.; Majd, H.; Richter, M.N.; Ghazizadeh, Z.; Zekavat, S.M.; Navickas, A.; Ramirez, J.T.; Asgharian, H.; Simoneau, C.R.; Bonser, L.R.; et al. Androgen Signaling Regulates SARS-CoV-2 Receptor Levels and Is Associated with Severe COVID-19 Symptoms in Men. Cell Stem Cell 2020, 27, 876–889.e812. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.; Schaumburg, B.; Mueller, Z.; Parplys, A.; Jarczak, D.; Roedl, K.; Nierhaus, A.; de Heer, G.; Grensemann, J.; Schneider, B.; et al. High estradiol and low testosterone levels are associated with critical illness in male but not in female COVID-19 patients: A retrospective cohort study. Emerging Microbes & Infections 2021, 10, 1807–1818. [Google Scholar] [CrossRef]
- Dhindsa, S.; Zhang, N.; McPhaul, M.J.; Wu, Z.; Ghoshal, A.K.; Erlich, E.C.; Mani, K.; Randolph, G.J.; Edwards, J.R.; Mudd, P.A.; et al. Association of Circulating Sex Hormones With Inflammation and Disease Severity in Patients With COVID-19. JAMA network open 2021, 4, e2111398. [Google Scholar] [CrossRef] [PubMed]
- Gebhard, C.E.; Hamouda, N.; Gebert, P.; Regitz-Zagrosek, V.; Gebhard, C.; Investigators, C. Sex versus gender-related characteristics: Which predicts clinical outcomes of acute COVID-19? Intensive Care Medicine 2022, 48, 1652–1655. [Google Scholar] [CrossRef]
- Durcan, E.; Turan, S.; Bircan, B.E.; Yaylamaz, S.; Demirel, O.; Demir, A.N.; Sulu, C.; Kara, Z.; Sahin, S.; Taze, S.S.; et al. TransCOVID: Does Gender-Affirming Hormone Therapy Play a Role in Contracting COVID-19? Journal of Sex & Marital Therapy 2022, 48, 415–426. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nature Reviews. Immunology 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Li, C.-L.; Li, C.-Y.; Lin, Y.-Y.; Ho, M.-C.; Chen, D.-S.; Chen, P.-J.; Yeh, S.-H. Androgen Receptor Enhances Hepatic Telomerase Reverse Transcriptase Gene Transcription After Hepatitis B Virus Integration or Point Mutation in Promoter Region. Hepatology (Baltimore, Md.) 2019, 69, 498–512. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-J.; Yeh, S.-H.; Liu, W.-H.; Lin, C.-C.; Huang, H.-C.; Chen, C.-L.; Chen, D.-S.; Chen, P.-J. Androgen pathway stimulates MicroRNA-216a transcription to suppress the tumor suppressor in lung cancer-1 gene in early hepatocarcinogenesis. Hepatology 2012, 56, 632–643. [Google Scholar] [CrossRef]
- A preliminary observation: Male pattern hair loss among hospitalized COVID-19 patients in Spain - A potential clue to the role of androgens in COVID-19 severity - PubMed. Available online: (accessed on.
- Wambier, C.G.; Vaño-Galván, S.; McCoy, J.; Gomez-Zubiaur, A.; Herrera, S.; Hermosa-Gelbard, Á.; Moreno-Arrones, O.M.; Jiménez-Gómez, N.; González-Cantero, A.; Fonda-Pascual, P.; et al. Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: The "Gabrin sign". Journal of the American Academy of Dermatology 2020, 83, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.G.; Zhong, X.; Liaw, B.; Tremblay, D.; Tsao, C.-K.; Galsky, M.D.; Oh, W.K. Does androgen deprivation therapy protect against severe complications from COVID-19? Annals of Oncology: Official Journal of the European Society for Medical Oncology 2020, 31, 1419–1420. [Google Scholar] [CrossRef]
- Lazzeri, M.; Duga, S.; Azzolini, E.; Fasulo, V.; Buffi, N.; Saita, A.; Lughezzani, G.; Paraboschi, E.M.; Hurle, R.; Nobili, A.; et al. Impact of chronic exposure to 5-alpha reductase inhibitors on the risk of hospitalization for COVID-19: A case-control study in male population from two COVID-19 regional centers of Lombardy, Italy. Minerva Urology and Nephrology 2022, 74, 77–84. [Google Scholar] [CrossRef] [PubMed]
- A Phase 2 Trial of the Effect of Antiandrogen Therapy on COVID-19 Outcome: No Evidence of Benefit, Supported by Epidemiology and In Vitro Data - PubMed. Available online: (accessed on.
- Nickols, N.G.; Mi, Z.; DeMatt, E.; Biswas, K.; Clise, C.E.; Huggins, J.T.; Maraka, S.; Ambrogini, E.; Mirsaeidi, M.S.; Levin, E.R.; et al. Effect of Androgen Suppression on Clinical Outcomes in Hospitalized Men With COVID-19: The HITCH Randomized Clinical Trial. JAMA network open 2022, 5, e227852. [Google Scholar] [CrossRef]
- Rambhatla, A.; Bronkema, C.J.; Corsi, N.; Keeley, J.; Sood, A.; Affas, Z.; Dabaja, A.A.; Rogers, C.G.; Liroff, S.A.; Abdollah, F. COVID-19 Infection in Men on Testosterone Replacement Therapy. The Journal of Sexual Medicine 2021, 18, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.B.O.; Leal, F.; Argenton, J.L.P.; Carvalheira, J.B.C. Impact of androgen deprivation therapy on mortality of prostate cancer patients with COVID-19: A propensity score-based analysis. Infectious Agents and Cancer 2021, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- Cinislioglu, A.E.; Cinislioglu, N.; Demirdogen, S.O.; Sam, E.; Akkas, F.; Altay, M.S.; Utlu, M.; Sen, I.A.; Yildirim, F.; Kartal, S.; et al. The relationship of serum testosterone levels with the clinical course and prognosis of COVID-19 disease in male patients: A prospective study. Andrology 2022, 10, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, G.; Di Stasi, V.; Inglese, F.; Beccaria, M.; Garuti, M.; Di Costanzo, D.; Spreafico, F.; Greco, G.F.; Cervi, G.; Pecoriello, A.; et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology 2021, 9, 88–98. [Google Scholar] [CrossRef]
- Camici, M.; Zuppi, P.; Lorenzini, P.; Scarnecchia, L.; Pinnetti, C.; Cicalini, S.; Nicastri, E.; Petrosillo, N.; Palmieri, F.; D'Offizi, G.; et al. Role of testosterone in SARS-CoV-2 infection: A key pathogenic factor and a biomarker for severe pneumonia. International journal of infectious diseases: IJID: Official publication of the International Society for Infectious Diseases 2021, 108, 244–251. [Google Scholar] [CrossRef]
- Li, C.; Ye, Z.; Zhang, A.J.X.; Chan, J.F.W.; Song, W.; Liu, F.; Chen, Y.; Kwan, M.Y.W.; Lee, A.C.Y.; Zhao, Y.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection by Intranasal or Intratesticular Route Induces Testicular Damage. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 2022, 75, e974–e990. [Google Scholar] [CrossRef] [PubMed]
- Pozzilli, P.; Lenzi, A. Commentary: Testosterone, a key hormone in the context of COVID-19 pandemic. Metabolism: Clinical and Experimental 2020, 108, 154252. [Google Scholar] [CrossRef]
- Costeira, R.; Lee, K.A.; Murray, B.; Christiansen, C.; Castillo-Fernandez, J.; Ni Lochlainn, M.; Capdevila Pujol, J.; Macfarlane, H.; Kenny, L.C.; Buchan, I.; et al. Estrogen and COVID-19 symptoms: Associations in women from the COVID Symptom Study. PLoS ONE 2021, 16, e0257051. [Google Scholar] [CrossRef]
- Montopoli, M.; Zorzi, M.; Cocetta, V.; Prayer-Galetti, T.; Guzzinati, S.; Bovo, E.; Rugge, M.; Calcinotto, A. Clinical outcome of SARS-CoV-2 infection in breast and ovarian cancer patients who underwent antiestrogenic therapy. Annals of Oncology: Official Journal of the European Society for Medical Oncology 2021, 32, 676–677. [Google Scholar] [CrossRef]
- Baker, M.E. What are the physiological estrogens? Steroids 2013, 78, 337–340. [Google Scholar] [CrossRef]
- Lange, C.A.; Gioeli, D.; Hammes, S.R.; Marker, P.C. Integration of rapid signaling events with steroid hormone receptor action in breast and prostate cancer. Annual Review of Physiology 2007, 69, 171–199. [Google Scholar] [CrossRef]
- Bashour, N.M.; Wray, S. Progesterone directly and rapidly inhibits GnRH neuronal activity via progesterone receptor membrane component 1. Endocrinology 2012, 153, 4457–4469. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ulrich, B.; Cho, J.; Park, J.; Kim, C.H. Progesterone promotes differentiation of human cord blood fetal T cells into T regulatory cells but suppresses their differentiation into Th17 cells. Journal of Immunology (Baltimore, Md.: 1950) 2011, 187, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Bereshchenko, O.; Bruscoli, S.; Riccardi, C. Glucocorticoids, Sex Hormones, and Immunity. Frontiers in Immunology 2018, 9, 1332. [Google Scholar] [CrossRef] [PubMed]
- Goddard, L.M.; Ton, A.N.; Org, T.; Mikkola, H.K.A.; Iruela-Arispe, M.L. Selective suppression of endothelial cytokine production by progesterone receptor. Vascular Pharmacology 2013, 59, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.B. COVID-19 and Progesterone: Part 1. SARS-CoV-2, Progesterone and its potential clinical use. Endocrine and Metabolic Science 2021, 5, 100109. [Google Scholar] [CrossRef]
- Ghandehari, S.; Matusov, Y.; Pepkowitz, S.; Stein, D.; Kaderi, T.; Narayanan, D.; Hwang, J.; Chang, S.; Goodman, R.; Ghandehari, H.; et al. Progesterone in Addition to Standard of Care vs Standard of Care Alone in the Treatment of Men Hospitalized With Moderate to Severe COVID-19: A Randomized, Controlled Pilot Trial. Chest 2021, 160, 74–84. [Google Scholar] [CrossRef]
- Yuan, L.; Zhu, H.; Wu, K.; Zhou, M.; Ma, J.; Chen, R.; Tang, Q.; Cheng, T.; Guan, Y.; Xia, N. Female sex hormone, progesterone, ameliorates the severity of SARS-CoV-2-caused pneumonia in the Syrian hamster model. Signal Transduction and Targeted Therapy 2022, 7, 47. [Google Scholar] [CrossRef]
- Chen, L.; Li, Q.; Zheng, D.; Jiang, H.; Wei, Y.; Zou, L.; Feng, L.; Xiong, G.; Sun, G.; Wang, H.; et al. Clinical Characteristics of Pregnant Women with Covid-19 in Wuhan, China. The New England Journal of Medicine 2020, 382, e100. [Google Scholar] [CrossRef]
- Ellington, S.; Strid, P.; Tong, V.T.; Woodworth, K.; Galang, R.R.; Zambrano, L.D.; Nahabedian, J.; Anderson, K.; Gilboa, S.M. Characteristics of Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status - United States, January 22-June 7, 2020. MMWR. Morbidity and mortality weekly report 2020, 69, 769–775. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, H.; Cai, J. Coronavirus disease 2019 in pregnancy. International journal of infectious diseases: IJID: Official publication of the International Society for Infectious Diseases 2021, 105, 721. [CrossRef]
- Knight, M.; Bunch, K.; Vousden, N.; Morris, E.; Simpson, N.; Gale, C.; O'Brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J.; et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: National population based cohort study. BMJ (Clinical research ed.) 2020, 369, m2107. [Google Scholar] [CrossRef]
- Leach, D.A.; Brooke, G.N.; Bevan, C.L. Roles of steroid receptors in the lung and COVID-19. Essays in Biochemistry 2021, 65, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Shulla, A.; Heald-Sargent, T.; Subramanya, G.; Zhao, J.; Perlman, S.; Gallagher, T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. Journal of Virology 2011, 85, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, P.; Wang, J.; Feng, J.; Zhou, H.; Li, X.; Zhong, W.; Hao, P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Science China. Life Sciences 2020, 63, 457–460. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e286. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Z.; Wang, Y.; Zhou, Y.; Ma, Y.; Zuo, W. Single-Cell RNA Expression Profiling of ACE2, the Receptor of SARS-CoV-2. American Journal of Respiratory and Critical Care Medicine 2020, 202, 756–759. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. International Journal of Oral Science 2020, 12, 8. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Chen, M.; Feng, Y.; Xiong, C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovascular Research 2020, 116, 1097–1100. [Google Scholar] [CrossRef]
- Douglas, G.C.; O'Bryan, M.K.; Hedger, M.P.; Lee, D.K.L.; Yarski, M.A.; Smith, A.I.; Lew, R.A. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology 2004, 145, 4703–4711. [Google Scholar] [CrossRef]
- Bernstein, K.E.; Khan, Z.; Giani, J.F.; Cao, D.-Y.; Bernstein, E.A.; Shen, X.Z. Angiotensin-converting enzyme in innate and adaptive immunity. Nature Reviews. Nephrology 2018, 14, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Iwata-Yoshikawa, N.; Okamura, T.; Shimizu, Y.; Hasegawa, H.; Takeda, M.; Nagata, N. TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. Journal of Virology 2019, 93, e01815–01818. [Google Scholar] [CrossRef]
- Hoffmann, M.; Hofmann-Winkler, H.; Smith, J.C.; Krüger, N.; Arora, P.; Sørensen, L.K.; Søgaard, O.S.; Hasselstrøm, J.B.; Winkler, M.; Hempel, T.; et al. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. EBioMedicine 2021, 65, 103255. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Schroeder, S.; Kleine-Weber, H.; Müller, M.A.; Drosten, C.; Pöhlmann, S. Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19. Antimicrobial Agents and Chemotherapy 2020, 64, e00754–00720. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Meyerholz, D.K.; Bartlett, J.A.; McCray, P.B. The TMPRSS2 Inhibitor Nafamostat Reduces SARS-CoV-2 Pulmonary Infection in Mouse Models of COVID-19. mBio 2021, 12, e0097021. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, M.; Damalanka, V.C.; Tartell, M.A.; Chung, D.H.; Lourenço, A.L.; Pwee, D.; Mayer Bridwell, A.E.; Hoffmann, M.; Voss, J.; Karmakar, P.; et al. A novel class of TMPRSS2 inhibitors potently block SARS-CoV-2 and MERS-CoV viral entry and protect human epithelial lung cells. Proceedings of the National Academy of Sciences of the United States of America 2021, 118, e2108728118. [Google Scholar] [CrossRef]
- A Phase 2 Randomized, Double-Blind, Placebo-controlled Trial of Oral Camostat Mesylate for Early Treatment of COVID-19 Outpatients Showed Shorter Illness Course and Attenuation of Loss of Smell and Taste - PubMed. Available online: (accessed on.
- Zhuravel, S.V.; Khmelnitskiy, O.K.; Burlaka, O.O.; Gritsan, A.I.; Goloshchekin, B.M.; Kim, S.; Hong, K.Y. Nafamostat in hospitalized patients with moderate to severe COVID-19 pneumonia: A randomised Phase II clinical trial. EClinicalMedicine 2021, 41, 101169. [Google Scholar] [CrossRef]
- Rossi, Á.D.; de Araújo, J.L.F.; de Almeida, T.B.; Ribeiro-Alves, M.; de Almeida Velozo, C.; Almeida, J.M.d.; de Carvalho Leitão, I.; Ferreira, S.N.; da Silva Oliveira, J.; Alves, H.J.; et al. Association between ACE2 and TMPRSS2 nasopharyngeal expression and COVID-19 respiratory distress. Scientific Reports 2021, 11, 9658. [Google Scholar] [CrossRef]
- Chappell, M.C.; Marshall, A.C.; Alzayadneh, E.M.; Shaltout, H.A.; Diz, D.I. Update on the Angiotensin converting enzyme 2-Angiotensin (1-7)-MAS receptor axis: Fetal programing, sex differences, and intracellular pathways. Frontiers in Endocrinology 2014, 4, 201. [Google Scholar] [CrossRef]
- Berletch, J.B.; Yang, F.; Xu, J.; Carrel, L.; Disteche, C.M. Genes that escape from X inactivation. Human Genetics 2011, 130, 237–245. [Google Scholar] [CrossRef]
- Tukiainen, T.; Villani, A.-C.; Yen, A.; Rivas, M.A.; Marshall, J.L.; Satija, R.; Aguirre, M.; Gauthier, L.; Fleharty, M.; Kirby, A.; et al. Landscape of X chromosome inactivation across human tissues. Nature 2017, 550, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Milsted, A.; Underwood, A.C.; Dunmire, J.; DelPuerto, H.L.; Martins, A.S.; Ely, D.L.; Turner, M.E. Regulation of multiple renin-angiotensin system genes by Sry. Journal of Hypertension 2010, 28, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Wang, X.-M.; Mannan, R.; Pitchiaya, S.; Zhang, Y.; Wotring, J.W.; Xiao, L.; Robinson, D.R.; Wu, Y.-M.; Tien, J.C.-Y.; et al. Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2. Proceedings of the National Academy of Sciences of the United States of America 2021, 118, e2021450118. [Google Scholar] [CrossRef]
- Baughn, L.B.; Sharma, N.; Elhaik, E.; Sekulic, A.; Bryce, A.H.; Fonseca, R. Targeting TMPRSS2 in SARS-CoV-2 Infection. Mayo Clinic Proceedings 2020, 95, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Asselta, R.; Paraboschi, E.M.; Mantovani, A.; Duga, S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging 2020, 12, 10087–10098. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.A.; Mohr, A.; Giotis, E.S.; Cil, E.; Isac, A.M.; Yates, L.L.; Barclay, W.S.; Zwacka, R.M.; Bevan, C.L.; Brooke, G.N. The antiandrogen enzalutamide downregulates TMPRSS2 and reduces cellular entry of SARS-CoV-2 in human lung cells. Nature Communications 2021, 12, 4068. [Google Scholar] [CrossRef]
- Song, H.; Seddighzadeh, B.; Cooperberg, M.R.; Huang, F.W. Expression of ACE2, the SARS-CoV-2 Receptor, and TMPRSS2 in Prostate Epithelial Cells. European Urology 2020, 78, 296–298. [Google Scholar] [CrossRef]
- Kalidhindi, R.S.R.; Borkar, N.A.; Ambhore, N.S.; Pabelick, C.M.; Prakash, Y.S.; Sathish, V. Sex steroids skew ACE2 expression in human airway: A contributing factor to sex differences in COVID-19? American Journal of Physiology. Lung Cellular and Molecular Physiology 2020, 319, L843–L847. [Google Scholar] [CrossRef]
- Stelzig, K.E.; Canepa-Escaro, F.; Schiliro, M.; Berdnikovs, S.; Prakash, Y.S.; Chiarella, S.E. Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. American Journal of Physiology. Lung Cellular and Molecular Physiology 2020, 318, L1280–L1281. [Google Scholar] [CrossRef]
- Liu, J.; Ji, H.; Zheng, W.; Wu, X.; Zhu, J.J.; Arnold, A.P.; Sandberg, K. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17β-oestradiol-dependent and sex chromosome-independent. Biology of Sex Differences 2010, 1, 6. [Google Scholar] [CrossRef]
- Lemes, R.M.R.; Costa, A.J.; Bartolomeo, C.S.; Bassani, T.B.; Nishino, M.S.; Pereira, G.J.d.S.; Smaili, S.S.; Maciel, R.M.d.B.; Braconi, C.T.; da Cruz, E.F.; et al. 17β-estradiol reduces SARS-CoV-2 infection in vitro. Physiological Reports 2021, 9, e14707. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, Q.; Xia, X.; Liu, K.; Yu, Z.; Tao, W.; Gong, W.; Han, J.-D.J. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell 2020, 19, e13168. [Google Scholar] [CrossRef]
- Keidar, S.; Gamliel-Lazarovich, A.; Kaplan, M.; Pavlotzky, E.; Hamoud, S.; Hayek, T.; Karry, R.; Abassi, Z. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circulation Research 2005, 97, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jessup, J.A.; Zhao, Z.; Da Silva, J.; Lin, M.; MacNamara, L.M.; Ahmad, S.; Chappell, M.C.; Ferrario, C.M.; Groban, L. Characterization of the cardiac renin angiotensin system in oophorectomized and estrogen-replete mRen2.Lewis rats. PLoS ONE 2013, 8, e76992. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Pineda, J.A.; Albaghdadi, M.; Jiang, W.; Vera-Lopez, K.J.; Nieto-Montesinos, R.; Alvarez, K.L.F.; Davila Del-Carpio, G.; Gómez, B.; Lindsay, M.E.; Malhotra, R.; et al. Structural and Functional Analysis of Female Sex Hormones against SARS-CoV-2 Cell Entry. International Journal of Molecular Sciences 2021, 22, 11508. [Google Scholar] [CrossRef]
- Fischer, M.; Baessler, A.; Schunkert, H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovascular Research 2002, 53, 672–677. [Google Scholar] [CrossRef]
- Chadchan, S.B.; Popli, P.; Maurya, V.K.; Kommagani, R. The SARS-CoV-2 receptor, angiotensin-converting enzyme 2, is required for human endometrial stromal cell decidualization†. Biology of Reproduction 2021, 104, 336–343. [Google Scholar] [CrossRef]
- Setlur, S.R.; Mertz, K.D.; Hoshida, Y.; Demichelis, F.; Lupien, M.; Perner, S.; Sboner, A.; Pawitan, Y.; Andrén, O.; Johnson, L.A.; et al. Estrogen-dependent signaling in a molecularly distinct subclass of aggressive prostate cancer. Journal of the National Cancer Institute 2008, 100, 815–825. [Google Scholar] [CrossRef]
- Reckelhoff, J.F. Gender differences in the regulation of blood pressure. Hypertension (Dallas, Tex.: 1979) 2001, 37, 1199–1208. [Google Scholar] [CrossRef]
- Targeting androgen regulation of TMPRSS2 and ACE2 as a therapeutic strategy to combat COVID-19 - PubMed. Available online: (accessed on.
- Baratchian, M.; McManus, J.M.; Berk, M.P.; Nakamura, F.; Mukhopadhyay, S.; Xu, W.; Erzurum, S.; Drazba, J.; Peterson, J.; Klein, E.A.; et al. Androgen regulation of pulmonary AR, TMPRSS2 and ACE2 with implications for sex-discordant COVID-19 outcomes. Scientific Reports 2021, 11, 11130. [Google Scholar] [CrossRef]
- Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer - PubMed. Available online: (accessed on.
- Lucas, J.M.; Heinlein, C.; Kim, T.; Hernandez, S.A.; Malik, M.S.; True, L.D.; Morrissey, C.; Corey, E.; Montgomery, B.; Mostaghel, E.; et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discovery 2014, 4, 1310–1325. [Google Scholar] [CrossRef]
- Mikkonen, L.; Pihlajamaa, P.; Sahu, B.; Zhang, F.-P.; Jänne, O.A. Androgen receptor and androgen-dependent gene expression in lung. Molecular and Cellular Endocrinology 2010, 317, 14–24. [Google Scholar] [CrossRef]
- Distinct mechanisms for TMPRSS2 expression explain organ-specific inhibition of SARS-CoV-2 infection by enzalutamide - PubMed. Available online: (accessed on.
- Bischof, E.; Wolfe, J.; Klein, S.L. Clinical trials for COVID-19 should include sex as a variable. The Journal of Clinical Investigation 2020, 130, 3350–3352. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet. Respiratory Medicine 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Cao, X. COVID-19: Immunopathology and its implications for therapy. Nature Reviews. Immunology 2020, 20, 269–270. [Google Scholar] [CrossRef]
- Xia, H.-J.; Zhang, G.-H.; Wang, R.-R.; Zheng, Y.-T. The influence of age and sex on the cell counts of peripheral blood leukocyte subpopulations in Chinese rhesus macaques. Cellular & Molecular Immunology 2009, 6, 433–440. [Google Scholar] [CrossRef]
- Melgert, B.N.; Oriss, T.B.; Qi, Z.; Dixon-McCarthy, B.; Geerlings, M.; Hylkema, M.N.; Ray, A. Macrophages: Regulators of sex differences in asthma? American Journal of Respiratory Cell and Molecular Biology 2010, 42, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Hewagama, A.; Patel, D.; Yarlagadda, S.; Strickland, F.M.; Richardson, B.C. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes and Immunity 2009, 10, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, M.; McClellan, B.; Allansmith, M. Influence of sex in immunoglobulin levels. Nature 1967, 214, 1224–1225. [Google Scholar] [CrossRef] [PubMed]
- Amadori, A.; Zamarchi, R.; De Silvestro, G.; Forza, G.; Cavatton, G.; Danieli, G.A.; Clementi, M.; Chieco-Bianchi, L. Genetic control of the CD4/CD8 T-cell ratio in humans. Nature Medicine 1995, 1, 1279–1283. [Google Scholar] [CrossRef]
- Meng, Y.; Wu, P.; Lu, W.; Liu, K.; Ma, K.; Huang, L.; Cai, J.; Zhang, H.; Qin, Y.; Sun, H.; et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: A retrospective study of 168 severe patients. PLoS pathogens 2020, 16, e1008520. [Google Scholar] [CrossRef] [PubMed]
- Verthelyi, D.; Klinman, D.M. Sex hormone levels correlate with the activity of cytokine-secreting cells in vivo. Immunology 2000, 100, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, C.M.; Cleary, J.; Dagtas, A.S.; Moussai, D.; Diamond, B. Estrogen alters thresholds for B cell apoptosis and activation. The Journal of Clinical Investigation 2002, 109, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Fuseini, H.; Yung, J.A.; Cephus, J.Y.; Zhang, J.; Goleniewska, K.; Polosukhin, V.V.; Peebles, R.S.; Newcomb, D.C. Testosterone Decreases House Dust Mite-Induced Type 2 and IL-17A-Mediated Airway Inflammation. Journal of Immunology (Baltimore, Md.: 1950) 2018, 201, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Kadihasanoglu, M.; Aktas, S.; Yardimci, E.; Aral, H.; Kadioglu, A. SARS-CoV-2 Pneumonia Affects Male Reproductive Hormone Levels: A Prospective, Cohort Study. The Journal of Sexual Medicine 2021, 18, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Trigunaite, A.; Dimo, J.; Jørgensen, T.N. Suppressive effects of androgens on the immune system. Cellular Immunology 2015, 294, 87–94. [Google Scholar] [CrossRef]
- Kadel, S.; Kovats, S. Sex Hormones Regulate Innate Immune Cells and Promote Sex Differences in Respiratory Virus Infection. Frontiers in Immunology 2018, 9, 1653. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Medicine 2020, 46, 846–848. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Correction to: Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Medicine 2020, 46, 1294–1297. [Google Scholar] [CrossRef]
- Pagano, M.T.; Peruzzu, D.; Busani, L.; Pierdominici, M.; Ruggieri, A.; Antinori, A.; D'Offizi, G.; Petrosillo, N.; Palmieri, F.; Piselli, P.; et al. Predicting respiratory failure in patients infected by SARS-CoV-2 by admission sex-specific biomarkers. Biology of Sex Differences 2021, 12, 63. [Google Scholar] [CrossRef]
- Infante, M.; Pieri, M.; Lupisella, S.; D'Amore, L.; Bernardini, S.; Fabbri, A.; Iannetta, M.; Andreoni, M.; Morello, M. Low testosterone levels and high estradiol to testosterone ratio are associated with hyperinflammatory state and mortality in hospitalized men with COVID-19. European Review for Medical and Pharmacological Sciences 2021, 25, 5889–5903. [Google Scholar] [CrossRef]
- Cadegiani, F.A.; McCoy, J.; Gustavo Wambier, C.; Goren, A. Early Antiandrogen Therapy With Dutasteride Reduces Viral Shedding, Inflammatory Responses, and Time-to-Remission in Males With COVID-19: A Randomized, Double-Blind, Placebo-Controlled Interventional Trial (EAT-DUTA AndroCoV Trial - Biochemical). Cureus 2021, 13, e13047. [Google Scholar] [CrossRef] [PubMed]
- Cadegiani, F.A.; McCoy, J.; Gustavo Wambier, C.; Vaño-Galván, S.; Shapiro, J.; Tosti, A.; Zimerman, R.A.; Goren, A. Proxalutamide Significantly Accelerates Viral Clearance and Reduces Time to Clinical Remission in Patients with Mild to Moderate COVID-19: Results from a Randomized, Double-Blinded, Placebo-Controlled Trial. Cureus 2021, 13, e13492. [Google Scholar] [CrossRef] [PubMed]
- Nicastri, E.; Marinangeli, F.; Pivetta, E.; Torri, E.; Reggiani, F.; Fiorentino, G.; Scorzolini, L.; Vettori, S.; Marsiglia, C.; Gavioli, E.M.; et al. A phase 2 randomized, double-blinded, placebo-controlled, multicenter trial evaluating the efficacy and safety of raloxifene for patients with mild to moderate COVID-19. EClinicalMedicine 2022, 48, 101450. [Google Scholar] [CrossRef]
- Woolf, P.D.; Hamill, R.W.; McDonald, J.V.; Lee, L.A.; Kelly, M. Transient hypogonadotropic hypogonadism caused by critical illness. The Journal of Clinical Endocrinology and Metabolism 1985, 60, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Hall, O.J.; Klein, S.L. Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal Immunology 2017, 10, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Hua, D.; Li, J.-P.; Zhang, X.-N.; Bai, L.; Cao, L.-B.; Guo, Y.; Zhang, M.; Dong, J.-Z.; Liang, X.-W.; et al. Modulation of innate immune response to viruses including SARS-CoV-2 by progesterone. Signal Transduction and Targeted Therapy 2022, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Tonetti, T.; Protti, A.; Langer, T.; Girardis, M.; Bellani, G.; Laffey, J.; Carrafiello, G.; Carsana, L.; Rizzuto, C.; et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: A multicentre prospective observational study. The Lancet. Respiratory Medicine 2020, 8, 1201–1208. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. The New England Journal of Medicine 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Card, J.W.; Carey, M.A.; Bradbury, J.A.; DeGraff, L.M.; Morgan, D.L.; Moorman, M.P.; Flake, G.P.; Zeldin, D.C. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. Journal of Immunology (Baltimore, Md.: 1950) 2006, 177, 621–630. [Google Scholar] [CrossRef]
- Diaz, G.A.; Parsons, G.T.; Gering, S.K.; Meier, A.R.; Hutchinson, I.V.; Robicsek, A. Myocarditis and Pericarditis After Vaccination for COVID-19. JAMA 2021, 326, 1210–1212. [Google Scholar] [CrossRef]
- da Silva, J.S.; Montagnoli, T.L.; Rocha, B.S.; Tacco, M.L.C.A.; Marinho, S.C.P.; Zapata-Sudo, G. Estrogen Receptors: Therapeutic Perspectives for the Treatment of Cardiac Dysfunction after Myocardial Infarction. International Journal of Molecular Sciences 2021, 22, 525. [Google Scholar] [CrossRef]
- Hale, S.L.; Birnbaum, Y.; Kloner, R.A. beta-Estradiol, but not alpha-estradiol, reduced myocardial necrosis in rabbits after ischemia and reperfusion. American Heart Journal 1996, 132, 258–262. [Google Scholar] [CrossRef]
- Manavathi, B.; Kumar, R. Steering estrogen signals from the plasma membrane to the nucleus: Two sides of the coin. Journal of Cellular Physiology 2006, 207, 594–604. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nature Medicine 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Ryan, D.H.; Ravussin, E.; Heymsfield, S. COVID 19 and the Patient with Obesity - The Editors Speak Out. Obesity (Silver Spring, Md.) 2020, 28, 847. [Google Scholar] [CrossRef] [PubMed]
- Dietz, W.; Santos-Burgoa, C. Obesity and its Implications for COVID-19 Mortality. Obesity (Silver Spring, Md.) 2020, 28, 1005. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O'Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ (Clinical research ed.) 2020, 369, m1966. [Google Scholar] [CrossRef] [PubMed]
- Kassir, R. Risk of COVID-19 for patients with obesity. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity 2020, 21, e13034. [CrossRef]
- Giagulli, V.A.; Castellana, M.; Pelusi, C.; Triggiani, V. Androgens, Body Composition, and Their Metabolism Based on Sex. Frontiers of Hormone Research 2019, 53, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Heine, P.A.; Taylor, J.A.; Iwamoto, G.A.; Lubahn, D.B.; Cooke, P.S. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proceedings of the National Academy of Sciences of the United States of America 2000, 97, 12729–12734. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C.; Bray, G.A.; Bourgeois, M.O.; Macchiavelli, R.; Rood, J.C.; Greeson, C.; Partington, C. Exogenous androgens influence body composition and regional body fat distribution in obese postmenopausal women--a clinical research center study. The Journal of Clinical Endocrinology and Metabolism 1996, 81, 2198–2203. [Google Scholar] [CrossRef] [PubMed]
- Battisti, S.; Pedone, C.; Napoli, N.; Russo, E.; Agnoletti, V.; Nigra, S.G.; Dengo, C.; Mughetti, M.; Conte, C.; Pozzilli, P.; et al. Computed Tomography Highlights Increased Visceral Adiposity Associated With Critical Illness in COVID-19. Diabetes Care 2020, 43, e129–e130. [Google Scholar] [CrossRef]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- West-Eberhard, M.J. Nutrition, the visceral immune system, and the evolutionary origins of pathogenic obesity. Proceedings of the National Academy of Sciences of the United States of America 2019, 116, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. The Lancet. Gastroenterology & Hepatology 2020, 5, 434–435. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nature Medicine 2020, 26, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Galasso, V.; Pons, V.; Profeta, P.; Becher, M.; Brouard, S.; Foucault, M. Gender differences in COVID-19 attitudes and behavior: Panel evidence from eight countries. Proceedings of the National Academy of Sciences of the United States of America 2020, 117, 27285–27291. [Google Scholar] [CrossRef]
- The Lancet, n. The gendered dimensions of COVID-19. Lancet (London, England) 2020, 395, 1168. [Google Scholar] [CrossRef]
- Tadiri, C.P.; Gisinger, T.; Kautzy-Willer, A.; Kublickiene, K.; Herrero, M.T.; Raparelli, V.; Pilote, L.; Norris, C.M.; Consortium, G.-F. The influence of sex and gender domains on COVID-19 cases and mortality. CMAJ: Canadian Medical Association journal = journal de l'Association medicale canadienne 2020, 192, E1041–E1045. [Google Scholar] [CrossRef]
| Viral Receptor |
Sex Hormone /Study Drug |
Species | Primary Findings | Sample | Refs. |
|---|---|---|---|---|---|
| ACE2 | Antiandrogen enzalutamide | Human | Decreases ACE2 expression | Heart cells and pulmonary tissue derived from human embryonic stem cells (hESC) | [8] |
| Testosterone | Human | Upregulation of ACE2 gene in men and women | Airway smooth muscle cells | [62] | |
| Estrogen | Human | Reduces ACE2, but not substantially | Airway smooth muscle cells | ||
| Antiandrogen enzalutamide | Human | Reducing TMPRSS2 expression | Human lung cells | [84] | |
| Estrogen | Mouse | Increases ACE2 expression | Thymus | [76] | |
| Testosterone | Mouse | Increase ACE2 expression | Kidney | ||
| Antagonist enzalutamide | Mouse | Moderately suppressible | Lung | [85] | |
| TMPRSS2 | Antagonist enzalutamide | Mouse | Did not decrease pulmonary TMPRSS2 | Lung | |
| Antiandrogen enzalutamide | Mouse | Reduced TMPRSS2 levels | Airway epithelial cells | [70] | |
| Leuprolide or Estradiol | Human | In males treated with leuprolide or estradiol, TMPRSS2 levels were markedly lower. | Epithelial cells | [87] | |
| ACE2 and TMPRSS2 | Androgen | Mouse | Decreased Tmprss2 and Ace2 expression in lung epithelial cells. | Lung epithelial cells | [67,72] |
| Castration | Mouse | Reduced levels of ACE2 and TMPRSS2 in lung, seminal vesicles and small intestine; ACE2 upregulated in kidney tissue, but not TMPRSS2 | Systemic | [84] |
| Research | STUDY DRUG | Species | Primary Findings | Refs. |
|---|---|---|---|---|
| Observational Research | Testosterone | Male | Men with lower plasma levels of Testosterone were more likely to have more severe ARDS and to experience a worsening of their status after hospitalization. | [106] |
| Exploratory retrospective study | Testosterone | Male | Plasma angiotensin 1-7 levels and neutrophil count were predictors of ARDS outcome only in women, while plasma Testosterone levels and lymphocyte count were only indicative in men. | [108] |
| Exploratory retrospective study | Estradiol | Female | Estrogen plasma concentrations were positively correlated with pulmonary function in COVID-19 women and negative correlated with pulmonary function in COVID-19 men. | |
| Exploratory retrospective study | Estradiol/Testosterone ratio | Male/Female | The non-survivors had a significantly higher median value for the Estradiol/Testosterone ratio. | [104] |
| Randomized controlled trial | Raloxifene | Male/Female | Increased white blood cell counts and accelerated viral clearing. | [112] |
| Experimental research | Progesterone | Hamsters | Inhibit proinflammatory cytokine overproduction and viral replication in lung. | [39] |
| Experimental research | Progesterone | Mice | Triggers downstream antiviral genes, stimulating cellular as well as mouse innate antiviral response. | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





