Submitted:
26 December 2023
Posted:
27 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
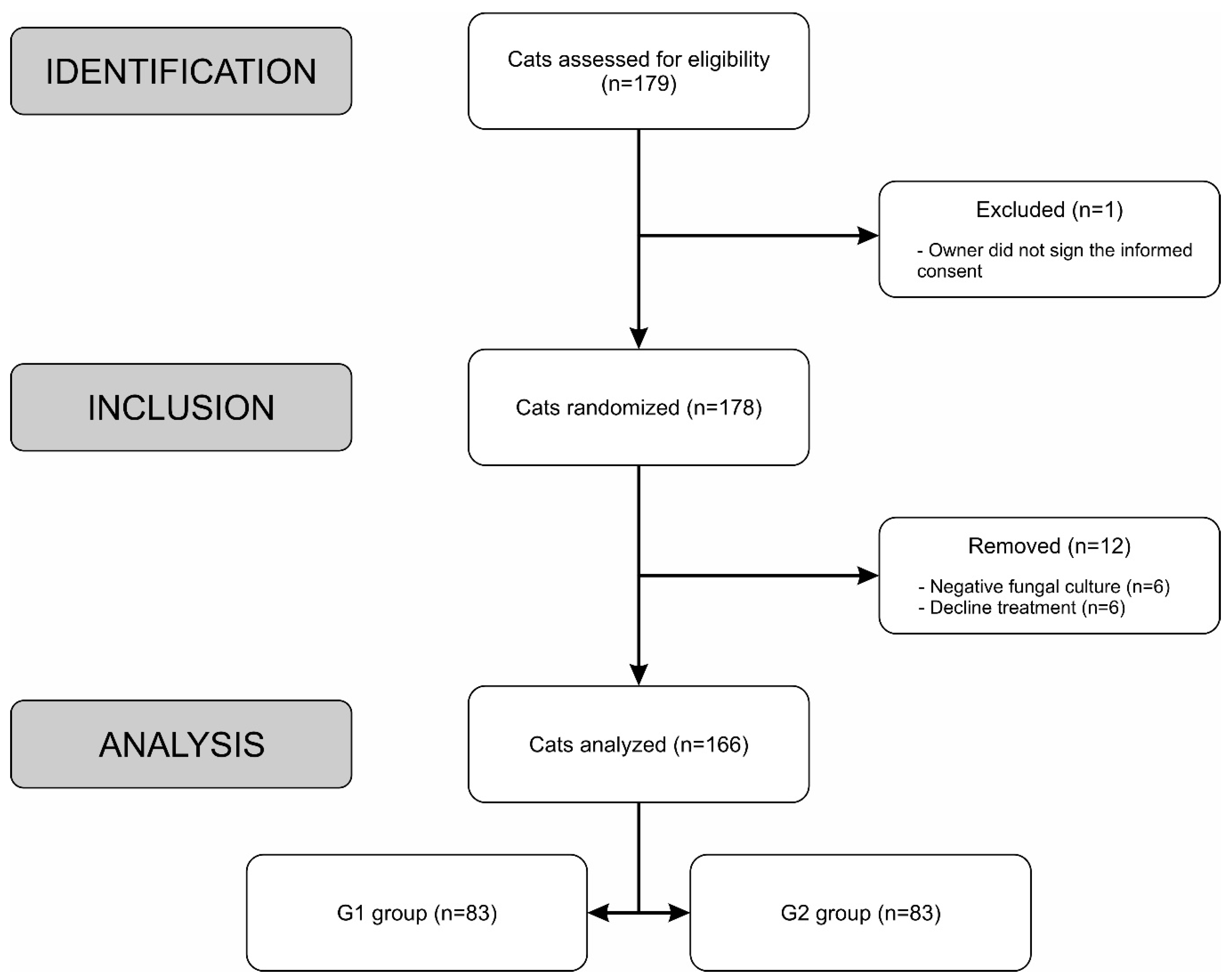
2.1. Inclusion, Exclusion and Elimination criteria
2.2. Study Procedure
2.3. Sample size and Randomization
2.4. Treatment
2.5. Follow-Up Procedures
2.6. Statistical analysis
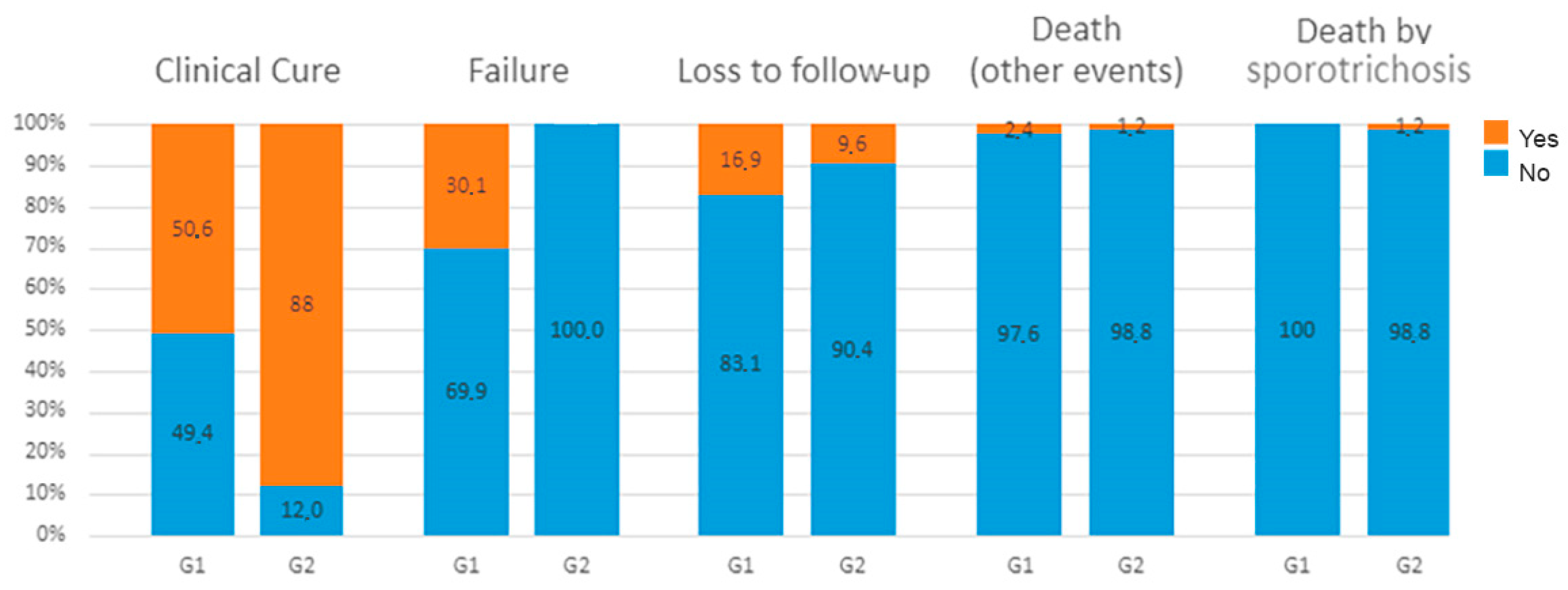
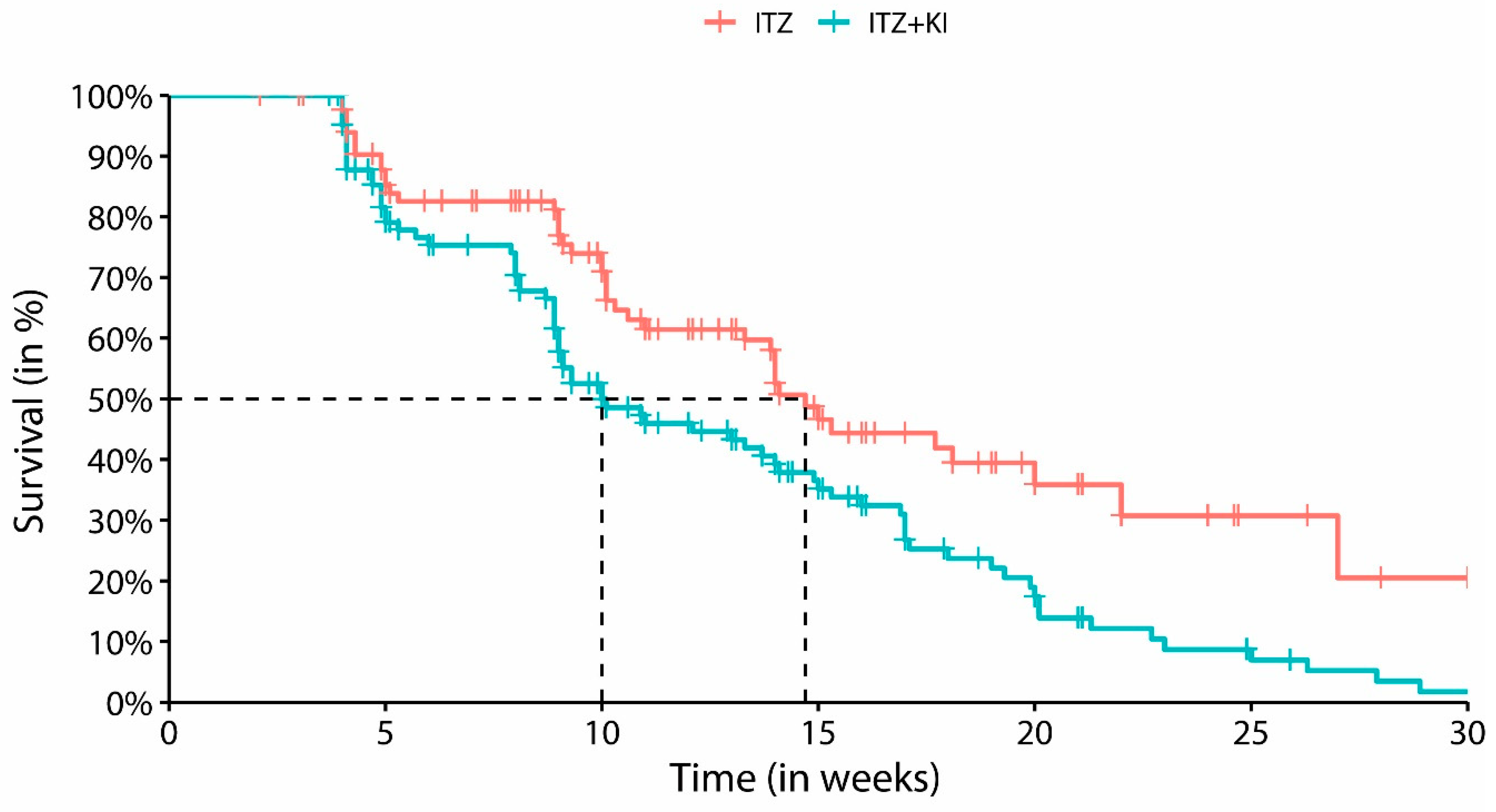
3. Results
3.1. Exploratory analysis
3.2. Cox regression model
| Predictors | Category | Clinical Cure | Crude HR (95% CI) | Adjusted HR (95% CI | |
|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | ||||
| Treatment group | ITZ | 42 (50.6) | 41 (49.4) | 1 | 1 |
| ITZ + KI | 73 (88) | 10 (12) | 1.77 (1.21-2.59) | 1.77 (1.2-2.62) | |
| Respiratory signs | Yes | 31 (58.5) | 22 (41.5) | 1 | 1 |
| No | 84 (74.3) | 29 (25.7) | 3 (1.74-5.17) | 2.09 (1.14-3.83) | |
| Mucosal lesions | Yes | 32 (54.2) | 27 (45.8) | 1 | 1 |
| No | 83 (77.6) | 24 (22.4) | 2.36 (1.56-3.58) | 1.74 (1.09-2.78) | |
| Distribution of skin lesions | L3 | 41 (59.4) | 28 (40.6) | 1 | 1 |
| L2 | 35 (79.5) | 9 (20.5) | 1.76 (1.12-2.78) | 2.08 (1.3-3.32) | |
| L1 | 39 (73.6) | 14 (26.4) | 1.79 (1.15-2.81) | 2.24 (1.39-3.61) | |
| Lymphangitis | Yes | 23 (79.3) | 6 (20.7) | 1 | 1 |
| No | 92 (67.2) | 45 (32.8) | 0.55 (0.34-0.87) | 0.46 (0.28-0.76) | |
| Neutering | No | 48 (58.5) | 34 (41.5) | 1 | 1 |
| Yes | 67 (82.7) | 14 (17.3) | 1.78 (1.22-2.58) | 1.61 (1.09-2.37) | |
3.3. Safety
| Aminotransferase levels | G1-ITZ (N=83) |
G2-ITZ+KI (N=83) |
Unit | Reference Value |
|---|---|---|---|---|
| Normal | 33 | 40 | U/L | AST (6-83) [52] ALT (26-43) [52] |
| Mild elevation (ALT and/or AST) | 43 |
39 | U/L | <5 times the upper reference range [53] |
| Moderate elevation (ALT) | 5 | 4 | U/L | 5–10 times the upper reference range [53] |
| Marked elevation (ALT) | 2 | 0 | U/L | >10 times the upper reference range [53] |
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Han, H.S.; Kano, R. Feline Sporotrichosis in Asia. Braz J Microbiol 2021, 52, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Goncalves, S.S.; de Carvalho, J.A.; Borba-Santos, L.P.; Rozental, S.; Camargo, Z.P. Current Progress on Epidemiology, Diagnosis, and Treatment of Sporotrichosis and Their Future Trends. J Fungi 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.S.; da Cruz Bahiense Rocha, I.; Rediguieri, B.C.; de Carvalho, J.A.; Maifrede, S.B.; Kruschewsky, W.L.L.; Falqueto, A.; Rodrigues, A.M. Human and Feline Sporotrichosis in a Reference Center of Southeastern Brazil: Genetic Differentiation, Diversity, and Antifungal Susceptibility of Sporothrix Species. JoF 2023, 9, 831. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Arias-Rodriguez, C.; Sánchez-Cifuentes, E.A.; Santa-Vélez, C.; Larrañaga-Piñeres, I.; Gaviria-Barrera, M.E.; Vásquez-Ochoa, L.A.; Montoya, D.; Jiménez-Alzate, M. del P. First Three Cases of Cat-associated Zoonotic Cutaneous Sporotrichosis in Colombia. Int J Dermatology 2022, 61, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Azevedo, M.I.; Amaral, C.I.; Grom, N.A.; Marinho, F.; de Oliveira, C.S.F.; de M. Soares, D.F.; Morais, M.H.F.; Brandão, S.T.; Menezes, R.C.; et al. Feline Sporotrichosis: Characterization of Cutaneous and Extracutaneous Lesions Using Different Diagnostic Methods. Feline Sporotrichosis: Characterization of Cutaneous and Extracutaneous Lesions Using Different Diagnostic Methods 2023, 61, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga-Valencia, M.S.; Ordoñez, A.R.; Cunha, F.R.; Guerrero-López, A.E. Esporotricosis en un gato doméstico. Reporte del primer caso en Ecuador. RC FCV-LUZ 2022, 32, 1–7. [Google Scholar] [CrossRef]
- Etchecopaz, A.; Toscanini, M.A.; Gisbert, A.; Mas, J.; Scarpa, M.; Iovannitti, C.A.; Bendezu, K.; Nusblat, A.D.; Iachini, R.; Cuestas, M.L. Sporothrix Brasiliensis: A Review of an Emerging South American Fungal Pathogen, Its Related Disease, Presentation and Spread in Argentina. J Fungi (Basel) 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.I.; Muncie, J.E. Sporotrichosis; Etiologic Considerations and Report of Additional Cases from New York. N Y State J Med 1952, 52, 2147–2153. [Google Scholar] [PubMed]
- Rees, R.K.; Swartzberg, J.E. Feline-Transmitted Sporotrichosis: A Case Study from California. Dermatol Online J 2011, 17, 2. [Google Scholar] [CrossRef]
- Reed, K.D.; Moore, F.M.; Geiger, G.E.; Stemper, M.E. Zoonotic Transmission of Sporotrichosis: Case Report and Review. Clinical Infectious Diseases 1993, 16, 384–387. [Google Scholar] [CrossRef]
- Della Terra, P.P.; Rodrigues, A.M.; Fernandes, G.F.; Nishikaku, A.S.; Burger, E.; de Camargo, Z.P. Exploring Virulence and Immunogenicity in the Emerging Pathogen Sporothrix Brasiliensis. PLoS Negl Trop Dis 2017, 11, e0005903. [Google Scholar] [CrossRef] [PubMed]
- Falcão, E.M.M.; Romão, A.R.; Magalhães, M.d.A.F.M.; de Lima Filho, J.B.; do Valle, A.C.F.; Bastos, F.I.; Gutierrez-Galhardo, M.C.; Freitas, D.F.S. A Spatial Analysis of the Spread of Hyperendemic Sporotrichosis in the State of Rio de Janeiro, Brazil. JoF 2022, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.F.S.; do Valle, A.C.F.; de Almeida Paes, R.; Bastos, F.I.; Galhardo, M.C.G. Zoonotic Sporotrichosis in Rio de Janeiro, Brazil: A Protracted Epidemic yet to Be Curbed. CLIN INFECT DIS 2010, 50, 453–453. [Google Scholar] [CrossRef] [PubMed]
- Angelo, D.F.D.S.; Rabello, V.B.D.S.; Maciel, M.A.S.; Atanázio, S.S.D.L.A.; Costa, M.C.L.D.; Silva, S.R.; Almeida-Paes, R.; Bernardes-Engemann, A.R.; Zancopé-Oliveira, R.M.; Clementino, I.J. Sporothrix Brasiliensis Infecting Cats in Northeastern Brazil: New Emerging Areas in Paraíba State. Cienc. Rural 2023, 53, e20220351. [Google Scholar] [CrossRef]
- Maschio-Lima, T.; Marques, M.D.R.; Lemes, T.H.; Brizzotti-Mazuchi, N.S.; Caetano, M.H.; de Almeida, B.G.; Bianco, L.M.; Monteiro, R.C.; Rodrigues, A.M.; de Camargo, Z.P.; et al. Clinical and Epidemiological Aspects of Feline Sporotrichosis Caused by Sporothrix Brasiliensis and in Vitro Antifungal Susceptibility. Vet Res Commun 2021, 45, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.M.E.; Maifrede, S.B.; Ribeiro, M.A.; Zancope-Oliveira, R.M. Molecular Identification of Sporothrix Species Involved in the First Familial Outbreak of Sporotrichosis in the State of Espírito Santo, Southeastern Brazil. Mem. Inst. Oswaldo Cruz 2013, 108, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Rabello, V.B.S.; Almeida-Silva, F.; Scramignon-Costa, B.d.S.; Motta, B. da S.; de Macedo, P.M.; Teixeira, M. de M.; Almeida-Paes, R.; Irinyi, L.; Meyer, W.; Zancopé-Oliveira, R.M. Environmental Isolation of Sporothrix Brasiliensis in an Area With Recurrent Feline Sporotrichosis Cases. Front. Cell. Infect. Microbiol. 2022, 12, 894297. [Google Scholar] [CrossRef]
- Almeida-Paes, R.; de Oliveira, M.M.; Freitas, D.F.; do Valle, A.C.; Zancope-Oliveira, R.M.; Gutierrez-Galhardo, M.C. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix Brasiliensis Is Associated with Atypical Clinical Presentations. PLoS Negl Trop Dis 2014, 8, e3094. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.F.; Santos, S.S.; Almeida-Paes, R.; de Oliveira, M.M.; do Valle, A.C.; Gutierrez-Galhardo, M.C.; Zancope-Oliveira, R.M.; Nosanchuk, J.D. Increase in Virulence of Sporothrix Brasiliensis over Five Years in a Patient with Chronic Disseminated Sporotrichosis. Virulence 2015, 6, 112–120. [Google Scholar] [CrossRef]
- Schechtman, R.C.; Falcão, E.M.M.; Carard, M.; García, M.S.C.; Mercado, D.S.; Hay, R.J. Sporotrichosis: Hyperendemic by Zoonotic Transmission, with Atypical Presentations, Hypersensitivity Reactions and Greater Severity. Anais Brasileiros de Dermatologia 2022, 97, 1–13. [Google Scholar] [CrossRef]
- Etchecopaz, A.N.; Lanza, N.; Toscanini, M.A.; Devoto, T.B.; Pola, S.J.; Daneri, G.L.; Iovannitti, C.A.; Cuestas, M.L. Sporotrichosis Caused by Sporothrix Brasiliensis in Argentina: Case Report, Molecular Identification and in Vitro Susceptibility Pattern to Antifungal Drugs. Journal de Mycologie Médicale 2020, 30, 100908. [Google Scholar] [CrossRef] [PubMed]
- Thomson, P.; González, C.; Blank, O.; Ramírez, V.; Río, C.D.; Santibáñez, S.; Pena, P. Sporotrichosis Outbreak Due to Sporothrix Brasiliensis in Domestic Cats in Magallanes, Chile: A One-Health-Approach Study. JoF 2023, 9, 226. [Google Scholar] [CrossRef]
- do Prado, C.M.; Razzolini, E.; Santacruz, G.; Ojeda, L.; Geraldo, M.R.; Segovia, N.; Pereira Brunelli, J.; Vicente, V.A.; Svoboda, W.K.; Queiroz-Telles, F. First Cases of Feline Sporotrichosis Caused by Sporothrix Brasiliensis in Paraguay. JoF 2023, 9, 972. [Google Scholar] [CrossRef] [PubMed]
- Kaadan, M.I.; Dennis, M.; Desai, N.; Yadavalli, G.; Lederer, P. One Health Education for Future Physicians: A Case Report of Cat-Transmitted Sporotrichosis. Open Forum Infectious Diseases 2020, 7, ofaa049. [Google Scholar] [CrossRef] [PubMed]
- Barnacle, J.R.; Chow, Y.J.; Borman, A.M.; Wyllie, S.; Dominguez, V.; Russell, K.; Roberts, H.; Armstrong-James, D.; Whittington, A.M. The First Three Reported Cases of Sporothrix Brasiliensis Cat-Transmitted Sporotrichosis Outside South America. Medical Mycology Case Reports 2023, 39, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, H.; Rodrigues, A.M.; Dias, M.A.G.; da Silva, E.A.; Bernardi, F.; de Camargo, Z.P. Feline Sporotrichosis Due to Sporothrix Brasiliensis: An Emerging Animal Infection in São Paulo, Brazil. BMC Vet Res 2014, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.O.; Poester, V.R.; Trápaga, M.R.; Stevens, D.A. Sporothrix Brasiliensis: Epidemiology, Therapy, and Recent Developments. JoF 2023, 9, 921. [Google Scholar] [CrossRef] [PubMed]
- Macêdo-Sales, P.A.; Souto, S.R.L.S.; Destefani, C.A.; Lucena, R.P.; Machado, R.L.D.; Pinto, M.R.; Rodrigues, A.M.; Lopes-Bezerra, L.M.; Rocha, E.M.S.; Baptista, A.R.S. Domestic Feline Contribution in the Transmission of Sporothrix in Rio de Janeiro State, Brazil: A Comparison between Infected and Non-Infected Populations. BMC Vet Res 2018, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Schubach, T.M.; Schubach, A.; Okamoto, T.; Barros, M.B.; Figueiredo, F.B.; Cuzzi, T.; Fialho-Monteiro, P.C.; Reis, R.S.; Perez, M.A.; Wanke, B. Evaluation of an Epidemic of Sporotrichosis in Cats: 347 Cases (1998-2001). J Am Vet Med Assoc 2004, 224, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Gremiao, I.D.F.; Martins da Silva da Rocha, E.; Montenegro, H.; Carneiro, A.J.B.; Xavier, M.O.; de Farias, M.R.; Monti, F.; Mansho, W.; de Macedo Assuncao Pereira, R.H.; Pereira, S.A.; et al. Guideline for the Management of Feline Sporotrichosis Caused by Sporothrix Brasiliensis and Literature Revision. Braz J Microbiol 2021, 52, 107–124. [Google Scholar] [CrossRef]
- Souza, E.W.; Borba, C.M.; Pereira, S.A.; Gremiao, I.D.F.; Langohr, I.M.; Oliveira, M.M.E.; de Oliveira, R.V.C.; da Cunha, C.R.; Zancope-Oliveira, R.M.; de Miranda, L.H.M.; et al. Clinical Features, Fungal Load, Coinfections, Histological Skin Changes, and Itraconazole Treatment Response of Cats with Sporotrichosis Caused by Sporothrix Brasiliensis. Sci Rep 2018, 8, 9074. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.A.; Passos, S.R.; Silva, J.N.; Gremiao, I.D.; Figueiredo, F.B.; Teixeira, J.L.; Monteiro, P.C.; Schubach, T.M. Response to Azolic Antifungal Agents for Treating Feline Sporotrichosis. Vet Rec 2010, 166, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.H.M.; Silva, J.N.; Gremiao, I.D.F.; Menezes, R.C.; Almeida-Paes, R.; Dos Reis, E.G.; de Oliveira, R.V.C.; de Araujo, D.; Ferreiro, L.; Pereira, S.A. Monitoring Fungal Burden and Viability of Sporothrix Spp. in Skin Lesions of Cats for Predicting Antifungal Treatment Response. J Fungi (Basel) 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Marimon, R.; Serena, C.; Gené, J.; Cano, J.; Guarro, J. In Vitro Antifungal Susceptibilities of Five Species of Sporothrix. Antimicrob Agents Chemother 2008, 52, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Koehler, A.; Pagani, D.M.; da Silva Hellwig, A.H.; Scroferneker, M.L. In-Vitro Antifungal Susceptibility of the Genus Sporothrix and Correlation with Treatment Options for Sporotrichosis: A Systematic Review. Reviews in Medical Microbiology 2021, 32, 219–227. [Google Scholar] [CrossRef]
- Nakasu, C.C.T.; Waller, S.B.; Ripoll, M.K.; Ferreira, M.R.A.; Conceição, F.R.; Gomes, A.d.R.; Osório, L.d.G.; de Faria, R.O.; Cleff, M.B. Feline Sporotrichosis: A Case Series of Itraconazole-Resistant Sporothrix Brasiliensis Infection. Braz J Microbiol 2021, 52, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Borba-Santos, L.P.; Gagini, T.; Ishida, K.; De Souza, W.; Rozental, S. Miltefosine Is Active against Sporothrix Brasiliensis Isolates with in Vitro Low Susceptibility to Amphotericin B or Itraconazole. Journal of Medical Microbiology 2015, 64, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Borba-Santos, L.P.; Rodrigues, A.M.; Gagini, T.B.; Fernandes, G.F.; Castro, R.; De Camargo, Z.P.; Nucci, M.; Lopes-Bezerra, L.M.; Ishida, K.; Rozental, S. Susceptibility of Sporothrix Brasiliensis Isolates to Amphotericin B, Azoles, and Terbinafine. Medical Mycology 2015, 53, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.S.; Cunha, S.C.; Moraes, V.A.; Leite, J.S.; Ferreira, A.M. Refractory Feline Sporotrichosis: A Comparative Analysis on the Clinical, Histopathological, and Cytopathological Aspects. Braz J Vet Res 2022, 42. [Google Scholar] [CrossRef]
- Rocha, R.F.D.B.; Schubach, T.M.P.; Pereira, S.A.; Dos Reis, E.G.; Carvalho, B.W.; Gremiao, I.D.F. Refractory Feline Sporotrichosis Treated with Itraconazole Combined with Potassium Iodide. J Small Anim Pract 2018, 59, 720–721. [Google Scholar] [CrossRef]
- de Souza, C.P.; Lucas, R.; Ramadinha, R.H.; Pires, T.B. Cryosurgery in Association with Itraconazole for the Treatment of Feline Sporotrichosis. Journal of Feline Medicine and Surgery 2016, 18, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Santi, J.P.; Santos, C.R.G.R.; Santos, A.S.d.; Souza, H.J.M. Intranasal Clotrimazole Spray 1% Associated with Oral Itraconazole for Nasal Feline Sporotrichosis: A Case Series. Braz. J. Vet. Med. 2022, 44, e004821. [Google Scholar] [CrossRef]
- Ribeiro, D.S.C.; Machado, L.J.; Pereira, J.G.; de Souza Baptista, A.R.; da Rocha, E.M.D.S. Laser Therapy in the Treatment of Feline Sporotrichosis: A Case Series. Braz. J. Vet. Med. 2023, 45, e005822. [Google Scholar] [CrossRef]
- Cabral, F.V.; Sellera, F.P.; Ribeiro, M.S. Feline Sporotrichosis Successfully Treated with Methylene Blue-Mediated Antimicrobial Photodynamic Therapy and Low Doses of Itraconazole. Photodiagnosis and Photodynamic Therapy 2022, 40, 103154. [Google Scholar] [CrossRef] [PubMed]
- Gremiao, I.; Schubach, T.; Pereira, S.; Rodrigues, A.; Honse, C.; Barros, M. Treatment of Refractory Feline Sporotrichosis with a Combination of Intralesional Amphotericin B and Oral Itraconazole. Aust Vet J 2011, 89, 346–351. [Google Scholar] [CrossRef]
- Reis, E.G.; Schubach, T.M.; Pereira, S.A.; Silva, J.N.; Carvalho, B.W.; Quintana, M.S.; Gremiao, I.D. Association of Itraconazole and Potassium Iodide in the Treatment of Feline Sporotrichosis: A Prospective Study. Med Mycol 2016, 54, 684–690. [Google Scholar] [CrossRef]
- Sargeant, J.M.; Plishka, M.; Ruple, A.; Selmic, L.E.; Totton, S.C.; Vriezen, E.R. Quality of Reporting of Clinical Trials in Dogs and Cats: An Update. J Vet Intern Med 2021, 35, 1957–1971. [Google Scholar] [CrossRef]
- Burns, P.B.; Rohrich, R.J.; Chung, K.C. The Levels of Evidence and Their Role in Evidence-Based Medicine: Plastic and Reconstructive Surgery 2011, 128, 305–310. 128. [CrossRef]
- Lloret, A. The Process of Evidence-Based Medicine. Journal of Feline Medicine and Surgery 2009, 11, 529–529. [Google Scholar] [CrossRef] [PubMed]
- Reis, E.G.; Gremiao, I.D.; Kitada, A.A.; Rocha, R.F.; Castro, V.S.; Barros, M.B.; Menezes, R.C.; Pereira, S.A.; Schubach, T.M. Potassium Iodide Capsule Treatment of Feline Sporotrichosis. J Feline Med Surg 2012, 14, 399–404. [Google Scholar] [CrossRef]
- Boechat, J.S.; Oliveira, M.M.E.; Gremião, I.D.F.; Almeida-Paes, R.; Machado, A.C.d.S.; Zancopé-Oliveira, R.M.; Oliveira, R.d.V.C.; Morgado, D.S.; Corrêa, M.L.; Figueiredo, A.B.F.; et al. Sporothrix Brasiliensis and Feline Sporotrichosis in the Metropolitan Region of Rio de Janeiro, Brazil (1998–2018). JoF 2022, 8, 749. [Google Scholar] [CrossRef]
- Kaneko, J.J.; Harvey, J.W. Appendixes. In Clinical Biochemistry of Domestic Animals; Elsevier, 2008; pp. 873–904 ISBN 978-0-12-370491-7.
- Center, S.A. Interpretation of Liver Enzymes. Vet Clin North Am Small Anim Pract 2007, 37, 297–333. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.A.; Codeço, C.; Freitas, D.F.S.; De Macedo, P.M.; Pereira, S.A.; Gremião, I.D.F.; Coelho, F.C. Mathematical Model of the Dynamics of Transmission and Control of Sporotrichosis in Domestic Cats. PLoS ONE 2023, 18, e0272672. [Google Scholar] [CrossRef] [PubMed]
- Morgado, F.N.; Schubach, A.O.; Barros, M.B.L.; Conceição-Silva, F. The in Situ Inflammatory Profile of Lymphocutaneous and Fixed Forms of Human Sporotrichosis. Med Mycol 2011, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Conceição-Silva, F.; Morgado, F. Immunopathogenesis of Human Sporotrichosis: What We Already Know. JoF 2018, 4, 89. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, S.S.; Zhong, S.X.; Liu, Y.Y.; Yao, L.; Huo, S.S. Report of 457 Sporotrichosis Cases from Jilin Province, Northeast China, a Serious Endemic Region. J Eur Acad Dermatol Venereol 2013, 27, 313–318. [Google Scholar] [CrossRef]
- Gupta, M.; Narang, T.; Kaur, R.J.; Manhas, A.; Saikia, U.N.; Dogra, S. A Prospective Case Series Evaluating Efficacy and Safety of Combination of Itraconazole and Potassium Iodide in Rhinofacial Conidiobolomycosis. Int J Dermatol 2016, 55, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Mendiratta, V.; Karmakar, S.; Jain, A.; Jabeen, M. Severe Cutaneous Zygomycosis Due to Basidiobolus Ranarum in a Young Infant. Pediatr Dermatol 2012, 29, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.H.M.; Meli, M.; Conceicao-Silva, F.; Novacco, M.; Menezes, R.C.; Pereira, S.A.; Sugiarto, S.; Dos Reis, E.G.; Gremiao, I.D.F.; Hofmann-Lehmann, R. Co-Infection with Feline Retrovirus Is Related to Changes in Immunological Parameters of Cats with Sporotrichosis. PLoS One 2018, 13, e0207644. [Google Scholar] [CrossRef]
- Rossi, C.N.; Odaguiri, J.; Larsson, C.E. Retrospective Assessment of the Treatment of Sporotrichosis in Cats and Dogs Using Itraconazole. Acta Scientiae Veterinariae 2013, 41. [Google Scholar]
- Mawby, D.I.; Whittemore, J.C.; Fowler, L.E.; Papich, M.G. Comparison of Absorption Characteristics of Oral Reference and Compounded Itraconazole Formulations in Healthy Cats. J Am Vet Med Assoc 2018, 252, 195–200. [Google Scholar] [CrossRef]
- Aceves, C.; Mendieta, I.; Anguiano, B.; Delgado-Gonzalez, E. Molecular Iodine Has Extrathyroidal Effects as an Antioxidant, Differentiator, and Immunomodulator. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.O.; Macedo, P.M.; Carvalhal, A.; Bernardes-Engemann, A.R. Use of Potassium Iodide in Dermatology: Updates on an Old Drug. An Bras Dermatol 2013, 88, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Ishikawa, S.; Ishii, E.; Koike, M.; Kaminaga, T.; Hamasaki, Y.; Sairenchi, T.; Kobashi, G.; Igawa, K. Anti-Inflammatory Effects of Potassium Iodide on SDS-Induced Murine Skin Inflammation. Journal of Investigative Dermatology 2020, 140, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Kang, J.; Deng, T.; Yang, X.; Chen, M. Exposure to DBP and High Iodine Aggravates Autoimmune Thyroid Disease Through Increasing the Levels of IL-17 and Thyroid-Binding Globulin in Wistar Rats. Toxicol Sci 2018, 163, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Edinboro, C.H.; Scott-Moncrieff, J.C.; Glickman, L.T. Feline Hyperthyroidism: Potential Relationship with Iodine Supplement Requirements of Commercial Cat Foods. J Feline Med Surg 2010, 12, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Kalarani, I.B.; Veerabathiran, R. Impact of Iodine Intake on the Pathogenesis of Autoimmune Thyroid Disease in Children and Adults. Ann Pediatr Endocrinol Metab 2022, 27, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Kawashima, A.; Ishido, Y.; Yoshihara, A.; Oda, K.; Hiroi, N.; Ito, T.; Ishii, N.; Suzuki, K. Iodine Excess as an Environmental Risk Factor for Autoimmune Thyroid Disease. Int J Mol Sci 2014, 15, 12895–12912. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global Epidemiology of Hyperthyroidism and Hypothyroidism. Nat Rev Endocrinol 2018, 14, 301–316. [Google Scholar] [CrossRef]
- Teng, W.; Shan, Z.; Teng, X.; Guan, H.; Li, Y.; Teng, D.; Jin, Y.; Yu, X.; Fan, C.; Chong, W.; et al. Effect of Iodine Intake on Thyroid Diseases in China. N Engl J Med 2006, 354, 2783–2793. [Google Scholar] [CrossRef]
| Variables | G1-ITZ (N=83) |
G2-ITZ+KI (N=83) |
|---|---|---|
| Sex, n (%) | ||
| Male | 58 (69.9%) | 64 (77.1%) |
| Female | 25 (30.1%) | 19 (22.9%) |
| Clinical form, n (%) | ||
| Cutaneous | 51 (61.4%) | 56 (67.5%) |
| Cutaneous/Mucosal | 32 (38.6%) | 27 (32.5%) |
| Distribution of skin lesions, n (%) | ||
| L1 | 27 (32.5%) | 26 (31.3%) |
| L2 | 22 (26.5%) | 22 (26.5%) |
| L3 | 34 (41.0%) | 35 (42.2%) |
| Respiratory signs, n (%) | 30 (36.1%) | 23 (27.7%) |
| Lymphadenomegaly, n (%) | 69 (83.1%) | 67 (80.7%) |
| Lymphangitis, n (%) | 16 (19.3%) | 13 (15.7%) |
| Access outdoors, n (%) | 68 (81.9%) | 62 (74.1%) |
| Neutering, n (%) | 36 (43.4%) | 45 (54.2%) |
| FIV Ab and FeLV Ag *, n (%) | 13 (8.07%) | 18 (11.18%) |
| Age in months, MD (IQR) ** | 24 (24-48) | 24 (15-36) |
| Weight in Kg, MD (IQR) ** | 4 (3.5-4.7) | 4 (3.6-4.5) |
| Time interval between the onset ofclinical signs in weeks, MD (IQR) ** | 8 (4-12) | 8 (4-12) |
| Variables | G1-ITZ (N=83) |
G2-ITZ+KI (N=83) |
| Sex, n (%) | ||
| Male | 58 (69.9%) | 64 (77.1%) |
| Female | 25 (30.1%) | 19 (22.9%) |
| Clinical form, n (%) | ||
| Cutaneous | 51 (61.4%) | 56 (67.5%) |
| Cutaneous/Mucosal | 32 (38.6%) | 27 (32.5%) |
| Distribution of skin lesions, n (%) | ||
| L1 | 27 (32.5%) | 26 (31.3%) |
| L2 | 22 (26.5%) | 22 (26.5%) |
| L3 | 34 (41.0%) | 35 (42.2%) |
| Respiratory signs, n (%) | 30 (36.1%) | 23 (27.7%) |
| Lymphadenomegaly, n (%) | 69 (83.1%) | 67 (80.7%) |
| Lymphangitis, n (%) | 16 (19.3%) | 13 (15.7%) |
| Access outdoors, n (%) | 68 (81.9%) | 62 (74.1%) |
| Neutering, n (%) | 36 (43.4%) | 45 (54.2%) |
| FIV Ab and FeLV Ag *, n (%) | 13 (8.07%) | 18 (11.18%) |
| Age in months, MD (IQR) ** | 24 (24-48) | 24 (15-36) |
| Weight in Kg, MD (IQR) ** | 4 (3.5-4.7) | 4 (3.6-4.5) |
| Time interval between the onset of clinical signs in weeks, MD (IQR) ** |
8 (4-12) | 8 (4-12) |
| Variable | Cats with increasing dose | KI mg/kg | |||
|---|---|---|---|---|---|
| n | % | range | |||
| Persistent Lesion | Skin | 15 | 53.6 | 2.5-9.5 | |
| Mucosal | 2 | 7.1 | 3.1-8.1 | ||
| Skin and mucosal | 11 | 39.3 | 2.5-12.5 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





