Submitted:
27 December 2023
Posted:
27 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of solid interpolyelectrolyte complexes (IPEC) and physical mixtures (PhM)
2.3. Preparation of tablets
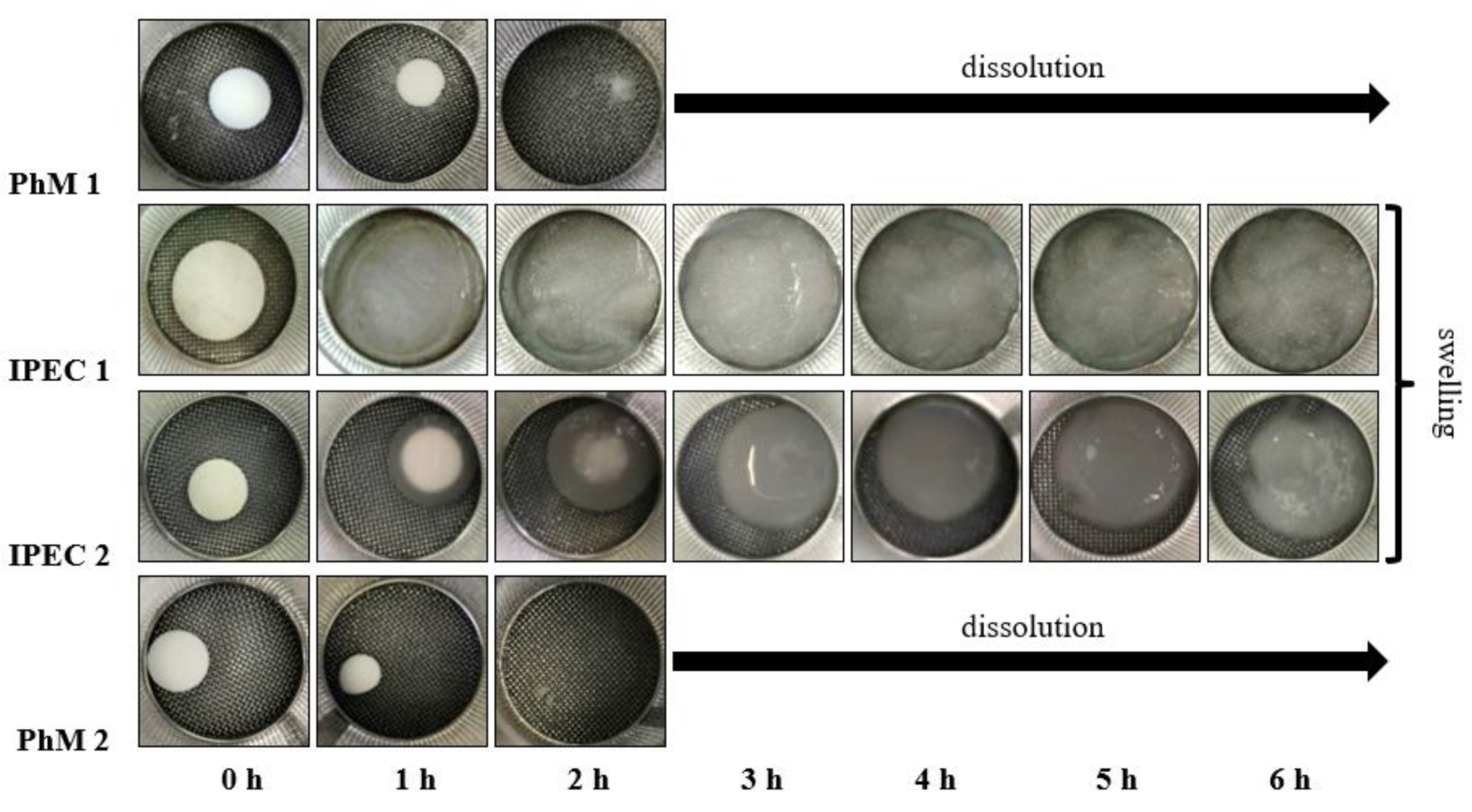
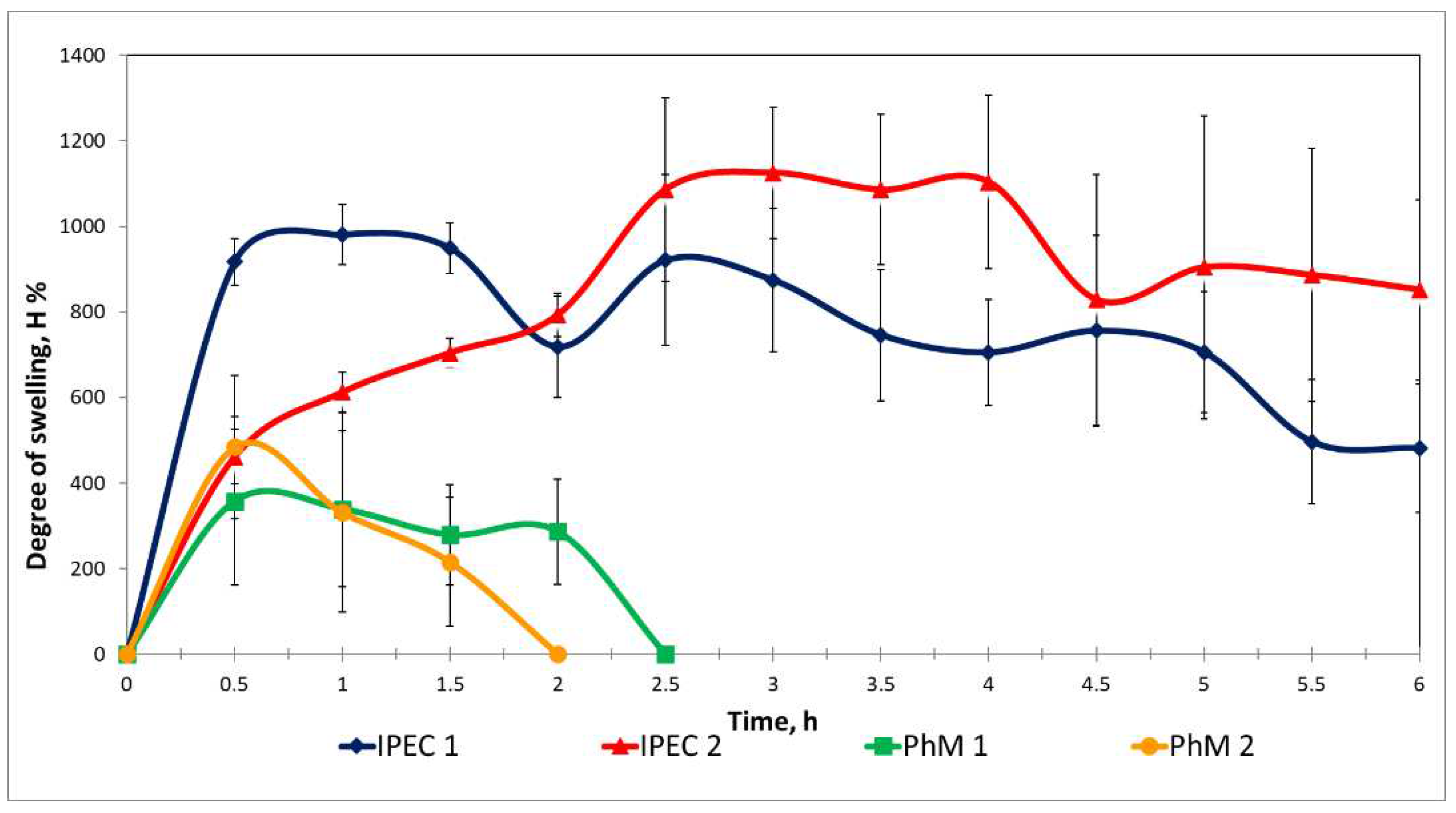
2.4. Determination of the degree of swelling of matrices.
2.5. Elemental Analysis
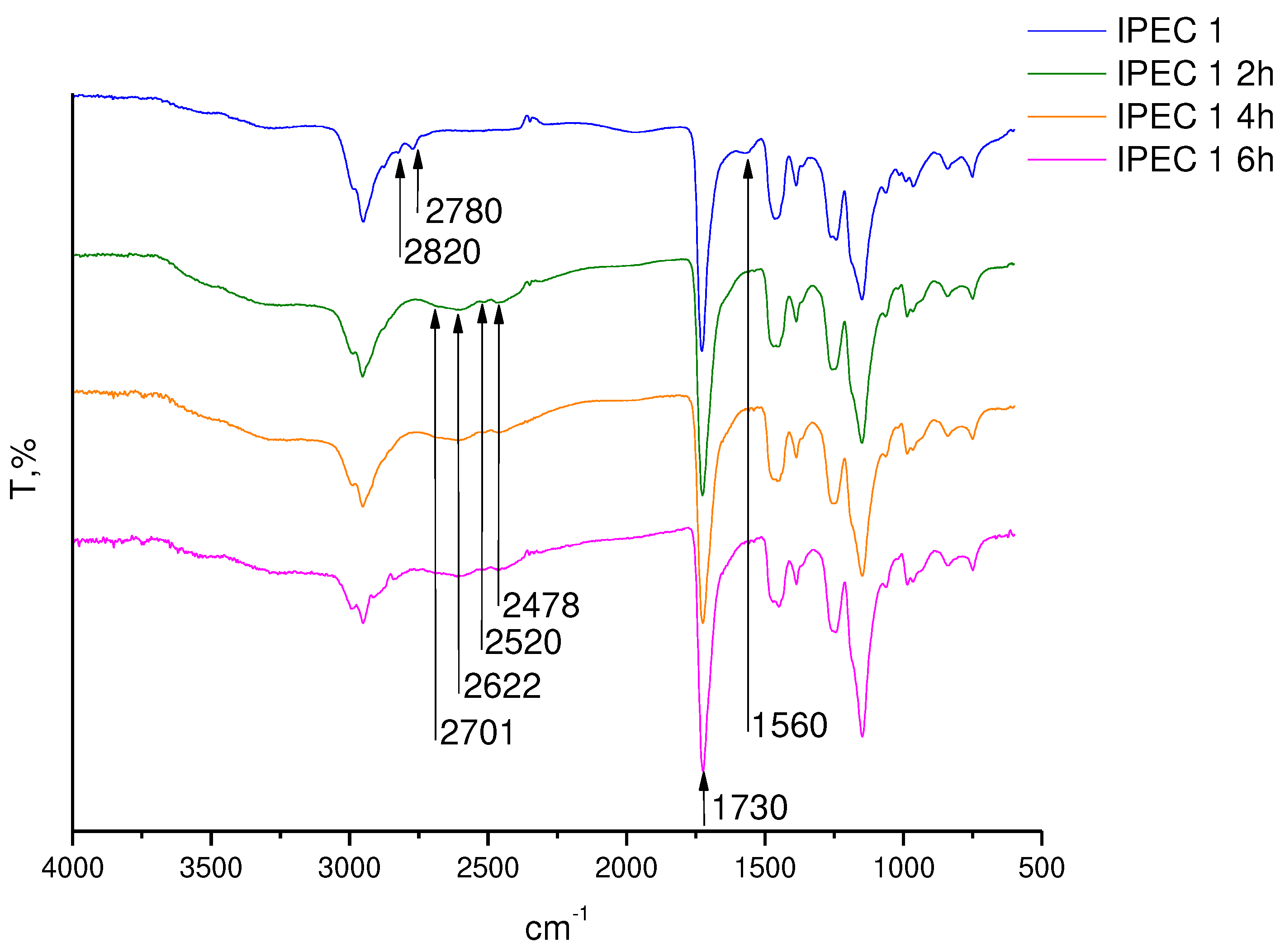
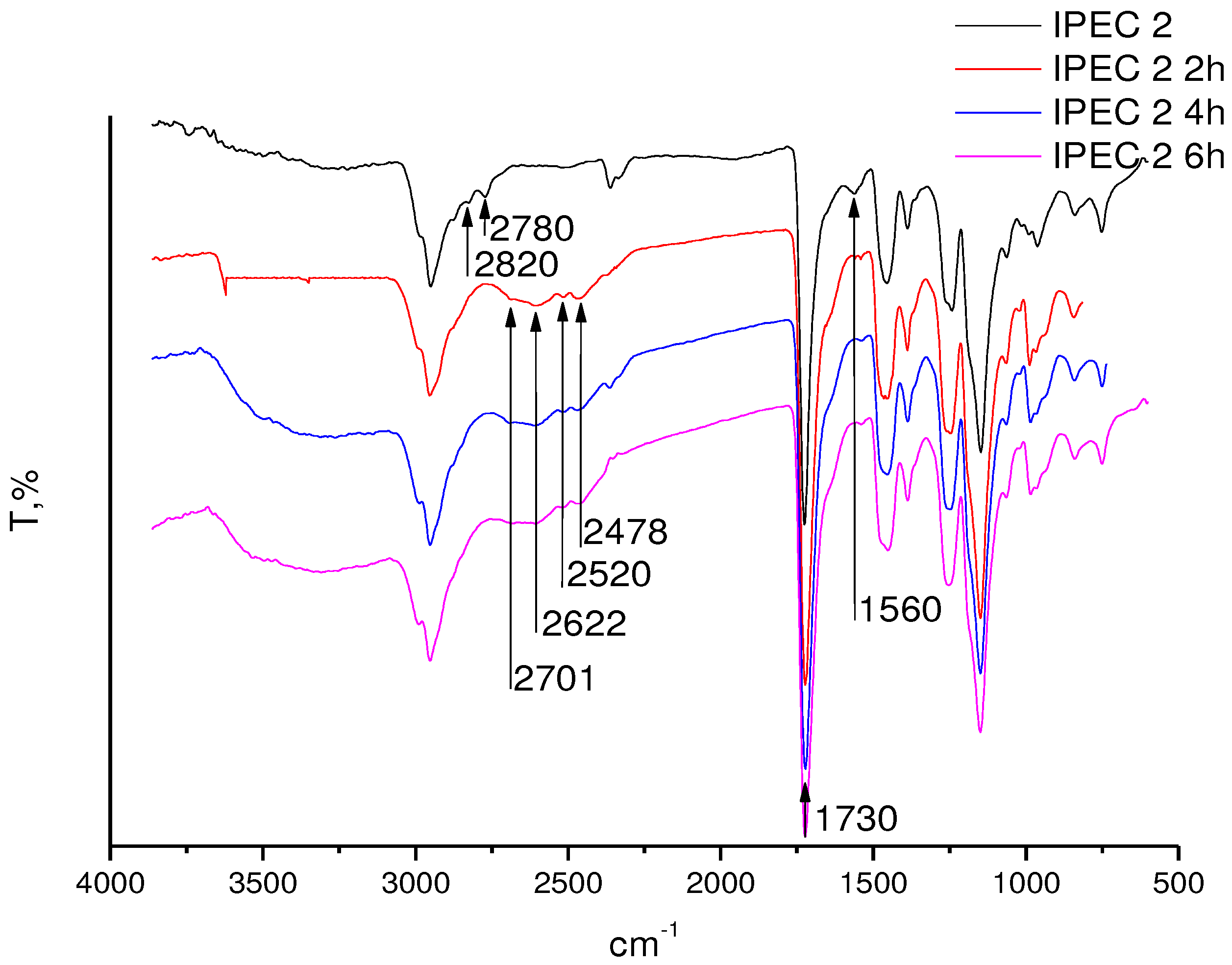
2.6. Fourier Transformed Infrared (ATR-FTIR) Spectroscopy
2.7. Thermal Analysis
2.8. Study of model drug release
2.9. Analysis of bioadhesive properties
2.10. Statistical analysis
3. Results and Discussion
3.1. Composition Study
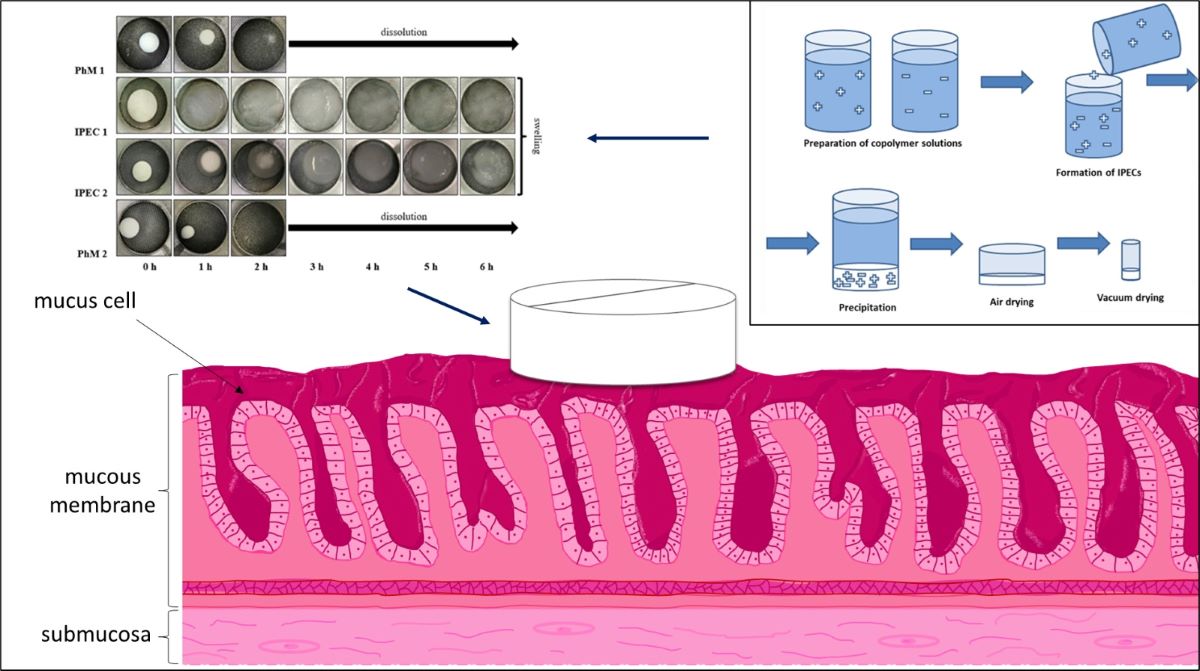
3.2. Assessment of the IPEC behavior in acidic medium
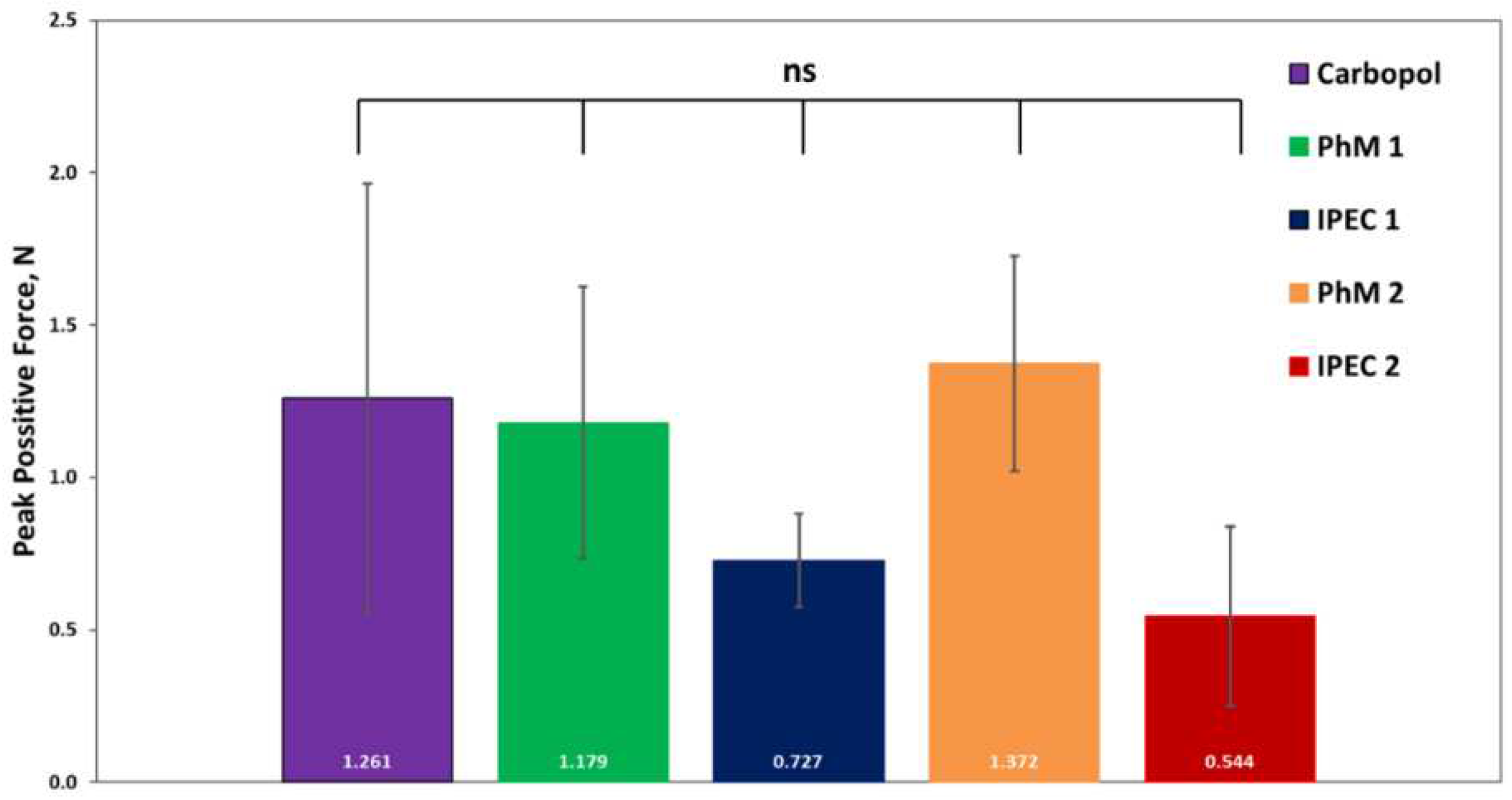
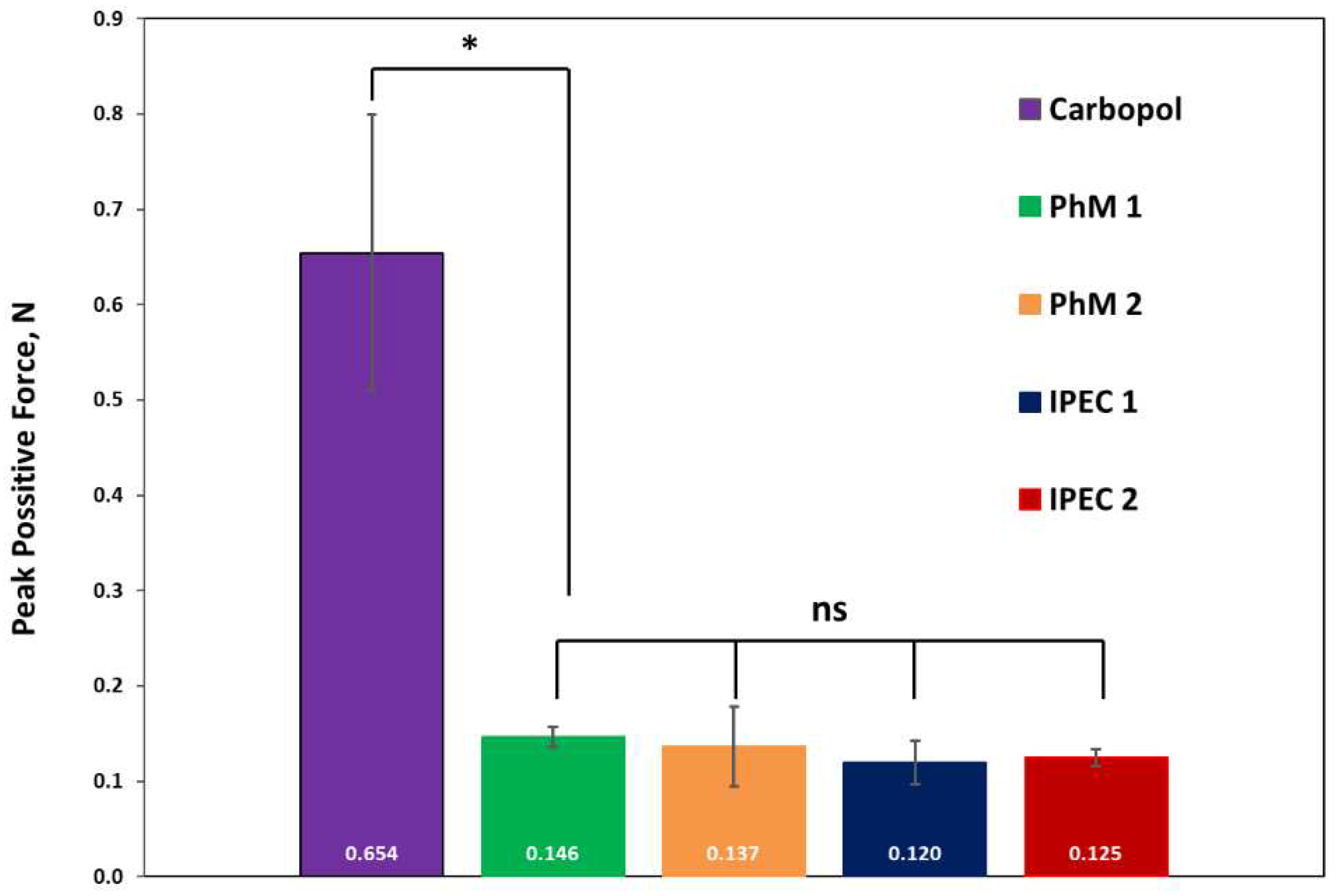
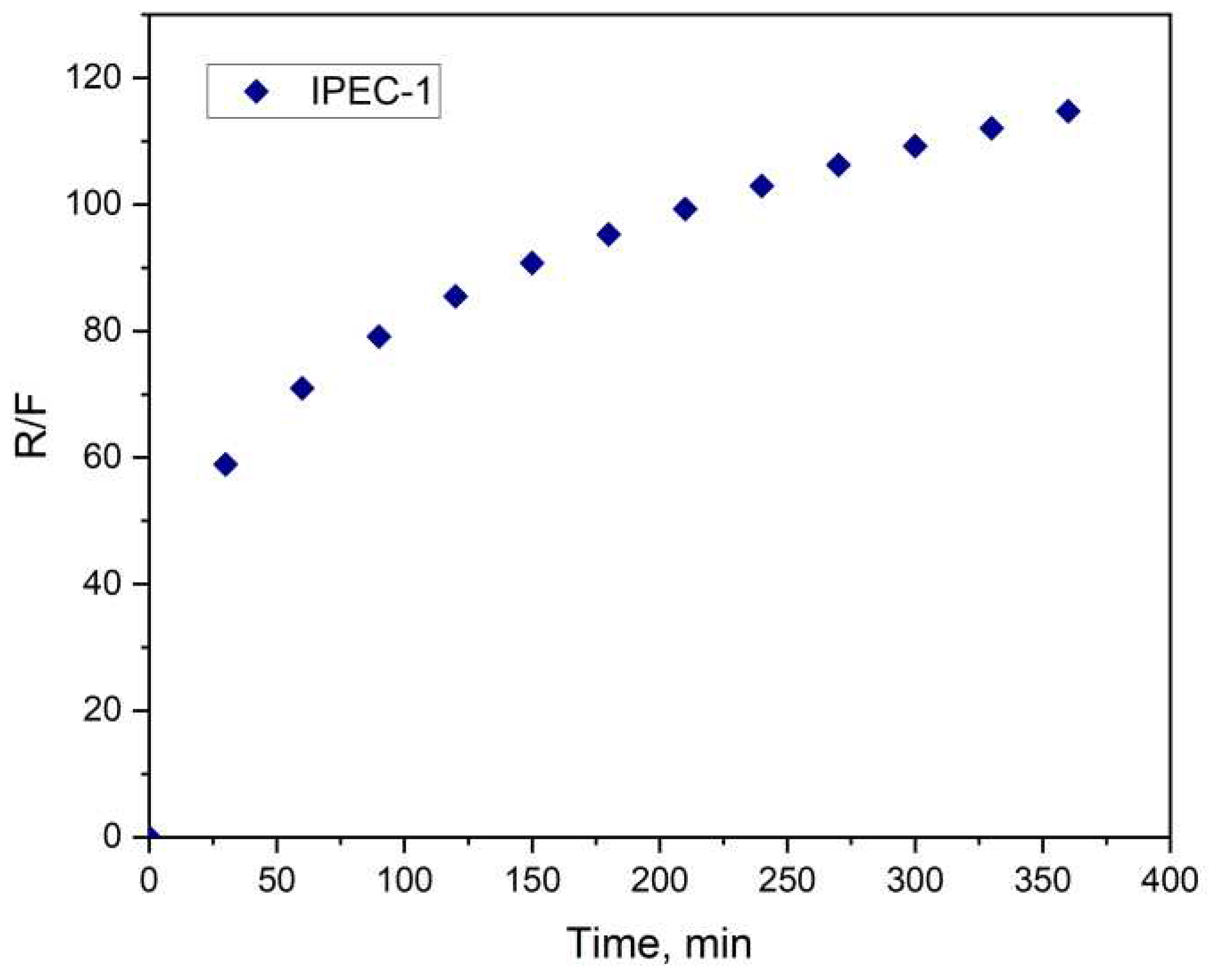
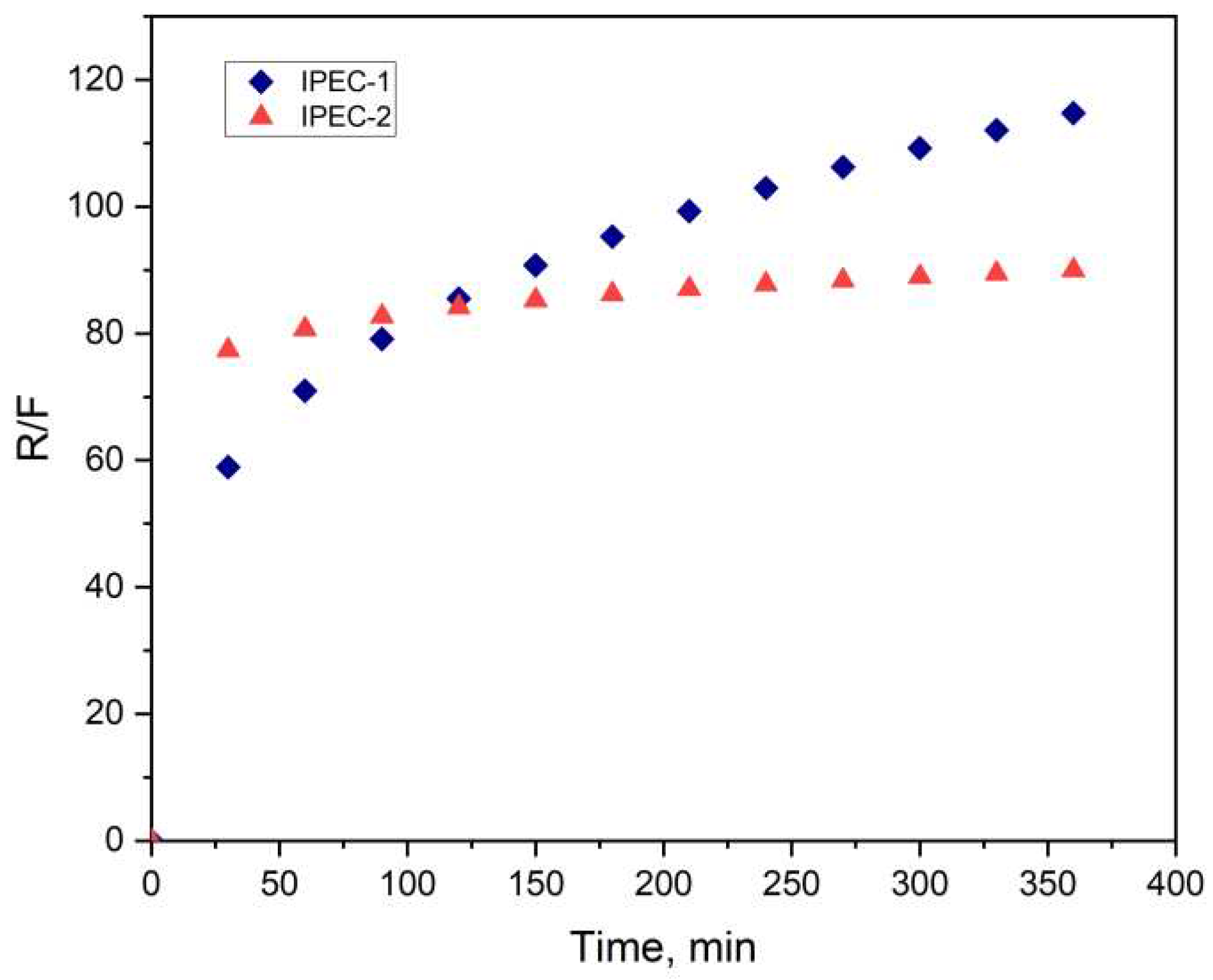
3.3. Analysis of bioadhesive properties
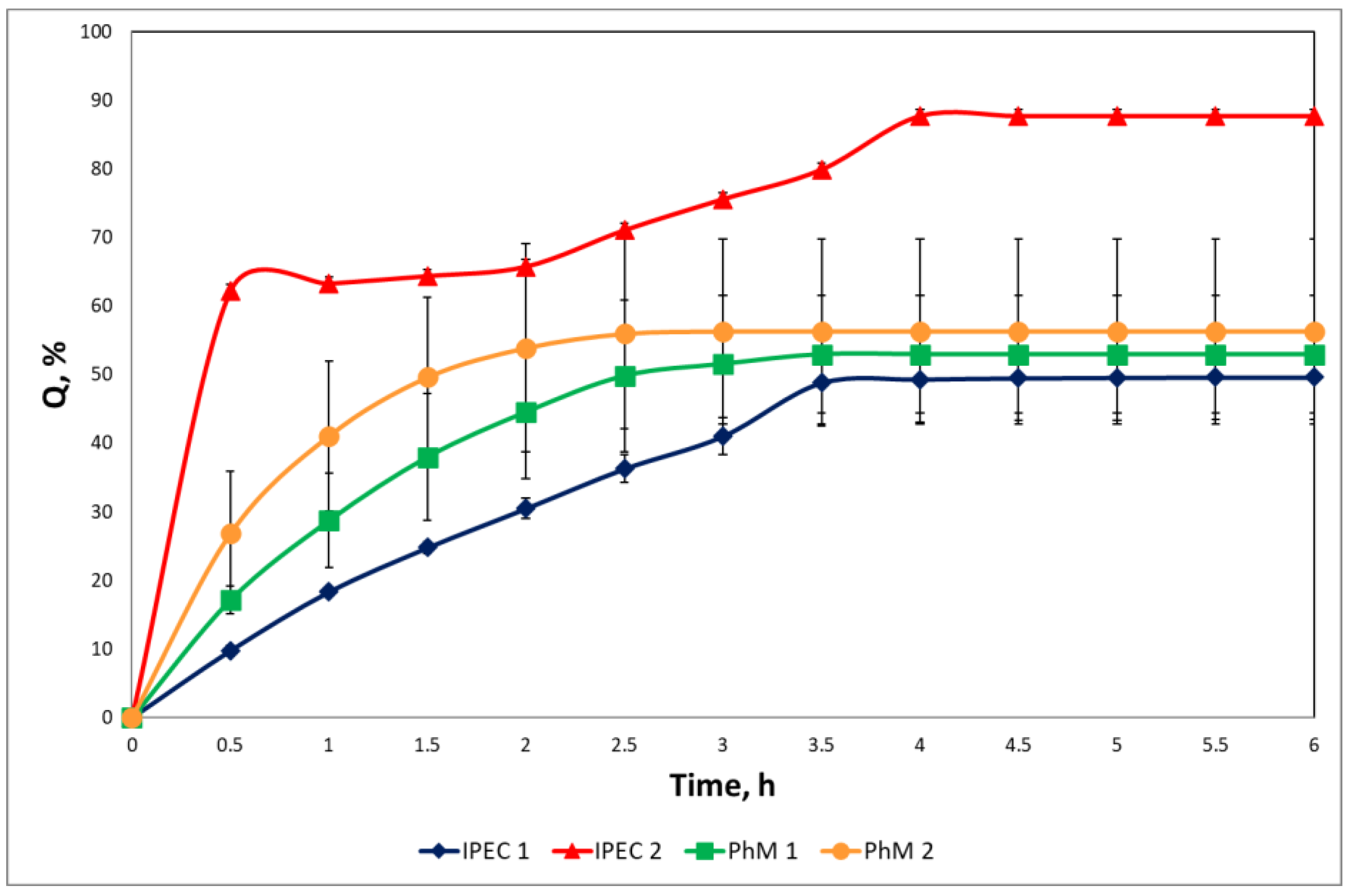
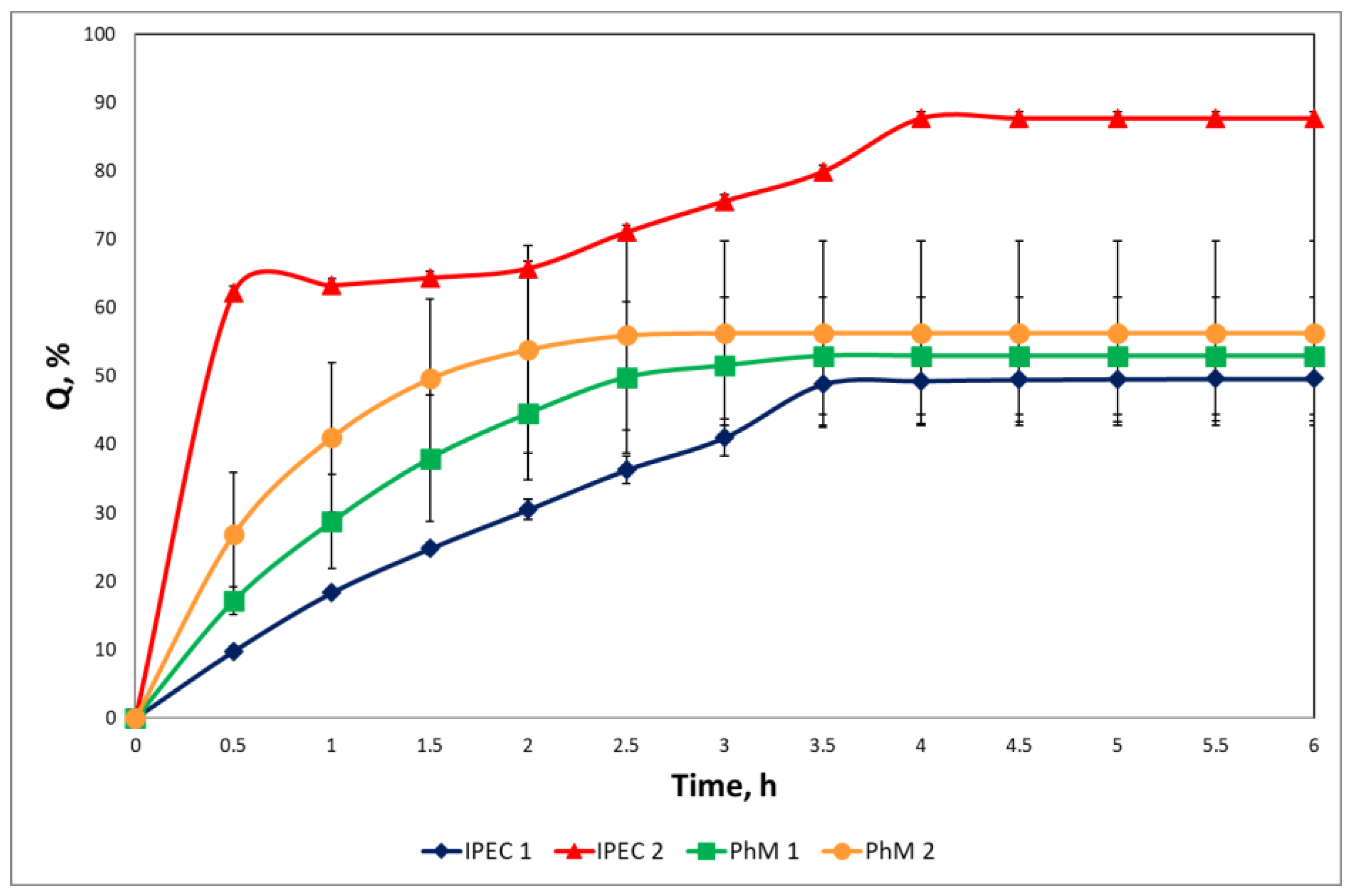
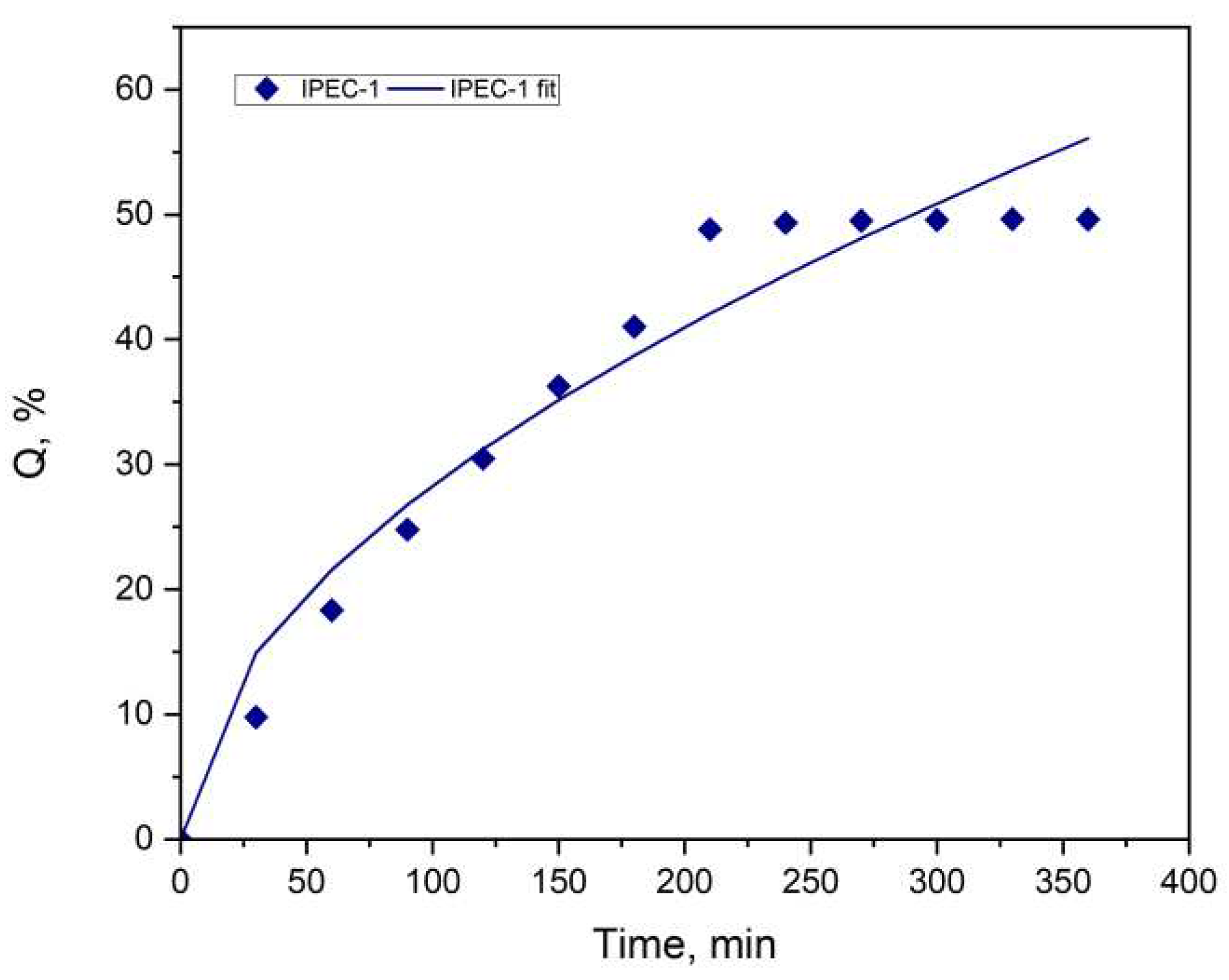
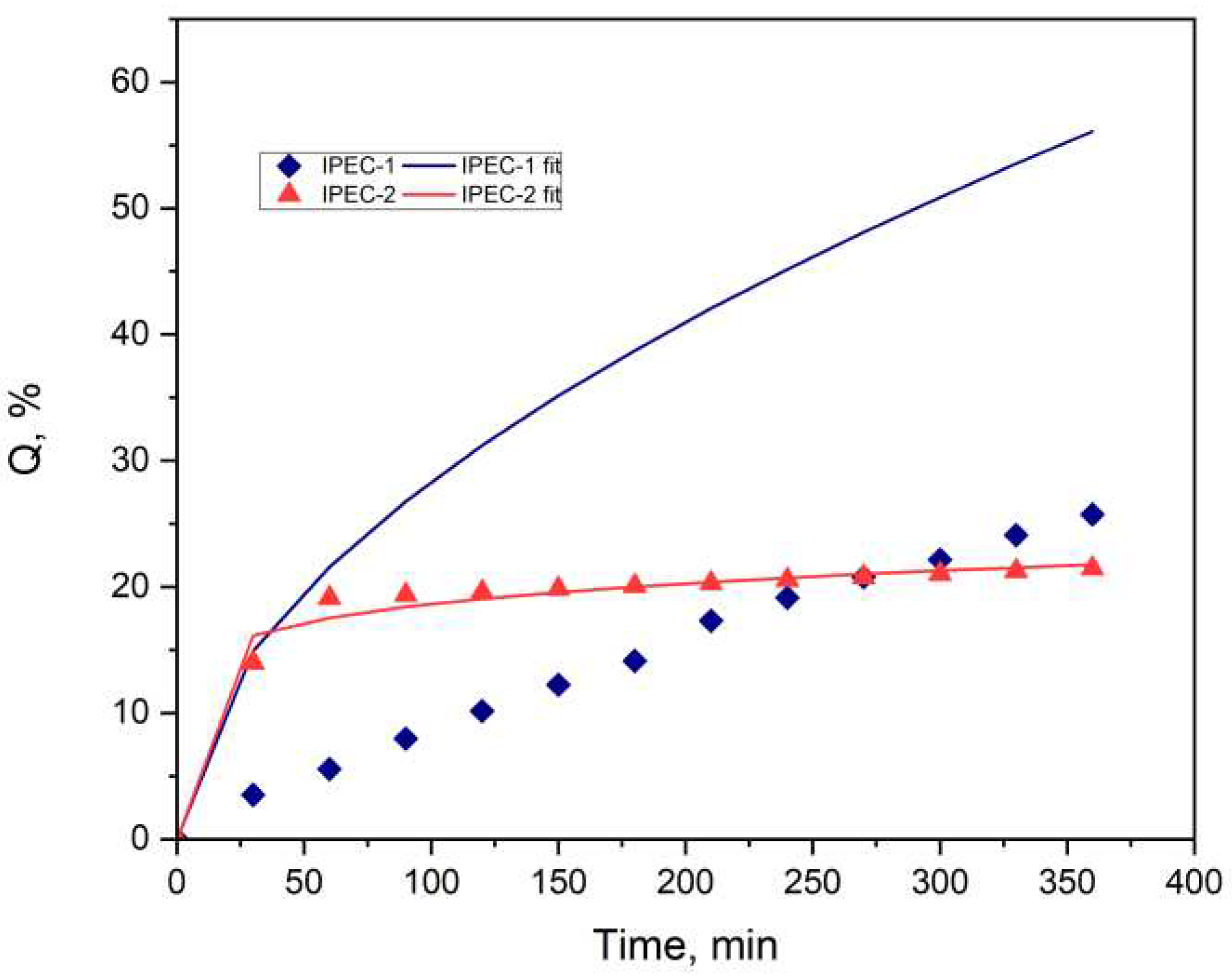
3.4. Study of drug release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alqahtani, A.A.; Mohammed, A.A.; Fatima, F.; Ahmed, M.M. Fused Deposition Modelling 3D-Printed Gastro-Retentive Floating Device for Propranolol HCl Tablets. Polymers 2023, 15, 3554. [CrossRef]
- Patel, M.; Shelke, S.; Shaikh, F.; Surti, N.; Panzade, P.; Panjwani, D. Gastroretentive Floating Microsponges of Mitiglinide: Design, Preparation, and Pharmacokinetic Evaluation. J. Pharm. Innov. 2023, 14. [CrossRef]
- Malladia, M.; Jukanti, R. Formulation development and evaluation of a novel bi-dependent clarithromycin gastroretentive drug delivery system using Box-Behnken design. J. Drug Deliv. Sci. Technol. 2016, 35, 134-145. [CrossRef]
- Uboldi, M.; Melocchi, A.; Moutaharrik, S.; Palugan, L.; Cerea, M.; Foppoli, A.; Maroni, A.; Gazzaniga, A.; Zema, L. Administration strategies and smart devices for drug release in specific sites of the upper GI tract. J. Contr. Rel. 2022, 348, 537–552. [CrossRef]
- Awasthi, R.; Kulkarni, G.T. Decades of research in drug targeting to the upper gastrointestinal tract using gastroretention technologies: Where do we stand? Drug Deliv. 2016, 23, 378–394. [CrossRef]
- Naseem, F.; Shah, S.U.; Rashid, S.A.; Farid, A.; Almehmadi, M.; Alghamdi, S. Metronidazole Based Floating Bioadhesive Drug Delivery System for Potential Eradication of H. pylori: Preparation and In Vitro Characterization. Polym. J. 2022, 14, 519. [CrossRef]
- Sravya, V.; Suresh, K.P.; Jagannath, P.V.; Sunitha, Ch. Formulate gastroretentive floating bioadhesive drug delivery system of nizatidine by direct compression technique. World J. Pharm. Sci. 2022, 10, 59–73. [CrossRef]
- Altreuter, D.H.; Kirtane, A.R.; Grant, T.; Kruger, C.; Traverso, G.; Bellinger, A.M. Changing the pill: developments toward the promise of an ultra-long-acting gastroretentive dosage form. Exp. Opin. Drug Deliv. 2018, 15, 189-1198. [CrossRef]
- Zhang, Ch.; Tang, J.; Liu, D.; Li, X.; Cheng, L.; Tang, X. Design and evaluation of an innovative floating and bioadhesive and multiparticulate drug delivery system based on hollow structure. Int. J. Pharm. 2016, 503, 41-55. [CrossRef]
- Patel, K.; Chouksey, R. A Recent Advantage on Gastroretentive Drug Delivery System: An Overview. Res. J. Pharm. Technol. 2023, 15, 36–44. [CrossRef]
- Pawar, V. K.; Kansal, Sh.; Asthana, Sh.; Chourasia, M. K. Industrial perspective of gastroretentive drug delivery systems: Physicochemical, biopharmaceutical, technological and regulatory consideration. Exp. Opin. Drug Deliv. 2012, 9, 551-565. [CrossRef]
- Tripathi, J.; Thapa, P.; Maharjan, R.; Jeong, S.H. Current State and Future Perspectives on Gastroretentive Drug Delivery Systems. Pharmaceutics 2019, 11, 193. [CrossRef]
- Singh, B.; Sharma, V.; Mohan, M.; Rohit; Sharma, P.; Ram, K. Design of ciprofloxacin impregnated dietary fiber psyllium-moringa gum-alginate network hydrogels via green approach for use in gastro-retentive drug delivery system, Bioact. Carbohydr. Diet. Fiber 2023, 29, 100345. [CrossRef]
- Farhaj, S.; Conway, B.R.; Ghori, M.U. Nanofibres in Drug Delivery Applications. Fibers 2023, 11, 21. [CrossRef]
- Jeong, S.H.; Park, K. Development of sustained release fast-disintegrating tablets using various polymer-coated ion-exchange resin complexes. Int. J. Pharm. 2008, 353, 195–204. [CrossRef]
- Das, S.; Kaur, S.; Rai, V. K. Gastro-retentive drug delivery systems: a recent update on clinical pertinence and drug delivery. Drug Deliv.Transl. Res. 2021, 11, 1849–1877. [CrossRef]
- Pal, R.; Pandey, P.; Nogai, L.; Arushi; Anand, A.; Suthar, Pa.; Keskar, M. S.; Kumar, V. The Future Perspectives and Novel Approach on Gastro Retentive Drug Delivery System (GRDDS) with Current State. Can. J. Clin. Pharmacol. 2023, 30, 594–613. [CrossRef]
- Zhu, X.; Qi, X.; Wu, Z.; Zhang, Z.; Xing, J.; Li, X. Preparation of multiple-unit floating-bioadhesive cooperative minitablets for improving the oral bioavailability of famotidine in rats, Drug Deliv. 2014, 21, 459–466. [CrossRef]
- Darbasizadeha, B.; Motasadizadehb, H.; Foroughi-Niac, B.; Farhadnejad, H. Tripolyphosphate-crosslinked chitosan/poly (ethylene oxide) electrospun nanofibrous mats as a floating gastro-retentive delivery system for ranitidine hydrochloride. J. Pharm. Biomed. Anal. 2018, 153, 63–75. [CrossRef]
- Meng, S.; Wang, S.; Piao, M.G. Prescription Optimization of Gastroretentive Furosemide Hollow-Bioadhesive Microspheres via Box-Behnken Design: In Vitro Characterization and in Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2022, 70, 103235. [CrossRef]
- Ngwuluka, N.C.; Choonara, Y.E.; Modi, G.; du Toit, L.C.; Kumar, P.; Ndesendo, V.M.K.; Pillay V.A. Design of an Interpolyelectrolyte Gastroretentive Matrix for the Site-Specific Zero-Order Delivery of Levodopa in Parkinson’s Disease. AAPS PharmSciTech. 2013, 14, 605–619. [CrossRef]
- Gallardo, D.; Skalsky, B.; Kleinebudde, P. Controlled release solid dosage forms using combinations of (meth)acrylate copolymer. Pharm. Dev. Technol. 2008, 13, 413–423. [CrossRef]
- Siepmann, F.; Siepmann, J.; Walther, M.; MacRae, R. J.; Bodmeier, R. Polymer blends for controlled release coatings. J. Control. Rel. 2008, 125, 1−15. [CrossRef]
- Mustafin, R.I. Interpolymer combinations of chemically complementary grades of Eudragit copolymers: A new direction in the design of peroral solid dosage forms of drug delivery systems with controlled release (review). Pharm. Chem. J. 2011, 45, 285–295. [CrossRef]
- Moustafine, R.I. Role of macromolecular interactions of pharmaceutically acceptable polymers in functioning oral drug delivery systems. Russ. J. Gen. Chem. 2014, 84, 364-367. [CrossRef]
- Moustafine, R.I.; Kabanova, T.V.; Kemenova, V.A.; Van den Mooter, G. Characteristics of interpolyelectrolyte complexes of Eudragit E100 with Eudragit L100. J. Control. Rel. 2005, 103, 191–198. [CrossRef]
- Moustafine, R. I.; Zaharov, I. M.; Kemenova, V. A. Physicochemical characterization and drug release properties of Eudragit E PO/Eudragit L100-55 interpolyelectrolyte complexes. Eur. J. Pharm. Biopharm. 2006, 63, 26−36. [CrossRef]
- Moustafine, R.I.; Bobyleva, V.L.; Bukhovets, A.V.; Garipova, V.R.; Kabanova, T.V.; Kemenova, V.A.; Van den Mooter, G. Structural transformations during swelling of polycomplex matrices based on countercharged (meth)acrylate copolymers (Eudragit® EPO/Eudragit® L100-55). J. Pharm. Sci. 2011, 100, 874−885. [CrossRef]
- Moustafine, R.I.; Bukhovets, A.V.; Sitenkov, A.Y.; Kemenova, V.A.; Rombaut, P.; Van den Mooter, G. Eudragit® E PO as a complementary material for designing oral drug delivery systems with controlled release properties: comparative evaluation of new interpolyelectrolyte complexes with countercharged Eudragit® L100 copolymers. Mol. Pharm. 2013, 10, 2630–2641. [CrossRef]
- Moustafine, R.I.; Sitenkov, A.Y.; Bukhovets, A.V.; Nasibullin, Sh.F.; Appeltans, B.; Kabanova, T.V.; Khutoryanskiy, V.V.; Van den Mooter, G. Indomethacin-containing interpolyelectrolyte complexes based on Eudragit® E PO/S 100 copolymers as a novel drug delivery system. Int. J. Pharm. 2017, 524, 121–133. [CrossRef]
- Sauer, D.; McGinity, J.W. Properties of theophylline tablets dry powder coated with Eudragit E PO and Eudragit L 100−55. Pharm. Dev. Technol. 2009, 16, 632−641. [CrossRef]
- Obeidat, W. M.; Abu Znait, A. H.; Sallam, A. A. Novel combination of anionic and cationic polymethacrylate polymers for sustained release tablet preparation. Drug Dev. Ind. Pharm. 2008, 34, 650−660. [CrossRef]
- Obeidat, W. M.; Abu Znait, A. H.; Sallam, A. A. Sustained release tablets containing soluble polymethacrylates: Comparison with tableted polymethacrylate IPEC polymers. AAPS PharmSciTech. 2010, 11, 54−63. [CrossRef]
- Bani-Jaber, A. H.; Alkawareek, M. J.; Al-Gousous, J. J.; Abu Helwa, A. Y. Floating and sustained-release characteristics of efferverscent tablets prepared with a mixed matrix of Eudragit L100-55 and Eudragit E PO. Chem. Pharm. Bull. 2011, 59. [CrossRef]
- Bani-Jaber, A. H.; Al-Aani, L.; Alkhatib, H.; Al-Khalidi, B. Prolonged intragastric drug delivery mediated by Eudragit E carrageenan polyelectrolyte matrix tablets. AAPS PharmSciTech. 2011, 12, 354−361. [CrossRef]
- Quinteros, D. A.; Manzo, R. H.; Allemandi, D. A. Interaction between Eudragit E100 and anionic drugs: Addition of anionic polyelectrolytes and their influence on drug release performance. J. Pharm. Sci. 2011, 100, 4664−4673. [CrossRef]
- Wulff, R., Leopold, C.S. Coatings from blends of Eudragit® RL and L55: A novel approach in pH-controlled drug release. Int. J. Pharm. 2014, 476, 78–87. [CrossRef]
- Wulff, R., Leopold, C.S. Coatings of Eudragit® RL and L55 blends: Investigations on the drug release mechanism. AAPS PharmSciTech. 2016, 17, 493–503. [CrossRef]
- Garcíaa, M.C.; Martinellic, M.; Ponced, N.E.; Sanmarcoe, L.M.; Aokie, M.P.; Manzo, R.H.; Jimenez-Kairuz, A.F. Multi-kinetic release of benznidazole-loaded multiparticulate drug delivery systems based on polymethacrylate interpolyelectrolyte complexes. Eur. J. Pharm. Sci. 2018, 120, 107–122. [CrossRef]
- Sester, C.; Ofridam, F.; Lebaz, N.; Gagnière, E.; Mangin, D.; Elaissari, A. pH-Sensitive methacrylic acid–methyl methacrylate copolymer Eudragit L100 and dimethylaminoethyl methacrylate, butyl methacrylate, and methyl methacrylate tri-copolymer Eudragit E100. Polym. Adv. Technol. 2020, 31, 440–450. [CrossRef]
- Bukhovets, A.V., Fotaki, N., Khutoryanskiy, V.V., Moustafine, R.I. Interpolymer complexes of Eudragit® copolymers as novel carriers for colon-specific drug delivery. Polymers 2020, 12, 1459. [CrossRef]
- Bukhovets, A.V.; Sitenkov, A.Y.; Moustafine, R.I. Comparative evaluation study of polycomplex carriers based on Eudragit® EPO/S100 copolymers prepared in different media. Polym. Adv. Technol. 2021, 32, 2761–2769. [CrossRef]
- Gordeeva, D.S.; Sitenkova, A.V.; Moustafine, R.I. Interpolyelectrolyte complexes based on Eudragit® copolymers as carriers for bioadhesive gastroretentive metronidazole delivery system. Drug Dev. Reg. 2020, 9, 72–76. [CrossRef]
- Lankalapalli, S.; Kolapalli, V.R.M. Polyelectrolyte complexes: A review of their applicability in drug delivery technology. Ind. J. Pharm. Sci. 2009, 71, 481–487. [CrossRef]
- De Robertis, S.; Bonferoni, M.C.; Elviri, L.; Sandri, G.; Caramella, C.; Bettini, R. Advances in oral controlled drug delivery: the role of drug—polymer and interpolymer non-covalent interactions. Exp. Opin. Drug Deliv. 2015, 12, 441–453. [CrossRef]
- Porfiryeva, N.N.; Nasibullin, S.F.; Abdullina, S.G.; Tukhbatullina, I.K.; Moustafine, R.I.; Khutoryanskiy, V.V. Acrylated Eudragit® E PO as a novel polymeric excipient with enhanced mucoadhesive properties for application in nasal drug delivery. Int. J. Pharm. 2019, 562, 241−248. [CrossRef]
- Porfiryeva, N.N.; Semina, I.I.; Salakhov, I.A.; Moustafine, R.I.; Khutoryanskiy, V.V. Mucoadhesive and mucus-penetrating interpolyelectrolyte complexes for nose-to-brain drug delivery. Nanomedicine: NBM 2021, 37, 102432. [CrossRef]
- Shin, S.; Kim, T.H.; Jeong, S.W.; Chung, S.E.; Lee, D.Y.; Kim, D.H.; Shin, B.S. Development of a gastroretentive delivery system for acyclovir by 3D printing technology and its in vivo pharmacokinetic evaluation in Beagle dogs. PLoS ONE 2017, 14, e0216875. [CrossRef]
- Farshforoush, P.; Ghanbarzadeh, S.; Gpganian, A.M.; Hamishehkar, H. Novel metronidazole-loaded hydrogel as a gastroretentive drug delivery system. Iran. Polym. J. 2017, 26, 895–901. [CrossRef]
- Peppas, N.; Sahlin, J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [CrossRef]
- Boddupalli, B.M.; Mohammed, Z.N.; Nath, R.A.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381−387. [CrossRef]
- Zhang, S.; Fang, M.; Zhang, Q.; Li, X.; Zhang, T. Evaluating the bioequivalence of metronidazole tablets and analyzing the effect of in vitro dissolution on in vivo absorption based on PBPK modeling. Drug Dev. Ind. Pharm. 2019, 45, 1646–1653. [CrossRef]
- 53. Sulistiawati; Dwipayanti, K.S.; Azhar, M.; Rahman, L.; Pakki, E.; Himawan, A.; Permana, A.D. Enhanced skin localization of metronidazole using solid lipid microparticles incorporated into polymeric hydrogels for potential improved of rosacea treatment: An ex vivo proof of concept investigation, Int. J. Pharm. 2022, 628, 122327. [CrossRef]
- Wu, F.; Cristofoletti, R.; Zhao, L.; Rostami-Hodjegan, A. Scientific considerations to move towards biowaiver for biopharmaceutical classification system classIII drugs: How modeling and simulation can help. Biopharm. Drug Dispos. 2021, 42, 118–127. [CrossRef]
- Mady, O.I.; Osman, M.A.; Sarhan, N.I.; Shatla, A.A.; Haggag, Y.A.; Bioavailability enhancement of acyclovir by honey: Analytical and histological evidence. J. Drug Deliv. Sci. Technol. 2023, 80, 104155. [CrossRef]
- Filippova, N.I.; Teslev, A.A. Application of mathematical modeling in the evaluation of in vitro drug release. Drug Dev. Reg. 2017, 4, 218–226.
- Unagollaa, J.M.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [CrossRef]
| Sample symbol | pH at which IPEC was obtained | IPEC composition | Tg value, °C |
|
| Z =[EPO]/[L100] | EPO:L100(mol/mol) | |||
| IPEC 1 | 6.0 | 1.02 | 1:0.98 | 146.6±0.3 |
| IPEC 2 | 6.5 | 1.49 | 1:0.67 | 117.4±0.2 |
| Sample | Glass transition, °C | Elemental analysis | |
| Тg | CompositionZ=EPO:L100 (mol/mol) | N,% | |
| IPEC 1 2 h | 165.8±0.1 | 1:1.17 | 2.84±0.16 |
| IPEC 1 4 h | 170.7±0.1 | 1:1.11 | 2.91±0.26 |
| IPEC 1 6 h | 170.6±0.1 | 1:1.08 | 3.11±0.10 |
| IPEC 2 2 h | 169.5±0.1 | 1:1.22 | 2.78±0.11 |
| IPEC 2 4 h | 172.3±0.5 | 1:1.47 | 2.54±0.12 |
| IPEC 2 6 h | 173.9±0.3 | 1:1.27 | 2.75±0.31 |
| Parameters | IPEC 1 |
| m | 0.2681 |
| K1 | 0.1000 |
| K2 | 2.3675 |
| R2 | 0.9762 |
| Parameters | IPEC 1 | IPEC 2 |
| m | 0.2681 | 0.0605 |
| K1 | 0.1000 | 0.1675 |
| K2 | 2.3676 | 10.5505 |
| R2 | 0.9813 | 0.9892 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





