Submitted:
25 December 2023
Posted:
26 December 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
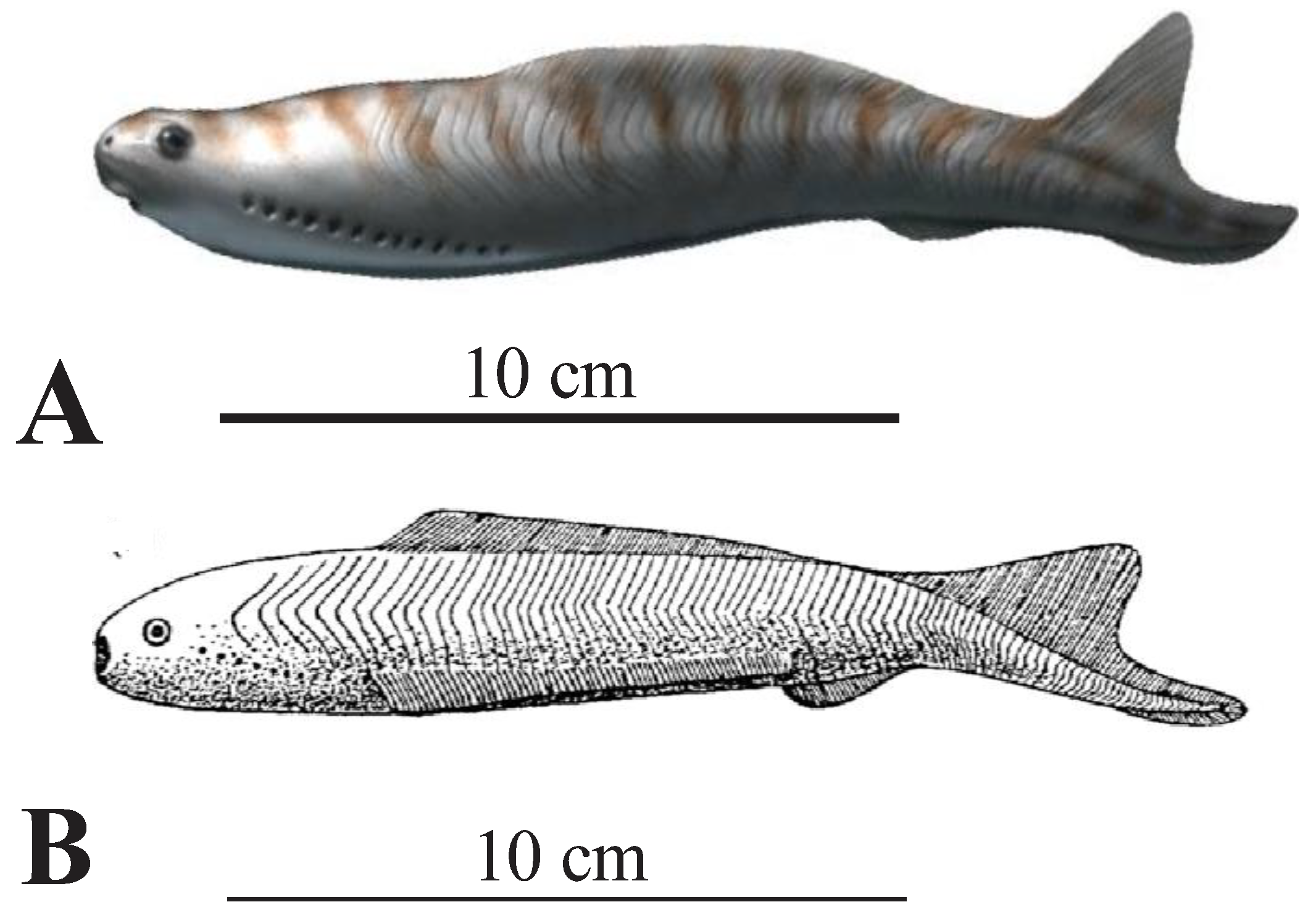
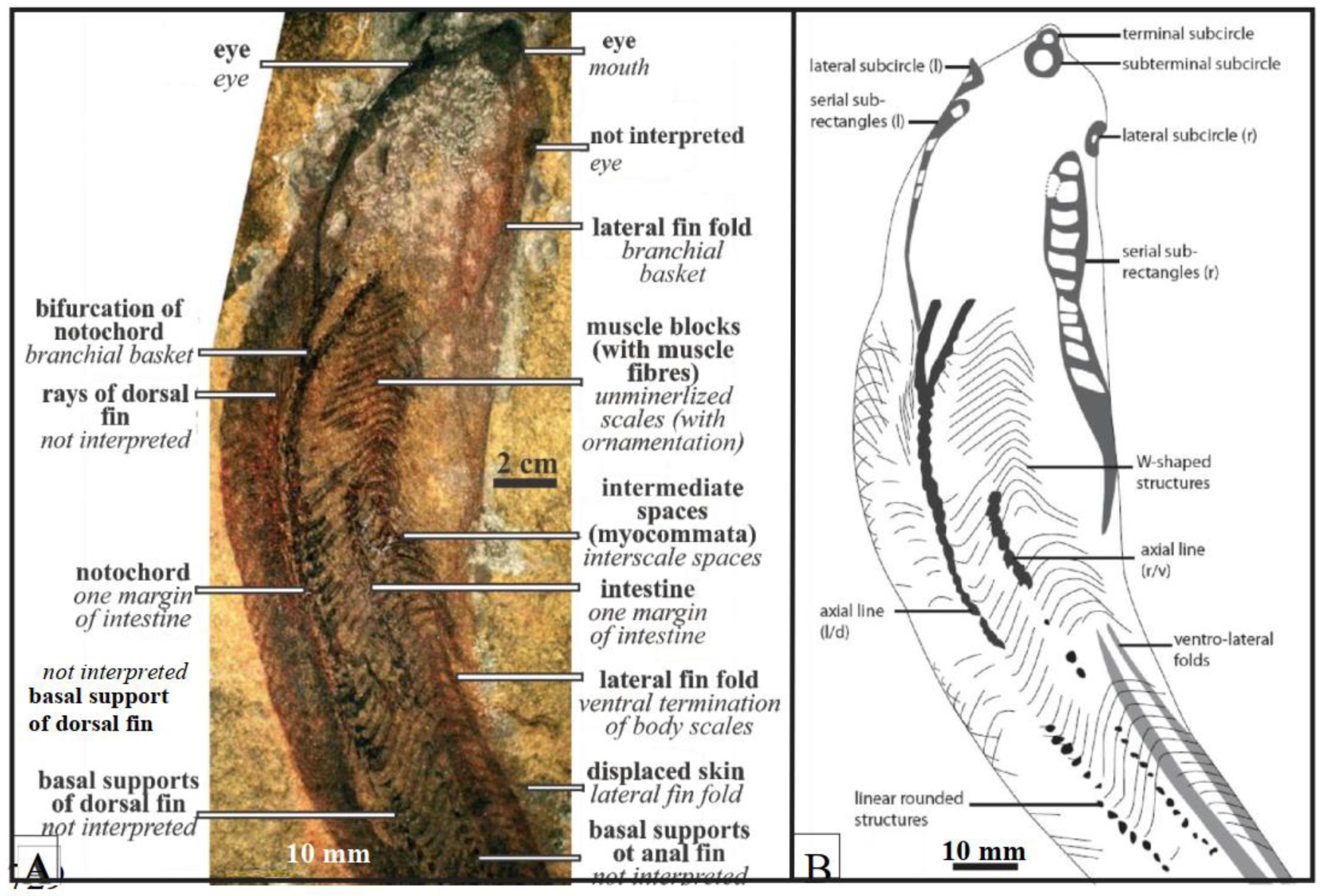
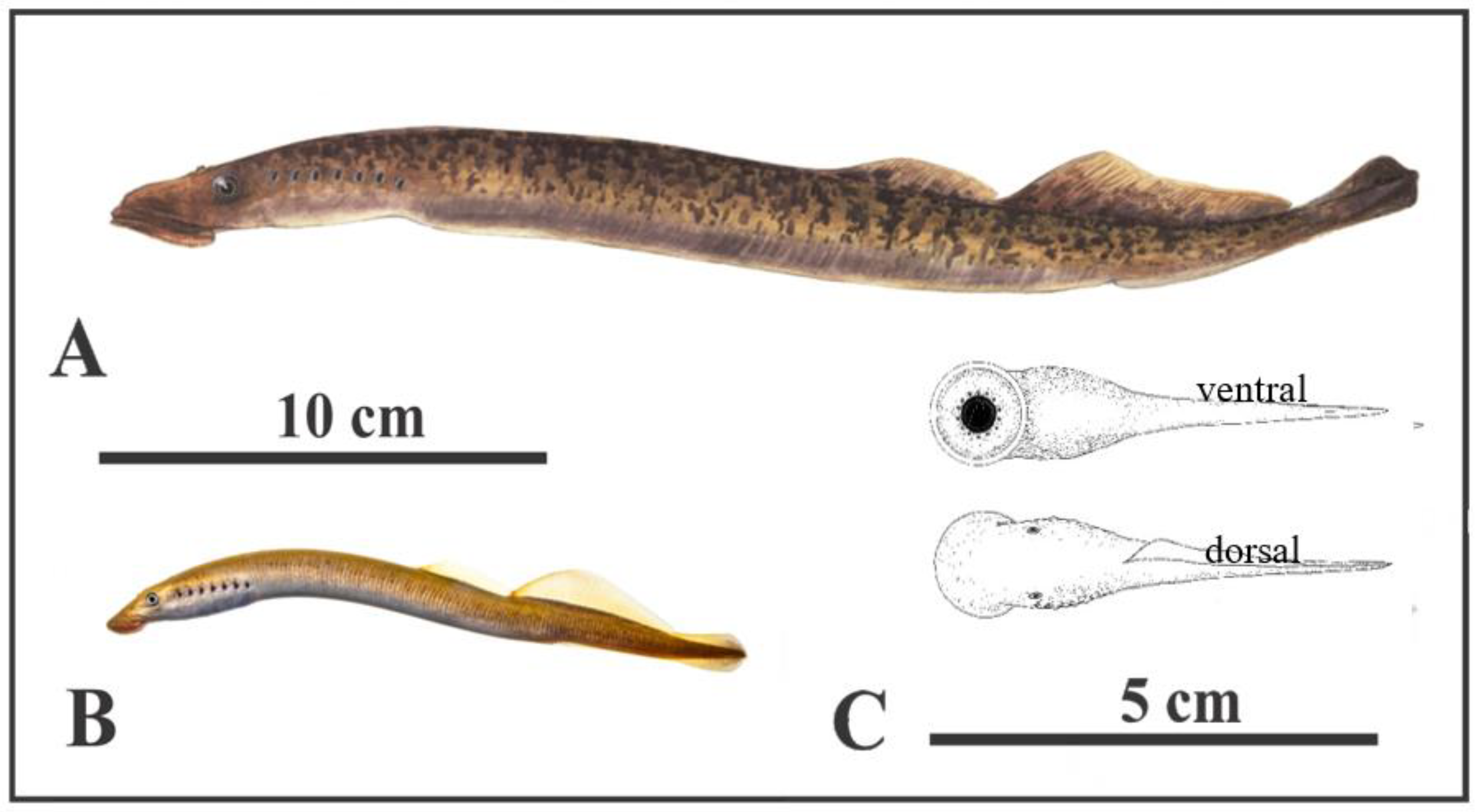
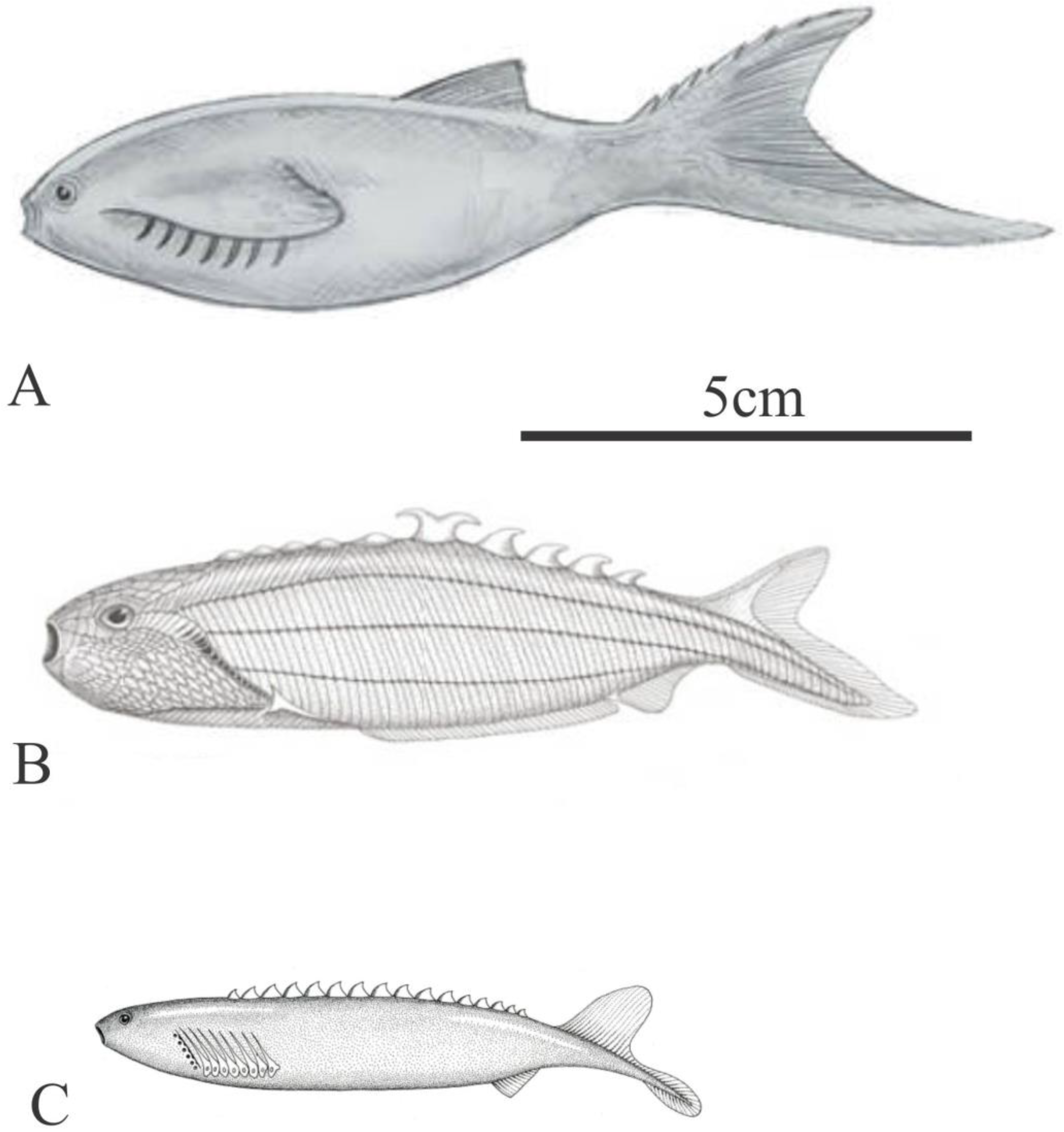
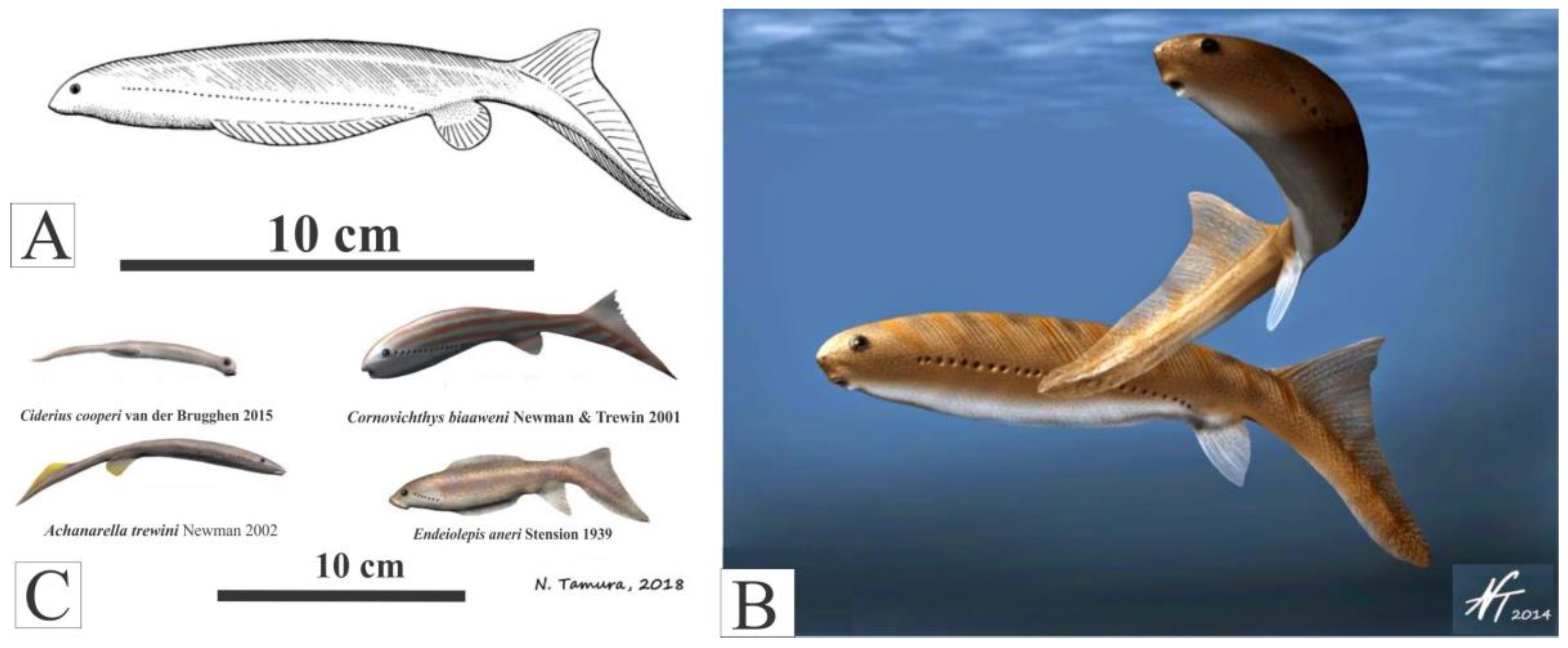
Anatomy
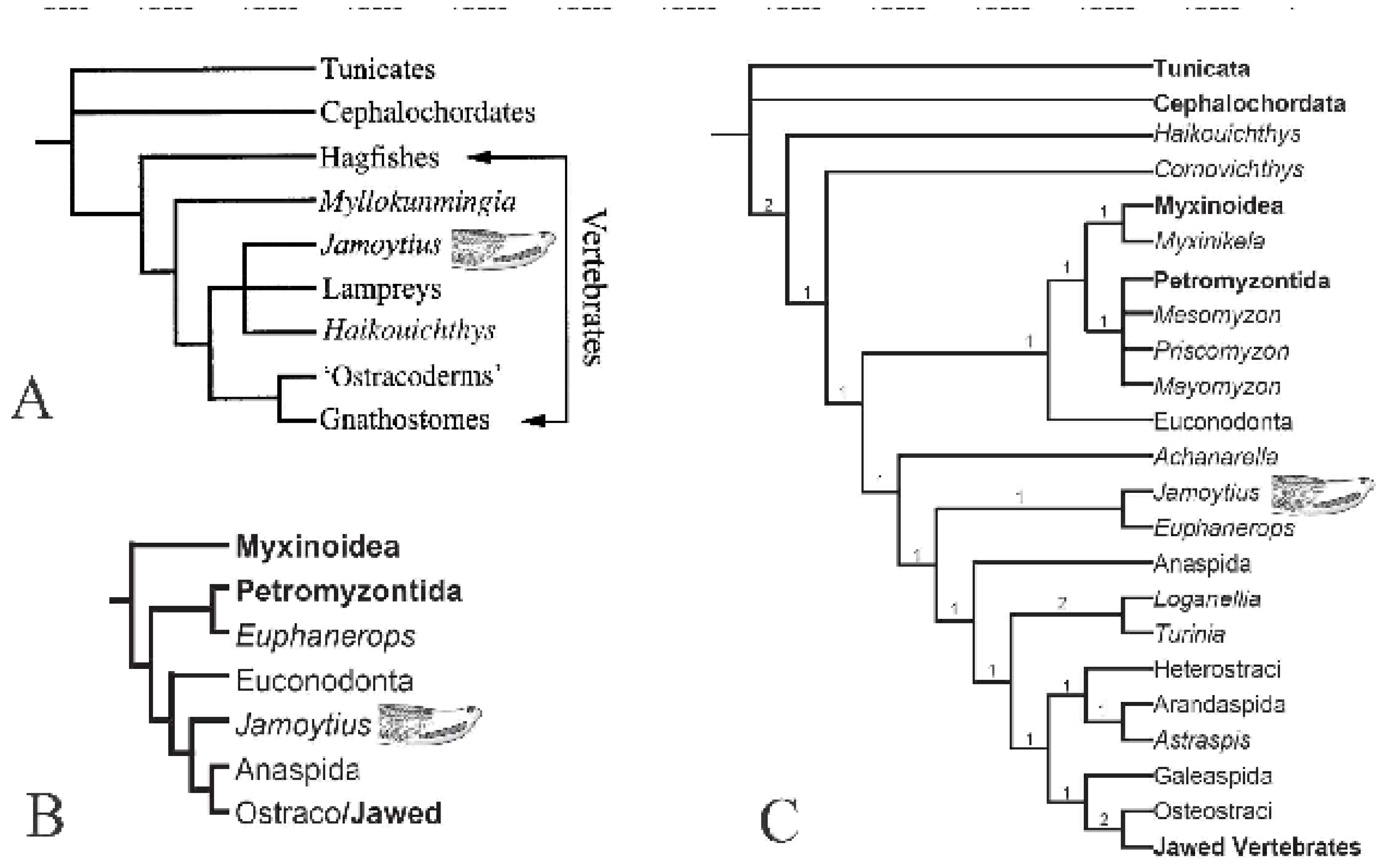
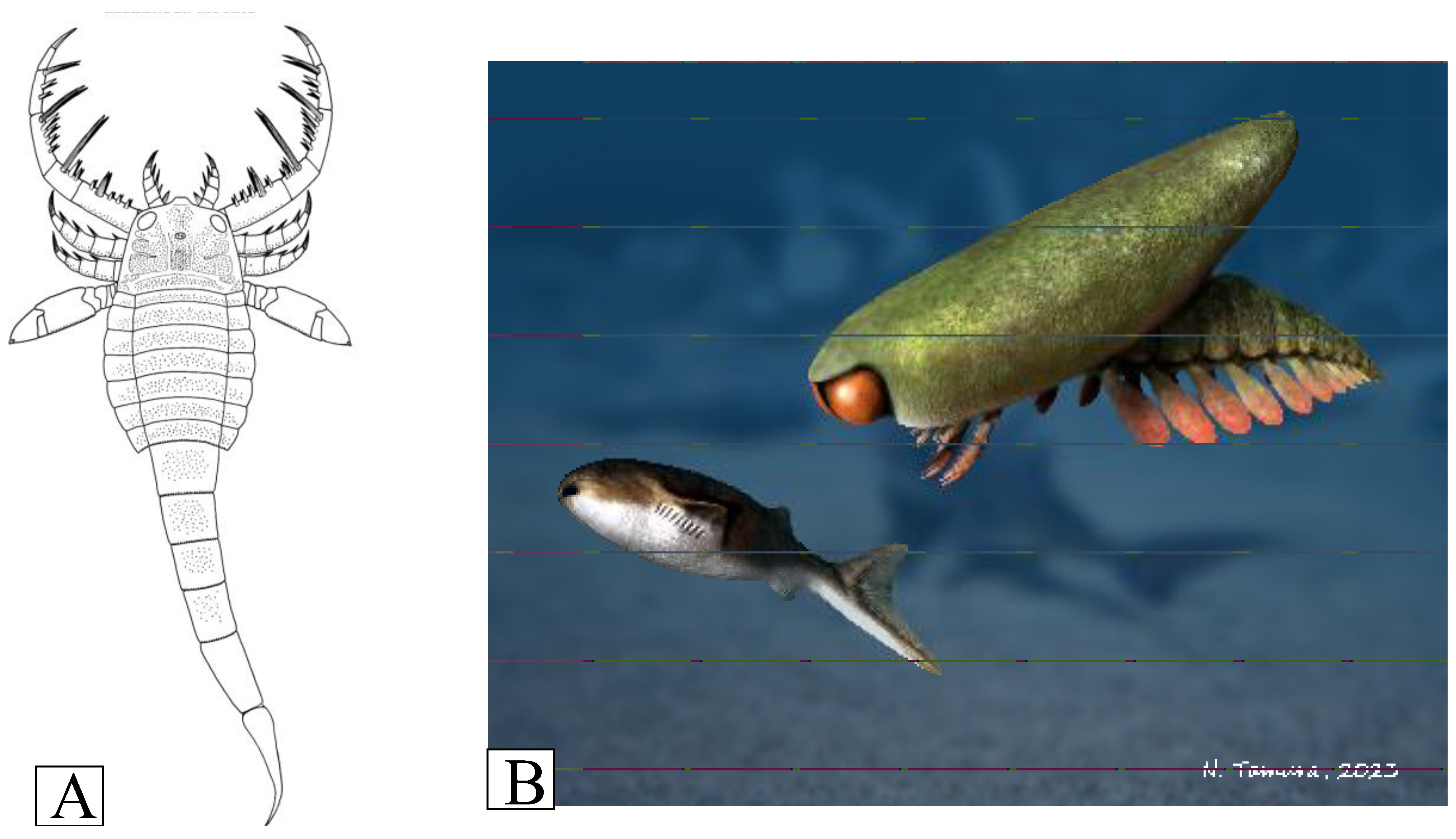
Mode of life of Jamoytius
2. Palaeoenvironments and paleoecology.
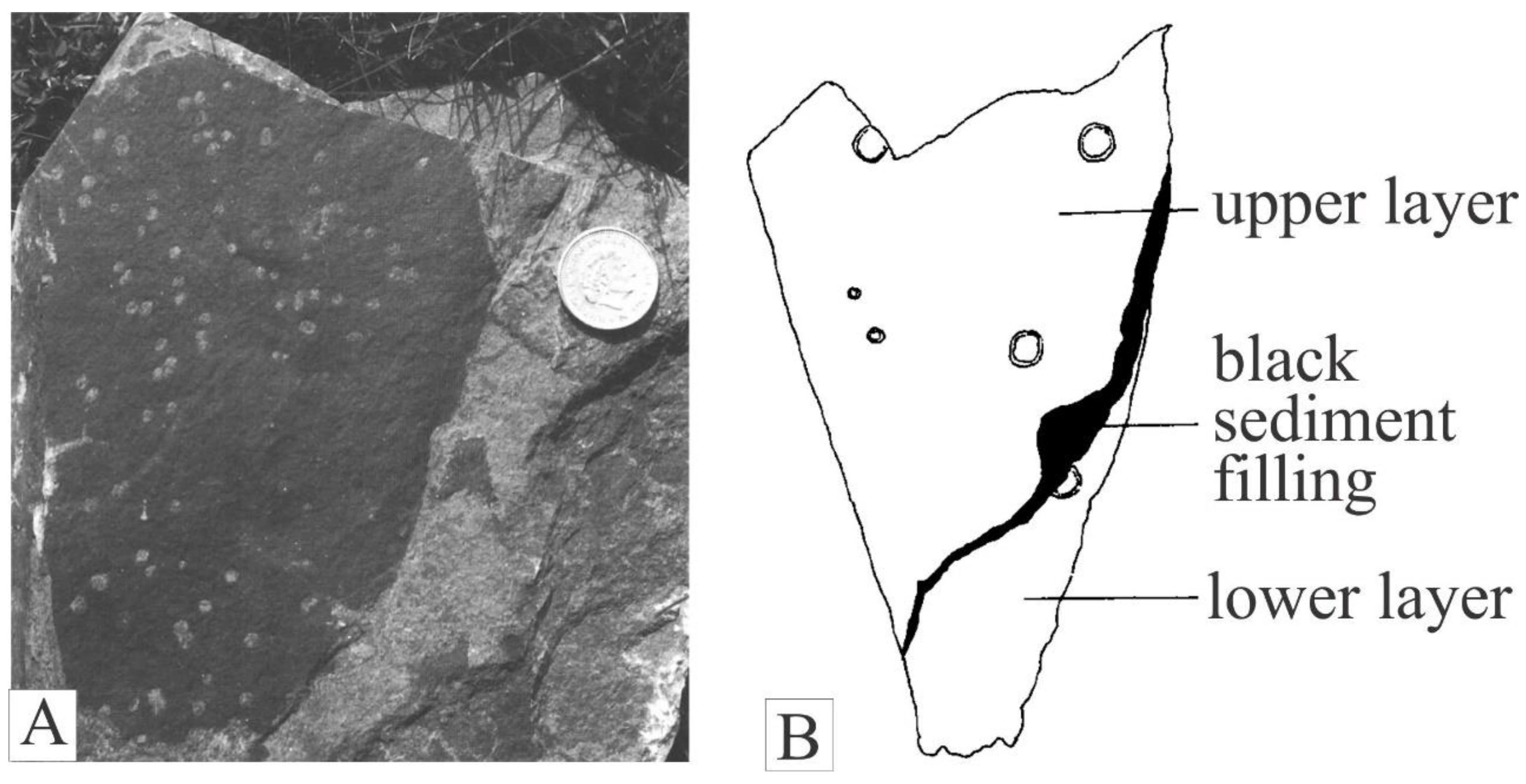
2.1. Sediments
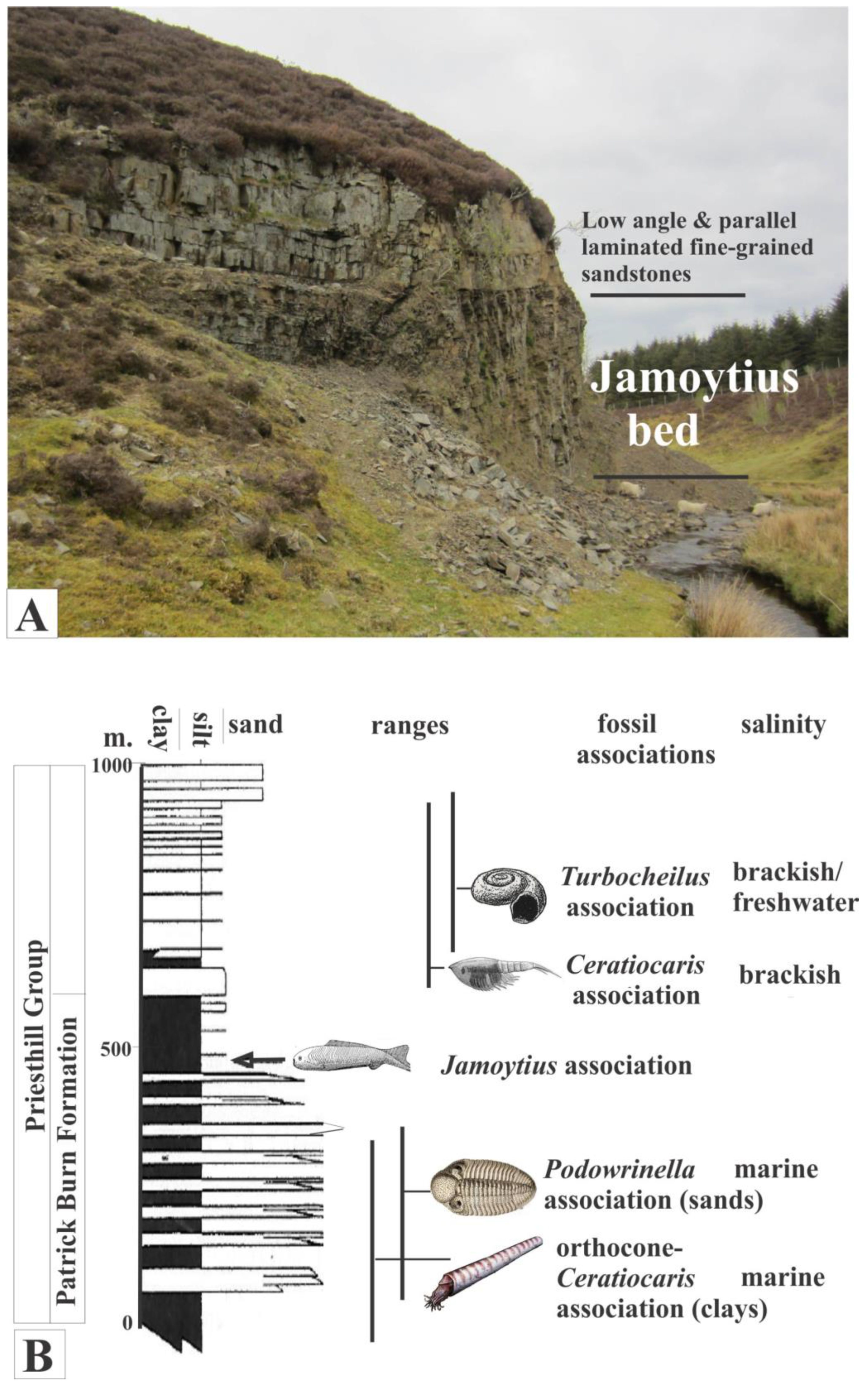
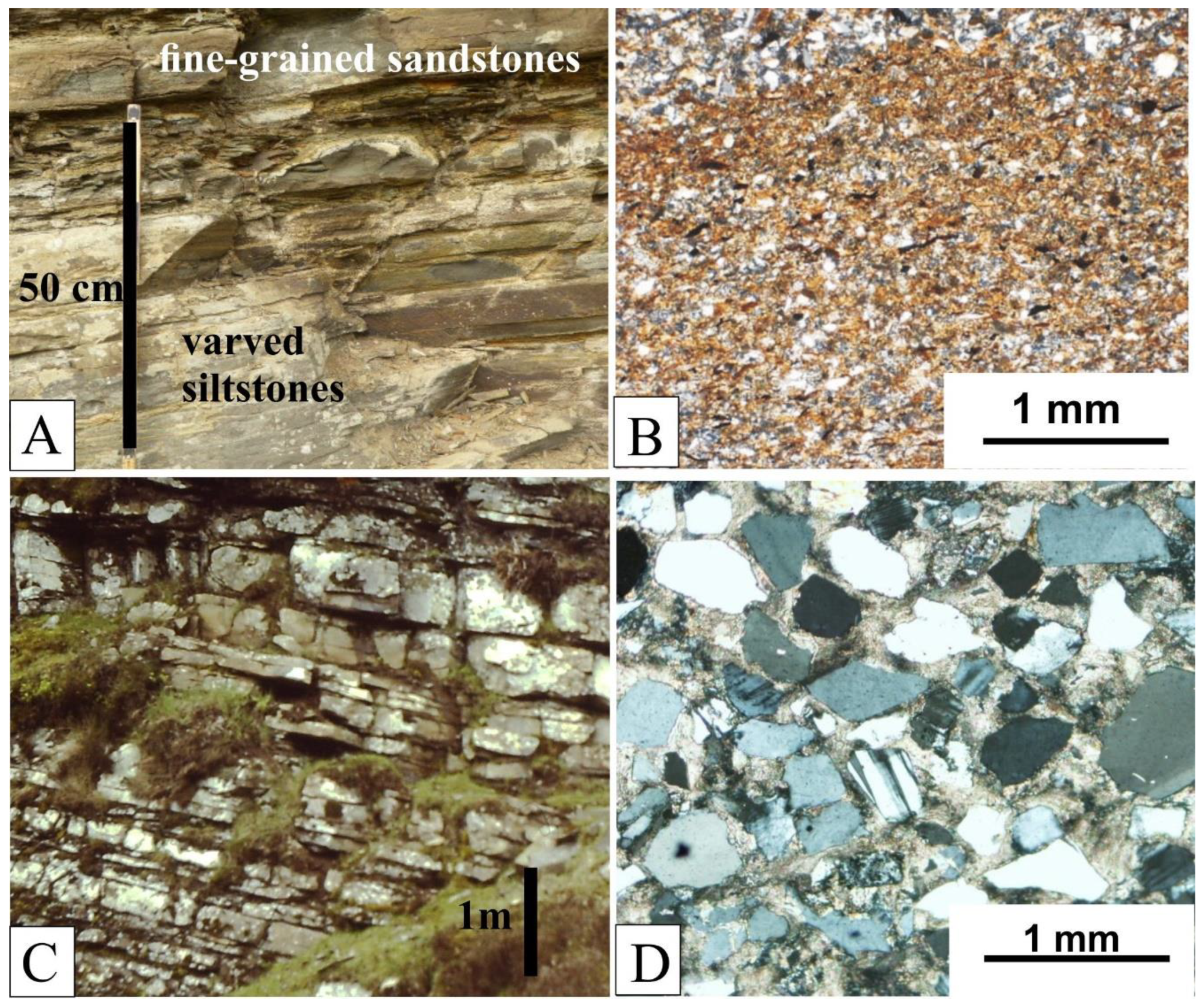
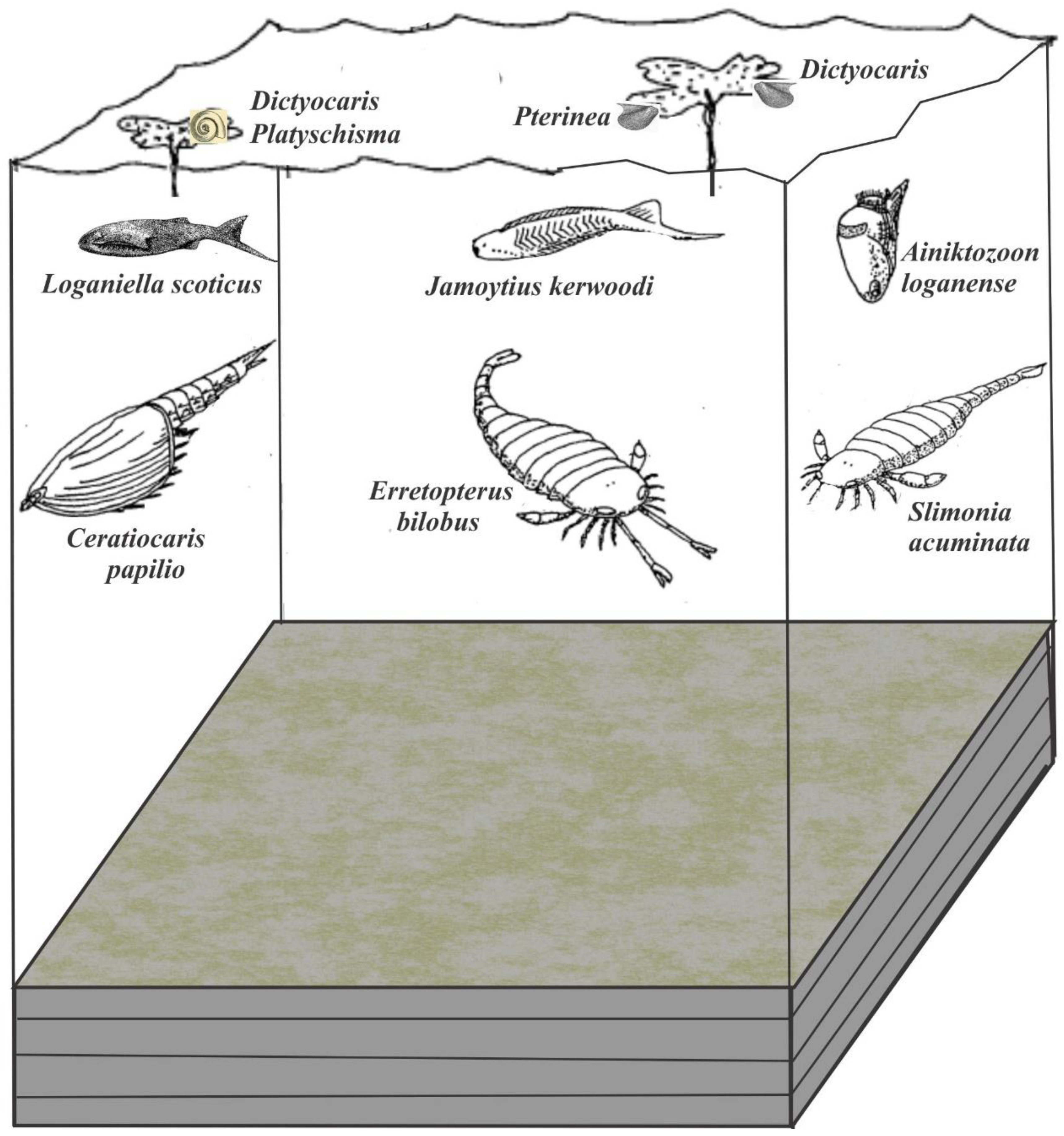
Palaeoecology
3. Discussion
4. Conclusions
5. Acknowledgements
References
- Albertoni, E.F., C. Palma-Silva & F.A. Esteves. 2003. Overlap of dietary niche and electivity of three shrimp species (Crustacea, Decapoda) in a tropical coastal lagoon (Rio de Janeiro, Brazil). Revista Brasileira de Zoologia 20, 135-140. [CrossRef]
- Anderson, L.I., 1999. A new specimen of the Silurian synziphosurine arthropod Cyamocephalus. Proceedings of the Geologist’s Association 110 (3), 211-216. [CrossRef]
- Armitage, S.J., Bristow, C./S., Drake, N.A., 2015. West African monsoon dynamics inferred from abrupt fluctuations of Lake Mega-Chad. Proceedings of the National Academy of Science 112(28), 8543-8548. [CrossRef]
- Athearn, W.D., 1965. Sediment Cores from the Cariaco Trench, Venezuela. Woods Hole Oceanographic Institution unpublished Technical Report 65-37, 20p.
- Blom, 2012. New Birkeniid anaspid from the Lower Devonian of Scotland and its phylogenetic implications. Palaeontology 55 (30, 641–652. [CrossRef]
- Blom, H., Märss, T., 2010. The interrelationships and evolutionary history of anaspid fishes. In: D.K. Elliott, J.G. Maisey, X. Yu, and D.Miao (eds.), Morphology, Phylogeny, and Paleobiogeography of Fossil Fishes, 45–58. Verlag Dr. F. Pfeil, Munich.
- Brownstein, C.D., Near, T.J., 2023. Phylogenetics and the Cenozoic Radiation of lampreys. Current Biology 33 (2), 397-404. [CrossRef]
- Chevrinais, M., Johanson, Z., Trinajstic, K., Long, J., Morel, C., Renaud, C., Cloutier, R., 2018. Evolution of vertebrate postcranial complexity: Axial skeleton regionalization and paired appendages in a Devonian jawless fish. Palaeontology. 61, 1-13. [CrossRef]
- Clarkson, E.N.K., Harper, D.T., 2016. Silurian of the Midland Valley of Scotland and Ireland. Geology Today 32(5), 195-200. [CrossRef]
- Cochran, P. A., 2008. Observations on giant American Brook Lamprey (Lampetra appendix). Journal of Freshwater Ecology 23, 161–164. [CrossRef]
- Denison, R. N., 1961. Feeding mechanisms of agnatha and early gnathostomes. American Zoologist 1, 177–182. [CrossRef]
- Dewey, J.F., Strachan R.A., 2003. Changing Silurian–Devonian relative plate motion in the Caledonides: sinistral transpression to sinistral transtension. Journal of the Geological Society, London 160, 219–229. [CrossRef]
- Dineley, D., 1999. Silurian fossils fish sites of Scotland. In: Dineley, D. & Metcalf, S (eds). Fossil Fishes of Great Britain. Chapter 2. Geological Conservation Review Series 16, 33-62. [CrossRef]
- Donoghue, P. C. J., Keating, J.N., 2014. Early vertebrate evolution. Palaeontology 57 (5), 879-893. [CrossRef]
- Donoghue, P. C. J., Purnell, M. A., 2009. Distinguishing heat from light in debate over controversial fossils. BioEssays 31(2), 178–189. [CrossRef]
- Donoghue, P. C. J., Smith, M. P., 2001. The anatomy of Turinia pagei (Powrie), and the phylogenetic status of the Thelodonti. Transactions of the Royal Society of Edinburgh: Earth Sciences 92, 15–37.
- Donoghue, P. C. J., Forey, P. L, Aldridge, R. J., 2000. Conodont affinity and chordate phylogeny. Biological Reviews 75, 191–251. [CrossRef]
- Donoghue, P.C.J., Smith, M. P., Sansom, I. J., 2003. The origin and early evolution of chordates: molecular clocks and the fossil record, in Donoghue, P. C. J., Smith, M. P. (Eds), Telling the evolutionary time: molecular clocks and the fossil record. CRC Press, London, pp.190–223.
- Ferrón, H.G., Botella, H., 2017. Squamation and ecology of thelodonts. PLoS One12(2), e0172781. [CrossRef]
- Fletcher, T., Altringham, J., Peakall, J., Wignall, P., Dorrell, R., 2014. Hydrodynamics of fossil fishes. Proceedings of the Royal Society B281,20140703. [CrossRef]
- Forey, P. L., 1984. Yet more reflections on agnatha-gnathostome relationships. Journal of Vertebrate Paleontology 4, 330–343. [CrossRef]
- Forey, P. L., 1995. Agnathans recent and fossil, and the origin of jawed vertebrates. Reviews in Fish Biology and Fisheries 5, 267–303. [CrossRef]
- Forey, P. L., Janvier, P. 1993. Agnathans and the origin of jawed vertebrates. Nature 361, 129–134. [CrossRef]
- Fuller, P., Neilson, M., 2015. Lethenteron appendix. USGS Nonindigenous Aquatic Species Database, Gainesville, FL. http://nas.er.usgs.gov/queries/FactSheet.aspx?speciesID=835.
- Gess, R. W., Coates, M. I., Rubidge, B. S., 2006. A lamprey from the Devonian period of South Africa. Nature 443, 981–984. [CrossRef]
- Gilmore, B., 1992 Scroll coprolites from the Silurian of Ireland and the feeding of early invertebrates. Palaeontology 35 (2), 319-333.
- González, C., Dupont, L.M., Behling, H., Wefer, G., 2008. Neotropical vegetation response to rapid climate changes during the last glacial period: Palynological evidence from the Cariaco Basin. Quaternary Research 69 (2), 217-230. [CrossRef]
- Harms, J.C., Southard, J.B., Spearing, D.R., Walker, R.G., 1975. Depositional Environments as Interpreted from Primary Sedimentary Structures and Stratification Sequences. Society for Economic Petrology and Mineralogy, Tulsa, Oklahoma. 161p. [CrossRef]
- Haug, C., Briggs, D.E.G., Mikulic, D.G., Kluessendorf, J., Huag, J.T., 2014. The implications of a Silurian and other thylacocephalan crustaceans for the functional morphology and systematic affinities of the group. BMC Evol Biol 14, 159. [CrossRef]
- Hughen, K.A., Overpeck, J.T., Peterson, L.C., Anderson, R.F., 1996a. The nature of varved sedimentation in the Cariaco Basin, Venezuela, and its paleoclimatic significance. Geological Society Special Publication 116, 171–183. [CrossRef]
- Hunter, J.R.S., 1884. Notes on a new fossil scorpion (Palaeophonus caledonicus) from the Upper Silurian Shales, Logan Water, Lesmahagow. Transactions of the Edinburgh Geological Society 5, 187 – 191. [CrossRef]
- James, K., 1990. The Venezuelan Hydrocarbon Habitat. Geological Society of London Special Publication 50. 9-35. [CrossRef]
- Janvier, P., 1981. The phylogeny of the Craniata, with reference to the significance of fossil ‘agnathans’. Journal of Vertebrate Paleontology 1, 121–159. [CrossRef]
- Janvier, P. 1996. Early Vertebrates. Oxford Monographs on Geology and Geophysics 33. Clarendon Press, Oxford. 393pp. [CrossRef]
- Janvier, P., 2008. Early Jawless Vertebrates and Cyclostome Origins. Zoological Science 25(10), 1045-1056. [CrossRef]
- Janvier, P., Arsenault, M., 2007. The anatomy of Euphanerops longaevus Woodward, 1900, an anaspid-like jawless vertebrate from the Upper Devonian of Miguasha, Quebec, Canada. Godiversitas 29, 143–216.
- Jennings, J.S. 1961. The geology of the eastern part of the Lesmahagow inlier. Univ. Edinburgh. Unpub. Ph.D. thesis, 306p.
- Keating, J.N., Donaghue, P.C.J., 2016. Histology and affinity of anaspids, and the early evolution of the vertebrate dermal skeleton. Proceedimngs of the royal society B283, 20152917. [CrossRef]
- Kermack, K.A. Kermack, K.A., 1943. The functional dignificance of the hypocercal Tail in Pteraspis rostrata. Journal of Experimental Biology 20 (1), 23-27. [CrossRef]
- Kuraku, S., Kuratani, S., 2006. Time Scale for Cyclostome Evolution Inferred with a Phylogenetic Diagnosis of Hagfish and Lamprey cDNA Sequences. Zoological Science 23 (12), 1053-1064. [CrossRef]
- Lammons, M.L., 2009. Mud and Mucus: Feeding selectivity in a suspension-feeding detritivorous fish. M.Sc., The College of William and Mary. 101p.
- Linsley, R. M., 1977. Some "laws" of gastropod shell form. Paleobiology 3, 196-206. [CrossRef]
- Lovelock, C.E., 1998. Sedimentary environments and biofacies of the Silurian inlier at Lesmahagow Midland Valley of Scotland. Unpub PhD thesis, University of Edinburgh, 448p.
- Mallat, J., 1984. Feeding ecology of the earliest vertebrates. Zoological Journal of the Linnean Society 82, 261–272. [CrossRef]
- Mallatt, J. (1985). Reconstructing the Life Cycle and the Feeding of Ancestral Vertebrates. In: Foreman, R.E., Gorbman, A., Dodd, J.M., Olsson, R. (eds) Evolutionary Biology of Primitive Fishes. NATO ASI Series, vol 103, 59-68. Springer, Boston, MA. [CrossRef]
- Mallat, J., 2023. Vertebrate origins are informed by larval lampreys (ammocoetes): a response to Miyashita et al., 2021. Zoological Journal of the Linnean Society 197, 287–321. [CrossRef]
- Miyashita, T., Coates, M., Farrar, R., Larson, P., Manning, P., Wogelius, R., Edwards, N., Anné, J., Bergmann, U., Palmer, A., Currie, P., 2019. Hagfish from the Cretaceous Tethys Sea and a reconciliation of the morphological-molecular conflict in early vertebrate phylogeny. Proceedings of the National Academy of Sciences. 116, e2146-2151. [CrossRef]
- Miyashita, T., Gess, R.W., Tietjen, K., Coates. M.I., 2021 Non-ammocoete larvae of Palaeozoic stem lampreys. Nature 591, 408–412. [CrossRef]
- Muller-Karger, F. E., Varela, R., Thunell, R., Scranton, M., Bohrer, R., Taylor, G., Capelo, J., Astor, Y., Tappa, E., Ho, T.Y., Walsh, J.J., 2001. Annual cycle of primary production in the Cariaco Basin: response to upwelling and implications for vertical export. Journal of Geophysical Research 106C3, 4527-4542. [CrossRef]
- Oleh, M., 2018. Laboratory Manual on General and Special Ichthyology. World News of Natural Sciences 18 (1), 1-51.
- Parrington, F. R. 1958. On the nature of the Anaspida. In T. S. Westoll, (ed.), Studies on fossil vertebrates. Athlone Press, London. p. 108-128.
- Peach, B.N., Horne, J. 1899. The Silurian rocks of Britain, Vol.1 Scotland. (with petrological chapters and notes by J.J.H. Teall). Memoirs of the Geological Survey of the United Kingdom. Glasgow, HMSO. 749 pp.
- Plotnick, R.E., 1999. Habitat of Llandoverian-Lochkovian eurypterids. In: A. J. Boucot, J. D. Lawson (eds.), Paleocommunities - a case study from the Silurian and Lower Devonian. p. 106-136.
- Potter, I. C., Gill, H. S., Renaud, C. B., Haoucher, D., 2015, The Taxonomy, Phylogeny, and Distribution of Lampreys. In: Docker, M. F. (ed.), Lampreys: Biology, Conservation and Control, Springer Netherlands, pp. 35–73. [CrossRef]
- Reeves, J.C., Sansom, R.S., 2023. Multivariate mapping of ontogeny, taphonomy and phylogeny to reconstruct problematic fossil taxa. Proceedings of the Royal Society B.290, 20230333. [CrossRef]
- Reeves, J.C., Wogelius, R., Keating, J., Sansom, R. S., 2023. Lasanius , an exceptionally preserved Silurian jawless fish from Scotland. Palaeontology 66, e12643. [CrossRef]
- Richardson, M.K., Admiraal, J., Wright, G.M., 2010. Developmental anatomy of lampreys. Biological Reviews 85 (1), 1-206. [CrossRef]
- Ritchie, A., 1960. A new interpretation of Jamoytius kerwoodi White. Nature 188, 647–649. [CrossRef]
- Ritchie, A., 1963. Palaeontological studies on Scottish Silurian fish beds. Upublished Ph.D. thesis, University of Edinburgh.170pp.
- Ritchie, A., 1968a. New evidence on Jamoytius kerwoodi White, an important ostracoderm from the Silurian of Lanarkshire, Scotland. Palaeontology 11, 21–39.
- Ritchie, A., 1968b. Lanarkopterus dolichoschelus (Störmer) gen. nov., a mixopterid eurypterid from the Upper Silurian of the Lesmahagow and Hagshaw Hills inliers, Scotland. Scottish Journal of Geology 4, 317 – 338. [CrossRef]
- Ritchie, A., 1984. Conflicting interpretations of the Silurian agnathan, Jamoytius. Scottish Journal of Geology 20, 249–256. [CrossRef]
- Rolfe, W.D.I., Beckett, E.C.M., 1984. Autecology of Silurian Xiphosurida, Scorpionida, Cirripedia, and Phyllocarida. Special Papers in Paleontology 32: 27–37.
- Sansom, R.S., Freedman, K., Gabbott, S.E., Aldridge, R.J., Purnell, M.A., 2010. Taphonomy and affinity of an enigmatic Silurian vertebrate, Jamoytius kerwoodi White. Palaeontology 53, 1393-1409. [CrossRef]
- Schmidt, M., Melzer, R.R., Plotnick, R.E., Bicknell, R.D.C., 2022. Spines and baskets in apex predatory sea scorpions uncover unique feeding strategies using 3D-kinematics. iScience 25 (1), 103662. [CrossRef]
- Schweitzer, M.H., Zheng, W., Moyer, A.E., Sjövall, P., Lindgren, J., 2018. Preservation potential of keratin in deep time. PloS one, 13:e0206569. [CrossRef]
- Scourfield, D.J., 1937. An anomalous fossil organism, possibly a new type of chordate, from the Upper Silurian of Lesmahagow, Lanarkshire – Ainiktozoon loganense, gen. et sp. nov. Proceedings of the Royal Society B. 121 (825): 533–547. [CrossRef]
- Shu, D., Luo, H-L., Conway-Morris, S., Zhang, X., Hu, S., Han, J., Zhu, M., Li, Y., L-Z., 1999. A Lower Cambrian vertebrate from South China. Nature. 402. 42-46. [CrossRef]
- Stanley, S.M., 1972. Functional morphology and evolution of byssally attached bivalves. Journal of Paleontology 46 (2), 165-212.
- Stensiö, E.A., 1958. Les cyclostomes fossiles ou ostracodermes. In: Grasse, P.-P. (editor) Traite de zoologie 13. Paris: Masson. p.173-125.
- Störmer, L., 1935. Dictyocaris salter, a large crustacean from the Upper Silunan and. Downtownian. Norsk Geologisk Tidsskrift 15, 267-298.
- Strahan, A., 1963. The behaviour of Mixinoids. Acta zoologica Stockholm 44, 73–102.
- Tarlo, L. B. H. 1967. Agnatha.. In W. B. Harland (ed.). The fossil record. London Geological Society, London, 629–636.
- Traquair R. H., 1899. Report on fossil fishes collected by the Geological Survey of Scotland in the Silurian rocks of the south of Scotland. Transactions of the Royal society of Edinburgh 39, 827-864. [CrossRef]
- Turner, S., 1982. A new articulated thelodont (Agnatha) from the Early Devonian of Britain. Palaeontology 25, 879-889.
- Turner, S., 1999. Early Silurian to Early Devonian thelodont assemblages and their possible ecological significance. In Boucot, A.J. and Lawson, J. (eds.). Palaeocommunities: a case study from the Silurian and Lower Devonian -International Geological Correlation Programme 53, Project Ecostratigraphy, Final Report. Cambridge University Press, Cambridge. pp 42-78.
- Turner, S., 2004. Early vertebrates: analysis from microfossil evidence. Recent Advances in the Origin and Early Radiation of Vertebrates G. Arratia, M. V. H. Wilson & R. Cloutier (eds.): pp. 67-94, 7 figs., 2 tabs. Verlag Dr. Friedrich Pfeil, München, Germany.
- van der Brugghen, G.,1995. Dictyocaris, een enigmatisch fossiel uit het Silur. Grondboor en Harner 1, 18-22.
- van der Brugghen, G., 2015. Ciderius cooperi gen. nov., sp. nov., the earliest known euphaneropid from the Lower Silurian of Scotland. Netherlands Journal of Geosciences 94, 1-10. [CrossRef]
- van der Brugghen, G., Schram, F.R., Marthill, D.M., 1997. The Fossil Ainiktozoon is an arthropod. Nature 385, 589–91.
- Walker, I., 2009. Omnivory and resource-sharing in nutrient-deficient Rio Negro waters: Stabilization of biodiversity? Acta Amazonica 39, 617-626. [CrossRef]
- Walton E. K.& Oliver, G. J. H. 1991. — Lower Palaeozoic stratigraphy, in Craig G. Y. (ed.), Geology of Scotland. 3rd ed. Scottish Academic Press, Edinburgh: 161-193.
- White, E. I., 1946. Jamoytius kerwoodi, a new chordate from the Silurian of Lanarkshire. Geological Magazine 83, 89– 97. [CrossRef]
- Wu, F., Janvier, P., Zhang, C., 2023. The rise of predation in Jurassic lampreys. Nature Communications 14 (1), 6652. [CrossRef]
- Zolitschka, B., Francus, P., Antti, E.K.O., Schimmelmann, A., 2015. Varves in lake sediments a review. Quaternary Science Reviews 117, 1-41. [CrossRef]
| Taxa | Feeding strategy | Frequency |
| Arthropods | ||
| Ceratiocaris papilio | nektonic omnivore | very common |
| Slimonia acuminata | nektonic scavenger | rare |
| Erretopterus bilobus | nektonic carnivore | very rare |
| Ainiktozoon loganense | unknown | common |
| Beyrichias sp. (1 specimen) detritivore | very rare | |
| Chordata | ||
| Loganellia scotica | nektonic detritus/herbivore? | common |
| Jamoytius kerwoodi | nektonic detritus/herbivore? | rare |
| Loganellia grossi | nektonic detritus/herbivore? | very rare |
| Cephalopoda | ||
| ?Orthocone indeterminate | nektonic carnivore | very rare |
| Small (2 specimens) | ||
| Gastropoda | ||
| Platyschisma helicites | mobile herbivore, grazer | rare |
| (7 specimens) | ||
| Bivalvia | ||
| Pteritonella sp. | Bysally attached suspension | rare |
| feeder | ||
| Unknown | ||
| Dictyocaris slimoni | plant primary producer? | very common |
| Plant | ||
| Tatia catena | primary producer | very rare |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





