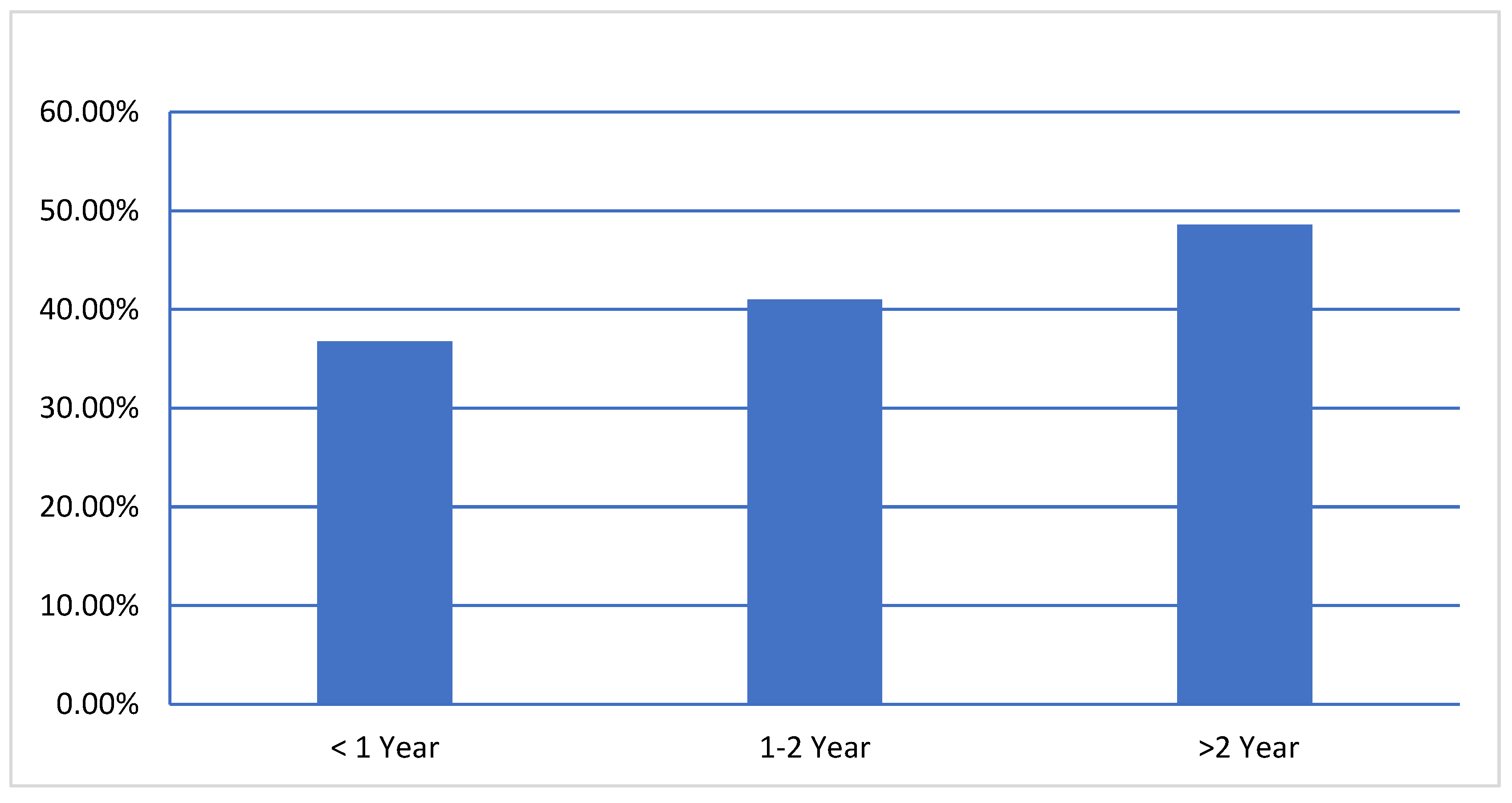1. Introduction
Bovine viral diarrhea (BVD) is a viral disease that can be considered as a highly contagious disease reported in many domestic and wildlife animals. However, BVD as a name is affecting mainly cattle and can cause significant and huge losses for cattle owners [
1]. BVD is caused by the BVD virus (BVDV) of the family Flaviviridae and belongs to the genus Pestivirus [
2,
3]. Two species of BVDV known as BVDV-1 and BVDV-2 as a result of their genetic and antigenic properties [
4,
5]. BVDV is back to 1949 when described for the first in New York City [
6,
7], however, another study is suggesting that BVDV has been circulating in cattle populations for long time [
8]. The clinical manifestations of BVDV range from subclinical to severe disease with a high mortality rate. The clinical signs include gastrointestinal disorders, respiratory and reproductive symptoms. The clinical and characteristics of BVDV infection varies among animal populations, and accordingly, the type of infection depend on the multiple factors, infecting viral strain as well as age, reproductive status, and immunological status of the animal of infected animals [
9]. Type of viral infection might be transient infection (TI) or persistent infection (PI).
Libya is located in North Africa and bordered by the Mediterranean Sea to the North, Tunisia and Algeria to the west, Niger and Chad to the south, Sudan to the Southeast, and Egypt to the East. The livestock production systems in the region are characterized by extensive management, in some cases by nomadic or transhumant systems, which exacerbate the spread of diseases. Disease spread also stems from uncontrolled movements of ruminants due to trade, in particular imports from infected countries [
10]. There are many TADs have been reported (stroked the country during the last couple of years, and meanwhile instability in this country has made the difficulties to implement surveillance and monitoring programs for emerging and re-emerging infectious diseases) in Libya with significant public health and socioeconomic impacts [
10], consequently, there is a scarcity of epidemiological data regarding the BVDV, therefore, this study was undertaken to investigate the seroprevalence of BVDV in cattle in Libya, and to determine the risk factors associated with BVDV infection. There is a lack of comprehensive research on the prevalence and impact of BVDV in North Africa.
In 1972, the first BVDV sample was isolated from a calf suffering from severe enteritis. Most of the BVDV reports from Egypt are based mainly on the detection of the virus by isolation or detection of viral antibodies. Therefore, there are only a few reports that elaborate on the subtyping of circulating BVDV in animal populations.
A study by Ait-Oudhia et al. [
11] conducted in Algeria found a high prevalence of BVDV antibodies in cattle, with 53.8% of animals testing positive. In another study, the prevalence was found to be about 59.9% [
12]. In Tunisia, a study by Sassi et al. [
13] found a lower prevalence of BVDV antibodies in cattle, with 11.8% of animals testing positive. The study noted that the prevalence of BVDV was higher in cattle raised for milk production than in those raised for meat production.
Similarly, a study by Fassi-Fihri et al. [
14] conducted in Morocco found a moderate prevalence of BVDV antibodies in cattle, with 33.3% of animals testing positive.
While there is limited research on BVDV in North Africa, the available studies suggest that the disease is a significant concern for the cattle industry in the region. The high prevalence of BVDV antibodies in Algerian cattle [
11], the higher prevalence in dairy cattle in Tunisia [
13], and the risk factors identified in Moroccan cattle [
14], all highlight the need for further research and effective management strategies to prevent and control BVDV in North Africa. Since there are no previous study on the BVDV in Libya, the objective of the study was conducted to investigate the level of sero-prevalence of BVDV in Libya and the associated risk factor.
2. Materials and Methods
2.1. Study Area and Study Design
The study was conducted in seven Libya regions.
Table 1 shows the seven regions with an estimated total number of cattle in each region.
2.2. Sampling Collection and Questionnaire Survey
A total of 1599 serum samples were collected randomly from herds of cattle which belonging to seven Libyan regions. The structured well designated questionnaire was use collected all relevant data regarding the risk factors associated with infection (age group, sex, and Region).
2.3. Samples Processing
Blood samples were collected from jugular vein. All samples are labeled for the identification purposes of each animal, and centrifugation of the serum stored until used. The samples were tested in Brescia, Italy, at IZSLER, an OIE/FAO reference laboratory by using screening ELISA assay for the detection of antibodies against BVDV.
After the wells were covered with antigen p80, the serum samples were incubated in them. Following the formation of the Ag- Ab complex, other steps including washing, adding conjugate, substrate, and stop solutions were carried out. After reading the optical density with an ELISA reader in a 450 nm wavelength for each sample, the S/N percentage (optical density /OD of the serum sample to OD of negative control) was calculated using the following formula:
Samples were considered positive, doubtful and negative for (S/N%) ≤40, 40< to ≤ 50%, and > 50% respectively.
2.4. Statistical Analysis
For each age group the prevalence and 95% confidence intervals (CI) were calculated using the Bayesian approach of Beta distribution. Chi-square in a univariable analysis was used for the association between the outcome variables including status of BVDV infection and risk factors. In addition, odds ratio was used to estimate the effect size as the association between the seroprevalence of BVDV and potential risk factors was analyzed using logistic regression. A p-value <0.05 was considered to be significant.
3. Results
In this study, the overall seroprevalence of BVDV was estimated to be 48.6% (95% Confidence interval (CL): 46.08%-50.98%). The results of the univariates analysis of independent variables were showed statistical differences, the result is significant at p < .05 as illustrated in
Table 2.
Considering the age groups, high seroprevalence of BVDV were reported in age groups
< 1 year 36.76% (95% CI, 29.81%-43.70%), 1-2 year 41% (95% CI, 37.07%-44.91%) and > 2 year 48.6% (95% CI, 45.13%-52.02%) (
Figure 1), significantly (P=.001), the animal age factor was associated with BVDV infection. The results showed the seroprevalence of BVDV was significantly higher in adult animals than in young animals.
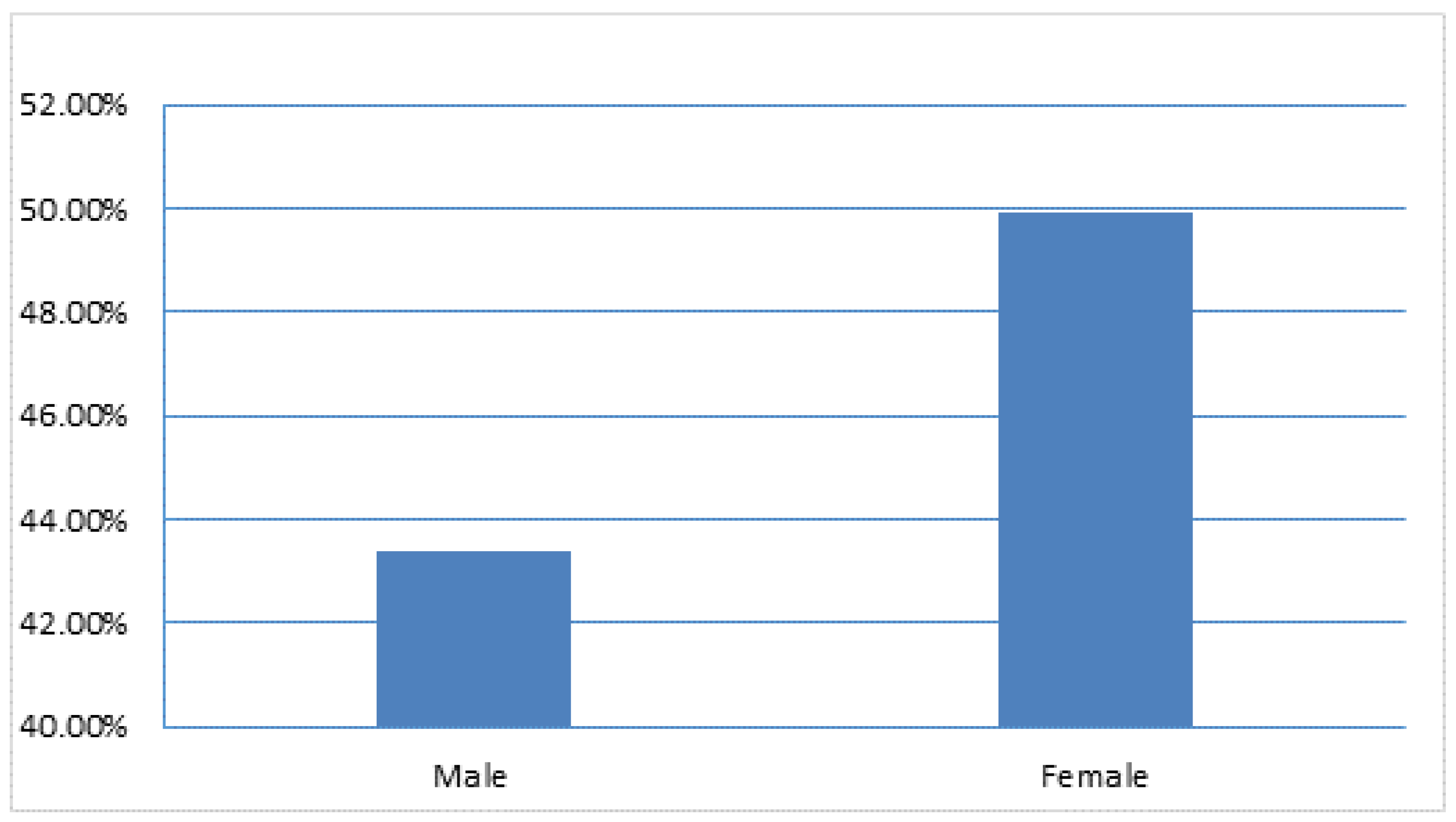
The results showed a high BVDV seroprevalence of 38.9% (95% CI; 32.10%-45.68%) and 49.9% (95% CI; 47.27%-52.51%) were reported in males and females respectively (
Figure 2). Significantly (P=.004), the gender factor was influenced by the seroprevalence of BVDV among the cattle.
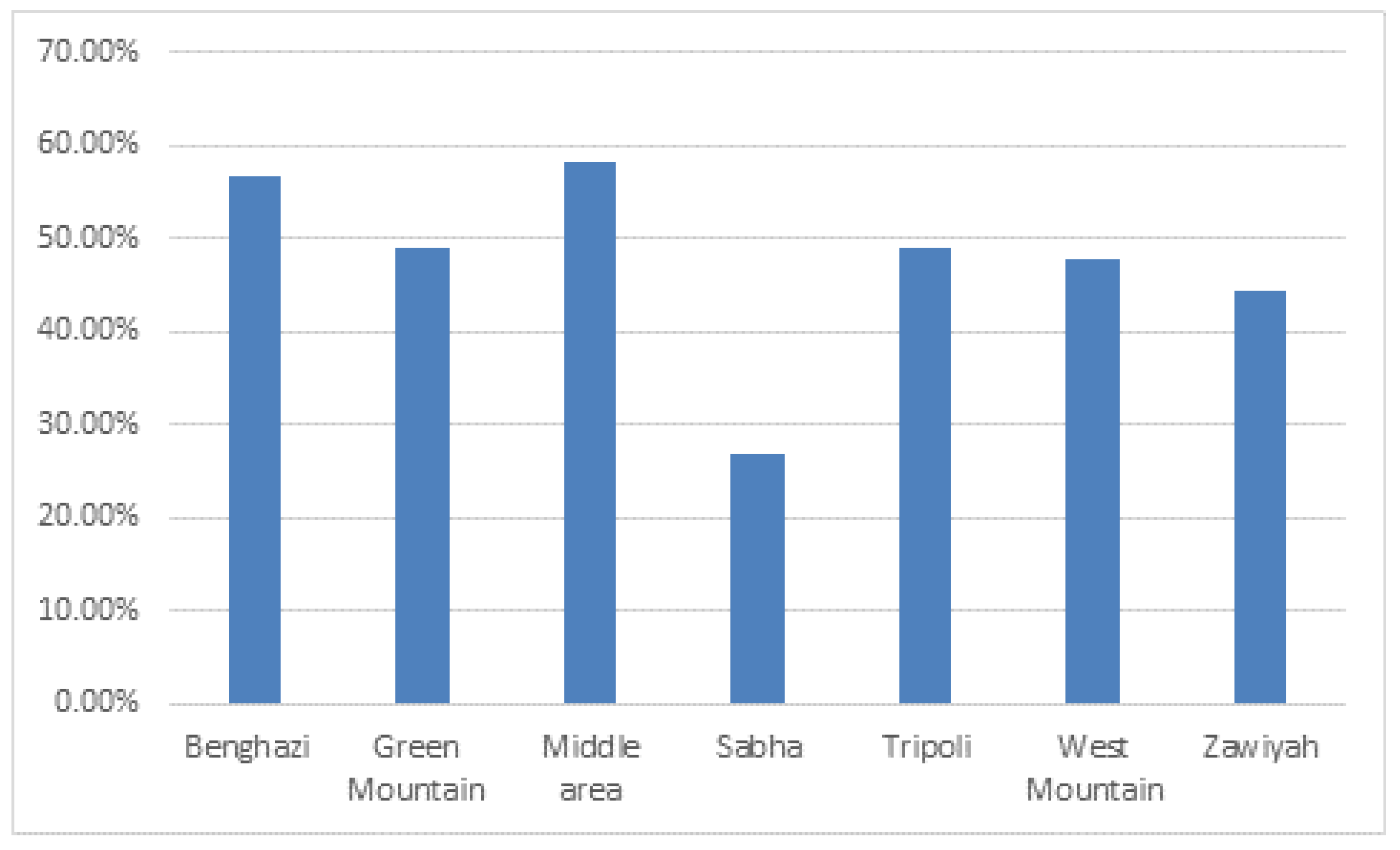
The results showed highest seroprevalence of BVDV in the middle area (58.2%; 95% CI, 45.15%-71.22%) and lowest seroprevalence in Sabha (Southern region) (27.03%; 95%, 12.72%-41.34%) followed by the Zawiyh region (44.4%; 95% CI, 38.45%-50.35%) (
Figure 3). Seroprevalence of BVDV was significantly associated with the geographical region (P= .033).
4. Discussion
The BVD is well known, described, and documented in the many kinds of literature conserving the almost North Africa and Mediterranean region. However, this is the first study to investigate BVDV among non-vaccinated cattle populations in Libya. Expectedly, this study reported a high seroprevalence of BVDV among dairy cattle in different parts of the country. In agreement to several studies reported high seroprevalence of BVDV in North Africa and Mediterranean regions [
15]. In line with other similar studies indicating different seroprevalence between males and females with higher seroprevalence in females, the significant difference between sex (P=.004) found in this study could be explained by the fact that fewer number of males present in cattle herds. Herd’s men sell the bulls after weaning resulting in higher numbers of older females than older males. The high seroprevalence of BVDV was estimated to be higher in adult animals than young. In agreement with several studies that reported frequently higher seroprevalence in adults [
16,
17]. Comparatively, the relatively high (36.76%) seroprevalence of BVDV in this study amongst young animals is considered another evidence of constant endemicity of BVD within the dairy cattle herds in the country. And in agreement with several studies from different parts of the world reported a significant association of BVDV infection with newborn animals are immunotolerant to and persistently infected (PI) with BVDV [
18,
19]. It's well-known that younger animal (calf) plays a crucial role in transmission and PI within bovine herds with BVDV. PI animals shed high titers of infectious BVDV from nasal and ocular secretions, urine, semen, colostrum/milk, and feces [
20,
21]. Unfortunately, our study was not designated to determine the PI in the pregnant dams and offspring calf among cattle populations. Consequently, the answer will remain unclear regarding the PI calf if could be playing a significant role in the epidemiology of BVDV within the cattle populations in Libya. Also, another question arises about impacts of BVDV on the reproductive performance among dairy cattle populations in Libya.
The present study reported the highest seroprevalence in the middle, Benghazi, and Green Mountain regions, followed by Western, Zawiyah, and Tripoli regions (
Figure 3). Comparatively, the lowest seroprevalence of BVDV was reported in Sabha (Southern region). The difference in seroprevalence values that have been reported among regions might be attributable to (influenced by) animal dynamics, density, animal housing system, and distribution of cattle at the national regional level (herd size per farm). The high seroprevalence rate indicated that BVD is constantly endemic in almost Libyan regions under the study. In spite, of the significant differences (P=0.033) in the BVDV seroprevalence values were demonstrated on the geographical level, conversely, seroprevalence was somewhat uniformly distributed in almost Libyan regions. And the highest seroprevalence values reported on the national regional level indicated a wide spatial distribution of BVDV infection among the most dairy cattle populations.
5. Conclusions
The present study revealed that BVDV infection is widespread among cattle populations in Libya. The results of this study would suggest that BVD is endemic in Libya, with a constant exposure to the infection of the animals during their life. More studies are still needed and one of the option s for the Libyan National Center for Animal Health to consider out of this study is to introduce vaccination against BVD as one of the control strategies to control this disease in Libya.
Author Contributions
Conceptualization, H.E., I.B., I.E. and A.D.; methodology, H.E., I.B., E.B., S.G., A.M., I.E. and A.D.; validation, I.B., A.M., I.E. and A.D.; formal analysis, H.E., I.B., E.B., S.G., A.M. and A.D.; investigation, H.E., I.B., A.M., I.E. and A.D.; data curation, A.M., I.E. and A.D.; writing—original draft preparation, H.E., I.E. and A.D.; writing—review and editing, H.E., I.B., A.M., I.E. and A.D.; supervision, I.B. and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NCAH, Libya and Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia Romagna (IZSLER), Brescia, Italy.
Institutional Review Board Statement
Blood samples were collected from horses and dogs with the prior consent of their owners. This research was approved by the Department of Microbiology and Parasitology at the Faculty of Veterinary Medicine, University of Tripoli. Sample collection was carried out following approval from the Ethical Committee at the National Center for Animal Health in Libya (NCAH-15-2019).
Data Availability Statement
Data are available in the article. Any additional required data can be provided upon reasonable request from corresponding authors.
Acknowledgments
The authors would like to thank the personnel of the National Center for Animal Health who helped with sample collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aguirre, I.M.; Quezada, M.P.; Celedón, M.O. Antigenic variability in bovine viral diarrhea virus (BVDV) isolates from alpaca (Vicugna pacos), llama (Lama glama) and bovines in Chile. Veterinary microbiology 2014, 168, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; Stapleton, J.T. Ictv Report Consortium ICTV Virus Taxonomy Profile: Flaviviridae. The Journal of general virology 2017, 98, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Walz, Paul, H.; Manuel, F. Chamorro, Shollie M. Falkenberg, Thomas Passler, Frank van der Meer, and Amelia R. Woolums. Bovine Viral Diarrhea Virus: An Updated American College of Veterinary Internal Medicine Consensus Statement with Focus on Virus Biology, Hosts, Immunosuppression, and Vaccination. J Vet Intern Med 2020, 34, 1690–1706. [CrossRef]
- Becher, P.; Avalos Ramirez, R.; Orlich, M.; Cedillo Rosales, S.; König, M.; Schweizer, M.; Stalder, H.; Schirrmeier, H.; Thiel, H.J. Genetic and antigenic characterization of novel pestivirus genotypes: implications for classification. Virology 2003, 311, 96–104. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.Q.; Lefkowitz, E.J.; Mushegian, A.R.; Adams, M.J.; Dutilh, B.E.; Gorbalenya, A.E.; Harrach, B.; Harrison, R.L.; Junglen, S.; Knowles, N.J.; et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2018). Archives of virology 2018, 163, 2601–2631. [Google Scholar] [CrossRef] [PubMed]
- OLAFSON, P.; MacCALLUM, A.D.; FOX, F.H. An apparently new transmissible disease of cattle. The Cornell veterinarian 1946, 36, 205–213. [Google Scholar] [PubMed]
- Stalder, H.; Bachofen, C.; Schweizer, M.; Zanoni, R.; Sauerländer, D.; Peterhans, E. Traces of history conserved over 600 years in the geographic distribution of genetic variants of an RNA virus: Bovine viral diarrhea virus in Switzerland. PLoS One 2018, 13, pe0207604. [Google Scholar] [CrossRef] [PubMed]
- Chernick, A.; van der Meer, F. Evolution of Bovine viral diarrhea virus in Canada from 1997 to 2013. Virology 2017, 509, 232–238. [Google Scholar] [CrossRef] [PubMed]
- OIE-World Organisation for Animal Health (2018): Bovine Viral Diarrhoea. Manual of Terrestrial Animals. P. 1075.
- FAO. 2013. Transboundary animal diseases : diseases with a strong social and economic impact. Newsletter of the FAO sub regional office for North Africa, 3rd quarter 2013.
- Ait-Oudhia, K.; Moulay, A.; Yahiaoui, M.; El Harrak, M.; Ait-Amrane, A. Prevalence and risk factors associated with bovine viral diarrhea virus (BVDV) in cattle in north-eastern Algeria. Tropical Animal Health and Production 2019, 51, 2071–2076. [Google Scholar]
- Guidoum KA, Benallou B, Pailler L, Espunyes, J.; Napp, S.; Cabezón, O. Ruminant pestiviruses in North Africa. Prev Vet Med. 2020, 184, 105–156. [CrossRef]
- Sassi, L.; Ben Hassine, T.; Jemli, M.H.; Gharbi, M.; Gribâa-Dridi, L. Seroprevalence and risk factors associated with bovine viral diarrhea virus in dairy cattle in Tunisia. Tropical Animal Health and Production 2016, 48, 1181–1186. [Google Scholar]
- Fassi-Fihri, O.; El Hicheri, K.; Idrissi, A.H.; Loutfi, C. Séroprévalence de la diarrhée virale bovine au Maroc. Revue Scientifique et Technique de l'OIE 2014, 33, 957–964. [Google Scholar]
- Yılmaz, V. Prevalence of antibodies to bovine viral diarrhea virus (BVDV) in blood and milk serum in dairy cattle in Kars district of Turkey. Indian J. Anim. Res 2016, 50, 811–815. [Google Scholar] [CrossRef]
- Nigussie, Z.; Mesfin, T.; Sertse, T.; Fulasa, T.; Regassa, F. Seroepidemiological Stud y of Bovine viral diarrhea (BVD) in three Agro-Ecological Zones in Ethiopia. Tropical Animal Health Production 2010, 42, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Handel, I.G.; Willoughby, K.; Land, F.; Koterwas, B.; Morgan, K.L.; Tanya, V.N.; Bronsvoort, B.M. Seroepidemiology of Bovine Viral Diarrhoea Virus (BVDV) in the Adamawa Region of Cameroon and Use of the SPOT Test to Identify Herds with PI Calves. Plos One 2011, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.W.; Hessman, B.E.; Ridpath, J.F.; Johnson, B.J.; Burge, L.J.; Kapil, S.; Braziel, B.; Kautz, K.; Reck, A. Multiple diagnostic tests to identify cattle with Bovine Viral Diarrhea Virus and duration of positive test results in persistently infected cattle. The Canadian Journal of Veterinary Research 2009, 73, 117–124. [Google Scholar] [PubMed]
- Akagami, M.; Seki, S.; Kashima, Y.; Yamashita, K.; Oya, S.; Fujii, Y.; Takayasu, M.; Yaguchi, Y.; Suzuki, A.; Ono, Y.; Ouchi, Y.; Hayama, Y. Risk factors associated with the within-farm transmission of bovine viral diarrhea virus and the incidence of persistently infected cattle on dairy farms from Ibaraki prefecture of Japan. Research in veterinary science 2020, 129, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.W.; Ridpath, J.F.; Ore, S.; Confer, A.W.; Saliki, J.T.; Burge, L.J.; Payton, M.E. Bovine viral diarrhoea virus (BVDV) subgenotypes in diagnostic laboratory accessions: distribution of BVDV1a, 1b, and 2a subgenotypes. Veterinary microbiology 2005, 111, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Konishi, M.; Tsutsui, T.; Yamamoto, T. Survey for detecting persiste ntly infected cattle with Bovine Viral Diarrhea in Japan. The Journal of Veterinary Medical Science 2016, 78, 1329–1331. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).