Introduction.
Chronic pulmonary infection and excessive inflammation are the leading causes of progressive pulmonary damage in cystic fibrosis (CF) lung disease. Managing chronic infection and limiting the excessive production of inflammatory mediators are the major therapeutic strategies for slowing the deterioration of lung function and improving survival in CF. Randomized controlled trials with azithromycin (AZM) showed improvement in lung function, fewer pulmonary exacerbations and hospital stays, reduced Pseudomonas aeruginosa colonization rates, and less intravenous antibiotic usage in patients with CF (1-3). However cellular and mechanistic evidence regarding the mode of action of AZM in CF was scarce. These improvements were most likely to come from immunomodulatory properties of macrolides (4). AZM significantly reduced pro-inflammatory cytokine release (IL-1β, CCL2, TNFα) and enhanced polarization of anti-inflammatory M2 macrophages in CF mice homozygous for the F508del mutation as compared with macrophages from wild-type mice (5). AZM has also been shown to improve phagocytosis ex-vivo in macrophages obtained from patients with chronic obstructive pulmonary disease (COPD) (6). However, the cellular mechanisms by which AZM exhibited immunomodulatory effects in CF remain largely unexplored.
Macrophages represent the first line of immune defense against invading pathogens in the lungs and are divided into lung-resident alveolar macrophages (AMs) and recruited monocyte-derived macrophages (MDMs). CF sputum analysis showed a shift from AMs (0.4%) to MDMs (19%) (7). When recruited to the lungs to deal with invading pathogens, MDMs play crucial roles in the initiation and resolution of pulmonary inflammation via their pro-(M1) or anti- (M2) inflammatory phenotypes, respectively (8). In the early stage of an inflammatory response, the M1 macrophage phenotype, activated via the classical pathway, uptakes the bacteria, initiates inflammation, releases pro-inflammatory mediators, and kill bacteria by phagocytosis (9). Later, inflammation-resolving M2 macrophages release anti-inflammatory cytokines and clear apoptotic cells by endocytosis to return to homeostasis (9). A dynamic equilibrium between M1 and M2 macrophages is crucial to maintain tissue homeostasis in the lung. We have previously shown defective phagocytosis by M1 macrophages and impaired polarization to anti-inflammatory (M2) phenotypes in monocyte-derived macrophages (MDMs) obtained from patients with CF (pwCF) (10).
The present study was undertaken to investigate whether defective macrophage function and polarization in CF were improved by AZM. We differentiated peripheral blood monocytes into MDMs, polarized them into the M1 phenotype, and studied macrophage functions, including P. aeruginosa uptake and killing ability as P. aeruginosa is the leading organism found in the lungs of pwCF. We also studied the relationship between bacterial uptake and ERK1/2 activation and whether AZM modulates ERK1/2 activation and thereby bacterial uptake.
The primary objective of this study was to assess whether AZM could ameliorate the defective macrophage function and polarization observed in CF. We therefore investigated the effect of AZM on the functions of unpolarized MDMs, LPS-induced M1 and IL-13-induced M2 macrophages. Bacterial killing ability was assessed against E. coli, P. aeruginosa and S. aureus, the latter two are also known as predominant bacteria in CF lungs. Additionally, this study explored ERK1/2 as the key molecular pathway required for bacterial killing and clearance.
Methods.
Study participants.
Buffy coats from healthy controls (HCs) (n=15) aged 20–40 years were obtained from the Australian Red Cross Blood Service. 17 adults and children with CF, carrying at least one F508del allele were recruited from the CF clinics at The Prince Charles Hospital (TPCH) and Queensland Children’s Hospital (QCH), Brisbane, Australia respectively (
Table 1).
Ex vivo macrophage differentiation and polarization
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats or peripheral blood obtained from pw CF using lymphoprep (AxisShiled, UK). Monocytes were enriched by CD14+ microbeads following the manufacturer’s instructions (Miltenyi, Germany). Macrophage differentiation and polarization were performed as previously described (9). Briefly, CD14+ monocytes were differentiated into unpolarized macrophages (MDMs) by 6-day stimulation with GM-CSF (50ng/ml, Miltenyi) in RPMI-1640 plus 10% FBS (ThermoFisher, US), 1% penicillin-streptomycin-amphotericin B (Lonza). Medium was refreshed on day 3 with GM-CSF. M1 and M2 polarizations were induced by E. coli LPS (20ng/ml, Sigma) and IL-13 (20ng/ml) respectively for 2 days. 5μg/ml of AZM (Sigma) was added to the culture medium during differentiation and before polarization.
AZM cytotoxicity.
AZM effects on the host cells are dose-dependent (4). Therefore, cytotoxicity of AZM at different concentrations was assessed on MDMs using membrane impermeant 7-AAD DNA binding dye staining.
P. aeruginosa uptake and killing.
Cells were infected with highly virulent
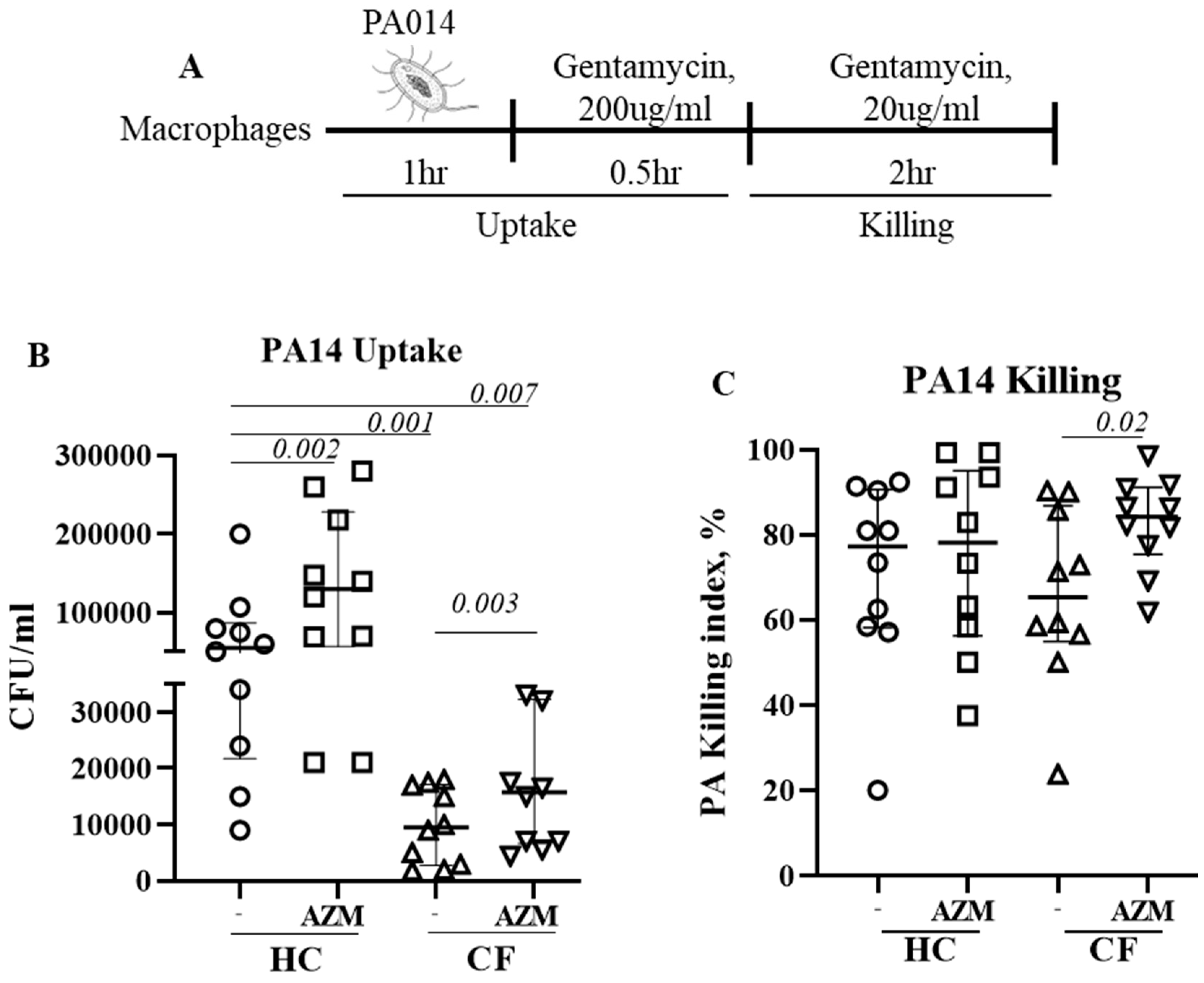
P. aeruginosa strain PA14 at MOI 10 for 1hr at 37C in absence of antibiotic. After 1hr, infection medium was removed and RPMI containing gentamycin (200μg/ml) was added for 1hr to kill any extracellular bacteria. This 2-hr time-point was defined as bacterial uptake (
Figure 1A). Cells were then left in low-dose gentamycin (20μg/ml) containing RPMI for next 2hr. This 2-hr time-point was considered for the killing of bacteria already taken in. Cells were then lysed with 1% saponin and plated them onto LB agar plate. The killing index was calculated as (CFU at 2hr – CFU at 4hr)*100/ CFU at 2hr.
Lysosome staining
Cells were infected with PA14 at MOI 10 for 1hr at 37C. After 1hr, infection medium was removed and RPMI containing gentamycin (200μg/ml) and 500nM of LysoTracker green (Thermofisher) was added for 30min to kill any extracellular bacteria and to stain lysosomes. Cells were acquired on BD Fortessa. Median fluorescence intensity (MFI) of LysoTracker green was calculated using FlowJo.
Immunoblotting.
MDMs stimulated with LPS for 30min were lysed by RIPA buffer containing protease inhibitor cocktail (Roche, US) and phosSTOP phosphatase inhibitors (Roche). Total protein was quantified using BCA protein assay (ThermoFisher). Immunoblotting was performed on 10% SDS-PAGE and proteins were transferred to PVDF membrane, blocked with 5% (w/v) skim milk in TBST and incubated overnight at 4
0C with primary antibodies (
Table 2). After washing the membrane, secondary antibody was added. The membrane was then developed with ECL substrate (Bio-Rad, US) and imaged using the ChemiPro Imaging System (Cleaver).
ERK1/2 inhibition
MDMs were pre-treated with 10 or 20μM of U0126, a potent ERK1/2 inhibitor (Sigma) for at least an hour prior to PA14 infection.
Flow cytometric analysis of cell surface receptors and M1/M2 markers.
Fully mature MDMs were analysed for the surface expression of TLR4, IL-13Rα1 and IL-4Rα, essential for M1 and M2 polarization respectively. The percentage of CD80+ M1 macrophages and that of CD209+ M2 macrophages were analysed by using anti- human CD80 PE and anti-human CD209 BV450. 7-AAD staining was performed to test cell viability (9). Data were acquired using BD LSR-Fortessa. All analyses were performed on Flowjo.
Phagocytosis and endocytosis.
Phagocytosis was studied by incubating M1 macrophages with pHrodo green E. coli and pHrodo red S. aureus bioparticles (LifeTech) at 37°C for 90 min. Endocytosis was determined by incubating M2 macrophages with AF647-dextran (10KD) (LifeTech) at 37°C for 90 min. Cells were then washed and acquired on BD Fortessa. Phagocytic or endocytic index was calculated by normalizing corresponding MFI with either CD80+ or CD209+ cells respectively as previously reported (11).
Cytokine quantification.
M1-specific pro-inflammatory cytokines, such as, IL-6, IL-8, IL-10 and TNFα were quantified in culture supernatant by alphaLISA (PerkinElmer).
Statistical analysis
The Wilcoxon signed-rank test was used to determine the statistical difference between two outcome variables with paired data. For between group comparison, the Wilcoxon rank-sum (Mann-Whitney) test was used for two groups, and the Kruskal-Wallis rank test was used for more than two groups. When the overall significance was observed for more than two groups, Dunn’s multiple comparisons (without adjustment) was used to determine the pair-wise significance difference. Statistical significance was determined at the 0.05 level. Data are presented as median (25th-75th percentile) unless stated otherwise. All analyses were performed using Graph-Pad 7 (San Diego, CA).
Results.
Azithromycin cytotoxicity.
Under light microscopy, MDMs treated with 50μg/ml of AZM were comparatively smaller in size than vehicle treated control MDMs. Flow cytometric analysis showed that MDMs treated with 2 and 5μg/ml of AZM showed minimal 7-AAD staining. With increasing AZM conc, 7-AAD staining increased indicating AZM cytotoxicity (Suppl.
Figure S1) Since a mean peak concentration of 3.89μg/ml has been reported in bronchial mucosa following AZM administration (12), we chose to use 5μg/ml of AZM for all subsequent experiments.
AZM increases the uptake and killing of P. aeruginosa by CF macrophages.
We previously observed impaired phagocytosis of
E. coli by LPS-induced CF M1 macrophages (10). In this study, we used unpolarized MDMs and observed the similar phenomenon. Uptake of
P. aeruginosa was significantly decreased in unpolarized CF MDMs compared to HC MDMs (
p 0.001). AZM treatment led to a significant increase of
P. aeruginosa uptake in both HC (
p 0.002) and CF MDMs (
p 0.003) (
Figure 1B), however uptake in AZM treated CF MDMs still remained significantly below than HC MDMs not exposed to AZM (
p 0.007). The killing index of
P. aeruginosa was enhanced in CF MDMs following AZM treatment (
p 0.02), but not in HCs (
Figure 1C). As lysosomal acidification, the key mechanism of MDMs to kill engulfed bacteria, was previously reported defective in CF MDMs by our and other groups (10, 13), we stained the lysosomes of
P. aeruginosa infected MDMs with LysoTracker with the aim whether AZM was able to alter lysosomal acidification. Median fluorescent intensity (MFI) of LysoTracker was slightly increased though not significantly in both HC and CF MDMs (Suppl.
Figure S2). Altogether, this study confirms the role of AZM in
P. aeruginosa uptake, but not in killing.
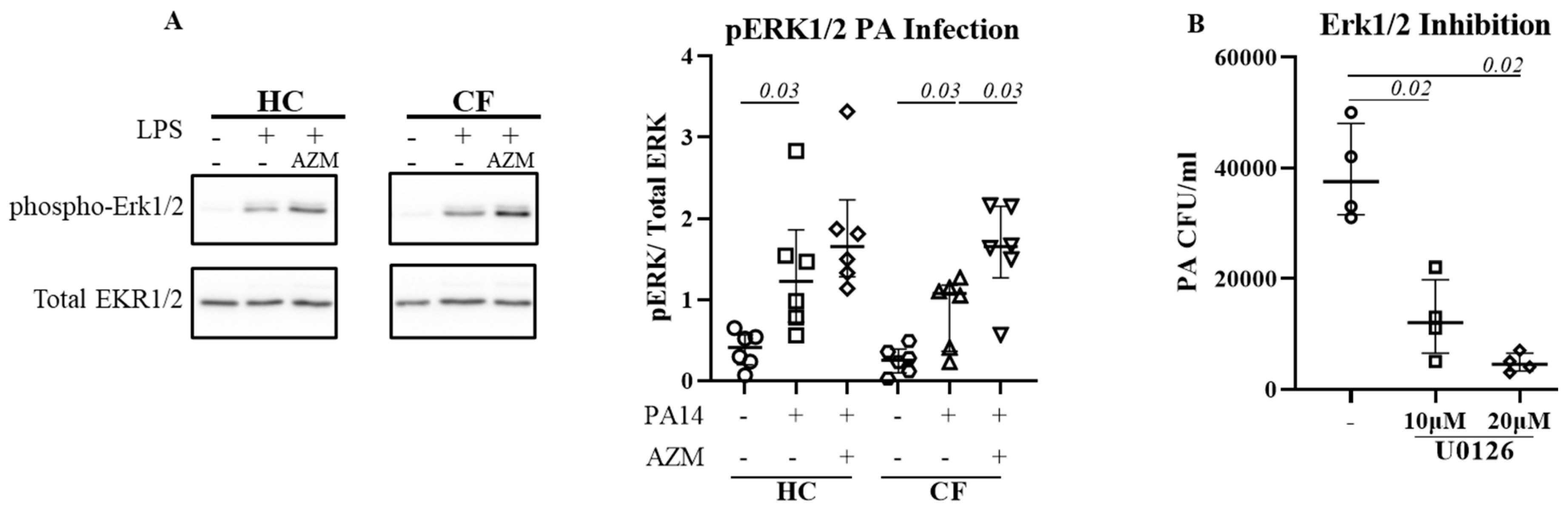
ERK1/2 activation is pivotal for P. aeruginosa uptake.
Activation of extracellular-signal-regulated kinase (ERK1/2) has been reported for efficient bacterial phagocytosis (14, 15). ERK1/2 activation was also observed in
P. aeruginosa-infected CF epithelial cells (16). We therefore analyzed for a direct link between ERK1/2 activation and
P. aeruginosa uptake in HC and CF MDMs. Infection with
P. aeruginosa led to significant activation of EKR1/2 in both HC (
p 0.03) and CF (
p 0.03) MDMs (
Figure 2A). AZM treatment increased ERK1/2 activation even more in both HC and CF (
p 0.03) MDMs. To confirm the role of ERK1/2 in
P. aeruginosa uptake, HC MDMs were treated with U0126, an ERK1/2 inhibitor, 1hr prior
P. aeruginosa infection. U0126 treatment led to a significant reduction of
P. aeruginosa uptake in HC MDMs in dose dose-dependent manner (
Figure 2B), confirming the role of ERK1/2 activation in bacterial uptake.
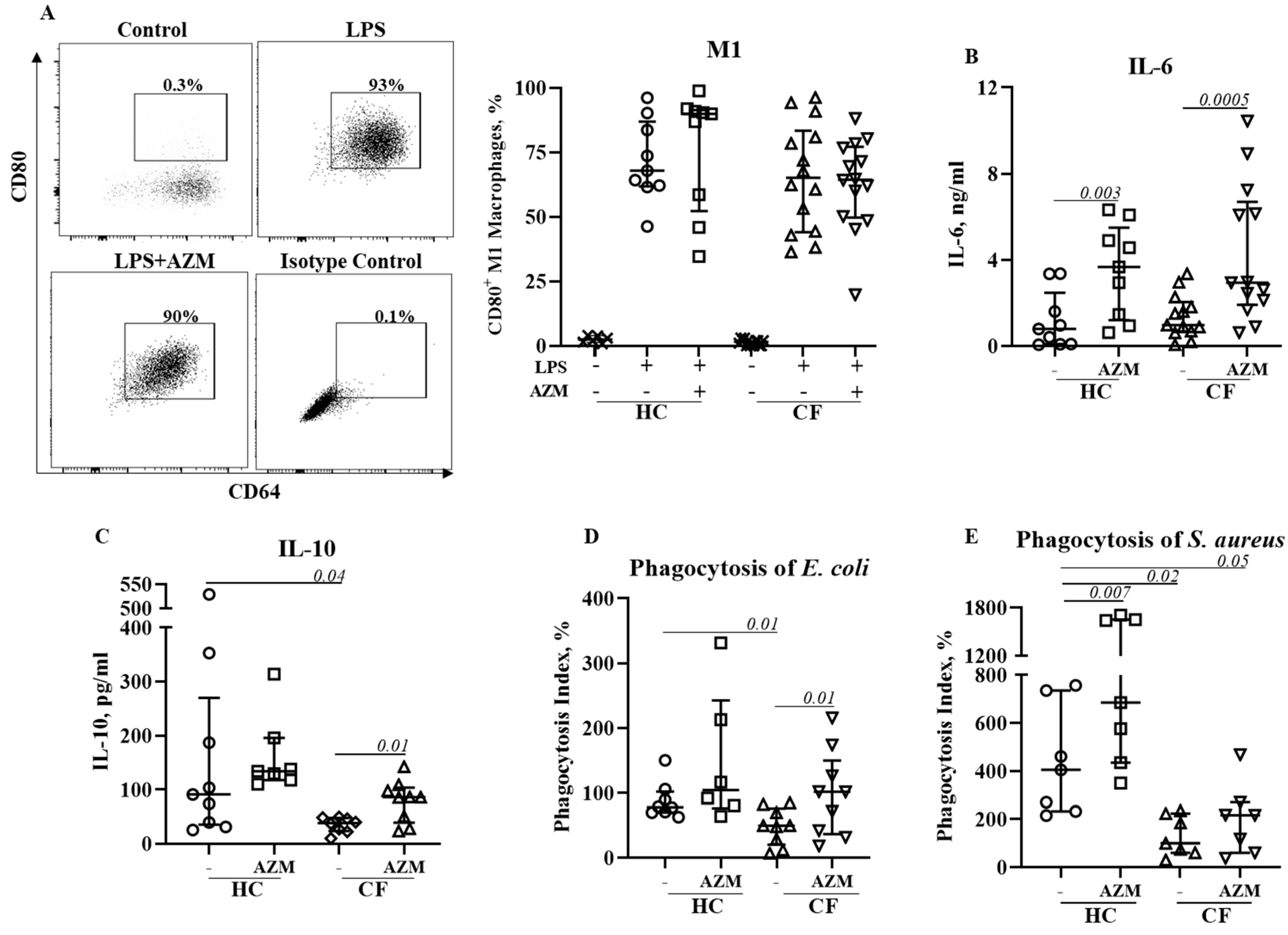
AZM neither reduced pro-inflammatory (M1) macrophage polarization nor pro-inflammatory cytokine secretion.
We then investigated the effect of AZM in LSP-stimulated M1 macrophage polarization and their cytokine release. M1 macrophage polarization was not affected by AZM in either HC or CF (
Figure 3A). IL-6 and IL-10 release was significantly increased in both HC and CF M1 macrophages following AZM treatment, however, IL-8 and TNF-α levels remained unchanged (
Figure 4B-E, IL-8, TNF-α data not shown). LPS-induced M1 macrophages are more effective phagocytic than unpolarized MDMs (9). We then tested the phagocytic ability of these AZM-treated M1 macrophages using pHrodo-labelled gram-negative
E. coli and gram-positive
S. aureus. Phagocytic index for both
E. coli and
S. aureus was significantly lower in CF M1 macrophages compared to HC M1 macrophages (
Figure 3D, E). This observation aligns with the data obtained from the PA14 infection data shown in
Figure 1B. AZM treatment led to enhanced phagocytic index for both bacteria in CF M1 macrophages (
Figure 3D, E). This data suggests that AZM enhances bacterial uptake irrespective of gram-positive and gram-negative bacterial strains. To understand the molecular mechanism of AZM treatment in M1 macrophages, we analyzed the activation of NFκB. Phosphorylation of NFκB (p65) was unaffected by AZM (Suppl
Figure S3).
Discussion.
Failure to clear bacteria from the lung and exaggerated inflammation are common in CF (17). Although RCTs trials with AZM showed improvement in lung function and reduced Pseudomonas aeruginosa colonization rates (1-3), cellular and mechanistic evidence regarding the mode of action of AZM in CF was scarce. We herein demonstrate that AZM significantly augments uptake of P. aeruginosa by both HC and CF MDMs, the killing index was significantly increased for CF MDMs too. However, lysosomal staining, an indication of lysosomal pH, remained unchanged. Mechanistically AZM enhanced uptake was associated with ERK1/2 pathway upregulation. Increased phagocytosis of E. coli and S. aureus was also observed by CF pro-inflammatory M1 macrophages. In addition, AZM partially restored anti-inflammatory M2 macrophage polarization in CF. Such enhanced uptake of both gram-positive and gram-negative bacteria that confers improved bacterial killing in CF and restoration of anti-inflammatory M2 polarization may contribute to the clinical improvement seen in pwCF treated with AZM.
Macrophages detect pathogens using pathogen recognition receptors (PRRs) which activate signalling pathways to internalize the pathogen into phagosomes. Phagosomes later fuse with acidic organelle lysosomes which contain reactive oxygen species (ROS), reactive nitrogen species (RNS), proteases and antimicrobial peptides to facilitate killing of engulfed pathogens. Elegant studies demonstrated that alveolar macrophages (AMs) do not proliferate and disappear during infection (18). Instead, monocyte-derived macrophages (MDMs) are recruited to the lungs to initiate inflammation and are then involved in bacterial killing and inflammation inception (19). MDMs were found higher in numbers in the CF lung compared with alveolar macrophages (7), confirming relevance to their use as macrophage models of CF pathogenesis. Using MDMs, we have previously demonstrated a CFTR-dependent defect in phagocytosis in CF macrophages (10). These functional deficiencies could contribute to exaggerated pulmonary inflammation by failing to kill and clear bacteria in the CF lungs.
Using unpolarized MDM, we initially analyzed uptake and killing of P. aeruginosa in HC and CF MDMs in presence or absence of AZM. Similar to our previous study with E. coli (10), we herein showed reduced uptake of P. aeruginosa by CF MDMs. AZM treatment significantly enhanced P. aeruginosa uptake in both HC and CF, however, uptake in AZM treated CF MDMs still remained significantly lower than in HC MDMs not exposed to AZM. Phagolysosomal acidification, the key killing machineries of MDMs, is tightly regulated by CFTR channel function, which was previously found lower in CF macrophages (13). In this study, lysosome staining using LysoTracker did not show any substantial increase in AZM-treated HC and CF MDMs compared to controls. This implies that AZM has no effect on CFTR channel function. The increased killing index by CF MDMs may be due to higher uptake of P. aeruginosa. Thus, we herein conclude that AZM enhances bacterial uptake in macrophages but has no role in enhancing phagolysosomal killing.
Bacterial uptake and phagolysosomal bacterial killing are complicated processes, with the involvement of different signalling pathways. Activation of extracellular-signal-regulated kinase (ERK1/2) has been reported for efficient phagocytosis (14, 15). Inhibition of the ERK1/2 pathway with U0126 resulted in reduced phagocytosis confirming the role ERK1/2 in phagocytosis (20). In addition, The ERK1/2 pathway is a known target of AZM (21). Similar to CF epithelial cells (16), ERK1/2 activation was observed after infection in both HC and CF MDMs and was enhanced following AZM treatment. ERK1/2 inhibition using U0126 diminished P. aeruginosa uptake in HC MDMs in a dose-dependent manner indicating a direct link between ERK1/2 activation in P. aeruginosa uptake.
The M1 pro-inflammatory MDM phenotype is associated with increased inflammatory response. Data from the present study on the effects of AZM on M1 polarization differ from a previous study conducted in a murine model of CF that reported reduced M1 polarization in peritoneal macrophages using NO production as a marker of polarization (5). However, we observed no reduction in the percentages of CD80+ M1 macrophages in both HC and CF M1 macrophages following AZM treatment. NFκB is a key molecular regulator of inflammation and induction of NFκB-dependent effector genes. We did not see any change in NFκB phosphorylation. Inhibition of NFκB phosphorylation was previously observed in human monocytes at a high dose of AZM (22), but we observed such higher doses of AZM is highly cytotoxic to MDMs. Using the dose of AZM that we used in this study, Haydar et al observed a significant inhibition of translocation of NFκB in bone-marrow derived macrophages (23). However, Haydar et al did not analyze cytokine release, whereas, we observed substantial increase in IL-6 and IL-10 release in HC and CF M1 macrophages following AZM treatment. These data are consistent with previous studies using macrophage cell lines showing that ERKs1/2 activation positively regulates IL-10 production (24). Since CF M1 macrophages exhibited enhanced phagocytosis of pHrodo E. coli and S. aureus bioparticles, it is important to address whether there is any role of IL-6 or IL-10 on bacterial phagocytosis. IL-6 has previously been reported to play role in phagocytosis (25). Recently Akoumianaki et al demonstrated requirement of IL-6 for ERK1/2 trafficking to phagosomes (26). Although other studies with epithelial cells reported reduced TNF-α and IL-8 release following AZM treatment, which was not observed in our study. Together, our data suggest that AZM induces IL-6 and IL-10 release which in turn promote bacterial phagocytosis by CF M1 macrophages.
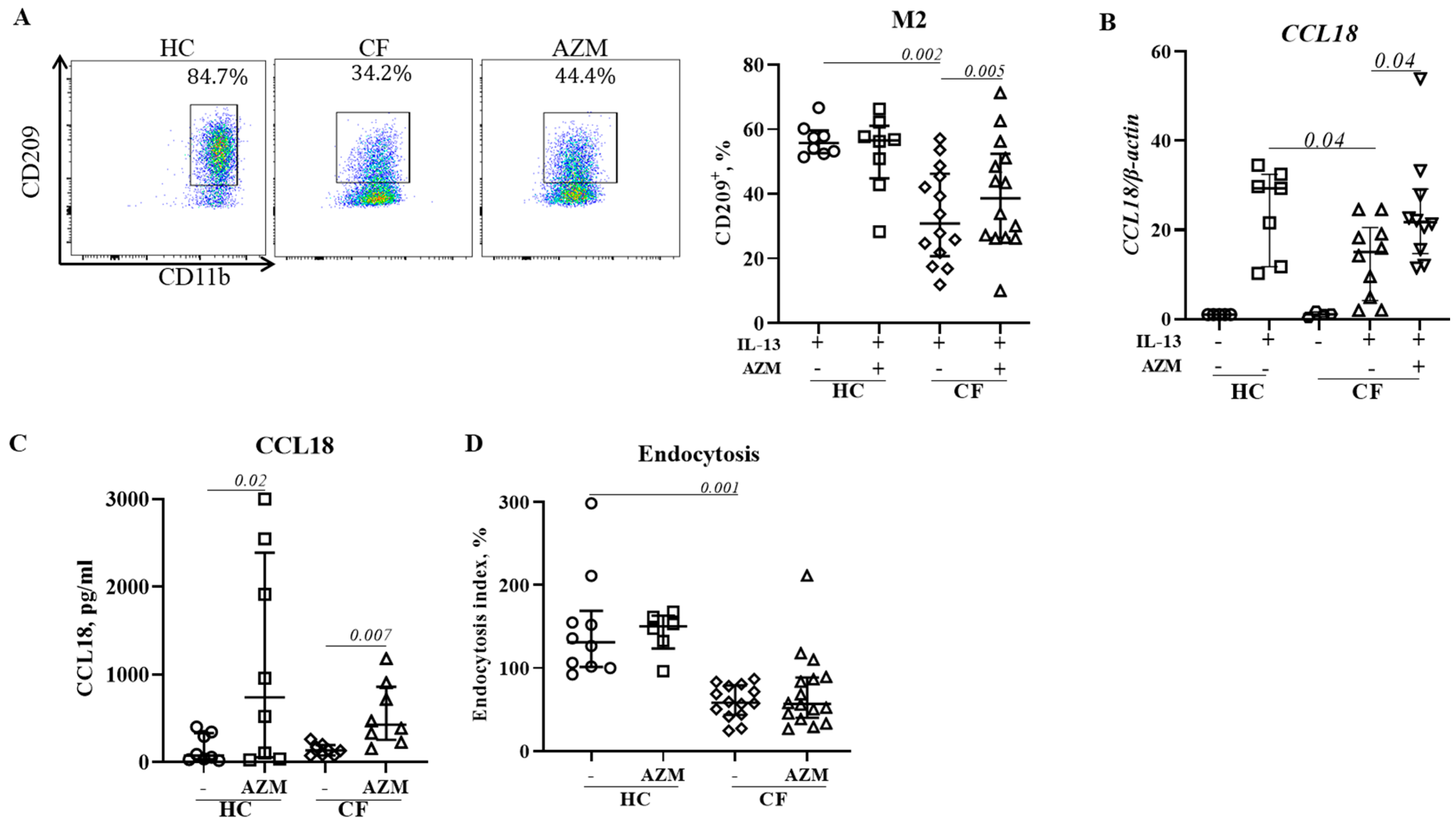
We previously reported impaired polarization of anti-inflammatory (M2) macrophages in CF (10). Haydar et al demonstrated that AZM was able to induce M2 macrophage polarization by inhibiting STAT1 and NFκB signalling pathways (23). Meyer et al showed a shift from pro-inflammatory M1 toward anti-inflammatory M2 macrophage polarization by AZM treatment in F508del-CF mice (5). In agreement with these studies, we observed an increase in M2 polarization in CF following AZM treatment. CCL18 release was found enhanced in both HC and CF M2 macrophages. CCL18 is known to recruit monocytes/macrophages and regulatory T cells to site of inflammation to maintain homeostasis. Pechkovsky et al showed that IL-10 enhanced CCL18 expression by M2 macrophages (27). In physiological conditions, it is possible that M1 macrophages clear the invading pathogen and release IL-10 which in turn induce M2 polarization. However, we did not find an increase in endocytosis in AZM treated CF M2 macrophages. AZM has previously been reported to selectively inhibit endocytosis by inhibiting endocytic uptake and transport of solutes along the endocytic pathway in the murine macrophage cell line J774 (28). This may explain why we did not observe any enhancement of endocytosis in CF M2 macrophages. Further research is required to identify the molecular mechanism of enhanced M2 macrophage polarization following AZM treatment.
A key question raised by our observation of enhanced bacterial uptake and improved M2 macrophage polarization was whether AZM induced CFTR protein expression and chloride channel activity or whether other mechanisms were involved. AZM has previously been reported to increase chloride efflux in CF epithelial cells without increasing CFTR protein or mRNA expression (29). In our observation, neither CFTR mRNA nor CFTR protein expression, as assessed by flow cytometry, was increased in AZM-treated MDMs when compared to control groups (data not shown). Therefore, the detailed molecular mechanism underlying of improved CF macrophage function and polarization still remained unclear and warrant further studies.
We observed some limitations in our study. While MDMs are appropriate cells to study (30), we assess them remote from their usual site of action. In addition, due to limited volume of blood that was collected from patients with CF, we did not have sufficient cells to perform all studies on cells from all patients. In summary, we provide evidence that the anti-inflammatory effects of AZM may be contributed to by increasing bacterial uptake by CF MDMs, increasing the proportion of CF MDMs able to polarize into the inflammation-resolving M2 phenotype and increasing the secretion of the anti-inflammatory cytokines IL-10 and CCL18. Mechanistically, increased bacterial uptake was mediated by activating the ERK1/2 pathway.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Cytotoxicity (7-AAD) of AZM in HC macrophages. AZM at different doses were given during macrophage differentiation. Cytotoxicity of AZM was assessed using 7-AAD DNA binding dye staining by flow cytometry. Each dot represents an independent donor. Data are shown as median (25%, 75%); Figure S2: Azithromycin has no effect on lysosomal acidification. Lysosomes of PA14 infected cells were stained with LystoTracker green. MFI of Lysotracker was measured by BD Fortessa. Each dot represents an individual healthy donor or pwCF. Data was shown as median (25%, 75%); Figure S3: Effect of AZM on NFκB activation. MDMs were differentiated in presence or absence of AZM and stimulated with LPS (20ng/ml) for 30min. Phosphorylation of NFκB was analyzed by western blot. Phosphorylation was normalized by the GAPDH level of unstimulated MDMs. Each symbol represents an individual donor or pwCF. Data are shown as median (25%, 75%).
Author Contributions
P.D.S., A.A.T. and E.F. generated the study concept. SCB and CEW were responsible for enrolment of the adult and children with CF respectively. A.A.T. designed all experiments. A.A.T. and D.K. conducted majority of the experiments including cell culture, cytokine quantification, flow cytometry, RNA extraction and qPCR. L.L. and J.L.S. contributed the concept of signalling pathway analysis. N.T. performed some of the western blot analysis. R.M.L. provided U0126 to A.A.T. N.B. performed all the statistical analysis. A.A.T. generated majority of the graphs and wrote the manuscript. All authors approved the manuscript.
Funding
A.A.T. and D.K. were supported by research grant from Cystic Fibrosis Foundation, USA (SLY16AO), fellowship from Children Health Foundation and Australian Cystic Fibrosis Innovation grant from Australian Cystic Fibrosis Research Trust. LL is supported by National Health and Medical Research Council of Australia (NHMRC) project grants (APP1159106) and an Australian Research Council Discovery Early Career Award (DE180100524). PDS and JLS are Leadership Fellows of the National Health and Medical Research Council (NHMRC), Australia.
Acknowledgments
We acknowledged to all study participants and the clinical teams who collected the venous blood from the study participants. We would also like to thank the Red Cross Blood Service, Brisbane for providing buffy coats as per request. A.A.T. and D.K. were supported by research grant from Cystic Fibrosis Foundation, USA (SLY16AO) and by Australian Cystic Fibrosis Innovation grant from Australian Cystic Fibrosis Research Trust. PDS and JLS are Leadership Fellows of the National Health and Medical Research Council (NHMRC), Australia.
References
- Stick SM, Foti A, Ware RS, Tiddens H, Clements BS, Armstrong DS, et al. The effect of azithromycin on structural lung disease in infants with cystic fibrosis (COMBAT CF): a phase 3, randomised, double-blind, placebo-controlled clinical trial. The Lancet Respiratory medicine. 2022, 10, 776–784. [CrossRef]
- Equi A, Balfour-Lynn IM, Bush A, and Rosenthal M. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet. 2002, 360, 978–984. [CrossRef]
- Clement A, Tamalet A, Leroux E, Ravilly S, Fauroux B, and Jais JP. Long term effects of azithromycin in patients with cystic fibrosis: A double blind, placebo controlled trial. Thorax. 2006, 61, 895–902. [CrossRef]
- Zarogoulidis P, Papanas N, Kioumis I, Chatzaki E, Maltezos E, and Zarogoulidis K. Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur J Clin Pharmacol. 2012, 68, 479–503. [CrossRef] [PubMed]
- Meyer M, Huaux F, Gavilanes X, van den Brule S, Lebecque P, Lo Re S, et al. Azithromycin reduces exaggerated cytokine production by M1 alveolar macrophages in cystic fibrosis. American journal of respiratory cell and molecular biology. 2009, 41, 590–602. [CrossRef]
- Hodge S, and Reynolds PN. Low-dose azithromycin improves phagocytosis of bacteria by both alveolar and monocyte-derived macrophages in chronic obstructive pulmonary disease subjects. Respirology. 2012, 17, 802–807. [CrossRef]
- Schupp JC, Khanal S, Gomez JL, Sauler M, Adams TS, Chupp GL, et al. Single-Cell Transcriptional Archetypes of Airway Inflammation in Cystic Fibrosis. American journal of respiratory and critical care medicine. 2020, 202, 1419–1429. [CrossRef] [PubMed]
- Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, et al. Transcriptome-Based Network Analysis Reveals a Spectrum Model of Human Macrophage Activation. Immunity. 2014.
- Tarique AA, Logan J, Thomas E, Holt PG, Sly PD, and Fantino E. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. American journal of respiratory cell and molecular biology. 2015, 53, 676–688. [CrossRef] [PubMed]
- Tarique AA, Sly PD, Holt PG, Bosco A, Ware RS, Logan J, et al. CFTR-dependent defect in alternatively-activated macrophages in cystic fibrosis. J Cyst Fibros. 2017.
- Hodge S, Tran HB, Hamon R, Roscioli E, Hodge G, Jersmann H, et al. Nonantibiotic macrolides restore airway macrophage phagocytic function with potential anti-inflammatory effects in chronic lung diseases. American journal of physiology Lung cellular and molecular physiology. 2017, 312, L678–L87. [CrossRef]
- Baldwin DR, Wise R, Andrews JM, Ashby JP, and Honeybourne D. Azithromycin concentrations at the sites of pulmonary infection. Eur Respir J. 1990, 3, 886–890. [CrossRef]
- Di A, Brown ME, Deriy LV, Li C, Szeto FL, Chen Y, et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nature cell biology. 2006, 8, 933–944. [CrossRef] [PubMed]
- Botelho RJ, Harrison RE, Stone JC, Hancock JF, Philips MR, Jongstra-Bilen J, et al. Localized diacylglycerol-dependent stimulation of Ras and Rap1 during phagocytosis. J Biol Chem. 2009, 284, 28522–28532. [CrossRef] [PubMed]
- Xin C, Kim J, Quan H, Yin M, Jeong S, Choi JI, et al. Ginsenoside Rg3 promotes Fc gamma receptor-mediated phagocytosis of bacteria by macrophages via an extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase-dependent mechanism. Int Immunopharmacol. 2019, 77, 105945. [CrossRef]
- Farias R, and Rousseau S. The TAK1-->IKKbeta-->TPL2-->MKK1/MKK2 Signaling Cascade Regulates IL-33 Expression in Cystic Fibrosis Airway Epithelial Cells Following Infection by Pseudomonas aeruginosa. Front Cell Dev Biol. 2015, 3, 87.
- Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, et al. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013, 368, 1963–1970. [CrossRef] [PubMed]
- Lagasse HA, Anidi IU, Craig JM, Limjunyawong N, Poupore AK, Mitzner W, et al. Recruited monocytes modulate malaria-induced lung injury through CD36-mediated clearance of sequestered infected erythrocytes. Journal of leukocyte biology. 2016, 99, 659–671. [CrossRef] [PubMed]
- Florentin J, Coppin E, Vasamsetti SB, Zhao J, Tai YY, Tang Y, et al. Inflammatory Macrophage Expansion in Pulmonary Hypertension Depends upon Mobilization of Blood-Borne Monocytes. J Immunol. 2018, 200, 3612–3625. [CrossRef] [PubMed]
- Shin OS, Miller LS, Modlin RL, Akira S, Uematsu S, and Hu LT. Downstream signals for MyD88-mediated phagocytosis of Borrelia burgdorferi can be initiated by TRIF and are dependent on PI3K. J Immunol. 2009, 183, 491–498. [CrossRef] [PubMed]
- Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, and Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacology & therapeutics. 2014, 143, 225–245.
- Vrancic M, Banjanac M, Nujic K, Bosnar M, Murati T, Munic V, et al. Azithromycin distinctively modulates classical activation of human monocytes in vitro. Br J Pharmacol. 2012, 165, 1348–1360. [CrossRef]
- Haydar D, Cory TJ, Birket SE, Murphy BS, Pennypacker KR, Sinai AP, et al. Azithromycin Polarizes Macrophages to an M2 Phenotype via Inhibition of the STAT1 and NF-kappaB Signaling Pathways. J Immunol. 2019, 203, 1021–1030. [CrossRef]
- Bouhamdan M, Bauerfeld C, Talreja J, Beuret L, Charron J, and Samavati L. MEK1 dependent and independent ERK activation regulates IL-10 and IL-12 production in bone marrow derived macrophages. Cell Signal. 2015, 27, 2068–2076. [CrossRef] [PubMed]
- Hoetzenecker W, Echtenacher B, Guenova E, Hoetzenecker K, Woelbing F, Bruck J, et al. ROS-induced ATF3 causes susceptibility to secondary infections during sepsis-associated immunosuppression. Nat Med. 2011, 18, 128–134.
- Akoumianaki T, Vaporidi K, Diamantaki E, Pene F, Beau R, Gresnigt MS, et al. Uncoupling of IL-6 signaling and LC3-associated phagocytosis drives immunoparalysis during sepsis. Cell host & microbe. 2021, 29, 1277–1293 e6.
- Pechkovsky DV, Prasse A, Kollert F, Engel KM, Dentler J, Luttmann W, et al. Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clinical immunology. 2010, 137, 89–101. [CrossRef] [PubMed]
- Tyteca D, Van Der Smissen P, Mettlen M, Van Bambeke F, Tulkens PM, Mingeot-Leclercq MP, et al. Azithromycin, a lysosomotropic antibiotic, has distinct effects on fluid-phase and receptor-mediated endocytosis, but does not impair phagocytosis in J774 macrophages. Exp Cell Res. 2002, 281, 86–100.
- Saint-Criq V, Rebeyrol C, Ruffin M, Roque T, Guillot L, Jacquot J, et al. Restoration of chloride efflux by azithromycin in airway epithelial cells of cystic fibrosis patients. Antimicrob Agents Chemother. 2011, 55, 1792–1793. [CrossRef]
- Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. The Journal of experimental medicine. 2017, 214, 2387–2404. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).