1. Introduction
Chronic kidney disease (CKD) is defined as structural or functional abnormalities in the kidneys presenting for more than three months. Decreased kidney clearance causes uremic toxins to accumulate in the body, thereby leading to damage to the kidneys, the cardiovascular system, the immune system, and the intestines [1,2]. In felines, the average prevalence of CKD is ~1−3% but increases to 80% in the geriatric feline population (> 15 years old) [3,4]. The common symptoms of CKD in cats include weight and muscle loss, vomiting, anorexia/hyporexia (up to 92% of cats), constipation, proteinuria, non-regenerative anemia, and hypokalemia [5–8]. Until now, there is no cure for CKD [9] with CKD progression controlled by limiting protein intake, decreasing uremic toxin absorption, dialysis, or kidney transplantation [5,9,10].
Indoxyl sulfate (IS), p-cresyl sulfate (PCS), and trimethylamine N-oxide (TMAO) are gut-derived uremic toxins (GDUTs) resulting from the breakdown of dietary protein by some proteolysis bacteria, such as Escherichia coli, Clostridium difficile, and Shigella, in the host intestine [9,11–13]. Accumulated circulating PCS and IS levels are strongly related to a deterioration in the estimated glomerular filtration rate and negatively correlated with kidney function [14,15]. Thus, microbiota-based strategies could provide a potential auxiliary therapeutic and preventative method for CKD.
The efficacy of probiotics to prevent/alleviate CKD has been intensively investigated in animal models. Probiotics not only enhance the homeostasis in the intestine but also reduce the production or retention of uremic toxins. Lactobacillus acidophilus NT decreased urinary protein excretion and urea nitrogen, IS, and PCS in the serum of 5/6 nephrectomy mice with mitigating systemic inflammation and kidney sclerosis [16]. In a cisplatin-induced CKD Lanyu pig model, the probiotic mix downregulated IS levels in serum, and reduced fibrosis and oxidative stress in the kidney by shifting the composition of gut microbiota toward the normal control group [17]. In clinical studies, CKD dogs and cats also showed improved kidney function after treatment with probiotics [18,19]. However, most studies lacked in-depth mechanism investigation.
In our previous study, Lactobacillus mixture (Lm, Lacticaseibacillus paracasei subsp. paracasei MFM 18 and Lactiplantibacillus plantarum subsp. plantarum MFM 30-3), isolated from traditional fermented milk exerted kidney-protective effects in CKD mice model [20] and human clinical trial [21]. The possible mechanism of Lm was also clarified, which involved Lm-mediated interconnection among the modification of microbial components, metabolic pathway and metabolite profiles.
Treating pets with probiotics in powder, capsule, or tablet form is challenging [22], so combining probiotics with feed or pet treats might provide a possible solution. However, certain factors, such as microorganism characteristics (thermal resistance, oxygen tolerance, acid, and bile resistance), processing conditions (including temperature, time, moisture/water activity, pressure, and pH), application method, and packaging and storage conditions, affect the probiotic survivability, which further influences their efficacy [23]. The efficacy of probiotics in pet foods is a new field of study, and inventions in the form of new application strategies, effective strain selection, and verification of the potential health benefits are necessary to ensure the product's effectiveness [24]. Thus, the present study evaluated the efficacy of Lm pet treats in feline CKD and elucidated the mechanisms underlying host-microbe interactions. This study clarified the possible mechanism of Lm in CKD cats and provided a possible novel way to treat cats with probiotics.
2. Materials and Methods
2.1. Bacterial strains
Lm consisted of L. paracasei subsp. paracasei MFM 18 and L. plantarum subsp. plantarum MFM 30-3 isolated from Mongolian fermented milk (MFM) in our lab. The Lm culture conditions were as previously described [20]. The freeze-dried Lm powder was produced by Grape King Bio, Ltd. (Taoyuan, Taiwan) with microcrystalline α-cellulose, magnesium stearate, silicon dioxide, and oligofructose as an excipient for the production of Lm pet treats. The total bacterial count in the Lm powder was 1.07 × 1011 CFU/g.
2.2. Production of Lm pet treats
The Lm powder was mixed with chicken oil and fish oil. Then, 1% of the Lm powder was spread-coated on the commercial pet feed made by Withpet Inc. (Taoyuan, Taiwan) as Lm pet treats. Three different flavors of Lm pet treats were produced including chicken and fish (CA), fish and mutton (CB), and chicken (CC). The nutrient composition of Lm pet treats is shown in
Table 1.
2.3. Safety and stability test of Lm pet treats
Lm pet treats were stored at room temperature. Harmful residue analysis and chemical stability were analyzed by Eurofins Food Testing Taiwan Ltd. (Kaohsiung, Taiwan). The pathogenic bacteria test was performed by the National Animal Industry Foundation (Taipei, Taiwan). Lm pet treats were homogenized with sodium chloride liquid every 2 weeks and the lactic acid bacteria count was evaluated on lactobacilli MRS agar (Neogen Corporation, Lansing, MI, U.S.A.).
2.4. Clinical CKD cat trial: a pilot study
The cat trial was conducted by a single-arm pilot study at the National Taiwan University Veterinary Hospital, Taiwan, from August to November 2021. The study was approved by the Institutional Animal Care and Use Committee of National Taiwan University (IACUC approval no: NTU-110-EL-00042). Before allowing their cats to participate in the study, all owners signed an informed consent form.
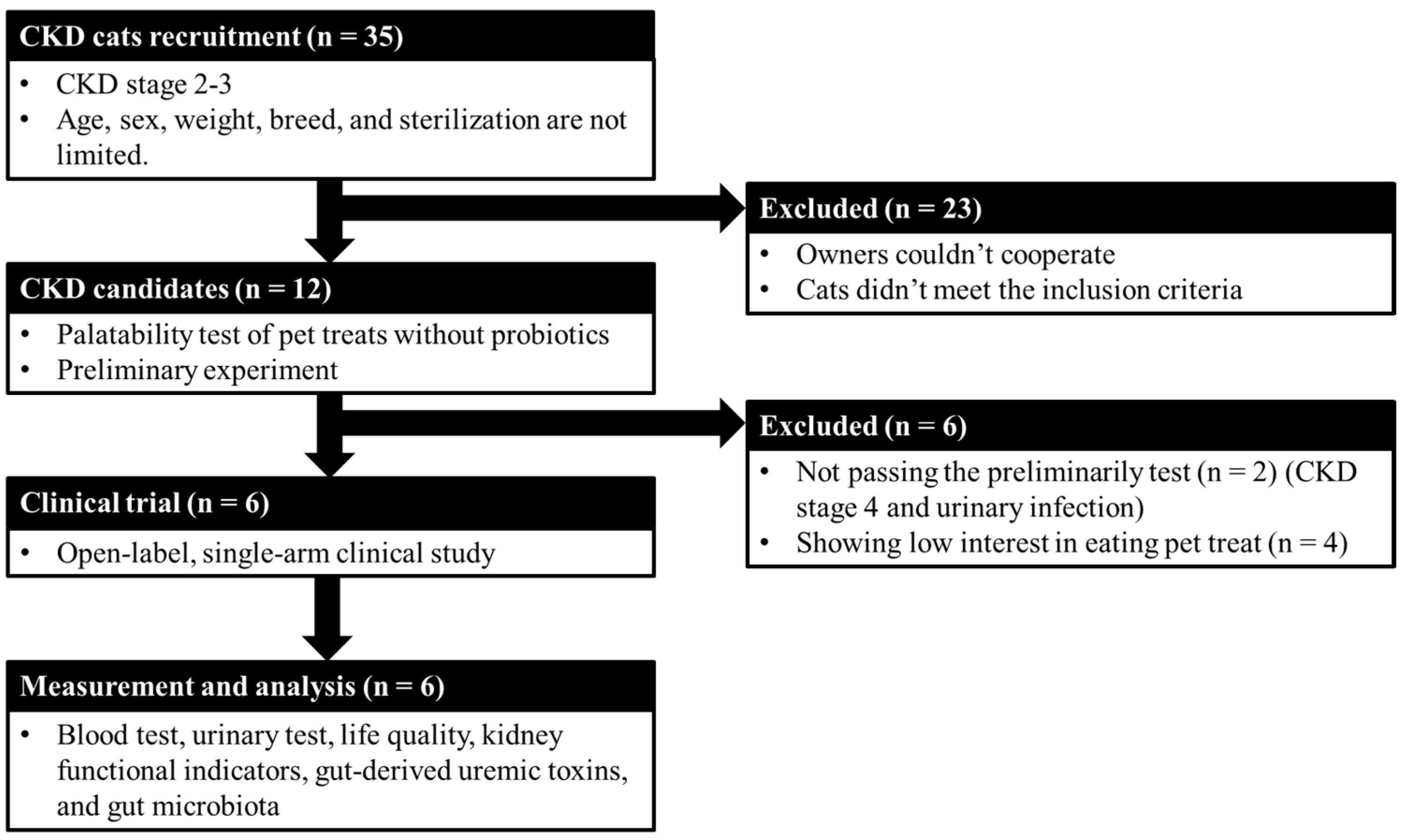
The study design is shown in
Figure 1. There were no limitations on the cats’ age, sex, breed, weight, and sterilization, but they were required to be CKD stages 2–3 and with 1.6–5.0 mg/dL creatinine or 18–38 μg/dL SDMA [24] and meet one of the following conditions for at least 3 months: abnormal urinary test {urine specific gravity > 1.035; persistent renal proteinuria [urine protein/urine creatinine ratio (UPC) > 0.4]}, or subclinical symptoms (e.g., polyuria, polydipsia, and dehydration). Cats were excluded if they had acute kidney disease, acute worsening azotemia, diabetes, hyperthyroidism, urinary tract infection, or another nonrenal disease (e.g., cardiac, hepatic, gastrointestinal, neoplastic diseases, or infection). Additionally, if cats were administered antibiotics 2 weeks before the beginning of the trial, they were also excluded. The cats were fed one bag of treats (10 g) by their owner daily for 8 weeks. Each bag of probiotic treats contained 2.79–3.93 × 10
8 CFU/cat/day of Lm. The cats maintained original dietary habits regular and their CKD therapy at the time of enrollment and during the study period. After the whole experiment, owners should answer a questionnaire about the life quality of the tested cats.
2.5. Biochemical measurements
Biochemical analyses of blood and urine were conducted by the veterinary hospital. Creatinine, blood urea nitrogen (BUN), complete blood count, and ions in blood were measured by a blood chemistry analyzer. Urinary tests were also performed using the urine analyzer, including UPC and specific gravity.
2.6. Uremic toxin analysis
The gut-derived uremic toxins, including IS, PCS, TMAO, and phenyl sulfate (PS), were determined in serum. Briefly, serum (50 µL) was mixed with 50 µL of internal control (1000 ng/mL of PCS-d7, IS-d4, PS-13C6, and 100 ng/mL TMAO-d9) and 400 µL of acetonitrile, then centrifuged at 15,000 rpm for 15 mins at 4℃. The supernatant (200 µL) was vacuum-concentrated and dissolved in 200 µL of 20% acetonitrile. The uremic toxins were measured by an LC-MS/MS system (TripleQuad5500, ABSCIEX, Framingham, MA, U.S.A.) with an ACQUITY UPLC BEH C18 Column (2.1 × 150 mm, 1.7 μm, waters). The mobile phase A was 1 mL methanol in 1000 mL ddH
2O, and the mobile phase B was 10 mM ammonium acetate in 1000 mL acetonitrile. The eluting gradient was as follows: 0.0→3.0 min (10%→95% B);3.0→4.0 min (95% B);4.0→4.1 min (95→10% B);4.1→6.0 min (0% B). The injection volume was 5 μL and the flow rate was 0.3 mL/min. The nebulizer gas pressure and drying gas pressure were both 55 psi, and the drying gas temperature was 550℃ in the positive electrospray ionization mode and negative electrospray ionization mode. The capillary voltage was 5.5 kV and –4.5 kV in positive and negative electrospray ionization mode, respectively. The model parameters of the multiple reaction monitoring of target uremic toxins are shown in
Table S1.
2.7. Microbiota analysis in feline feces
The fecal genomic DNA samples were extracted and stored at –20℃. The DNA concentration was measured by a Qubit 4.0 Fluorometer (Thermo Scientific, Rockford, IL, U.S.A.) before third-generation sequencing assays were performed by BIOTOOLS Co., Ltd. (Taipei, Taiwan). The 16S whole-length sequencing was amplified by universe primers 27F: 5’-AGRGTTYGATYMTGGCTCAG-3’, and 1492R: 5’ RGYTACCTTGTTACGACTT-3’. In this study, Hifi reads with read quality (RQ) >30 were retained and the DADA2 package (dada2_1.20) in R software was used to denoise. The reads after denoising are called ASVs (amplicon sequence variants) and one ASV was regarded as one species cluster. QIIME2 (v2021.4; http;//qiime2.org/) was used to process and analyze the representative sequencing of the same ASVs. The NCBI 16S ribosomal RNA database (2020.7) was used to identify the taxonomy classification. The observed species, Shannon-Wiener diversity index, and Pielou’s evenness were analyzed through QIIME2 as the α diversity indexes. Beta diversity used principal coordinate analysis (PCoA) to compare the differences between microbiota composition before and after cats administrating Lm pet treats.
Specific bacteria, including
Bifidobacterium,
Lactobacillus, and
Enterobacteriaceae, were quantified by qPCR. Each reaction included 5 μL 2× KAPA SYBR FAST qPCR Master Mix (Kapa Biosystems, Wilmington, MA, U.S.A.), 0.2 μL 10 μM forward primer, 0.2 μL 10 μM reverse primer (
Table S2), 2 μL template DNA, and 2.6 μL ddH
2O.
The PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) database were applied to compare the gene information to predict metabolic function changes. In Spearman correlation analysis, kidney function indexes (BUN and creatinine), uremic toxins (IS, PS, TMAO, and PCS), and KEGG level 3 pathways were correlated with the bacterial biomarkers in the third-generation sequencing analysis.
2.8. Statistical analysis
All data were presented as mean ± SEM. Based on the results of the Shapiro-Wilk normality test, the statistical analyses were performed by the Wilcoxon sign rank test or ratio paired t-test by GraphPad Prism v9.3.1 (GraphPad Software Inc., Boston, MA, U.S.A.) and Statistical Analysis System v9.4 (SAS Institute Inc., Cary, NC, U.S.A.). Considering the limited sample size in this pilot study and the resulting limitations in statistical power, it is possible that the effects of the Lm intervention may be underestimated. The treatment effect of the Lm pet treats in clinical measurements was assessed in conjunction with 90% confidence intervals (CIs) to explain its clinical significance [25,26].
3. Results
3.1. Lm pet treats were safe and chemical- and bacterial-stable.
The chemical and microbial analyses of the Lm pet treats showed no evidence of pathogenic bacteria or harmful residues, therefore the treats met all the relevant regulatory standards (
Table S3). When stored at room temperature, there were no distinct changes in moisture, acid value, and peroxide value (POV) of the Lm pet treats (
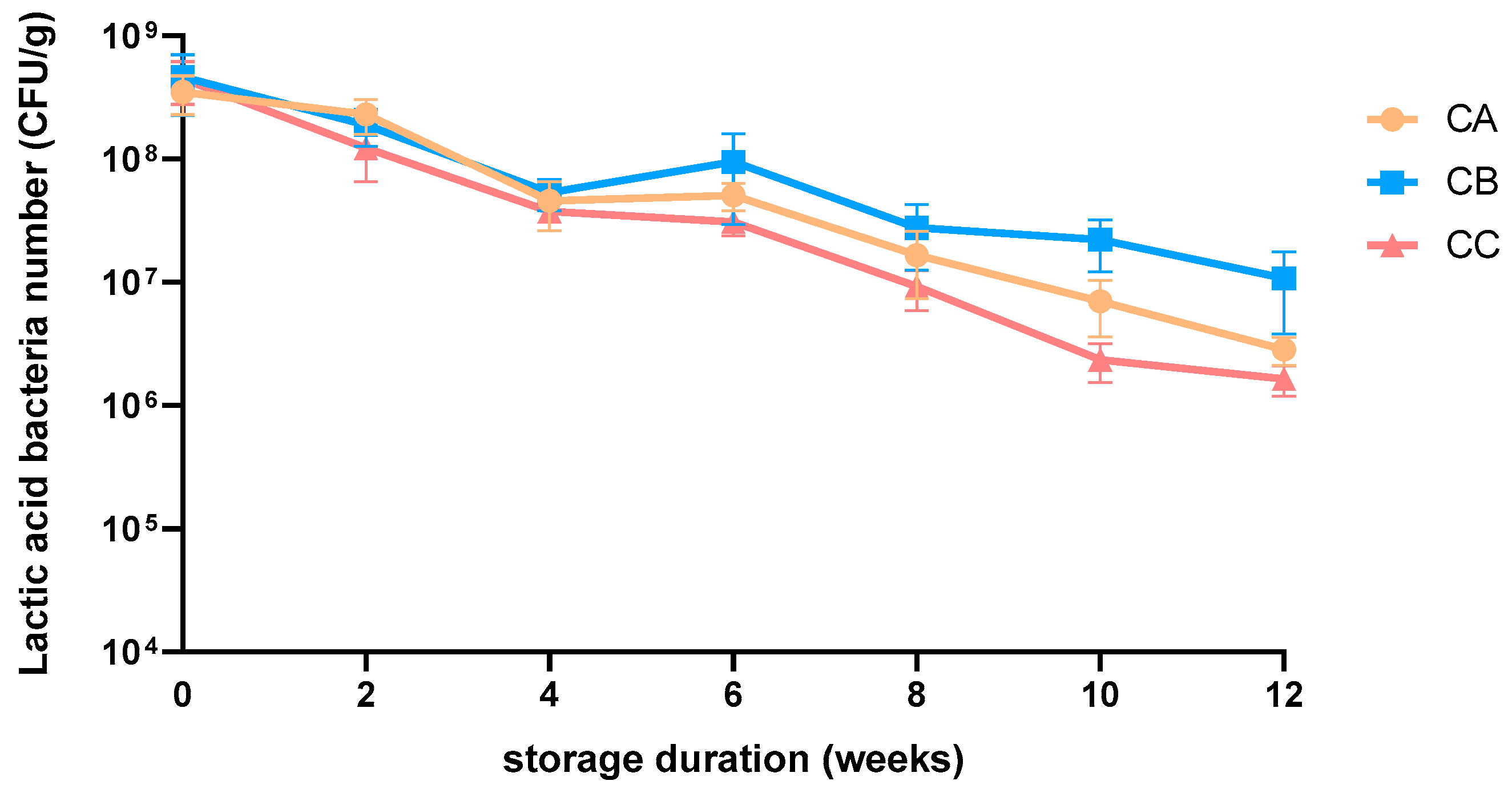
Table S4) after 8 weeks of storage. The average lactic acid bacteria count of the three flavors of Lm pet treats declined gradually from 4.3 × 10
8 CFU/g to 5.4 × 10
6 CFU/g after 12 weeks of storage (
Figure 2). The effective dose of Lm in CKD cats (3–5 kg) was determined as 2.79–3.93 × 10
8 CFU/cat/day [20,28]. The amount of Lm pet treats should be calculated independently for cats heavier than 5 kg to meet the requirement. The Lm pet treats were produced monthly and sent to owners directly to maintain the Lm viable dose.
3.2. Clinical trial on CKD feline: a polit study
3.2.1. Study population
The current study included 35 cats with CKD that were comprehensively evaluated to gain previous clinical diagnosis, dietary and medical histories, and availability of clinical samples. Their owners provided consent for them to participate in this study, and their CKD stage and thyroxine levels were confirmed to ensure their suitability for study participation. Of the 12 cats with CKD enrolled in this clinical study, 6 completed the study and 6 dropped out due to unexpected complications including palatability of pet treats and a urinary tract infection, which may influence the evaluation of kidney function. The ratio of male to female cats was 1:1, with a median age of 13.00 ± 4.86 (range 8–19 years) and a median weight of 4.4 kg (range 3.1–6.3 kg). One cat was CKD stage 2 and five were stage 3.
3.2.2. Lm pet treats could improve/maintain life quality and kidney function in CKD cats.
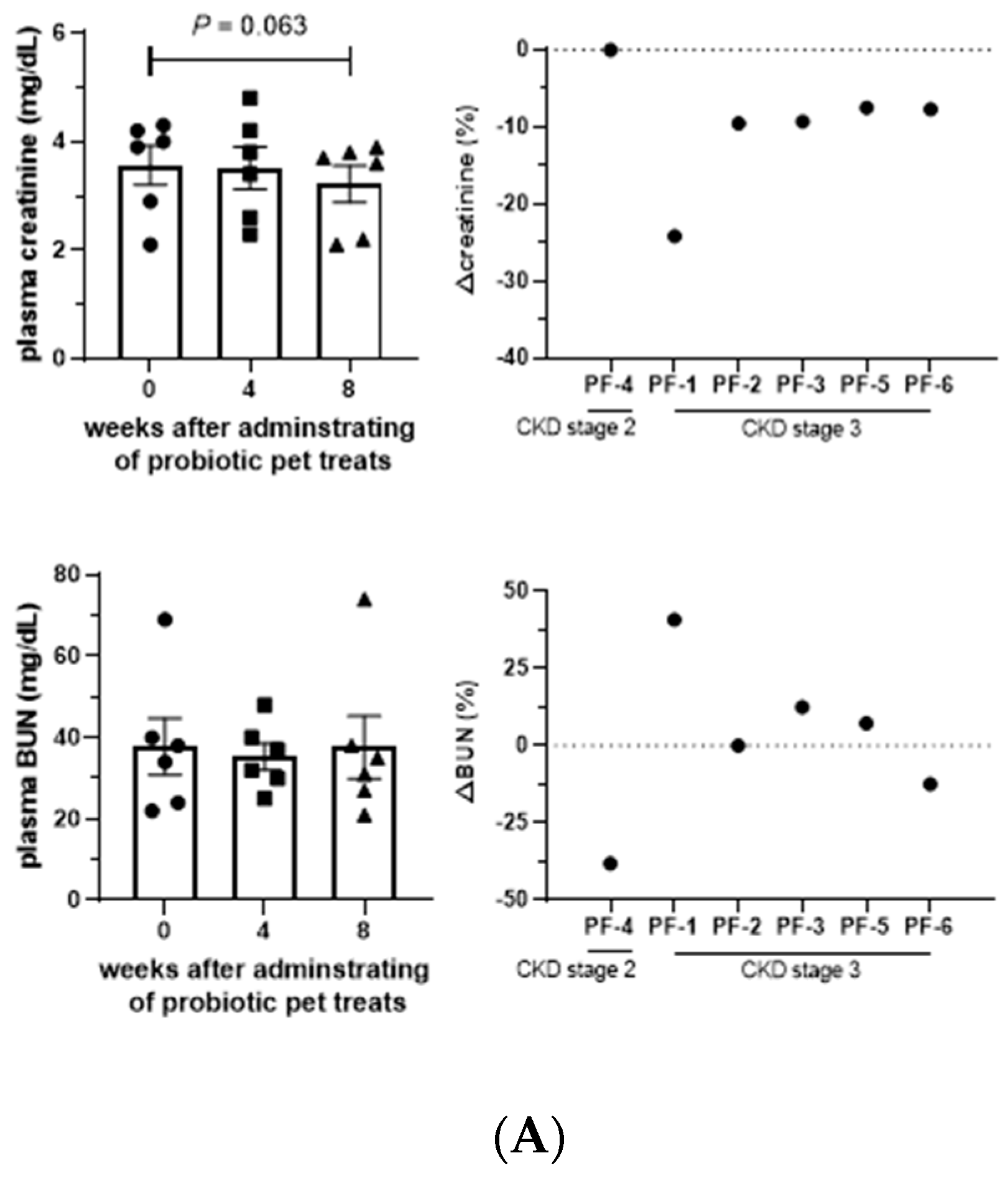
Serum and urine biochemical parameters were maintained during the administration of Lm treats, except serum phosphate, which was significantly elevated (
P < 0.05) after taking Lm treats. The body weights of tested cats also remained stable (
Table S5), indicating no adverse effects of Lm in cats with CKD (
Table S6). Creatinine, a key indicator of kidney function, showed the 90% CIs of each measurement shifted towards a lower distribution with a
P-value = 0.06 after 8 weeks of Lm treatment (
Table 2), with all cats experiencing a reduction in creatine, signifying a potential alleviatory effect of Lm treats on CKD progression (
Figure 3A). BUN levels were also reduced or maintained in 50% of the cats.
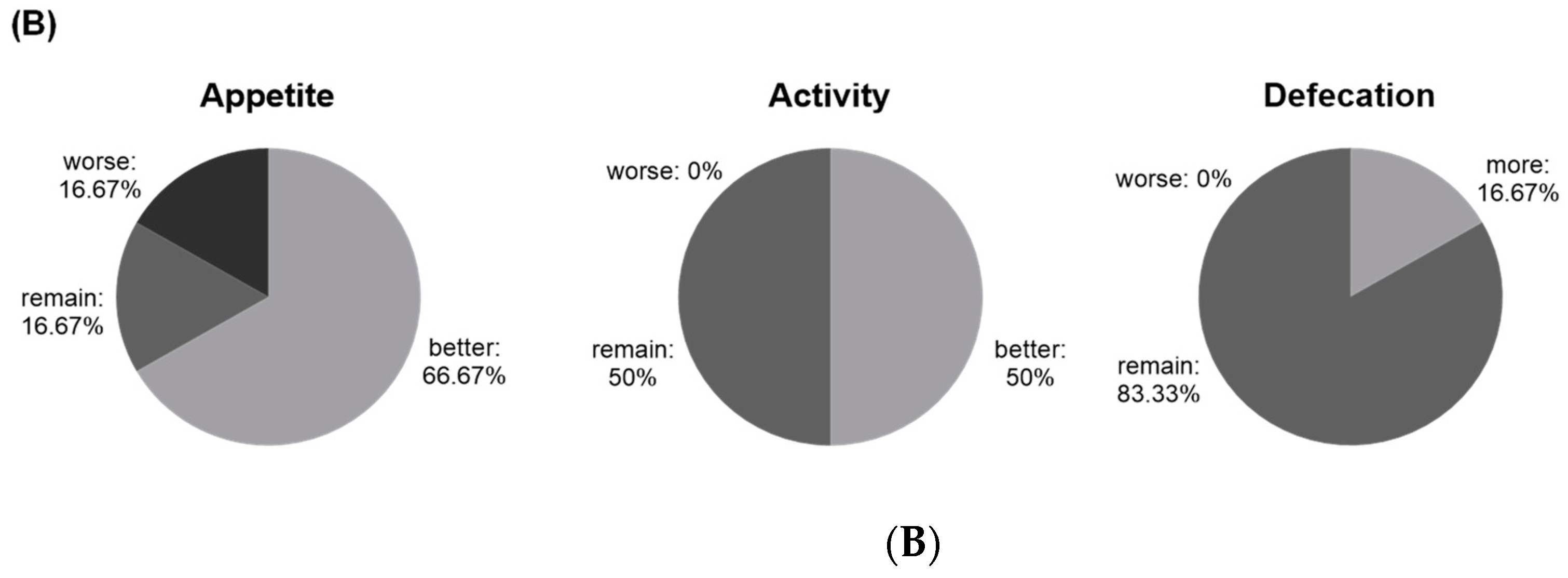
After two months of Lm treatment, 66.67% (4/6 cats) of CKD cats had a better appetite with 100% (6/6 cats) of cats improving/maintaining their activity. One cat had a higher defecation frequency, and the others sustained the frequency (83.33%) (
Figure 3B). Feedback from cat owners also reported that feline stool shape and color improved after the Lm treatment.
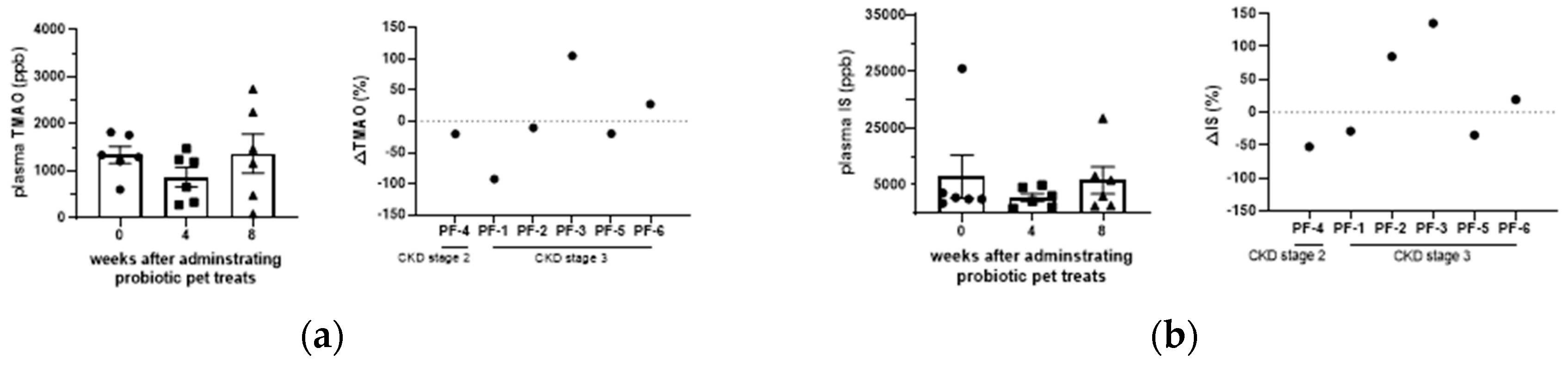
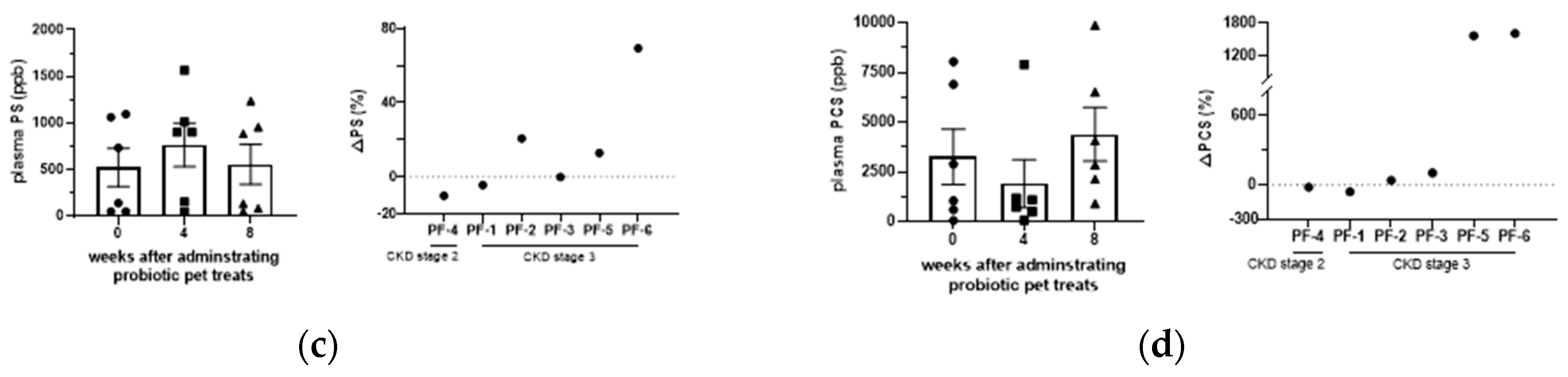
3.3. Effect of GDUTs in serum after the administration of Lm treats.
Although serum TMAO, IS, PCS, and PS were not significantly different between groups (
P > 0.05), GDUTs were further evaluated based on CIs (90%) to assess the treatment effect. The CIs in the present study suggest potential clinical significance in TMAO, IS, and PCS (
Table 2) after 4 weeks of Lm treatment. A comparison of the percentage changes in individual cats, 66%, 50%, and 50% of the CKD cats decreased or maintained their serum levels of TMAO, IS, and PCS, respectively, after 8 weeks of Lm treat treatment (
Figure 4).
3.4. Lm pet treats modified fecal microbiota of CKD cats.
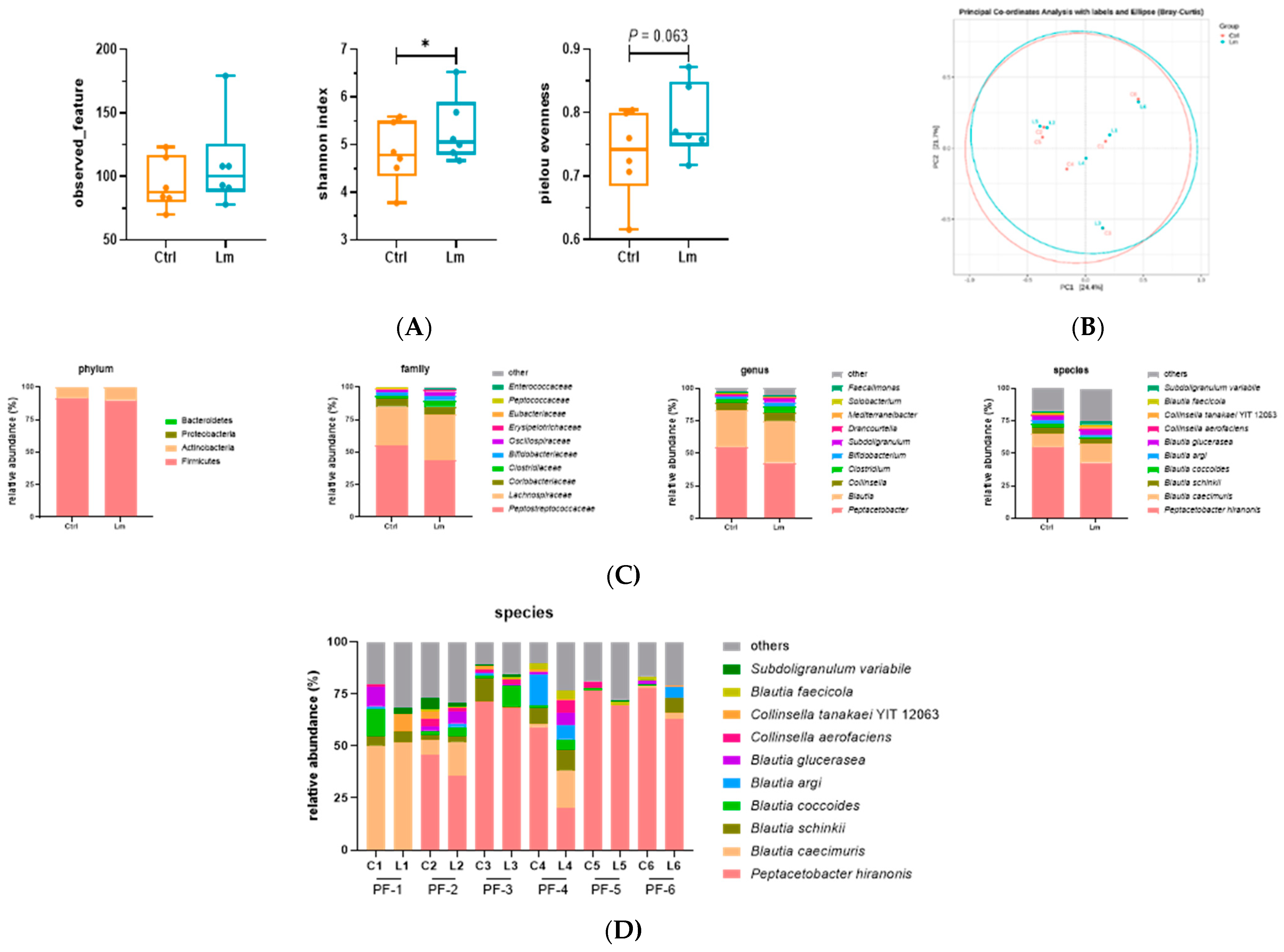
16S full-length sequencing was applied to explore the link between Lm treatment and the gut microbiota composition, showing that the Shannon index and alpha diversity index significantly increased after the Lm treatment (
P < 0.05), while the Pielou evenness increased (
P = 0.06) (
Figure 5A). Conversely, beta diversity in the PCoA plot showed only a slight shift, indicating 24.4% and 21.7% of the total gut microbiota composition in PC1 and PC2, respectively (
Figure 5B). These data suggested that Lm pet treats upregulated the richness of microbial species and enhanced evenness among these species. It also maintained the core gut microbiome composition in felines.
Regarding the gut microbial configuration before and after administrating Lm treats, there were four dominant phyla including Firmicutes (~90%), Actinobacteria (~10%), Proteobacteria, and Bacteroidetes (
Figure 5C), and ten prominent families, with
Peptostreptococcaceae being the most dominant family (from 55.0% to 43.5%), followed by
Lachnospiraceae (from 30.0% to 35.0%),
Coriobacteriaceae (from 5.8% to 6.62%),
Clostridiaceae (from 2.4% to 4.6%),
Bifidobacteriaceae (from 2.6% to 3.0%),
Oscillospiraceae (from 1.7% to 3.7%),
Erysipelotrichaceae (from 0.7% to 1.2%),
Eubacteriaceae (from 0.7% to 0.3%),
Pepcococcaceae (from 0.6% to 0.2%), and
Enterococaceae (from 0.0% to 0.5%).
Peptacetobacter (from 55.0% to 42.7%) was the most abundant at the genus level, followed by
Blautia (from 28.2% to 32.3%),
Collinsella (from 5.7% to 6.6%),
Clostridium (from 2.4% to 4.6%),
Bifidobacterium (from 2.6% to 3.0%),
Subdoligranulum (from 0.9% to 2.1%),
Drancourtella (from 0.5% to 1.1%),
Mediterraneibacter (from 0.7% to 0.9%),
Solobacterium (from 0.5% to 0.8%) and
Faecalimonas (remain 0.5%) (
Figure 5C).
A total of 121 bacterial species were identified in the feline gut microbiome and the top ten were
Peptacetobacter hiranonis (from 55.0% to 42.7%),
Blautia caecimuris (from 10.1% to 14.9%),
Blautia schinkii (from 4.3% to 3.3%),
Blautia coccoides (from 3.1% to 1.6%),
Blautia argi (from 3.0% to 1.3%),
Blautia glucerasea (from 2.5% to 4.2%),
Collinsella aerofaciens (from 1.5% to 1.3%),
Collinsella tanakaei (from 1.1% to 1.7%),
Blautia faecicola (from 1.0% to 2.3%), and
Subdoligranulum variabile (from 0.9% to 2.1%) (
Figure 5C).
There were also variations in the individual fecal microbiomes at the species level (
Figure 5D), for example, the PF-1 cat had a high proportion of
Blautia, while the other 5 cats had more
P. hiranonis. However, they showed a similar change in fecal microbiota after treatment with Lm pet treats (
Figure 6A).
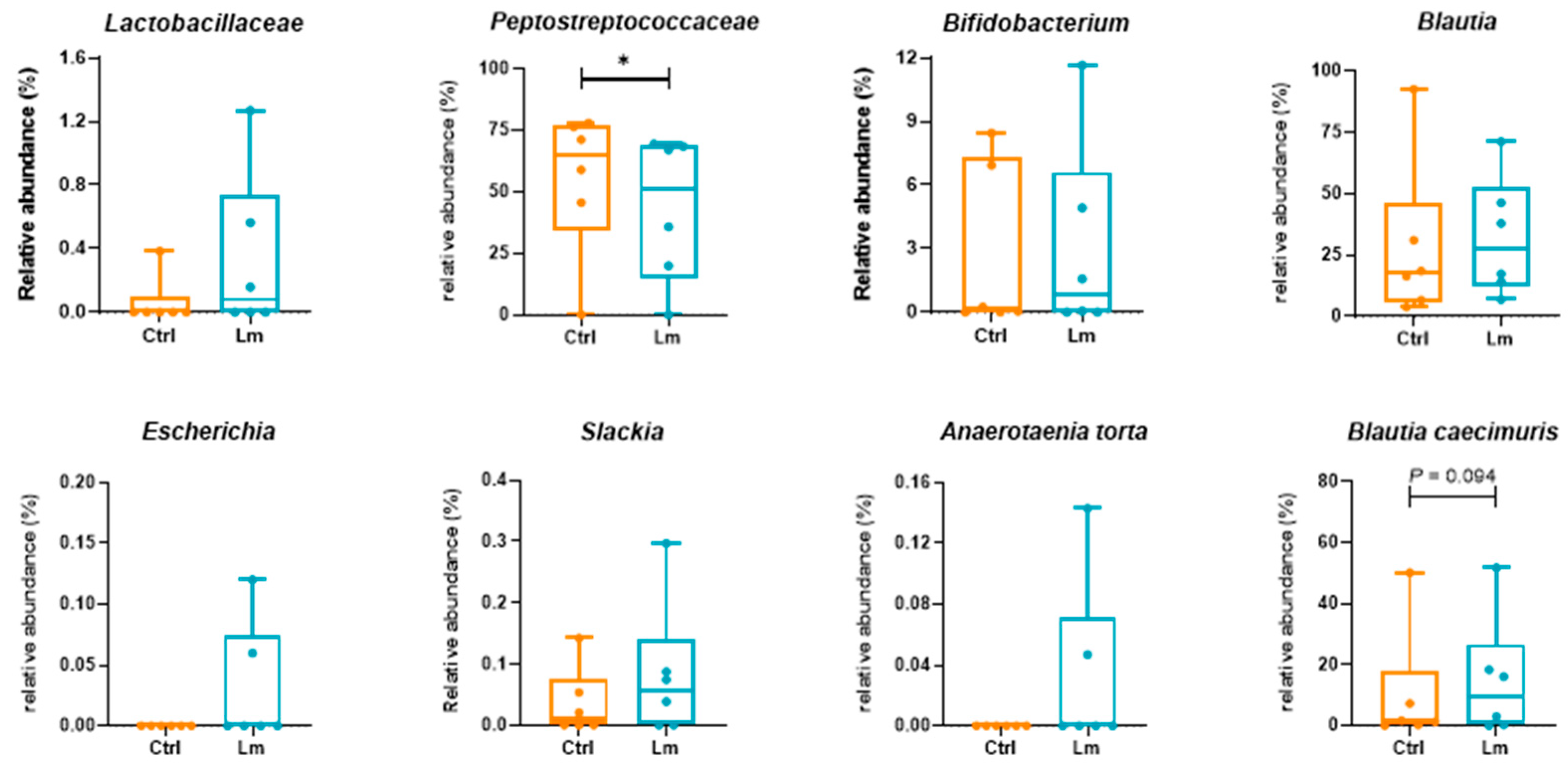
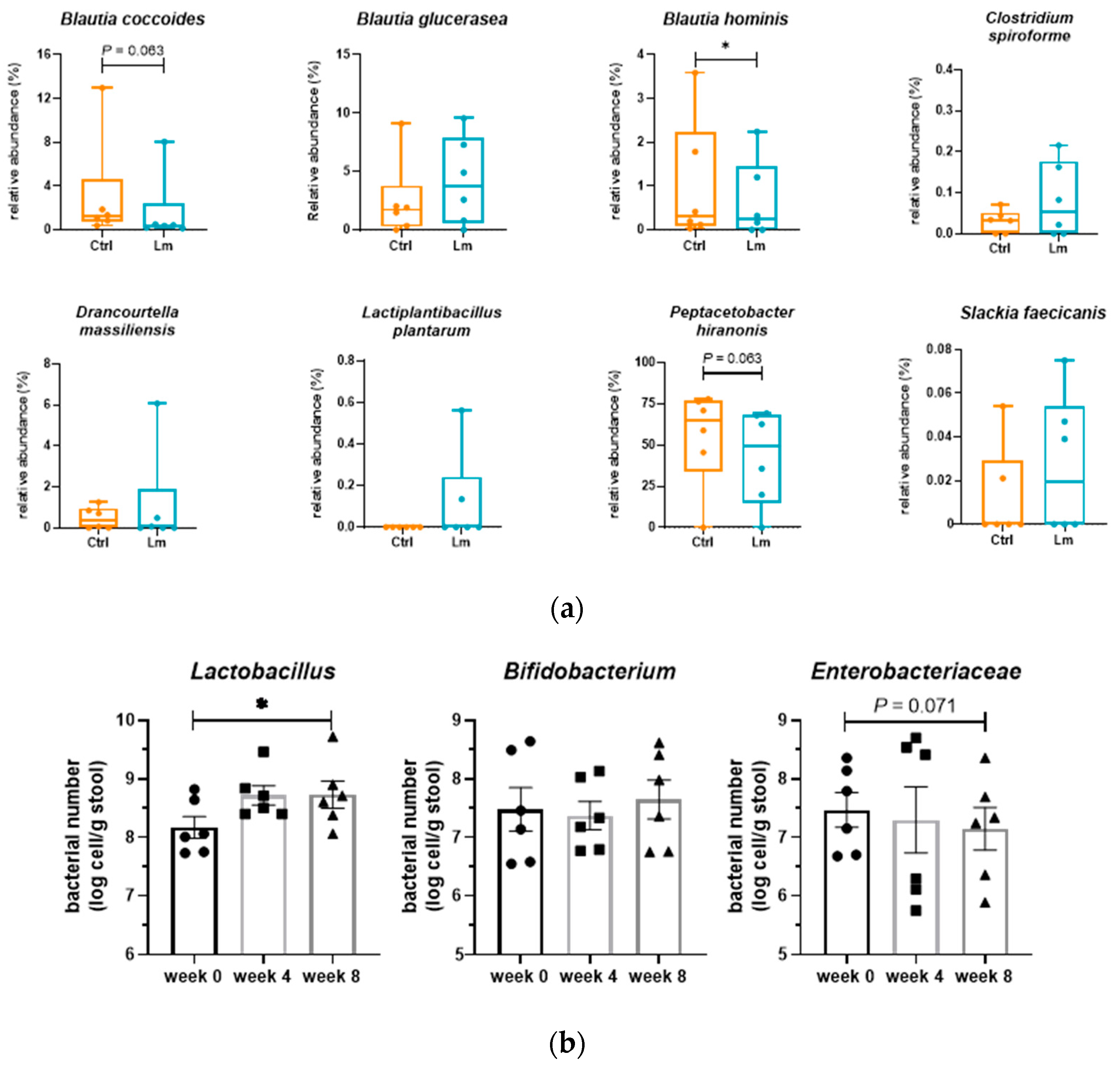
Peptostreptococcaeae significantly reduced (
P < 0.05), while
Lactobacillaceae and
Bifidobacterium increased from week 0 to week 8. At the species level,
Blautia hominis (
P < 0.05),
B. coccoides (
P = 0.063), and
P. hiranonis (
P = 0.063) reduced but genus
Blautia increased after the trial. Besides, one of the bacteria strains (
L. plantarum) of Lm strains was detected in two cats after the clinical trial.
Bacteria related to CKD in fecal microbiota were quantified by qPCR (
Figure 6B) showing that
Lactobacillus and
Enterobacteriaceae were significantly upregulated (
P < 0.05) and downregulated (
P = 0.071) after 8 weeks of Lm pet treats administration, respectively.
3.5. Lm pet treats altered the gut microbial function.
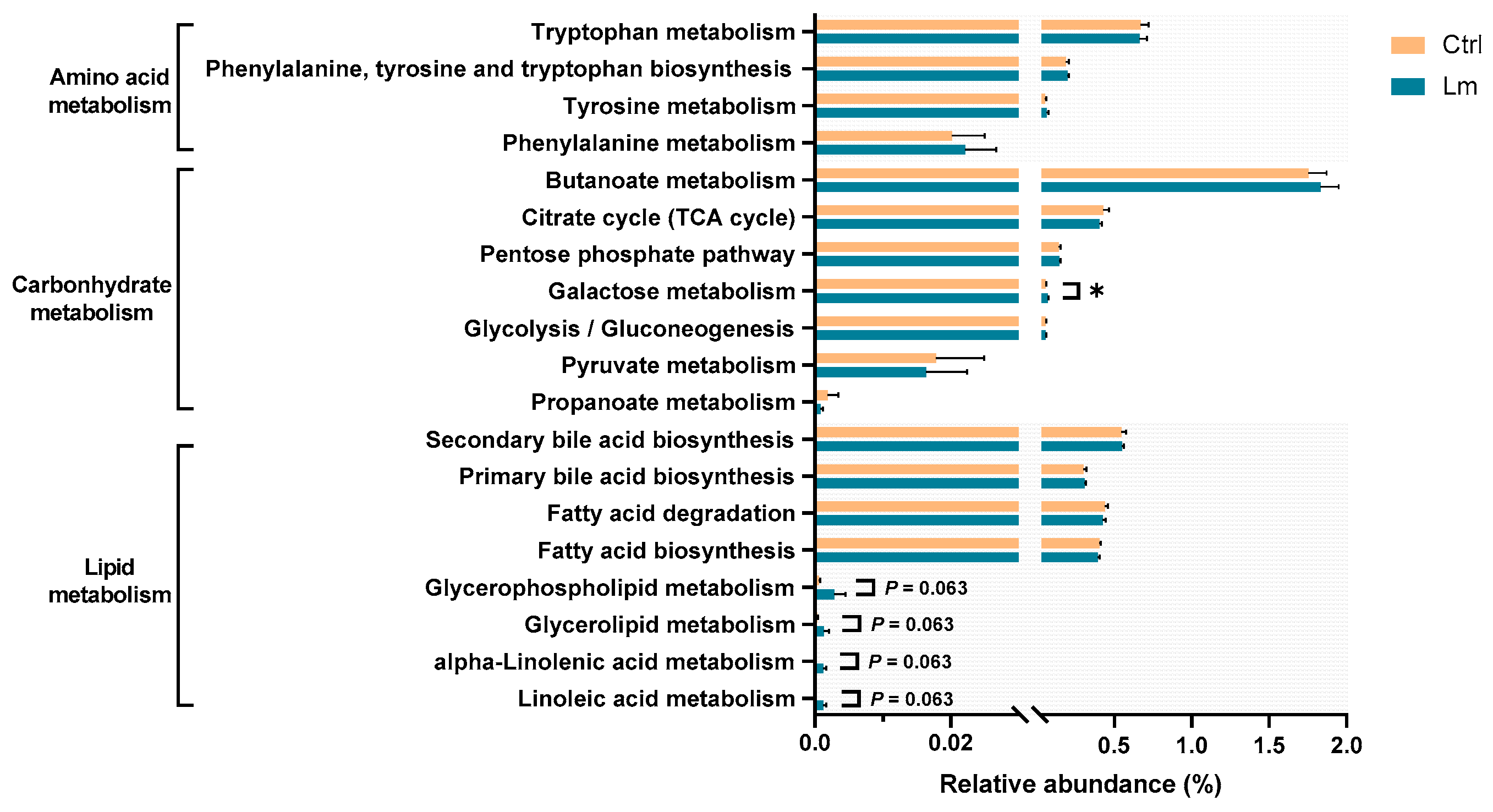
Genetic functional prediction was performed (
Figure 7) revealing that the pathways related to producing uremic toxins, including “Tyrosine metabolism (ko00350),” “Phenylalanine metabolism (ko00360),” “Tryptophan metabolism (ko00380),” and “Phenylalanine, tyrosine and tryptophan biosynthesis (ko00400),” were not different after Lm treatment, whereas the carbohydrate-related pathway, “Galactose metabolism (ko00052)” was significantly higher (
P < 0.05). After the clinical trial, the lipid metabolism pathways “Glycerolipid metabolism (ko00561),” “Glycerophospholipid metabolism (ko00564),” “Linoleic acid metabolism (ko00591),” and “Alpha-linolenic acid metabolism (ko00592)” were upregulated (
P < 0.1).
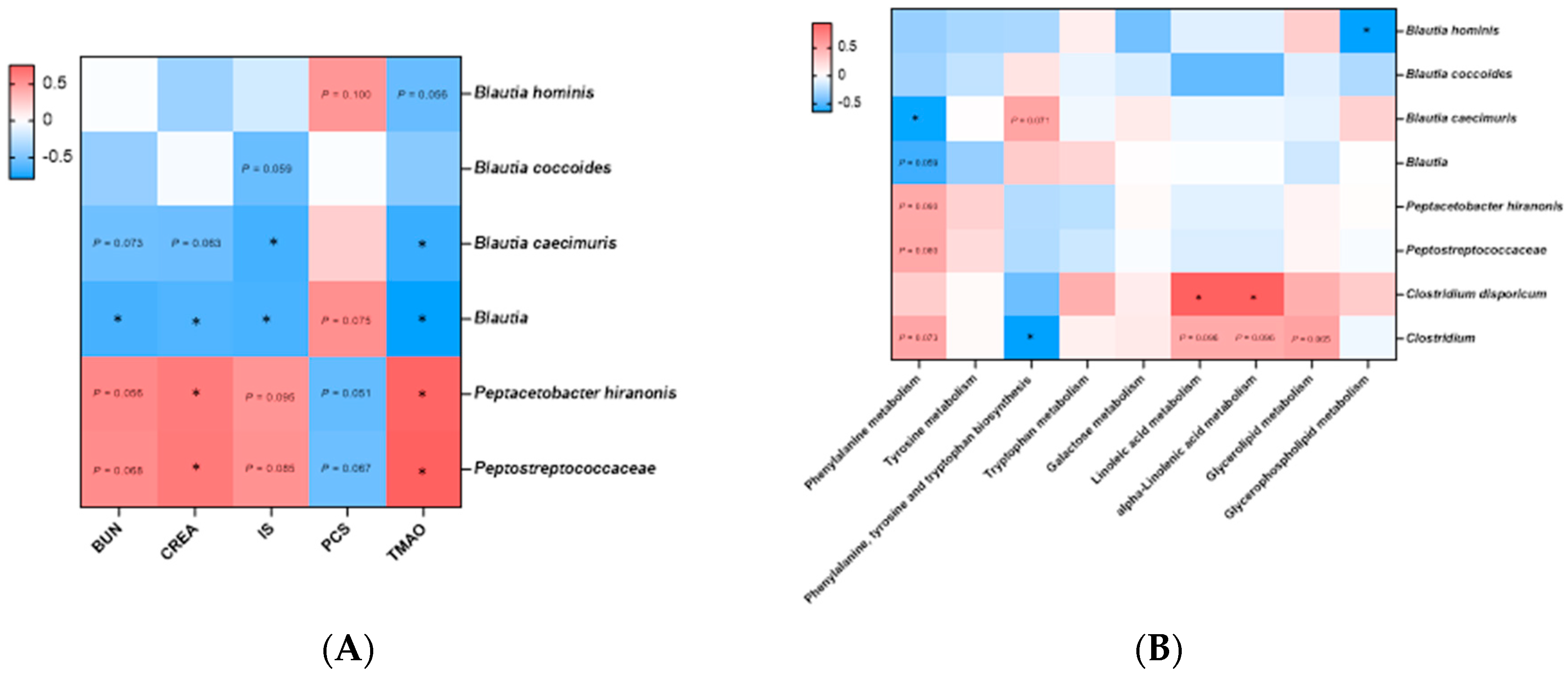
A Spearman’s correlation network was first constructed between eight bacterial species and 5 CKD risk factors that were significantly different before and after administration of the Lm treats (
Figure 8A). All bacteria belonging to the genus
Blautia were negatively correlated with BUN and creatinine, particularly genus
Blautia (
P < 0.05). In uremic toxins analyses,
B. caecimuris and
Blautia were significantly negatively correlated with IS and TMAO (
P < 0.05), whereas
Peptostreptococcaceae and
P. hiranonis demonstrated a significant positive correlation with kidney function indicators and uremic toxins, especially creatinine and TMAO (
P < 0.05). The correlation network between bacterial species and KEGG pathways revealed 2 and 3 positive and negative relationships with KEGG level 3 pathways, respectively (
P < 0.05,
Figure 8B).
B. caecimuris and
Clostridium were negatively correlated with phenylalanine metabolism and phenylalanine, tyrosine and tryptophan biosynthesis pathways, respectively (
Figure 8B).
4. Discussion
In the present study, a novel low-temperature oil-spreading approach was developed to produce pet food coated with Lactobacillus mix (Lm, L. plantarum subsp. plantarum MFM 30−3 and L. paracasei subsp. paracasei MFM 18). The produced Lm pet treats were chemically and bacterially stable with a 4-month shelf life at room temperature. Pet food production designed to ensure food safety and to extend shelf-life negatively impacts the survival of probiotics [24]. Additional verification of the potential health benefits is crucial to ensure the efficacy of probiotics in pet foods. We demonstrated the CKD alleviatory effect of Lm probiotic treats in an open-label, single-arm pilot study of cats with stage 2–3 CKD. Downregulation of harmful GDUTs and CKD serum indicators (CRE and BUN) with improved life quality (appetite, activity, and defecation frequency) of CKD cats were observed after two months of the Lm pet treats intervention. This is in line with our previous studies using an adenine-induced CKD mouse model [20] and CKD patient clinical trial [21]. This finding provides evidence that Lm probiotics could be applied with various matrices without negatively impacting their health benefits. However, the study sample size was restricted by the willingness of cat owners to take part, the palatability of the pet treats, and rigorous candidate screening. The different diets could also cause inconsistent changes in microbiota composition, so reaching statistical significance might be difficult. Statistical significance indicates the reliability of the study results, which is dependent on the study's sample size [29], whereas, clinical significance reflects its impact on clinical practice, which emphasizes the estimated effect size and its precision (such as confidence interval) [30]. To evaluate the actual treatment effect of Lm pet treats on CKD cats, the clinical significance was interpreted in this study results with 90% confidence intervals and P < 0.1 [26,27].
Intensive studies have shown numerous outcomes to disclose the physiological functions of probiotics in human patients with CKD, including reduced uremic toxins and related precursors, modulation of gut microbiota, regulation of immune capacity, protection of the gastrointestinal tract, and improved gastrointestinal symptoms [31–35], but few studies have evaluated the effect of probiotics and probiotic pet food in feline CKD. Serum creatinine is one of the main evaluation indicators because it is the major parameter to calculate the estimated Glomerular filtration rate (eGFR) and to ensure the stability of renal function [36]. Maintenance or reduction of serum creatinine levels in all of the tested cats after the administration of Lm pet treats suggests an alleviating effect on CKD feline. A previous clinical study with CKD stage 2–4 cats showed that the BUN and creatinine levels of most cats decreased after intaking synbiotics for two months [18], but this effect was not observed in the CKD counterparts with prebiotics mixed with or sprinkled onto the cat food.
The improved life quality of CKD cats, including appetite, activity, and defecation frequency, was also observed after two months of Lm treatment. Cats and patients with CKD have an increased risk of constipation, which would further impact their life quality [8,37,38]. Constipation may lead to enhancing fermentation of unmetabolized amino acids and peptides in the colon, resulting in the generation and absorption of more uremic toxins precursors [9]. Thus, a higher frequency of defecation and moister and softer feces would promote body waste discharge rather than accumulation. Therefore, Lm pet treats could be a novel way to supplement probiotics and yield positive effects on CKD cats’ life quality and kidney function.
Gut dysbiosis in patients with CKD contributes to deteriorating CKD progression [10,39–41]. The feline fecal bacteria diversity and abundance were restored with the Lm pet treats intervention. Most bacterial taxa that showed lower abundances after the Lm treatment belonged to the proteolytic families (Peptostreptococcaceae and Enterobacteriaceae). Peptostreptococcaceae and Enterobacteriaceae, which possess GDUT precursor-producing enzymes contributing to indole, phenol, and TMAO production in humans, were more abundant in CKD patients than in healthy participants [42–44]. The correlation analysis is consistent with the findings of the feline gut microbiome. Peptostreptococcaceae was positively correlated to kidney function indicators and uremic toxins. The beneficial bacteria, Lactobacillus, in feline feces was elevated after administrating Lm pet treats. Lactobacillaceae (especially Lactobacillus), which are butyric acid-producing bacteria, are important for intestinal homeostasis [45–47]. Additionally, after consuming probiotic pet treats for 8 weeks, L. plantarum was detected in the feces of 2 tested cats, indicating that Lm could be preserved in the intestine. The abundance of Lm strains may affect its efficacy in alleviating kidney function, but additional study is necessary to recognize the factors impacting probiotic colonization.
Interestingly, the Blautia species demonstrated different changes in abundance in CKD cats after administrating Lm pet treats, but they were all negatively correlated with renal function indicators and GDUT. The less abundant genus Blautia has been reported in the gut microbiota of CKD patients and chronic renal failure (CRF) rats [43,48], and it is also negatively correlated with plasma uremic toxins [49]. However, Blautia was rich in patients with CKD stage 5 [43], suggesting that different strains of Blautia might cause different effects. It is also worth noting the effect of diet on gut microbiota, with the higher abundance of Blautia and Peptostreptococcaceae in the feline fecal microbiota associated with consuming kibbled meals and canned food, respectively [42]. This might explain why there was a difference in gut microbial composition between PF-1 and the other cats.
Alteration of gut microbial composition was associated with significant changes in KEGG microbial functions, with the phenylalanine pathway being most related to the CKD alleviatory effect of Lm pet treats. Abnormal phenylalanine metabolism has been detected in patients with diabetic kidney disease [50]. However, few studies have exposed the effect of probiotics on downstream microbial functions in patients and cats with CKD. The analyses of feline gut microbiota, KEGG microbial functions, and renal function indicators clarified the possible downstream mechanisms of CKD alleviating effects of Lm pet treats involving the downstream functional phenylalanine pathway.
5. Conclusions
Lm pet treats provide an alternative and effective adjuvant treatment for alleviating CKD progression with life quality improvement of CKD cats. Administration of Lm pet treats modulated feline microbiota (Peptostreptococcaceae, Lactobacillus, Blautia, and Enterobacteriaceae), further regulating microbial functions involved in phenylalanine metabolism, contributing to downregulating deleterious IS, PCS, and TMAO. The abundance of Lm strains may influence their efficacy in improving gut-derived metabolites and kidney function. Although large-scale prospective longitudinal clinical studies are needed to confirm this finding, the current study provides potential adjuvant therapeutic insights into probiotic pet foods or treats for pets with CKD. To the best of our knowledge, this study is the pioneer to directly evaluate CKD alleviating efficacy in pet treats.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1. Model parameters of multiple reaction monitoring of the target; Table S2. qPCR primers of different microorganisms; Table S3. Harmful residue and pathogenic bacteria analyses of probiotic pet treats; Table S4. Chemical stability analysis of probiotic pet treats; Table S5. Weight changes in CKD cats; Table S6. Serum and urinary biochemical parameters of CKD cats during the whole trial.
Author Contributions
Conceptualization, Min-Ju Chen and Ya-Jane Lee; methodology, Min-Ju Chen, Ya-Jane Lee, and Hsiao-Wen Huang; software, Hsiao-Wen Huang; validation, Ching-Wen Tsai and Hsiao-Wen Huang; formal analysis, Ching-Wen Tsai and Hsiao-Wen Huang; investigation, Ching-Wen Tsai and Hsiao-Wen Huang; resources, Tsu-Cheng Hsu; data curation, Ching-Wen Tsai; writing—original draft preparation, Ching-Wen Tsai; writing—review and editing, Min-Ju Chen; visualization, Ching-Wen Tsai; supervision, Min-Ju Chen and Ya-Jane Lee; project administration, Ching-Wen Tsai and Hsiao-Wen Huang; funding acquisition, Min-Ju Chen. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by Council of Agriculture of Taiwan, Executive Yuan, Republic of China (110AS-1.6.1-AD-U1).
Institutional Review Board Statement
The animal study was approved by the Institutional Animal Care and Use Committee of National Taiwan University (IACUC approval no: NTU-110-EL-00042).
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its
Supplementary materials. Raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank the Department of Chemistry, National Taiwan University for assisting with uremic toxins measurements, and BIOTOOLS Co., Ltd, in Taiwan for supporting NGS analysis.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ASVs, amplicon sequence variants; BUN, blood urea nitrogen; CA, chicken and fish; CB, fish and mutton; CC, chicken; CKD, chronic kidney disease; CRF, chronic renal failure; ESRD, end-stage renal disease; IACUC, Institutional Animal Care and Use Committee of National Taiwan University; IS, Indoxyl sulfate; KEGG, Kyoto Encyclopedia of Genes and Genomes; Lm, Lactobacillus mixture; MFM, Mongolian fermented milk; PCoA, principal coordinate analysis; PCS, p-cresyl sulfate; PICRUSt, Phylogenetic Investigation of Communities by Reconstruction of Unobserved States; POV, peroxide value; qPCR, real-time polymerase chain reaction; SCFA, short-chain fatty acid; TMAO, trimethylamine N-oxide; UPC, urine protein/urine creatinine ratio
References
- Lu, P.-H.; Yu, M.-C.; Wei, M.-J.; Kuo, K.-L. The Therapeutic strategies for uremic toxins control in chronic kidney disease. Toxins. 2021, 13, 573. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M. H.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P. J.; Cozzolino, M.; Juillard, L.; Kashani, K.; Kaushik, M.; Kawanishi, H.; Massy, Z.; Sirich, T. L.; Zuo, L.; Ronco, C. Classification of uremic toxins and their role in kidney failure. Clin. J. Am. Soc. Nephrol. 2021, 16, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Roura, X. Risk factors in dogs and cats for development of chronic kidney disease. Available online: https://www.iris-kidney.com/education/education/risk_factors.html (accessed on June 2023).
- Chen, H.; Dunaevich, A.; Apfelbaum, N.; Kuzi, S.; Mazaki-Tovi, M.; Aroch, I.; Segev, G. Acute on chronic kidney disease in cats: etiology, clinical and clinicopathologic findings, prognostic markers, and outcome. J. Vet. Intern. Med. 2020, 34, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Polzin, D. J. Chronic kidney disease in small animals. Vet. Clin. North Am. Small Anim. Pract. 2011, 41, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Bartges, J.W. Chronic kidney disease in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 2012, 42, 669–vi. [Google Scholar] [CrossRef] [PubMed]
- Parker, V. J. Nutritional management for dogs and cats with chronic kidney disease. Vet. Clin. North Am. Small Anim. Pract. 2021, 51, 685–710. [Google Scholar] [CrossRef]
- Jones, S. E.; Quimby, J. M.; Summers, S. C.; Adams, S. M.; Caney, S. M.; Rudinsky, A. J. Survey of defecation habits in apparently healthy and chronic kidney disease cats. J. Feline Med. Surg. 2022, 24, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Meijers, B. K.; Bammens, B. R.; Verbeke, K. Uremic toxins originating from colonic microbial metabolism. Kidney Int. 2009, 76, S12–S19. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Mafra, D.; Fouque, D. Probiotics and chronic kidney disease. Kidney Int. 2015, 88, 958–966. [Google Scholar] [CrossRef]
- Lim, Y. J.; Sidor, N. A.; Tonial, N. C.; Che, A.; Urquhart, B. L. Uremic toxins in the progression of chronic kidney disease and cardiovascular disease: mechanisms and therapeutic targets. Toxins. 2021, 13, 142. [Google Scholar] [CrossRef]
- Subramaniam, S.; Fletcher, C. Trimethylamine N-oxide: breathe new life. Br. J. Pharmacol. 2018, 175, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Carlström, M.; Moretti, C. H.; Weitzberg, E.; Lundberg, J. O. Microbiota, diet and the generation of reactive nitrogen compounds. Free Radical Biol. Med. 2020, 161, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Wu, I. W.; Hsu, K. H.; Lee, C. C.; Sun, C. Y.; Hsu, H. J.; Tsai, C. J.; Tzen, C. Y.; Wang, Y. C.; Lin, C. Y.; Wu, M. S. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol., Dial., Transplant. 2011, 26, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, C. J.; McWhinney, B. C.; Sipinkoski, B.; Johnson, L. A.; Rossi, M.; Campbell, K. L.; Ungerer, J. P. Reference ranges and biological variation of free and total serum indoxyl- and p-cresyl sulphate measured with a rapid UPLC fluorescence detection method. Clin. Chim. Acta. 2013, 419, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Yoshifuji, A.; Wakino, S.; Irie, J.; Tajima, T.; Hasegawa, K.; Kanda, T.; Tokuyama, H.; Hayashi, K.; Itoh, H. Gut Lactobacillus protects against the progression of renal damage by modulating the gut environment in rats. Nephrol. Dial. Transplant. 2016, 31, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Li, K.-Y.; Wang, P. J.; Huang, H. W.; Chen, M. J. Alleviating chronic kidney disease progression through modulating the critical genus of gut microbiota in a cisplatin-induced Lanyu pig model. J. Food Drug Anal. 2020, 28, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, R. A preliminary clinical evaluation of Kibow Biotics,® a probiotic agent, on feline azotemia. J. Am. Holistic Vet. Med. Assoc. 2006, 24, 23–27. [Google Scholar]
- Lippi, I.; Perondi, F.; Ceccherini, G.; Marchetti, V.; Guidi, G. Effects of probiotic VSL#3 on glomerular filtration rate in dogs affected by chronic kidney disease: a pilot study. Can. Vet. J. 2017, 58, 1301–1305.2. [Google Scholar]
- Huang, H.; Li, K.; Lee, Y.; Chen, M. Preventive effects of Lactobacillus mixture against chronic kidney disease progression through enhancement of beneficial bacteria and downregulation of gut-derived uremic toxins. J. Agric. Food Chem. 2021, 69, 7353–7366. [Google Scholar] [CrossRef]
- Chan, W. N.; Ho, D. R.; Huang, Y. C.; Lin, J. H.; Liu, Y. L.; Chen, M. J.; Chen, C. S. A pilot study of nephrogenic probiotics to further improve an already stabilized graft function after kidney transplantation. Transplant. Proc. 2023, 55, 2090–2094. [Google Scholar] [CrossRef]
- Rishniw, M.; Wynn, S. G. Azodyl, a synbiotic, fails to alter azotemia in cats with chronic kidney disease when sprinkled onto food. J. Feline Med. Surg. 2011, 13, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.J.; Huang, Y.Y.; Lo, S.H.; Hsu, T.F.; Huang, W.Y.; Huang, S.L.; Lin, Y.S. Effects of pH on the shape of alginate particles and its release behavior. Int. J. Polym. Sci. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Acuff, H.; G. Aldrich, C. A Review of application strategies and efficacy of probiotics in pet food. IntechOpen 2023. [CrossRef]
- International Renal Interest Society. IRIS staging of CKD (modified 2023). Available online: http://www.iris-kidney.com/pdf/2_IRIS_Staging_of_CKD_2023.pdf (accessed on 28 February 2023).
- Ranganathan, P.; Pramesh, C. S.; Buyse, M. Common pitfalls in statistical analysis: clinical versus statistical significance. Perspect. Clin. Res. 2015, 6, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M. R.; Wykoff, C. C.; Thabane, L.; Bhandari, M.; Chaudhary, V.; Retina Evidence Trials InterNational Alliance (R.E.T.I.N.A.) Study Group. The clinician's guide to p values, confidence intervals, and magnitude of effects. Eye (London, U. K.) 2022. 36, 341–342. [CrossRef]
- Nair, A.; Morsy, M. A.; Jacob, S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev. Res. 2018, 79, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Moore, M. J.; Goldstein, D.; Hamm, J.; Figer, A.; Hecht, J. R.; Gallinger, S.; Au, H. J.; Murawa, P.; Walde, D.; Wolff, R. A.; Campos, D.; Lim, R.; Ding, K.; Clark, G.; Voskoglou-Nomikos, T.; Ptasynski, M.; Parulekar, W.; National Cancer Institute of Canada Clinical Trials Group. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007, 25, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K. F.; Altman, D. G.; Moher, D.; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Cosola, C.; Rocchetti, M. T.; di Bari, I.; Acquaviva, P. M.; Maranzano, V.; Corciulo, S.; Di Ciaula, A.; Di Palo, D. M.; La Forgia, F. M.; Fontana, S.; De Angelis, M.; Portincasa, P.; Gesualdo, L. An innovative synbiotic formulation decreases free serum indoxyl sulfate, small intestine permeability and ameliorates gastrointestinal symptoms in a randomized pilot trial in stage IIIb-IV CKD patients. Toxins 2021, 13, 334. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, H.; Chen, L.; Liang, S. S.; Shi, K.; Meng, W.; Xue, J.; He, Q.; Jiang, H. Effect of probiotics on the intestinal microbiota of hemodialysis patients: a randomized trial. Eur. J. Nutr. 2020, 59, 3755–3766. [Google Scholar] [CrossRef]
- Mitrović, M.; Stanković-Popović, V.; Tolinački, M.; Golić, N.; Soković Bajić, S.; Veljović, K.; Nastasijević, B.; Soldatović, I.; Svorcan, P.; Dimković, N. The impact of synbiotic treatment on the levels of gut-derived uremic toxins, inflammation, and gut microbiome of chronic kidney disease patients-a randomized trial. J. Renal Nutr. 2023, 33, 278–288. [Google Scholar] [CrossRef]
- Wang, I. K.; Yen, T. H.; Hsieh, P. S.; Ho, H. H.; Kuo, Y. W.; Huang, Y. Y.; Kuo, Y. L.; Li, C. Y.; Lin, H. C.; Wang, J. Y. Effect of a probiotic combination in an experimental mouse model and clinical patients with chronic kidney disease: a pilot study. Front. Nutr. 2021, 8, 661794. [Google Scholar] [CrossRef] [PubMed]
- Lim, P. S.; Wang, H. F.; Lee, M. C.; Chiu, L. S.; Wu, M. Y.; Chang, W. C.; Wu, T. K. The Efficacy of Lactobacillus-containing probiotic supplementation in hemodialysis patients: a randomized, double-blind, placebo-controlled trial. J. Renal Nutr. 2021, 31, 189–198. [Google Scholar] [CrossRef]
- Shahbaz H.; Gupta M. Creatinine Clearance. StatPearls [Internet]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK544228/2 (accessed on 13 December 2023).
- Sumida, K.; Molnar, M. Z.; Potukuchi, P. K.; Thomas, F.; Lu, J. L.; Matsushita, K.; Yamagata, K.; Kalantar-Zadeh, K.; Kovesdy, C. P. Constipation and incident CKD. J. Am. Soc. Nephrol. 2017, 28, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Ikee, R.; Sasaki, N.; Yasuda, T.; Fukazawa, S. Chronic kidney disease, gut dysbiosis, and constipation: a burdensome triplet. Microorg. 2020, 8, 1862. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the gastrointestinal tract. Med. Sci. (Basel) 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C. D. Non-alcoholic fatty liver disease: an emerging driving force in chronic kidney disease. Nat. Rev. Nephrol. 2017, 13, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xie, S.; Lv, D.; Wang, P.; He, H.; Zhang, T.; Zhou, Y.; Lin, Q.; Zhou, H.; Jiang, J.; Nie, J.; Hou, F.; Chen, Y. Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci. Rep. 2017, 7, 2870. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, E. N.; Young, W.; Butowski, C. F.; Moon, C. D.; Maclean, P. H.; Rosendale, D.; Cave, N. J.; Thomas, D. G. The fecal microbiota in the domestic cat (Felis catus) is influenced by interactions between age and diet; a five year longitudinal study. Front. Microbiol. 2018, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Su, S.; Li, Y.; Zhu, Z.; Guo, J.; Zhu, Y.; Guo, S.; Qian, D.; Duan, J. Danshen can interact with intestinal bacteria from normal and chronic renal failure rats. Biomed. Pharmacother. 2019, 109, 1758–1771. [Google Scholar] [CrossRef]
- Bäckhed, F. Meat-metabolizing bacteria in atherosclerosis. Nat. Med. 2013, 19, 533–534. [Google Scholar] [CrossRef]
- Wong, J.; Piceno, Y. M.; DeSantis, T. Z.; Pahl, M.; Andersen, G. L.; Vaziri, N. D. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef]
- Sampaio-Maia, B.; Simões-Silva, L.; Pestana, M.; Araujo, R.; Soares-Silva, I. J. The role of the gut microbiome on chronic kidney disease. Adv. Appl. Microbiol. 2016, 96, 65–94. [Google Scholar] [CrossRef] [PubMed]
- Rukavina Mikusic, N. L.; Kouyoumdzian, N. M.; Choi, M. R. Gut microbiota and chronic kidney disease: evidences and mechanisms that mediate a new communication in the gastrointestinal-renal axis. Pfluegers Arch. 2020, 472, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Fan, Y.; Li, A.; Shen, Q.; Wu, J.; Ren, L.; Lu, H.; Ding, S.; Ren, H.; Liu, C.; Liu, W.; Gao, D.; Wu, Z.; Guo, S.; Wu, G.; Liu, Z.; Yu, Z.; Li, L. Alterations of the human gut microbiome in chronic kidney disease. Adv. Sci. 2020, 7, 2001936. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Han, P.; Ma, S.; Peng, R.; Wang, C.; Kong, W.; Cong, L.; Fu, J.; Zhang, Z.; Yu, H.; Wang, Y.; Jiang, J. Abnormal metabolism of gut microbiota reveals the possible molecular mechanism of nephropathy induced by hyperuricemia. Acta Pharm. Sin. B. 2020, 10, 249–261. [Google Scholar] [CrossRef]
- Grabrucker, S.; Marizzoni, M.; Silajdžić, E.; Lopizzo, N.; Mombelli, E.; Nicolas, S.; Dohm-Hansen, S.; Scassellati, C.; Moretti, D. V.; Rosa, M.; Hoffmann, K.; Cryan, J. F.; O’Leary, O. F.; English, J. A.; Lavelle, A.; O’Neill, C.; Thuret, S.; Cattaneo, A.; Nolan, Y. M. Microbiota from Alzheimer’s patients induce deficits in cognition and hippocampal neurogenesis. Brain 2023, 146, 4916–4934. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Miyamoto, Y.; Takada, T.; Matsumoto, K.; Oyaizu, H.; Tanaka, R. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl. Environ. Microbiol. 2002, 68, 5445–5451. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Tsuji, H.; Asahara, T.; Kado, Y.; Nomoto, K. Sensitive quantitative detection of commensal bacteria by rRNA-targeted reverse transcription-PCR. Appl. Environ. Microbiol. 2007, 73, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Rinttilä, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef]
- Kikuchi, E.; Miyamoto, Y.; Narushima, S.; Itoh, K. Design of species-specific primers to identify 13 species of Clostridium harbored in human intestinal tracts. Microbiol. Immunol. 2002, 46, 353–358. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).