Submitted:
11 December 2023
Posted:
22 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
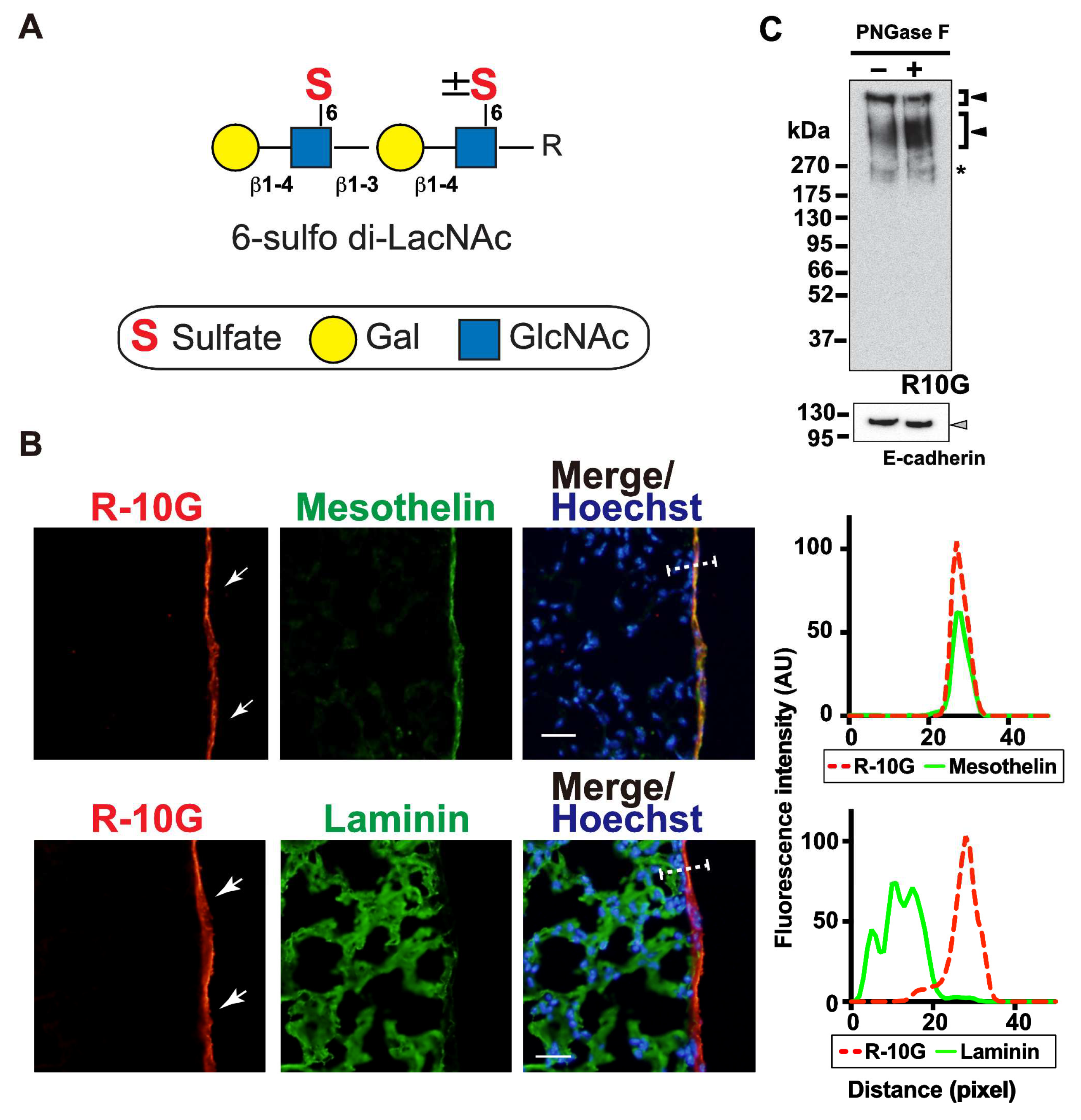
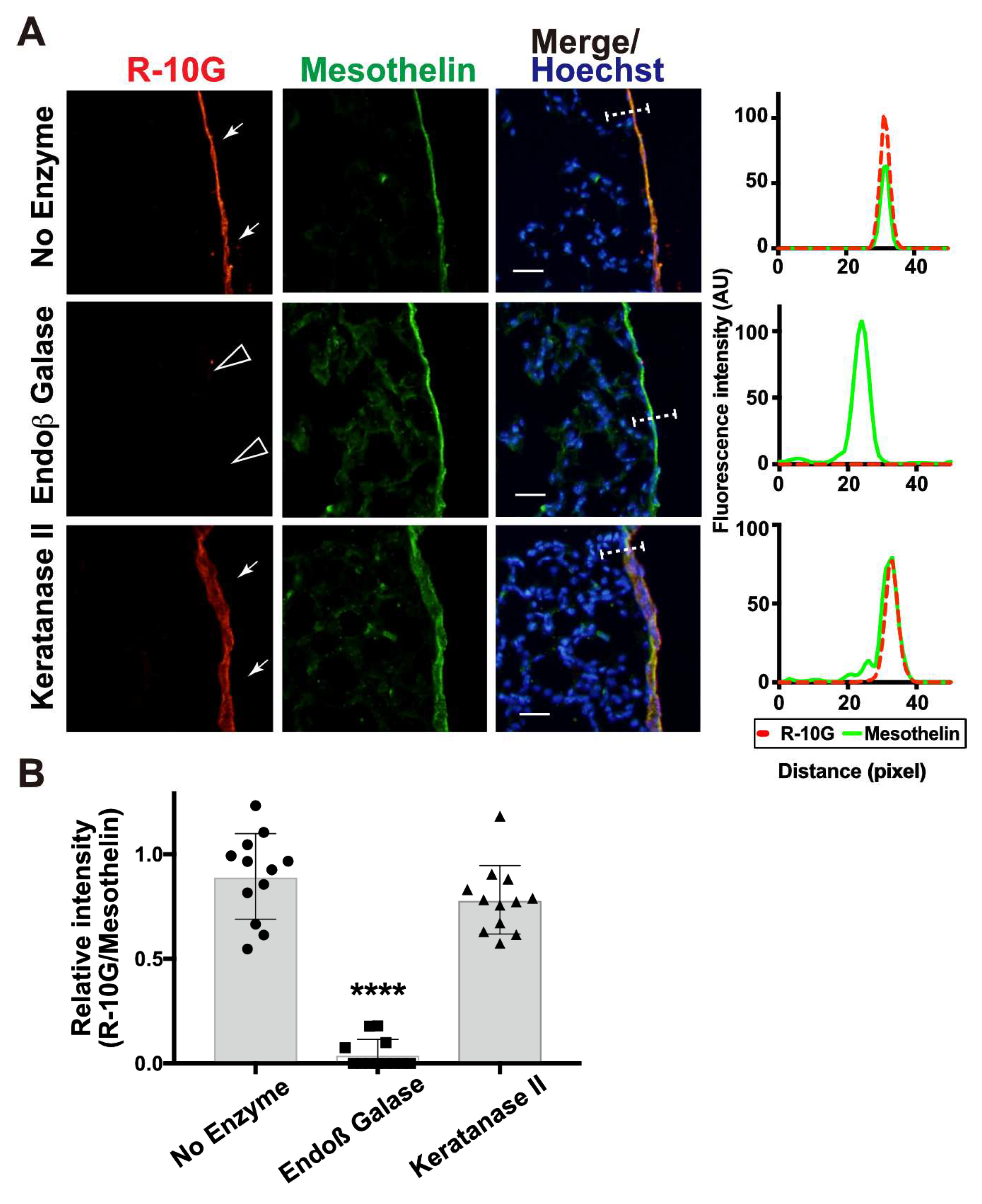
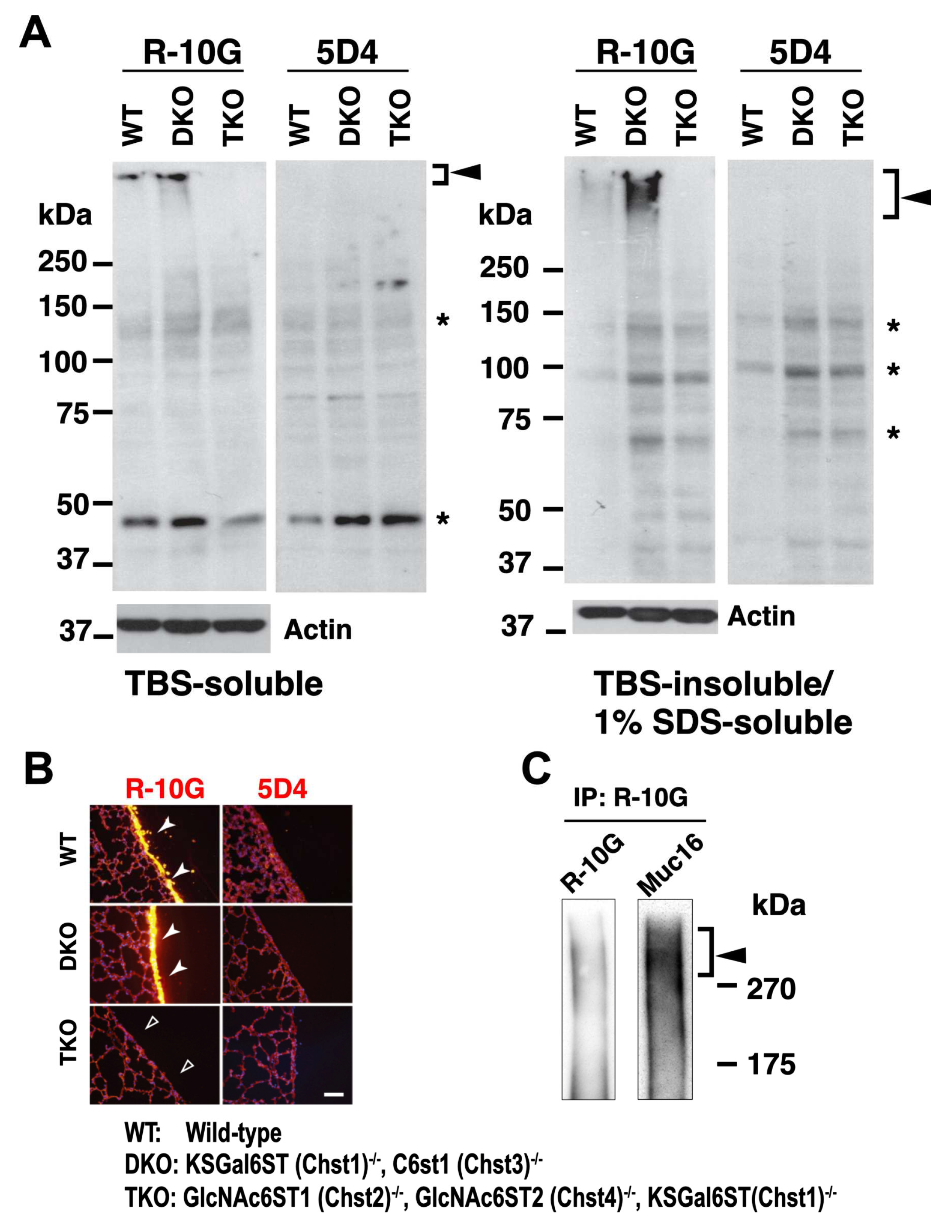
2.1. R10G-reactive sulfated glycans are abundant in the mouse pleural mesothelium
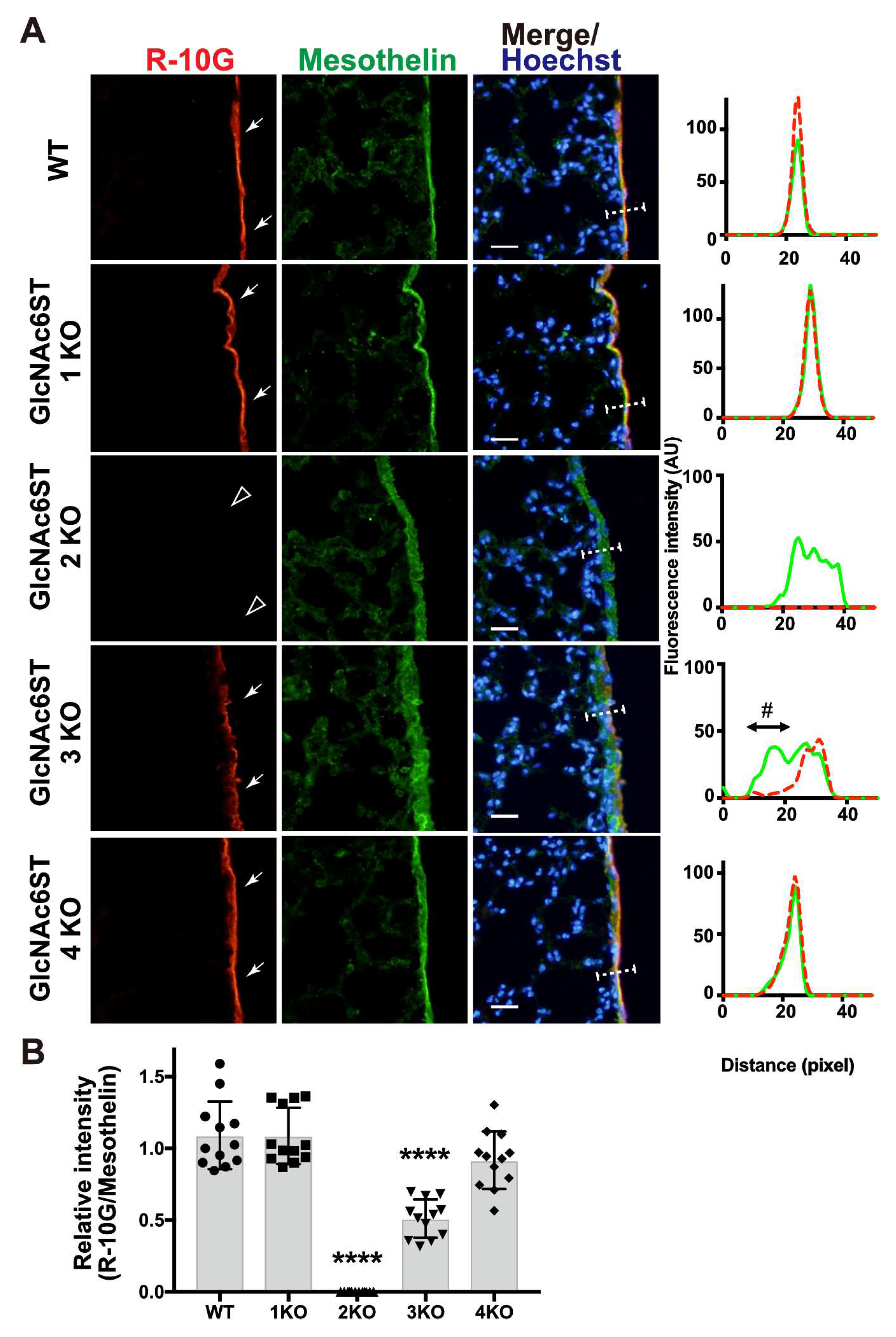
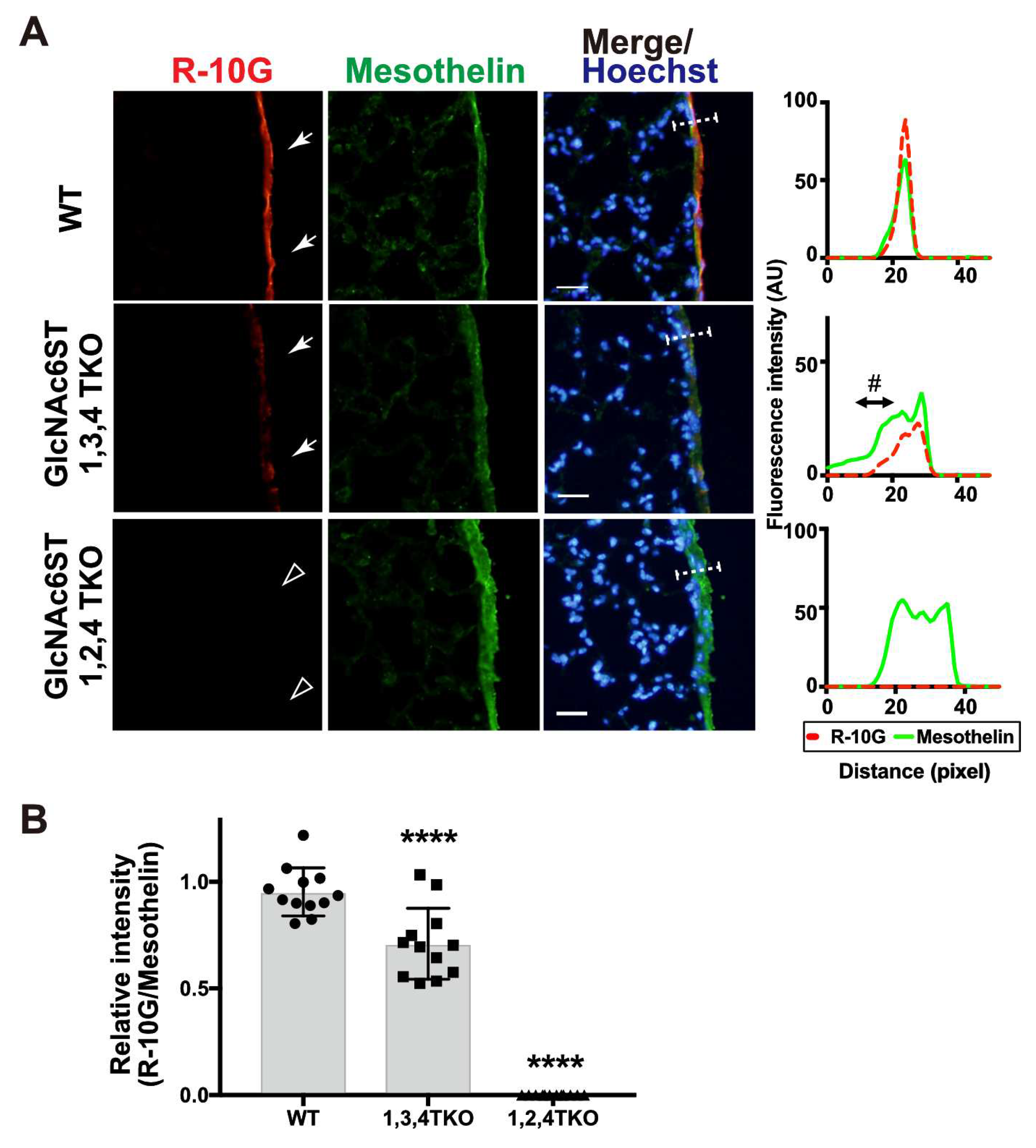
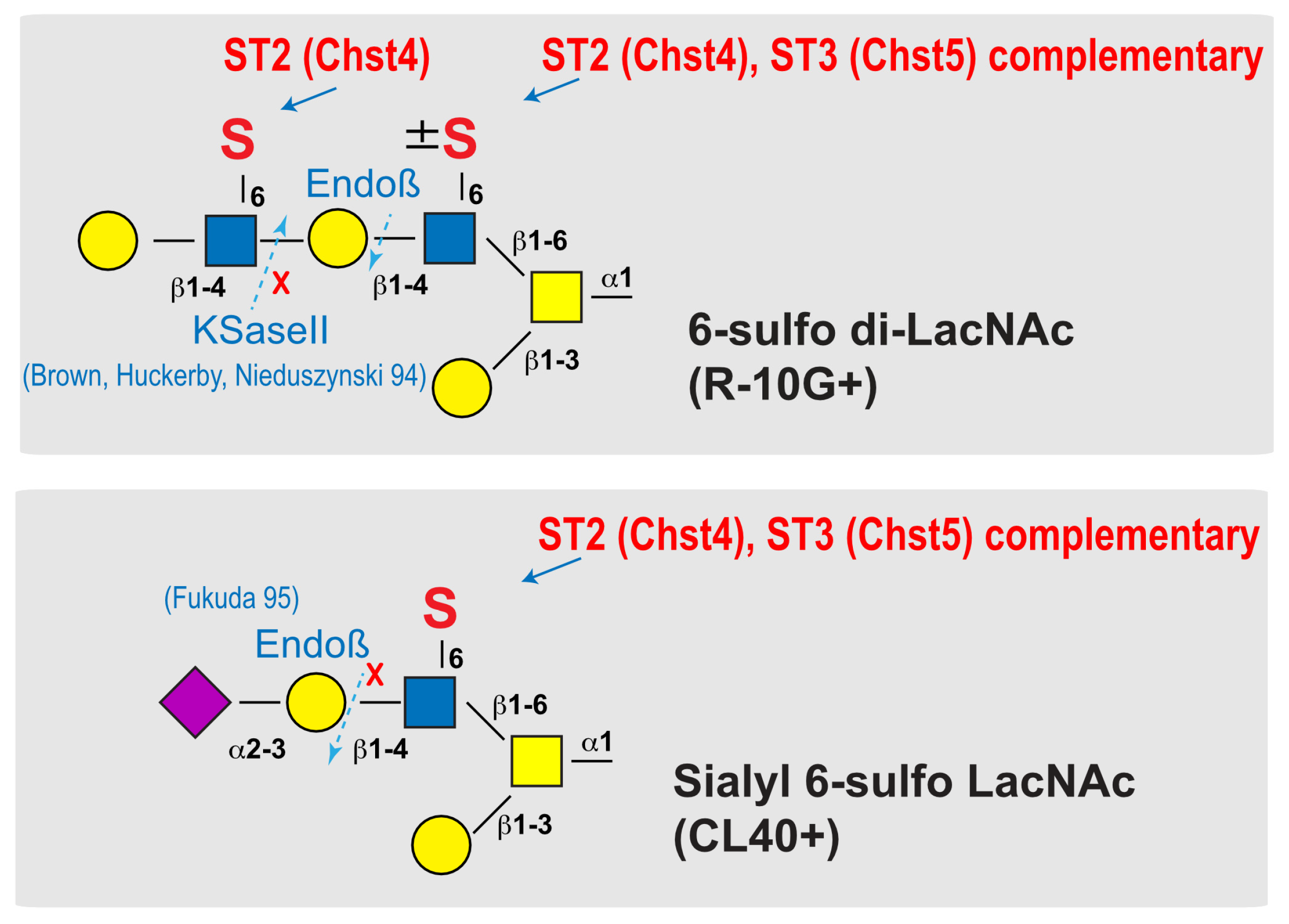
2.2. GlcNAc6ST2 is required and sufficient for the synthesis of R10G-reactive KS glycans in the mouse pleural mesothelium
3. Discussion
4. Materials and Methods
4.1. Antibodies and enzymes
4.2. Mice
4.3. Mouse tissues
4.4. Immunohistochemistry and fluorescence microscopy
4.5. Immunoprecipitation
4.6. WGA bead-bound precipitation
4.7. Immunoblots
4.8. Statistical analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Arata-Kawai, H.; Singer, M.S.; Bistrup, A.; Zante, A.; Wang, Y.Q.; Ito, Y.; Bao, X.; Hemmerich, S.; Fukuda, M.; Rosen, S.D. Functional contributions of N- and O-glycans to L-selectin ligands in murine and human lymphoid organs. Am J Pathol 2011, 178, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Takeda-Uchimura, Y.; Ikezaki, M.; Akama, T.O.; Nishioka, K.; Ihara, Y.; Allain, F.; Nishitsuji, K.; Uchimura, K. Complementary Role of GlcNAc6ST2 and GlcNAc6ST3 in Synthesis of CL40-Reactive Sialylated and Sulfated Glycans in the Mouse Pleural Mesothelium. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Uchimura, K.; Rosen, S.D. Sulfated L-selectin ligands as a therapeutic target in chronic inflammation. Trends Immunol 2006, 27, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Narentuya; Takeda-Uchimura, Y.; Foyez, T.; Zhang, Z.; Akama, T.O.; Yagi, H.; Kato, K.; Komatsu, Y.; Kadomatsu, K.; Uchimura, K. GlcNAc6ST3 is a keratan sulfate sulfotransferase for the protein-tyrosine phosphatase PTPRZ in the adult brain. Sci Rep 2019, 9, 4387. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, K.; Tateyama, D.; Toyoda, H.; Kawasaki, N.; Hashii, N.; Nakao, H.; Matsumoto, S.; Nonaka, M.; Matsumura, H.; Hirose, Y.; et al. A novel antibody for human induced pluripotent stem cells and embryonic stem cells recognizes a type of keratan sulfate lacking oversulfated structures. Glycobiology 2013, 23, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Nakao, H.; Nagai, Y.; Kojima, A.; Toyoda, H.; Kawasaki, N.; Kawasaki, T. Binding specificity of R-10G and TRA-1-60/81, and substrate specificity of keratanase II studied with chemically synthesized oligosaccharides. Glycoconj J 2017, 34, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Silva, L.M.; Liu, Y.; Zhang, Y.; Gao, C.; Zhang, F.; Fu, L.; Peng, Y.; Linhardt, R.; Kawasaki, T.; et al. Glycan Markers of Human Stem Cells Assigned with Beam Search Arrays. Mol Cell Proteomics 2019, 18, 1981–2002. [Google Scholar] [CrossRef] [PubMed]
- Nakao, H.; Yamaguchi, T.; Kawabata, K.; Higashi, K.; Nonaka, M.; Tuiji, M.; Nagai, Y.; Toyoda, H.; Yamaguchi, Y.; Kawasaki, N.; et al. Characterization of novel antibodies that recognize sialylated keratan sulfate and lacto-N-fucopentaose I on human induced pluripotent cells: comparison with existing antibodies. Glycobiology 2023, 33, 150–164. [Google Scholar] [CrossRef]
- Patnode, M.L.; Cheng, C.W.; Chou, C.C.; Singer, M.S.; Elin, M.S.; Uchimura, K.; Crocker, P.R.; Khoo, K.H.; Rosen, S.D. Galactose 6-O-sulfotransferases are not required for the generation of Siglec-F ligands in leukocytes or lung tissue. J Biol Chem 2013, 288, 26533–26545. [Google Scholar] [CrossRef]
- Takeda-Uchimura, Y.; Uchimura, K.; Sugimura, T.; Yanagawa, Y.; Kawasaki, T.; Komatsu, Y.; Kadomatsu, K. Requirement of keratan sulfate proteoglycan phosphacan with a specific sulfation pattern for critical period plasticity in the visual cortex. Exp Neurol 2015, 274, 145–155. [Google Scholar] [CrossRef]
- Fukuda, M.N.; Matsumura, G. Endo-beta-galactosidase of Escherichia freundii. Purification and endoglycosidic action on keratan sulfates, oligosaccharides, and blood group active glycoprotein. J Biol Chem 1976, 251, 6218–6225. [Google Scholar] [CrossRef]
- Fukuda, M.N. Endo-β-Galactosidases and Keratanase. Current Protocols in Molecular Biology 1995, 32, 17.17.16-17.17.13. [Google Scholar] [CrossRef]
- Uchimura, K.; Gauguet, J.M.; Singer, M.S.; Tsay, D.; Kannagi, R.; Muramatsu, T.; von Andrian, U.H.; Rosen, S.D. A major class of L-selectin ligands is eliminated in mice deficient in two sulfotransferases expressed in high endothelial venules. Nat Immunol 2005, 6, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Petryniak, B.; Hiraoka, N.; Mitoma, J.; Huckaby, V.; Nakayama, J.; Uchimura, K.; Kadomatsu, K.; Muramatsu, T.; Lowe, J.B.; et al. N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat Immunol 2005, 6, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Uchimura, K.; Kadomatsu, K.; El-Fasakhany, F.M.; Singer, M.S.; Izawa, M.; Kannagi, R.; Takeda, N.; Rosen, S.D.; Muramatsu, T. N-acetylglucosamine 6-O-sulfotransferase-1 regulates expression of L-selectin ligands and lymphocyte homing. J Biol Chem 2004, 279, 35001–35008. [Google Scholar] [CrossRef]
- Hemmerich, S.; Bistrup, A.; Singer, M.S.; van Zante, A.; Lee, J.K.; Tsay, D.; Peters, M.; Carminati, J.L.; Brennan, T.J.; Carver-Moore, K.; et al. Sulfation of L-selectin ligands by an HEV-restricted sulfotransferase regulates lymphocyte homing to lymph nodes. Immunity 2001, 15, 237–247. [Google Scholar] [CrossRef]
- Hayashida, Y.; Akama, T.O.; Beecher, N.; Lewis, P.; Young, R.D.; Meek, K.M.; Kerr, B.; Hughes, C.E.; Caterson, B.; Tanigami, A.; et al. Matrix morphogenesis in cornea is mediated by the modification of keratan sulfate by GlcNAc 6-O-sulfotransferase. Proc Natl Acad Sci U S A 2006, 103, 13333–13338. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Jung, S.; Zhao, J.; Kasinath, V.; Ichimura, T.; Joseph, J.; Fiorina, P.; Liss, A.S.; Shah, K.; Annabi, N.; et al. Simultaneous targeting of primary tumor, draining lymph node, and distant metastases through high endothelial venule-targeted delivery. Nano Today 2021, 36. [Google Scholar] [CrossRef]
- Habuchi, O.; Hirahara, Y.; Uchimura, K.; Fukuta, M. Enzymatic sulfation of galactose residue of keratan sulfate by chondroitin 6-sulfotransferase. Glycobiology 1996, 6, 51–57. [Google Scholar] [CrossRef]
- Fukuta, M.; Inazawa, J.; Torii, T.; Tsuzuki, K.; Shimada, E.; Habuchi, O. Molecular cloning and characterization of human keratan sulfate Gal-6-sulfotransferase. J Biol Chem 1997, 272, 32321–32328. [Google Scholar] [CrossRef]
- Patnode, M.L.; Yu, S.Y.; Cheng, C.W.; Ho, M.Y.; Tegesjo, L.; Sakuma, K.; Uchimura, K.; Khoo, K.H.; Kannagi, R.; Rosen, S.D. KSGal6ST generates galactose-6-O-sulfate in high endothelial venules but does not contribute to L-selectin-dependent lymphocyte homing. Glycobiology 2013, 23, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, H.; Foyez, T.; Ohtake-Niimi, S.; Takeda-Uchimura, Y.; Michikawa, M.; Kadomatsu, K.; Uchimura, K. KSGal6ST is essential for the 6-sulfation of galactose within keratan sulfate in early postnatal brain. J Histochem Cytochem 2014, 62, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Caterson, B.; Christner, J.E.; Baker, J.R. Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. The Journal of biological chemistry 1983, 258, 8848–8854. [Google Scholar] [CrossRef]
- Angelidis, I.; Simon, L.M.; Fernandez, I.E.; Strunz, M.; Mayr, C.H.; Greiffo, F.R.; Tsitsiridis, G.; Ansari, M.; Graf, E.; Strom, T.M.; et al. An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat Commun 2019, 10, 963. [Google Scholar] [CrossRef] [PubMed]
- Takeda-Uchimura, Y.; Nishitsuji, K.; Ikezaki, M.; Akama, T.O.; Ihara, Y.; Allain, F.; Uchimura, K. Beta3Gn-T7 Is a Keratan Sulfate beta1,3 N-Acetylglucosaminyltransferase in the Adult Brain. Front Neuroanat 2022, 16, 813841. [Google Scholar] [CrossRef] [PubMed]
- Uchimura, K.; El-Fasakhany, F.M.; Hori, M.; Hemmerich, S.; Blink, S.E.; Kansas, G.S.; Kanamori, A.; Kumamoto, K.; Kannagi, R.; Muramatsu, T. Specificities of N-acetylglucosamine-6-O-sulfotransferases in relation to L-selectin ligand synthesis and tumor-associated enzyme expression. J Biol Chem 2002, 277, 3979–3984. [Google Scholar] [CrossRef] [PubMed]
- Werlang, C.A.; Chen, W.G.; Aoki, K.; Wheeler, K.M.; Tymm, C.; Mileti, C.J.; Burgos, A.C.; Kim, K.; Tiemeyer, M.; Ribbeck, K. Mucin O-glycans suppress quorum-sensing pathways and genetic transformation in Streptococcus mutans. Nat Microbiol 2021, 6, 574–583. [Google Scholar] [CrossRef]
- Brown, G.M.; Huckerby, T.N.; Nieduszynski, I.A. Oligosaccharides derived by keratanase II digestion of bovine articular cartilage keratan sulphates. Eur J Biochem 1994, 224, 281–308. [Google Scholar] [CrossRef] [PubMed]
- Foyez, T.; Takeda-Uchimura, Y.; Ishigaki, S.; Narentuya; Zhang, Z.; Sobue, G.; Kadomatsu, K.; Uchimura, K. Microglial keratan sulfate epitope elicits in central nervous tissues of transgenic model mice and patients with amyotrophic lateral sclerosis. Am J Pathol 2015, 185, 3053–3065. [Google Scholar] [CrossRef]
- Bochner, B.S.; Alvarez, R.A.; Mehta, P.; Bovin, N.V.; Blixt, O.; White, J.R.; Schnaar, R.L. Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem 2005, 280, 4307–4312. [Google Scholar] [CrossRef]
- Tateno, H.; Crocker, P.R.; Paulson, J.C. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6’-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology 2005, 15, 1125–1135. [Google Scholar] [CrossRef]
- Propster, J.M.; Yang, F.; Rabbani, S.; Ernst, B.; Allain, F.H.; Schubert, M. Structural basis for sulfation-dependent self-glycan recognition by the human immune-inhibitory receptor Siglec-8. Proc Natl Acad Sci U S A 2016, 113, E4170–4179. [Google Scholar] [CrossRef]
- Bull, C.; Nason, R.; Sun, L.; Van Coillie, J.; Madriz Sorensen, D.; Moons, S.J.; Yang, Z.; Arbitman, S.; Fernandes, S.M.; Furukawa, S.; et al. Probing the binding specificities of human Siglecs by cell-based glycan arrays. Proc Natl Acad Sci U S A 2021, 118. [Google Scholar] [CrossRef]
- Jung, J.; Enterina, J.R.; Bui, D.T.; Mozaneh, F.; Lin, P.H.; Nitin; Kuo, C.W.; Rodrigues, E.; Bhattacherjee, A.; Raeisimakiani, P.; et al. Carbohydrate Sulfation As a Mechanism for Fine-Tuning Siglec Ligands. ACS Chem Biol 2021, 16, 2673–2689. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Vos, G.M.; Huang, C.; Chapla, D.; Kimpel, A.L.M.; Moremen, K.W.; de Vries, R.P.; Boons, G.-J. Exploiting Substrate Specificities of 6-O-Sulfotransferases to Enzymatically Synthesize Keratan Sulfate Oligosaccharides. JACS Au 2023. [Google Scholar] [CrossRef]
- Kiwamoto, T.; Katoh, T.; Evans, C.M.; Janssen, W.J.; Brummet, M.E.; Hudson, S.A.; Zhu, Z.; Tiemeyer, M.; Bochner, B.S. Endogenous airway mucins carry glycans that bind Siglec-F and induce eosinophil apoptosis. J Allergy Clin Immunol 2015, 135, 1329–1340 e1329. [Google Scholar] [CrossRef]
- Gonzalez-Gil, A.; Porell, R.N.; Fernandes, S.M.; Wei, Y.; Yu, H.; Carroll, D.J.; McBride, R.; Paulson, J.C.; Tiemeyer, M.; Aoki, K.; et al. Sialylated keratan sulfate proteoglycans are Siglec-8 ligands in human airways. Glycobiology 2018, 28, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gil, A.; Li, T.A.; Porell, R.N.; Fernandes, S.M.; Tarbox, H.E.; Lee, H.S.; Aoki, K.; Tiemeyer, M.; Kim, J.; Schnaar, R.L. Isolation, identification, and characterization of the human airway ligand for the eosinophil and mast cell immunoinhibitory receptor Siglec-8. J Allergy Clin Immunol 2021, 147, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, O.; Gong, L.; Zhang, J.; Hansen, J.K.; Hassan, R.; Lee, B.; Ho, M. A binding domain on mesothelin for CA125/MUC16. J Biol Chem 2009, 284, 3739–3749. [Google Scholar] [CrossRef]
- Rump, A.; Morikawa, Y.; Tanaka, M.; Minami, S.; Umesaki, N.; Takeuchi, M.; Miyajima, A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem 2004, 279, 9190–9198. [Google Scholar] [CrossRef]
- Gubbels, J.A.; Belisle, J.; Onda, M.; Rancourt, C.; Migneault, M.; Ho, M.; Bera, T.K.; Connor, J.; Sathyanarayana, B.K.; Lee, B.; et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer 2006, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Belisle, J.A.; Horibata, S.; Jennifer, G.A.; Petrie, S.; Kapur, A.; Andre, S.; Gabius, H.J.; Rancourt, C.; Connor, J.; Paulson, J.C.; et al. Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes. Mol Cancer 2010, 9, 118. [Google Scholar] [CrossRef]
- Das, S.; Batra, S.K. Understanding the Unique Attributes of MUC16 (CA125): Potential Implications in Targeted Therapy. Cancer Res 2015, 75, 4669–4674. [Google Scholar] [CrossRef]
- Hanson, R.L.; Hollingsworth, M.A. Functional Consequences of Differential O-glycosylation of MUC1, MUC4, and MUC16 (Downstream Effects on Signaling). Biomolecules 2016, 6. [Google Scholar] [CrossRef]
- Saldova, R.; Struwe, W.B.; Wynne, K.; Elia, G.; Duffy, M.J.; Rudd, P.M. Exploring the glycosylation of serum CA125. Int J Mol Sci 2013, 14, 15636–15654. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Sakai, Y.; Hoshino, H.; Umeda, Y.; Kawashima, H.; Sekido, Y.; Ishizuka, T.; Kobayashi, M. Sulfated Glycans Recognized by S1 Monoclonal Antibody can Serve as a Diagnostic Marker for Malignant Pleural Mesothelioma. Lung 2022, 200, 339–346. [Google Scholar] [CrossRef]
- Uchimura, K.; Kadomatsu, K.; Nishimura, H.; Muramatsu, H.; Nakamura, E.; Kurosawa, N.; Habuchi, O.; El-Fasakhany, F.M.; Yoshikai, Y.; Muramatsu, T. Functional analysis of the chondroitin 6-sulfotransferase gene in relation to lymphocyte subpopulations, brain development, and oversulfated chondroitin sulfates. J Biol Chem 2002, 277, 1443–1450. [Google Scholar] [CrossRef]
- Hosono-Fukao, T.; Ohtake-Niimi, S.; Hoshino, H.; Britschgi, M.; Akatsu, H.; Hossain, M.M.; Nishitsuji, K.; van Kuppevelt, T.H.; Kimata, K.; Michikawa, M.; et al. Heparan sulfate subdomains that are degraded by Sulf accumulate in cerebral amyloid ss plaques of Alzheimer’s disease: evidence from mouse models and patients. Am J Pathol 2012, 180, 2056–2067. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




